Supplemental digital content is available in the text.
Key Words: AGING, SPLIT-BELT WALKING, MARGIN OF STABILITY, LOCOMOTION
ABSTRACT
Introduction
The ability to adapt dynamic balance to perturbations during gait deteriorates with age. To prevent age-related decline in adaptive control of dynamic balance, we must first understand how adaptive control of dynamic balance changes across the adult lifespan. We examined how adaptive control of the margin of stability (MoS) changes across the lifespan during perturbed and unperturbed walking on the split-belt treadmill.
Methods
Seventy-five healthy adults (age range, 18–80 yr) walked on an instrumented split-belt treadmill with and without split-belts. Linear regression analyses were performed for the mediolateral (ML) and anteroposterior (AP) MoS, step length, single support time, step width, double support time, and cadence during unperturbed and perturbed walking (split-belt perturbation), with age as predictor.
Results
Age did not significantly affect dynamic balance during unperturbed walking. However, during perturbed walking, the ML MoS of the leg on the slow belt increased across the lifespan due to a decrease in bilateral single support time. The AP MoS did not change with aging despite a decrease in step length. Double support time decreased and cadence increased across the lifespan when adapting to split-belt walking. Age did not affect step width.
Conclusions
Aging affects the adaptive control of dynamic balance during perturbed but not unperturbed treadmill walking with controlled walking speed. The ML MoS increased across the lifespan, whereas bilateral single support times decreased. The lack of aging effects on unperturbed walking suggests that participants’ balance should be challenged to assess aging effects during gait. The decrease in double support time and increase in cadence suggests that older adults use the increased cadence as a balance control strategy during challenging locomotor tasks.
Control of dynamic balance maintains erect posture during walking. There is a need for continuous, online supervision of dynamic balance because the vertical projection of the center of mass (CoM) is outside the base of support (BoS) for approximately 80% of the gait cycle during walking (1). Adaptive control of dynamic balance helps cope with internal and external perturbations that the locomotor system encounters in daily life (2). With adaptive control, we refer to the recalibration of motor control in response to perturbations which reestablishes reliable and efficient task performance (3,4). The ability to adapt dynamic balance deteriorates with age, due to age-related changes in the sensorimotor system (5,6). This decline in adaptive control puts older adults at a higher risk for falls, as about 60% of outdoor falls among community-dwelling older adults are perturbation-related falls (7). To prevent age-related decline in adaptive control of dynamic balance during gait, we must first understand how adaptive control of dynamic balance changes across the adult lifespan.
One way to examine adaptability of dynamic balance is to have a continuous change in the speed of one of the two belts while walking on a split-belt treadmill. The difference in speed between the two belts initially causes an asymmetry in gait and requires adjustments in the movements of each leg. That is, adaptive control of dynamic balance is necessary to avoid a loss of balance and continue walking (4,8). Split-belt gait is an attractive perturbation paradigm to probe locomotor adaptability because it is sufficiently challenging yet virtually all healthy adults are able to complete the task (9,10).
Stable locomotion necessitates a spatial margin between the extrapolated CoM (XCoM), a parameter that incorporates CoM position and velocity, and the BoS, called the margin of stability (MoS) (4,8,11,12). A positive MoS indicates stable gait and a negative MoS indicates unstable walking, with a possible loss of balance that would require a corrective step (13). The MoS can be influenced by multiple gait variables (14,15). The anteroposterior (AP) MoS tends to increase with decreasing walking speed, by which AP XCoM excursion is reduced, or by increasing step length, by which the AP BoS is increased (15). The mediolateral (ML) MoS can become larger by decreasing bilateral single support time, by which ML XCoM excursion is reduced, and by increasing step width, by which the ML BoS is increased (12,14,15). Understanding adaptive control during gait of MoS therefore requires an understanding of changes in the variables that can influence MoS, that is, gait speed, step length, single support time, cadence, and step width.
Adaptive control of dynamic balance may deteriorate with age. In the present study, a lifespan approach will be used to investigate age-related changes in adaptive control of dynamic balance. A previous lifespan study (16) and a review (17) showed a gradual decline in ML local dynamic stability after age 40 yr and a gradual decline in AP root mean square trunk acceleration while walking. These studies provide novel insights into age-related change in balance control but did not control for walking speed, which declines with increasing age and affects MoS. Older adults’ gait is characterized by shorter and wider steps and increased stance and double support times (5,18), and these spatiotemporal parameters are closely related to walking speed (19). Therefore, to fully capture changes in adaptive control of dynamic balance across the lifespan, we used a fixed treadmill speed. Furthermore, although not directly related to MoS, adaptive balance mechanisms could involve a shift in the time spent in single support time and double support time with age. With aging, there could be a shortening of single support time and a prolongation of double support time. Alternatively, cadence could be increased to cope with timing differences.
