Abstract
Introduction
Mimicking movements of others makes both the imitating and imitated partners feel closer. Oxytocin may increase focus on others and has been shown to increase automatic imitation in healthy controls (HC). However, this has not been replicated, and oxytocin’s effects on automatic imitation have not been demonstrated in clinical populations. This study attempts to replicate effects on HC and examine effects on people with comorbid posttraumatic stress disorder and alcohol use disorder (PTSD-AUD).
Methods
Fifty-four males with PTSD-AUD and 43 male HC received three intranasal treatment conditions (placebo, oxytocin 20 International Units (IU), and oxytocin 40 IU) in a randomized order, across three separate testing days, as part of a double-blind, crossover parent study. At 135 minutes post-administration, each performed the imitation-inhibition task, which quantifies automatic imitation as the congruency effect (CE). After exclusions, the final analyzed data set included 49 participants with PTSD-AUD and 38 healthy controls.
Results
In HC, oxytocin 20 IU demonstrated a statistically significant increase in CE, and 40 IU showed a trend-level increase. In PTSD-AUD, oxytocin did not significantly increase CE. Post-hoc analysis showed the PTSD-AUD group had higher CE than HC on placebo visits.
Discussion
Our data suggest PTSD-AUD is associated with higher automatic imitation than HC in the absence of oxytocin administration. We successfully replicated findings that oxytocin increases automatic imitation in HC. This demonstrates an unconscious motor effect induced by oxytocin, likely relevant to more complex forms of imitative movements, which have the potential to improve social connection. We did not find a significant effect of oxytocin on automatic imitation in PTSD-AUD. Future research should examine imitation in both sexes, at peak oxytocin levels, and on increasingly complex forms of imitation.
Keywords: Oxytocin; stress disorders, post traumatic; Alcoholism; imitative behavior; inhibition, psychological; Administration, Intranasal
1. Introduction
Humans automatically and unconsciously mimic the movements of others, such as facial expressions and posture (Chartrand and Lakin, 2013). This likely contributes to social connection as mimicry leads to both imitating and imitated partners feeling more liked by, and closer to, the other (Stel and Vonk, 2010). One aspect of the tendency to mimic can be quantified (Chartrand and Lakin, 2013; Heyes, 2011; Leighton et al., 2010) by the imitation-inhibition task, which focuses on automatic imitation of simple finger movements. More imitation on this task has been linked to prosocial states and traits, where focus is more on others than the self. For example, priming with prosocial statements or cartoon movies of helping others increases automatic imitation (Wang and Hamilton, 2013), while priming with self-referential statements decreases automatic imitation (Spengler et al., 2010). Similarly, ratings of self-centered, narcissistic traits are inversely correlated with automatic imitation (Obhi et al., 2014). Despite its social relevance, little is known about how to pharmacologically manipulate automatic imitation, or whether it is abnormal in psychiatric disorders with social deficits.
The oxytocin system is critical to pair bonding and infant-parent attachment. Oxytocin administration in humans may also shift focus from self to other (Zhao et al., 2016), implying it could increase the tendency to imitate other people’s movements. Indeed, intranasal oxytocin increased synchrony, the intersection of imitation and timing, in body language (Ramseyer et al., 2019), in dance movements (Josef et al., 2019), and on a similar finger task (Gebauer et al., 2016). De Coster et al. (2014) showed that a single intranasal oxytocin administration also increases automatic imitation of finger movements in undergraduates, even when the element of timing is removed. However, this result has not been replicated, and oxytocin’s effects on automatic imitation in clinical populations have not yet been investigated. Individuals with comorbid posttraumatic stress disorder and alcohol use disorder (PTSD-AUD) have poor social connection (Grant et al., 2015; Ouimette et al., 2006) and evidence of aberrant self-other processing (Cracco et al., 2020; Frewen et al., 2017; Hudson et al., 2020). Thus, they may have low automatic imitation, which could be increased by oxytocin. Determining if automatic imitation is abnormal in PTSD-AUD, and if a single administration of oxytocin increases imitation in this population, could reveal a promising intervention.
