Abstract
Objectives
Serum levels of potassium (K+) appear to be significantly lower in severe cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the clinical significance of this is unknown. The objective was to investigate whether hypokalemia acts as a biomarker of severity in coronavirus disease 2019 (COVID-19) pneumonia and is associated with major clinical outcomes.
Methods
A retrospective cohort study of inpatients with COVID-19 pneumonia (March 3 to May 2, 2020) was performed. Patients were categorized according to nadir levels of K+ in the first 72 h of admission: hypokalemia (K+ ≤3.5 mmol/l) and normokalemia (K+ >3.5 mmol/l). The main outcomes were all-cause mortality and the need for invasive mechanical ventilation (IMV); these were analyzed by multiple logistic regression (odds ratio (OR), 95% confidence interval (CI)).
Results
Three hundred and six patients were enrolled. Ninety-four patients (30.7%) had hypokalemia and these patients showed significantly higher comorbidity (Charlson comorbidity index ≥3, 30.0% vs 16.3%; p = 0.02) and CURB65 scores (median (interquartile range): 1.5 (0.0–3.0) vs 1.0 (0.0–2.0); p = 0.04), as well as higher levels of some inflammatory parameters at baseline. After adjustment for confounders, hypokalemia was independently associated with requiring IMV during the admission (OR 8.98, 95% CI 2.54–31.74). Mortality was 15.0% (n = 46) and was not influenced by low K+. Hypokalemia was associated with longer hospital and ICU stays.
Conclusions
Hypokalemia is prevalent in patients with COVID-19 pneumonia. Hypokalemia is an independent predictor of IMV requirement and seems to be a sensitive biomarker of severe progression of COVID-19.
Keywords: COVID19 pneumonia, Hypokalemia, Mortality, Mechanical ventilation, Cohort study
Introduction
Early studies reported various electrolyte abnormalities at admission in patients who later presented the severe form of coronavirus disease 2019 (COVID-19). Electrolyte imbalance may not only affect patient care, but could provide insights into the pathophysiology of COVID-19. In a pooled analysis of five studies with a total sample size of 1415 patients, serum levels of potassium were reported to be significantly lower in COVID-19 patients with severe disease, (−0.12 mmol/l, 95% CI − 0.18 to −0.07 mmol/l) (Lippi et al., 2020).
Recently, Chen and colleagues published data showing a high prevalence of hypokalemia among a cohort of patients with COVID-19 in Wenzhou, China, and suggested the presence of disordered renin–angiotensin system (RAS) activity, which is altered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and that this may be associated with treatment outcomes (Chen et al., 2020a). Unfortunately, the authors limited the analysis to the description of the correlation between hypokalemia and clinical and laboratory parameters, without evaluating outcomes.
To provide insights into this issue, the prevalence of hypokalemia during hospitalization and its impact on major outcomes was examined retrospectively in patients hospitalized with COVID-19 in Alicante, Spain, one of the European countries most affected by the SARS-CoV-2 outbreak.
Materials and methods
Study design
This was a retrospective cohort study of patients with COVID-19 pneumonia hospitalized in an academic center in Spain. The study period was from March 3 to May 2, 2020. The Ethics Committee of HGUA-ISABIAL approved the study; as the study was retrospective, the need to obtain informed consent from participants was waived. The research was conducted according to the principles of the World Medical Association Declaration of Helsinki.
The diagnosis of COVID-19 required a positive reverse transcriptase PCR (RT-PCR) test for SARS-CoV-2, mainly in nasopharyngeal aspirates. Patients who did not undergo testing, those for whom testing was unavailable, and those with a negative result were not included in this study. Only one positive test was necessary for the patient to be included in the analysis. Criteria for hospital admission included advanced age, significant comorbidities, severe symptoms or poor clinical status, hypoxemia on room air (oximetry <94%, PaO2:FiO2 <300 mmHg), and/or significant radiological pulmonary opacities. Antiviral agents, anti-inflammatory drugs, and non-invasive ventilation were administered according to individual assessment under the hospital protocol. Intravenous tocilizumab (TCZ) was reserved for severe cases at admission and those with a progressive clinical deterioration.
Variables and data collection
Explanatory variables—associated factors
Data on patient demographic characteristics and coexisting conditions (based on International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes) were included in this analysis. Clinical information included age, sex, underlying coexisting conditions as noted in either the inpatient or outpatient electronic health record, and Charlson comorbidity index (CCI). Coexisting conditions included chronic obstructive pulmonary disease (COPD), immunosuppression, and a history of hypertension, diabetes mellitus, or underlying cardiovascular disease (including coronary artery disease, stroke, and peripheral vascular disease). Drug therapy for high blood pressure recorded at the time of hospital admission was also included, i.e., angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors.