The present study aims to determine the effects of aging on: 1) AP and ML MoS during unperturbed walking at a fixed treadmill speed; 2) AP and ML MoS when gait is perturbed during split-belt walking; 3) the variables (step length, single support time, step width) that influence the MoS; and 4) the variables double support time and cadence to investigate a possible shift in timing as a balance mechanism.
Figure 1 shows the hypotheses in graphical format. We hypothesize that: 1) AP MoS and ML MoS will, respectively, gradually decrease and increase with age during unperturbed walking; 2) when gait is perturbed by split-belt walking, AP MoS will decrease and ML MoS will gradually increase with age; 3) if there are age-related changes in gait parameters when walking speed is fixed, we expect step length and single support time to gradually decrease and step width to gradually increase with age; and 4) An increase in double support time or cadence with age.
FIGURE 1.
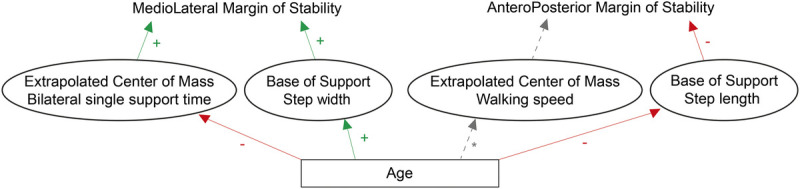
Graphical representation of the hypothesized changes across the lifespan. During unperturbed and perturbed walking, we expect step width to increase and bilateral single support time to decrease with increasing age. These age-related changes will cause the ML MoS to increase. With increasing age, step length will decrease, which will cause the AP MoS to decrease. The * next to the line from age to walking speed indicates that although there is a well-documented effect of age on walking speed, walking speed is fixed throughout the experiment, and thus there are no effects on walking speed.
METHODS
Participants
Healthy adults, 12 to 13 per decade, participated in the study (N = 75; age range, 18–79 yr). The table in Supplemental Digital Content 1 shows participant characteristics (see Table, Supplemental Digital Content 1, participant characteristics, http://links.lww.com/MSS/B961). Male and female participants were included if they were able to walk without walking aids and understood verbal instructions. Exclusion criteria were: 1) previous experience with split-belt walking; 2) orthopedic surgery in the last 2 yr; 3) neurological disorders; 4) (neuro)psychiatric disorders; 5) use of medication that might affect gait. The local ethical committee of the Center for Human Movement Sciences of the University Medical Center Groningen approved the study protocol. Before the measurements, all participants signed a written informed consent.
Instrumentation and protocol
Participants walked on an instrumented split-belt treadmill (Motek, Amsterdam, The Netherlands). The embedded force plates measured three-dimensional ground reaction forces and the center of pressure (CoP) at 1000 Hz.
Participants walked on the split-belt treadmill for 22 min, starting with the belts tied at 0.7 m·s−1 and 1.4 m·s−1 (Slow and Fast Baseline) for 3 min at each speed. Next, participants walked for 10 min with split-belts with one belt on 1.4 m·s−1, whereas the speed of the other belt was kept at 0.7 m·s−1. The first five steps of this adaptation phase are from here on referred to as initial perturbation and steps 6 to 30 are termed early change (20). After 16 min, participants returned to tied-belt walking at 0.7 m·s−1. The fast and slow speeds were randomly assigned to the left or right side per participant during split-belt walking. Participants were instructed to look straight ahead and not touch the handrails throughout the experiment (21).
Data analysis
All data were analyzed off-line with a custom Matlab code (R2015b; MathWorks, Natick, MA). Vertical ground reaction forces were filtered with a 15-Hz second-order low-pass Butterworth filter and missing data were interpolated. Gait events were determined at the moment when the vertical ground reaction forces crossed the threshold of 50 N on either force plate. To determine balance parameters, the CoM velocity and CoM position were obtained from the ground reaction forces which were divided by body mass to obtain the CoM acceleration (m·s−2). The CoM acceleration was double-integrated to obtain CoM position (m) and was high-pass filtered (0.2 Hz cutoff) to prevent integration drift. For the absolute CoM position, the previously obtained CoM position was supplemented with the 0.2-Hz low-pass Butterworth filtered CoP signal (22,23).
The XCoM position was determined with Equation 1 for both the AP as the ML directions (11). With l as leg length (m) and g as gravitational acceleration (9.81 m·s−1).
| [1] |
The MoS was defined as the distance between the CoP position and the XCoM position at contralateral toe-off for each step. The AP MoS is calculated along the x axis and the ML MoS along the z axis (4).
