Abstract
Background
A tetravalent live attenuated dengue vaccine, Dengvaxia, sensitised naïve recipients to severe dengue illness upon a subsequent natural dengue infection and is suspected to be due to antibody-dependent enhancement (ADE). ADE has also been implicated in the severe neurological outcomes of Zika virus (ZIKV) infection. It has become evident that cross-reactive antibodies targeting the viral pre-membrane protein and fusion-loop epitope are ADE-competent. A pre-clinical tetravalent dengue sub-unit vaccine candidate, DSV4, eliminates these ADE-competent epitopes.
Methods
We compared protective efficacy and ADE-competence of murine polyclonal antibodies induced by DSV4, Dengvaxia and an ‘in house’ tetravalent mixture of all four laboratory DENV strains, TV DENV, using established mouse models.
Findings
DSV4-induced antibodies, known to be predominantly type-specific, provided significant protection against lethal DENV challenge, but did not promote ADE of either DENV or ZIKV infection in vivo. Antibodies elicited by Dengvaxia and TV DENV, which are predominantly cross-reactive, not only failed to offer protection against lethal DENV challenge, but also promoted ADE of both DENV and ZIKV infection in vivo.
Interpretation
Protective efficacy against DENV infection may be linked to the induction of neutralising antibodies which are type-specific rather than cross-reactive. Whole virus-based dengue vaccines may be associated with ADE risk, despite their potent virus-neutralising capacity. Vaccines designed to eliminate ADE-competent epitopes may help eliminate/minimise ADE risk.
Funding
This study was supported partly by ICGEB, India, the National Biopharma Mission, DBT, Government of India, Sun Pharmaceutical Industries Limited, India, and NIAID, NIH, USA.
Keywords: Dengue virus, Zika virus, Dengue VLP vaccine, DSV4, Dengvaxia, LAV, Antibody-dependent enhancement, AG129, C57BL/6 Stat2−/−
Research in Context.
Evidence before this study
Dengvaxia, a tetravalent live attenuated vaccine for dengue, approved for use in many dengue-endemic countries, has been found to pose a risk to dengue-naïve individuals. It has been argued that this vaccine can induce cross-reactive (CR) antibodies, which may have enhanced the severity of a subsequent natural dengue infection. It has been proposed that, pre-existing CR antibodies in dengue-endemic populations may have exacerbated the severity of Zika virus infections, leading to neuropathological manifestations. This phenomenon, underlying severe flaviviral disease pathogenesis, is known as antibody-dependent enhancement (ADE). Recent work has shown that antibodies elicited by the viral pre-membrane (prM) protein and the fusion loop epitope (FLE) can cause ADE. This prompted us to design the tetravalent dengue subunit vaccine, DSV4, which does not contain these detrimental prM- and FLE-determinants.
Added value of this study
In this study, we provide experimental evidence confirming the suspicion that Dengvaxia can indeed induce ADE-competent antibodies. Using well-established mouse models of ADE, we demonstrate that antibodies elicited by Dengvaxia can enhance not only dengue infection, but also infection by Zika virus. On the contrary, the DSV4 dengue vaccine candidate, designed to eliminate prM and FLE, and elicit predominantly type-specific (TS) anti-dengue virus neutralising antibodies, did not manifest ADE of either dengue virus or Zika virus infection. Our work suggests that TS, rather than CR neutralising antibodies, may be important in conferring protection against DENV infection.
Implications of all the available evidence
Despite the capacity to neutralise dengue and Zika viruses potently, CR antibodies elicited by Dengvaxia promote ADE of infection by both viruses. On the other hand, TS antibodies, particularly directed to dengue virus envelope domain III, do not possess ADE competence towards either heterotypic dengue viruses or Zika virus. This work provides credible evidence to support the development of dengue vaccines devoid of epitopes which can elicit CR ADE-competent antibodies.
Alt-text: Unlabelled box
1. Introduction
Dengue is a viral disease spread among humans by Aedes mosquitoes. Nearly half the global population is at risk of being infected by any one or more of four distinct serotypes of dengue viruses (DENV-1, -2, -3 and -4), all of which are members of the genus Flavivirus, of the family Flaviviridae [1]. DENVs are estimated to be responsible for approximately a million infections around the world on a daily basis [2].
A safe, effective and affordable vaccine to prevent DENV infections continues to be an unmet public health need. One complicating factor in the design of a safe DENV vaccine is the phenomenon of antibody-dependent enhancement (ADE), by which antibodies raised against one serotype of DENV can enhance, rather than mitigate, subsequent infection by a different serotype. Cross-reactive (CR) antibodies form immune complexes (ICs) with heterologous flaviviruses and enable their entry into Fc gamma receptor (Fcγ-R)-bearing cells, leading to increased tissue virus load and more severe disease. Cross-reactivity between antibodies of different DENV serotypes is thus not always protective [[3], [4], [5]].
In most clinically apparent cases, DENVs cause a mild self-limiting febrile illness. However, a small proportion of DENV infections result in severe dengue. This is a potentially fatal disease, characterised by increased capillary permeability leading to plasma leakage and shock [6]. A systematic meta-analysis identified several clinical signs including nausea, vomiting, abdominal pain, and gastrointestinal bleeding, among others, as being significantly associated with severe dengue disease [7]. Retrospective dengue epidemiological studies in Taiwan [8] and Brazil [9] identified gastrointestinal bleeding as a significant predictor of fatal dengue. Severe dengue has often been associated with sequential infection with DENVs of different serotypes. It is believed that this is because of the ADE phenomenon referred to above, with antibodies to the initial DENV serotype augmenting infection by the subsequent DENV serotype [3,4].
Besides laboratory experimentation with cell lines [10,11] and animals [[12], [13], [14]], multiple lines of evidences from human studies support the occurrence of ADE in severe dengue disease. Historically, the first indication which linked CR antibodies to ADE was the observation that infants born to dengue-immune mothers manifested severe dengue upon primary DENV infection [15]. The role of CR antibodies in severe dengue was strengthened by quite comprehensive Cuban epidemiological studies. These studies found that DENV-naive Cubans, sensitised by exposure to DENV-1 in 1977, experienced severe dengue during DENV-2 epidemics, 4 years later in 1981, and 20 years later, in 1997 [16]. The 1981 epidemic was initially presumed to be due to a serotype other than DENV-1, based on a more severe dengue-like clinical presentation. Subsequent virus isolation from acute phase sera followed by immunofluorescence and plaque reduction neutralisation test identified it definitively to be due to DENV-2 [17]. Several follow-up studies established the link between clinical outcome and secondary infection [18,19]. More recently, the results of a 12-year study of a large paediatric cohort in Nicaragua showed that the titres of pre-existing CR antibodies, measured using an inhibitory ELISA protocol, predict the risk of ADE in humans [20]. Essentially similar conclusions were evident from a recent analysis of haemagglutination inhibition (HI) titres (a measure of CR antibodies) determined during a five-year Thai paediatric cohort study reported in 2002 [21]. This analysis defined pre-existing HI titres of 1:40 as a cut-off parameter below which the risk of severe dengue was 7.4 times more likely compared to dengue-naïve children [22].
During the past decade, it has become evident that the antibodies induced during natural DENV infection target predominantly two viral structural proteins, the envelope (E) and the pre-membrane (prM) proteins [23]. While only a minority (up to 10%) of the naturally-induced anti-DENV antibodies are type-specific (TS), a majority are CR antibodies. An analysis of the memory B cell responses in DENV-infected individuals showed that ~60% of the human antibody response is directed towards the viral prM protein. Up to 30% of anti-DENV antibodies target the fusion loop epitope (FLE) on the viral E protein. Anti-DENV prM and FLE antibodies are CR antibodies capable of recognising all four DENV serotypes. However, these are either poor or weak neutralisers, but potent promoters of DENV infectivity [11,[24], [25], [26]]. The Nicaraguan study mentioned above actually analysed anti-DENV antibodies, capable of outcompeting the binding of well-characterised pan-DENV mAbs (specific to prM, FLE etc), in their cohorts to correlate CR antibody titres to ADE in humans [20].
The ADE phenomenon mandates that an important feature of any successful dengue vaccine must be that it confers simultaneous protection against all four DENV serotypes. In the last two decades, significant efforts to create tetravalent dengue vaccines have focused on developing mixtures of live attenuated vaccine (LAV) virus strains representing all four DENV serotypes, based on either yellow fever vaccine 17D (YF17D) vector [27,28] or attenuated DENV vector backbones [[29], [30], [31], [32]]. The first tetravalent LAV, Dengvaxia, was launched for public use recently [33]. This is a mixture of four chimeric YF17D-dengue viruses (CYD-1 through CYD-4), each carrying the prM and E genes of one DENV serotype, in place of the corresponding YF17D structural genes [27].
Though the expectation was that Dengvaxia would elicit robust immunity against all four DENVs and circumvent ADE, it did not. It has been proposed, but not experimentally proven, that Dengvaxia perhaps did not elicit the right T cell responses, as it lacks DENV non-structural proteins [34]. However, it is clear that Dengvaxia mimicked a monotypic infection in dengue-naive recipients, and sensitised them to severe dengue during a subsequent natural DENV infection [35,36]. A likely interpretation may be that Dengvaxia-induced anti-prM and anti-FLE antibodies in dengue-naïve recipients exacerbated a natural DENV infection following vaccination.
In this regard, we recently reported the development of a tetravalent dengue subunit vaccine, DSV4, which lacks prM and FLE. DSV4 displays the E domain III (EDIII), shown to elicit DENV-neutralising TS antibodies, of all the four DENVs, on a virus-like particle (VLP) platform [37]. Passively transferred sera from DSV4-immunised BALB/c mice into AG129 mice (which lack interferon α/β and γ receptors) afforded significant protection from lethal challenge with DENV-4 (strain 703-4). Importantly, consistent with its lack of prM and FLE, DSV4-induced mouse and monkey antibodies did not cause ADE of DENV infection in AG129 mice.
Despite the finding that Dengvaxia sensitised dengue-naïve individuals to severe dengue disease, the capacity of Dengvaxia-induced antibodies to enhance a sub-lethal DENV infection in AG129 mice has not been investigated to date. The CR nature of anti-prM and anti-FLE antibodies implies that ADE of flavivirus infection can extend beyond the four DENV serotypes. The occurrence of ZIKV outbreaks associated with more severe neuropathology, in regions of high DENV endemicity, suggests a role for anti-DENV antibodies in severe ZIKV pathogenesis [38,39]. This notion receives support from recent laboratory observations showing that anti-DENV FLE antibodies can interact with Fcγ-R to mediate ZIKV uptake into susceptible cells [40] and that passively transferred human anti-DENV plasma significantly increased placental damage, foetal growth restriction and foetal resorption, in ZIKV-infected pregnant C57BL/6 Stat2−/− mice [41].
This study was designed to address some of the questions pertaining primarily to the ADE potential of DENV- and DENV vaccine-induced antibodies using available mouse ADE models of flavivirus infection. To this end, we generated polyclonal immune sera by vaccinating BALB/c mice with our experimental VLP vaccine DSV4 [37]. For comparison we also generated murine immune sera against Dengvaxia and a physical tetravalent DENV mixture (TV DENV) comprised of laboratory strains of the four DENV serotypes. As BALB/c mice do not support viral replication, we also generated immune sera to Dengvaxia in DENV-sensitive CD11c-Ifnar1−/− mice [42], in some experiments, to evaluate LAV-induced antibodies produced in the context of ongoing viral replication. We investigated the potential of all resultant immune sera to: (i) mediate in vivo ADE of DENV and ZIKV infection, and (ii) offer protection against lethal DENV challenge. We found that antibodies elicited by DSV4, offered significant protection from lethal DENV-2 challenge and did not promote ADE of either DENV or ZIKV in vivo. In contrast, Dengvaxia- and TV DENV-induced antibodies, which did not possess protective efficacy against lethal DENV-2 challenge, vigorously promoted ADE of both DENV and ZIKV in vivo.
2. Methods
2.1. Ethics statement
Animal experiments using BALB/c, AG129 and CD11c-Ifnar1−/− mice, (ICGEB/IAEC/02042019/RGP-3), performed at the International Centre for Genetic Engineering & Biotechnology (ICGEB), New Delhi, were strictly compliant with the ‘Committee for the Purpose of Control and Supervision of Experiments on Animals’ guidelines issued by the Government of India. Experiments using C57BL/6 Stat2−/− mice (IACUC Approved Protocol # LA11-00147) were carried out at Icahn School of Medicine at Mount Sinai, and complied with the US federal regulations and the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals. Additional details are provided in the Supplementary File (Methods S1).
2.2. Immunogens
The yeast produced VLP-based DENV EDIII vaccine candidate DSV4 has been described before [37]. The tetravalent LAV Dengvaxia was procured from Bangkok, Thailand. When reconstituted in sterile 1x phosphate buffered saline (PBS), the vaccine contains 4.5-6.0 log10 CCID50 (=50% Cell Culture Infectious Dose) of each serotype per human dose of 0.5 ml. TV DENV was prepared ‘in house’ by mixing titred stocks of the WHO reference strains [43], DENV-1 (West Pac 74), DENV-2 (S-16803), DENV-3 (CH53489) and DENV-4 (TVP360), to obtain 2 × 106 Fluorescence Activated Cell Sorting (FACS) Infectious Units (FIUs) of each DENV serotype per ml.
A single dose of DSV4 contained 20 µg purified DSV4 VLPs adsorbed onto 500 µg alhydrogel in 100 µl 1x PBS. A single dose of Dengvaxia contained one-tenth the human dose (3.5-5.0 log10 CCID50) in a final volume of 50 µl 1x PBS (or one-twentieth the human dose for CD11c-Ifnar1−/− mice). A single dose of TV DENV contained 105 FIUs of each DENV serotype in a final volume 50 µl 1x PBS.
2.3. Immunisation of mice
Groups (n=20) of 4-6 weeks old BALB/c mice were administered three intramuscular (i.m.) doses of each of the three immunogens, at three-week intervals, and bled two weeks after the final dose for seroanalysis. Immune sera from each group were pooled together (after ensuring that all single sera had comparable total antibody titres). A group of mice was mock-immunised with alhydrogel alone, following the same schedule, and the resultant sera (after pooling) also included in all subsequent experiments for comparison.
