Abstract
Sandflies are insects of public health interest due to their role as vectors of parasites of the genus Leishmania, as well as other pathogens. Psychodopygus carrerai carrerai is considered an important sylvatic vector of Leishmania (Viannia) braziliensis in Amazonia. In this study, sandflies were collected in a forested area in the Xapuri municipality, in the State of Acre (Northern Brazil). Two Ps. carrerai carrerai females were found parasitized with a larval form of a filarial worm, one in the labium of the proboscis, the other after the head was squashed, suggesting they were infective larvae. Sandflies were identified through morphological characters as well as amplification and sequencing of the cytochrome oxidase gene (COI). This was the first sequence obtained for Ps. carrerai carrerai for this marker. The obtained nematodes were also characterized through direct sequencing of a fragment of COI and 12S genes, both mitochondrial, and ITS1, a nuclear marker. Phylogenetic analyses revealed that the filarial nematodes belong to a species without sequences for these markers in the database, part of family Onchocercidade and closely related to genus Onchocerca (12S tree). Although sandfly infection with nematodes including members of the Onchocercidae has been reported in the Old World, this is the first report of sandfly infection by a member of the Onchocercidae family in the New World, to the best of our knowledge. Considering that the phylogenetic relationships and location in the insect, it can be expected that this is a parasite of mammals and the transmission cycle should be clarified.
Subject terms: Entomology, Parasitic infection
Introduction
Phlebotomine sandflies are dipterans of medical significance due to their role in the transmission of the aetiological agents of leishmaniasis and bartonellosis, as well as of arboviruses1. The greatest sandfly diversity in the Americas is found in the Amazon region2. An important sylvatic vector of American cutaneous leishmaniasis in the Bolivian Amazon rainforest3 is the sandfly Psychodopygus carrerai carrerai (Barretto, 1946). This species is anthropophilic and associated with primary forest environments, where it has been found to be naturally infected by Leishmania (Viannia) braziliensis. In addition to Leishmania, other protozoa, such as trypanosomatids from the genera Endotrypanum and Trypanosoma4 and from the phyla Apicomplexa, Ascogregarina and Psychodiella5,6 have been reported to infect sandflies. Infections by nematodes have rarely been reported7. Nonetheless, Madathamugadia wanjii (fam. Onchocercidae) in Phlebotomus duboscq8, Didilia sp., Didilia ooglypta, members of the Tylenchoidea superfamily, in Phlebotomus papatasi and Phlebotomus sergenti9–12 and members of the Steinernematidae family in Phlebotomus tobbi13 have been reported in the Old World. In South America, natural infections by Anandarema phlebotophaga (Allantonematidae: Tylenchida)14 and other nematodes, tentatively assigned to family Steinernematidae15, have been observed in colonies of Lutzomyia longipalpis. Additionally, in Argentina, natural populations of Pintomyia fischeri have been found infected by Tylenchid nematodes16. Unfortunately, few DNA sequences are available from these nematodes found in sandflies, which means that phylogenetic comparison between isolates and reports is very difficult.
Family Onchocercidae includes worms of the genus Onchocerca, which comprises 28 species. In humans, Onchocerca volvulus causes onchocerciasis17, also known as river blindness, while O. lupi is a mainly zoonotic parasite of dogs18,19. Other species such as O. gutturosa, O. gibsoni, O. cervicalis and O. ochengi are of veterinary importance in ruminants, horses and dogs20. Species of this genus are usually transmitted by simulid species (blackflies). Other members of this family include the agents of lymphatic filariasis (Wuchereria bancrofti and species of the genus Brugia) and mainly zoonotic parasites, of the genera Dirofilaria, Mansonella, Acanthocheilonema, among others21.
In a recent survey of American cutaneous leishmaniasis (ACL) and sandflies vectors performed in Xapuri, two Ps. carrerai carrerai females were found parasitized with a filarial larval form, whose infection is here reported. Xapuri is a municipality located in Acre State, Brazil, and it is an area of high prevalence of ACL with a high diversity of sandflies species22,23. There is no record of parasitic diseases caused by filarids in the municipality of Xapuri. However, in the surrounding municipalities, the occurrences of Mansonella ozzardi and Onchocerca volvulus infecting indigenous and riverside populations have been reported24–26, and recently, Wuchereria bancrofti, the filarial worm of lymphatic filariasis, transmitted by mosquitoes, has been identified in immigrants from Haiti27.
