Abstract
A dietary intervention study was assessed to determine if different sources of starch in homemade diets could significantly modify fecal microbiome of dogs. Twenty-seven adult dogs were enrolled and fed a diet based on a mixture of rice and pasta with fresh raw meat (CD). After 90 d, 8 dogs continued to receive CD diet, 10 dogs received a diet made of a raw meat and a complementary food with rice as the main source of starch (B1), and 9 dogs were fed a diet with the same raw meat and a complementary food with potato as the main source of starch (B2). Samples of feces were collected from each dog in the mornings at the beginning of the study and after 15 d and analyzed for pH, ammonia N (N–NH3) and total N, short chain fatty acids (SCFA) and lactic acid. Relative abundance of fecal microbiota was assessed by sequencing and annotating the V3–V4 regions of the 16S rRNA. Total starch intake was similar between diets but differed in the in vitro rate digestion and in the resistant starch, which was higher in B2 than in B1 and CD diets. Dogs fed B2 diet showed lower (P < 0.05) N–NH3 and pH but higher (P < 0.05) molar proportion of lactic acid. Linear discriminant analysis of the genera relative abundances indicated a significant (P < 0.01) increase of SMB53 genus at the end of the study in B1 diet and of Megamonas genus in B1 and B2 diets in comparison to CD diet. These results suggest that changes of starch source in a raw meat-based diet have limited effects on fecal microbiome in healthy dogs, but underline a high variability of microbiota among dogs.
Keywords: Diets, Starch fraction, Microbiome, Nutrition, Canis lupus familiaris
1. Introduction
Studies of microbial population in the feces with non-culturable techniques have attracted the scientific community in the last decade, allowing a deeper investigation of the interactions among gut microbiome, diet and intestinal functions in human and animals (Maria et al., 2017, Middelbos et al., 2010, Nagpal et al., 2018, Panasevich et al., 2015, Sandri et al., 2014). Gut microbial ecology has been associated with several human patho-physiological conditions (Jiminez et al., 2016) and this feature has also been reported to companion animals (Suchodolski et al., 2012, Xu et al., 2016). Modification of gut microbiome in dogs has been investigated also in relation to dietary factors (Kerr et al., 2013, Middelbos et al., 2010, Panasevich et al., 2013, Panasevich et al., 2015, Roehe et al., 2016, Sandri et al., 2017, Stercova et al., 2016), suggesting that the variability of microbial population can be associated to specific ingredients or nutrients. These studies have allowed to gather more insight on the composition of fecal microbiota, useful in digestibility trials (Algya et al., 2018, Kieler et al., 2017) and in diet formulation.
Canis lupus familiaris is considered an opportunistic carnivore and domestication has improved the ability to digest starch through an increase of amylase gene copy number variation and therefore in modulating its enzymatic activity (Arendt et al., 2014). Due to this enzymatic ability of the dog, the pet industry can include a high content of starch in the formulation of extruded foods, a process which require this carbohydrate for flashing, expansion and texturizing. The source of starch and the thermal process affect the percentage of rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS) and influence the starch fermentation characteristics (Chiofalo et al., 2019, Murray et al., 2001, Peixoto et al., 2017). An in vitro study with fecal inoculum from dogs (Murray et al., 1999) showed that the source of starch and the percentage of RDS, SDS and RS affect the productions of short chain fatty acid and lactic acid. The degree of gelatinization and the sorce of starch also influenced the short chain fatty acid and lactic acid in vivo (Bazolli et al., 2015). In particular low degree of gelatinization was associated to a higher production of butyrate and the starch from corn and sorghum led to an increase of lactate. In humans, the SDS and RS fractions are considered nutraceuticals. The SDS is slowly digested into the small intestine and has a low glycemic index whilst the RS is not digestible in the small intestine and is fermented by lactobacilli, bifidobacteria and streptococci in the bowel, exerting a healthy activity (Magallanes-Cruz et al., 2017). Also in dogs, the level of starch in the diet and its gelatinization can affect the postprandial glucose and insulin concentration (Hewson-Hughes et al., 2011) and the percentage of RS fraction in the diet of dogs increased the production of butyrate and affected the insulin sensitivity and the gut health (Ribeiro et al., 2019). The level of RS does not always lead to higher butyrate production (Beloshapka et al., 2013, Peixoto et al., 2017). However, the starch-to-lipid ratio in the diet did caused a shift of microbial communities in the feces of dogs. Several other factors affect the gut microbiome as the extent of thermal treatments of food (Algya et al., 2018) and the administration of raw meat (Beloshapka et al., 2011, Bermingham et al., 2017, Kim et al., 2017, Sandri et al., 2017), prebiotics (Swanson et al., 2002) and source of protein (Sandri et al., 2019).
The present study aimed to determine if the association of different sources of starch to a raw meat-based diet could modulate the microbial community and the end products fermentation in dog feces.
2. Materials and methods
2.1. Ethics
All protocols, procedures and the care of the animals complied with the Italian (DL n.116, 27/1/1992) and European (Directive, 2010/63/EU) legislations on animal experiments and the study was approved by the ethical committee of the University of Udine (OBPA, Prot. N. 2/2017, approved on 01/03/2017). The personnel of the shelter was instructed to feed their pets as usual, without any food reward. At the end of the study, dogs returned to the homemade diet regularly fed before in the shelter.
2.2. Animals and housing
The study was conducted in late winter in North-East Italy, with an average temperature of 10 to 15 °C and 60% to 70% relative humidity during the whole period. Thirty adult dogs housed in a shelter, healthy, as confirmed by clinical examination, and not under drug treatments from last 4 months were selected. Dogs were housed in individual pens with beds from around from 18:00 until 09:00 and were individually fed in the morning before leaving the pen. Dogs were free to move in the shelter area during the day (from 09:00 until 18:00), where they had access to a recreational park where water was always available. At the beginning and at the end of the study, dogs were weighed and body condition score evaluated by an experienced person (Laflamme, 1997). In Appendix Table 1, individual records of the dogs are reported.
