Abstract
The ADP-ribosylation factor (ARF) superfamily of regulatory GTPases, including both the ARF and ARF-like (ARL) proteins, control a multitude of cellular functions, including aspects of vesicular traffic, lipid metabolism, mitochondrial architecture, the assembly and dynamics of the microtubule and actin cytoskeletons, and other pathways in cell biology. Considering their general utility, it is perhaps not surprising that increasingly ARF/ARLs have been found in connection to primary cilia. Here, we critically evaluate the current knowledge of the roles four ARF/ARLs (ARF4, ARL3, ARL6, ARL13B) play in cilia and highlight key missing information that would help move our understanding forward. Importantly, these GTPases are themselves regulated by guanine nucleotide exchange factors (GEFs) that activate them and by GTPase-activating proteins (GAPs) that act as both effectors and terminators of signaling. We believe that the identification of the GEFs and GAPs and better models of the actions of these GTPases and their regulators will provide a much deeper understanding and appreciation of the mechanisms that underly ciliary functions and the causes of a number of human ciliopathies.
Keywords: ARF GTPases, cilia
INTRODUCTION
Primary cilia are found on the vast majority of mammalian cells and play essential roles in signaling, particularly important during development, and in highly specialized cell types responsible for sensory perception and signaling, most notably retinal and olfactory epithelial cells. Mutations in almost 200 genes have been linked to ciliopathies (with another ~50 candidates under investigation), which can result from defects in the structure of cilia, or from altered protein content in cilia or traffic to and within cilia, with consequent defects in ciliary signaling (132). Recent reviews provide comprehensive summaries of current knowledge of ciliary biogenesis and intraflagellar transport (IFT), and of the many factors known to regulate ciliogenesis, ciliary homeostasis, and cilia-dependent signaling (81, 107, 118, 128). Briefly, the cilium consists of a microtubule backbone called the axoneme, covered by a lipid bilayer that is continuous with the plasma membrane, although maintaining a distinct composition of membrane proteins and means of their localization. Protein content in the cilium is controlled by the transition zone at the base of the cilium that, through incompletely understood mechanisms, separates the cilium from the cytoplasm and plasma membrane. Traffic to the cilium is exquisitely controlled during ciliogenesis and throughout the lifetime of the cilium. Proteins are targeted to cilia via a number of different routes and mechanisms that are different for small soluble, large soluble, and membrane proteins. Small proteins (<40 kDa) are thought to diffuse through the transition zone while larger proteins rely on transporters such as importin-β1 (87). Membrane proteins destined for the cilium are synthesized in the endoplasmic reticulum, transported through the Golgi to the trans-Golgi network (TGN) where they are sorted into transport vesicles that are targeted to the plasma membrane at the ciliary base. The mechanisms through which they then enter the cilium through lateral transport remain under active investigation. One of several pathways used by a subset of ciliary proteins is a VxP ciliary targeting sequence (CTS) that was first identified in the transmembrane rhodopsin and later found to be conserved in additional transmembrane ciliary proteins, as well as in soluble proteins such as ADP-ribosylation factor-like 13B (ARL13B) (34). However, not all ciliary proteins contain this sequence, and the VxP motif is not necessarily a predictor of ciliary traffic, since analysis of VxP prevalence in the mammalian genome results in ~40% of all proteins having this motif. Once inside the cilium, transmembrane and soluble proteins may freely diffuse or be carried in a directional manner along the axoneme by the intraflagellar transport system.
In this review we focus on the members of the ADP-ribosylation factor (ARF) superfamily, both the ARF and ARF-like (ARL) GTPases, and our current understanding of their role(s) in cilia and at centrosomes/basal bodies, including traffic to and within cilia as well as links to ciliogenesis and ciliary signaling. Below, we summarize current understanding and models of the actions of four ARF superfamily members with links to cilia (ARF4, ARL3, ARL6, and ARL13B), with additional comparisons between ARL3 and its closest paralog, ARL2. A more complete treatment of the entire ARF superfamily and the challenges facing researchers studying them can be found in Sztul et al. (151). Before describing the current models of each ARF/ARL GTPase function in cilia, we have included a short summary of relevant ARF family GTPase biology, to point out what we consider some of the underlying causes of concern or potential weaknesses in current models.
Studies of ciliary biology are technically challenging for a number of reasons but not the least is their small size, relative to the cell body. In contrast to the exaggerated cilia of retinal cells (e.g., rods), termed outer segments, primary cilia are smaller (1–10 µm) and are not readily purified from mammalian sources. Furthermore, we have limited (though quickly growing) understanding of the regulation of protein import and export through the transition zone. A couple of studies have provided an important service in alerting researchers to the risks of overexpressing proteins and overwhelming the regulatory systems in place, resulting in potentially misinterpreting data (90, 176). Perhaps less appreciated in the field are the concerns raised by NH2-terminal truncation or epitope tagging of ARF family GTPases at either end (82). This can result in altered localizations, changes in nucleotide-binding properties and thus regulation of activities, and in affinity for binding partners, including effectors, that bind directly to the NH2-terminus. While the use of exogenous engineered proteins is practically unavoidable, the resulting concerns should be taken into account in designing experiments and certainly when discussing results gained from their use. Indeed, it is important to understand the technical challenges in studies of ciliogenesis and cilia function to better appreciate the impressive progress made in the last decade.
ARF AND ARLs
ARF superfamily regulatory GTPases (like the heterotrimeric G proteins and monomeric RAS superfamilies) all share a “GTPase domain” with highly conserved structures, the ability to bind guanine nucleotides, assume different conformations depending upon which nucleotide is bound (GDP or GTP), and bind with altered affinities to protein partners as a consequence of the different conformations. As a result, these “molecular switches” commonly regulate signaling pathways that are defined by 1) the GTPase, 2) the guanine nucleotide exchange factor (GEF) that promotes release of GDP and binding of GTP (activation step), 3), the effector that binds preferentially to the activated GTPase and propagates the biological signal to downstream partners, and 4) the GTPase-activating protein (GAP) that also binds preferentially to the activated species and promotes hydrolysis of bound GTP to return it to its GDP-bound (inactive) state.
The ARF superfamily is composed of 30 monomeric GTPases in mammals, including 6 ARFs (sharing >65% identity), 22 ARLs (typically sharing 40–60% identity to each other or to ARFs), and 2 secretion-associated RAS-related proteins (SARs), that act as molecular switches to control a wide range of cellular functions (17, 30, 37, 71, 77, 86, 121, 124, 151). The family is ancient, with more than a dozen GTPases present in the last eukaryotic common ancestor and expressed widely among eukaryotes, attesting to their fundamental and highly conserved roles in eukaryotic cell biology. Mammalian cells also may express as many as 15 ARF GEFs, which can be divided into five families based on sequence relatedness and domain organization, but all sharing a highly conserved catalytic Sec7 domain (16, 19, 151, 170). The ARF GEFs promote GDP/GTP exchange on ARFs but do not act on ARLs in in vitro GEF assays. The only documented ARL GEF activity is that of ARL13B, acting as a GEF in vitro for ARL3 (54, 75; see section on ARL13B). There are also 23 known mammalian ARF GAPs, each sharing the ARF GAP domain, with most active (at least in vitro) on each of the six mammalian ARFs but almost none acting on ARLs (71, 131, 151). There are only four proteins with demonstrated GAP activity against any ARLs; the three ELMOD proteins and retinitis pigmentosa 2 (RP2) (14, 38, 76, 158, 171). Thus, there are very likely to be yet unidentified ARL GEFs and ARL GAPs whose identities are central to developing models for understanding of ARL functions and pathways (151).
At least some ARLs involved in ciliary biology are “atypical” in that they deviate in primary sequence within the canonical G motifs. Thus, it is worth considering how they may differ in mechanisms and why caution is required in interpreting data from (over)expression and mutagenesis studies that are based on what is known for other GTPases, even those in the same family. By far the most common way of testing GTPase cellular functions is to express point mutants that are designed to mimic a stably activated (aka “dominant active”) or inactivated (aka “dominant negative”) form of the GTPase. All regulatory GTPases share the G domain, which contains five highly conserved motifs that in the folded protein form the guanine nucleotide-binding site (13, 146, 169). The G-3 motif is most often present as DVGGQ in both ARF and heterotrimeric G protein families and DTAGQ in RAS. The glutamine (Q) has consistently been found to be essential for GTP hydrolysis, both spontaneous and GAP driven, through the coordination of a water molecule required to break the β-γ phosphate bond. Mutation of this glutamine, most often to leucine, is sufficient to dramatically impede GTP hydrolysis, resulting in a much prolonged activated state. The equivalent residue in ARF1 is Q71L and in RAS is Q61L, with the latter being particularly important because of its links to human cancers (17, 177). Because the Q mutants are activated by GEFs, but do not readily hydrolyze their GTP, they have prolonged interactions with effectors. In addition, they may bind GAPs without undergoing inactivation and thereby may also inhibit GAP’s inactivating activity on endogenous wild-type GTPases, resulting in a dominant active phenotype. However, ARL10, ARL13 (A and B), ARL15, and ARL16 have diverged in the G-3 motif and lack this “catalytic glutamine,” depriving researchers of this valuable tool but offering the likelihood of novel means of regulation that is predicted to inform future models of signaling by these GTPases. Whether the lack of this glutamine in the wild-type proteins results in a chronically activated state or if these GTPases use a different mechanism for GTP hydrolysis and inactivation is unknown.
The other common and valuable point mutant used to study regulatory GTPases is often termed dominant negative, dominant inactive, or (incorrectly) as GDP locked. These are typically point mutants in residues in serine or threonine at the end of the G-1 motif, GX4GK(S/T) (T31N in ARF1, T27N in ARF6, and S17N in RAS). These residues make direct contact with the bound nucleotide and magnesium, and when mutated result in decreased affinity for both GTP and GDP, although typically the effect is greater on GTP (26, 110, 119). Because the T31 mutants typically have reduced affinity for both GTP and GDP, they are actually likely to be nucleotide free (110). Thus, rather than being “GDP-locked” they are probably apo-GTPases. These forms of GTPases are found as intermediates in the GEF reaction and thus may act as transition state inhibitors of GEFs, tying them up and preventing the activation of the endogenous (wild-type) GTPases, explaining their dominant effects in cells (26, 43, 110). Importantly, when more than one GTPase shares a common GEF, it could cause misinterpretations of the effects of such a point mutant in one GTPase while, in fact, activities of another GTPase are inhibited. Furthermore, because some (e.g., ARL2 and ARL13B) GTPases depend on a bound nucleotide for protein stability, such mutants are often found expressed to lower levels than wild-type or other mutants.
Another unusual aspect of ARF family GTPases is that they display highly varied affinities for guanine nucleotides. For example, ARFs release GDP slowly in aqueous solution (Kd values in the nM range) and display a strong preference for a hydrophobic environment to promote GDP dissociation. In contrast, at least some purified ARLs (including ARL2 and ARL13B) exchange GTP and GDP readily and without apparent regard to the environment, displaying affinities for guanine nucleotides in the micromolar range (15, 75).
