Abstract
Background:
Impairments in postural control in Huntington’s disease (HD) have important consequences for daily functioning. This observational study systematically examined baseline postural control and the effect of sensory attenuation and sensory enhancement on postural control across the spectrum of HD.
Methods:
Participants (N=39) included healthy controls, and individuals in premanifest (pHD) and manifest stages (mHD) of HD. Using wearable sensors, postural control was assessed according to: (1) postural set (sit vs stand); (2) sensory attenuation using clinical test of sensory integration; and (3) sensory enhancement with gaze fixation. Outcomes included sway smoothness, amplitude, and frequency.
Results:
Based on postural set, pHD reduced postural sway in sitting relative to standing, while mHD had pronounced sway in standing and sitting, highlighting a baseline postural deficit. During sensory attenuation, postural control in pHD deteriorated relative to controls when proprioceptive demands were high (eyes closed on foam), while mHD had significant deterioration of postural control when proprioception was attenuated (eyes open and closed on foam). Finally, gaze fixation improved sway smoothness, amplitude and frequency in pHD; however no benefit was observed in mHD.
Conclusions:
Systematic examination of postural control revealed a fundamental postural deficit in mHD, which further deteriorates when proprioception is challenged. Meanwhile, postural deficits in pHD are detectable when proprioceptive challenge is high. Sensory enhancing strategies using gaze fixation to benefit posture may be useful when introduced well before motor diagnosis. These findings encourage further examination of wearable sensors as part of routine clinical assessments in HD.
Keywords: Huntington’s disease, postural control, wearable sensors, sensory modulation
1. Introduction
Huntington’s disease (HD) is an autosomal-dominant neurodegenerative disease characterized by an abnormal expansion of the trinucleotide CAG, which results in motor, cognitive, and behavioral disturbances. Among motor signs of manifest HD (mHD) are postural control impairments, characterized by increased postural sway in standing,1 excessive translations of the center of pressure during weight shifting,2 and delayed response to postural perturbations.3 Impaired postural control has consequences on functional tasks such as stepping, turning,4 and walking,5 and is associated with risk for falls.5 Given the impact of postural control impairments on activities of daily living, it is important to examine mechanisms underlying postural control impairments in HD as this may guide the design of interventions to improve balance and stability. Among motor impairments in HD, balance and postural control may improve with physical therapy,6 thus, highlighting the potential immediate translation of knowledge into more meaningful interventions that could improve postural function.
Balance and postural control are examined using clinical tests7,8 or quantified with posturography. Observational studies2,9 have used dynamic posturography to examine postural control in HD. While useful, both clinical and quantified measures have limitations: clinical scales may have limited resolution especially in pre-manifest stages,10 and posturography equipment is not commonly accessible in clinical settings. With continued advancements in computing and wearable technology, portable and more affordable wearable sensor systems offer opportunities to better quantify posture, as these enable tracking disease over time and measuring response to intervention within and outside the laboratory.11 Indeed, some studies have utilized sensor-based instrumentation in HD.1,12,13 Our study systematically examined the integrity of postural control system across the spectrum of HD using wearable sensors by examining sensory modulation in different positions, and in states where sensory cues are attenuated and enhanced.
Individuals in the mHD stage are less stable compared to controls not only under conditions where sensory information is attenuated but also during quiet standing when sensory information is salient.3,9,14 Because postural control is modulated by task demands related to the postural set, we first examined whether standing is inherently more unstable than sitting. Effective modulation of postural control requires the integration of sensory input. Deficits in sensorimotor integration15 are common in basal ganglia disorders, such as HD.16 In mHD, stance is impaired in challenging sensory conditions3,9 on the Sensory Organization Testing (SOT), a computerized test of postural control that manipulates visual and proprioceptive cues. For individuals in the premanifest stage (pHD), prior to motor diagnosis, quantitative assessment of postural control during conditions with attenuated sensory cues has revealed measurable deficits2,13,14 which are not evident on clinical assessment.10 The accumulating evidence on postural deficits in pHD9, 12 point to subtle changes in postural control that may reflect early motor changes in HD. However, results across studies are inconsistent-assessment using force plates indicated larger sway velocities and distances in pHD relative to controls during eyes-open and eyes-closed stance.9 Measuring posture with wearable inertial sensors demonstrated that impairments in pHD were present only when standing with eyes-closed and feet together;12 however, SOT failed to detect postural impairments in pHD relative to controls.9 These inconsistent findings may be due to differences in methodology.
Postural control in Parkinson’s disease (PD), another basal ganglia disorder, has been characterized through measures across domains such as smoothness, amplitude, and frequency.17,18 These domains of postural control are relatively independent of each other,18,19 and may represent distinct underlying neural processes.18 The global scores of SOT may not capture the components of postural control that are deficient in pHD. Therefore, evaluating postural control across domains of postural control under different sensory challenges may improve precision of assessment, especially in pHD. The Modified Clinical Test for Sensory Integration and Balance (mCTSIB)20 is a common bedside test that assesses standing balance through a range of sensory challenges, which may detect postural deficits in pHD. Unlike the SOT, this test is easy to administer and does not require expensive equipment. The use of wearable sensors to quantify the mCTSIB may enable more fine-grained analysis of postural control, as has been demonstrated in PD.15 This approach may be useful in pHD but is yet to be examined. In this study, we assessed postural control in pHD and mHD by instrumenting the mCTSIB with wearable inertial sensors.21
Finally, there is clinical interest in understanding compensatory strategies available in HD to assist with postural stability. Sensory enhancement through explicit utilization of visual cues has been shown to improve motor performance in PD and HD. For example, patients with PD demonstrate increased reliance on visual information compared to controls on foot placement during walking.22 In HD, overt gaze (i.e. directed attention to gaze) has been shown to benefit performance of arm movements.23 Gaze fixation has also been shown to benefit postural control in populations with vestibular hypofunction24 and PD,22 however this has not yet been examined in HD. Gaze (i.e. vestibulo-ocular reflex) is considered to be largely preserved in HD even in the late stages of the disease,25 thus, if operational, this may be a viable strategy to improve postural control. In this study, we examined the effects of gaze fixation on postural sway in HD.
In summary, this study examined postural control in pHD and mHD with the following aims: (1) to examine baseline postural control by comparing postural sets of standing and sitting; (2) to examine sensory modulation of postural control with the instrumented mCTSIB; (3) to examine the effects of gaze fixation on postural sway, and (4) examine the relationship between postural control and disease severity in HD. Test-retest reliability was also assessed.
