Abstract
Extracellular vesicles (EVs) have emerged as key mediators of cell-cell communication during homeostasis and in pathology. Central nervous system (CNS)-derived EVs contain cell type-specific surface markers and intralumenal protein, RNA, DNA, and metabolite cargo that can be used to assess the biochemical and molecular state of neurons and glia during neurological injury and disease. The development of EV isolation strategies coupled with analysis of multi-plexed biomarker and clinical data have the potential to improve our ability to classify and treat traumatic brain injury (TBI) and resulting sequelae. Additionally, their ability to cross the blood–brain barrier (BBB) has implications for both EV-based diagnostic strategies and for potential EV-based therapeutics. In the present review, we discuss encouraging data for EV-based diagnostic, prognostic, and therapeutic strategies in the context of TBI monitoring and management.
Keywords: biomarkers, diagnostics, exosomes, extracellular vesicles, inflammation, outcome, prognostics, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a complex, debilitating condition and global public health concern affecting an estimated 69 million people worldwide each year.1 TBI results in a diverse set of physical, cognitive, sensory, and emotional symptoms caused by a dynamic combination of the damage to tissue from the initial injury and secondary cellular and biochemical processes that follow the injury.2–4 The successful management of TBI, which varies greatly between individual patients, demands accurate diagnostics and personalized therapeutic strategies to promote neurological recovery and improved outcome for the unique combination of damage in each patient.
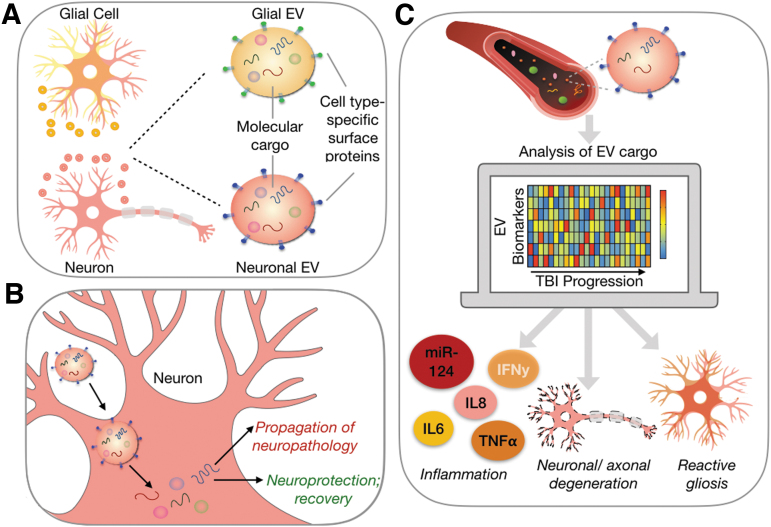
Extracellular vesicles (EVs), including exosomes, have generated enormous enthusiasm as new opportunities for both diagnosing and guiding the treatment of TBI. EVs derived from the central nervous system (CNS) participate in neuron-glial communication, neuroinflammation, and propagation of pathogenic proteins such as amyloid ß, all processes that drive the neurodegenerative microenvironment of evolving TBI pathology.5–7 The direct involvement of EVs in TBI progression and their expression of brain cell type-specific surface markers (Fig. 1A) makes them valuable potential biomarkers (Fig 1B).8–10 Because EVs can cross the blood–brain barrier (BBB) and remain intact, they are ideal biomarkers of processes of CNS injury and recovery.11 EV cargo can reflect the biomolecular state of their cells of origin,12–14 and therefore brain-derived EVs provide a direct window into the pathology and progression of TBI (Fig. 1C). Additionally, the internalization of EVs by recipient cells holds therapeutic potential for treatment of TBI. For example, EVs derived from mesenchymal stem cells demonstrate therapeutic potential by promoting cellular and functional recovery following TBI in animal models.15–19 Other studies utilize EVs' ability to cross the BBB to develop EV-based nanocarrier systems that deliver anti-inflammatory or neurotrophic factors into the inflamed CNS to treat neurodegenerative disease.20,21
FIG. 1.
EVs are promising biomarkers of traumatic brain injury. (A) EVs are nanoscale mediators of cell-cell communication possessing cell-type specific surface markers and a wide variety of molecular cargo. (B) EVs have been indicated in both processes of disease progression and recovery after TBI through their effects on target cells. (C) EVs are released by cells of the brain into the interstitial fluid where they can gain access to circulation allowing for a non-invasive assessment of the injured brain. A multi-dimensional, dynamic assessment of EV cargo isolated from multiple brain cell types could increase granularity of TBI monitoring by identifying underlying mechanisms of pathology. EV, extracellular vesicle; TBI, traumatic brain injury.
In this review we discuss the challenges to accurately diagnosing, prognosing, and treating TBI in the clinic, and how EVs provide a promising new opportunity to tackle these issues. In particular, we will focus on the role of previous work as setting the stage for future opportunities to use EVs as biomarkers to guide the treatment of TBI.22,14
The fundamental challenges of developing biomarkers for TBI
The variability of injury severity and the heterogeneity of the underlying mechanisms of TBI progression present challenges to both clinical trial design and development of accurate diagnostic, prognostic, and predictive molecular biomarkers of TBI.23–33 Once considered primarily an acute condition, mild TBI (mTBI) is now recognized as a progressive disease34,35 that leads to the development of sequelae ranging from mood36 and sleep37 disturbances to post-traumatic epilepsy38 in 10–20% of patients.35 For moderate and severe TBI, survivors are left with significant neurological impairments that contribute career transitions, disruptions in lifestyle, and significant economic burden.39–41
Dysfunction of the neurovascular unit following TBI arises through the injury and increased reactivity of the endothelial cells, astrocytes, pericytes, and microglia that regulate blood flow to the surrounding parenchyma. Microstructural endophenotypes associated with TBI, such as vascular damage, axonal shearing, and intracranial hemorrhage, each have varying contributions to molecular and functional pathology across patients.42 Gliosis following TBI triggers complement and inflammasome activation and chemokine and cytokine release, which occur to recruit immune cells to sites of damage to clear cellular debris and return the brain to homeostasis.43–45 When left unresolved, however, the continued release of free radicals and reactive oxygen species that occurs during the inflammatory response has damaging effects on surrounding neurons and endothelial cells. The consequences of unresolved neuroinflammation include edema, BBB disruption, exacerbation of excitotoxicity, increased parenchymal metabolic demand, and decreased cerebral blood flow.46–48 The complex interplay between damaged brain cell types thus drives the progression of neurodegeneration that contributes to the development of cognitive deficits post-TBI.41,49,50
The current clinical classification schemes for TBI fail to completely encompass underlying causes of the pathologies. For this reason, it is difficult to use these clinical classifications alone to effectively treat TBI. For example, TBIs can be classified on the conditions associated with the injury (falls, assaults, collisions, etc.), functional outcomes such as the Glasgow Coma Scale (GCS), or by structural damage indicated by imaging techniques.51 The score of the GCS, a scale that assesses the patient's level of consciousness through motor, eye, and verbal responses, duration of unconsciousness, and extent of amnesia, is used to categorize the patient's injuries as either mild, moderate, or severe.52 However, these terms are currently being challenged as insufficient representations of TBI heterogeneity.53 Additionally, imaging is often performed to identify cerebral lesions and abnormalities to assess severity, operability, and to determine injury localization to guide surgical planning if necessary.54 Traditional imaging modalities, however, offer little guidance for therapeutic treatment.
