Discovery of how sleep function shifts from neural reorganization to repair by combining mathematical theory and data analysis.
Abstract
Sleep serves disparate functions, most notably neural repair, metabolite clearance and circuit reorganization. Yet the relative importance remains hotly debated. Here, we create a novel mechanistic framework for understanding and predicting how sleep changes during ontogeny and across phylogeny. We use this theory to quantitatively distinguish between sleep used for neural reorganization versus repair. Our findings reveal an abrupt transition, between 2 and 3 years of age in humans. Specifically, our results show that differences in sleep across phylogeny and during late ontogeny (after 2 or 3 years in humans) are primarily due to sleep functioning for repair or clearance, while changes in sleep during early ontogeny (before 2 or 3 years) primarily support neural reorganization and learning. Moreover, our analysis shows that neuroplastic reorganization occurs primarily in REM sleep but not in NREM. This developmental transition suggests a complex interplay between developmental and evolutionary constraints on sleep.
INTRODUCTION
The pervasiveness of sleep during development and throughout the animal kingdom suggests that it is a biological process that is necessary for survival. Although we spend approximately a third of our life asleep, its explicit physiological and evolutionary function remains unclear with myriad hypotheses being postulated. Two of the leading hypotheses are that sleep enables (i) the repair and clearance needed to correct and prevent neuronal damage (1–7) and (ii) the neural reorganization necessary for learning and synaptic homeostasis (8–13). These hypotheses are compelling because neither of these processes can be easily achieved in waking states, and there is supporting empirical evidence that they occur during sleep.
For instance, prolonged sleep deprivation can lead to death in rats (14), dogs (15), fruit flies (16), and even humans (17). These extreme cases are believed to result from damage to neuronal cells, caused by metabolic processes, which would normally be remedied by the clearance of damaging agents and repair that occurs primarily during sleep (2, 5). Moreover, a recent hypothesis related to neuronal damage from metabolic processes is that sleep drives metabolic clearance from the brain (2). Because the brain lacks a penetrating lymphatic system, cerebrospinal fluid recirculates through the brain and removes interstitial proteins, likely through meningeal lymphatics (18). Furthermore, the concentration of β-amyloid (Aβ) is higher in the awake state than during sleep, suggesting that wakefulness is associated with producing (Aβ) (3), while sleep is associated with its clearance. This view is further supported by the 60% increase in interstitial space associated with sleep (2).
There is also substantial and direct evidence that sleep promotes neuroplastic reorganization related to learning and consolidating memory and also regulates synaptic rescaling. For instance, neuron firing sequences that encode spatial maps learned during awake periods are replayed during sleep (11, 19–21). In addition, sleep facilitates the growth of learning-associated synapses and the homeostatic weakening and pruning of seldom-used synapses (12). More generally, two recent studies demonstrate that sleep regulates the cycling of proteins related to synaptic functioning (22, 23).
Comparative, developmental, physiological, and human studies have all been fruitfully used to address questions about the nature of sleep. However, because data are seldom analyzed in a way that connects them with mathematical models or quantitative predictions, conclusions about the function of sleep have remained slow to evolve. In this context, we develop a general theory for the function of sleep that provides a framework for addressing several fundamental questions, such as what are the relative roles of repair and reorganization during sleep? And do these change during ontogenetic development?
An important quantitative observation is that sleep times systematically decrease with body mass across mammals (1, 24). Moreover, the fraction of time spent in rapid eye movement (REM) sleep (also referred to as active sleep) does not change with brain or body mass (24). Since increasing body mass strongly correlates with decreasing mass-specific metabolic rate (i.e., metabolic rate per unit mass) and, therefore, to a decreasing rate of cellular damage, this strongly suggests that less sleep time is needed for repair and maintenance in larger animals. These empirical observations led two of us (24) to develop a quantitative mechanistic theory for understanding the origins and function of sleep across species based on the central role played by metabolism in both damage and repair. This work suggested novel analyses of the empirical data on brain size and brain metabolic rate, both of which depend nonlinearly on body size [with an exponent of approximately 3/4; (24)], and showed that both brain size and brain metabolic rate are better predictors of sleep time than body size. This provided strong evidence that sleep is primarily associated with repair of the brain rather than with the other parts of the body. Specifically, we predicted that the ratio of sleep time to awake time should decrease with brain size as a power law whose exponent is and, consequently, that it should decrease with body weight with an exponent of , both in good agreement with data. The scaling exponent of for brain size corresponds to the same scaling as mass-specific metabolic rate in the brain. The theory also provides a quantitative understanding for why the proportion of REM sleep does not change with either brain or body mass.
The major focus of this paper is to address the intriguing question as to whether these general relationships for sleep times remain valid during growth, implying that ontogeny recapitulates phylogeny, or whether new patterns emerge during development, reflecting a different dynamical origin for sleep. More pointedly, during both development and across species, sleep times systematically decrease with brain and body size. But do they do so at similar rates? And are they attributable to the same underlying dynamics? If new patterns emerge, what do those patterns reveal about neurological development and the growth of the brain? To answer these questions, we derive a quantitative ontogenetic sleep model across species that combines both ontogeny and phylogeny in a single framework and use this model to guide the analysis of human sleep data from birth to adult. We compare our new findings with previous empirical and theoretical results for how sleep changes across phylogeny to ask whether explanations for why a mouse sleeps roughly five times more than a whale can also be used to explain why babies sleep roughly twice as long as adults.
Although previous studies focus on total sleep time, how it is partitioned between REM and non-rapid eye movement (NREM), and how these change during growth (25, 26), we are unaware of any systematic quantitative mechanistic models for how or why these change as children grow. Here, we combine the most comprehensive published data on sleep throughout human development and across species with a new mechanistic model to elucidate the function of sleep, reveal how it markedly changes during early growth, and show how this is related to brain development.
In the following section, we develop a framework for modeling neural repair/metabolite clearance and reorganization during sleep and show how the brain metabolic rate depends on the number of synaptic connections between neurons. Moreover, we propose a general quantitative model for how sleep time changes with brain mass ontogenetically. Next, we describe the sources and collection of our data and the statistical and numerical methods for how the data were analyzed. We collate and integrate data for total sleep time, REM sleep time, brain weight, body weight, cerebral metabolic rate, and synaptic density based on a systematic review of the literature. The resulting dataset spans from 0 to 15 years of age and cumulatively represents about 400 data points. We then use the empirical data to find patterns of sleep during ontogeny, compare them with phylogenetic patterns, and test predictions from our theoretical framework. In so doing, we:
1) develop distinct quantitative theories for both neural repair/clearance and neural reorganization;
2) use extensive human sleep and brain data from birth to adult to cleanly test and discriminate between these theories;
3) provide strong evidence of a remarkably sharp transition at about 2.4 years of age in the primary purpose of sleep from being for neural reorganization that occurs during the active sleep/REM cycle in early development to neural repair and metabolite clearance in late development.