2. Methods
2.1. Participants
We enrolled 39 participants including 11 healthy controls, 17 pHD, and 11 mHD participants. Participants with expanded CAG repeats (mHD and pHD) were recruited from the Huntington’s Disease Center at Columbia University, while controls were gene-negative family members of HD participants, and members of the university. The Institutional Review Board approved this protocol, and informed consent was obtained before participation. The inclusion criteria for pHD and mHD participants were: (1) age 21-65 years; (2) able to walk 20 meters without assistive device; (3) genetic confirmation and Diagnostic Confidence Score (DCS) of <4 for pHD ; and (4) genetic confirmation or known family history and DCS of 4 for mHD. Clinical diagnosis of HD was confirmed by a neurologist certified in the administration of the Unified Huntington Disease Rating scale (UHDRS),26 based on a clinical motor exam and DCS of 4. Control participants were: (1) age 21-65 years; (2) have independent and unrestricted walking ability; and (3) absence of neurological, orthopedic, or medical condition that impacts balance and mobility.
2.2. Instrument
Postural control was quantified using a tri-axial inertial sensor (APDM, Eugene, OR, USA) that was secured on the low back at L5 segment using a belt strap. Postprocessing of data was performed with proprietary software (Mobility Lab™, APDM, Eugene, OR, USA).17
2.3. Protocol
2.3.1. Postural set: Sit vs stand
To examine the effects of postural demands of standing relative to sitting, participants were asked to sit still without back support, arms folded, with both feet together and fully supported on ground. The following conditions were tested: (a) EO-Sit: eyes open sitting; and (b) EC-Sit: eyes closed sitting. Standing conditions with eyes open and closed (EO-firm, EC-firm) are described below in mCTSIB.
2.3.2. mCTSIB
To examine sensory modulation of postural control, the Instrumented Sway paradigm20,27 was used. Participants were asked to stand still for 30 seconds with feet together and arms folded across their chest. The following testing conditions were based on the mCTSIB:21,27 (a) EO-firm: eyes open stand on firm surface; (b) EC-firm: eyes closed stand on firm surface; (c) EO-foam: eyes open stand on foam surface; and (d) EC-foam: eyes closed stand on foam surface. We used medium-density foam (Airex® Balance Pad, AIREX AG, Switzerland) consistent with other protocols.27 The tests were performed with participants facing a blank wall to minimize salient visual cues.
2.3.3. Gaze fixation
To examine the effects of gaze fixation on postural sway, participants were asked to stand still for 30 seconds while fixing their gaze on a visual target, an “X” printed on a card positioned 1.5 m from participant,20 and 5 cm below eye level. Conditions for gaze fixation were: (a) Gaze-firm: gaze fixation on firm surface; and (b) Gaze-foam: gaze fixation on foam surface. To ensure adherence to instructions, a researcher experienced in gaze assessment (FP) monitored gaze position during testing. Additionally, video recordings of eye-head movements were obtained and reviewed to confirm adherence to task.
Data collection was performed within a single session, and familiarization trials were provided prior to testing. Three trials were recorded per condition. Trials were pseudo-randomized between mCTSIB and Sit vs Stand, and were performed prior to Gaze trials to minimize potential learning effects from gaze fixation.
2.4. Postural measures
Postural control domains of smoothness, amplitude, and frequency were characterized by measuring sway Jerk, Total Sway Area, and Total Power, respectively. These measures were selected because they have been found to be sensitive and reliable measures of postural dysfunction in other movement disorders.17,19,21 Sway in anteroposterior (AP) and mediolateral (ML) directions were obtained for Jerk and Total Power. Jerk (m2/s5) is the time derivative of acceleration and refers to smoothness of sway17. Increased Jerk may signify less fluid postural adjustments. Total Sway Area (m2/s5) is the two-dimensional planar sway area in transverse plane, computed as the area included in acceleration per unit time17. An increase in Total Sway Area signifies greater magnitude of sway. Total Power ((m/s2)2*Hz−) refers to total power of a specific plane, and provides a measure of the frequency of sway17. Total Power, which characterizes the shape of power spectral density28 of the acceleration signal, may conceptually refer to how frequent postural adjustments are made within a given time.
2.5. Disease severity in HD
Disease severity was measured by CAG-age product score (CAP) and Total Motor Score (TMS). CAP score was calculated as the age at completion of study multiplied by the difference between CAG length minus 33.60.29 TMS refers to the sum of motor assessment items in the UHDRS,26 and reflects the severity of motor signs in HD. CAP score and TMS of pHD and mHD were used in correlation analyses with postural control measures.
2.6. Data analysis
Descriptive statistics were used to assess differences in clinical and demographic information. Based on the Shapiro-Wilk test, postural sway data were not normally distributed; therefore, nonparametric statistics were used. First, to examine differences in sitting and standing (EO-Sit vs EO-firm; EC-Sit vs EC-firm) (Aim 1), the Wilcoxon signed-ranks test was used. Next, to examine within-group differences in mCTSIB (Aim 2), Friedman’s ANOVA was used. Between-group differences in sensory modulation were examined based on differences in the change in postural control that occurs with sensory challenge. This change score was calculated as the difference in sway when sensory input was attenuated (i.e. EC-firm, EO-foam, EC-foam) relative to baseline condition (i.e. EO-firm). The Kruskal-Wallis test was used to examine between-group differences on change scores. The significance was set at p<0.05, with Bonferroni corrections to account for multiple comparisons. To examine the effect of gaze on postural sway (Aim 3), the Wilcoxon signed-ranks test was used to differentiate sway with versus without explicit gaze instruction (Gaze-firm vs EO-firm; Gaze-foam vs EO-foam). To examine the relationship between postural control and disease severity (Aim 4), Spearman correlation was used to examine association between postural measures sensitive in both pHD and mHD with CAP score29 and total motor score.26 Finally, test-retest reliability of sensors was examined using intraclass correlations (ICC)30 of log-transformed values during EO-firm. All analyses utilized absolute values, while Kruskal-Wallis test utilized change scores in sway as described above. Statistical analyses were performed in IBM SPSS software (Version 25.0, Armonk, NY, USA), and data organization, visualization, and ICC30,31 with Matlab (Mathworks, Natick, MA, USA). Sampling size estimates were determined a priori based on effect size of 0.40, study power of 0.80, and significance level at 0.05, performed using G*Power (Version 3.1.9.2).
