Abstract
The zebrafish (Danio rerio) has emerged as a popular model organism in developmental biology and pharmacogenetics due to its attribute of pathway conservation. Coupled with the availability of robust genetic and transgenic tools, transparent embryos and rapid larval development, studies of zebrafish allow detailed cellular analysis of many dynamic processes. In recent decades, the cellular and molecular mechanisms involved in the process of gonad development have been the subject of intense research using zebrafish models. In this mini-review, we give a brief overview of these studies, and highlight the essential genes involved in sex determination and gonad development in zebrafish.
Keywords: Gonad development, Zebrafish, Genes
1. Introduction
The existence of two differentiated sexes is common among animals, and is critically important for sexual reproduction and the survival of a species. However, the mechanisms of sex determination are amazingly variable and poorly understood. In contrast to most other highly conserved developmental processes, no single gene network controls sex determination in all species. Consequently, a series of current studies have been working on this important open question: how is the highly diverse system suited to achieving reproductive fitness and success [1]? Zebrafish are increasingly popular as research organisms, especially in studies of embryonic development and as disease models [2], [3]. In the extensive literature on embryonic development and biology of zebrafish, some attention has been given to their cellular reproductive processes. Recent progress in Cas9-related transgenic technology and single cell RNA-seq analysis also provides the opportunity to understand the cellular events of sex determination and gonad development in specific fish [4], [5]. In turn, these data allow us to compare gonad development in zebrafish with that in other animals. Here we summarize current knowledge of the gene networks involved in sex determination and gonad development in zebrafish.
2. Sex determination and gonad development in zebrafish
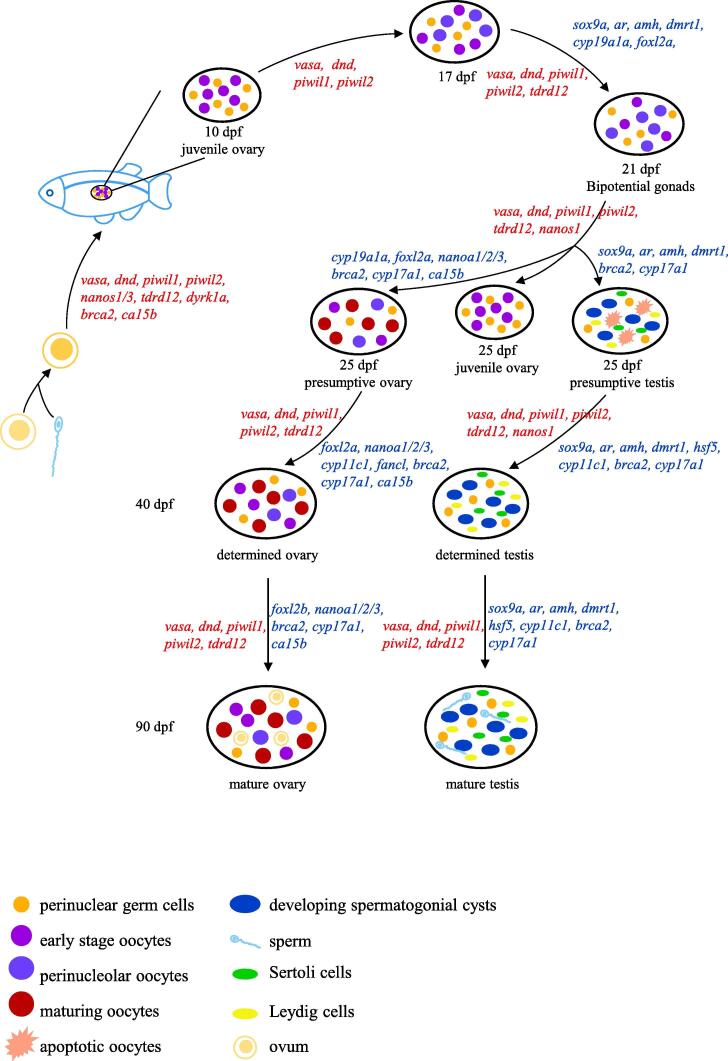
In vertebrates, the male and female reproductive tracts are derived from the same embryonic tissue [6]. In other words, the gonad begins as a bipotential tissue. Sex determination refers to the key events involved in the differentiation of gonads into testis or ovary (Fig. 1). In zebrafish, the development of gonads first passes through an ovary-like stage. Early oocytes can be found in all gonads at 10 days post fertilization (dpf) [7], [8]. While no signs of mitotic activity are typically recorded until 15 dpf [9], early diplotene oocytes can be observed with a clear juvenile ovarian structure [7], [10], suggesting early vertebrate oogenesis. Selman et al. described the oogenesis process in zebrafish as having five stages, defined according to their morphology and karyotype in the ovary [11], [12]. Based on this, sex differentiation is marked by the appearance of perinuclear oocytes, which occurs as early as 17 dpf [13]. The two patterns of differentiation appear in the gonads by 21 dpf, and meiosis is initiated in some gonads at 22 dpf [7]. Depending on multiple signaling networks, the juvenile ovaries maintain the oogenic pathway in about half of the individuals; the other half undergo apoptosis to initiate the “juvenile ovary to testis” gonad transformation process, ultimately transforming into testes [7], [8], [14]. By 25 dpf, gonads are still bipotential [7]. Three morphological states of gonads, including juvenile ovary with perinucleolar oocytes, presumptive ovary with maturing oocytes, and presumptive testis with apoptotic oocytes and developing spermatogonial cysts, can be observed by 31 dpf [15]. At 40 dpf, female gonads contain germ cells at various stages of oogenesis, whereas in other gonads, degenerative oocytes are observed and considered the first indication of spermatogenic activity [7], [15].
Fig. 1.
Gene networks during gonad development in zebrafish. Different genes play important roles in different stages to complete the complex life process of sex determination and gonad development. Graphics with different colors or shapes represent different types of cells.
