Summary
Damage-associated molecular patterns are signalling molecules involved in inflammatory responses and restoration of homeostasis. Chronic release of these molecules can also promote inflammation in the context of liver disease. Herein, we provide a comprehensive summary of the role of damage-associated molecular patterns as danger signals in liver injury. We consider the role of reactive oxygen species and reactive nitrogen species as inducers of damage-associated molecular patterns, as well as how specific damage-associated molecular patterns participate in the pathogenesis of chronic liver diseases such as alcohol-related liver disease, non-alcoholic steatohepatitis, liver fibrosis and liver cancer. In addition, we discuss the role of damage-associated molecular patterns in ischaemia reperfusion injury and liver transplantation and highlight current studies in which blockade of specific damage-associated molecular patterns has proven beneficial in humans and mice.
Keywords: Alcohol-related liver disease, Hepatocellular carcinoma, Liver fibrosis, Liver transplantation, Non-alcoholic steatohepatitis, Oxidative stress
Introduction
Detection of threats such as pathogens and cellular damage is critical to organismal survival. One mechanism of detection is the secretion of endogenous molecules to the extracellular environment, which cell-surface receptors recognise as a danger signals or “alarmins”, requiring initiation and persistence of innate immune responses. These relocated host cell-derived activators, called damage-associated molecular patterns (DAMPs), are a key aspect of inflammation.1
Dying cells passively release DAMPs following injury, trauma, ischaemia or infection-induced necrosis. In the liver, passive release occurs mostly in lipid-laden, damaged, apoptotic, necroptotic or necrotic hepatocytes.2–4 DAMPs are also actively released via secretory lysosomes in immune cells5–7 or in stressed parenchymal and non-parenchymal cells.8 These molecules are sensed via pattern recognition receptors (PRRs) and the NOD-like receptor protein 3 (NLRP3) or inflammasome, all of which trigger release of chemokines and other mediators to provoke initial proinflammatory responses that fight infection and cellular damage.2,9–17 While this response can be beneficial (i.e., resolving danger), sustained release of DAMPs has adverse effects in chronic liver disease.
Indeed, further injury can result when DAMPs are activated by reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are also released in response to injury and inflammation.18–23 The second wave of cell injury and death enhances release of second-line DAMPs, triggering a more complex and pronounced reaction. In the liver, inflammatory responses such as activation of Kupffer cells (KCs) and extravasation and activation of monocyte-derived macrophages (MFs) and neutrophils24 prompt release of tumour necrosis factor-α (TNFα) and other proinflammatory cytokines that activate the NF-κB pathway in hepatocytes to exacerbate damage.25 Thus, while physiological levels of ROS, RNS and DAMPs contribute to liver homeostasis, their uncontrolled production and release activate signalling cascades that, if left unchecked, exacerbate liver damage.
The rapid increase in circulating levels of DAMPs reflects the severity of liver injury; therefore, these molecules could be promising biomarkers and/or potential therapeutic targets to prevent liver damage. However, the number of clinical trials targeting DAMPs, some of which are disease-specific, is still very limited; hence, a careful review of the main DAMPs that contribute to chronic liver disease is warranted.
ROS and RNS induce DAMPs and events involved in chronic liver disease
ROS and RNS are typically generated by healthy cells during biological and metabolic processes.26 The liver generates and is exposed to free radicals via mitochondrial metabolism27 and activation of membrane-bound NADPH oxidase (NOX),28–30 cytoplasmic inducible nitric oxide synthase (iNOS)31,32 and microsomal cytochrome P450.33,34 Maintaining a balance between free radical production and antioxidant defence is crucial in the regulation of cellular homeostasis.26,35 Likewise, physiological levels of free radicals are indispensable to preserve the immune response against pathogens and to regulate cell proliferation in response to growth factors.26,35
Yet, the production of free radicals can also promote inflammatory disease. In the liver, excessive oxidative and nitrosative stress not only contributes to increased production of DAMPs, but also correlates with pathogenesis of chronic liver diseases such as alcohol-related liver disease (ALD), non-alcoholic steatohepatitis (NASH), fibrosis and hepatocellular carcinoma (HCC)36–49 (Table 1 and Fig. 1). Thus, an initial injury response can promote subsequent chronic inflammatory processes and further cell and tissue damage.
Table 1.
ROS and RNS induce events involved in chronic liver disease.
| Effect(s) | Reference(s) | |
|---|---|---|
| ALD | ||
| ROS | Mitochondrial dysfunction; Proinflammatory; Profibrogenic | 51,76,77 |
| RNS | ONOO− induced liver injury | 22,60,61 |
| NASH | ||
| ROS | Lipid peroxidation; Proinflammatory | 43,44,69,71 |
| RNS | De novo lipogenesis; Proinflammatory | 45 |
| Fibrosis | ||
| ROS | TGFβ signalling; HSC activation | 46,78 |
| RNS | iNOS induces MMP9; DNA damage; Profibrogenic | 62,63 |
| HCC | ||
| ROS | Oxidative DNA damage; DNA adducts; Proinflammatory; Oncogenic; Increase telomerase activity, telomere length and HCC tumour growth; Protein oxidation | 47–49 |
| RNS | iNOS promotes HCC stem cell phenotype | 32 |
ALD, alcohol-related liver disease; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Fig. 1. ROS and RNS induce DAMPs and events involved in chronic liver disease.
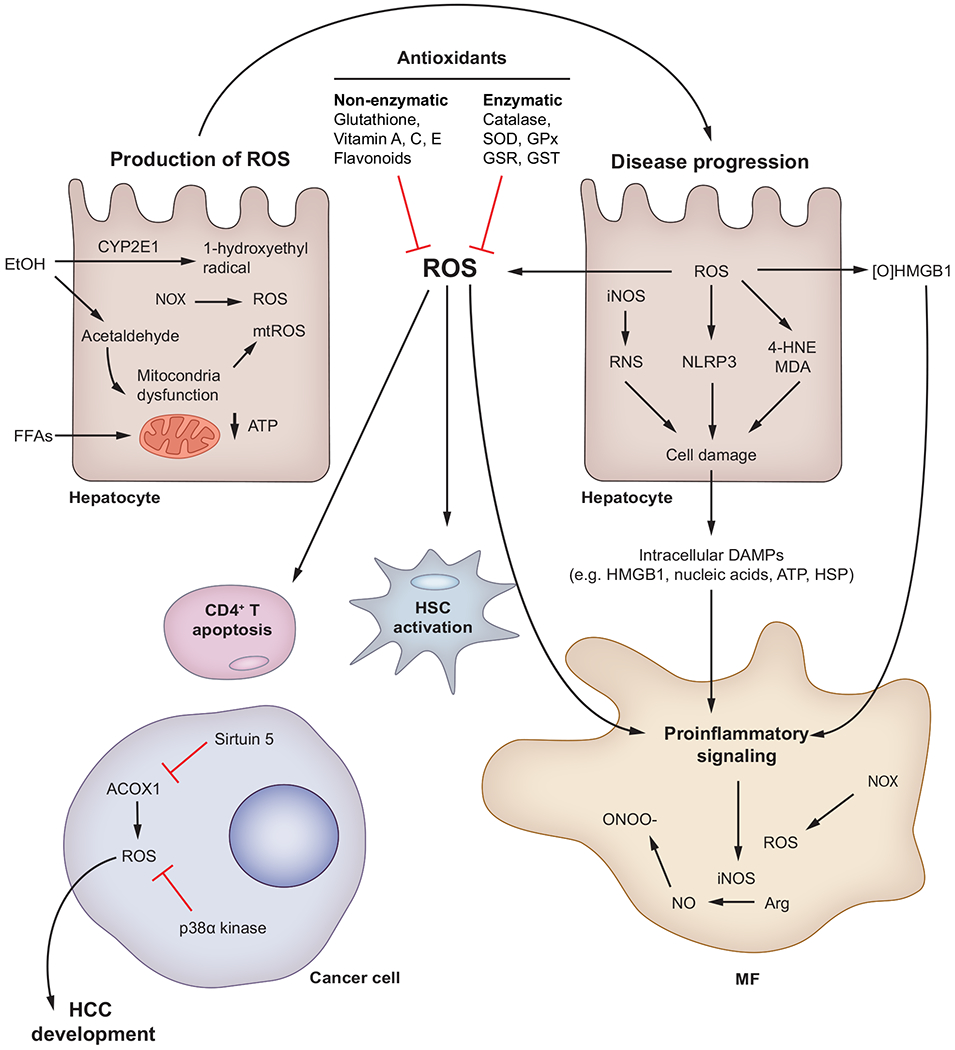
ROS are produced mostly in hepatocytes and MFs by CYP2E1, mitochondrial injury and NOX. ROS participate in progression of chronic liver disease, causing hepatocyte damage, inflammation, HSC activation and CD4+ T cell apoptosis. Peroxisomal ROS and kinases contribute to HCC development and resolution, respectively. RNS are generated in hepatocytes and MFs due to activation of iNOS. Excess NO reacts with ROS to generate damaging RNS such as ONOO−. Enzymatic and non-enzymatic antioxidant defence systems balance the generation of ROS and play an important role in resolution of liver disease. 4-HNE, 4-hydroxynonenal; ACOX1, acetyl-CoA oxidase; CYP2E1, cytochrome P450 2E1; DAMP(s), damage-associated molecular pattern(s); EtOH, ethanol; FFAs, free fatty acids; GPx, glutathione peroxidase; GSR, glutathione-disulfide reductase; GST, glutathione S-transferase; HCC, hepatocellular carcinoma; HSC(s), hepatic stellate cell(s); iNOS, inducible nitric oxide synthase; MDA, malondialdehyde; MF(s), macrophages; mtROS, mitochondrial ROS; NLRP3, NOD-like receptor protein-3; NO, nitric oxide; NOX, NADPH oxidase; [O]HMGB1, disulfide High-mobility group box-1; ONOO−, peroxynitrite; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase.
Mitochondrial dysfunction is a key factor in the pathogenesis of fatty liver diseases.43,50,51 Acetaldehyde, the end-product of alcohol metabolism, causes structural and functional alterations in mitochondria that lower production of ATP and increase generation of ROS.52 Further, diets enriched in fructose and fat, together with insulin resistance, enhance flux of free fatty acids (FFAs) to the mitochondria for β-oxidation. This flux increases mitochondrial membrane permeability, proton leakage and ROS production, lowering ATP levels.53 In NASH, CD4+ T cells have increased mitochondrial mass, facilitating production of mitochondrial ROS (mtROS), although treatment with antioxidants increases CD4+ T cells, delaying the progression of NAFLD and HCC.54
ROS increase production of DAMPs, such as osteopontin (OPN)55 and high-mobility group box 1 (HMGB1), and induce oxidative modifications that enhance immunostimulatory properties.56,57 In KCs and MFs, membrane-bound NOX is the major source of ROS and NOX-deficient (p47phox−/−) mice are protected from ALD.58,59 Alcohol stimulates cytoplasmic iNOS, the major source of RNS, and increases production of peroxynitrite (ONOO−) and, thus, oxidative and nitrosative stress.60,61 Indeed, iNos−/− mice are protected from ALD,59 while lack of iNOS decreases carbon tetrachloride (CCl4)-induced fibrosis.62,63
Alcohol is oxidised in hepatocytes by the microsomal cytochrome P450 2E1 (CYP2E1) and generates 1-hydroxyethyl radical, a major driver of alcohol-induced liver injury.64,65 Mice lacking Cyp2e1 display less alcohol-induced liver injury.64,66 Moreover, ROS and RNS bind to proteins and generate neo-antigens that elicit immune responses.52,67 In NASH and ALD, lipid peroxidation end-products such as 4-hydroxynonenal and malondialdehyde bind DNA and proteins68,69 to form carcinogenic exocyclic etheno-DNA adducts70,71 and protein-adducts,67,72,73 both of which enhance injury. ROS also regulate proangiogenic and profibrogenic responses in hepatic stellate cells (HSCs).20,74–78
Importantly, peroxisomal ROS and kinases are implicated in HCC. The deacetylase sirtuin 5 suppresses activity of peroxisomal acetyl-CoA oxidase-1 (ACOX1), lowers generation of H2O2 and reduces oxidative DNA damage in in vivo models of HCC.79 In addition, liver-specific ablation of the stress-activated protein kinase p38α enhances ROS, whereas its re-introduction prevents fibrosis and HCC by limiting ROS.80
ROS and RNS do not bind receptors; instead, most cells react to them by transmitting signals to organelles including the nucleus.81–85 In MFs, the adaptor Kelch ECH associating protein 1 (KEAP1) senses ROS and transduces signals to nuclear factor erythroid 2-related factor-2 (NRF2) to regulate production of cytokines.81,84 In myeloid and lymphoid cells, I-kappa-B kinase (IKK) senses ROS and transduces signals to activate NF-κB and regulate the inflammatory response.85,86 The NLRP3 inflammasome, a key player in chronic liver disease, is also stimulated by ROS.82
Pre-clinical and clinical trials have investigated the efficacy of antioxidants in acute and chronic liver disease, as the antioxidant defence is usually depleted.87,88 Vitamin E alone or in combination with the lipid-lowering agent atorvastatin alleviates progression of steatosis to NASH in animal models.89–91 However, in a randomised clinical trial of patients with alcoholic hepatitis (AH), vitamin E alone or in combination with corticosteroids failed to confer a benefit.92,93 Nonetheless, a clinical trial (NCT01792115) is currently evaluating the most effective dose of vitamin E for the treatment of NAFLD. N-acetylcysteine, a precursor of glutathione, is the only FDA-approved antioxidant for treatment of acetaminophen-induced hepatotoxicity.94 Another option to reduce oxidative stress is dietary restriction, as high-calorie intake is associated with increased mtROS and reduced activity of antioxidant enzymes.95
DAMPs in alcohol-related liver disease
In ALD, the type of cell death determines the release of DAMPs.96 Apoptosis is the most common and is associated with the release of DAMPs from hepatocytes.97 Necrosis is typically observed in severe acute AH,98 where hepatocytes undergo swelling, autolysis and death without significant signal transduction.99 Necroptosis, which resembles necrosis, is the regulated version of necrotic cell death through the RIPK1-RIPK3 heterodimer scaffold complex that leads to the release of intracellular contents.100 In both necrosis and necroptosis, multiple DAMPs are secreted into the extracellular space and initiate an inflammatory response100,101 (Table 2 and Fig. 2).