Dengvaxia alone was also administered to a group (n=20) of 6-8 weeks old CD11c-Ifnar1−/− mice, using the same route and schedule as above. In parallel, a mock-immunised group (n=20) of this mouse strain was administered adjuvant alone. Sera were collected from a subset of mice on day 4 (for the determination of viral RNA levels), and from all mice at 2 weeks after the 3rd dose for seroanalysis. One separate group (n=3) of CD11c-Ifnar1−/− mice was immunised with UV-inactivated (302 nm, 1 h, with intermittent mixing) Dengvaxia, and bled 4 days later, to serve as a control during quantitative analysis of viral RNA.
The immunisation schedule used was based on previous experiments, which showed that flavivirus VLP immunogens administered to BALB/c mice in three doses, spread over 28 days (days 0, 14 and 28) or 90 days (days 0, 30 & 90), result in comparable immunogenicity [44].
2.4. Time-resolved fluorometry-based immunoassay (TRF-IA)
Streptavidin coated 96-well microplates (KaiSA96 plates, from Kaivogen Oy, Turku, Finland) were pre-blocked with 150 µl of 1% bovine serum albumin (BSA) in 1x PBS and incubated at room temperature (RT) for 1 h, with slow shaking. Blocked plates were coated with biotinylated recombinant insect cell-expressed E proteins (Meridian Life Science, Inc., Tennessee, USA; catalogue #s, DENV-1 E: R01659; DENV-2 E, R01660; DENV-3 E, R01661; DENV-4 E, R01662; and ZIKV E, R01635) or ‘in-house’ yeast-expressed EDIII proteins, expressed and purified as reported [45], using 25 ng antigen/50 µl/well. Coated plates were washed three times with wash buffer (5 mM Tris, pH 7.75, 0.9% NaCl, 0.02% NaN₃ and 0.05% Tween 20). Washed plates were incubated with different murine sera (mock-immune, α-DSV4, α-Dengvaxia or α-TV DENV immune sera), at 1:500 dilution in assay buffer (50 mM Tris, pH 7.75, 150 mM NaCl, 0.05% NaN₃, 0.5% BSA, 20 µM diethylenetriaminepentaacetic acid, 0.01% Tween-40, 20 mg/l Cherry red) for 60 min at RT. After washing the plates, 50 ng/50 µl/well Europium (Eu3+)-labelled anti-Mouse IgG were added and incubated for 45 min at RT, with slow shaking. The plates were washed four times with wash buffer, followed by the addition of enhancement solution (100 µl/well). Plates were incubated for 15 min with slow shaking at RT, and read by Eu3+-(time resolved fluorometry) TRF protocol (excitation at 340 nm and emission at 615 nm).
2.5. Determination of neutralising antibody (nAb) titres in sera
DENV- and ZIKV-nAb titres in immune sera were determined using the FACS-based neutralisation assay, as described [43,46]. Equal quantities of DENVs 1-4 or ZIKV (5 × 103 FIUs each) were separately pre-incubated with serial dilutions of heat inactivated (56°C/30 min), filter-sterilised immune sera and infected on to Vero cells in 96-well plates. Two hours later, the inoculum was removed and the cells rinsed (to remove any non-specifically attached virus) and fed with fresh maintenance medium. One day later, cells were trypsinised, fixed and stained with specific mAb to detect infected cells. DENV-infected cells were detected by staining the fixed cells with mAb 2H2-Alexa 488 conjugate. ZIKV-infected cells were detected using mAb 4G2 with goat anti-mouse IgG-phycoerythrin conjugate. Stained cells were counted in either a FACSverse or FACScalibur cytometer, and the data analysed using Flowjo software. The FACS neutralisation titre (FNT50) is defined as the reciprocal of the immune serum dilution which decreased the number of virus-infected Vero cells by 50% with reference to the number of virus-infected cells in the absence of immune serum, taken as 100%.
2.6. In vitro DENV ADE assay
Serial two-fold dilutions of heat inactivated immune sera or mAb 4G2 (40 µl/well, DMEM+2% ΔFBS) were pre-incubated with equal volumes of suitably diluted stocks of DENV-1, -2, -3 and -4 (40 µl/well), separately for 1 h at 37°C. The amount of each DENV used was calculated to result in a multiplicity of infection (MOI) of 0.1. This low MOI was used to ensure that the DENVs do not infect the FcγIIR-expressing K562 cells to any discernible extent in the absence of anti-DENV antibodies. After 1 h pre-incubation, the resultant ICs were mixed with K562 cells (5 × 104 cells/20 μl/well), and allowed to incubate in a humidified 10% CO2 incubator at 37°C. After 1 h, the cells were washed once (to remove any unattached virus, antibody or ICs) with DMEM+2% ΔFBS and suspended in fresh DMEM+2% ΔFBS (200 µl/well) and returned to the incubator for 24 h. Cells were fixed with 4% formaldehyde (50 µl/well, 10 min, RT), permeabilised (with BD Perm/Wash), blocked with normal mouse serum (NMS) (40 µl/well, 20 min, RT) and stained with prM-specific 2H2-Alexa 488 conjugate (20 µl/well, 1 h, 37°C). Cells were analysed in BD FACS-Verse (BD Biosciences).
2.7. In vivo DENV ADE assay: passive transfer method
Groups (n=5) of AG129 mice (6-8 weeks old) were administered 10 µg mAb 4G2 (in 200 µl 1x PBS) or 20 µl immune serum (Mock, α-DSV4, α-Dengvaxia or α-TV DENV) intraperitoneally (i.p.), and challenged intravenously (i.v.) 2 h later, with DENV-2 S221 (2 × 104 FIUs/mouse). An additional group of mice which received neither a passive transfer of antibody/immune serum nor the sub-lethal virus challenge (Uninfected) was included for comparison. Mice were monitored daily for survival, clinical signs of illness and body weight loss, for a period of 15 days. Clinical signs were scored using a 5-point system, with the maximum score denoting death: 0.5, mild ruffled fur; 1.0, ruffled fur; 1.5, compromised eyes; 2, compromised eyes with hunched back; 2.5, loose stools; 3.0, limited movement; 3.5, no movement on stimulus/hind leg paralysis; 4.0, euthanised if cumulative score ≥3.5.
2.8. In vivo DENV ADE assay: IC inoculation method
A sub-lethal dose of DENV-2 S221 (2 × 104 FIUs) was pre-incubated with ~5 µl each of the different immune sera for 1 h on ice to obtain fully neutralised ICs (nIC 100%). Partially neutralised ICs (nIC 30%) were generated by incubating the sub-lethal dose of the virus separately with α-DSV4, α-Dengvaxia or α-TV DENV immune sera for 1 h on ice. Fully neutralised nICs were also generated using mAb 4G2 (sub-lethal dose of virus + 10 µg mAb, 1 h on ice). All the different 30% and 100% nICs were administered i.v. into groups (n=12) of AG129 mice (6-8 week old) and monitored daily, for up to 15 days for survival, and scored for clinical signs of illness (5-point scoring system, described above) and body weight loss.
Subsets of mice (n=3) from each group were euthanised on day 4 post-nIC inoculation, for determination of DENV RNA levels in serum and small intestinal tissue by reverse transcription-quantitative polymerase chain reaction (RT-qPCR), as described below. Euthanised mice were perfused extensively with 1x PBS (~50 ml), small intestines excised, and flushed thoroughly with 1x PBS (~30 ml) to remove luminal contents, portions stored in ~0.6 ml RNAlater RNA stabilisation reagent at 4°C (for viral genomic RNA) or in 1x PBS at -80°C (for cytokine determinations, see below), and photographed to document any visible haemorrhage.
To quantify the extent of vascular leakage, subsets of mice (n=3) from the various nIC groups were administered 1% sterile Evan's Blue dye (100 µl/mouse, in 1x PBS, i.v.) at day 3.5 post-nIC inoculation. Mice were euthanised 2 h later. After extensive perfusion and removal of intestinal luminal contents with 1x PBS, the small intestines were collected into formamide (50 mg wet tissue/500 µl formamide). Tissues in formamide were extracted at 52°C for 24 h, and clarified by low speed centrifugation. The absorbance of the clarified supernatants was measured against formamide blank at 620 nm in a visible spectrophotometer. Fold increase in absorbance of a given tissue of nIC-inoculated mice with reference to the same tissue from uninfected mice was taken as a measure of vascular leakage.
Viral RNA from AG129 mouse serum (140 µl of 1:15 dilution) and total RNA from small intestinal tissue (30 mg wet weight) were purified using QIAmp viral RNA isolation Minikit (Qiagen Catalogue # 52904) and RNeasy Plus Minikit (Qiagen catalogue #74134), respectively, as per the manufacturer's instructions. Purified serum viral RNA and total small intestinal tissue RNA were obtained in final volumes of 60 µl and 100 µl, respectively.
For cytokine determinations, small intestinal tissue (~50 mg wet weight) was extracted in 0.5 ml 1x PBS in a Polytron homogeniser and clarified by centrifugation. Tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the clarified small intestinal extracts were determined using commercial ELISA kits (Invitrogen, catalogue #s KMC3011 and KMC0061, respectively), with reference to murine recombinant biotinylated TNF-α and IL-6 standards, respectively, according to the manufacturer's protocols.
2.9. Determination of the protective efficacy of DSV4 and Dengvaxia
Groups of 6-8 week old AG129 mice were injected i.p. with immune sera from BALB/c mice vaccinated with DSV4 (n=4) or Dengvaxia (n=3). One group (n=3) of AG129 mice received passive transfer of immune sera from CD11c-Ifnar1−/− mice immunised with Dengvaxia. The immune sera were administered at two dosage levels, 100 and 300 µl/mouse (administered in a final volume of 400 µl/mouse, made up with 1x PBS). Two additional groups received passive transfers of either mAb 3H5 or mAb 4G2 (each at 100 µg/mouse, in a final volume of 400 µl/mouse). Two hours following passive transfer, mice were bled (for the determination of circulating DENV-2 nAb titres) and challenged immediately with a lethal dose (1 × 105 FIU/mouse) of DENV-2 S221. One group (n=3, Virus Control, VC) of mice that received neither mAb nor polyclonal serum was challenged in parallel. A final group (n=3) of mice that received neither passively transferred antibodies nor the lethal DENV-2 S221 challenge dose (Uninfected group) was included for comparison. All mice were monitored for 15 days post-infection for survival, development of clinical symptoms (clinical scoring as described in Section 2.7) and weight loss.
2.10. In vivo ZIKV ADE assay
ADE of ZIKV infection was evaluated in groups (n=6-8) of 4-5-week-old C57BL/6 Stat2−/− mice as described earlier [46,47]. Mice were injected with 20 µl of the different immune sera (in 200 µl 1x PBS, i.p.). A control group received pooled DENV-seropositive human plasma (20 µl/mouse). This pool was created using samples collected during an earlier study [41]. All mice were challenged with 5 × 103 PFUs of ZIKV PRVABC59, given intradermally (i.d.), and monitored for survival, clinical signs of illness, body weight loss and body temperature change, for up to 15 days. Body temperature was measured daily at the same time using a rectal thermometer (Braintree Scientific) [47]. A group of mice which received no treatment was monitored in parallel for comparison. A 4-point clinical scoring method was used to grade signs of illness: 0, no symptom; 0.5, mild ruffling of fur; 1.0, ruffled fur; 1.5, compromised eyes; 2.0, hunched back position; 2.5, very limited movement/one leg paralysis; 3.0, no movement and paralysis of both hind legs; 4.0, dead or euthanised.
A subset of ZIKV-challenged mice, from each group, was bled on day 4 post-challenge for determination of viremia by RT-qPCR. ZIKV RNA was purified from 50 µl plasma, using the QIAmp viral RNA isolation Minikit, as above. Mice from these subsets were euthanised on day 6 and vital internal organs harvested for determination of tissue virus load by RT-qPCR. Tissues (ranging in wet weight from 5–300 mg depending on the tissue) were homogenised in 0.5–1.0 ml 1x PBS, using a Beadblaster 24 microtube homogeniser (Benchmark Scientific). Total RNA was prepared by treating 100 µl tissue homogenate with Trizol (Ambion, catalogue #15596018) and purified using Direct-zol RNA kit (Zymo Research, catalogue #R2072).
A portion of each organ was also formalin-fixed and paraffin-embedded for identification of ZIKV RNA by fluorescence in situ hybridization (FISH) using RNAscope (Advanced Cell Diagnostics) on 5 µm paraffin-embedded tissue slices in accordance with the vendor's protocol (ACD catalogue# 323110), using the ZIKV probe V-ZIKVsph2015 (catalogue# 467871) and visualised by fluorescence microscopy, essentially as described previously [41,48].
2.11. Viral RNA quantification by RT-qPCR
DENV-2 and ZIKV genomic RNA in serum/plasma and tissue homogenates of infected mice were determined using SYBR green-based RT-qPCR. Viral RNA was purified from 50 µl plasma (for ZIKV RNA) or 10 µl serum (for DENV-2 RNA) and obtained in a final volume of 60 µl in both instances.
Purified serum/plasma viral RNA (10 µl) or total RNA (10 ng) from tissues was reverse transcribed (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, catalogue #4368814 for ZIKV RNA; iScript Select cDNA synthesis kit, Bio-Rad catalogue #1708897 for DENV-2 RNA) with either random primers (ZIKV RNA) or DENV-2 specific reverse primer in a final reaction volume of 20 µl (for both ZIKV and DENV-2 RNAs). Two µl each of the ZIKV and DENV-2 cDNA products were subjected to qPCR using ZIKV NS5-specific primers, and DENV-2 5’ untranslated region (UTR)/Capsid-specific primers, respectively.
