Abstract
Aging is associated with gradual decline in numerous physiological processes, including a reduction in metabolic functions and immunological system. The circadian rhythm plays a vital role in health, and prolonged clock disruptions are associated with chronic diseases. The relationships between clock genes, aging, and immunosenescence are not well understood. Inflammation is an immune response triggered in living organisms in response to the danger associated with pathogens and injury. The term ‘inflammaging’ has been used to describe the chronic low-grade-inflammation that develops with advancing age and predicts susceptibility to age-related pathologies. Equilibrium between pro-and anti-inflammatory cytokines is needed for healthy aging and longevity. Sedentary and poor nutrition style life indices a disruption in circadian rhythm promoting an increase in pro-inflammatory factors or leads for chronic low-grade inflammation. Moreover, signals mediated by pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, might accentuate of the muscle loss during aging. Circadian clock is important to maintain the physiological functions, as maintenance of immune system. A strategy for imposes rhythmicity in the physiological systems may be adopted of exercise training routine. The lifelong regular practice of physical exercise decelerates the processes of aging, providing better quality and prolongation of life. Thus, in this review, we will focus on how aging affects circadian rhythms and its relationship to inflammatory processes (inflammaging), as well as the role of physical exercise as a regulator of the circadian rhythm, promoting aging with rhythmicity.
Keywords: Aging, Inflammaging, Immunosenescence, Circadian rhythms, Clock genes
Graphical abstract
1. Introduction
Aging is characterized by a gradual decline in numerous physiological processes, including a reduction in metabolic function [1], increase in fat mass, reduction in lean mass [2], a decline in immune function leading to increased susceptibility to disease [3], and reduced cognitive performance [4,5]. Moreover, sleep and circadian rhythm activity measured under natural environmental conditions showed significant age-related alterations. Both sleep and activity rhythms become fragmented and attenuated in the elderly. The degeneration of the circadian timing system likely contributes to the age-related changes in sleep and the circadian rhythm [[6], [7], [8]].
Circadian rhythms exert control in several biological processes, such as sleep-wake cycles, body temperature, food intake, secretion of hormones and enzymes, glucose homeostasis, and regulation of the cell cycle. The control of circadian rhythms is regulated by central and peripheral clocks [9].
The term circadian rhythm was defined by Halberg et al. in 1959 to describe the association between the endogenous oscillation of many physiological factors and the earth's daily rotation [10,11].The circadian rhythm can be controlled by endogenous oscillators, which govern the rhythmic pattern of several physiological and behavioral functions [12]. Some environmental cycles adjust the oscillator, which generates the circadian rhythm, causing it to oscillate with the same cycle period [13]. The circadian timing system is vulnerable to aging, showing alterations with advanced age. The major circadian changes observed in aging include the reduction of the amplitude and earlier timing of the phase of daily rhythms. These alterations were observed in sleep-wake cycles, melatonin production, and body temperature [14].
Furthermore, the immune system exhibits rhythm in the control of its tasks. The first evidence of rhythmicity in the immune response was reported in the 1950s and showed that the host defense against pathogens (endotoxin) and mortality were dependent on the time of the day that the endotoxin was injected in mice [15]. Another study showed that the complex interactions between biological and physiological rhythms affect the immune response. For example, shift workers exhibited an elevated risk in the development of different diseases characterized by chronic inflammation, such as cancer, cardiovascular diseases, type 2 diabetes, and obesity [16]. Hence, several researchers have tried to understand the role of clock genes and the central clock in innate and adaptive immune systems, and we review this topic below.
Thus, in this review, we focus on the circadian rhythm by aging and its relationship to inflammatory processes (inflammaging), as well as the role of physical exercise as a modulator of the circadian rhythm, promoting aging with rhythmicity.
2. Mechanisms of the circadian rhythm
The circadian rhythm is controlled by circadian clock that are present in almost all mammalian tissues. The central clock is located in the suprachiasmatic nucleus of the hypothalamus (SCN), a small region of the brain containing 10,000–15,000 neurons [17]. The SCN clock can operate autonomously without any external input from stimuli but can be entrained by environmental stimuli, such as light. Clocks outside the SCN are referred to as peripheral clocks, but they are synchronized by the central clock to temporally ensure physiological coordination. In addition, peripheral clocks in liver or skeletal muscle can be synchronized by the availability of metabolites, feeding time, or physical exercise [18]. Peripheral clocks are present in almost all mammalian tissues, including the liver, heart, lungs, kidneys, and immune cells, in which circadian rhythms are maintained [19].
The circadian clock is controlled by interactions between the feedbacks looping of circadian genes in the nucleus of all cells of the body. The fundamental mechanism of rhythm maintenance is similar in central and peripheral clocks [19].
As master regulators, Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT-like protein 1 (BMAL1) form heterodimers, which interact to form a transcriptional activator complex that stimulates the transcription of the Period (PER1, PER2, and PER3) and Cryptochrome (CRY1and CRY2) genes (negative feedback). In turn, CRY and PER form a complex, which acts as the negative arm to inhibit the transcriptional activity of CLOCK/BMAL1 heterodimers [17,19,20]. Another regulatory mechanism is induced by the CLOCK/BMAL1 heterodimeric complex that activates the transcription of nuclear orphan receptors REV-ERBs (α and β) and RORs (α, β, and γ isoforms) [21,22]. REV-ERB and ROR subsequently compete for retinoic acid-related orphan receptor response elements present in the BMAL1 promoter. ROR activates the transcription of BMAL1 [[23], [24], [25]], whereas REV-ERB suppresses its transcription [17,19,20]. Thus, the circadian oscillation of BMAL1 is both positively and negatively regulated by RORs and REV-ERBs [19].
We observe the deregulation on central and peripheral clocks on the aging that affect directly on quality of life and life expectancy. Thus, we detailed bellow the consequences of aging on circadian rhythm and the early aging that may be observed in adults with disruption of circadian rhythms.
3. Aging and circadian rhythms disruption
The aging process can be characterized as the progressive impairment of homeostasis at genomic, cellular, tissue, and whole organism levels, which reduces survival and fertility while increasing the risk of disease and death [23].
The circadian rhythm plays a vital role in health, and prolonged clock disruptions are associated with negative health consequences. With advancing age, the circadian system undergoes significant changes that affect behavioral rhythms, temperature regulation, and hormones release, and these changes in circadian rhythms are related to poor quality sleep [14]. These changes in sleep quality and total sleep time are directly related to higher daytime sleepiness, poorer quality of life of the elderly, and cognitive impairment [14].
One of the factors observed with advancing age is a change in sleep architecture. Studies show that in humans, changes in sleep can begin in the fourth decade of life, leading to a decrease in total sleep time of up to 30 min every 10 years [26]. When compared with young adults, older adults experience more night awakenings, have longer latencies to fall asleep, and spend less time in stage 3 and rapid eye movement sleep [14,24].