2.3. Diets
All dogs were fed a homemade diet based on boiled wheat pasta (macaroni type) and boiled rice in a ratio of 1:1 and fresh raw beef meat muscle (bottom sirloin) for 3 months before the beginning of the study. Dogs did not receive extra food from the personnel of the shelter, at least during the 15 d of the study. The diet was under the supervision of an animal scientist and was formulated to cover the nutrient requirements of adult dogs at maintenance (NRC, 2006). Dogs were divided in 3 groups of 10 subjects each, balanced for age, sex and live weight. Control group continued to receive the same diet (CD), whilst the second group was fed a diet with about 70% (wt/wt) raw bottom sirloin beef meat and 30% (wt/wt) of a dry complementary food, specifically formulated with rice as the main source of starch (diet B1). In the third group, a complementary food based on potato substituted the previous one based on rice (diet B2). The complementary foods were manufactured and provided by Nutrigene srl (www.nutrigenefood.com; Udine, Italy). The B1 diet contained raw meat, rice flour, chickpeas flour, oat flakes, dry ground carrots, algae-derived omega 3 fatty acids and mineral–vitamin complex. In B2 diet, potato substituted rice and beet pulp substituted oat flakes. Rice, chickpeas, and potato were individually treated in autoclave, then dried with high intensity hot air and milled to a mean particle size of 500 μm. Oat flakes and beet pulp were milled to the same mean particle size. Vitamins, macro and micro elements were added to cover, in association with 70% (wt/wt) of raw beef meat the nutritional requirements according to NRC recommendations (NRC, 2006). The ingredients and the nutritional additives were cold mixed and packed in 1-kg bags. The raw bottom sirloin meat came from a unique batch and was purchased from a local slaughterhouse. The meat was frozen at −20 °C and thawed every day. Thus, B1 and B2 diets were prepared by mixing the complementary foods with raw meat and by adding tap water up to obtain a wet meal (approximatively, the ratio of water to complementary food was 2:1 [wt/wt]). The diets were offered to dogs for 15 d in the morning before moving into the recreational park. At the beginning (T0) and at the end of the study (T15), before the morning meal, dogs were weighed and body condition score (BCS), on a scale from 1 to 9, was assessed. The amounts of the diets were adjusted for initial live weight, according to NRC (2006) recommendations. During the study, due to adoptions, the final numbers of dogs for each group were 8 for CD, 10 for B1 and 9 for B2 (Appendix Table 1).
2.4. Chemical and enzymatic analysis of diets
Samples of the 3 diets, complementary foods (CD, B1 and B2) and raw meat were collected and analyzed for dry matter, ash, crude protein, crude fat and crude fiber (AOAC, 2000), as reported in Table 1. Total starch (TS) was measured with the Megazyme enzymatic kit (cod K-TSTA; Bray, Ireland). A 2-steps in vitro enzymatic hydrolysis was used to measure starch digestibility of the 3 diets (Giuberti et al., 2012). About 800 mg of ground samples were weighed in 50-mL tubes with glass balls. The samples were treated with 5 mL of HCl solution (0.05 mol/L) containing pepsin (5 mg/mL; Sigma P-7000, Sigma–Aldrich Co., Milan, Italy) at 37 ℃ for 30 min under agitation. At the end of incubation, the pH was adjusted by adding 20 mL of 0.1 mol/L sodium acetate buffer to the value of 5.2, and 5 mL of an enzymatic mixture was added. The mixture contained pancreatin (Merck 7130, Merck KGaA, Darmstadt, Germany), amyloglucosidase (Sigma A-7095, Sigma–Aldrich Co., Milan, Italy) and invertase (Sigma I-4504, Sigma–Aldrich Co., Milan, Italy), to ensure an amylase activity of around 7,000 U/mL. The incubation was carried out for 240 min, taking an aliquot at 0 min and after 15, 30, 60, 90, 120, 180 and 240 min. Absolute ethanol was immediately added to the samples and the glucose concentration was determined at 510 nm with a glucose oxidase kit (GODPOD 4058, Giesse Diagnostic snc, Rome, Italy).
Table 1.
Chemical compositions, starch fractions, and energy content of the food ingredients administered to the dogs in the dietary intervention study.1
| Item | CD | B1 | B2 | Meat |
|---|---|---|---|---|
| Chemical compositions, % DM basis | ||||
| Dry matter | 91.1 | 93.0 | 92.5 | 35.5 |
| Crude protein | 10.0 | 11.9 | 9.4 | 49.6 |
| Crude lipids | 3.9 | 4.1 | 3.2 | 41.4 |
| Crude fiber | 1.9 | 1.8 | 2.2 | – |
| TDF | 5.2 | 6.1 | 6.3 | – |
| Ash | 6.0 | 7.1 | 8.0 | 2.3 |
| Starch | 66.8 | 67.4 | 67.3 | – |
| Starch fractions, % of starch content | ||||
| RDS | 60.3 | 68.9 | 46.2 | – |
| SDS | 30.1 | 20.9 | 39.0 | – |
| RS | 9.5 | 10.2 | 14.0 | |
| ME, kcal/kg DM | 3,696 | 3,675 | 3,579 | 5,865 |
TDF = total dietary fibre; RDS = rapidly digestible starch; SDS = slowly digestible starch; RS = resistant starch; ME = metabolizable energy.
CD refers to a complementary food made of mix of pasta and rice in a ratio 1:1; B1 refers to a complementary food made of rice as main source of starch; B2 refers to a complementary food made of potato as main source of starch; Meat refers to beef raw meat.
2.5. Collection of fecal samples
Samples of feces were collected from each dog at T0 and T15, when each dog still stayed in its individual pen. Starting from 07:00, the first stool defecated from each dog was collected with sterile gloves in hermetic sterile plastic bags and frozen in liquid nitrogen, then stored at −80 °C until analysis. A subsample of frozen stools was carefully cleaned from external contaminations with a sterile blade, then was manually ground to a fine powder in liquid nitrogen using a sterile mortar and pestle. Three aliquots were obtained, placed in sterile polypropylene tubes and stored at −80 °C for N fractions, pH, short chain fatty acids (SCFA), lactic acid and DNA analysis.
2.6. Fecal pH, N fractions, SCFA and lactic acid analysis
Total N was measured with a Kjeldahl apparatus. Ammonia nitrogen was also measured with a Kjeldahl, after distillation followed by titration of distillate with sulphuric acid. The determination of pH was conducted with a pH meter (Mettler Toledo InLab Expert Pro) starting from 2 g of faeces mixed with deionized water 1:1 (wt/vol). The concentration of SCFA (acetic, propionic, butyric, isobutyric, valeric, isovaleric) and lactic acid of fecal samples was measured by HPLC according to the procedure previously described by Sandri et al. (2017). Individual SCFA and lactic acid concentrations were calculated with reference to a standard solution of 50.0 mmol/L lactic acid, 89.0 mmol/L acetic acid, 77.8 mmol/L propionic acid, 86.6 mmol/L butyric acid and isobutyric acid, 94.0 mmol/L valeric acid and isovaleric acid in 0.05 mol/L H2SO4 (Sigma–Aldrich Co., Milan, Italy). Quantification was calculated using an external calibration curve based on the standards described above. Total acid (TA) was determined as a sum of SCFA and lactic acid; single acid concentration was expressed as molar percentage of the TA.
2.7. Fecal DNA extraction, sequencing and taxonomic annotation
Microbial DNA of the feces was extracted from 150-mg samples using a Fecal DNA MiniPrep kit (Zymo Research; Irvine, CA, USA), following the manufacturer's instructions, including a bead beating step. Pre-amplification concentration of DNA in the samples was measured with a Qubit 3 Fluorometer (Thermo Scientific; Waltham, MA, USA). DNA was fragmented and 16S rRNA V3 and V4 regions amplified for library preparation, adding also the Indexes for sequencing, using a Nextera DNA Library Prep kit (Illumina; San Diego, CA, USA), following manufacturer's instructions and primers. Amplicons were then sequenced with a MiSeq (Illumina; San Diego, CA, USA) in 2 × 300 paired-end mode, following the standard procedures.