Signs and symptoms at admission, the extent of opacities on the lung surface on X-ray or computed tomography, laboratory data (including C-reactive protein, procalcitonin, ferritin, interleukin 6, lactate dehydrogenase, D-dimer, troponin T, and brain natriuretic peptide), and treatments used (including diuretics) were obtained from the electronic medical records during admission.
According to the usual procedure at HGUA-ISABIAL, determination of the potassium level was conducted on plasma in the emergency laboratory, while it was conducted on serum during the hospital admission. The patients were classified into groups according to the nadir potassium level (K+) during the first 72 h of hospital admission: hypokalemia was considered a K+ level of ≤3.5 mmol/l and included severe hypokalemia (<3 mmol/l) and mild hypokalemia (3–3.5 mmol/l)); normokalemia was considered K+ of >3.5 mmol/l) (Chen et al., 2020b).
Outcomes
The primary outcome of interest was the association between explanatory variables and the endpoint of all-cause in-hospital mortality. The secondary outcome of interest was the association between explanatory variables and the requirement for invasive mechanical ventilation (IMV) in the intensive care unit (ICU).
Statistical analysis
The clinical features were compared among the three groups; categorical variables were recorded as the frequency (percentage) and continuous variables as the median (interquartile range (IQR)). The Mann-Whitney U-test and Chi-square test were used for group comparisons. The correlation between K+ nadir levels and K+ at admission was analyzed by Spearman’s rho.
The study population was categorized into two groups: maximum care (ICU and intubation as needed) and patients with limited therapeutic effort (LTE). The attending team agreed the suitable approach for each individual with their family, considering patient and disease characteristics. Since patients with LTE were not candidates for the ICU or for invasive measures, only the maximum care population was included in the logistic regression models for IMV.
Multiple logistic regression models were built to explore the association between hypokalemia and the clinical outcomes all-cause mortality, ICU admission, and IMV, and the odds ratios (OR) and 95% confidence intervals (CI) were estimated. Variables were included as covariates if they showed significant associations in simple models. Continuous variables were categorized on their 75th percentiles within each population, to show the impact of severe, extreme values on the outcomes, except for those in which severity is defined by the lowest levels, such as the lymphocyte count, where the 25th percentiles were used. For the following variables, standard categorizations were followed: age ≥65 years, CCI ≥ 3, estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 (by CKD-EPI formula), oximetry <94%, PaO2:FiO2 <300 mmHg (ARDS Definition Task Force et al., 2012), and CURB65 score ≥3 (Chalmers et al., 2010).
IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA) was used for the analyses; p < 0.050 defined statistical significance.
Results
Three hundred and six patients were enrolled; their median age was 65.0 years (IQR 51.0–77.0), 57.8% were male, and 53.3% had a CCI ≥ 3 (see Table 1 ). Regarding concomitant comorbidity, 46.2% were hypertensive, 38.8% were obese, and 21.1% had diabetes. The median evolution of the disease at the time of the first consultation was 7.0 days (IQR 4.0–11.0 days), and fever (72.1%) and dry cough (61.1%) were the most frequent clinical features.
Table 1.
Characteristics of the study population according to the potassium levelsa.
| Normokalemia (n = 212) | Mild hypokalemia (n = 76) | Severe hypokalemia (n = 18) |
p-Valuesb |
||||
|---|---|---|---|---|---|---|---|
| p1 | p2 | p3 | p4 | ||||
| Demographics | |||||||
