Abstract
Background
Spontaneous coronary artery dissection (SCAD) is a rare condition, mainly affecting young women. Cases in male patients are rare, especially with recurrence.
Case summary
A 59-year-old male non-elite athlete presented as an ST-elevation myocardial infarction following a 5-km run. Urgent coronary angiogram was normal, but cardiac magnetic resonance showed a myocardial infarction. Four years later, he experienced similar chest pain with no ST-elevation on electrocardiogram and a mild troponin rise. Urgent coronary angiogram was initially thought normal but subsequent close inspection confirmed a Type 2b SCAD. Cardiac magnetic resonance showed a small additional myocardial infarction contained within an area of acute myocardial oedema.
Discussion
Spontaneous coronary artery dissection is more common in young women compared to men and recurrent dissection has been rarely reported in the literature. Cohort studies have shown the rate of recurrent dissection to be 13–16%, but most of the patients in these cohorts are female. Poor data exists on the best treatment of SCAD in men, but given the presence of intramural thrombus, dual antiplatelet therapy was discontinued on the presumption that it may exacerbate an intramural bleeding process.
Keywords: Coronary artery dissection, Male, Athlete, Recurrent, Case report, MRI, Coronary angiogram, Myocardial infarction
Learning points
Spontaneous coronary artery dissection is rare in male patients.
It must be considered in cases where no atherosclerotic disease is identified on angiogram.
Cardiac magnetic resonance can aid confidence in identifying the affected vessel by confirming and locating the infarct.
Introduction
Spontaneous coronary artery dissection (SCAD) is a rare condition, predominantly affecting young women, caused by a tear between the layers of the coronary arteries.1,2 The clinical presentation is often indistinguishable from myocardial infarction. Spontaneous coronary artery dissection has associations with several conditions, including fibromuscular dysplasia, connective tissue disease, and hormone-dependent states (pregnancy, oral contraceptive pill use). Recurrent SCAD is exceptionally rare, with a limited number of cases in the literature.3–5
Timeline
| November 2014 |
|
| December 2018 |
|
| March 2019 |
|
Case presentation
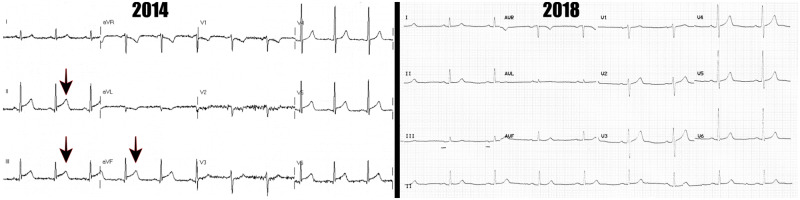
A 59-year-old non-elite male athlete6 presented in 2014, after experiencing sudden onset chest pain at the end of a 5-km run. There was a background history of hypertension, but no other risk factors. Examination revealed a normotensive patient with a heart rate of 68. Heart and lungs were clear to auscultation. Electrocardiogram showed infero-lateral ST-elevation (see Figure 1) with an associated high-sensitivity troponin I rise of 47 865 ng/L (upper limit of normal laboratory range = 40 ng/L). His cholesterol was 4.3 mmol/L with normal LDL, HDL, and triglycerides.
Figure 1.
Electrocardiograms from 2014 to 2018, respectively. Note the ST-elevation in 2014 (arrowed).
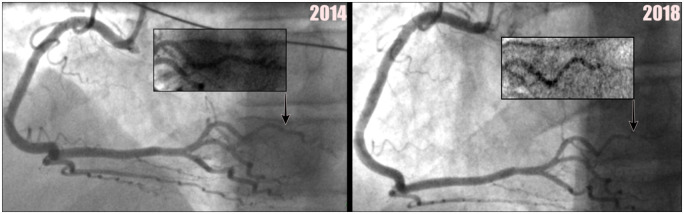
Emergency coronary angiogram performed in 2014 demonstrated unobstructed coronary arteries (see Figures 2 and 3). Echocardiogram showed good left and right ventricular systolic function [left ventricular end-diastolic diameter (LVEDD) = 4.7 cm] with mild hypokinesia in the mid-cavity lateral wall and no significant valvular heart disease. The patient was treated for a diagnosis of myocardial infarction with non-occlusive coronary arteries (MINOCA) or myocarditis and commenced on dual antiplatelet therapy of long-term aspirin 75 mg o.d. (once daily) and ticagrelor 90 mg o.d. (for 1 year), as well as simvastatin 40 mg o.d. He was also changed from enalapril 10 mg to ramipril 5 mg o.d.