The ZIKV cDNA product was subjected to qPCR with the primers: 5’ TGC GTT GTG AAG CCA ATT GAT GAT AGG 3’ (forward); and 5’ TGT GTG TCC TTC CTA ACT TTT CCC ATA TCA TTC 3’ (reverse) in conjunction with Roche SYBR Green qPCR kit (catalogue #04887352001), in a LightCycler 480 machine (Roche Life Sciences).
The qPCR with DENV-2 cDNA product was performed using the primers: 5’-AGT TGT TAG TCT ACG TGG ACC GA-3’ (forward) and 5’-CGC GTT TCA GCA TAT TGA AAG-3’ (reverse) in conjunction with iTaq Universal SYBR Green Super Mix (BioRad, catalogue #1725124) in a StepOnePlus Real-Time PCR system (Applied Biosystems).
In vitro-transcribed ZIKV NS5 RNA and synthetic DENV-2 5’ UTR/Capsid RNA were analysed as standards, in parallel, to calculate ZIKV and DENV-2 genome equivalents/ml plasma or serum, respectively (correcting for background using plasma/serum RNA from uninfected mice, processed simultaneously in the RT-qPCR experiment).
2.12. Statistical analysis
Unpaired two-tailed t-test and two-way ANOVA (with Bonferroni's correction for multiple comparisons) were used to determine statistical significance of the difference between data sets. Kaplan-Meier survival curves were analysed by the Log-Rank test for significance. Probability (p) levels ≤0.05 were considered significant and p values <0.005 considered very significant. All statistical calculations were performed using GraphPad Prism (v8.0) software.
2.13. Role of funding source
This study was supported partly by ICGEB, India, the National Biopharma Mission, Department of Biotechnology, Government of India (Grant No. BT/NBM0011/01/17), Sun Pharmaceutical Industries Limited, India, and the National Institute of Allergy and Infectious Diseases, NIH, USA (R21AI144844). Three authors (HB, SK & AAL), whose roles are indicated in the contributors section, are from Sun Pharmaceuticals Industries Limited, which partly funded this work. All other funders (ICGEB, Department of Biotechnology, Govt. of India and NIAID, USA) had no role in any aspect of the work carried out or the decision to write and submit the manuscript for publication.
2.14. Material and data availability
Material used in this study may be available at the discretion of ICGEB and SPIL. All data generated or analysed during this study are included in this article and its supplementary Information files.
3. Results
3.1. DSV4, Dengvaxia and TV-DENV elicit tetravalent anti-E DENV antibodies
BALB/c mice were immunised with DSV4, Dengvaxia and TV DENV using a three dose schedule over a six week period and bled two weeks after the final dose (Fig. 1a). We devised an ‘in-house’ TRF-IA protocol to identify antibodies to the recombinant E and EDIII proteins of each of the four DENV serotypes, in these three BALB/c immune sera. All three immune sera were found to contain antibodies reactive to recombinant E proteins corresponding to each of the four DENV serotypes. However, the levels of anti-E antibodies were significantly higher in anti-DSV4 antiserum (α-DSV4) in comparison to anti-Dengvaxia (α-Dengvaxia) and anti-TV DENV antisera (α-TV DENV), which were comparable to each other, for all four serotypes (Supplementary file, Fig. S1a). When the same TRF-IA was performed to assess the proportion of anti-E antibodies reactive to EDIII (Supplementary file, Fig. S1b), we found that DSV4 elicited predominantly EDIII-directed antibodies, an observation consistent with its design [37]. In contrast, EDIII was a relatively very minor target of the antibody responses elicited by Dengvaxia and TV DENV.
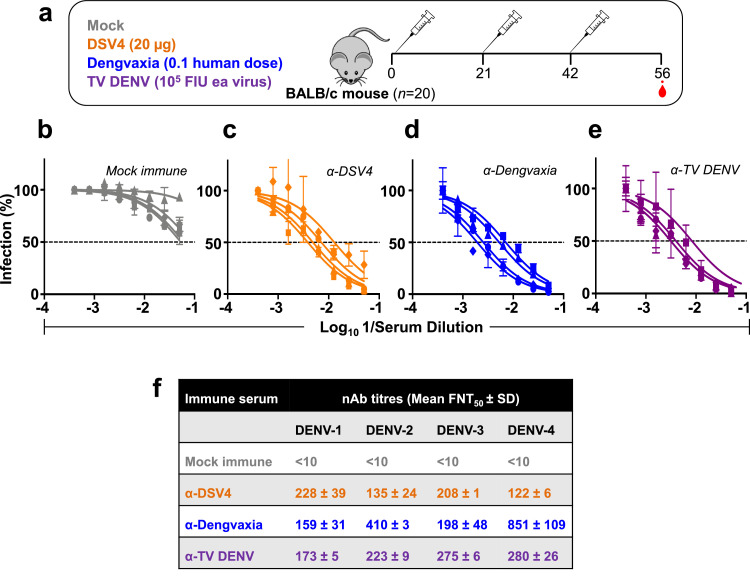
Fig. 1.
Generation of immune sera in BALB/c mice. (a) Schematic representation of the vaccination schedule. Groups of BALB/c mice (n=20) were immunised (i.m.) with three doses of (i) DSV4 (20 μg/dose, formulated in alum); (ii) Dengvaxia (1/10th the human dose); or (iii) TV DENV (a tetravalent mixture containing WHO reference strains of DENV-1, DENV-2, DENV-3 and DENV-4, 105 FIU each). Mice were bled 2 weeks after the last dose for seroanalysis. A fourth group of mice (Mock, n=20) did not receive any vaccine (these received PBS plus alum). (b-e) The graphs depict DENV nAb titration curves determined using mock-immune serum (grey curves, panel ‘b’), α-DSV4 antiserum (orange curves, panel ‘c’), α-Dengvaxia antiserum (blue curves, panel ‘d’) and α-TV DENV antiserum (purple curves, panel ‘e’), against DENV-1 (squares), DENV-2 (circles), DENV-3 (triangles) and DENV-4 (diamonds). (f) The mean nAb titres (± SD, standard deviation) against the four DENV serotypes (DENV-1, -2, -3 and -4), for each immune serum, based on the DENV neutralisation curves shown in panels ‘b-e’. The nAb titres in the mock immune sera, depicted as <10, denote that there was no discernible DENV neutralisation, even at the lowest serum dilution tested, which was 1:10 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Given that we pooled all 20 sera from each immunisation group in the TRF-IA experiment, it is likely that high responders may have masked non-responders. That this was not a major concern was evident from an analysis of several randomly selected single sera and comparing their TRF-IA reactivities to pooled sera (Supplementary file, Fig. S2). Once again, it was evident from the single sera data as well, that almost all of the antibody response elicited by DSV4 was focused on EDIII, unlike that induced by Dengvaxia or TV DENV vaccination.
We next determined the DENV-neutralising activity of these three immune sera (pooled) using a flow cytometry-based assay [37,43]. All three manifested neutralising activity towards each of the four DENV serotypes (Fig. 1c–f). Despite the large difference in total anti-E IgG titres seen for DSV4, in comparison to the remaining two (Dengvaxia and TV DENV), serotype-wise neutralising antibody (nAb) titres induced by all three tetravalent vaccines were more or less comparable to each other, with the exception of Dengvaxia, which elicited relatively higher nAb titres to DENV-4 (Fig. 1f), for unknown reasons.
We have shown earlier that DSV4 elicits predominantly EDIII-directed TS antibodies to each DENV serotypes based on specific deletion of EDIII of a given serotype in the gene design of DSV4 and by selective depletion of anti-EDIII antibodies of a given serotype from α-DSV4 immune serum. In contrast, selective depletion experiments of immune sera from donors after the 3rd dose of Dengvaxia revealed that the vaccine preferentially elicited DENV-4 TS nAbs, which were able to cross-neutralise the remaining three DENV serotypes as well [49].
Taken together, these data reflect that while EDIII-directed anti-E antibodies account for the major neutralising activity in α-DSV4 immune serum, non-EDIII antibodies are mainly responsible for the neutralising activity of the α-Dengvaxia and α-TV DENV immune sera.
3.2. DSV4 can be distinguished from Dengvaxia and TV DENV by in vivo, but not by in vitro ADE assays
Several investigators have shown that ADE activity of human [50,51], macaque [52] and murine [53] polyclonal anti-DENV antibodies in Fcγ receptor-bearing cell line-based assays can be evaluated directly, in appropriately diluted immune sera, without the need to purify the IgG fraction. We examined the ability of each of the three immune sera to promote entry of each of the four DENV serotypes into FcγII receptor-bearing K562 cells (Fig. 2a–d). None of the DENV serotypes infect these cells in the absence of anti-DENV antibodies (Fig. 2a). However, in the presence of suitably diluted immune sera from all three groups of vaccinated BALB/c mice, infection of K562 cells by DENVs of all four serotypes could be discerned. In the case of α-DSV4 immune serum (Fig. 2b), the peak enhancement titre was 1:1080, with 5–19% infected cells. On the other hand, both α-Dengvaxia (Fig. 2c) and α-TV DENV (Fig. 2d) immune sera mediated DENV infection of K562 cells to comparable extents, at lower dilutions. The peak enhancement titre was 1:120 for α-Dengvaxia immune serum (with 14-18% DENV-infected cells) as well as α-TV DENV immune serum (with 16–21% DENV-infected cells), as summarised in Supplementary file, Table S1. Collectively, the data from the in vitro studies, using the K562 assay system, revealed that all three immunogens, regardless of whether they are live virus-based (Dengvaxia and TV DENV) or recombinant protein-based (DSV4), manifest comparable ADE potential.
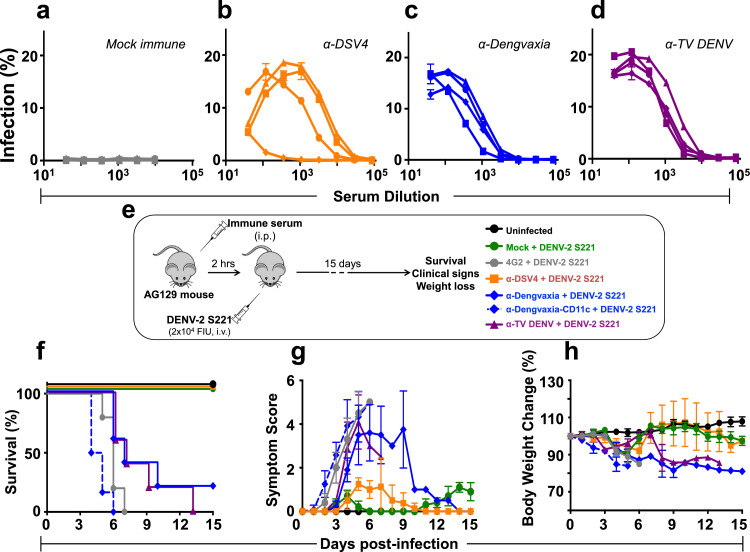
Fig. 2.
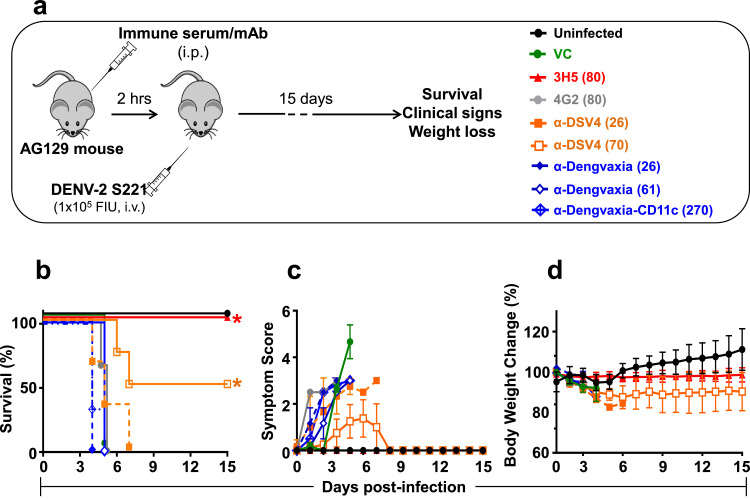
Analysis of the capacity of anti-DSV4, anti-Dengvaxia and anti-TV DENV immune sera to enhance DENV infection in vitro and in vivo. (a–d) The graphs depict infection profiles of K562 cells by DENV-1 (squares), DENV-2 (circles), DENV-3 (triangles) and DENV-4 (diamonds) as a function of dilution of mock- (grey curves, panel ‘a’), α-DSV4- (orange curves, panel ‘b’), α-Dengvaxia- (blue curves, panel ‘c’) and α-TV DENV (purple curves, panel ‘d’) immune sera (raised in BALB/c mice), determined by flow cytometry. (e-h) Groups of AG129 mice (n=5) were administered either mAb 4G2 (grey curves), mock- (green curves), DSV4- (orange curves), Dengvaxia- (blue curves) or TV DENV- (purple curves) immune sera by passive transfer (10 µg mAb or 20 µl immune serum in a final volume of 200 µl 1x PBS), then challenged with a sub-lethal dose of DENV-2 S221 (panel ‘e’) and monitored for survival (panel ‘f’), clinical signs (panel ‘g’) and body weight change (panel ‘h’) over the next 15 days. A group of mice which received no treatment (Uninfected, black curves) was monitored in parallel for comparison. Survival data (in panel ‘f’) were analysed by Log-Rank (Mantel-Cox) test for significant difference in survival rates. Survival of mice which received either mock immune serum or α-DSV4 before challenge was not significantly different from that of Uninfected mice (100% survival). In comparison, survival was significantly reduced (p<0.05) in mice which received α-Dengvaxia immune serum and very significantly reduced (p<0.002) in mice which received α-TV DENV immune serum or mAb 4G2. In panel ‘g’, clinical scores were based on a 5 point system, with the maximum score denoting death: 0.5, mild ruffled fur; 1.0, ruffled fur; 1.5, compromised eyes; 2, compromised eyes with hunched back; 2.5, loose stools; 3.0, limited movement; 3.5, no movement/hind leg paralysis; 4.0, euthanised if cumulative score was 5. In panel ‘h’, body weight was monitored twice a day in the morning and evening, and the mean taken for plotting. Note: in panels ‘e-h’ a second pool of anti-Dengvaxia immune serum (α-Dengvaxia-CD11c+DENV-2 S221, dashed blue curves), raised by immunising CD11c-Ifnar1−/− mice, was also tested (all other murine immune sera are from BALB/c mice) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Unlike in the K562 cell-based system wherein DENV entry is solely dependent on the presence of anti-DENV antibodies, DENV entry into cells in vivo occurs via both antibody-dependent and antibody-independent pathways. This underlines the relevance of assessing ADE in vivo as well. In this regard, immune-compromised mice strains, such as the interferon (IFN) α/β receptor-lacking C57BL/6 mice [54] and the IFN α/β and γ-receptor double knockout mouse (AG129) [55] are dengue-sensitive and may serve as tools in evaluating ADE in vivo.
