Abstract
Neurodegenerative diseases are debilitating and currently incurable conditions causing severe cognitive and motor impairments, defined by the progressive deterioration of neuronal structure and function, eventually causing neuronal loss. Understand the molecular and cellular mechanisms underlying these disorders are essential to develop therapeutic approaches. MicroRNAs (miRNAs) are short non-coding RNAs implicated in gene expression regulation at the post-transcriptional level. Moreover, miRNAs are crucial for different processes, including cell growth, signal transmission, apoptosis, cancer and aging-related neurodegenerative diseases. Altered miRNAs levels have been associated with the formation of reactive oxygen species (ROS) and mitochondrial dysfunction. Mitochondrial dysfunction and ROS formation occur in many neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases. The crosstalk existing among oxidative stress, mitochondrial dysfunction and miRNAs dysregulation plays a pivotal role in the onset and progression of neurodegenerative diseases. Based on this evidence, in this review, with a focus on miRNAs and their role in mitochondrial dysfunction in aging-related neurodegenerative diseases, with a focus on their potential as diagnostic biomarkers and therapeutic targets.
Keywords: miRNAs, mitochondrial dysfunction, reactive oxygen species, neurodegenerative disease, aging
1. Introduction
The brain is particularly susceptible to oxidative stress because of the elevated level of oxygen consumption, with a consequent high ATP need and low antioxidant defenses. During aging and neurodegenerative disorders, a progressive neuronal loss occurs parallel with cell dysfunctions due to mitochondrial impairment, oxidative imbalance and altered metabolism [1,2]. Neurodegenerative disease, including Alzheimer’s (AD), Parkinson’s (PD), Huntington disease (HD) and amyotrophic lateral sclerosis (ALS) are debilitating and presently incurable illnesses leading to significant motor and cognitive decline principally during aging [3]. The mechanisms underlying neurodegeneration are still unclear, but it would be relevant for developing new therapeutic approaches. Mitochondria are critical regulators of neuronal cell death and survival, especially during aging; mitochondrial energy impairment induces decreased ATP production, altered calcium buffering, and improved reactive ROS production [4,5,6]. Low ROS concentrations are essential for normal cellular signaling, whereas higher levels and long-time exposure cause damage to cellular macromolecules such as DNA, lipids and proteins, and accelerates the mutation rate of mitochondrial DNA (mtDNA) [1,7]. In the last decades, accumulated evidence suggests that mitochondrial dysfunction occurring during aging and oxidative stress contributes to the physiological decline that appears with aging and aging-related neurodegeneration [8]. Recently, numerous investigations focused on the miRNAs role in mitochondrial dysfunction and neurodegeneration. By binding to the 3′ untranslated region (UTR) of the mRNA of specific genes, miRNAs inhibit the translation of particular genes [9] and exert a considerable role in protein expressions, genes regulation, and phenotypic alterations in human disorders. miRNAs also were shown to target genes involved in ROS production and mitochondrial impairment [10]. Senescence is characterized by mitochondrial damage, and miRNAs can regulate the senescence process by monitoring mitochondria autophagy through the expression of miR-210, miR-376a, miR-486-5p, miR-494, and miR-542-5p, which regulate the autophagy with the nmTOR-related mechanism.
In contrast, miR-101 inhibited autophagy by targeting several proautophagic proteins. Additionally, miR-210 is upregulated in senescence and acts by preventing the translation of electron transport chain (ETC) machinery [11]. Further, miR-494 causes senescence, probably controlling ATP synthesis by the ETC and cell cycle, and it is a potential biomarker for mitochondrial dysfunction [12].
Other miRNAs, including miR-34a and miR-335 enhance ROS generation, leading to the downregulation of mitochondrial antioxidative enzymes. MiR-23a/b reduces ATP production by targeting proteins implicated in ATP synthesis through amino acid catabolism [13]. The mitochondrial protein Sirtuin 4 (SIRT4) is overexpressed under stress, and miR-15b inhibits SIRT4, counteracting senescence, and the related mitochondrial impairment [14]. Additionally, oxidative stress can be due to the aberrant expression of miRNAs. For instance, miR-146a downregulates the NOX4 subunit of NADPH oxidase. This miRNA is reduced during senescence, inducing to elevated NADPH oxidase activity, with a consequent increase of oxidative stress [15]. In another study, in oxidative stress-treated neurons and a senescence mouse model, miR-329, miR-193b, miR-20a, miR-296 and miR-130b appear overexpressed [16]. These miRNAs are implicated in numerous functions, including cell survival, cancer, apoptosis and signal transmission, mainly dependent on mitogen-activated protein kinase signaling pathway [17]. In this review we will discuss the characteristics and the role of miRNAs in neurodegenerative diseases and their potential as diagnostic biomarkers and/or therapeutic targets.
2. miRNA Biology and Regulation
MiRNAs are conserved small non-coding RNAs that exert a significant part in the post-transcriptional control of gene expression [18]. At present, over 2000 miRNAs have been identified and are available on http://www.mirbase.org. Researchers showed that miRNAs are differentially expressed depending on cell kinds and tissues. miRNAs are assumed to affect different cellular activities, comprising of aging, replicative senescence, cell proliferation and development [19]. Two-thirds of these miRNAs are located in the intronic portion of genes, while the others are in the coding portion. Mature miRNA are formed in different steps: initially, by cooperating with RNA polymerase II, the miRNA genes are transcribed to the miRNA primary transcript (pri-miRNA) presenting some attributes of RNA polymerase II among which 5′ cap structure and 3′ poly (A) tail [20]. Pri-miRNA is cleaved in its precursor (pre-miRNA) by intranuclear RNase III Drosha. The pre-miRNA is transferred into the cytoplasm by Exportin 5, the Ran-GTP-dependent nucleo-cytoplasmic transporter, situated in the cell membrane. Exportin 5, binding pre-miRNA containing a 3′ poly (A) tail, keeps the integrity of this region through the transport from the nucleus to the cytoplasm. In the cytoplasm, the pre-miRNA is cleaved by RNase III Dicer to form mature miRNA with 21-23 nucleotides [21]. The miRNA is taken to the RNA-induced silencing complex (RISC) to create the miRNA RISC complex (miRISC), which identifies and links the 3′ untranslated region (3′UTR) of mRNA through a miRNA-specific sequence. Argonaute protein (Ago-1 and Ago-2) is crucial for miRISC-mediated silencing [22,23,24]. According to the current studies, mature miRNAs are sorted into exosomes (exosomal-miRNA, ex-miRNA) [25]. Exosomes are generated by early endosomes (EEs) formed from the inner plasma membrane. EEs cooperate with the Golgi complex for the generation of late endosomes or multivesicular bodies (MVBs), which, in turn, led to intraluminal vesicle (ILVs) formation (i.e., exosomes) across the internal budding of the plasma membrane [26,27]. The MVBs can be degraded by merging with lysosomes or can be released as exosome into the extracellular space by fusing with the plasma membrane. Ras-associated binding (RAB) proteins control the MVBs trafficking to the plasma membrane and in the secretion of exosomes (ex-miRNA) [28]. Cerebrospinal fluid (CSF) and peripheral blood are the main sources of exosomes from the nervous system [29]. The analysis of these specific ex-miRNAs can suggest the physiological situation of the nervous system and offer a potential biomarker for neurodegenerative diseases. Different factors influence the levels of miRNAs in common fluids, including inflammatory factors, lifestyle, sex and ethnicity [30,31,32]. Alterations of the nervous system microenvironment influence the nature of ex-miRNA released by nerve cells, and miRNAs composition is substantially modified as well, suggesting that ex-miRNAs are valuable as early diagnostic markers for neurodegenerative disease. MiRNAs are controlled by processes comparable to other RNAs, such as epigenetic repression, transcriptional activation or inhibition and degradation rates. Intronic miRNAs are mainly controlled by their host gene, and processed from the intron, but may have different promoter regions. Upstream signaling begins the transcription of miRNA genes and generates reaction loops by targeting their transcription factors [33]. The half-life of miRNAs is usually extended; indeed, they persist for 5 or more days, even if certain miRNAs have a quick turnover [34]. With a high-throughput RNA sequencing analysis, Shao and collaborators [35] reported several brain-specific miRNAs, including miR134, miR-135, let-7g, miR-101, miR-181a-b, miR-191, miR-124, miR-let-7c, let-7a, miR-29a and miR-107 [36]. The majority of these miRNAs regulate synaptic plasticity, neurotransmitter release and neurite growth; for this reason, it is crucial to study how these miRNAs can participate in the onset and progression of neurodegenerative diseases. As previously said, the involvement of miRNAs in different important processes in neurodegeneration has been strongly recognized [37,38]. MiRNAs participate in neurodegenerative disorders mainly by three pathways: (1) involvement in neuroinflammation through a toll-like receptor (TLR) or regulating TLR mRNA expression; (2) block of the translation of proteins or degrading proteins via target regulation-related gene mRNA and (3) miRNA formation disorder [39] (Table 1).
Table 1.
The most representative miRNAs involved in neurodegenerative diseases.
