Abstract
Physiological organ cross-talk is necessary to maintain equilibrium and homeostasis. Heart and kidney are the essences of this equilibrium. Organ failure in either of these organs can perturb the bidirectional communication between them, impinging this unpleasant vascular and cellular milieu on other distant organs. Cardiorenal syndrome (CRS) type I occurs due to acute deterioration of cardiac function, ultimately causing acute kidney injury (AKI). This syndrome is an intricate condition with neurohormonal and inflammatory aspects. Inflammation creates a vicious circle filled with the innate and adaptive immune systems, pro-inflammatory cytokines, chemokines to actuate hemodynamic compromise in CRS type I patients. Pro-inflammatory cytokines not only aggravate fluid retention and venous congestion but also initiate apoptosis and oxidative stress. The immune response's primary motive is to elicit the heart and kidney to produce cytokines, intensifying the inflammatory process. Despite the possible standard of care, patient mortality, treatment cost, readmissions are extreme in CRS type I, and inflammation certainly has critical inferences warranting future research in humans.
Keywords: cardiorenal syndrome type i, inflammation, acute kidney injury, cytokines, toll-like receptors, innate immune system, apoptosis, oxidative stress
Introduction and background
“Physiological interaction between organs is necessary for the optimal equilibrium and functioning of the organism” [1]. The human body continuously strives to achieve one critical goal: homeostasis. The human body has an intricate communication system, collectively termed as “organ cross-talk.” Irrespective of their distance, the synergy between each organ, tissue, and cell is vital to attain equilibrium and a stable internal environment. This communication is in the form of hormones, proteins, mechanical stimuli, and electrical signals. Altering this cross-talk produces some drastic consequences, adaptive or maladaptive. Cardiorenal syndromes are such instances where specific maladaptive changes alter the bidirectional link between heart and kidneys. The term cardiorenal syndromes constitute of five subtypes: in type I (acute cardiorenal syndrome), rapid cardiac dysfunction causes acute kidney injury (AKI) while in type II (chronic cardiorenal syndrome), chronic cardiac disorders invoke chronic kidney disease. On the same spectrum, in type III (acute nephrocardiac syndrome) and type IV (chronic nephrocardiac syndrome), AKI and chronic kidney disease surmount to acute heart failure. In type V, nonetheless, a systemic illness elicits simultaneous damage of heart and kidneys [2]. This article focuses on cardiorenal syndrome type I (CRS type I). Etiology of CRS type 1 stems from sudden and abrupt pump failure due to conditions such as cardiogenic shock, acute decompensated heart failure (ADHF), acute coronary syndrome, and cardiac surgery [2-4]. In patients with acute decompensated heart failure and acute heart failure, CRS type 1 represents an astonishing 25% to 33% of patients and 27% to 45% of patients in the hospital, respectively [5-9]. Most patients first manifest laboratory evidence of AKI - an increase in serum creatinine greater than 0.3 mg/dL - and gradually reveal symptoms of kidney dysfunction three to five days after admission [10-12]. Although AKI is an independent predictor of mortality, it also has significant implications for prognosis, treatment cost, hospitalization duration, readmissions, cardiovascular events, and rapid progression to chronic kidney disease in CRS type I patients [12-14]. As the disease evolves, underlying pathology holds the key to unravel promising therapeutic and diagnostic benefits. Like its counterparts, the pathophysiology of CRS type I is multifactorial. There are hemodynamic mechanisms - arterial underfilling, decreased cardiac output, increased venous congestion, hyponatremia, and non-hemodynamic mechanisms - activation of the sympathetic nervous system and renin-angiotensin-aldosterone-system (RAAS) including the uncontrolled formation of reactive oxygen species (ROS) and nitric oxide [15-17]. One of the most speculated non-hemodynamic mechanisms of CRS type I is the immune system. The immune response is a powerful defense system adapted by the human body that has drastic repercussions if gone rogue. A savior and savage, immune system orchestrates organ healing and damage mediated by many components [18]. The normal immune response occurs with antigen recognition/presentation, activation of the complement and innate/ adaptive immune systems, and finally, resolution of the response. Interestingly, studies have shown that the function of the immune system is skewed in CRS type I partly because of improper inflammatory stimulation and inhibition [4]. Recent evidence shows that patients with severe heart failure and AKI demonstrate high pro-inflammatory cytokines, chemokine up-regulation, neutrophil migration and extravasation, toll-like receptor expression, and unregulated apoptosis leading to oxidative stress [4,19]. In CRS type I, the immune response induces a high-pressure system, fabricates mechanical stress in the vasculature, and promotes additional inflammation [20]. Numerous researches are underway to elaborate on the influence of the immune system in CRS type I, but the disease’s complex nature poses a significant limitation. Recent studies analyzed the overzealous immune system in CRS type I, and how it evokes multiple organ damage. Nevertheless, a few crucial aspects remain in question: Does the immune system govern other etiologic factors in CRS type I? What triggers the immune system in CRS type I? Is immune response the culprit for patient deterioration and high mortality? Will there be any therapeutic benefit from immunomodulation? This review primarily centers on immune system dominance over CRS type I pathogenesis.
Review
Discussion
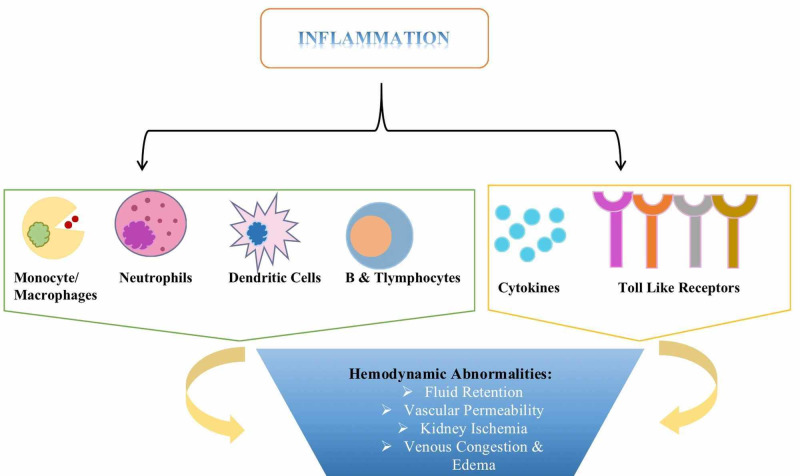
CRS type I is characterized by a multifactorial etiology; however, there is a causal relationship between the factors that promote functional deterioration of the heart and kidney. Changes at the cellular level are fundamental for detrimental tissue remodeling and aberrant organ cross-talk. As shown in Figure 1, inflammation instigates an environment to advance these microscopic changes through its integral units- cells, cytokines, and toll-like receptors (TLRs) [4]. These components mediate the hemodynamic abnormalities of CRS type I. Fluid reabsorption is a strictly controlled process in the pulmonary interstitium, and immune activation interferes with the reabsorption process causing fluid overload in the lungs [21,22]. Inflammation creates a framework for third spacing that contributes to many of the hemodynamic abnormalities in the kidney. A few of the underlying mechanisms are insufficient renal perfusion with a subsequent decrease in glomerular filtration rate (GFR), ischemic damage to the tubular epithelium, and peritubular edema [4].