| Age (median), years | 64.0 (46.8–77.0) | 65.0 (58.3–75.0) | 73.5 (53.5–80.0) | 0.266 | 0.448 | 0.241 | 0.123 |
| Comorbidities | |||||||
| Hypertension, % | 46.2 | 57.9 | 50.0 | 0.085 | 0.544 | 0.756 | 0.100 |
| ARA2/ACEI, % | 28.6 | 36.8 | 22.2 | 0.181 | 0.239 | 0.565 | 0.337 |
| Diabetes, % | 21.4 | 22.4 | 22.2 | 0.900 | 0.989 | 0.937 | 0.859 |
| Obesity, % | 38.3 | 31.0 | 17.6 | 0.376 | 0.273 | 0.091 | 0.108 |
| Cardiovascular disease, % | 13.8 | 9.2 | 16.7 | 0.266 | 0.356 | 0.738 | 0.445 |
| Charlson comorbidity index ≥3, % | 49.0 | 59.2 | 77.8 | 0.129 | 0.143 | 0.019* | 0.027* |
| Clinical presentation | |||||||
| Clinical duration, daysc | 7.0 (4.0–11.0) | 5.5 (3.0–8.0) | 3.0 (1.0–7.5) | 0.513 | 0.098 | 0.029* | 0.088 |
| Fever, % | 72.1 | 76.3 | 55.6 | 0.409 | 0.077 | 0.138 | 0.968 |
| Dry cough, % | 64.9 | 57.9 | 29.4 | 0.450 | 0.033* | 0.004* | 0.045* |
| Dyspnea, % | 57.2 | 48.7 | 38.9 | 0.253 | 0.454 | 0.133 | 0.093 |
| Diarrhea, % | 24.0 | 28.0 | 22.2 | 0.450 | 0.620 | 0.864 | 0.597 |
| Confusion, % | 11.2 | 14.7 | 22.2 | 0.511 | 0.434 | 0.170 | 0.239 |
| Fatigue, % | 39.9 | 43.2 | 35.3 | 0.565 | 0.549 | 0.709 | 0.765 |
| Myalgia/arthralgia, % | 29.7 | 21.6 | 11.8 | 0.244 | 0.358 | 0.115 | 0.075 |
| Anosmia/dysgeusia, % | 13.6 | 11.0 | 5.9 | 0.627 | 0.530 | 0.362 | 0.387 |
| Initial assessment | |||||||
| Oximetry on room air (%) | 95.0 (92.0–97.0) | 94.0 (91.0–97.0) | 94.5 (90.5–96.8) | 0.195 | 0.604 | 0.889 | 0.227 |
| PaO2:FiO2 | 333.3 (276.1–400.0) | 324.4 (277.3–371.4) | 323.0 (276.0–407.0) | 0.665 | 0.684 | 0.494 | 0.456 |
| Respiratory rate, breaths/min | 18.0 (16.0–24.0) | 18.0 (15.3–25.0) | 17.0 (16.0–27.0) | 0.775 | 0.848 | 0.890 | 0.862 |
| Systolic BP, mmHg | 130.0 (115.8–141.3) | 126.0 (108.5–153.5) | 132.0 (115.3–159.0) | 0.903 | 0.408 | 0.272 | 0.493 |
| Diastolic BP, mmHg | 78.5 (68.0–89.0) | 73.0 (64.0–84.0) | 81.5 (72.8–91.5) | 0.032* | 0.059 | 0.317 | 0.170 |
| Heart rate, beats/min | 94.0 (80.0–106.0) | 94.0 (83.0–103.0) | 96.5 (79.8–106.5) | 0.665 | 0.628 | 0.817 | 0.766 |
| CURB65 | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 2.0 (0.3–2.8) | 0.069 | 0.924 | 0.258 | 0.045* |
| eGFR, ml/min/m2 | 83.0 (56.2–90.0) | 78.7 (53.6–90.0) | 76.1 (37.5–90.0) | 0.972 | 0.335 | 0.293 | 0.586 |
| eGFR <60 mL/min/m2, % | 26.8 | 28.9 | 47.1 | 0.891 | 0.149 | 0.075 | 0.331 |
| Lymphocytes, 109 cells/liter | 1.09 (0.79–1.41) | 0.88 (0.64–1.31) | 1.08 (0.49–1.54) | 0.025* | 0.758 | 0.478 | 0.018* |
| C-reactive protein, mg/dl | 5.7 (2.5–11.9) | 7.7 (2.9–14.4) | 7.8 (2.0–15.2) | 0.256 | 0.931 | 0.623 | 0.231 |
| Procalcitonin, ng/mL | 0.10 (0.05–0.18) | 0.13 (0.06–0.20) | 0.22 (0.06–0.36) | 0.240 | 0.327 | 0.129 | 0.075 |
| Ferritin, mg/l | 618.0 (293.0–1184.0) | 961.0 (470.3–1442.5) | 796.5 (353.5–2935.0) | 0.008* | 0.905 | 0.160 | 0.002* |
| Interleukin 6, pg/mL | 21.50 (9.25–46.75) | 47.0 (18.50–69.75) | 28.0 (4.50–105.50) | 0.001* | 0.824 | 0.358 | 0.002* |
| Lactate dehydrogenase, U/l | 269.0 (218.0–360.0) | 274.0 (238.0–371.0) | 265.0 (240.0–413.0) | 0.218 | 0.971 | 0.686 | 0.198 |
| D-dimer, mg/mL | 0.59 (0.39–1.20) | 0.75 (0.46–1.52) | 0.85 (0.62–2.73) | 0.198 | 0.287 | 0.051 | 0.046* |
| Troponin T, ng/l | 9.0 (6.0–20.5) | 11.5 (7.0–22.8) | 27.0 (11.0–55.0) | 0.384 | 0.025* | 0.005* | 0.033* |
| Brain natriuretic peptide, pg/mL | 133.0 (30.8–693.0) | 176.0 (48.0–905.0) | 1502.0 (81.0–2964.0) | 0.312 | 0.072 | 0.014* | 0.040* |
| Creatine phosphokinase, U/l | 78.0 (51.0–143.5) | 87.5 (63.0–144.3) | 108.5 (34.3–449.5) | 0.422 | 0.715 | 0.597 | 0.317 |
| Opacities >50% of lung surface on X-ray, % | 24.7 | 19.4 | 18.8 | 0.438 | 0.953 | 0.592 | 0.327 |
ARA2/ACEI, angiotensin II receptor antagonists/angiotensin-converting enzyme inhibitors; BP, blood pressure; eGFR, estimated glomerular filtration rate. Data shown as % or median (interquartile range), unless specified otherwise. *p < 0.05, statistically significant difference.
