Abstract
Even if the reader has only browsed through the previous chapters, he ought to have received my feeling that ozone has an enormous therapeutic potential that, so far, has been either disregarded, if not obstructed by world medical authorities. Reasons for delaying the use of ozone are multiple: while quacks and inexpert ozonetherapists are at fault for poor work, other aspects such as commercial and pharmaceutical interests, prejudice, lack of knowledge and a myopic medical vision have done their best to block a substantial and rapid progress.
Keywords: Endothelial Progenitor Cell, Bone Marrow Stem Cell, Alpha Lipoic Acid, Ozone Dose, Chronic Oxidative Stress
Even if the reader has only browsed through the previous chapters, he ought to have received my feeling that ozone has an enormous therapeutic potential that, so far, has been either disregarded, if not obstructed by world medical authorities. Reasons for delaying the use of ozone are multiple: while quacks and inexpert ozonetherapists are at fault for poor work, other aspects such as commercial and pharmaceutical interests, prejudice, lack of knowledge and a myopic medical vision have done their best to block a substantial and rapid progress.
Before examining the usefulness of ozone in various diseases (10.1007/978-90-481-9234-2_9), I would like to summarize the number of biological effects induced by this gas on the body after stimulation of blood, skin, subcutis, muscles and gut lumen. Blood is obviously the best vehicle for transmitting the messages generated by ozone but other tissues have a cooperative relevance.
My words should not be misunderstood in the sense that I always give principal importance to orthodox medicine integrated, when necessary, by ozonetherapy. We shall see that there are vascular diseases such as chronic ulcers and never-healing wounds, where ozone therapy is essential, while in other diseases it has a useful but only an integrative role.
Vasodilation caused by an increased release of NO, nitrosothiols (Joyner and Dietz, 1997; Kashiba et al., 1999) and autacoids can save ischaemic areas in the limbs, heart, brain, kidneys and lungs. An increased supply and release of oxygen and nutrients is crucial for recovering moribund cells, so that a timely intervention can avoid irreversible damages and possibly death.
Release of an array of growth factors from platelets and endothelial cells, while almost impossible to describe in pharmacological and kinetic details, shows its importance by examining every day the extraordinarily rapid healing of necrotic ulcers, particularly enhanced by the topical application of ozonated water and oil.
At least everyone agrees on the disinfectant properties of ozone over the majority of pathogens but, in Western countries, the mental aptitude to profitably use ozone, particularly in chronic infections (large abscesses, peritonitis, osteomyelitis, etc.) is still primordial. How many thousands of patients with septic and toxic shock could have been saved if physicians had accepted my advice to treat them vigorously with ozone therapy?
In spite of the fact that my first interest in ozone was borne out by the finding that oxidants can induce release of cytokines such as TNF alpha (Bocci and Paulesu, 1990), much work remains to be done to fully envisage the activating or/and modulating effect of ozone on the immune system after several months of therapy. Nonetheless, we have gained some evidence that ozonetherapy can be a useful adjuvant for patients with HCV and HIV infections. In this regard, all the hype made by charlatans about the direct intravenous administration of ozone as a route able to “cure” AIDS is highly deplorable, mostly because it served to exploit the good faith of desperate patients. Truly enough, this does not happen only in this field because, in the last decade, too much noise was also made by official medicine regarding gene therapy of tumours (Wadhwa et al., 2002; Noguchi, 2003) and more recently antiangiogenesis. In spite of huge investments to produce many antiangiogenesis antibodies, it appears that survival of metastatic cancer patients is prolonged only of a few months because cancer cells are able to evade even that inhibition. A similar sad story is repeated every day when performing a raving, high-intensity chemotherapy, which often destroys the last resources of the patient. In the cancer section (10.1007/978-90-481-9234-2_9), I will expand the concept that, while an initial, well-focused chemotherapy can be profitable for getting rid of the bulk or residual tumour, to stubbornly continue the administration of palliative cytotoxic drugs (owing to chemoresistance) is wrong, because the prolongation of a few months survival is paid dearly by the suffering patients.
When I name ozone “the wonder drug” of the twenty-first century, I am not making an overstatement as a foolish retaliation to an unjustified scepticism, but because I have good reasons to believe that prolonged ozonetherapy can allow four extraordinary phenomena:
-
(A)
the induction of oxidative shock proteins (OSP),
-
(B)
the upregulation of antioxidant enzymes,
-
(C)
hence, the reduction, if not the normalization of the oxidative stress and
-
(D)
the probable release of bone marrow staminal cells (BMSC).
Everyone aware of the current biological trends will agree that these are not farfetched ideas.
Regarding points (A and B), the teleological significance of the OSP appears well demonstrated in bacteria, fungi, plants and mammals. These results are truly fascinating (Jolly and Morimoto, 2000).
Any change of the external environment or internal “milieu” disturbs cell homeostasis, but if the stress is tolerable, or graduated in intensity, the cell can adapt to it and survive. If it is too violent, the cell programmes its own death, or apoptosis (Jacobson, 1996). The great number of stresses includes hyperthermia, hyperoxia, hypoxia, ischaemia, excessive ROS and LOPs production, heavy metals, ethanol, hypoglycemia, pH modifications, viral, bacterial and parasitic infections, antibiotics, malignancy, radiation, metabolic inhibitors, amino acid analogs and most likely mental stress and hormonal derangement. Obviously, OZONE HAS TO BE INCLUDED: heat stress proteins (HSP70) are expressed after ozone inhalation (Su and Gordon, 1997) and an attenuation of ozone-induced inflammation has been recorded after repeated daily exposure (Christian et al., 1998). In relation to the variety of stresses, the cell either upregulates or synthesizes probably a hundred or more new proteins like HSPs, glucose-regulated proteins (GRPs) and OSPs, which allow the cell to resist against new and even more intensive stresses. As it has been observed in the cytokine field, also in this case there is an apparent redundancy, with the final aim of establishing “stress tolerance” and insuring cell survival. Already Paracelsus (1493–1541) had this intuition and in the “Nature of Disease” wrote that “the body possesses the high art of wrecking but also restoring health”. The Romans, twenty centuries ago, already guessed the power of the “vis medicatrix naturae”, or in other words, of the natural ability of the organism to heal itself when appropriately stimulated. The modern pharmacological approach, although useful, can often have a too narrow aim.