Two groups have independently established the utility of the AG129 mouse as an in vivo model system which upon a sub-lethal infection with a mouse-adapted DENV-2 strain, in the presence of anti-DENV antibodies, recapitulates the essential hallmarks of ADE-mediated severe dengue disease in humans [13,14]. These hallmark features include high viral burden, cytokine storm, thrombocytopenia, increased vascular leakage, intestinal haemorrhage, and death.
We used the AG129 model to evaluate the in vivo ADE potential of α-DSV4, α-Dengvaxia and α-TV DENV immune sera. Groups (n=5) of these mice received different immune sera by passive transfer (20 µl/mouse, i.p), challenged 2 h later with a sub-lethal dose of the mouse-adapted challenge strain DENV-2 S221 [14] and monitored for 15 days (Fig. 2e). As a positive control, one group of AG129 mice received the CR mAb 4G2, documented to cause ADE in the AG129 model [13,14]. As a negative control, an uninfected group (which received neither the passive transfer of any immune serum or the sub-lethal virus challenge) was monitored throughout the experiment.
Data on survival, clinical signs and body weight change of the different groups are presented in Fig. 2 (panels f-h). Mice that had received mock-immune serum before challenge with the sub-lethal dose of DENV-2 S221 (‘Mock + DENV-2 S221’ group, green curves) displayed 100% survival, similar to the mice in the ‘uninfected’ group (black curves), but with very mild disease in terms of clinical signs and body weight loss. Survival between these two groups was not statistically significant (p>0.9999, Mantel-Cox test). As reported earlier, prior transfer of 10 µg of the CR mAb 4G2 resulted in escalation of the subsequent sub-lethal virus challenge, resulting in 100% mortality by day 7, preceded by very severe clinical symptoms and weight loss (‘4G2 + DENV-2 S221’ group, grey curves). Compared to the ‘Mock + DENV-2 S221’ group, survival in the ‘4G2 + DENV-2 S221’ group was very significantly reduced (p=0.002). Interestingly, all mice which received prior passive transfer of α-DSV4 immune serum also survived the sub-lethal challenge, with only mild symptoms and without discernible body weight loss (‘α-DSV4 + DENV-2 S221’ group, orange curves). In striking contrast, the prior transfer of α-Dengvaxia (‘α-Dengvaxia + DENV-2 S221’ group, blue curves) and α-TV DENV (‘α-TV DENV + DENV-2 S221’ group, purple curves) immune sera, sensitised the mice to the sub-lethal viral challenge, leading to drastically increased mortality accompanied by severe clinical symptoms and body weight loss. Compared to survival of AG129 mice in the ‘α-DSV4 + DENV-2 S221’ group, survival in the ‘α-Dengvaxia + DENV-2 S221’ (p=0.0249) and ‘α-TV DENV + DENV-2 S221’ (p=0.0045) groups, were significantly reduced (Mantel-Cox test). The data suggest that antibodies induced by DSV4 do not cause ADE in the AG129 model, whereas, antibodies elicited by Dengvaxia and TV DENV do.
The immunogenicity of Dengvaxia and TV DENV, both based on live viruses, is dependent on their capacity to replicate. However, as the BALB/c mouse does not support DENV replication, it is unlikely that Dengvaxia and TV-DENV may have replicated to any significant extent. Thus, the antibodies elicited by Dengvaxia and TV-DENV, may be qualitatively different from those elicited in the context of viral replication. This difference may perhaps underlie the observation that BALB/c antibodies elicited by Dengvaxia and TV DENV promote ADE in AG129 mice. To address this concern, we immunised CD11c-Ifnar1−/− mice with Dengvaxia (as a representative live virus-based tetravalent formulation). This mouse strain lacks Ifnar1 selectively in CD11c-expressing DCs alone and supports DENV-2 replication [42]. After demonstrating that the CYD-2 component of Dengvaxia replicates in this mouse, we collected immune sera and found that it elicits potent nAbs against all four DENVs (Supplementary File Fig. S3). This immune serum, designated as α-Dengvaxia-CD11c (Fig. 2, panels ‘f-h’, dashed blue curves), also displayed significant ADE-promoting capacity. This leads to the conclusion that antibodies induced by the live virus formulations, irrespective of their replication potential in the immunised host, behave similarly in terms of their ADE-promoting capacity.
In the passive transfer experiment above, it may be inferred that circulating nAb titres after passive transfer, would have been very low (based on nAb titres shown in Fig. 1e and Supplementary File Fig. S3 and the low volumes of immune sera transferred). It is thus possible to explain the above data with the argument that circulating nAb levels were low enough to result in ADE in mice receiving α-Dengvaxia and α-TV DENV immune sera, and perhaps vanishingly low to be of any consequence, in the case of mice receiving α-DSV4. This raises the question whether ADE in AG129 mice may be observed if nAbs are present at levels adequate to neutralise the sub-lethal dose of DENV-2 S221. Clearly, given the nAb titres detected in the murine immune sera we generated, the passive transfer strategy to achieve adequate circulating nAb titres is not feasible. We therefore utilised an alternate approach reported earlier, which bypasses the need to achieve high circulating nAb titres [56].
This approach is based on first generating ICs in vitro by pre-incubating the sub-lethal DENV-2 S221 dose with the desired amount of immune serum to achieve either partial or full neutralisation of the virus and then injecting the ICs intravenously into AG129 mice. It has been reported earlier [56] that injection of ICs made with mAb 4G2 at levels adequate to fully neutralise the sub-lethal dose of DENV-2 S221, into the AG129 mouse, recapitulates all the characteristic hallmarks of severe dengue in humans described in the passive transfer ADE model [13,14]. It was also reported that under the experimental conditions used, ICs made using UV-inactivated DENV-2 S221 did not cause any mortality, ruling out the possibility that the validity of this approach is compromised by type III hypersensitivity reaction to IC inoculation [56].
Results of an AG129 mouse ADE assay based on the IC inoculation approach are presented in Fig. 3. A sub-lethal dose of DENV-2 S221, with or without pre-incubation with mock-immune serum (virus control, ‘VC’, green curves) is well-tolerated by AG129 mice, which exhibit no mortality and very mild illness and body weight loss compared to mice which did not receive any ICs (‘Uninfected’, black curves). Fully neutralised ICs made by pre-incubating the sub-lethal dose of DENV-2 S221 with 10 µg mAb 4G2 were injected into another control group (‘4G2 ICs’ group, grey curves). These mice suffered 100% mortality very rapidly accompanied by severe signs of illness, consistent with the findings of the passive transfer experiment above (Fig. 3b). Mortality in the ‘4G2 ICs’ group compared to the ‘VC’ group was very significantly high, based on the Mantel-Cox test of the survival curves (p=0.001).
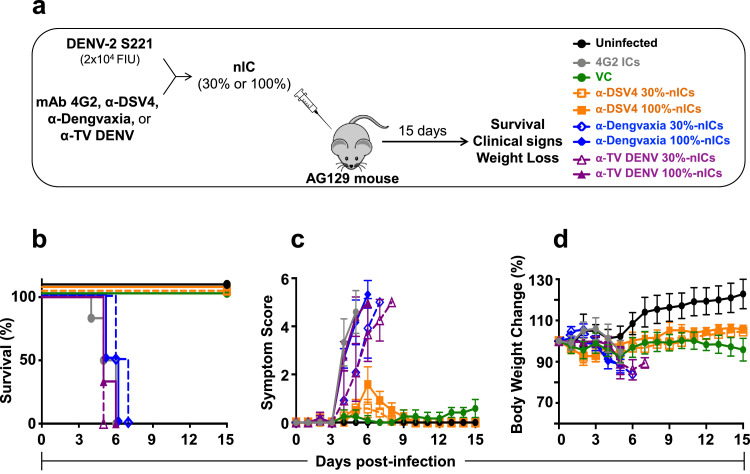
Fig. 3.
Evaluation of in vivo ADE capacity of BALB/c immune sera by inoculation of nICs into AG129 mice. Groups of AG129 mice (n=6) were given intravenous (retro-orbital) injections of nICs, generated by pre-incubating 20,000 FIUs of DENV-2 S221 with α-DSV4 antiserum, α-Dengvaxia antiserum or α-TV DENV antiserum, on day 0 (panel ‘a’). The amounts of antisera used were such that the virus in the nIC was either fully (100%, using ~5 µl immune serum) or partially (30%, using 0.08–0.32 µl immune serum) neutralised (based on residual infectivity measured using the Vero cell-based FACS assay). In parallel, control groups of mice which received no treatment (Uninfected), only the virus in the absence of any antiserum (virus control, VC) or nICs (containing 100% neutralised virus) generated using the FLE-specific CR mAb 4G2 (10 µg), were also included. The mice were monitored for survival (panel ‘b’, Kaplan-Meir survival analysis), clinical symptoms (panel ‘c’) and body weight change (panel d’), for up to 15 days post nIC inoculation. In panels ‘b–d’, the data for the different groups are indicated as follows: the Uninfected (black curves), VC (green curves) and mAb4G2-IC (grey curves) groups are shown by solid curves in different colours as indicated. For groups receiving nICs made using α-DSV4 (orange curves), α-Dengvaxia (blue curves) or α-TV DENV (purple curves) antisera, those receiving the 100% and 30% neutralised ICs, are indicated by solid and dashed curves, respectively. Survival data (panel ‘b’) were analysed by Log-Rank (Mantel-Cox) test for significant difference in survival rates. Survival of mice in α-DSV4 30% and 100% nIC groups was not significantly different from that of uninfected mice. In comparison, survival of mice in all the other experimental groups was significantly compromised (p≤0.001). Clinical scoring in panel ‘c’ and body weight measurements in panel ‘d’, were as described in Fig. 2 legend (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
We pre-incubated sub-lethal doses of DENV-2 S221 with calculated volumes of immune sera (based on their DENV-2 S221-specific neutralisation titres) from vaccinated mice to obtain neutralised ICs (nICs) containing either 100% neutralised (100% nICs) or 30% neutralised (30% nICs) DENV-2 S221. The data revealed that AG129 mice administered either 30% nICs (dashed orange curve) or 100% nICs (solid orange curve) made using α-DSV4 immune sera did not show any mortality, similar to the ‘VC’ and ‘Uninfected’ groups (Fig. 3b). Of note, this is consistent with the lack of any possible type III hypersensitivity reaction. It is noteworthy that the 30% nICs made with α-DSV4 immune serum, to simulate sub-neutralising antibody concentrations, did not escalate the sub-lethal DENV-2 S221 infection. However, these two groups (‘α-DSV4 30%’ and ‘α-DSV4 100% nICs’ groups) of mice showed mild clinical signs, but no notable body weight loss (Fig. 3c,d, dashed and solid orange curves).
On the other hand, mice receiving nICs, made using α-Dengvaxia (blue curves) and α-TV DENV (purple curves) immune sera, regardless of whether the DENV-2 S221 virus in the nICs was 100% neutralised (solid curves) or 30% neutralised (dashed curves), manifested high mortality (Fig. 3b), severe clinical signs (Fig. 3c) and weight loss (Fig. 3d), akin to those in the ‘4G2 nIC’ group. Survival was significantly decreased in mice in the ‘α-Dengvaxia 30%%nICs’ (p=0.0009), ‘α-Dengvaxia 100%%nICs’ (p=0.0009), ‘α-TV DENV 30% nICs’ (p=0.0009), ‘α-TV DENV 100% nICs’ (p=0.0006), and the ‘4G2 nICs’ (p=0.001) groups, compared to either of the ‘α-DSV4 nICs’ groups, by the Mantel-Cox test. It may be plausible that the severe mortality associated with 30% nICs containing α-Dengvaxia (dashed blue curve) and α-TV DENV (dashed purple curve) immune sera, may be the consequence of partial neutralisation of the DENV-2 S221 virus. However, the 100% mortality evident in the groups receiving 100% nICs, made with α-Dengvaxia (solid blue curves) and α-TV DENV (solid purple curves) immune sera is intriguing, because the DENV-2 S221 in these ICs is fully neutralised. A supplementary media file showing videos of AG129 mice administered different 100% nICs is provided online (Movie S1).
Collectively, we draw two conclusions from the data presented above: (i) the passive transfer and nIC inoculation approaches to the AG129 ADE assay yield comparable results; and (ii) though all three immune sera possess comparable DENV-2 neutralising antibody titres (Fig. 1e), there is a qualitative difference between the antibodies in α-DSV4 immune serum (mainly EDIII-directed TS antibodies) [37] and the α-Dengvaxia and α-TV DENV immune sera (mainly prM- as well as FLE-directed CR antibodies plus TS antibodies), in terms of the ADE potential they manifest in the AG129 mouse model.
3.3. α-Dengvaxia and α-TV DENV immune sera cause ADE by increasing serum viremia and intestinal haemorrhage
A characteristic hallmark of ADE in the AG129 mouse model is the increased DENV viremia and virus load in the small intestine, associated with elevated levels of pro-inflammatory cytokines, TNF-α and IL-6, and evidence of severe small intestinal haemorrhage [13,14,56]. We examined these parameters signifying ADE on day 4 post-nIC inoculation in parallel groups of AG129 mice, administered the same types of nICs described in Fig. 3a. Compared to the ‘VC’ group, serum viremia was in general elevated in the ‘α-Dengvaxia’ and ‘α-TV DENV’ nIC groups, regardless of whether the virus was partially or fully neutralised (Fig. 4a). The maximum elevation in serum viremia was observed in the ‘α-Dengvaxia 30% nICs’ group, compared to the ‘VC’ group (p=0.0002, two-way ANOVA). Serum viremia in the ‘α-DSV4 30% nICs’ and ‘α-DSV4 100% nICs’ groups were comparable and lower than that in the ‘VC’ group. However, this was not statistically significant.
