Abstract
Background
Currently, 2 coprimary end points are used by health authorities to determine the effectiveness of therapeutic interventions in patients with Crohn’s disease (CD): symptomatic remission (patient-reported outcome assessment) and endoscopic remission (ileocolonoscopy). However, there is lack of accepted biomarkers to facilitate regulatory decision-making in the development of novel therapeutics for the treatment of CD.
Methods
With support from the Helmsley Charitable Trust, Critical Path Institute formed the Crohn’s Disease Biomarkers preconsortium (CDBpC) with members from the pharmaceutical industry, academia, and nonprofit organizations to evaluate the CD biomarker landscape. Biomarkers were evaluated based on biological relevance, availability of biomarker assays, and clinical validation data.
Results
The CDBpC identified the most critical need as pharmacodynamic/response biomarkers to monitor disease activity in response to therapeutic intervention. Fecal calprotectin (FC) and serum C-reactive protein (CRP) were identified as biomarkers ready for the regulatory qualification process. A number of exploratory biomarkers and potential panels of these biomarkers was also identified for additional development. Given the different factors involved in CD and disease progression, a combination of biomarkers, including inflammatory, tissue injury, genetic, and microbiome-associated biomarkers, will likely have the most utility.
Conclusions
The primary focus of the Inflammatory Bowel Disease Regulatory Science Consortium will be development of exploratory biomarkers and the qualification of FC and CRP for IBD. The Inflammatory Bowel Disease Regulatory Science Consortium, focused on tools to support IBD drug development, will operate in the precompetitive space to share data, biological samples for biomarker testing, and assay information for novel biomarkers.
Keywords: Crohn’s disease, inflammatory bowel disease, ulcerative colitis, biomarker, biomarker qualification, regulatory science
There is a lack of biomarkers to facilitate regulatory decision-making in the drug development process for CD therapeutics. Authors have conducted an evaluation of potential biomarkers and plan to launch a consortium to qualify novel CD biomarkers.
INTRODUCTION
Crohn’s Disease
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterized by debilitating and chronic relapsing and remitting inflammation of the gastrointenstinal (GI) tract of unknown etiology. Crohn’s disease can affect any part of the GI tract from mouth to anus but is primarily localized to the terminal ileum.1 It can affect the entire thickness of the bowel wall and leave unaffected areas between patches of diseased tissue.2 Crohn’s disease manifests with discrete periods of acute worsening of clinical symptoms (abdominal pain and diarrhea) and signs (elevated inflammatory markers, endoscopic and radiological findings) followed by periods of clinical remission. Nevertheless, there is often ongoing subclinical inflammation that occurs even during these periods when patients are asymptomatic, resulting in clinical symptoms that do not correlate with endoscopic or radiological findings. In fact, many patients with CD have continued disease activity in the absence of clinical manifestations. Inflammatory bowel disease (IBD) includes CD, which can affect the entire GI tract, and ulcerative colitis (UC), which mainly affects the large bowel. Factors that may play a role in the development of IBD include genetics, environmental factors, and an abnormal immune response. These diseases affect from 1.6 to 3.1 million Americans3 and more than 6.8 million worldwide.4
Diagnosis of IBD is based on patient symptoms (diarrhea, abdominal pain) and follow-up laboratory testing, radiology, and endoscopy. The prevalence of IBD increased in many areas of the world from 1990 to 2017.4 In 2015, estimates suggested that 1.3% of the US adult population had been diagnosed with IBD,5 and the prevalence increased by 123% in adults in the United States from 2007 to 2016.6 In the US pediatric population, IBD prevalence increased by 133% between 2007 and 2016.6
Currently, 2 coprimary end points are used by health authorities to determine the effectiveness of therapeutic interventions in patients with IBD: symptomatic remission (patient-reported outcome assessment) and endoscopic remission (ileocolonoscopy [ICS]).7, 8 However, ICS is a relatively invasive measure that can be burdensome to patients requiring bowel preparation and sedation and is resource-intensive from a clinical trial perspective, thus limiting serial measurements. Additionally, ICS in some cases is insufficient in capturing the extent of disease in CD patients, as there is often a submucosal component that can be missed when assessing the bowel surface and patchy presentation that can often lead to misleading interpretation of biopsies.9, 10 A minimally invasive biomarker would enable more frequent serial measurements of the whole GI tract, would provide more meaningful disease monitoring, and would be likely to reduce the cost of disease monitoring. Moreover, the inflammatory response can travel beyond the ileum (jejunum and duodenum) and thus beyond the observation of conventional ICS.11, 12 Additional procedures can also be conducted during clinical trials including capsule endoscopy, balloon-assisted enteroscopy, ultrasound, and radiological assessments including computerized tomography (CT) scan, magnetic resonance enterography (MRE), and magnetic resonance imaging (MRI). However, these procedures currently lack collective regulatory endorsement.
There is no cure for IBD. Treatment options are based on controlling the symptoms and controlling inflammation.13 Some of the drugs that are available or in the development phase include aminosalicylates, corticosteroids, other immune modulators, antitumor necrosis factor (TNF) agents,14 monoclonal antibodies against adhesion molecules/integrins15 or the p40 subunit of interleukins (IL-12/IL-23),16 and JAK-STAT pathway inhibitors.17
Although biomarkers are currently accepted to screen for CD and monitor for inflammation, there is a need for regulatory accepted, minimally invasive biomarkers that enable drug developers to follow patients during clinical trials when serial measurements are important to evaluation of a new therapy. With the knowledge that the scientific evidence in the literature could support regulatory qualification of CD biomarkers, Critical Path Institute formed a preconsortium to evaluate the CD biomarker landscape and potentially build a formal consortium to qualify biomarkers with regulatory agencies.
The Crohn’s Disease Biomarkers pre-Consortium (CDBpC)
The Crohn’s disease Biomarkers pre-Consortium (CDBpC) was formed with members from pharmaceutical industry, academia, and nonprofit organizations, with support from the Helmsley Charitable Trust, to evaluate the minimally invasive CD biomarker landscape and determine which biomarkers may be ready for regulatory qualification. The team first determined the drug development needs in CD and paired context-of-use (COU) statements with each need. By definition, a COU statement is “a concise description of the biomarker’s specified use in drug development.” 18 The COU includes 2 components: (1) the BEST biomarker category19 and (2) the biomarker’s intended use in drug development. An assessment of CD biomarkers with clinical data was undertaken utilizing PubMed and clinicaltrials.gov, in addition to the experience of CDBpC members. Biomarkers of interest were then evaluated for their regulatory ready status and paired with COU statements to determine the path forward for qualification through the formation of a full consortium. The objective of this preconsortium was to develop a broad understanding of the CD fluid biomarker landscape, with the goal of ultimately initiating a consortium to qualify identified biomarkers with regulatory authorities to support their broad use in CD drug development. In addition, after discussions with stakeholders, the full consortium will also pursue further validation of exploratory biomarkers through sharing of knowledge and data, generation and/or sharing of biological samples, and assay development for novel biomarkers. Exploratory biomarkers are those biomarkers with less evidence to support their clinical use. In some cases, these biomarkers will only have reported preclinical evidence of their utility but, due to the biomarker’s association with the disease biology or pathology, are candidates for further evaluation. Although the focus of CDBpC was primarily on CD, the team decided that the full consortium would be broadened to include inflammatory bowel diseases (Inflammatory Bowel Diseases Regulatory Sciences Consortium, IBD-RSC) with identified biomarkers being considered for use in both CD and UC and an additional mandate to conduct a UC landscape assessment.