Although GCS and imaging-based biomarkers of TBI are standard clinical measures, molecular biomarkers derived from liquid biopsy overcome many of the limitations associated with these measures. Computed tomography (CT) imaging, the initial modality used during acute diagnosis of TBI, provides only an assessment of macro-scale anatomical changes most characteristic of severe TBI such as brain bleeds and lesions, but cannot resolve other TBI endophenotypes such as inflammation, gliosis, and diffuse axonal injury.23,55 Even though magnetic resonance imaging (MRI) can be used to assess regions of increased brain activity, altered cerebral blood flow, and endophenotypes such as axonal and microvascular pathology, its high cost and inaccessibility limits its use for repeated monitoring of TBI progression.56 Additionally, both GCS and imaging techniques are inadequate for characterization and prognosis of mTBI, which may present with a “healthy” appearing GCS score, and no obvious structural damage.34,57 Indeed, mTBI presents a unique diagnostic challenge because it often includes microscopic axonal and vascular injury patterns that affect biochemical, metabolic, and cellular homeostasis, but these patterns are difficult to detect completely. With the widespread distribution of damage, this damage at the microscopic level may play a role in the development of long-term neurological deficits exhibited in post-concussion syndrome.58
As an alternative to imaging and behavioral diagnostics, liquid biopsy is minimally invasive and can provide an accessible metric for the biochemical and molecular changes occurring in neurons and glia throughout the course of TBI. Blood-based biomarkers that cross the BBB even during periods of relative integrity could be particularly beneficial for the assessment of mTBI and would also provide a cost-effective alternative to imaging techniques for assessing TBI of all severities. To this end, several past studies correlate blood-based biomarkers of TBI pathology with patient outcome, often classifying patients with TBI based on specific endophenotypes.59
The state of the art of blood-based biomarkers for TBI
To date, the most widely studied blood-based biomarkers of TBI have been proteins that are released into circulation as a result of acute pathological processes occurring within 24 h of injury.60,61 For example, glial fibrillary acidic protein (GFAP), an intermediate filament protein expressed in astrocytes, and ubiquitin c-terminal hydrolase L1 (UCHL1), a neuronal cytosolic protein, successfully identify injury severity in acute TBI.62 The general concept of this approach is that injured astrocytes and neurons release GFAP and UCHL1, respectively, into interstitial fluid where these proteins gain access to cerebrospinal fluid (CSF) and then enter systemic circulation through the potentially compromised BBB.63,64 In human studies, receiver operating characteristic (ROC) analysis of GFAP and UCHL1 yielded area under the curve (AUC) of 0.87 and 0.91 for GFAP and UCHL1, respectively, in discriminating patients with mTBI from healthy controls.62 Further, these markers yield AUCs of 0.71 and 0.84 for GFAP and UCHL1, respectively, for discriminating mTBI patients with and without CT scan abnormalities,62,63 demonstrating their use in assessing injury severity.
Although serological markers such as GFAP and UCHL1 hold promise as acute TBI diagnostics of injury severity, several limitations exist. Investigators showed that these markers—like GCS score and imaging modalities—are inadequate for predicting recovery 6 months after mTBI with AUCs of only 0.51 and 0.61 for GFAP and UCHL1, respectively.62 Additionally, these protein markers show transient elevations in the peripheral circulation, likely a result of BBB disruption caused by neuroinflammation and gliosis,65,66 edema,67 and microvascular disruption.68 In addition, serological markers (e.g., GFAP and UCHL1) are not directly indicative of these underlying pathological processes, a potential reason for their limited utility as predictive biomarkers of specific TBI sequelae. Lastly, UCHL1 is not CNS-specific and is expressed by cells of the peripheral nervous system, some tumor cells, cells of the endocrine system, and smooth muscle cells. As a result, the lack of cell-specificity for UCHL1 may limit its use in blood as a TBI-specific biomarker.69,70
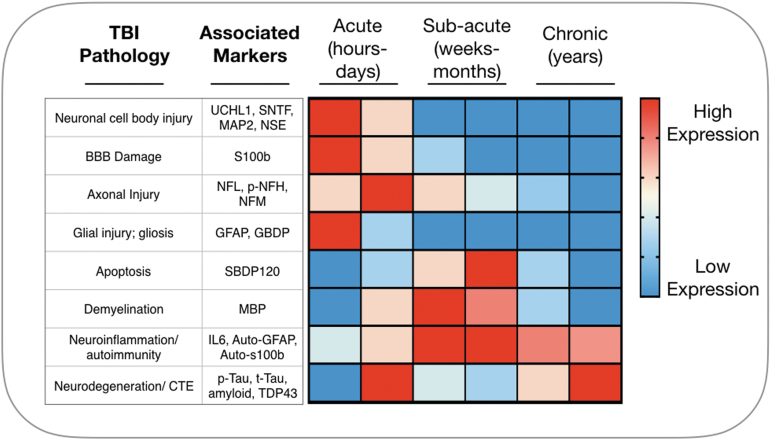
Similar to GFAP and UCHL1, the serological and CSF presence of other brain-derived proteins such as neurofilament light polypeptide (NFL),71 neuron-specific enolase (NSE),72 myelin basic protein (MBP),73 calpain-cleaved αII-spectrin N-terminal fragment (SNTF),74 tau,75 microtubule-associated protein 2 (MAP2),76 and others following cell injury and death have been linked to specific phases of injury progression (Fig. 2), but they have demonstrated limited diagnostic and prognostic potential.77 These proteins exhibit low concentration in circulation (fM–pM), rapidly degrade in the liver and kidney,71 and bind to plasma protein; all of these factors decrease their bioavailability. Further, the unique combination of pathological mechanisms and recovery trajectories for each TBI makes it unlikely that a single protein biomarker will be diagnostically and prognostically useful for all patients with TBI.78 Together, the small number of candidate serum protein markers, in combination with their relative lack of molecular specificity for determining prognostic outcomes has led many to search for alternative measurement platforms for predicting outcome after TBI.
FIG. 2.
The current gold standard in blood-based biomarkers of TBI are brain-derived markers of acute neuronal and glial cell injury. The release of brain-derived proteins into systemic circulation is dependent on BBB disruption and the extent of cell injury and death.39 These markers are thus more indicative of injury severity than of specific underlying mechanisms of TBI progression. BBB, blood–brain barrier; TBI, traumatic brain injury.
Monitoring TBI pathology with EV-based biomarkers
EVs are membranous vesicles released from essentially all cell types of the brain possessing different sizes and origins (Table 1). Apoptotic bodies are large EVs (50–4000 nm) containing DNA from dying cells that are released by cells undergoing apoptosis that can then be taken up by neighboring cells.79,80 Microparticles (MPs), another subset of EVs, are formed from outward budding of the plasma membrane and are 100–1000 nm in diameter. MP membranes contain lipid microdomains containing cholesterol, phospholipids, and receptors and are heterogeneous in shape.81 Exosomes are distinguished from apoptotic bodies and MPs by their smaller size (30–100 nm), origin, homogeneous shape, and by the presence of endosome-specific surface markers and RNA and protein cargos.82 Unlike other EVs that are released from the plasma membrane, exosome production begins with plasma membrane invagination to form endosomes. Invagination of endosomes leads to formation of intraluminal vesicles (ILVs) within multi-vesicular bodies (MVBs). MVBs can then be trafficked to lysosomes for degradation or to the plasma membrane to release ILVs into the extracellular space. Once ILVs are released into the extracellular space they are termed exosomes.82
Table 1.
Characteristics of Extracellular Vesicles
| Vesicle type | Markers and cargo | Size and origin | Sources |
|---|---|---|---|
| Apoptotic bodies | Integrins, selectins, cell-specific markers; proteins, MHC 1 and 2, lipid rafts, targeting and adhesion proteins, RNAs (mRNAs, miRNAs, circRNAs, lncRNAs) from apoptotic cells | 40-4000 nm diameter; budding of plasma membrane by cells undergoing apoptosis | Henson et al.79; Bergsmedh et al.80 |
| Microparticles/Microvesicles | Integrins, selectins, cell-specific markers; proteins, MHC 1 and 2, lipid rafts, targeting and adhesion proteins, RNAs (mRNAs, miRNAs, circRNAs, lncRNAs) | 100-1000 nm; budding of plasma membrane by healthy cells | Del Conde et al.81 |
| Exosomes | Surface markers, integrins; proteins, MHC 1 and 2, lipid rafts, targeting and adhesion proteins, RNAs (mRNAs, miRNAs, circRNAs, lncRNAs) | 30-100 nm; formation of MVBs within endosomes and subsequent release of intralumenal vesicles into extracellular space | Denzer et al.82 |
EVs are a heterogeneous population of membranous structures derived through a variety of mechanisms. Although there is overlap in vesicle size and expression of vesicular proteins across the different types of EVs, generally exosomes are distinguished from microparticles and apoptotic bodies by their smaller size and endocytic origin.59–62
circRNA, circular RNA; EV, extracellular vesicle; lncRNA, long non-coding RNA; mRNA, messenger RNA; miRNA, microRNA; MVB, multi-vesicular bodies.