I believe that the future of ozonetherapy rests in part on the pedestal of OSP, but it will be necessary to demonstrate how best it can be obtained, its relevance and amplitude.The concept is old and it has been named in different ways only because it has been observed in different pathological conditions: Murry et al. (1986) pioneered the concept of “ischaemic preconditioning” for the heart, which after undergoing a brief, non-lethal period of ischaemia can become resistant to infarction from a subsequent ischaemic insult. Goldman (1996) has introduced the term “hormesis” for explaining “the beneficial effect of a low level exposure to an agent that is harmful at high levels”, e.g. very low doses of radiation induce an adaptive response to a high dose in human lymphocytes (Olivieri et al., 1984; Wolff, 1996). Calabrese and Baldwin (2001) and Calabrese (2002, 2009) have presented numerous examples of stimulatory responses following stimuli below the toxicological threshold. This concept echoes Aristotle’s thought (384–322 B.C.): “Principium quantitate minimum, potestate autem maximum” i.e., a minimal amount of a drug (ozone!) displays potent effects.
“Oxidative preconditioning” has been achieved by warm ischaemia or hyperthermia (Kume et al., 1996; Yamamoto et al., 2000), transitory limb ischaemia (Sun et al., 1999), AHT (Bocci, 1996a, c) and RI of ozone (León et al., 1998; Barber et al., 1999; Peralta et al., 1999, 2000; Borrego et al., 2004; Gonzalez, 2004; Madej et al., 2007). However, when ozone is used, the term “ozone tolerance” or “adaptation to COS” seems more appropriate because it specifies the inducing agent. We face a real paradox, since ozone, the “toxic gas”, can be turned into a useful drug able to readjust an otherwise irreversible state of chronic oxidative stress.
There are several pathologies, such as atherosclerosis, diabetes, ischemia, hyperhomocysteinaemia, neurodegeneration, nephropaties, chronic viral infections, autoimmune diseases and cancer where a vicious imbalance between oxidants and antioxidants becomes firmly established, leading more or less rapidly to death. Today we are also concerned about the obesity epidemic as a serious health-risk factor.
How can modern medicine correct this?
Let us first consider the orthodox strategies to reduce oxidative stress in these diseases (Bocci et al., 2009). Owing to the great variety of metabolic disorders, approaches aims to:
Inhibit xanthine oxidase to reduce formation of superoxide and hydrogen peroxide using allopurinol (Farquharson et al., 2002).
Inhibit NAD(P)H oxidase (Lambeth, 2004). A direct action remains an unsolved pharmacological problem and moreover there is a risk of an increased bacterial infection.
Inhibit the renin-angiotensin system. Angiotensin-converting enzyme (ACE) inhibitors and Ang-II receptor antagonists are broadly used drugs for effectively reducing blood pressure and interestingly they can also reduce oxidative stress by inhibiting NAD(P)H oxidase. On the other hand, Ca2+ channel blockers, beta blockers and alpha receptor blockers are antihypertensive but do not improve the antioxidant status in patients (Baykal et al., 2003). Administration of diuretics is helpful but is transitory.
Inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the key enzyme of cholesterol biosynthesis. There are now a variety of lipophilic and hydrophilic statins able to lower serum cholesterol levels, increase the number of hepatic LDL receptors and modulate pathophysiologic processes in patients with acute coronary syndromes (Spencer et al., 2004). Statins have proved to be much more than simple lipid lowering agents (Liao, 2002) because, by blocking the synthesis of critical isoprenoid intermediates, they express several other effects such as: the inhibition of NAD(P)H oxidase, the increased expression of endothelial NO synthase and of tissue-type plasminogen activator, while the expression of plasminogen activator inhibitor and endothelin-1 are inhibited. Thus the multiplicity of hepatic and extrahepatic effects, by reducing inflammation, tumour progression (Katano et al., 2004) and an excessive immune reactivity (Vollmer et al., 2004) have raised statins at the level of a “miracle drug” comparable to the old penicillin (Roberts, 1996). Statins seem also able to mobilize bone marrow-derived endothelial progenitor cells (Llevadot et al., 2001) and practically every month a new beneficial effect is discovered. However, even with statins there are two problems: one, is their cost, which limits their use to an unacceptable minority of patients (Topol, 2004) and the second is the danger of rabdomyolysis. The risk is fairly rare but high doses of statins associated with an infection can cause this dangerous pathology. In order to reduce the risk and to maintain the advantage of low cholesterol LDL levels, it was suggested to associate oral Ezetimibe with only 20–40 mg statin daily. Ezetimibe inhibits the intestinal absorption of cholesterol but even this combination does not appear totally safe because it may represent a procangerogenic stimulus and therefore the latest suggestion is to use Niacin instead of Ezetimibe.
- Inhibit the excess of oxidants production by administration of either antioxidant vitamins or of a “healthy diet” enriched with polyphenols and flavonoids (red wine, olive oil, etc.). It is also known that administration of thiol-containing compounds (NAC and alpha lipoic acid) can inhibit LDL oxidation. This seems an easy solution but does ADMINISTRATION OF ANTIOXIDANTS really work? This is a recurrent and fashionable theme, often discussed by vitaminologists and by charlatans, who may intoxicate patients with megadoses of selenium, zinc, iron and vitamins A, and E. Authoritative scientists have often posed the question as to whether supplementation with antioxidants (Antioxidant therapy, AT) reduces oxidative damage in humans. The conclusion is that an equilibrated dose may be essential during growth and useful in oxidative stress-related conditions, but there is little evidence that it can be a definitive remedy (Hennekens et al., 1994; Packer et al., 1997; Zino et al., 1997; Clinton, 1998; Halliwell, 1999a, b; McCall and Frei, 1999; Pryor, 2000; Polidori et al., 2001, 2004; Bender, 2002; Vivekananthan et al., 2003; Seifried et al., 2003; Ames, 2004; Victor et al., 2006). An excessive amount may modulate the synthesis of HSPs and actually reduce the synthesis of HO-1 (Peng et al., 2000). If we wish to tackle this problem realistically, we must consider:
- the uncertainty of intestinal absorption;
- the individual variability of metabolism and excretion;
- the variable and often reduced uptake of antioxidants by the cell;
- the possible reduced synthesis of GSH (observed in HIV infection);
- the potential toxicity of excessive doses;
- the inability of antioxidants to stimulate the synthesis of antioxidant enzymes;
-
if not, to inhibit this process.Thus the problem of antioxidant supplementation must be seriously considered and, while it is certainly useful to administer a correct and equilibrated amount, it cannot do miracles.
Inhibit production of superoxide by long-term administration of L-arginine (Enwonwu, 1989; Morris et al., 2000), which is the substrate for NO synthesis.
Inhibit the excessive production of superoxide by SOD mimetics (Fontana et al., 1999), because the administration of an exogenous enzyme, unable to enter into the cell, has shown to be useless. Induction of SOD by gene transfer is fashionable but, until we are able to control transgene expression and the homogenous distribution of the vector all over the vascular system, it remains a theoretical possibility difficult to realize.