CD BIOMARKER LANDSCAPE ANALYSIS
Drug Development Need
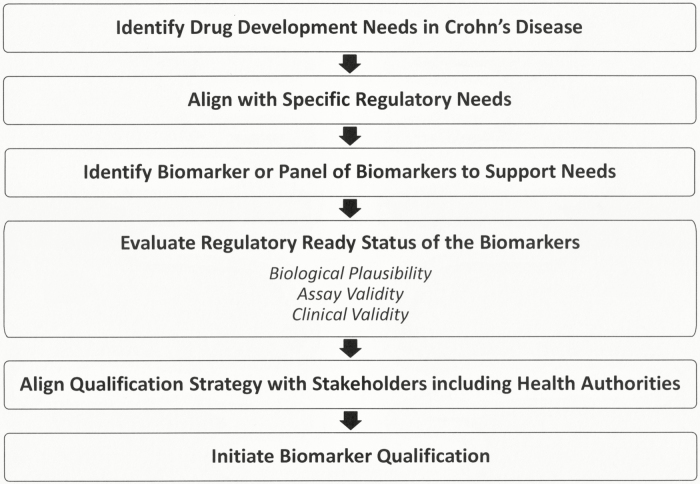
One of the first questions that was addressed through discussions with pharmaceutical companies working in the CD space and regulatory authorities was the drug development gaps in CD (Fig. 1). Meeting the specific needs of the pharmaceutical industry and regulators is essential to the choice of biomarker to pursue. In some cases, there will be a need for a biomarker because no biomarker or other measure exists to serve that requirement. There will also be cases where a biomarker that is currently in use has limitations and can be improved upon with the use of a new biomarker. Such limitations can include the sensitivity, selectivity, specificity, and cost. A single biomarker may provide the necessary information to support a drug development need, but it is also possible and more likely that a panel of biomarkers may provide more or better information.
FIGURE 1.
CD biomarker landscape assessment.
Five drug development gaps were identified that could be overcome using biomarkers. The gaps include a lack of biomarkers to identify the presence of disease or condition of interest (diagnostic and prognostic biomarker); identify disease course in patients with CD (disease activity biomarker); predict long-term therapeutic benefit of investigational drugs (predictive biomarker); monitor patients and their response to new therapeutics (pharmacodynamic biomarker); and quantify therapeutic response to investigational drugs that correlate with currently accepted end points (surrogate end point). In CD, the primary drug development need was identified as minimally invasive, pharmacodynamic biomarkers that could be measured serially during clinical trials to monitor patient response to therapies.
Defining Context-of-use Statements
Context-of-use statements were then developed for each identified drug development gap. Each biomarker qualification effort should identify a single COU that is supported by available data. The FDA has recognized biomarkers as drug development tools (DDTs) with the potential to promote innovation in the drug development process with significant public health impact.20 Context-of-use statements were identified by the CDBpC team for predictive, prognostic, and pharmacodynamic/response biomarkers. In each case, the novel biomarker will be compared with endoscopic healing—the current standard for evaluation of patients with CD—in order to demonstrate the utility of the novel biomarker.
Three final COU statements were agreed upon to fill the identified CD drug development needs:
A prognostic biomarker to enable enrichment for patients with CD at high risk for progressive increase in disease activity for inclusion in clinical trials
A predictive biomarker for long-term endoscopic remission, following induction therapy in patients in clinical trials with CD
A pharmacodynamic/response biomarker to identify when a patient with CD has achieved endoscopic remission following therapeutic intervention in clinical trials
Further discussion pinpointed that the most critical need is for pharmacodynamic/response biomarkers to monitor disease activity in response to treatment which, if adequately validated to correlate with currently accepted clinical trial outcomes, can ultimately act as surrogate end points. In discussions with the FDA, a minimally invasive pharmacodynamic biomarker was most attractive to reduce the need for repeated ICS during the conduct of clinical trials. Moreover, the FDA has raised the need for pharmacodynamic biomarkers not only in adult patients but also in pediatric patients with CD, where ICS is more challenging.
Assessment of Biomarkers
A landscape assessment of drug development tools (ie, biomarkers) being utilized in CD was performed during the initial stages of the preconsortium process. Information including the biological relevance, available assays, and clinical data associated with reported CD biomarkers was pulled from literature, stakeholder participant experience, and clinicaltrials.gov. This information was then compiled into a searchable document (https://c-path.org/ibd_cd_summary). As new biomarkers were discussed or identified through continuing review of the literature and discussions among CDBpC stakeholders, these were added. The biomarkers were then assessed using a predefined checklist of regulatory ready components (available at https://c-path.org/ibd_cd_summary) including biological plausibility, assay information, and available clinical data. Regulatory ready biomarkers are defined as those biomarkers with available clinical data that are currently being utilized in clinical trials and have available measurement assay information, suggesting that they could be candidates for regulatory qualification. In addition, regulatory ready biomarkers fill an identified drug development gap, align with specific regulatory needs, and improve upon currently used biomarkers. Improvement on current biomarkers can include reduced cost, reduced patient burden, and increased selectivity or sensitivity.
Biologic plausibility describes the cell- or organ-specific expression pattern of the biomarker, the association of the biomarker with disease or injury, and changes in the biomarker that can be measured in different disease states or injury. Assay information includes reported assays (including any assays that may already be FDA-cleared), the biological matrix, biomarker stability, any reported assay interferents, and reported medically relevant cutoffs and baseline values. Also included in the regulatory ready checklist are the outstanding questions that need to be evaluated to prepare for qualification. These include assay variability, intra-individual variability, and specificity. Critically important to assess the status of a novel biomarker is the availability of adequate, relevant, clinical data sets amenable for data integration to support qualification. In some cases, data will be unavailable or nonexistent, and these data will have to be generated by the consortium from existing samples or newly collected samples generated for this specific biomarker project and must be feasible for the consortium from a resource, timing, and operational perspective. Key publications are also cited in the regulatory ready checklist to enable qualification document preparation when warranted. These considerations enabled the list of identified CD biomarkers to be divided into 2 categories: biomarkers with the potential for qualification and exploratory biomarkers.