Results
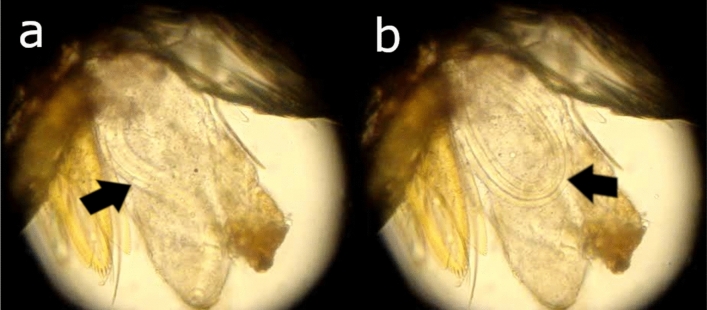
A total of 2,643 specimens of Ps. carrerai carrerai (2,233 females and 410 males) were collected, of which 139 females were dissected for trypanosomatids detection. Two females were found to be parasitized by a nematode larva. One of these nematodes was found on July 31, 2015, in the labium of the proboscis of the sandfly, while the other was only observed after the insect head was squashed on March 10, 2016 (Fig. 1 and Supplementary video). Ecological analyses of the phlebotomine fauna have been published elsewhere23.
Figure 1.
Microscopy photograph of the head of a specimen Ps. carrerai carrerai female with the presence of an Onchocercidae worm larva. (A) Larvae in a linear conformation, and (B) Larvae in a round conformation.
The two sandflies samples were unambiguously identified as Ps. carrerai carrerai, based on morphological criteria. The PCR amplification of COI, using primers LCO1490 and HCO2198, from these two sandflies resulted in amplicons with the expected molecular weight (658 bp). The obtained sequences (GenBank MG029462 and MG029463) were identical for both specimens and had, upon a BLAST search, 90% nucleic acid identity with at least three different species of sandflies: Ps. hirsutus hirsutus, Lutzomyia carrerai thula, and Psathyromyia pascalei (Table 1).
Table 1.
Sequencing results showing the BLAST (Megablast option, except where indicated) homology of sandfly and filarial parasite sequences obtained from Xapuri—Rio Branco in relation to sequences found in GenBank.
| Molecular marker | Query sequence | Match | Identity | GenBank accession |
|---|---|---|---|---|
| COI |
Sandfly (658 bp) |
Psychodopygus (syn. Lutzomyia) hirsutus | 595/658 (90%) | KP112991.1 |
| Psychodopygus (syn. Lutzomyia) thula | 592/657 (90%) | KC921238.1 | ||
| Psathyromyia (syn. Lutzomyia) pascalei | 589/658 (90%) | KP112959.1 | ||
|
Parasite (649 bp) |
Onchocerca gibsoni | 587/648 (91%) | AJ271616.1 | |
| Onchocerca volvulus | 587/649 (90%) | KC167355.1 | ||
| Onchocerca ochengi | 586/649 (90%) | KX181290.2 | ||
| 12S |
Parasite (465 bp) |
Onchocerca takaokai | 439/471/ (93%) | AB972364.1 |
| Onchocerca flexuosa | 429/465 (92%) | AP017692.1 | ||
| Onchocerca sp type A | 431/470 (92%) | AB518879.1 | ||
| Onchocerca cervipedis | 422/461 (92%) | JX075208.1 | ||
| ITS |
Parasite (376 bp) |
Onchocerca fasciata | 156/174 (90%) | JQ316671.1 |
| Onchocerca volvulus | 145/161 (90%) | EU272179.1 | ||
| Onchocerca sp. 1 WS-2017 | 292/373 (78%) BLASTn | MG192126.1 | ||
| Onchocerca dewittei japonica | 284/360 (79%) BLASTn | MG192134.1 |
The COI sequences (649 bp; GenBank MG029460 and MG029461) for both parasite samples, which were also identical, and the 12S (465 bp; MH049488) and ITS-1 (376 bp; MH049489) sequences derived from filarial nematoids had maximum BLASTn identity results of 90–93% with species of family Onchocercidae, in particular species of the genus Onchocerca (Table 1). The alignments of the sequences used for phylogenetic analyses from COI, 12S and ITS1 genes are presented in Supplementary Informations 1, 2 and Supplementary Fig. S2, respectively.