3. Gene networks for reproductive development in zebrafish
The sex determination mechanism can be mainly divided into two types, genetic sex determination (GSD) and environmental sex determination (ESD). The GSD can either have a monogenic or a polygenic basis. XX/XY system and ZZ/ZW system are two typical systems of GSD. There are various sex determination mechanisms in fish, including GSD, ESD or GSD-ESD interactions. Zebrafish do not have the so-called “heteromorphic sex chromosomes” that determine sex in humans and several other species. Zebrafish displays polygenic sex determination and also affected by the environment. Laboratory strains lack a clear sex-linked locus of sex chromosomes, but wild strains from India have locus on Chr4 that acts as a ZZ/ZW sex chromosome system with environmental influence [16]. Researchers have known that environmental factors, such as water temperature for example, can alter the sex ratio in zebrafish populations [17]. But the gene networks involved in sex determination have remained unclear and sometimes controversial. Here we summarize the genes critical for sex determination and gonad development in zebrafish (Table 1).
Table 1.
Summary of genes required for gonad development.
| Gene | Zebrafish | Mouse | Human |
|---|---|---|---|
| vasa | Important for germ line specification, survival, migration and maintenance, required for fertility in adult zebrafish [21] | Required for male but not essential for female during germ cell development, crucial for premeiotic differentiation in spermatogenesis [63] | Expressed in germ line, necessary for germ cell maintenance [64] |
| dnd1 | Required for PGC migration and survival [20] | Expressed in PGCs, Dnd1‐knockouts lead to germ‐cell‐free, sterile gonads [65] | Has been identified ESTs and genomic sequences encoding closely related genes in human [20] |
| piwil1 | Essential for germ line maintenance, unessential for germ cell specification and early maintenance [23] | Essential for spermatogenesis [66] | Expressed in spermatocytes and spermatids [67] |
| piwil2 | Required for germ cell differentiation and meiosis [24] | Much more predominant in female germ cells than in males [68] | Expressed in testis or embryonic cells, also important for the pathological process of various malignant tumors [69] |
| nanos1 | Essential for PGCs survival, indispensable for maintaining the oocyte production [27] | Not concerned with germ cells development, expressed in maturating spermatids and oocytes [70] | Important for mRNA translation within chromatoid body, represses apoptosis in human germ cells [71], [72] |
| tdrd12 | Important for germ cell development and maintenance [26] | Important for spermatogenesis [73], [74] | Involved in spermatogenesis [75] |
| ca15b | Required for PGCs development in early embryos and perhaps has an important role in oogenesis [29] | No detailed information | No detailed information |
| dyrk1a | Overexpression will alters the expression of some important factors (e.g. piwii1), leading to dysplasia of PGCs [30] | Involved in the migration and maintenance of PGCs [76] | Associated with Down Syndrome, related to cell proliferation and has other multiple functions [77] |
| sox9a | Important for male testis determination [31], [35] | Reduced expression in the ovary and growing expression in the testis, heterozygous mutations do not lead to XY sex reversal [78], [79], [80] | Heterozygous SOX9 mutations cause partial or complete XY sex reversal in the context of the skeletal malformation syndrome campomelic dysplasia [81], [82] |
| dmrt1 | Unessential for ovary development, indispensable for testis development [37] | Required for maintaining spermatogonial stem cells (SSCs) during steady state spermatogenesis, important for recovery of spermatogenesis after germ cell depletion [83] | Required for human testis differentiation, dmrt1 deficiency is related to focal testicular dysgenesis and sex-reversal [84], [85] |
| amh | Important for regression of Müllerian ducts, controls the balance between proliferation and differentiation of germ cells in males [38] | Involved in Sertoli cell development, facilitate the expression of regulating factors in spermatogenesis [86] | Impacts a variety of fundamental processes within the ovaries and testes [87] |
| ar | Interact with androgens, essential for male development and maintenance, key to spermatogenesis and maintenance of ovarian function [41] | Essential for male reproductive development and spermatogenesis [88] | Crucial for spermatogenesis, AR mutations lead to disorders in male reproductive and developmental [59] |
| hsf5 | Essential for proper spermatogenesis and fertility in males [40] | Essential for spermatogenesis [89] | Expression of HSF5 protein is restricted to spermatocytes and round spermatids [90] |
| cyp19a1a | Encodes an aromatase limiting the rate of transformation from testosterone to estrogen,plays duple roles during sex differentiation in zebrafish [43] | No detailed information | Irregular expression related to the development of polycystic ovary syndrome (PCOS) [91] |
| foxl2 | Crucial for ovary development and maintenance, foxl2a and foxl2b make a cooperation to conduct ovary development and maintenance [45] | Indispensable for follicular development and female fertility maintenance, continuous expression important for “ovarian somatic cells to testicular cells” transformation [92] | Required for granulosa cell development, mutations lead to granulosa cell tumors (GCTs) [93] |
| nanos2 | A marker for germline stem cell, expressed in both ovarian and testicular pre-meiotic germ cells [47] | Expressed only in male gonocytes, inhibits meiosis and promotes male‐type differentiation [94] | Testis-specific, expressed in prenatal germ cells and late stages of spermatogenesis [95] |
| nanos3 | Only found in oocytes, nanos3 mutants perform loss of <20um germ cells in juvenile ovary [47] | Important for germ cell development [96] | Expressed in embryonic stem cells, essential for maintaining normal germ cell numbers [97] |
| brca2 | Critical for ovarian development [51], [52], required for the development of embryonic kidney podocytes [53], [54] | Limited information is available due to most homozygous Brca2 mouse mutants display severe embryonic lethal phenotypes [60] | Involved in FA, breast and ovarian cancer [98] |
| fancl | Important for ovarian differentiation and development [46] | Necessary for germ cell proliferation maturation of oocytes, but not for the proliferation or maturation of spermatogonia in adulthood [46], [58] | Involved in FA [99], [100] |
| cyp17a1 | Required for ovarian differentiation and maintaining male-typical SSCs and mating behaviors [55] | Deletion of Cyp17a1 leads to infertility and sexual behavior defects due to the insufficiency of androgen [101] | Essential for the production of androgens and glucocorticoids, involved in prostate cancer [102] |
| cyp11c1 | Necessary for oocytes maturation, testicular development and spermatogenesis [56], [57] | Involved in congenital adrenal hyperplasia [103] | Involved in congenital adrenal hyperplasia and abnormalities in gonad [104], [105] |
3.1. Germ cell-specific genes
Correct specification and maintenance of germ cells is necessary for sexual reproduction in multicellular organisms. While germ cell specification varies among different species, the molecular factors are primarily conserved [18].