Table 2.
DAMPs are involved in chronic liver injury and restoration of homeostasis.
| DAMP | Receptor(s) | Effect(s) | Reference(s) |
|---|---|---|---|
| ALD | |||
| mtDNA | TLR9 | Proinflammatory; Profibrogenic | 105 |
| Uric acid | NLR | Proinflammatory | 15 |
| ATP | P2RX7 | Proinflammatory | 110 |
| HSP90 | Oxidative stress; Prosteatotic; Proinflammatory | 115 | |
| HMGB1 | RAGE/TLR4 | Prosteatotic | 118 |
| Hyaluronic acid | TLRs | Profibrogenic | 122 |
| LCN2 | LCN2R | Proinflammatory | 125 |
| PGE2 | PGE2 receptor | Immunosuppression; Prosteatotic | 128 |
| NASH | |||
| mtDNA | TLR9 | Prosteatotic; Profibrogenic | 2,138 |
| ssRNA | TLR7 | Monocyte-derived macrophage activation; IFNγ and TNFα production; T cell recruitment | 142,143 |
| AGE | RAGE | Increase during NASH progression | 157 |
| mtROS | NLRP3 | Prosteatotic; Profibrogenic | 144,145 |
| Biglycan | TLR2/4 | Increase during NASH progression | 146 |
| Galectin-3 | TLR2/4 | Promote fibrosis and hepatocyte ballooning | 150,151 |
| Fibrinogen | TLR4 | Form deposits to promote fatty liver disease | 149 |
| Cholesterol crystals | NLRP3 | Prosteatotic | 153,154 |
| Fibrosis | |||
| HMGB1 | RAGE | Hepatic stellate cell activation; Collagen type I production; Endoplasmic reticulum stress | 55,118,166 |
| OPN | Integrin αVβ3 | Increases HMGB1; HSC activation via PI3K/pAkt/NF-κB; Ductular reaction | 55,168 |
| HSP90 | TLR2/TLR4 | HSC activation | 169,170 |
| HSP47 | TLRs | Profibrogenic | 172,173 |
| IL-33 | IL-33R | Profibrogenic via NF-κB and MAPKs; Proinflammatory (Th2 cytokines) | 176–179 |
| ATP adenosine | P2rX7, A2AR, A2BR | MF release of IL1β and HMGB1; Profibrogenic | 181,182 |
| HCC | |||
| HMGB1 | RAGE, TLR9 | Tumour initiation and progression | 190,209,210 |
| OPN | Integrins, CD44 | Tumour growth, metastasis and immune escape | 200,219 |
| S100A1 | RAGE | Increases in HCC and correlates with poor survival | 220 |
| S100A4 | RAGE | Tumour growth and metastasis | 221,222 |
| S100A8 | RAGE | Tumour growth and metastasis | 223,224 |
| S100A9 | RAGE | Tumour initiation and progression | 204,223 |
| mtDNA | TLR9 | HMGB1 binding to TLR9 | 210 |
| Extracellular ATP | P2 | HCC cell migration | 226 |
| Calreticulin | N/A | Tumour growth and invasion | 227 |
| Histones | TLR4 | HCC metastasis | 229 |
| IRI< | |||
| ATP | P1, P2 | IRI; Graft rejection | 259,269 |
| DNA | TLRs | Accumulates following machine perfusion | 16 |
| Histones | TLR9, NLRP3 | IRI | 260,261 |
| HMGB1 | TLR4, RAGE | Circulating HMGB1 exacerbates hepatic IRI; Intracellular HMGB1 protects from IRI | |
| HSP 70 HSP 27 |
TLRs, LOX-1 | Protects from hepatic IRI; Inhibits graft rejection | 262,263,270 |
| IL-33 | ST2 | IRI; NETs | 255,257 |
| PGE2 | PG receptor | Induced in recipients with good graft function | 264 |
ALD, alcohol-related liver disease; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; IRI, ischaemia reperfusion injury; LT, liver transplantation; AGE, advanced glycation end-products.
Fig. 2. DAMPs promote inflammation, steatosis and hepatocyte injury in ALD.
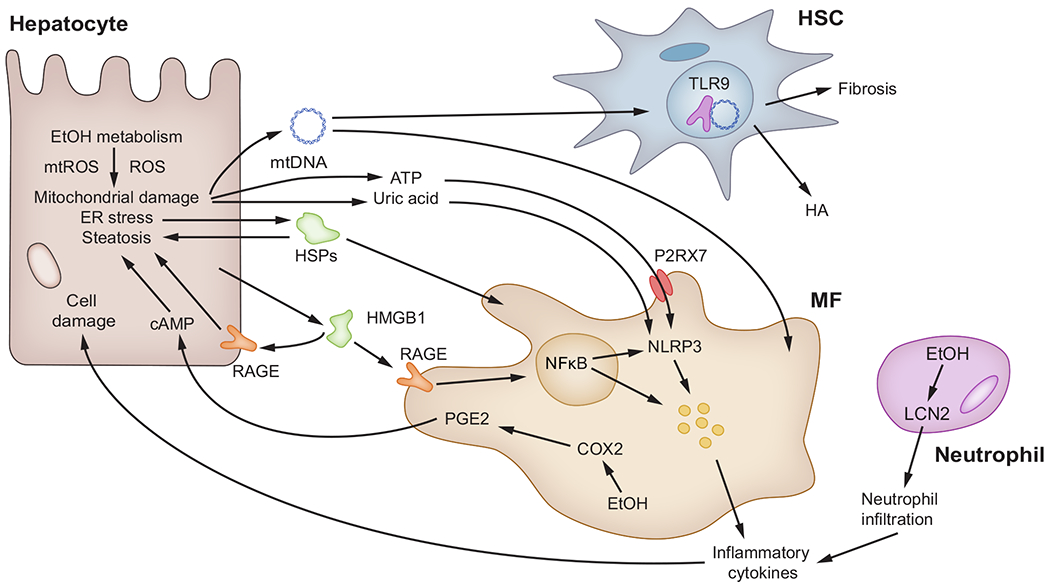
Ethanol-induced hepatocyte injury causes release of DAMPs, including mitochondrial DAMPs (mtDNA and ATP), uric acid, HSPs and HMGB1 from damaged hepatocytes. Most of these DAMPs are recognised by MFs through RAGE, TLRs and P2RX7 and activate NF-κB and the NLRP3 inflammasome. These result in release of proinflammatory cytokines that trigger cellular injury and steatosis. HSCs release HA and are responsive to mtDNA which activates them. MFs produce PGE2 that causes steatosis via cAMP activation. Neutrophils produce LCN2 and respond to it by infiltrating the liver to exacerbate cellular injury by releasing proinflammatory cytokines. cAMP, cyclic adenosine monophosphate; COX2, cyclooxygenase-2; DAMP(s), damage-associated molecular pattern(s); EtOH, ethanol; HA, hyaluronic acid; HMGB1, high-mobility group box-1; HSC(s), hepatic stellate cell(s); HSPs, heat shock proteins; LCN2, lipocalin-2; MF(s), macrophage(s); mtDNA, mitochondrial DNA; mtROS, mitochondrial ROS; NFκB, nuclear factor kappa B; NLRP3, NOD-like receptor protein-3; P2RX7, purinergic receptor P2X7; PGE2, prostaglandin E2; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; TLR9, toll-like receptor 9.
Mitochondrial DAMPs
Mitochondrial DNA (mtDNA) and ATP maintain the mitochondrial structure and aid in energy metabolism.102,103 Chronic alcohol abuse increases mtROS and causes mtDNA oxidation.104,105 Moreover, alcohol depolarises mitochondria, disrupts mitophagy and leads to the release of mitochondrial DAMPs (mtDAMPs) into the cytosol, before they are eventually secreted from hepatocytes into the extracellular space.105 Once released, mtDAMPs promote proinflammatory and profibrotic events that lead to ALD progression.105
Metabolic DAMPs
Alcohol-induced hepatocyte damage leads to the release of metabolic DAMPs, such as uric acid (following the degradation of nucleic acids) and ATP.106 Uric acid acts as an antioxidant by scavenging ROS and ONOO− in the plasma.107–109 Uric acid and ATP levels are elevated in serum and liver tissue from alcoholic patients and alcohol-fed mice15; both uric acid and ATP mediate cross-talk between hepatocytes and immune cells, enhancing inflammation.15 Further, pharmacological depletion of uric acid and blockade of ATP protect against ALD,110 suggesting they are candidate targets to prevent disease progression.
Stress-induced DAMPs
Cellular stress increases expression of heat shock proteins (HSPs), which act as chaperones for refolding, disaggregation and degradation of polypeptides.111 When the chaperone activity of HSP90 is abnormal, it promotes alcohol-induced injury by enhancing hepatic lipid accumulation, MF-mediated inflammation and cellular stress.112–114 Pharmacological inhibition of HSP90 promotes reversal of alcohol-induced liver injury.115
HMGB1 is an architectural protein that plays a physiological role. It binds chromatin to facilitate bending and participates in nucleosome formation, DNA replication and DNA repair.116,117 HMGB1 also acts as a DAMP, serving as a ligand for the receptor for advanced glycation end-products (RAGE) and for toll-like receptor 4 (TLR4).8,118 Liver biopsies from alcoholic patients show a robust increase in HMGB1 expression and translocation, which correlate with disease stage. Similar findings are observed in chronic ethanol-fed mice.8 Further, ablation of Hmgb1 in hepatocytes protects mice from alcohol-induced liver injury by elevating LDL and VLDL export and increasing the levels of carnitine palmitoyltransferase-1, phosphorylated 5′ AMP-activated protein kinase-α and phosphorylated peroxisome proliferator-activated receptor-α.8
Non-parenchymal cells also release DAMPs in ALD. For instance, hyaluronic acid (HA) produced by HSCs and hepatocytes is abundant in the extracellular matrix (ECM) of alcoholic patients.119,120 Individuals with ALD have increased serum HA levels, which correlate with progression of ALD and fibrosis.121,122 In addition, lipocalin-2 (LCN2), an acute-phase protein increased in patients with AH,123 acts as an alarmin by recruiting neutrophils to the liver.124–126
Prostaglandin E2 (PGE2) is a potent vasodilator. In patients with advanced AH, upregulation of cyclooxygenase-2 (COX2) in MFs and KCs elevates plasma levels of PGE2, which causes immunosuppression and thus increased susceptibility to infection.127–129 Moreover, KC-derived PGE2 increases cAMP in hepatocytes and triglyceride accumulation in livers from alcoholic patients.130
DAMPs in non-alcoholic steatohepatitis
NASH is characterised by increased steatosis, lobular inflammation and the presence of chicken-wire fibrosis.131,132 During NASH progression, excessive lipid accumulation, ROS generation and endoplasmic reticulum (ER) stress damage hepatocytes. This damage triggers regulated cell death primarily through apoptosis and pyroptosis, which involves the formation of plasma membrane pores by the gasdermin family of proteins, largely induced by activation of proinflammatory caspases.36,99,133,134 Regulated cell death results in secondary necrosis and release of intracellular materials into the extracellular space, where they act as DAMPs recognised by PRRs.135 TLRs and NLRs sense multiple DAMPs (Table 2 and Fig. 3) that mediate inflammation and fibrosis during NASH progression.136–140
Fig. 3. Intrahepatic and extrahepatic DAMPs contribute to NASH.