The last feature of ARF superfamily GTPases worth mentioning here is that, when aligned to members of other GTPase families (Rab, Ras, Rho, etc.), the ARFs typically have an NH2-terminal extension of ~10–15 residues. The NH2-terminus is N-myristoylated in all ARFs and ARL5s, N-acetylated on ARL3, and palmitoylated on cysteine 8 and 9 of ARL13B. These modifications promote membrane association and in the case of at least the ARFs do so in an activation-dependent fashion. While GDP-bound ARFs are soluble, the binding of GTP requires release of the NH2-terminal helix from a hydrophobic groove on the surface of the GTPase, leading to the translocation of the GTPase from cytosol to membrane as part of the activation process (104, 106, 139). In contrast, despite having an NH2-terminal glycine (site of N-myristoylation), ARL2 is not myristoylated, and, despite having amphipathic α-helices at their NH2-termini, ARLs have not been shown to translocate onto membranes upon activation. Rather, the NH2-terminal helices (or at least residues found within them) have been found to make critical direct contact with effectors, essentially serving as a third effector loop in addition to the two canonical ones present in all regulatory GTPases (5, 6, 180). These NH2-terminal helices are also important in displacement of cargos bound to other binding partners (see below discussion of mechanisms of ARL3 and ARL2). For these reasons, deletion or tagging of the NH2-terminus of any ARF/ARL proteins can have dramatic changes in activity, interactions, and binding properties.
The ARF family is best known for the roles of ARF1–5 in membrane traffic, particularly at the Golgi, and of ARF6 in cytokinesis and actin organization at or near the plasma membrane (reviewed in Refs. 96, 134, and 151). Only one ARF, ARF4, has been implicated in ciliary traffic by acting at the TGN. In contrast, at least four ARLs regulate aspects of cilia biology. Early work showed the importance of ARL13/ARL13B to ciliogenesis in zebrafish (called scorpion or hi459), Caenorhabditis elegans, and mammalian development with links to Joubert syndrome (18, 20, 21, 150) and of ARL6 (BBS3) to Bardet-Biedl syndrome (23, 41, 92) and ciliary traffic linked to the BBSome (84, 167). ARL3 was first implicated in flagellar biogenesis in Leishmania and later linked to kidney and photoreceptor development along with Joubert syndrome (1, 29, 136). ARL2 is the closest paralog of ARL3 (52% identity), with each localizing in part to centrosomes and sharing a subset of effectors (156, 182). ARL2 is also implicated in ciliary stability and development in photoreceptors, but is discussed below only in context with ARL3 (172). Each of these four ARLs localizes to centrosomes and/or cilia and function at least in part at those sites. We describe the current status of our understanding of the cilia-related actions of each of these GTPases below.
ARF4
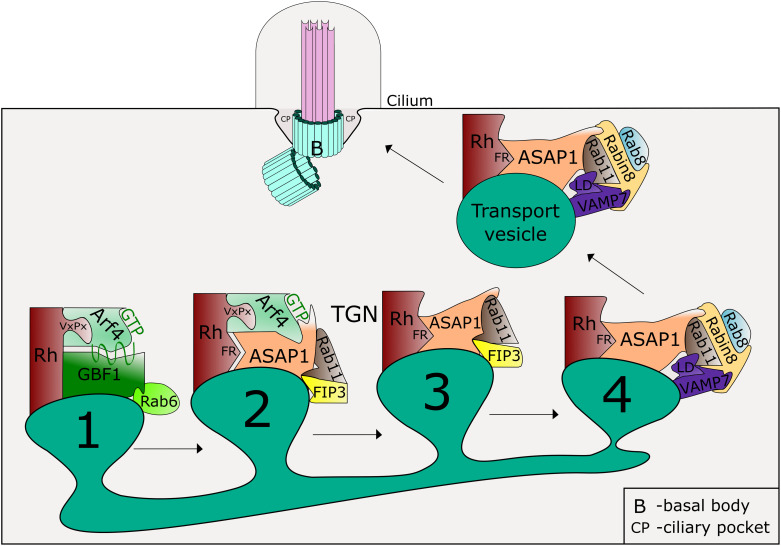
The study of ARF4 function in ciliary traffic began with the identification of ARF4 as an interactor of rhodopsin, a major component of the outer segments of vertebrate photoreceptor cells (35). This and subsequent work from the Deretic laboratory formed the basis for a model in which ARF4 binds to a recently synthesized ciliary cargo protein and facilitates its traffic from the TGN to the cilium by nucleating the formation of a series of protein complexes that include ARF4, ArfGAP with SH3 domain, ankyrin repeat and PH domain 1 (ASAP1), Rab11 family interacting protein 3 (FIP3; also known as Rab11-FIP3 as it is an effector of Rab11), Rab11, Rab8, and Rabin8 (see Fig. 1). Cargos include rhodopsin, polycystin1/2 (PC1/PC2), and fibrocystin.
Fig. 1.
Model for ADP-ribosylation factor 4 (ARF4) role in rhodopsin sorting at the trans-Golgi network (TGN). The process begins with the recruitment of the guanine nucleotide exchange factor (GEF) Golgi brefeldin A-resistant guanine nucleotide exchange factor 1 (GBF1) to TGN membranes by the small GTPase Rab6, GBF1-mediated activation of ARF4 (ARF4-GTP), which recognizes and binds to the ciliary targeting sequence (VxPx motif) of rhodopsin (Rh) (step 1). The activated ARF4 recruits Arf GTPase-activating protein (GAP) with SH3 domain, ankyrin repeat and PH domain 1 (ASAP1), which also binds to rhodopsin through the phenylalanine arginine motif (FR) and recruits Rab11 and Rab11 family-interacting protein 3 (FIP3, step 2). ASAP1 with the aid of FIP3 promotes the hydrolysis of GTP on ARF4, which is released from the complex (step 3). Following the recruitment of Rab8 and the Rab8 GEF Rabin8, FIP3 dissociates from the complex and is replaced by the SNARE VAMP7. VAMP7 interacts with Rabin8 through the SNARE domain and with Rab11 and Rab8 through longin domain (LD) (step 4). This process sorts rhodopsin into transport vesicles targeted to the base of the cilium and ensures the fusion of these vesicles with the target membrane.
In this model (Fig. 1), traffic from the TGN to cilia is initiated by the activation of ARF4 by the GEF, Golgi brefeldin A resistant guanine nucleotide exchange factor 1 (GBF1), and the binding of the activated ARF4 to rhodopsin through a COOH-terminal VxP CTS in rhodopsin, which initiates vesicle budding (35, 115). The initiating recruitment of GBF1 to TGN membrane has been proposed to be mediated by the small GTPase Rab6 (32, 33). ARF4-GTP and rhodopsin bind ASAP1 (an ARF GAP/effector), which in turn binds FIP3. Rab11 and FIP3 act together with ASAP1 to recruit Rab8 and a GEF for Rab8, Rabin8 (63–66, 70, 135, 144, 160, 163, 164, 168). Together these components are proposed to form a number of complexes (see Fig. 1) that ensure the sorting of ciliary proteins into ciliary vesicles at the TGN and the subsequent VAMP3-mediated fusion of such vesicles at the plasma membrane or the ciliary pocket membrane. FIP3 increases the GAP activity of ASAP1, resulting in inactivation and release of ARF4 from the membrane (115, 163). Thus, the role of ARF4 in ciliary traffic at the TGN is analogous to the roles of ARF1/3 at the Golgi in facilitating the sorting of select proteins into COPI-coated retrograde vesicles (2, 145, 152). The key roles of FIP3, Rab11, Rab8, and Rabin8 in ciliogenesis and in the transport of vesicular cargo to the cilium have been documented by numerous groups and extensively reviewed (12, 49, 91, 125, 129, 162, 166, 173).
The ARF4-nucleated complex was also reported to regulate ciliary delivery of PC1 (an 11 trans-membrane domain protein, mutations in which cause autosomal dominant polycystic kidney disease) and PC2 (50, 157). Each has a VxP motif analogous to that of rhodopsin in their cytosolic tail, and this motif has been reported to be required for their ciliary traffic (50, 165). How strong is the model for ARF4 regulating ciliary traffic of rhodopsin, PC1/PC2, or other cargos? A number of recent studies raise uncertainty about ARF4 functioning at the TGN to ensure the sorting of these cargoes into carrier vesicles destined to the cilia and/or the formation of an ARF4-based ciliary targeting complex. First, the results showing a requirement for ARF4 in ciliary traffic of PC1 must be interpreted with caution, since these studies used a PC1 chimera. Subsequent studies that examined the traffic of full-length PC1 showed that the VxP motif is dispensable for ciliary delivery, that full-length PC1 targeting to cilia is not perturbed by ARF4 depletion, and that endogenous PC1 does not bind ARF4 in collecting duct cells (88, 89, 149, 165). Such differences might be due to the requirement for PC2 in traffic of full-length PC1 but not the chimeric PC1 protein (109, 149).
Second, the proposed central role of ARF4 in ciliary traffic of rhodopsin, PC1, and PC2 implies that genetic ablation of ARF4 should result in ciliary defects, analogous to the phenotypes caused by certain mutations in rhodopsin, PC1, or PC2 (retinal degeneration and polycystic kidneys, respectively). Mice deleted for ARF4 died in midgestation (embryonic day E10.5), at about the same time as mice with defects in ciliogenesis (45). However, the lethality appears to be due to visceral endoderm defects caused by dysfunction in the traffic and localization of megalin, a plasma membrane scavenger receptor important for uptake of vitamins, lipoproteins, and signaling molecules (reviewed in Ref. 114) that also binds ARF4 (45). Scanning electron microscopy of the embryos showed that nodal cilia form and appear normal, suggesting that ARF4 is not essential for ciliogenesis per se (45). The normal-looking cilia were functional in breaking left/right symmetry. Whether cilia were fully functional in the ARF4−/− mice was not tested, and it remains possible that these mice had ciliary traffic defects. See Table 1 for a partial listing of tools that can be used to assess cilia structure and function.
Table 1.