Aging is related to the declined secretion of hormones, such as melatonin. Melatonin is a hormone produced by the pineal gland. During daylight, intense light blocks pineal melatonin production. However, as light intensity declines with dusk, the retina sends information through the retino-hypothalamic tract to the SCN in which this information is transmitted to the pineal gland to initiate melatonin production and release [25,27]. Melatonin release regulates core body temperature, promotes sleep onset, modulates the activity of intrinsically photosensitive retinal ganglion cells that provide time-keeping signals to the SCN [14,28], and controls the circadian oscillator system [29].
Furthermore, a decline in the secretion of melatonin is related to multiple alterations in the immune system that can be taken to the pro-inflammatory side [25,30], and studies have shown the relationship between melatonin and age-associated neuropathologies, such as Alzheimer's or Parkinson's disease [25].
In addition, the expression of the clock genes are found beyond of the central clock (SNC), as in a number of extra-SCN sites in the mouse brain, including the hippocampus, amygdala and the paraventricular, arcuate and dorsomedial nucleus of the hypothalamus, therefore the amplitude and phase of expression in these regions might be altered with the aging [31,32]. In the SCN, Clock, Bmal1 and Per2 have been showed decreases in the rhythm amplitude or its expression levels with the age advanced [31,33].
As previously discussed, the circadian clock consists of input pathways perceived mainly by the SCN and other brain areas; this signaling via SCN will synchronize peripheral tissues. Similar to the differential physiological impacts of aging on organ and tissue function, aging also appears to affect peripheral clocks to several degrees [34].
In peripheral tissue may be disturbance in the oscillations of the genes that control the circadian clock affected by aging. For example, the circadian clock regulates many genes involved in the responses to oxidative stress and cellular redox [35,36], and this mechanism can changes with aging [37].
Whereas, the aging is characterized by disruption on circadian rhythms, the opposite is true too. The chronic deraignment on circadian clock reduces the longevity on Drosophila [38,39]. In humans, the shift-workers show loss of circadian rhythms and consequently higher risk to develop metabolic abnormalities and early aging observed by shorter of leukocytes telomere length [40].
In accordance, the other life habits that they are able to induce the loss of circadian rhythm and cause the early aging. Obesity is the most well established risk factor able to induce the early aging. Thus, the obese young adults with higher insulin concentrations showed telomere shortening, an important parameter to show the non-chronological age [41]. Furthermore, when metabolic syndrome was present during the pregnancy the child showed 14% of reduction on telomere length [42]. Regarding to vascular aging, the obesity accelerate the early vascular aging in young obese [43,44].
Drugs, in special chemotherapy and radiotherapy are able to induce shorter of telomere and reduction on activity in fibroblast. The telomere dysfunction leads to reduction on life expectancy and genomic instability on somatic cell [45]. In this sense, doxorubicin is able to induce early senescence on human cardiac progenitor cells [46].
Moreover, the doxorubicin induces the senescence of mesenchymal stem cells with reduction on telomere length and activity, and induces the senescence associated secretory phenotype (SASP) that it is a trigger to immunesenescence [47]. Our group recently demonstrated that the doxorubicin treatment in vivo and in vitro disrupt the clock gene expression with concomitantly immunesupression [48].
The immune system modifications throw the life cause direct effect on aging process. Thus we discuss bellow the role of immune system and the circadian rhythms in aging.
4. Immune system, circadian rhythms and aging
Many parameters of the immune system show rhythmicity, including circulating white blood cells, hormones (cortisol and catecholamine), and cytokine production [49]. In humans, the peak of mature leukocytes (except for effector CD8T lymphocytes) occurs at night, whereas the secretion of cortisol, catecholamine, and pro-inflammatory cytokines reaches the maximum concentration during the day [49].
Furthermore, the activity of immune cells is tightly regulated by circadian rhythms. The response of lipopolysaccharide (LPS) is stronger at the end of the rest period or beginning of the active period than in the early rest period [50]. Moreover, recently was demonstrated that the sepsis causes disruption on circadian rhythms associated with sepsis severity [51]. In addition, the phagocytosis ability of intraperitoneal macrophages shows circadian rhythms. Specifically, their phagocytic activity is enhanced at the end of the rest period in comparison with all other times of the day [52]. CRY1 and CRY2 double knockout caused several disturbs on many aspects of immunity and the mice showed increase on autoimmune diseases [53].
The relationships between clock genes, aging, and immunosenescence are not fully clarified, but there is the emergency in clarify this complex interplay to improve the immunity in elderly. Monocytes and macrophages show great rhythmicity with a high amplitude of clock gene expression [50]. Our group observed that the control of clock genes in macrophages is very well-controlled [48].
On the past year, we observed the intrinsic pathway between circadian rhythm, clock gene on the function of group 3 innate lymphoid cells (ILC3) and gut homeostasis [54,55]. Godinho-Silva and colleagues showed that conditional depletion of BMAIL1, a master a circadian activation on hematopoietic cells affected severely the ILC3 causing hyperinflammation, gut disbiosis and disorder on lipid metabolism, and the environmental cues as light-dark cycles, feeding schedule and microbiota impact in clock machinery in ILC3 and consequently on intestinal homeostasis [55]. In accordance, the BMAL1 conditional deletion on ROR-γ T cells showed the disruption on ILC3 with increased pro-apoptotic and inflammatory pathways [56]. Noteworthy, that the gastrointestinal tract show a concentration 400 times higher of melatonin that the pineal gland, what it exemplify the interplay between circadian clock and gut homeostasis [57]. This may explain the gut derangement during in the aging process.
The rhythmicity of the immune response in aging is impaired, but the cause of this is unclear. It was observed that the elderly showed dysregulation of circadian rhythms in T helper lymphocytes [58]. Furthermore, the neuroendocrine-immune axis lost circadian rhythm expression in elderly people [59,60]. Another cause of immunosenescence can be related with reduction in melatonin secretion since this hormone is able to induce the production of macrophages, granulocytes, and lymphocytes [30].
Many alterations are found in the immune system during aging. For example, humans lose the better response to immunization, with a decrease in the effectiveness of vaccines and an increase in autoimmunity diseases and inflammaging (see below) [59].
The role of clock genes in innate immunity is more clear, but few studies have shown the role of clock genes in adaptive immunity [61]. T-cells express clock genes, and this clock machinery is highly associated with cytokine responses [62]. Interestingly, in the last year, Nobis and colleagues showed that the effectiveness of vaccines is dependent on the time of day. A more pronounced response occurred after the vaccination in the middle of the day, and the deletion of BMAL1 in dendritic cells or CD8 lymphocytes abrogated this circadian rhythmicity response to vaccination [63].
A perspective very important in this moment, during the COVID-19 pandemic, it is evaluate the clock genes expression in dendritic cells and lymphocytes that may explain the impair on vaccination response making possible that induction of regulation of clock genes expression in immune cells, so mitigate the effects of immunosenescence on efficacy of vaccination.