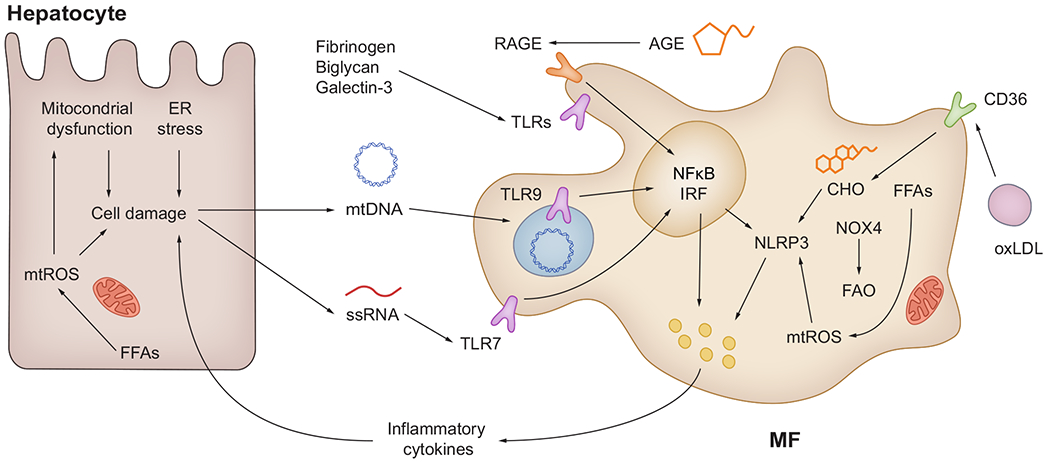
Damaged hepatocytes are the major source of intrahepatic DAMPs (mtDNA and ssRNA). ECM components such as biglycan, fibrinogen and galectin-3 can also act as DAMPs to active TLRs. MFs and dendritic cells recognise DAMPs through RAGE, TLRs and NLRP3 signalling. Extrahepatic DAMPs (AGE, FFAs and oxidised LDLs) are delivered via circulation and can bind RAGE and CD36, contributing to steatohepatitis. AGE, advanced glycation end-products; CHO, cholesterol; DAMP(s), damage-associated molecular pattern(s); ER endoplasmic reticulum; FAO, fatty acid oxidation; FFA(s), free fatty acid(s); IRF, interferon-regulatory factor; MF(s), macrophage(s); mtDNA, mitochondrial DNA; mtROS, mitochondrial ROS; NFkB, nuclear factor kappa B; NLRP3, NOD-like receptor protein-3; NOX4, NADPH oxidase 4; oxLDL, oxidized low-density lipoproteins; ssRNA, single-stranded RNA; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; TLR(s), Toll-like receptor(s).
Intrahepatic DAMPs
Mitochondrial damage and subsequent cell death release immunogenic mtDNA.141 TLR9, a mtDNA receptor, is internalised in intracellular organelles such as endosomes and recognises phagocytosed unmethylated CpG DNA fragments,138,141 which are rare in host genomic DNA but abundant in mtDNA.141 Unmethylated CpG DNA fragments are elevated in serum from obese patients, together with upregulated TLR9 expression.141 Mice with global or myeloid cell-specific ablation of Tlr9 fed either a high-fat (HF) diet or a high-fat, fructose and cholesterol (HFHC) diet show reduced liver steatosis, inflammation and fibrosis.138,141 Likewise, treatment with the TLR9 antagonist IRS954 attenuates NASH, suggesting a possible therapeutic avenue.141 Further, single-stranded RNA (ssRNA) binds TLR7 and triggers an inflammatory response in MFs and dendritic cells.142 Ablation of Tlr7 attenuates progression of NASH in a methionine and choline-deficient diet mouse model by suppressing TNFα and interferon-γ (IFNγ) production and CD4+ T cell recruitment.142,143
mtROS also act as DAMPs and contribute to NASH progression. Hepatocyte-specific ablation of Nox4 attenuates inflammation and fibrosis in the HF and choline-deficient L-amino acid-defined (CDAA) murine models.144 In MFs, NOX4 accelerates β-oxidation of long-chain FFAs causing oxidative stress and polarisation toward a more proinflammatory phenotype.145 NLRP3 is the intracellular PRR that responds to these ROS145; it is upregulated in the livers of patients with NASH and ablation of Nlrp3 prevents NASH progression in mice.140 Likewise, treating mice with GKT137831, a NOX1/4 inhibitor currently being tested in clinical trials, reduces ROS and activation of NLRP3 in palmitate-treated bone marrow-derived MFs and decreases inflammation in the CDAA murine model of NASH.144,145 Notably, MCC950, an NLRP3 inhibitor, improves NAFLD and fibrosis in obese diabetic mice.140
ECM-derived DAMPs
The ECM is dynamic and supports tissue homeostasis.146,147 Active ECM remodelling is observed in both patients with NASH and mouse models of NASH.146,148 The deposition of fibrin and fibrinogen into the ECM occurs in the liver of patients with NASH and mice fed a HF diet. Additionally, mice overexpressing mutated fibrinogen are protected from fatty liver disease.149 Although no functional studies were performed, proteomics analysis revealed a sustained increase in biglycan, a potential ligand for TLRs, in hepatic ECM from mouse models of NASH.146 Further, galectin-3, a secreted lectin regulating matrix-to-cell interactions, promotes progression of NASH by interacting with the interleukin-33 (IL33)/ST2 axis.150 Although a clinical trial (NCT02462967) of belapectin, an inhibitor of galectin-3, did not improve fibrosis in patients with NASH, a significant decrease in hepatocyte ballooning was observed.151
Extrahepatic DAMPs
Cholesterol species act as surfactants to maintain the plasma membrane and excessive cholesterol intake and hypercholesterolemia are risk factors for NASH.152 Cholesterol crystals are delivered by oxidised LDLs through CD36 and activate the NLRP3 inflammasome in MFs.153 Moreover, the cholesterol-lowering drugs ezetimibe and atorvastatin suppress NLRP3 expression and inflammation in an HFHC mouse model of NASH, while targeting CD36 protects mice from NASH.154,155
Advanced glycation end-products (AGEs) are generated via the non-enzymatic Amadori reaction between a reducing sugar (e.g., glucose) and proteins, lipids or nucleic acids.156 Diabetic patients have increased AGEs due to hyperglycaemia.156 In addition, population genetics suggest that a polymorphism in the AGE receptor (RAGE) gene and circulating soluble RAGE (encoded by AGER) are associated with the risk of NASH.157 In addition, a HFHC mouse model shows that dietary supplementation with AGEs aggravates inflammation and ROS production in KCs, exacerbating NASH-induced liver injury.158 However, global knockout of Ager in Ldlr−/− mice minimally affects progression of NASH under short- or long-term HFHC diet feeding.159 The role of RAGE in NASH remains inconclusive as these studies used mice of different sex.158,159
DAMPs in liver fibrosis
Chronic liver injury leads to pathological scarring and fibrosis.160,161 DAMPs such as HMGB1, OPN, HSPs, IL33 and ATP activate HSCs, the main source of fibrillar collagen, the main ECM component in fibrosis162,163 (Table 2 and Fig. 4).
Fig. 4. DAMPs activate HSCs and contribute to fibrosis.
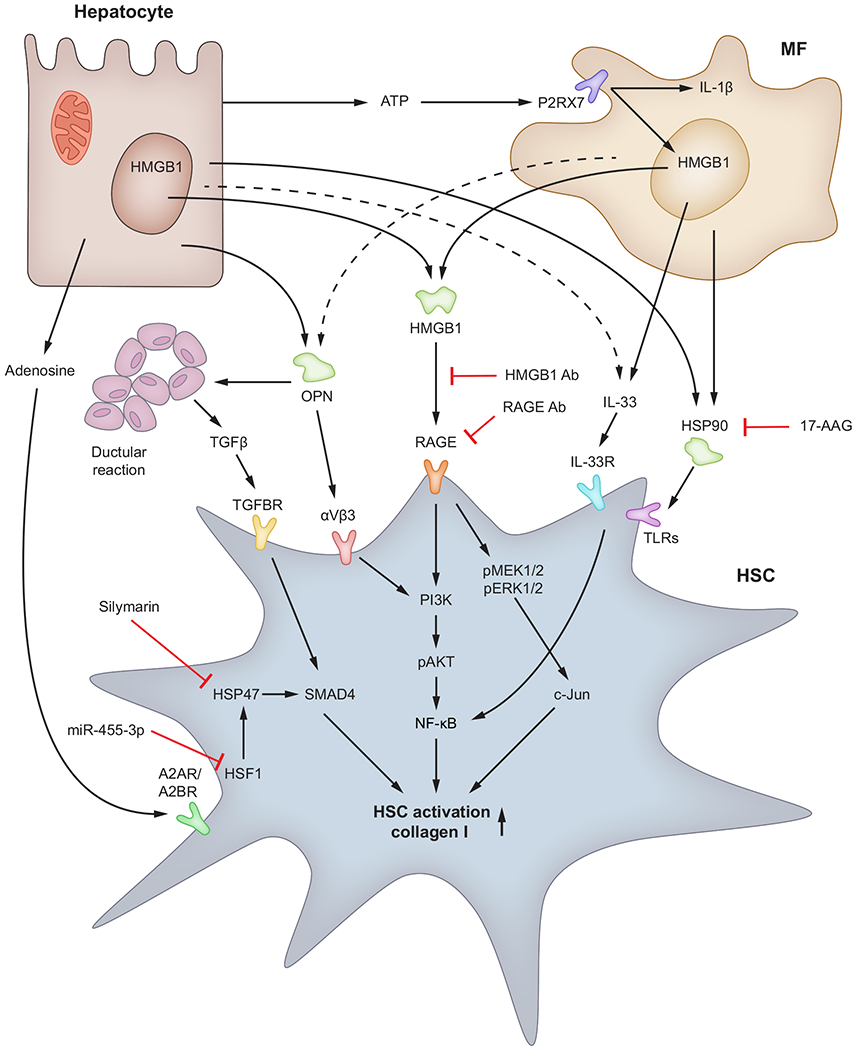
In addition to being a significant source of ROS, hepatocytes produce adenosine, OPN and HMGB1, which target HSCs through A2AR/A2BR, αvβ3 integrin and RAGE, respectively and activate HSCs to promote scar deposition. MFs are also a significant source of ROS due to NOX activation and they produce HMGB1, OPN, IL33 and HSPs, which signal through RAGE, αvβ3 integrin, IL33R and TLRs, respectively, in HSCs to magnify the fibrogenic response. The contribution of biliary epithelial cells to HSC activation is significant as they produce TGFβ, which enhances collagen type I synthesis. HMGB1, OPN, IL-33, HSPs, ATP and adenosine, through interaction with their receptors on HSCs, signal via MEK1/2/c-Jun, PI3k/pAKT/NF-κB and TGFBR/Smad4 pathways to enhance collagen type I. Ab, antibody; DAMP(s), damage-associated molecular pattern(s); HMGB1, high-mobility group box-1; HSC(s), hepatic stellate cell(s); HSF, heat shock factor; HSPs, heat shock proteins; IL1, interleukin-1; IL33R, IL33 receptor; MF(s), macrophage(s); NOX, NADPH oxidase; OPN, osteopontin; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; TGFβ, transforming growth factor β; TGFBR, transforming growth factor beta receptor; TLR(s), Toll-like receptor(s).
Hepatic expression and serum levels of HMGB1 correlate with fibrosis stage in patients with chronic HCV or HBV infection, primary biliary cirrhosis and AH, as well as in mouse models of fibrosis based on administration of CCl4 or thioacetamide and in the bile duct ligation model.118,164,165 HMGB1 activates HSCs166 and induces ER stress (unpublished observations). Our laboratory demonstrated that ablation of Hmgb1 in hepatocytes and myeloid cells as well as neutralisation of HMGB1 and RAGE protects mice from fibrosis.118 In addition, HMGB1 signals through RAGE in HSCs to upregulate collagen type I expression via the pMEK1/2/pERK1/2/pc-Jun signalling pathway. We showed that pMEK1/2 is upstream of pAkt and enhances collagen type I as well.55 In addition, nilotinib, a tyrosine kinase inhibitor, ameliorates CCl4-induced fibrosis in rats by attenuating Hmgb1/Rage expression and oxidative stress.167
OPN, a matrix-bound protein sensitive to oxidant stress and highly induced upon liver damage emerges as a key DAMP in the pathogenesis of fibrosis by increasing HMGB1 and collagen type I expression in HSCs through RAGE.55 OPN itself upregulates collagen type I through integrin αvβ3 engagement and PI3K/pAkt/NFκB signalling. Moreover, OPN drives ductular reaction and contributes to periportal scarring and fibrosis via TGFβ signalling.168
HSP90 is involved in the activation and survival of HSCs.169,170 The HSP90 inhibitor 17-AAG induces apoptosis and reduces activation of HSCs in a thioacetamide model of fibrosis.171 HSP47, a collagen-specific chaperone, plays a key role in the deposition of collagen around fibrotic areas and is thus involved in fibrosis.172,173 Moreover, inhibitors of HSP47 such as lactoferrin and silymarin prevent HSC activation.174 Overexpression of heat shock factor 1 (HSF1) in HSCs activates them and increases cell proliferation by inducing HSP47 and upregulating the TGFβ/SMAD4 signalling pathway. Notably, miR-455-3p alleviates HSC activation and fibrosis by suppressing its target gene, Hsf1.175
IL33 is constitutively present in the nucleus and binds DNA.176 Hepatic IL33 expression is increased in mice with portal fibrosis and in liver biopsies from fibrotic patients.177,178 In chronic liver injury, IL33 binds the IL33 receptor (IL33R) and activates NF-κB and MAPKs to enhance profibrogenic responses.179 IL33 binding to its receptor also produces proinflammatory and T helper 2 (Th2) cytokines. Recombinant IL33 increases hepatic inflammation and activates HSCs – an effect abrogated by ablation of Il33r or pharmacological inhibition of MAPK signalling.177,180
To fuel various processes, cells transport ATP into the extracellular space via pannexin-1, converting ATP to AMP and adenosine. Extracellular ATP activates MFs through the P2X7 receptor; activated MFs release IL1β and HMGB1 that trigger inflammation and fibrogenesis.181 Extracellular adenosine interacts with the A2A (A2AR) or A2B (A2BR) G-coupled protein receptors to directly stimulate fibroblast production of ECM and increase fibrosis.182 Deletion of Cd73 or Cd9, involved in adenosine production and blockade of A2A or A2B prevents fibrosis in mice.183 In addition, mice lacking adenosine deaminase have a marked increase in extracellular adenosine and develop fibrosis, which is prevented by blockade of A2A and A2B.184
DAMPs in liver cancer
Liver cancer represents the common end-stage of chronic liver disease. About 90% of HCCs arise from cirrhosis185 and mouse models of liver cancer show greater tumour incidence when exposed to chemically induced fibrosis.186,187 DAMPs participate in both initiation and progression of liver cancer (Table 2 and Fig. 5).