Tools for assessing mammalian cilia and ciliary function
| Antibody | Transgenic Reporter Mice | |
|---|---|---|
| Ciliated cells, % | γ-Tubulin (Sigma Aldrich) with acetylated α-tubulin (Sigma Aldrich) or ARL13B (Proteintech) or ACIII (since tubulin is not acetylated in neurons; Lifespan Biosciences) |
Cilia-GFP (123) (Mouse Genome Informatics,
MGI:5524281) ARL13B-mCherry (7); centrin2-GFP (59) (MGI:5645691; MGI:3793421) FUCCI2a (mCherry-hCdt1 and mVenus-hGem); ARL13B-cerulean (46) (MGI:6193732) |
| Cilia length | ||
| General shape |
| General structure (antibody) | |
|---|---|
| Axoneme | Acetylated α-tubulin (Sigma Aldrich); IFT-B components: IFT88, IFT81, IFT20, IFT27, IFT52, IFT57 (Proteintech); IFT-A components: IFT122, IFT43 (Proteintech) |
| Ciliary membrane | INPP5E, ARL13B (Proteintech) |
| Transition zone | CEP290, MKS1, NPHP4 (Proteintech) |
| Basal body | γ-Tubulin (Sigma Aldrich), centrin (EMD Millipore) |
| Distal appendage | CEP164 (Sigma Aldrich) |
| Centriole | TTBK2 (Sigma Aldrich), CP110 (Proteintech) |
| Ciliary vesicle | RAB11, RAB8a (Cell Signaling) |
| Signaling | ||||
|---|---|---|---|---|
| Antibody | Reporter Construct | qRT-PCR | Transgenic Reporter Mice | |
| Vertebrate hedgehog | SMO (Santa Cruz) | 8X Gli-eGFP (69) (Addgene no. 84602) |
Gli1 Ptch1 Cell-specific targets (i.e., cyclinD1) |
Gli1-LacZ (4)
(MGI:2449767) Ptch1-LacZ (53) (MGI:1857447) |
| Wnt | β-Catenin (DSHB) | BAT Red (Addgene no. 20674) TOPFlash (Addgene no. 12456) |
Axin2 (or in situ via RNAScope) Find cell-specific targets at http://web.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes |
BATgal (111) (MGI:3697064) |
| Calcium | 5HT6-mCherry-G-GECO1.0 (148) (Addgene no. 47500) |
Arl13b-mCherry-GECO1.2 (31) (MGI:5898420) | ||
Listed are antibodies and reporter constructs along with mouse alleles that are useful to assess general mammalian ciliary structure and function. Table prioritizes commercially available reagents to provide a basic framework for investigators and is not intended to be exhaustive. By staining basal body using γ-tubulin, the location of the cilium can be identified so that cilium itself or ciliary compartment or structure can be assessed by costaining with appropriate ciliary marker and imaging. Transgenic mouse lines expressing fluorescent cilia proteins can be used for in vivo analysis. The FUCCI2; Arl13b-cerulean line enables cilia to be live imaged relative to the stage of the cell cycle. To examine the function of cilia, several signaling pathways linked to cilia can be analyzed. Three examples are included. Vertebrate hedgehog (Hh) signaling is the best characterized signaling pathway linked to the cilium. Components of the vertebrate Hh pathway such as smoothened (SMO) enrich dynamically within cilia in response to pathway stimulation. The pathway can be stimulated with ligand (N-Shh; Fisher) or smoothened agonist, SAG (VWR), and blocked with cyclopamine (Toronto Research Chemicals); the downstream transcription output can be monitored with endogenous transcriptional targets (Gli1, Ptch1 by qRT-PCR and Gli1-lacZ, Ptch1-lacZ in vivo) or reporter constructs, such as 8XGli-eGFP. For Wnt signaling, β-catenin localizes to the nucleus when the pathway is stimulated and induces transcription of Axin2, which can be monitored by qRT-PCR or RNAScope. β-Catenin binds TCF/LEF-binding sites that are used in Wnt reporter constructs such as TOPFlash (for transfection), BAT Red (for transduction), or BATgal (for in vivo). Finally, the calcium flux in cilia can be monitored in vitro or in vivo using genetically encoded calcium indicator for optical imaging (GECO) reporters that are targeted to cilia. Targeting sensitive biosensors to cilia is a current active area in the field. ARL, ADP-ribosylation factor-like; IFT, intraflagellar transport; INPP5E, inositol polyphosphate-5-phosphatase E; DSHB, Developmental Studies Hybridoma Bank.
To more clearly examine ARF4 function in ciliary traffic of rhodopsin and PC1/PC2, a conditional null ARF4 allele was generated and combined with either retina- or kidney collecting duct-specific Cre recombinases. Retina Cre produced a chimeric retina where ~80% of cells lacked ARF4. No evidence of retinal degeneration or rhodopsin mislocalization was observed, as would have been expected if ARF4 is critical for rhodopsin transport (126). Kidney collecting duct Cre reduced ARF4 in the total kidney to 40% of controls. Because the collecting duct makes up <50% of the kidney, this strongly suggests that most collecting duct cells lacked ARF4. No evidence of cyst formation was observed, as would be expected if ciliary delivery of PC1/PC2 was significantly decreased (126). Consistent with ARF4 not controlling ciliary traffic, patients with changes in ARF4 function display phenotypes linked to cancer, not ciliopathies (80, 174).
Third, a role for ARF4 in ciliary traffic is questioned by studies of fibrocystin, a ciliary protein encoded by the PKHD1 gene (mutations in PKHD1 cause autosomal recessive polycystic kidney disease). Fibrocystin contains a cilia-targeting motif in its cytosolic tail that, when fused to the extracellular domain of CD8, localizes it to cilia, and fibrocystin and ARF4 coimmunoprecipitated when coexpressed in inner medullary collecting duct (IMCD) cells (the interaction required the intact CTS) (44, 45). However, when the fibrocystin-CD8 chimera was arrested at the TGN by a low temperature (19°C) block, and then released and followed during its traffic to cilia, ARF4 depletion did not affect the amount of the chimera reaching the cilium in 4 h (albeit the rate of delivery was delayed at earlier time points) (45, 52). Based on these considerations, it appears that, while ARF4 is clearly essential for mammalian development, the proposed model of its action at the TGN to sort ciliary cargoes and assemble a ciliary transport complex is less certain.
ARF4 and ARF5 are ~90% identical, and each is activated by GBF1 at the TGN, raising the possibility that ARF5 also may play a role in ciliary traffic of proteins (24, 108). Depletion of either ARF4 or ARF5 from primary keratinocytes inhibited Notch signaling (components of Notch signaling are localized to the centrosome), suggesting that each ARF can affect ciliary function but do not provide insight into the mechanism of such inhibition (40). ARF4 and ARF5 display quite distinct phenotypes when deleted in mice, since mice deleted of ARF5 have normal life expectancy and no defects have been reported in any of their tissues, in contrast to ARF4 null mice, which are embryonic lethal (67). The lack of retinal or kidney dysfunction in ARF5−/− mice argues against ARF5 regulating a key ciliary function on its own, although it may share some relevant activities with ARF4.
ARL3 AND ARL2
ARL3 and ARL2 are the closest paralogs to each other and, despite having some distinct functions in cells, display a number of similar or homologous actions in regulating cargo traffic to cilia. Each has been predicted to have been present in the last eukaryotic common ancestor, and both are expressed widely among eukaryotes (97). The first evidence linking ARL3 to cilia came from Leishmania where it is required for ciliogenesis and localizes to the flagellum (29). Soon afterward, ARL3 (as well as ARL6 and IFT genes) appeared in screens for cilia genes in other organisms (3, 97). A large phenotypic screen in mice led to the generation and characterization of ARL3 null mice, demonstrating that ARL3 deletion causes ciliopathy-like phenotypes, including retinal degeneration and kidney disease resulting in neonatal lethality (136). In humans, mutations in ARL3 are causative in the ciliopathies retinitis pigmentosa (61, 147) and Joubert syndrome (1).
ARL2 localizes to centrosomes (where it regulates recruitment of the γ-tubulin ring complex), to cytosol [where it complexes with tubulin cofactor D and β-tubulin to regulate tubulin folding], and inside mitochondria (where it regulates mitochondrial fusion) (10, 28, 47, 48, 120, 142, 143, 155, 182). The links between ARL2 and cilia are less well established than ARL3, although recently ARL2 was linked to photoreceptor outer segment stability (172).
Early biochemical and yeast two-hybrid assays identified protein-binding partners of both ARL3 and ARL2 that informed a model of each GTPase regulating the unloading of lipidated cargos at cilia. Prominent among these partners are the homologous proteins phosphodiesterase 6δ (PDE6δ) and UNC119 (UNC119 lipid-binding chaperone, aka HRG4), and RP2. Each is present in photoreceptor cells and has appeared in screens with links to cilia, suggesting that they may function within a common or homologous pathway(s) to cilia (3, 97, 103). While PDE6δ and UNC119 share sequence homology and are modeled to act in cells to transport prenylated or N-myristoylated proteins, respectively, RP2 shares weak homology to tubulin cochaperone C, a tubulin GAP. Yeast two-hybrid screens identified both ARL2 and ARL3 as interactors of PDE6δ, with higher binding to the activated GTP-bound forms, consistent with PDE6δ acting in cells as an effector of these GTPases (57, 58, 102). PDE6δ contains a hydrophobic pocket that binds prenyl groups, which allows it to reversibly extract prenylated proteins from membranes and escort them to other membranes in the cell (25, 113). Both ARL2 and ARL3 are able to release prenylated cargos from PDE6δ in in vitro assays. Rheb, a cargo of PDE6δ, localizes to intracellular membranes when expressed as a GFP fusion protein, but, when coexpressed with PDE6δ, both are found in the cytosol. Coexpression of activating mutants of ARL2 or ARL3 causes the partial redistribution of Rheb-GFP back onto membranes, consistent with the model that they act in cells to unload prenylated cargos of PDE6δ (22, 74).
Like PDE6δ, UNC119 (HRG4) was identified through yeast two-hybrid screening with activating mutants of ARL2 or ARL3, suggesting that it is an effector of these GTPases (156). Despite sharing 23% primary sequence identity with PDE6δ overall and 40% identity in the COOH-terminal third of the protein, UNC119 did not display a preference for binding the activated conformation of ARL3, as did PDE6δ, although it does preferentially bind the active form of ARL2 (156). UNC119 localizes to photoreceptor synapses and inner segments, and its deletion in mice results in defects in rod outer segments (72). Similar to PDE6δ, UNC119 has a hydrophobic pocket, but it shows clear preference for binding N-myristoylated, rather than farnesylated, proteins. This function is important for targeting proteins to cilia (171, 178). Despite the sequence homology and functional similarities, PDE6δ and UNC119 differ in the molecular mechanisms by which the GTPases facilitate cargo release. Activated ARL2/3 binding to PDE6δ results in allosteric narrowing of the hydrophobic prenyl-binding pocket, resulting in the expulsion of the lipid moiety and cargo release (74). In contrast, the myristoyl binding pocket of UNC119 widens upon binding to ARL3-GTP, accommodating the NH2-terminal amphipathic helix of ARL3 with consequent release of cargo (73).
The specificity of cargos released by ARL2 or ARL3 is regulated in several ways, the first of which is intrinsic to the interaction between the cargos and the carrier proteins. Different cargos have differing affinities for PDE6δ and UNC119, dependent on the residues in the −1 and −3 positions, relative to the farnesylated cysteine for PDE6δ and on the +2 and +3 positions from the myristoylated glycine for UNC119 (42, 79). Only activated ARL3 is able to release the high-affinity cargos. Typically cargos targeted for the cilium are high affinity, and therefore are not released by ARL2 in the cytosol and are thought to be specifically released by ARL3 in the cilium.
The RP2 gene was linked to retinitis pigmentosa, and its gene product was identified as a binding partner of ARL3-GTP (9, 137). RP2 shares homology with tubulin-folding cofactor C and also acts as a GAP with high specificity for ARL3 and very little activity with ARL2 (9, 158). ARL3 is able to bind both UNC119 and RP2 at the same time, resulting in a system in which ARL3-GTP first binds and releases cargo from UNC119, and then RP2 binds the UNC119-ARL3-GTP complex and induces the hydrolysis of GTP by ARL3, which results in the separation of the complex, since the affinity of UNC119 and RP2 for ARL3-GDP is low (159). This system is relevant for the targeting of the ciliary protein NPHP3 (171).
In addition to ARL3’s role in release of lipidated cargos into the ciliary compartment, ARL3 also regulates ciliary protein traffic through the Golgi, through an as yet unknown mechanism. ARL3 localizes to the Golgi (182), and expression of the dominant active ARL3 (ARL3[Q71L]), RP2 depletion, or depletion of ARL3 disrupts the Golgi and results in defects in traffic of ciliary proteins (and likely other proteins) through the Golgi (39, 89, 182).