Inhibit the increase of homocysteine levels in the plasma because the auto-oxidation of its sulfhydryl group generates superoxide and hydrogen peroxide that can become cytotoxic for the endothelium, Hyperhomocysteinaemia can be kept under control by the daily administration of folic acid plus vitamins B6 and B12 (Das, 2003) and by increasing the plasma level of adenosine (Riksen et al., 2003).
Inhibit platelet aggregation with aspirin, ticlopidine clopidogrel and the like.
Inhibit the synthesis of pro-inflammatory autacoids by the daily administration (2 g) of n-3 PUFAs present in fish oil, which enhance the generation of 3-series PGs and 5-series LTs, which are anti-inflammatory (Belluzzi et al., 1996; Mori et al., 2003).
Inhibit hyperglycaemia by carefully regulating caloric intake with abundance of fresh vegetables and adopt a correct life style without smoking and find time for at least 30 min of a moderate physical exercise (Fontana et al., 2004).
Inhibit inflammation by using either corticosteroids or non-steroidalanti-inflammatory drugs (NSAID). Both types of drugs can be used for a limited time owing to undesirable adverse effects.
Inhibit the formation of advanced glycation end products (AGEs). These toxic compounds, deposited in arterial wall can induce oxidative stress and accelerate the progression of diabetes type II, atherosclerosis, renal and retinal damage. Hyperglycemia, obesity and a wrong life style must be severely controlled.
I have just summarized the most relevant therapeutic strategies that orthodox medicine offers for reducing the chronic oxidative stress: with the exception of the statin and antihypertensive agents, the use of them separately makes little sense and cannot solve the problem. Even if it implies taking daily six or more tablets, this long-term cocktail-type therapy is recommended in spite of the cost. If the patient is compliant, the actual evidence is that the morbidity and mortality of seriously-ill patients diminish markedly, suggesting that this multiform treatment can slow down the involution.
Is any point in suggesting ozone therapy? Ozone cannot remove the primary causes of these diseases, but is able to reverse the chronic oxidative stress (Fig. 8.1).
Fig. 8.1.
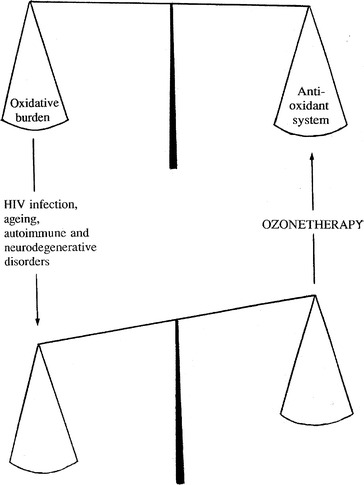
The normal and pathological redox balance. The scheme suggests that, by upregulating the expression of OSP and antioxidant enzymes, ozonetherapy may favour normalization of the impaired redox balance
Can ozone alone do as much as the above listed thirteen treatments? This is unlikely and it seems wise to consider ozonetherapy as an integrative support.
I envisage the ozone treatment as a transitory and calculated oxidative stress resulting in a sort of “therapeutic shock” for the ailing organism. Ozone realizes this shock because it generates a number of messengers that can reach all cells in the organism. How can this happen? First of all, it is necessary to distinguish local from parenteral treatments. Among the latter, major AHT, the “glucose or saline peroxide” infusion and EBOO are reasonably precise, and both the infused hydrogen peroxide, but especially LOPs with a long half-life, are the most important putative agents. BOEX and RI are somewhat imprecise approaches, but nonetheless likely to put LOPs, generated on the cutaneous and mucosal gut surface, into the circulation. Thus, during and immediately after one of these treatments, cells throughout the body will suddenly receive a pulse of LOPs and newly generated autacoids. As it was already mentioned in 10.1007/978-90-481-9234-2_4, these compounds are heterogeneous and undergo dilution and metabolism (Vasiliou et al., 2000). Over a certain level they are cytotoxic, while below micro-or nanomolar levels they can act as physiological messengers by interacting with several cellular enzymes (Forman et al., 2008) and this is a very good reason starting ozonetherapy with low ozone doses scaled up slowly and cautiously. One possible way to interrupt the cell anergy, due to a chronic oxidative stress, may be an adequate and atoxic stimulation of the cell via a few LOP molecules. If the cell is still able to transduce the message to the nucleus, via phosphorylation of protein kinases and the like, it may represent the alarm signal able to reactivate gene expression, leading to the synthesis of OSPs and antioxidant enzymes. While a too high LOPs concentration or a too advanced disease will end with the cell death, a very low and gradual stimulation may favour a re-equilibration of the oxidant-antioxidant balance, as shown in Fig. 8.1. If the idea is correct, ozonetherapy should start at concentrations just above the threshold level, which is in line with the old concept “start low, go slow”. Experiments in laboratory animals (León et al., 1998; Barber et al., 1999; Peralta et al., 1999, 2000; Borrego et al., 2004; Stadlbauer et al., 2008) treated daily with either RI or intraperitoneal insufflation of ozone have shown a really surprising adaptation to COS, with consequent resistance to prolonged ischaemia or toxic compounds or allograft rejection, within a short treatment. Of course experimental results in rats are not the last word because in a healthy volunteer (Fig. 8.2), as well as in HIV patients, I found that it took from 2 to 4 weeks (5–9 major AHTs; twice weekly) to detect an increased plasma level of SOD and a concomitant decrease of the TBARS level.
Fig. 8.2.

An AMRD patient’s response to a single (left side) or intermittent (right side) infusion of ozonated AHT (300 g blood treated with an ozone dose of 21 mg/session). MDA, malonyldialdehyde (◊) and Mn-SOD (U/ml plasma ○) are reported on the ordinate. Arrows indicate the time of blood reinfusion
Which proteins and enzymes are important in correcting the COS? This problem has been extensively investigated in the last 15 years and it has been shown that hyperoxia and ROS can induce increased levels of SODs, GSH-Pxs, GSSGR and catalase (Heng et al., 1987; Rahman et al., 1991; Shull et al., 1991; Doroshow, 1995; Hernandez et al., 1995; Bocci, 1996a; Tacchini et al., 1996; Sagara et al., 1998; Wang et al., 1998; Barber et al., 1999; Chen et al., 2000; Csonka et al., 2000). All of these data have been extremely encouraging for demonstrating the effects of ozonetherapy.