Fig. 5. Role of DAMPs in initiation and progression of HCC.
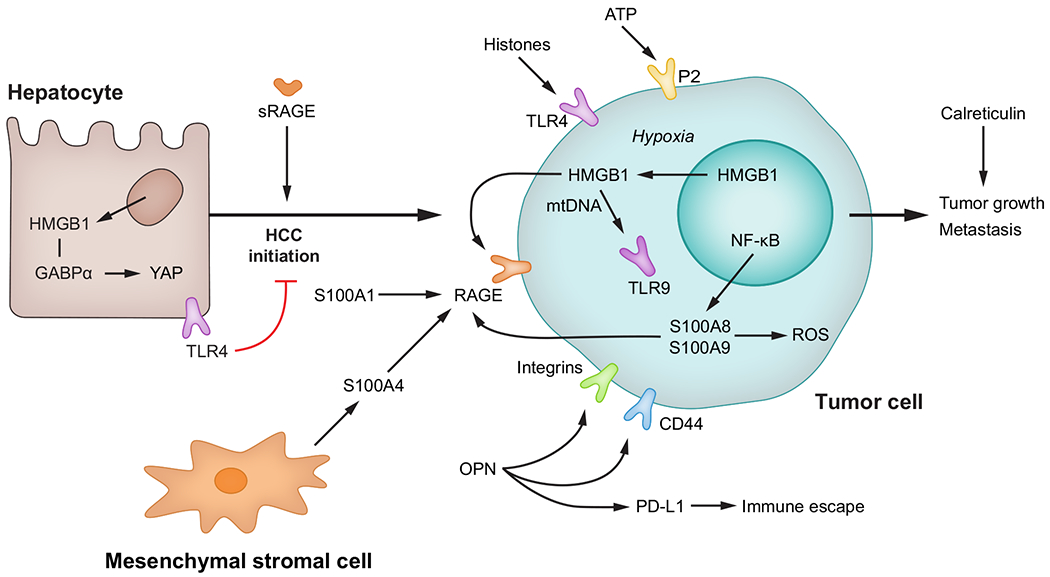
HMGB1 participates in initiation of HCC by GABPα-mediated activation of YAP signalling, while TLR4 represses it. In tumour cells, intracellular HMGB1, through mtDNA and TLR9 signalling as well as S100A8 and S100A9 via ROS production, contributes to tumour growth and metastasis. Extracellular DAMPs such as HMGB1, OPN, S100A1, S100A4, ATP, histones and calreticulin contribute to tumour progression. DAMP(s), damage-associated molecular pattern(s); GABPα, GA binding protein transcription factor subunit-α; HCC, hepatocellular carcinoma; HMGB1, high-mobility group box-1; mtDNA, mitochondrial DNA; OPN, osteopontin; RAGE, receptor for advanced-glycation end-products; ROS, reactive oxygen species; sRAGE, soluble RAGE; TLR(s), Toll-like receptor(s); YAP, Yes-associated protein.
Initiation of HCC
HMGB1 is increased in the liver188–190 and serum191 in human HCC and is associated with tumour stage and poor outcome (meta-analysis in192). In the diethylnitrosamine (DEN) murine model of HCC, HMGB1 expression correlates with tumourigenesis,193 yet hepatocyte-specific ablation of Hmgb1 only reduces tumour burden when combined with CCl4-induced liver injury194 or in the early stages of tumourigenesis.195,196 This effect is mediated by activation of Yes-associated protein 1 (YAP), a key driver of hepatocellular carcinogenesis, as HMGB1 binds to the transcription factor GABPα and enhances YAP signalling in vivo and in vitro.195
Further, OPN expression is significantly increased in patients with HCC, correlating with tumour stage and survival.197,198 However, the role of OPN in HCC initiation is not fully understood, as global ablation of Opn in the DEN model provided inconsistent results.199–201
Proteins of the S100 family act as intracellular Ca2+ sensors and extracellular DAMPs that bind RAGE202 and are frequently dysregulated in various cancers.203 Ablation of S100a9 decreases tumour burden in the DEN model,204 whereas ablation of S100a4 does not prevent HCC caused by hepatic deletion of Pten.205
To date, the role of RAGE in HCC initiation remains unknown but truncated soluble isoforms of RAGE negatively correlate with HCC risk in human HBV and HCV infection.206 In addition to RAGE, HMGB1 also interacts with TLR4. This receptor has a protective role in HCC initiation, as Tlr4 ablation increases tumour burden in the DEN model.207
Progression of HCC
HMGB1 induces proliferation, migration and invasion in HCC cells.190,208,209 In an orthotropic model, Hmgb1 ablation decreases tumour growth.190,195 Mechanistically, under hypoxic conditions HMGB1 translocates from the nucleus to the cytosol and binds TLR9208–210; in a mtDNA-mediated fashion.210 TLR9 activation helps tumour cells adapt to hypoxia, leading to mitochondrial biogenesis, tumour-associated MF invasion, tumour growth and metastasis.190,196,210–212 Two studies suggest that HMGB1 induces HCC progression by activating RAGE.208,209 In HCC cell lines, RAGE signalling triggers proliferation,213,214 angiogenesis,215 tolerance to hypoxia216 and migration.217
OPN induces tumour proliferation, invasion and metastasis in vivo and in vitro activating integrins and CD44.218,219 Importantly, OPN is associated with PDL1 levels in human and mouse HCC, suggesting a role in immune escape.200 Thus, targeting OPN in human HCC could be a promising approach as a second line of treatment after immunotherapy.
Among S100 proteins, S100A1 is upregulated in human HCC and correlates with poor survival and reduced apoptosis.220 S100A4 secretion by mesenchymal stromal cells induces HCC proliferation, invasion, epithelial-to-mesenchymal transition and metastasis in humans.221,222 Further, S100A8 and S100A9 trigger ROS production and promote cell survival in HCC cells in vitro.223 S100A8 induces cell proliferation, migration, invasion and tumour growth in vivo and the extent of methylation decreases in human HCC and correlates with patient survival.224 S100A9 also induces cell proliferation and invasion through RAGE signalling.225
New emerging DAMPs are also thought to play a role in HCC progression. Extracellular ATP induces HCC cell migration through activation of the purinergic 2 (P2) receptor, whose expression correlates with worse patient outcome.226 Ablation of calreticulin decreases HCC cell growth and invasion.227 Histones, found in the nuclei of eukaryotic cells, are involved in gene regulation but can be released into the circulation under stress conditions and act as DAMPs.228 Histone secretion and subsequent activation of TLR4 induce HCC metastasis in an orthotopic mouse model.229
DAMPs in other liver cancers
Little is known about the role of DAMPs in other liver cancers, although HMGB1 is increased and associated with poor survival in intrahepatic cholangiocarcinoma230 and perihilar cholangiocarcinoma.231
DAMPs in ischaemia reperfusion injury and liver transplantation
Patients who progress to end-stage liver disease may require liver transplantation (LT); multiple steps during LT induce the release of DAMPs, which mediate graft injury. Damage to the liver graft results in early allograft dysfunction,232 rejection233 and even recurrence of HCC.234 Unfortunately, all these events negatively affect recipient outcomes and limit the use of marginal organs that could increase the donor source. Consequently, DAMPs are not only early markers of graft injury but also potential therapeutic targets to prevent graft dysfunction. A list of DAMPs involved in ischaemia reperfusion injury (IRI) and LT is provided in Table 2 and Fig. 6.
Fig. 6. The role of HMGB1 in hepatic IRI and LT.
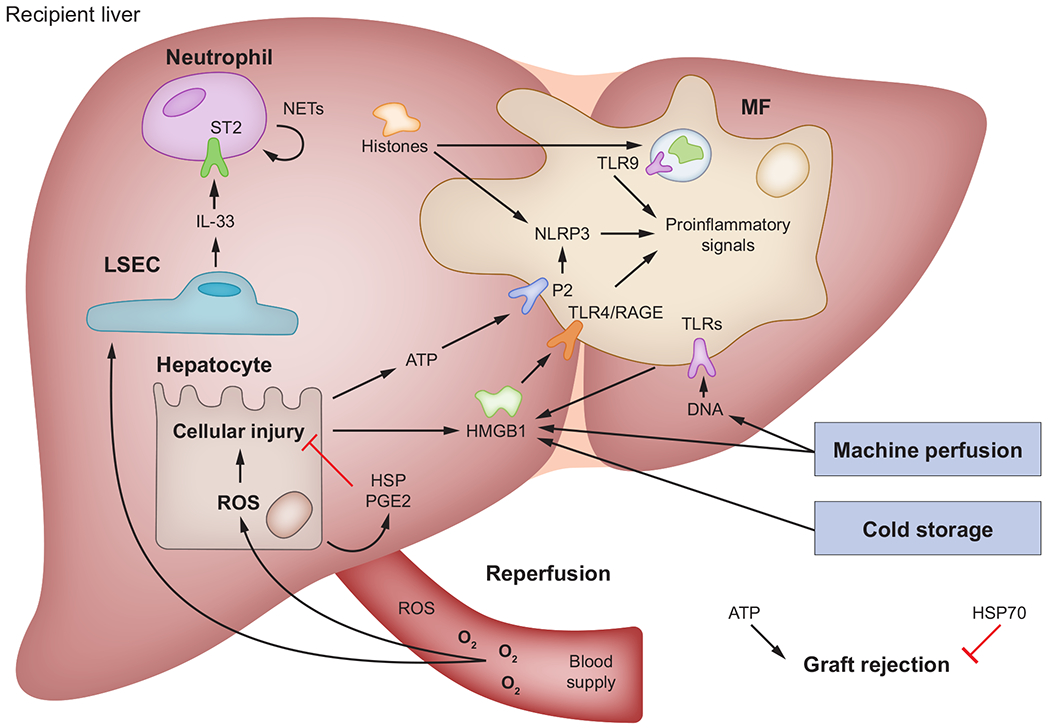
Multiple steps during LT release DAMPs that, in turn, are involved in graft injury and immune rejection. First, methods for preserving liver grafts, such as cold storage and machine perfusion, induce release of DAMPs into the perfusate, which then become flushed into circulation after perfusion. Second, the oxidative burst during graft reperfusion damages hepatocytes and actives Kupffer cells and MFs to release various DAMPs, which mediate graft injury and immune response through selective receptors. Third, in addition to DAMPs that induce a harmful response, HSP and PGE2 protect the liver graft from injury and inhibit immune rejection. DAMP(s), damage-associated molecular pattern(s); HMGB1, high-mobility group box-1; HSP(s), heat shock proteins; IL, interleukin; IRI, ischaemia reperfusion injury; LSEC, liver sinusoidal endothelial cell: LT, liver transplantation; MF(s), macrophages; NETs, neutrophil extracellular traps; NLRP3, NOD-like receptor protein-3; PGE2, prostaglandin E2; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; ST2, suppression of tumorigenicity 2; TLR(s), Toll-like receptor(s).