The dose-response curve for intranasal oxytocin’s effects on automatic imitation is also unknown. Therefore, we examined the effects of two dosages of intranasal oxytocin on automatic finger imitation in individuals with PTSD-AUD and age-matched healthy controls (HC). We 1) assessed baseline differences between PTSD-AUD and HC groups, 2) attempted to replicate oxytocin’s effects on automatic imitation in HC, and 3) examined oxytocin’s effects on automatic imitation in individuals with PTSD-AUD, expecting oxytocin to increase automatic imitation in both groups.
2. Materials and Methods
2.1. Participants
Fifty-four males with PTSD-AUD (mean age 50.48 ± 13.02) and 43 male HC (48.46 ± 13.31) recruited from the San Francisco Bay Area received the intervention. No participants had contraindications to receiving intranasal oxytocin (see supplement S2.1–2). PTSD-AUD participants met criteria for both PTSD and AUD on the Clinician Administered PTSD Scale and the Structured Clinical Interview for DSM-5. We excluded individuals in legally mandated alcohol treatment programs, or with acute alcohol withdrawal, suicidal ideation with plan within 30 days, or a suicide attempt within six months. HC had no history of PTSD or substance use disorders, though mild AUD was allowed. All gave written informed consent and were compensated for time and travel. For parent study details, see Stauffer et al. (2019).
2.2. Procedure
As the oxytocin literature suffers from mixed results with poor replication, in order to separate exploratory from confirmatory analyses, we pre-registered our methods, hypotheses, and analytic plan: https://aspredicted.org/vy72m.pdf. While this was after data collection, the first author and statistician remained blinded when the statistical analysis plan for this arm of the study was chosen and pre-registered. We used a placebo-controlled, double-blind, crossover design. Across three testing days (“visits”), separated by a washout period of at least one week, participants received each of three intranasal treatment conditions (placebo, oxytocin 20 International Units [IU], and oxytocin 40 IU) in a randomized order. We conducted unrelated tasks, such as a cue-induced-craving of alcohol paradigm and a fear-potentiated acoustic startle paradigm, published separately, and then, ~135 minutes after intranasal administration, performed the imitation-inhibition task (see Fig.SI; adapted from De Coster et al., 2014 and Brass et al., 2000) to quantify automatic imitation.
During the imitation-inhibition task, participants first depressed two keyboard keys with the index and middle fingers of their right hand, and were told to lift and replace their index finger as fast as possible whenever the screen displayed a ‘1’, or their middle finger whenever the screen displayed a ‘2.’ They were told to respond only to this number, even though hand images would also appear. Then, during each trial, the screen displayed a ‘1’ or ‘2’ between the index and middle finger of hand images that, like participants, made index or middle finger lifting movements. The screen randomly displayed three trial types: a) neutral trials, where the hand was stationary, b) congruent trials, where the hand performed the same movement that the participant was instructed to perform, and c) incongruent trials, where the hand performed the finger movement different from what the participant was instructed to perform. Participants completed 12 practice trials and then 50 experimental trials, ~5 minutes in total (Cracco et al., 2018). Reaction times (RT) during incorrect responses were excluded (see S2.2).
The congruency effect (CE), a measure of automatic imitation, represents the RT difference between incongruent and congruent trials. Because slower incongruent RT can represent “trouble inhibiting imitation” of the other, and faster congruent RT can represent “easily imitating”, higher CE encompasses two ways to express “more imitation” (Wang and Hamilton, 2013).
2.3. Statistical Analysis Plan
We used a multilevel model to evaluate the main effects of intranasal oxytocin dosage (placebo vs. oxytocin 20 IU, and placebo vs. oxytocin 40 IU) and group (PTSD-AUD vs. HC) on our primary outcome, CE. Given that we had strong group-specific a priori hypotheses, we used the same model to assess group differences in CE on the placebo day only, as well as to examine oxytocin effects on CE in the diagnostic groups separately. We expected: 1) the PTSD-AUD group’s placebo-day CE would be lower compared to HC; and that oxytocin administration would increase CE in 2) HC, and 3) in PTSD-AUD.