The immunosenescence can be better characterized and marked in cells of the adaptive immune system [64,65]. One of the main changes in the adaptive immune system is the replacement of naive T and B cells by memory cells [65,66]. The changes in the aging adaptive immune system occur in the T cell compartment [67], this increase in the number of memory T cells and, at the later, that of B cells may be due to a continuous chronic antigenic stimulation of inflammatory process [68,69]. Therefore, with advancing age, a deterioration of immunity can be observed, while some cells remain unchanged or others tend to have greater activity or hyperactivity [[70], [71], [72]].
Moreover, the immunosenescence should be defined by senescence phenotype on lymphocytes, with increase on end stage of differentiation of CD4 and CD8 T cells and B lymphocytes, and reduction on naïve circulating lymphocytes. Moreover, the chronological or non-chronological aging present inflammaging that will be discussed in the next topic.
5. Clock genes deregulation and inflammaging on elderly
Inflammation is an immune response triggered in living organisms in response to the danger associated with pathogens and injury. Inflammation may vary over 24 h. For example, in sepsis, inflammation shows a large diurnal variation [73]. On the other hand, studies have shown the relationship between clock genes and inflammatory processes. For example, CLOCK can directly interact with the p65 subunit of NF-kΒ to enhance its transcriptional activity on the promoters of inflammatory genes [74]. Additionally, the deletion of CRY proteins augments both basal and inducible inflammatory responses, as CRY1 and CRY2 knockout mice show increased expression of TNF-alpha [75].
However, BMAL1 may promote an anti-inflammatory effect when in a heterodimer with CLOCK, thus preventing the interaction of CLOCK with the p65 subunit of NF-kΒ, and BMAL1 deficiency may result in chronic inflammation [74]. Moreover, NF-kB activation is under the control of a feedback loop represented by the clock genes BMAL1/CLOCK and their transcriptional regulators RORα (positive) and REV-ERBα (negative). Recently, Zhao et al. (2019) showed that the forced increased expression of REV-ERBα reduced the secretion of pro-inflammatory cytokines induced by LPS via the inhibition of TLR-4-NF-kB activation [76].
Interestingly, cytokines are able to modify clock genes. TNF-α in synovial cells culture reduces the expression of PER2 and REV-ERBα [77]. Moreover, this cytokine increased the expression and association between BMAL1 and RORα [78]. The underlying mechanisms involved in the alteration of clock genes after cytokine stimuli are not well understood, but this is a two-way road.
A balance between pro- and anti-inflammatory cytokines is needed for healthy aging and longevity. A disruption in circadian rhythm may alter this balance, favoring an increase in pro-inflammatory factors or causing chronic low-grade inflammation. In the last two decades, the term ‘inflammaging’ has been used to describe the chronic low-grade inflammation that develops with advancing age and predicts susceptibility to age-related pathologies [79].
Chronic inflammation is associated with many age-related physiologic or pathophysiologic processes and diseases. Even in healthy aging, serum concentrations of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-12, IL-15, IL-17, IL-18, TNF-α, and IFN-γ) are significantly increased in comparison with younger individuals [80]. In the same way that the concentrations of pro-inflammatory cytokines are increased in the elderly, anti-inflammatory cytokines are also increased (IL-1Ra, IL-4, and IL-10) [80]. This balance between pro- and anti-inflammatory cytokines occurs in an attempt to protect tissues. The imbalance between the action of pro- and anti-inflammatory cytokines is related to unhealthy aging and the development of some age-related pathological conditions [81].
Other factors that contribute to chronic low-grade inflammation are physical inactivity/sedentary [82] that contributes for augmented visceral adipose tissue. The prevalence of physical inactivity and sedentarism is quite high in the aging population [83] (see next topic).
The different adipose tissue depots exert impact to inflammation in aging, and are related to metabolic alterations and immune cell infiltration, particularly in visceral adipose tissue. For example, the macrophages trapped within fat depots are able to release pro-inflammatory cytokines, such as IL-6 and TNF-α [84]. Further, a recent study in mice showed that in the absence of obesity, visceral adipose tissue possesses a pronounced anti-inflammatory phenotype during aging, which is further enhanced by exercise [85].
In addition, studies have been proposed that obesity could be considered an accelerated model of aging. The hallmarks of aging may be compared with the physiological stresses induced by obesity. This compare is related cause a range of cellular and whole-body deteriorations that maintenance the pathophysiology of adipose tissue accumulation and dysfunction [65,86,87].
Regarding the clock genes, obesity and inflammation, the ROR family (RORα and RORγ) are very important on immunometabolism response. RORγ, for instance, is essential to T lymphocyte differentiation on Th17 subset [88]. Moreover, ROR family is very expressed in metabolic peripheral organs (liver, skeletal muscle, kidney and adipose tissue) and regulates the lipid metabolism, in special de lipogenesis, triacylglycerol formation and storage and cholesterol metabolism [89,90]. RORα or RORγ knockout mice showed resistance to weight-gain and metabolic syndrome in high fed diet model, while the obesity induces the increased of RORα or RORγ on liver and adipose tissue [90]. Moreover, recently, Hams et al. (2020) showed that humans and mice obese showed raise mRNA expression of RORα. In accordance, the myeloid cells the specific deletion of RORα on myeloid cells is sufficient to reduction of inflammation, insulin resistance and weight gain on high fatty diet fed mice [91].
In summary, the immunosenescence founded in obese and elderly people show many similarities. The low-grade inflammation, reduction on anti-viral response and inflammaging are close associated with increase on visceral adiposity that is very common in obese and aging process. Moreover, the physical inactivity induces the fat accumulation on visceral adipose tissue. On the other hand, the high physical fitness induces the reduction and protection against the fat accumulation in this depot [92]. Thus, the visceral adiposity is in the center of co-morbidities related with, obesity poor aging (Fig. 1 ), and physical activity is able to delay this process as will be explained in the next topics.
Fig. 1.
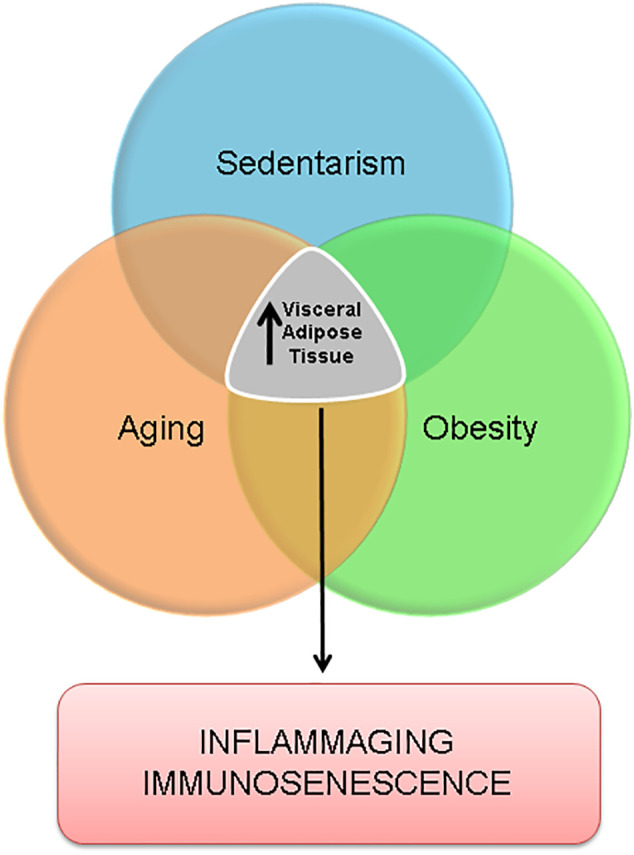
Aging, obesity and sedentarism have in common the increase in visceral adipose tissue that can lead to a state of inflammaging and immunosenescence.