Last, we explain our conclusions and discuss remaining questions and future directions.
Framework for predicting sleep times and testing sleep functions
Our conceptual, quantitative framework for how sleep changes as brains increase in size and age through development is grounded in key hypotheses about the dominant function of sleep being for neural repair/clearance and/or reorganization. We explain simple mathematical equivalencies that lead to specific, baseline predictions for scaling exponents that encapsulate how ratios of REM, NREM, and total sleep times change with brain size.
Theory of sleep for neural repair
We previously constructed a mathematical theory that focused on neural repair in adult brains and empirically tested a suite of predictions for how characteristic times for sleep change with brain and body size across species (24). The theory, which we first briefly review, has been supported by experiments and results over the past several years (2). It has long been postulated, and there is increasing empirical and theoretical evidence favoring it, that neural repair or clearance of metabolic wastes is an important function of sleep. One theory, for instance, suggests that sleep plays the role of regulating oxidative stress in the brain by restoring and repairing neurons damaged by this oxidative stress (7). It has also been found that the production of oxidating agents in the brain during awake time promotes sleep (27).
The basis of our theory is that the total amount of damage incurred and/or the accumulation of damaging agents during wakefulness must be reversed or counteracted by repair during sleep. Unlike other organs, it is crucial for the continuing functionality of the entire organism for the neurological damage to be faithfully repaired. The total damage that is generated during awake time is proportional to the mass-specific metabolic rate of the brain, Bb (effectively, the average metabolic rate of a cell), multiplied by the total time awake, tA. To counteract this, the total amount of repair or clearance accomplished during sleep is the power density, PR, allocated to repair or clearance during sleep multiplied by the total brain volume Vb( ∝ Mb) and total sleep time, tS. Assuming that nearly all damage must be repaired or cleared in order for the brain to continue to function normally and with high fidelity throughout growth and adulthood, the total damage or accumulation of damaging agents must be balanced by the total repair or clearance. This leads to
| (1) |
where c is a constant that incorporates the efficiency of repair processes together with the fraction of metabolic rate that leads to damage via free radicals, metabolic waste, or vessel damage. PR is a local, cellular quantity and is assumed to be independent of body or brain size. Consequently, the predicted scaling exponent for sleep times is completely determined by the scaling of brain metabolic rate, , and therefore by its scaling exponent, α. For simplicity, we have here also assumed that all damage or accumulation of damaging agents occurs during wakefulness and that all repair and clearance occurs during sleep. This theory can straightforwardly be generalized to include damage during sleep and thereby to show that the dominant scaling relationships do not change. This leads to an estimate that damage rates during sleep are about 1/3 of those during wakefulness (24).
Equation 1 predicts that the ratio of sleep time to awake time follows a simple power law relationship, which is well supported below by data. The theory predicts that sleep time, tS, by itself does not obey pure power law behavior with respect to brain size. Rather, it is the ratios of sleep to awake times or REM times that do (section S3). Because of this functional form, traditional plots in the literature for either tS or ln (tS) versus ln (Mb) are predicted to have much greater variance than for corresponding ratios and to be much more difficult to interpret.
Another key prediction of this theory based on neural repair is the invariance of the fraction of REM sleep
| (2) |
This pattern strongly holds across species (24). Consequently, testing whether it remains valid during development will help reveal whether sleep during growth is primarily driven by neural repair or by some other function such as neural reorganization.
Theory of sleep for neural reorganization
During early development the brain is undergoing extensive changes in size, architecture, and cellular makeup. One of the major changes is in synaptic plasticity, which is greatest during early development after which it declines to a baseline adult level (28). This corresponds to neural reorganization, somatocortical pathway development, and pruning that underlie the experience-dependent plasticity and learning necessary for adult behavior (29). Sleep is required for the consolidation and optimization of learning and governs underlying synaptic processes including synapse formation, sizing, and pruning (12, 13).
We now develop the theory for those aspects of neural reorganization related to sleep by focusing on the fundamental need to process information. Analogous to the theory for repair, the basis of the theory is an accounting and balancing of the rate of information being sensed by the body with the rate at which it is being processed by the brain. A key component of this model is that the amount of information needed to be processed is sensed through the entire body because stimuli are received from all parts of the body via pain, heat, cold, pressure, etc. On the other hand, the number of inputs that can be processed is constrained by the brain and its metabolic rate, Bb. The brain metabolic rate sets the pace for synaptic changes that incorporate the information collected by the peripheral nervous system. This crucial insight that information input is associated with the entire body, whereas its processing is localized in the brain, leads to a mismatch in the scaling of all of the sleep processes because brain size and brain metabolic rate scale nonlinearly and differently with both body size and whole-body metabolic rate, B (section S4) (30).
A core question is whether synaptic plasticity and information processing are occurring during just one or both of the two main stages of sleep: NREM and REM. Studies show that both are important for learning and memory, although their relative roles remain a topic of intense debate (31–34). Evidence suggests that REM may be more associated with local circuit changes reflecting memory consolidation, while during NREM, global synaptic homeostasis and inter-region memory transfer may dominate (13, 34). Other evidence (33, 35, 36) suggests that synaptic pruning and reconnection primarily take place during REM sleep, while other results and arguments have posited that NREM sleep is when pruning and reorganizing occur (31).
Given this controversy, we derive separate predictions assuming either the primacy of REM or NREM sleep for neural reorganization. Consequently, our theory provides a quantitative test and a means for distinguishing between these two opposing hypotheses for the importance of REM versus NREM sleep by analyzing developmental sleep data. For simplicity, we present our equations in terms of REM sleep, since the NREM predictions are obtained by simply swapping NREM for REM everywhere in the following equations.
Assuming (i) that local neural reorganization associated with changes in synaptic density primarily occurs during REM sleep, (ii) that idealized synaptogenesis occurs uniformly across the brain, and (iii) that information exchange is directly tied to energy use, we relate the amount of information sensed by the body during wakefulness when the organism is being exposed to myriad stimuli to the amount being processed by the brain during sleep.
Defining ΔEI→σ as the energy needed to convert a unit of information acquired by sensory systems to synaptic changes in the brain, and fI as the fraction of the total metabolic rate required for sensing that information, then information is being transmitted to the brain at a rate given by (fIB)/ΔEI→σ. Consequently, the total amount of information generated while awake is proportional to (fIBtA)/ΔEI→σ.