In summary (see Fig. 2), among its other cellular actions, ARL3 regulates protein traffic to cilia. In cells depleted of ARL3, cilia contain altered protein content resulting in defects in ciliary signaling (94). The best-characterized role of ARL3 is in release of lipidated cargoes from the carrier proteins UNC119 and PDE6δ. ARL3 activity is tightly regulated in this system, both by its GAP, RP2, and its GEF, ARL13B (discussed below). Defects in ARL3, its regulators, and its effectors, as well as some of their known cargoes, result in ciliopathies, including retinitis pigmentosa and Joubert syndrome; however, it is not understood precisely how these mutations result in the specific phenotypes seen in these diseases.
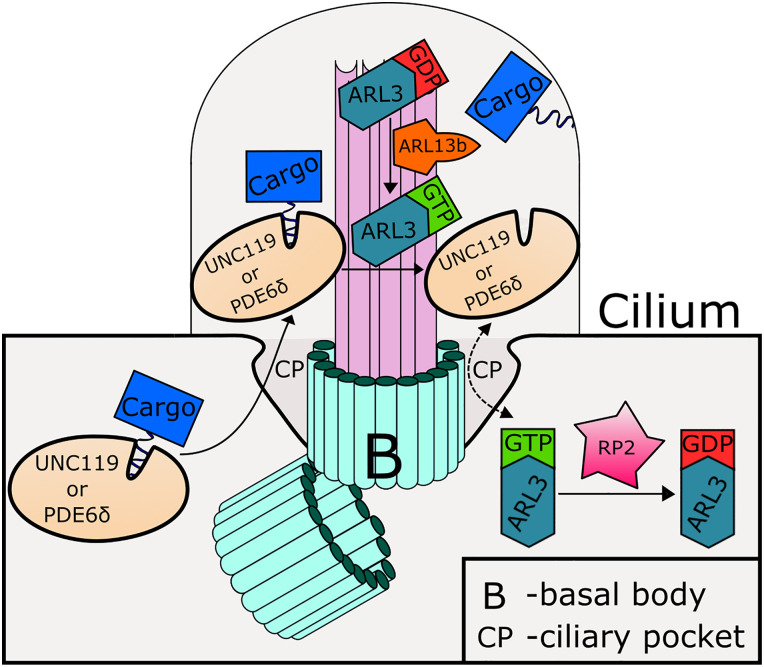
Fig. 2.
Model for ADP-ribosylation factor-like 3 (ARL3) and ARL13B function in regulating the release of lipidated cargoes in the cilium. Myristoylated and prenylated cargoes are carried to the cilium by UNC119 lipid-binding chaperone (UNC119) and phosphodiesterase 6δ (PDE6δ), respectively. Inside the cilium, these cargoes are released by ARL3-GTP that has been activated by cilia-localized ARL13B. Outside the cilium, ARL3 is deactivated by the GTPase-activating protein (GAP) retinitis pigmentosa 2 (RP2).
ARL13B
The ARF family GTPase with the clearest function in primary cilia due to its links to Joubert syndrome, mammalian development, and ciliogenesis, is ARL13/ARL13B (18, 20, 21). Mammals express two paralogs, ARL13A/B, while lower eukaryotes have a single ARL13 gene/protein present in all ciliated organisms. Initially linked to cilia in zebrafish, study of ARL13/ARL13B has since expanded to several other model systems, including mice and C. elegans (20, 98, 150). ARL13B function appears to be conserved in these systems both at the cellular and organismal level. In the absence of ARL13B, primary cilia are short and have defects in B tubule closure of the axoneme (20, 98). In zebrafish, mice, and humans, disruption of ARL13B causes defects of the kidney, eye, limb, and nervous system (18, 20, 150). Work on ARL13B has been extensively reviewed in a variety of contexts, most recently in 2019; therefore, we keep our discussion here general, with a focus on the most recent discoveries and ARL13B’s function in the context of other ARLs (36, 62, 101, 179).
ARL13B is an outlier in the ARF superfamily in several respects: 1) it has a COOH-terminal extension of a couple hundred residues (termed the C-terminal domain, CTD) making it about two times the size of other family members, 2) the CTD contains a VxP CTS that is essential for ARL13B ciliary localization, 3) the protein migrates aberrantly in SDS gels (to about 70–75 kDa, instead of the predicted ~47 kDa), 4) it has a glycine instead of glutamine in the G-3 motif, raising question as to the mechanism of GTP hydrolysis, 5) it has relatively low affinity for guanine nucleotides, 6) it is the only family member that is palmitoylated near its NH2-terminus, and 7) the CTD is heavily phosphorylated by casein kinase 2 (at least in vitro) (75). Thus, models of mechanisms of ARL13B action that are based on ARF biologies are risky, at least until we gain a better appreciation of these aspects of the protein.
ARL13B acts as a GEF for ARL3 (54, 75). Like the discovery of the other ARL3 interaction partners, ARL13B was identified as an ARL3 interactor through a yeast two-hybrid screen (54). In vitro, ARL13B in its active GTP-bound form stimulates the release of GDP from ARL3 ~70-fold, and expression of ARL13B-GFP increases the amount of ARL3-GTP in IMCD3 and HEK cells, indicating that ARL13B is able to act as a GEF in a cellular context (54). Consistent with the cell biological importance of this function, some Joubert syndrome mutations in ARL3 or ARL13B disrupt the interaction of these proteins or impair ARL13B’s GEF activity. With the presence of the ARL3 GEF, ARL13B, in the cilium, and the ARL3 GAP, RP2, excluded from the cilium, it is reasonable to predict that the ciliary compartment is enriched in ARL3-GTP, allowing ARL3 to specifically release high-affinity cargos of PDE6δ and UNC119 in the cilium (see Fig. 2).
The molecular interplay between ARL13B and ARL3 has been primarily characterized in vitro; however, there is some evidence from in vivo studies that support this model in cells. When ARL3 was specifically knocked out in the retina, basal bodies dock but outer segments fail to form, leading to photoreceptor degeneration (55). A strikingly similar phenotype is observed with ARL13B retina-specific deletion, but the photoreceptors degenerate at a faster rate than in the ARL3 deletion. The elevated severity of the phenotype of the ARL13B deletion suggests that ARL13B regulates this process by both ARL3-dependent and -independent pathways (56). Interestingly, these phenotypes are nearly identical to retina-specific deletion of Kif3a (a kinesin with links to IFT, ciliogenesis, and polycystic kidney disease), indicating that ARL3 and ARL13B may be regulating aspects of IFT that are essential for outer segment formation (83). This is further supported by observations in C. elegans where ARL3 or ARL13 depletion disrupts IFT (98). Furthermore, the ARL3 GAP RP2 regulates ciliary tip localization of two other kinesins, Kif7 and Kif17, suggesting that IFT is regulated in still poorly understood ways by ARL13B, ARL3, and RP2 (138).
In spite of numerous studies of ARL13B, the details of its function in cilia are still somewhat unclear. In addition to its function as a GEF for ARL3, ARL13B also acts in cilia independently of ARL3. ARL13B regulates IFT, although the molecular details here remain to be elucidated (21, 98, 122). ARL13B also regulates protein composition of cilia through a pathway independent of IFT but dependent on SUMOylation of ARL13B (99, 130). And finally, ARL13B has been shown to regulate endocytic traffic (8).
ARL13B is a key regulator of vertebrate hedgehog (Hh) signaling in the cilium, although it was recently discovered that ARL13B does not have to be in the cilium to regulate this pathway (51, 112). Mutation of the VxP CTS of ARL13B (V358A) results in its exclusion from the cilium even upon overexpression (51, 60, 112). Yet, this mutant is able to rescue the Hh signaling defects observed in cells and mice lacking ARL13B, although it does not restore the morphological defects of the ARL13B-deficient cilia (51, 112). Interestingly, while ARL13B[V358A] retains normal GEF activity for ARL3 in vitro, localization of ARL3 to cilia is lost in mouse embryonic fibroblasts (MEFs) expressing the cilia-excluded ARL13B[V358A] (51).
There is limited knowledge of the specific ciliary cargos that are dependent on ARL13B-mediated activation of ARL3. One exception is inositol polyphosphate-5-phosphatase E (INPP5E), a prenylated protein that binds independently to ARL13B and ARL3, and mutations in which have been linked to Joubert syndrome (11). Early clues to INPP5E ciliary targeting came from the findings that its localization was PDE6δ- and ARL13B-dependent, but ARL3-independent (68). Based on these results, a model was proposed in which the prenylated COOH-terminus of INPP5E binds PDE6δ, and a subsequent interaction of the INPP5E CTS with ARL13B releases INPP5E from PDE6δ into the cilium (with the caveat that prenylation of INPP5E appeared dispensable for ciliary targeting in this system). This model was challenged by Thomas et al. (154), who found that the PDE6δ interaction with INPP5E is dependent on farnesylation and in contrast to Humbert et al. (68) that farnesylation is required for ciliary targeting. Both groups agree that depletion of ARL3 is not sufficient to disrupt INPP5E ciliary localization, which is perplexing considering ARL3’s established role in releasing farnesylated cargos in the cilium and its ability to release INPP5E from PDE6δ in vitro. This model was further complicated in 2016, when Fansa et al. (42) showed that ARL3 depletion did in fact disrupt GFP-INPP5E ciliary localization. Most recently, results described in Kösling et al. (93) supported the requirement of INPP5E farnesylation for ciliary targeting and proposed a model in which INPP5E is carried to the cilium by PDE6δ, released into the ciliary compartment by ARL3-GTP, and retained there by ARL13B.
In addition to acting as a GEF for ARL3, ARL13B also functions independently of ARL3, regulating IFT, tubulin in the ciliary axoneme, and the exocyst complex during ciliogenesis (95, 133, 140). Further work must be done to determine the extent to which the different actions of ARL13B are truly independent, which of its actions are happening inside versus outside the cilium, and whether some of those actions may regulate overlapping pathways.
ARL6
ARL6 (aka BBS3) was identified in the early 1990s as a gene linked to both Bardet-Biedl syndrome (BBS) and nonsyndromic retinitis pigmentosa, each of which is closely linked to defects in primary or sensory cilia (Fig. 3). Later genetic testing and analyses in multiple model organisms further strengthened such links, with mutations found in BBS patients correlated with ARL6 protein instability (23, 41, 92).
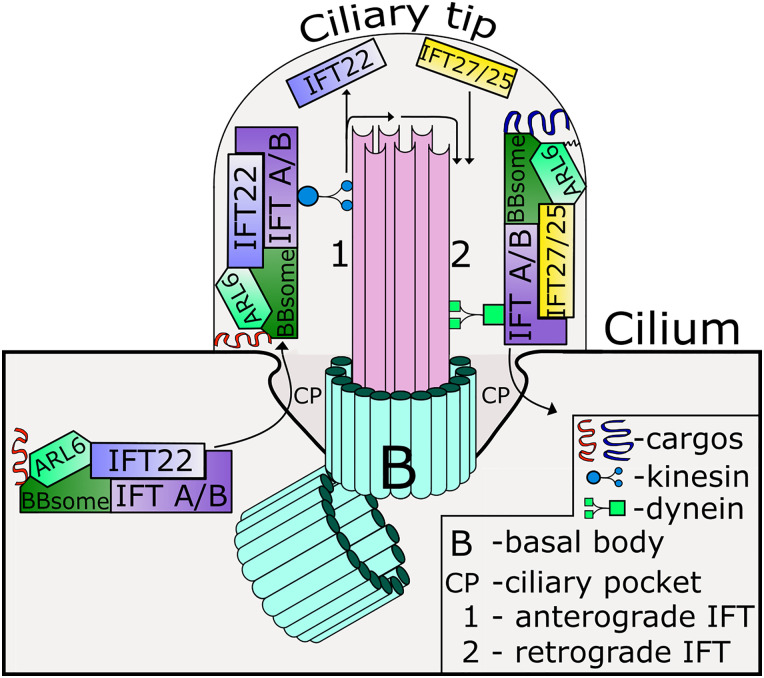
Fig. 3.