Regulatory Ready Biomarkers
The potential regulatory ready biomarkers had numerous literature citations, and data were already being collected in clinical trials, suggesting that clinical data exist to support assessment of qualification for a specific COU. Ideally, a regulatory ready biomarker would have both clinical and analytical validation completed (Table 1). In addition, there would be information about the biomarker assay(s), and in some cases, these have been approved as safe and effective in vitro diagnostics by the FDA. Regulatory ready biomarkers would be considered for qualification with regulatory authorities to “make DDTs publicly available for a specific context of use to expedite drug development and review of regulatory applications.” 20 The purpose of qualification of these biomarkers is to answer key questions that remain unanswered in published reports about the specific COU of the biomarker, any outstanding questions about assay variability, and any questions about baseline values in specific populations and to confirm or determine medically significant changes in the biomarker that can be measured during the course of a clinical trial. Qualification also provides certainty to drug developers that regulatory authorities will accept a biomarker for use in a clinical trial for the qualified COU without further testing. Fecal calprotectin (FC) and serum C-reactive protein (CRP) both have been deemed regulatory ready for the prognostic, predictive, and pharmacodynamic/response COUs outlined. Fecal lactoferrin was also discussed as a potentially regulatory ready CD biomarker. However, given fecal lactoferrin’s similarity to FC as a neutrophil-derived inflammatory protein with comparable performance to FC and lack of available clinical data, the team decided to focus its efforts on FC.
TABLE 1.
Regulatory Ready Checklist for FC and CRP as Pharmacodynamic/Response Biomarker in CD
| Fecal Calprotectin | Serum C-Reactive Protein | |
|---|---|---|
| Biological Plausibility | Expression in neutrophils, a primary inflammatory component of CD | Increases in blood with inflammation |
| Association with CD | Increases with CD severity | Increases with CD severity |
| Assay status | FDA cleared diagnostic assays are available | FDA cleared diagnostic assays are available |
| Improvement over current tools | Decreased cost and less invasive than ICS | Decreased cost and less invasive than ICS |
| Clinical Data | Significant clinical validation data available (~50 clinical trials found in clinicaltrials.gov) | Significant clinical validation data available (~50 clinical trials found in clinicaltrials.gov) |
| Information gaps for qualification | • Ramifications of variable baseline must be understood • Relevant cutoff values must be defined • Measurement methodology must be evaluated and standardized |
• Relevant cutoff values must be defined • Ramifications of biomarker sensitivity and specific on utility must be understood |
Though the regulatory ready status of FC and CRP has been confirmed, questions about the specifics of how these biomarkers should be used in clinical trials (such as biomarker testing frequency, medically significant cut points, impact of disease location, sample collection, and pre-analytical considerations) will be answered during the qualification process through evaluation of a composite database of shared data on the use of each biomarker.
Fecal calprotectin
Calprotectin is a 36 kilodalton zinc-binding antimicrobial protein expressed in neutrophils currently being used to screen for intestinal inflammation and to decide whether to perform a colonoscopy to assess CD. Neutrophils, normally present in the blood, can be found in the gut and gut contents during CD due to release of chemokines during intestinal inflammation and the associated disruption of the epithelial barrier.21 Plasma levels of calprotectin have been reported to be elevated in several conditions including IBD, whereas fecal levels seem to be more specific to IBD. In a meta-analysis of diagnostic accuracy studies, including 6 studies in adults and 7 studies in children, fecal calprotectin was compared with endoscopy as the reference standard.22 The authors found that the pooled sensitivity of FC testing to select patients for further evaluation by endoscopy was 0.93 (95% confidence interval, 0.85–0.97), and the pooled specificity was 0.96 (0.79–0.99) in the adult studies; in children and teenagers, the pooled sensitivity was 0.92 (0.84–0.96) and the pooled specificity 0.76 (0.62–0.86). The specificity of FC for children and teenagers was found to be lower than for adults.
Many studies have been published describing the utility of FC as a biomarker for CD.23 In fact, there are studies suggesting FC could serve as a prognostic, predictive, and/or pharmacodynamic/response biomarker. For example, an increased level of FC measured during routine monitoring has been shown to be prognostic for progression of CD regardless of symptoms or CD location.24 Children with CD being treated with infliximab and in clinical remission were tested for FC levels at baseline and in follow-up visits. The study demonstrated that FC levels of >250 μg/g predicted a relapse in the next 3 months.25 Studies also suggest that FC can be used as a minimally invasive marker of mucosal healing in IBD.26, 27 However cutoff levels across studies are dependent on the test used to measure FC.26
The regulatory ready status of FC is supported by the large amount of published data and clinical trial data repeatedly demonstrating its clinical utility across multiple uses. The expression of calprotectin in neutrophils supports the biological plausibility of FC as a marker of IBD. Many studies have demonstrated a change in FC in response to disease worsening (increased FC) or healing (decreased FC).22, 28 However, there are still questions that could be answered by combining data from a number of studies in preparation for a regulatory qualification of FC. The most critical questions involve the measurement of FC. Fecal calprotectin can be measured with a variety of commercially available and FDA-cleared assays, most of which use an enzyme-linked immunosorbent assay (ELISA); however, these tests are approved for diagnosis of disease. Fecal calprotectin is reported to be stable for up to 72 hours at room temperature.29 However, the use of different extraction methods and different antibodies can affect the data collected.30, 31 For example, baseline levels of 10 to 200 µg/mg of FC have been reported with higher basal levels in specific populations or those from areas with poor sanitation.30 Levels over 250 µg/mg are thought to be indicative of active disease.29, 32, 33 However, assay differences and intrasubject and intersubject variability contribute to the inability to absolutely determine baseline FC levels. There may also be a difference in the level of FC depending on the time of day that the sample was collected, so morning samples are recommended in some studies.29, 31 In summary, variability in data depending on FC sample collection and the assay utilized suggest that this information will need to be evaluated in the biomarker qualification process. Biological variability and cutoff values are critical knowledge gaps that will need to be understood so that FC can be used with confidence as a pharmacodynamic/response biomarker in clinical trials.
The identified questions, especially those about FC biological variability and cutoff values of medical concern, suggest the need to build a comprehensive database of relevant calprotectin clinical studies to be able to analyze these data together in the context of a qualification effort to support use of FC in clinical trials. This aggregated database may lead to prospective studies that are necessary to answer key questions about the use of FC. Greater than 50 clinical trials were identified in a recent search of clinicaltrials.gov using “inflammatory bowel diseases” and “fecal calprotectin” as search terms. A successful qualification will provide a regulatory-endorsed tool that can be used with confidence to support development of new treatments for CD. This information may also be relevant for utilization of FC as a biomarker in clinical practice.