Step length was defined as the AP distance between the CoPx position of the left and right leg at heel-strike. Step length was normalized for leg length by dividing step length through leg length (24). Single support time was defined as the time from contralateral toe-off to contralateral heel-strike (14). Double support time was defined as the time from unilateral heel-strike to contralateral toe-off. Step width was specified as the difference between minimum/maximum ML CoP position of the ipsilateral single support, and minimum/maximum ML CoP of the consecutive contralateral single support for each step. Cadence was defined as the number of steps per minute.
Balance parameters were averaged over a number of steps for each of the four phases, fast baseline (last five steps), slow baseline (last five steps), initial perturbation (first five steps of adaptation), and early change (steps 6–30 of the adaptation) (20). During the baselines (fast, slow), all variables (except step width) were calculated for the left and right legs, and during split-belt walking (initial perturbation, early change), variables were calculated for the leg on the fast belt (fast leg) and the leg on the slow belt (slow leg).
Statistical analysis
To determine the effects of aging on AP and ML MoS during unperturbed walking (aim 1), linear regression analyses were performed for both parameters (AP/ML MoS) at baseline (fast, slow; controlled walking speed) with age as the predictor. To address the second research question, that is, effects of aging on adaptive control of the MoS, linear regression analyses were performed for AP and ML MoS during initial perturbation and early change with age as predictor. To address research questions 3 and 4, a similar linear regression analyses were performed on the variables that can influence the MoS (step length, single support time, double support time, step width, and cadence) during the four phases (fast baseline, slow baseline, initial perturbation, early change) with age as predictor. The data of all participants were checked for outliers through inspection of the normal probability plots of the residuals. No data were excluded from the analyses. To account for the 48 linear regression analyses that were performed, the critical P value was set at a Bonferroni-corrected α = 0.05/48 tests = 0.001042. The variance explained by the linear regression models was expressed with a coefficient of determination (R2).
If effects of age are found for AP or ML MoS in the linear regression analysis, a multiple linear regression analysis will be performed with the variables that can influence the AP/ML MoS to determine which of these variables explains most of the changes in the MoS with age. Because effects of age were only found for the ML MoS of the slow leg during the initial perturbation and early change phases, we performed a multiple linear regression where the ML MoS of the slow leg is predicted by single support time, double support time, and step width.
RESULTS
Tied-belt walking at fixed speeds
When walking on tied-belts during the fast baseline, cadence increased with 0.14 steps per minute per year from 18 to 79 yr (F[1,73] = 13.03, P < 0.001; Table 1). During the Slow Baseline, double support time of the left leg significantly decreased with 0.0007 s·yr−1 from 18 to 79 yr (F[1,73] = 12.55, P < 0.001) and cadence significantly increased with 0.18 steps per minute per year from 18 to 79 yr (F[1,73] = 11.77, P < 0.001).
TABLE 1.
Statistics (R2 and P values) of the linear regression models of the balance parameters with age as predictor for the four phases.
| Fast Baseline | Slow Baseline | Initial Perturbation | Early Change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | ||
| AP MoS | |||||||||
| Left | 0.0007 | 0.81 | 0.0003 | 0.88 | Fast | 0.05 | 0.05 | 0.08 | 0.02 |
| Right | 0.02 | 0.27 | 0.006 | 0.52 | Slow | 0.06 | 0.03 | 0.10 | 0.005 |
| Step length | |||||||||
| Left | 0.01 | 0.35 | 0.005 | 0.54 | Fast | 0.07 | 0.02 | 0.19 | <0.001 |
| Right | 0.00002 | 0.97 | 0.02 | 0.22 | Slow | 0.13 | 0.0013 | 0.15 | <0.001 |
| ML MoS | |||||||||
| Left | 0.07 | 0.02 | 0.12 | 0.003 | Fast | 0.03 | 0.17 | 0.09 | 0.008 |
| Right | 0.02 | 0.18 | 0.04 | 0.07 | Slow | 0.16 | <0.001 | 0.22 | <0.001 |
| Single support | |||||||||
| Left | 0.14 | 0.00109 | 0.09 | 0.008 | Fast | 0.08 | 0.01 | 0.26 | <0.001 |
| Right | 0.13 | 0.0015 | 0.04 | 0.07 | Slow | 0.17 | <0.001 | 0.31 | <0.001 |
| Double support | |||||||||
| Left | 0.04 | 0.10 | 0.15 | <0.001 | Fast | 0.09 | 0.008 | 0.22 | <0.001 |
| Right | 0.01 | 0.34 | 0.10 | 0.007 | Slow | 0.18 | <0.001 | 0.21 | <0.001 |
| Step width | 0.04 | 0.08 | 0.05 | 0.06 | — | 0.03 | 0.14 | 0.07 | 0.03 |
| Cadence | 0.15 | <0.001 | 0.14 | <0.001 | — | 0.23 | <0.001 | 0.36 | <0.001 |
R2 and P values are highlighted in bold if the regression line was significant after Bonferroni correction (α = 0.001042). During the fast and slow baseline, the variables were calculated for the left and right leg. During initial perturbation and early change, the variables were calculated for the fast and slow leg.