The Quantitative Insights Into Microbial Ecology (QIIME 2) (Caporaso et al., 2010) was used to process the raw sequences, which were uploaded to NCBI Sequence Read Archive (Bioproject ID PRJNA529651). After demultiplexing, sequenced reads that passed the quality check (Phred score ≥ 30) were annotated for 16S rRNA against the Greengenes database. Chimeras were also detected and then filtered from the reads and the remaining sequences were clustered into operational taxonomic units by using an open reference approach in QIIME 2.
The 16S rRNA annotated sequences were normalized to ‰ abundance profiles (relative abundance [RA]) for each sample and each taxonomic level. Taxa with RA lower than 10‰ were excluded from the statistical analysis. Shannon α-biodiversity (H′) index was calculated at the genus level including all taxa according to the equation H′ = sum [Pi × ln (Pi)] , where Pi was the frequency of every genus within the sample. Evenness index (J′) was calculated as J′ = H′/ln(S), where S was the total number of genera within each sample. Beta diversity was evaluated with the phylogeny based on UniFrac (Lozupone and Knight, 2005) distance metric and visualized using principal coordinate analysis plots.
2.8. Computation and statistical analysis
The proportion of starch digested in vitro at each time interval (DCt) was calculated according to the following equation and using a factor of 0.9 to convert mono-to polysaccharide:
| DCt = (Amount of glucose present at time t × 0.9)/Total starch. |
The following first-order exponential model was used to describe the kinetic of starch digestion:
| Ct = C0 + C∞ × [1 – e−(k× 100)t] , |
where Ct was starch digested at time t (%/TS); C0 was starch digested at 0 min (%/TS), C∞ was the potential digestibility of starch (%/TS); k is the digestion rate (/min) and t is the incubation time (min). Data were fitted with the nonlinear regression procedure, with the minimum least square method. Using the parameter of the model, RDS (%/TS), SDS (%/TS) were calculated. Firstly, RDS and digestible starch were calculated with the first-order equation, fixing the time of incubation to 20 or 120 min, respectively. Slowly digestible starch was then computed as SDS = DS - RDS and resistant starch (RS) was estimated as the following equation (Hung et al., 2016): RS = 100 - C0 – C∞ .
Linear Mixed Model was used to analyze the data of SCFA, lactic acid, pH and N fractions, including the fixed effect of time of sampling (2 levels, T0 and T15), treatment (3 levels, CD, B1 and B2), the interaction of time of sampling and treatment, with the subject (dog) as random factor repeated over the time of sampling.
The analysis of similarity was performed to test whether the microbial communities differed significantly between CD, B1 and B2 diets at T0 and T15 using the ‘Vegan’ package in R (Version 3.2.1). All these statistical analyses were performed with XLSTAT (Addinsoft, 2019). For the RA data, linear discriminant analysis effect size (LEfSe) was conducted to determine the differentially abundant microbial taxa in feces (Segata et al., 2011).
3. Results and discussion
The rate of starch in vitro digestion (Table 2) was much higher in rice containing diet (7.2%/min, B1) and lower in potato diet (3.8%/min, B2), with an intermediate value for the CD (5.3%/min). Moreover, the starch fractions varied between the 3 complementary foods, confirming the highest RDS and SDS in rice and potato, respectively.
Table 2.
Parameters of the model1 fitting the in vitro digestion of starch and starch fractions (% of starch content) of the diets offered to the dog in the dietary intervention study.2
| Item | CD | B1 | B2 |
|---|---|---|---|
| C0 | 3.3 | 1.5 | 0 |
| C∞ | 87.2 | 88.3 | 86.0 |
| k, %/min | 5.3 | 7.2 | 3.8 |
| Model fitting | |||
| r2 | 0.949 | 0.920 | 0.987 |
| RMSE | 433.71 | 670.05 | 125.01 |
| Starch fraction | |||
| RDS | 60.3 | 68.9 | 46.2 |
| SDS | 30.1 | 20.9 | 39.0 |
| RS | 9.5 | 10.2 | 14.0 |
r2 = coefficient of determination; RMSE = residual mean square error of the model; RDS = rapidly digestible starch; SDS = slowly digestible starch; RS = resistant starch.
Model: Ct = C0 + C∞ × [1-e−(k×100) ×t], where t = time of incubation; Ct = starch digested at time t; C0 = starch digested at 0 min; C∞ = potential digestibility of starch, k = rate of starch digestion.
CD, control diet, made with pasta and rice as main source of starch in a ratio 1:1 and raw meat; B1, diet with a complementary food made of rice as main source of starch and raw meat; B2, diet with a complementary food made of potato as main source of starch and raw meat.
The average amounts of food offered to the 3 groups of dogs are reported in Table 3 and the individual data of animals in Appendix Table 1. CD diet was substituted with B1 and B2 diets in 1 d and this rapid change did not cause diarrhea or differences in the appearance of feces. Moreover, B1 and B2 diets were highly palatable and dogs ate all the offered daily amounts. The total amounts of starch provided to dogs was similar between the 3 diets, but due to the different digestion rate, RDS and SDS, the RS intakes was higher in B2 than B1 and CD diets.
Table 3.
Average dietary, nutrient and metabolizable energy (ME) intakes of the experimental diets administered to the dogs during the 15 d of the study.1
| Item | CD | B1 | B2 |
|---|---|---|---|
| Average dietary intakes, g/d as fed | |||
| Complementary food | 103 | 108 | 104 |
| Raw meat | 256 | 254 | 240 |
| Total daily amount | 360 | 362 | 366 |
| Dry matter | 186 | 191 | 190 |
| Average dietary Average nutrient intakes, g/d | |||
| Crude protein | 55.9 | 56.7 | 54.9 |
| Crude lipids | 42.4 | 41.4 | 41.3 |
| Crude fiber | 2.2 | 1.8 | 2.1 |
| TDF | 9.7 | 11.7 | 12.0 |
| Ash | 7.8 | 9.2 | 9.9 |
| Starch | 72.7 | 72.8 | 71.3 |
| RDS | 43.8 | 50.2 | 32.9 |
| SDS | 21.9 | 15.2 | 27.8 |
| RS | 6.9 | 7.4 | 10.0 |
| ME, kcal/d | 862 | 875 | 867 |
TDF = total dietary fibre; RDS = rapidly digestible starch; SDS = slowly digestible starch; RS = resistant starch; ME = metabolizable energy.
CD, control diet, made with pasta and rice as main source of starch in a ratio 1:1 and raw meat; B1, diet with a complementary food made of rice as main source of starch and raw meat; B2, diet with a complementary food made of potato as main source of starch and raw meat.
In Table 4, the effects of diet, time of sampling and their interaction on the variables measured in the feces are reported. The administration of B2 diet, with potato starch, significantly reduced the concentration of N–NH3 (P < 0.05) and pH (P < 0.01), and increased the molar concentration of lactic acid (P < 0.01). Indeed, the molar concentration of lactic acid decreased in B1 group at T15 in comparison to T0 (P < 0.05). The concentration of butyric acid was affected by sampling time (P < 0.05). Moisture, ash and total N contents were unaffected by dietary treatments and time of sampling.