In summary, FC is currently being utilized in clinical trials as a diagnostic, prognostic, predictive, and pharmacodynamic/response biomarker. Fecal calprotectin has the potential to enable longitudinal assessment of disease during clinical trials. However, the exact clinical methodology for utilizing FC and the ability to compare data across studies are lacking. The continued use of FC could proceed as is, with each study requiring regulatory approval for the use of FC, or the application and quantitation of FC could be standardized through a qualification effort, allowing drug developers to use this biomarker with regulatory certainty.
Serum C-reactive Protein
C-reactive protein is produced in the liver in response to inflammation induced by IL-1, IL-6 or TNF-α and increases in the blood with many inflammatory conditions.34 In a meta-analysis intended to determine the diagnostic accuracy of serum CRP and other CD biomarkers, the literature was found to be highly variable.35 The authors found that CRP has a relatively high specificity for active disease in patients with a diagnosis of CD. However, they also found that although the specificity of CRP is high, its sensitivity is low, and therefore, a negative CRP test does not rule out IBD.35 Because CRP is increased in many inflammatory diseases, its overall specificity for CD and UC is low in a non-IBD enriched population.36 There are also indications that genetic factors may contribute to CRP increases during active IBD. This has been attributed to specific genetic variants in patients who do not exhibit an increase in CRP levels during active disease.37
Like FC, there are studies published on the utility of serum CRP in IBD, suggesting it could be useful as a pharmacodynamic/response biomarker. C-reactive protein has been shown to be highly correlated with perienteric inflammation but not inflammation of the bowel wall.38 This differential response to intestinal inflammation likely drives the lower than expected sensitivity of CRP in patients with IBD. In a recent study, UC patients who were primary nonresponders to a novel Janus kinase inhibitor, tofacitinib, exhibited a higher baseline CRP level.39 C-reactive protein levels have also been measured as a pharmacodynamic/response biomarker in patients transitioning to an infliximab biosimilar treatment to monitor efficacy at 3 months40 and up to 24 months, along with trough levels of drug.41 A low baseline level of CRP and a lower CRP level after 14 weeks of infliximab treatment was associated with a higher probability of continued response or remission in the ACCENT 1 study.42 A high CRP level (20 mg/L) has also been used to predict treatment failure after long-term thiopurine treatment for CD.43
The regulatory ready status of CRP is supported by its biological plausibility as an accepted inflammatory marker in many different diseases.34 C-reactive protein increases have been seen in patients with CD and may be useful in predicting clinical relapse, particularly when CRP is part of a biomarker panel.44 More than 80 CRP assays are cleared for use by the FDA, many for cardiac indications, highlighting the potential for a lack of specificity for CD. C-reactive protein has a half-life of 19 hours, and the sample handling and stability and other assay characteristics are clear, given that there has been a large number of reported/FDA-cleared assays.34
Questions that remain to be addressed for CRP as a CD biomarker include its poor specificity for IBD, suggesting that it may be most useful in combination with other more specific biomarkers such as FC,36 relevant cutoffs,34 and the reliability of the response of CRP in CD patients with CRP genetic variants.37 Though baseline levels of serum CRP are generally reported to be <1 mg/L, medically relevant cutoff values vary from 5 to 200 mg/L.34 Despite its potential limitations as a CD biomarker, CRP has been measured in over 50 completed clinical trials and is a component of several commercially available biomarker panels for CD. Therefore, there is clinical data that, if standardized and combined in a single database, could potentially be utilized to evaluate the utility of CRP as a CD biomarker. Any proven utility could be documented and submitted to the FDA for potential qualification of CRP as a biomarker for CD.
Exploratory Biomarkers
Even though many clinical trials are including inflammatory biomarkers FC and CRP in their studies, numerous exploratory biomarkers are also being included in clinical trials by drug developers and clinical investigators. Exploratory biomarkers cover inflammation, tissue injury, and the microbiome but lack sufficient evidence to support their use and enable their use in regulatory decision-making (Table 2). The IBD-RSC plan to assess exploratory IBD (CD and UC) biomarkers includes a precompetitive consortia-based identification of biomarkers, with in-kind contributions from members, of existing data, existing samples, and assay information, which may also include a plan to run prospective studies or test banked samples where there is not enough existing or accessible data for an exploratory biomarker qualification effort. Prioritized biomarkers will meet an intended application that answers a drug development need and have clearly defined, reasonable resources required to generate the evidence necessary to support the biomarker’s intended application.
TABLE 2.
Exploratory CD Biomarkers
| Biomarker | Function | Class |
|---|---|---|
| IL-6 IL-22, IL-2345,46,80,81 | Cytokine | Inflammation |
| NGAL52 | Inflammatory mediator | Inflammation |
| miR-21, miR-31, miR-146a, and miR-37556,60 | microRNA | Inflammation |
| TREM-182 | Triggering receptor on myeloid cells | Inflammation |
| pASCA65 | Autoimmune antibodies targeting neutrophils | Inflammation |
| Oncostatin M57 | Cytokine | Inflammation |
| lower Firmicutes; higher Faecalibacterium60, 62 | Distinct signature in CD phenotypes | Microbiome |
| OmpC, ANCA, I2, A4-Fla2, Fla-X, Cbir165,83,84 | Antibodies to microbial antigens | Microbiome |
| Pro-C4, C4M, C3M, ECM1, BGM, EL-NE, C5M, Pro-C569 | Extracellular matrix proteins | Tissue Injury |
| MMP-3, MMP-9, MMP-1463,70 | Matrix metalloproteases | Tissue Injury |
Biomarkers of inflammation
Given the prominent role of inflammation and the immune system in the pathogenesis of CD and the fact that the 2 regulatory-ready CD biomarkers FC and CRP are immune markers, many of the exploratory biomarkers currently being investigated play a role in immune function. Interleukins and antimicrobial antibodies are among the most studied.