Severe hypokalemia (K+ <3 mmol/l), mild hypokalemia (K+ 3–3.5 mmol/l), and normokalemia (K+ >3.5 mmol/l). Hypokalemia was defined as K+ ≤3.5 mmol/l (i.e., severe hypokalemia plus mild hypokalemia).
The Mann–Whitney U-test and Chi-square test were used for group comparisons: p1, mild hypokalemia vs normokalemia; p2, mild hypokalemia vs severe hypokalemia; p3, severe hypokalemia vs normokalemia; p4, hypokalemia vs normokalemia.
Days of symptoms before admission.
At the time of admission (in the emergency department), median K+ was 4.1 mmol/l (IQR 3.8–4.4 mmol/l); two patients (0.6%) presented with severe hypokalemia and 39 (12.7%) with mild hypokalemia.
The median (IQR) nadir K+ level in the first 72 h of hospital admission was 3.8 mmol/l (3.4–4.1 mmol/l). Ninety-four patients (30.7%) had hypokalemia, of whom 18 (5.9%) were classified as having severe hypokalemia and 76 (24.8%) as having mild hypokalemia. Two hundred and twelve patients (69.2%) presented normokalemia.
There was a positive correlation between K+ nadir levels and the K+ value on admission (Spearman’s rho = 0.636, p < 0.001).
Of the study population, 75/306 (24.5%) received diuretic treatment in the first 72 h of hospital admission. Of these, 78.7% were given loop diuretics (mainly furosemide), 16% thiazide diuretics, and 5.3% others. The use of diuretics in the first 72 h of hospital admission, globally as a group or by subtype, did not influence the onset of hypokalemia (25 diuretics/94 hypokalemia (26.5%) vs 50 diuretics/210 normokalemia (23.8%), p = 0.603) or severe hypokalemia (5 diuretics/18 severe hypokalemia (27.7%) vs 70 diuretics/286 non-severe hypokalemia (24.4%), p = 0.753).
The prevalence of hypokalemia was 60.4% (29/48) among patients admitted to the ICU and 65.8% (25/38) among those requiring IMV. Clinical outcomes of the study population according to the potassium levels are represented in Table 2 .
Table 2.
Clinical outcomes of the study population according to the potassium levelsa.
| Normokalemia (n = 212) | Mild hypokalemia(n = 76) | Severe hypokalemia (n = 18) |
p-Valuesb |
||||
|---|---|---|---|---|---|---|---|
| p1 | p2 | p3 | p4 | ||||
| Critical outcomes | |||||||
| ICU, % | 9.0 | 30.3 | 33.3 | 0.001* | 0.800 | 0.002* | 0.001* |
| IMV, % | 6.2 | 25.0 | 33.3 | 0.001* | 0.472 | 0.001* | 0.001* |
| Mortality rate, % | 16.2 | 15.8 | 16.7 | 0.977 | 0.927 | 0.958 | 0.959 |
| Length of stay | |||||||
| Length of admission, days | 7 (4–11) | 11 (8–18) | 16.5 (7.0–25.75) | 0.000* | 0.790 | 0.004* | 0.000* |
| Length of ICU, days | 8 (3.5–13.5) | 11 (7–31.5) | 15.0 (9.5–36.5) | 0.037* | 0.493 | 0.055* | 0.018* |
ICU, intensive care unit; IMV, invasive mechanical ventilation. Data shown as % or median (interquartile range), unless specified otherwise. *p < 0.05, statistically significant difference.
Severe hypokalemia (K+ <3 mmol/l), mild hypokalemia (K+ 3–3.5 mmol/l), and normokalemia (K+ >3.5 mmol/l). Hypokalemia was defined as K+ ≤3.5 mmol/l (i.e., severe hypokalemia plus mild hypokalemia).
The Mann–Whitney U-test and Chi-square test were used for group comparisons: p1, mild hypokalemia vs normokalemia; p2, mild hypokalemia vs severe hypokalemia; p3, severe hypokalemia vs normokalemia; p4, hypokalemia vs normokalemia.
The median length of stay was 9 days (IQR 5–14 days) and the median follow-up was 105 days (IQR 100–109 days).
Hypokalemia, baseline clinical features, and QTc monitoring
Characteristics of the study population according to the K + levels are represented in Table 1.