This information has to be processed during sleep by synapses (37). On average, each synapse processes information at a rate ν that, like all processes directly linked to brain metabolism, is expected to scale inversely with its mass-specific metabolic rate Bb/Mb, that is, inversely with cellular metabolic rate (24). Assuming first that local synaptic changes occur during REM sleep, tR, the total information processed is NσtRν ∝ NσBbtR/Mb, where Nσ is the total number of synapses in the brain. We neglect terms related to synapses being formed and pruned within that same sleep-wake cycle because this number will be very small over such a relatively short time scale. Last, equating the information processed during sleep with information sensed while awake and rearranging terms, we have
| (3) |
To determine how this ratio scales with brain and body size, we first recognize that the parameters fI and ΔEI→σ represent energies and percentages that are typically invariant with respect to size, in contrast to the scaling of biological rates and times (38). To express our result in terms of brain size, Mb, we note that across species (24) and throughout development (fig. S1), brain size scales nonlinearly with body size as approximately Mb ∝ M3/4. Combining this with the canonical allometric relationship for whole-body metabolic rate, B ∝ M3/4 (valid through ontogeny), gives B ∝ Mb. In the following section, we further argue that Nσ ∝ Bb, thereby predicting the scaling of the ratio of REM sleep time to awake time
| (4) |
where α is the scaling exponent that relates brain metabolic rate to brain size. This can be reexpressed in terms of the ratio of REM sleep time to total sleep time [which is invariant across species (24)]
| (5) |
Thus, an empirical determination of how the ratio tA/tS scales with brain size provides a prediction for the fraction of time spent in REM sleep across development. As we shall show below, these relationships provide a good description of the data and an important test of the theory. Furthermore, by simply switching NREM sleep time, tNR, with REM sleep time, tR, in the above equations, we also have the prediction for the complementary case that assumes the primacy of NREM sleep for neural reorganization and information processing. This will be markedly different and distinguishable from the predictions for REM sleep, hence providing a clear indication for when these processes occur during the sleep cycle.
Developmental changes in cerebral metabolic rate, synapses, and white matter
The theory for neural repair and reorganization developed above is fundamentally driven by the brain’s metabolic rate. To make the scaling relationships for sleep fully predictive, the only remaining unknown is the scaling exponent, α, that relates brain metabolic rate to brain size. Across species, the brain can be treated as a nearly autonomous organ with its own vascular system supplied primarily by a single carotid artery, much in the same way that the vascular system of the entire body is supplied through a single aorta. Following the theoretical derivation of the scaling relationship of metabolic rate for the whole body, this predicts , consistent with data for mature mammals (24).
However, during early ontogeny, the brain is undergoing rapid changes in size and synaptogenesis that require a relaxation of the power optimization and other constraints that determine how metabolic rate scales with size for mature organisms. Therefore, the canonical theory for the scaling of metabolic rate needs to be reformulated for the brain to recognize that ontogeny may not recapitulate phylogeny. Neural signaling and computation in the brain are extremely costly, accounting for 80 to 90% of its metabolic expenditure (39, 40). These signals and computations are implemented through patterns of neuronal synaptic connectivity. It is therefore natural to focus on the number of synapses as a major driver of brain metabolic rate rather than the number of neurons (28). The primary function of these connections is to regulate electrical signals through axons that transmit information through neural networks to gather and process information to learn and react (41). Crucially, as the number of synapses quickly increases in early development, they bring along associated increases in glial cells that are also highly metabolically active. Following this initial increase in the number of synapses, they are subsequently pruned away as part of the process of learning and reorganization in a way that is consistent with the Hebbian maxim that neurons that fire together, wire together.
Consequently, cerebral metabolic rate at early developmental stages is proportional to the total number of synapses already present plus the rate at which energy needs to be supplied to grow new ones. This is consistent with prior work showing the invariance of cerebral metabolic rate per synapse across development for mammals. Since most neurons in the adult brain are already present at or soon after birth, with only an extremely slow increase in their number during development and adulthood (42), the metabolic rate devoted to existing synapses at any given time is much greater than that needed to create new ones.
The increase in the number of synapses after birth largely represents the wiring together of preexisting neurons, further emphasizing the dependence of changes in metabolic rate on synapse number rather than neuron number.
As a result, we predict that the metabolic rate of the brain should scale approximately linearly with the total number of synapses or, equivalently, that its mass-specific metabolic rate should scale linearly with synaptic density. In addition, after birth, the increase in brain mass derives largely from the increase in glial cell and neuronal spine mass within gray matter and through the myelination of axons within white matter (43). The primary function of glial cells is to support synaptic activity, so increases in white matter are driven by increasing synaptic demand. Analogously, increases in myelination reflect the need for increased speed and bandwidth of axonal information transfer as the number of synapses per axon increases. Hence, we expect synapse number to scale approximately linearly with white matter volume, Vw
| (6) |
| (7) |
Previous studies across species have shown that the volume of white matter increases superlinearly (scaling exponent >1) with that of gray matter, Vg, across species (44). If a similar result holds during development, which we test below, this would predict superlinear scaling (α > 1) for brain metabolic rate with brain size. This result means that brain metabolic rate per gram of tissue or per cell is actually increasing during development, in marked contrast to all other scaling relationships between metabolic rate and brain or body size.
The reconciliation between such a superlinear scaling across development and a sublinear allometric scaling across species can be understood in two ways. First, in adults, the number of connections scales linearly with the number of neurons across species, representing a roughly constant adult synaptic density that is realized after pruning is complete (45). Second, for adults, the number of neurons in the brain scales nonlinearly and approximately as the 3/4 power with brain size across species (24).
Marked phase transition in sleep function related to brain development
As discussed below, a major transition in brain development (46, 47) and growth occurs around 2 to 3 years old in humans that is associated with the stabilization of synaptic growth and connectivity (28, 45, 48, 49). In our theory, sleep is inextricably linked with brain development, function, and metabolic rate. Consequently, we predict a sharp and marked transition in the scaling of sleep function at about this age. Such a transition reflects a fundamental change in brain development that occurs shortly after the peak in synaptic density when connectivity patterns in the brain begin to stabilize. At this point, sleep function shifts from being dominated by neural reorganization toward neural repair and maintenance. Because the fraction of REM sleep is predicted to change with size in the regime when neural reorganization dominates but be invariant for neural repair, we might expect a classic phase transition, analogous to when water freezes to ice. Mathematically, this would reveal itself as a discontinuity in the first derivative in the fit. Below, we present an analysis of the data to confirm this remarkable prediction and show that it occurs at about 2.4 years old. To our knowledge, this sharp transition in sleep function, its simultaneity with the transition in rates of synaptogenesis and synapse density, and the associated scaling behavior have neither been previously predicted nor documented. This is remarkable given that this shift likely signals a profound shift in the function of sleep and the behavior of sleep processes.