Interleukin 6 (IL-6) is a pro-inflammatory cytokine implicated in autoimmunity and regulation of T and B cells, along with production of CRP and other acute phase reactants. Interleukin 6 production and signaling are increased in CD-inflamed mucosa.45 There is also some evidence that measurement of IL-6 in serum could function as a clinical response biomarker in patients treated with biologic therapies.46
Interleukin 23 is a pro-inflammatory cytokine composed of 2 subunits, P40 which is also a component of IL-12, and p19 which is exclusive to IL-23. Currently, IL-23 is being targeted for therapeutic intervention in CD. Interleukin 23 induces release of IL-22 and other cytokines. In turn, IL-22 induces the release of calprotectin subunits S100A8 and S100A9 and activation of JAK/STAT pathway proteins that are encoded by IBD susceptibility genes.47 Interleukin 23 inhibition has been shown to decrease fecal calprotectin, fecal CRP, and IL-22 serum levels.48 This suggests that IL-22 could be a pharmacodynamic/response biomarker and/or prognostic biomarker for selecting patients in clinical trials of new IL-23 based treatments.48, 49
Neutrophil gelatinase–associated lipocalin (NGAL), like the regulatory ready biomarkers CRP and FC, is an antimicrobial protein marker for neutrophil infiltration and activation expressed in inflammatory diseases including IBD. Fecal levels of NGAL were first suggested as a biomarker of IBD in 1999; however, fecal NGAL did not reflect disease activity in that study.50 More recently, fecal levels of epithelial cell–derived NGAL have been shown to increase in active CD with high sensitivity and specificity that correlates well with calprotectin increases.50, 51 The gene LCN2, which encodes NGAL, has also been shown to be upregulated in the terminal ileum in patients with active CD.52 Studies suggest that NGAL is stable for up to 7 days at room temperature in fecal samples.51
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression and are thought to be important for immune function.53 Based on differential expression, studies have suggested that serum miRNA panels can differentiate between CD and UC patients using miR-21, miR-31, miR-146a, and miR-375,54 in addition to differentiating between healthy controls, IBD patients, and active and inactive CD patients.55 In addition, increases in specific serum miRNAs may be predictive of fibrosis.56 Kits for miRNA isolation and databases for results analysis are commercially available and suggest that a regulatory path to advance these potential IBD biomarkers may be available.
Other inflammatory markers that may be useful in predicting patient response to CD therapies include high baseline tissue levels of Oncostatin M, which may predict failure of anti-TNF therapy57 and a decrease in whole blood TREM-1 as a predictor of endoscopic remission in patients treated with anti-TNF therapy.58
Biomarkers of microbiota dysfunction
Gut microbiota is responsible for maintaining homeostasis, protecting against pathogens, and regulating immune function, among other things.59 Additionally, the fecal microbiota community in IBD patients has been shown to be atypical and of decreased diversity, with lower levels of Firmicutes, for example.59, 60 The microbiota community of an individual can be identified and quantified using next generation sequencing of bacterial ribosomal RNA (rRNA) from biopsies or fecal samples. A unique microbiota signature could be a biomarker of disease and may also be useful to identify patients who achieve remission after onset of therapy. For example, in addition to baseline microbiota differences in responders vs nonresponders with higher Faecalibacterium being of particular importance, patients who responded to ustekinumab saw an increase in the diversity of their microbiota community.61 Another recent study also suggests that lower abundance of diverse microbial families in patients could predict stricturing and penetrating disease phenotypes.62
A dysregulated immune response that produces antibodies to intestinal microbes is one theory for the pathogenesis of CD. Antibodies have been found against Saccharomyces cerevisiae (ASCA), perinuclear antineutrophil cytoplasmic antibodies (pANCAs), Escherichia coli outer membrane porin C (OmpC), Pseudomonas fluorescens–associated sequence I2, bacterial flagellin CBir1, Lachnospiraceae-derived flagellin A4-Fla2, and bacterial flagellin Fla-X.63, 64 In fact, antimicrobial antibodies may be present in patients for years before diagnosis of CD, suggesting that they may function as susceptibility/risk biomarkers.65, 66 In CD, increased circulating antibodies to microbial antigens suggest the likelihood of more complications in these patients.64, 65
Biomarkers of tissue injury
Disruption of the mucosal barrier is discernable by endoscopy as ulceration, but it is likely that molecular changes of tissue injury may be a more sensitive indicator of barrier disruption. Therefore, like antimicrobial antibodies, tissue injury biomarkers may also be useful as predictive, prognostic, and monitoring biomarkers of CD. To qualify minimally invasive biomarkers of tissue injury or tissue fibrosis for CD, foundational work on imaging biomarkers that will identify deep submucosal tissue injury and/or fibrosis will be necessary. Potential tissue injury biomarkers include extracellular matrix (ECM) proteins and matrix metalloproteinases (MMPs). In a dextran sulfate sodium (DSS)-induced colitis model in rats, fragments of basement membrane collagen type 4 (Pro-C4 and C4M) and interstitial matrix collagen type 3 (C3M) were elevated in serum compared with baseline values.67 Other ECM proteins have also shown promise as biomarkers in CD. Extracellular matrix 1 protein has been hailed as a serum biomarker that could be used to stratify CD patients at high risk of developing fibrostenotic complications.68 Five blood-based biomarkers of ECM turnover, MMP-3 and -9, degraded biglycan (BGM), neutrophil elastase degraded elastin (EL-NE), MMP-9 degraded type 5 collagen (C5M), and type 5 pro-collagen (Pro-C5) were evaluated for their ability to discriminate CD patients and healthy controls. In this study, C5M, Pro-C5, and EL-NE were shown to be the most accurate combination of biomarkers to distinguish between CD patients and healthy controls.69
Matrix metalloproteases, which are capable of degrading extracellular matrix and are involved in the repair and maintenance of the extracellular matrix, have also shown promise as CD biomarkers. For example, increased fecal MMP-9 was able to detect endoscopic activity in both CD and UC patients with a high sensitivity of >90%.70 In another study, decreased serum MMP-9 and increased serum MMP-14 were the strongest factors differentiating IBD patients from healthy controls.63
There are commercially available ELISA kits for single MMPs, panels of MMPs, collagen fragments, and ECM proteins, and a number of clinical and preclinical studies suggests the utility of these potential CD biomarkers. Some of these tissues remodeling proteins are also included in commercially available CD biomarker panels. Therefore, a closer look at the available data and development of a COU for a single biomarker or a panel of tissue-remodeling biomarkers would constitute the next step in assessing their regulatory ready status.
Biomarkers Used in Panels
Combinations of biomarkers targeting different biological processes and biomarker panels are being utilized in commercial and academic laboratories to predict, diagnose, and monitor treatment effects in CD patients (Table 3). It is likely, given the different factors involved in CD and disease progression, that a combination of biomarkers including inflammatory, tissue injury, genetic, and microbiome-associated biomarkers will have the most utility. We provide examples of some of the relevant biomarker panel approaches from industry and academia.
TABLE 3.