We are continuing to investigate the levels of antioxidant enzymes, G-6PD (Puskas et al., 2000) and some OSPs inducible by hydrogen peroxide and ozone (Jornot et al., 1991; Cardile et al., 1995; Kiang and Tsokos, 1998), before, during and after ozonetherapy. We are particularly interested in analysing the pattern of HO-1 (or HSP-32) because even a gentle exposure of blood to ozone (40 mcg/ml) appears to release traces of haeme. Its breakdown generates beneficial molecules, such as traces of CO acting synergically with NO as vasodilator, bilirubin acting as a lipophilic antioxidant (Abraham et al., 1996), as well as free Fe2+ which, if not promptly chelated, may act as a pro-oxidant (Dong et al., 2000; Nath et al., 2000; Ryter and Tyrrell, 2000; Snyder and Baranano, 2001). On the whole, HO-1 is becoming a most interesting enzyme (Galbraith, 1999; Zuckerbraun and Billiar, 2003; Bocci et al., 2007), involved in protecting the skin (Reeve and Tyrrell, 1999), in avoiding acute haeme toxicity and iron overload (Nath et al., 2000), in suppressing endothelial cell apoptosis (Brouard et al., 2000), in blocking the growth of vascular smooth muscle cells (Durante, 2003), in rejection of mouse to rat cardiac transplants (Sato et al., 2001) and in protecting heart, liver, kidneys, lungs against ischaemia/reperfusion and hyperoxia injury (Csonka et al., 1999; Amersi et al., 1999; Otterbein, 1999; Miyazono et al., 2002; Choi et al., 2003; Wagner et al., 2003; Seixas et al., 2009).
Thus, there is already supporting evidence that the adaptation to COS can be realized with ozonetherapy. Before starting ozonetherapy, we should at least once determine the TAS of each patient. If this is not possible and if the patient is in a critical condition (cachexia, anorexia, great pain, etc.), I feel it is necessary to give a daily well-balanced and reliable supplementation of antioxidants 1 week before ozonetherapy, calibrated at a correct level (Bocci, 2007). Moreover, in the case of a demanding approach, such as EBOO performed in critical patients, we can start with short treatment periods (20 min only, followed by 30, 40, 50 and finally 60 min, corresponding to the 1st, 2nd, 3rd, 4th and 5th treatment, respectively). We regularly prescribe the following daily oral supplementation:
0.5 g of vitamin C (morning). This dose saturates the body (Levine et al., 1996). While a megadose (40–80 g daily) of intravenously infused ascorbate can be useful in cancer or infections, in this particular case may be only partly absorbed, may quench ozone activity, may act as an oxidant and, most likely, be rapidly eliminated with highly acidified urine. Only in critical patients, this dosage can be doubled (Polidori et al., 2004).
0.6 g of NAC (either morning or evening) (Bridgeman et al., 1991; Hack et al., 1998) as the precursor of GSH. I would like to remind that exogenous (oral or/and IV) administration of GSH, with a few notable exceptions (hepatic poisoning, etc.) is a biochemical and pharmacological nonsense. In particular situations this dosage has been increased four-fold (Hack et al., 1998; Tepel et al., 2003).
an approved multivitamin complex (Recommended dosage allowance, RDA) including vitamin E, alpha lipoic acid and selenium;
a rich dietary intake of fresh fruit and vegetables.
This antioxidant regimen can be maintained throughout the therapy and will allow us to progressively increase the ozone dose without risk. My belief is that, unless we are able to ACTIVELY increasing the intracellular antioxidant capacity, even if body fluids are flooded with exogenous antioxidants, there is no hope to rehabilitate the cell and to achieve a therapeutic result.
I wish I could give a definitive answer whether ozone therapy can do as well or even better than the treatments previously discussed and it is pointless to debate this issue unless we can compare them in a randomized clinical trial. This is certainly an impossible task for our means and orthodox medicine will never entertain it because statins alone represent a colossal “business”. For the time being and the sake of the patient, I can only suggest accepting orthodox therapy associated with the least invasive ozone therapy for obtaining the maximal effect with a minimal discomfort.
The final point regards the exciting possibility to improve the oxygenation of ischaemic tissues by promoting angiogenesis. It has been shown already that autologous bone marrow stem cells (BMSC) or/ and endothelial progenitor cells (EPC) can play a role in accelerating angiogenesis of the human myocardium thus improving the perfusion of the infarct zone leading to regeneration (Strauer et al., 2001; Orlic et al., 2001; Schwartz and Curfman, 2002; Aicher et al., 2003).
First of all let us consider how conventional medicine has tried to solve this problem. Two main approaches have been used: The first consists in collecting autologous BMSC and transplanting them via intracoronary or transendocardial routes. The invasiveness of this method may limit its clinical application. The second exploits the release of SC in the circulation after administration of granulocyte-colony stimulating factor (G-CSF). After collection of enriched haemopoietic stem cells (using CD34 as a marker of SC) from the circulation, these have been infused via intracoronary route. This method is fairly practical but there is a risk of in-stent restenosis (Kang et al., 2004). Thus, although both approaches can improve myocardial perfusion, they don’t seem ideal procedures.
Ozone therapy could be advantageous because it rapidly improves the oxygenation and the metabolism of ischaemic tissues and could itself mobilise endogenous SC, thus avoiding the need to collect and to transfuse cells. The hypothesis that ozonetherapy may enhance the release of SC from bone marrow was put forward some time ago (Bocci, 2002) for explaining the surprisingly long lasting remission in two of seven cardiopathic patients after EBOO’s treatment, when the usual therapeutic effect lasts only a few months. It became obvious to imagine that a sort of myocardial repair could have occurred if BMSC have homed in the infarct zone and regenerated the necrotic myocardium but, regrettably, an appropriate evaluation could not be performed. It is also possible, although less likely, that in situ cardiomyocyte replication allowed replacement of the myocardial scar tissue. In a brilliant review, von Harsdorf et al. (2004) have discussed this possibility as the “newt” approach that has been clearly shown in amphibians.
Even if the location of SC remains elusive, it seems that every organ (liver, brain, skeletal muscle, skin, endothelium and cancer as well) is gifted with these cells but the real trove seems to be the bone marrow that contains about 1% of haematopoietic and some 0.05% of mesenchymal stem cells (MSC). It has been demonstrated (Barakat et al., 2004) that in rats, after intraperitoneal injection of ozone at variable concentrations (4.0, 40.0 and 75.0 mcg/ml), an induction of neoangiogenesis can be achieved in both skeletal and cardiac muscle with the medium ozone concentration. If this happens during prolonged ozone therapy, it remains undetermined, but it is one of the most exciting avenues of research. After all, almost every day, we notice a far more rapid healing of cutaneous ulcers in patients with chronic limb ischaemia undergoing ozonetherapy, so why couldn’t the skin reconstruction mirror the heart repair!