METHODS
Empirical data
To conduct empirical tests of the predictions of our models, we surveyed the literature for available data on sleep times, REM sleep times, brain size, brain metabolic rate, body size, body metabolic rate, and other relevant factors for humans during growth from birth to adulthood. Together, compiled data contain about 400 points, mostly corresponding to an age range of 0 to 15 years. Each type of data—sleep time, brain weight, and REM sleep time—has the same source and identical methods. That is, there is no difference between the source or measurement method for the data across age. The study of Galland et al. (25) contained 105 data points for sleep times of humans from ages 0 to 12 years. The study took data from multiple individuals and provided error bars on sleep times as the means ± 1.96 SDs to approximate 95% confidence intervals. Further data (40 data points) were obtained by Dekaban et al. (50) for brain weight for 0 to 20 year olds. Because we do not consider the effects of gender differences, we combine these data by calculating the mean of the female and male brain weights and body weights. Data for the percentage of REM sleep time across ontogeny and before 18 years old were found by Roffwarg et al. (26). In addition, sleeping metabolic rate (SMR) values from 0 to 1 year old are taken by Reichman et al. (51). They performed repeated measurements of SMR at 1.5, 3, 6, 9, and 12 months of age in 43 healthy infants. To better test for connections between white matter, synaptic densities, and cerebral metabolic rate, we also use ontogenetic data for cerebral metabolic rate (28 data points) and synaptic density (12 data points) for 0 to 15 years old by Feinberg et al. (28), as well as data for white matter and gray matter volume (52) from 0 to about 3 years old. Because numerical values or tabular data were rarely published for these studies, the software DataThief was used to collect data from graphs. Moreover, when plotting one dataset against another, the ages were not always completely aligned, so we used interpolation to obtain values at the same age for those cases. We want to emphasize that each data type—sleep time, brain weight, REM sleep time, and metabolic rate—is cited from a separate single source. However, some of the sources are themselves compilations of previous studies meaning even for a single data type, data may come from different groups and studies. See the original sources for full details. As explained further in the Discussion, these differences among groups and studies do not introduce any bias in the transition points we identify.
Data analysis
To illuminate patterns in these data, we test for relationships between sleep time, brain size, and metabolic rate in humans. More specifically, we analyze the data from these different sources by constructing plots, calculating correlations between variables, and measuring slopes and exponents to test whether empirical values match our theoretical predictions.
We focus our analysis on ages 0 to 12 years old because the data show that the relevant variables mostly stabilize after 12 years old. In doing our analysis, we note that this period itself can be split into two distinct regimes and discuss how the relative roles of repair/clearance and reorganization shift during this time. By dividing the data into two separate regimes, the logarithmic plots closely follow a straight line for each of these two regimes. Because biological and physical changes are typically continuous, we require that the line before and the line after the transition connect to each other in a continuous fashion. We first choose this intersection point (x0, y0), and we then use two lines y = k1(x − x0) + y0 and y = k2(x − x0) + y0 to fit the data. We determine the best value of k1 and k2, as well as the intersection point (x0, y0), through a minimization of the sum of the squared errors (SSEs) (see section S5).
RESULTS
Brain metabolic rate is fundamental to our theories of sleep for neural repair and for neural reorganization. We thus begin by analyzing our collected dataset to determine the scaling relationships between brain metabolic rate, total number of synapses, volume of white matter, and brain size (see Eq. 6). These will be used to test our predictions and determine the exponent, α, needed to complete the quantitative predictions for sleep times expressed in Eqs. 1 to 5. We divide the analysis of sleep data into two distinct sleep phases based on the predicted and statistically determined transition that occurs between 2 and 3 years of age. Figure 1 shows three plots that evaluate our main predictions for these quantities in the early development phase before this transition.
Fig. 1. Scaling of brain metabolism and connections with brain mass (kilogram) and age before transition.
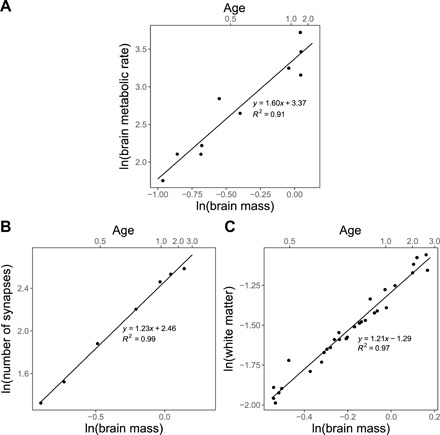
(A) Plot of the logarithm of cerebral metabolic rate versus the logarithm of brain mass before transition with measured slope of 1.60. (B) Plot of the logarithm of number of synapses versus the logarithm of brain mass before transition with measured slope of 1.23. (C) Plot of the logarithm of white matter volume versus the logarithm of brain mass before transition with measured slope of 1.21. Also, shown on the top horizontal axes is the corresponding age in years.
First, Fig. 1A shows a logarithmic plot of the brain’s SMR versus its mass (kilogram). This reveals a remarkable superlinear behavior with an exponent, α = 1.60 ± 0.40, confirming our prediction of superlinear scaling based on the scaling of white and gray matter. Most notably, it runs strongly counter to all the normal patterns of allometric scaling relationships across species that are invariably sublinear (i.e., with exponents of <1) (38). This results from the brain becoming increasingly energetically more costly during development and stands in marked contrast to the energetics of all other tissues and organs in the body where economies of scale dominate. In that case, the larger the organism (or organ), the less metabolic power is required per unit mass of tissue. Superlinear scaling, on the other hand, means exactly the opposite: The larger the organism (or, in this case, the brain), the more metabolic power required per unit mass of tissue or per cell.
Second, Fig. 1B shows a similar logarithmic plot for the number of synapses versus brain mass. The number of synapses is simply the product of synaptic density, ρσ—usually measured with respect to a local section of gray matter volume—and the volume of gray matter in the brain, Vg: Nσ = ρσVg (Fig. 1B and section S6) yields a scaling exponent of 1.23 ± 0.09, which is consistent with our prediction (Eq. 6), from the scaling of brain metabolic rate and with the scaling of white matter with gray matter across species.
Last, we evaluate our predictions based on a much more comprehensively measured property, namely, the volume of white matter as a function of brain mass. Figure 1C shows a plot for this relationship that reveals a scaling exponent of 1.21 ± 0.08, consistent with our predictions and the other two estimates of α.
These results show that the value of the superlinear exponent α is in the range from 1.20 to 1.60. We now use this in Eqs. 1 to 5 to predict how sleep time ratios, such as tS/tA and tR/tS, scale with brain and body size. Recall that the predictions depend on whether sleep function is dominated by neural repair or neural reorganization. If it is based on neural repair, Eq. 1 predicts that tS/tA scales with an exponent between 0.20 and 0.60 and that the fraction of REM sleep tR/tS is invariant with respect to brain mass. In contrast, if neural reorganization dominates, we predict from (4) that tR/tA scales with an exponent between −1.20 and − 0.40 if driven primarily by REM sleep, whereas if it is driven primarily by NREM sleep, tNR/tA scales with an exponent between −1.20 and −0.40. This provides a remarkably clean test for discerning between different underlying mechanisms for sleep, and whether they occur during REM or NREM sleep.