Commercially Available CD Biomarker Panels
| Panel Name | Biomarkers | Matrix | Purpose | Company | References |
|---|---|---|---|---|---|
| PROMETHEUS IBD sgi Diagnostic | ASCA, OMPC, CBir2, A4-Fla2, FlaX, pANCA, ATG16L1, ECM1, NKX2, STAT3, ICAM1, VCAM1, VEGF, CRP, SAA | Blood | IBD vs non-IBD and UC vs CD | Prometheus Laboratories Inc. | https://www.prometheusbiosciences.com/ibd-sgi/ |
| PROMETHEUS Monitr Crohn’s Disease Test | Ang 1, 2; CEACAM 1, hsCRP; EMMPRIN; IL-7; MMP1, 2, 3, 9; SAA 1; TGF-α; VCAM 1 | Serum | Monitor muscosal healing | Prometheus Laboratories Inc. | https://www.prometheusbiosciences.com/monitor/ |
| PROMETHEUS Crohn’s Prognostic | ASCA; OMPC; CBir1; pANCA; NOD2 SNP 8, 12 and 13 | Blood | Probability of disease progression | Prometheus Laboratories Inc. | https://www.prometheusbiosciences.com/crohns-prognostic/ |
| Fingerprint Technology Assays | P1NP, Pro-C3, Pro-C5, Pro-C6; C1M, C3M, C5M and C6M | Serum | Complications for CD | Nordic Bioscience A/S | https://www.nordicbioscience.com/biomarkers-research/nordic-bioscience-assays/ |
| PredictSURE IBD | 15 genes (+2 reference genes) related to CD8+T cells | Blood | Diagnosis; aggressive or milder disease | PredictImmune Ltd | https://www.predictimmune.com/predictsure-ibd-2/ |
Prometheus Laboratories Inc. has several commercially available biomarker panels for CD. PROMETHEUS IBD sgi Diagnostic test includes serologic, genetic, and inflammatory markers designed to differentiate IBD from non-IBD and CD from UC. There is also the PROMETHEUS Crohn’s Prognostic, a CD prognostic panel that measures serologic and genetic makers designed to provide information on potential development of disease progression and complications in CD patients. The PROMETHEUS Monitr Crohn’s Disease Test assesses mucosal healing in CD patients by measuring 13 biomarkers of mucosal damage and repair.
Nordic Bioscience A/S also has a panel of serological biomarkers, comprising assays from its fingerprint technology portfolio related to tissue injury and including collagen fragments and MMP degraded proteins to determine active inflammation, structuring, and penetrating CD.71
PredictSURE IBD is a Conformité Européenne (CE)-marked, commercially available biomarker panel real-time polymerase chain reaction (qPCR) molecular test from PredictImmune Ltd. Based on earlier studies suggesting that gene expression in CD8+ T cells could predict UC and CD patient prognosis,72, 73 the PredictSURE IBD test uses whole blood to analyze expression of 17 genes (2 of which are reference genes). The test is able to stratify patients as IBDhi patients with more aggressive disease and IBDlo patients with less aggressive disease.74 The stratification is thought to aid in determining treatment course and might also be used to select patients for clinical trials. There are still questions about the utility of this test in patients who are on current treatments, and there are no data yet on whether use of this test will improve clinical outcomes.74 However, a clinical trial called the PRECIOUS Study currently recruiting patients to evaluate the PredictSURE IBD test in the US population (NCT NCT03952364) is expected to be completed in 2021, notwithstanding unforeseen issues associated with the coronavirus 2019 (COVID-19) pandemic.
D’Haens et al recently published a multicenter, multinational study measuring endoscopic activity in CD patients using 13 blood proteins.75 The endoscopic healing index (EHI) is composed of markers of mucosal damage, compromised barrier function, wound healing, growth factors, cytokines, adhesion molecules, and pathological angiogenesis and includes angiopoietin 1 and 2 (ANG1, ANG2), CRP, serum amyloid A1 (SAA1), IL-7, ECM protein inducer (EMMPRIN), matrix metalloproteases (MMP-1, MMP-2, MMP-3, MMP-9), TGF-α, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and vascular cell adhesion molecule 1 (VCAM1). Biomarkers were eliminated from the original 47 for analytical issues and because they did not increase EHI performance. The EHI assay was validated using 2 cohorts, with a cutoff of 20 having a high sensitivity for lack of endoscopic inflammation and a cutoff of 50 having a high specificity for endoscopic inflammation.75 Next steps outlined by the authors for assessing the EHI performance include further testing diagnostic accuracy for histologic inflammation and cross-sectional imaging, assessing the prognostic ability of the EHI for future relapse, and assessing the reasons for performance differences in different CD populations to ensure generalizability across disease populations. A variety of panels supporting different stages, severities, and patient populations may be necessary once the value of each biomarker and combinations of biomarkers are more clearly understood.
In another recent article, a panel of serum antibodies and proteins was identified that was able to predict which asymptomatic patients would be diagnosed with CD within 5 years.66 Another group also demonstrated that a panel of antibodies including ASCA IgG, ASCA IgA, pANCA, antibodies against Escherichia coli outer membrane porin C, and flagellin CBir1 were most accurate as predictors of CD diagnosis.76 And a combination of SAA, IL-6, IL-8, and Eotaxin-1 was shown to predict endoscopic disease in CD.77
The literature also contains examples of tools that utilize both clinical variables and serological and genetic biomarkers to predict patients who will be diagnosed with CD complications. Siegel et al first developed the model in pediatric patients for the purpose of predicting and communicating risk of complications in individual patients with CD.78 This model included disease location and ASCA, Cbir1, OmpC, and pANCA serological biomarkers. In 2016, Siegel at al refined the PROSPECT model and developed a web-based tool for prediction of risk in adult patients with CD.79 The final model included disease location; serological biomarkers ASCA, CBir1, and ANCA; NOD2 variants SNP8, SNP12, and SNP13; and an interaction term between perianal disease and ASCA to account for the unrealistic degree of risk predicted in patients with high ASCA and high perianal disease. There is a need to apply this model in populations beyond North America and potentially include other disease variables such as inflammatory markers.79 However, access to a predictive model, such as the PROSPECT model, could aid in determining the need for early intensive therapy with current medications and selecting patients for clinical trials of new medications, thus limiting costs and side effects and promoting drug development for CD.
SUMMARY AND NEXT STEPS
Currently, 2 coprimary end points are used by health authorities to determine the effectiveness of therapeutic interventions in patients with CD: symptomatic remission (PRO assessment) and endoscopic remission by ileocolonoscopy (ICS). It is universally accepted that there is a need for minimally invasive monitoring of IBD patient enrichment and stratification and efficacy of treatments. The CDBpC was formed with members from industry, academia, and nonprofit organizations, with support from the Helmsley Charitable Trust, to evaluate the CD minimally invasive biomarker landscape and determine which biomarkers were ready for regulatory qualification. This team identified the primary drug development need as minimally invasive, fluid-based biomarkers that could be measured serially during clinical trials to monitor patient response to therapies. The preconsortium work also identified 2 regulatory ready biomarkers for CD, FC, and CRP, for which clinical data are available to support the biomarker for a specific COU. Furthermore, a list of exploratory biomarkers of interest was also identified, and the team suggested including UC in future biomarker work. The ultimate purpose of the CDBpC was to plan for the launch of a larger consortium (IBD-RSC) dedicated to IBD biomarker regulatory qualification to ensure that these biomarkers are available to drug developers for a specific COU.