vasa and dnd1 (previous name: dnd) are always used as germ cells specific markers. Maternally supplied vasa and dnd1 are detected during the cleavage stage. It has been shown that the expression of these two genes is critical for germ cell migration, survival and maintenance [19], [20], [21]. Piwi genes have been identified as regulatory proteins responsible for stem cell and germ cell differentiation. In zebrafish, Piwi homologs, piwil1 and piwil2 are expressed in both male and female gonads. piwil1 is expressed in primary germ cells in the embryonic genital ridge and adult gonads, where it is co-localized with vasa. Houwing et al. reported that the absence of piwil1 triggered germ cell apoptosis during larval development. In these adult fish with reduced piwil1 function, germ cells were maintained but displayed abnormal levels of apoptosis. piwil2 is expressed in primordial germ cells (PGCs) from 3 dpf and found in the gonads of both adult males and females [22], [23]. As with piwil1, the loss of piwil2 also has marked effects, resulting in the failure of germ cells to differentiate into mature oocytes or sperm [24]. nanos1, which encodes a maternal mRNA, is expressed in PGCs during an early embryonic developmental stage, and is essential for the survival and migration of PGCs [25]. Tudor domain-related proteins (Tdrds), functioning as Piwi-interacting proteins, have been demonstrated to be involved in spermatogenesis. Dai et al. showed that tdrd12-deficient fish displayed reduced numbers of germ cells and germ cells eventually lost by 35 dpf. Furthermore, meiosis defects were also observed in tdrd12 mutant-derived germ cells [26].
The correct expression of these genes is essential for healthy formation of PGCs and normal development of gonads. Homozygotic knockout zebrafishes or mutants of these germ cell-specific genes (vasa, dnd1, piwil1, piwil2, nanos1 and tdrd12) are usually exclusively male with threadlike sterile testes; while these fish can mate with females and stimulate spawning, the eggs are unfertilized [21], [23], [26], [27], [28].
In addition, ca15b is expressed in PGCs in early embryos, whose expression is similar to vasa, suggesting ca15b is required for PGCs development [29] Besides, dyrk1a expression begins in the 2-cell phase and exists in all cells at the blastocyst stage in zebrafish, which is in turn related to the normal formation of PGCs. Overexpression of dyrk1a leads to decreased expression of two important factors related to the development of PGCs, ca15b and piwil1, resulting in decreased number of PGCs and disordered migration. Zebrafish dyrk1a is highly conserved with human DYRK1A, and this work may provide a possible mechanism for germ cell defects in Down syndrome patients [30].
3.2. Testis-associated genes
The correct differentiation and development of the testes is a fundamental prerequisite for reproduction. The genes, sox9a, dmrt1, amh, ar and hsf5 play important roles in the “ovary to testis” stage and later testicular maturation stage in zebrafish, all of which are essential for proper differentiation and development of testes [31], [32], [33], [34].
The expression of SRY-box transcription factor 9a (sox9a) in zebrafish reaches its first high peak at 18 dpf; the next peak is consistent with the ar expression peak at 22 dpf, when the bipotential gonads initiate differentiation to ovary or testis [35]. sox9a has been shown to be an upstream positive regulator of amh and an upstream negative regulator of cyp19a1a in the testes [36]. Webster et al. demonstrated that doublesex and mab-3 related transcription factor 1 (dmrt1) is indispensable for the development of the testis but unnecessary for ovary development; dmrt1 also facilitates male development through transcriptional regulation of the amh and foxl2 genes [37]. Anti-Müllerian hormone (amh) is known to be a crucial factor for male gonad development. It is important for regression of Müllerian ducts, and for controlling the balance between proliferation and differentiation of germ cells in males [38]. The androgen receptors (ar) interact with androgens to exert functions in male development. The transcripts of ar peak at 16 dpf and 22 dpf, and are subsequently maintained at a high stable level across the presumptive male development stage, suggesting that it is essential for male differentiation and maintenance [35], [39]. Saju et al. found that heat shock transcription factor family member 5 (hsf5), whose expression increased from 35dpf, plays an important role in early stage of spermatogenesis and fertility in males [40].
In summary, homozygotic knockout zebrafishes or mutants of these testis-associated genes (dmrt1, amh and ar) usually display a female-biased sex ratio [37], [38], [41]. In particular, abnormal expression of sox9a can block the ovary-testis transformation in zebrafish juvenile. Male gonads lacking dmrt1 expression lead to impaired amh gene expression, with the result that the expression of the foxl2 gene is no longer restrained. Decreased oocyte apoptosis and intersex gonad development is observed with testicular dysgenesis and failed spermatogenesis. In contrast, female ovary development is normal in these dmrt1 mutants [37]. Hypertrophic testes with more germ cells and fewer spermatozoa are observed in homozygous amh mutated males, on account of uncontrollable proliferation and defective differentiation of the germ line [38]. ar-null male zebrafishes appear with female secondary sex characteristics and are sterile, having smaller testes and blocked spermatogenesis [41]. hsf5−/− mutant males are infertile with decreased sperm count, abnormal sperm morphology and decreased sperm motility, however, females are fertile [40].
3.3. Ovary-related genes
The ovaries, producing ova and secreting estrogen, are the core of the female gonads. Genes such as cyp19a1a, foxl2, fancl, ca15b, nanos1, nanos2, and nanos3 jointly regulate the proper formation and function of the ovaries.