Table 4.
Mean values of pH, moisture, ash, nitrogen fractions and short chain fatty acids measured in the fecal samples during the study (data on fresh fecal matter).1
| Item | T0 |
T15 |
Time (T) | Diet (D) | T × D | Mean | MSE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | CD | B1 | B2 | CD | ||||||
| pH | 6.53B | 6.80A | 6.39B | 6.88A | 6.42B | 6.34B | ns | ns | ∗∗∗ | 6.57 | 0.05 |
| Moisture, % | 70.24 | 68.8 | 65.8 | 70.67 | 72.95 | 70.06 | ns | ns | ns | 69.89 | 0.79 |
| Total N, % | 1.25 | 1.36 | 1.33 | 1.31 | 1.08 | 1.26 | ns | ns | ns | 1.27 | 0.04 |
| N–NH3, % | 0.09 | 0.08 | 0.10a | 0.08a | 0.05b | 0.09a | ns | ∗ | ∗ | 0.08 | 0 |
| Ash, % | 8.29 | 9.63 | 10.47 | 9.97 | 5.30 | 9.10 | ns | ns | ns | ||
| N–NH3: N ratio | 7.14 | 6.82 | 7.93 | 6.2 | 5.16 | 7.47 | ns | ns | ns | 6.8 | 0.36 |
| Lactic, mmol/g | 53.0A | 28.7B | 43.8B | 39.0B | 55.2A | 39.1B | ns | ns | ∗∗ | 42.86 | 3.57 |
| Acetic, mmol/g | 143.1 | 120.8 | 92 | 124.2 | 105.9 | 93.5 | ns | ns | ns | 114.26 | 7.24 |
| Propionic, mmol/g | 104.5 | 90.7 | 76.2 | 84.8 | 82.1 | 93.8 | ns | ns | ns | 88.7 | 6.63 |
| Isobutyric, mmol/g | 13.4 | 11.5 | 5.4 | 7.3 | 4.4 | 4.5 | ∗ | ∗∗ | ns | 7.91 | 0.87 |
| Butyric, mmol/g | 32 | 26.8 | 21.1 | 32.8 | 20.3 | 22.5 | ∗ | ns | ns | 26.59 | 1.76 |
| Isovaleric, mmol/g | 0.6 | 0 | 0.3 | 1.6 | 0.5 | 0 | ns | ns | ns | 0.49 | 0.16 |
| Valeric, mmol/g | 0.8 | 0.6 | 0.2 | 0.7 | 0.4 | 0.6 | ns | ns | ns | 0.56 | 0.07 |
| TA, mmol/g | 347.4 | 279.2 | 238.9 | 290.3 | 268.8 | 254.0 | ns | ns | ns | 281.36 | 14.44 |
| Lactic, % | 16.5a | 11.5b | 18.1a | 15.5a | 23.5a | 15.3b | ns | ns | ∗ | 16.54 | 1.4 |
| Acetic, % | 40 | 43.1 | 39.9 | 41.7 | 38.5 | 37.7 | ns | ns | ns | 40.24 | 1.1 |
| Propionic, % | 28.7 | 31.3 | 30.5 | 26.5 | 28.6 | 36.0 | ns | ns | ns | 30.09 | 1.17 |
| Isobutyric, % | 4.3 | 4.4 | 2.7 | 2.7 | 1.8 | 1.9 | ns | ∗ | ns | 3.01 | 0.35 |
| Butyric, % | 10.1 | 9.5 | 8.6 | 12.6 | 7.2 | 8.9 | ∗ | ns | ns | 9.73 | 0.58 |
| Isovaleric, % | 0.2 | 0 | 0.1 | 0.7 | 0.2 | 0 | ns | ns | ns | 0.2 | 0.07 |
| Valeric, % | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | ns | ns | ns | 0.2 | 0.02 |
MSE = mean square error of the model; N–NH3 = ammonia nitrogen; TA = total acids; ns = not significant.
∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
A, B On a same row, different superscripts denote differences between means for P < 0.01.
a, b On a same row, different superscripts denotes differences between means for P < 0.05.
CD, control diet, made with pasta and rice as main source of starch in a ratio 1:1 and raw meat; B1, diet with a complementary food made of rice as main source of starch and raw meat; B2, diet with a complementary food made of potato as main source of starch and raw meat; T0, beginning of the study (sampling time T0); T15, after 15 d of administration of experimental diets (sampling time T15).
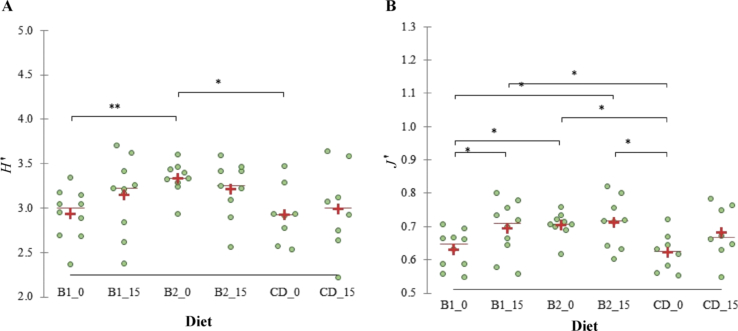
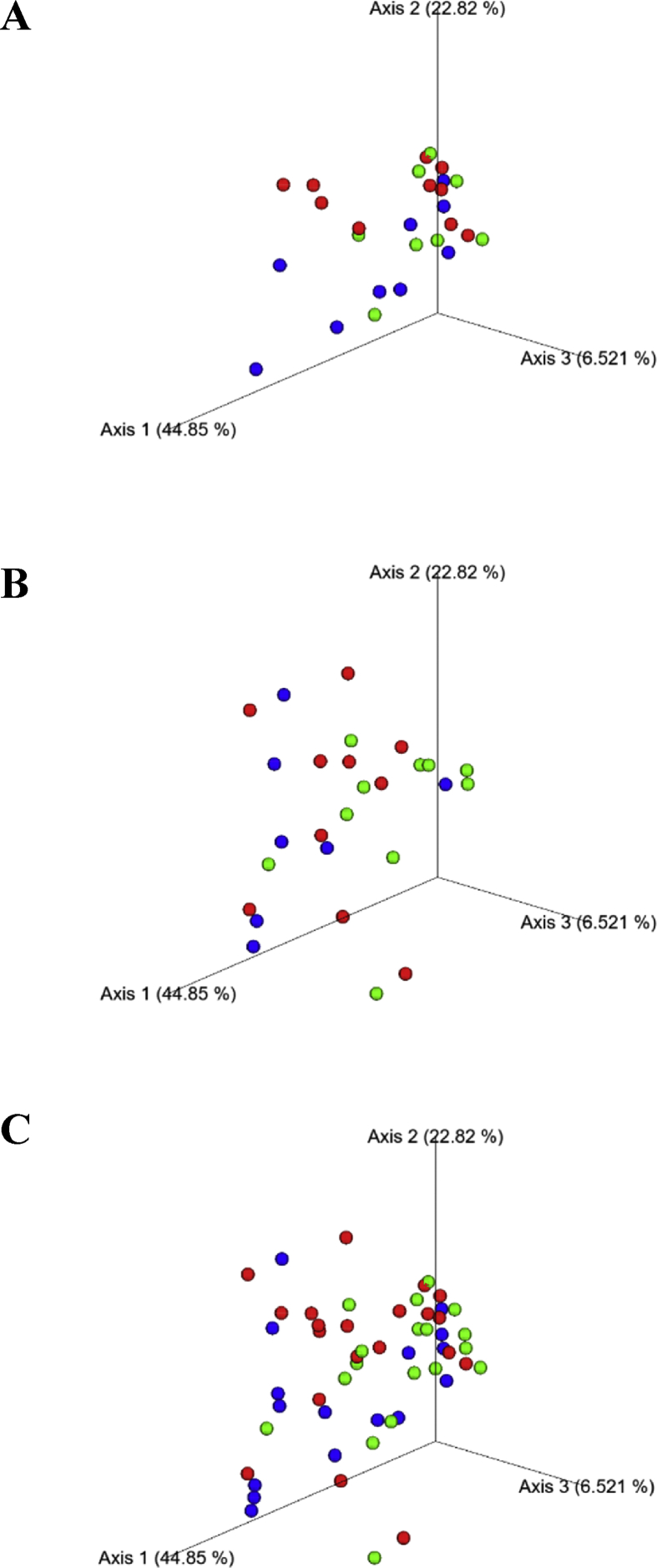
The alpha diversity, calculated as H′, did not significantly vary within each diet from T0 to T15 (Fig. 1) and differences between diets were observed at T0, but not at T15. The J′ value significantly increased in the B1 diet from T0 to T15 (P < 0.05); other differences within groups were not observed. The UniFrac distances of microbiota showed in the principal coordinate analysis reported separately at T0 and T15 or taken together (Fig. 2) indicated that diets and time of sampling did not have a significant impact on the microbial communities. This was also confirmed by the analysis of similarity test, which was not significant (P > 0.05).