| miRNAs | Role In | Target | Action | References |
|---|---|---|---|---|
| miR-26b ↑ | AD | Rb1 | Upregulation of Rb1/E2F cell cycle and proapoptotic transcriptional targets | [43] |
| miR-206 ↑ | AD | BDNF | Binds and downregulates specifically the 3-UTR region of BDNF | [44] |
| miR-34a ↑ | AD | VAMP2, SYT1, HCN1, NR2A, GLUR1, BCL2, | Targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity | [45] |
| miR-126 ↑ | AD | IRS-1, PIK3R2 | Downregulated elements in the GF/PI3K/AKT and ERK signaling cascades | [46] |
| miR-9 ↓ | AD | Aβ | Increases production of γ-secretase and maintains neurons in sustaining Aβ production | [47] |
| miR-221 ↓ | AD | ADAM10 | Indirectly represses the 1 expression through the suppression of TIMP3 gene | [48] |
| miR-98 ↑ | AD | HEY2 | Reduces oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 protein | [49] |
| miR-330 ↓ | AD | VAV1 | Reduces oxidative stress and mitochondrial dysfunction through the MAPK pathway | [50] |
| miR-19 ↓ | AD | BACE1 | Alters PTEN/AKT/p53 pathway | [51] |
| miR-30 ↓ | AD | P53, DRP1 | Involved in regulating mitochondrial dynamics via Drp1 through p53 | [17] |
| miR-375 ↓ | AD | p53 | Increases the expression of TP53 gene through the 3-UTR region | [52] |
| miR-7 ↓ | PD | α-Synuclein, VDAC1 | Binds and downregulates specifically the 3-UTR region of synuclein | [53] |
| miR-124 ↓ | PD | FOXOa2 | Binds and downregulates specifically to the 3-UTR region of FOXO gene its | [54] |
| miR-153 ↓ | PD | α-Synuclein | Bind specifically and downregulates the 3-UTR region of synuclein | [55] |
| miR-443 ↑ | PD | FGF20 | Increases FGF20 mRNA translation that increases α-synuclein expression | [56] |
| miR-494 ↑, miR-34b/c ↓ | PD | DJ-1 | Binds and downregulates specifically the 3-UTR region of DJ.1 | [57,58] |
| miR-205 ↓ | PD | LRKK2 | Suppresses the expression of LRRK2 protein through the 3-UTR region | [59] |
| miR-27a ↓, miR-105 ↓ | PD | ATP5G3 (complex V) | TNF-α regulates the expression of miR-27a which regulates the transcript levels of ATP5G3 |
[60] |
| miR-103 ↑ | PD | Complex I | TNF-α regulates the expression of mir-103 which regulates the transcript levels of complex I | [60] |
| miR 4639 5p ↓ | PD | DJ-1 | Suppresses the expression of PARK7 protein through the 3-UTR region | [61] |
| miR-21 ↑ | PD | BCL-2, PTEN | Suppresses the expression of BCL-2 and PTEN protein through the 3-UTR region | [62] |
| miR-331-5p ↑ | PD | NRP2 | Downregulation through the NRP2 gene | [63] |
| miR-132 ↓ | HD | p250GAP, ACHE | Enhances neurite outgrowth and breakdown of the neurotransmitter acetylcholine through the dysregulation by p250GAP/ACHE | [64] |
| miR-125b ↓, miR-196a ↓, miR-146a ↓ | HD | mHTT | CAG length-dependent changes in miRNA expression in brain | [65,66,67] |
| miR-9 ↑, miR-124 ↑, miR-29a ↑ |
HD | REST | Suppresses the expression of REST protein through the 3-UTR region | [68,69] |
| miR-129-5p ↑, miR-133a ↓ | ALS | SOD1 | Suppresses the expression of SOD1 protein through the 3-UTR region | [70,71] |
| miR-27a-b, miR-335-5p ↑ |
ALS | Caspase 3/7 | Alters mitochondrial dynamics and activates caspase 3/7 | [72] |
| miR-151b ↓ | ALS | PINK1, UCP2, PKM | Determine the downregulation of the expression of these genes | [73] |
| miR-221-3p ↓ | ALS | BAX, ITGA5, PRKCD, | Determine the downregulation of the expression of these genes | |
| miR-130a-3p ↓ | ALS | ABCG1, LGALS3, CTDSP1 | Determine the downregulation of the expression of these genes |
↑ = miRNA upregulated in related disease; ↓ = miRNA downregulated in related disease. AD = Alzheimer’s disease; PD = Parkinson’s disease; HD = Huntington’s disease; ALS = Amyotrophic lateral sclerosis.
In the brain there is a wide variety of miRNAs, mainly localized in specific tissues (Table 2); moreover, some of them show a precise pattern of expression during brain development [40,41]. MiRNAs also have a functional role in different brain activities, thus it is relevant to deepen our knowledge regarding the signaling pathway involved in a neurodegenerative disease [42] (Table 1).
Table 2.
Specificity and tissue localization of the most important miRNAs involved in neurodegenerative diseases.
| miRNAs | Specificity | Localization | References |
|---|---|---|---|
| miR-26b ↑ | AD, PD | CNS, Blood | [43] |
| miR-206 ↑ | AD, ALS | CSF | [44] |
| miR-34a ↑ | AD, PD, HD | CNS, Blood | [45] |
| miR-126 ↑ | AD, PD | CNS | [46] |
| miR-9 ↓ | AD | Cortex | [47] |
| miR-221 ↓ | AD | Cortex | [48] |
| miR-98 ↑ | AD | Cortex | [49] |
| miR-330 ↓ | AD, HD | CNS | [50] |
| miR-19 ↓ | AD, PD | CNS | [51] |
| miR-30 ↓ | AD, PD | Blood, CSF | [17] |
| miR-375 ↓ | AD | CSF | [53] |
| miR-7 ↓ | PD | CNS | [52] |
| miR-124 ↓ | PD, AD | CNS | [54] |
| miR-133b ↓ | PD, HD, ALS | CNS, Cortex | [74] |
| miR-153 ↓ | PD | CNS | [55] |
| miR-443 ↑ | PD | CNS | [56] |
| miR-494 ↑ | PD | CSF | [57] |
| miR-34b/c ↓ | PD, AD, HD | CNS, Blood | [58] |
| miR-205 ↓ | PD | CNS | [59] |
| miR-27a ↓ | PD, ALS | CNS | [60] |
| miR-105 ↓ | PD, AD | CSF | [60] |
| miR-103 ↑ | PD, AD | CNS, Blood | [60] |
| miR 4639 5p ↓ | PD | CNS | [61] |
| miR-21 ↑ | PD, AD | Lymphocytes, CSF, Blood | [62] |
| miR-331-5p ↑ | PD | CNS, Blood | [63] |
| miR-132 ↓ | HD, ALS, AD | CNS, CSF, Cortex, Cerebellum | [64] |
| miR-125b ↓ | HD, AD | CNS, Hippocampus | [65] |
| miR-146a ↓ | HD, AD, ALS | CSF, Spinal cord | [66] |
| miR-196a ↓ | HD | Cortex | [67] |
| miR-9 ↑ | HD, AD, ALS | CNS | [68] |
| miR-124 ↑ | HD, AD | CSF, Hippocampus, Cortex | [69] |
| miR-29a ↑ | HD, AD, PD | Cortex, Blood | [69] |
| miR-129-5p ↑ | ALS. PD | Cerebellum, CSF | [70] |
| miR-27a/b ↓ | ALS | Monocytes CD14 + CD16- | [72] |
| miR-335-5p ↑ | ALS | CNS | [72] |
| miR-151b ↓ | ALS, HD, AD | Cortex, CNS | [73] |
| miR-221-3p ↓ | ALS | CNS | [73] |
| miR-130a-3p ↓ | ALS | CNS | [73] |
↑ = miRNA upregulated in related disease; ↓ = miRNA downregulated in related disease. AD = Alzheimer’s disease; PD = Parkinson’s disease; HD = Huntington’s disease; ALS = Amyotrophic lateral sclerosis; CNS = central nervous system; CSF = cerebrospinal fluid.
3. miRNA and Aging
Aging is a physiological aspect of all live beings and an additional factor in the progression of neurodegenerative disorders. Studies previously performed identified numerous genes responsible for selective degeneration of neurons in different neurodegenerative disorders [75], such as PD, AD, ALS and HD. A key factor for the aging process is cellular senescence, neuroinflammation, telomere shortening, change in homeostasis, among which oxidative injury, mitochondrial aberration, somatic and germline DNA alterations, which all may exacerbate the aging process. Cellular mechanisms of aging and age-related disorders are not fully known. Xurde et al. [76] evaluated cellular and molecular hallmarks that define the aging phenotype. These “hallmarks of aging” have been distributed into three groups: primary hallmarks (genomic instability, telomere shortening, epigenetic mutations and loss of proteostasis), antagonistic hallmarks (altered nutrient-sensing, mitochondrial aberration and cellular senescence) and integrative hallmarks (changed intercellular communication and stem cell collapse). Nevertheless, recent discoveries revealed that miRNAs are possible sensors of aging and cellular senescence [10].
Cellular senescence includes the permanent interruption of growth and programmed cell death. During the senescence process, numerous tumor suppressors and death signalings, such as p53, p21 and p16, inhibit many of the normal functions of the cell, and this system is generally regulated by miRNA expression [77,78]. Studies showed that miRNAs, which controls genes participating in the DNA injury checkpoint response and the insulin signaling pathways, influence life expectancy and aging processes [79]. One of the ways used to manipulate aging in vivo is the decrease the insulin IGF-1 signaling, the main insulin pathway known to regulate aging together with PI3K/Akt pathway; these two pathways can control aging and are altered in neurodegenerative disorders, including PD, AD and HD [80]. Kim and collaborators [46] identified a miR-126, target of IGF-1/PI3K signaling and participates in PD pathogenesis. Additional findings from Kim et al. demonstrated that miR-126 plays a crucial role in the link existing among metabolic dysfunction and neurotoxicity by controlling IGF/PI3K signaling in the aged brain and the pathogenesis of neurodegenerative diseases, including PD and AD [46].
Further, elevated expression of miR-126 in rats and humans brains was observed, suggesting its involvement in brain functions [81]. In addition, the inhibition of miR-126 is protective against the staurosporine (apoptosis inducer and non-selective inhibitor of protein kinases) and Aβ1–42 deleterious effect. During aging, the expression of miR-126 rises, and neurons result more vulnerable to staurosporine or Aβ1–42. Further, the regulation of IGF/PI3K signaling in neurons by miR-126 plays an essential mechanistic connection between metabolic alteration and neurotoxicity during aging, PD and AD [46].
Zhang and collaborators [82] reported that miRNAs present in the exosomes of hypothalamic stem/progenitor cells control brain aging, and, during aging, a decrease in the expression of exosomal miRNAs occurs. Additionally, treatment with healthy hypothalamic stem cells or progenitor cell-secreted exosomes were able to counteract the aging progression, partly via the release of exosomal miRNAs [82]. Mitochondrial dysfunction and protein misfolding represent two main events in the majority of the neurodegenerative disorders; this suggests that the molecular mechanisms involved in the onset and progression of these diseases may show comparisons with aging [75]. Lang and collaborators observed that the inhibition of miR-15b leads to increased mitochondrial ROS, decreased mitochondrial membrane potential and modulation of the mRNA levels of nuclear-encoded mitochondrial genes and components of the senescence-associated secretory phenotype (SASP) in a SIRT4-dependent way. Indeed, miR-15b is a negative regulator of stress-induced SIRT4 expression, thus avoiding the senescence-associated mitochondrial aberration and controlling the SASP and organ aging, i.e., photoaging of human skin. Insight of this, the researchers identified miR-15b as a new regulator of stress-induced increase of SIRT4 expression. They linked the miR-15b–SIRT4 axis to senescence-associated mitochondrial dysfunction/redox homeostasis and SASP regulation.
The identification of miRNAs commonly present during aging and neurodegeneration support the knowledge of the complex mechanisms underlying the development of neurodegenerative diseases. One significant result, obtained from a study on AD patients, was the increase in DNA methylation in the temporal cortex, which directly targets the CpG islands of miRNAs. One of the major improvements in neurodegenerative research was the finding regarding neuronal plasticity, which suggests that the aging and neurodegenerative process can be controlled by genetic, nutritional and pharmacological factors [83]. The expression of miRNAs in the brain is controlled by different environmental toxicants (metal/pesticides), the abuse of drugs (ethanol/nicotine) and other components [84,85].
Singh et al. [75] demonstrated that knocking down the Dicer gene in SH-SY5Y, a human neuroblastoma cell line, enhances its sensitivity for ethanol treatment. MiRNA profiling studies in SH-SY5Y cells upon acute and chronic quantities of ethanol showed remarkable alterations in the miRNA profile. The miRNA profiling analyses identified significantly elevated miR-497 and miR-302b levels, upon 72 h of ethanol exposure. Elevated levels of miR-497 led to the downregulation of B-cell lymphoma 2 (BCl2), an anti-apoptotic protein, which led to enhanced apoptosis of neuronal cells [84].