Low expression or no expression of sox9a prevent bipotential gonads from developing into male gonads; following this, gonads initiate differentiation to females with increased expression of cyp19a1a. The gene cyp19a1a encodes an aromatase that limits the rate of transformation from testosterone to estrogen, and plays dual roles during sex differentiation: low expression of cyp19a1a is needed for testis differentiation, while high expression of cyp19a1a is vital for ovarian differentiation and maintenance of female sex [42], [43]. foxl2 is crucial for ovary development and maintenance in mammals. There are two foxl2 homologous genes in zebrafish [44], named foxl2a and foxl2b. The expression of foxl2a is higher than foxl2b during ovary differentiation and development, and the expression of foxl2b is more abundant in mature ovaries. The functions of foxl2a and foxl2b are complementary, and they cooperate to regulate ovary development and maintenance, with foxl2b playing a more specific role in ovary maintenance and preventing male transformation [45]. fancl, a member of the Fanconi/BRCA DNA repair pathway, is expressed in developing oocytes and spermatocytes. In particular, fancl is important for ovarian differentiation and development by progressing oocytes through meiosis, and meanwhile, maintaining cyp19a1a expression and down-regulating amh expression [46]. Besides, as we mentioned before, ca15b is expressed in PGCs in early embryos. Wang et al. also found that ca15b is expressed in oocytes in adult females, suggesting ca15b perhaps has an important role in oogenesis [29]. In addition, the expression of nanos1 is required for maintaining oocyte production during female gonad development [27]. nanos2 and nanos3 are important for the maintenance of germline stem cells (GSCs) in the ovaries [47], [48].
Abnormal expression of these ovary-related genes can each lead to respective abnormal phenotypes. The biosynthesis of estrogen is defective in cyp19a1a-null mutant zebrafishes, and although male cyp19a1a mutants are fertile, the effect on estrogen causes ovary-like gonads to disappear during sex differentiation and leads to all males having delayed male sex differentiation. In addition, dysregulated cyp19a1a expression gives rise to expression of other genes, such as sox9a, amh, dmrt1 and foxl2, at abnormal stages during the gonad differentiation period [43]. foxl2a mutant homozygotes have a normal 1:1 sex ratio, while foxl2b disruption causes partial sex reversal in adult females. Both foxl2a−/− and foxl2b−/− mutants perform normal ovarian differentiation and oocyte development initially. However, after maturity, the testis-associated genes (sox9a, amh and dmrt1) are increasingly expressed, while in contrast, the ovary-related gene cyp19a1a is decreasingly expressed in foxl2a−/− or foxl2b−/− female mutants. Accelerated ovarian aging and abnormal meiosis of oocytes are detected in foxl2a−/− female adult zebrafishes, which is similar to conditions associated with premature ovarian failure (POF) in human patients [45], [49]. In zebrafish, some of the foxl2b−/− homozygous female mutants undergo sex reversal from 180 dpf. Moreover, the foxl2a and foxl2b concurrently deficient females appear to undergo complete sex reversal [45]. fancl homozygous mutants develop as all fertile males due to female-to-male sex reversal. Oocytes are absent caused by abnormal apoptosis, and the expression of female-specific genes (e.g., cyp19a1a) is failed to maintaining and the expression of male-specific genes (e.g., amh) is upregulated, further leading to masculinized testes [46]. In addition, nanos1 mutant females are completely sterile at 6 months post fertilization (mpf) [27], while nanos3 mutant adult females are initially fertile, but transition into sterility after 5 mpf [47].
3.4. Other important genes
In addition to the genes described above, there are some genes that play important roles in the development of both male and female zebrafishes, regulating the development of their gonads. Here we introduce the functions of brca2, cyp17a1 and cyp11c1.
Zebrafish fancd1(brca2) and fancl are homologous to human FANCD1(BRCA2) and FANCL and conserved in both structure and function, respectively [50]. They are two of Fanconi Anemia (FA) genes contributed to DNA repair and maintaining genome stability. The function of fancl has been described above. brca2 is expressed both in the developing oocytes and in mature oocytes in adult zebrafish, however, in the testis, it is expressed in spermatogonia and developing spermatocytes, but not in mature sperm. brca2 is required for establishing or maintaining oocyte nuclear architecture and critical for ovarian development. All homozygous brca2 adult mutants are males due to oocytes failed to progress through meiosis at juvenile stage, resulting in female-to-male sex reversal. Adult males are infertile owing to abnormal meiosis of spermatocytes, subsequently leading to apoptosis of spermatocytes. Meanwhile, they are more susceptible to suffer testicular tumors [15], [51], [52]. And recently, Kroeger et al. and Drummond and Wingert found brca2 is required for the development of zebrafish embryonic kidney podocytes in recent years respectively, which are given a new model to further study BRCA2 functions in disease [53], [54]. Besides, mutation of tp53 can rescue the female-to-male sex reversal phenotype of both brca2 and fancl mutants, but cannot rescue fertility in brca2 mutants [46], [51].
cyp17a1, participated in the steroidogenic pathway producing estrogens and androgens, is important for sexual trait development in zebrafish. Androgens is required for testicular development, maintaining male-typical secondary sex characters (SSCs) and normal mating behaviors. Meanwhile, estrogens are indispensable for ovarian differentiation. cyp17a1-deficient zebrafishes are all males due to the biosynthesis of estrogens is blocked. Simultaneously, males perform female-like characters, including light yellow anal fin coloration and dark black body pigmentation, and loss of parallel swimming and grasping leading to failed female spawning, which resulted from impairing androgen synthesis [55].
Zebrafish cyp11c1, encoded 11β-hydroxylase, is important for the biosynthesis of 11-ketotestosterone (11-KT) and cortisol. Zhang et al. found cyp11c1 is expressed both in the testis and ovaries at 30 and 35 dpf, and then specifically expressed in the testis after 45 dpf. The sex ratio of cyp11c1−/− fishes has no significant difference compared to wildtype. Zhang et al. considered that endogenous cortisol synthesized by cyp11c1 might be necessary for oocytes maturation, because exogenous cortisol treatment could partially rescue the defective mature processes of oocytes and decreased oviposition in females [56]. cyp11c1−/− males perform delayed and prolonged juvenile ovary-to-testis transition process [56], and show smaller testes, delayed spermatogenesis, reduced spermatogenesis efficiency and decreased spermatogenesis quantity, which can be rescued by 11-KT or testosterone treatment [57]. Meanwhile, the expression of cyp17a1, amh and dmrt1 was significantly decreased during testis development of cyp11c1−/− males. In addition, functional gametes can be produced in both cyp11c1−/− males and females, but offsprings cannot be produced due to defective mating behavior [56].