In Fig. 2, we analyze data and provide strong statistical evidence for the existence and sharpness of the transition from early to late development. To identify the location of the transition and measure its sharpness, we focus on two independent measures of sleep that are related to total sleep time and the NREM/REM sleep trade-off, tS/tA and tNR/tA. To determine the transition point in brain mass for each of these sleep ratios, we choose all possible break points in the data for Mb and calculate the corresponding SSEs of the residuals from the two best-fit straight lines on either side of each possible break point. As observed in Fig. 2, there are unique and sharp minima at almost exactly the same value of Mb in both plots, corresponding to the same age in development. On the basis of these results, we identify the transition point to be at 2.4 years old, consistent with the age range of 2 to 3 years old that corresponds to many known transitions in brain development (46–48).
Fig. 2. Identification of transition from reorganization to repair.
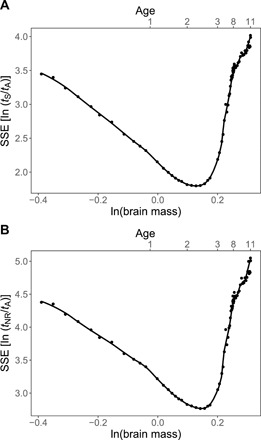
Plots of the sum of squared errors for the residuals of the two best-fit lines to data for (A) ln (tS/tA) and (B) ln (tNR/tA) on either side of a break point in the lines that corresponds to that value of the logarithm of brain mass (Mb). The minimum of each curve is identified as the transition point that divides sleep function into early and late developmental stages as described by our theory. These minima are unique and have values of Mb = 1.14 kg for the transition in tS/tA and Mb = 1.15 kg for the transition in tNR/tA, corresponding to ages of 2.4 to 2.5 years old, respectively. Also, shown on the top horizontal axes is the corresponding age in years. SSEs, sum of squared errors.
In Fig. 3, we present plots of the various sleep time ratios versus brain mass, demonstrating clearly that all of the data for sleep time ratios exhibit a clear transition from early to late development. Using our compiled developmental data for sleep in humans, we test predictions from the theory and are thereby able to determine the underlying mechanisms of sleep.
Fig. 3. Scaling and transition points for sleep time ratios.
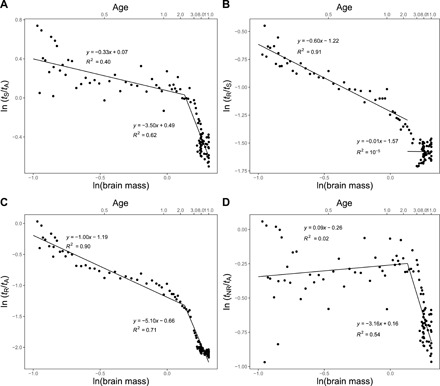
(A) Plot of the logarithm of the ratio of total sleep time to total awake time per day versus the logarithm of brain mass with measured slope of −0.33 before transition and −3.50 after. (B) Plot of the logarithm of the ratio of REM sleep time to total sleep time per day versus the logarithm of brain mass with measured slope of −0.60 before transition and −0.01 after. (C) Plot of the logarithm of the ratio of REM sleep time to total awake time per day versus the logarithm of brain mass with measured slope of −1.00 before transition and −5.10 after. (D) Plot of the logarithm of the ratio of NREM sleep time to total awake time per day versus the logarithm of brain mass with measured slope of 0.09 before transition and −3.16 after. Also, shown on the top horizontal axes is the corresponding age in years.
Figure 3A shows that the scaling exponent for the ratio of sleep to awake time, tS/tA, during early development (<2.4 years) is −0.33 ± 0.07, in the opposite direction (decreasing with size rather than increasing) and strongly at odds with the predictions from neural repair. Similarly, in Fig. 3B, we see that the scaling exponent for the fraction of REM sleep, tR/tS, is −0.60 ± 0.06 during early development, again in complete contradiction to the invariance predicted from neural repair. On the other hand, from Fig. 3C, the ratio of REM sleep time to awake time, tR/tA, has an exponent of −1.00 ± 0.05, consistent with the prediction that assumes sleep function is primarily driven by neural reorganization during REM sleep. Last, as a consistency check on this, Fig. 3D reveals that the corresponding exponent for the ratio of NREM sleep time to awake time, tNR/tA, is 0.09 ± 0.09, consistent with it being an invariant and strongly counter to the predictions assuming that sleep function is primarily driven by neural reorganization during NREM sleep.
As a further test of our predictions, we return to Eq. 5. Because the observed scaling of tS/tA has an exponent of −0.33, the theory based on REM sleep being for neural reorganization would predict that the exponent for the fraction of REM sleep, tR/tS, should be between −0.87 and − 0.07. This differs substantially from the invariance predicted from neural repair and implies that the empirically measured exponent of −0.60 provides additional support for sleep function during early development being tied to neural reorganization in REM sleep.
To summarize, when theoretical predictions are confronted with empirical data, the only consistent mechanism is that sleep function throughout early development is primarily driven by neural reorganization during REM sleep. Equally importantly, all other mechanisms are soundly rejected as can be seen by comparing measured scaling exponents and their confidence intervals from Figs. 1 to 3 with predictions from our theory (see Table 1).
Table 1. Early development (<2.4 years).
Summary of the key empirical results and theoretical tests of our model for the various ratios of sleep times in the first column during the period of early development (<2.4 years old). The second column contains the values and 95% confidence intervals for the scaling exponents as determined from direct empirical data, whereas the third through fifth columns contain the ranges of predicted values for the scaling exponents based on theories that sleep function is primarily for neural reorganization in either REM (third column) or NREM (fourth column) sleep or that it is primarily for neural repair (fifth column). The range of predicted values is calculated in each case using the three best-fit estimates for the scaling exponent α from Fig. 1. NA denotes that the corresponding theory makes no prediction for that specific variable. Predictions that match data are in bold. For these data, the predictions of the theory that sleep function during early development is primarily for neural reorganization in REM sleep are all supported, whereas the predictions of the theory that, during early development, sleep function is either primarily for neural repair or for neural reorganization during NREM sleep are all rejected.
| Ratio |
Measured exponent |
REM reorganization prediction |
NREM reorganization prediction |
Repair prediction |
| tS/tA | −0.33 ± 0.07 | NA | NA | 0.20 to 0.60 |
| tR/tS | −0.60 ± 0.06 | −0.87 to −0.07 | NA | 0 |
| tR/tA | −1.00 ± 0.05 | −1.20 to −0.40 | NA | NA |
| tNR/tA | 0.09 ± 0.09 | NA | −1.20 to −0.40 | NA |
The result that REM sleep time takes up about 50% of total sleep time for newborns, whereas people older than 50 years spend only about 14 to 15% of their sleep time in REM (26), is a particularly marked result. This ontogenetic change is a fundamentally different pattern than that observed phylogenetically, i.e., across species, in which the fraction of time spent in REM sleep does not change from mice to whales. Yet, the ontogenetic change is consistent across phylogeny (26, 53, 54). The decline in the fraction of REM sleep strongly suggests the decreasing importance of reorganization as a function for sleep beyond about human age 2.4 years old and, correspondingly, the ascendance of repair and/or clearance as the primary function in later development (Table 2). That is, as we grow, the dominance of sleep by processes for neural reorganization transitions to the dominance by neural repair and clearance. To test this, we fit the data for tR/tS after the transition point (Fig. 3B) and find that it has a slope not significantly different from 0 and, therefore, consistent with it being an invariant as it is across adult mammals.