Figure 1. Various inflammatory components instrumental in the hemodynamic pathophysiology of cardiorenal syndrome (CRS) type I.
Inflammation superimposes itself on CRS type I and plays a pivotal role in the pathophysiology of the disease. There are four forms of CRS: in type I and II, recent cardiac injury incites AKI and chronic kidney disease. However, in types III and IV, acute exacerbation of chronic cardiac disease promotes AKI and the sudden worsening of chronic kidney disease [4]. Excluding the first subtype, the remaining three subtypes are acute on chronic decompensation of heart or kidney disease. A noteworthy characteristic of chronic diseases is a subtle yet concealed and active immune response. Hence, when CRS type I manifests symptomatically, a formerly well established inflammatory activation gets magnified and sets in stone a series of events, including hemodynamic consequences that increase mortality in these patients.
Innate Immune System
The first line of defense from foreign antigens is the innate immune system. Cells of the innate immune system are perpetually circulating in the blood monitoring for any signs of insults in the organ systems. Nevertheless, during chronic illnesses, tissue injury releases ROS and reactive nitrogen species (RNS) along with mitochondrial products provoking the inflammatory response [5]. In terms of CRS type I and as Figure 2 illustrates, neutrophils, dendritic cells (DCs), monocytes/macrophages are integral to the dysfunctional immune response. Notably, after an ischemia-reperfusion injury, neutrophils infiltrate tissues, produce harmful proteases, ROS, and myeloperoxidase (MPO) enzyme debilitating organ function [23,24]. Table 1 reveals numerous studies on components of the innate immune system and their interpretation of CRS type I pathology.
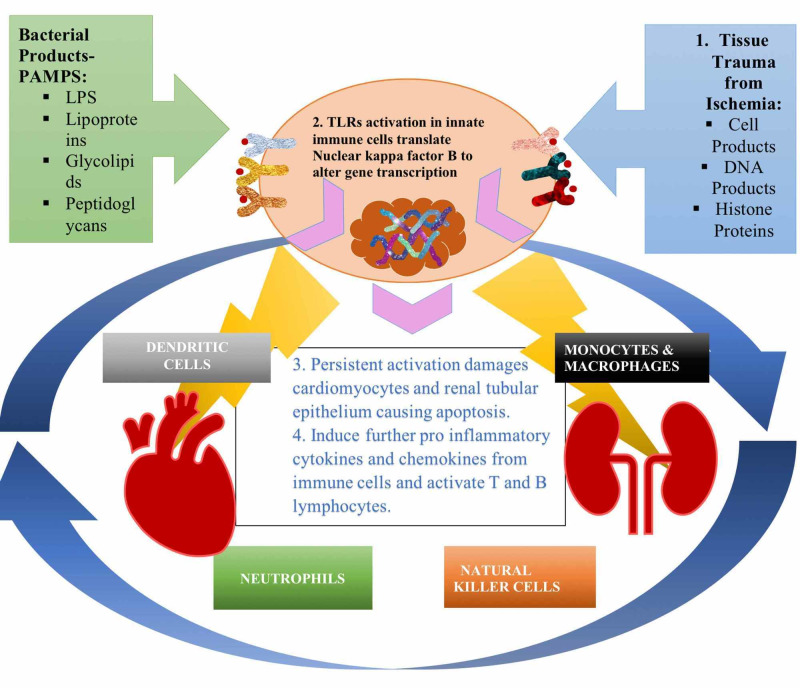
Figure 2. Mechanisms that activate toll-like receptors (TLRs) and their impact in cardiorenal syndrome (CRS) type I pathophysiology.
1. Tissue trauma releases products called endogenous ligands that stimulate TLRs. This pathologic trigger is the earliest immune response in AKI injury that sets a cascade of events in motion, which eventually causes massive tissue damage, 2. Amplification of pro-inflammatory cytokines occurs through translocation of transcription factor nuclear factor-kappa B to the nucleus, 3. Sustained TLR activation and inflammatory response leads to apoptosis of renal tubular epithelium, 4. TLRs fuel further cytokine release from neutrophils, monocyte/macrophages, neutrophils, and natural killer cells, resulting in the adaptive immune system activation.
Table 1. Studies detailing the effects of the innate immune system in cardiorenal syndrome (CRS) type I.
ACE- angiotensin-converting enzyme, AKI- acute kidney injury, IL- interleukin, TLR- toll-like receptors, TNF- tumor necrosis factor, GM-CSF- granulocyte/monocyte-colony stimulating factor, G-CSF- granulocyte-colony stimulating factor.
| AUTHOR & YEAR OF PUBLICATION | TYPE OF STUDY | PURPOSE OF STUDY | CONCLUSION |
| Clementi et al. [1] 2019 | Review | Neurohormonal, endocrine, immune dysregulation and inflammation in CRS | To insinuate the need for novel drug therapies that cover new mechanisms in CRS. |
| Virzì et al. [5] 2014 | Review | The Hemodynamic and Non-hemodynamic Cross-talk in CRS type I | To elaborate molecular, cellular, and subcellular features for advancing treatments |
| Virzì et al. [18] 2014 | Review | Heart--kidney cross-talk and role of humoral signaling in critical illness | Damaged cardiac myocytes and renal tubular epithelium promote activation of innate and adaptive immune systems strengthening the humoral response. |
| Pasare and Medzhitov [25] 2004 | Review | Toll-like receptors: linking innate and adaptive immunity | TLRs are essential for immune cell maturation and the apt recognition of internal and external molecular pathogens |
| de kleijn and Pasterkamp [26] 2003 | Review | Toll-like receptors in cardiovascular diseases | TLRs play a crucial role in initiating cardiovascular diseases especially atherosclerosis formation |
| Chao [27] 2009 | Review | Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart | TLRs respond to tissue injury and are important sources of cardiac ischemia-reperfusion injury. These receptors also cardioprotectors regulating cell survival in heart. |
| Eissler et al. [28] 2011 | Animal study | Hypertension augments cardiac Toll-like receptor-4 expressions and activity | Hypertension actives the innate immune system through TLR. ACE inhibitors inhibit inflammation only at high doses |
| Allam et al. [29] 2012 | In vitro study | Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4 | Histone neutralization can reduce ischemia-reperfusion injury from dying renal epithelial cells. |
| Kinsey and Okusa [30] 2008 | Review | Inflammation in Acute Kidney Injury | Ischemia-reperfusion injury stimulates the immune system. TLR, chemokines. Cytokines amplify this response. Further research is needed to categorize the function of each component of the immune system in AKI. |
| Dong et al. [31] 2007 | Animal study | Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury | Renal dendritic cells are localized to renal peritubular space and respond to innate immunity enrolling further circulating immune cells into the kidney. |
| Virzì et al. [32] 2018 | Review | The role of dendritic and endothelial cells in CRS | Heart and kidney dendritic cells are involved in tissue remodeling. Additionally, endothelial cells act as antigen-presenting cells and act as a bridge between innate and adaptive immune systems. |
| Zhang et al. [33] 1993 | Animal study | Interstitial dendritic cells of the rat heart. Quantitative and ultrastructural changes in experimental myocardial infarction | These dendritic cells instigate additional lymphocytes and reduce in quantity with the formation of a scar. |
| Naito et al. [34] 2008 | Animal study | Differential effects of GM-CSF and G-CSF on the infiltration of dendritic cells during early left ventricular remodeling after myocardial infarction | Induction of MI in rat models is characterized by dendritic cells infiltration, increased interferon-gamma and TLR4 expression with decreased IL-10 levels |