3. Results
3.1. Participants
Table 1 presents a summary of clinical data of participants. Age, height, weight, and Activities Specific Balance Confidence scores were not different across groups (p>0.05). Scores on the Berg Balance Scale (BBS) were lower in mHD relative to other groups (p<.05). mHD demonstrated greater motor impairment (based on Total Motor Score), and lower level of independence (based on the Independence Score) relative to pHD (p<.05). The CAG-Age Product (CAP) score, a measure of disease burden, and DCS, a rating of clinician’s confidence on presence of unequivocal signs of HD, were significantly higher in mHD than pHD (p<.05). It is worth noting that our pHD sample included a range of DCS levels, with 24% in “unimpaired” (DCS=0) and 76% in “impaired” states (DCS=1,2,3), as operationally defined in other longitudinal studies in HD.32,33
Table 1:
Demographic and clinical data reported as mean (standard deviation)
| Control | pHD | mHD | Sig | |
|---|---|---|---|---|
| N | 11 | 17 | 11 | |
| Age, y | 43.8 (11.4) | 41.1 (9.3) | 50 (11.8) | .108 |
| Height, m | 1.72 (1.03) | 1.72 (.08) | 1.70 (.01) | .757 |
| Weight, kg | 76.2 (16.5) | 77 (10.32) | 67.9 (13) | .175 |
| ABC | 98.6 (3.1) | 91.3 (12.4) | 84.6 (23.7) | .103 |
| BBS | 55.3 (1.4) | 55.2 (1.3) | 51.6 (4.3) | <.001ab |
| CAP | NT | 356.3 (100.5) | 465 (104.4) | .019b |
| UHDRS | ||||
| TMS | NT | 6.7 (4.9) | 30.5 (11) | <.001b |
| TFC | NT | 12.5 (1.7) | 10.8 (2.1) | .790 |
| FA | NT | 24.5 (2) | 23 (2) | .121 |
| IS | NT | 98.4 (6.3) | 89 (9.9) | .010b |
| DCS | NT | 1.53 (1.12) | 4 (0) | <.001b |
Abbreviations: pHD, Premanifest Huntington’s disease, mHD, Manifest Huntington’s disease ABC, Activities-Specific Balance Confidence Scale; BBS, Berg Balance Score; CAP, CAG-Age-Product; UHDRS, Unified Huntington’s Disease Rating Scale; TMS, Total motor score; TFC, Total functional capacity; FA, Functional assessment; IS, Independence scale; DCS, Diagnostic confidence score; NT, Not tested Significant between group differences (p < .05) based on ANOVA with multiple comparisons with Bonferonni corrections
Control vs mHD
pHD vs mHD
3.2. Test-retest reliability
Test-retest reliability of APDM sensors were examined, as summarized in Supplementary Table A. Controls had moderate to good reliability34 for all measures of interest (ICC: 0.501 - 0.815), except for Jerk ML with poor reliability (ICC: 0.473). Both pHD and mHD had good reliability34 for all measures of interest (ICC: 0.764 – 0.887), except for Total Power AP for pHD with moderate reliability (ICC: 0.644).
3.3. Comparing postural sets of standing and sitting
Comparisons between sway in sitting and standing were assessed. In the ML direction, all groups reduced postural sway during sitting versus standing regardless of visual condition (p<0.05) (see Supplementary Figure B). Conversely, postural sway in AP and transverse directions were differently impacted across groups and was more reflective of the disease process, as shown in Figure 1. First, controls minimized postural sway in sitting compared to standing during EC conditions (Jerk AP, Total Sway Area) (p<0.05), and EO condition (Total Sway Area) (p<0.05). For controls, Total Power AP was not sensitive to changes in postural set (p>0.05). Meanwhile, pHD had reduced sway in virtually all measures of Jerk, Total Sway Area, and Total Power in sitting compared to standing in all visual conditions (p<0.05). This was mainly due to increased sway in standing while postural sway in sitting was within similar range as those of controls. Finally, mHD demonstrated postural sway (Jerk, Total Sway Area, Total Power) that was interestingly not different between sitting and standing in the AP or transverse directions (p>0.05).
Figure 1:
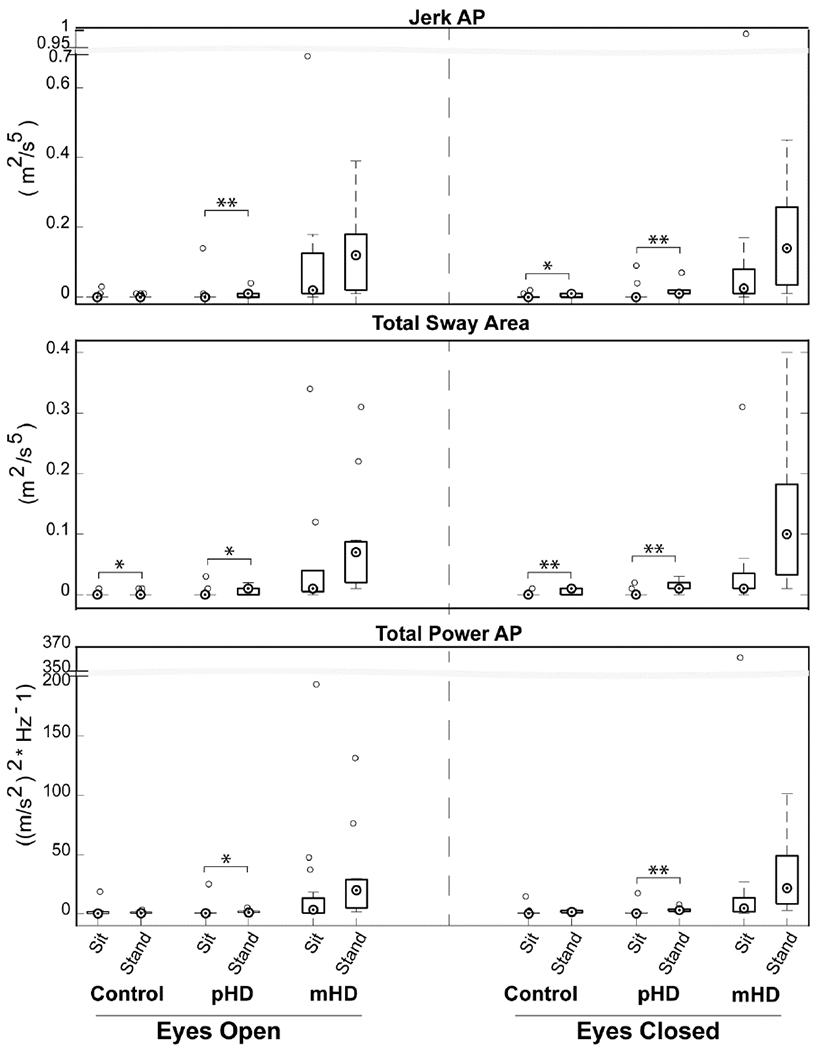
Modulation of postural control based on postural set. Boxplot central mark indicates median, and bottom and top edges indicate 25th and 75th percentiles, respectively. Whiskers extend to extreme data that are not outliers. Open circles are outliers. *Abbreviations: pHD, Premanifest Huntington’s disease; mHD, Manifest Huntington’s disease; AP, Anteroposterior p-value: *: <0.05; **: <0.001
3.4. Sensory modulation of postural control
3.4.1. Within-group difference
Each group demonstrated postural sway that was different across conditions of the mCTSIB (p<0.05) for all measures of Jerk, Total Sway Area, and Total Power. Postural sway systematically increased from EO-Firm → EC-Firm → EO-Foam → EC-Foam (see Supplementary Table C).