We removed visits with CE greater than three SD above or below the mean for each dosage group (4 removed) and those with a non-response rate of over 50% for all trials on a specific visit (1 removed). We log-transformed our non-normal outcome of CE (z-skew = 6.1 corrected to 2.69, and z-kurtosis = 4.1 corrected to 2.13; Kim, 2013). The final analyzed data set included 138 visits from 49 participants with PTSD-AUD and 107 visits from 38 healthy controls. We included a treatment order variable, and group-by-dosage and group-by-order interaction terms in the models. We included candidate confounders in final models only if these predicted the outcome in univariate analysis at the p < 0.10 level. Statistical analysis was done using R (version 1.1463; R Foundation for Statistical Computing, Vienna, Austria) and the “nlme: Linear and Nonlinear Mixed Effects Models.R” package (version 3.1-131).
3. Results
3.1. Pre-registered Analysis
Congruency effect model, overall group:
Age significantly increased CE in univariate regression and was included in the multilevel model. Race and positive urine toxicologies (THC, cocaine, benzodiazepines, amphetamines, and methamphetamines) were not significant univariate predictors. The multilevel model revealed that oxytocin increased logCE in the overall sample 5.2% beyond placebo (converted from ∆logCE = 0.02), equivalent to a 6 msec increase in our average subject (95%CI, 0.11-11.76 msec; see S3.1–1). Both dosages had similar positive effect sizes on logCE, with p< 0.05 for 20 IU and p <0.10 for 40 IU (Table 1a). There was no effect of treatment order, and no significant group-by-dosage interactions in our models, though one combination showed a trend.
Table 1.
Summary of Multilevel Model Regression Analyses of CE
| Model 1a. [Both groups] Predictors of Log10(CE) |
||||
|---|---|---|---|---|
| Variable | Level | Effect(∆lgCE) | SE | P |
| OXT Dose* | (ref. = Placebo) | - | - | - |
| 20 IU* | 0.02 | 0.01 | 0.05 | |
| 40 IU | 0.02 | 0.01 | 0.08 | |
| Group | (ref. = Control) | - | - | - |
| PTSD-AUD | 0.02 | 0.03 | 0.47 | |
| Age* | 0.00 | 0.00 | >0.01 | |
| PTSD-AUD x 20 IU Dose | −0.01 | 0.01 | 0.56 | |
| PTSD-AUD x 40 IU Dose | −0.03 | 0.02 | 0.08 | |
| Crossover Order | >0.34 | |||
| Group x Order | >0.34 | |||
| Model 1b.† [Placebo visit only] Predictors of Log10(CE) |
||||
| Variable | Level | Effect(∆lgCE) | SE | P |
| OXT Dose | (Placebo only) | - | - | - |
| Group* | (ref. = Control) | - | - | - |
| PTSD-AUD* | 0.04 | 0.01 | 0.01 | |
| Age* | <0.01 | <0.01 | <0.01 | |
| Model 1c. [HC only] Predictors of Log10(CE) |
||||
| Variable | Level | Effect(∆lgCE) | SE | P |
| Group | (HC only) | - | - | - |
| OXT Dose* | (ref. = Placebo) | - | - | - |
| 20 IU* | 0.02 | 0.01 | 0.04 | |
| 40 IU | 0.02 | 0.01 | 0.06 | |
| Age* | <0.01 | <0.01 | <0.01 | |
| Crossover Order | >0.31 | |||
| Model 1d. [PTSD-AUD only] Predictors of Log10(CE) |
||||
| Variable | Level | Effect(∆lgCE) | SE | P |
| Group | (PTSD-AUD only) | - | - | - |
| OXT Dose | (ref. = Placebo) | - | - | - |
| 20 IU | 0.01 | 0.01 | 0.20 | |
| 40 IU | −0.01 | 0.01 | 0.51 | |
| Age* | <0.01 | <0.01 | <0.01 | |
| Crossover Order | > 0.15 | |||
Abbrev.: CE=congruency effect; Effect = per unit effect size (regression coefficient); ∆lg=change in log units; SE= standard error; OXT= oxytocin; IU= international units; PTSD= posttraumatic stress disorder; AUD= alcohol use disorder. †Post-hoc analysis.