EVs exhibit multiple properties that make them ideal potential biomarkers of neurological disease. Unlike surrogate protein biomarkers of acute TBI pathology, EVs are directly implicated in driving both homeostatic processes and processes of recovery and pathology in the injured CNS throughout disease progression.6,7 For example, a 2008 study demonstrated for the first time that exosome-mediated transport of RNAs and proteins between cells can occur, and these transferred exosomes were associated with neuropathology in glioblastoma.83 EVs cross the BBB into peripheral circulation and have been isolated from nearly all bodily fluids including blood and CSF,11,84 allowing for non-invasive assessment of the state of the injured and recovering CNS. Nearly all cells of the brain release EVs with cargo molecules reflective of their origin cells that cross the BBB and remain protected from degradation.6 Thus, isolation of EVs derived from multiple brain cell types followed by targeted assessment of specific EV cargo could improve our ability to identify specific TBI endophenotypes such as neuroinflammation, axonal injury, and neurodegeneration.
Most efforts to develop EV-based biomarkers of TBI have focused on using the presence of traditionally studied proteins such as UCHL1, tau, and amyloid ß found within EVs as diagnostics and as predictors of outcome and neurological deficits following TBI.85,86 Additionally, EV-based predictive biomarker work has focused on chronic traumatic encephalopathy (CTE) and neurodegeneration, the most debilitating potential outcomes of TBI. CTE is a condition that could result from repetitive mTBI; a single, severe TBI; or a series of impacts that do not cause concussion. CTE is characterized by unique disbursement of tau pathology that distinguishes it from other neurodegenerative conditions such as Alzheimer's disease (AD).87 Elevations in plasma and CSF levels of tau occur acutely after TBI,88 but although plasma and CSF proteins have not served as adequate biomarkers of CTE,89,90 many believe EV-associated proteins may provide a more accurate assessment of neurodegeneration.
These studies demonstrate that measuring a diverse array of brain-derived EV molecular cargo may provide a more dynamic view of the pathological processes that drive TBI sequelae such as CTE. For example, although increased neuron-derived exosome levels of neurofunctional proteins such as UCHL1 and occludin have been transiently observed in acute mTBI, elevations in exosome levels of pathogenic isoforms of tau and amyloid are exhibited in both acute and chronic mTBI91 and in veterans with histories of 3+ TBIs.86 Additionally, exosome tau correlates with post-TBI severity, progression, and neuropsychiatric and behavioral symptoms in US veterans.85,86 Moreover, exosomal tau levels distinguish controls from former National Football League players, a group with increased risk of developing CTE,92 with 82% sensitivity and 100% specificity.89 In addition to the potentially predictive abilities of exosomal tau, exosomal interleukin 10 (IL-10) levels correlate with behavioral symptoms following TBI in military personnel. These studies demonstrate the potential for EVs to serve as biomarkers of TBI progression that persist from the acute to the chronic phases of injury, and the potential utility of analyzing multiple types of exosomal cargo to fully capture the nature of TBI progression into specific sequelae.
Although neuron-derived exosomal levels of neurodegenerative proteins appear frequently in past efforts to develop EV-based diagnostics, EVs from other cell types are now implicated in other processes post-injury.6,93 For example, microglial-derived exosome-associated miR-124 plays a role in recovery post-injury by promoting neurite outgrowth,94 inhibiting neuronal autophagy,95 reducing expression of pro-inflammatory mediators,94,96 and increasing expression of anti-inflammatory factors.96 Conversely, protein and microRNA (miRNA) cargo from astrocyte-derived EVs promotes the peripheral acute cytokine response, a process that occurs in the periphery to induce transmigration of peripheral leukocytes into the brain following injury.97–99
Additionally, astrocyte-derived EVs isolated from patients with AD exhibit increased expression of ßACE1 and pathological tau and amyloid ß isoforms, implicating them in processes of neurodegeneration.100 EVs isolated from TBI and spinal cord injury patient CSF express inflammasome proteins such as ASC, NLRP1, and caspase, further implicating EVs in activation of inflammatory signaling following CNS injury.101 Lastly, a recent study found that complement protein levels were 12- to 35-fold higher in astrocyte-derived exosomes isolated from patient plasma than in neuron-derived exosomes, and that these alterations persisted for 1–4 years following injury.102 These studies demonstrate not only the potential role of EVs in mediating neuroinflammation, but also the practicality of acquiring biomarkers from multiple biofluid components, including from different EV populations. Thus, as our understanding of the role EVs play in neuropathology begins to expand, so too does the potential for identifying EV-based biomarkers of the specific TBI endophenotypes that contribute to patient outcome and target individualized therapeutic interventions.
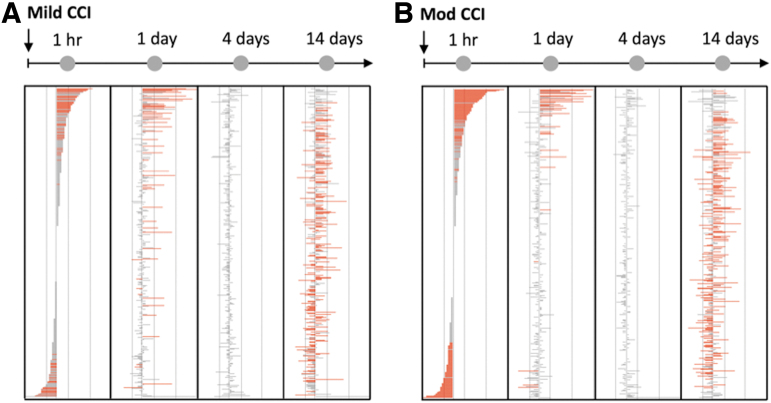
In addition to the study of specific EV-associated cargo, multi-dimensional transcriptomic and proteomic analyses of EVs expand the focus from conventional markers of neuronal and glial damage to a more directed, dynamic, and individualized assessment of processes of brain injury and recovery. Mass spectrometry analysis of protein cargo from EVs derived from TBI patient CSF exhibit elevations not only in conventionally studied cytoskeletal markers such as MAP2 and GFAP, but also differential expression of neurite outgrowth-related and synaptic proteins, homeostatic and cell signaling regulators, and proteins involved in cell death and proteolysis (Fig. 3).103 Using miRNA sequencing of GluR2+ EVs, another study illustrated that brain-derived EVs isolated from a mild (Fig. 4A) and moderate (Fig. 4B) TBI mouse model exhibited differential expression of miRNAs involved in 56 different pathways including long-term potentiation, neurotransmitter signaling, and intracellular signaling pathways, in addition to unique pathways implicated in processes such as cancer.104 These studies demonstrate the expansive diversity of molecular cargo expressed in easily accessible samples of EVs that could provide a wealth of molecular and biochemical information for assessing TBI diagnosis, progression, and target pathways for therapeutic intervention.
FIG. 3.
CNS-derived EVs express molecular cargo involved in a wide variety of cellular functions. Proteomic analysis of microvesicles isolated from the CSF of patients with TBI revealed differential expression of proteins involved in a wide variety of signaling pathways.81 These data provide rationale for the analysis of multiple types of EV cargo for assessment of underlying mechanisms of TBI pathology. CSF, cerebrospinal fluid; CNS, central nervous system; EV, extracellular vesicle; TBI, traumatic brain injury.
FIG. 4.
Brain-derived EVs exhibit dynamic changes in miRNA in a mouse model of injury. Brain-derived EVs isolated from the plasma of mice undergoing both (A) mild CCI and (B) moderate CCI exhibited differential expression of miRNAs compared with sham injured mice.82 These data further demonstrate the potential of brain-derived EVs for monitoring the course of TBI progression. CCI, controlled cortical impact; EV, extracellular vesicle; miRNA, microRNA; TBI, traumatic brain injury.
Although EVs have demonstrated promise as diagnostic and predictive biomarkers of TBI, there remain many understudied avenues within this field. First, the correlation between specific EV populations (e.g., vesicles released from reactive astrocytes) and the burden of specific TBI endophenotypes (e.g., reactive gliosis) is not available; completing a study of this correlation could greatly improve clinical trial design and development of targeted therapeutics. More broadly, because EVs are shed from nearly all brain cell types, they could be used to identify and distinguish neuronal pathology, gliosis, and axonal and vascular damage by using an appropriate EV surface marker selection followed by a targeted analysis of specific classes of EV cargo. However, it is likely that the cellular localization of a miRNA or protein of interest has an impact on the amount and timing of its packaging into EVs. There remains an incomplete understanding of how some molecules are designated for internalization into EVs over others and how CNS injury alters this process.