Donor livers release DAMPs
Although IRI is the most common cause of injury during LT,235 release of DAMPs occurs before organ procurement and the IRI insult.236 The majority of deceased organs in the western world are donations after brain death.237 Experimental studies on the response to brain death show that DAMPs are released and stimulate secretion of proinflammatory cytokines by activating TLRs238; consequently, DAMPs affect distant organs and act as the first insult to the liver graft.239,240
Liver graft preservation releases DAMPs
LT involves cold ischaemia and warm IRI. Damage due to cold ischaemia occurs during organ perfusion and cold storage. This step is designed to protect parenchymal cells by slowing metabolism and stabilising them.241 However, KCs and MFs are more sensitive to cold ischaemia and release DAMPs when activated.242 For instance, clinical studies show high levels of HMGB1 in liver graft effluent after cold storage,243 which correlates with post-operative early allograft dysfunction.244
Normothermic machine perfusion aims to provide a more physiological environment to preserve liver grafts before implantation.245 However, despite promising clinical trial results, a recent study shows that HMGB1 and extracellular DNA increase during normothermic machine perfusion under different temperature conditions and correlate with TLR activation, suggesting that DAMPs act as inflammatory mediators during machine perfusion.16
Effects of DAMPs during liver graft reperfusion
Liver graft implantation requires a period of portal flow occlusion to allow anastomosis of vessels. Warm ischaemia arises when the liver graft returns to normothermic conditions.246 When blood flow is re-established, the subsequent oxidative burst directly damages hepatocytes that then release DAMPs, which are also secreted by KCs and MFs.247 As the major player in graft injury, IRI is inevitable during LT. DAMPs, such as HMGB1,248 HSP,249 extracellular ATP250 and extracellular DNA,16 are involved in IRI and mediate graft injury.
IRI in mice increases HMGB1 levels after 1 hour and lasts for 24 hours, indicating that HMGB1 is an early biomarker of graft injury.251,252 Indeed, neutralizing antibodies against HMGB1 or TLR4 lessen IRI,252,253 whereas Hmgb1ΔHep show aggravated hepatic IRI and DNA damage.254 These findings indicate that HMGB1 is essential for intracellular homeostasis and acts as a danger signal when it is released into the circulation.
IL33 is a nuclear protein released into the extracellular space during cell injury. IL33 promotes neutrophil infiltration, migration and formation of neutrophil extracellular traps (NETs) by binding to its receptor, suppression of tumorigenicity 2 (ST2).255 Although it was reported that NETs formation is beneficial for the host defence against pathogens,256 recent studies found that IL33 secreted by liver sinusoidal endothelial cells promotes NETs formation and eventually exacerbates inflammation and liver injury.257 Other DAMPs such as HMGB1 and histones induce NETs through TLR signalling. In addition, HMGB1 and histones reside in NETs and can recruit more neutrophils to further aggravate IRI.258
Further, ATP released from injured or stressed cells acts as a DAMP by binding to P2, activating the inflammasome in MFs via pannexin-1 and contributing to liver damage during IRI.259
Circulating histones significantly elevate and exacerbate liver damage following IRI signalling through TLR9. However, histone neutralisation and Tlr9 ablation ameliorate injury in vivo.260 During IRI, histones activate the NLRP3 inflammasome in KCs by generating ROS in a TLR9-dependent manner.261
Although HSP90 and HSP47 participate in the pathogenesis of ALD and fibrosis, HSP70 protects rat livers from IRI by reducing hepatic inflammatory and oxidative damage.262 Overexpression of HSP27 in mice protects from hepatic IRI by reducing necrosis, apoptosis and neutrophil infiltration.263 Likewise, PGE2 levels are significantly higher in the plasma of LT recipients with good graft function.264 Although HSP70 and PGE2 are considered danger signals, their protective effects against liver IRI and graft injury suggest a re-evaluation of their role as DAMPs in the setting of LT 265,266
Role of DAMPs in immune rejection
DAMPs induce innate and adaptive immune responses that result in immune rejection.258 After organ reperfusion, in addition to accumulated DAMPs generated by cold storage and IRI, alloantibodies from recipients lead to non-infectious injury and release of DAMPs, which persist after resolution of IRI.236 Many of these DAMPs bind to TLRs and drive the immune reaction toward the allograft. Further, lung and heart transplantation demonstrate a link between the release of DAMPs and acute rejection.267,268 Although the liver is an immunotolerant organ, the effect of DAMPs in LT rejection has been reported.269
CD39 is essential to maintain homeostatic levels of ATP. Cd39−/− mice exhibit increased liver-infiltrating CD8+ T cells, stronger response to donor alloantigens and reduced recipient survival rates after major histocompatibility complex mismatched LT.269 This outcome reinforces the involvement of extracellular ATP in post-transplant rejection.
HSPs also protect against LT rejection. Indeed, a retrospective clinical analysis shows significantly lower HSP70 mRNA levels in graft biopsy samples from LT recipients who developed graft dysfunction caused by rejection.270
Resolving the effects of DAMPs
Reducing release, promoting clearance and inhibiting DAMP signalling have been proposed to reduce graft injury and improve recipient outcomes. Treatment with recombinant soluble thrombomodulin attenuates liver graft injury by binding to HMGB1 and preventing the proinflammatory response.271 A similar effect is achieved by inhibiting TLR4, an HMGB1 receptor.252,253 In addition, preconditioning with low concentrations of HMGB1 before LT protected against hepatic IRI.272 Enhancement of extracellular ATP clearance by activating the P1 receptor A2A on bone marrow-derived cells also protects livers from IRI.273 Further, since NETs play a role in IRI, studies have examined inhibition of NETs during IRI. Peptidylarginine-deiminase-4 (PAD4) is required for formation of NETs and inhibition of PAD4 alleviates liver IRI in mice.274 However, considering the role of NETs in host defence, inhibition of NETs should be given special consideration due to the risk of infection in LT recipients.
Along with reducing harmful DAMPs, enhancing protective DAMPs holds promise for reducing hepatic IRI. Activation of HSP70 protects against hepatic IRI,249 an effect attributed to iNOS. Although nitrosative stress promotes ALD, fibrosis and HCC,22,32,59 it protects against hepatic IRI by activating HSP70. While specific HSPs are pathogenic in some liver diseases, the protective effect of HSP70 reported in these studies262 suggests that the role of HSPs as DAMPs during hepatic IRI should be re-evaluated. HSPs could provide a potential target to attenuate liver injury after LT.
Concluding remarks
Overall, these studies suggest that DAMPs induced by ROS and RNS, as well as DAMPs that signal through receptors and are produced by injured hepatocytes or non-parenchymal cells during ALD, NASH, fibrosis and HCC drive liver injury by increasing oxidative stress, lipid accumulation, inflammation and fibrosis. To our knowledge, there are not many existing clinical trials successfully targeting DAMPs to prevent onset and progression of chronic liver diseases. Blocking specific DAMPs alone or in combination could be a promising strategy to improve patient survival in the future, as HCC is the second leading cause of cancer-related deaths worldwide.275,276 While LT aims to rescue patients with end-stage liver disease, this surgical procedure is associated with significant release of DAMPs along with DAMP-induced graft injury and immune rejection. A thorough understanding of the role of each DAMP in LT is essential to improve graft quality and recipient outcomes, which could eventually be achieved by targeting specific DAMPs or controlling their signalling.
Key point.
Damage-associated molecular patterns are signalling molecules involved in inflammatory responses and restoration of homeostasis.
Chronic release of these molecules promotes inflammation in the context of liver disease.
Reactive oxygen species and reactive nitrogen species induce damage-associated molecular patterns.
Specific damage-associated molecular patterns participate in pathogenesis of chronic liver diseases such as alcohol-related liver disease, non-alcoholic steatohepatitis, liver fibrosis and liver cancer.
Damage-associated molecular patterns play a role in ischaemia reperfusion injury and liver transplantation.
Blockade of specific damage-associated molecular patterns has proven beneficial in humans and mice.
Acknowledgement
The authors are very grateful to Wei Chen for his careful review of the manuscript.
Financial support
American Association for the Study of Liver Diseases, Pinnacle Research Award G3156 (X. G.); US Public Health Service Grant R01 DK111677 from the National Institute of Diabetes and Digestive and Kidney Diseases (N. N.); US Public Health Service Grant R01AA025907 from the National Institute on Alcohol Abuse and Alcoholism (N. N.). US Department of Veterans Affairs Grant I01BX005093 (N. N.)
Abbreviations
- A2AR
adenosine A2A receptor
- A2BR
adenosine A2B receptor
- ACOX1
acetyl-CoA oxidase
- AGE
advanced-glycation end-products
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- CCl4
carbon tetrachloride
- CDAA
choline-deficient amino acid-defined
- COX2
cyclooxygenase-2
- CYP2E1
cytochrome P450 2E1
- DAMP(s)
damage-associated molecular pattern(s)
- DEN
diethylnitrosamine
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- FFA(s)
free fatty acid(s)
- GABPα
GA binding protein transcription factor subunit-α
- HA
hyaluronic acid
- HCC(s)
hepatocellular carcinoma(s)
- HF
high-fat
- HFHC
high-fat, fructose and cholesterol
- HMGB1
high-mobility group box-1
- HSC(s)
hepatic stellate cell(s)
- HSF1
heat shock factor-1
- HSP
heat shock protein
- IFNγ
interferon-γ
- IKK
I-κappa-B kinase
- IL33R
IL33 receptor
- iNOS
inducible nitric oxide synthase
- IRI
ischaemia reperfusion injury
- KEAP1
Kelch ECH associating protein-1
- KC(s)
Kupffer cell(s)
- LCN2
lipocalin-2
- LT
liver transplantation
- MAPK
mitogen-activated protein kinase
- MF(s)
macrophage(s)
- mtDAMP(s)
mitochondrial DAMPs
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NET(s)
neutrophil extracellular trap(s)
- NLRP3
NOD-like receptor protein-3
- NO
nitric oxide
- NOD
nucleotide-binding oligomerisation domain
- NOX
NADPH oxidase
- NRF2
nuclear factor erythroid 2-related factor-2
- ONOO−
peroxynitrite
- OPN
osteopontin
- P2
purinergic-2
- P2RX7
purinergic receptor P2X7
- PAD4
peptidyl-arginine-deiminase-4
- pAKT
phosphorylated protein kinase-B
- pc-Jun
phosphorylated c-Jun
- pERK1/2
phosphorylated extracellular signal-regulated kinase
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- pMEK
phosphorylated mitogen-activated protein kinase
- PRR(s)
pattern recognition receptor(s)
- RAGE
receptor for advanced glycation end-products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- ssRNA
single-stranded RNA
- ST2
suppression of tumourigenicity-2
- TGFβ
transforming growth factor-β
- Th2
T helper-2
- TLR(s)
toll-like receptor(s)
- TNFα
tumor necrosis factor-α
- YAP1
Yes-associated protein-1
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.04.033.