As previously mentioned, miRNAs are released into peripheral body fluids through microvesicles and exosomes. These ‘circulatory’ miRNAs are present in at least 12 different human body fluids, comprising of saliva, cerebrospinal fluid, blood, amniotic fluid, urine and breast milk [86]. Circulatory miRNAs are abundant, stable and change with chronological age and during the development of age-related pathologies, triggering fascination as non-invasive “biomarkers of aging” [87].
Recent studies evaluated differential miRNA expression in different peripheral fluids, including serum [19,88], plasma [89,90] and saliva [91], comparing young and aged people. Hooten and collaborators [92] reported circulating miRNAs in serum of old (mean age of 64.6 years) and young patients (mean age of 30.5 years), detecting a decrease of miR-181a-5p, miR-1248 and miR-151a-3p during aging [92]. Another study profiling miRNAs in serum from adults of various ages found miR-29b, miR-106b, miR-130b, miR142-5p and miR-340 to be downregulated during aging, while miR-92a, miR-222 and miR-375 were upregulated [30]. Another study evaluating the differences in plasma between 374 young and old humans and miR-126-3p, miR30c-5p, miR-30b-5p, miR-210, miR-142-3p and let-7a-5p were increased in the aged patient, while the miR-93-5p result decreased [90].
4. miRNA Dysregulation and Mitochondrial Dysfunction in Alzheimer’s
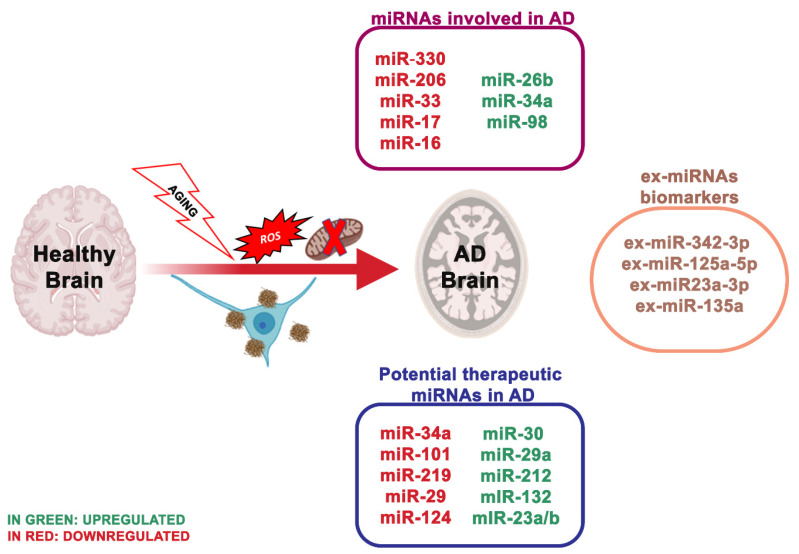
AD is one of the most prevalent diseases that affect the aged people, characterized by memory loss, impaired cognitive function and various neuropsychiatric disturbances. Two varieties of AD exist, sporadic and familial. Early-onset AD, recognized as familial AD, is a sporadic form of the disease observed in 1–2% of all AD cases. Numerous studies reported some biochemical and genetic alterations that participate to the onset, progressive degeneration and neuronal death in AD [93]: deposition of amyloid β (Aβ) peptide, proteasome inhibition [94], oxidative stress [95], mitochondrial dysfunction [96], hyperphosphorylation of tau protein and heritable mutations in presenilin 1, presenilin 2 [97] and Aβ precursor protein (APP) genes [98] (illustrated in Figure 1).
Figure 1.
The most essential miRNAs involved in Alzheimer’s disease.
Compromised energy uptake and defects in mitochondrial respiration are essential characteristics of brain tissue altered by neurodegeneration, but also of peripheral cells (platelets and fibroblasts) in AD patients. Specifically, decreased cerebral metabolism has been shown in the temporoparietal cortices of AD patients, and these variations lead to both neuropsychological impairment and atrophy, as demonstrated by neuroimaging analyses [99]. In the last years, numerous researchers tried to understand the underlying mechanism of brain metabolism decline during AD and to identify a possible connection of the reductions in mitochondrial enzyme activities with premorbid cognitive level and with plaque counts [100].
Variations in mtDNA levels, typically assessed as the mitochondrial genome to the nuclear genome ratio and the mtDNA levels in body tissues and fluids, represent a biomarker of mitochondrial aberration [100]. Chen et al. 2018 [49] examined, in scopolamine-treated mice, an animal model of AD, the effect of miR-98 on Aβ-protein, oxidative stress and altered mitochondria activity via the Notch signaling pathway by targeting hairy and enhancer of split (Hes)-related with YRPW motif protein 2 (HEY2). The data obtained in humans revealed that the expression of miR-98 was significantly different in patients with AD respect with the controls; in the AD group, when miR-98 was lower, and the expression of HEY2 was elevated, the Notch-HEY2 pathway was stimulated [38]. The Notch1 pathway is a cellular cascade with primary roles in brain development and the adult brain; the increased activation of this pathway following brain damage is deleterious for neuronal survival [101]. MiR-98 targeting HEY2 prevented the activity of the Notch pathway, promoting to the inhibition of the production of Aβ and the increase of oxidative stress and mitochondria dysfunction in AD mice. An earlier study showed that miR-98-5p regulated the expression of Sorting Nexin 6 (SNX6) and was crucial for Aβ deposits [102]. miR-98 also led to AD-like disorder by targeting insulin-like growth factor 1 and, in turn, supporting the production of Aβ, thus implying that miR-98 is vital in the development of the pathology of AD [103]. In the sight of this, additional studies are necessary to validate the impacts of miR-98 in the regulation of AD mice by targeting HEY2 through the Notch signaling pathway before its consideration as an appropriate treatment for AD. Zhou et al. [50] described the effect of miRNA targeting proto-oncogene vav (VAV) on Aβ production, oxidative stress and mitochondrial malfunction in AD mice through the MAPK signaling pathway.
MiR-330 appeared decreased in neuronal cells of AD mice, and proto-oncogene VAV1 was negatively controlled by miR-330. In contrast with the healthy animals, the positive protein expression rate of VAV1 was considerably higher in the AD group. Increased level of miR-330 reduced the expression of VAV1, c-Jun N-terminal kinase (JNK1), extracellular signaling kinase 1 (ERK1), mitogen-activated protein kinase (P38) and Aβ. Still, it enhanced the expression of low-density lipoprotein receptor-related protein-1 (LRP-1) and cyclooxygenase [104]. AD mice showed high Aβ production with reduced Cu/Zn Super Oxide Dismutase 1 (SOD1) levels. Moreover, miR-195 was related to mitochondrial dysfunction by mitofusin 2 deregulation. Thus, the overexpression of miR-330 in AD supports the oxidative stress, and mitochondrial dysfunction by targeting VAV1 through the MAPK signaling pathway. Another research group reported that miR-30 family members prevent mitochondrial fission targeting p53, which stimulates mitochondrial fission by transcriptionally upregulating dynamin-related protein 1 (Drp1) expression [105]. Further, miRNAs represent potential, non-invasive peripheral biomarkers in aging and other age-related disorders, including AD [106]; in fact, several miRNAs [107] were strongly influenced by age either before or during Aβ plaque deposits.
The latest studies regarding miRNAs as biomarkers are moving towards the use of ex-miRNAs [108]. Ex-miRNAs are more stable, more reliable and change upon aging, diets and pathologic states. Lugli and collaborators [109], isolated exosomes from AD patients plasma and healthy group to analyze miRNA expression using high-throughput sequencing technology. Notably, ex-miR-342-3p levels were substantially reduced in AD patients. These data were also confirmed by Rani et al. [110]; they also revealed lower levels of ex-miR-125a-5p, ex-miR-125b5p and ex-miR-451a levels in AD, and the reduction of these ex-miRNAs is associated with the extent of cognitive impairment. Another ex-miRNA study, including 10 AD patients and 15 controls, through next-generation sequencing technology, identified reduced levels of ex-miR23a-3p, ex-let-7i-5p, ex-miR-126-3p and ex-miR-151a-3p in AD patients; indicating that these altered ex-miRNAs levels in the plasma showed diagnostic value for AD [111]. Yang et al. [112], through quantitative RT-PCR measured the serum levels of three ex-miRNAs, ex-miR-135a, ex-miR-193b and ex-miR-384 in 101 patients with mild cognitive impairment and 107 patients with dementia of Alzheimer’s type (DAT). The levels of ex-miR-135a and ex-miR-384 were increased in DAT patients, while ex-miR-193b resulted reduced. Furthermore, the combination of the three miRNAs would be better for the early diagnosis of AD than any single one.
The serum level of ex-miR223 was also deregulated in AD patients; for instance, Wei et al. [113] indicated that ex-miR-223 level was substantially reduced in AD and may represent a biomarker to differentiate AD patients from healthy individuals.
Researchers have also attempted to differentiate young and late-onset AD (YOAD/LOAD). McKeever and collaborators noticed that CSF-derived ex-miR-125b-5p was enhanced in YOAD patients, whereas ex-miR-16-5p, ex-miR-451a and ex-miR-605-5p were reduced. Additionally, the different levels of ex-miR-125b-5p, ex-miR-451a and ex-miR-605-5p were comparable in both LOAD and YOAD patients [114]. Another study examined the levels of free miRNAs, and ex-miRNAs in the CSF of AD patients and, interestingly, ex-miR-9-5p and ex-miR-598 levels were substantially reduced. Nevertheless, free miR-9-5p and miR-598 were found in up to 50% and 75% of healthy control CSF samples, respectively, but they did not appear in any AD CSF sample. This variation indicated that ex-miRNAs might be valuable as possible biomarkers of AD [115].