Table 2. Late development (>2.4 years).
Summary of the key empirical results and theoretical tests of our model for the various ratios of sleep times in the first column during the period of late development (>2.4 years old). The second column contains the values and 95% confidence intervals of the scaling exponents as determined from direct empirical data, whereas the third through fifth columns contain the ranges of predicted values for the scaling exponents based on theories that sleep function is primarily for neural reorganization in either REM (third column) or NREM (fourth column) sleep or that it is primarily for neural repair (fifth column). The 95% confidence intervals for the predictions are derived from the confidence intervals determined for the scaling exponent α = − 1.70 ± 1.66 in later development (fig. S2). NA denotes that the corresponding theory makes no prediction for that specific variable. Predictions that match data are in bold. For these data, the predictions of the theory that sleep function during early development is primarily or neural repair and clearance are all supported, whereas the predictions of the theory that, during early development, sleep function is primarily for neural reorganization in REM sleep or NREM sleep are all rejected.
| Ratio |
Measured exponent |
REM reorganization prediction |
NREM reorganization prediction |
Repair prediction |
| tS/tA | −3.50 ± 0.23 | NA | NA | −2.70 ± 1.66 |
| tR/tS | −0.01 ± 0.52 | 8.90 ± 3.55 | NA | 0 |
| tR/tA | −5.10 ± 0.20 | 5.40 ± 3.32 | NA | NA |
| tNR/tA | −3.16 ± 0.26 | NA | 5.40 ± 3.32 | NA |
Moreover, if we try to fit a line through these data to connect it with the line at our transition point, we obtain an R2 value of −0.33 (the negative sign being due to the fixing of the y intercept), indicating a terrible fit. Together, this means that in later development, as well as across species, the scaling of the fraction of REM sleep is consistent with the prediction of it being invariant based on the importance of neural repair and clearance for sleep function. Furthermore, the fits indicate that there is an actual discontinuity in the slope (i.e., first derivative) of this property, corresponding in physics terminology to a true phase transition and indicative of a seismic change in sleep and brain function at this early age of around 3 years old.
As further support for the idea that sleep function in later development is for neural repair and clearance, we measure the scaling exponent of brain metabolic rate, α, beyond this transition (section S7) and find a value of −1.70 ± 1.66. Using this in Eq. 1 predicts that tS/tA should scale in this regime with an exponent of −2.70 ± 1.66, which is consistent with the value of −3.50 ± 0.12 measured in Fig. 3A. Together, this provides a compelling evidence in favor of sleep function being primarily for neural repair and clearance during later development, beyond about 3 years old, and also into adulthood and across species (24).
In summary, our main results are as follows:
1) To identify the exact transition point in the function of sleep from reorganization to repair in the brain and recognize that it tightly corresponds to transitions in brain development;
2) To quantitatively demonstrate that this transition, which occurs at 2.4 years old, is remarkably sharp and analogous to a phase transition, or tipping point, as when water freezes to ice;
3) To show that the evidence supports the REM reorganization theory of sleep before this early transition and strongly excludes both NREM-based reorganization and repair-driven mechanisms.
4) To show that theories for the function of sleep in late development that are based on neural reorganization during either REM or NREM sleep are strongly excluded by the data.
DISCUSSION
Sleep is such an engrained and necessary part of our lives that we often take its functions and origins for granted. Presuming that sleep evolved to serve some primary function, it is almost certain that multiple physiological functions have piggybacked onto this pervasive and time-consuming feature of animal life. Here, by deriving a novel theory, compiling comprehensive data on sleep and brain development, and quantitatively comparing sleep ontogeny with sleep phylogeny, we illuminate the dominant functions of sleep and how they change through development. Infants spend a much greater percentage of time in REM sleep compared with older children and adults. This finding suggests that REM sleep is likely crucial for the initial growth of babies and perhaps especially for the regulation of synaptic weights throughout the nervous system (55). These substantial changes in percent REM sleep across human growth are in stark contrast with the constant percentage of REM sleep observed across an enormous range in brain and body size for adult mammals (24). The large change in percent REM sleep across development is thus a key indicator that the function of sleep, and particularly of REM sleep, is very different during development than in adults. It shows that ontogeny does not recapitulate phylogeny because ontogeny does not show qualitatively similar patterns to phylogeny for REM sleep. Rather, it differs from it in the most fundamental of ways (e.g., invariance versus rapid change) and exhibits a phase transition between early and late development.
In our analysis, we divide development into two regimes: an early period of high plasticity accompanied by ongoing synaptogenesis and increasing myelination followed by a later period of declining plasticity, slow synaptic pruning, and increasing white matter integrity and stabilizing connectivity. Our new theory, mathematical models, and data analysis provide compelling evidence that these fundamental differences arise because sleep is used primarily for neural reorganization until about 2 to 3 years of age, at which point, there is a critical transition, and the function shifts sharply toward sleep being for repair and clearance. We identify the specific turning point as occurring at an unexpectedly precise age of around 2.4 years old, reflecting a critical physiological or cerebral developmental change. In all cases, we see a sharp shift in the scaling of sleep during this period of early development that, to our knowledge, has never been conceptually or quantitatively connected to a shift in sleep function.
As discussed in Methods, we compile the most comprehensive and accurate data that exists in the literature. For each data type, we cite a single source, but notably, some of those sources are compilations themselves that drew data from different previous studies. There is no evidence, however, for these differences leading to any changes or systematic biases at 2.4 years of age that we identify as the transition point. Moreover, the fact that we identify the same transition point for different data types from different studies is evidence that our results are not dependent on a particular data type or group and therefore signals the robustness and strength of our results in terms of replicability. Future work should further analyze this transition point by targeting the collection of data for sleep times, brain size, and brain metabolism around this critical age.
When looking at functional brain development in humans, Johnson (49) found that the first 2 years of life is the period that most of the pronounced advances in brain structure and behavior occur. Brains develop extremely dynamically in the first 2 years (48), and most brain structures have the overall appearance of adults by the age of around 2. One notable exception is the delayed development of the prefrontal cortex, the onset of which perhaps corresponds with a surge in REM sleep around later puberty, which would be predicted by our theory. Overall brain size increases markedly during the first 2 years of life and reaches 80 to 90% of adult size by the age of 2. All of the main fiber tracts are observed by 3 years old (49), and in frontal brain regions, white matter changes most rapidly during the first 2 years. White matter is associated with cognitive function, so the rapid change of white matter by the age of 2 helps to partly explain why reorganization might dominate before 2.4 years of age and then transition to a different stage. Other critical periods and transitions in early development with regard to learning and brain function are well known and of great interest, including the acquisition of language (46–48).