4. Future prospects
Compared to asexual reproduction that cells simply create carbon copies of themselves, sexual reproduction allows the introduction of genetic diversity into a population. As an ancient feature of life on earth, it led to the impression that sex determination mechanisms are old and conserved. However, males and females are determined by diverse mechanisms, whereas proper activation essential for successful sex determination differs in various taxa. Indeed, a series of studies are working on these important open questions: What are the mechanisms determine sexes? What are the ways in which they manifest? Why so many ways of doing sex determination for one seemingly common result? What are the molecular players linking rapid turnover of sex determination in few systems?
The rapid development of genomics has allowed researchers to analyze this novel biological system at the molecular level. In addition, a full understanding of the diversity of gender determination mechanisms will require us to expand the breadth of study systems. As discussed, the process of sex determination in zebrafish undergoes great change after some genes are knocked out or mutated. In studies of mice, however, individuals with homologous gene knockouts did not show significant male or female sexual bias (such as Fancl mutant mice) [58], suggesting a significant difference in sex determination between zebrafish and mammals with a strong genetic sex-determining mechanism. In human populations, when we look at the correlation between these gene mutants and clinical cases, patients carrying these mutants often show issues with gonadogenesis, including hypogonadism and infertility. For example, AR mutations in human patients display androgen insensitivity syndrome (AIS), premature ovarian failure (POF) or other gonadal problems [59]. These data suggest that although there are differences in the mechanism of sex determination between zebrafishes and mammals, zebrafish still hold great promise as a model organism. In particular, zebrafish may be a good complementary model to study gene functions when homozygous lethality occurs in knockout mice. For example, most homozygous Brca2 mouse mutants display severe embryonic lethal phenotypes [60]. Therefore, studies of gonad development in zebrafish can complement those of mammalian gonad development, and potentially provide entirely new ideas.
In addition, many groups have in recent years conducted Single-cell RNA-seq, an unbiased approach that has extended our understanding of heterogeneous tissues in embryonic studies [61], [62]. There is no doubt that this technique will benefit studies of gonad development. The analysis of stage-specific gene expression profiles of individual gametogenic cells may provide unbiased and novel insights into their molecular details. Comparative and functional genomic data will allow researchers to figure out how new master sex-determination genes are incorporated into existing genetic networks and control sexual development. In this respect, zebrafish have many advantages as experimental animals. For instance, zebrafish provide relatively large fertilized eggs on a daily basis under simple laboratory conditions. In addition, the embryos and early larvae are transparent, allowing detailed cellular analysis of many dynamic processes.
Taken together, these advantages mean that future work with zebrafish aimed at understanding complex gonad development will likely support and improve our knowledge of physiopathology, with diagnostic as well as therapeutic potential in humans.
CRediT authorship contribution statement
Mengling Ye: Writing - original draft. Ye Chen: Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2018YFC1004803), Natural Science Foundation of China (31671305), and the Fundamental Research Funds for the Central Universities.
References
- 1.Capel B. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat Rev Genet. 2017;18:675–689. doi: 10.1038/nrg.2017.60. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson R.N., van Eeden F.J. The zebrafish as a model of vascular development and disease. Prog Mol Biol Transl Sci. 2014;124:93–122. doi: 10.1016/B978-0-12-386930-2.00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Gore A.V., Pillay L.M., Venero Galanternik M., Weinstein B.M. The zebrafish: a fintastic model for hematopoietic development and disease. Wiley Interdiscip Rev Dev Biol. 2018;7 doi: 10.1002/wdev.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hruscha A., Krawitz P., Rechenberg A., Heinrich V., Hecht J. Efficient Crispr/Cas9 genome editing with low off-target effects in zebrafish. Development. 2013;140:4982–4987. doi: 10.1242/dev.099085. [DOI] [PubMed] [Google Scholar]
- 5.Macosko E.Z., Basu A., Satija R., Nemesh J., Shekhar K. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas-Herald A.K., Bashamboo A. Gonadal development. Endocr Dev. 2014;27:1–16. doi: 10.1159/000363608. [DOI] [PubMed] [Google Scholar]
- 7.Uchida D., Yamashita M., Kitano T., Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- 8.Maack G., Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003;62:895–906. [Google Scholar]
- 9.Grace E.O., Shirley H., Barry C.F. Early gonad development in zebrafish (Danio rerio) Afr J Biotechnol. 2014;13:3433–3442. [Google Scholar]
- 10.Wang X.G., Bartfai R., Sleptsova-Freidrich I., Orban L. The timing and extent of ‘juvenile ovary’ phase are highly variable during zebrafish testis differentiation. J Fish Biol. 2007;70:33–44. [Google Scholar]
- 11.Wu X., Shen W., Zhang B., Meng A. The genetic program of oocytes can be modified in vivo in the zebrafish ovary. J Mol Cell Biol. 2018;10:479–493. doi: 10.1093/jmcb/mjy044. [DOI] [PubMed] [Google Scholar]