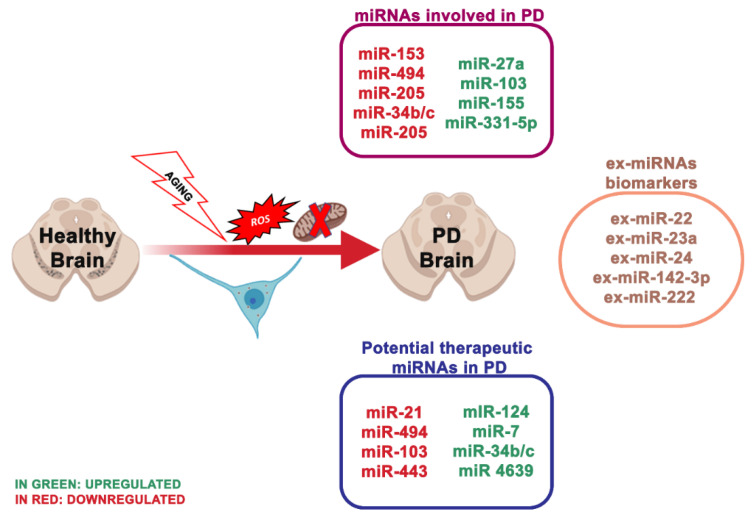
4.1. miRNA Dysregulation and Mitochondrial Dysfunction in Parkinson
PD is the second most frequent neurodegenerative disorder concerning 1% of the population over 55 years [116]. It is clinically described by motor symptoms, involving resting tremor, muscle stiffness, bradykinesia and postural instability. In PD, the imbalance between mitochondrial dysfunction and ROS production results in oxidative stress and neuronal degeneration [1]. In neuronal cells, ROS can injure macromolecules, comprising of nucleic acids, lipids and proteins, inducing dopaminergic (DA) neuron degeneration and neuronal network alteration, eventually leading to PD [117]. It is well known that oxidative stress is involved in PD. There is fascination in identifying how miRNAs are interested in the pathological processes and how they are involved in oxidative stress, mitochondrial dysfunction [118], α-synuclein aggregation [119], neuroinflammation [120] and dysregulation of the endogenous antioxidant system [121] (Figure 2). miRNAs control the gene expression in the cortex of PD patients, and the cortical expression patterns of miRNAs accurately discern PD from healthy brains [122]. Six miRNAs are correlated with the dopaminergic phenotype in PD [39]:
miR-133b that controls the transcriptional activator Pitx3, which is a crucial component in the development of the DA neuronal phenotype in vivo [74];
miR-7, which suppressed α-synuclein in human neuroblastoma cells, and it may be inhibited by oxidative stress in vitro and in vivo [53].
miR153, conserved across vertebrate species, and inhibited from α-synuclein [55];
miR-433, related to a mutation of its binding region in the 3′UTR region of the FGF20 gene. miR-433 prevented the translation of the FGF 20 gene in vitro. Single nucleotide polymorphism in FGF20 shows that the genetic variability of FGF20 may represent a PD risk [56].
miR-205, transfecting miR-205 in the neurons expressing a PD-related LRKK2 R1441G mutant avoided the neural development defects [123].
miR-124 controls the transcription activator FoxA2, relevant in midbrain dopaminergic cell development in both rodents and humans [124], and its role is necessary for dopaminergic neurons survival in PD mice [54].
Figure 2.
The most essential miRNAs involved in Parkinson’s disease.
Some of these miRNAs described are also implicated in the destruction of mitochondrial homeostasis. Once mitochondrial dysfunction occurs, leakage of ETC may directly stimulate ROS production, thus intensifying the neuronal impairment. Mitochondrial impairment and decreased complex I activity were detected in the substantia nigra pars compacta (SNpc) and frontal cortex of PD patients [125]. Wang et al. [126] demonstrated that miR-124 regulates the apoptosis and autophagy process in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model of PD. In contrast, elevated miR-124 levels impede the expression of the protein bcl-2-like protein 11 (Bim), reducing the translocation of its downstream protein bcl-2-like protein 4 (Bax) to mitochondria and lysosome, consequently inhibiting mitochondria apoptotic signaling pathways and stabilizing the impaired autophagic activity.
The encoding gene of protein DJ-1 (PARK-7) can induce autosomal recessive PD with reduced DJ-1 levels in the SNpc. Several investigations revealed that DJ-1 could directly combine to the subunits of complex I and sustain its function as an integral mitochondrial protein [127]. Mitochondrial morphology and dynamics are altered in DJ-1-knockdown neurons [128]. An exciting study suggested that miR-494 may exacerbate oxidative stress-induced neuronal damage by reducing DJ-1 expression [57]. Another study suggested that decreased miR34b/c levels parallel with reduced expression of DJ-1 impact to mitochondrial impairment in PD patients’ brains, confirmed in miR-34b/c-depleted cells. MiR-34b/c may regulate DJ-1 expression in an indirect manner; however, the specific mechanism is still unclear [58]. Another protein of interest in mitochondrial dynamics is leucine-rich repeat kinase 2 (LRRK2). Increased LRRK2 protein levels can alter the mitochondrial dynamics and integrity via dynamin-like protein (DLP1), and, notably, miR-205 expression is significantly reduced in PD patient brains, parallel with higher LRRK2 protein levels [129,130]. Further studies showed that miR-205 inhibits LRRK2 protein expression in primary neurons by targeting the 3′-UTR of the LRRK2 gene [59]. SH-SY5Y cells, upon tumor necrosis factor-α treatment, showed improved miR-27a and miR-103 levels, that may inhibit the expression of the functional units of complex I. Elevated levels of miR-155 and miR-27a may lead to mitochondrial dysfunction and oxidative stress, reducing the transcript levels of the ATP synthase membrane subunit c locus 3 (ATP5G3), a subunit of complex V [60].
Additionally, miR-7 stabilizes mitochondrial membrane potential by inhibiting the expression of the voltage-dependent anion channel 1 (VDAC1), one element of the mitochondrial permeability transition pore, which may represent a possible target for decreasing the mitochondrial dysfunction in PD [131]. Another essential protein involved in mitochondrial homeostasis is the peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1α), a crucial activator for mitochondrial genes, reduced in PD patients [132].
In consideration of the vital role of miRNAs in PD pathogenesis and progression, small RNA molecules seem an encouraging means in PD therapy, leading to potential molecular targets and supporting improved and personalized therapeutic approaches [133]. Valdes et al. [134] developed small double-stranded RNA molecules that mimic miRNAs and can function as gain-of-function tools for specific miRNAs. This miRNA can mimic reduced target proteins by interacting with the 3′UTR of the mRNA of a specific target gene. Thus, they can regulate PD-related risk genes and proteins associated with PD. However, this strategy may have possible risks of off-target effects and the possibility of undesirable interfering with other genes.
It has been demonstrated that L-DOPA treatment led to modified miRNA profiles in PD patients [135], indicating that small drugs may modify miRNA profiles in neurodegeneration, improving the disease progression. Ebert and colleagues [136] developed miRNA sponges for inhibiting various miRNA concomitantly; these miRNA sponges have a heptameric sequence able to target a complete miRNA family.
The ex-miRNA analysis is more reliable and can better reflect PD severity respect common markers for PD (DJ-1, α-synuclein, etc.). The ex-miRNAs analyses in patients with PD demonstrated that the miR-331-5p expression level was drastically increased compared to control samples. In contrast, the miR-505 expression result diminished in PD patients, thus, these two ex-miRNAs may be valuable for the early diagnosis of PD [137]. In another study, the ex-miRNA content of the PD patients serum was compared with healthy individuals and, notably, the expression of ex-miR-22, ex-miR-23a, ex-miR-24, ex-miR-142-3p and ex-miR-222 was considerably higher in the serum of PD patients [138]. Ravanidis et al. [108] applied a specially designed panel of miRNAs to evaluate the miRNA profile of genetic and idiopathic PD patients with healthy individuals. Notably, 8 of the 20 assessed miRNAs were strongly increased in PD patients, 15/20 miRNAs appeared deregulated in the genetic cohorts. Despite numerous studies on the role of miRNAs in PD, it is still difficult to fully clarify their function in neurodegenerative pathologies since the underlying factors of PD interact with each other [139], making it hard to elucidate the role of related miRNAs.
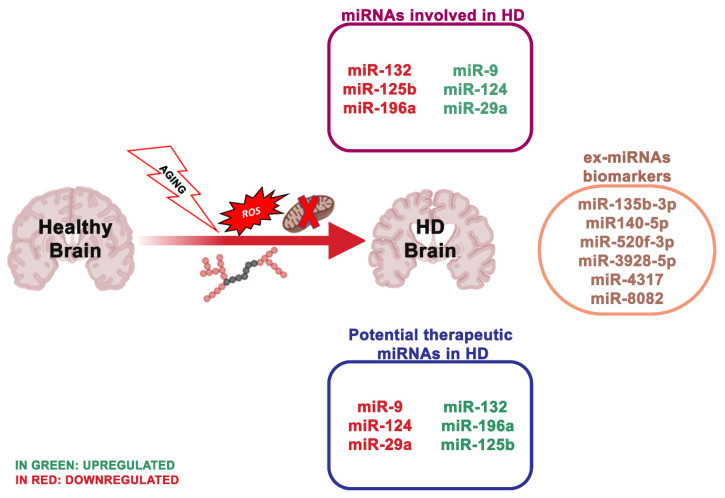
4.2. miRNA Dysregulation and Mitochondrial Dysfunction in Huntington’s Disease
HD is a dominantly inherited neurodegenerative disease clinically described by cognitive impairment, gradual movement condition and psychiatric problems [140]. The main characteristic of HD is the striatal medium spiny neurons (MSNs) degeneration, but also of deep-layer cortical pyramidal neurons. HD is due to the CAG trinucleotide repeat expansion encoding an extended polyglutamine (polyQ) section, adjacent to the N-terminus of Huntingtin (HTT) [141] (Figure 3). Healthy people have CAG repeat lengths that vary between 6 and 35, while HD patients show repeat lengths superior to 36 on one HTT allele, with the length of the CAG repeats inversely related to the age of HD onset [142]. To date, there is no cure to counteract the onset or advancement of HD.
Figure 3.
The most essential miRNAs involved in Huntington’s disease.
How altered mitochondria and bioenergetic impairment in HD developed is still unclear [143]. Mutant huntingtin (mHTT) can directly interact with the outer mitochondrial membrane (OMM) [144,145,146], supporting that mHTT directly led to mitochondrial dysfunction. In addition, this relation is due to the capability of mHTT to reduce the expression of a key protein of mitochondrial homeostasis, PGC-1α [147]. mHTT inhibits the PGC-1α pathway, which in turn avoids the activation of downstream pathways, whereas PGC-1α ectopic expression resulted neuroprotective in transgenic HD mice and the 3-NPA mouse model [65]. The upregulation and downregulation of some neuroactive miRNAs can influence mitochondrial homeostasis through the regulation of PGC-1. We can, therefore, hypothesize that by regulating the miRNAs implicated in mitochondrial dysfunctions, we could indirectly act on the interaction between mHTT and OMM, which appears to be the basis of mitochondrial dysfunctions in the HD.
Numerous researchers are focused on the study of therapies able to lower the HTT expression in the brain. The study by Stanek et al. [148] offers additional proof regarding the importance of astrocytes in the HD pathology. Indeed, astrocytes are fundamental for neural circuits; however, it is still unknown if they participate in the mechanism to initiate HD. Increasing studies reported that the presence of mHTT in astrocytes induces reduced expression of glutamate transporters and aberrant glutamate uptake, which is sufficient to led neurodegeneration in medium spiny neurons of the striatum [149]. Impaired neuronal excitability and excitotoxicity linked with HD may, therefore, be a result of altered astrocyte function [150].