Congruency effect model, HC group only:
the same model, limited to HC, revealed that logCE was significantly increased by oxytocin 20 IU, and by 40 IU at a trend level (see Table 1c; Figure 1b). Congruency effect model PTSD-AUD group only: the same model, limited to PTSD-AUD participants, revealed that neither dose of oxytocin significantly increased logCE (Table 1d; Figure 1c).
Fig. 1.
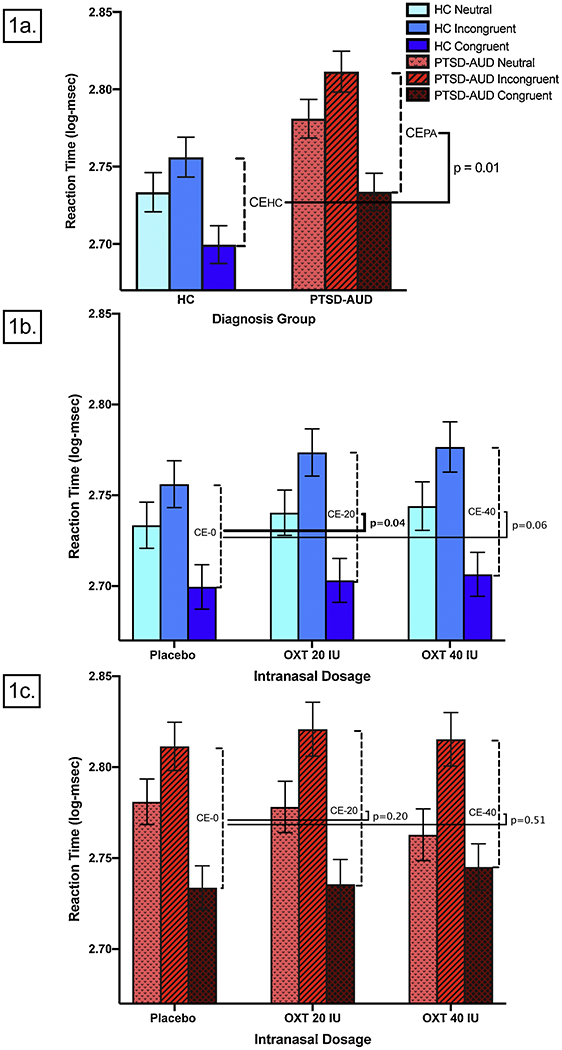
(1a) Placebo-visit logCE was higher in PTSD-AUD compared to HC.† (1b) Oxytocin increased logCE in the HC group. (1c) Oxytocin did not increase logCE in the PTSD-AUD group. Abbrev.: HC= healthy controls; PTSD= post-traumatic stress disorder; AUD=Alcohol Use Disorder; CE= congruency effect; CEHC= CE in HC; CEPA= CE in PTSD-AUD; IU= international units; OXT = oxytocin. Neutral= average RT for neutral trials; Incongruent= average RT for incongruent trials; Congruent= average RT for congruent trials (see 2.2). Vertical bars indicate standard error of the mean (SEM). †Post-hoc analysis. [Note to editors: please use color for this figure; this and all screenshots shown here are placeholders. Please use separately uploaded PDFs of figures and tables.]
3.2. Post-hoc Analysis
Placebo visits:
the same model, limited to placebo visits only, revealed that the PTSD-AUD group, compared to HC, had significantly higher CE (see Table 1b; Figure 1a) on the placebo visit.
Equivalence:
To confirm oxytocin did not increase CE in PTSD-AUD, we used the “Two One-Sided Test” (TOST) to evaluate equivalence (Lakens, 2017). Aiming to detect equivalence with 80% power in this dependent sample, and allowing for alpha of 0.1, we determined the tightest range within which we could determine equivalence was effect size d = −0.4 to 0.4, requiring n = 42 dependent pairs (present study: n = 46). Within this range, for the PTSD-AUD group, TOST showed equivalence between placebo and both dosages’ effects on log CE [M(±SD); placebo: 2.65 (±0.07) log-msec vs. oxytocin 20 IU: 2.66 (±0.07) log-msec; p < 0.05; and vs. oxytocin 40 IU: 2.64 (±0.07) log-msec; p < 0.02].