Fig. 1.
Determination of microorganisms in dog feces. (A) Shannon index of biodiversity (H′) and (B) evenness (J′) calculated on the microbial genera measured in the feces of dogs fed a control diet (CD), rice based diet (B1) or potato based diet (B2), at the beginning of the study (sampling time T0) and after 15 d of administration of the diets (sampling time T15). Green dots denotes dogs, red line the median and the red cross the mean for each group. Legend of x-axis: B1_0, raw meat with a complementary food made of rice as the main source of starch at T0; B1_15, raw meat with a complementary food made of rice as the main source of starch at T15; B2_0, raw meat with a complementary food made of potato as the main source of starch at T0; B2_15, raw meat with a complementary food made of potato as the main source of starch at T15; CD_0, raw meat with a complementary food made of pasta and rice as the main source of starch at T0; CD_15, raw meat with a complementary food made of pasta and rice as the main source of starch at T15. ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 2.
Principal coordinates analysis (PCoA) of microbial communities from the fecal samples of dogs. This figure shows a 3D PCoA plot based on weighted UniFrac distances of 16S rRNA genes. (A) the samples collected at the beginning of the study (sampling time T0). (B) the samples collected after 15 d of administration of experimental diets (sampling time T15). (C) shows all the samples collected at T0 and T15. Green dots refer to control diet (CD), made with pasta and rice as main sources of starch in a ratio 1:1 and raw meat; red dots refer to a rice-based diet (B1), with a complementary food made of rice as the main source of starch and raw meat; blue dots refers to a potato-based diet (B2), with a complementary food made of potato as the main source of starch and raw meat. Each dot was an individual and analysis of similarity did not reveal clustering between the 3 groups (P > 0.05).
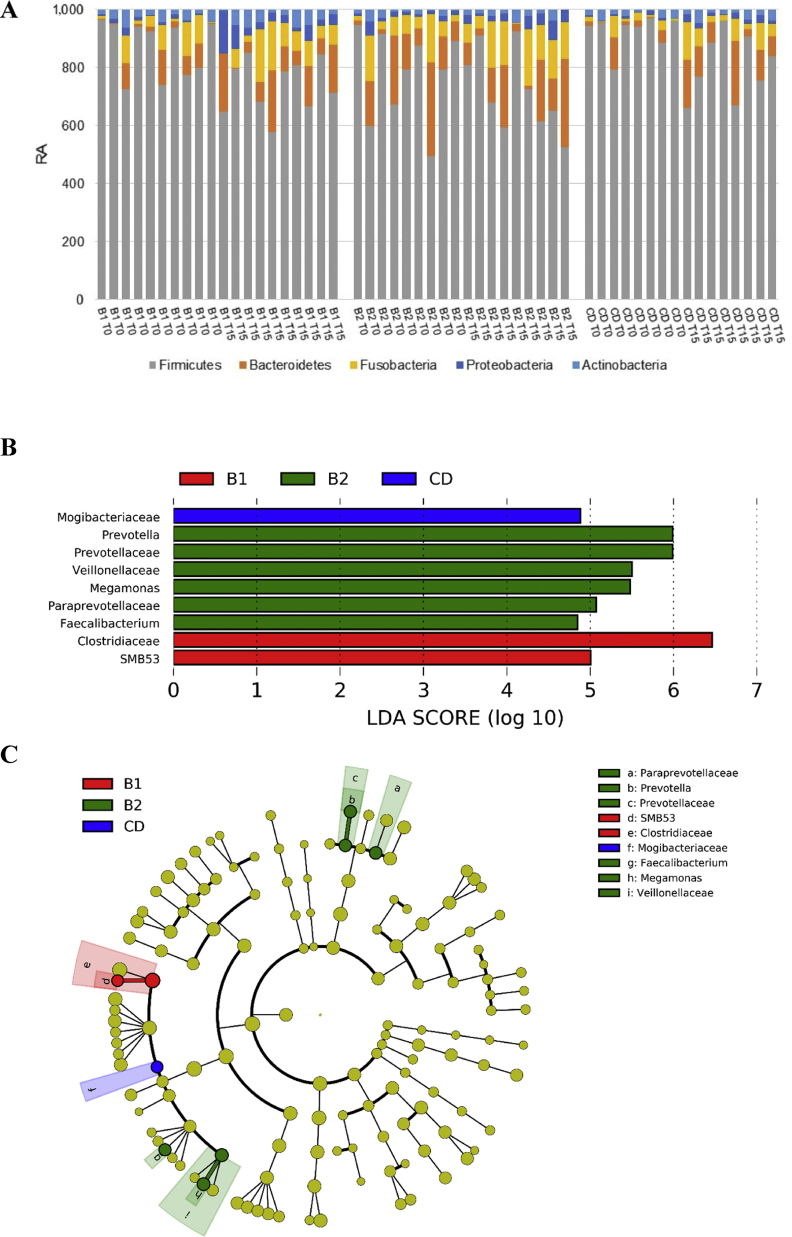
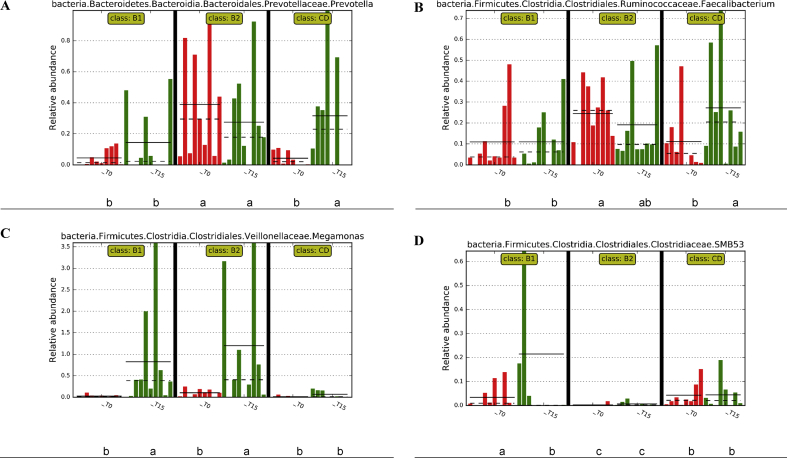
The most abundant phylum in the fecal samples was the Firmicutes, followed by the Bacteroidetes and the Fusobacteria, whilst the Actinobacteria and Proteobacteria were less represented. The variations of these phyla from samples collected at T0 in comparison to those collected at T15 were not significant and also the change of diet did not cause a significant variation of the RA of the phyla (Fig. 3). The LEfSe analysis of the fecal microbiota indicated a significant (P < 0.01) increase of the family Clostridiaceae and its genus SMB53 for the B1 diet. Families Paraprevotellaceae, Prevotellaceae and genus Prevotella, family Veillonellaceae and genus Megamonas and genus Faecalibacterium were more abundant in the B2 diet. A significant higher RA for the family Mogibacteriacee was observed in CD diet. The cladogram reported in Fig. 3C illustrates taxa significantly affected by diet. The individual RA of the genera significantly differed among the 3 diets (Fig. 4). The RA of Faecalibacterium, a member of the phylum Firmicutes, and of Prevotella, a member of the phylum Bacteroidetes, increased in the CD diet at T15, but showed a high individual variability, in particular at T0 between diets. The change of diet (i.e. from T0 to T15) increased the RA of genus Megamonas in B1 and B2 diets and of genus SMB53 in B1 diet.
Fig. 3.
Composition of the fecal microbial communities at different taxonomic levels measured in the fecal samples of dogs. (A) The composition of the fecal microbial community at the phylum level of dogs, (B) the histogram and (C) the cladogram of the linear discriminant analysis (LDA) scores for taxa differentially abundant (P < 0.01) between diets. RA = relative abundance; CD = control diet, made with pasta and rice as main sources of starch in a ratio 1:1 and raw meat; B1 refers to a rice-based diet with a complementary food made of rice as the main source of starch and raw meat; B2 refers to a potato-based diet with a complementary food made of potato as the main source of starch and raw meat; T0 refers to sampling time at the beginning of the study; T15 refers to sampling time which was after 15 d of administration of experimental diets.
Fig. 4.