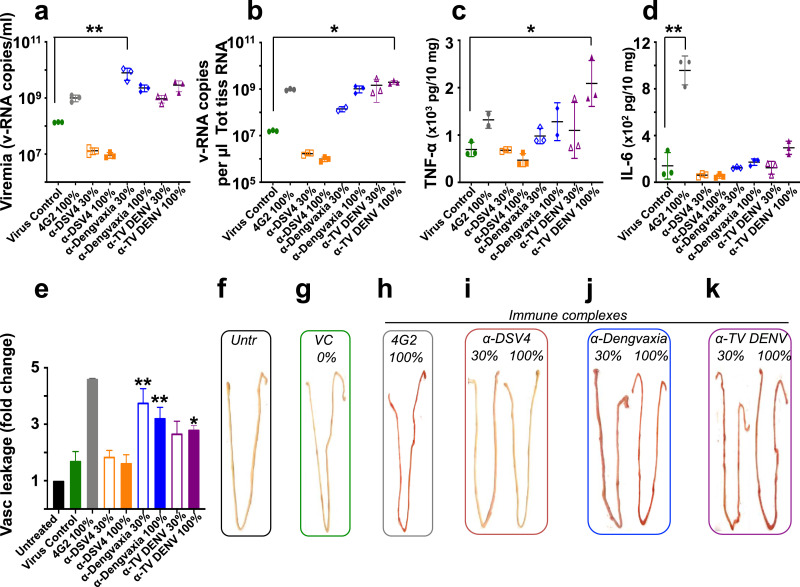
Fig. 4.
Analysis of serum viremia, virus load, cytokine production and vascular leakage in the small intestines of nIC-inoculated AG129 mice. Groups of mice (n=3) corresponding to each of the experimental groups described in Fig. 3 were set up in parallel and euthanised on day 4 post-nIC inoculation for serum viremia determination (panel ’a’), determination of virus load (panel ‘b’), TNF-α (panel ‘c’) and IL 6 (panel ‘d’) production and vascular leakage (quantitative analysis in panel ‘e’; qualitative analysis in panel ‘f-k’) in the small intestines. Vascular leakage was quantified by measuring the amount of Evan's blue extravasation into the small intestines by spectrophotometry. Vascular leakage is expressed as fold change in small intestinal dye content with reference to that in the ‘Untreated’ mice, taken as 1.0. The single and double asterisks (panels ‘a-e’; in all cases, the test groups are compared to the ‘Virus Control’ group) denote statistically significant and very significant differences, respectively, with reference to the virus control group (using the two-way ANOVA test with Bonferroni correction). In all other nIC groups, differences with reference to the cognate virus control group were not significant. The percent neutralisation of the challenge virus is indicated as 0 (panel ‘g’), 30 or 100 (panels ‘h-k’) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
A more or less similar pattern of relative virus loads was discernible in the small intestines of mice in the different groups (Fig. 4b). Tissue viral loads in small intestines of ‘α-DSV4 30% nICs’ and ‘α-DSV4 100% nICs’ groups were comparable to each other and lower than that in the ‘VC’ group, which in turn was lower than those in the remaining four groups. However, the highest tissue viral load was seen in the ‘α-TV DENV 100% nICs’ group, compared to the ‘VC’ group (p=0.005, two-way ANOVA). Collectively, the viremia and tissue virus load data suggest that antibodies in α-DSV4 immune serum not only do not promote ADE in AG129 mice, but also tend to suppress virus replication. That this occurs even when DENV-2 S221 was only partially neutralised (‘α-DSV4 30% nICs’ group), points to the protective capacity of antibodies in the α-DSV4 immune serum. This contrasts sharply with the elevated viremia and tissue virus loads in the ‘α-Dengvaxia’ and ‘α-TV DENV’ nIC groups, even when the virus is 100% neutralised, and underlines the significance of the quality of antibodies in the immune sera, in determining the outcome of a sub-lethal DENV-2 infection.
Small intestinal TNF-α levels generally mirrored the relative pattern observed for serum viremia and tissue virus loads, in all of the different groups, with elevated levels in the groups receiving 30% and 100% nICs made using immune sera from Dengvaxia- and TV DENV-vaccinated mice (Fig. 4c). The highest levels of TNF-α production were found in the small intestines of mice in the ‘α-TV DENV 100% nICs’ group. In comparison to this, TNF-α levels in small intestinal extracts from the ‘α-DSV4 30% nICs’ (p=0.0015) and ‘α-DSV4 100% nICs’ (p=0.0003) groups were significantly lower (two-way ANOVA). With regard to IL-6 production in the small intestine, the highest levels were observed in the ‘4G2 100% nICs’ group (p<0.05). The elevation in IL-6 levels, relative to those in the ‘VC’ group, was muted in the groups receiving 30% and 100% nICs made using α-Dengvaxia and α-TV DENV immune sera. In the groups receiving nICs made with α-DSV4 immune serum, IL-6, like TNF-α, was lower than that in the ‘VC’ group (Fig. 4d). The reason for the wide difference in IL-6 levels between the ‘4G2 100% nICs’ group on the one hand, and all remaining groups, on the other, is not clear. It is likely a reflection of monoclonal versus polyclonal antibody differences.
Finally, we examined the mice for evidence of intestinal haemorrhage, using the Evan's blue dye method to measure plasma extravasation. The results are expressed as fold increase in vascular leakage, using the Evan's blue levels in the intestines of untreated mice as the baseline (Fig. 4e). This revealed that the levels of small intestinal plasma leakage in both ‘α-DSV4 30% nICs’ and ‘α-DSV4 100% nICs’ groups were comparable to each other, and similar to that in the ‘VC’ group, attesting to lack of escalation of the sub-lethal DENV-2 S221 infection. In contrast to this, small intestines from mice which had received 30% and 100% nICs made using α-Dengvaxia and α-TV DENV immune sera, showed increased plasma leakage. Compared to the ‘VC’ group, plasma leakage in the ‘α-Dengvaxia 30% nICs’ (p<0.001), ‘α-Dengvaxia 100% nICs’ (p=0.0016), and ‘α-TV DENV 100% nICs’ (p=0.0284) groups was significantly elevated (Two-way ANOVA with Bonferroni's correction).
These findings (Fig. 4e) are mirrored by the visual appearance of small intestines obtained from one representative mouse of each experimental group (Fig. 4f–k). Small intestines from mice in the ‘α-DSV4 30% nICs’ and ‘α-DSV4 100% nICs’ groups were visually indiscernible from those of mice in the ‘Uninfected’ and ‘VC’ groups. In contrast prominent signs of haemorrhage are clearly visible in the small intestines of mice form all other groups. Taken together, the data show that antibodies in α-DSV4 immune serum, even at sub-neutralising levels, did not cause significant ADE in vivo. In striking contrast, antibodies in α-Dengvaxia and α-TV DENV immune sera, even at levels adequate to fully neutralise the sub-lethal dose of DENV-2 S221, possess the potential to cause ADE, characterised by increase in viremia, small intestinal virus load and TNF-α levels, and vascular leakage, leading to death. Intuitively, it would appear that antibodies lacking ADE activity may likely offer protective efficacy whereas ADE-promoting antibodies are unlikely to do so.
3.4. Antibodies induced by DSV4, but not Dengvaxia, protect against lethal DENV-2 challenge
Next, we tested the protective efficacy of the antibodies in α-Dengvaxia and α-DSV4 immune sera in a lethal challenge experiment (Fig. 5a). Groups of AG129 mice (n=3 or 4) received by passive transfer, α-Dengvaxia or α-DSV4 immune sera, each at two dosage levels, 100 and 300 µl per mouse, to result in two different pre-challenge DENV-2 nAb titres (low titre of ~25 and moderate titre of ~60–70) in the circulation. Paucity of adequate α-DSV4 immune sera precluded passive transfers to achieve higher circulating nAb titres. In parallel, two more groups received passive transfer of the CR mAb 4G2 and the TS mAb 3H5 (100 µg each/mouse, which resulted in circulating DENV-2 nAb titres of 80 each). We also included another group which received the previously described α-Dengvaxia-CD11c immune serum (achieving a high circulating nAb titre of 270). All these mice were challenged 2 h post-passive transfer with a lethal dose of DENV-2 S221 (105 FIU/mouse), established in preliminary experiments. One group, which did not receive any mAb or immune serum, received only the lethal challenge dose (‘VC’ group). All groups, including a group which received neither passive mAb/immune serum transfer nor the lethal virus dose (‘Uninfected’ group), were monitored for survival, clinical signs and body weight change for up to 15 days post-challenge (Fig. 5b–d).
Fig. 5.
The capacity of α-DSV4 and α-Dengvaxia immune sera to confer protection against lethal DENV-2 S221 challenge. (a) Schematic representation of the experimental design. Groups of AG129 mice (n=3 or 4) were administered (i.p) BALB/c immune sera (in two dosage levels, 100 µl and 300 µl/mouse) or mAbs (100 µg/mouse). The immune sera used were anti-DSV4 (α-DSV4, orange) and anti-Dengvaxia (α-Dengvaxia, blue) and the mAbs used were a DENV-2 TS mAb (3H5, red) and a pan-DENV CR mAb (4G2, grey). Two hours after passive transfer the mice were bled (for the determination of circulating DENV-2 nAb titers, shown in parenthesis in the legends in panel ‘a’), and then challenged shortly thereafter with a lethal dose (105 FIU/mouse) of DENV-2 S221. All groups were monitored over the next 15 days for survival (Kaplan-Meir survival curves, panel ‘b’), clinical symptoms (panel ‘c’) and body weight change (panel ‘d’). In parallel, one group received just the challenge virus (VC, green), and another group received neither passive antibody transfer nor the challenge virus (Uninfected, black). In panel ‘b-d’, data for mice which received 100 µl and 300 µl immune sera are shown using dashed and solid curves, respectively, in the indicated colours. Survival data (panel ‘b’) were analysed by Log-Rank (Mantel-Cox) test for significant difference in survival rates [the asterisks denote that survival in the 3H5 and α-DSV4 (70) groups were significantly higher in comparison to the 4G2 group (p<0.05), for both]. Clinical scoring in panel ‘c’ and body weight measurements in panel ‘d’, were as described in Fig. 2 legend. Note: a second pool of anti-Dengvaxia immune serum (α-Dengvaxia-CD11c, dashed blue curves), raised by immunising CD11c-Ifnar1−/− mice, was also tested for its efficacy against lethal DENV-2 S221 challenge (other murine immune sera are from BALB/c mice) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Mice in the ‘VC’ group developed severe clinical symptoms rapidly and succumbed completely to the lethal challenge by post challenge day 5. Mice in the ‘4G2’ group also showed rapid and 100% mortality, though the severity of the symptoms, which became apparent earlier, was lesser, compared to the ‘VC’ group. In contrast, all mice in ‘3H5’ group were fully protected from the lethal challenge with no discernible clinical symptoms or body weight change. Survival of mice in the ‘3H5’ group was essentially indistinguishable from that in the ‘Uninfected’ group (p>0.9999, Mantel-Cox text). It is interesting that in the case of mice in the ‘3H5’ and ‘4G2’ groups, circulating DENV-2 nAb titres were the same at 80, yet, survival outcomes were diametrically opposite.
Mice in the two ‘α-Dengvaxia’ groups with circulating DENV-2 nAb titres of 26 and 61, were similar to the ‘VC’ and ‘4G2’ groups in that they also manifested 100% mortality quite rapidly. Interestingly, elevating the circulating DENV-2 nAb titre to 270 using α-Dengvaxia-CD11c immune serum did not offer any protective advantage. It is also noteworthy that in the latter instance anti-Dengvaxia antibodies were produced in the context of ongoing viral replication (using the CD11c-Ifnar1−/− mice). Altogether, antibodies in α-Dengvaxia immune sera, regardless of the mouse host (BALB/c or CD11c-Ifnar1−/−) in which they were raised, over a ten-fold range of circulating DENV-2 nAb titres (26–270), failed to offer any protection against lethal DENV-2 challenge. The survival of mice in all three ‘α-Dengvaxia’ groups was significantly compromised, in comparison to that of mice in the ‘3H5’ or ‘Uninfected’ groups (p<0.05, Mantel-Cox test).
In contrast, mice in the two α-DSV4 groups manifested a dose-dependent protective effect. Low circulating DENV-2 nAb titre of 26 slightly delayed mortality compared to that in the ‘VC’ and ‘4G2’ groups. However, survival was not significantly different from that observed in the ‘VC’ or ‘4G2’ groups (p=0.6149, Mantel-Cox test). On the other hand, increasing the circulating DENV-2 nAb titres to a moderate level (DENV-2 nAb titre of 70), suppressed symptoms to much milder levels and improved survival dramatically, with 50% survival at the end of 15 days. The survival of mice in the ‘α-DSV4’ group at a circulating DENV-2 nAb titre of 70 was significantly better than that in the ‘4G2’ group, at a comparable circulating DENV-2 nAb titre of 80 (p=0.014) and similar to that in the ‘Uninfected’ group (p=0.1869).
Collectively, the data demonstrate that antibodies in α-DSV4 immune serum do possess the potential to offer statistically significant protection against lethal DENV-2 S221 challenge when circulating DENV-2 nAb titres ≥70. At comparable (nAb titre=61) and much higher (nAb titre=270) circulating DENV-2 nAb titres, antibodies in α-Dengvaxia immune sera failed to offer any protection. It is also evident that the quality of the antibodies elicited by Dengvaxia in two mice hosts, one that precludes and the other that permits viral replication, is similar in terms of the survival outcome in the lethal DENV-2 S221 challenge assay.