References
Author names in bold designate shared co-first authorship
- [1].Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469–478. [DOI] [PubMed] [Google Scholar]
- [2].Handa P, Vemulakonda A, Kowdley KV, Uribe M, Mendez-Sanchez N. Mitochondrial DNA from hepatocytes as a ligand for TLR9: drivers of nonalcoholic steatohepatitis? World J Gastroenterol 2016;22:6965–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martin-Murphy BV, Holt MP, Ju C. The role of damage associated mo-lecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 2010;192:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi S, Verstegen MMA, Mezzanotte L, de Jonge J, Lowik C, van der Laan LJW. Necroptotic cell death in liver transplantation and underlying diseases: mechanisms and clinical perspective. Liver Transpl 2019;25:1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pardo M, Budick-Harmelin N, Tirosh B, Tirosh O. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic Biol Med 2008;45:1073–1083. [DOI] [PubMed] [Google Scholar]
- [6].Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev 2017;280:74–82. [DOI] [PubMed] [Google Scholar]
- [7].Campwala H, Fountain SJ. Constitutive and agonist stimulated ATP secretion in leukocytes. Commun Integr Biol 2013;6:e23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem 2014;289:22672–2269 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Farrell GC, Haczeyni F, Chitturi S. Pathogenesis of NASH: how metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Adv Exp Med Biol 2018;1061:19–44. [DOI] [PubMed] [Google Scholar]
- [10].Lu L, Zhou H, Ni M, Wang X, Busuttil R, Kupiec-Weglinski J, et al. Innate immune regulations and liver ischemia-reperfusion injury. Transplantation 2016;100:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S. Toll like receptor 4 and hepatocellular carcinoma; a systematic review. Life Sci 2017;179: 80–87. [DOI] [PubMed] [Google Scholar]
- [12].Bauernfeind F, Niepmann S, Knolle PA, Hornung V. Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J Immunol 2016;197:2900–2908. [DOI] [PubMed] [Google Scholar]
- [13].He Y, Li S, Tang D, Peng Y, Meng J, Peng S, et al. Circulating peroxiredoxin-1 is a novel damage-associated molecular pattern and aggravates acute liver injury via promoting inflammation. Free Radic Biol Med 2019;137:24–36. [DOI] [PubMed] [Google Scholar]
- [14].Nyakundi BB, Toth A, Balogh E, Nagy B, Erdei J, Ryffel B, et al. Oxidized hemoglobin forms contribute to NLRP3 inflammasome-driven IL-1beta production upon intravascular hemolysis. Biochim Biophys Acta Mol Basis Dis 2019;1865:464–475. [DOI] [PubMed] [Google Scholar]
- [15].Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol 2015;98:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scheuermann U, Zhu M, Song M, Yerxa J, Gao Q, Davis RP, et al. Damage-associated molecular patterns induce inflammatory injury during machine preservation of the liver: potential targets to enhance a promising technology. Liver Transpl 2019;25:610–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiahou Z, Wang X, Shen J, Zhu X, Xu F, Hu R, et al. NMI and IFP35 serve as proinflammatory DAMPs during cellular infection and injury. Nat Commun 2017;8:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jaeschke H Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 2011;26(Suppl 1):173–179. [DOI] [PubMed] [Google Scholar]
- [19].van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol 2013;28:394–400. [DOI] [PubMed] [Google Scholar]
- [20].Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology 2002;35:62–73. [DOI] [PubMed] [Google Scholar]
- [21].Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem 2002;277:9853–9864. [DOI] [PubMed] [Google Scholar]
- [22].Urtasun R, Cubero FJ, Vera M, Nieto N. Reactive nitrogen species switch on early extracellular matrix remodeling via induction of MMP1 and TNFalpha. Gastroenterology 2009;136:1410–1422.e1–4. [DOI] [PubMed] [Google Scholar]
- [23].Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 2012;55:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mihm S Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci 2018;19:3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Su L, Li N, Tang H, Lou Z, Chong X, Zhang C, et al. Kupffer cell-derived TNF-alpha promotes hepatocytes to produce CXCL1 and mobilize neutrophils in response to necrotic cells. Cell Death Dis 2018;9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247. [DOI] [PubMed] [Google Scholar]
- [27].Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014;94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim SY, Jeong JM, Kim SJ, Seo W, Kim MH, Choi WM, et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun 2017;8:2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liang S, Kisseleva T, Brenner DA. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol 2016;7:1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cordero-Herrera I, Kozyra M, Zhuge Z, McCann Haworth S, Moretti C, Peleli M, et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc Natl Acad Sci U S A 2019;116:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Iwakiri Y, Kim MY. Nitric oxide in liver diseases. Trends Pharmacol Sci 2015;36:524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang R, Li Y, Tsung A, Huang H, Du Q, Yang M, et al. iNOS promotes CD24(+)CD133(+) liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc Natl Acad Sci U S A 2018;115:E10127–E10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arauz J, Ramos-Tovar E, Muriel P. Redox state and methods to evaluate oxidative stress in liver damage: from bench to bedside. Ann Hepatol 2016;15:160–173. [DOI] [PubMed] [Google Scholar]
- [34].Xu J, Ma HY, Liang S, Sun M, Karin G, Koyama Y, et al. The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatol Commun 2017;1:1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 2004;55:373–399. [DOI] [PubMed] [Google Scholar]
- [36].Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis 2015;6:e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Muriel P Role of free radicals in liver diseases. Hepatol Int 2009;3:526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clin-ical evidence. J Dig Dis 2012;13:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim HG, Huang M, Xin Y, Zhang Y, Zhang X, Wang G, et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J Hepatol 2019;71:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab 2017;28:250–260. [DOI] [PubMed] [Google Scholar]
- [41].Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal 2017;26:519–54 [DOI] [PubMed] [Google Scholar]
- [42].Musso G, Cassader M, Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov 2016;15:249–274. [DOI] [PubMed] [Google Scholar]
- [43].Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 2012;52:59–69. [DOI] [PubMed] [Google Scholar]
- [44].Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014;59:886–897. [DOI] [PubMed] [Google Scholar]
- [45].Navarro LA, Wree A, Povero D, Berk MP, Eguchi A, Ghosh S, et al. Arginase 2 deficiency results in spontaneous steatohepatitis: a novel link between innate immune activation and hepatic de novo lipogenesis. J Hepatol 2015;62:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Torok NJ. Dysregulation of redox pathways in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2016;311:G667–G674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ko E, Kim JS, Ju S, Seo HW, Chang Y, Kang JA, et al. Oxidatively modified protein-disulfide isomerase-associated 3 promotes Dyskerin Pseudouridine synthase 1-mediated Malignancy and survival of hepatocellular carcinoma cells. Hepatology 2018;68:1851–1864. [DOI] [PubMed] [Google Scholar]
- [48].Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology 2018;67:1378–1391. [DOI] [PubMed] [Google Scholar]
- [49].Lin CY, Hu CT, Cheng CC, Lee MC, Pan SM, Lin TY, et al. Oxidation of heat shock protein 60 and protein disulfide isomerase activates ERK and migration of human hepatocellular carcinoma HepG2. Oncotarget 2016;7:11067–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol Commun 2018;2:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mukhopadhyay P, Horvath B, Rajesh M, Varga ZV, Gariani K, Ryu D, et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol 2017;66:589–600. [DOI] [PubMed] [Google Scholar]
- [52].Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16. [DOI] [PubMed] [Google Scholar]
- [53].Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 2018;155:629–647. [DOI] [PubMed] [Google Scholar]
- [54].Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Arriazu E, Ge X, Leung TM, Magdaleno F, Lopategi A, Lu Y, et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut 2017;66:1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008;29:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 2011;32:157–164. [DOI] [PubMed] [Google Scholar]
- [58].Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 2000;106:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 2003;125:1834–1844. [DOI] [PubMed] [Google Scholar]
- [60].Chamulitrat W, Spitzer JJ. Nitric oxide and liver injury in alcohol-fed rats after lipopolysaccharide administration. Alcohol Clin Exp Res 1996;20:1065–1070. [DOI] [PubMed] [Google Scholar]
- [61].Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, et al. Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alpha-tocopherol than alpha-tocopherol/ascorbate. J Biol Chem 2000;275:10812–10818. [DOI] [PubMed] [Google Scholar]
- [62].Anavi S, Eisenberg-Bord M, Hahn-Obercyger M, Genin O, Pines M, Tirosh O. The role of iNOS in cholesterol-induced liver fibrosis. Lab Invest 2015;95:914–924. [DOI] [PubMed] [Google Scholar]
- [63].Aram G, Potter JJ, Liu X, Torbenson MS, Mezey E. Lack of inducible nitric oxide synthase leads to increased hepatic apoptosis and decreased fibrosis in mice after chronic carbon tetrachloride administration. Hepatology 2008;47:2051–2058. [DOI] [PubMed] [Google Scholar]
- [64].Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 2008;44:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Knecht KT, Adachi Y, Bradford BU, Iimuro Y, Kadiiska M, Xuang QH, et al. Free radical adducts in the bile of rats treated chronically with intragastric alcohol: inhibition by destruction of Kupffer cells. Mol Pharmacol 1995;47:1028–1034. [PubMed] [Google Scholar]
- [66].Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol 2013;58:395–398. [DOI] [PubMed] [Google Scholar]
- [67].Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology 1996;23:872–880. [DOI] [PubMed] [Google Scholar]
- [68].Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009;50:453–461. [DOI] [PubMed] [Google Scholar]
- [69].Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, et al. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun 1993;194:1044–1050. [DOI] [PubMed] [Google Scholar]
- [70].Mueller S, Peccerella T, Qin H, Glassen K, Waldherr R, Flechtenmacher C, et al. Carcinogenic etheno DNA adducts in alcoholic liver disease: correlation with cytochrome P-4502E1 and fibrosis. Alcohol Clin Exp Res 2018;42:252–259. [DOI] [PubMed] [Google Scholar]
- [71].Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol 2002;37:56–62. [DOI] [PubMed] [Google Scholar]
- [72].Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kharbanda KK, Shubert KA, Wyatt TA, Sorrell MF, Tuma DJ. Effect of malondialdehyde-acetaldehyde-protein adducts on the protein kinase C-dependent secretion of urokinase-type plasminogen activator in hepatic stellate cells. Biochem Pharmacol 2002;63:553–562. [DOI] [PubMed] [Google Scholar]
- [74].Bataller R, Lemon SM. Fueling fibrosis in chronic hepatitis C. Proc Natl Acad Sci U S A 2012;109:14293–14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37:1043–1055. [DOI] [PubMed] [Google Scholar]
- [76].Araujo Junior RF, Garcia VB, Leitao RF, Brito GA, Miguel Ede C, Guedes PM, et al. Carvedilol improves inflammatory response, oxidative stress and fibrosis in the alcohol-induced liver injury in rats by regulating Kuppfer cells and hepatic stellate cells. PLoS One 2016;11:e0148868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Su X, Wang Y, Zhou G, Yang X, Yu R, Lin Y, et al. Probucol attenuates ethanol-induced liver fibrosis in rats by inhibiting oxidative stress, extracellular matrix protein accumulation and cytokine production. Clin Exp Pharmacol Physiol 2014;41:73–80. [DOI] [PubMed] [Google Scholar]
- [78].Fabregat I, Caballero-Diaz D. Transforming growth factor-beta-induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front Oncol 2018;8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen XF, Tian MX, Sun RQ, Zhang ML, Zhou LS, Jin L, et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep 2018;19:e45124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sakurai T, Kudo M, Umemura A, He G, Elsharkawy AM, Seki E, et al. p38alpha inhibits liver fibrogenesis and consequent hepatocarcinogenesis by curtailing accumulation of reactive oxygen species. Cancer Res 2013;73:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev 2017;9:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016;41:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem 2007;282:30667–30672. [DOI] [PubMed] [Google Scholar]
- [84].Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 2006;8:1791–1806. [DOI] [PubMed] [Google Scholar]
- [86].Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffray E, et al. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J Cell Biol 2000;148:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Besse-Patin A, Leveille M, Oropeza D, Nguyen BN, Prat A, Estall JL. Estrogen signals through peroxisome proliferator-activated receptor-gamma coactivator 1alpha to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology 2017;152:243–256. [DOI] [PubMed] [Google Scholar]
- [88].Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. [DOI] [PubMed] [Google Scholar]
- [89].Karimian G, Kirschbaum M, Veldhuis ZJ, Bomfati F, Porte RJ, Lisman T. Vitamin E attenuates the progression of non-alcoholic fatty liver disease caused by partial Hepatectomy in mice. PLoS One 2015;10:e014312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Klaebel JH, Skjodt M, Skat-Rordam J, Rakipovski G, Ipsen DH, Schou-Pedersen AMV, et al. Atorvastatin and vitamin E accelerates NASH resolution by dietary intervention in a preclinical guinea pig model. Nutrients 2019;11:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Presa N, Clugston RD, Lingrell S, Kelly SE, Merrill AH Jr, Jana S, et al. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice. Biochim Biophys Acta Mol Basis Dis 2019;1865:14–25. [DOI] [PubMed] [Google Scholar]
- [92].Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol 2004;40:40–46. [DOI] [PubMed] [Google Scholar]
- [93].Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 2007;47:277–283. [DOI] [PubMed] [Google Scholar]
- [94].Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 2010;51:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med 2014;66:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Barnes MA, Roychowdhury S, Nagy LE. Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1. Redox Biol 2014;2:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Crawford JM. Histologic findings in alcoholic liver disease. Clin Liver Dis 2012;16:699–716. [DOI] [PubMed] [Google Scholar]