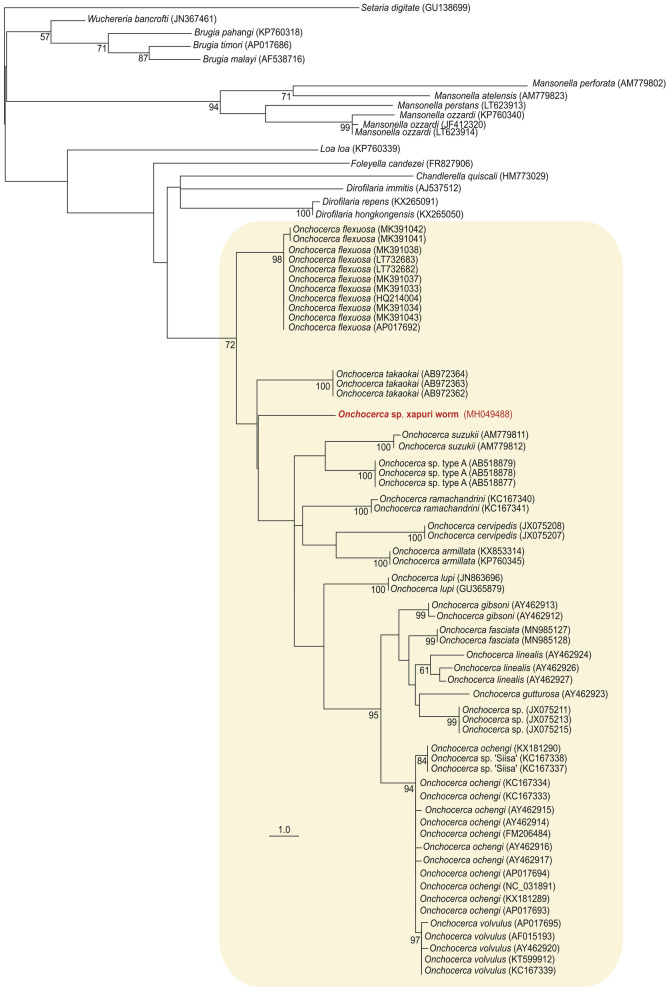
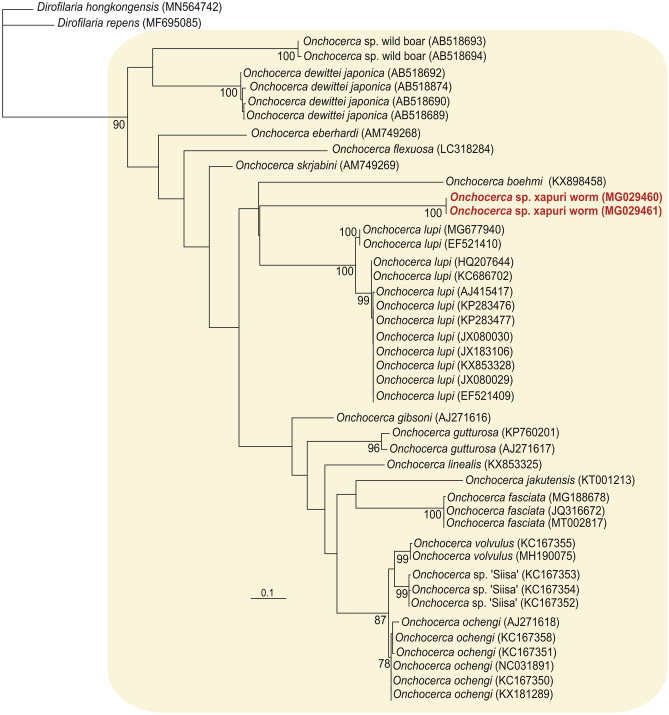
The alignment of the 12S gene was performed using different species from the Onchocercidae family acoording to Crainey et al.28 and using Setaria diditate as an outgroup. For the COI gene, the alignment was made with different Onchocerca and Dirofilaria species (external group). Phylogenetic analyses with mitochondrial genes (12S and COI) clustered the new sequence from the Xapuri worm in the clade of Onchocerca species with 72% and 99% bootstrap, respectively. This clade was more closely related to the genus Dirofilaria (Figs. 2 and 3). In the network tree from all ITS-1 (Supplementary Fig. S1) available in the Genbank sequences from Family Onchocercidae and related species as outgroup, the Xapuri worm sequences clustered with Family Onchocercidae (bootstrap support > 80%). However with the analysis of this gene it was not possible to identify which genus or species it belongs to.
Figure 2.
Phylogenetic tree based on the 12S gene of the new Onchocerca sp. Xapuri worm in Onchocercidae family. Phylogenetic tree inferred by maximum likelihood (403 characters, – Ln = 2,743.160330) of 12S sequences from 58 isolates belonging to the genus Onchocerca (yellow box) and 17 sequences that representing other genera/species from Onchocercidae family. Numbers at nodes are support values derived from 1,000 replicates in maximum likelihood analyses. Codes within parenthesis are GenBank accession numbers.
Figure 3.