Patients with hypokalemia (i.e., K+ ≤3.5 mmol/l) had significantly higher comorbidity rates, CURB65 severity score for community-acquired pneumonia, and serum levels of ferritin, interleukin 6 (IL-6), D-dimer, troponin T, and brain natriuretic peptide at baseline. Furthermore, patients with hypokalemia and severe hypokalemia had a lower frequency of dry cough at the time of hospitalization. There was no difference in respiratory function parameters at admission (i.e., oximetry on room air, PaO2:FiO2, and respiratory rate).
Eighty-two patients received TCZ (Moreno-Pérez et al., 2020). In this subpopulation, the degree of hypokalemia was not associated with a higher Brescia-COVID Respiratory Severity Scale (BCRSS) (p = 0.347).
None of the patients in the cohort had ventricular arrhythmias.
A subpopulation of 78 patients treated with hydroxychloroquine, who had high-risk features, underwent close electrocardiographic monitoring. There was no difference in K+ between patients who presented some degree of QTc prolongation during follow-up when compared to those who did not (median: 4.0 (IQR 3.7–4.2) mmol/l vs 4.1 (IQR 3.8–4.3) mmol/l, p = 0.42).
Hypokalemia and treatment
The patients with hypokalemia also required the administration of TCZ (44.1% (41 TCZ/93 hypokalemia) vs 19.5% (41 TCZ/210 non-hypokalemia), p = 0.001) and corticosteroids (44.3% (39 corticosteroids/88 hypokalemia) vs 25.9% (53 corticosteroids/205 non-hypokalemia), p = 0.002) in a higher percentage.
Of the 94 patients with hypokalemia, 50% received K+ supplements, with a median maximum daily dosage of 40 mmol (IQR 30–60 mmol) and a median accumulated dose of 172 mmol (IQR 80–390 mmol).
Hypokalemia and clinical outcomes
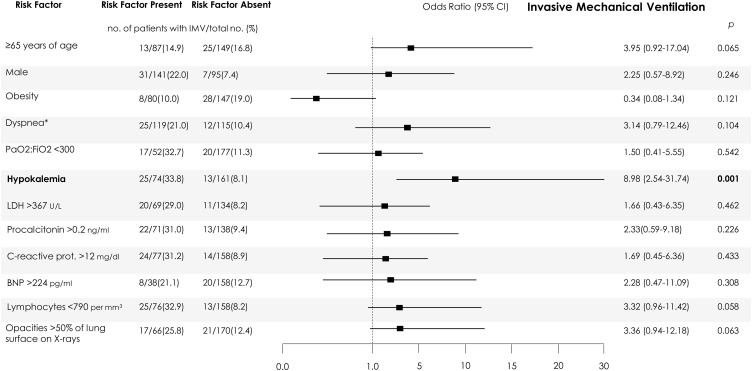
In patients who were candidates for the ICU (not LTE) (238/306), severe hypokalemia and mild hypokalemia (combined or not) were associated with ICU admission and the requirement for IMV: OR 4.48 (95% CI 2.35–8.53) and OR 5.49 (95% CI 2.66–11.33), for hypokalemia, respectively. After adjustment (Figure 1 ), only hypokalemia was independently associated with requiring IMV during the admission. Age ≥65 years, lymphocyte count <0.79 109 cells/liter, and extensive pulmonary opacities on chest X-ray showed a trend towards significance.
Figure 1.
Independent predictors of invasive mechanical ventilation in the maximum care population.
Numbers and percentages of patients with each risk factor who had the outcomes (risk factor present) and of patients without each risk factor with a favorable evolution (risk factor absent) are shown. The 95% confidence intervals (CIs) of the odds ratios have been adjusted for multiple testing. R2 models = 0.51 for invasive mechanical ventilation. Maximum care population refers to patients suitable for intensive measures and ICU admission, if necessary. Independent predictors associated with the outcomes are shown in bold. *On admission. LDH, lactate dehydrogenase; prot., protein; BNP, brain natriuretic peptide. For the purpose of logistic regression, variables were categorized regarding their 75th percentiles within each subpopulation, to show the impact of severe extreme values on the outcomes, except for those in which severity is defined by the lowest levels, such as lymphocyte count, where the 25th percentiles were used. For the following variables, standard categorizations were followed: age ≥65 years, PaO2:FiO2 <300 mmHg.
Mortality was 15.0% (n = 46) and was not influenced by low potassium levels.
Severe hypokalemia and hypokalemia were associated with a longer length of hospital and ICU stay.
Hypokalemia in the emergency area was not associated with clinical outcomes.
The final outcomes of ICU admission, IMV requirement, and a fatal outcome were not influenced by K+ replacement.