The IBD-RSC will pursue exploratory IBD biomarkers through sharing of knowledge and data, generation and/or sharing of biological samples, and assay development for novel biomarkers to evaluate interest in regulatory endorsement of FC and CRP utilizing existing data. Exploratory biomarkers of interest include inflammatory markers, and markers of microbiota dysfunction, and tissue injury and may be a combination of biomarkers or a biomarker panel covering different biological processes involved in the pathogenesis of IBD. Working together with IBD stakeholders, including regulators, and sharing data and information in the precompetitive space will be critical to developing new tools to support drug development and potentially patient care for patients with IBD.
ACKNOWLEDGMENTS
The CDBpC Team would like to the thank the Helmsley Charitable Trust for their support of the preconsortium. In addition, they would like to thank these members of CDBpC who contributed to review of the CD biomarker landscape: John Braun, James Bugni, James Butler, Laurence Cheng, Yehuda Chowers, Laurie Churchill, Ted Lee Denson, Joshua Friedman, Fayez Ghishan, Caren Heller, Robert Hinton, Andres Hurtado-Lorenzo, Ash Jain, Heng-Hong Li, Sharin Roth, David Rubin, Mirko Sikirica, Shannon Telesco, Harland Winter and Garabet Yeretssian.
Supported by: Disclosure of funding from National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI). The CDBpC Team preConsortium work was funded by the Helmsley Charitable Trust. JB is the recipient of National Institutes of Health grants NIH DK046763 and NIH DK085691. NVC holds a Research Scholar Award from the American Gastroenterological Association and has received research and consulting support from Takeda and UCB, research support from R-Biopharm and consulting support from Janssen, Pfizer, Progenity and Prometheus.
Conflicts of Interest: JA is an employee of Takeda. CC is an employee of AbbVie and participant in stock retirement plan. SD is an employee of Takeda. JDG is an employee and stockholder in Pfizer Inc. BL is employed by Robarts Clinical Trials.
REFERENCES
- 1. Caprilli R. Why does Crohn’s disease usually occur in terminal ileum? J. Crohns Colitis. 2008;2:352–356. [DOI] [PubMed] [Google Scholar]
- 2. NIDDK. Crohn’s Disease. 2017. Accessed May 28, 2020. https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/all-content.
- 3. Crohn’s and Colitis Foundation. About Crohn’s Disease and Ulcerative Colitis. 2018. Accessed April 17, 2020. https://www.crohnscolitisfoundation.org/sites/default/files/legacy/assets/pdfs/IBDoverview.pdf.
- 4. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 6. Ye Y, Manne S, Treem WR, et al. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007–2016. Inflamm Bowel Dis. 2019;26:619–625. [DOI] [PubMed] [Google Scholar]
- 7. European Medicines Agency. Guideline on the Development of New Medicinal Products for the Treatment of Crohn’s Disease. 2018. Accessed April 17, 2020. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-new-medicinal-products-treatment-crohns-disease-revision-2_en.pdf.
- 8. US Food and Drug Administration. Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry. 2016. Accessed April 17, 2020. https://www.fda.gov/media/99526/download.
- 9. Jauregui-Amezaga A, Rimola J, Ordás I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;64:1397–1402. [DOI] [PubMed] [Google Scholar]
- 10. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. [DOI] [PubMed] [Google Scholar]
- 11. Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–1768. [DOI] [PubMed] [Google Scholar]
- 12. Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. [DOI] [PubMed] [Google Scholar]
- 13. Weisshof R, El Jurdi K, Zmeter N, et al. Emerging therapies for inflammatory bowel disease. Adv Ther. 2018;35:1746–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verstockt B, Ferrante M, Vermeire S, et al. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 16. Clarke K, Chintanaboina J. Allergic and immunologic perspectives of inflammatory bowel disease. Clin Rev Allergy Immunol. 2019;57:179–193. [DOI] [PubMed] [Google Scholar]
- 17. Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323–337. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. Biomarker Qualification Context of Use. 2018. Accessed May 20, 2020. https://www.fda.gov/drugs/cder-biomarker-qualification-program/context-use.
- 19. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. 2016. Accessed May 4, 2020. http://www.ncbi.nlm.nih.gov/books/NBK338448/.
- 20. FDA. CDER Biomarker Qualification Program. FDA. 2019. Accessed October 19, 2019. http://www.fda.gov/drugs/drug-development-tool-qualification-programs/cder-biomarker-qualification-program.
- 21. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. [DOI] [PubMed] [Google Scholar]
- 22. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vernia F, Di Ruscio M, Stefanelli G, et al. Is fecal calprotectin an accurate marker in the management of Crohn’s disease? J Gastroenterol Hepatol. 2020;35:390–400. [DOI] [PubMed] [Google Scholar]
- 24. Kennedy NA, Jones G-R, Plevris N, et al. Association between level of fecal calprotectin and progression of Crohn’s disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foster AJ, Smyth M, Lakhani A, et al. Consecutive fecal calprotectin measurements for predicting relapse in pediatric Crohn’s disease patients. World J Gastroenterol. 2019;25:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Amico F, Bonovas S, Danese S, et al. Review article: faecal calprotectin and histologic remission in ulcerative colitis. Aliment Pharmacol Ther. 2020;51:689–698. [DOI] [PubMed] [Google Scholar]
- 27. Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. [DOI] [PubMed] [Google Scholar]
- 28. Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reenaers C, Bossuyt P, Hindryckx P, et al. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol J. 2018;6:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2017;13:53–56. [PMC free article] [PubMed] [Google Scholar]
- 31. Brookes MJ, Whitehead S, Gaya DR, et al. Practical guidance on the use of faecal calprotectin. Frontline Gastroenterol. 2018;9:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bressler B, Panaccione R, Fedorak RN, et al. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2015;29:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–1415. [DOI] [PubMed] [Google Scholar]
- 34. Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 36. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moran CJ, Kaplan JL, Winter HS. Genetic variation affects C-reactive protein elevations in Crohn’s disease. Inflamm Bowel Dis. 2018;24:2048–2052. [DOI] [PubMed] [Google Scholar]
- 38. Colombel JF, Solem CA, Sandborn WJ, et al. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multi-centre UK experience. J Crohns Colitis. 2020. [DOI] [PubMed] [Google Scholar]
- 40. Bhat S, Altajar S, Shankar D, et al. Process and clinical outcomes of a biosimilar adoption program with infliximab-Dyyb. J Manag Care Spec Pharm. 2020;26:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guerra Veloz MF, Belvis Jiménez M, Valdes Delgado T, et al. Long-term follow up after switching from original infliximab to an infliximab biosimilar: real-world data. Therap Adv Gastroenterol. 2019;12:1756284819858052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568–576. [DOI] [PubMed] [Google Scholar]