6. Inflammaging and absence of contractile activity/inactivity physical
The promotion of catabolic signals mediated by pro-inflammatory cytokines, such as TNF-α and IL-6, induces muscle loss during aging. Chronic low-grade inflammation is related to low muscle regeneration capacity through satellite cells in older adults, which contributes to muscle loss and sarcopenia [93,94].
Nevertheless, when and where the aging-related physiological and biochemical changes? These questions remain unclear. However, these dysfunctions, such as changes in circadian rhythm, hormonal changes, worsening sleep quality, and chronic inflammation associated with a sedentary lifestyle, contribute to a substantial loss of life quality. In addition to the factors listed above, including chronic low-grade inflammation and hormonal changes, a sedentary lifestyle and the consumption of low-quality food, such as processed foods, also contribute to sarcopenia [95].
All of these changes are part of the life cycle, but the question is how to delay these changes as much as possible for prolonged and healthy aging. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis, and management published in 2018 recommend physical activity as the primary treatment of sarcopenia [96].
Clock genes are important in the maintenance of the physiological functions of skeletal muscle. Approximately 17% of the skeletal muscle transcriptome shows circadian rhythms, including clock genes, as well as genes related to metabolism, cell cycle, cytoskeletal organization, stress response, and differentiation, and the overexpression of CLOCK caused a significant modification in the pattern of these gene expression profiles [97]. Moreover, the deletion of BMAL-1 in the whole body or specifically in skeletal muscle induces early aging with the development of sarcopenia and reduction in metabolism, oxidative stress responses, and physical activity [98]. However, it is necessary to elucidate how clock genes may be related to physical activity and the preservation of muscle mass in aging.
Physical exercise is associated with a lower rate of developing chronic diseases, such as cardiovascular disease, type 2 diabetes mellitus, sarcopenia, obesity, and others. In addition, lifelong exercise is related to increased longevity. It is not so recent that studies showed the relationship between physical exercise and inflammation [99].
In healthy populations, exercise training has been shown to increase the level of anti-inflammatory cytokines (e.g., IL-10), reduce overall TNF-α expression, decrease pro-inflammatory adipokines, and reduce the expression of Toll-like receptors on monocytes and macrophages [100,101]. Furthermore, a 12-month program of moderate-intensity physical activity (combination of aerobic, strength, balance, and flexibility exercises, 40–60 min, 3 times/week) results in reduced systemic concentrations of IL-6 in elderly individuals [102]. However, IL-6 could be a dual effect, when produced by skeletal muscle during contractile activity, IL-6 is known by the anti-inflammatory effect. It has been consistently demonstrated that the plasma concentration of IL-6 increases during exercise. This increase is followed by the appearance of IL-1ra and IL-10 the anti-inflammatory cytokine [[103], [104], [105]], and this anti-inflammatory profile.
Recently, Minuzzi et al. (2019) showed that lifelong training helps to maintain the balance of pro- and anti-inflammatory cytokines, together with IL-10 levels close to those found in young adults [106], and this will be discussed in next section.
7. Exercise as a time-conditioning effector
The lifelong regular practice of physical exercise decelerates the processes of aging, providing better quality and prolongation of life. Lifelong exercise is characterized by regular exercise throughout many years (20 or more).
Interestingly, immunosenescence observed in aging can be enhanced by obesity and sedentarism. This can be explained by similar conditions as the development of chronic low-grade inflammation. The therapy for react in this situation is increases physical fitness status, with enrollment of exercise training [65,92]. Thus, lifelong athletes show decreased inflammaging and immunosenescence [106,107].
Moreover, the age-related dysfunction in muscle is caused, at least in part, by an increase in inflammatory cytokines observed during inflammaging [108]. Thus, the inhibition of pro-inflammatory markers can help to preserve muscle mass. It is interesting because aging modified the profile of myokines secreted by skeletal muscle, which induced defect in the communication between skeletal cells and immune cells and initiated the vicious inflammatory cycle [108].
Minuzzi et al. (2018) showed that maintaining high levels of aerobic fitness during the natural course of aging may help prevent the accumulation of senescent T-cells [107]. Moreover, lifelong training helps to maintain the balance of pro- and anti-inflammatory cytokines, together with IL-10 levels close to those found in young adults [106]. In the Minuzzi's study, the master athletes trained approximately 4 h per week, with intensity the training sessions classified as moderate.
Physical exercise is a well-known anti-inflammatory therapy and induces a “health profile of myokine secretion”. For example, lifelong participation in exercise training delays the senescence process. Furthermore, one bout of exercise is able to induce the release of myokines in the circulation. Lavin et al. showed that in lifelong athletes who trained in aerobic modality, one bout of resistance training induces higher anti-inflammatory myokines than in sedentary elderly people. Thus, this study showed a better adaptive response after one acute bout of exercise in lifelong athletes [108]. The schedule of exercise training routine in the Lavin's study show that master athletes trained approximately ~5 days for week, 7 h per week, with intensity the training sessions classified as moderate.
The same group of researchers analyzed women with an average age of 72 who trained in the last 48 years and found that lifelong training did not benefit type MHC I and IIa muscle fiber size but promoted adaptations of the contractile function that increased the strength of type I fibers and preserved the energy of type IIa fibers through different contractile mechanisms [109]. In addition, a greater intensity of training throughout life provided increased protection against the infiltration of adipose tissue in muscle [110].
Another study showed that more than 50 years of aerobic exercise fully preserved capillarization and aerobic enzymes regardless of intensity and suggested that skeletal muscle metabolic fitness may be easier to maintain with lifelong aerobic exercise [111]. In addition, other benefit of lifelong exercise is the reduction in the decline of maximum oxygen consumption (VO2max). This concept describe show the subject is able to transfer oxygen from the atmosphere to working muscles [112]. This is the measure of cardiorespiratory ability, and this function is severely reduced with aging [113]. However, lifelong exercise has attenuated the decline in VO2max that is related to aging [114,115].