MiRNAs deregulation has been described in HD in vitro models, transgenic HD animals and human HD brains [67,151]. The study of Langfelder et al. [152] was the first to explain Htt-CAG length-dependent alterations in miRNA expression in the brain regions differentially susceptible to HD. In particular, a high number (n = 159), of miRNAs resulted transformed in the striatum and a smaller number in the cerebellum (n = 102), hippocampus (n = 51) and cortex (n = 45). Notably, the number of deregulated miRNAs in the cerebellum was double that in the cortex and hippocampus, even though the cerebellum is fairly unaffected in HD. A larger study evaluated miRNA expression in HD patients, revealing multiple differentially expressed miRNAs, some of which modulating neuron survival [153]. The results obtained by Hoss et al. [154] indicated numerous miRNA changes in the HD brain, and most of these are associated with clinical manifestations of HD, where the signal is impartial of the size of the CAG repeat extension. Other research used a next-generation sequence analysis of small non-coding RNAs to analyze 26 HD patients and 36 healthy individuals. Nine hundred and thirty eight miRNAs were found, and 75 of these were differentially expressed. Concordant with these results, the downregulation of miR-132-3p in human HD parietal cortical tissue and brains of R6/2 and YAC128 HD mouse models have been detected [69,155]. miR-132 is greatly enhanced in the brain [40,156], and its expression alters neuronal morphogenesis and sustains neurite outgrowth by inhibiting the GTPase-activating protein p250GAP [64]. An additional target of miR-132 is the acetylcholinesterase (ACHE), an enzyme involved in the degradation of the neurotransmitter acetylcholine at the synapse [157]. ACHE is strongly implicated in cognitive performances, and ACHE inhibitors are FDA-approved for the treatment of AD [157]. Thus, reduced miR-132 levels may negatively influence brain health, via the p250 GAP (reducing its inhibition) and ACHE deregulation.
Lee et al. [158] created and tested an exosome-based delivery method (Ex-124) for miRNA delivery in a R6/2 transgenic HD mouse model. Unfortunately, this method was not able to ameliorate the motor symptom; however, this research is still valuable. There is no reported study exploring ex-miRNAs as biomarkers for HD diagnosis.
A study by Reed et al. [159] suggested that six miRNAs: miR-135b-3p, miR140-5p, miR-520f-3p, miR-8082, miR-4317 and miR-3928-5p were considerably elevated in presymptomatic HD patients, thus, representing valuable biomarkers for the early diagnosis of the disease. Another study revealed that the plasma level of miR-34b was sharply reduced in presymptomatic HD patients compared with controls, indicating that miR-34b may be a novel biomarker of HD, which can be steadily expressed in the plasma and identified before clinical symptoms [151].
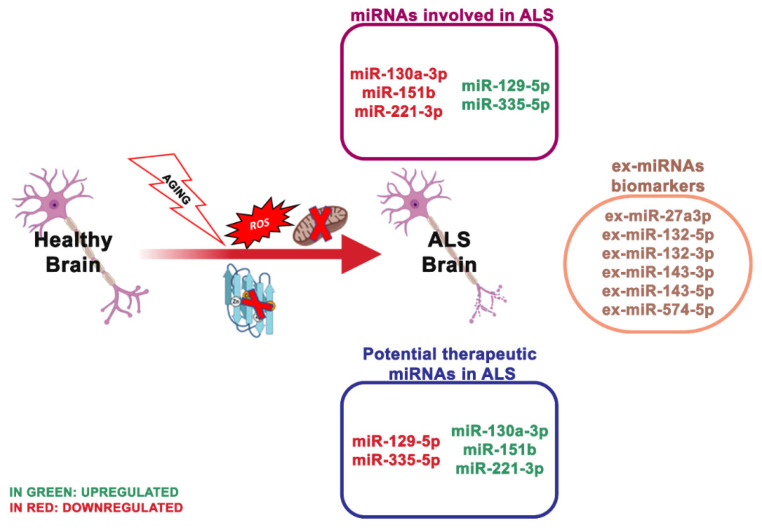
4.3. miRNA Dysregulation and Mitochondrial Dysfunction in ALS
ALS is a group of intricate multi-factorial neurodegenerative disorders. It is due to the selective deficiency of upper and lower motor neurons, thus leading to gradual skeletal muscle atrophy and death by respiration collapse after 2–5 years from the onset. ALS incidence is 2 per 100,000 persons per year. Mutations in ALS causing genes are related to the disorder in roughly 70% of the familial forms of ALS (FALS) and 15% of the sporadic forms of ALS (SALS) [160]. The familial ALS forms are only around 5% of cases. However, most cases are sporadic, which are phenotypically interchangeable from familial types, implying that there are shared pathways causing neuronal death [161].
Well-explored genetic causes of ALS are mutations or deletion of the SOD1 gene (Figure 4). Recently, through advanced genomic screening tools, other genes related to ALS have been recognized, including TAR DNA-binding protein 43 (TARDBP), encoding TDP-43; fused in sarcoma (FUS) and chromosome 9 open reading frame 72 (C9ORF72). Notably, TDP-43 and FUS are RNA-binding proteins that function in mRNA and miRNA biogenesis [162,163]. Loffreda and collaborators [70] reported that miR-129-5p was upregulated in various models of SOD1 linked ALS and in peripheral blood mononuclear cells (PBMCs) of SALS patients. This study showed that upon an antisense oligonucleotide (ASO) inhibitor of miR-129-5p, ALS SOD1 (G93A) mice ameliorated the neuromuscular phenotype. In light of this, miR-129-5p may be a therapeutic target to treat ALS patients.
Figure 4.
The most essential miRNAs involved in ALS’s disease.
Different mechanisms are responsible for the neuronal death occurring in ALS, including impaired metabolism, neuroinflammation, oxidative imbalance, mitochondrial dysfunction, glutamate excitotoxicity, growth factor defects and defective axonal transport [164,165]. Still, the pathogenic molecular mechanisms underlying ALS onset and progression are not entirely known.
To date, there is no available cure for this disorder. Increasing evidence reported that also the variation of RNA metabolism, including miRNA processing, is an essential pathogenetic component and a potential target for ALS.
Recently, the miRNA expression in ALS serum was analyzed and the assumption that changes of some miRNA involve the mitochondrial physiology of neuronal cells was tested [72]. The screening of numerous miRNAs in serum from patients and controls showed that miR335-5p was strongly decreased in ALS patients, and this was confirmed in an independent validation cohort.
MiR-335-5p targets 2544 genes [166], and its downregulation is involved in neuronal plasticity and memory processes in mice [167]. The downregulation of miR-335-5p induced mitophagy in SH-SY5Y cells and helped the concept that dysregulated miRNAs may participate in the pathogenesis of neuronal degeneration in ALS.
ALS lacks specific biomarkers, thus, the clinical diagnosis is problematic, with a high rate of misdiagnosis [30]. Notably, the serum levels of miR-1234-3p and miR-1825 resulted in being substantially reduced in ALS patients, and the miR-1825 decline was detected in both sporadic and familial ALS patients. At the same time, the miR-1234-3p reduction was limited to patients with sporadic ALS [168].
Plasma levels of miR-130a-3p, miR-151b and miR-221-3p resulted in also being reduced in sporadic ALS patients and positively associated with sporadic ALS progression, indicating that these miRNAs may be valuable even for monitoring the disorder progress [73]. A study of serum-derived ex-miRNA analyzed 10 ALS patients and 20 controls and revealed that ex-miR-27a-3p levels were considerably reduced in ALS patients. The researchers assumed that ex-miR-27a3p might represent a possible diagnostic biomarker of ALS [169].
Another investigation analyzed the expression of ex-miRNAs in the CSF and serum of 22 patients with sporadic ALS and compared them with control individuals. Ex-miR-132-5p, ex-miR-132-3p and ex-miR-143-3p resulted in being considerably reduced, while ex-miR-143-5p and ex-miR574-5p resulted in being elevated in ALS patients, indicating that these ex-miRNAs are possible biomarkers for ALS [170].
5. Discussion and Future Perspective
The above-reported findings undoubtedly show the intrinsic relation existing between oxidative stress, mitochondrial dysfunction and miRNAs in aging and neurodegenerative diseases.
From the data discussed above, it appears to be clear that any disease is characterized by specific miRNAs, with the consequent downregulation or upregulation of specific genes, but that also each condition analyzed shares some miRNA with the others, thus indicating an overlapping of some pathways. This is conceivable if the common presence of oxidative stress and mitochondrial impairment in diverse diseases is considered.
Several studies have underlined the essential roles of miRNAs in the regulation of genes involved in mitochondrial integrity and oxidative stress in different brain pathological conditions. However, some limits still exist in these studies, mainly related to the experimental model considered, and the results should be carefully examined. In fact, even though extremely useful, the in vitro studies may be affected by the culturing conditions, or the in vivo experimental model may express different miRNAs with respect to humans. Additionally, the type of cell studied (neuron or glia) or the specific brain area is relevant in identifying specific miRNAs related to a specific pathology. Even using stem cells, some problems of specificity may be present. Finally, some points still need to be addressed, particularly the understanding of the underlying molecular mechanisms, i.e., how the causal factors involving oxidative stress, mitochondrial dysfunction and miRNAs are specifically connected to a specific pathology, need further investigations.
However, once that these problems will be resolved with the improvement of the technological platforms, it appears clear that miRNAs may represent in the future valuable biomarkers for the different neurodegenerative conditions. In this regard, the analysis of specific ex-miRNAs from CSF and peripheral blood can offer potential biomarkers for neurodegenerative diseases to be easily accessible. Additionally, different factors may influence the composition of miRNAs in common fluids, including inflammatory factors, lifestyle, sex and ethnicity [30,31,32]. The alteration of the brain microenvironment may in turn influence the nature of ex-miRNA released by neural cells, and miRNAs composition results substantially modified as well, thus, suggesting that ex-miRNAs may be considered valuable early diagnostic markers for neurodegenerative disease.
The technological improvements in the bioavailability and stability of miRNAs, with the development of well-defined patient cohorts will substantially lead to a valid diagnostic and therapeutic miRNA-based approach for neurodegenerative diseases.
Acknowledgments
The Authors thank Ken and Ann Douglas Charitable Foundation for the support.