3.5. α-Dengvaxia- and α-TV DENV, but not α-DSV4 antisera, neutralise ZIKV in vitro
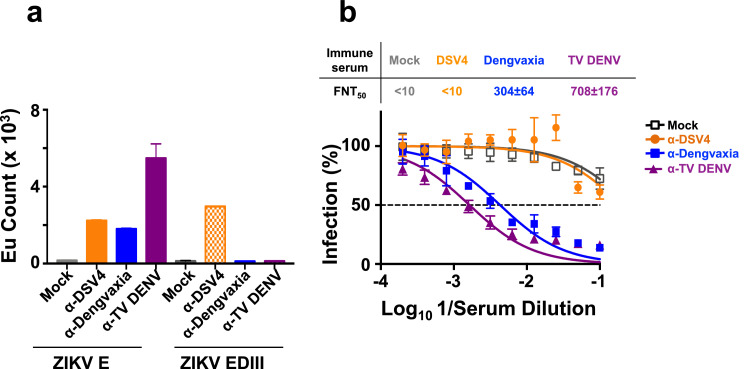
As it is regarded that pre-existing anti-DENV antibodies may have had a role to play in the ZIKV outbreaks during 2015/2016 [38,39], we next examined if antibodies induced by DSV4, Dengvaxia and TV-DENV can interact with ZIKV. Pooled immune sera from BALB/c mice vaccinated with DSV4, Dengvaxia and TV-DENV were tested for reactivity towards recombinant ZIKV E and EDIII antigens in the TRF-IA (Fig. 6a). All three immune sera manifested cross-reactivity towards ZIKV E. Of these, maximal cross-reactivity towards ZIKV E was manifested by α-TV DENV immune serum. The cross-reactivity of α-TV DENV immune serum was significantly higher (unpaired two-tailed t test) than that of α-Dengvaxia (p=0.0203) and α-DSV4 (p=0.0259) immune sera. Importantly, the cross-reactivity of α-DSV4 immune serum towards ZIKV E was exclusively directed towards its EDIII. In contrast, only a very small fraction of ZIKV E-reactive antibodies in α-Dengvaxia and α-TV DENV immune sera displayed reactivity towards ZIKV EDIII (2–7%). These observations were consistent when several randomly selected individual sera from the three immunisation groups were tested in the TRF-IA for reactivity against recombinant ZIKV E and ZIKV EDIII proteins (Supplementary File Fig. S4).
Fig. 6.
The capacity of α-DSV4, α-Dengvaxia and α-TV DENV immune sera to bind to recombinant ZIKV E and EDIII proteins and neutralise ZIKV in vitro. (a) TRF-IA of pooled BALB/c mock-immune sera (mock, grey), anti-DSV4 (α-DSV4, orange), anti-Dengvaxia (α-Dengvaxia, blue) and anti-TV DENV (α-TV-DENV, purple) antisera using insect cell-expressed recombinant ZIKV E protein (ZIKV E) and yeast-expressed ZIKV EDIII (ZIKV EDIII) as the capture antigens. Bound total antibodies were detected using anti-mouse IgG-Eu3+ conjugate by TRF. (b) The graph depicts nAb titration curves determined using mock-immune serum (grey curve), α-DSV4 antiserum (orange curve), α-Dengvaxia antiserum (blue curve) and α-TV DENV antiserum (purple curve), against ZIKV PRVABC59. The mean ZIKV nAb titers (FNT50 ± SD) are shown above the graph (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
As the next step we measured the ability of these immune sera to neutralise ZIKV using the FACS-based assay as done with DENVs. The data revealed that, despite its ability to bind to ZIKV E, α-DSV4 immune serum failed to neutralise ZIKV (FNT50 <10). On the other hand, α-Dengvaxia and α-TV DENV immune sera were capable of neutralising ZIKV efficiently, with mean FNT50 titres of 304 and 708, respectively (Fig. 5b). This suggests that the cross-neutralising activity against ZIKV is associated with the non-EDIII-directed (directed to E epitopes outside EDIII) antibodies present in the α-Dengvaxia and α-TV DENV immune sera.
3.6. α-Dengvaxia- and α-TV DENV, but not α-DSV4 antisera, promote ADE of ZIKV infection in vivo
That α-DSV4 immune serum which cross-reacted with ZIKV E, but was not capable of neutralising ZIKV, offers a situation wherein ADE of ZIKV infection is potentially possible. Would α-DSV4 immune serum enhance ZIKV infection? Also, what would the effect of the ZIKV E CR antibodies, present in α-Dengvaxia and α-TV DENV immune sera, be on ZIKV infection? To address these questions, we used the C57BL/6 Stat2−/− mouse, which has been established as a model system to assess ADE of ZIKV infection in vivo. This mouse is sensitive to infection by ZIKV strain PRVABC59, but manifests only mild illness. However, in the presence of passively transferred human anti-DENV antibodies, the mild illness caused by ZIKV PRVABC59 turns severe, marked by enhanced clinical symptoms, body weight loss and high fatality, comparable to that caused by the pathogenic ZIKV MR766 strain [47].
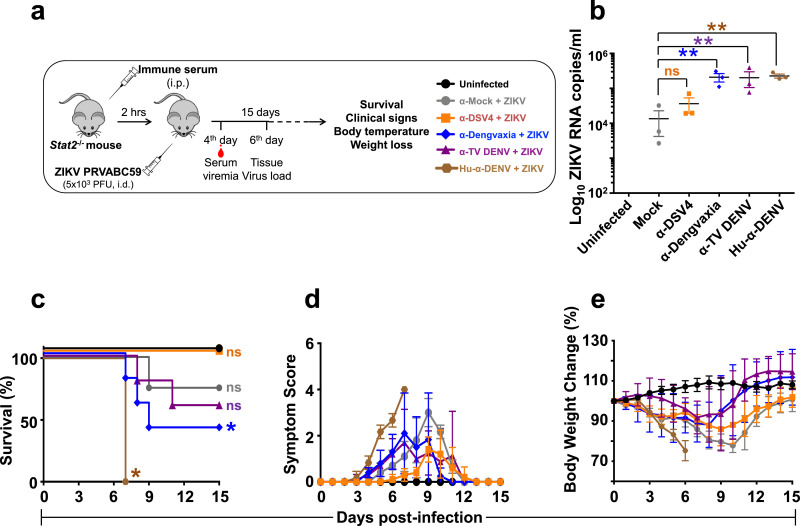
Groups of Stat2−/− mice (n=6-8), which received passive transfer of mock, α-DSV4, α-Dengvaxia, α-TV DENV murine immune sera or human DENV-positive (Hu-α-DENV) plasma, were challenged 2 h later with ZIKV PRVABC59 and monitored for 15 days (Fig. 7a). One group of Stat2−/− mice which received neither immune serum nor ZIKV challenge (‘Uninfected’) was monitored in parallel as a reference. A subset of animals was bled on day 4 post ZIKV challenge for determination of ZIKV RNA levels in plasma by RT-qPCR (Fig. 7b). Plasma ZIKV RNA levels in mice which had received Hu-α-DENV plasma was significantly elevated compared to those in mice which received mock-immune serum (p<0.0001). Plasma ZIKV RNA levels in mice which had received α-Dengvaxia or α-TV DENV immune sera were comparable to those in mice which had received Hu-α-DENV plasma. In contrast, plasma ZIKV RNA levels in mice which received α-DSV4 were similar to those in mice which received mock-immune serum (p>0.9999). This indicates that while the CR antibodies in α-Dengvaxia, α-TV DENV murine immune sera contributed to increased ZIKV replication, those in α-DSV4 immune serum did not.
Fig. 7.
The capacity of α-DSV4, α-Dengvaxia and α-TV immune sera to cause ADE of ZIKV infection in C57BL/6 Stat2−/− mice. (a) Groups (n=6-8) of C57BL/6 Stat2−/− mice were administered mock- (grey), α-DSV4- (orange), α-Dengvaxia- (blue), α-TV DENV- (purple) immune sera or dengue patient plasma (‘Hu-α-DENV’, brown), by passive transfer (20 µl/mouse), two hours before infection with ZIKV strain PRVABC59. A subset (n=3) of challenged mice were bled on day 4 (see panel ‘b’) and euthanised on day 6 post ZIKV challenge (see Fig. 8). The rest of the challenged mice were monitored for up to 15 days (see panels ‘c-e’). (b) Day 4 post infection ZIKV viral RNA in serum, isolated from a subset (n=3) of mice of each of the experimental groups shown in panel ‘a’, was quantified by RT-qPCR as described in methods. ZIKV RNA levels were determined with reference to an in vitro-transcribed RNA standard. Each data point denotes viremia level in a single mouse. The horizontal lines represent the mean viremia levels. ‘ns’ (not significant) and the twin asterisk symbol (very significant), denote statistical significance between the groups indicated, determined by two-way ANOVA with Bonferroni's correction. The rest of the infected mice were monitored for survival (panel ‘c’, ‘ns’ and the single asterisk denote ‘not significant’ and ‘significant’ survival differences for each of the groups with reference to survival of ‘Uninfected’ mice which was 100%), clinical signs (panel ‘d’) and body weight change (panel ‘e’), over the next 15 days. A group of mice which received no treatment (Uninfected, black curves) was monitored in parallel for comparison. In panel ‘d’, clinical scores were based on a 4-point system to grade signs of illness: 0, no symptom; 0.5, mild ruffling of fur; 1.0, ruffled fur; 1.5, compromised eyes; 2.0, hunched back position; 2.5, very limited movement/one leg paralysis; 3.0, no movement and paralysis of both hind legs; 4.0, dead or euthanised. In panel ‘e’, body weight was monitored daily at the same time and expressed as a percent of initial body weight (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
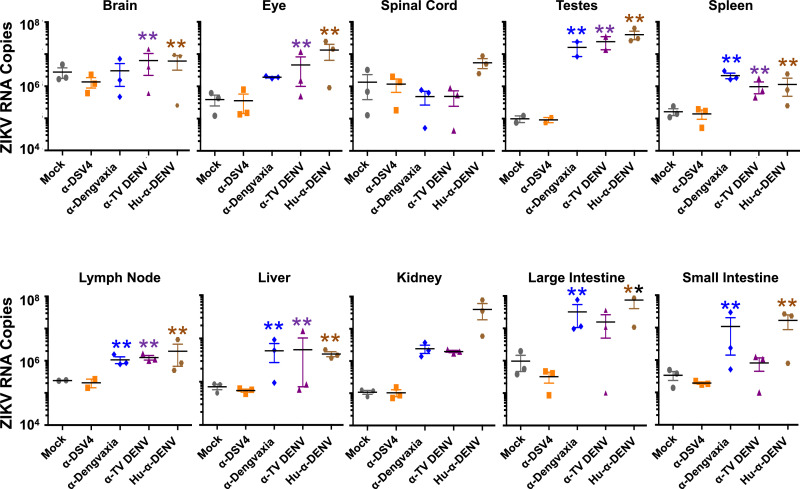
The same subsets of Stat2−/− mice, corresponding to the different immune sera passive transfer groups above, were euthanised on day 6 post-ZIKV infection for determination of ZIKV virus burden in ten tissues (brain, eye, spinal cord, testes, spleen, lymph node, liver, kidney, large and small intestines). This was done by RT-qPCR for ZIKV RNA in tissue homogenates and the results summarised in Fig. 8. The data were analysed by two-way ANOVA with Bonferroni's correction and the p values are summarised in Table S2 (Supplementary File). As expected, ZIKV RNA levels in the ‘Hu-α-DENV’ group of mice was significantly (p<0.0001) elevated across tissues, with the exception of spinal cord (in which the increase was not statistically significant), compared to those in corresponding tissues of ZIKV-challenged mice, which had received prior passive transfer of mock-immune serum.
Fig. 8.
Determination of systemic virus loads in several organs of C57BL/6 Stat2−/− mice infected with ZIKV PRVABC59 in the presence of α-DSV4, α-Dengvaxia and α-TV DENV immune sera. A subset of mice (n=3) from the different immune sera passive transfer groups in the experiment described in Fig. 7, were euthanised on day 6 following ZIKV infection. Equal amounts of total RNA, extracted from the indicated tissues of these mice, were reverse-transcribed to cDNA and subjected to qPCR. Viral RNA genomes were quantified with reference to an in vitro-transcribed RNA standard. Each data point denotes tissue RNA level in a single mouse. The horizontal lines represent the mean tissue virus load. The double asterisks indicate statistical significance (two-way ANOVA with Bonferroni correction) of the data for group above which they are placed compared to that of the ‘Mock’ group. All other groups did not differ significantly in comparison to the mock group.
In striking contrast, in ZIKV-challenged mice which had received prior passive transfer of α-DSV4 immune serum, ZIKV RNA levels in all ten tissues examined were not significantly different from those in the corresponding tissues of mice which had been administered mock immune serum before ZIKV challenge (p>0.9999, for all ten tissues). Prior transfer of α-Dengvaxia or α-TV DENV immune sera were associated with significantly elevated ZIKV RNA levels in liver, testes, lymph nodes and spleen, but not in spinal cord and kidney. ZIKV challenge was accompanied by splenomegaly, the degree of which was significantly minimised by prior passive transfer of α-DSV4 immune sera, but not by α-Dengvaxia or α-TV DENV immune sera (Supplementary file, Fig. S5). In addition, while mice in the ‘α-Dengvaxia’ group had significantly high ZIKV RNA levels in large (p=0.0003) and small (p<0.0001) intestines, compared to those in these organs of mice in the mock immune group, they were comparable in the small and large intestines of both these groups. Likewise, ZIKV RNA levels in the brains (p<0.0001) and eyes (p=0.0011) of mice in the ‘α-TV DENV’ group were elevated significantly, in comparison to those in these two organs of mice in the ‘mock immune’ group. Fluorescence in situ hybridisation of tissue slices from organs corresponding to the different passive transfer groups are presented in the Supplementary file (Fig. S6).