- 43. Cornish JS, Wirthgen E, Däbritz J. Biomarkers predictive of response to thiopurine therapy in inflammatory bowel disease. Front Med (Lausanne). 2020;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Musci JO, Cornish JS, Däbritz J. Utility of surrogate markers for the prediction of relapses in inflammatory bowel diseases. J Gastroenterol. 2016;51:531–547. [DOI] [PubMed] [Google Scholar]
- 45. Nikolaus S, Waetzig GH, Butzin S, et al. Evaluation of interleukin-6 and its soluble receptor components sIL-6R and sgp130 as markers of inflammation in inflammatory bowel diseases. Int J Colorectal Dis. 2018;33:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caviglia GP, Rosso C, Stalla F, et al. On-treatment decrease of serum interleukin-6 as a predictor of clinical response to biologic therapy in patients with inflammatory bowel diseases. J Clin Med. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mizoguchi A, Yano A, Himuro H, et al. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 49. Schmechel S, Konrad A, Diegelmann J, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. [DOI] [PubMed] [Google Scholar]
- 50. Nielsen OH, Gionchetti P, Ainsworth M, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999;94:2923–2928. [DOI] [PubMed] [Google Scholar]
- 51. Thorsvik S, Damås JK, Granlund AV, et al. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J Gastroenterol Hepatol. 2017;32:128–135. [DOI] [PubMed] [Google Scholar]
- 52. Thorsvik S, Bakke I, van Beelen Granlund A, et al. Expression of neutrophil gelatinase-associated lipocalin (NGAL) in the gut in Crohn’s disease. Cell Tissue Res. 2018;374:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bazzoni F, Rossato M, Fabbri M, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schaefer JS, Attumi T, Opekun AR, et al. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iborra M, Bernuzzi F, Correale C, et al. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moret-Tatay I, Iborra M, Cerrillo E, et al. Possible biomarkers in blood for Crohn’s disease: oxidative stress and MicroRNAs-current evidences and further aspects to unravel. Oxid Med Cell Longev. 2016;2016:2325162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. West NR, Hegazy AN, Owens BMJ, et al. ; Oxford IBD Cohort Investigators . Erratum: Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:788. [DOI] [PubMed] [Google Scholar]
- 58. Verstockt B, Verstockt S, Dehairs J, et al. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. Ebiomedicine. 2019;40:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 60. Rojas-Feria M, Romero-García T, Fernández Caballero-Rico JÁ, et al. Modulation of faecal metagenome in Crohn’s disease: Role of microRNAs as biomarkers. World J Gastroenterol. 2018;24:5223–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Doherty MK, Ding T, Koumpouras C, et al. Fecal microbiota signatures are associated with response to ustekinumab therapy among Crohn’s disease patients. mBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dovrolis N, Drygiannakis I, Filidou E, et al. Gut microbial signatures underline complicated Crohn’s disease but vary between cohorts; an in silico approach. Inflamm Bowel Dis. 2019;25:217–225. [DOI] [PubMed] [Google Scholar]
- 63. Coufal S, Galanova N, Bajer L, et al. Inflammatory bowel disease types differ in markers of inflammation, gut barrier and in specific anti-bacterial response. Cells. 2019;8:719. doi: 10.3390/cells8070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elkadri AA, Stempak JM, Walters TD, et al. Serum antibodies associated with complex inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1499–1505. [DOI] [PubMed] [Google Scholar]
- 65. Choung RS, Princen F, Stockfisch TP, et al. ; PREDICTS Study Team . Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016;43:1300–1310. [DOI] [PubMed] [Google Scholar]
- 66. Torres J, Petralia F, Sato T, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 y before diagnosis. Gastroenterology. 2020. [DOI] [PubMed] [Google Scholar]
- 67. Lindholm M, Manon-Jensen T, Madsen GI, et al. Extracellular matrix fragments of the basement membrane and the interstitial matrix are serological markers of intestinal tissue remodeling and disease activity in dextran sulfate sodium colitis. Dig Dis Sci. 2019;64:3134–3142. [DOI] [PubMed] [Google Scholar]
- 68. Wu J, Lubman DM, Kugathasan S, et al. Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric Crohn’s disease. Am J Gastroenterol. 2019;114:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mortensen JH, Manon-Jensen T, Jensen MD, et al. Ulcerative colitis, Crohn’s disease, and irritable bowel syndrome have different profiles of extracellular matrix turnover, which also reflects disease activity in Crohn’s disease. Plos One. 2017;12:e0185855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Buisson A, Vazeille E, Minet-Quinard R, et al. Fecal matrix metalloprotease-9 and lipocalin-2 as biomarkers in detecting endoscopic activity in patients with inflammatory bowel diseases. J Clin Gastroenterol. 2018;52:e53–e62. [DOI] [PubMed] [Google Scholar]
- 71. van Haaften WT, Mortensen JH, Karsdal MA, et al. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn’s disease. Aliment Pharmacol Ther. 2017;46:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121:4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKinney EF, Lyons PA, Carr EJ, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–591, 1p following 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Biasci D, Lee JC, Noor NM, et al. A blood-based prognostic biomarker in IBD. Gut. 2019;68:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology. 2020;158:515–526.e10. [DOI] [PubMed] [Google Scholar]
- 76. van Schaik FDM, Oldenburg B, Hart AR, et al. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut. 2013;62:683–688. [DOI] [PubMed] [Google Scholar]
- 77. Bourgonje AR, von Martels JZH, Gabriëls RY, et al. A combined set of four serum inflammatory biomarkers reliably predicts endoscopic disease activity in inflammatory bowel disease. Front Med (Lausanne). 2019;6:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Siegel CA, Siegel LS, Hyams JS, et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn’s disease. Inflamm Bowel Dis. 2011;17:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Siegel CA, Horton H, Siegel LS, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther. 2016;43:262–271. [DOI] [PubMed] [Google Scholar]
- 80. Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Powell N, Pantazi E, Pavlidis P, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Verstockt B, Verstockt S, Blevi H, et al. TREM-1, the ideal predictive biomarker for endoscopic healing in anti-TNF-treated Crohn’s disease patients? Gut. 2019;68:1531–1533. [DOI] [PubMed] [Google Scholar]
- 83. Amcoff K, Joossens M, Pierik MJ, et al. Concordance in anti-ompc and anti-i2 indicate the influence of genetic predisposition: results of a European study of twins with Crohn’s disease. J Crohns Colitis. 2016;10:695–702. [DOI] [PubMed] [Google Scholar]
- 84. Ahmed Z, Lysek M, Zhang N, et al. Association between serological markers and Crohn’s disease activity. J Clin Med Res. 2020;12:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]