The idea that ozone therapy could mobilize BMSC is supported by some biochemical data: several years ago, we demonstrated that LOPs present in human ozonized plasma induced NO synthase (NOs) in human endothelial cells and we measured a significant release of NO and nitrosothiols (Valacchi and Bocci, 2000). These compounds are of fundamental importance in the physiology of the vascular bed because they enhance vasodilation and inhibit platelet-leukocyte aggregation-adhesion and muscle cell proliferation (Joyner and Dietz, 1997; Kashiba et al., 1999; Stamler, 2004). Aicher et al. (2003) have added the crucial finding that the induction of endothelial NOs is essential for neovascularization because NO activates matrix metalloproteinase-9 (MMP-9) indispensable for SC mobilization.
In conclusion this process can be distinguished in four phases:
-
i.
MOBILIZATION or RELEASE of BMSC, MSC and EPC. Reinfusion of ozonated blood represents an acute, precisely calculated stress able to stimulate the bone marrow by means of LOPs and possibly autacoids, growth factors and cytokines. The sudden homeostatic change in the bone marrow microenvironment caused by these messengers (particularly NO) may well be an effective way for enhancing the output of stem cells.
-
ii.
THE JOURNEY TO THE TARGET: circulatory BMSC, MSC and EPC do not get lost in the vast expanse of the vascular bed and eventually home in an injuried site that likely is an ischaemic and/or an infarcted area.
-
iii.
HOMING may be determined by chemoattractive mechanisms as a damaged tissue may release chemoattracting factors or express new receptors where SC can dock.
-
iv.
INCORPORATION and TISSUE REPAIR, given due time, can occur via proliferation and appropriate differentiation of SC, thanks to improved oxygenation and presence of growth factors in the microenvironment. If this is correct, even a small number of SC can be eventually sufficient to reconstruct the infarcted zone.
Although humans have not the power to regenerate the organs, except the liver, the present state of the art is encouraging for the heart and can also help to spare amputations of limbs in some patients. An astonishing result observed in one of our patients at the 4th stage of POAD after ozone therapy has led us to believe that only the new formation of an efficient circulatory network could have allowed the recovery from an apparently irreversible damage. However, highly compromised patients with advanced dysmetabolic syndrome appear unable to recover. There can be little doubts that, besides a correct timing and efficacy of the therapy, genetic, metabolic and neuro-endocrine factors play an important role in the final outcome because only a minority of patients (Grade IV) have a positive response. Results obtained with prostanoids’ infusion are inferior to ozone therapy (Di Paolo et al., 2005) suggesting that ozone deserves to be thouroughly examined. It will not be easy but we will try our best to investigate with refined instrumental analysis if this repair process really occurs in vasculopathic patients treated with ozone therapy. If ozonetherapy really offers an advantage over the more elaborate administration of staminal cells via special routes (Strauer and Kornowski, 2003), it ought to be seriously investigated because we could easily and inexpensively help a far larger number of critical patients.
A final remark regards the duration of an ozonetherapeutic treatment and if it allows to “cure” a disease. Around 80 A.D. Tacitus wrote “nature infirmitatis humanae tardiora sunt remedia quam mala” or, on the basis of the nature of human frailty, remedies work more slowly than illnesses. This remains true today for both orthodox medicine and ozonetherapy. With this complementary approach it takes some time to notice a real improvement and this depends very much on the state of the patient, age, type of disease, the quality of the treatment and also on the capacity of the ozonetherapist. Moreover ozonetherapy only rarely can “cure” a disease but it can correct or block its progression and the benefit can often be conserved with a maintenance therapy.
Conclusions
This chapter was written for outlining the number of potential benefits obtainable with ozonetherapy. There is a real possibility that, by combining the use of the best medical drugs (statin, platelet-antiaggregants and hypertension inhibitors) with ozonetherapy, we can really defeat the infamous chronic oxidative stress (COS) with all its negative consequences. As slowly we move on and observe the validity of ozone therapy in new diseases, we are surprised of the breadth of action of this approach and its atoxicity against the blackest predictions. It is regrettable that for lack of organization and resources (practically nothing in comparison to orthodox medicine!), basic and clinical researches progress at a snail’s pace. Yet they allow putting forward new exciting scientific ideas that would indicate the ozone capability of restoring health, if we can prove their exactness.
References
- Abraham N. G., Drummond G. S., Lutton J. D., Kappas A. The biological significance and physiological role of heme oxygenase. Cell. Physiol. Biochem. 1996;6:129–168. [Google Scholar]
- Aicher A., Heeschen C., Mildner-Rihm C., Urbich C., Ihling C., Technau-Ihling K., Zeiher A. M., Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Natl. Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Amersi F., Buelow R., Kato H., Ke B., Coito A. J., Shen X. D., Zhao D., Zaky J., Melinek J., Lassman C. R., Kolls J. K., Alam J., Ritter T., Volk H. D., Farmer D. G., Ghobrial R. M., Busuttil R. W., Kupiec-Weglinski J. W. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J. Clin. Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N. A role for supplements in optimizing health: the metabolic tune-up. Arch. Biochem. Biophys. 2004;423:227–234. doi: 10.1016/j.abb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Barakat S., Seif-El Nasr A., Ardel-Maksoud N., El-Ebiary F., Amer H., Zaghloul A., Thabet S. Induktion der angiogenese durch medizinisches ozon. In: Viebahn-Hansler R., Knoch H. G., editors. Ozon-Handbuch Grundlagen Pravention Therapie. Landsberg: Ecomed; 2004. [Google Scholar]
- Barber E., Menéndez S., León O. S., Barber M. O., Merino N., Calunga J. L., Cruz E., Bocci V. Prevention of renal injury after induction of ozone tolerance in rats submitted to warm ischaemia. Mediators Inflamm. 1999;8:37–41. doi: 10.1080/09629359990702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykal Y., Yilmaz M. I., Celik T., Gok F., Rehber H., Akay C., Kocar I. H. Effects of antihypertensive agents, alpha receptor blockers, beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, on oxidative stress. J. Hypertens. 2003;21:1207–1211. doi: 10.1097/00004872-200306000-00022. [DOI] [PubMed] [Google Scholar]