Phylogenetic tree based on the COI gene of the new Onchocerca sp. Xapuri worm. Phylogenetic tree inferred by maximum likelihood (649 characters, – Ln = 3,152.161339) of COI sequences from 43 isolates belonging to the genus Onchocerca (yellow box) and 2 sequences of Dirofilaria spp. used as outgroup. Numbers at nodes are support values derived from 1,000 replicates in maximum likelihood analyses. Codes within parenthesis are GenBank accession numbers.
Discussion
During a survey of Leishmania vectors in forest areas of Xapuri, Acre state, Brazilian Amazon, filarial worms were detected in phlebotomine sandflies. The vector species was unambiguously identified morphologically as Ps. carrerai carrerai, based on spermatheca characteristics, thorax coloration and labrum length. Barcoding analysis using COI sequences confirmed that the samples were more closely related to Ps. hirsutus hirsutus, but public databases lack Ps. carrerai carrerai sequences. As such, this was the first COI sequence deposited in GenBank for Ps. carrerai carrerai. COI DNA barcoding will become a more accurate tool to identify species of sandflies in Brazil as more sequences are added to the databases, as shown by Pinto et al.29. Therefore, the new sequences generated in this study for Ps. carrerai carrerai will be important for future identification based on molecular data.
It was not possible to identify morphologically the two samples of filarid worms because of field work conditions and lack of a nematode specialist during sandfly collection. Indeed, previous studies have demonstrated similarities among Onchocerca species, for example: O. gibsoni and O. volvulus have highly similar cuticle morphologies and chromosomal data17,30. According to Gasser31, some of the aspects that are taken into account to identify parasites are morphological features, the host they infect, and their geographical origin. However, these criteria are often insufficient for specific identification and there are limitations of traditional approaches as microscopic analysis. Molecular approaches have provided powerful alternative tools to overcome these complications.
The BLAST searches of three genetic markers (one nuclear—ITS-1—and two mitochondrial—COI and 12S) and phylogenetic trees based on mitochondrial genes sequences support the classification of Xapuri worm in the Onchocerca genus (Table 1, Figs. 2 and 3). However, the phylogenetic relationship of this isolate with the other species of the genus Onchocerca varied according to the analyzed gene. In the 12S phylogenetic tree, Xapuri worm it was closer to O. takaokai. But in the COI tree it was more related to O. lupi. Although there are many sequences of Onchocerca spp. deposited in the GenBank database, many species do not have sequences of both genes (12S and COI). Further studies are needed to verify whether this parasite is more related to a species of Onchocerca already described, or if it is a new species of the genus. For this reason it was denominated as Onchocerca sp. Xapuri worm.
To the best of our knowledge, this is the first record of sandflies carrying filarial worms of the family Onchocercidae in the Americas, and only the second in the world, the other being of Madathamugadia wanjii (fam. Onchocercidae) in Phlebotomus duboscq8. The genus Onchocerca is common in South America, and in Brazil, the most prevalent species of this genus are O. gutturosa in cattle and O. cervicalis in horses20,32, both with veterinary importance. In the Amazon region, human onchocerciasis caused by O. volvulus was described in the 1960s33. However, other filarids of similar medical and clinical importance, such as Mansonella ozzardi and Ma. perstans, have also been found in sympatry in this region24,25,34. Additionally, other atypical filarids are in circulation and result in unknown clinical aspects35,36. To distinguish sympatric filarial species from the Amazon Region, Tang et al.34 proposed a system based on amplification of the internal transcribed spacer (ITS-1). In this analysis, the size of the yielded amplicons varies for each Amazonian filariae species, Ma. perstans (312 bp), Ma. ozzardi (305 bp) and O. volvulus (344 bp). The ITS-1 fragment of the parasite here investigated by the same system had a different band size, 416 bp (including primers), which also differs from the other parasites as Loa loa (344 bp), Wuchereria bancrofti (301 bp) or Dirofilaria immitis (276 bp). ITS-1primers were designed to target a highly conserved genomic region among filarial species and represents an important region for gene splicing.
Another important point to be considered in the phylogenetic analyzes of these filarids is the occurrence of mtDNA pseudogenes, commonly known as Numts, something common found in M. ozzardi COI sequences, as shown by the study by Crainey et al.28. According to the authors, the 12S gene seems to be more reliable for diagnosis and phylogenetic studies of this parasite. They also emphasized of the need for a better screening of these mitochondrial sequences of cryptic pseudogenes before being deposited in public domains. Our all COI sequences (649 bp) do not contain indels or stop codons, and there is no evidence of being considered a pseudogene.The location where both larvae were found (head) and the position in the labrum, as well as the very active and oscillatory movement of the larva found in Ps. carrerai carrerai in the field are characteristic of infective L337 (Supplementary Video). However, it cannot be affirmed with certainty that the larva recovered was L3, given that it was not possible to perform morphological studies. The highly anthropophilic behaviour of this phlebotomine sandfly suggests that humans may be exposed to such nematodes.