- 12.Selman K., Wallace R.A., Sarka A., Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:203–224. doi: 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- 13.Sun D., Zhang Y., Wang C., Hua X., Zhang X.A. Sox9-related signaling controls zebrafish juvenile ovary-testis transformation. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradhan A., Khalaf H., Ochsner S.A., Sreenivasan R., Koskinen J. Activation of Nf-Kappab protein prevents the transition from juvenile ovary to testis and promotes ovarian development in zebrafish. J Biol Chem. 2012;287:37926–37938. doi: 10.1074/jbc.M112.386284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shive H.R., West R.R., Embree L.J., Azuma M., Sood R. Brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:19350–19355. doi: 10.1073/pnas.1011630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson C.A., High S.K., McCluskey B.M., Amores A., Yan Y.L. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 2014;198:1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos D., Luzio A., Coimbra A.M. Zebrafish sex differentiation and gonad development: a review on the impact of environmental factors. Aquat Toxicol. 2017;191:141–163. doi: 10.1016/j.aquatox.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson E.A., Wessel G.M. Vasa genes: emerging roles in the germ line and in multipotent cells. BioEssays. 2010;32:626–637. doi: 10.1002/bies.201000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon C., Kawakami K., Hopkins N. Zebrafish vasa homologue Rna is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- 20.Weidinger G., Stebler J., Slanchev K., Dumstrei K., Wise C. Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- 21.Hartung O., Forbes M.M., Marlow F.L. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol Reprod Dev. 2014;81:946–961. doi: 10.1002/mrd.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan C.H., Lee T.C., Weeraratne S.D., Korzh V., Lim T.M. Ziwi, the zebrafish homologue of the drosophila Piwi: co-localization with vasa at the embryonic genital ridge and gonad-specific expression in the adults. Gene Expr Patterns. 2002;2:257–260. doi: 10.1016/s1567-133x(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 23.Houwing S., Kamminga L.M., Berezikov E., Cronembold D., Girard A. A role for Piwi and Pirnas in germ cell maintenance and transposon silencing in zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Houwing S., Berezikov E., Ketting R.F. Zili is required for germ cell differentiation and meiosis in zebrafish. Embo J. 2008;27:2702–2711. doi: 10.1038/emboj.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koprunner M., Thisse C., Thisse B., Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X., Shu Y., Lou Q., Tian Q., Zhai G. Tdrd12 is essential for germ cell development and maintenance in zebrafish. Int J Mol Sci. 2017;18:1127. doi: 10.3390/ijms18061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Draper B.W., McCallum C.M., Moens C.B. Nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol. 2007;305:589–598. doi: 10.1016/j.ydbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Fujii W., Naito K., Yoshizaki G. Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol Reprod Dev. 2017;84:1100–1111. doi: 10.1002/mrd.22870. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Teng Y., Xie Y., Wang B., Leng Y. Characterization of the carbonic anhydrases 15b expressed in Pgcs during early zebrafish development. Theriogenology. 2013;79:443–452. doi: 10.1016/j.theriogenology.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Lin Z., Liu M., Wang H., Sun H. Overexpression of Dyrk1a, a down syndrome candidate gene, impairs primordial germ cells maintenance and migration in zebrafish. Sci Rep. 2017;7:15313. doi: 10.1038/s41598-017-15730-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang E.F., Pai C.I., Wyatt M., Yan Y.L., Postlethwait J. Two Sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001;231:149–163. doi: 10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- 32.Matson C.K., Murphy M.W., Sarver A.L., Griswold M.D., Bardwell V.J. Dmrt1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi A., Stewart C.A., Wang Y., Fujioka K., Thomas N.C. Beta-catenin is essential for mullerian duct regression during male sexual differentiation. Development. 2011;138:1967–1975. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen A., Andersen O., Bjerregaard P., Rasmussen L.J. Identification and characterisation of an androgen receptor from zebrafish Danio Rerio. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:561–568. doi: 10.1016/j.cbpc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen A., Morthorst J.E., Andersen O., Rasmussen L.J., Bjerregaard P. Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod Biol Endocrinol. 2008;6:25. doi: 10.1186/1477-7827-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Mari A., Yan Y.L., Bremiller R.A., Wilson C., Canestro C. Characterization and expression pattern of zebrafish anti-mullerian hormone (Amh) relative to Sox9a, Sox9b, and Cyp19a1a, during gonad development. Gene Expr Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Webster K.A., Schach U., Ordaz A., Steinfeld J.S., Draper B.W. Dmrt1 is necessary for male sexual development in zebrafish. Dev Biol. 2017;422:33–46. doi: 10.1016/j.ydbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Q., Mei J., Li Z., Zhang X., Zhou L. Distinct and cooperative roles of Amh and Dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics. 2017;207:1007–1022. doi: 10.1534/genetics.117.300274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain M.S., Larsson A., Scherbak N., Olsson P.E., Orban L. Zebrafish androgen receptor: isolation, molecular, and biochemical characterization. Biol Reprod. 2008;78:361–369. doi: 10.1095/biolreprod.107.062018. [DOI] [PubMed] [Google Scholar]
- 40.Saju J.M., Hossain M.S., Liew W.C., Pradhan A., Thevasagayam N.M. Heat shock factor 5 is essential for spermatogenesis in zebrafish. Cell Rep. 2018;25:3252–3261. doi: 10.1016/j.celrep.2018.11.090. [DOI] [PubMed] [Google Scholar]
- 41.Yu G., Zhang D., Liu W., Wang J., Liu X. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget. 2018;9:24320–24334. doi: 10.18632/oncotarget.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X.G., Orban L. Anti-mullerian hormone and 11 beta-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev Dyn. 2007;236:1329–1338. doi: 10.1002/dvdy.21129. [DOI] [PubMed] [Google Scholar]
- 43.Yin Y., Tang H., Liu Y., Chen Y., Li G. Targeted disruption of aromatase reveals dual functions of Cyp19a1a during sex differentiation in zebrafish. Endocrinology. 2017;158:3030–3041. doi: 10.1210/en.2016-1865. [DOI] [PubMed] [Google Scholar]