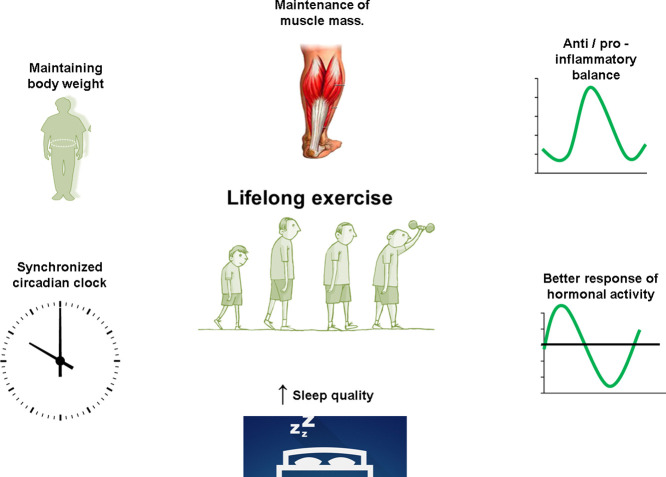
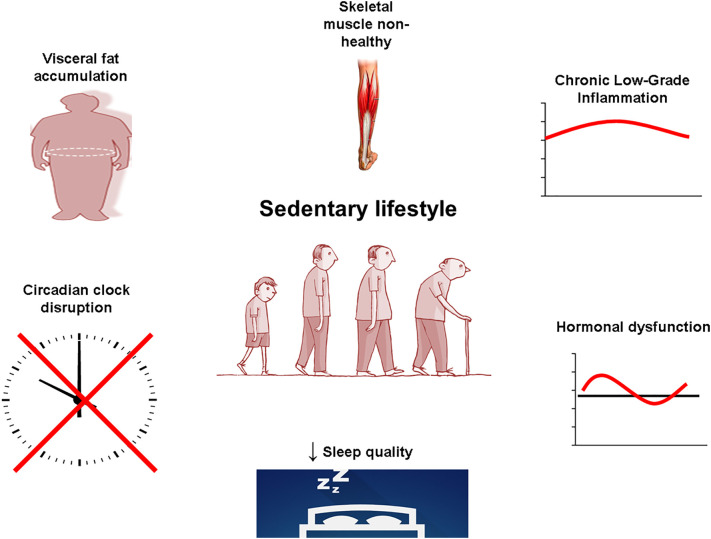
Our group has recently found a relationship between the genes that control the circadian rhythm in EM CD4+ T-cells (but not in EM CD8+ T-cells) and the production of anti-inflammatory cytokines in individuals who trained throughout their lives (Teixeira et al. data not published). Taken together, many scientific evidences suggestion the exercise as a “Time-conditioning Effector” [116]. News avenues are open for better understating of the essential role of lifelong exercise as “the best” synchronizator of clock genes, promoting efficient signaling between immunological cells, skeletal muscle, brain, adipose tissue, and others tissue/organs (Fig. 2, Fig. 3 ).
Fig. 2.
A sedentary lifestyle is related to the accumulation of visceral fat, chronic low-grade inflammation, hormonal dysfunction, worsening sleep quality, skeletal muscle non-healthy, and circadian clock disruption.
Fig. 3.
Lifelong exercise is related to the maintenance of body weight, the anti/pro-inflammatory balance, better response of hormonal activity, improvement in sleep quality, maintenance of muscle mass, and maintenance of the circadian rhythm throughout life.
8. Conclusion
Thus, lifelong exercise prevents an exacerbates of pro-inflammatory processes, promotes better immune function, prevents the decline in VO2max related to aging, improves the hormonal profile, and improves the quality of sleep. Together, this helps to maintain the circadian rhythm throughout life and can assist in healthy aging, increasing the quality of life through regular physical exercise. However, the interactions between clock genes as central players of this game should be proved by molecular and physiological approaches that show the role of them in the slow aging in lifelong athletes.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
To the funding sources, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil) process number 2015/16777-0, 2016/01409-8, 2018/24187-6, 2019/09854-9 and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
The present study was conducted in tribute of the 15-year anniversary of the death of Professor Luis Fernando Bicudo Pereira Costa Rosa (GG), an eminent exercise immunology researcher in Brazil, whose ideas, erudition, critical sense and honesty continue to inspire us. In special with this interaction between neuro-immune-endocrine axis influenced by physical activity.
References
- 1.Krems C., Luhrmann P.M., Strassburg A., Hartmann B., Neuhauser-Berthold M. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur. J. Clin. Nutr. 2005;59:255–262. doi: 10.1038/sj.ejcn.1602066. doi:1602066 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Atlantis E., Martin S.A., Haren M.T., Taylor A.W., Wittert G.A. Lifestyle factors associated with age-related differences in body composition: the Florey Adelaide male aging study. Am. J. Clin. Nutr. 2008;88:95–104. doi: 10.1093/ajcn/88.1.95. doi:88/1/95 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Haynes L., Maue A.C. Effects of aging on T cell function. Curr. Opin. Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. S0952-7915(09)00089-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks G., Nolan P.M., Peirson S.N. Reciprocal interactions between circadian clocks and aging. Mamm. Genome. 2016;27:332–340. doi: 10.1007/s00335-016-9639-610.1007/s00335-016-9639-6. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson R.D., Barnes C.A. Impact of aging brain circuits on cognition. Eur. J. Neurosci. 2013;37:1903–1915. doi: 10.1111/ejn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijk D.J., Duffy J.F. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann. Med. 1999;31:130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 7.D.J. Dijk, J.F. Duffy, E. Riel, T.L. Shanahan, C.A. Czeisler, Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms, J. Physiol.. 516 ( Pt 2 (1999) 611–627. [DOI] [PMC free article] [PubMed]
- 8.Huang Y.L., Liu R.Y., Wang Q.S., Van Someren E.J., Xu H., Zhou J.N. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol. Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. doi:S0031938402007333 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Zee P.C., Attarian H., Videnovic A. Circadian rhythm abnormalities. Contin. (Minneap Minn) 2013;19:132–147. doi: 10.1212/01.CON.0000427209.21177.aa00132979-201302000-00014. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halberg F., Halberg E., Barnum C.P., Bittner J.J. 1959. Photoperiodism and Related Phenomena in Plants and Animals, AAAS 1959. [Google Scholar]
- 11.Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. nri3386 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi J.S., Zatz M. Regulation of circadian rhythmicity. Science (80-.) 1982;217:1104–1111. doi: 10.1126/science.6287576. [DOI] [PubMed] [Google Scholar]
- 13.Kondratov R.V., Chernov M.V., Kondratova A.A., Gorbacheva V.Y., Gudkov A.V., Antoch M.P. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.109950317/15/1921. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood S., Amir S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 2017;127:437–446. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HALBERG F., JOHNSON E.A., BROWN B.W., BITTNER J.J. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- 16.Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht U., Eichele G. The mammalian circadian clock. Curr. Opin. Genet. Dev. 2003;13:271–277. doi: 10.1016/s0959-437x(03)00055-8. doi:S0959437X03000558 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Tahara Y., Shibata S. Entrainment of the mouse circadian clock: effects of stress, exercise, and nutrition. Free Radic. Biol. Med. 2018;119:129–138. doi: 10.1016/j.freeradbiomed.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Robinson I., Reddy A.B. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014;588:2477–2483. doi: 10.1016/j.febslet.2014.06.005. doi:S0014-5793(14)00446-3 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Sahar S., Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat. Rev. Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. nrc2747 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Eide E.J., Woolf M.F., Kang H., Woolf P., Hurst W., Camacho F., Vielhaber E.L., Giovanni A., Virshup D.M. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. doi:25/7/2795 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volek J.S., Freidenreich D.J., Saenz C., Kunces L.J., Creighton B.C., Bartley J.M., Davitt P.M., Munoz C.X., Anderson J.M., Maresh C.M., Lee E.C., Schuenke M.D., Aerni G., Kraemer W.J., Phinney S.D. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65:100–110. doi: 10.1016/j.metabol.2015.10.028. doi:S0026-0495(15)00334-0 [pii] [DOI] [PubMed] [Google Scholar]