Psychodopygus carrerai carrerai was collected in Xapuri, in a primary forest area in the presence of wild animals that included rodents, marsupials, felids and others. In the surrounding region, it was possible to find rural properties with limited cattle, horse and sheep breeding, which suggests that sandflies could be feeding on these animals, which could, therefore, possibly be hosts of the Onchocerdidae nematode described here. However, further investigations are necessary to determine if this is an insect or a zoonotic parasite and its possible host. More studies are also needed to characterize the filarids in circulation in this region, as well as to elucidate the vectorial capacity and competence of sandflies to transmit these parasites.
Methods
Study area and sandfly sampling
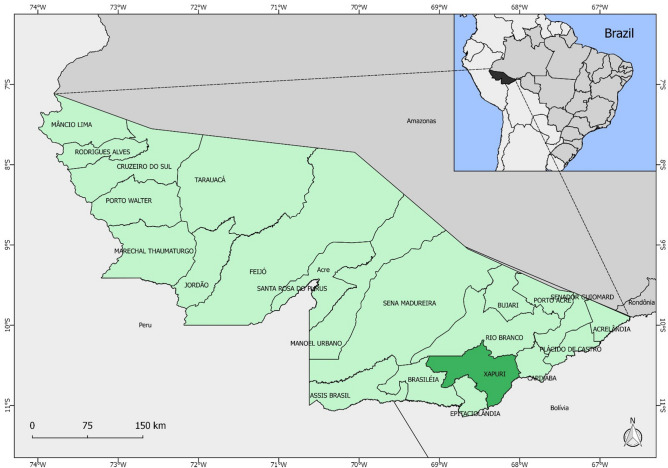
Sandflies collections were undertaken in a forested area of the Xapuri municipality, which is approximately 175 km from Rio Branco, the capital of Acre state, Northern Brazil. Xapuri is situated in the Vale do Acre mesoregion and is bordered by the municipalities of Rio Branco, Brasiléia, Epitaciolândia and Capixaba, as well as the Amazonian border with Bolivia (Fig. 4). The local economy is based on rubber extraction and Brazil nuts38.
Figure 4.
Map of Brazil highlighting the state of Acre and the Xapuri municipality.
Two modified Shannon traps in black and white colours illuminated by LED lights (light emitting diodes—2 W) were installed in a primary forest area in which ecological and environmental tourism activities, such as hiking and tree climbing, are offered. The insects were collected during periods of varying length once a month between August 2013 and July 2015 and in March 2016.
Sandflies were maintained in separate vials for an interval of two hours and then taken to the field laboratory. Female insects were immobilized with ethyl acetate and dissected under a stereomicroscope on slides in a drop of sterile saline solution for exposure of the digestive tract and genitalia. Then, slides were covered with a coverslip and observed under the microscope to investigate the presence of flagellates in their guts. The guts were searched for natural infection by tripanosomatids and spermathecae for the identification of the sandfly species at a 400 X magnification. The identification of the insects collected in this study was performed following Galati’s identification keys39.
The insects with unidentified parasite in the proboscides were filmed and photographed, stored in absolute ethanol for molecular analysis, and then sent to the Oswaldo Cruz Foundation (FIOCRUZ) in Recife, Pernambuco, Brazil.
Molecular identification of the species
DNA extraction and amplification
Before the DNA extraction, the two insects selected in this study were differently processed: from one, the unidentified parasite was separated from the insect, while the other was processed along with the parasite. Parasites and insects were submitted to individual DNA extraction, following protocol described in Ayres et al.40. Four different PCR reactions were performed with the same template, i.e., PCR I, which consisted of cytochrome oxidase subunit I (COI) using primers LCO1490 and HCO219841 that targeted the insect (710 bp product size), and PCR II, which used a different set of primers (COIintF and COIintR), targeting a 689 bp DNA fragment of the parasite42. The PCR III and IV amplified only filarial DNA using respectively, primers FIL-2F and FIL-2R for 416 bp fragment of first internal transcribed spacer 1 (ITS-1)34 and primers 12SF and 12SdegR for 504 bp of 12S rDNA43. PCR reactions were performed in a 25-µL final volume containing the following: 1 × high-fidelity PCR buffer, 2 mM MgSO4, 0.5 U Platinum® Taq DNA Polymerase High-Fidelity (Invitrogen™), 200 µM dNTP, 0.4 µM of each primer, and 6.5 ng of template DNA. The PCR I program consisted of the following: 94 °C for 3 min; 35 cycles at 94 °C for 45 s, 55 °C for 1 min, 72 °C for 45 s; and one final extension step at 72 °C for 10 min. The program for the PCR II consisted of the following: 94 °C for 3 min; 40 cycles at 94 °C for 45 s, 52 °C for 45 s, and 72 °C for 90 s; and a final extension step at 72 °C for 10 min. PCR III and IV the program consisted of the following: 94 °C for 3 min; 35 cycles at 94 °C for 45 s, 50 °C for 45 s, and 72 °C for 45 s; and a final extension step at 72 °C for 5 min. The PCR products were analysed in a 2.5% agarose gel stained with ethidium bromide and visualized under UV light.