Next, we monitored the survival, clinical signs and body weight change in the rest of the Stat2−/− mice (Fig. 7c–e). We also monitored body temperature of the mice daily (Supplementary file, Fig. S7). Consistent with earlier work [47], passive transfer of Hu-α-DENV plasma into Stat2−/− mice, ahead of ZIKV PRVABC59 infection, resulted in 100% mortality by day 7, preceded by severe clinical signs and body weight loss (brown curves). This is consistent with the significantly high virus replication observed above. Interestingly, we also observed that these mice manifested progressive hypothermia. Mice receiving passive transfer of mock immune serum, upon subsequent ZIKV PRVABC59 infection, suffered 25% mortality, manifesting clinical signs of disease and weight loss around days 8–10, but recuperating thereafter (grey curves). Also, these mice displayed transient lower body temperatures preceding the onset of other clinical symptoms. Interestingly, passive transfer of α-DSV4 immune serum did not escalate subsequent ZIKV PRVABC59 infection and survival was essentially comparable to that of uninfected mice (p>0.9999, Mantel-Cox test). Mice in α-DSV4 group not only did not suffer any fatality, but also exhibited relatively milder clinical signs and body weight loss (orange curves), as well as comparatively milder transient body temperature changes, compared to the group which received mock immune serum, suggesting that the ZIKV EDIII CR antibodies in α-DSV4 immune serum actually minimise disease severity.
In striking contrast, the CR antibodies in α-Dengvaxia (blue curves) and α-TV DENV (purple curves) immune sera sensitised the mice to severe ZIKV disease, resulting in up to 50% mortality with clinical signs and body weight loss being more severe than in the mock immune serum group. The transient hypothermia in these two groups of mice was relatively greater in magnitude compared to mice in the α-DSV4 group, but similar to that in the ‘mock immune serum’ group. Survival in the α-Dengvaxia group was significantly reduced relative to that in either the ‘Uninfected’ group (p<0.0001, Mantel-Cox test) or the group which received mock immune serum (p<0.0001, Mantel-Cox test). This was also true for the survival of mice in the α-TV DENV group. A video of different groups of ZIKV challenged mice is provided in a supplementary media file online (Supplementary file, Movie S2). These data suggest that antibodies in α-Dengvaxia and α-TV DENV immune sera are qualitatively different from those in the α-DSV4 immune serum, from the viewpoint of their in vivo ADE potential. While α-DSV4 antibodies did not cause significant ADE of ZIKV infection in Stat2−/− mice, antibodies in α-Dengvaxia and α-TV DENV did.
4. Discussion
We examined the role of polyclonal anti-DENV antibodies in protection and pathogenesis of dengue using the AG129 mouse model. We also examined potential pathogenesis of ZIKV infection in another mouse model (C57BL/6 Stat2−/−). The polyclonal anti-DENV antibodies were generated using three different immunogens, a VLP subunit vaccine candidate in pre-clinical development (DSV4) [37], the licensed tetravalent LAV for dengue (Dengvaxia) [33] and a mixture of four laboratory strains of DENV (TV DENV) as a surrogate for other DENV based LAVs currently in advanced clinical development [29], [30], [31], [32].
To address the concern that Dengvaxia and TV DENV, which rely on their replicative potential to elicit a complete and balanced immune response, may not elicit an appropriate immune response in the BALB/c mouse, as it does not permit significant DENV replication, we also raised immune sera against Dengvaxia in a slightly immune-compromised mouse model (CD11c-Ifnar1−/−) demonstrated to support viral replication (see below). It is expected that in this host viral replication would activate T cell responses as well and lead to a more natural immune response.
To enable a head-to-head comparison of the three immunogens, we strived to optimise the vaccine dosages and schedules empirically and obtained more or less comparable nAb titres to the four DENV serotypes. Using TRF-IAs in conjunction with recombinant E and EDIII antigens, we found that DSV4-induced antibodies were exclusively EDIII-specific. This is consistent with the design of DSV4 which contains the EDIII moieties of the four DENVs displayed on the surface of VLPs [37]. TRF-IAs showed that Dengvaxia- and TV DENV-elicited antibodies, which were highly reactive towards the E proteins of the 4 DENV serotypes, were only marginally reactive towards EDIII, suggesting that they are predominantly focused to EDI and EDII, consistent with the observation that in DENV particles the EDIII is not well-exposed [57], a situation which must hold for Dengvaxia and TV DENV.
All three BALB/c immune sera enhanced infection of K562 cells by all four DENVs (at low MOI), when diluted appropriately, yielding typical bell-shaped dose response curves. However, it is relevant to note that the K562 assay does have certain limitations. DENV entry into K562 cells, which do not appear to contain any specific DENV attachment factors, is restricted to just Fcγ-RII (CD32) receptors, only in the presence of anti-flavivirus CR antibodies. But, DENV entry in vivo takes place by both antibody-independent and -dependent pathways, in the presence of both activating and inhibitory Fcγ-Rs. Therefore, it is reasonable to expect that the in vivo ADE assay, based on mice (which carry Fcγ-Rs comparable to human orthologues [58]), is likely to more closely reflect the physiological situation, compared to the in vitro ADE assay based on K562 cells. The validity of this notion draws support from the finding of several other investigators that ADE is demonstrable in vivo in sub-lethally DENV-infected AG129 mouse in the presence of anti-DENV antibodies, which causes a disease resembling severe dengue in humans. In this model, a sub-lethal DENV infection is escalated to a potentially fatal disease associated with pathological signs such as elevated serum viremia, elevated virus load in the small intestine, accompanied by increased cytokine production, especially TNF-α, small intestinal haemorrhage and ultimately mortality [13,14,56].
We examined the in vivo ADE potential of the three BALB/c immune sera using the AG129 mouse model. We restricted our focus to ADE of a mouse-adapted strain of DENV-2 alone, as this is the serotype for which the AG129 mouse-based ADE model system has been established. DENV isolates for the other three serotypes capable of causing severe dengue disease in the AG129 mouse, manifesting similar pathological signs have been identified [[59], [60], [61]]. But these are all lethal challenge models which need to be optimised and characterised to assess their potential utility for ADE studies.
We observed that a sub-lethal DENV-2 S221 infection of AG129 mice was enhanced significantly by α-Dengvaxia and α-TV DENV immune sera, but not by α-DSV4 immune serum. This outcome was observed in AG129 mice using either prior passive transfer approach or nIC inoculation approach (regardless of whether the sub-lethal virus dose was partially or fully neutralised). That the nIC approach did not cause artefactual results (for example due to type III hypersensitivity) is apparent from the following: (i) the nIC survival data mirror the passive transfer survival data; (ii) nICs made with α-DSV4 immune serum did not affect survival; and (iii) nICs made using UV-inactivated DENV-2 S221 + mAb 4G2 did not kill AG129 mice [56].
The AG129 mice exposed to sub-lethal DENV-2 S221 infection in presence of α-Dengvaxia and α-TV DENV immune sera manifested all the pathological signs of typical ADE-mediated lethal disease in AG129 mice documented in earlier reports. None of these pathological changes were discernible in sub-lethally challenged AG129 mice in the presence of α-DSV4 immune serum. Notably, the behaviour of α-Dengvaxia immune sera generated in CD11c-Ifnar1−/− mice (in which the CYD-2 component of Dengvaxia was found to replicate and elicit potent nAbs) behaved essentially similarly in that it also promoted ADE efficiently. Apparently, murine polyclonal α-Dengvaxia antibodies behave similarly irrespective of whether they are raised in the context of ongoing virus replication (CD11c-Ifnar1−/−) or not (BALB/c). This was corroborated by the behaviour of α-Dengvaxia antibodies in efficacy experiments as well (see below).
It is to be noted that the volume of immune sera used in the passive transfer (20 µl/mouse), 100% nIC (5 µl/mouse) and 30% nIC (0.08 µl/mouse) inoculation experiments can be estimated to result in dilutions of ~100, ~400, and ~25,000 folds, respectively, in the mouse circulation (assuming a total blood volume ~2 ml/mouse). These dilutions roughly represent the peak dilution, followed by an intermediate dilution and a high dilution, along the descending arms of the bell-shaped dose-response curves of the K562 ADE assay. In the K562 assay, all three immune sera behaved similarly in a serum dilution-dependent manner. But the same three immune sera apparently deviated from this behaviour in the AG129 assay in that ADE was evident for immune sera produced by immunisation with live virus-based immunogens (Dengvaxia and TV DENV), but not for the non-replicating protein immunogen DSV4. The precise reason for the differential behaviour of the three immune sera in the K562 versus AG129 assay is unknown and remains to be addressed.
Another intriguing finding in the nIC inoculation experiment was that risk of AG129 mice experiencing severe disease and increasing mortality was not related to the degree of neutralisation of the sub-lethal dose of DENV-2 S221. While 30% neutralisation of the sub-lethal dose of the challenge virus with α-DSV4 did not affect survival, even 100% neutralisation of the same dose achieved either with α-Dengvaxia or α-TV DENV immune sera resulted in 0% survival. This is reminiscent of an earlier study on the in vivo ADE potential of TS and CR DENV mAbs using the nIC inoculation approach [56]. This study reported that mAb 3H5 (a DENV-2 TS antibody), at sub-neutralising concentrations, lacked significant DENV-2 ADE activity in vivo, while mAbs 4G2 and 6B6C-1 (pan-DENV CR antibodies), even at neutralising concentrations, sensitised AG129 mice to significant ADE.
We are tempted to speculate that the α-DSV4 immune serum does not mediate ADE in vivo at sub-neutralising levels because it contains predominantly TS antibodies. We have shown previously that DSV4 elicits predominantly TS antibodies, which lack in vivo ADE potential [37]. In the 30% nICs made with α-DSV4 immune serum, the sub-lethal dose of 2 × 104 FIU DSV4 would be reduced to ~1.4 × 104 FIU (after 30% neutralisation), and continue to be a sub-lethal dose. On the other hand, the α-Dengvaxia and α-TV DENV immune sera, despite neutralising the sub-lethal DENV-2 dose completely (100% nICs), do so utilising predominantly CR antibodies, especially anti-prM and anti-FLE antibodies, and thus end up escalating the sub-lethal infection to highly lethal levels in vivo. While our speculation above needs to be experimentally investigated, the in vivo ADE data, however, are in line with (i) the design of DSV4, which does not contain prM and FLE and the fact that Dengvaxia and TV DENV are based on viruses that encode both these components; and (ii) the findings of other investigators that antibodies to prM and FLE are ADE-competent [11,[24], [25], [26]].
The functional dichotomy in the in vivo ADE behaviour of polyclonal murine antibodies, elicited by the protein-based immunogen (DSV4) on the one hand, and the live virus-based immunogens (Dengvaxia and TV DENV) on the other, was also evident, albeit to a lesser degree, in their relative capacity to offer protection against lethal DENV-2 challenge. At the highest circulating DENV-2 nAb titre (FNT50=70), which we could achieve by prior passive transfer of α-DSV4 immune serum, we observed delayed and attenuated clinical symptoms accompanied by 50% protection against lethal DENV-2 S221 challenge. At a lower circulating DENV-2 nAb titre (FNT50=26), survival was incrementally extended by α-DSV4 immune serum, compared to lethally challenged control mice. This signals a dose-dependent protective effect of α-DSV4 antibodies and suggests that achieving higher circulating DENV-2 nAb titres with α-DSV4 immune sera may likely confer higher levels of protective efficacy. At similar circulating DENV-2 nAb titres (FNT50=61) achieved using α-Dengvaxia immune sera, there was absolutely no protection. Significantly, a >4-fold increase in circulating DENV-2 nAb titre (FNT50=270), achieved using ‘α-Dengvaxia-CD11c’ immune sera, also failed to offer any protection.
The outcome of the two control groups in the efficacy experiment above provided new insights. One control group received the TS mAb 3H5 and the other, the CR mAb 4G2, by prior passive transfer, before lethal challenge. In both cases we fortuitously achieved identical circulating DENV-2 nAb titres (FNT50=80). Upon lethal challenge, the ‘3H5’ and ‘4G2’ groups showed 0% and 100% mortality, respectively. That both mAbs achieved the same nAb titre in the circulation, yet one (3H5) was protective while the other (4G2) was not, is likely linked to the protective mAb being TS and the non-protective mAb being CR, with respect to the challenge virus. In a broad sense, the efficacy outcomes with mAbs 3H5 and 4G2 appears to align with the efficacy outcomes with α-DSV4 and α-Dengvaxia immune sera. These polyclonal immune sera achieved fairly similar circulating DENV-2 nAb titres (FNT50 titres of 70 and 61, for α-DSV4 and α-Dengvaxia, respectively), which in turn were quite close to those achieved using the two mAbs (FNT50=80). Yet, α-DSV4 immune sera manifested 50% protective efficacy, while α-Dengvaxia immune sera did not confer any protection.
Taken together, the efficacy data lead to a more nuanced view of the role of nAbs in protection against lethal DENV-2 challenge, in that it is not just nAb titre per se, but the specificity (TS vs CR) of the nAbs may also be a critical determinant. The mAb 3H5 data strongly suggest that TS antibodies may have a dominant role in protection. That α-DSV4 immune sera, despite achieving a circulating DENV-2 nAb titre close to that obtained with mAb 3H5, was only half as effective may be due to polyclonal versus monoclonal antibody differences.
In theory, anti-DENV antibodies are expected to be capable of interacting with ZIKV, given the phylogenetic proximity of ZIKV with DENVs. In line with this, the occurrence of severe ZIKV-associated neuropathology in areas where there is a high seroprevalence of DENV has been noted [38,39]. However, there is no epidemiological evidence demonstrating that pre-existing anti-DENV antibodies may cause severe ZIKV illness. Nevertheless, laboratory experiments reported by many investigators support the potential capacity of anti-DENV antibodies to promote ADE of ZIKV infection in vivo [40,41].
We found that while α-DSV4 immune sera failed to neutralise ZIKV, α-Dengvaxia and α-TV DENV immune sera possessed potent ZIKV-neutralising activity. This is perhaps explained by the fact that DSV4 elicits predominantly DENV TS antibodies lacking ZIKV cross-neutralising activity, whereas the virus-based immunogens presumably elicit predominantly CR antibodies which possess ZIKV cross-neutralising capacity.