Relative abundances of genera measured in the fecal samples of dogs. (A) Prevotella, (B) Faecalibacterium, (C) Megamonas, and (D) SMB53. Dogs were fed control diet (CD), rice-based diet (B1) or potato-based diet (B2) and samples collected at sampling time T0 and sampling time T15. Different letters a, b and c, below the graph of each genus denote the mean which significantly differed (P < 0.01) between diets and times of sampling. CD = control diet, made with pasta and rice as main source of starch in a ratio 1:1 and raw meat; B1 = diet with a complementary food made of rice as main source of starch and raw meat; B2 = diet with a complementary food made of potato as main source of starch and raw meat; T0 = sampling time at the beginning of the study; T15 = sampling time which was after 15 d of administration of experimental diets.
The aim of the study was to investigate if the supplementation of raw meat with the same amount of starch but differing for in vitro digestion influences fecal microbiome. The significant variations of pH, N–NH3 and lactic acid (Table 4) observed in B2 diet can be related to the variable amounts of RDS, SDS and RS between diets. According to the in vitro kinetic parameters, B2 diet contained higher amount of RS with a lower rate of digestion. Murray et al. (1999) found in dogs that the ileal digestibility of starch varied between extruded kibbles containing barley, corn, potato, rice, sorghum or wheat starches, although the total tract digestibility of starch was similar. A large amount of starch is enzymatically digested in the small intestine of dogs and the small amount escaping from the ileum is utilized by commensal bacteria in the large intestine (Maria et al., 2017). It is likely that the unavailable fraction of starch is mainly composed by RS, which can be completely fermented in the cecum (Haenen et al., 2013). Goudez et al. (2011) investigated the effects of RS from corn or potato on fecal quality of dogs and reported that the highest concentrations of RS in the kibble negatively affects the fecal quality in dogs of large size, independently from the source of starch. However, it has also been reported that the fermentation of RS in the bowel increases the total concentration of SCFA and butyrate and reduces the pH in the intestine of pigs (Haenen et al., 2013), humans (Martinez et al., 2010) and dogs (Simpson, 1998). In the present study B2 diet did not cause significant variations of TA (sum of total SCFA and lactate) or butyrate (Table 4), but the decrease of pH and the increase of lactic acid in diets with higher amount of RS agreed with the data reported in other studies (Beloshapka et al., 2013, Peixoto et al., 2017).
The observed decrease of N–NH3 concentration in feces after the administration of B2 diet likely was related to the different flows of nutrients. Fecal N–NH3 concentration depends on the amount and quality of protein intakes (Algya et al., 2018) and fiber (Maria et al., 2017), and its reduction was observed in diets, which provide high percentage of RS (Peixoto et al., 2017). The lowest rate of digestion and the highest RS percentage of potato starch (i.e. B2 diet) can have increased the amount of starch reaching the large intestine with a shift of the microbial community and activity. Conversely, the lower amount of RS in the B1 diet likely led to a reduction of protein utilization by gut bacteria, as observed by the highest N–NH3 and isobutyric acid concentrations, but the lack of a significant interaction between diet × time of sampling indicated that the effect was maybe related to the dogs and not to the diets. Furthermore, diets had a mild influence on alpha (Fig. 1) and beta biodiversity (Fig. 2), since they did not change after the substitution of CD diet with B1 and B2 diets. Only the J′ significantly increased in the B1 diet, suggesting that rice had some effect on the microbial community as a whole. Also, in the study of Schauf et al. (2018), the administration of diet differing for starch and lipid concentrations did not modify the alpha diversity. The change of diet led to a significant variation of RA at a family taxonomic level (Fig. 3), but a wide individual variability of RA was observed, as already reported by Garcia-Mazcorro et al. (2012). The variations observed at a genus taxonomic level were limited and again a large individual variation within diets and time of sampling was shown (Fig. 4). The genetic of the host is a factor that largely influences the gut microbial community, at least in humans (Goodrich et al., 2017) and livestock (Roehe et al., 2016, Sandri et al., 2018).