- [99].Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. Cell Mol Life Sci 2016;73:2137–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev 2010;90:1165–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 2012;26:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 2017;17:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fromenty B, Grimbert S, Mansouri A, Beaugrand M, Erlinger S, Rotig A, et al. Hepatic mitochondrial DNA deletion in alcoholics: association with microvesicular steatosis. Gastroenterology 1995;108:193–200. [DOI] [PubMed] [Google Scholar]
- [105].Lemasters JJ, Zhong Z. Mitophagy in hepatocytes: types, initiators and role in adaptive ethanol metabolism. Liver Res 2018;2:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology 2016;150:1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008;27:608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014;63:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Iracheta-Vellve A, Petrasek J, Satishchandran A, Gyongyosi B, Saha B, Kodys K, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 2015;63:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mandrekar P Signaling mechanisms in alcoholic liver injury: role of transcription factors, kinases and heat shock proteins. World J Gastroenterol 2007;13:4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Saha B, Momen-Heravi F, Furi I, Kodys K, Catalano D, Gangopadhyay A, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology 2018;67:1986–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther 2005;315:8–15. [DOI] [PubMed] [Google Scholar]
- [114].Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact 2011;192:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol 2014;61:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog 2009;48:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A 2008;105:10320–10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ge X, Arriazu E, Magdaleno F, Antoine DJ, Dela Cruz R, Theise N, et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology 2018;68:2380–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Saikia P, Roychowdhury S, Bellos D, Pollard KA, McMullen MR, McCullough RL, et al. Hyaluronic acid 35 normalizes TLR4 signaling in Kupffer cells from ethanol-fed rats via regulation of microRNA291b and its target Tollip. Sci Rep 2017;7:15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].He Y, Gao B. A small specific-sized hyaluronic acid ameliorates alcoholic liver disease by targeting a small RNA: new hope for therapy? Hepatology 2017;66:321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Stickel F, Poeschl G, Schuppan D, Conradt C, Strenge-Hesse A, Fuchs FS, et al. Serum hyaluronate correlates with histological progression in alcoholic liver disease. Eur J Gastroenterol Hepatol 2003;15:945–950. [DOI] [PubMed] [Google Scholar]
- [122].Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol 2005;3:167–174. [DOI] [PubMed] [Google Scholar]
- [123].Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631–646. [DOI] [PubMed] [Google Scholar]
- [124].Wieser V, Tymoszuk P, Adolph TE, Grander C, Grabherr F, Enrich B, et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J Hepatol 2016;64:872–880. [DOI] [PubMed] [Google Scholar]
- [125].Dubuquoy L Lipocalin 2 highlights the complex role of neutrophils in alcoholic liver disease. J Hepatol 2016;64:770–772. [DOI] [PubMed] [Google Scholar]
- [126].Cai Y, Jogasuria A, Yin H, Xu MJ, Hu X, Wang J, et al. The detrimental role played by lipocalin-2 in alcoholic fatty liver in mice. Am J Pathol 2016;186:2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med 2014;20:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Arroyo V, Moreau R. Tying up PGE2 with albumin to relieve immune-suppression in cirrhosis. Nat Med 2014;20:467–469. [DOI] [PubMed] [Google Scholar]
- [129].Choe WH, Baik SK Prostaglandin E2 -mediated immunosuppression and the role of albumin as its modulator. Hepatology 2015;61:1080–1082. [DOI] [PubMed] [Google Scholar]
- [130].Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, et al. Kupffer cell-derived prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol 2000;279:G100–G106. [DOI] [PubMed] [Google Scholar]
- [131].Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- [132].Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [133].Kim JY, Garcia-Carbonell R, Yamachika S, Zhao P, Dhar D, Loomba R, et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell 2018;175:133–145.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820. [DOI] [PubMed] [Google Scholar]
- [136].Aragones G, Colom-Pellicer M, Aguilar C, Guiu-Jurado E, Martinez S, Sabench F, et al. Circulating microbiota-derived metabolites: a “liquid biopsy? Int J Obes (Lond) 2020;44:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cengiz M, Ozenirler S, Elbeg S. Role of serum toll-like receptors 2 and 4 in non-alcoholic steatohepatitis and liver fibrosis. J Gastroenterol Hepatol 2015;30:1190–1196. [DOI] [PubMed] [Google Scholar]
- [138].Mridha AR, Haczeyni F, Yeh MM, Haigh WG, Ioannou GN, Barn V, et al. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin Sci (Lond) 2017;131:2145–2159. [DOI] [PubMed] [Google Scholar]
- [139].O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 2013;13:453–460. [DOI] [PubMed] [Google Scholar]
- [140].Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 2016;126:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Roh YS, Kim JW, Park S, Shon C, Kim S, Eo SK, et al. Toll-like receptor-7 signaling promotes nonalcoholic steatohepatitis by inhibiting regulatory T cells in mice. Am J Pathol 2018;188:2574–2588. [DOI] [PubMed] [Google Scholar]
- [143].Kim S, Park S, Kim B, Kwon J. Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci Rep 2016;6:27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, et al. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 2015;149:468–480.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabon MA, et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med 2016;22:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [146].van Koppen A, Verschuren L, van den Hoek AM, Verheij J, Morrison MC, Li K, et al. Uncovering a predictive molecular signature for the onset of NASH-related fibrosis in a translational NASH mouse model. Cell Mol Gastroenterol Hepatol 2018;5:83–98.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hennig EE, Mikula M, Goryca K, Paziewska A, Ledwon J, Nesteruk M, et al. Extracellular matrix and cytochrome P450 gene expression can distinguish steatohepatitis from steatosis in mice. J Cell Mol Med 2014;18:1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Decaris ML, Li KW, Emson CL, Gatmaitan M, Liu S, Wang Y, et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 2017;65:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kopec AK, Abrahams SR, Thornton S, Palumbo JS, Mullins ES, Divanovic S, et al. Thrombin promotes diet-induced obesity through fibrin-driven inflammation. J Clin Invest 2017;127:3152–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J Gastroenterol 2016;22:9706–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 2020;158:1334–1345.e5. [DOI] [PubMed] [Google Scholar]
- [152].McGettigan B, McMahan R, Orlicky D, Burchill M, Danhorn T, Francis P, et al. Dietary lipids differentially shape nonalcoholic steatohepatitis progression and the transcriptome of Kupffer cells and infiltrating macrophages. Hepatology 2019;70:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 2013;14:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ioannou GN, Van Rooyen DM, Savard C, Haigh WG, Yeh MM, Teoh NC, et al. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J Lipid Res 2015;56:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol 2018;69:705–717. [DOI] [PubMed] [Google Scholar]
- [156].Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes 1994;43:676–683. [DOI] [PubMed] [Google Scholar]
- [157].Mehta R, Shaw G, Masschelin P, Felix S, Otgonsuren M, Baranova A, et al. Polymorphisms in the receptor for advanced glycation end-products (RAGE) gene and circulating RAGE levels as a susceptibility factor for non-alcoholic steatohepatitis (NASH). PLoS One 2018;13:e0199294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Leung C, Herath CB, Jia Z, Andrikopoulos S, Brown BE, Davies MJ, et al. Dietary advanced glycation end-products aggravate non-alcoholic fatty liver disease. World J Gastroenterol 2016;22:8026–8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bijnen M, Beelen N, Wetzels S, Gaar JV, Vroomen M, Wijnands E, et al. RAGE deficiency does not affect non-alcoholic steatohepatitis and atherosclerosis in Western type diet-fed Ldlr(-/-) mice. Sci Rep 2018;8:15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol 2015;39(Suppl 1):S60–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 2014;111:E3297–E3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Inkaya AC, Demir NA, Kolgelier S, Sumer S, Demir LS, Ural O, et al. Is serum high-mobility group box 1 (HMGB-1) level correlated with liver fibrosis in chronic hepatitis B? Medicine (Baltimore) 2017;96:e7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hu YB, Hu DP, Fu RQ. Correlation between high mobility group box-1 protein and chronic hepatitis B infection with severe hepatitis B and acute-on-chronic liver failure: a meta-analysis. Minerva Med 2017;108:268–276. [DOI] [PubMed] [Google Scholar]
- [166].He Q, Fu Y, Ding X, Li D, Wang Z, Tian D, et al. High-mobility group box 1 induces endoplasmic reticulum stress and activates hepatic stellate cells. Lab Invest 2018;98:1200–1210. [DOI] [PubMed] [Google Scholar]
- [167].Khanjarsim V, Karimi J, Khodadadi I, Mohammadalipour A, Goodarzi MT, Solgi G, et al. Ameliorative effects of nilotinib on CCl4 induced liver fibrosis via attenuation of RAGE/HMGB1 gene expression and oxidative stress in rat. Chonnam Med J 2017;53:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R, et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 2014;63:1805–1818. [DOI] [PubMed] [Google Scholar]
- [169].Yu Z, Jv Y, Cai L, Tian X, Huo X, Wang C, et al. Gambogic acid attenuates liver fibrosis by inhibiting the PI3K/AKT and MAPK signaling pathways via inhibiting HSP90. Toxicol Appl Pharmacol 2019;371:63–73. [DOI] [PubMed] [Google Scholar]
- [170].Zhang F, Hao M, Jin H, Yao Z, Lian N, Wu L, et al. Canonical hedgehog signalling regulates hepatic stellate cell-mediated angiogenesis in liver fibrosis. Br J Pharmacol 2017;174:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Myung SJ, Yoon JH, Kim BH, Lee JH, Jung EU, Lee HS. Heat shock protein 90 inhibitor induces apoptosis and attenuates activation of hepatic stellate cells. J Pharmacol Exp Ther 2009;330:276–282. [DOI] [PubMed] [Google Scholar]
- [172].Ito S, Ogawa K, Takeuchi K, Takagi M, Yoshida M, Hirokawa T, et al. A small-molecule compound inhibits a collagen-specific molecular chaperone and could represent a potential remedy for fibrosis. J Biol Chem 2017;292:20076–20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Ito S, Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol 2017;62:142–151. [DOI] [PubMed] [Google Scholar]
- [174].Rizk FH, Sarhan NI, Soliman NA, Ibrahim MAA, Abd-Elsalam M, Abd-Elsalam S. Heat shock protein 47 as indispensible participant in liver fibrosis: possible protective effect of lactoferrin. IUBMB Life 2018;70:795–805. [DOI] [PubMed] [Google Scholar]
- [175].Wei S, Wang Q, Zhou H, Qiu J, Li C, Shi C, et al. miR-455–3p alleviates hepatic stellate cell activation and liver fibrosis by suppressing HSF1 expression. Mol Ther Nucleic Acids 2019;16:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 2012;188:3488–3495. [DOI] [PubMed] [Google Scholar]
- [177].Tan Z, Liu Q, Jiang R, Lv L, Shoto SS, Maillet I, et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol Immunol 2018;15:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Liu J, Yang Y, Zheng C, Chen G, Shen Z, Zheng S, et al. Correlation of interleukin-33/ST2 receptor and liver fibrosis progression in biliary atresia patients. Front Pediatr 2019;7:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013;39:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Gao Y, Liu Y, Yang M, Guo X, Zhang M, Li H, et al. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget 2016;7:33649–33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Toki Y, Takenouchi T, Harada H, Tanuma S, Kitani H, Kojima S, et al. Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1beta, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem Biophys Res Commun 2015;458:771–776. [DOI] [PubMed] [Google Scholar]
- [182].Ferrari D, Gambari R, Idzko M, Muller T, Albanesi C, Pastore S, et al. Purinergic signaling in scarring. FASEB J 2016;30:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Feig JL, Mediero A, Corciulo C, Liu H, Zhang J, Perez-Aso M, et al. The antiviral drug tenofovir, an inhibitor of pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PLoS One 2017;12:e0188135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wang G, Wang H, Singh S, Zhou P, Yang S, Wang Y, et al. ADAR1 prevents liver injury from inflammation and suppresses interferon production in hepatocytes. Am J Pathol 2015;185:3224–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35–S50. [DOI] [PubMed] [Google Scholar]
- [186].Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 2018;15:536–554. [DOI] [PubMed] [Google Scholar]
- [187].Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer 2015;15:653–66 . [DOI] [PubMed] [Google Scholar]
- [188].Kawahara N, Tanaka T, Yokomizo A, Nanri H, Ono M, Wada M, et al. Enhanced coexpression of thioredoxin and high mobility group protein 1 genes in human hepatocellular carcinoma and the possible association with decreased sensitivity to cisplatin. Cancer Res 1996;56:5330–5333. [PubMed] [Google Scholar]
- [189].Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem 2010;337:251–258. [DOI] [PubMed] [Google Scholar]
- [190].Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology 2012;55:1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis 2008;40:446–452. [DOI] [PubMed] [Google Scholar]
- [192].Zhang L, Han J, Wu H, Liang X, Zhang J, Li J, et al. The association of HMGB1 expression with clinicopathological significance and prognosis in hepatocellular carcinoma: a meta-analysis and literature review. PLoS One 2014;9:e110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Yan HX, Wu HP, Zhang HL, Ashton C, Tong C, Wu H, et al. p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release. J Hepatol 2013;59:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Hernandez C, Huebener P, Pradere JP, Antoine DJ, Friedman RA, Schwabe RF. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J Clin Invest 2019;129:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Chen R, Zhu S, Fan XG, Wang H, Lotze MT, Zeh HJ 3rd, et al. High mobility group protein B1 controls liver cancer initiation through yes-associated protein -dependent aerobic glycolysis. Hepatology 2018;67:1823–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Tohme S, Yazdani HO, Liu Y, Loughran P, van der Windt DJ, Huang H, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology 2017;66:182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Sieghart W, Wang X, Schmid K, Pinter M, Konig F, Bodingbauer M, et al. Osteopontin expression predicts overall survival after liver transplantation for hepatocellular carcinoma in patients beyond the Milan criteria. J Hepatol 2011;54:89–97. [DOI] [PubMed] [Google Scholar]