Abbreviations
| (qRT)-PCR | Real-Time Quantitative Reverse Transcription PCR |
| 3-NPA | Beta-Nitropropionic acid |
| ACHE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AGO | Argonaute protein |
| ALS | Amyotrophic lateral sclerosis |
| APP | Amyloid precursor protein |
| ASO | Antisense oligonucleotide |
| ATP5G3 | ATP synthase membrane subunit c locus 3 |
| Aβ1–42 | Amyloid β-Peptide 1-42 |
| BAX | bcl-2-like protein 4 |
| BCl2 | B-cell lymphoma 2 |
| BIM | bcl-2-like protein 11 |
| C9ORF72 | Chromosome 9 open reading frame 72 |
| COX | Cyclooxygenase |
| CpG | Regions with a high frequency of CpG sites |
| CSF | Cerebrospinal fluid |
| CNS | central nervous system |
| DA | Dopaminergic neuron |
| DAT | Dopamine active transporter |
| DRP1 | dynamin-related protein 1 |
| EEs | Early endosomes |
| ERK1 | Extracellular Signal-Regulated Kinase |
| ETC | electrons transport chain |
| ex-miRNA | Exosome miRNA |
| FALS | Familial forms of ALS |
| FGF20 | Fibroblast Growth Factor 20 |
| FOXA2 | Forkhead box protein A2 |
| FUS | Fused in Sarcoma |
| HD | Huntington’s disease |
| HEY2 | Hairy/enhancer-of-split related with YRPW motif protein 1 |
| IGF-1 | Insulin-like growth factor 1 |
| iPD | Idiopathic Parkinson’s disease |
| JNK1 | c-Jun N-terminal kinase |
| LES | Late endosomes |
| LOAD | Late onset Alzheimer’s disease |
| LRP-1 | Low density lipoprotein receptor-related protein 1 |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MAPK | mitogen-activated protein kinase |
| mHTT | Mutant human huntingtin |
| miRISC | miRNA RISC complex |
| MPP+ | 1-Methyl-4-phenylpyridine |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MVBs | Multivesicular bodies |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NOTCH1 | Notch homolog 1, translocation-associated |
| OMM | Outer mitochondrial membrane |
| P38 | P38 mitogen-activated protein kinases |
| PARIS | Zinc finger protein 746 |
| PBMCs | Peripheral blood mononuclear cells |
| PD | Parkinson’s disease |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K/Akt | Phosphatidylinositol-3-Kinase and Protein Kinase B |
| PINK1 | PTEN-induced kinase 1 |
| PITX3 | Pituitary homeobox 3 |
| polyQ | Poly glutamine |
| pri-miRNA | Primary transcript |
| R1441G | disease-linked leucine-rich repeat kinase 2 |
| RAB | Ras-associated binding |
| RISC | RNA Induced Silencing Complex |
| SALS | Sporadic form of ALS |
| SASP | Senescence-associated secretory phenotype |
| SIRT4 | Sirtuin 4 |
| SNpc | substantia nigra pars compacta |
| SNX6 | Sorting Nexin 6 |
| SOD1 | Superoxide dismutase [Cu-Zn] |
| TARDBP | TAR DNA-binding protein 43 |
| TLR | Toll-like receptor |
| TNFα | Tumor necrosis factor-α |
| UTR | Untranslated region |
| VAV1 | Proto-oncogene vav |
| VDAC1 | Voltage-dependent anion-selective channel 1 |
| YOAD | Young onset Alzheimer’s disease |
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Castelli V., Benedetti E., Antonosante A., Catanesi M., Pitari G., Ippoliti R., Cimini A., d’Angelo M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019;12:132. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castelli V., Melani F., Ferri C., d’Angelo M., Catanesi M., Grassi D., Benedetti E., Giordano A., Cimini A., Desideri G. Neuroprotective activities of bacopa, lycopene, astaxanthin, and vitamin B12 combination on oxidative stress-dependent neuronal death. J. Cell. Biochem. 2020 doi: 10.1002/jcb.29722. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal M.F. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl. 3):S39–S48. doi: 10.1002/ana.10479. [DOI] [PubMed] [Google Scholar]
- 5.Catanesi M., d’Angelo M., Antonosante A., Castelli V., Alfonsetti M., Benedetti E., Desideri G., Ferri C., Cimini A. Neuroprotective potential of choline alfoscerate against β-amyloid injury: Involvement of neurotrophic signals. Cell Biol. Int. 2020 doi: 10.1002/cbin.11369. [DOI] [PubMed] [Google Scholar]
- 6.d’Angelo M., Castelli V., Catanesi M., Antonosante A., Dominguez-Benot R., Ippoliti R., Benedetti E., Cimini A. PPARγ and Cognitive Performance. Int. J. Mol. Sci. 2019;20:5068. doi: 10.3390/ijms20205068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 9.Llave C., Xie Z., Kasschau K.D., Carrington J.C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- 10.Williams J., Smith F., Kumar S., Vijayan M., Reddy P.H. Are microRNAs true sensors of ageing and cellular senescence? Ageing Res. Rev. 2017;35:350–363. doi: 10.1016/j.arr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puisségur M.-P., Mazure N.M., Bertero T., Pradelli L., Grosso S., Robbe-Sermesant K., Maurin T., Lebrigand K., Cardinaud B., Hofman V., et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn J.Y., Datta S., Bandeira E., Cano M., Mallick E., Rai U., Powell B., Tian J., Witwer K.W., Handa J.T., et al. Release of extracellular vesicle miR-494-3p by ARPE-19 cells with impaired mitochondria. Biochim. Biophys. Acta Gen. Subj. 2020:129598. doi: 10.1016/j.bbagen.2020.129598. [DOI] [PubMed] [Google Scholar]
- 13.Gao P., Tchernyshyov I., Chang T.-C., Lee Y.-S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang A., Grether-Beck S., Singh M., Kuck F., Jakob S., Kefalas A., Altinoluk-Hambüchen S., Graffmann N., Schneider M., Lindecke A., et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany N. Y.) 2016;8:484–505. doi: 10.18632/aging.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasa-Nicotera M., Chen H., Tucci P., Yang A.L., Saintigny G., Menghini R., Mahè C., Agostini M., Knight R.A., Melino G., et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R., Zhang Q., Niu J., Lu K., Xie B., Cui D., Xu S. Screening of microRNAs associated with Alzheimer’s disease using oxidative stress cell model and different strains of senescence accelerated mice. J. Neurol. Sci. 2014;338:57–64. doi: 10.1016/j.jns.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P.H., Williams J., Smith F., Bhatti J.S., Kumar S., Vijayan M., Kandimalla R., Kuruva C.S., Wang R., Manczak M., et al. MicroRNAs, Aging, Cellular Senescence, and Alzheimer’s Disease. Prog. Mol. Biol. Transl. Sci. 2017;146:127–171. doi: 10.1016/bs.pmbts.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Reddy P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta. 2016;1862:1617–1627. doi: 10.1016/j.bbadis.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung H.J., Suh Y. MicroRNA in Aging: From Discovery to Biology. Curr. Genom. 2012;13:548–557. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen B.R. Transcription and processing of human microRNA precursors. Mol. Cell. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y.S., Nakahara K., Pham J.W., Kim K., He Z., Sontheimer E.J., Carthew R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/S0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu J., Yang J., Cho W.C., Zheng Y. Argonaute proteins: Structural features, functions and emerging roles. J. Adv. Res. 2020;24:317–324. doi: 10.1016/j.jare.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 24.Okamura K. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D., Li Y.-P., Li Y.-X., Zhu X.-H., Du X.-G., Zhou M., Li W.-B., Deng H.-Y. Effect of Regulatory Network of Exosomes and microRNAs on Neurodegenerative Diseases. Chin. Med. J. 2018;131:2216–2225. doi: 10.4103/0366-6999.240817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourembanas S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 27.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Pant S., Hilton H., Burczynski M.E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Zhang L. Circulating Exosomal miRNA as Diagnostic Biomarkers of Neurodegenerative Diseases. Front. Mol. Neurosci. 2020;13:53. doi: 10.3389/fnmol.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci C., Marzocchi C., Battistini S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells. 2018;7:219. doi: 10.3390/cells7110219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roser A.E., Caldi Gomes L., Schünemann J., Maass F., Lingor P. Circulating miRNAs as Diagnostic Biomarkers for Parkinson’s Disease. Front. Neurosci. 2018;12:625. doi: 10.3389/fnins.2018.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohr A., Mott J. Overview of MicroRNA Biology. Semin. Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gantier M.P., McCoy C.E., Rusinova I., Saulep D., Wang D., Xu D., Irving A.T., Behlke M.A., Hertzog P.J., Mackay F., et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 2011;39:5692–5703. doi: 10.1093/nar/gkr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao N.-Y., Hu H., Yan Z., Xu Y., Hu H., Menzel C., Li N., Chen W., Khaitovich P. Comprehensive survey of human brain microRNA by deep sequencing. BMC Genom. 2010;11:409. doi: 10.1186/1471-2164-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlakha Y.K., Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol. Cancer. 2014;13:33. doi: 10.1186/1476-4598-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson P.T., Wang W.-X., Rajeev B.W. MicroRNAs (miRNAs) in Neurodegenerative Diseases. Brain Pathol. 2008;18:130–138. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bian S., Sun T. Functions of Noncoding RNAs in Neural Development and Neurological Diseases. Mol. Neurobiol. 2011;44:359–373. doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan L., Yu J.-T., Tan L. Causes and Consequences of MicroRNA Dysregulation in Neurodegenerative Diseases. Mol. Neurobiol. 2015;51:1249–1262. doi: 10.1007/s12035-014-8803-9. [DOI] [PubMed] [Google Scholar]
- 40.Lagos-Quintana M. Identification of Novel Genes Coding for Small Expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 41.Kapsimali M., Kloosterman W.P., de Bruijn E., Rosa F., Plasterk R.H., Wilson S.W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao F.-B. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31:20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Absalon S., Kochanek D.M., Raghavan V., Krichevsky A.M. MiR-26b, Upregulated in Alzheimer’s Disease, Activates Cell Cycle Entry, Tau-Phosphorylation, and Apoptosis in Postmitotic Neurons. J. Neurosci. 2013;33:14645–14659. doi: 10.1523/JNEUROSCI.1327-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian N., Cao Z., Zhang Y. MiR-206 decreases brain-derived neurotrophic factor levels in a transgenic mouse model of Alzheimer’s disease. Neurosci. Bull. 2014;30:191–197. doi: 10.1007/s12264-013-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar S., Raymick J., Imam S. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016;17:904. doi: 10.3390/ijms17060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim W., Lee Y., McKenna N.D., Yi M., Simunovic F., Wang Y., Kong B., Rooney R.J., Seo H., Stephens R.M., et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging. 2014;35:1712–1721. doi: 10.1016/j.neurobiolaging.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krichevsky A.M. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manzine P.R., Pelucchi S., Horst M.A., Vale F.A.C., Pavarini S.C.I., Audano M., Mitro N., Di Luca M., Marcello E., Cominetti M.R. microRNA 221 Targets ADAM10 mRNA and is Downregulated in Alzheimer’s Disease. JAD. 2017;61:113–123. doi: 10.3233/JAD-170592. [DOI] [PubMed] [Google Scholar]