Effects of aging on adaptive control of the MoS during split-belt walking
During adaptation to walking with split-belts, the regression models for ML MoS of the slow leg revealed an increase of 0.035 cm·yr−1 from 18 to 79 yr in both the initial perturbation (F[1,73] = 13.46, P < 0.001) and early change phase (F[1,73] = 20.25, P < 0.001; Table 1, Fig. 2). Age explained respectively 16% and 22% of the variance in ML MoS slow during initial perturbation and early change. Aging did not affect AP MoS (P > 0.05).
FIGURE 2.
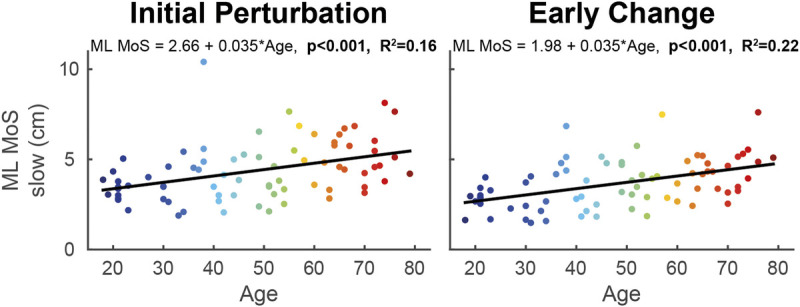
Linear regression models of ML MoS of the slow leg with age as predictor during the initial perturbation (steps 1–5 of split-belt walking; left panel) and early change (steps 6–30; right panel) phases. The color of the data points changes with increasing age. The formula of the regression line is given at the top of each graph. When the models were significant, the P value and R2 value of the model are given in bold.
Effects of aging on the variables that control the MoS
During early change, step length of the fast leg (F[1,73] = 19.52, P < 0.001; Table 1, Fig. 3) and step length of the slow leg (F[1,73] = 22.24, P < 0.001) decreased with 0.002 m·yr−1 across the lifespan.
FIGURE 3.
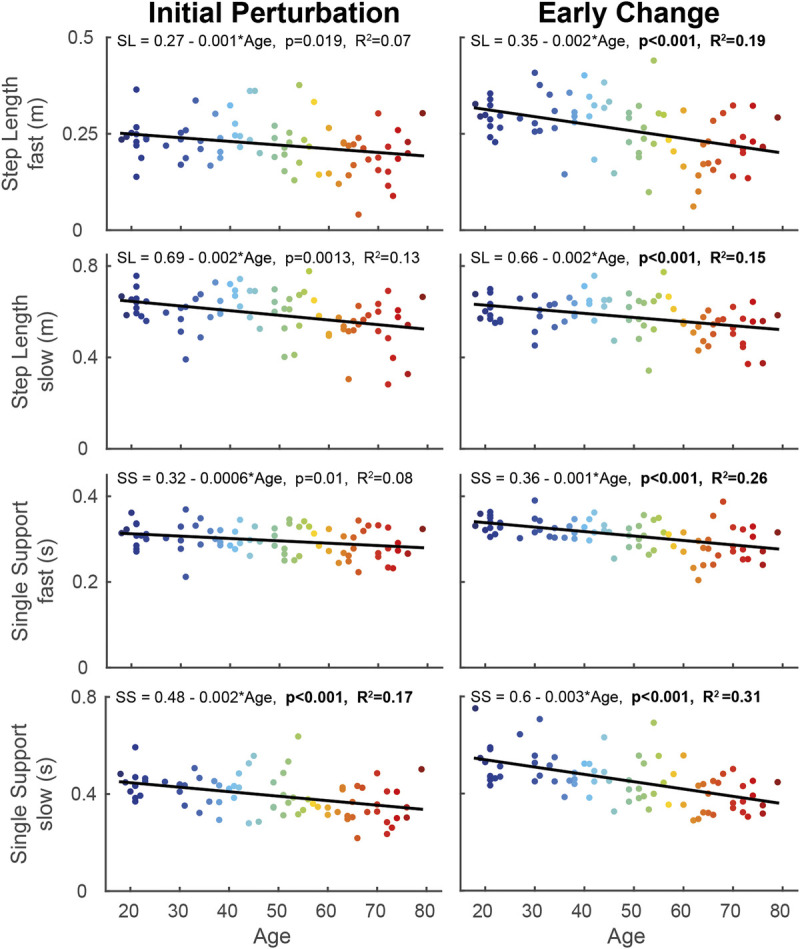
Linear regression models of step length and single support time of the fast and slow leg, with age as predictor during the initial perturbation (steps 1–5 of split-belt walking; left panel) and early change (steps 6–30 of split-belt walking; right panel) phases. The color of the data points changes with increasing age. The formula of the regression line is given at the top of each graph. When the models were significant, the P value and R2 value of the model are given in bold. SL, step length; SS, single support time.