Second, the use of EVs as predictive markers of specific TBI sequelae outside of CTE and neurodegeneration such as epilepsy, sleep disturbances, and specific mood and emotional disorders has been understudied. Last, the development of a TBI diagnostic that combines information from multiple biomarker types (e.g., serologic proteins, EV-associated proteins, and miRNAs) that provide orthogonal information about the nature of brain injury could further increase specificity of TBI assessment. Success in these areas of biomarker development could transform the way TBI is currently assessed and treated clinically by allowing for increased granularity of TBI diagnosis and a more personalized approach to TBI management.
From bench to bedside: Clinical implications of EV-based diagnostics
Brain-derived EVs shed by injured neurons and glia that cross the BBB and enter systemic circulation provide a unique opportunity to assess the complexity and heterogeneity of CNS injury through a multi-dimensional analysis of pathogenic and protective molecules obtained non-invasively. However, clinical utilization of EV-based biomarkers for TBI diagnostics requires innovative approaches for the rapid isolation of specific EV populations such that point-of-care systems may be introduced directly to clinicians. The most commonly used strategies of EV isolation are time-consuming, requiring days of ultracentrifugation, and often lead to samples contaminated with cellular debris.105,106 To address these problems, miniaturized microfluidic platforms developed recently greatly increase the sensitivity and specificity of EV isolation. Several recent reviews outline the progress made with microchip development for isolation of EVs.107,108 These low-cost platforms allow for more time-efficient, higher-throughput isolation of brain-derived EVs. When combined with the advancements made in next-generation sequencing and digital enzyme-linked immunosorbent assay (ELISA) techniques for biomarker detection, these platforms allow for the rapid quantification of hundreds of biomarkers from relatively small volumes of sample.
In addition to the advancements required in EV isolation strategies, deciphering this complex wealth of molecular information requires computational tools to combine multiple biomarkers into signatures of specific disease states to increase the specificity of TBI diagnosis.109 To address these challenges, machine learning is commonly used to detect signatures of pathology from liquid biopsy approaches.109,110 Rather than relying on a single molecular biomarker, a technique that does not address the heterogeneity of TBI pathology across individuals, machine learning provides a method for combining measurements of different molecular biomarkers across patients that together can be used as signatures to discriminate specific TBI endophenotypes.78 If assessed at different time-points throughout TBI progression, the machine learning algorithms provide a method for monitoring patient recovery and response to therapeutic intervention. Machine learning analysis can be accomplished through the use of any number of different algorithms such as LASSO and random forest with results that often outperform the sensitivity and specificity of single biomarkers.109,111,112
Several studies have used machine learning approaches with clinical data to successful predict patient outcome.113,114 In a 2018 clinical study of more than 500 pediatric patients with TBI, investigators developed an artificial neural network model using machine learning to combine CT scan parameters (e.g., presence of hemorrhage) with clinical measures (e.g., blood glucose level and GCS score)114 to discriminate between favorable and unfavorable outcome at 6 months with an AUC of 0.9774 compared with an AUC of 0.748, the highest AUC obtained using a score based on a logistic regression model.
The few studies using machine learning techniques to analyze multi-plexed liquid biopsy and diagnostic data for disease diagnostics demonstrate the promise that this approach holds. In a study by Ko and colleagues, the investigators demonstrated the potential diagnostic utility of machine learning analysis of brain-derived EVs isolated with a microfluidic platform.115 Brain-derived EVs were isolated based on their expression of GluR2, an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit, followed by analysis of the exosomal miRNA. By using machine learning to combine multiple differentially expressed miRNA biomarkers into a blood-based diagnostic, investigators were able to distinguish a heterogeneous population of mice injured at different severities and time-points from control mice with an AUC of 1 and accuracy of 99%. Further, investigators were able to use this approach to accurately predict specific features of injury including severity, time elapsed since injury, and different histories of injury. Lastly, this approach was used to discriminate patients with TBI from controls with an AUC of 0.9. Several studies have also demonstrated the promise of machine learning analysis of EV markers for the diagnosis of certain cancers.116–120
Despite the promise that machine learning techniques hold for improving the assessment of TBI, precautions must be taken to reduce the risk of overfitting, a common challenge with small data sets (n < 50) in which a similar number of biomarkers are measured.121 Additionally, the use of machine learning with clinical data requires unique strategies for protecting patient privacy and confidentiality. Another major concern among neurologists and pharmacologists is that the results of machine learning analysis of liquid biopsy data may not be easily correlated with actual mechanisms of pathology,122 and it is unclear whether this would limit the utility of this approach for guiding specific therapeutic interventions. For example, it is possible that biomarkers deemed significant by an algorithm may result from processes downstream from the actual pathology being assessed, and for this reason caution should be taken when applying machine learning approaches.109
More specifically, deciphering and monitoring neurotrauma using an EV-based approach could prove difficult depending on the phase of injury and the timing of onset of specific pathologies. Some studies have demonstrated that at various time-points following TBI, there are alterations in EV cargo such as miRNAs102,104 and specific proteins such as complement components, but how these changes relate to and signify specific mechanisms of TBI progression has yet to be determined. These questions necessitate studies that directly correlate the TBI-induced activation and progression of pathological cascades occurring during inflammation and neurodegeneration with the downstream packaging and release of EVs.
Another concern is the potential difficulty with relating the EV level of a biomarker of interest to its level in the brain-derived cell of origin. It remains unclear how certain protein and miRNA cargo are packaged into EVs and whether, for a specific cargo type, this loading occurs immediately after injury or on a longer time scale. There may also be differences in the ability of a specific biomarker of interest to cross the BBB while packaged into EVs compared with the same molecule released directly from its cell of origin. Additionally, processes of preferential loading and release of damaged proteins via EVs could result in a higher EV concentration of a biomarker compared with its concentration in its cell of origin.
Despite these challenges, investigators have shown that even when EV cargo does not exactly reflect biomarker alterations of its host cells, EV analysis can still provide useful information for characterizing disease. A study by Hoshino and associates found that exosomes isolated from metastatic cells expressed a unique integrin not representative of the host tumor cell integrin expression.123 The authors concluded that this finding is consistent with a process of selective packaging into EVs as a result of the disease state. These data suggest that, when combined with other techniques such as imaging and GCS evaluation, EVs could provide a more specific assessment of the underlying mechanisms of pathology and recovery following CNS injury.
Other clinical uses of EVs: EV-based therapeutic strategies
Despite decades of pre-clinical and clinical research, the successful development of pharmacological strategies for improving patient outcome following TBI remains limited. The range of injury severity, variation in onset of pathology across patients, the complexity of the underlying mechanisms of TBI progression, and the presence of comorbidities all contribute to the difficulty in developing personalized approaches for treating TBI sequelae.124–126 Increased understanding of the complexity of TBI pathology renewed interest in the development of mechanism-based approaches to targeting TBI pathology. Targets of therapeutic intervention explored include excitotoxicity and neuronal death, inflammation, axonal injury, cognitive enhancement, augmentation of endogenous systems of neuroprotection, and cellular therapies, among other approaches.126–127
Recent studies indicate that stem cells promote neurogenesis and functional recovery in neurological conditions such as Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis (ALS), with mesenchymal stem cells (MSCs) as the most widely studied platform.128–133 However, the primary mechanism of MSC-mediated regeneration and neurogenesis is not through engraftment and differentiation of MSCs themselves but through paracrine-mediated signaling through molecules secreted by MSCs, including their secreted EVs.134,135 For example, several studies have demonstrated that administration of cell-free exosomes isolated from MSCs is sufficient for promoting therapeutic effects following TBI.15,16,136,137
Administration of MSC-derived EVs promotes recovery following TBI through a combination of mechanisms. A study by Ni and co-workers demonstrated that MSC-derived exosomes administered following controlled cortical impact (CCI) in mice led to decreased expression of pro-apoptotic factor BAX and pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and IL-1b while promoting expression of anti-apoptotic factor BCL2 compared with vehicle control.16 Treatment with MSC-derived exosomes also promoted decreased microglial inducible nitric oxide synthase (iNOS) expression and increased expression of CD206 and arginase-1, two anti-inflammatory molecules. In another study, administration of MSC-derived EVs following CCI in rats promoted angiogenesis and neurogenesis.137 Finally, MSC-derived EVs also promote increased expression of neurotrophic factors such as nerve growth factor (NGF).138
In addition to delivering stem-cell derived exosomes for repair of the injured brain, the crossing of exosomes across the BBB also point to an opportunity for using exosomes as a delivery vehicle for therapeutic molecules. Even with systemic intravenous (IV) administration, a method producing nearly 100% bioavailability for pharmacologics targeting tissues outside the CNS, the BBB acts as a major obstacle preventing the entry of 98% of drugs.139 BBB dysfunction in combination with the inflammation associated with TBI promotes migration of peripheral immune cells across the BBB.140 The ability of naive macrophages able to cross the BBB through diapedesis requires the use of certain proteins such as lymphocyte function-associated antigen-1 (LFA-1), which can also be found in the exosomes isolated from these cells.141
The discovery that EVs can cross the BBB has led to innovative studies in which they are used to deliver potentially therapeutic agents to the inflamed brain. In one such study, exosomes from naive macrophages were used to deliver brain-derived neurotrophic factor (BDNF) to the inflamed brain resulting in greater accumulation of BDNF in the brain than when BDNF was injected alone.20 In another study, the same group demonstrated that administration of exosomes loaded with catalase-encoded plasmids led to inhibition of inflammation and reactive oxygen species (ROS) production.21
The lack of success in clinical trials for potential TBI therapies is partially due to the inability of animal models to fully replicate the complexity of TBI, but also likely due to the inability of targeting one pathological process to be effective for the treatment of all patients. Although these results seem promising, the investigation of the therapeutic potential of MSC-derived EVs is still in its infancy, and these results must still be replicated before translation to clinical trials. If successful replication of this work is completed across different TBI animal models, MSC-derived EVs hold potential as multi-faceted pharmacologics targeting multiple mechanisms of TBI pathology to promote regeneration and functional recovery.