Model for ADP-ribosylation factor-like 6 (ARL6) action in regulating cargo transport in the cilium. ARL6 and the BBSome (where BBS is Bardet-Biedl syndrome) bind ciliary cargo and the intraflagellar transporter (IFT) 22 and IFT A/B complex to enter the cilium [anterograde transport (1)]. This complex is carried along the axoneme to the ciliary tip by kinesin motors. At the tip of the cilium, the GTP of ARL6 is hydrolyzed, the cargo is released, and the IFT trains are remodeled. IFT22 leaves the complex, whereas IFT27/25 is recruited. The remodeled complex containing BBSome, IFT27/25, and IFT A/B binds protein cargos at the ciliary tip and carries them out of the cilium [retrograde transport (2)]. IFT22 binds preferentially to activated (GTP-bound) ARL6 while IFT27 prefers to bind the nucleotide-free or apo-ARL6.
Almost everything we know about ARL6 function has emerged from the explosion of interest and understanding of primary ciliary biology. This began with the seminal work in which the BBSome was first purified and later shown to be involved in traffic of membrane proteins at primary cilia and as an effector of ARL6 (84, 117). This latter study describes probably the best biochemical analyses of ARL6 functions and provides convincing evidence that ARL6 is acting at cilia to promote membrane association of the BBSome, perhaps analogous to the actions of ARF1/3 in the recruitment of coat complexes at the Golgi and other membrane surfaces (30, 104–106, 127). While the data supporting direct binding of ARL6-GTP and the BBSome is strong, their other molecular interactions and links to traffic within cilia, specifically linked to IFT and the carriers IFT-A and IFT-B, are murkier.
Recently, Xue et al. (175) provided evidence supporting a role for IFT22 (RABL5) in binding ARL6 and the BBSome, and in so doing to recruit all to the ciliary base prior to their entry into cilia. IFT22 is a small (22-kDa) GTPase (although not in the ARF or RAS superfamilies), and is one of nine components of the IFT-B complex, but may also act separately (153). Knockdown of IFT22 results in loss of BBSome staining in cilia because of defects in its import (175). At the same time, loss of IFT22 resulted in decreased levels of ciliary ARL6, while knockdown of ARL6 had no effect on levels of IFT22, leading to the conclusion that binding to IFT22 is required for stability of ARL6 (175). Thus, two different GTPases from different families work together in aspects of ciliary biology, as already has been documented for RAB family GTPases known to play important ciliary roles, most of which we cannot discuss here because of space considerations (12, 101, 141).
While the Xue et al. (175) study argues convincingly for ARL6 acting in concert with IFT22 in BBSome entry to cilia, an earlier study demonstrated a role for ARL6 acting with IFT27 (RABL4) in BBSome-cargo export (100). As predicted by each of these models, knockdown of IFT22 results in the loss of ARL6 and BBSome staining inside cilia while knockdown of IFT27 causes a hyperaccumulation of each in cilia (100, 175). In contrast to IFT22, which binds preferentially to activated (GTP-bound) ARL6, IFT27 prefers to bind the nucleotide-free or apo-ARL6. However, in each case, ARL6 binding to the IFT protein increased its stability in cells. Because ARL6 does not appear to bind the intact IFT-B complex, it is IFT27, or more likely the IFT27/25 heterodimer, that binds ARL6. The rate of BBSome export is significantly increased in response to knockdown of IFT27. Furthermore, the export of the G protein-coupled receptor GPR161 in response to Hh signaling requires ARL6 and IFT27, all consistent with the ARL6 and IFT27 GTPases being involved in export of BBSome and cargos from cilia. Because IFT27 binds to the apo-ARL6 when not complexed with other IFT-B subunits and remodeling of IFT trains occurs at the ciliary tip, this also predicts the site of action for ARL6-IFT27 to be at the ciliary tip to promote cargo export. This contrasts to ARL6-IFT22-BBSomes acting together to enter cilia at the base (100).
We consider the studies described above to be important indicators of the actions of ARL6 in cilia, with more work required to bring the molecular mechanisms into better focus. We lack detailed biochemical analyses of ARL6 nucleotide-binding and hydrolysis properties and so base several interpretations on analogies to other GTPases. Note that the Jacobs et al. (78) study describing such properties of “ADP-ribosylation factor (ARF)-like 4, 6, and 7” are not relevant to this discussion, since it was published before the current consensus nomenclature for the ARF family and present data for what today are known as ARL4A, -C, and -D (85). Many studies of ARL6, BBSomes, IFT, and cilia use model genetic systems, since they have proven to be of fundamental importance to our evolving understanding of ciliary biology and signaling. Human and Chlamydomonas ARL6 share 51% identity, in contrast to ARF1’s that share 90% identity. Perhaps more importantly, the highly conserved (in all GTPases) glutamine in the G-3 motif of the guanine nucleotide-binding pocket that is critical in the hydrolysis of GTP (Q73 in human ARL6) is an alanine in Chlamydomonas and thus might be predicted to have very different nucleotide-binding and hydrolysis properties from the mammalian orthologs. This makes correlations between organisms somewhat risky and qualified.
SUMMARY
The last decade has seen rapid increases in our understanding of molecular details of cilia biogenesis, ciliary signaling in development, and how each of these deepen our understanding of the ciliopathies that can result from their dysfunction. Members of the ARF superfamily regulate key ciliary events, but, despite progress, the precise roles of these small GTPases remain incompletely understood. In this review we set out to evaluate current models of how and where ARF/ARL GTPases act with respect to ciliary function. Future studies will need to address many remaining questions, the most pressing of which we highlight below.
First, the role of ARF4 in cilia must be dissected from its other functions within the cell. The model of ARF4 action in ciliary traffic at the TGN (Fig. 1) appears analogous to the best-known functions of other ARFs (especially ARF1) in secretory traffic, i.e., to sort a subset of cargo proteins for packaging into a vesicle targeted to a specific destination. However, ARF4 predominantly localizes to the cis-Golgi, and it is possible that it influences ciliary traffic from that location by regulating IFT20 traffic or that of other proteins, rather than function at the TGN (24, 181). ARF4 may also regulate ciliary function indirectly, as when ARF4 controls the TGN localization and function of presenilin-2, a protease required to process Notch receptors and release the Notch intracellular domains (NICD) necessary for signaling (40). Thus, the model that the sole or even the most important role of ARF4 in ciliary biology is to sort proteins and assemble ciliary vesicles at the TGN requires additional study and confirmation from several laboratories.
Second, our knowledge of the regulatory proteins (GEFs, GAPs, and effectors) that act on and with ARF/ARL GTPases within the ciliary context is extremely limited. There are 15 ARF GEFs and close to 30 ARF GAPs, with most or possibly all able to act on multiple ARFs (but not ARLs) (reviewed in 131, 151, 161). Which of those act in ciliary-related pathways remains largely unknown. Identification of the GEFs, GAPs, and effectors of the ARLs affecting ciliary biology may well require purification of novel proteins or far more extensive testing of known ones.
Third, we lack an even basic understanding of how the ciliary ARF/ARLs and their regulatory proteins may themselves be regulated by posttranslational modifications. For example, the known ARF GEFs and GAPs are extensively phosphorylated, but whether such modifications are important for their ciliary function is unknown. The COOH-terminal domain of ARL13B also has been shown to be phosphorylated by casein kinase 2 in vitro, yet with unknown significance in vivo (75).
Fourth, the number of pathways by which proteins reach cilia, and the extent to which ARF/ARLs regulate all or some of them, or allow coordination between them, is not understood. It is easiest to assume that all or most transmembrane proteins traffic through a single pathway, but that might be an oversimplification. Recent work implicates at least three distinct pathways that may deliver proteins to the cilium. These three pathways emerged from studies in IMCD3 cells, where newly synthesized ciliary proteins were first arrested at the Golgi and then released and followed during their traffic to cilia over 6 h, under conditions of depletion of various combinations of IFT20, exocyst, and biogenesis of lysosome-related organelles complex-1 (BLOC-1) (116). One pathway to the cilium was taken by Smoothened (Smo, a G protein-coupled receptor involved in hedgehog signaling) and was independent of IFT20, exocyst, or BLOC-1 complex. In contrast, fibrocystin (a single-span membrane protein) used a pathway requiring IFT20 and the exocyst, but not BLOC-1. The third pathway was used by PC2 (a multispanning transmembrane protein) and was regulated by IFT20, exocyst, and the BLOC-1 complex and most likely involved passage through an endosomal compartment. The role(s) of ARF family GTPases in these pathways is unknown.
Fifth, differences in ciliary traffic may exist between different tissues and cell types, as well as different cilia themselves, but we have limited understanding of any variances between ciliary traffic in neurons versus kidney versus the retina. We have depended largely on the use of cells and tissues with highly exaggerated cilia (e.g., retinal cells), which may skew the regulatory aspects of ARF family GTPases. Furthermore, different GTPases might be preferentially expressed in certain types of ciliated cells to regulate specific ciliary traffic. Also, the expansion of GTPases in mammals might reflect more specific ciliary roles that arose during evolution. For example, class II ARFs include ARF4 and ARF5. Epidermal shRNA depletion of either ARF4 or ARF5 in embryos caused polydactyly, a sign of ciliary defect, suggesting that each of these ARFs may have ciliary roles. Phylogenetic analyses to identify conserved ciliary proteins also suggest that other ARF superfamily GTPase may act in ciliary processes.
Based on the increase in studies of ciliary biogenesis and function over the last 15 years, it is likely that our understanding of ciliary traffic will expand significantly over the next few years, including the identification of all the ARF/ARLs regulating ciliary function and their mechanisms of action. In vitro biochemical reconstitution assays, cellular analyses using ARF/ARL mutants, and organismal studies using both model systems and clinical data are needed to further discover the many ways ARF/ARL GTPases control ciliary traffic and function. Such studies are essential to provide important new insight to inform our understanding of ciliopathies and are likely to offer novel targets and approaches to therapies.
GRANTS
This work was supported by National Institute of General Medical Sciences Grants R01GM122802 to E.S., R35GM122568 to R.A.K., and R35GM122549 to T.C., National Institute of Child Health and Human Development Grant F31HD096815 to S.F., and National Science Foundation Grant MCB-1615607 to E.S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.K. prepared figures; S.F., D.K., T.C., R.A.K., and E.S. drafted manuscript; S.F., D.K., T.C., R.A.K., and E.S. edited and revised manuscript; S.F., D.K., T.C., R.A.K., and E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors regret the omission of citations of outstanding work in the field that could not be cited or discussed in greater detail due to space limitations. We thank Dr. Greg Pazour for many insightful suggestions.