| Vaduganathan et al. [35] 2013 | Review | The immunological axis in heart failure: the importance of the leukocyte differential | High lymphocyte counts in acute and chronic heart failure patients are associated with unfavorable prognosis. In particular, elevated monocyte counts are also indicative of severe outcomes in heart failure |
| Wrigley et al. [36] 2011 | Review | The role of monocytes and inflammation in the pathophysiology of heart failure | Persistent activation of monocytes during heart failure augments tissue injury and are implicated in disease progression |
| Satoh et al. [37] 2008 | Review | Immune modulation: role of the inflammatory cytokine cascade in the failing human heart. | Cytokines and TNF-alpha induce changes in monocyte phenotype, myocardial cell death, and advanced matrix metalloproteinase activity to enhance ventricular remodeling |
| Pastori et al. [38] 2015 | In vitro study 40 patients | CRS type I: A Defective Regulation of Monocyte Apoptosis Induced by Pro-inflammatory and Proapoptotic Factors | Inflammatory cytokines in the plasma of CRS patients induce the production of more cytokines leading to unregulated apoptosis. |
TLRs induce activation of the innate immune response. They are transmembrane proteins located on the plasma membrane of leukocytes and lymphocytes cardinal for mounting an immune response. Ten subtypes of TLRs dimerize with each other to recognize pathogen-associated molecular patterns (PAMPs) traversing through our bodies, depicted in Figure 2. These receptors create a functional change in the immune cells activating them to secrete cytokines (IL-1 beta, IL-6, and tumor necrosis factor (TNF)-alpha) and chemokines (keratinocyte chemoattractant-1 and monocyte chemoattractant protein-1) [25]. During CRS type I and especially AKI, TLRs play a critical role in influencing inflammatory activation and poor bidirectional communication. Studies have shown that TLRs are involved in various cardiac pathologies, such as acute myocardial infarction, cardiac failure, ischemic myocardial injury, and myocardial dysfunction [26-28].
Virzì et al. discuss the aftermath in AKI after inducing the TLR pathway; Figure 2 reveals the critical events related to TLRs activation [18]. Similarly, Clementi and colleagues mentioned that TLRs incite innate and adaptive immune systems, coordinating the deleterious cardiorenal cross-talk [1]. Allam et al. defined another perspective for TLR signaling where histones released from dying tubular epithelium initiate cytokine production and exacerbate kidney injury [29]. These studies suggest that TLRs are essential components of immunity and serve as bridges between innate and adaptive immune systems. Furthermore, inflammation is one of the earliest responses to tissue injury that damages the bidirectional communication in CRS type I and sets the platform for ensuing pathogenesis.
DCs are antigen-presenting cells (APC) of the innate immune system that provoke adaptive immune cells. As noted in Figure 2, they depend on TLRs for maturation and always scrutinize for antigens in many tissues. These cells interact with other subtypes using CD40-CD40 ligand and produce many pro-inflammatory substances, including interleukin (IL)-12, IL-6, TNF-alpha, and monocyte chemoattractant protein-1 [30,31]. Clementi et al. elucidated the vital role of renal dendritic cells in the inflammatory response and, after maturation, increase MHC II expression levels and costimulatory molecules [1]. Additionally, Virzì et al. emphasized that DCs maturation and antigen presentation regulates CD4+ and CD8+ T lymphocyte function and proliferation [5]. In other articles, however, Virzì and colleagues also shed light on the unclear contribution of DCs in AKI, including their part in the pathophysiology of CRS type I [18,32]. The majority of the data regarding DCs in heart and kidney illnesses arises from murine models where research-induced ischemic- reperfusion injury mimics in vivo effects [33,34].
Virzì and Clementi extrapolated their ideas about the role of dendritic cells in CRS type I from mouse models. Since these researchers chronicled this emerging evidence of DCs in CRS type I, there has been no further research or clinical trials to document the effects of DCs in CRS dysfunction. Although animal studies cannot be applied to humans precisely, these studies prove that inflammatory cells are principal for the dysfunction associated with cardiorenal syndromes, warranting future research in patients.
The monocyte/macrophage system is another potent immune regulator. They produce cytokines that enhance the immune response and function as APCs to the B and T lymphocytes. Notably, in heart failure, monocytes are the primary cells for cytokine generation and are also responsible for most of the inflammatory process in heart diseases [35-37]. Concerning CRS type I, there are dual points of observation for monocytes. Virzì et al., in their review, acknowledged the presence of monocytes/macrophages in myocardial pathologies; however, they also asserted the uncertain contribution of these cells in repair or damage of the myocardium [5].
Additionally, while the in vitro study led by Pastori et al. supported the pro-inflammatory effect of monocytes, they also endorsed the theory of monocyte apoptosis from cytokine laced plasma of CRS type I patients [38]. Specifically, Clementi et al. also stressed the significance of high inflammatory levels associated with acute heart failure caused monocytes’ death and oxidative stress [1]. Even though in vitro studies are the weakest evidence, they laid the groundwork to enunciate the vicious milieu created by the immune system’s components.
Provocation of Humoral Signaling and Its Repercussions
This moiety of the immune system includes water-soluble mediators that principally strive to reinforce initial immune response. Standard humoral signaling components include cytokines, chemokines, interferons, and growth factors. Cytokines such as interleukins establish powerful communication strategies between white blood cells while chemokines facilitate chemotaxis, especially for neutrophils, and interferons have antiviral properties [5]. The continuous presence of these mediators magnifies immune response, precipitates inter/intracellular changes, deteriorates organ function, and impairs organ cross-talk [5]. Table 2 enumerates on recent studies, detailing humoral signaling function in CRS type I.
Table 2. Studies elucidating the effects of humoral signaling on cardiorenal syndrome (CRS) type I pathogenesis.