3.4.2. Between-group differences
Figure 2 summarizes the sensory modulation of postural control based on change scores in the mCTSIB relative to the baseline condition. For all measures, between-group differences were noted in conditions that involved standing on Foam surface (p<0.05). Postural sway while standing on Firm surface was not different across groups (p>0.05), except for Total Power ML (p<0.05).
Figure 2:
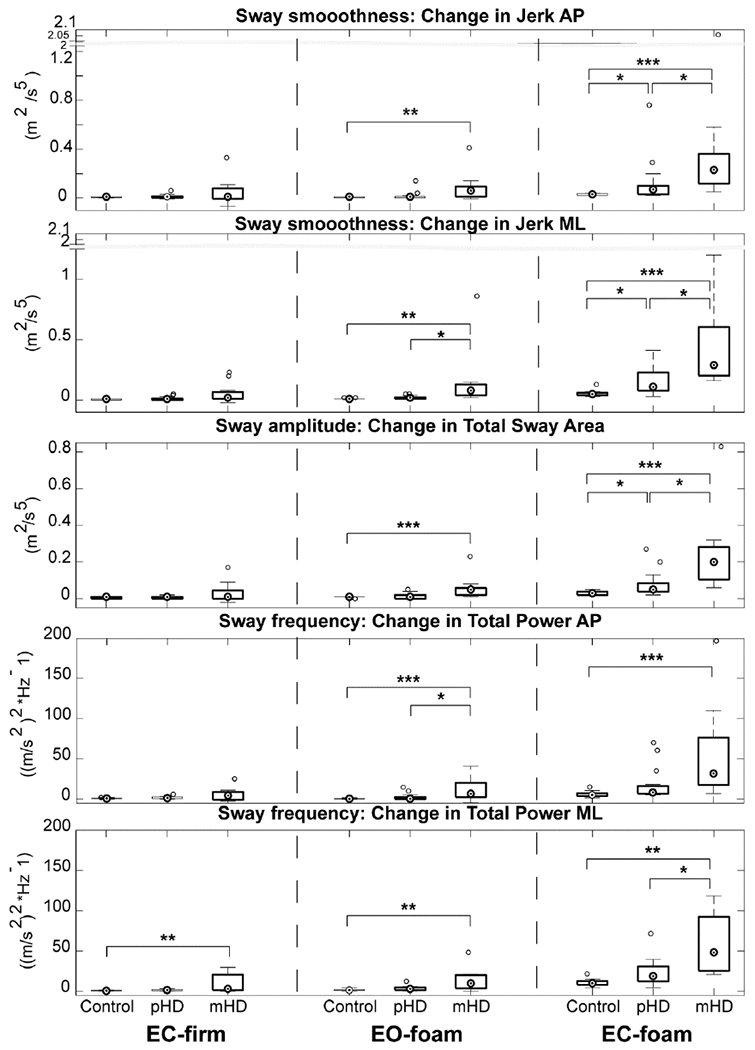
Modulation of postural control based on sensory attenuation using mCTSIB. Values are change scores relative to EO-firm. Abbreviations: pHD, Premanifest Huntington’s disease; mHD, Manifest Huntington’s disease, AP, Anteroposterior, ML, Mediolateral; p-value: *: < 0.05; **:<0.001; ***; <0.001
Based on pairwise comparisons, pHD demonstrated larger increases in Jerk-AP and -ML, and Total Sway Area compared to controls only during EC-Foam (p<0.05). On the other hand, mHD had larger increases in sway for all measures of interest relative to controls during Foam conditions EO-foam and EC-foam, as well as EC-firm condition for Total Power-MF (p<0.05).
To determine differences in postural control between pHD and mHD, pairwise comparisons were examined. Generally, mHD demonstrated larger increases in sway than pHD especially during EC-foam for Jerk-AP, -ME, Total Sway Area, and Total Power-ML, and to a lesser extent during EO-foam for measures of Jerk-ML and Total Power-AP (p<0.05).
3.5. Effect of gaze fixation on postural control
All participants complied with gaze fixation instructions confirmed by in-session observations and video recordings. Measures that demonstrated significant changes are shown in Figure 3. In both Firm and Foam conditions, healthy controls reduced postural sway with gaze fixation relative to baseline for Total Power-ML, while gaze fixation also reduced Jerk-ML and Total Sway Area during Foam condition (p<0.05).
Figure 3:
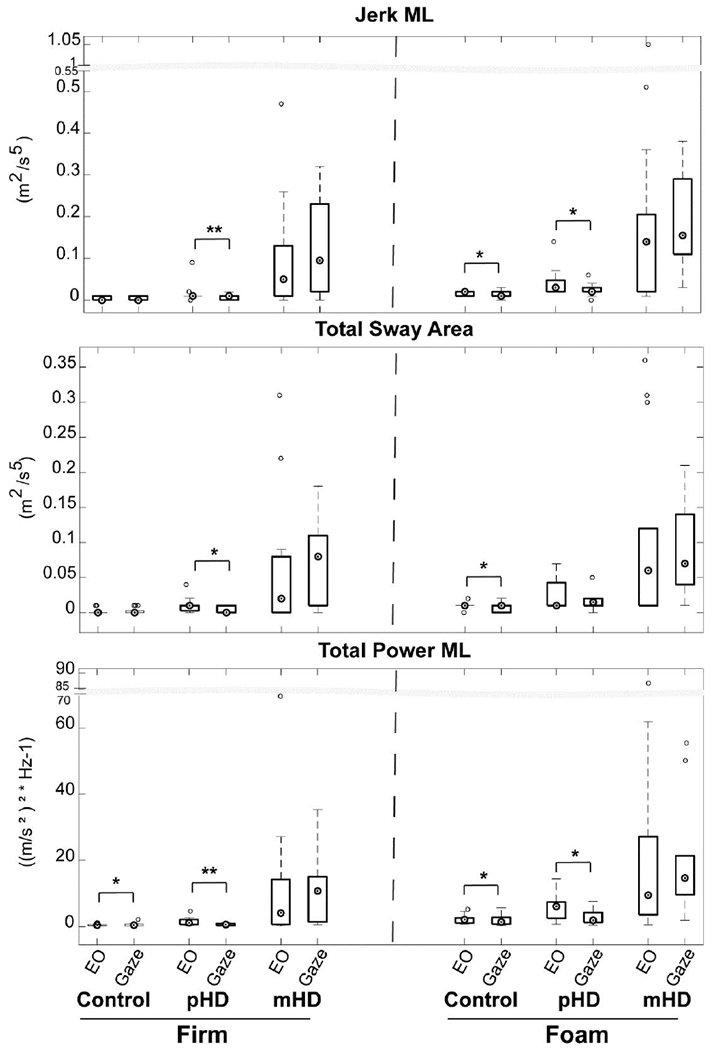
Modulation of postural control based on sensory enhancement with gaze fixation
Abbreviations: pHD, Premanifest Huntington’s disease, mHD, Manifest Huntington’s disease, EO, Eyes open; ML: Mediolateral; p-value: *: < 0.05, **: < 0.01
Similarly, the pHD group responded to gaze fixation by reducing sway in both Firm and Foam conditions, such that Jerk-ML and Total Power-ML were reduced relative to baseline condition (p<0.05). During Firm condition, Total Sway Area was also reduced with gaze fixation (p<0.05). Finally, the mHD group failed to show improvements in postural sway with gaze fixation (p<0.05). Gaze fixation did not change sway in AP direction (Jerk-AP, Total Power-AP) for all groups (p>0.05).