Sequencing and sequence analysis
PCR products were excised from the gel and purified using the GFX™ PCR DNA and Gel Band Purification Kit (GE Healthcare). Amplicons were sequenced in both directions using the PCR primers described above. Sequencing reactions were performed on an ABI Prism 3500xL Genetic Analyzer (Applied Biosystems). Quality assessment, edition, assembly and multiple alignments of data derived from sequencing were performed with CodonCode Aligner (v.3.7.1) and BioEdit/ClustalW44. The BLAST tool (www.ncbi.nlm.nih.gov/BLAST) was used to confirm the sequence identities by comparing our sequences to those deposited in GenBank database.
Phylogenetic analysis
Up to 500 homologous sequences were obtained from a BLAST search for COI, ITS-1 and 12S parasite sequences and aligned in BioEdit 7.2.544. Regions of unreliable alignment were removed from ITS-1 alignments, as were sequences that were too short for all regions. Phylogenetic trees for 12S and COI genes were inferred by maximum likelihood (ML) method. The ML analyses were performed using RAxML v.7.045 using the GTRGAMMA model, gamma shape parameter and proportion of invariable sites. Model parameters were estimated in RAxML over the duration of tree search. Nodal supports were estimated with 1,000 replicates in RAxML using the rapid bootstrapping algorithm. The network genealogy for ITS1 was perfomed by SplitsTree4 using the NeighborNet method46.
Supplementary information
Acknowledgements
The authors are grateful to the Program for Technological Development in Tools for Health-PDTIS/Fiocruz-PE genomic platform for sequencing assistance. The authors also wish to express their thanks to Mr. Jailson Ferreira de Souza of the Endemic Management of the Xapuri Municipality for his contribution to the field work. This study was supported by the Fundação de Amparo à Pesquisa do Estado do Acre (FAPAC; Grant Agreement Number PPSUS nº774445); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES/Brasil Sem Miséria—Programa de Pós-graduação em Biociências e Biotecnologia em Saúde.
Author contributions
A.F.B., E.A.B.G. and C.O.C. designed the study project. A.L.A., A.R., C.F.J.A. and M.H.S.P. performed the molecular analyses and sequencing. A.F.B., E.A.B.G., M.M.A. and C.O.C. performed the fieldwork and the phlebotomine identification. I.L.M. performed the phylogenetic analysis. All authors wrote the manuscript. All authors have read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72065-9.
References
- 1.Sherlock IA. Importância médico-veterinária, importância dos flebotomíneos. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. [Google Scholar]
- 2.Aguiar GM, Medeiros WM. Distribuição e hábitats. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. [Google Scholar]
- 3.Le Pont F, Breniere RS, Mouchet J, Desjeux P. Leishmaniose en Bolivie. III. Psychodopygus carrerai carrerai (Barretto, 1946) nouveau vecteur de Leishmania braziliensis braziliensis en milieu sylvatique de région subandine basse. C. R. Acad. Sci. 1988;III(307):279–282. [Google Scholar]
- 4.Arias JR, et al. Flagellate infections of Brazilian sand flies (Diptera: Psychodidae): isolation in vitro and biochemical identification of Endotrypanum and Leishmania. Am. J. Trop. Med. Hyg. 1985;34:1098–1108. doi: 10.4269/ajtmh.1985.34.1098. [DOI] [PubMed] [Google Scholar]
- 5.Lantova L, Volf P. Mosquito and sand fly gregarines of the genus Ascogregarina and Psychodiella (Apicomplexa: Eugregarinorida, Aseptatorina): overview of their taxonomy, life cycle, host specificity and pathogenicity. Infect. Gen. Evol. 2014;28:616–627. doi: 10.1016/j.meegid.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Rocha LS, Santos CB, Falqueto A, Brazil RP. Natural infection of Evandromyia lenti (Mangabeira) (Diptera: Psychodidae) by Psychodiella chagasi (Adler & Mayrink) (Apicomplexa: Lecudinidae) J. Vec. Ecol. 2015;40:419–421. doi: 10.1111/jvec.12184. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JJ, Rosa AT, Souza A, Cruz AC. Os flebotomíneos brasileiros como hospedeiros e vetores de determinadas espécies. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. [Google Scholar]