Single support time of the fast leg decreased with 0.001 s·yr−1 from 18 to 79 yr in early change (F[1,73] = 24.99, P < 0.001; Table 1, Fig. 3). Single support of the slow leg decreased across the lifespan with 0.002 s·yr−1 in the initial perturbation (F[1,73] = 14.45, P < 0.001) and with 0.003 s in the early change phase (F[1,73] = 32.19, P < 0.001). No significant age effects were found for step width.
Double support of the fast leg decreased with 0.0006 s·yr−1 from 18 to 79 yr in early change (F[1,73] = 20.29, P < 0.001; Table 1, Fig. 4). The regression models for double support of the slow leg showed that double support time decreased with 0.001 s·yr−1 across the lifespan in both the initial perturbation (F[1,73] = 16.36, P < 0.001) and the early change phase (F[1,73] = 19.88, P < 0.001).
FIGURE 4.
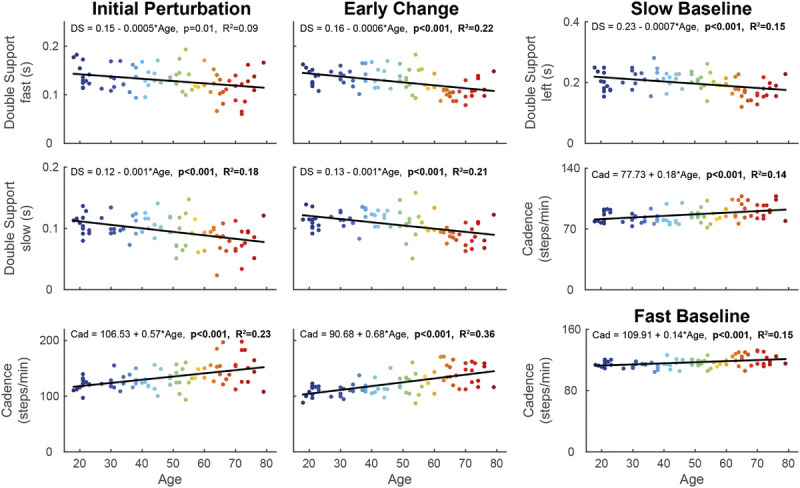
Linear regression models of double support time of the fast and slow leg and cadence, with age as predictor during the initial perturbation (steps 1–5 of split-belt walking; left panel), early change (steps 6–30 of split-belt walking; middle panel), and fast/slow baseline (right panel). The color of the data points changes with increasing age. The formula of the regression line is given at the top of each graph. When the models were significant, the P value and R2 value of the model are given in bold. DS, double support time; Cad, cadence.
Cadence increased across the lifespan with 0.57 steps per minute per year in initial perturbation (F[1,73] = 21.29, P < 0.001) and with 0.68 steps per minute in early change (F[1,73] = 40.66, P < 0.001; Table 1, Fig. 4).
Multiple linear regression for ML MoS of the slow leg
Multiple linear regression was used to predict ML MoS of the slow leg based on single support time, double support time, and step width during initial perturbation and early change. During initial perturbation, a significant regression equation was found (F[3,71] = 132.17, P < 0.001), with an R2 of 0.85. Both single support time and step width were significant predictors of ML MoS, but double support time was not. The participants’ predicted ML MoS is equal to 1.90 to 10.4SS + 0.32SW. During early change, a significant regression equation was found (F[3,71] = 118.45; P < 0.001), with an R2 of 0.83. Single support time, double support time, and step width were all significant predictors of ML MoS. The participants’ predicted ML MoS is equal to −8.12SS + 11.40DS + 0.28SW during early change.
DISCUSSION
We examined how adaptive control of dynamic balance, quantified by the MoS, changed across the lifespan during perturbed and unperturbed walking on a split-belt treadmill. Aging did not affect the ML and AP MoS during unperturbed walking. When balance was challenged by split-belt walking, aging affected the adaptive control of the ML MoS for the leg on the slow belt. This indicates that gait must be perturbed to assess age-related changes in adaptive control of dynamic balance, because these effects might not surface during unperturbed walking. Interestingly, step length, single support time, and double support time decreased across the lifespan when adapting to split-belt walking, whereas cadence increased with age. The cadence data suggest that as healthy humans age, they shorten their steps and their single and double support time to cope with balance perturbations. Figure 5 summarizes an interpretation of the results.
FIGURE 5.