3.6. Relationship between postural control and disease burden
Correlation analyses were performed for CAP score (measure of disease burden) and TMS with measures that were capable of revealing deficits in both pHD and mHD relative to controls, which were Jerk-AP, Jerk-ML, and Total Sway Area during EC-foam (Table 2). Data were not available on CAP score for 2 pHD and 2 mHD, and TMS for 1 mHD, therefore were treated as missing data in analyses. The selected postural measures had moderate positive correlations with CAP scores (p<0.001), and moderate to strong positive correlations with TMS (p<0.001). These correlations were based on absolute values of postural sway, and correlations were similarly present when using change scores (not reported).
Table 2:
Correlation between postural impairment and disease severity
| Posture | CAP Score | TMS |
|---|---|---|
| (EC-foam) | rs, p-value | rs, p-value |
| Jerk-AP | 0.716, <0.001 | 0.678, <0.001 |
| Jerk-ML | 0.657, <0.001 | 0.823, <0.001 |
| Total Sway Area | 0.685, <0.001 | 0.794, <0.001 |
Abbreviations: CAP, CAG Age Product, TMS, Total Motor Score from UHDRS AP, Anteroposterior; ML, Mediolateral
4. Discussion
In this study, we used commercially available wearable sensors to assess the integrity of the postural control system across the spectrum of HD. Several metrics of these sensors demonstrated good reliability in assessing standing posture in HD. Postural control was assessed in different postural sets (sit vs stand), in response to sensory attenuation, and sensory cues (gaze fixation) to improve postural control. Our results reveal that mHD have a baseline postural impairment that is indifferent to postural set. Instrumentation of the mCTSIB, where sensory challenges were imposed, revealed early and measurable postural deficits that begin in the pHD stage, and worsen in mHD stage. When sensory information was enhanced through gaze fixation, improved postural responses were observed in pHD but not in mHD. These findings have important implications for the management of HD.
4.1. Baseline postural control according to postural set: Sit vs stand
Studies have shown that mHD are significantly less stable than healthy controls even in baseline sensory conditions,3,9,14 similar to this study. Our study examined whether there is a fundamental deficit in standing regardless of the sensory challenge, and if similar patterns exist in pHD. Sitting and standing were compared. Standing presents greater challenges due to heightened neural and biomechanical demands35,36 relative to sitting. As such, all groups, including healthy controls, demonstrated larger postural sway in standing relative to sitting, particularly for sway measures in ML direction. This may be explained by differences in biomechanical constraints rendered by narrow base of support in the frontal plane in standing (i.e. feet together) relative to sitting.
Interestingly, the sway patterns in the AP direction between sitting and standing were more reflective of postural control impairments in HD than ML measures. Figure 4 provides an exemplar measure, Jerk, which demonstrates this behavior. Controls regulated posture by improving sway smoothness and reducing sway magnitude in sitting compared to standing, especially when vision was attenuated. On the other hand, pHD had significantly reduced sway in sitting compared to standing in all measures regardless of sensory condition (Figure 1). This generalized benefit in improving sway in sitting can be attributed to the two- to fourfold increases in sway in standing in pHD relative to controls, while postural sway in sitting was similar to controls. In other words, the significant difference between sitting and standing in pHD is brought about by aberrant sway in standing contrasted by preserved postural control in sitting.
Figure 4:
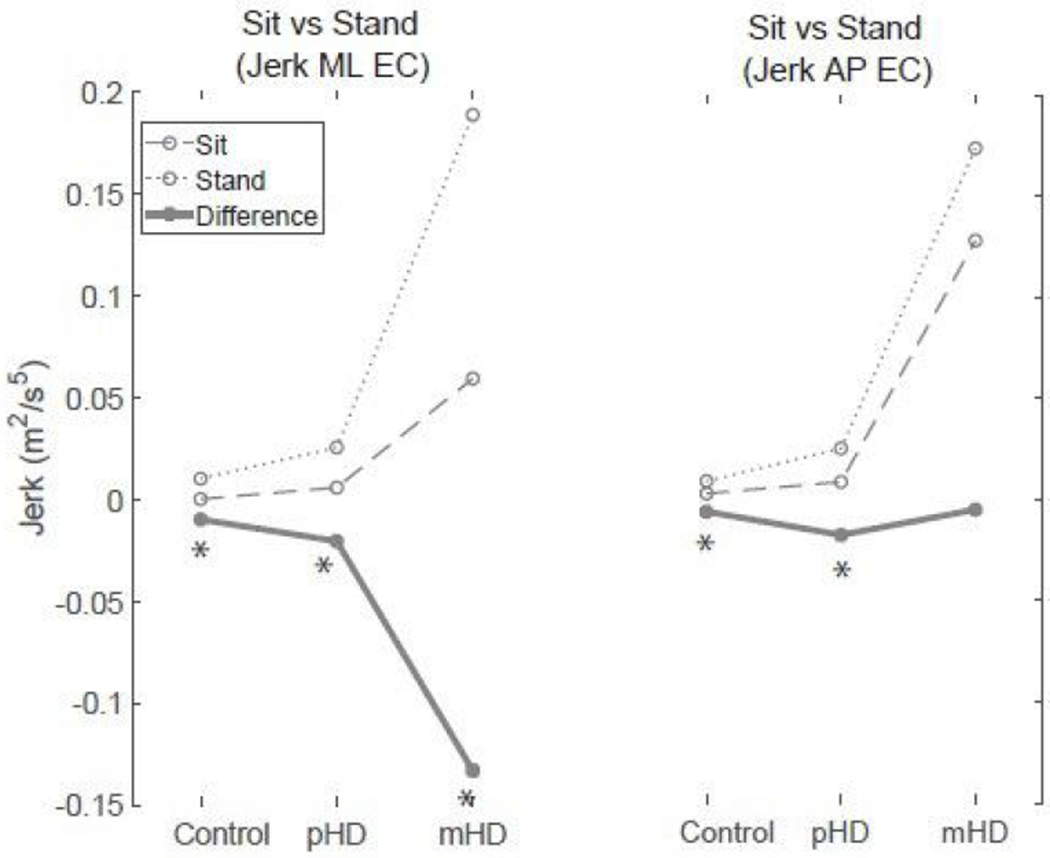
Comparison between sitting and standing based on Jerk as representative measure. Difference represents the decrement in postural control in standing relative to sitting (sway in sitting minus sway in standing).