Conclusion
EVs have demonstrated great potential for guiding the field of TBI diagnostics and therapeutic intervention. As our understanding of the roles that EVs play in TBI pathology is only beginning to grow, there are many opportunities to drive this field into new arenas. For example, in addition to markers of TBI diagnosis, prognosis, and outcome, EVs also have the potential to be used to advance personalized medicine approaches for TBI therapy by performing companion diagnostics for drugs being investigated in clinical trials. To identify what treatment is likely to be most efficacious for an individual patient with TBI, biomarkers are needed to identify what specific targetable pathological processes contribute most to that patient's disease. Thus, biomarkers are essential for advancement of personalized medicine in TBI management.
An example from cancer therapy illustrates the utility of companion biomarkers in the evaluation of potential therapies and the value of increased granularity of disease classification. The first companion biomarker to be widely used in cancer therapy was the estrogen receptor (ER) for breast cancer.142,143 Administration of tamoxifen to ER-positive breast cancer patients decreases recurrence rates by almost 50%.144 Further, analysis of HER2 levels in ER-negative breast cancer patients can direct clinicians to administer Herceptin, a drug that has prolonged disease progression (from 4.6 to 7.4 months), increased drug response rate (from 32% to 50%), and increased survival (from 20 to 25 months).145 Importantly, although both ER-positive and ER-negative breast cancers share similarities and affect the same tissue, each is driven by different hormone signaling cascades, expresses different biomarkers, and exhibits different responses to different therapies.
Similarly, the increased granularity in TBI diagnosis achieved by accurate assessment of distinct TBI endophenotypes may result in improved ability to develop companion biomarkers and targeted TBI therapies. Rather than grouping vastly dissimilar instances of “moderate” TBI via GCS score, for example, by using biomarkers to separate patients into categories such as “moderate TBI with microvascular damage,” or “moderate TBI with diffuse axonal injury” we may come closer to developing effective TBI therapies and prognostic biomarkers for each condition.
Although the field has progressed, there is still much about the function of EVs in TBI pathology that remains unknown. There are many different populations of EVs released from all cell types of the brain; current research in the TBI field focuses on a subset of these cell types. Further, it is unclear how different populations of EVs interact within the same cell type to promote recovery or injury progression. More broadly, the role of exosomes in driving interactions between the CNS and the periphery following injury is largely unknown. Based on these and other remaining questions in the field, we expect that the continued study of EVs will result in innovations in TBI diagnostics and therapeutic strategies.
Funding Information
Funding was provided by the New Jersey Commission on Brain Injury Research (CSCR14IRG005) to DI and DM, National Institutes of Health (NS 088276) to DM, Allen Foundation to DM and DI, Health Research Formula Fund (4100077073) from Commonwealth of Pennsylvania to DI, the Pennsylvania Department of Health Commonwealth Universal Research Enhancement Program, the National Institutes of Health (1R21CA182336-01A1) to DI, and DI was supported by an American Cancer Society-CEOs Against Cancer-CA Division Research Scholar Grant (RSG-15-227-01-CSM), the National Science Foundation's CAREER Award (#1554200), The Hartwell Individual Research Award, and the Congressionally Directed Medical Research Program Award (W81XWH-19-2-0002).
Author Disclosure Statement
David Issadore is a founder and holds equity in Chip Diagnostics, a start-up company spun out of his lab.
References
- 1. Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung YC., Punchak M., Agrawal A., Adeleye A.O., Shrime M.G., Rubiano A.M., Rosenfield J.V., and Park K.B. (2019). Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097 [DOI] [PubMed] [Google Scholar]
- 2. Werner C., and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 3. Kaur P., and Sharma S. (2018). Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 16, 1224–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prins M., Greco T., Alexander D., and Giza C.C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis. Model Mech. 6, 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sardar Sinha M., Ansell-Schultz A., Civitelli L., Hildesjö C., Larsson M., Lannfelt L., Ingelsson M., and Hallbeck M. (2018). Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 136, 41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frühbeis C., Fröhlich D., Kuo W.P., and Krämer-Albers E.M. (2013). Extracellular vesicles as mediators of neuron-glia communication. Front, Cell Neurosci. 7, doi: 10.3389/fncel.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y., Boza-Serrano A., Dunning C.J.R., Clausen B.H., Lambertsen K.L., and Deierborg T. (2018). Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflammation. 15, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mondello S., Thelin E.P., Shaw G., Salzet M., Visalli C., Cizkova D., Kobeissy F., and Buki A. (2018). Extracellular vesicles: pathogenetic, diagnostic and therapeutic value in traumatic brain injury. Expert Rev. Proteomics 15, 451–461 [DOI] [PubMed] [Google Scholar]
- 9. Hazelton I., Yates A., Dale A., Roodselaar J., Akbar N., Ruitenberg M.J., Anthony D.C., and Couch Y. (2018). Exacerbation of acute traumatic brain injury by circulating extracellular vesicles. J. Neurotrauma 35, 639–651 [DOI] [PubMed] [Google Scholar]
- 10. Paolicelli R.C., Bergamini G., and Rajendran L. (2019). Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405, 148–157 [DOI] [PubMed] [Google Scholar]
- 11. Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A., Riazifar M., Pham V., Digman M.A., Pone E.J., and Zhao W. (2016). Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng. 9, 509–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko J., Hemphill M.A., Gabrieli D., Wu L., Yelleswarapu V., Lawrence G., Pennycooke W., Singh A., Meaney D., and Issadore D. (2016). Smartphone-enabled optofluidic exosome diagnostic for concussion recovery. Sci. Rep. 6, 31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karnati H.K., Garcia J.H., Tweedie D., Becker R.E., Kapogiannis D., and Greig N.H. (2018). Neuronal enriched extracellular vesicle proteins as biomarkers for traumatic brain injury. J. Neurotrauma 36, 975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor D.D., and Gercel-Taylor C. Exosome platform for diagnosis and monitoring of traumatic brain injury. (2014). Philos. Trans. R. Soc. B. Biol. Sci. 369, 20130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiong Y., Mahmood A., and Chopp M. (2017). Emerging potential of exosomes for treatment of traumatic brain injury. Neural. Regen. Res. 12,19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni H., Yang S., Siaw-Debrah F., Hu J., Wu K., He Z., Yang J., Pan S., Lin X., Ye H., Xu Z., Wang F., Jin K., Zhuge Q., and Huang L. (2019). Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front. Neurosci. 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams A.M., Dennahy I.S., Bhatti U.F., Halaweish I., Xiong Y., Chang P., Nikolian V.C., Chtraklin K., Brown J., Zhang Y., Zhang Z.G., Chopp M., Buller B., and Alam H.B. (2018). Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma. 36, 54–60 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., and Xiong Y. (2015). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim D.K., Nishida H., An S.Y., Shetty A.K., Bartosh T.J., Prockop D.J. (2016). Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. U. S. A. 113, 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan D., Zhao Y., Banks W.A., Bullock K.M., Haney M., Batrakova E., and Kabanov A.V. (2017). Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., and Batrakova E.V. (2015). Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Controlled Release 207, 18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chopp M., and Zhang Z.G.Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. (2015). Expert Opin. Emerg. Drugs 20, 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruce E.D., Konda S., Dean D.D., Wang E.W., Huang J.H., and Little D.M. (2015). Neuroimaging and traumatic brain injury: state of the field and voids in translational knowledge. Mol. Cell Neurosci. 66, 103–113 [DOI] [PubMed] [Google Scholar]