IL- interleukin
| AUTHOR & YEAR OF PUBLICATION | TYPE OF STUDY & # OF PATIENTS | PURPOSE OF STUDY | CONCLUSION |
| Clementi et al. [1] 2019 | Review | Neurohormonal, endocrine, immune dysregulation and inflammation mechanisms in CRS | To insinuate the need for novel drug therapies that cover new mechanisms in CRS. |
| Ronco et al. [4] 2012 | Review | CRS type I: Pathophysiological Crosstalk Leading to Combined Heart and Kidney Dysfunction in Acutely Decompensated Heart Failure | Multifactorial mechanisms lead to progressive heart and kidney dysfunction. New diagnostic and therapeutic strategies decrease the morbidity associated with CRS type I. |
| Virzì et al. [5] 2012 | Review | The Hemodynamic and Non-hemodynamic Cross-talk in CRS type I | To elaborate molecular, cellular, and sub-cellular features for advancing treatments. |
| Virzì et al. [18] 2014 | Review | Heart--kidney cross-talk and role of humoral signaling in critical illness | Damaged cardiac myocytes and renal tubular epithelium promote activation of innate and adaptive immune systems strengthening the humoral response. |
| Colombo et al. [20] 2012 | Review | Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with cardiorenal syndrome | To highlight the sustained inflammatory response that is responsible for the functional deterioration of patients with CRS. Existing anti-inflammatory treatment methods have been disappointing to date necessitating new studies. |
| Pastori et al. [38] 2015 | In vitro study, 40 patients | CRS type I: A Defective Regulation of Monocyte Apoptosis Induced by Pro-inflammatory and Proapoptotic Factors | Inflammatory cytokines (IL-6, IL-18) in the plasma of CRS patients induce the production of more cytokines leading to unregulated apoptosis. |
| Virzì et al. [39] 2018 | In vitro study, 53 patients | Levels of Pro-inflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without CRS type I | High levels of Pro-inflammatory cytokines, oxidative stress, and biomarkers are the crux of CRS type I pathophysiology. |
| Virzì et al. [40] 2012 | In vitro study, 15 patients | CRS type I may be Immunologically Mediated: A Pilot Evaluation of Monocyte Apoptosis | Inflammation is the basis for organ damage and impaired apoptosis in CRS type I patients. CRS type I patients plasma can trigger apoptosis in monocytes. |
| Pastori et al. [41] 2015 | In vitro studies, 29 patients | CRS type I: Activation of Dual Apoptotic Pathways | Inflammation induced apoptosis of renal tubular epithelial cells, which is a fundamental pathogenic mechanism in CRS type I and a potential future therapeutic target. |
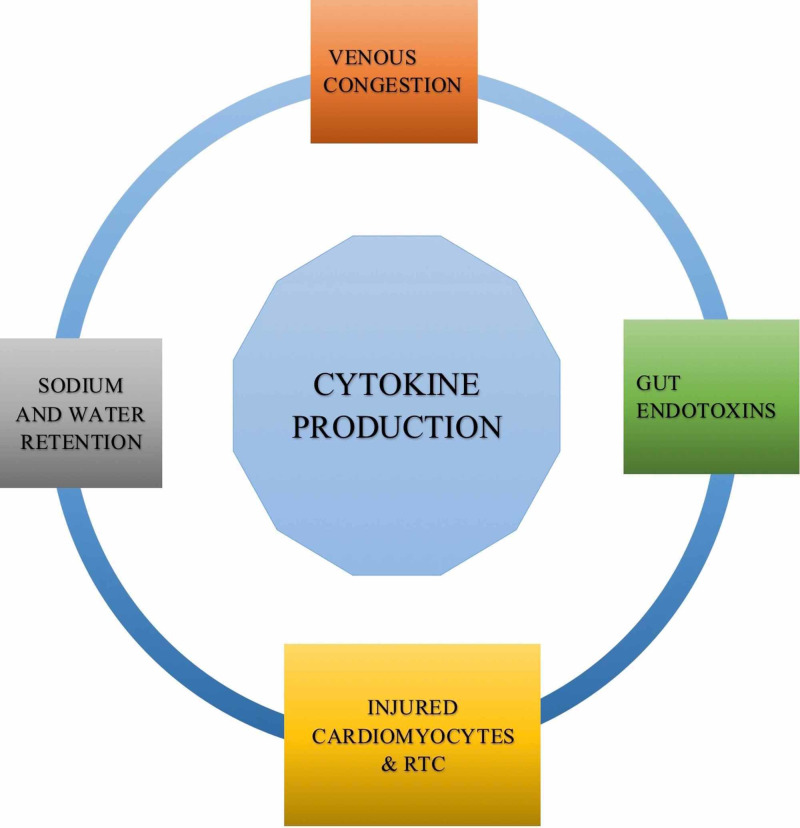
Various pathological aspects in CRS type I govern cytokine production, as depicted in Figure 3. Virzì et al. stressed that the release of humoral factors from damaged cells supervises TLRs activation and increases cytokine generation- TNF-alpha, IL-1, IL-4, IL-6, IL-13, and IL-17, which are fatal in cardiac critical care units and instigate organ damage in CRS type I [18]. At the same time, Clementi et al. discussed that venous congestion in CRS type I fosters cytokine release, crippling renal function [1]. Furthermore, Colombo et al. hinted at the prospect of sodium and water retention, which aggravates fluid overload, congestion, and inflammatory cytokine production [20].
Figure 3. Sources of cytokine production in cardiorenal syndrome (CRS) type I.
Independently, Ronco et al. and Colombo et al. put forward another exciting aspect- gut endotoxins induce cytokines in CRS type I [4,20]. Colombo et al. proposed that fluid overload in CRS causes bowel edema which in turn destroys intestinal villi endothelial cells, releasing lipopolysaccharide (LPS) from gut bacteria [20]. Ronco et al. elaborated on this idea and ascertained that low intestinal perfusion in CRS type I and heart failure liberates LPS promoting IL-1, IL-6, and TNF-alpha generation [4]. These reviews validate the ideology where multiple pathways exist for inflammation to debilitate organ function and impair the physiological equilibrium. Therefore, targeting one mechanism or organ system does not compensate for the systemic imbalance.