- [198].Yuan RH, Jeng YM, Chen HL, Lai PL, Pan HW, Hsieh FJ, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 2006;209:549–558. [DOI] [PubMed] [Google Scholar]
- [199].Lee SH, Park JW, Woo SH, Go DM, Kwon HJ, Jang JJ, et al. Suppression of osteopontin inhibits chemically induced hepatic carcinogenesis by induction of apoptosis in mice. Oncotarget 2016;7:87219–87231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carci-noma to anti-PD-L1 blockade. Gut 2019;68:1653–1666. [DOI] [PubMed] [Google Scholar]
- [201].Fan X, He C, Jing W, Zhou X, Chen R, Cao L, et al. Intracellular Osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res 2015;75:86–97. [DOI] [PubMed] [Google Scholar]
- [202].Donato R RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med 2007;7:711–724. [DOI] [PubMed] [Google Scholar]
- [203].Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer 2015;15:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].De Ponti A, Wiechert L, Schneller D, Pusterla T, Longerich T, Hogg N, et al. A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett 2015;369:396–404. [DOI] [PubMed] [Google Scholar]
- [205].Jiao J, Gonzalez A, Stevenson HL, Gagea M, Sugimoto H, Kalluri R, et al. Depletion of S100A4(+) stromal cells does not prevent HCC development but reduces the stem cell-like phenotype of the tumors. Exp Mol Med 2018;50:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology 2013;57:2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wang Z, Yan J, Lin H, Hua F, Wang X, Liu H, et al. Toll-like receptor 4 activity protects against hepatocellular tumorigenesis and progression by regulating expression of DNA repair protein Ku70 in mice. Hepatology 2013;57:1869–1881. [DOI] [PubMed] [Google Scholar]
- [208].Chen M, Liu Y, Varley P, Chang Y, He XX, Huang H, et al. High-mobility group box 1 promotes hepatocellular carcinoma progression through miR-21-mediated matrix metalloproteinase activity. Cancer Res 2015;75:1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Chen RC, Yi PP, Zhou RR, Xiao MF, Huang ZB, Tang DL, et al. The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines. Mol Cell Biochem 2014;390:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol 2015;63:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Bao D, Zhao J, Zhou X, Yang Q, Chen Y, Zhu J, et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 2019;38:5007–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Eiro N, Altadill A, Juarez LM, Rodriguez M, Gonzalez LO, Atienza S, et al. Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma: relationship with clinicopathological characteristics and prognosis. Hepatol Res 2014;44:769–778. [DOI] [PubMed] [Google Scholar]
- [213].Sakuraoka Y, Sawada T, Okada T, Shiraki T, Miura Y, Hiraishi K, et al. MK615 decreases RAGE expression and inhibits TAGE-induced proliferation in hepatocellular carcinoma cells. World J Gastroenterol 2010;16:5334–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Li J, Wu PW, Zhou Y, Dai B, Zhang PF, Zhang YH, et al. Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy. Cell Death Dis 2018;9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Takino J, Yamagishi S, Takeuchi M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol 2012;18:1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hiwatashi K, Ueno S, Abeyama K, Kubo F, Sakoda M, Maruyama I, et al. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol 2008;15:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Yang Y, Zhao LH, Huang B, Wang RY, Yuan SX, Tao QF, et al. Pioglitazone, a PPARgamma agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol Carcinog 2015;54:1584–1595. [DOI] [PubMed] [Google Scholar]
- [218].Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappaB-HIF-1alpha pathway. Oncotarget 2015;6:6627–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Zhao J, Dong L, Lu B, Wu G, Xu D, Chen J, et al. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology 2008;135:956–968. [DOI] [PubMed] [Google Scholar]
- [220].Guo Q, Wang J, Cao Z, Tang Y, Feng C, Huang F. Interaction of S100A1 with LATS1 promotes cell growth through regulation of the Hippo pathway in hepatocellular carcinoma. Int J Oncol 2018;53:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [221].Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, et al. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology 2013;57:2274–2286. [DOI] [PubMed] [Google Scholar]
- [222].Dou C, Liu Z, Xu M, Jia Y, Wang Y, Li Q, et al. miR-187–3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett 2016;381:380–390. [DOI] [PubMed] [Google Scholar]
- [223].Nemeth J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology 2009;50:1251–1262. [DOI] [PubMed] [Google Scholar]
- [224].Liu K, Zhang Y, Zhang C, Zhang Q, Li J, Xiao F, et al. Methylation of S100A8 is a promising diagnosis and prognostic marker in hepatocellular carcinoma. Oncotarget 2016;7:56798–56810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Wu R, Duan L, Cui F, Cao J, Xiang Y, Tang Y, et al. S100A9 promotes human hepatocellular carcinoma cell growth and invasion through RAGE-mediated ERK1/2 and p38 MAPK pathways. Exp Cell Res 2015;334:228–238. [DOI] [PubMed] [Google Scholar]
- [226].Khalid M, Brisson L, Tariq M, Hao Y, Guibon R, Fromont G, et al. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells. Oncotarget 2017;8:37278–37290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Feng R, Ye J, Zhou C, Qi L, Fu Z, Yan B, et al. Calreticulin down-regulation inhibits the cell growth, invasion and cell cycle progression of human hepatocellular carcinoma cells. Diagn Pathol 2015;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Murai S, Yamaguchi Y, Shirasaki Y, Yamagishi M, Shindo R, Hildebrand JM, et al. A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat Commun 2018;9:4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Chen R, Xie Y, Zhong X, Fu Y, Huang Y, Zhen Y, et al. Novel chemokine-like activities of histones in tumor metastasis. Oncotarget 2016;7:61728–61740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Xu YF, Ge FJ, Han B, Yang XQ, Su H, Zhao AC, et al. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol 2015;21:3256–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Xu YF, Liu ZL, Pan C, Yang XQ, Ning SL, Liu HD, et al. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene 2019;38:868–880. [DOI] [PubMed] [Google Scholar]
- [232].Agopian VG, Harlander-Locke MP, Markovic D, Dumronggittigule W, Xia V, Kaldas FM, et al. Evaluation of early allograft function using the liver graft assessment following transplantation risk Score model. JAMA Surg 2018;153:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Hofer A, Jonigk D, Hartleben B, Verboom M, Hallensleben M, Hubscher SG, et al. DSA are associated with more graft injury, more fibrosis, and upregulation of rejection-associated transcripts in subclinical rejection. Transplantation 2020;104:551–561. [DOI] [PubMed] [Google Scholar]
- [234].Li CX, Man K, Lo CM. The impact of liver graft injury on cancer recurrence posttransplantation. Transplantation 2017;101:2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Liu Y, Lu T, Zhang C, Xu J, Xue Z, Busuttil RW, et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J Hepatol 2019;71:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Todd JL, Palmer SM. Danger signals in regulating the immune response to solid organ transplantation. J Clin Invest 2017;127:2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Michel SG, Madariaga MLL, LaMuraglia GM 2nd, Villani V, Sekijima M, Farkash EA, et al. The effects of brain death and ischemia on tolerance induction are organ-specific. Am J Transplant 2018;18:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Sung PH, Lee FY, Lin LC, Chen KH, Lin HS, Shao PL, et al. Melatonin attenuated brain death tissue extract-induced cardiac damage by suppressing DAMP signaling. Oncotarget 2018;9:3531–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Floerchinger B, Ge X, Lee YL, Jurisch A, Padera RF, Schmid C, et al. Graft-specific immune cells communicate inflammatory immune responses after brain death. J Heart Lung Transplant 2012;31:1293–1300. [DOI] [PubMed] [Google Scholar]
- [240].Floerchinger B, Yuan X, Jurisch A, Timsit MO, Ge X, Lee YL, et al. Inflammatory immune responses in a reproducible mouse brain death model. Transpl Immunol 2012;27:25–29. [DOI] [PubMed] [Google Scholar]
- [241].Belaschk E, Rohn S, Mukiibi R, Reutzel-Selke A, Tang P, Sawitzki B, et al. Isolation, characterization and cold storage of cells isolated from diseased explanted livers. Int J Artif organs 2017;40:294–306. [DOI] [PubMed] [Google Scholar]
- [242].Olschewski P, Hunold G, Eipel C, Neumann U, Schoning W, Schmitz V, et al. Improved microcirculation by low-viscosity histidine- tryptophan-ketoglutarate graft flush and subsequent cold storage in University of Wisconsin Solution: results of an orthotopic rat liver transplantation model. Transpl Int 2008;21:1175–1180. [DOI] [PubMed] [Google Scholar]
- [243].Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl 2008;14:1517–1525. [DOI] [PubMed] [Google Scholar]
- [244].Houben P, Hohenberger R, Yamanaka K, Buchler MW, Schemmer P. Evaluation of graft effluent high mobility group box-1 (HMGB-1) for prediction of outcome after liver transplantation. Ann Transplant 2018;23:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557:50–56. [DOI] [PubMed] [Google Scholar]
- [246].Orci LA, Lacotte S, Delaune V, Slits F, Oldani G, Lazarevic V, et al. Effects of the gut-liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J Hepatol 2018;68:978–985. [DOI] [PubMed] [Google Scholar]
- [247].Andersohn A, Garcia MI, Fan Y, Thompson MC, Akimzhanov AM, Abdullahi A, et al. Aggregated and hyperstable damage-associated molecular patterns are released during ER stress to modulate immune function. Front Cell Dev Biol 2019;7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].van Golen RF, Reiniers MJ, Marsman G, Alles LK, van Rooyen DM, Petri B, et al. The damage-associated molecular pattern HMGB1 is released early after clinical hepatic ischemia/reperfusion. Biochim Biophys Acta Mol Basis Dis 2019;1865:1192–1200. [DOI] [PubMed] [Google Scholar]
- [249].Qiao Y, Zhang X, Zhao G, Liu Z, Yu M, Fang Z, et al. Hepatocellular iNOS protects liver from ischemia/reperfusion injury through HSF1-dependent activation of HSP70. Biochem Biophys Res Commun 2019;512:882–888. [DOI] [PubMed] [Google Scholar]
- [250].Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology 2010;51:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Song Z, Humar B, Gupta A, Maurizio E, Borgeaud N, Graf R, et al. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J Pineal Res 2018;65:e12486. [DOI] [PubMed] [Google Scholar]
- [252].Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005;201:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Koh WU, Kim J, Lee J, Song GW, Hwang GS, Tak E, et al. Remote Ischemic preconditioning and diazoxide protect from hepatic ischemic reperfusion injury by inhibiting HMGB1-induced TLR4/MyD88/NF-kappaB signaling. Int J Mol Sci 2019;20:5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Huang H, Nace GW, McDonald KA, Tai S, Klune JR, Rosborough BR, et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology 2014;59:1984–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Barrientos L, Bignon A, Gueguen C, de Chaisemartin L, Gorges R, Sandre C, et al. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J Immunol 2014;193:5689–5698. [DOI] [PubMed] [Google Scholar]
- [256].Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 2012;198:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Yazdani HO, Chen HW, Tohme S, Tai S, van der Windt DJ, Loughran P, et al. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015;62:600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J 2015;282:259–270. [DOI] [PubMed] [Google Scholar]
- [260].Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 2011;54:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Huang H, Chen HW, Evankovich J, Yan W, Rosborough BR, Nace GW, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 2013;191:2665–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Wu HH, Huang CC, Chang CP, Lin MT, Niu KC, Tian YF. Heat shock protein 70 (HSP70) reduces hepatic inflammatory and oxidative damage in a rat model of liver ischemia/reperfusion injury with Hyperbaric oxygen preconditioning. Med Sci Monit 2018;24:8096–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Chen SW, Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury. Transplantation 2009;87:1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Motino O, Frances DE, Casanova N, Fuertes-Agudo M, Cucarella C, Flores JM, et al. Protective role of hepatocyte cyclooxygenase-2 expression against liver ischemia-reperfusion injury in mice. Hepatology 2019;70:650–665. [DOI] [PubMed] [Google Scholar]
- [265].van Eden W, Spiering R, Broere F, van der Zee R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 2012;17:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Hangai S, Ao T, Kimura Y, Matsuki K, Kawamura T, Negishi H, et al. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc Natl Acad Sci U S A 2016;113:3844–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest 2015;125:4255–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Johnsson C, Tufveson G. Serum hyaluronan-α potential marker ofcardiac allograft rejection? J Heart Lung Transplant 2006;25:544–549. [DOI] [PubMed] [Google Scholar]
- [269].Yoshida O, Dou L, Kimura S, Yokota S, Isse K, Robson SC, et al. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl Immunol 2015;32:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Flohe S, Speidel N, Flach R, Lange R, Erhard J, Schade FU. Expression of HSP 70 as a potential prognostic marker for acute rejection in human liver transplantation. Transpl Int 1998;11:89–94. [DOI] [PubMed] [Google Scholar]
- [271].Kashiwadate T, Miyagi S, Hara Y, Akamatsu Y, Sekiguchi S, Kawagishi N, et al. Soluble thrombomodulin ameliorates ischemia-reperfusion injury of liver grafts by modulating the proinflammatory role of high-mobility group box 1. Tohoku J Exp Med 2016;239:315–323. [DOI] [PubMed] [Google Scholar]
- [272].Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, et al. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol 2006;176:7154–7158. [DOI] [PubMed] [Google Scholar]
- [273].Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol 2005;174:5040–5046. [DOI] [PubMed] [Google Scholar]
- [274].Claushuis TAM, van der Donk LEH, Luitse AL, van Veen HA, van der Wel NN, van Vught LA, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Kleb-siella pneumoniae-induced pneumonia-derived sepsis. J Immunol 2018;201:1241–1252. [DOI] [PubMed] [Google Scholar]
- [275].Engelmann C, Adebayo D, Oria M, De Chiara F, Novelli S, Habtesion A, et al. Recombinant alkaline phosphatase prevents acute on chronic liver failure. Sci Rep 2020;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Engelmann C, Sheikh M, Sharma S, Kondo T, Loeffler-Wirth H, Zheng YB, et al. Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure. J Hepatol 2020;73:102–112. [DOI] [PubMed] [Google Scholar]