Abbreviations: pHD, Premanifest Huntington’s disease mHD, Manifest Huntington’s disease; AP: Anteroposterior; ML, Mediolateral *:Significant difference between Sit and Stand (p < 0.05)
In mHD, there was exaggerated AP sway in standing and in sitting (Figure 4 as example), consistent with prior work.1 This suggests a persistent instability that is indifferent to the postural set in mHD. It is possible that this impairment may be due to impaired trunk control, as leg muscle activity has been shown to be appropriately regulated in sitting relative to standing in HD.36 Finally, the influence of trunk involuntary movements cannot be excluded, as whole body involuntary movements are evident in both sitting and standing in HD.37 However this is unlikely because the instability observed in our sample was specific to the AP direction, while involuntary movements in HD are irregular, non-stereotyped and random.38 Interestingly, findings by Reyes et al. also found direction-specific abnormalities with disease progression involving AP instability during limits of stability testing.9 The AP-specific instability in both pHD and mHD warrant further investigation on direction-specific postural abnormalities in HD. Altogether, our results describe the progression of postural impairment in HD that may begin with deficits in postural control in standing in pHD and worsens in mHD impacting both standing and sitting. Our findings suggest that there is a fundamental deficit in postural control in mHD that is present regardless of postural set.
4.2. Sensory attenuation and instrumented mCTSIB in pHD and mHD and correlations
Our study examined how pHD and mHD respond to sensory attenuation. To our knowledge, this is the first study to assess the use of wearable sensors in instrumenting the mCTSIB in both pHD and mHD. This test has been shown to be a meaningful measure of sensory organization in PD27 and mHD,21 however its value in pHD remains unknown. Quantifying postural control in the pHD stage can reveal the earliest impairments which may influence timing of treatment.33 Our results show that the instrumented mCTSIB reveals early deficits in postural control in pHD, and can further characterize the impairment in mHD. In our sample, all pHD and mHD participants successfully completed the mCTSIB, except for 1 mHD who required assistance, but was able to complete the task on a subsequent attempt. Thus, clinical implementation of the non-instrumented mCTSIB using only a stopwatch would fail to capture these postural anomalies in pHD and mHD, and that using wearable sensors during mCTSIB is capable of characterizing underlying postural deficits in HD.
Postural control relies on visual, proprioceptive, and vestibular information. During mCTSIB, sensory re-organization is needed in response to attenuation of sensory cues. Because mHD demonstrate postural instability independent of sensory attenuation as seen in our study and others,3,9,14 we therefore assessed the sensory modulation process based on how much change or degradation in postural control occurs when sensory information was attenuated. Our results show that pHD had significant degradation in postural sway smoothness and magnitude compared to controls when vision and proprioception were both attenuated during EC-foam. Other sensory conditions were not sufficient in revealing these deficits in pHD. On the other hand, mHD had significant degradation in postural control compared to controls in all domains of postural control (smoothness, amplitude and frequency) during EO-foam and EC-foam conditions. The postural degradation that occurs particularly during foam conditions suggests that processing of proprioceptive input is particularly difficult in mHD, as seen in other basal ganglia disorders.15 In pHD, the instability present only during EC-foam suggests that postural control deficits can be detected when proprioceptive demands of the task are high. Therefore, clinical examination of postural control should include concurrent attenuation of vision and proprioception (EC-foam) for pHD, whereas attenuation of proprioception alone may be sufficient in mHD. The use of change scores may be valuable in appraising the modulation process that occurs with manipulation of sensory cues.
Sway measures that were sensitive in detecting postural deficits in both pHD and mHD (Jerk-AP, Jerk-ML, and Total Sway Area) during EC-foam were examined for their association with disease severity based on CAP score and TMS. These postural measures had moderate positive correlation with CAP score, and moderate to high positive correlation with TMS (Table 2). The strength of relationships found in this current study, along with other studies,9,14 suggest that postural control impairments may reflect the progression of HD.
While there is accumulating evidence on postural impairments in pHD,13, 14 the study by Reyes et al. did not detect impairment in stance postural control regardless of sensory condition based on the SOT.9 The discrepancy with our results may be due to differences in measurement techniques between SOT and accelerometer-based measurements.13 The SOT uses peak-to-peak sway values based on the inverted pendulum model which is highly dependent on the ankle strategy, based on the assumption that ankle rotations are the primary reasons for postural sway.39 In contrast, stance control in aging and neurological conditions involve multi-segment coordination that includes hip strategy,40–42 where adjustments at the hip joint are generated to control center of mass translations, and that individuals with HD may be utilizing this given the increase in pelvis and trunk excursions in standing.1 Pelvic and trunk excursions may not be detected during the SOT. The ability of accelerometry-based measurements in detecting the subtle postural changes in pHD may be advantageous over other techniques, as seen in PD.43
4.3. Sensory enhancement through gaze fixation
While sensory perturbation paradigms such as the mCTSIB are valuable in evaluating the integrity of the postural control system, it is also worth examining how malleable the postural control system is in explicitly utilizing sensory cues to improve postural stability. Sensory-enhancing strategies using visual cues may influence postural stability. With overt gaze fixation, ocular muscles interact with neck muscles that complement head stabilization enabling a steady view of the environment.44 Further, visual fixation may focus attention23 in HD, thereby improving postural control.
In this study, controls reduced their postural sway with gaze stabilization in conditions involving attenuated and non-attenuated proprioceptive information (Foam, Firm). Likewise, the pHD group responded favorably to overt gaze by improving postural sway smoothness and frequency in attenuated and non-attenuated proprioceptive states, and sway amplitude during non-attenuated condition. However, overt gaze in mHD had no effect in improving postural control. While useful in upper extremity movement tasks in HD,45 visual cues may have limited influence on postural control due to its high reliance on cognitive processing for the transformation of retinal slip information into appropriate postural responses. Given the well-documented deficits in cognition and attention in mHD, overt gaze fixation which may rely on these processes was not beneficial for mHD. Because gaze reflexes are largely functional in HD even in later stages,25 the benefit exhibited by prodromal group offers a potential strategy for training. Future work is needed to test hypothesis-driven treatment strategies for postural control in HD.