4. Discussion
We examined oxytocin’s effects on automatic imitation of finger movements in both a clinical and a control population. We found that: 1) PTSD-AUD was associated with higher automatic imitation than HC on the placebo day; and 2) intranasal oxytocin increased automatic imitation in the HC group, replicating prior work (De Coster et al., 2014). Given our pre-registered analysis, this replication supports the hypothesis that oxytocin administration increases automatic imitation in healthy individuals. We also found that 3) oxytocin did not alter automatic imitation in PTSD-AUD. Therefore, our data does not support oxytocin as a treatment for imitative abnormalities in PTSD-AUD.
Further strengthening our finding that oxytocin increases automatic imitation in HC, we saw a similar magnitude of increase from both the 20 and 40 IU oxytocin dosages. While the mechanism remains unclear, DeCoster et al., (2014) proposed oxytocin may alter self-other distinction, thereby increasing automatic imitation. Oxytocin has already been shown to shift focus to the “other,” not only on imitation tasks, but also on decision-making tasks (Zhao et al., 2016). Oxytocin administration has also been shown to alter neural networks involved in self-other distinction and focus; the same networks that are activated with imitation changes on our imitation-inhibition task (Brass et al., 2009). Regardless of mechanism, our results imply that oxytocin administration increases motor imitation on an automatic (i.e., unconscious) level. This automatic motor effect may be one of many effects oxytocin induces when increasing more complex imitation, such as synchrony in body language (Ramseyer et al., 2019) and dance (Josef et al., 2019). Better understanding how and when oxytocin affects complex motor imitation, which increases closeness and empathy, brings us one step closer to understanding oxytocin’s nuanced effects on social connection.
Given problematic social relationships in both PTSD and AUD (Grant et al., 2015; Ouimette et al., 2006), we expected less placebo-day imitation in PTSD-AUD compared to HC group, but we found the opposite. This may explain why oxytocin did not increase imitation in the PTSD-AUD group; patients may have been at a ceiling level. Additionally, as attachment style modulates oxytocin’s effects (Bartz et al., 2011), and as trauma often affects attachment, PTSD-AUD participants may suffer from attachment disruption that blunts oxytocin’s effects. Other confounding influences in the PTSD-AUD population compared to HC, including higher distractibility, mistrust, sensitivity to threat and incongruent behavior, may also have contributed to higher CE and insensitivity to oxytocin. Further work is necessary to clarify baseline characteristics of, and moderators of oxytocin’s effects on, automatic imitation in PTSD-AUD.
Our study has several limitations. Our analytic plan was pre-registered after data collection. Though pre-registration is ideally done prior to data collection, it is increasingly viewed as a continuum of beneficial efforts undertaken at varying times (Benning et al., 2019), including using preexisting data (Nosek et al., 2018). Our first author and statistician remained blinded and our pre-registration defined the analytic plan before observing outcomes. Thus, we suggest this remains a strength, albeit a limited strength. We tested automatic imitation at 135 minutes post-administration, which is later than in most prior work and may be after oxytocin’s peak effects. However, oxytocin is likely still elevated in the CSF at this time (Spengler et al., 2017; Striepens et al., 2013), timing of oxytocin effect varies greatly by brain region, and effects may occur in a non-linear fashion (Martins et al., 2020); also see S4–2). In our overall model, oxytocin significantly affected CE without significant group-by-dosage interactions, though there was a trend for this interaction with the 40 IU dosage. However, we did find group-specific effects when each group was examined separately. We were likely underpowered to detect group-by-dosage interactions. Additionally, our study included only men to decrease the heterogeneity of a relatively small sample. Given male-female differences in both the oxytocin system and automatic imitation tendencies, larger studies should examine the influence of sex. Lastly, we used the placebo visit to examine group differences, but this may not represent a true baseline.