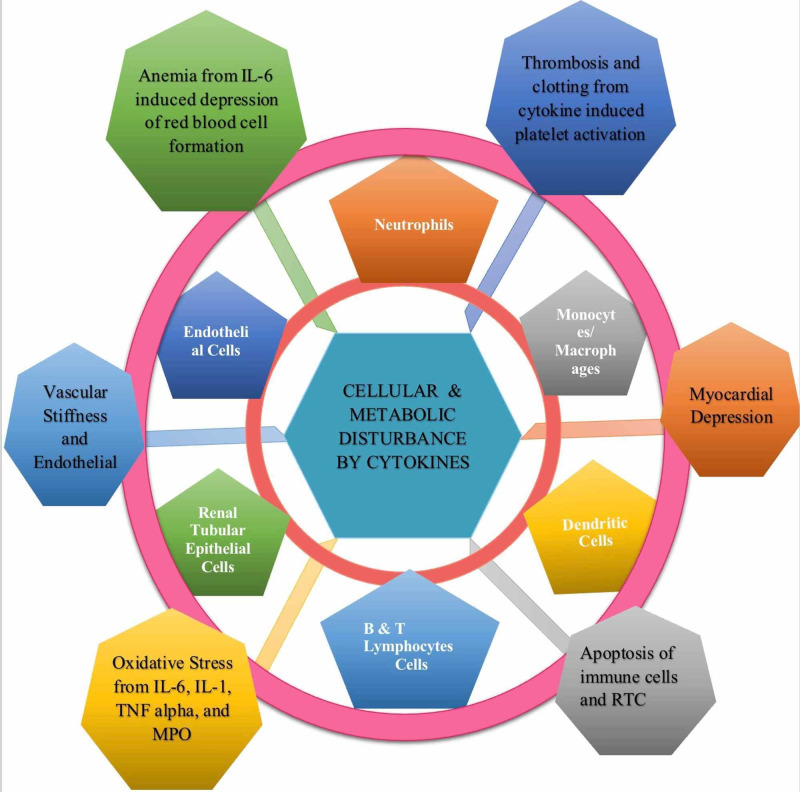
Humoral mediation proves that inflammation not only disturbs communication between organs but also influences metabolism in CRS type I. Figure 4 demonstrates cytokine impact on multiple cells and on homeostasis. Clementi and colleagues described the inverse relation between IL-6 and hemoglobin; pro-inflammatory cytokines cause anemia by bone marrow suppression, erythropoietin receptor down-regulation, red blood cell precursors destruction, and iron sequestration [1]. Additionally, they noted the interrelation between clotting and inflammation; immune response damages renal vessels and activates platelets, resulting in additional cytokine and chemokine production [1]. In another study, Colombo et al. explained the cardio depressant actions of TNF-alpha through increased nitric oxide [20]. Ronco and colleagues emphasized this concept of cytokine and TLRs’ role in diminishing myocardial excitability, altering resting membrane potential, modulating substrate metabolism, and responding to the sympathetic nervous system [4]. Moreover, Colombo et al. delineated the vascular abnormalities of inflammation, such as immune system induced arterial stiffness and endothelial permeability [20]. All these reviews remark on various neurohormonal effects of inflammation, and therefore, it is the principal element mediating the pathogenesis of CRS type I.
Figure 4. Heterogenous sequela of cytokines on different tissues and on homeostasis.
MPO- myeloperoxidase, IL- interleukin, TNF- tumor necrosis factor, RTC- renal tubular cells
Apoptosis is a highly regulated, essential physiologic process. Cell death is dependent on timing, and just like inflammation, it can create havoc if stimulated inappropriately. Two apoptosis pathways are intrinsic pathways that result from disturbance to intracellular homeostasis, and the extrinsic pathway results from the coupling of death receptors on the plasma membrane [18]. Virzì et al. deciphered that TLR stimulation instigates caspases to initiate pathological apoptosis of renal tubular epithelial cells [18]. In another article, Virzì and colleagues affirmed that low perfusion and hypoxia evoked cellular metabolism changes, ultimately causing cell death [39].
Pro-inflammatory cytokines have a dual purpose where they provoke other immune cells to produce additional inflammatory markers, creating an environment for cell death and altering organ fate. Virzì et al. incubated monocytes with CRS type I plasma and identified a two-fold increase in caspase-8 levels, a three-fold surge in IL-6, and a ten-fold hike in TNF-alpha levels [40]. Likewise, Pastori et al. analyzed this theory in their in vitro study, where they incubated monocytes and renal tubular epithelial cells in CRS type I plasma. They found high IL-6, IL-18, and TNF-alpha levels that increased humoral mediators and caspase 8 and 3 levels in monocytes, and also discerned that CRS type I plasma initiated dual apoptotic pathways [38,41].
In vitro studies justify the notion that inflammation alters organ destiny compared to other compensatory mechanisms. These studies also create a compelling foundation for future observational studies or clinical trials targeting the immune system as a treatment strategy.
Oxidative Stress
Oxidative stress and inflammation are intertwined, and their clinical ramifications are a subject of interest in the medical literature. A delicate equilibrium exists between essential and excessive amounts of an element. Notably, every tissue needs a certain amount of oxidative species to protect itself, while excessive amounts can cause dysfunction, injury, and organ failure [5,42]. An ongoing inflammatory process in CRS type I renders the heart and kidney incapable of controlling oxidative stress [5]. Similar to multiple humoral signaling pathways, there are different mechanisms to bring about oxidative stress in CRS type I. Table 3 elaborates on oxidative stress-induced CRS type I pathology.
Table 3. Studies explicating the importance of oxidative stress in cardiorenal syndrome (CRS) type I pathophysiology.
RAS- renin-angiotensin system, SNS- sympathetic nervous system, ESRD- end stage renal disease, MPO- myeloperoxidase
| AUTHOR & YEAR OF PUBLICATION | TYPE OF STUDY & # OF PATIENTS | PURPOSE OF STUDY | CONCLUSION |
| Lullo et al. [2] 2017 | Review | Update on the pathophysiology of CRS types 1-5 | To elucidate the burden of pathophysiology in CRS types 1-5 on the functioning of the heart and kidney. |
| Virzì et al. [5] 2014 | Review | The Hemodynamic and Non-hemodynamic Cross-talk in CRS type I | To elaborate molecular, cellular, and subcellular features for advancing treatments. |
| McCullough [13] 2011 | Review | Cardiorenal syndrome: pathophysiology to prevention | Importance of catalytic iron in organ injury and other biomarkers for diagnosis, treatment, and prognosis. |
| Bongartz et al. [16] 2004 | Review | The severe Cardiorenal syndrome: Guyton Revisited | To unravel the harmful consequences of RAS, RNS &ROS, inflammation, and SNS. |
| Virzì et al. [39] 2018 | In vitro study, 53 patients | Levels of Pro-inflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without CRS type I | High levels of Pro-inflammatory cytokines, oxidative stress, and biomarkers are the crux of CRS type I pathophysiology. |
| Virzì et al. [42] 2015 | In vitro study, 23 patients | Oxidative stress: Dual Pathway Induction in CRS Type I Pathogenesis | To understand the imbalances of reactive oxygen species and nitrogen species in CRS type I and their implications in activating the inflammatory cascade. |
| Maruyama et al. [43] 2004 | Review | Inflammation and oxidative stress in ESRD-the role of myeloperoxidase | Inflammation increases cardiovascular risk in end-stage renal disease. Furthermore, MPO associated oxidative stress is a significant risk factor for vascular dysfunction. |
Lullo and companions drew attention to significant elevations in ROS, RNS, IL-6 levels, and MPO, which generates ROS through hydrogen peroxide and nitrogen dioxide [2,42,43]. This hypothesis was endorsed by Virzì et al. when they observed higher MPO levels, an enzyme present in neutrophil granules, in CRS type I plasma [39]. Their findings also confirmed a strong correlation between oxidative stress, immune response, IL-6, and IL-18 [39].
Virzì et al. [42], organized another in vitro study and presented five fundamental points on oxidative stress: 1. There was a dramatic rise in ROS and RNS species in CRS type I plasma compared to acute heart failure patients, 2. Hydrogen peroxide and nitric oxide augment IL-6 levels in CRS type I patients, 3. In CRS type I, inflammation reduces the metabolism of free fatty acids decreasing myocardial ATP levels, increasing glycolysis, and enhancing ROS, 4. ROS disturbs cardiomyocyte contractility, calcium processing, and ion transport affecting cardiac and renal function, 5. Diabetes was a strong risk factor for oxidative stress as hyperglycemia substitutes other pathways for glucose metabolism.