- 24. Dean P.J., and Sterr A. (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 12, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mouzon B.C., Bachmeler C., Ojo J.O., Acker C.M., Ferguson S., Paris D., Ait-Ghezala G., Crynen G., Davies P., Mullan M., Stewart W., and Crawford F. (2017). Lifelong behavioral and neuropathological consequences of repetitive mild traumatic brain injury. Ann. Clin. Transl. Neurol. 5, 64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 27. Carroll L.J., Cassidy J.D., Peloso P.M., Borg J., von Holst H., Holm L., Paniak C., and Pépin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre task force on mild traumatic brain injury. (2004). J. Rehabil. Med, Suppl. 43, 84–105 [DOI] [PubMed] [Google Scholar]
- 28. Godbolt A.K., Cancelliere C., Hincapié C.A., Marras C., Boyle E., Kristman V.L., Coronado V.G., and Cassidy J.D. (2014). Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: results of the International Collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 95, Suppl. 3, S245–S256 [DOI] [PubMed] [Google Scholar]
- 29. Lee Y.K., Hou S.W., Lee C.C., Hsu C.Y., Huang Y.S., and Su Y.C. (2013). Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 8, e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nordström A., and Nordström P. (2018). Traumatic brain injury and the risk of dementia diagnosis: a nationwide cohort study. PLoS Med. 15, e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marras C., Hincapié C.A., Kristman V.L., Cancelliere C., Soklaridis S., Li A., Borg J., afGeijerstam J.L., and Cassidy J.D. (2014). Systematic review of the risk of Parkinson's disease after mild traumatic brain injury: results of the International Collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 95, Suppl. 3, S238–S244 [DOI] [PubMed] [Google Scholar]
- 32. Lehman E.J., Hein M.J., Baron S.L., and Gersic C.M. (2012). Neurodegenerative causes of death among retired National Football League players. Neurology 79, 1970–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savica R., Parisi J.E., Wold L.E., Josephs K.A., and Ahlskog J.E. (2012). High school football and risk of neurodegeneration: a community-based study. Mayo Clin. Proc. 87, 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kochanek P.M., Berger R.P., Bayir H., Wagner A.K., Jenkins L.W., and Clark R.S. (2008). Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care. 14, 135–141 [DOI] [PubMed] [Google Scholar]
- 35. McAllister T.W. (2011). Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 13, 287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jorge R.E., and Arciniegas D.B. (2014). Mood disorders after TBI. Psychiatr. Clin. North Am. 37, 13–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viola-Saltzman M., and Watson N.F. (2012). Traumatic brain injury and sleep disorders. Neurol. Clin. 30, 1299–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verellen R.M., and Cavazos J.E. (2010). Post-traumatic epilepsy: an overview. Therapy 7, 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., Manley G.T., and Gruen R.L. (2012). Early management of severe traumatic brain injury. Lancet 380, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 40. Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 41. Tagliaferri F., Compagnone C., Korsic M., Servadei F., and Kraus J. (2006). A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien) 148, 255–268; discussion 268 [DOI] [PubMed] [Google Scholar]
- 42. Mckee A.C., and Daneshvar D.H. (2015). The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 127, 45–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sewell D., Nacewicz B., Liu F., Macvilay S., Erdei A., Lambris J.D., Sandor M., and Fabry Z. (2004). Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J. Neuroimmunol. 155, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y., Meng J., Bi F., Li H., Chang C., Ji C., and Liu W. (2019). NEK7 regulates NLRP3 inflammasome activation and neuroinflammation post-traumatic brain injury. Front. Mol. Neurosci. 12, doi: 10.3389/fnmol.2019.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dinet V., Petry K.G., and Badaut J. (2019). Brain-immune interactions and neuroinflammation after traumatic brain injury. Front. Neurosci. 12, 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glushakova O.Y., Johnson D., Hayes R.L. (2014). Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma 31, 1180–1193 [DOI] [PubMed] [Google Scholar]
- 47. Allan S.M., Parker L.C., Collins B., Davies R., Luheshi G.N., and Rothwell N.J. (2000). Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat. Proc. Natl. Acad. Sci. U. S. A. 97, 5580–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sankar S.B., Pybus A.F., Liew A., Sanders B., Shah K.J., Wood L.B., and Buckley E.M. (2019). Low cerebral blood flow is a non-invasive biomarker of neuroinflammation after repetitive mild traumatic brain injury. Neurobiol. Dis. 124, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pop V., Sorensen D.W., Kamper J.E., Ajao D.O., Murphy M.P., Head E., Hartman R.E., and Badaut J. (2013). Early brain injury alters the blood-brain barrier phenotype in parallel with ß-amyloid and cognitive changes in adulthood. J. Cereb. Blood Flow Metab. 33, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hay J.R., Johnson V.E., Young A.M., Smith D.H., and Stewart W. (2015). Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 74, 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levin H.S., and Diaz-Arrastia R. (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 14, 506–517 [DOI] [PubMed] [Google Scholar]
- 52. Hawryluk G.W., and Manley G.T. (2015). Classification of traumatic brain injury: past, present, and future. Handb. Clin. Neurol. 127, 15–21 [DOI] [PubMed] [Google Scholar]
- 53. Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., Manley G.T., and Workshop Scientific Team and Advisory Panel Members. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee B., and Newberg A. (2005). Neuroimaging in traumatic brain imaging. NeuroRx 2, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marshall S.A., and Riechers R.G. (2012). Diagnosis and management of moderate and severe traumatic brain injury sustained in combat. Mil. Med. 177, 76–85 [DOI] [PubMed] [Google Scholar]
- 56. Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain J. Neurol. 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 57. Bazarian J.J., McClung J., Shah M.N., Cheng Y.T., Flesher W., and Kraus J. (2005). Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 19, 85–91 [DOI] [PubMed] [Google Scholar]
- 58. Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang K.K., Yang Z., Zhu T., Shi Y., Rubenstein R., Tyndall J.A., and Manley G.T. (2018). An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 18, 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Agoston D.V., Shutes-David A., and Peskind E.R. (2017). Biofluid biomarkers of traumatic brain injury. Brain Inj. 31, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 61. Strathmann F.G., Schulte S., Goerl K., and Petron D.J. (2014). Blood-based biomarkers for traumatic brain injury: evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin. Biochem. 47, 876–888 [DOI] [PubMed] [Google Scholar]
- 62. Diaz-Arrastia R., Wang K.K., Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., Okonkwo D.O., Manley G.T., and TRACK-TBI Investigators. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma. 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Okonkwo D.O., Yue J.K., Puccio A.M., Panczykowski D.M., Inoue T., McMahon P.J., Sorani M.D., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., and Manley G.T., and TRACK-TBI Investigators. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang Z., and Wang K.K. (2015). Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 38, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chodobski A., Zink B.J., and Szmydynger-Chodobska J. (2011). Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2, 492–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burda J.E., and Sofroniew M.V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaz R., Sarmento A., Borges N., Cruz C., and Azevedo I. (1997). Ultrastructural study of brain microvessels in patients with traumatic cerebral contusions. Acta Neurochir. (Wien) 139, 215–220 [DOI] [PubMed] [Google Scholar]