- 23.N.J. Hunt, S.W.S. Kang, G.P. Lockwood, D.G. Le Couteur, V.C. Cogger, Hallmarks of aging in the liver., Comput. Struct. Biotechnol. J. 17 (2019) 1151–1161. doi: 10.1016/j.csbj.2019.07.021. [DOI] [PMC free article] [PubMed]
- 24.Dijk D.J., Duffy J.F., Czeisler C.A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 25.Hardeland R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Cauter E., Plat L., Leproult R., Copinschi G. Alterations of circadian rhythmicity and sleep in aging: endocrine consequences. Horm. Res. 1998;49:147–152. doi: 10.1159/000023162. [DOI] [PubMed] [Google Scholar]
- 27.Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 28.Arendt J. Melatonin and human rhythms. Chronobiol. Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 29.Hardeland R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12377. [DOI] [PubMed] [Google Scholar]
- 30.Cardinali D.P., Esquifino A.I., Srinivasan V., Pandi-Perumal S.R. Melatonin and the immune system in aging. Neuroimmunomodulation. 2008;15:272–278. doi: 10.1159/000156470. [DOI] [PubMed] [Google Scholar]
- 31.Wyse C.A., Coogan A.N. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- 32.De Nobrega A.K., Luz K.V., Lyons L.C. Resetting the aging clock: implications for managing age-related diseases. Adv. Exp. Med. Biol. 2020;1260:193–265. doi: 10.1007/978-3-030-42667-5_9. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T.J., Nakamura W., Tokuda I.T., Ishikawa T., Kudo T., Colwell C.S., Block G.D. Age-related changes in the circadian system unmasked by constant conditions. ENeuro. 2015;2 doi: 10.1523/ENEURO.0064-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welz P.-S., Benitah S.A. Molecular connections between circadian clocks and aging. J. Mol. Biol. 2020;432:3661–3679. doi: 10.1016/j.jmb.2019.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Giebultowicz J.M. Circadian regulation of metabolism and healthspan in Drosophila. Free Radic. Biol. Med. 2018;119:62–68. doi: 10.1016/j.freeradbiomed.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan N., Davis A.J., Giebultowicz J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klichko V.I., Chow E.S., Kotwica-Rolinska J., Orr W.C., Giebultowicz J.M., Radyuk S.N. Aging alters circadian regulation of redox in Drosophila. Front. Genet. 2015;6:83. doi: 10.3389/fgene.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boomgarden A.C., Sagewalker G.D., Shah A.C., Haider S.D., Patel P., Wheeler H.E., Dubowy C.M., Cavanaugh D.J. Chronic circadian misalignment results in reduced longevity and large-scale changes in gene expression in Drosophila. BMC Genomics. 2019;20:14. doi: 10.1186/s12864-018-5401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt L.C., Jiao J., Wang Y.-D., Finkelstein D., Rao D., Curley M., Robles-Murguia M., Shirinifard A., Pagala V.R., Peng J., Fan Y., Demontis F. Circadian gene variants and the skeletal muscle circadian clock contribute to the evolutionary divergence in longevity across Drosophila populations. Genome Res. 2019;29:1262–1276. doi: 10.1101/gr.246884.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavanello S., Stendardo M., Mastrangelo G., Casillo V., Nardini M., Mutti A., Campisi M., Andreoli R., Boschetto P. Higher number of night shifts associates with good perception of work capacity and optimal lung function but correlates with increased oxidative damage and telomere attrition. Biomed. Res. Int. 2019;2019:8327629. doi: 10.1155/2019/8327629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangge H., Herrmann M., Almer G., Zelzer S., Moeller R., Horejsi R., Renner W. Telomere shortening associates with elevated insulin and nuchal fat accumulation. Sci. Rep. 2020;10:6863. doi: 10.1038/s41598-020-63916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAninch D., Bianco-Miotto T., Gatford K.L., Leemaqz S.Y., Andraweera P.H., Garrett A., Plummer M.D., Dekker G.A., Roberts C.T., Smithers L.G., Grieger J.A. The metabolic syndrome in pregnancy and its association with child telomere length. Diabetologia. 2020 doi: 10.1007/s00125-020-05242-0. [DOI] [PubMed] [Google Scholar]
- 43.Ryder J.R., Northrop E., Rudser K.D., Kelly A.S., Gao Z., Khoury P.R., Kimball T.R., Dolan L.M., Urbina E.M. Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Sanchez M., Gomez-Sanchez L., Patino-Alonso M.C., Cunha P.G., Recio-Rodriguez J.I., Alonso-Dominguez R., Sanchez-Aguadero N., Rodriguez-Sanchez E., Maderuelo-Fernandez J.A., Garcia-Ortiz L., Gomez-Marcos M.A. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: early vascular ageing study. J. Hypertens. 2020;38:1110–1122. doi: 10.1097/HJH.0000000000002373. [DOI] [PubMed] [Google Scholar]
- 45.Li P., Hou M., Lou F., Björkholm M., Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int. J. Biochem. Cell Biol. 2012;44:1531–1540. doi: 10.1016/j.biocel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Piegari E., De Angelis A., Cappetta D., Russo R., Esposito G., Costantino S., Graiani G., Frati C., Prezioso L., Berrino L., Urbanek K., Quaini F., Rossi F. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res. Cardiol. 2013;108:334. doi: 10.1007/s00395-013-0334-4. [DOI] [PubMed] [Google Scholar]
- 47.Özcan S., Alessio N., Acar M.B., Mert E., Omerli F., Peluso G., Galderisi U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 2016;8:1316–1329. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira A.A.S., Biondo L.A., Silveira L.S., Lima E.A., Batatinha H.A., Diniz T.A., Oliveira De Souza C., Comin J., Neto J.C.R. Doxorubicin modulated clock genes and cytokines in macrophages extracted from tumor-bearing mice. Cancer Biol. Ther. 2020:1–10. doi: 10.1080/15384047.2019.1702400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheiermann C., Gibbs J., Ince L., Loudon A. Clocking in to immunity. Nat. Rev. Immunol. 2018;18:423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 50.Labrecque N., Cermakian N. Circadian clocks in the immune system. J. Biol. Rhythm. 2015;30:277–290. doi: 10.1177/0748730415577723. 0748730415577723 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Coiffard B., Diallo A.B., Culver A., Mezouar S., Hammad E., Vigne C., Nicolino-Brunet C., Dignat-George F., Baumstarck K., Boucekine M., Leone M., Mege J.-L. Circadian rhythm disruption and Sepsis in severe trauma patients. Shock. 2019;52:29–36. doi: 10.1097/SHK.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M., Shimba S., Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. doi:JST.JSTAGE/bpb/30.621 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Cao Q., Zhao X., Bai J., Gery S., Sun H., Lin D.-C., Chen Q., Chen Z., Mack L., Yang H., Deng R., Shi X., Chong L.-W., Cho H., Xie J., Li Q.