- 44.Crespo B., Lan-Chow-Wing O., Rocha A., Zanuy S., Gomez A. Foxl2 and Foxl3 are two ancient paralogs that remain fully functional in teleosts. Gen Comp Endocrinol. 2013;194:81–93. doi: 10.1016/j.ygcen.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y.J., Wang Y., Li Z., Zhou L., Gui J.F. Sequential, divergent, and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebrafish. Genetics. 2017;205:1551–1572. doi: 10.1534/genetics.116.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Mari A., Canestro C., Bremiller R.A., Nguyen-Johnson A., Asakawa K. Sex Reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beer R.L., Draper B.W. Nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene Nanos2 in the zebrafish ovary. Dev Biol. 2013;374:308–318. doi: 10.1016/j.ydbio.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Cao Z., Mao X., Luo L. Germline stem cells drive ovary regeneration in zebrafish. Cell Rep. 2019;26:1709–1717. doi: 10.1016/j.celrep.2019.01.061. [DOI] [PubMed] [Google Scholar]
- 49.Pal L., Santoro N. Premature ovarian failure (Pof): discordance between somatic and reproductive aging. Ageing Res Rev. 2002;1:413–423. doi: 10.1016/s1568-1637(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 50.Titus T.A., Selvig D.R., Qin B., Wilson C., Starks A.M. The Fanconi Anemia gene network is conserved from zebrafish to human. Gene. 2006;371:211–223. doi: 10.1016/j.gene.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Marí A., Postlethwait J.H. The role of fanconi anemia/brca genes in zebrafish sex determination. Methods Cell Biol. 2011;105:461–490. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Marí A., Wilson C., Titus T.A., Cañestro C., BreMiller R.A. Roles of Brca2 (Fancd1) in oocyte nuclear architecture, gametogenesis, gonad tumors, and genome stability in zebrafish. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroeger P.T., Jr., Drummond B.E., Miceli R., McKernan M., Gerlach G.F. The zebrafish kidney mutant zeppelin reveals that Brca2/fancd1 is essential for pronephros development. Dev Biol. 2017;428:148–163. doi: 10.1016/j.ydbio.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drummond B.E., Wingert R.A. Scaling up to study Brca2: the zeppelin zebrafish mutant reveals a role for Brca2 in embryonic development of kidney mesoderm. Cancer Cell Microenviron. 2018;5 doi: 10.14800/ccm.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhai G., Shu T., Xia Y., Lu Y., Shang G. Characterization of sexual trait development in Cyp17a1-deficient zebrafish. Endocrinology. 2018;159:3549–3562. doi: 10.1210/en.2018-00551. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q., Ye D., Wang H., Wang Y., Hu W. Zebrafish Cyp11c1 knockout reveals the roles of 11-ketotestosterone and cortisol in sexual development and reproduction. Endocrinology. 2020;161:bqaa048. doi: 10.1210/endocr/bqaa048. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Q., Xiao H., Shi H., Wang T., Sun L. Loss of Cyp11c1 causes delayed spermatogenesis due to the absence of 11-ketotestosterone. J Endocrinol. 2020;244:487–499. doi: 10.1530/JOE-19-0438. [DOI] [PubMed] [Google Scholar]
- 58.Lu B., Bishop C.E. Late onset of spermatogenesis and gain of fertility in Pog-deficient mice indicate that Pog is not necessary for the proliferation of spermatogonia. Biol Reprod. 2003;69:161–168. doi: 10.1095/biolreprod.102.014654. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb B., Beitel L.K., Nadarajah A., Paliouras M., Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 60.Evers B., Jonkers J. Mouse models of Brca1 and Brca2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 61.Petropoulos S., Edsgard D., Reinius B., Deng Q., Panula S.P. Single-cell Rna-Seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell. 2016;165:1012–1026. doi: 10.1016/j.cell.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevant I., Nef S. Single cell transcriptome sequencing: a new approach for the study of mammalian sex determination. Mol Cell Endocrinol. 2018;468:11–18. doi: 10.1016/j.mce.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka S.S., Toyooka Y., Akasu R., Katoh-Fukui Y., Nakahara Y. The mouse homolog of drosophila vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- 64.Castrillon D.H., Quade B.J., Wang T.Y., Quigley C., Crum C.P. The human vasa gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youngren K.K., Coveney D., Peng X., Bhattacharya C., Schmidt L.S. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vourekas A., Zheng Q., Alexiou P., Maragkakis M., Kirino Y. Mili and Miwi target Rna repertoire reveals pirna biogenesis and function of Miwi in spermiogenesis. Nat Struct Mol Biol. 2012;19:773–781. doi: 10.1038/nsmb.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hempfling A.L., Lim S.L., Adelson D.L., Evans J., O'Connor A.E. Expression patterns of Henmt1 and Piwil1 in human testis: implications for transposon expression. Reproduction. 2017;154:363–374. doi: 10.1530/REP-16-0586. [DOI] [PubMed] [Google Scholar]
- 68.Kabayama Y., Toh H., Katanaya A., Sakurai T., Chuma S. Roles of Miwi, Mili and Pld6 in small Rna regulation in mouse growing oocytes. Nucleic Acids Res. 2017;45:5387–5398. doi: 10.1093/nar/gkx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu Y., Zhang K., Li C., Yao Y., Tao D. Piwil2 suppresses P53 by inducing phosphorylation of signal transducer and activator of transcription 3 in tumor cells. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haraguchi S., Tsuda M., Kitajima S., Sasaoka Y., Nomura-Kitabayashid A. Nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech Dev. 2003;120:721–731. doi: 10.1016/s0925-4773(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 71.Ginter-Matuszewska B., Kusz K., Spik A., Grzeszkowiak D., Rembiszewska A. Nanos1 and Pumilio2 bind Microrna biogenesis factor Gemin3, within chromatoid body in human germ cells. Histochem Cell Biol. 2011;136:279–287. doi: 10.1007/s00418-011-0842-y. [DOI] [PubMed] [Google Scholar]
- 72.Janecki D.M., Ilaslan E., Smialek M.J., Sajek M.P., Kotecki M. Human Nanos1 represses apoptosis by downregulating pro-apoptotic genes in the male germ cell line. Int J Mol Sci. 2020;21:3009. doi: 10.3390/ijms21083009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim M., Ki B.S., Hong K., Park S.P., Ko J.J. Tudor domain containing protein Tdrd12 expresses at the acrosome of spermatids in mouse testis. Asian-Australas J Anim Sci. 2016;29:944–951. doi: 10.5713/ajas.15.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey R.R., Tokuzawa Y., Yang Z., Hayashi E., Ichisaka T. Tudor domain containing 12 (Tdrd12) is essential for secondary Piwi interacting Rna biogenesis in mice. Proc Natl Acad Sci USA. 2013;110:16492–16497. doi: 10.1073/pnas.1316316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Babakhanzadeh E., Khodadadian A., Rostami S., Alipourfard I., Aghaei M. Testicular expression of Tdrd1, Tdrd5, Tdrd9 and Tdrd12 in Azoospermia. BMC Med Genet. 2020;21:33. doi: 10.1186/s12881-020-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leffler A., Ludwig M., Schmitt O., Busch L.C. Germ cell migration and early development of the gonads in the trisomy 16 mouse–an animal model for down's syndrome. Ann Anat. 1999;181:247–252. doi: 10.1016/S0940-9602(99)80039-9. [DOI] [PubMed] [Google Scholar]