- 49.Chen F., Zhao Y., Chen H. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int. J. Mol. Med. 2018 doi: 10.3892/ijmm.2018.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y., Wang Z.-F., Li W., Hong H., Chen J., Tian Y., Liu Z.-Y. Protective effects of microRNA-330 on amyloid β-protein production, oxidative stress, and mitochondrial dysfunction in Alzheimer’s disease by targeting VAV1 via the MAPK signaling pathway. J. Cell. Biochem. 2018;119:5437–5448. doi: 10.1002/jcb.26700. [DOI] [PubMed] [Google Scholar]
- 51.Zhu M., Huang C., Ma X., Wu R., Zhu W., Li X., Liang Z., Deng F., Zhu J., Xie W., et al. Modulation of miR-19 in Aluminum-Induced Neural Cell Apoptosis. JAD. 2016;50:1149–1162. doi: 10.3233/JAD-150763. [DOI] [PubMed] [Google Scholar]
- 52.De Santis R., Santini L., Colantoni A., Peruzzi G., de Turris V., Alfano V., Bozzoni I., Rosa A. FUS Mutant Human Motoneurons Display Altered Transcriptome and microRNA Pathways with Implications for ALS Pathogenesis. Stem Cell Rep. 2017;9:1450–1462. doi: 10.1016/j.stemcr.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Junn E., Lee K.-W., Jeong B.S., Chan T.W., Im J.-Y., Mouradian M.M. Repression of -synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kittappa R., Chang W.W., Awatramani R.B., McKay R.D.G. The foxa2 Gene Controls the Birth and Spontaneous Degeneration of Dopamine Neurons in Old Age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doxakis E. Post-transcriptional Regulation of α-Synuclein Expression by mir-7 and mir-153. J. Biol. Chem. 2010;285:12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh N., Ohta H. Roles of FGF20 in dopaminergic neurons and Parkinson’s disease. Front. Mol. Neurosci. 2013;6:15. doi: 10.3389/fnmol.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong R., Wang Z., Zhao Z., Li H., Chen W., Zhang B., Wang L., Wu L., Li W., Ding J., et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol. Aging. 2014;35:705–714. doi: 10.1016/j.neurobiolaging.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 58.Miñones-Moyano E., Porta S., Escaramís G., Rabionet R., Iraola S., Kagerbauer B., Espinosa-Parrilla Y., Ferrer I., Estivill X., Martí E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011;20:3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 59.Cho H.J., Liu G., Jin S.M., Parisiadou L., Xie C., Yu J., Sun L., Ma B., Ding J., Vancraenenbroeck R., et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013;22:608–620. doi: 10.1093/hmg/dds470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prajapati P., Sripada L., Singh K., Bhatelia K., Singh R., Singh R. TNF-α regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim. Biophys. Acta Mol. Basis Dis. 2015;1852:451–461. doi: 10.1016/j.bbadis.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Chen C.-M., Orefice L.L., Chiu S.-L., LeGates T.A., Hattar S., Huganir R.L., Zhao H., Xu B., Kuruvilla R. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc. Natl. Acad. Sci. USA. 2017;114:E619–E628. doi: 10.1073/pnas.1615792114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gumireddy K., Young D.D., Xiong X., Hogenesch J.B., Huang Q., Deiters A. Small-Molecule Inhibitors of MicroRNA miR-21 Function. Angew. Chem. Int. Ed. 2008;47:7482–7484. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunsberger H.C., Weitzner D.S., Rudy C.C., Hickman J.E., Libell E.M., Speer R.R., Gerhardt G.A., Reed M.N. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. J. Neurochem. 2015;135:381–394. doi: 10.1111/jnc.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vo N., Klein M.E., Varlamova O., Keller D.M., Yamamoto T., Goodman R.H., Impey S. From The Cover: A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui L., Jeong H., Borovecki F., Parkhurst C.N., Tanese N., Krainc D. Transcriptional Repression of PGC-1α by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Sinha M., Ghose J., Bhattarcharyya N.P. Micro RNA -214,-150,-146a and-125b target Huntingtin gene. RNA Biol. 2011;8:1005–1021. doi: 10.4161/rna.8.6.16035. [DOI] [PubMed] [Google Scholar]
- 67.Cheng P.-H., Li C.-L., Chang Y.-F., Tsai S.-J., Lai Y.-Y., Chan A.W.S., Chen C.-M., Yang S.-H. miR-196a Ameliorates Phenotypes of Huntington Disease in Cell, Transgenic Mouse, and Induced Pluripotent Stem Cell Models. Am. J. Hum. Genet. 2013;93:306–312. doi: 10.1016/j.ajhg.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martí E., Pantano L., Bañez-Coronel M., Llorens F., Miñones-Moyano E., Porta S., Sumoy L., Ferrer I., Estivill X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010;38:7219–7235. doi: 10.1093/nar/gkq575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson R., Zuccato C., Belyaev N.D., Guest D.J., Cattaneo E., Buckley N.J. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol. Dis. 2008;29:438–445. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Loffreda A., Nizzardo M., Arosio A., Ruepp M.-D., Calogero R.A., Volinia S., Galasso M., Bendotti C., Ferrarese C., Lunetta C., et al. miR-129-5p: A key factor and therapeutic target in amyotrophic lateral sclerosis. Prog. Neurobiol. 2020;190:101803. doi: 10.1016/j.pneurobio.2020.101803. [DOI] [PubMed] [Google Scholar]
- 71.Dobrowolny G., Bernardini C., Martini M., Baranzini M., Barba M., Musarò A. Muscle Expression of SOD1G93A Modulates microRNA and mRNA Transcription Pattern Associated with the Myelination Process in the Spinal Cord of Transgenic Mice. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Luna N., Turon-Sans J., Cortes-Vicente E., Carrasco-Rozas A., Illán-Gala I., Dols-Icardo O., Clarimón J., Lleó A., Gallardo E., Illa I., et al. Downregulation of miR-335-5P in Amyotrophic Lateral Sclerosis Can Contribute to Neuronal Mitochondrial Dysfunction and Apoptosis. Sci. Rep. 2020;10:4308. doi: 10.1038/s41598-020-61246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liguori M., Nuzziello N., Introna A., Consiglio A., Licciulli F., D’Errico E., Scarafino A., Distaso E., Simone I.L. Dysregulation of MicroRNAs and Target Genes Networks in Peripheral Blood of Patients With Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018;11:288. doi: 10.3389/fnmol.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J., Inoue K., Ishii J., Vanti W.B., Voronov S.V., Murchison E., Hannon G., Abeliovich A. A MicroRNA Feedback Circuit in Midbrain Dopamine Neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh T., Yadav S. Role of microRNAs in neurodegeneration induced by environmental neurotoxicants and aging. Ageing Res. Rev. 2020;60:101068. doi: 10.1016/j.arr.2020.101068. [DOI] [PubMed] [Google Scholar]
- 76.Caravia X.M., López-Otín C. Regulatory Roles of miRNAs in Aging. Adv. Exp. Med. Biol. 2015;887:213–230. doi: 10.1007/978-3-319-22380-3_11. [DOI] [PubMed] [Google Scholar]
- 77.Borgdorff V., Lleonart M.E., Bishop C.L., Fessart D., Bergin A.H., Overhoff M.G., Beach D.H. Multiple microRNAs rescue from Ras-induced senescence by inhibiting p21(Waf1/Cip1) Oncogene. 2010;29:2262–2271. doi: 10.1038/onc.2009.497. [DOI] [PubMed] [Google Scholar]
- 78.Overhoff M.G., Garbe J.C., Koh J., Stampfer M.R., Beach D.H., Bishop C.L. Cellular senescence mediated by p16INK4A-coupled miRNA pathways. Nucleic Acids Res. 2014;42:1606–1618. doi: 10.1093/nar/gkt1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Lencastre A., Pincus Z., Zhou K., Kato M., Lee S.S., Slack F.J. MicroRNAs both promote and antagonize longevity in C. elegans. Curr. Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quesada A., Lee B.Y., Micevych P.E. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev. Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sonntag K.C., Woo T.-U.W., Krichevsky A.M. Converging miRNA functions in diverse brain disorders: A case for miR-124 and miR-126. Exp. Neurol. 2012;235:427–435. doi: 10.1016/j.expneurol.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Kim M.S., Jia B., Yan J., Zuniga-Hertz J.P., Han C., Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benedetti E., Cristiano L., Antonosante A., d’Angelo M., D’Angelo B., Selli S., Castelli V., Ippoliti R., Giordano A., Cimini A. PPARs in Neurodegenerative and Neuroinflammatory Pathways. CAR. 2018;15:336–344. doi: 10.2174/1567205014666170517150037. [DOI] [PubMed] [Google Scholar]
- 84.Yadav S., Pandey A., Shukla A., Talwelkar S.S., Kumar A., Pant A.B., Parmar D. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J. Biol. Chem. 2011;286:37347–37357. doi: 10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balaraman S., Winzer-Serhan U.H., Miranda R.C. Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcohol. Clin. Exp. Res. 2012;36:1669–1677. doi: 10.1111/j.1530-0277.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., How Huang K., Jen Lee M., Galas D.J., Wang K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar S., Vijayan M., Bhatti J.S., Reddy P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017;146:47–94. doi: 10.1016/bs.pmbts.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Sawada S., Akimoto T., Takahashi M., Sakurai R., Shinkai S., Ushida T., Fujiwara Y., Suzuki K. Effect of Aging and Sex on Circulating MicroRNAs in Humans. AAR. 2014;3:152–159. doi: 10.4236/aar.2014.32023. [DOI] [Google Scholar]
- 89.Olivieri F., Bonafè M., Spazzafumo L., Gobbi M., Prattichizzo F., Recchioni R., Marcheselli F., Sala L.L., Galeazzi R., Rippo M.R., et al. Age- and glycemia-related miR-126-3p levels in plasma and endothelial cells. Aging. 2014;6:771–786. doi: 10.18632/aging.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ameling S., Kacprowski T., Chilukoti R.K., Malsch C., Liebscher V., Suhre K., Pietzner M., Friedrich N., Homuth G., Hammer E., et al. Associations of circulating plasma microRNAs with age, body mass index and sex in a population-based study. BMC Med. Genom. 2015;8:61. doi: 10.1186/s12920-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Machida T., Tomofuji T., Ekuni D., Maruyama T., Yoneda T., Kawabata Y., Mizuno H., Miyai H., Kunitomo M., Morita M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015;16:21294–21309. doi: 10.3390/ijms160921294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hooten N.N., Fitzpatrick M., Wood W.H., De S., Ejiogu N., Zhang Y., Mattison J.A., Becker K.G., Zonderman A.B., Evans M.K. Age-related changes in microRNA levels in serum. Aging. 2013;5:725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prasad K.N. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer’s disease. Mech. Ageing Dev. 2016;153:41–47. doi: 10.1016/j.mad.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Bonet-Costa V., Pomatto L.C.-D., Davies K.J.A. The Proteasome and Oxidative Stress in Alzheimer’s Disease. Antioxid. Redox Signal. 2016;25:886–901. doi: 10.1089/ars.2016.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butterfield D.A., Boyd-Kimball D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018;62:1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.García-Escudero V., Martín-Maestro P., Perry G., Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid. Med. Cell. Longev. 2013;2013:162152. doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Šimić G., Babić Leko M., Wray S., Harrington C., Delalle I., Jovanov-Milošević N., Bažadona D., Buée L., de Silva R., Di Giovanni G., et al. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules. 2016;6:6. doi: 10.3390/biom6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williamson J., Goldman J., Marder K.S. Genetic aspects of Alzheimer disease. Neurologist. 2009;15:80–86. doi: 10.1097/NRL.0b013e318187e76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blass J.P. Brain metabolism and brain disease: Is metabolic deficiency the proximate cause of Alzheimer dementia? J. Neurosci. Res. 2001;66:851–856. doi: 10.1002/jnr.10087. [DOI] [PubMed] [Google Scholar]