4.4. Domains of postural control
Postural control is a complex process where several domains are involved. Since there is no single measure to comprehensively describe stance postural control in healthy and impaired states,18,46 our study included measures from domains of smoothness, amplitude, and frequency. Postural impairments for the mHD group on the mCTSIB were detected in all measures across domains, perhaps owing to the severity of postural impairment, indicating that sway is jerky, amplified, and has higher frequencies compared to healthy controls. However, the value of measuring sway across different domains can be appreciated more effectively in pHD-measures of smoothness (Jerk-AP, -ML), and amplitude (Total Sway Area) were sensitive to postural instabilities but not frequency measures (Total Power-AP, -ML), particularly during combined attenuation of vision and proprioception. Based on effects of gaze fixation, only Total Power-ML detected improvements in postural control for pHD. The current work highlights the importance of assessing different domains of postural control in HD. Future large-sample studies are needed to examine the underlying relationships in postural measures in HD by using multivariate statistical analyses (e.g. exploratory factorial analysis) as has been performed in PD.18
4.5. Clinical implications
As wearable sensors are becoming more accessible in clinical settings, it is imperative for clinicians to use their quantitative metrics to guide clinical management of people living with HD. APDM sensors bring precision of lab-based assessments to clinical practice,17 without the burden of post-processing data by using clinician-friendly interface47 that allows quick retrieval of data immediately after testing. The enhanced resolution from quantitative assessments may support personalized and precision medicine.48 Our study demonstrates that these wearable sensors used during mCTSIB have generally good reliability in pHD and mHD, and are sensitive to subtle postural changes as early as pHD, and to behavioral interventions such as gaze fixation. Moreover, these sensors were capable of differentiating postural control even in group samples that were not widely divergent from each other between premanifest and earlier phases (Stage 1 and 2 using TFC49) of HD. Longitudinal data from quantified mCTSIB obtained from pHD to mHD may improve disease progression models which is needed for testing pharmacologic and behavioral interventions. With testing that typically takes <10 minutes, this practical consideration supports the integration of quantified balance assessments in routine examination. From a rehabilitation standpoint, training postural control under different sensory conditions, and to enhance it through gaze fixation training appear to be viable strategies. However hypothesis-driven interventional studies are needed to examine its therapeutic effects.
4.6. Limitations
The current study has limitations related to its design and method. First, our small sample size limits generalization of findings. Second, we did not quantify gaze fixation by measuring eye movements. While clinical observations and video recordings of gaze were performed, it is possible that we missed subtle ocular abnormalities which may moderate postural responses. Additionally, performance of gaze tasks may be influenced by cognitive level, for which data were not available. Future studies may need to stratify participants by ocular measures. Finally, while our work demonstrated the feasibility and reliability of using wearable sensors during mCTSIB, future validation studies may be needed to assess other clinimetric properties.
5. Conclusions
Quantification of postural control is urgently needed in HD. The consensus that treatment should be initiated at the earliest stage possible33 is challenged by limited knowledge of when motor deficits emerge in the course of HD. This study found that there indeed is a baseline postural deficit in mHD that is further exacerbated with sensory challenge, thus highlighting the need for earlier assessments in the course of HD. Examination of posture in pHD revealed presence of postural deficits. These deficits however appear to be responsive to sensory enhancing strategies, and thus provide further rationale for earlier intervention. The feasibility and reliability of these wearable sensors in detecting early motor changes in HD highlight their potential role in motor assessments during routine clinical examination.
Supplementary Material
7. Acknowledgements
This work was partially supported by the National Institutes of Health [grant no. K01 HD060912].
Footnotes
6 Declaration of conflicting interests
The authors declare that there is no conflict of interest.
References
- 1.Kegelmeyer DA, Kostyk SK, Fritz NE, et al. Quantitative biomechanical assessment of trunk control in Huntington’s disease reveals more impairment in static than dynamic tasks. Journal of the Neurological Sciences. 2017;376:29–34. [DOI] [PubMed] [Google Scholar]
- 2.Blanchet M, Prince F, Chouinard S, Messier J. Postural stability limits in manifest and premanifest Huntington’s disease under different sensory conditions. Neuroscience. 2014;279:102–112. [DOI] [PubMed] [Google Scholar]
- 3.Tian JR, Herdman SJ, Zee DS, Folstein SE. Postural control in Huntington ‘ s Disease ( HD ). Acta Otolaryngology (Stockholm). 1991;Suppl 481:333–336. [DOI] [PubMed] [Google Scholar]
- 4.Panzera R, Salomonczyk D, Pirogovosky E, et al. Postural deficits in Huntington ‘ s disease when performing motor skills involved in daily living. Gait & Posture. 2011;33(3):457–461. [DOI] [PubMed] [Google Scholar]
- 5.Grimbergen YAM, Knol MJ, Bloem BR, Kremer BPH, Roos RAC, Munneke M. Falls and Gait Disturbances in Huntington ‘ s Disease. Movement Disorders. 2008;23(7):970– 976. [DOI] [PubMed] [Google Scholar]
- 6.Fritz NE, Rao AK, Kegelmeyer D, et al. Physical therapy and exercise interventions in Huntington ‘ s Disease : A mixed methods systematic review. Journal of Huntington’s Disease. 2017;6(3):217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piira A, Walsem MR, Mikalsen G, Nilsen KH, Knutsen S, Frich JC. Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s Disease: A prospective intervention study. PLoS Currents. 2013(SEP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse M, Quinn L, Debono K, et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. Journal of Neurologic Physical Therapy. 2013;37(4):149–158. [DOI] [PubMed] [Google Scholar]
- 9.Reyes A, Salomonczyk D, Teo Wp, et al. Computerised dynamic posturography in premanifest and manifest individuals with Huntington’s Disease. Scientific Reports. 2018(September):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao AK, Louis ED, Marder KS. Clinical assessment of mobility and balance impairments in pre-symptomatic Huntington’s disease. Gait & Posture. 2009;30(3):391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porciuncula F, Roto AV, Kumar D, et al. Wearable movement sensors for rehabilitation : A focused review of technological and clinical advances. PM&R. 2018;10(9):S220–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrzejewski KL, Dowling AV, Stamler D, Felong TJ, Harris DA. Wearable Sensors in Huntington Disease : A Pilot Study. Journal of Huntington’s Disease. 2016;5:199–206. [DOI] [PubMed] [Google Scholar]