REFERENCES
- 1.Alkanderi S, Molinari E, Shaheen R, Elmaghloob Y, Stephen LA, Sammut V, Ramsbottom SA, Srivastava S, Cairns G, Edwards N, Rice SJ, Ewida N, Alhashem A, White K, Miles CG, Steel DH, Alkuraya FS, Ismail S, Sayer JA. ARL3 mutations cause Joubert Syndrome by disrupting ciliary protein composition. Am J Hum Genet 103: 612–620, 2018. doi: 10.1016/j.ajhg.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakel EC, Schwappach B. Formation of COPI-coated vesicles at a glance. J Cell Sci 131: jcs209890, 2018. doi: 10.1242/jcs.209890. [DOI] [PubMed] [Google Scholar]
- 3.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539, 2004. doi: 10.1016/S0092-8674(04)00412-X. [DOI] [PubMed] [Google Scholar]
- 4.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129: 4753–4761, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bailey LK, Campbell LJ, Evetts KA, Littlefield K, Rajendra E, Nietlispach D, Owen D, Mott HR. 1H, 13C and 15N resonance assignments for binder of Arl2, BART. Biomol NMR Assign 3: 33–36, 2009. doi: 10.1007/s12104-008-9135-3. [DOI] [PubMed] [Google Scholar]
- 6.Bailey LK, Campbell LJ, Evetts KA, Littlefield K, Rajendra E, Nietlispach D, Owen D, Mott HR. The structure of binder of Arl2 (BART) reveals a novel G protein binding domain: implications for function. J Biol Chem 284: 992–999, 2009. doi: 10.1074/jbc.M806167200. [DOI] [PubMed] [Google Scholar]
- 7.Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol 17: 113–122, 2015. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barral DC, Garg S, Casalou C, Watts GF, Sandoval JL, Ramalho JS, Hsu VW, Brenner MB. Arl13b regulates endocytic recycling traffic. Proc Natl Acad Sci USA 109: 21354–21359, 2012. doi: 10.1073/pnas.1218272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartolini F, Bhamidipati A, Thomas S, Schwahn U, Lewis SA, Cowan NJ. Functional overlap between retinitis pigmentosa 2 protein and the tubulin-specific chaperone cofactor C. J Biol Chem 277: 14629–14634, 2002. doi: 10.1074/jbc.M200128200. [DOI] [PubMed] [Google Scholar]
- 10.Bhamidipati A, Lewis SA, Cowan NJ. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J Cell Biol 149: 1087–1096, 2000. doi: 10.1083/jcb.149.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036, 2009. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blacque OE, Scheidel N, Kuhns S. Rab GTPases in cilium formation and function. Small GTPases 9: 76–94, 2018. doi: 10.1080/21541248.2017.1353847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349: 117–127, 1991. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 14.Bowzard JB, Cheng D, Peng J, Kahn RA. ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem 282: 17568–17580, 2007. doi: 10.1074/jbc.M701347200. [DOI] [PubMed] [Google Scholar]
- 15.Bowzard JB, Sharer JD, Kahn RA. Assays used in the analysis of Arl2 and its binding partners. Methods Enzymol 404: 453–467, 2005. doi: 10.1016/S0076-6879(05)04040-1. [DOI] [PubMed] [Google Scholar]
- 16.Bui QT, Golinelli-Cohen MP, Jackson CL. Large Arf1 guanine nucleotide exchange factors: evolution, domain structure, and roles in membrane trafficking and human disease. Mol Genet Genomics 282: 329–350, 2009. doi: 10.1007/s00438-009-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burd CG, Strochlic TI, Setty SR. Arf-like GTPases: not so Arf-like after all. Trends Cell Biol 14: 687–694, 2004. doi: 10.1016/j.tcb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG; International Joubert Syndrome Related Disorders Study Group . Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83: 170–179, 2008. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic 8: 1476–1485, 2007. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 20.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 12: 767–778, 2007. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Cevik S, Hori Y, Kaplan OI, Kida K, Toivenon T, Foley-Fisher C, Cottell D, Katada T, Kontani K, Blacque OE. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol 188: 953–969, 2010. doi: 10.1083/jcb.200908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra A, Grecco HE, Pisupati V, Perera D, Cassidy L, Skoulidis F, Ismail SA, Hedberg C, Hanzal-Bayer M, Venkitaraman AR, Wittinghofer A, Bastiaens PI. The GDI-like solubilizing factor PDEδ sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol 14: 148–158, 2011. doi: 10.1038/ncb2394. [DOI] [PubMed] [Google Scholar]
- 23.Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3). Am J Hum Genet 75: 475–484, 2004. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun J, Shapovalova Z, Dejgaard SY, Presley JF, Melançon P. Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1. Mol Biol Cell 19: 3488–3500, 2008. doi: 10.1091/mbc.e08-04-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. The delta subunit of type 6 phosphodiesterase reduces light-induced cGMP hydrolysis in rod outer segments. J Biol Chem 276: 5248–5255, 2001. doi: 10.1074/jbc.M004690200. [DOI] [PubMed] [Google Scholar]
- 26.Cool RH, Schmidt G, Lenzen CU, Prinz H, Vogt D, Wittinghofer A. The Ras mutant D119N is both dominant negative and activated. Mol Cell Biol 19: 6297–6305, 1999. doi: 10.1128/MCB.19.9.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov 13: 828–851, 2014. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham LA, Kahn RA. Cofactor D functions as a centrosomal protein and is required for the recruitment of the gamma-tubulin ring complex at centrosomes and organization of the mitotic spindle. J Biol Chem 283: 7155–7165, 2008. doi: 10.1074/jbc.M706753200. [DOI] [PubMed] [Google Scholar]
- 29.Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci 113: 2065–2074, 2000. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358, 2006. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 31.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660, 2016. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deretic D, Lorentzen E, Fresquez T. The ins and outs of the Arf4-based ciliary membrane-targeting complex. Small GTPases 1–12, 2019. doi: 10.1080/21541248.2019.1616355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deretic D, Papermaster DS. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci 106: 803–813, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci USA 95: 10620–10625, 1998. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci USA 102: 3301–3306, 2005. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dilan T, Ramamurthy V. The dynamic and complex role of the Joubert Syndrome-associated ciliary protein, ADP-ribosylation factor-like GTPase 13B (ARL13B) in photoreceptor development and maintenance. Adv Exp Med Biol 1185: 501–505, 2019. doi: 10.1007/978-3-030-27378-1_82. [DOI] [PubMed] [Google Scholar]
- 37.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12: 362–375, 2011. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.East MP, Bowzard JB, Dacks JB, Kahn RA. ELMO domains, evolutionary and functional characterization of a novel GTPase-activating protein (GAP) domain for Arf protein family GTPases. J Biol Chem 287: 39538–39553, 2012. doi: 10.1074/jbc.M112.417477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet 19: 1358–1367, 2010. doi: 10.1093/hmg/ddq012. [DOI] [PubMed] [Google Scholar]
- 40.Ezratty EJ, Pasolli HA, Fuchs E. A Presenilin-2-ARF4 trafficking axis modulates Notch signaling during epidermal differentiation. J Cell Biol 214: 89–101, 2016. doi: 10.1083/jcb.201508082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, Green JS, Lewis RA, van Haelst MM, Parfrey PS, Baillie DL, Beales PL, Katsanis N, Davidson WS, Leroux MR. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet 36: 989–993, 2004. doi: 10.1038/ng1414. [DOI] [PubMed] [Google Scholar]
- 42.Fansa EK, Kösling SK, Zent E, Wittinghofer A, Ismail S. PDE6δ-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity. Nat Commun 7: 11366, 2016. doi: 10.1038/ncomms11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol 8: 3235–3243, 1988. doi: 10.1128/MCB.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol 188: 21–28, 2010. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Follit JA, San Agustin JT, Jonassen JA, Huang T, Rivera-Perez JA, Tremblay KD, Pazour GJ. Arf4 is required for mammalian development but dispensable for ciliary assembly. PLoS Genet 10: e1004170, 2014. doi: 10.1371/journal.pgen.1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford MJ, Yeyati PL, Mali GR, Keighren MA, Waddell SH, Mjoseng HK, Douglas AT, Hall EA, Sakaue-Sawano A, Miyawaki A, Meehan RR, Boulter L, Jackson IJ, Mill P, Mort RLA. A cell/cilia cycle biosensor for single-cell kinetics reveals persistence of cilia after G1/S transition is a general property in cells and mice. Dev Cell 47: 509–523.e5, 2018. doi: 10.1016/j.devcel.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis JW, Goswami D, Novick SJ, Pascal BD, Weikum ER, Ortlund EA, Griffin PR, Kahn RA. Nucleotide binding to ARL2 in the TBCD∙ARL2∙β-tubulin complex drives conformational changes in β-tubulin. J Mol Biol 429: 3696–3716, 2017. doi: 10.1016/j.jmb.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis JW, Newman LE, Cunningham LA, Kahn RA. A trimer consisting of the tubulin-specific chaperone D (TBCD), regulatory GTPase ARL2, and β-tubulin is required for maintaining the microtubule network. J Biol Chem 292: 4336–4349, 2017. doi: 10.1074/jbc.M116.770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G, Posor Y, Maffucci T, Marengo S, Haucke V, Falasca M, Perez-Morga D, Boletta A, Merlo GR, Hirsch E. PI3K class II α controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell 28: 647–658, 2014. doi: 10.1016/j.devcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119: 1383–1395, 2006. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 51.Gigante ED, Taylor MR, Ivanova AA, Kahn RA, Caspary T. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife 9: 9, 2020. doi: 10.7554/eLife.50434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert CE, Sztul E, Machamer CE. Commonly used trafficking blocks disrupt ARF1 activation and the localization and function of specific Golgi proteins. Mol Biol Cell 29: 937–947, 2018. doi: 10.1091/mbc.E17-11-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277: 1109–1113, 1997. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 54.Gotthardt K, Lokaj M, Koerner C, Falk N, Gießl A, Wittinghofer A. A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins (Abstract). eLife 4: 11859, 2015. doi: 10.7554/eLife.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanke-Gogokhia C, Wu Z, Gerstner CD, Frederick JM, Zhang H, Baehr W. Arf-like protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J Biol Chem 291: 7142–7155, 2016. doi: 10.1074/jbc.M115.710954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanke-Gogokhia C, Wu Z, Sharif A, Yazigi H, Frederick JM, Baehr W. The guanine nucleotide exchange factor Arf-like protein 13b is essential for assembly of the mouse photoreceptor transition zone and outer segment. J Biol Chem 292: 21442–21456, 2017. doi: 10.1074/jbc.RA117.000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanzal-Bayer M, Linari M, Wittinghofer A. Properties of the interaction of Arf-like protein 2 with PDEdelta. J Mol Biol 350: 1074–1082, 2005. doi: 10.1016/j.jmb.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 58.Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC. The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J 21: 2095–2106, 2002. doi: 10.1093/emboj/21.9.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higginbotham H, Bielas S, Tanaka T, Gleeson JG. Transgenic mouse line with green-fluorescent protein-labeled Centrin 2 allows visualization of the centrosome in living cells. Transgenic Res 13: 155–164, 2004. doi: 10.1023/B:TRAG.0000026071.41735.8e. [DOI] [PubMed] [Google Scholar]
- 60.Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 23: 925–938, 2012. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holtan JP, Teigen K, Aukrust I, Bragadóttir R, Houge G. Dominant ARL3-related retinitis pigmentosa. Ophthalmic Genet 40: 124–128, 2019. doi: 10.1080/13816810.2019.1586965. [DOI] [PubMed] [Google Scholar]