Discussion
This study confirmed the high prevalence of hypokalemia (one-third) in patients with COVID-19, highlighting its association with critically ill patients, i.e. the requirement for IMV (two-thirds) and longer length of hospital and ICU stays. The degree of hypokalemia was associated with some clinical features that reflected the severity of the disease, such as the CURB65 index and inflammatory markers at admission. These associations, together with the fact that nadir hypokalemia in the first 72 h of hospital admission was the only predictor of IMV requirement after adjusting for confounders, establish K+ levels as a sensitive dynamic biomarker of severe COVID-19 and suggest the disruption of the RAS system by SARS-CoV-2 (Kunutsor and Laukkanen, 2020).
Three notable aspects reinforced the development of hypokalemia as an independent early marker of an unfavorable evolution of COVID-19, without an etiopathogenic role. First, hypokalemia in the emergency area was not associated with clinical outcomes. Thus, K+ levels seem to show a dynamic behavior that parallels the evolution of the disease. Second, the therapeutic approaches initiated at admission did not influence the appearance of hypokalemia. The greater use of corticosteroids in patients with low K+ levels was conditioned by the severity of the underlying condition; of the 39 patients with hypokalemia who received corticosteroids, 26 (67%) received them after 72 h of admission, without any difference in the prevalence of hypokalemia when compared to those who received them within the first 72 h of admission (p = 0.24). Also, the use of diuretics (either as a group or focusing on loop diuretics) showed no impact on the appearance of hypokalemia or severe hypokalemia. Lastly, the final outcomes of ICU admission, IMV requirement, and a fatal outcome were not influenced by K+ replacement.
Limited data are available on serum K+ levels in patients with COVID-19 (Lippi et al., 2020). Previously, Chen et al. (2020) reported a 54% prevalence of hypokalemia in a younger cohort, with only three of the 175 patients included requiring IMV. They also reported that only 10 patients had plasma K+ >4 mmol/l, which is the recommended concentration for patients with myocardial dysfunction, compared to 60.9% of the present study population at the time of admission, which remained above the target in 37.2% of patients throughout their hospital stay. Considering the differences between the two studies, genetic factors such as population polymorphisms and the chosen therapeutic approach could explain these discrepancies. East Asian populations have much higher allele frequency in the expression quantitative trait loci variants associated with higher angiotensin-converting enzyme 2 (ACE2) expression in tissues, which may suggest a variable susceptibility or response to SARS-CoV-2 of different populations under similar conditions (Cao et al., 2020, Devaux et al., 2020), and could partially explain the differences in hypokalemia prevalence in Asian and European populations.
Unfortunately, other evidence of the impact of electrolyte disturbances on adverse outcomes is limited to cohort studies that were not designed to assess this association. Huang et al. (Huang et al., 2020), in a case series study of 41 patients, reported a mean serum K+ level of 4.2 mmol/l and found higher K+ levels in patients in the ICU (mean 4.6 mmol/l) than in patients who were not admitted to the ICU (mean 4.1 mmol/l), suggesting that elevated serum K+ is associated with the severity of illness. Chen et al. (2020) described hyperkalemia as a common complication observed more frequently in patients who died (37% of 113 who died versus 14% of 161 patients who recovered), while not providing data on the prevalence of hypokalemia.
In non-COVID-19 populations, the presence of hypokalemia at admission in hospitalized patients is not common. Neither is it prevalent in patients with severe bacterial pneumonia. Thongprayoon et al. (2019) studied 73 983 hospitalized patients (without end-stage kidney disease) and the mean admission K+ level was 4.2 ± 0.5 mmol/l; 0.9% had K+ <2.9 mmol/l and 5% had K+ 3.0–3.4 mmol/l. Regardless of the main diagnosis, both hypokalemia and hyperkalemia were associated with an increased 1-year mortality rate when compared to the normokalemia group, and this mortality risk increased in accordance with the severity of the hypokalemia or hyperkalemia.
This U-shaped risk relationship between potassium levels and all-cause and cardiovascular mortality is maintained in ambulatory patients across the range of kidney function (Kovesdy et al., 2018). In a meta-analysis of 27 international cohorts, at the individual data level, compared with a reference K+ level of 4.2 mmol/l, the adjusted hazard ratio for all-cause mortality was 1.22 (95% CI 1.15–1.29) at 5.5 mmol/l and 1.49 (95% CI 1.26–1.76) at 3.0 mmol/l. Patient risks were similar regardless of eGFR, use of RAS inhibitors, and across cohorts, and pointed in the same direction as the present study data.
The primary hypothesis is that hypokalemia is likely linked to a decreased activity of receptor ACE2 (Guo et al., 2020). SARS-CoV-2 is thought to infect host cells through ACE2 to cause COVID-19 (Hoffmann et al., 2020), promoting ACE2 depletion and an imbalance of the RAS and ACE2/angiotensin 1–7 axis, with marked elevations of deleterious angiotensin II levels. These changes would promote vasoconstriction and proinflammatory–profibrotic effects, and lead to an increased reabsorption of sodium and water, thereby increasing blood pressure and potassium excretion (K+) (Weir and Rolfe, 2010).