In sum, using a pre-registered approach, we replicated the finding that intranasal oxytocin increases automatic imitation in HC. Oxytocin’s increase of automatic imitation of finger movements demonstrates an unconscious motor effect likely relevant to more complex forms of imitative movements, which have the potential to improve social connection. We also found, contrary to expectation, that PTSD-AUD was associated with higher CE compared to HC under the placebo condition, and that oxytocin did not increase imitation in our clinical group. Future research should investigate oxytocin’s effects on automatic imitation in both sexes, at peak oxytocin levels, and on increasingly complex forms of imitation.
Supplementary Material
Highlights.
Higher automatic imitation in co-morbid PTSD and alcohol use disorder (PTSD-AUD) than controls
Oxytocin administration increased automatic imitation in controls, replicating prior findings
Oxytocin administration did not increase automatic imitation in the PTSD-AUD group
Acknowledgements
Steven Batki, MD; Jessica Buffington, BA; Andreas Kuffer, Ph.D; David Leung, BS; Daniel Mathalon, PhD, MD; Thomas Neylan, MD; Andrea Niles, PhD; Evan Sheh, BA
Funding:
This work was supported in part by the National Institute of Mental Health [R25MH060482]; the Department of Veterans Affairs Clinical Science Research & Development [Federal Award Identification Number IK2CX001495]; and Department of Defense Awards [W81XWH-12-2-0048; W81XWH-13-2-007]. Funders had no role in study design; in data collection, analysis and interpretation; in the writing of the manuscript; or in the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of competing interest: None
References
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E, 2011. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc. Cogn. Affect. Neurosci 6, 556–563. 10.1093/scan/nsq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Bachrach RL, Smith EA, Freeman AJ, Wright AGC, 2019. The registration continuum in clinical science: A guide toward transparent practices. J. Abnorm. Psychol 128, 528–540. 10.1037/abn0000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschlager A, Prinz W, 2000. Compatibility between Observed and Executed Finger Movements: Comparing Symbolic, Spatial, and Imitative Cues. Brain Cogn. 44, 124–143. 10.1006/brcg.2000.1225 [DOI] [PubMed] [Google Scholar]
- Brass M, Ruby P, Spengler S, 2009. Inhibition of imitative behaviour and social cognition. Philos. Trans. R. Soc. B Biol. Sci 364, 2359–2367. 10.1098/rstb.2009.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand TL, Lakin JL, 2013. The Antecedents and Consequences of Human Behavioral Mimicry. Annu. Rev. Psychol 64, 285–308. 10.1146/annurev-psych-113011-143754 [DOI] [PubMed] [Google Scholar]
- Cracco E, Bardi L, Desmet C, Genschow O, Rigoni D, De Coster L, Radkova I, Deschrijver E, Brass M, 2018. Automatic imitation : a meta-analysis. Psychol. Bull 144, 453–500. 10.1037/bul0000143 [DOI] [PubMed] [Google Scholar]
- Cracco E, Hudson AR, Van Hamme C, Maeyens L, Brass M, Mueller SC, 2020. Early interpersonal trauma reduces temporoparietal junction activity during spontaneous mentalising. Soc. Cogn. Affect. Neurosci. nsaa015 10.1093/scan/nsaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster L, Mueller SC, T’Sjoen G, De Saedeleer L, Brass M, 2014. The influence of Oxytocin on automatic motor simulation. Psychoneuroendocrinology 50, 220–226. 10.1016/j.psyneuen.2014.08.021 [DOI] [PubMed] [Google Scholar]
- Frewen P, Thornley E, Rabellino D, Lanius R, 2017. Neuroimaging the traumatized self: fMRI reveals altered response in cortical midline structures and occipital cortex during visual and verbal self-and other-referential processing in women with PTSD. Eur. J. Psychotraumatology 8, 1314164 10.1080/20008198.2017.1314164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer L, Witek MAG, Hansen NC, Thomas J, Konvalinka I, Vuust P, 2016. Oxytocin improves synchronisation in leader-follower interaction. Sci. Rep 6, 38416 10.1038/srep38416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J, Yajima M, 2012. Why We (Usually) Don’t Have to Worry About Multiple Comparisons. J. Res. Educ. Eff 5, 189–211. 10.1080/19345747.2011.618213 [DOI] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C, 2011. Automatic imitation. Psychol. Bull 137, 463–483. 10.1037/a0022288 [DOI] [PubMed] [Google Scholar]