We showed previously that C57BL/6 Stat2−/− mice are sensitive to ZIKV infection manifesting virus spread to the brain, spinal cord, testes and other organs [62]. One of the ZIKV strains tested, PRVABC59, was found to cause mild illness, which could be escalated to severe and fatal disease, in the presence of human DENV antibodies [47]. We used this mouse model to evaluate the ZIKV ADE potential of BALB/c-induced antibodies to DSV4, Dengvaxia and TV DENV. We found that prior passive transfer of α-DSV4 immune sera did not enhance the pathogenicity of the ZIKV PRVABC59 challenge. In striking contrast, passive transfer of α-Dengvaxia and α-TV DENV immune sera, led to statistically significant pathology upon ZIKV PRVABC59 challenge. This outcome was essentially similar to what we observed when we evaluated ADE of DENV infection using the AG129 mouse model.
Though we did not specifically examine the role of T cells in protection and pathogenesis, some evidence for this may be discernible in certain observations. In ADE experiments wherein we observed 100% survival in AG129 mice administered a sub-lethal dose of DENV-2 (or C57BL/6 Stat2−/− mice administered sub-pathogenic ZIKV), in the presence of α-DSV4 immune serum, there was evidence of mild illness. It is likely that this may be linked to the absence of DENV-specific T cells. It may also be argued that the 50% protective efficacy seen with passively transferred α-DSV4 immune serum against lethal DENV-2 challenge may have been higher if DENV-specific T cells had been present. The nature of T cell responses DSV4 can evoke need to be investigated for better understanding of the immunogenicity and efficacy of DSV4. DSV4, by virtue of being a VLP immunogen, can potentially be cross-presented on MHC class I molecules [63], to induce T cell responses as well. Further work based on adoptive transfer of T cells from immunised BALB/c mice into AG129 mice may provide additional data on the role of T cells to complement the findings in this study.
One important limitation of this study is that the data and the conclusions are based on experiments with immune-compromised murine models of enhancement and protective efficacy. This limitation is imposed by the fact that wild-type mice do not permit DENV and ZIKV to replicate in them. In the context of DENV infection, evidence in the literature suggests that both T cells [[64], [65], [66], [67], [68]] and antibodies [23,69] play important roles in protection as well as pathogenesis. But these aspects are difficult to investigate using the AG129 mouse model as its lack of IFN α/β and γ receptors compromise the integrity of its immune response. This limitation applies to the Stat2−/− mice as well. A way around this, adopted in the field, as we have done, is to vaccinate immunocompetent mice, and investigate the immune sera in naïve AG129 mice exposed to DENV infection (or naïve Stat2−/− mice exposed to ZIKV infection). It is to be noted that passive immunisation lacks memory B (and T) cells and the associated anamnestic responses.
Another limitation of the AG129 mouse model is that ADE of DENV infection cannot be caused by sequential DENV infection as it happens in nature. However, naïve AG129 mice receiving CR anti-DENV antibodies or sera at sub-protective concentrations by passive transfer manifest ADE of DENV infection [13,14,56]. As the naïve AG129 mice lack a memory response, this model is closer to ADE occurring in infants after primary infection due to their receiving passive transfer of maternal anti-DENV antibodies, rather than to classic secondary infection-associated ADE. That this model relies on mouse-adapted DENV, rather than natural DENV isolates, is another likely limitation.
All these limitations notwithstanding, the ADE of DENV infection that happens in AG129 mice is accompanied by a disease characterised by several hallmark features of severe dengue in humans. Likewise, the pregnant Stat2−/- mouse is a relevant model for examining ADE of ZIKV infection as mentioned earlier, as it manifests foetal abnormalities. The approach based on passive transfer of immune sera which allows separation of other aspects of the secondary immune response, enables us to focus on the role of vaccine-induced antibodies to be investigated in isolation. As mentioned above a similar strategy can provide data on the role of T cells as well.
In summary, this study demonstrated that α-DSV4 immune sera exclusively recognised DENV EDIII moieties, neutralised DENVs and ZIKV in vitro, did not significantly enhance a sub-lethal DENV-2 or sub-pathogenic ZIKV challenge and conferred partial protection against a lethal DENV-2 challenge in vivo. On the other hand, α-Dengvaxia and α-TV DENV immune sera, not only neutralised DENVs and ZIKV in vitro, but also enhanced both DENV and ZIKV infection in vivo. Collectively, our data provide a clear indication that the quality of antibodies (TS vs CR) could contribute to protection against DENV infection as well as to pathogenesis of both DENV and ZIKV infections. Our findings do not exclude a potential role for T cells in these phenomena.
Contributors
Literature search: SS; figures: RS, HB, SS, NK; study design: SS, NK, JKL, FK; data collection: RS, HB, JAB, RA, VR, RKS, AP, GB; procurement of Dengvaxia: SK; data analysis and interpretation: SS, NK, JKL, FK; writing first draft: SS and NK; final editing: SS, NK, AAL; Approval of final version: all authors.
Declaration of Competing Interest
RS, RA, VR, RKS, AP and SS received salary support from the International Centre for Genetic Engineering & Biotechnology (ICGEB), New Delhi. JKL received salary support through an NIAID grant. AAL reports personal fees from Sun Pharmaceutical Industries Limited (SPIL) as a consultant on exclusivity basis for dengue vaccine development. NK reports grants from ICGEB, New Delhi and SPIL. HB and SK report grants from the National Biopharma Mission, BIRAC, DBT, Ministry of Science & Technology, Government of India, and personal fees outside this work, as employees of SPIL. VR and NK have a US patent (US20170240601A1) licensed to SPIL. JAB, GB and FK have no competing interests to declare.
Acknowledgement
The authors acknowledge Ms. Sarla Yadav for her help with TRF-IA.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102991.
Contributor Information
Sathyamangalam Swaminathan, Email: swami@icgeb.res.in.
Navin Khanna, Email: navin@icgeb.res.in.
Appendix. Supplementary materials
References
- 1.Pierson TC, Diamond MS. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Wolters Kluwer and Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 747–794. [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 4.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 5.Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJC, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Reviews. 2015;268:340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons CP, Farrar JJ, Chau NV, Willis B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 7.Huy NT, Giang TV, Thuy DHD. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2412. doi: 10.1371/journal.pntd.0002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh CY, Chen PL, Chuang KT. Symptoms associated with adverse dengue fever prognoses at the time of reporting in the 2015 dengue outbreak in Taiwan. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto RC, de Castro DB, de Albuquerque BC. Mortality predictors in patients with severe dengue in the state of Amazonas, Brazil. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0161884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt WE, McCown JM, Gentry MK, Russell PK. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect Immun. 1982;36:1036–1041. doi: 10.1128/iai.36.3.1036-1041.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejnirattisai W, Jumnainsong A, Onsirisakul N. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalvez AP, Engle RE, St. Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Nat Acad Sci USA. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balsitis SJ, Williams KL, Lachica R. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 16.Guzman MG, Kouri G, Valdes L, Bravo J, Vazquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11:223–227. doi: 10.1590/s1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 17.Kouri G, Mas P, Guzman MG, Soler M, Goyenechea A, Morier L. Dengue hemorrhagic fever in Cuba, 1981: rapid diagnosis of the etiologic agent. Bull Pan Am Health Organ. 1983;17:126–132. [PubMed] [Google Scholar]
- 18.Kouri GP, Guzman MG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull WHO. 1989;67:375–380. [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–184. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 20.Katzelnick L, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endy TP, Chunsuttiwat S, Nisalak Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 22.Salje H, Cummings DAT, Rodriguez-Barraquer I. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018;557:719–723. doi: 10.1038/s41586-018-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rey FA, Stiasny K, Vaney MC, Dellarole M, Heinz FX. The bright and dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep. 2018;19:e45302. doi: 10.15252/embr.201745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltramello M, Williams KL, Simmons CP. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe Jr JE. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following Infection. J Virol. 2012;86:2665–2675. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SA, de Alwis AR, Kose N, Jadi RS, de Silva AM, Crowe JE. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol. 2014;88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP. Preclinical and clinical development of YF17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 28.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. New Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 29.Osorio JE, Wallace D, Stinchcomb DT. A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone. Exp Rev Vac. 2016;15:497–508. doi: 10.1586/14760584.2016.1128328. [DOI] [PubMed] [Google Scholar]
- 30.Biswal S, Reynales H, Saez-Llorens X. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. New Engl J Med. 2019;381:2009–2019. doi: 10.1056/NEJMoa1903869. [DOI] [PubMed] [Google Scholar]
- 31.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29:7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine: What makes this vaccine different from the Sanofi-Pasteur CYDTM vaccine? Exp Rev Vac. 2016;15:509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy B, Noriega F, Ochiai RL. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev Vaccine. 2017;16:671–684. doi: 10.1080/14760584.2017.1335201. [DOI] [PubMed] [Google Scholar]
- 34.Halstead SB. Which dengue vaccine approach is the most promising, and should we be concerned about enhanced disease after vaccination? There is only one true winner. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine. 2017;35:6355–6358. doi: 10.1016/j.vaccine.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 36.Sridhar S, Luedtke A, Langevin E. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 37.Ramasamy V, Arora U, Shukla R. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessler J, Chaisson LH, Kucrka LM. Assessing the global threat from Zika virus. Science. 2016;353:aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culshaw A, Mongkolsapaya J, Screaton GR. The immunopathology of dengue and Zika virus infections. Current Opin Immunol. 2017;48:1–6. doi: 10.1016/j.coi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Stettler K, Beltramello M, Espinosa DA. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 41.Brown JA, Singh G, Acklin JA. Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity. 2019;50:1–12. doi: 10.1016/j.immuni.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zust R, Toh YX, Valdes I. Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol. 2014;88:7276–7285. doi: 10.1128/JVI.03827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol. 2007;45:3777–3780. doi: 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanmugam RK, Ramasamy V, Shukla R, Arora U, Swaminathan S, Khanna N. Pichia pastoris-expressed Zika virus envelope domain III on a virus-like particle platform: design, production and immunological evaluation. Pathogens Dis. 2019;77:ftz026. doi: 10.1093/femspd/ftz026. [DOI] [PubMed] [Google Scholar]
- 45.Kaushik N, Rohila D, Arora U, Raut R, Lamminmaki U, Khanna N, Batra G. Casamino acids facilitate the secretion of recombinant dengue virus serotype-3 envelope domain III in Pichia pastoris. BMC Biotechnol. 2016;16:12. doi: 10.1186/s12896-016-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla R, Shanmugam RK, Ramasamy V. Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement. EBioMed. 2020;54 doi: 10.1016/j.ebiom.2020.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bardina SV, Bunduc P, Tripathi S. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao B, Parnell LA, Diamond MS, Mysorekar IU. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J Exp Med. 2017;214:2303–2313. doi: 10.1084/jem.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henein S, Swanstrom J, Byers AM. Dissecting antibodies induced by a chimeric yellow fever-dengue, live attenuated, tetravalent dengue vaccine (CYD-TDV) in naïve and dengue-exposed individuals. J Infect Dis. 2017;125:351–358. doi: 10.1093/infdis/jiw576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Libraty DH, Acosta LP, Tallo V. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Alwis R, Williams KL, Schmid MA. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito M, Mukai R, Takasaki T, Kotaki A, Kurane I. Antibody-dependent enhancement of dengue virus infection in vitro by undiluted sera from monkeys infected with heterotypic dengue virus. Arch Virol. 2010;155:1617–1624. doi: 10.1007/s00705-010-0741-x. [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Li M, Wang Y, Hao P, Jin X. Elaboration of tetravalent antibody response against dengue viruses using a subunit vaccine comprised of a single consensus dengue envelope sequence. Vaccine. 2017;35:6308–6320. doi: 10.1016/j.vaccine.2017.09.063. [DOI] [PubMed] [Google Scholar]
- 54.Orozco S, Schmid MA, Parameswaran P. Characterization of a model of lethal dengue 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J Gen Virol. 2010;93:2152–2157. doi: 10.1099/vir.0.045088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe S, Chan KWK, Wang J, Rivino JL, Lok SM, Vasudevan SG. Dengue virus infection with highly neutralizing levels of cross-reactive antibodies causes acute lethal small intestinal pathology without a high level of viremia in mice. J Virol. 2015;89:5847–5861. doi: 10.1128/JVI.00216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn RJ, Zhang W, Rossmann MG. Structure of dengue virus: implications for Flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dekkers G, Bentlage AEH, Stegmann TC. Affinity of human IgG subclasses to mouce Fc gamma receptors. MABS. 2017;9:767–773. doi: 10.1080/19420862.2017.1323159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarathy VV, White M, Li L. A lethal murine infection model for dengue virus 3 in AG129 mice deficient in type I and II interferon receptors leads to systemic disease. J Virol. 2015;89:1254–1266. doi: 10.1128/JVI.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milligan GN, Sarathy VV, Infante E. A dengue virus type 4 model of disseminated lethal infection in AG129 mice. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milligan GN, Sarathy VV, White MM. A lethal model of disseminated dengue virus type 1 infection in AG129 mice. J Gen Virol. 2017;98:2507–2519. doi: 10.1099/jgv.0.000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathi S, Balasubramaniam VRMT, Brown JA. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Weiskopf D, Angelo MA, de Azeredo EL. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci USA. 2013;110:E2046–E2053. doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiskopf D, Cerpas C, Angelo MA. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J Infect Dis. 2015;212:1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiskopf D, Bangs DJ, Sidney J. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci USA. 2015;112:E4256–E4263. doi: 10.1073/pnas.1505956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mongkolsapaya J, Duangchinda T, Dejnirattisai W. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 68.Duangchinda T, Dejnirattisai W, Vasanawathana S. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci USA. 2010;107:16922–16927. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acosta EG, Bartenschlager R. Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development. Expert Rev Vaccines. 2016;15:467–482. doi: 10.1586/14760584.2016.1121814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.