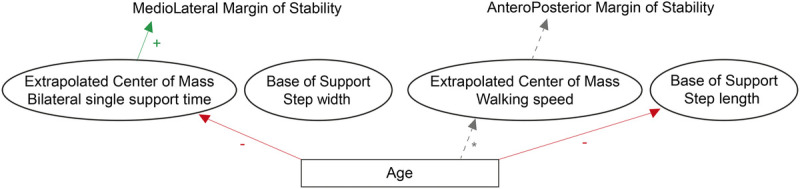
Graphical representation of the changes across the lifespan during perturbed walking (split-belt walking). With increasing age, the ML MoS of the slow leg increased due to decreased bilateral single support time. Step length decreased with age, but did not cause the AP MoS to change across the lifespan. The asterisk next to the line from age to walking speed indicates that although there is a well-documented effect of age on walking speed, walking speed is fixed throughout the experiment, and thus there are no effects on walking speed.
Changes in adaptive control of dynamic balance during walking across the lifespan
Adaptive control is important for dynamic balance during walking, as a recalibration of motor control in response to perturbations is necessary to maintain ambulant. A decline in adaptive control of dynamic balance with increasing age would make older adults more vulnerable to adverse outcomes, such as falls. During unperturbed walking with controlled walking speed, no age-related changes were found for the ML and AP MoS. The effects found were that left leg double support time decreased and cadence increased with age, suggesting that increasing cadence might be an age-specific strategy for the control of dynamic balance. Because walking speed is controlled, increasing cadence might resemble a similar strategy as decreasing walking speed with advancing age. Older adults could increase cadence as a way to reduce the propulsive impulse and to reduce the braking impulse (25). With increasing age, it may be more difficult to produce a propulsive impulse because there is an age-related distal-to-proximal shift (26) that may change the way older adults can use the plantarflexors, which provide forward propulsion (27). With increasing age, adults may reduce the braking impulse to more easily facilitate the contralateral forward propulsion and to reduce internal balance perturbations.
During perturbed walking, we observed that step length decreased 12 cm on average from 18 to 79 yr for both the fast and slow legs. This age-related decrease in step length, however, did not affect the AP MoS. As walking speed was similar for all participants, this, interestingly, means that the changes in step length were canceled out by reduced AP XCoM excursion, but not through reduced walking speed. This indicates that excursion of whole-body AP momentum may be reduced with age, leading to a reduction in AP XCoM excursion with age. Another possibility could be that the changes in step length are canceled out by the shortening of the single and double support duration.
During perturbed walking, the ML MoS of the slow leg increased 2 cm from age 18 to 79 yr. Single and double support time of both legs decreased with age, cadence increased with age, and no changes were seen across the lifespan for step width. The results suggest that the changes in ML MoS were due to age-related changes in bilateral single support times, as bilateral single support times are known to affect the ML MoS (14). This is confirmed by the multiple linear regression analysis, which showed that single support time and step width predict the changes in the ML MoS. No age-related changes were found for step width, but any change in step width might be limited in this study, as walking on a split-belt treadmill already causes adults to walk with wider steps (28). Because step width did not change with age, the changes in ML MoS are due to single support time. No age-related changes were found for step width, but any change in step width might be limited in this study, as walking on a split-belt treadmill already causes adults to walk with wider steps (28). The data, thus, suggest that changes in single support time in the slow and fast leg coincide with changes in adaptive control of the ML MoS across the lifespan. Future research is necessary to assess whether participants actively modify single support time to control the MoS, or if such changes are instead a side effect of changes in the temporal regulation of the gait cycle. The increase in ML MoS with advancing age may feel counterintuitive. Balance usually deteriorates with increasing age, and thus it may be expected that the ML MoS decreases with age. However, the magnitude of the ML MoS does not reflect balance quality per se, as a positive ML MoS is a condition for dynamic stability (11), but a large positive ML MoS may very well be a strategy to compensate poor balance control at the cost of a wide and inefficient gait pattern. This compensation for poor balance control is important, as it may offer an opportunity for fall prevention. This larger ML MoS might be utilized to prevent for example perturbation-related falls, which are a large part of the outdoor falls (7).
Unexpectedly, the age-related changes in the ML MoS, step length, single support time, double support time, and cadence were linear. These findings contrast the previous balance research that found either exponential changes (17) or quadratic changes after the age of 40 yr (16). The difference may be that both these studies did not control for walking speed, which affects how the age-related changes on other parameters progress.