The lower prevalence of dry cough in patients with hypokalemia and severe hypokalemia, in turn, supports the role of compromised ACE2 activity; whether this is a true or random relationship should be explored in future research. ACE2 is involved in the synthesis of bradykinins; in fact, patients with angiotensin converting enzyme inhibitors and activated ACE2 have higher concentrations of angiotensin 1–7 and bradykinins, which contribute to dry cough (Nicolau et al., 2020). Thus, the low frequency of dry cough in patients with hypokalemia could result from the low ACE2 activity mediated by SARS-CoV-2.
The role of gastrointestinal symptoms in the development of hypokalemia is not supported by the present study results, in accordance with cohort data from China (Chen et al., 2020a).
Aside from being a marker of severity, the clinical relevance of hypokalemia in this clinical setting lies in the fact that it would potentially contribute to myocardial dysfunction, ventricular arrhythmia (Bielecka-Dabrowa et al., 2012), and respiratory muscle dysfunction. Nonetheless, the impact of hypokalemia on myocardial and respiratory function in COVID-19 patients is not clear, and direct evidence demonstrating that SARS-CoV-2 infects the human heart and decreases ACE2 expression is currently lacking. In these regards, Shi et al. (Shi et al., 2020) reported no difference in median potassium level between patients with and without cardiac injury, in a cohort of 416 hospitalized patients with COVID-19.
Regarding the limitations of this study, this was an observational, retrospective, single-center study, and the collection of data was not standardized in advance. The long follow-up and no losses reinforce the present data. Multiple factors can condition changes in K+ during hospitalization, including the use of beta-lactam antibiotics and hydroxychloroquine (Heijden et al., 2019, Clemessy et al., 1995). Nonetheless, the prevalence of hypokalemia in patients who received ceftriaxone (the main beta-lactam prescribed in our series) was similar to that reported for the whole cohort (36% vs 30%), and for patients who received hydroxychloroquine (94%), the dose prescribed (200 mg every 12 h) was less than the 10 mg/kg/day described in the literature as causing hypokalemia (Clemessy et al., 1995). Finally, the absence of acid–base metabolism data hampers the interpretation of the results.
In summary, the high prevalence of hypokalemia among patients with COVID-19 in this Mediterranean cohort suggests the presence of a disorder in RAS activity that is linked to severe SARS-CoV-2 infection. Besides, this sensitive biomarker may reflect the progression of COVID-19. Hypokalemia was found to be independently associated with the requirement for IMV after adjusting for confounders and should be closely monitored to guide timely treatment. Considering the implications of serum K+ concentrations in this disease, further investigations are necessary.
Author contributions
Oscar Moreno-Pérez, Esperanza Merino, Miguel Perdiguero: conception and design, analysis and interpretation of the data, drafted the article, provided intellectual content of critical importance to the work described, approved the final version to be published. Jose-Manuel Leon-Ramirez, Laura Fuertes-Kenneally, Mariano Andres, Mar Garcia-Navarro, Paloma Ruiz-Torregrosa, Vicente Boix, Joan Gil: interpreted the data, revised the article, provided intellectual content of critical importance to the work described, approved the final version to be published.
Funding sources
There were no sources of funding for this research work.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgement
The members of the COVID19-ALC Research Group are: Santos Asensio, Cleofé Fernandez, Alfredo Candela, Mª del Mar García, Rosario Sánchez, Sergio Reus, Paloma Ruiz, Raquel García-Sevila, María-Ángeles Martínez, María-Mar García-Mullor, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabil, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, Mari-Carmen López-González, Blanca Figueres.