The significant increase of Megamonas in the B2 and B1 diets at T15 (Fig. 4), can be related to dietary modifications. This genus is predominant in the family of Veillonellaceae and is responsive to dietary changes (Garcia-Mazcorro et al., 2012, Sandri et al., 2018). Beloshapka et al. (2013) reported that Megamonas increased with the inclusion of inulin in the diet, suggesting a higher fermentation activity in the bowel. According to Kieler et al. (2017), members of Megamonas produce acetic and propionic acids and the reduction of RA of this genus is considered positive for obese dogs, because it limits the amounts of energy substrates produced by colonic fermentation. However, Sandri et al. (2018) found that RA of Megamonas correlates negatively with acetate and positively with lactate, confirming the significant increase of lactic acid (Table 4) in potato-based diet (B2). For the CD diet, the significantly increased at T15 in comparison to T0 of Faecalibacterium, a producer of SCFA (Minamoto et al., 2015), did not correspond to a significant variation of fatty acids in feces. Nevertheless, Faecalibacterium and Prevotella are considered beneficial for the gut health, and a decrease of their RA has been observed in dogs affected by inflammatory bowel disease (Suchodolski, 2015). However, these genera showed a high individual variability also at T0 (Fig. 4), when dogs were fed with the same diet, making the observed changes of RA at T15 in the CD diet not easy to interpret. The gut microbiota is a highly complex ecosystem, where the interactions among microbial communities, more than the variation of a single microorganism, probably play a major role in the regulation of gut health.
4. Conclusions
The study investigated the effect that diets based on raw meat and supplemented with different sources of in vitro starch digestion fractions have on fecal microbiome of healthy dogs. The results underlined that the variation of starch fractions had minor influence on the microbiota profile and on the end products of fermentation, suggesting that each dog presents a uniqueness of fecal microbiome, which is almost resilient to slight dietary modifications, particularly in older dogs. Among the interesting variations, the potato base diet enhanced the molar proportion of lactic acid and caused a decrease of pH and N–NH3 concentrations, but the change of RA of microbiota was limited, and a fecal microbial signature of a specific diet was not observed.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was made funded from Department of Agrofood, Environmental and Animal Science of the University of Udine, Grant PRID2017.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.03.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Addinsoft . 2019. XLSTAT Statistical and data analysis solution.https://www.xlstat.com Boston, MA, USA. [Google Scholar]
- Algya K.M., Cross T.L., Leuck K.N., Kastner M.E., Baba T., Lye L., de Godoy M.R.C., Swanson K.S. Apparent total tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked, and raw diets. J Anim Sci. 2018;96:3670–3683. doi: 10.1093/jas/sky235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) 17th ed. 2000. Official methods of analysis. Gaithersburg, MD, USA. [Google Scholar]
- Arendt M., Fall T., Lindblad-Toh K., Axelsson E. Amylase activity is associated with AMY2B copy numbers in dog: implications for dog domestication, diet and diabetes. Anim Genet. 2014;45:716–722. doi: 10.1111/age.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazolli R.S., Vasconcellos R.S., de-Oliveira L.D., Sá F.C., Pereira G.T., Carciofi A.C. Effect of the particle size of maize, rice, and sorghum in extruded diets for dogs on starch gelatinization, digestibility, and the fecal concentration of fermentation products. J Anim Sci. 2015;93:2956–2966. doi: 10.2527/jas.2014-8409. [DOI] [PubMed] [Google Scholar]
- Beloshapka A.N., Dowd S.E., Duclos L., Swanson K.S. Comparison of fecal microbial communities of healthy adult dogs fed raw meat-based or extruded diets using 454 pyrosequencing. J Anim Sci. 2011;89(E-Suppl. 1):284. [Google Scholar]
- Beloshapka A.N., Dowd S.E., Suchodolski J.S., Steiner J.M., Duclos L., Swanson K.S. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol Ecol. 2013;84:532–541. doi: 10.1111/1574-6941.12081. [DOI] [PubMed] [Google Scholar]
- Bermingham E.N., Maclean P., Thomas D.G., Cave N.J., Young W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. Peerj. 2017;5:e3019. doi: 10.7717/peerj.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiofalo B., De Vita G., Presti V.L., Cucinotta S., Gaglio G., Leone F., Di Rosa A.R. Grain free diets for utility dogs during training work: evaluation of the nutrient digestibility and faecal characteristics. Anim Nutr. 2019;5:297–306. doi: 10.1016/j.aninu.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mazcorro J.F., Dowd S.E., Poulsen J., Steiner J.M., Suchodolski J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen. 2012;1:340–347. doi: 10.1002/mbo3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuberti G., Gallo A., Masoero F. Plasma glucose response and glycemic indices in pigs fed diets differing in in vitro hydrolysis indices. Animal. 2012;6–7:1068–1076. doi: 10.1017/S1751731111002345. [DOI] [PubMed] [Google Scholar]
- Goodrich J.K., Davenport E.R., Clark A.G., Ley R.E. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;27:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudez R., Weber M., Biourge V., Nguyen P. Influence of different levels and sources of resistant starch on faecal quality of dogs of various body sizes. Br J Nutr. 2011;106(Suppl 1):S211–S215. doi: 10.1017/S0007114511003345. [DOI] [PubMed] [Google Scholar]
- Haenen D., Zhang J., Souza da Silva C., Bosch G., van der Meer I.M., van Arkel J., van den Borne J.J., Pérez Gutiérrez O., Smidt H., Kemp B., Müller M., Hooiveld G.J. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- Hewson-Hughes A.K., Gilham M.S., Upton S., Colyer A., Butterwick R., Miller A.T. The effect of dietary starch level on postprandial glucose and insulin concentrations in cats and dogs. Br J Nutr. 2011;106(Suppl 1):S105–S209. doi: 10.1017/S0007114511001887. [DOI] [PubMed] [Google Scholar]