- 68. Schwarzmaier S.M., Kim S.W., Trabold R., and Plesnila N. (2010). Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J. Neurotrauma 27, 121–130 [DOI] [PubMed] [Google Scholar]
- 69. Yang H., Zhang C., Fang S., Ou R., Li W., and Xu Y. (2015). UCH-LI acts as a novel prognostic biomarker in gastric cardiac adenocarcinoma. Int. J. Clin. Exp. Pathol. 8, 13957–13967 [PMC free article] [PubMed] [Google Scholar]
- 70. Bedekovics T., Hussain S., Feldman A.L., and Galardy P.J. (2015). UCH-L1 is induced in germinal center B-cells and identifies patients with aggressive germinal center diffuse large B-cell lymphoma. Blood 127, 1564–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zetterberg H., Smith D.H., and Blennow K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Scarna H., Delafosse B., Steinberg R., Debilly G., Mandrand B., Keller A., and Pujol J.F. (1982). Neuron-specific enolase as a marker of neuronal lesions during various comas in man. Neurochem. Int. 4, 405–411 [DOI] [PubMed] [Google Scholar]
- 73. Berger R.P., Adelson P.D., Pierce M.C., Dulani T., Cassidy L.D., and Kochanek P.M. (2005). Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. 103(1 Suppl.), 61–68 [DOI] [PubMed] [Google Scholar]
- 74. Johnson V.E., Stewart W., Weber M.T., Cullen D.K., Siman R., and Smith D.H. (2016). SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol. (Berl) 131,115–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Castellani R.J., and Perry G. (2019). Tau biology, tauopathy, traumatic brain injury, and diagnostic challenges. J. Alzheimers Dis. 67, 447–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Papa L., Robicsek S.A., Brophy G.M., Wang K.K., Hannay H.J., Heaton S., Schmalfuss I., Gabrielli A., Hayes R.L., and Robertson C.S. (2017). Temporal profile of microtubule-associated protein 2: a novel indicator of diffuse brain injury severity and early mortality after brain trauma. J. Neurotrauma 35, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dadas A., Washington J., Diaz-Arrastia R., and Janigro D. (2018). Biomarkers in traumatic brain injury (TBI): a review. Neuropsychiatr. Dis. Treat. 14, 2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pineda J.A., Wang K.K., and Hayes R.L. (2004). Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 14, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Henson P.M., Bratton D.L., and Fadok V.A. (2001). Apoptotic cell removal. Curr. Biol. CB. 11, R795–R805 [DOI] [PubMed] [Google Scholar]
- 80. Bergsmedh A., Szeles A., Henriksson M., Bratt A., Folkman M.J., Spetz A.L., and Holmgren L. (2001). Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. U. S. A. 98, 6407–6411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Del Conde I., Shrimpton C.N., Thiagarajan P., and López J.A. (2005). Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604–1611 [DOI] [PubMed] [Google Scholar]
- 82. Denzer K., Kleijmeer M.J., Heijnen H.F., Stoorvogel W., and Geuze H.J. (2000). Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113 (Pt. 19), 3365–3374 [DOI] [PubMed] [Google Scholar]
- 83. Salzer U., Hinterdorfer P., Hunger U., Borken C., and Prohaska R. (2002). Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 99, 2569–2577 [DOI] [PubMed] [Google Scholar]
- 84. van Niel G., Raposo G., Candalh C., Boussac M., Hershberg R., Cerf-Bensussan N., and Heyman M. (2001). Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121, 337–349 [DOI] [PubMed] [Google Scholar]
- 85. Gill J., Mustapic M., Diaz-Arrastia R., Lange R., Gulyani S., Diehl T., Motamedi V., Osier N., Stern R.A., and Kapogiannis D. (2018). Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 32, 1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kenney K., Qu B.X., Lai C., Devoto C., Motamedi V., Walker W.C., Levin H.S., Nolen T., Wilde E.A., Diaz-Arrastia R., Gill J., and CENC Multisite Observational Study Investigators. (2018). Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 32, 1276–1284 [DOI] [PubMed] [Google Scholar]
- 87. McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rubenstein R., Chang B., Yue J.K., Chiu A., Winkler E.A., Puccio A.M., Diaz-Arrastia R., Yuh E.L., Mukherjee P., Valadka A.B., Gordon W.A., Okonkwo D.O., Davies P., Agarwal S., Lin F., Sarkis G., Yadikar H., Yang Z., Manley G.T., Wang K.K.; the TRACK-TBI Investigators, Cooper S.R., Dams-O-Connor K., Borrasso A.J., Inoue T., Maas A.I.R., Menon D.K., Schnyer D.M., and Vassar M.J. (2017). Comparing plasma phospho tau, total tau, and phospho tau–total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol. 74, 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stern R.A, Tripodis Y., Baugh C.M., Fritts N.G., Martin B.M., Chaisson C., Cantu R.C., Joyce J.A., Shah S., Ikezu T., Zhang J., Gercel-Taylor C., and Taylor D.D. (2016). Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J. Alzheimers Dis. 51, 1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Peskind E.R., Kraemer B., and Zhang J. (2015). Biofluid biomarkers of mild traumatic brain injury: whither plasma tau. JAMA Neurol. 72, 1103–1105 [DOI] [PubMed] [Google Scholar]
- 91. Goetzl E.J., Elahi F.M., Mustapic M., Kapogiannis D., Pryhoda M., Gilmore A., Gorgens K.A., Davidson B., Granholm A.C., and Ledreux A. (2019). Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 33, 5082–5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tharmaratnam T., Iskandar M.A., Tabobondung T.C., Tobbia I., Gopee-Ramanan P., and Tabobondung T.A. (2018). Chronic traumatic encephalopathy in professional American football players: where are we now? Front. Neurol. 9, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gupta A., and Pulliam L. (2014). Exosomes as mediators of neuroinflammation. J. Neuroinflammation 11, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huang S., Ge X., Yu J., Han Z., Yin Z., Li Y., Chen F., Wang H., Zhang J., and Lei P. (2018). Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 32, 512–528 [DOI] [PubMed] [Google Scholar]
- 95. Li D., Huang S., Yin Z., Zhu J., Ge X., Han Z., Tan J., Zhang S., Zhao J., Chen F., Wang H., and Lei P. (2019). Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem. Res. 44,1903–1923 [DOI] [PubMed] [Google Scholar]
- 96. Yang Y., Ye Y., Kong C., Su X., Zhang X., Bai W., and He X. (2019). MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res. 44, 811–828 [DOI] [PubMed] [Google Scholar]
- 97. Dickens A.M., Tovar-y-Romo L.B., Yoo S.W., Trout A.L., Bae M., Kanmogne M., Megra B., Williams D.W., Wiltwer K.W., Gacias M., Tabatadze N., Cole R.N., Casaccia P., Berman ,J.W., Anthony D.C., and Haughey N.J. (2017). Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 10, eaai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Campbell S.J., Anthony D.C., Oakley F., Carlsen H., Eisharkawy A.M., Blomhoff R., and Mann D.A. (2008). Hepatic nuclear factor kappa B regulates neutrophil recruitment to the injured brain. J. Neuropathol. Exp. Neurol. 67, 223–230 [DOI] [PubMed] [Google Scholar]
- 99. Campbell S.J., Zahid I., Losey P., Law S., Jiang Y., Bilgen M., van Rooijen N., Morsali D., Davis A.E., and Anthony D.C. (2008). Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology 55, 780–787 [DOI] [PubMed] [Google Scholar]
- 100. Goetzl E.J., Mustapic M., Kapogiannis D., Eitan E., Lobach I.V., Goetzl L., Schwartz J.B., and Miller B.L. (2016). Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB J. 30, 3853–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de RiveroVaccari J.P., Brand F. 3rd., Adamczak S., Lee S.W., Perez-Barcena J., Wang M.Y., Bullock M.R., Dietrich W.D., Keane R.W. (2016). Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 136 (Suppl 1.), 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goetzl E.J., Yaffe K., Peltz C.B., Ledreux A., Gorgens K., Davidson B., Granholm A.C., Mustapic M., Kapogiannis D., Tweedie D., Greig N.H. (2020). Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB J. 34, 3359–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Manek R., Moghieb A., Yang Z., Kumar D., Kobessiy F., Sarkis G.A., Raghavan V., Wang K.K. (2018). Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol. Neurobiol. 55, 6112–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ko J., Hemphill M., Yang Z., Beard K., Sewell E., Shallcross J., Schweizer M., Sandsmark D.K., Diaz-Arrastia R., Kim J., Meaney D., and Issadore D. (2019). Multi-dimensional mapping of brain-derived extracellular vesicle microRNA biomarker for traumatic brain injury diagnostics. J Neurotrauma, doi: 10.1089/neu.2018.6220 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ko J., Carpenter E., and Issadore D. (2016). Detection and isolation of circulating exosomes and microvesicles for cancer monitoring and diagnostics using micro-/nano-based devices. Analyst 141, 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xu R., Greening D.W., Zhu H.J., Takahashi N., and Simpson R.J. (2016). Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest. 126, 1152–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Contreras-Naranjo J.C., Wu H.J., and Ugaz V.M. (2017). Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17, 3558–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Iliescu F.S., Vrtačnik D., Neuzil P., and Iliescu C. (2019). Microfluidic technology for clinical applications of exosomes. Micromachines 10, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ko J., Baldassano S.N., Loh P.L., Kording K., Litt B., and Issadore D. (2018). Machine learning to detect signatures of disease in liquid biopsies: a user's guide. Lab Chip 18, 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fatima M., and Pasha M. (2017). Survey of machine learning algorithms for disease diagnostic. J. Intell. Learn. Syst. Appl. 09, 1–16 [Google Scholar]