Contributor Information
On behalf of COVID19-ALC Research Group:
Santos Asensio, Cleofé Fernandez, Alfredo Candela, Mª del Mar García, Rosario Sánchez, Sergio Reus, Paloma Ruiz, Raquel García-Sevila, María-Ángeles Martínez, María-Mar García-Mullor, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, and Elena Vives
References
- ARDS Definition Task Force, Marco Ranieri V., Rubenfeld Gordon D., Taylor Thompson B., Ferguson Niall D., Caldwell Ellen, Fan Eddy, Camporota Luigi, Slutsky Arthur S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa Agata, Mikhailidis Dimitri P., Jones Linda, Rysz Jacek, Aronow Wilbert S., Banach Maciej. The Meaning of Hypokalemia in Heart Failure. Int J Cardiol. 2012;158(1):12–17. doi: 10.1016/j.ijcard.2011.06.121. [DOI] [PubMed] [Google Scholar]
- Cao Yanan, Li Lin, Feng Zhimin, Wan Shengqing, Huang Peide, Sun Xiaohui, Wen Fang, Huang Xuanlin, Ning Guang, Wang Weiqing. Comparative Genetic Analysis of the Novel Coronavirus (2019-NCoV/SARS-CoV-2) Receptor ACE2 in Different Populations. Cell Discovery. 2020;6(1):11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers James D., Singanayagam Aran, Akram Ahsan R., Mandal Pallavi, Short Philip M., Choudhury Gourab, Wood Victoria, Hill Adam T. Severity Assessment Tools for Predicting Mortality in Hospitalised Patients with Community-Acquired Pneumonia. Systematic Review and Meta-Analysis. Thorax. 2010;65(10):878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- Dong Chen, Li Xiaokun, Song Qifa, Hu Chenchan, Su Feifei, Dai Jianyi, Ye Yinghai, Huang Jianping, Zhang Xiaoming. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Network Open. 2020;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Tao, Wu Di, Chen Huilong, Yan Weiming, Yang Danlei, Chen Guang, Ma Ke. Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019: Retrospective Study. BMJ. 2020 doi: 10.1136/bmj.m1091. March, m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemessy J.-L., Borron S.W., Baud F.J., Favier C., Hantson P.E., Vicaut E. Hypokalaemia Related to Acute Chloroquine Ingestion. Lancet. 1995;346(8979):877–880. doi: 10.1016/S0140-6736(95)92711-5. [DOI] [PubMed] [Google Scholar]
- Devaux Christian A., Rolain Jean-Marc, Raoult Didier. ACE2 Receptor Polymorphism: Susceptibility to SARS-CoV-2, Hypertension, Multi-Organ Failure, and COVID-19 Disease Outcome. J Microbiol Immunol Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Junyi, Huang Zheng, Lin Li, Jiagao Lv. Coronavirus Disease 2019 (COVID‐19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin‐Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijden Charlotte D.C.C., Duizer Marleen L., Fleuren Hanneke W.H.A., Veldman Bart A., Sprong Tom, Dofferhoff Anton T.S.M., Kramers Cornelis. Intravenous Flucloxacillin Treatment Is Associated with a High Incidence of Hypokalaemia. Br J Clin Pharmacol. 2019;85(12):2886–2890. doi: 10.1111/bcp.13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann Markus, Kleine-Weber Hannah, Schroeder Simon, Krüger Nadine, Herrler Tanja, Erichsen Sandra, Schiergens Tobias S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Chaolin, Wang Yeming, Li Xingwang, Ren Lili, Zhao Jianping, Hu Yi, Zhang Li. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdy Csaba P., Matsushita Kunihiro, Sang Yingying, Brunskill Nigel J., Carrero Juan J., Chodick Gabriel, Hasegawa Takeshi. Serum Potassium and Adverse Outcomes across the Range of Kidney Function: A CKD Prognosis Consortium Meta-Analysis. Eur Heart J. 2018;39(17):1535–1542. doi: 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor Setor K., Laukkanen Jari A. Renal Complications in COVID-19: a systematic review and meta-analysis. Annals of Medicine. 2020:1–9. doi: 10.1080/07853890.2020.1790643. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi Giuseppe, South Andrew M., Michael Henry Brandon. Electrolyte Imbalances in Patients with Severe Coronavirus Disease 2019 (COVID-19) An Clin Biochem: Int J Lab Med. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez Oscar, Andres Mariano, Leon-Ramirez Jose-Manuel, Sánchez-Payá José, Rodríguez Juan Carlos, Sánchez Rosario, García-Sevila Raquel, Boix Vicente, Gil Joan, Merino Esperanza. Experience with Tocilizumab in Severe COVID-19 Pneumonia after 80 Days of Follow-up: A Retrospective Cohort Study. J Autoimmunity. 2020:102523. doi: 10.1016/j.jaut.2020.102523. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau Lucas A.D., Magalhães Pedro J.C., Vale Mariana L. What Would Sérgio Ferreira Say to Your Physician in This War against COVID-19: How about Kallikrein/Kinin System? Med Hypotheses. 2020;143(October):109886. doi: 10.1016/j.mehy.2020.109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Shaobo, Qin Mu, Shen Bo, Cai Yuli, Liu Tao, Yang Fan, Gong Wei. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprayoon Charat, Cheungpasitporn Wisit, Hansrivijit Panupong, Mao Michael A., Medaura Juan, Bathini Tarun, Chewcharat Api, Erickson Stephen B. Admission Serum Potassium Levels in Hospitalized Patients and One-Year Mortality. Medicines. 2019;7(1):2. doi: 10.3390/medicines7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir Matthew R., Rolfe Mark. Potassium Homeostasis and Renin-Angiotensin-Aldosterone System Inhibitors. Clin J Am Soc Nephrol. 2010;5(3):531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]