- Belluzzi A., Brignola C., Campieri M., Pera A., Boschi S., Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. N. Engl. J. Med. 1996;334:1557–1560. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- Bender D. A. Daily doses of multivitamin tablets. BMJ. 2002;325:173–174. doi: 10.1136/bmj.325.7357.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V. Does ozone therapy normalize the cellular redox balance? Med. Hypotheses. 1996;46:150–154. doi: 10.1016/s0306-9877(96)90016-x. [DOI] [PubMed] [Google Scholar]
- Bocci V. Ozone: a mixed blessing. New mechanisms of the action of ozone on blood cells make ozonated major autohaemotherapy (MAH) a rational approach. Forsch. Komplementärmed. 1996;3:25–33. [Google Scholar]
- Bocci V. Ossigeno-ozono terapia. Milano: Casa Editrice Ambrosiana; 2000. pp. 1–324. [Google Scholar]
- Bocci V. Oxygen-ozone therapy, a critical evaluation. Dordrecht: Kluwer Academic Publischer; 2002. [Google Scholar]
- Bocci V. Può l’Ossigeno-Ozonoterapia migliorare la prognosi della bronco-pneumopatia cronica ostruttiva? Giorn. Ital. Mal. Tor. 2007;61:434–446. [Google Scholar]
- Bocci V., Paulesu L. Studies on the biological effects of ozone 1. Induction of interferon gamma on human leucocytes. Haematologica. 1990;75:510–515. [PubMed] [Google Scholar]
- Borrego A., Zamora Z. B., Gonzalez R., Romay C., Menendez S., Hernandez F., Montero T., Rojas E. Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators Inflamm. 2004;13:13–19. doi: 10.1080/09629350410001664806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman M. M., Marsden M., MacNee W., Flenley D. C., Ryle A. P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax. 1991;46:39–42. doi: 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard S., Otterbein L. E., Anrather J., Tobiasch E., Bach F. H., Choi A. M., Soares M. P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. J. Hormesis: changing view of the dose-response, a personal account of the history and current status. Mutat. Res. 2002;511:181–189. doi: 10.1016/s1383-5742(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Calabrese E. J., Baldwin L. A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese E. J. Getting the dose-response wrong: why hormesis became marginalized and the threshold model accepted. Arch. Toxicol. 2009;83:227–247. doi: 10.1007/s00204-009-0411-5. [DOI] [PubMed] [Google Scholar]
- Cardile V., Jiang X., Russo A., Casella F., Renis M., Bindoni M. Effects of ozone on some biological activities of cells in vitro. Cell Biol. Toxicol. 1995;11:11–21. doi: 10.1007/BF00769988. [DOI] [PubMed] [Google Scholar]
- Chen Z., Oberley T. D., Ho Y., Chua C. C., Siu B., Hamdy R. C., Epstein C. J., Chua B. H. Overexpression of CuZnSOD in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radic. Biol. Med. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Choi B. M., Pae H. O., Kim Y. M., Chung H. T. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810–823. doi: 10.1053/jhep.2003.50114. [DOI] [PubMed] [Google Scholar]
- Christian D. L., Chen L. L., Scannell C. H., Ferrando R. E., Welch B. S., Balmes J. R. Ozone-induced inflammation is attenuated with multiday exposure. Am. J. Respir. Crit. Care Med. 1998;158:532–537. doi: 10.1164/ajrccm.158.2.9709023. [DOI] [PubMed] [Google Scholar]
- Clinton S. K. Lycopene: chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Csonka C., Pataki T., Kovacs P., Muller S. L., Schroeter M. L., Tosaki A., Blasig I. E. Effects of oxidative stress on the expression of antioxidative defense enzymes in spontaneously hypertensive rat hearts. Free Radic. Biol. Med. 2000;29:612–619. doi: 10.1016/s0891-5849(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Csonka C., Varga E., Kovacs P., Ferdinandy P., Blasig I. E., Szilvassy Z., Tosaki A. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic. Biol. Med. 1999;27:119–126. doi: 10.1016/s0891-5849(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Das U. N. Folic acid says NO to vascular diseases. Nutrition. 2003;19:686–692. doi: 10.1016/s0899-9007(02)01044-4. [DOI] [PubMed] [Google Scholar]
- Di Paolo N., Bocci V., Salvo D. P., et al. Extracorporeal blood oxygenation and ozonation (EBOO). A controlled trial in patients with peripheral artery disease. Int. J. Artif. Organs. 2005;28:1039–1050. doi: 10.1177/039139880502801012. [DOI] [PubMed] [Google Scholar]
- Dong Z., Lavrovsky Y., Venkatachalam M. A., Roy A. K. Heme oxygenase-1 in tissue pathology: the Yin and Yang. Am. J. Pathol. 2000;156:1485–1488. doi: 10.1016/S0002-9440(10)65019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H. Glutathione peroxidase and oxidative stress. Toxicol. Lett. 1995;82/83:395–398. doi: 10.1016/0378-4274(95)03570-2. [DOI] [PubMed] [Google Scholar]
- Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J. Cell Physiol. 2003;195:373–382. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]
- Enwonwu J. W. Increased metabolic demand for arginine in sickle cell anemia. Med. Sci. Res. 1989;17:997–998. [Google Scholar]
- Farquharson C. A., Butler R., Hill A., Belch J. J., Struthers A. D. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Fontana L., McNeill K. L., Ritter J. M., Chowienczyk P. J. Effects of vitamin C and of a cell permeable superoxide dismutase mimetic on acute lipoprotein induced endothelial dysfunction in rabbit aortic rings. Br. J. Pharmacol. 1999;126:730–734. doi: 10.1038/sj.bjp.0702331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Meyer T. E., Klein S., Holloszy J. O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H. J., Fukuto J. M., Miller T., et al. The chemistry of cell signalling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch. Biochem. Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. Heme oxygenase: who needs it? Proc. Soc. Exp. Biol. Med. 1999;222:299–305. doi: 10.1177/153537029922200313. [DOI] [PubMed] [Google Scholar]
- Goldman M. Cancer risk of low-level exposure. Science. 1996;271:1821–1822. doi: 10.1126/science.271.5257.1821. [DOI] [PubMed] [Google Scholar]
- Hack V., Breitkreutz R., Kinscherf R., Rohrer H., Bartsch P., Taut F., Benner A., Droge W. The redox state as a correlate of senescence and wasting and as a target for therapeutic intervention. Blood. 1998;92:59–67. [PubMed] [Google Scholar]
- Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic. Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Vitamin C: poison, prophylactic or panacea? Trends Biochem. Sci. 1999;24:255–259. doi: 10.1016/s0968-0004(99)01418-8. [DOI] [PubMed] [Google Scholar]