Furthermore, Bongartz stressed that oxidative stress provokes IL-6, IL-1, and TNF-alpha, and hinders renal compensatory mechanisms [16]. Another take on ROS generation is iron, and McCullough put forward this initiative in his review. He rationalized that unstable iron converts into hydroxyl radicals, which result in cellular dysfunction and apoptosis [13]. All these studies on oxidative stress corroborate the theory inflammation has a spectrum of molecular manifestations that depress heart and kidney function in CRS type I. These disturbances clinically deteriorate patients making them refractory to treatment.
Biomarkers
In chronic diseases, biomarkers are crucial elements. Their rising levels indicate occult pathologies before apparent evidence of symptoms. In CRS, these markers indicate heart and renal dysfunction and anticipate renal injury in cardiac disease [44]. Additionally, they predict prognosis, assess treatment efficacy, and aid in treatment strategy [44]. Inflammatory biomarkers are the earliest markers to spike; however, clinicians do not prefer to utilize them. Table 4 mentions studies about the diagnostic purpose of inflammatory biomarkers in CRS type I.
Table 4. Studies manifesting evidence of biomarker contribution in cardiorenal syndrome (CRS) type I diagnosis.
| AUTHOR & YEAR OF PUBLICATION | TYPE OF STUDY & # OF PATIENTS | PURPOSE OF STUDY | CONCLUSION |
| Ronco et al. [6] 2009 | Review | Cardio-Renal Syndromes: a report from the consensus of the Acute Dialysis Quality Initiative | To define the epidemiology, biomarkers, preventive strategies that can direct future research, including clinical trials. |
| McCullough [13] 2011 | Review | Cardiorenal syndrome: pathophysiology to prevention | Importance of catalytic iron in organ injury and other biomarkers for diagnosis, treatment, and prognosis. |
| Virzì et al. [39] 2018 | In vitro study, 53 patients | Levels of Pro-inflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without CRS type I | High levels of Pro-inflammatory (IL-6, IL-18) cytokines, oxidative stress, and biomarkers are the crux of CRS type I pathophysiology. |
| Fu et al. [44] 2018 | Review | Biomarkers in Cardiorenal Syndromes | To identify the importance of various biomarkers in renal injury caused by cardiac dysfunction. |
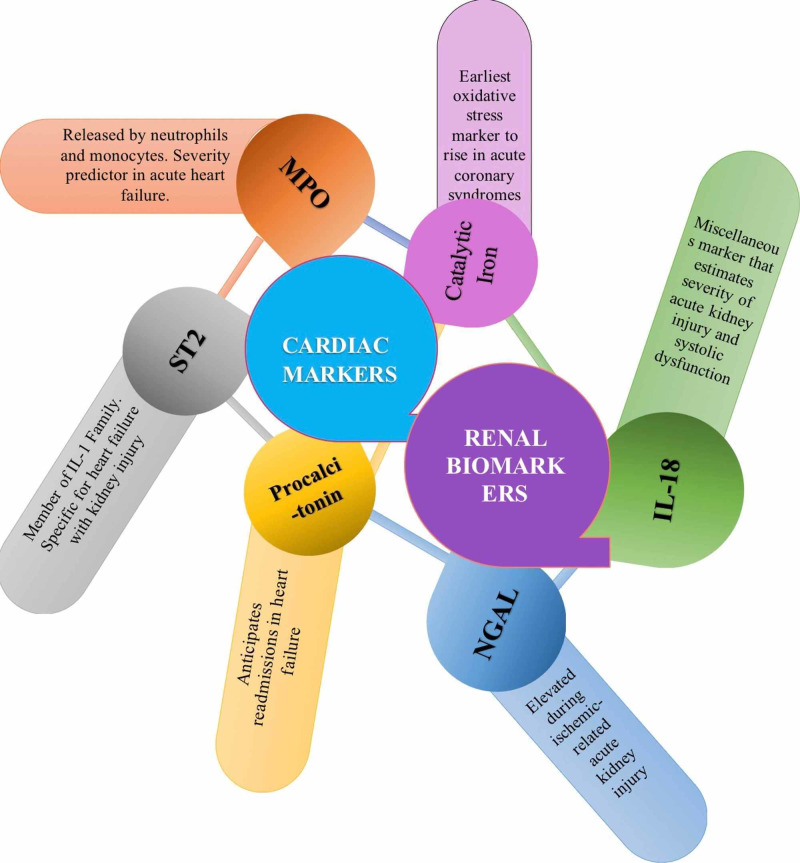
Fu et al. [44], enlightened biomarkers function in their review. They focused on inflammatory and non-inflammatory markers, but for this review, we shall solely enumerate on inflammatory markers in CRS, as seen in Figure 5. Cardiac inflammatory markers include: 1. MPO- released by neutrophils and monocytes, predicts disease severity in acute heart failure and kidney disease, 2. Suppression of tumorigenicity 2 (ST2)- a member of the IL-1 family, is specific for the prognosis of heart failure with kidney malfunction, 3. Procalcitonin- usually elevated after bacterial infection, but also assess for readmissions in heart failure. Renal inflammatory biomarkers include: 1. Neutrophil gelatinase-associated lipocalin (NGAL)- increases in ischemic AKI and anticipates worsening renal failure, 2. IL-18- a marker of blood vessel stiffness that exacerbates kidney injury and foresees mortality in systolic dysfunction. Figure 5 represents an overview of cardiac and renal inflammatory markers.
Figure 5. Inflammatory cardiac and renal biomarkers during acute heart failure and acute kidney injury (AKI).
MPO- myeloperoxidase, NGAL- neutrophil gelatinase-associated lipocalin, IL- interleukin, ST2- suppression of tumorigenicity 2
Moreover, McCullough elaborated on NGAL, IL-18, and catalytic iron in CRS [13]. As Figure 5 also demonstrates, catalytic iron is the root of the lethal hydroxyl radical and rises before serum troponin in acute coronary syndromes. NGAL or siderocalin, the first renal marker to be detected in blood and urine after AKI, is a scavenger of catalytic iron limiting oxidative stress. IL-18 increases 48 hours before creatinine and has an area under the operating curve of >90% for sensitivity and specificity in ischemic AKI. Virzì and assistants in their in vitro study emphasized the importance of high NGAL levels in CRS type I patients on the third day of admission [39]. Similarly, Ronco et al. prioritized NGAL because one urinary NGAL measurement in AKI patients renders a sensitivity of 90% and specificity of 99% [6].
In the medical literature, most inflammatory markers like C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) are non-specific markers of inflammation in chronic diseases. Nonetheless, these studies stress on inflammatory biomarkers that specifically pertain to cardiac or renal damage. Compared to non-inflammatory markers, NGAL and IL-18’s statistics validate their accuracy in CRS.