Our ontogenetic findings differ markedly from previous phylogenetic findings both in terms of the magnitude and sometimes, the direction of changes and the corresponding scaling exponents. These results are quite unexpected, yet by transitioning from our model for neural reorganization to one for repair and clearance, we are able to simultaneously explain the scaling of sleep time across species and across growth. Repair/clearance (6) and reorganization both occur throughout growth, and in analyzing data and building our theory, we hypothesized and showed how each of these dominates during specific developmental stages: Reorganization dominates at early ages, whereas repair and clearance dominate at later stages. Our theory explains the scaling with brain and body mass in these two different regions for quite different reasons. For neural reorganziation, the scaling arises because of the mismatch between the sublinear scaling of whole-body metabolic rate, which drives information transfer to the brain, and the superlinear scaling of synapse number and white matter volume. In contrast, the scaling arises from the repair and clearance mechanisms because of a mismatch in their being proportional to brain mass, while the damage rate is proportional to brain metabolic rate that scales nonlinearly with brain mass. These multiple origins of the scaling of sleep properties during different periods of life history is crucial because it allows us to match our different theories to the proposed functions for sleep. Another crucial difference is that the scaling exponents manifest as a steep, superlinear increase in brain metabolic rate with brain size during early development followed by a subsequent decline in later development.
Given the relative simplicity of the theory, the various sleep and cerebral properties predicted by our model match empirical data unexpectedly well. We are not yet able to predict all measured sleep properties, but our agreement for such a diverse set of characteristics during ontogenetic development and across phylogeny in adults is impressive. This lends credence to our assumptions and to the quantitative, mathematical framework that we developed.
One of our most compelling findings is not only that there is a transition but also how sharp that transition is, leading to complete reversals in direction for scaling relationships and also in percent REM changing instead of being invariant as it is across species. Although sleep always involves a loss of consciousness and characteristic electrical activity, our results suggest that the underlying dynamics of sleep may change fundamentally around 2 to 3 years of age. During early development, when substantial synaptogenesis is occurring, connections between neurons are likely transitioning from more short-range (e.g., spatially localized circuits or networks) to more long-range connections (e.g., whole brain) (56). Moreover, connections are much more plastic in early development, while they are much more solidified in later development. From this perspective, the brain is in a more fluid state at birth and “cools off” during early ontogeny until a critical point is reached at 2 to 3 years of age, which corresponds to a more crystallized state of brain structure and dynamics.
In addition, it is important to recognize that brain regions exhibit substantial heterogeneity in development and that this heterogeneity likely affects the ontogeny of sleep, possibly in a way that depends on specific brain regions. For instance, sensory areas reach peak synaptic density, myelination, and gray matter maturation before prefrontal regions, the last cortical regions to fully develop (57). The hippocampus undergoes particularly fast, early development in the first few years of life. However, the hippocampus also continues complex subregional development until around age 14 (58). Consistent with the implications of our theory, there is some evidence that differences in regional cortical maturation rates correlate with differences in sleep brain wave pattern development (59). At present, our model assumes uniform, average rates of synaptogenesis and REM and NREM changes. In future work, we hope that incorporating heterogeneity into our model will improve its explanatory and predictive power.
A central feature of our approach is that it is quantitative, computational, predictive, and can be readily tested with empirical data. Our findings point toward new and exciting questions that require more studies. An open question is whether the same ontogenetic patterns in sleep exist for other species. Humans are known to be unusual in the amount of brain development that occurs after birth (42). Therefore, it is conceivable that the phase transition described here for humans may occur earlier in other species, possibly even before birth. Fetuses sleep a very large amount of the time (60), but it may be exceedingly difficult to take precise measurements of metabolic rate or brain mass and thus to observe this shift in other species before birth. Measurements for growth and development in rats, zebra finches, drosophila, Caenorhabditis elegans, and many other species (16) are needed to test how well our theory generalizes and the extent to which these shifts really are phase transitions.
Supplementary Material
Acknowledgments
Funding: V.M.S. acknowledges funding from an NSF DBI CAREER Award (1254159), and G.B.W. would like to thank the NSF under the grant PHY1838420, the Eugene and Clare Thaw Charitable Trust, and Toby Shannan and CAF Canada for their generous support. Author contributions: G.B.W. and V.M.S. conceived and designed the research. J.C. performed the research, compiled the data, performed the computations, and analyzed the data. J.C., A.B.H., G.B.W., and V.M.S. developed the theory. J.C., G.B.W., and V.M.S. wrote the paper. A.B.H. helped conceive and guide neuroscience and scaling aspects of research and edited the paper. G.P. helped guide neuroscience and sleep aspects of research and edited paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All data used and associated units are available at https://github.com/jycao9/Sleep.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/38/eaba0398/DC1
REFERENCES AND NOTES
- 1.Siegel J. M., Clues to the functions of mammalian sleep. Nature 437, 1264–1271 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie L., Kang H., Xu Q., Chen M. J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D. J., Nicholson C., Iliff J. J., Takano T., Deane R., Nedergaard M., Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman R. J., Munsell L. Y., Morris J. C., Swarm R., Yarasheski K. E., Holtzman D. M., Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 12, 856–861 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herculano-Houzel S., Decreasing sleep requirement with increasing numbers of neurons as a driver for bigger brains and bodies in mammalian evolution. Proc. Biol. Sci. 282, 20151853 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zada D., Bronshtein I., Lerer-Goldshtein T., Garini Y., Appelbaum L., Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat. Commun. 10, 895 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf A., Song S. M., Talbot C. B., Miesenböck G., A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature 568, 230–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reimund E., The free radical flux theory of sleep. Med. Hypotheses 43, 231–233 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Feinberg I., Campbell I. G., Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 72, 56–65 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Orban P., Rauchs G., Balteau E., Degueldre C., Luxen A., Maquet P., Peigneux P., Sleep after spatial learning promotes covert reorganization of brain activity. Proc. Natl. Acad. Sci. U.S.A. 103, 7124–7129 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickgold R., Hobson J. A., Fosse R., Fosse M., Sleep, learning, and dreams: Off-line memory reprocessing. Science 294, 1052–1057 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Skaggs W. E., McNaughton B. L., Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Maquet P., The role of sleep in learning and memory. Science 294, 1048–1052 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Tononi G., Cirelli C., Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A., Gilliland M. A., Bergmann B. M., Winter J. B., Physiological correlates of prolonged sleep deprivation in rats. Science 221, 182–184 (1983). [DOI] [PubMed] [Google Scholar]