- 111. Kenny L.C., Dunn W.B., Ellis D.I., Myers J., Baker P.N., GOPEC Consortium, and Kell D.B. (2005). Novel biomarkers for pre-eclampsia detected using metabolomics and machine learning. Metabolomics 1, 227 [Google Scholar]
- 112. Pinto J.V., Passos I.C., Gomes F., Reckziegel R., Kapczinski F., Mwangi B., and Kauer-Sant'Anna M. (2017). Peripheral biomarker signatures of bipolar disorder and schizophrenia: a machine learning approach. Schizophr. Res. 188, 182–184 [DOI] [PubMed] [Google Scholar]
- 113. Penny W., and Frost D. Neural networks in clinical medicine. (1996). Med. Decis. Making 16, 386–398 [DOI] [PubMed] [Google Scholar]
- 114. Hale A.T., Stonko D.P., Brown A., Lim J., Voce D.J., Gannon S.R., Le T.M., and Shannon C.N. (2018). Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg. Focus 45, E2. [DOI] [PubMed] [Google Scholar]
- 115. Ko J., Hemphill M., Yang Z., Sewell E., Na Y.J., Sandsmark D.K., Haber M., Fisher S.A., Torre E.A., Svane K.C., Omelchenko A., Firestein BL., Diaz-Arrastia R., Kim J., Meaney D.F., and Issadore D. (2018). Diagnosis of traumatic brain injury using miRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip 18, 3617–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen C., Zong S., Liu Y., Wang Z., Zhang Y., Chen B., and Cui Y. (2019). Profiling of exosomal biomarkers for accurate cancer identification: combining DNA-PAINT with machine- learning-based classification. Small. 15, 1901014. [DOI] [PubMed] [Google Scholar]
- 117. Ko J., Bhagwat N., Yee SS., Ortiz N., Sahmoud A., Black T., Aiello N.M., McKenzie L., O'Hara M., Redlinger C., Romeo J., Carpenter E.L., Stanger B.Z., and Issadore D. (2017). Combining machine learning and nanofluidic technology to diagnose pancreatic cancer using exosomes. ACS Nano. 11, 11182–11193 [DOI] [PubMed] [Google Scholar]
- 118. Ebrahimkhani S., Vafaee F., Hallal S., Wei H., Lee M.Y.T., Young P.E., Satgunaseelan L., Beadnall H., Barnett M.H., Shivalingam B., Suter C.M., Buckland M.E., and Kaufman K.L. (2018). Deep sequencing of circulating exosomal microRNA allows non-invasive glioblastoma diagnosis. NPJ Precis. Oncol. 2, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T. Jr., Carter B.S., Krichevsky A.M., and Breakfield X.O. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shao H., Chung J., Lee K., Balaj L., Min C., Carter B.S., Hochberg F.H., Breakfield X.O., Lee H., and Weissleder R. (2015). Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 6, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heitzer E., Haque I.S., Roberts C.E.S, and Speicher M.R. (2019). Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20, 71–88 [DOI] [PubMed] [Google Scholar]
- 122. Perakis S., and Speicher M.R. (2017). Emerging concepts in liquid biopsies. BMC Med. 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A.E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J.M., Dumont-Cole V.D., Kramer K., Wexler L.H., Narendran A., Schwartz G.K., Healey J.H., Sandstrom P., Labori K.J., Kure E.H., Grandgenett P.M., Hollingsworth M.A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S.K., Jarnagin W.R., Brady M.S., Fodstad O., Muller V., Pantel K., Minn A.J., Bissel M.J., Garcia B.A., Kang Y., Rajasekhar V.K., Ghajar C.M., Matei I., Peinado H., Bromberg J., and Lyden D. (2015). Tumor exosome integrins determine organotropic metastasis. Nature 527, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McAllister T.W. (2008). Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry 7, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Smith D.H., Hicks R., and Povlishock J.T. (2013). Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Margulies S., Anderson G., Atif F., Badaut J., Clark R., Empey P., Guseva M., Hoane M., Huh J., Pauly J., Raghupathi R., Scheff S., Stein D., Tang H., and Hicks M. (2016). Combination therapies for traumatic brain injury: retrospective considerations. J. Neurotrauma 33, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., and Salzer W. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kochanek P.M., Jackson T.C., Ferguson N.M., Carlson S.W., Simon D.W., Brockman E.C., Ji J., Bayir H., Poloyac S.M., Wagner A.K., Kline A.E., Empey P.E., Clark R.S., Jackson E.K., and Dixon C.E. (2015). Emerging therapies in traumatic brain injury. Semin. Neurol. 35, 83–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Furno D.L., Mannino G., and Giuffrida R. (2018). Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell Physiol. 233, 3982–3999 [DOI] [PubMed] [Google Scholar]
- 130. Ahmed H.H., Salem A.M., Atta H.M., Eskandar E.F., Farrag A.R., Ghazy M.A., Salem N.A., and Aglan H.A. (2016). Updates in the pathophysiological mechanisms of Parkinson's disease: emerging role of bone marrow mesenchymal stem cells. World J. Stem Cells 8, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sakthiswary R., and Raymond A.A. (2012). Stem cell therapy in neurodegenerative diseases. Neural. Regen. Res. 7, 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Danielyan L., Beer-Hammer S., Stolzing A., Schäfer R., Siegel G., Fabian C., Kahle P., Biedermann T., Lourhmati A., Buadze M., Novakovic A., Proksch B., Gleiter C.H., Frey W.H., and Schwab M. (2014). Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer's and Parkinson's disease. Cell Transplant. 23 (Suppl 1.), S123–S139 [DOI] [PubMed] [Google Scholar]
- 133. Deda H., Inci M.C., Kürekçi A.E., Sav A., Kayihan K., Ozgün E., Ustünsoy G.E., and Kocabay S. (2009). Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy 11, 18–25 [DOI] [PubMed] [Google Scholar]
- 134. Caplan A.I., and Dennis J.E. (2006). Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 98, 1076–1084 [DOI] [PubMed] [Google Scholar]
- 135. Caplan A.I. (2016). MSCs: the new medicine, in: Stem Cells in Regenerative Medicine. John Wiley & Sons, Ltd.; pps. 415–422 [Google Scholar]
- 136. Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., and Chopp M. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang Y., Chopp M., Zhang Z.G., Katawoski M., Xin H., Qu C., Ali M., Mahmood A., and Xiong Y. (2017). Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 111, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mahmood A., Lu D., and Chopp M. (2004). Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21, 33–39 [DOI] [PubMed] [Google Scholar]
- 139. Pardridge W.M. (2012). Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Obermeier B., Daneman R., and Ransohoff R.M. (2013). Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Muller W.A. (2013). Getting leukocytes to the site of inflammation. Vet. Pathol. 50, 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Duffy M.J., and Crown J. (2013). Companion biomarkers: paving the pathway to personalized treatment for cancer. Clin. Chem. 59, 1447–1456 [DOI] [PubMed] [Google Scholar]
- 143. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C., Godwin J., Gray R., Clarke M., Cutter D., Darby S., McGale P., Pan H.C., Taylor C., Wang Y.C., Dowsett M., Ingle J., and Peto R. (2011). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chia S.K., and Wolff A.C. (2011). With maturity comes confidence: EBCTCG tamoxifen update. Lancet 378, 747–749 [DOI] [PubMed] [Google Scholar]
- 145. Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., Baselga J., and Norton L. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 [DOI] [PubMed] [Google Scholar]