- Hudson AR, De Coster L, Spoormans H, Verbeke S, Van der Jeught K, Brass M, Mueller SC, 2020. Childhood Abuse and Adult Sociocognitive Skills: Distinguishing Between Self and Other Following Early Trauma. J. Interpers Violence 088626052090619. 10.1177/0886260520906190 [DOI] [PubMed] [Google Scholar]
- Josef L, Goldstein P, Mayseless N, Ayalon L, Shamay-Tsoory SG, 2019. The oxytocinergic system mediates synchronized interpersonal movement during dance. Sci. Rep 9, 1894 10.1038/s41598-018-37141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-Y, 2013. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod 38, 52 10.5395/rde.2013.38.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D, 2017. Equivalence Tests: A Practical Primer for t Tests, Correlations, and Meta-Analyses. Soc. Psychol. Personal. Sci 8, 355–362. 10.1177/1948550617697177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Bird G, Orsini C, Heyes C, 2010. Social attitudes modulate automatic imitation. J. Exp. Soc. Psychol 46, 905–910. 10.1016/j.jesp.2010.07.001 [DOI] [Google Scholar]
- Martins DA, Mazibuko N, Zelaya F, Vasilakopoulou S, Loveridge J, Oates A, Maltezos S, Mehta M, Wastling S, Howard M, McAlonan G, Murphy D, Williams SCR, Fotopoulou A, Schuschnig U, Paloyelis Y, 2020. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat. Commun 11, 1160 10.1038/s41467-020-14845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek BA, Ebersole CR, DeHaven AC, Mellor DT, 2018. The pre-registration revolution. Proc. Natl. Acad. Sci 115, 2600–2606. 10.1073/pnas.1708274114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obhi SS, Hogeveen J, Giacomin M, Jordan CH, 2014. Automatic imitation is reduced in narcissists. J. Exp. Psychol. Hum. Percept. Perform 40, 920–928. 10.1037/a0034056 [DOI] [PubMed] [Google Scholar]
- Ouimette P, Goodwin E, Brown PJ, 2006. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addict. Behav 31, 1415–1423. 10.1016/j.addbeh.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Ramseyer F, Ebert A, Roser P, Edel M, Tschacher W, Briine M, 2019. Exploring nonverbal synchrony in borderline personality disorder: A double-blind placebo-controlled study using oxytocin. Br. J. Clin. Psychol. bjc 12240 10.1111/bjc.12240 [DOI] [PubMed] [Google Scholar]
- Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, Hurlemann R, 2017. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol. Psychiatry 82, 885–894. https://doi.Org/10.1016/j.biopsych.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Spengler S, Brass M, Kiihn S, Schiitz-Bosbach S, 2010. Minimizing motor mimicry by myself: self-focus enhances online action-control mechanisms during motor contagion. Conscious. Cogn 19, 98–106. https://doi.Org/10.1016/j.concog.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Meinzer NK, Morrison T, Wen J, Radanovich L, Leung D, Niles A, O’Donovan A, Batki SL, Woolley JD, 2019. Effects of Oxytocin Administration on Cue-Induced Craving in Co-occurring Alcohol Use Disorder and PTSD: A Within-Participant Randomized Clinical Trial. Alcohol. Clin. Exp. Res 43, 2627–2636. 10.1111/acer.14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stel M, Vonk R, 2010. Mimicry in social interaction: Benefits for mimickers, mimickees, and their interaction. Br. J. Psychol 101, 311–323. 10.1348/000712609X465424 [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wiillner U, Maier W, Hurlemann R, 2013. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep 3 10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hamilton A.F.de C., 2013. Understanding the role of the “self” in the social priming of mimicry. PloS One 8, e60249 10.1371/journal.pone.0060249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Yao S, Li Q, Geng Y, Ma X, Luo L, Xu L, Kendrick KM, 2016. Oxytocin blurs the self-other distinction during trait judgments and reduces medial prefrontal cortex responses. Hum. Brain Mapp 37, 2512–2527. 10.1002/hbm.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