- Heng H., Rucker R. B., Crotty J., Dubick M. A. The effects of ozone on lung, heart, and liver superoxide dismutase and glutathione peroxidase activities in the protein-deficient rat. Toxicol. Lett. 1987;38:225–237. doi: 10.1016/0378-4274(87)90003-8. [DOI] [PubMed] [Google Scholar]
- Hennekens C. H., Buring J. E., Peto R. Antioxidant vitamins – benefits not yet proved. N. Engl. J. Med. 1994;330:1080–1081. doi: 10.1056/NEJM199404143301510. [DOI] [PubMed] [Google Scholar]
- Hernandez F., Menendez S., Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic. Biol. Med. 1995;19:115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- Jolly C., Morimoto R. I. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Jornot L., Mirault M. E., Junod A. F. Differential expression of hsp70 stress proteins in human endothelial cells exposed to heat shock and hydrogen peroxide. Am. J. Respir. Cell Mol. Biol. 1991;5:265–275. doi: 10.1165/ajrcmb/5.3.265. [DOI] [PubMed] [Google Scholar]
- Joyner M. J., Dietz N. M. Nitric oxide and vasodilation in human limbs. J. Appl. Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Kang H. J., Kim H. S., Zhang S. Y., Park K. W., Cho H. J., Koo B. K., Kim Y. J., Soo Lee D., Sohn D. W., Han K. S., Oh B. H., Lee M. M., Park Y. B. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- Kashiba M., Kasahara E., Chien K. C., Inoue M. Fates and vascular action of S-nitrosoglutathione and related compounds in the circulation. Arch. Biochem. Biophys. 1999;363:213–218. doi: 10.1006/abbi.1998.1055. [DOI] [PubMed] [Google Scholar]
- Katano H., Pesnicak L., Cohen J. I. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas, Proc. Natl. Acad. Sci. USA. 2004;101:4960–4965. doi: 10.1073/pnas.0305149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang J. G., Tsokos G. C. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol. Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Kume M., Yamamoto Y., Saad S., Gomi T., Kimoto S., Shimabukuro T., Yagi T., Nakagami M., Takada Y., Morimoto T., Yamaoka Y. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J. Lab. Clin. Med. 1996;128:251–258. doi: 10.1016/s0022-2143(96)90026-8. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- León O. S., Menéndez S., Merino N., Castillo R., Sam S., Pérez L., Cruz E., Bocci V. Ozone oxidative preconditioning: a protection against cellular damage by free radicals. Mediators Inflamm. 1998;7:289–294. doi: 10.1080/09629359890983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Conry-Cantilena C., Wang Y., Welch R. W., Washko P. W., Dhariwal K. R., Park J. B., Lazarev A., Graumlich J. F., King J., Cantilena L. R. Vitamin C pharmacokinetics in health volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J. K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llevadot J., Murasawa S., Kureishi Y., Uchida S., Masuda H., Kawamoto A., Walsh K., Isner J. M., Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow – derived endothelial progenitor cells. J. Clin. Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madej P., Plewka A., Madej J. A., et al. Ozone therapy in induced endotoxemic shock. II. The effect of Ozone therapy upon selected histochemical reactions on organs of rats in endotoxemic shock. Inflammation. 2007;30:69–86. doi: 10.1007/s10753-007-9023-5. [DOI] [PubMed] [Google Scholar]
- McCall M. R., Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic. Biol. Med. 1999;26:1034–1053. doi: 10.1016/s0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- Miyazono M., Garat C., Morris K. G., Jr, Carter E. P. Decreased renal heme oxygenase-1 expression contributes to decreased renal function during cirrhosis. Am. J. Physiol. Renal. Physiol. 2002;283:F1123–F1131. doi: 10.1152/ajprenal.00363.2001. [DOI] [PubMed] [Google Scholar]
- Mori T. A., Woodman R. J., Burke V., Puddey I. B., Croft K. D., Beilin L. J. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic. Biol. Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Morris C. R., Kuypers F. A., Larkin S., Sweeters N., Simon J., Vichinsky E. P., Styles L. A. Arginine therapy: a novel strategy to induce nitric oxide production in sickle cell disease. Br. J. Haematol. 2000;111:498–500. doi: 10.1046/j.1365-2141.2000.02403.x. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nath K. A., Haggard J. J., Croatt A. J., Grande J. P., Poss K. D., Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am. J. Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi P. Risks and benefits of gene therapy. N. Engl. J. Med. 2003;348:193–194. doi: 10.1056/NEJMp020184. [DOI] [PubMed] [Google Scholar]
- Olivieri G., Bodycote J., Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- Orlic D., Kajstura J., Chimenti S., Limana F., Jakoniuk I., Quaini F., Nadal-Ginard B., Bodine D. M., Leri A., Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Roy S., Sen C. K. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv. Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- Peng J., Jones G. L., Watson K. Stress proteins as biomarkers of oxidative stress: effects of antioxidant supplements. Free Radic. Biol. Med. 2000;28:1598–1606. doi: 10.1016/s0891-5849(00)00276-8. [DOI] [PubMed] [Google Scholar]
- Peralta C., Leon O. S., Xaus C., Prats N., Jalil E. C., Planell E. S., Puig-Parellada P., Gelpi E., Rosello-Catafau J. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: antioxidant-prooxidant balance. Free Radic. Res. 1999;31:191–196. doi: 10.1080/10715769900300741. [DOI] [PubMed] [Google Scholar]
- Peralta C., Xaus C., Bartrons R., Leon O. S., Gelpi E., Rosello-Catafau J. Effect of ozone treatment on reactive oxygen species and adenosine production during hepatic ischemia-reperfusion. Free Radic. Res. 2000;33:595–605. doi: 10.1080/10715760000301121. [DOI] [PubMed] [Google Scholar]
- Polidori M. C., Mecocci P., Levine M., Frei B. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch. Biochem. Biophys. 2004;423:109–115. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Polidori M. C., Stahl W., Eichler O., Niestroj I., Sies H. Profiles of antioxidants in human plasma. Free Radic. Biol. Med. 2001;30:456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic. Biol. Med. 2000;28:141–164. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Puskas F., Gergely P., Jr, Banki K., Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–1361. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- Rahman I., Clerch L. B., Massaro D. Rat lung antioxidant enzyme induction by ozone. Am. J. Physiol. 1991;260:L412–L418. doi: 10.1152/ajplung.1991.260.6.L412. [DOI] [PubMed] [Google Scholar]