- 8.Bain O, Petit G, Paperna I, Finkelman S, Killick-Kendrick M. A new filaria of a lizard transmitted by sandflies. Mem. Inst. Oswaldo Cruz. 1992;87:21–29. [Google Scholar]
- 9.Shortt HE, Swaminath CS. Monocystis mackiei n. sp. parasitic in Phlebotomus argentipes. Ann. Brun. Indian. J. Med. Res. 1927;15:539–553. [PubMed] [Google Scholar]
- 10.Killick-Kendrick R, Killick-Kendrick M, Qala I, Nawi NA, Ashford RW, Tang Y. Preliminary observations on a tetradonematid nematode of phlebotomine sandflies of Afghanistan. Ann. Parasitol. Hum. Comp. 1989;64:332–339. [Google Scholar]
- 11.Tang Y, Killick-Kendrick R, Hominick WM. Life cycle of Didilia ooglypta (Nematoda: Tetradonematidae), a parasite of phlebotomine sandflies of Afghanistan. Nematologia. 1997;42:491–503. [Google Scholar]
- 12.Dinesh, D.S., Kumar, V. & Das, P. Infestation of Nematodes in Phlebotomus argentipes Annandale and Brunetti (Diptera: Psycodidae), Bihar, India. Glob. J. Med. Res. 13 (2013).
- 13.Karakus M, Arserim SK, Töz SÖ, Özbel Y. Detection of entomopathogen nematode [EPN-Sand Flies (Phlebotomus tobbi)] Caught in the Wild in Aydin, Kusadasi Town and its assessment as a biological control agent. Türkiye. Parazitol. Derg. 2013;37:36–39. doi: 10.5152/tpd.2013.09. [DOI] [PubMed] [Google Scholar]
- 14.Poinar GO, Ferro C, Morales A, Tesh RB. Anandranema phlebotophaga n. gen., n. sp. (Allantonematidae: Tylenchida), a new nematode parasite of phlebotomine sand flies (Psychodidae: Diptera) with notes on experimental infections of these insects with parasitic rhabditoids. Fundam. Appl. Nematol. 1993;16:11–16. [Google Scholar]
- 15.Secundino NFC, et al. Preliminary description of a new entomoparasitic nematode infecting Lutzomyia longipalpis sand fly, the vector of visceral leishmaniasis in the New World. J. Invertebr. Pathol. 2002;80:35–40. doi: 10.1016/s0022-2011(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 16.Fernández MS, et al. Parasitism by tylenchid nematodes in natural populations of Pintomyia fischeri (Diptera: Psychodidae: Phlebotominae) in Argentina. SM. Trop. Med. J. 2016;1:1001. [Google Scholar]
- 17.Morales-Hojas R, Cheke RA, Post RJ. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J. Helminthol. 2006;80:281–290. [PubMed] [Google Scholar]
- 18.Otranto D, et al. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasit Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otranto D, et al. Case report: first evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am. J. Trop. Med. Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz PST, Mattos MJT, Hoffman RP, Marques SMT. Oncocercose bovina, equina e canina: revisão bibliográfica. Vet. Foco. 2012;10:35–52. [Google Scholar]
- 21.Bain O, Babayan S. Behaviour of filariae: morphologicaland anatomical signatures of their life style within the arthropod and vertebrate hosts. Filaria J. 2003;2:16. doi: 10.1186/1475-2883-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brilhante AF, Melchior LAK, Nunes VLB, Cardoso CO, Galati EAB. Epidemiological aspects of American cutaneous leishmaniasis (ACL) in an endemic area of forest extractivist culture in western Brazilian Amazonia. Rev. Inst. Med. Trop. Sao Paulo. 2017;59:1–9. doi: 10.1590/S1678-9946201759012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brilhante AF, et al. Attractiveness of black and white modified Shannon traps to phlebotomine sandflies (Diptera, Psychodidae) in the Brazilian Amazon Basin, an area of intense transmission of American cutaneous leishmaniasis. Parasite. 2017;24:1–13. doi: 10.1051/parasite/2017021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelley AJ. Human onchocerciasis in Brazil: an overview. Cad. Saúde Pública. 2002;18:1167–1177. doi: 10.1590/s0102-311x2002000500009. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros JF, Py-Daniel V, Barbosa UC, Ogawa GM. Ocorrência da Mansonella ozzardi (Nematoda, Onchocercidae) em comunidades ribeirinhas do rio Purus, Município de Boca do Acre, Amazonas, Brasil. Cad. Saúde Pública. 2009;25:1421–1426. doi: 10.1590/s0102-311x2009000600024. [DOI] [PubMed] [Google Scholar]