Age-related changes in adaptive control of dynamic balance were most prominent in parameters of the limb that stood on the slow belt during split-belt walking. For the slow leg, ML MoS increased, whereas step length, single support time, and double support time decreased. Indeed, during split-belt walking, the slow leg has a longer stance time than the fast leg (29), and therefore, there is more time available to adjust balance parameters. Alternatively, with increasing age, humans may unload the perturbed leg (on the fast belt) more. The majority of weight-bearing during adaptive walking is then performed with the leg on the slow belt. Although both the single support time of the fast and slow leg decreased with age, the single support time is still longer on the slow versus fast side during split-belt walking.
Age-related changes were more prominent during early change (steps 6–30 of split-belt adaptation) than during initial perturbation (first five steps of split-belt adaptation). With increasing age, adults may need more steps to overcome the initial perturbation of changing belt speeds, whereas young adults start adapting to split-belt walking during early change. Such a longer lower rate of adaptation was previously found for the rate of adaptation of step length asymmetry (30,31). This could explain the most prominent age-related changes in the early change phase. Further research is necessary to investigate the rate of adaptation of dynamic balance, for instance by assessing the number over steps necessary to overcome the initial perturbation.
Controlling walking speed affects age-related changes in other gait parameters
Walking speed tends to decrease in old age (32). Because walking speed affects AP MoS, we fixed walking speed. We found no changes across the lifespan in AP MoS, ML MoS, single support time, step length, and step width during unperturbed treadmill walking. In overground walking studies with self-selected gait speed, age-related changes were reported, such as changes in step length, stride length, step width, stance time, and double support time (32–34). The difference between the present study and these overground studies could be due to the between-subject differences in walking speed in the overground studies. Therefore, the results of the current study emphasize the need to control walking speed to accurately assess age-related changes in adaptive control of dynamic balance. Because we controlled for walking speed, this allows us to unambiguously interpret changes in other gait parameters due to age, with only age-related changes in double support time and cadence.
The lack of age-related changes in balance parameters during normal walking could also be due to the fact that walking at comfortable speed or in a controlled laboratory environment is not challenging enough to show age effects on dynamic balance in a healthy population. Age-related changes were seen during perturbed walking but not during unperturbed walking. This suggests that when older adults face a task that more specifically requires a skill, in this case, maintaining dynamic balance, like walking with a visual perturbation (35), reacting to an ankle resistance perturbation while walking (36) or during split-belt walking (30,31), age-related changes in dynamic balance emerge that are not seen during unperturbed walking due to the age-related deteriorations in this particular skill. This should be taken into account in future studies designed to decipher how balance and gait changes across the healthy lifespan.
CONCLUSIONS
Aging affects the adaptive control of dynamic balance during perturbed treadmill walking. The ML MoS increased across the lifespan due to a decrease in bilateral single support times, that is, increased cadence, whereas aging did not affect AP MoS despite changes in step length. Furthermore, the age-related decreases in double support time along with the increases in cadence that were observed suggest that older adults prefer an increase of cadence as balance control strategy during challenging locomotor tasks. The finding that aging affects the adaptive control of dynamic balance during perturbed but not unperturbed treadmill walking suggests that adult’s balance should be challenged by a task that targets this specific skill to assess aging effects in dynamic balance. This may provide insight into the specific skill, testing dynamic balance, which should be the focus of, for example, fall prevention programs.
Supplementary Material
Acknowledgments
The authors would like to thank Lotte Knol, Timon Louwsma, Jorien Nijboer, and Rob van Renen for their help during the measurements, and Wim Kaan, Anniek Heerschop, Dirk van der Meer and Emyl Smid for their technical support. Lastly, the authors would like to thank the participants.
The authors declare no conflict of interest. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The present study does not constitute an endorsement by the American College of Sports Medicine.
There are no sources of funding to declare.
Availability of data and material: The data set used and analyzed in the current study is available from the corresponding author on reasonable request.