- 62.Hor CH, Goh EL. Small GTPases in hedgehog signalling: emerging insights into the disease mechanisms of Rab23-mediated and Arl13b-mediated ciliopathies. Curr Opin Genet Dev 56: 61–68, 2019. doi: 10.1016/j.gde.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans 37: 1032–1036, 2009. doi: 10.1042/BST0371032. [DOI] [PubMed] [Google Scholar]
- 64.Horgan CP, Oleksy A, Zhdanov AV, Lall PY, White IJ, Khan AR, Futter CE, McCaffrey JG, McCaffrey MW. Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic 8: 414–430, 2007. doi: 10.1111/j.1600-0854.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 65.Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun 319: 83–94, 2004. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- 66.Horgan CP, Zurawski TH, McCaffrey MW. Purification and functional properties of Rab11-FIP3. Methods Enzymol 403: 499–512, 2005. doi: 10.1016/S0076-6879(05)03044-2. [DOI] [PubMed] [Google Scholar]
- 67.Hosoi N, Shibasaki K, Hosono M, Konno A, Shinoda Y, Kiyonari H, Inoue K, Muramatsu SI, Ishizaki Y, Hirai H, Furuichi T, Sadakata T. Deletion of class II ADP-ribosylation factors in mice causes tremor by the Nav1.6 loss in cerebellar purkinje cell axon initial segments. J Neurosci 39: 6339–6353, 2019. doi: 10.1523/JNEUROSCI.2002-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci USA 109: 19691–19696, 2012. doi: 10.1073/pnas.1210916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, Solow-Cordero DE, Jiang J, Rowitch DH, Chen JK. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA 106: 14132–14137, 2009. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell 19: 4224–4237, 2008. doi: 10.1091/mbc.e08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic 8: 1465–1475, 2007. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 72.Ishiba Y, Higashide T, Mori N, Kobayashi A, Kubota S, McLaren MJ, Satoh H, Wong F, Inana G. Targeted inactivation of synaptic HRG4 (UNC119) causes dysfunction in the distal photoreceptor and slow retinal degeneration, revealing a new function. Exp Eye Res 84: 473–485, 2007. doi: 10.1016/j.exer.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ismail SA, Chen YX, Miertzschke M, Vetter IR, Koerner C, Wittinghofer A. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J 31: 4085–4094, 2012. doi: 10.1038/emboj.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ismail SA, Chen YX, Rusinova A, Chandra A, Bierbaum M, Gremer L, Triola G, Waldmann H, Bastiaens PI, Wittinghofer A. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat Chem Biol 7: 942–949, 2011. doi: 10.1038/nchembio.686. [DOI] [PubMed] [Google Scholar]
- 75.Ivanova AA, Caspary T, Seyfried NT, Duong DM, West AB, Liu Z, Kahn RA. Biochemical characterization of purified mammalian ARL13B protein indicates that it is an atypical GTPase and ARL3 guanine nucleotide exchange factor (GEF). J Biol Chem 292: 11091–11108, 2017. doi: 10.1074/jbc.M117.784025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ivanova AA, East MP, Yi SL, Kahn RA. Characterization of recombinant ELMOD (cell engulfment and motility domain) proteins as GTPase-activating proteins (GAPs) for ARF family GTPases. J Biol Chem 289: 11111–11121, 2014. doi: 10.1074/jbc.M114.548529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson CL, Bouvet S. Arfs at a glance. J Cell Sci 127: 4103–4109, 2014. doi: 10.1242/jcs.144899. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs S, Schilf C, Fliegert F, Koling S, Weber Y, Schürmann A, Joost HG. ADP-ribosylation factor (ARF)-like 4, 6, and 7 represent a subgroup of the ARF family characterization by rapid nucleotide exchange and a nuclear localization signal. FEBS Lett 456: 384–388, 1999. doi: 10.1016/S0014-5793(99)00759-0. [DOI] [PubMed] [Google Scholar]
- 79.Jaiswal M, Fansa EK, Kösling SK, Mejuch T, Waldmann H, Wittinghofer A. Novel biochemical and structural insights into the interaction of myristoylated cargo with Unc119 and their release by Arl2/3. J Biol Chem 291: 20766–20778, 2016. doi: 10.1074/jbc.M116.741827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang SY, Jang SW, Ko J. Regulation of ADP-ribosylation factor 4 expression by small leucine zipper protein and involvement in breast cancer cell migration. Cancer Lett 314: 185–197, 2012. doi: 10.1016/j.canlet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 81.Jensen VL, Leroux MR. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr Opin Cell Biol 47: 83–91, 2017. doi: 10.1016/j.ceb.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 82.Jian X, Cavenagh M, Gruschus JM, Randazzo PA, Kahn RA. Modifications to the C-terminus of Arf1 alter cell functions and protein interactions. Traffic 11: 732–742, 2010. doi: 10.1111/j.1600-0854.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang L, Wei Y, Ronquillo CC, Marc RE, Yoder BK, Frederick JM, Baehr W. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J Biol Chem 290: 12765–12778, 2015. doi: 10.1074/jbc.M115.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141: 1208–1219, 2010. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 172: 645–650, 2006. doi: 10.1083/jcb.200512057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kahn RA, Volpicelli-Daley L, Bowzard B, Shrivastava-Ranjan P, Li Y, Zhou C, Cunningham L. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans 33: 1269–1272, 2005. doi: 10.1042/BST0331269. [DOI] [PubMed] [Google Scholar]
- 87.Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol 14: 431–437, 2012. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim H, Kang AY, Ko AR, Park HC, So I, Park JH, Cheong HI, Hwang YH, Ahn C. Calpain-mediated proteolysis of polycystin-1 C-terminus induces JAK2 and ERK signal alterations. Exp Cell Res 320: 62–68, 2014. doi: 10.1016/j.yexcr.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K, Germino GG, Weinman EJ, Watnick T, Qian F. Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 5: 5482, 2014. doi: 10.1038/ncomms6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA 106: 21666–21671, 2009. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knödler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107: 6346–6351, 2010. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi T, Hori Y, Ueda N, Kajiho H, Muraoka S, Shima F, Kataoka T, Kontani K, Katada T. Biochemical characterization of missense mutations in the Arf/Arl-family small GTPase Arl6 causing Bardet-Biedl syndrome. Biochem Biophys Res Commun 381: 439–442, 2009. doi: 10.1016/j.bbrc.2009.02.087. [DOI] [PubMed] [Google Scholar]
- 93.Kösling SK, Fansa EK, Maffini S, Wittinghofer A. Mechanism and dynamics of INPP5E transport into and inside the ciliary compartment. Biol Chem 399: 277–292, 2018. doi: 10.1515/hsz-2017-0226. [DOI] [PubMed] [Google Scholar]
- 94.Lai CK, Gupta N, Wen X, Rangell L, Chih B, Peterson AS, Bazan JF, Li L, Scales SJ. Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell 22: 1104–1119, 2011. doi: 10.1091/mbc.e10-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell 22: 4694–4703, 2011. doi: 10.1091/mbc.e10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li R, Peng C, Zhang X, Wu Y, Pan S, Xiao Y. Roles of Arf6 in cancer cell invasion, metastasis and proliferation. Life Sci 182: 80–84, 2017. doi: 10.1016/j.lfs.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Kelly WG, Logsdon JM Jr, Schurko AM, Harfe BD, Hill-Harfe KL, Kahn RA. Functional genomic analysis of the ADP-ribosylation factor family of GTPases: phylogeny among diverse eukaryotes and function in C. elegans. FASEB J 18: 1834–1850, 2004. doi: 10.1096/fj.04-2273com. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol 189: 1039–1051, 2010. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Zhang Q, Wei Q, Zhang Y, Ling K, Hu J. SUMOylation of the small GTPase ARL-13 promotes ciliary targeting of sensory receptors. J Cell Biol 199: 589–598, 2012. doi: 10.1083/jcb.201203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell 31: 265–278, 2014. doi: 10.1016/j.devcel.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell 103: 209–221, 2011. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- 102.Linari M, Hanzal-Bayer M, Becker J. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Lett 458: 55–59, 1999. doi: 10.1016/S0014-5793(99)01117-5. [DOI] [PubMed] [Google Scholar]
- 103.Liu Q, Tan G, Levenkova N, Li T, Pugh EN Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 6: 1299–1317, 2007. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Kahn RA, Prestegard JH. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol 17: 876–881, 2010. doi: 10.1038/nsmb.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Kahn RA, Prestegard JH. Interaction of Fapp1 with Arf1 and PI4P at a membrane surface: an example of coincidence detection. Structure 22: 421–430, 2014. doi: 10.1016/j.str.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Kahn RA, Prestegard JH. Structure and membrane interaction of myristoylated ARF1. Structure 17: 79–87, 2009. doi: 10.1016/j.str.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long H, Huang K. Transport of ciliary membrane proteins. Front Cell Dev Biol 7: 381, 2020. doi: 10.3389/fcell.2019.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lowery J, Szul T, Styers M, Holloway Z, Oorschot V, Klumperman J, Sztul E. The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN). J Biol Chem 288: 11532–11545, 2013. doi: 10.1074/jbc.M112.438481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo C, Wu M, Su X, Yu F, Brautigan DL, Chen J, Zhou J. Protein phosphatase 1α interacts with a novel ciliary targeting sequence of polycystin-1 and regulates polycystin-1 trafficking. FASEB J 33: 9945–9958, 2019. doi: 10.1096/fj.201900338R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macia E, Luton F, Partisani M, Cherfils J, Chardin P, Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci 117: 2389–2398, 2004. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- 111.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100: 3299–3304, 2003. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mariani LE, Bijlsma MF, Ivanova AA, Suciu SK, Kahn RA, Caspary T. Arl13b regulates Shh signaling from both inside and outside the cilium. Mol Biol Cell 27: 3780–3790, 2016. doi: 10.1091/mbc.e16-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marzesco AM, Galli T, Louvard D, Zahraoui A. The rod cGMP phosphodiesterase delta subunit dissociates the small GTPase Rab13 from membranes. J Biol Chem 273: 22340–22345, 1998. doi: 10.1074/jbc.273.35.22340. [DOI] [PubMed] [Google Scholar]
- 114.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med 39: 219–228, 2007. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 115.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 28: 183–192, 2009. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Monis WJ, Faundez V, Pazour GJ. BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol 216: 2131–2150, 2017. doi: 10.1083/jcb.201611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213, 2007. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 118.Nachury MV, Mick DU. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol 20: 389–405, 2019. doi: 10.1038/s41580-019-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nassar N, Singh K, Garcia-Diaz M. Structure of the dominant negative S17N mutant of Ras. Biochemistry 49: 1970–1974, 2010. doi: 10.1021/bi9020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Newman LE, Schiavon CR, Turn RE, Kahn RA. The ARL2 GTPase regulates mitochondrial fusion from the intermembrane space. Cell Logist 7: e1340104, 2017. doi: 10.1080/21592799.2017.1340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol 15: 396–404, 2003. doi: 10.1016/S0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 122.Nozaki S, Katoh Y, Terada M, Michisaka S, Funabashi T, Takahashi S, Kontani K, Nakayama K. Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. J Cell Sci 130: 563–576, 2017. doi: 10.1242/jcs.197004. [DOI] [PubMed] [Google Scholar]