Non-Immune Cells With Inflammatory Profile
In CRS type I, two cell lines succumb to immune attack and gradually change their course: endothelial cells (EC) and renal tubular epithelium. Endothelial cells are the medium between blood and tissues controlling vascular, inflammatory, and coagulation stability [32]. Vascular dysfunction emanates with endothelial remodeling that affects their function and the organs they serve. On the other hand, renal tubular epithelium, delicate tissue of the kidney, has different coping mechanisms that feed-forward into the inflammatory response and subsequently loses its structure and function [45]. Table 5 documents evidence on the perpetual vicious circle created by inflammation using endothelium and renal tubular epithelium.
Table 5. Studies validating additional immune responses from the endothelium and renal tubular epithelium in cardiorenal syndrome (CRS) type I.
| AUTHOR & YEAR OF PUBLICATION | TYPE OF STUDY & # OF PATIENTS | PURPOSE OF STUDY | CONCLUSION |
| Clementi et al. [1] 2019 | Review | Neurohormonal, endocrine, immune dysregulation and inflammation mechanisms in CRS | To insinuate the need for novel drug therapies that cover new mechanisms in CRS. |
| Virzì et al. [18] 2014 | Review | Heart--kidney cross-talk and role of humoral signaling in critical illness | Damaged cardiac myocytes and renal tubular epithelium promote activation of innate and adaptive immune systems strengthening the humoral response. |
| Colombo et al. [20] 2012 | Review | Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with cardiorenal syndrome | To highlight the sustained inflammatory response that is responsible for the functional deterioration of patients with CRS. Existing anti-inflammatory treatment methods have been disappointing to date necessitating new studies. |
| Virzì et al. [32] 2018 | Review | The role of dendritic and endothelial cells in CRS | Heart and kidney Dendritic cells are involved in tissue remodeling. Additionally, endothelial cells act as antigen-presenting cells and act as a bridge between innate and adaptive immune systems. |
| Bonventre [45] 2003 | Review | Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. | The renal epithelium can regenerate and replace dead tubular cells after ischemic-repercussion injury. |
| Laxmanan et al. [46] 2005 | In vitro study | CD40: a mediator of pro- and anti-inflammatory signals in renal tubular epithelial cells. | CD40 serves a dual purpose, promoting inflammatory ROS and anti-inflammatory heme oxygenase-1. Finding the balance between these two effects has a therapeutic purpose in inflammatory renal disease. |
Clementi and colleagues, postulated endothelial transformation effects on the heart and kidney. In AKI, endothelial injury boosts inflammation, thrombosis, and vasoconstriction while in heart failure, it influences afterload and preload [1]. Virzì et al., in their review, analyzed the cytokine-induced accumulation of MHC class I and adhesion molecules- ICAM-1, ICAM-2, VCAM-1, E-selectin, P-selectin on ECs that function as unconventional APCs [32]. Colombo and companions further clarified TNF-alpha and IL-1 beta as the primary cytokines that actuate cell adhesion molecules on endothelium and also diminish nitric oxide-mediated vasodilation [20]. Vasoconstriction compromises hemodynamics of the heart and kidneys, worsening venous congestion that stimulates endothelium to produce additional cytokines [20].
On the contrary, tubular epithelium alters cell surface molecules and stimulatory pathways to withstand inflammatory stress. As noted by Virzì et al., endothelial permeability opens the gate for immune cell invasion into the renal interstitium, causing epigenetic and DNA changes [18]. As manifested by Laxmanan et al. in their in vitro study, epigenetic alterations encourage the cells to express more inflammatory receptors like CD40/CD40-ligand [46]. Inflammation recruits other cells into the immune response, thereby creating a vicious circle. These recent studies prop up the feed-forward mechanism, magnifying the anomalous type I cardiorenal cross-talk.
We were not able to find any randomized controlled trials and observational studies relevant to this topic. Therefore, we could not establish causal relationships delineating risk factors and inflammation-related incidence and prevalence. General questions that could guide future research include: 1. Could the combination testing of inflammatory biomarkers confirm the dominance of inflammation? 2. Can these markers also be used to categorize the severity profile of CRS type I patients? 3. Does the inflammatory remodeling of cardiac and renal parenchyma guide future research to define diagnostic and therapeutic protocols? 4. Should patients with cardiac abnormalities have a regular checkup of inflammatory markers? 5. What is the specific treatment for CRS type I? 6. What are the treatment options to control the inflammatory process in CRS type I patients?
Limitations
Unfortunately, most of the evidence in this paper comes from other reviews, animal studies, and in vitro studies from patients in Italy. There were few reviews from the United States, but the data for in vitro studies came from CRS type I patients in Italy. Pertinent data from in vitro studies is inapplicable to clinical practice due to limited patients and high bias. We formulated only mere associations about inflammatory control on CRS type I pathology. Another limitation of this paper is the lack of human studies to establish certain prognostic factors like NGAL, ST2, and IL-18 as risk factors in CRS type I patients.
Conclusions
CRS type I challenges clinicians and complicates patient recovery. Neurohormonal mechanisms have been the interest of diagnosis and treatment in clinical care, but the disease’s mortality and morbidity are sorely high, indicating a hidden medium playing along. Inflammation governs the hemodynamics, end-organ dysfunction, and severity of CRS type I. AKI is a dreadful aspect of this disease extending hospitalization periods, cost of care, and risk of adverse events.