Figure 2.
This demonstrates a normal coronary angiogram of right coronary artery from 2014 with a normal posterior left ventricular branch (seen 2014 image), with subsequent coronary angiogram of the right coronary artery from 2018, showing a dissection see in the posterior left ventricular branch (seen 2018 image). Close-up views are also provided.
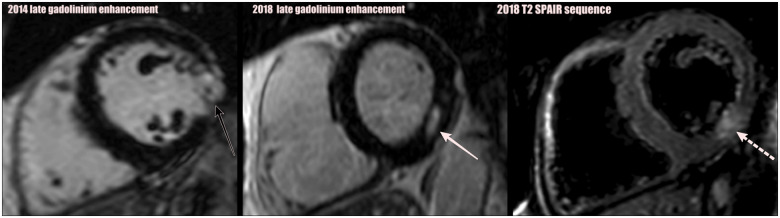
Cardiac magnetic resonance performed 4 months later showed evidence of a small transmural myocardial infarction in the lateral wall at mid-cavity level (see Figure 4 late gadolinium enhancement—solid black arrow). Holter monitor showed no evidence of arrhythmia.
Figure 4.
This figure shows mid-cavity views from 2014 to 2018, showing both short axis of the right and left ventricle. The late gadolinium enhancement magnetic resonance imaging images from 2014, demonstrating transmural infarction of the mid-cavity inferolateral segment of left ventricular wall (black arrow). Late gadolinium enhancement magnetic resonance imaging images from 2018 showing a new area of mid-wall infarct (solid white arrow). 2018 T2-SPAIR shows T2-weighted SPAIR with fat saturation images, showing signal increase in the mid inferior wall, in keeping with acute injury with a component of oedema (broken white arrow).
In 2018, the patient re-presented with chest pain for 12 h, after a morning run. On examination, the patient was normotensive with a heart rate of 55. Auscultation of the heart and lung revealed no significant findings. Electrocardiogram in 2018 was unremarkable (Figure 1) and high-sensitivity troponin I measured 4500 ng/L. His cholesterol was 3.2 mmol/L again with normal LDL, HDL, and triglycerides.
Urgent coronary angiogram in 2018 did not identify any obvious coronary atherosclerotic culprit (Figures 2 and 3). Echocardiogram showed an ejection fraction of 63% and an LVEDD of 5.3 cm (resting heart rate—54). He was continued on ramipril 5 mg o.d. and aspirin 75 mg o.d. Ticagrelor 90 mg o.d. was re-commenced for 3 months.
Cardiac magnetic resonance was performed 7 days after acute presentation and showed a new small area of subendocardial infarct in the mid inferolateral segment. Figure 4 shows late gadolinium enhancement on magnetic resonance imaging (MRI) in 2018, with an area of infarct showing late enhancement (solid white arrow). Figure 4 also shows T2-SPAIR (fat saturated) sequence shows the oedema surrounding the acute infarct (area shown with broken white arrow), which is situated immediately above the left ventricular slices containing the old infarct from 2014.
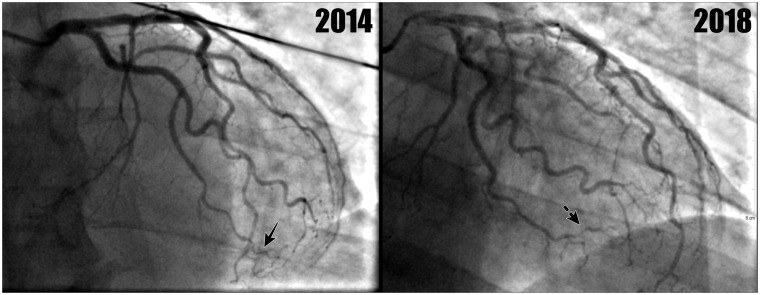
Subsequent review of the 2018 coronary angiogram (Figure 2) identified a dissection in the posterior left ventricular branch, classified as a Type 2b SCAD.2 Less clear cut changes were also noted in the distal and terminal branches of the left circumflex, suggestive of distal intra-mural coronary haematoma formation, which can be seen on the 2018 angiogram (Figure 3).