Footnotes
D. V. and T. J. W. B. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. 2nd ed University of Waterloo Press; 1991. 143 p. [Google Scholar]
- 2.Patla AE. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag. 2003;22(2):48–52. [DOI] [PubMed] [Google Scholar]
- 3.Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 2009;629:405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buurke TJW, Lamoth CJC, Vervoort D, Van der Woude LHV, den Otter R. Adaptive control of dynamic balance in human gait on a split-belt treadmill. J Exp Biol. 2018;221(13):jeb174896. [DOI] [PubMed] [Google Scholar]
- 5.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70(6):340–7. [DOI] [PubMed] [Google Scholar]
- 6.Woollacott MH, Tang P-F. Balance control during walking in the older adult: research and its implications. Phys Ther. 1997;77(6):646–60. [DOI] [PubMed] [Google Scholar]
- 7.Luukinen H, Herala M, Koski K, Honkanen R, Laippala P, Kivelä SL. Fracture risk associated with a fall according to type of fall among the elderly. Osteoporos Int. 2000;11(7):631–4. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Finley JM. Characterizing dynamic balance during adaptive locomotor learning. In: 2017 International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2017. pp. 50–3. [DOI] [PubMed] [Google Scholar]
- 9.Vervoort D, den Otter AR, Buurke TJW, Vuillerme N, Hortobágyi T, Lamoth CJC. Effects of aging and task prioritization on split-belt gait adaptation. Front Aging Neurosci. 2019;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vervoort D, den Otter AR, Buurke TJW, Vuillerme N, Hortobágyi T, Lamoth CJC. Do gait and muscle activation patterns change at middle-age during split-belt adaptation? J Biomech. 2020;99:109510. [DOI] [PubMed] [Google Scholar]
- 11.Hof AL, Gazendam MGJ, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38(1):1–8. [DOI] [PubMed] [Google Scholar]
- 12.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25(2):250–8. [DOI] [PubMed] [Google Scholar]
- 13.Hof AL. The “extrapolated center of mass” concept suggests a simple control of balance in walking. Hum Mov Sci. 2008;27(1):112–25. [DOI] [PubMed] [Google Scholar]
- 14.Buurke TJW, Lamoth CJC, Van der Woude LHV, Hof AL, den Otter R. Bilateral temporal control determines mediolateral margins of stability in symmetric and asymmetric human walking. Sci Rep. 2019;9(1):12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hak L, Houdijk H, Beek PJ, van Dieën JH. Steps to take to enhance gait stability: the effect of stride frequency, stride length, and walking speed on local dynamic stability and margins of stability. PLoS One. 2013;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait Posture. 2015;41(1):170–4. [DOI] [PubMed] [Google Scholar]
- 17.Iosa M, Fusco A, Morone G, Paolucci S. Development and decline of upright gait stability. Front Aging Neurosci. 2014;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrager MA, Kelly VE, Price R, Ferrucci L, Shumway-Cook A. The effects of age on medio-lateral stability during normal and narrow base walking. Gait Posture. 2008;28(3):466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. J Biomed Eng. 1985;7(4):282–8. [DOI] [PubMed] [Google Scholar]
- 20.Roemmich RT, Bastian AJ. Two ways to save a newly learned motor pattern. J Neurophysiol. 2015;113:3519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buurke TJW, Lamoth CJC, van der Woude LHV, den Otter R. Handrail holding during treadmill walking reduces locomotor learning in able-bodied persons. IEEE Trans Neural Syst Rehabil Eng. 2019;27(9):1753–9. [DOI] [PubMed] [Google Scholar]
- 22.Schepers HM, van Asseldonk E, Buurke JH, Veltink PH. Ambulatory estimation of center of mass displacement during walking. IEEE Trans Biomed Eng. 2009;56(4):1189–95. [DOI] [PubMed] [Google Scholar]
- 23.Hof AL. Comparison of three methods to estimate the center of mass during balance assessment. J Biomech. 2005;38(10):2134–5. [DOI] [PubMed] [Google Scholar]
- 24.Hof AL. Scaling gait data to body size. Gait Posture. 1996;4(3):222–3. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg EJ, Kautz SA, Neptune RR. Can treadmill walking be used to assess propulsion generation? J Biomech. 2008;41(8):1805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88(5):1804–11. [DOI] [PubMed] [Google Scholar]
- 27.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–98. [DOI] [PubMed] [Google Scholar]
- 28.Zeni JA, Jr, Higginson JS. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clin Biomech (Bristol, Avon). 2010;25(4):383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94(4):2403–15. [DOI] [PubMed] [Google Scholar]
- 30.Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. J Neurophysiol. 2012;108(4):1149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sombric CJ, Harker HM, Sparto PJ, Torres-Oviedo G. Explicit action switching interferes with the context-specificity of motor memories in older adults. Front Aging Neurosci. 2017;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samson MM, Crowe A, De Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging (Milano). 2001;13(1):16–21. [DOI] [PubMed] [Google Scholar]
- 33.McKay MJ Baldwin JN Ferreira P, et al. Spatiotemporal and plantar pressure patterns of 1000 healthy individuals age 3-101 years. Gait Posture. 2017;58:78–87. [DOI] [PubMed] [Google Scholar]
- 34.Herssens N, Verbecque E, Hallemans A, Vereeck L, Van Rompaey V, Saeys W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture. 2018;64:181–90. [DOI] [PubMed] [Google Scholar]
- 35.Francis CA, Franz JR, O’Connor SM, Thelen DG. Gait variability in healthy old adults is more affected by a visual perturbation than by a cognitive or narrow step placement demand. Gait Posture. 2015;42(3):380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrum C, Epro G, Meijer K, Zijlstra W, Brüggemann GP, Karamanidis K. Locomotor stability and adaptation during perturbed walking across the adult female lifespan. J Biomech. 2016;49(7):1244–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