-Z., Müschen M., Atkins A.R., Liddle C., Yu R.T., Alkan S., Said J.W., Zheng Y., Downes M., Evans R.M., Koeffler H.P. Circadian clock cryptochrome proteins regulate autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12548–12553. doi: 10.1073/pnas.1619119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q., Robinette M.L., Billon C., Collins P.L., Bando J.K., Fachi J.L., Sécca C., Porter S.I., Saini A., Gilfillan S., Solt L.A., Musiek E.S., Oltz E.M., Burris T.P., Colonna M. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aay7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godinho-Silva C., Domingues R.G., Rendas M., Raposo B., Ribeiro H., da Silva J.A., Vieira A., Costa R.M., Barbosa-Morais N.L., Carvalho T., Veiga-Fernandes H. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. 2019;574:254–258. doi: 10.1038/s41586-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teng F., Goc J., Zhou L., Chu C., Shah M.A., Eberl G., Sonnenberg G.F. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aax1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esteban-Zubero E., López-Pingarrón L., Alatorre-Jiménez M.A., Ochoa-Moneo P., Buisac-Ramón C., Rivas-Jiménez M., Castán-Ruiz S., Antoñanzas-Lombarte Á., Tan D.-X., García J.J., Reiter R.J. Melatonin’s role as a co-adjuvant treatment in colonic diseases: a review. Life Sci. 2017;170:72–81. doi: 10.1016/j.lfs.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Mazzoccoli G., Vendemiale G., De Cata A., Tarquini R. Change of gammadeltaTCR-expressing T cells in healthy aging. Int. J. Immunopathol. Pharmacol. 2011;24:201–209. doi: 10.1177/039463201102400124. [DOI] [PubMed] [Google Scholar]
- 59.Mazzoccoli G., Cata A.D.E., Carughi S., Greco A., Inglese M., Perfetto F., Tarquini R. A possible mechanism for altered immune response in the elderly. In Vivo. 2010;24:471–487. [PubMed] [Google Scholar]
- 60.Fonken L.K., Frank M.G., Gaudet A.D., Maier S.F. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain Behav. Immun. 2018;73:133–148. doi: 10.1016/j.bbi.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis A.M., Fagundes C.T., Yang G., Palsson-McDermott E.M., Wochal P., McGettrick A.F., Foley N.H., Early J.O., Chen L., Zhang H., Xue C., Geiger S.S., Hokamp K., Reilly M.P., Coogan A.N., Vigorito E., FitzGerald G.A., O’Neill L.A. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7231–7236. doi: 10.1073/pnas.1501327112. doi:1501327112 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bollinger T., Leutz A., Leliavski A., Skrum L., Kovac J., Bonacina L., Benedict C., Lange T., Westermann J., Oster H., Solbach W. Circadian clocks in mouse and human CD4+ T cells. PLoS One. 2011;6:e29801. doi: 10.1371/journal.pone.0029801PONE-D-11-10211. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobis C.C., Dubeau Laramee G., Kervezee L., Maurice De Sousa D., Labrecque N., Cermakian N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl. Acad. Sci. U. S. A. 2019;116:20077–20086. doi: 10.1073/pnas.1905080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aw D., Silva A.B., Palmer D.B. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trim W., Turner J.E., Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front. Immunol. 2018;9:169. doi: 10.3389/fimmu.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J. Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tu W., Rao S. Mechanisms underlying T cell Immunosenescence: aging and cytomegalovirus infection. Front. Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp. Gerontol. 2014;54:1–5. doi: 10.1016/j.exger.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and Inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Müller L., Pawelec G. Aging and immunity - impact of behavioral intervention. Brain Behav. Immun. 2014;39:8–22. doi: 10.1016/j.bbi.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 72.Müller L., Di Benedetto S., Pawelec G. The immune system and its Dysregulation with aging. Subcell. Biochem. 2019;91:21–43. doi: 10.1007/978-981-13-3681-2_2. [DOI] [PubMed] [Google Scholar]
- 73.Mul Fedele M.L., Aiello I., Caldart C.S., Golombek D.A., Marpegan L., Paladino N. Differential thermoregulatory and inflammatory patterns in the circadian response to LPS-induced septic shock. Front. Cell. Infect. Microbiol. 2020;10:100. doi: 10.3389/fcimb.2020.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spengler M.L., Kuropatwinski K.K., Comas M., Gasparian A.V., Fedtsova N., Gleiberman A.S., Gitlin I.I., Artemicheva N.M., Deluca K.A., Gudkov A.V., Antoch M.P. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. 1206274109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. 1209965109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao W., Cui L., Huang X., Wang S., Li D., Li L., Sun Y., Du M. Activation of rev-erbalpha attenuates lipopolysaccharide-induced inflammatory reactions in human endometrial stroma cells via suppressing TLR4-regulated NF-kappaB activation. Acta Biochim. Biophys. Sin. Shanghai. 2019;51:908–914. doi: 10.1093/abbs/gmz078. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida K., Hashiramoto A., Okano T., Yamane T., Shibanuma N., Shiozawa S. TNF-alpha modulates expression of the circadian clock gene Per2 in rheumatoid synovial cells. Scand. J. Rheumatol. 2013;42:276–280. doi: 10.3109/03009742.2013.765031. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida K., Nakai A., Kaneshiro K., Hashimoto N., Suzuki K., Uchida K., Hashimoto T., Kawasaki Y., Tateishi K., Nakagawa N., Shibanuma N., Sakai Y., Hashiramoto A. TNF-alpha induces expression of the circadian clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochem. Biophys. Res. Commun. 2018;495:1675–1680. doi: 10.1016/j.bbrc.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 79.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 80.Minciullo P.L., Catalano A., Mandraffino G., Casciaro M., Crucitti A., Maltese G., Morabito N., Lasco A., Gangemi S., Basile G. Inflammaging and anti-Inflammaging: the role of cytokines in extreme longevity. Arch. Immunol. Ther. Exp. 2016;64:111–126. doi: 10.1007/s00005-015-0377-3. [DOI] [PubMed] [Google Scholar]
- 81.Popa-Wagner A., Buga A.-M., Dumitrascu D.I., Uzoni A., Thome J., Coogan A.N. How does healthy aging impact on the circadian clock? J. Neural Transm. 2017;124:89–97. doi: 10.1007/s00702-015-1424-2. [DOI] [PubMed] [Google Scholar]