- 15.Tobler I., Sigg H., Long-term motor activity recording of dogs and the effect of sleep deprivation. Experientia 42, 987–991 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Kayser M. S., Yue Z., Sehgal A., A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 344, 269–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorino A. S., Sleep, genes and death: Fatal familial insomnia. Brain Res. Rev. 22, 258–264 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Ahn J. H., Cho H., Kim J.-H., Kim S. H., Ham J.-S., Park I., Suh S. H., Hong S. P., Song J.-H., Hong Y.-K., Jeong Y., Park S.-H., Koh G. Y., Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Ego-Stengel V., Wilson M. A., Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie K., Wilson M. A., Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–156 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Todorova R., Zugaro M., Isolated cortical computations during delta waves support memory consolidation. Science 366, 377–381 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Brüning F., Noya S. B., Bange T., Koutsouli S., Rudolph J. D., Tyagarajan S. K., Cox J., Mann M., Brown S. A., Robles M. S., Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 366, eaav3617 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Noya S. B., Colameo D., Brüning F., Spinnler A., Mircsof D., Opitz L., Mann M., Tyagarajan S. K., Robles M. S., Brown S. A., The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 366, eaav2642 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Savage V. M., West G. B., A quantitative, theoretical framework for understanding mammalian sleep. Proc. Natl. Acad. Sci. U.S.A. 104, 1051–1056 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galland B. C., Taylor B. J., Elder D. E., Herbison P., Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Med. Rev. 16, 213–222 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Roffwarg H. P., Muzio J. N., Dement W. C., Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619 (1966). [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M., Ikeda-Sagara M., Okada T., Clement P., Urade Y., Nagai T., Sugiyama T., Yoshioka T., Honda K., Inoué S., Brain oxidation is an initial process in sleep induction. Neuroscience 130, 1029–1040 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Feinberg I., Thode H. C. Jr., Chugani H. T., March J. D., Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J. Theor. Biol. 142, 149–161 (1990). [DOI] [PubMed] [Google Scholar]
- 29.Chechik G., Meilijson I., Ruppin E., Neuronal regulation: A mechanism for synaptic pruning during brain maturation. Neural Comput. 11, 2061–2080 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Elgar M. A., Pagel M. D., Harvey P. H., Sleep in mammals. Anim. Behav. 36, 1407–1419 (1988). [Google Scholar]
- 31.Poe G. R., Sleep is for forgetting. J. Neurosci. 37, 464–473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vyazovskiy V. V., Delogu A., NREM and REM sleep: Complementary roles in recovery after wakefulness. Neuroscientist 20, 203–219 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Sara S. J., Sleep to remember. J. Neurosci. 37, 457–463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poe G. R., Nitz D. A., McNaughton B. L., Barnes C. A., Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Li W., Ma L., Yang G., Gan W.-B., REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 20, 427–437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.T. J. Walter, REM Illumination: Memory Consolidation (Lotus Magnus, 2007). [Google Scholar]
- 37.D. O. Hebb, The Organization of Behavior: A Neuropsychological Theory (John Wiley and Sons, 1962). [Google Scholar]
- 38.W. A. Calder, Size, Function, and Life History (Courier Corporation, 1984). [Google Scholar]
- 39.Attwell D., Laughlin S. B., An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Lennie P., The cost of cortical computation. Curr. Biol. 13, 493–497 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Fields R. D., White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clancy B., Darlington R. B., Finlay B. L., Translating developmental time across mammalian species. Neuroscience 105, 7–17 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Stiles J., Jernigan T. L., The basics of brain development. Neuropsychol. Rev. 20, 327–348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K., Sejnowski T. J., A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 97, 5621–5626 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huttenlocher P. R., Synaptic density in human frontal cortex––developmental changes and effects of aging. Brain Res. 163, 195–205 (1979). [DOI] [PubMed] [Google Scholar]
- 46.Gopnik A., Choi S., Baumberger T., Cross-linguistic differences in early semantic and cognitive development. Cogn. Dev. 11, 197–225 (1996). [Google Scholar]
- 47.D. M. Singleton, L. Ryan, Language Acquisition: The Age Factor (Multilingual Matters, 2004), vol. 9. [Google Scholar]
- 48.Knickmeyer R. C., Gouttard S., Kang C., Evans D., Wilber K., Smith J. K., Hamer R. M., Lin W., Gerig G., Gilmore J. H., A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28, 12176–12182 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson M. H., Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Dekaban A. S., Sadowsky D., Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann. Neurol. 4, 345–356 (1978). [DOI] [PubMed] [Google Scholar]
- 51.Reichman C. A., Shepherd R. W., Trocki O., Cleghorn G. J., Davies P. S. W., Comparison of measured sleeping metabolic rate and predicted basal metabolic rate during the first year of life: Evidence of a bias changing with increasing metabolic rate. Eur. J. Clin. Nutr. 56, 650–655 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Groeschel S., Vollmer B., King M. D., Connelly A., Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int. J. Dev. Neurosci. 28, 481–489 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Kayser M. S., Biron D., Sleep and development in genetically tractable model organisms. Genetics 203, 21–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szeto H. H., Hinman D. J., Prenatal development of sleep-wake patterns in sheep. Sleep 8, 347–355 (1985). [DOI] [PubMed] [Google Scholar]
- 55.Del Rio-Bermudez C., Blumberg M. S., Active sleep promotes functional connectivity in developing sensorimotor networks. Bioessays 40, 1700234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohmann C., Kessels H. W., The developmental stages of synaptic plasticity. J. Physiol. 592, 13–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw P., Kabani N. J., Lerch J. P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J. L., Giedd J. N., Wise S. P., Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keresztes A., Bender A. R., Bodammer N. C., Lindenberger U., Shing Y. L., Werkle-Bergner M., Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc. Natl. Acad. Sci. U.S.A. 114, 9212–9217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.M. Ringli, R. Huber, Progress in Brain Research (Elsevier, 2011), vol. 193, pp. 63–82. [DOI] [PubMed] [Google Scholar]
- 60.Okai T., Kozuma S., Shinozuka N., Kuwabara Y., Mizuno M., A study on the development of sleep-wakefulness cycle in the human fetus. Early Hum. Dev. 29, 391–396 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Kerkhoff A. J., Enquist B. J., Elser J. J., Fagan W. F., Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob. Ecol. Biogeogr. 14, 585–598 (2005). [Google Scholar]
- 62.Savage V. M., Gillooly J. F., Woodruff W. H., West G. B., Allen A. P., Enquist B. J., Brown J. H., The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282 (2004). [Google Scholar]
- 63.Thoman E. B., Waite S. P., Desantis D. T., Denenberg V. H., Ontogeny of sleep and wake states in the rabbit. Anim. Behav. 27, 95–106 (1979). [DOI] [PubMed] [Google Scholar]
- 64.Vogel G. W., Feng P., Kinney G. G., Ontogeny of REM sleep in rats: Possible implications for endogenous depression. Physiol. Behav. 68, 453–461 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Ibuka N., Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav. Brain Res. 11, 185–196 (1984). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/38/eaba0398/DC1