- 77.Kay L.J., Smulders-Srinivasan T.K., Soundararajan M. Understanding the multifaceted role of human down syndrome kinase Dyrk1a. Adv Protein Chem Struct Biol. 2016;105:127–171. doi: 10.1016/bs.apcsb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Morais da Silva S., Hacker A., Harley V., Goodfellow P., Swain A. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 79.Kent J., Wheatley S.C., Andrews J.E., Sinclair A.H., Koopman P. A male-specific role for Sox9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 80.Bi W., Huang W., Whitworth D.J., Deng J.M., Zhang Z. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foster J.W., Dominguez-Steglich M.A., Guioli S., Kwok C., Weller P.A. Campomelic dysplasia and autosomal sex reversal caused by mutations in an Sry-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 82.Wagner T., Wirth J., Meyer J., Zabel B., Held M. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the Sry-related gene Sox9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 83.Zhang T., Oatley J., Bardwell V.J., Zarkower D. Dmrt1 is required for mouse spermatogonial stem cell maintenance and replenishment. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy M.W., Lee J.K., Rojo S., Gearhart M.D., Kurahashi K. An ancient protein-DNA interaction underlying metazoan sex determination. Nat Struct Mol Biol. 2015;22:442–451. doi: 10.1038/nsmb.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macdonald J., Kilcoyne K.R., Sharpe R.M., Kavanagh A., Anderson R.A. Dmrt1 repression using a novel approach to genetic manipulation induces testicular dysgenesis in human fetal gonads. Hum Reprod. 2018;33:2107–2121. doi: 10.1093/humrep/dey289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rehman Z.U., Worku T., Davis J.S., Talpur H.S., Bhattarai D. Role and mechanism of Amh in the regulation of sertoli cells in mice. J Steroid Biochem Mol Biol. 2017;174:133–140. doi: 10.1016/j.jsbmb.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 87.David B., Seifer R.T. Nova Science; New York: 2016. Anti-MüLlerian Hormone: Biology, Role in Ovarian Function and Clinical Significance; p. 339. [Google Scholar]
- 88.O'Hara L., Smith L.B. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab. 2015;29:595–605. doi: 10.1016/j.beem.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Hemati A., Modarressi M.H., Kolivand S., Azarnia M. Heat shock factor 5 is essential for spermatogenesis in mice: detected by a new monoclonal antibody. Iran J Basic Med Sci. 2020;23:293–297. doi: 10.22038/IJBMS.2019.38615.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chalmel F., Lardenois A., Evrard B., Mathieu R., Feig C. Global human tissue profiling and protein network analysis reveals distinct levels of transcriptional germline-specificity and identifies target genes for male infertility. Hum Reprod. 2012;27:3233–3248. doi: 10.1093/humrep/des301. [DOI] [PubMed] [Google Scholar]
- 91.Franks S., Stark J., Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 92.Uhlenhaut N.H., Jakob S., Anlag K., Eisenberger T., Sekido R. Somatic sex reprogramming of adult ovaries to testes by Foxl2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 93.Cluzet V., Devillers M.M., Petit F., Chauvin S., François C.M. Aberrant granulosa cell-fate related to inactivated P53/Rb signaling contributes to granulosa cell tumors and to Foxl2 downregulation in the mouse ovary. Oncogene. 2020;39:1875–1890. doi: 10.1038/s41388-019-1109-7. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki A., Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kusz K.M., Tomczyk L., Sajek M., Spik A., Latos-Bielenska A. The highly conserved Nanos2 protein: testis-specific expression and significance for the human male reproduction. Mol Hum Reprod. 2009;15:165–171. doi: 10.1093/molehr/gap003. [DOI] [PubMed] [Google Scholar]
- 96.Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 97.Julaton V.T., Reijo Pera R.A. Nanos3 function in human germ cell development. Hum Mol Genet. 2011;20:2238–2250. doi: 10.1093/hmg/ddr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neveling K., Endt D., Hoehn H., Schindler D. Genotype-phenotype correlations in Fanconi anemia. Mutat Res. 2009;668:73–91. doi: 10.1016/j.mrfmmm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Meetei A.R., de Winter J.P., Medhurst A.L., Wallisch M., Waisfisz Q. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 100.Auerbach A.D. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y., Yao Z.X., Bendavid C., Borgmeyer C., Han Z. Haploinsufficiency of cytochrome P450 17alpha-hydroxylase/17,20 Lyase (Cyp17) causes infertility in male mice. Mol Endocrinol. 2005;19:2380–2389. doi: 10.1210/me.2004-0418. [DOI] [PubMed] [Google Scholar]
- 102.Kmeťová Sivoňová M., Jurečeková J., Tatarková Z., Kaplán P., Lichardusová L. The role of Cyp17a1 in prostate cancer development: structure, function, mechanism of action, genetic variations and its inhibition. Gen Physiol Biophys. 2017;36:487–499. doi: 10.4149/gpb_2017024. [DOI] [PubMed] [Google Scholar]
- 103.Mullins L.J., Peter A., Wrobel N., McNeilly J.R., McNeilly A.S. Cyp11b1 null mouse, a model of congenital adrenal hyperplasia. J Biol Chem. 2009;284:3925–3934. doi: 10.1074/jbc.M805081200. [DOI] [PubMed] [Google Scholar]
- 104.Curnow K.M., Slutsker L., Vitek J., Cole T., Speiser P.W. Mutations in the Cyp11b1 gene causing congenital adrenal hyperplasia and hypertension cluster in Exons 6, 7, and 8. Proc Natl Acad Sci U S A. 1993;90:4552–4556. doi: 10.1073/pnas.90.10.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karnak I., Senocak M.E., Göğüş S., Büyükpamukçu N., Hiçsönmez A. Testicular enlargement in patients with 11-hydroxylase deficiency. J Pediatr Surg. 1997;32:756–758. doi: 10.1016/s0022-3468(97)90027-0. [DOI] [PubMed] [Google Scholar]