- 82.Hojbjerre L., Sonne M.P., Alibegovic A.C., Nielsen N.B., Dela F., Vaag A., Bruun J.M., Stallknecht B. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34:2265–2272. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harvey J.A., Chastin S.F.M., Skelton D.A. Prevalence of sedentary behavior in older adults: a systematic review. Int. J. Environ. Res. Public Health. 2013;10:6645–6661. doi: 10.3390/ijerph10126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedersen M., Bruunsgaard H., Weis N., Hendel H.W., Andreassen B.U., Eldrup E., Dela F., Pedersen B.K. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech. Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 85.Ziegler A.K., Damgaard A., Mackey A.L., Schjerling P., Magnusson P., Olesen A.T., Kjaer M., Scheele C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pérez L.M., Pareja-Galeano H., Sanchis-Gomar F., Emanuele E., Lucia A., Gálvez B.G. “Adipaging”: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016;594:3187–3207. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tzanetakou I.P., Katsilambros N.L., Benetos A., Mikhailidis D.P., Perrea D.N. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res. Rev. 2012;11:220–229. doi: 10.1016/j.arr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., Watowich S.S., Tian Q., Jetten A.M., Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mongrain V., Ruan X., Dardente H., Fortier E.E., Cermakian N. Clock-dependent and independent transcriptional control of the two isoforms from the mouse Rorgamma gene. Genes Cells. 2008;13:1197–1210. doi: 10.1111/j.1365-2443.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 90.Jetten A.M., Kang H.S., Takeda Y. Retinoic acid-related orphan receptors α and γ: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Front. Endocrinol. (Lausanne). 2013;4:1. doi: 10.3389/fendo.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hams E., Roberts J., Bermingham R., Hogan A.E., O’Shea D., O’Neill L., Fallon P.G. Role for retinoic acid-related orphan receptor alpha (RORα) expressing macrophages in diet-induced obesity. Front. Immunol. 2020;11:1966. doi: 10.3389/fimmu.2020.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pedersen B.K. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J. Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. doi:jphysiol.2009.179515 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Londhe P., Guttridge D.C. Inflammation induced loss of skeletal muscle. Bone. 2015;80:131–142. doi: 10.1016/j.bone.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alway S.E., Myers M.J., Mohamed J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014;6:246. doi: 10.3389/fnagi.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsson L., Degens H., Li M., Salviati L., Il Lee Y., Thompson W., Kirkland J.L., Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dent E., Morley J.E., Cruz-Jentoft A.J., Arai H., Kritchevsky S.B., Guralnik J., Bauer J.M., Pahor M., Clark B.C., Cesari M., Ruiz J., Sieber C.C., Aubertin-Leheudre M., Waters D.L., Visvanathan R., Landi F., Villareal D.T., Fielding R., Won C.W., Theou O., Martin F.C., Dong B., Woo J., Flicker L., Ferrucci L., Merchant R.A., Cao L., Cederholm T., Ribeiro S.M.L., Rodriguez-Manas L., Anker S.D., Lundy J., Gutierrez Robledo L.M., Bautmans I., Aprahamian I., Schols J.M.G.A., Izquierdo M., Vellas B. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J. Nutr. Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 97.Miller B.H., McDearmon E.L., Panda S., Hayes K.R., Zhang J., Andrews J.L., Antoch M.P., Walker J.R., Esser K.A., Hogenesch J.B., Takahashi J.S. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chatterjee S., Ma K. Circadian clock regulation of skeletal muscle growth and repair. F1000Research. 2016;5:1549. doi: 10.12688/f1000research.9076.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cartee G.D., Hepple R.T., Bamman M.M., Zierath J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 101.Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. 98/4/1154 [pii] [DOI] [PubMed] [Google Scholar]
- 102.Nicklas B.J., Hsu F.-C., Brinkley T.J., Church T., Goodpaster B.H., Kritchevsky S.B., Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fischer C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 104.Pedersen B.K. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sport. Exerc. 2012;44:392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 105.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. doi:88/4/1379 [pii] [DOI] [PubMed] [Google Scholar]
- 106.Minuzzi L.G., Chupel M.U., Rama L., Rosado F., Munoz V.R., Gaspar R.C., Kuga G.K., Furtado G.E., Pauli J.R., Teixeira A.M. Lifelong exercise practice and immunosenescence: master athletes cytokine response to acute exercise. Cytokine. 2019;115:1–7. doi: 10.1016/j.cyto.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Minuzzi L.G., Rama L., Chupel M.U., Rosado F., Dos Santos J.V., Simpson R., Martinho A., Paiva A., Teixeira A.M. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc. Immunol. Rev. 2018;24:72–84. [PubMed] [Google Scholar]
- 108.Lavin K.M., Perkins R.K., Jemiolo B., Raue U., Trappe S.W., Trappe T.A. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J. Appl. Physiol. 2020;128:87–99. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gries K.J., Minchev K., Raue U., Grosicki G.J., Begue G., Finch W.H., Graham B., Trappe T.A., Trappe S. Single-muscle fiber contractile properties in lifelong aerobic exercising women. J. Appl. Physiol. 2019;127:1710–1719. doi: 10.1152/japplphysiol.00459.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chambers T.L., Burnett T.R., Raue U., Lee G.A., Finch W.H., Graham B.M., Trappe T.A., Trappe S. Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J. Appl. Physiol. 2020;128:368–378. doi: 10.1152/japplphysiol.00426.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gries K.J., Raue U., Perkins R.K., Lavin K.M., Overstreet B.S., D’Acquisto L.J., Graham B., Finch W.H., Kaminsky L.A., Trappe T.A., Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J. Appl. Physiol. 2018;125:1636–1645. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joyner M.J., Coyle E.F. Endurance exercise performance: the physiology of champions. J. Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleg J.L., Morrell C.H., Bos A.G., Brant L.J., Talbot L.A., Wright J.G., Lakatta E.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 114.Valenzuela P.L., Maffiuletti N.A., Joyner M.J., Lucia A., Lepers R. Lifelong endurance exercise as a countermeasure against age-related [formula: see text] decline: physiological overview and insights from masters athletes. Sports Med. 2019 doi: 10.1007/s40279-019-01252-0. [DOI] [PubMed] [Google Scholar]
- 115.Radak Z., Torma F., Berkes I., Goto S., Mimura T., Posa A., Balogh L., Boldogh I., Suzuki K., Higuchi M., Koltai E. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019;132:33–41. doi: 10.1016/j.freeradbiomed.2018.10.444. [DOI] [PubMed] [Google Scholar]
- 116.Costa Rosa L.F. Exercise as a Time-conditioning Effector in Chronic Disease: a Complementary Treatment Strategy. Evid Based Complement Alternat Med. 2004;1(1):63–70. doi: 10.1093/ecam/neh018. [DOI] [PMC free article] [PubMed] [Google Scholar]