- 123.O’Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, Yoder BK. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia 2: 8, 2013. doi: 10.1186/2046-2530-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pasqualato S, Renault L, Cherfils J. Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep 3: 1035–1041, 2002. doi: 10.1093/embo-reports/kvf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol 85: 115–149, 2008. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 126.Pearring JN, San Agustin JT, Lobanova ES, Gabriel CJ, Lieu EC, Monis WJ, Stuck MW, Strittmatter L, Jaber SM, Arshavsky VY, Pazour GJ. Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet 13: e1006740, 2017. doi: 10.1371/journal.pgen.1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Popoff V, Langer JD, Reckmann I, Hellwig A, Kahn RA, Brügger B, Wieland FT. Several ADP-ribosylation factor (Arf) isoforms support COPI vesicle formation. J Biol Chem 286: 35634–35642, 2011. doi: 10.1074/jbc.M111.261800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prevo B, Scholey JM, Peterman EJG. Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery. FEBS J 284: 2905–2931, 2017. doi: 10.1111/febs.14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qin H. Regulation of intraflagellar transport and ciliogenesis by small G proteins. Int Rev Cell Mol Biol 293: 149–168, 2012. doi: 10.1016/B978-0-12-394304-0.00010-5. [DOI] [PubMed] [Google Scholar]
- 130.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol 15: 1695–1699, 2005. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 131.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal 16: 401–413, 2004. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18: 533–547, 2017. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Revenkova E, Liu Q, Gusella GL, Iomini C. The Joubert syndrome protein ARL13B binds tubulin to maintain uniform distribution of proteins along the ciliary membrane. J Cell Sci 131: jcs212324, 2018. doi: 10.1242/jcs.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodrigues FF, Harris TJC. Key roles of Arf small G proteins and biosynthetic trafficking for animal development. Small GTPases 10: 403–410, 2019. doi: 10.1080/21541248.2017.1304854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schonteich E, Pilli M, Simon GC, Matern HT, Junutula JR, Sentz D, Holmes RK, Prekeris R. Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol 86: 417–431, 2007. doi: 10.1016/j.ejcb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS. ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol 168: 1288–1298, 2006. doi: 10.2353/ajpath.2006.050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schwahn U, Lenzner S, Dong J, Feil S, Hinzmann B, van Duijnhoven G, Kirschner R, Hemberger M, Bergen AA, Rosenberg T, Pinckers AJ, Fundele R, Rosenthal A, Cremers FP, Ropers HH, Berger W. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet 19: 327–332, 1998. doi: 10.1038/1214. [DOI] [PubMed] [Google Scholar]
- 138.Schwarz N, Lane A, Jovanovic K, Parfitt DA, Aguila M, Thompson CL, da Cruz L, Coffey PJ, Chapple JP, Hardcastle AJ, Cheetham ME. Arl3 and RP2 regulate the trafficking of ciliary tip kinesins. Hum Mol Genet 26: 3451, 2017. doi: 10.1093/hmg/ddx245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seidel RD III, Amor JC, Kahn RA, Prestegard JH. Structural perturbations in human ADP ribosylation factor-1 accompanying the binding of phosphatidylinositides. Biochemistry 43: 15393–15403, 2004. doi: 10.1021/bi0490385. [DOI] [PubMed] [Google Scholar]
- 140.Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, Fogelgren B, Caspary T, Lipschutz JH, Barral DC. Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27: 308–320, 2016. doi: 10.1091/mbc.e15-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seixas E, Barros M, Seabra MC, Barral DC. Rab and Arf proteins in genetic diseases. Traffic 14: 871–885, 2013. doi: 10.1111/tra.12072. [DOI] [PubMed] [Google Scholar]
- 142.Sharer JD, Shern JF, Van Valkenburgh H, Wallace DC, Kahn RA. ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter. Mol Biol Cell 13: 71–83, 2002. doi: 10.1091/mbc.01-05-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shern JF, Sharer JD, Pallas DC, Bartolini F, Cowan NJ, Reed MS, Pohl J, Kahn RA. Cytosolic Arl2 is complexed with cofactor D and protein phosphatase 2A. J Biol Chem 278: 40829–40836, 2003. doi: 10.1074/jbc.M308678200. [DOI] [PubMed] [Google Scholar]
- 144.Shiba T, Koga H, Shin HW, Kawasaki M, Kato R, Nakayama K, Wakatsuki S. Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc Natl Acad Sci USA 103: 15416–15421, 2006. doi: 10.1073/pnas.0605357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shiba Y, Randazzo PA. ArfGAP1 function in COPI mediated membrane traffic: currently debated models and comparison to other coat-binding ArfGAPs. Histol Histopathol 27: 1143–1153, 2012. doi: 10.14670/HH-27.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem 66: 639–678, 1997. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 147.Strom SP, Clark MJ, Martinez A, Garcia S, Abelazeem AA, Matynia A, Parikh S, Sullivan LS, Bowne SJ, Daiger SP, Gorin MB. De novo occurrence of a variant in ARL3 and apparent autosomal dominant transmission of retinitis pigmentosa. PLoS One 11: e0150944, 2016. doi: 10.1371/journal.pone.0150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10: 1105–1107, 2013. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Su X, Wu M, Yao G, El-Jouni W, Luo C, Tabari A, Zhou J. Regulation of polycystin-1 ciliary trafficking by motifs at its C-terminus and polycystin-2 but not by cleavage at the GPS site. J Cell Sci 128: 4063–4073, 2015. doi: 10.1242/jcs.160556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 151.Sztul E, Chen PW, Casanova JE, Cherfils J, Dacks JB, Lambright DG, Lee FS, Randazzo PA, Santy LC, Schürmann A, Wilhelmi I, Yohe ME, Kahn RA. ARF GTPases and their GEFs and GAPs: concepts and challenges. Mol Biol Cell 30: 1249–1271, 2019. doi: 10.1091/mbc.E18-12-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Szul T, Sztul E. COPII and COPI traffic at the ER-Golgi interface. Physiology (Bethesda) 26: 348–364, 2011. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- 153.Taschner M, Weber K, Mourão A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J 35: 773–790, 2016. doi: 10.15252/embj.201593164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thomas S, Wright KJ, Le Corre S, Micalizzi A, Romani M, Abhyankar A, Saada J, Perrault I, Amiel J, Litzler J, Filhol E, Elkhartoufi N, Kwong M, Casanova JL, Boddaert N, Baehr W, Lyonnet S, Munnich A, Burglen L, Chassaing N, Encha-Ravazi F, Vekemans M, Gleeson JG, Valente EM, Jackson PK, Drummond IA, Saunier S, Attié-Bitach T. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutat 35: 137–146, 2014. doi: 10.1002/humu.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tian G, Thomas S, Cowan NJ. Effect of TBCD and its regulatory interactor Arl2 on tubulin and microtubule integrity. Cytoskeleton (Hoboken) 67: 706–714, 2010. doi: 10.1002/cm.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA. ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors: characterizing ARL1-binding proteins. J Biol Chem 276: 22826–22837, 2001. doi: 10.1074/jbc.M102359200. [DOI] [PubMed] [Google Scholar]
- 157.Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye Cp, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 158.Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol 15: 373–380, 2008. doi: 10.1038/nsmb.1396. [DOI] [PubMed] [Google Scholar]
- 159.Veltel S, Kravchenko A, Ismail S, Wittinghofer A. Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Lett 582: 2501–2507, 2008. doi: 10.1016/j.febslet.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 160.Vetter M, Stehle R, Basquin C, Lorentzen E. Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat Struct Mol Biol 22: 695–702, 2015. doi: 10.1038/nsmb.3065. [DOI] [PubMed] [Google Scholar]
- 161.Vitali T, Girald-Berlingeri S, Randazzo PA, Chen PW. Arf GAPs: A family of proteins with disparate functions that converge on a common structure, the integrin adhesion complex. Small GTPases 10: 280–288, 2019. doi: 10.1080/21541248.2017.1299271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Walia V, Cuenca A, Vetter M, Insinna C, Perera S, Lu Q, Ritt DA, Semler E, Specht S, Stauffer J, Morrison DK, Lorentzen E, Westlake CJ. Akt regulates a Rab11-effector switch required for ciliogenesis. Dev Cell 50: 229–246.e7, 2019. doi: 10.1016/j.devcel.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang J, Deretic D. The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci 128: 1375–1385, 2015. doi: 10.1242/jcs.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J 31: 4057–4071, 2012. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH II, Deretic D, Wandinger-Ness A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 22: 3289–3305, 2011. doi: 10.1091/mbc.e11-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA 108: 2759–2764, 2011. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wiens CJ, Tong Y, Esmail MA, Oh E, Gerdes JM, Wang J, Tempel W, Rattner JB, Katsanis N, Park HW, Leroux MR. Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. J Biol Chem 285: 16218–16230, 2010. doi: 10.1074/jbc.M109.070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 16: 849–860, 2005. doi: 10.1091/mbc.e04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wittinghofer A, Vetter IR. Structure-function relationships of the G domain, a canonical switch motif. Annu Rev Biochem 80: 943–971, 2011. doi: 10.1146/annurev-biochem-062708-134043. [DOI] [PubMed] [Google Scholar]
- 170.Wright J, Kahn RA, Sztul E. Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation. Cell Mol Life Sci 71: 3419–3438, 2014. doi: 10.1007/s00018-014-1602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, Jackson PK. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 25: 2347–2360, 2011. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wright ZC, Loskutov Y, Murphy D, Stoilov P, Pugacheva E, Goldberg AFX, Ramamurthy V. ADP-ribosylation factor-like 2 (ARL2) regulates cilia stability and development of outer segments in rod photoreceptor neurons. Sci Rep 8: 16967, 2018. doi: 10.1038/s41598-018-35395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu CT, Chen HY, Tang TK. Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat Cell Biol 20: 175–185, 2018. doi: 10.1038/s41556-017-0018-7. [DOI] [PubMed] [Google Scholar]
- 174.Wu Q, Ren X, Zhang Y, Fu X, Li Y, Peng Y, Xiao Q, Li T, Ouyang C, Hu Y, Zhang Y, Zhou W, Yan W, Guo K, Li W, Hu Y, Yang X, Shu G, Xue H, Wei Z, Luo Y, Yin G. MiR-221-3p targets ARF4 and inhibits the proliferation and migration of epithelial ovarian cancer cells. Biochem Biophys Res Commun 497: 1162–1170, 2018. doi: 10.1016/j.bbrc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 175.Xue B, Liu YX, Dong B, Wingfield JL, Wu M, Sun J, Lechtreck KF, Fan ZC. Intraflagellar transport protein RABL5/IFT22 recruits the BBSome to the basal body through the GTPase ARL6/BBS3. Proc Natl Acad Sci USA 117: 2496–2505, 2020. doi: 10.1073/pnas.1901665117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye F, Nager AR, Nachury MV. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol 217: 1847–1868, 2018. doi: 10.1083/jcb.201709041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang CJ, Rosenwald AG, Willingham MC, Skuntz S, Clark J, Kahn RA. Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J Cell Biol 124: 289–300, 1994. doi: 10.1083/jcb.124.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, Inana G, Whitby FG, Jorgensen EM, Hill CP, Tong L, Baehr W. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci 14: 874–880, 2011. doi: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res 319: 2316–2322, 2013. doi: 10.1016/j.yexcr.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang T, Li S, Zhang Y, Zhong C, Lai Z, Ding J. Crystal structure of the ARL2-GTP-BART complex reveals a novel recognition and binding mode of small GTPase with effector. Structure 17: 602–610, 2009. doi: 10.1016/j.str.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 181.Zhao X, Lasell TK, Melançon P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol Biol Cell 13: 119–133, 2002. doi: 10.1091/mbc.01-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou C, Cunningham L, Marcus AI, Li Y, Kahn RA. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell 17: 2476–2487, 2006. doi: 10.1091/mbc.e05-10-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]