Figure 3.
This shows left coronary arterial system from 2014 to 2018. The latter shows subtle changes suggestive of distal intra-mural coronary haematoma formation in the distal circumflex branches (broken arrow). The 2014 image demonstrates the same vessel (solid arrow) which was normal and is displayed for comparison.
The patient’s aspirin 75 mg o.d. and ticagrelor 90 mg o.d. were subsequently stopped, to reduce the risk of intramural bleeding. A beta-blocker was not used due to the patient’s low resting heart rate.
Agitated saline contrast echocardiogram was performed to exclude a patent foramen ovale, which could have raised the possibility of coronary embolism. This test excluded any inter-atrial communication (see Figure 5). An implantable loop recorder did not reveal any arrhythmic episodes. A CT angiogram of the arterial tree from the circle of Willis to the popliteal arteries was performed to screen for fibromuscular dysplasia. This showed mild ectasia of the ascending aorta with moderate aorto-iliac atherosclerosis. However, no evidence of fibromuscular dysplasia was found. Blood results demonstrated negative anticardiolipin antibodies and negative lupus anticoagulant.
Figure 5.
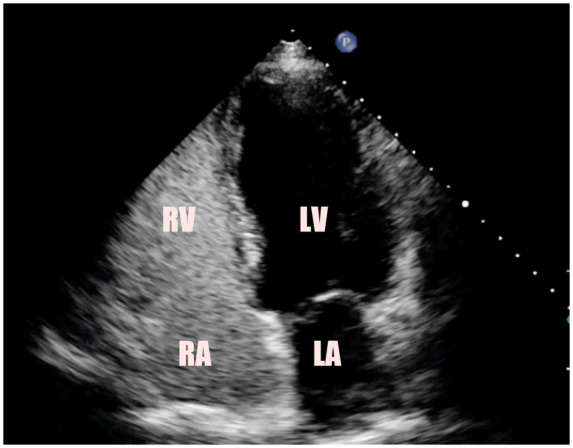
This figure shows echocardiography four-chamber view of the heart with agitated bubbles in the right atrium and right ventricle. No bubbles are identified in the left atrium or left ventricle, confirming that the atrial septum is intact. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
On further discussion, the patient’s younger sister suffered a carotid artery dissection at the age of 45, which resulted in a fatal stroke 7 days after presentation.
On latest follow-up in 2019, the patient was doing well. There were no further episodes of chest pain and excellent compliance with medications.
Discussion
A recurrence rate of 13–16% of SCAD has been reported in the literature but this data emerges mostly from female patient cohorts.1 There is very limited data on the risk of recurrence in men. Our patient is an exemplary case, given he is male, middle-aged, has a family history of vascular dissection, as well as both events being triggered by intense physical exercise. The SCAD was also located in small distal coronary branches, and these events may possibly involving two separate territories.
While no abnormality was identified on the initial angiogram, the detection of Type 2 SCAD is often not appreciated or misdiagnosed.7 The presence of this aetiology in the second event is highly suggestive of SCAD as the pathophysiology of the primary event.
While coronary angiography remains the most common modality to diagnose SCAD, intracoronary imaging has been shown to be a useful adjunct in cases of subtle dissection.8 Intravascular ultrasound was not used in this case due to the risk of further vessel wall damage give the distal nature of the dissection.
Cardiac MRI is used as the initial diagnostic investigation for evaluating the case of MINOCA. It allows assessment of infarct size, as well as myocardial dysfunction,9 as well as differentiating infarcts from other causes of troponin rise.
Computed tomography coronary angiogram may be of benefit in diagnosis of larger coronary vessels and in follow-up of patients who have proven dissection8 but has lower temporal resolution than coronary angiography. In this case, the presence of a small calibre vessel dissection limits its usefulness and therefore, it was not performed.