- Reeve V. E., Tyrrell R. M. Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc. Natl. Acad. Sci. USA. 1999;96:9317–9321. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riksen N. P., Rongen G. A., Blom H. J., Russel F. G., Boers G. H., Smits P. Potential role for adenosine in the pathogenesis of the vascular complications of hyperhomocysteinemia. Cardiovasc. Res. 2003;59:271–276. doi: 10.1016/s0008-6363(03)00462-0. [DOI] [PubMed] [Google Scholar]
- Roberts W. C. The underused miracle drugs: the statin drugs are to atherosclerosis what penicillin was to infectious disease, Am. J. Cardiol. 1996;78:377–378. doi: 10.1016/s0002-9149(96)00441-9. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Tyrrell R. M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Dargusch R., Chambers D., Davis J., Schubert D., Maher P. Cellular mechanisms of resistance to chronic oxidative stress. Free Radic. Biol. Med. 1998;24:1375–1389. doi: 10.1016/s0891-5849(97)00457-7. [DOI] [PubMed] [Google Scholar]
- Sato K., Balla J., Otterbein L., Smith R. N., Brouard S., Lin Y., Csizmadia E., Sevigny J., Robson S. C., Vercellotti G., Choi A. M., Bach F. H., Soares M. P. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Curfman G. D. Can the heart repair itself? N. Engl. J. Med. 2002;346:2–4. doi: 10.1056/NEJM200201033460102. [DOI] [PubMed] [Google Scholar]
- Seifried H. E., McDonald S. S., Anderson D. E., Greenwald P., Milner J. A. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- Shull S., Heintz N. H., Periasamy M., Manohar M., Janssen Y. M. W., Marsh J. P., Mossman B. T. Differential regulation of antioxidant enzymes in response to oxidants. J. Biol. Chem. 1991;266:24398–24403. [PubMed] [Google Scholar]
- Snyder S. H., Baranano D. E. Heme oxygenase: a font of multiple messengers. Neuropsychopharmacology. 2001;25:294–298. doi: 10.1016/S0893-133X(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Spencer F. A., Allegrone J., Goldberg R. J., Gore J. M., Fox K. A., Granger C. B., Mehta R. H., Brieger D. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Ann. Intern. Med. 2004;140:857–866. doi: 10.7326/0003-4819-140-11-200406010-00006. [DOI] [PubMed] [Google Scholar]
- Stadlbauer T. H. W., Eisele A., Heidt M. C., et al. Preconditioning with ozone abrogates acute rejection and prolongs cardiac allograft survival in rats. Transplant. Proc. 2008;40:974–977. doi: 10.1016/j.transproceed.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. S-nitrosothiols in the blood: roles, amounts, and methods of analysis. Circ. Res. 2004;94:414–417. doi: 10.1161/01.RES.0000122071.55721.BC. [DOI] [PubMed] [Google Scholar]
- Strauer B. E., Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–934. doi: 10.1161/01.cir.0000057525.13182.24. [DOI] [PubMed] [Google Scholar]
- Strauer B. E., Brehm M., Zeus T., Gattermann N., Hernandez A., Sorg R. V., Kogler G., Wernet P. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch. Med. Wochenschr. 2001;126:932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- Su W. Y., Gordon T. In vivo exposure to ozone produces an increase in a 72-kDa heat shock protein in guinea pigs. J. Appl. Physiol. 1997;83:707–711. doi: 10.1152/jappl.1997.83.3.707. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Lu F. J., Huang W. C., Hou S. M., Tsuang Y. H., Hang Y. S. Antioxidant status following acute ischemic limb injury: a rabbit model. Free Radic. Res. 1999;31:9–21. doi: 10.1080/10715769900300561. [DOI] [PubMed] [Google Scholar]
- Tacchini L., Pogliaghi G., Radice L., Bernelli-Zazzera A., Cairo G. Post-transcriptional control of increased hepatic catalase gene expression in response to oxidative stress. Redox Report. 1996;2:273–278. doi: 10.1080/13510002.1996.11747061. [DOI] [PubMed] [Google Scholar]
- Tepel M., van der G. M., Statz M., Jankowski J., Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
- Topol E. J. Intensive statin therapy – a sea change in cardiovascular prevention. N. Engl. J. Med. 2004;350:1562–1564. doi: 10.1056/NEJMe048061. [DOI] [PubMed] [Google Scholar]
- Vasiliou V., Pappa A., Petersen D. R. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem. Biol. Interact. 2000;129:1–19. doi: 10.1016/s0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Victor V. M., McCreath K. J., Rocha M. Recent progress in pharmacological research of antioxidants in pathological conditions, cardiovascular health. Recent Pat. Antinfect. Drug Discov. 2006;1:17–31. doi: 10.2174/157489106775244136. [DOI] [PubMed] [Google Scholar]
- Vivekananthan D. P., Penn M. S., Sapp S. K., Hsu A., Topol E. J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- Vollmer T., Key L., Durkalski V., Tyor W., Corboy J., Markovic-Plese S., Preiningerova J., Rizzo M., Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R., Poole-Wilson P. A., Dietz R. Regenerative capacity of the myocardium: implications for treatment of heart failure. Lancet. 2004;363:1306–1313. doi: 10.1016/S0140-6736(04)16006-6. [DOI] [PubMed] [Google Scholar]
- Wadhwa P. D., Zielske S. P., Roth J. C., Ballas C. B., Bowman J. E., Gerson S. L. Cancer gene therapy: scientific basis. Annu. Rev. Med. 2002;53:437–452. doi: 10.1146/annurev.med.53.082901.104039. [DOI] [PubMed] [Google Scholar]
- Wagner M., Cadetg P., Ruf R., Mazzucchelli L., Ferrari P., Redaelli C. A. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003;63:1564–1573. doi: 10.1046/j.1523-1755.2003.00897.x. [DOI] [PubMed] [Google Scholar]
- Wang P., Chen H., Qin H., Sankarapandi S., Becher M. W., Wong P. C., Zweier J. L. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc. Natl. Acad. Sci. USA. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. Aspects of the adaptive response to very low doses of radiation and other agents. Mutat. Res. 1996;358:135–142. doi: 10.1016/s0027-5107(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Yamamoto Y., Yamagami K., Kume M., Kimoto S., Toyokuni S., Uchida K., Fukumoto M., Yamaoka Y. Heat-shock preconditioning reduces oxidative protein denaturation and ameliorates liver injury by carbon tetrachloride in rats. Res. Exp. Med. (Berl). 2000;199:309–318. doi: 10.1007/s004339900040. [DOI] [PubMed] [Google Scholar]
- Zino S., Skeaff M., Williams S., Mann J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ. 1997;314:1787–1791. doi: 10.1136/bmj.314.7097.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerbraun B. S., Billiar T. R. Heme oxygenase-1: a cellular Hercules. Hepatology. 2003;37:742–744. doi: 10.1053/jhep.2003.50139. [DOI] [PubMed] [Google Scholar]
- Seixas E., Gozzelino R., Chora A. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl. Acad. Sci. USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]