A hyperactive immune system sabotages cardiorenal cross-talk and sets the ground for injurious cellular remodeling. In CRS type I, inflammation facilitates this damage through its components, altering homeostasis and metabolism, breaking barriers, and disintegrating communication. Since inflammation can modulate heart and kidneys’ identity, a swift change in the diagnostic and therapeutic approach of CRS type I is necessary. In summary, the high prevalence of this syndrome entails future human clinical trials not only to catch the disease at an appropriate time but also to discover new treatment options to curb inflammation.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Neurohormonal, endocrine, and immune dysregulation and inflammation in cardiorenal syndrome. Clementi A, Virzì GM, Battaglia GG, Ronco C. Cardiorenal Med. 2019;9:265–273. doi: 10.1159/000500715. [DOI] [PubMed] [Google Scholar]
- 2.Pathophysiology of the cardio-renal syndromes types 1-5: an uptodate. Di Lullo L, Bellasi A, Barbera V, et al. Indian Heart J. 2017;69:255–265. doi: 10.1016/j.ihj.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Haase M, Müller C, Damman K, Murray PT, Kellum JA, Ronco C, McCullough PA. Contrib Nephrol. 2013;182:99–116. doi: 10.1159/000349969. [DOI] [PubMed] [Google Scholar]
- 4.Cardiorenal syndrome type 1: pathophysiological cross-talk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. Ronco C, Cicoira M, McCullough PA. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 5.The hemodynamic and non-hemodynamic cross-talk in cardiorenal syndrome type 1. Virzì GM, Clementi A, Brocca A, de Cal M, Vescovo G, Granata A, Ronco C. Cardiorenal Med. 2014;4:103–112. doi: 10.1159/000362650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acute Dialysis Quality Initiative (ADQI) Consensus Group Cardiorenal syndromes: an executive summary from the Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Ronco C, McCullough PA, Anker SD, et al. Contrib Nephrol. 2010;165:54–67. doi: 10.1159/000313745. [DOI] [PubMed] [Google Scholar]
- 7.Acute kidney injury and outcomes in acute decompensated heart failure: evaluation of the RIFLE criteria in an acutely ill heart failure population. Hata N, Yokoyama S, Shinada T, et al. Eur J Heart Fail. 2010;12:32–37. doi: 10.1093/eurjhf/hfp169. [DOI] [PubMed] [Google Scholar]
- 8.Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Cruz DN. Adv Chronic Kidney Dis. 2013;20:56–66. doi: 10.1053/j.ackd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Metra M, Nodari S, Parrinello G, et al. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Cardiorenal interactions: insights from the ESCAPE trial. Nohria A, Hasselblad V, Stebbins A, et al. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. Forman DE, Butler J, Wang Y, et al. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Plasma cytokine levels predict mortality in patients with acute renal failure. Simmons EM, Himmelfarb J, Sezer MT, et al. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Cardiorenal syndromes: pathophysiology to prevention. McCullough PA. Int J Nephrol. 2010;2011:762590. doi: 10.4061/2011/762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Adams KF Jr, Fonarow GC, Emerman CL, et al. Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Newsome BB, Warnock DG, McClellan WM, et al. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 16.The severe cardiorenal syndrome: ‘Guyton revisited’. Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 17.Preservation of glomerular filtration rate in human heart failure by activation of the renin-angiotensin system. Packer M, Lee WH, Kessler PD. Circulation. 1986;74:766–774. doi: 10.1161/01.cir.74.4.766. [DOI] [PubMed] [Google Scholar]
- 18.Heart-kidney crosstalk and role of humoral signaling in critical illness. Virzì G, Day S, de Cal M, Vescovo G, Ronco C. Crit Care. 2014;18:201. doi: 10.1186/cc13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organ cross-talk: the role of the kidney. Li X, Hassoun HT, Santora R, Rabb H. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 20.Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Colombo PC, Ganda A, Lin J, et al. Heart Fail Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mechanisms of pulmonary edema clearance. Gokhan MM, Sznajder JI. Am J Physiol Lung Cell Mol Physiol. 2005;289:0. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 22.Fluid overload in acute heart failure: re-distribution and other mechanisms beyond fluid accumulation. Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 23.New targets for heart-failure therapy: endothelin, inflammatory cytokines, and oxidative stress. Givertz MM, Colucci WS. Lancet. 1998;352:0. doi: 10.1016/s0140-6736(98)90017-4. [DOI] [PubMed] [Google Scholar]
- 24.Cardio-renal syndrome type 3: epidemiology, pathophysiology, and treatment. Chuasuwan A, Kellum JA. Semin Nephrol. 2012;32:31–39. doi: 10.1016/j.semnephrol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Toll-like receptors: linking innate and adaptive immunity. Pasare C, Medzhitov R. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Toll-like receptors in cardiovascular diseases. De Kleijn D, Pasterkamp G. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- 27.Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Chao W. Am J Physiol Heart Circ Physiol. 2009;296:0. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hypertension augments cardiac Toll-like receptor 4 expression and activity. Eissler R, Schmaderer C, Rusai K, et al. Hypertens Res. 2011;34:551–558. doi: 10.1038/hr.2010.270. [DOI] [PubMed] [Google Scholar]
- 29.Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. Allam R, Scherbaum CR, Darisipudi MN, et al. J Am Soc Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inflammation in acute kidney injury. Kinsey GR, Li L, Okusa MD. Nephron Exp Nephrol. 2008;109:0. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 32.The role of dendritic and endothelial cells in cardiorenal syndrome. Virzì GM, Zhang J, Nalesso F, Ronco C, McCullough PA. Cardiorenal Med. 2018;8:92–104. doi: 10.1159/000485937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Interstitial dendritic cells of the rat heart. Quantitative and ultrastructural changes in experimental myocardial infarction. Zhang J, Yu ZX, Fujita S, Yamaguchi ML, Ferrans VJ. Circulation. 1993;87:909–920. doi: 10.1161/01.cir.87.3.909. [DOI] [PubMed] [Google Scholar]
- 34.Differential effects of GM-CSF and G-CSF on infiltration of dendritic cells during early left ventricular remodeling after myocardial infarction. Naito K, Anzai T, Sugano Y, et al. J Immunol. 2008;181:5691–5701. doi: 10.4049/jimmunol.181.8.5691. [DOI] [PubMed] [Google Scholar]
- 35.The immunological axis in heart failure: importance of the leukocyte differential. Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, Gheorghiade M. Heart Fail Rev. 2013;18:835–845. doi: 10.1007/s10741-012-9352-9. [DOI] [PubMed] [Google Scholar]
- 36.The role of monocytes and inflammation in the pathophysiology of heart failure. Wrigley BJ, Lip GY, Shantsila E. Eur J Heart Fail. 2011;13:1161–1171. doi: 10.1093/eurjhf/hfr122. [DOI] [PubMed] [Google Scholar]
- 37.Immune modulation: role of the inflammatory cytokine cascade in the failing human heart. Satoh M, Minami Y, Takahashi Y, Nakamura M. Curr Heart Fail Rep. 2008;5:69–74. doi: 10.1007/s11897-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 38.Cardiorenal syndrome type 1: a defective regulation of monocyte apoptosis induced by pro-inflammatory and proapoptotic factors. Pastori S, Virzì GM, Brocca A, de Cal M, Clementi A, Vescovo G, Ronco C. Cardiorenal Med. 2015;5:105–115. doi: 10.1159/000371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levels of proinflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without cardiorenal syndrome type 1. Virzì GM, Breglia A, Brocca A, de Cal M, Bolin C, Vescovo G, Ronco C. Cardiorenal Med. 2018;8:321–331. doi: 10.1159/000492602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardiorenal syndrome type 1 may be immunologically mediated: a pilot evaluation of monocyte apoptosis. Virzì GM, Torregrossa R, Cruz DN, et al. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardiorenal syndrome type 1: activation of dual apoptotic pathways. Pastori S, Virzì GM, Brocca A, et al. Cardiorenal Med. 2015;5:306–315. doi: 10.1159/000438831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oxidative stress: dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Virzì GM, Clementi A, de Cal M, et al. Oxid Med Cell Longev. 2015;2015:391790. doi: 10.1155/2015/391790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inflammation and oxidative stress in ESRD-the role of myeloperoxidase. Maruyama Y, Lindholm B, Stenvinkel P. http://pubmed.ncbi.nlm.nih.gov/15599890/ J Nephrol. 2004;17:0. [PubMed] [Google Scholar]
- 44.Biomarkers in cardiorenal syndromes. Tan K, Sethi SK. Transl Res. 2014;164:122–134. doi: 10.1016/j.trsl.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. Bonventre JV. J Am Soc Nephrol. 2003;14:0. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 46.CD40: a mediator of pro- and anti-inflammatory signals in renal tubular epithelial cells. Laxmanan S, Datta D, Geehan C, Briscoe DM, Pal S. J Am Soc Nephrol. 2005;16:2714–2723. doi: 10.1681/ASN.2005010045. [DOI] [PubMed] [Google Scholar]