- 13.Dalton A, Khalil H, Busse M, Rosser A, Deursen R, OLaighin G. Analysis of gait and balance through a single triaxial accelerometer in presymptomatic and symptomatic Huntington’s disease. Gait & Posture. 2013;37(1):49–54. [DOI] [PubMed] [Google Scholar]
- 14.Beckmann H, Bohlen S, Saft C, et al. Objective assessment of gait and posture in premanifest and manifest Huntington disease — A multi-center study. Gait & Posture. 2018;62:451–457. [DOI] [PubMed] [Google Scholar]
- 15.Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurology.2014;13(1):100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbruzzese G, Berardelli A. Sensorimotor integration in movement control. Movement Disorders. 2003;18(3):231–240. [DOI] [PubMed] [Google Scholar]
- 17.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: A sensitive, valid and reliable measure of postural control. Journal of NeuroEngineering and Rehabilitation. 2012;9(l):l–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horak FB, Mancini M, Carlson-kuhta P, Nutt JG, Salarian A. Balance and gait represent independent domains of mobility in Parkinson disease. Physical Therapy. 2016;96(9):1364—1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M. How to select balance measures sensitive to Parkinson ‘s Disease from body-worn inertial sensors — separating the trees from the forest. Sensors. 2019;19(3320):1—18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horak FB. Clinical Measurement of Postural Control in Adults. Physical Therapy. 1987;67(12):1881–1885. [DOI] [PubMed] [Google Scholar]
- 21.Purcell NL, Goldman JG, Ouyang B, Bernard B, O’Keefe JA. The effects of dual-task cognitive interference and environmental challenges on balance in Huntington’s disease. Movement Disorders Clinical Practice. 2019;6(3):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitório R, Gobbi LTB, Lirani-silva E, Moraes R, Almeida QJ. Synchrony of gaze and stepping patterns in people with Parkinson ‘ s disease. Behavioural Brain Research. 2016;307:159–164. [DOI] [PubMed] [Google Scholar]
- 23.Georgiou N, Bradshaw JL, Phillips JG, Chiu E. Effect of directed attention in Huntington’s Disease. Journal of Clinical and Experimental Neuropsychology. 1997;19(3):367–377. [DOI] [PubMed] [Google Scholar]
- 24.Lacour M, Dosso NY, Heuschen S, Thiry A. How eye Movements stabilize posture in patients with bilateral vestibular hypofunction. Frontiers in Neurology. 2018;9(744):1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leigh RJ, Newman SA, Folstein SE, et al. Abnormal ocular motor control in Huntington’s disease. Neurology. 1983;33:1268–1275. [DOI] [PubMed] [Google Scholar]
- 26.Huntington Study Group . Unified Huntington’s Disease Rating Scale: Reliability - and- Consistency. Movement Disorders. 1996;11(2):136–142. [DOI] [PubMed] [Google Scholar]
- 27.Freeman L, Gera G, Horak FB, Blackinton MT, Besch M, King L. Instrumented Test of Sensory Integration for Balance: A Validation Study. Journal of Geriatric Physical Therapy. 2018;41(2):77—84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer C, Peterka RJ. A New Interpretation of Spontaneous Sway Measures Based on a Simple Model of Human Postural Control. Journal of Neurophysiology. 2005;93:189–200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Jane S. Indexing disease progression at study entry with individuals at-risk for Huntington’s disease. American Journal of Medical Genetics. 2011;156(7):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Methods. 1996;1(1):30–46. [Google Scholar]
- 31.Salarian A 2020.Intraclass Correlation Coefficient (ICC). (https://www.mathworks.com/matlabcentral/fileexchange/22099-intraclasscorrelation-coefficient-icc), MATLAB Central File Exchange. Retrieved May 15, 2020.
- 32.Liu D, Long JD, Zhang Y, et al. Motor onset and diagnosis in Huntington disease using the diagnostic confidence level. Journal of Neurology 2015;262(12):2691– 2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: The Predict-HD Study. Archives of Neurology. 2006;63(6):883. [DOI] [PubMed] [Google Scholar]
- 34.Portney A, Watkins M. Foundations ofClinical Research Applications to Practice. Upper Saddle, NJ: Pearson & Prentice Hall; 3rd ed. 2009. [Google Scholar]
- 35.Tokuno CD, Taube W, Cresswell AG. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiologica. 2009;195(3):385–395. [DOI] [PubMed] [Google Scholar]
- 36.Huttunen J, Homberg V. EMG responses in leg muscles to postural perturbations in Huntington’s disease. Journal of Neurology Neurosurgery and Psychiatry. 1990;53(1):55– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann RK, Edwards R, Zhou J, Fenney A, Jog M, Duval C. Comparing movement patterns associated with Huntington’s chorea and Parkinson’s dyskinesia. Experimental Brain Research. 2012;218(4):639—654. [DOI] [PubMed] [Google Scholar]
- 38.Bhidayasiri R, Truong DD. Chorea and related disorders. Postgraduate Medical Journal. 2004;80(947):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morasso P, Cherif A, Id JZ. Quiet standing : The Single Inverted Pendulum model is not so bad after all. PLoS ONE. 2019:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neuroscience Lettters. 2005;377:75– 80. [DOI] [PubMed] [Google Scholar]
- 41.Boonstra TA, Schouten AC, Kooij HVD. Identification of the contribution of the ankle and hip joints to multi-segmental balance control. Journal of NeuroEngineering and Rehabilitation. 2013;10(23):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekkers EMJ, Dockx K, Heremans E, Vercruysse S, Sabine MP. The contribution of proprioceptive information to postural control in elderly and patients with Parkinson ‘ s disease with a history of falls. Frontiers in Human Neuroscience. 2014;8(November):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitney SL, Roche JL, Marchetti GF, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait & Posture. 2011;33(4):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoula Z, Le Tt. Effects of distance and gaze position on postural stability in young and old subjects. Experimental Brain Research. 2006;173:438–445. [DOI] [PubMed] [Google Scholar]
- 45.Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA. Reliance on advance information and movement sequencing in Huntington’s Disease. Movement Disorders. 1995;10(4):472–481. [DOI] [PubMed] [Google Scholar]
- 46.Rocchi L, Chiari L, Cappello A. Feature selection of stabilometric parameters based on principal component analysis. Medical and Biological Engineering and Computing. 2004;42(September 2003). [DOI] [PubMed] [Google Scholar]
- 47.Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of Bioengineering and Biomedical Science. 2014;(Suppl 1):1—15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhawan AP. Collaborative paradigm of preventive, personalized, and precision medicine with point-of-care technologies. IEEE Journal of Translational Engineering in Health and Medicine. 2016;4(February). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoulson I, Fahn S. Huntington disease: Clinical care and evaluation. Neurology. 1979;29(January):1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