- 26.Adami YL, Rodrigues G, Alves MC, Moraes MAP, Banic DM, Maia-Herzog M. New records of Mansonella ozzardi: a parasite that is spreading from the state of Amazonas to previously uninfected areas of the state of Acre in the Purus River region. Mem. Inst. Oswaldo Cruz. 2014;109:87–92. doi: 10.1590/0074-0276130243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes LV, et al. Lymphatic filariasis: surveillance action among immigrants from endemic areas, Acre State, Brazilian Amazon. Asian Pac. J. Trop. Dis. 2016;6:521–526. [Google Scholar]
- 28.Crainey JL, Marín MA, Silva TRRD, et al. Mansonella ozzardi mitogenome and pseudogene characterisation provides new perspectives on filarial parasite systematics and CO-1 barcoding. Sci. Rep. 2018;8:6158. doi: 10.1038/s41598-018-24382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto IS, et al. DNA barcoding of neotropical sand flies (Diptera, Psychodidae, Phlebotominae): Species identification and discovery within Brazil. PLoS ONE. 2015;10:e0140636. doi: 10.1371/journal.pone.0140636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bino Sundar ST, Dhivya B, Jyothimol G, Anandaraja R, Ramesh P, Balachandran C, Thangapandiyan M, Soundararajan C, Gomathinayagami S, Latha BR, Harikrishnan TJ. Incidence of Onchocerca gibsoni in subcutaneousnodules of cross bred Jersey cows: case report. J. Parasit. Dis. 2016;40:1–3. doi: 10.1007/s12639-016-0831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasser RB. Molecular technologies in parasitology, with an emphasis on genomic approaches for investigating parasitic nematodes. Parassitologia. 2006;48:9–11. [PubMed] [Google Scholar]
- 32.Santos ASO, Costa RS, Costa RFR, Lemos LS, Carvalho ECQ. Anatomopatologia de bursite cervical (oncocercose) encontrada em bovinos abatidos sob inspeção estadual no estado do Rio de Janeiro. Arq. Bras. Med. Vet. Zootec. 2014;66:579–582. [Google Scholar]
- 33.Bearzoti P, Lane E, Menezes Júnior J. Relato de um caso de oncocercose adquirida no Brasil. Rev. Paul. Med. 1967;70:102. [Google Scholar]
- 34.Tang THT, López-Vélez R, Lanza M, Shelley AJ, Rubio JM, Luz SLB. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon Region. Mem. Inst. Oswaldo Cruz. 2010;105:823–828. doi: 10.1590/s0074-02762010000600016. [DOI] [PubMed] [Google Scholar]
- 35.Rimarachín D, Flores F, Vásquez J, Rocha A, Forshey BM, Morrison AC. Prevalencia de microfilariasis en la población humana de Iquitos y zonas peri urbanas, Loreto, Perú. Cienc. Amazón. 2016;6:3–15. [Google Scholar]
- 36.Ta-Tang TH, et al. Atypical Mansonella ozzardi microfilariae from an endemic area of Brazilian Amazonia. Am. J. Trop. Med. Hyg. 2016;95:629–632. doi: 10.4269/ajtmh.15-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paily KP, Hoti SL, Das PK. A review of the complexity of biology of lymphatic filarial parasites. J. Parasit. Dis. 2009;33:3–12. doi: 10.1007/s12639-009-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acre. Governo do Estado do Acre, 2016. https://www.ac.gov.br/wps/portal/acre/Acre/estado-acre/municipios. Accessed 21 Mar 2016.
- 39.Galati EAB. Classificação de phlebotominae. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2018. p. 618. [Google Scholar]
- 40.Ayres CF, Romao TP, Melo-Santos MA, Furtado AF. Genetic diversity in Brazilian populations of Aedes albopictus. Mem. Inst. Oswaldo Cruz. 2002;97:871–875. doi: 10.1590/s0074-02762002000600022. [DOI] [PubMed] [Google Scholar]
- 41.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Bio. Biotechnol. 1994;5:294–299. [PubMed] [Google Scholar]
- 42.Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- 43.Lefoulon E, et al. Shaking the tree: multi-locus sequence typing usurps current onchocercid (filarial nematode) phylogeny. PLoS Negl. Trop. Dis. 2015;9:1–11. doi: 10.1371/journal.pntd.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 45.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 46.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.