Management of these patients remains a matter of future research. The current preferred strategy is conservative management, including use of beta-blockers, angiotensin-converting enzyme inhibitors, antiplatelet therapy, and statin therapy but other approaches have included emergent revascularization and coronary artery bypass grafting.1
The overall mortality in women is relatively low, ranging from 0% to 5% over 3–6 years.10 Such data is not known for male patients. Since intramural haematoma is central to the pathophysiology of Type 2b dissection and can extend dissection,1 dual antiplatelet therapy was stopped in this case. He self-rehabilitated very rapidly and returned to running. We did not restart the patient on antiplatelet therapy, as the risk of true coronary artery disease was considered low, given no significant atherosclerotic disease was seen on his coronary angiogram.
On the basis of the significant evidence of beneficial effects of exercise in the prevention of occlusive coronary artery disease,11counter-balanced by a relatively smaller risk of further recurrences of myocardial infarction in the presence of unobstructed coronary arteries due to spontaneous coronary dissection of small branches (which appear to characterize more the male patients), we agreed with continuation of the medium-distance running strategy, involving a maximum 10 km runs. This was after extensive consultation with the patient, who did not wish to cease running activities.
Spontaneous coronary artery dissection has been reported as a cause of up to 8% of sudden cardiac arrest and death in athletes12 and has been associated with power sport.3 However, our patient was not interested in undertaking power sport activities. Also, poor compliance with detraining in athletes is a documented phenomenon.13
Conclusion
This case is uncommon, given the rarity of recurrent SCAD in male patients. The diagnosis should be suspected in cases of MINOCA with evidence of hypokinesia or infarct on echocardiogram and/or cardiac MRI. The diagnosis of SCAD is clinically important, as its management can significantly differ from that of atherosclerotic coronary artery disease.
Lead author biography

John J. Fitzpatrick is a Specialist Registrar in Clinical Radiology in Aberdeen Royal Infirmary. He has an interest in Interventional Radiology and Cardiothoracic imaging.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Saw J, Mancini GBJ, Humphries KH.. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 2. Adlam D, Maas A, Vrints C, Alfonso F.. Spontaneous coronary artery dissection. Eur Heart J 2016;37:3073–3074. [DOI] [PubMed] [Google Scholar]
- 3. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ. et al. Percutaneous coronary intervention for acute spontaneous coronary artery dissection is associated with reduced rates of technical success. Circulation 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 4. Papireddy MR, Nandish S, Mishkel GJ.. Recurrent spontaneous coronary artery dissection: first case report in men with three episodes of spontaneous coronary dissection in separate vascular territories. Catheter Cardiovasc Interv 2016;87:E192–E196. [DOI] [PubMed] [Google Scholar]
- 5. Rosengarten JA, Dana A.. Recurrent spontaneous coronary artery dissection: acute management and literature review. Eur Heart J Acute Cardiovasc Care 2012;1:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lorenz DS, Reiman MP, Lehecka BJ, Naylor A.. What performance characteristics determine elite versus nonelite athletes in the same sport? Sports Health 2013;5:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandrasekhar J, Thakkar J, Starovoytov A, Mayo J, Saw J.. Spontaneous coronary artery dissection—a review. Cardiovasc Diagn Ther 2015;10: 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE. et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasupathy S, Tavella R, McRae S, Beltrame JF.. Myocardial infarction with non-obstructive coronary arteries—diagnosis and management. Eur Cardiol 2015;10:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macaya F, Salinas P, Gonzalo N, Fernández-Ortiz A, Macaya C, Escaned J.. Spontaneous coronary artery dissection: contemporary aspects of diagnosis and patient management. Open Heart 2018;5:e000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stensel D. Primary prevention of CVD: physical activity. BMJ Clin Evid 2009;2009:0218. [PMC free article] [PubMed] [Google Scholar]
- 12. Peterson DF, Siebert DM, Kucera KL, Thomas LC, Maleszewski JJ, Lopez-Anderson M.. Etiology of sudden cardiac arrest and death in US Competitive Athletes: a 2-year prospective surveillance study. Clin J Sport Med Actions 2018;doi:10.1097/JSM.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 13. Sharma S, Merghani A, Mont L.. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J 2015;36:1445–1453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.