- Hung P.V., Vien N.V., Lan-Phi N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016;191:67–73. doi: 10.1016/j.foodchem.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Jiminez J.A., Uwiera T.C., Abbott D.W., Uwiera R.R.E., Inglis G.D. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016;8:67. doi: 10.1186/s13099-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K.R., Forster G., Dowd S.E., Ryan E.P., Swanson K.S. Effects of dietary cooked navy bean on the fecal microbiome of healthy companion dogs. PLoS One. 2013;8(9):e74998. doi: 10.1371/journal.pone.0074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieler I.N., Shamzir Kamal S., Vitger A.D., Nielsen D.S., Lauridscen C., Bjornvard C.R. Gut microbiota composition may relate to weight loss rate in obese pet dogs. J Vet Med Sci. 2017;3:252–262. doi: 10.1002/vms3.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., An J.U., Kim W., Lee S., Cho S. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the illumine MiSeq platform. Gut Pathog. 2017;9:68. doi: 10.1186/s13099-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10–15. [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–e8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallanes-Cruz P.A., Flores-Silva P.C., Bello-Perez L.A. Starch structure influences its digestibility: a review. J Food Sci. 2017;82:2016–2023. doi: 10.1111/1750-3841.13809. [DOI] [PubMed] [Google Scholar]
- Maria A.P.J., Ayane L., Putarov T.C., Loureiro B.A., Neto B.P., Casagrande M.F., Gomes M.O.S., Glória M.B.A., Carciofi A.C. The effect of age and carbohydrate and protein sources on digestibility, fecal microbiota, fermentation products, fecal IgA, and immunological blood parameters in dogs. J Anim Sci. 2017;95:2452–2466. doi: 10.2527/jas.2016.1302. [DOI] [PubMed] [Google Scholar]
- Martinez I., Kim J., Duffy P.R., Schlegel V.L., Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbos I.S., Vester Boler B.M., Qu A., White B.A., Swanson K.S., Fahey G.C. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto Y., Otoni C.C., Steelman S.M., Büyükleblebici O., Steiner J.M., Jergens A.E., Suchodolski J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microb. 2015;6:33–47. doi: 10.1080/19490976.2014.997612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.M., Fahey G.C., Jr., Merchen N.R., Sunvold G.D., Reinhart G.A. Evaluation of selected high-starch flours as ingredients in canine diets. J Anim Sci. 1999;77:2180–2186. doi: 10.2527/1999.7782180x. [DOI] [PubMed] [Google Scholar]
- Murray S.M., Flickinger E.A., Patil A.R., Merchen N.R., Brent J.L., Jr., Fahey G.C., Jr. In vitro fermentation characteristics of native and processed cereal grains and potato starch using ileal chyme from dogs. J Anim Sci. 2001;79:435–444. doi: 10.2527/2001.792435x. [DOI] [PubMed] [Google Scholar]
- Nagpal R., Wang S., Solberg Woods L.C., Seshie O., Chung S.T., Shively C.A., Register T.C., Craft S., McClain D.A., Yadav H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol. 2018;9:2897. doi: 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . The National Academies Press; Washington, DC, USA: 2006. Nutrient requirements of dogs and cats. [Google Scholar]
- Panasevich M.R., Kerr K.R., Dilger R.N., Fahey G.C., Guérin-Deremaux L., Lynch G.L., Wils D., Suchodolski J.S., Steer J.M., Dowd S.E., Swanson K.S. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br J Nutr. 2015;113:125–133. doi: 10.1017/S0007114514003274. [DOI] [PubMed] [Google Scholar]
- Panasevich M.R., Rossoni Serao M.C., de Godoy M.R.C., Swanson K.S., Guérin-Deremaux L., Lynch G.L., Wils D., Fahey G.C., Dilger R.N. Potato fiber as a dietary fiber source in dog foods. J Anim Sci. 2013;91(11):5344–5352. doi: 10.2527/jas.2013-6842. [DOI] [PubMed] [Google Scholar]
- Peixoto M.C., Ribeiro É.M., Maria A.P.J., Loureiro B.A., di Santo L.G., Putarov T.C., Yoshitoshi F.N., Pereira G.T., Sá L.R.M., Carciofi A.C. Effect of resistant starch on the intestinal health of old dogs: fermentation products and histological features of the intestinal mucosa. J Anim Physiol Anim Nutr. 2017;102:e111–e121. doi: 10.1111/jpn.12711. [DOI] [PubMed] [Google Scholar]
- Ribeiro É.M., Peixoto M.C., Putarov T.C., Monti M., Pacheco P.D.G., Loureiro B.A., Pereira G.T., Carciofi A.C. The effects of age and dietary resistant starch on digestibility, fermentation end products in faeces and postprandial glucose and insulin responses of dogs. Arch Anim Nutr. 2019;73:485–504. doi: 10.1080/1745039X.2019.1652516. [DOI] [PubMed] [Google Scholar]
- Roehe R., Dewhurst R.J., Duthie C.A., Rooke J.A., McKain N., Ross D.W., Hyslop J.J., Waterhouse A., Freeman T.C., Watson M., Wallace R.J. Bovine host genetic variation influences rumen microbial methane production with best selection criterion for low methane emitting and efficiently feed converting hosts based on metagenomic gene abundance. PLoS Genet. 2016;12:e1005846. doi: 10.1371/journal.pgen.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M., Dal Monego S., Conte G., Sgorlon S., Stefanon B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet Res. 2017;13:65. doi: 10.1186/s12917-017-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M., Licastro D., Dal Monego S., Sgorlon S., Stefanon B. Investigation of rumen metagenome in Italian Simmental and Italian Holstein cows using a whole-genome shotgun sequencing technique. Ital J Anim Sci. 2018;17:890–898. [Google Scholar]
- Sandri M., Sgorlon S., Conte G., Serra A., Dal Monego S., Stefanon B. Substitution of a commercial diet with raw meat complemented with vegetable foods containing chickpeas or peas affects faecal microbiome in healthy dogs. Ital J Anim Sci. 2019;18(1):1205–1214. [Google Scholar]
- Sandri M., Manfrin C., Pallavicini A., Stefanon B. Microbial biodiversity of the liquid fraction of rumen content from lactating cows. Animal. 2014;8:572–579. doi: 10.1017/S1751731114000056. [DOI] [PubMed] [Google Scholar]
- Schauf S., de la Fuente G., Newbold C.J., Salas-Mani A., Torre C., Abecia L., Castrillo C. Effect of dietary fat to starch content on fecal microbiota composition and activity in dogs. J Anim Sci. 2018;96:3684–3698. doi: 10.1093/jas/sky264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W.J. Diet and large intestinal disease in dogs and cats. J Nutr. 1998;128:2717–2722. doi: 10.1093/jn/128.12.2717S. [DOI] [PubMed] [Google Scholar]
- Stercova E., Kumprechtova D., Auclair E., Novakova J. Effects of live yeast dietary supplementation on nutrient digestibility and fecal microflora in beagle dogs. J Anim Sci. 2016;94(7):2909–2918. doi: 10.2527/jas.2016-0584. [DOI] [PubMed] [Google Scholar]
- Suchodolski J.S., Dowd S.E., Wilke V., Steiner J.M., Jergens A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the Duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 2012;7:e39333. doi: 10.1371/journal.pone.0039333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet J. 2015;215:30–37. doi: 10.1016/j.tvjl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Swanson K.S., Grieshop C.M., Flickinger E.A., Bauer L.L., Healy H.P., Dawson K.A., Merchen N.R., Fahey G.C., Jr. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J Nutr. 2002;132:980–989. doi: 10.1093/jn/132.5.980. [DOI] [PubMed] [Google Scholar]
- Xu J., Verbrugghe A., Lourenço M., Janssens G.P., Liu D.J., Van de Wiele T., Eeckhaut V., Van Immerseel F., Van de Maele I., Niu Y., Bosch G., Junius G., Wuyts B., Hesta M. Does canine inflammatory bowel disease influence gut microbial profile and host metabolism? BMC Vet Res. 2016;12:114. doi: 10.1186/s12917-016-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.