- 100.Malik A.N., Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–492. doi: 10.1016/j.mito.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 101.Brai E., Alina Raio N., Alberi L. Notch1 hallmarks fibrillary depositions in sporadic Alzheimer’s disease. Acta Neuropathol. Commun. 2016;4:64. doi: 10.1186/s40478-016-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Q., Li X., Wang L., Zhang Y., Chen L. miR-98-5p Acts as a Target for Alzheimer’s Disease by Regulating Aβ Production Through Modulating SNX6 Expression. J. Mol. Neurosci. 2016;60:413–420. doi: 10.1007/s12031-016-0815-7. [DOI] [PubMed] [Google Scholar]
- 103.Hu Y.-K., Wang X., Li L., Du Y.-H., Ye H.-T., Li C.-Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bull. 2013;29:745–751. doi: 10.1007/s12264-013-1348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang R., Zhou H., Jiang L., Mao Y., Cui X., Xie B., Cui D., Wang H., Zhang Q., Xu S. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res. 2016;1652:135–143. doi: 10.1016/j.brainres.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 105.Li J., Donath S., Li Y., Qin D., Prabhakar B.S., Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/annotation/4050116d-8daa-4b5a-99e9-34cdd13f6a26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan M.M., Guévremont D., Mockett B.G., Abraham W.C., Williams J.M. Circulating Plasma microRNAs are Altered with Amyloidosis in a Mouse Model of Alzheimer’s Disease. JAD. 2018;66:835–852. doi: 10.3233/JAD-180385. [DOI] [PubMed] [Google Scholar]
- 107.Reddy P.H., Tonk S., Kumar S., Vijayan M., Kandimalla R., Kuruva C.S., Reddy A.P. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2017;483:1156–1165. doi: 10.1016/j.bbrc.2016.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ravanidis S., Bougea A., Papagiannakis N., Maniati M., Koros C., Simitsi A., Bozi M., Pachi I., Stamelou M., Paraskevas G.P., et al. Circulating Brain-Enriched MicroRNAs for Detection and Discrimination of Idiopathic and Genetic Parkinson’s Disease. Mov. Disord. 2020;35:457–467. doi: 10.1002/mds.27928. [DOI] [PubMed] [Google Scholar]
- 109.Lugli G., Cohen A.M., Bennett D.A., Shah R.C., Fields C.J., Hernandez A.G., Smalheiser N.R. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS ONE. 2015;10:e0139233. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rani A., O’Shea A., Ianov L., Cohen R.A., Woods A.J., Foster T.C. miRNA in Circulating Microvesicles as Biomarkers for Age-Related Cognitive Decline. Front. Aging Neurosci. 2017;9:323. doi: 10.3389/fnagi.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gámez-Valero A., Campdelacreu J., Vilas D., Ispierto L., Reñé R., Álvarez R., Armengol M.P., Borràs F.E., Beyer K. Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl. Neurodegener. 2019;8:31. doi: 10.1186/s40035-019-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang T.T., Liu C.G., Gao S.C., Zhang Y., Wang P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018;31:87–96. doi: 10.3967/bes2018.011. [DOI] [PubMed] [Google Scholar]
- 113.Wei H., Xu Y., Xu W., Zhou Q., Chen Q., Yang M., Feng F., Liu Y., Zhu X., Yu M., et al. Serum Exosomal miR-223 Serves as a Potential Diagnostic and Prognostic Biomarker for Dementia. Neuroscience. 2018;379:167–176. doi: 10.1016/j.neuroscience.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 114.McKeever P.M., Schneider R., Taghdiri F., Weichert A., Multani N., Brown R.A., Boxer A.L., Karydas A., Miller B., Robertson J., et al. MicroRNA Expression Levels Are Altered in the Cerebrospinal Fluid of Patients with Young-Onset Alzheimer’s Disease. Mol. Neurobiol. 2018;55:8826–8841. doi: 10.1007/s12035-018-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riancho J., Vázquez-Higuera J.L., Pozueta A., Lage C., Kazimierczak M., Bravo M., Calero M., Gonalezález A., Rodríguez E., Lleó A., et al. MicroRNA Profile in Patients with Alzheimer’s Disease: Analysis of miR-9-5p and miR-598 in Raw and Exosome Enriched Cerebrospinal Fluid Samples. JAD. 2017;57:483–491. doi: 10.3233/JAD-161179. [DOI] [PubMed] [Google Scholar]
- 116.Grasso M., Piscopo P., Crestini A., Confaloni A., Denti M.A. Circulating microRNAs in Neurodegenerative Diseases. Exp. Suppl. 2015;106:151–169. doi: 10.1007/978-3-0348-0955-9_7. [DOI] [PubMed] [Google Scholar]
- 117.Sanders L.H., Timothy Greenamyre J. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 2013;62:111–120. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subramaniam S.R., Chesselet M.-F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu L.J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., Wong J., Takenouchi T., Hashimoto M., Masliah E. α-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am. J. Pathol. 2000;157:401–410. doi: 10.1016/S0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tansey M.G., McCoy M.K., Frank-Cannon T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., León R. Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Hoss A.G., Labadorf A., Beach T.G., Latourelle J.C., Myers R.H. microRNA Profiles in Parkinson’s Disease Prefrontal Cortex. Front. Aging Neurosci. 2016;8:36. doi: 10.3389/fnagi.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gui Y., Liu H., Zhang L., Lv W., Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6:37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin W., Metzakopian E., Mavromatakis Y.E., Gao N., Balaskas N., Sasaki H., Briscoe J., Whitsett J.A., Goulding M., Kaestner K.H., et al. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev. Biol. 2009;333:386–396. doi: 10.1016/j.ydbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Parker W.D., Parks J.K., Swerdlow R.H. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang H., Ye Y., Zhu Z., Mo L., Lin C., Wang Q., Wang H., Gong X., He X., Lu G., et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016;26:167–176. doi: 10.1111/bpa.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayashi T., Ishimori C., Takahashi-Niki K., Taira T., Kim Y., Maita H., Maita C., Ariga H., Iguchi-Ariga S.M.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 128.Irrcher I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M., Lutz A.K., Rousseaux M.W.C., Bevilacqua L., Jahani-Asl A., et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 129.Mortiboys H., Johansen K.K., Aasly J.O., Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 130.Wang X., Yan M.H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S.G., Perry G., Casadesus G., Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chaudhuri A.D., Choi D.C., Kabaria S., Tran A., Junn E. MicroRNA-7 Regulates the Function of Mitochondrial Permeability Transition Pore by Targeting VDAC1 Expression. J. Biol. Chem. 2016;291:6483–6493. doi: 10.1074/jbc.M115.691352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng B., Liao Z., Locascio J.J., Lesniak K.A., Roderick S.S., Watt M.L., Eklund A.C., Zhang-James Y., Kim P.D., Hauser M.A., et al. PGC-1, A Potential Therapeutic Target for Early Intervention in Parkinson’s Disease. Sci. Transl. Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y., Yang Z., Le W. Tiny But Mighty: Promising Roles of MicroRNAs in the Diagnosis and Treatment of Parkinson’s Disease. Neurosci. Bull. 2017;33:543–551. doi: 10.1007/s12264-017-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valdés P., Schneider B.L. Gene Therapy: A Promising Approach for Neuroprotection in Parkinson’s Disease? Front. Neuroanat. 2016;10:123. doi: 10.3389/fnana.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Serafin A., Foco L., Zanigni S., Blankenburg H., Picard A., Zanon A., Giannini G., Pichler I., Facheris M.F., Cortelli P., et al. Overexpression of blood microRNAs 103a, 30b, and 29a in L-dopa-treated patients with PD. Neurology. 2015;84:645–653. doi: 10.1212/WNL.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 136.Ebert M.S., Neilson J.R., Sharp P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yao Y.-F., Qu M.-W., Li G.-C., Zhang F.-B., Rui H.-C. Circulating exosomal miRNAs as diagnostic biomarkers in Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2018;22:5278–5283. doi: 10.26355/eurrev_201808_15727. [DOI] [PubMed] [Google Scholar]
- 138.Barbagallo C., Mostile G., Baglieri G., Giunta F., Luca A., Raciti L., Zappia M., Purrello M., Ragusa M., Nicoletti A. Specific Signatures of Serum miRNAs as Potential Biomarkers to Discriminate Clinically Similar Neurodegenerative and Vascular-Related Diseases. Cell. Mol. Neurobiol. 2020;40:531–546. doi: 10.1007/s10571-019-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie Y., Chen Y. microRNAs: Emerging Targets Regulating Oxidative Stress in the Models of Parkinson’s Disease. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walker F.O. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 141.Finkbeiner S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andrew S.E., Goldberg Y.P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M.A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 1993;4:398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 143.Franco-Iborra S., Vila M., Perier C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 2018;12:342. doi: 10.3389/fnins.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gutekunst C.-A., Li S.-H., Yi H., Ferrante R.J., Li X.-J., Hersch S.M. The Cellular and Subcellular Localization of Huntingtin-Associated Protein 1 (HAP1): Comparison with Huntingtin in Rat and Human. J. Neurosci. 1998;18:7674–7686. doi: 10.1523/JNEUROSCI.18-19-07674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choo Y.S. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 146.Orr A.L., Li S., Wang C.-E., Li H., Wang J., Rong J., Xu X., Mastroberardino P.G., Greenamyre J.T., Li X.-J. N-Terminal Mutant Huntingtin Associates with Mitochondria and Impairs Mitochondrial Trafficking. J. Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Puigserver P., Spiegelman B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 148.Stanek L.M., Bu J., Shihabuddin L.S. Astrocyte transduction is required for rescue of behavioral phenotypes in the YAC128 mouse model with AAV-RNAi mediated HTT lowering therapeutics. Neurobiol. Dis. 2019;129:29–37. doi: 10.1016/j.nbd.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 149.Bradford J., Shin J.-Y., Roberts M., Wang C.-E., Li X.-J., Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. PNAS. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Skotte N.H., Andersen J.V., Santos A., Aldana B.I., Willert C.W., Nørremølle A., Waagepetersen H.S., Nielsen M.L. Integrative Characterization of the R6/2 Mouse Model of Huntington’s Disease Reveals Dysfunctional Astrocyte Metabolism. Cell Rep. 2018;23:2211–2224. doi: 10.1016/j.celrep.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 151.Gaughwin P.M., Ciesla M., Lahiri N., Tabrizi S.J., Brundin P., Björkqvist M. Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington’s disease. Hum. Mol. Genet. 2011;20:2225–2237. doi: 10.1093/hmg/ddr111. [DOI] [PubMed] [Google Scholar]
- 152.Langfelder P., Gao F., Wang N., Howland D., Kwak S., Vogt T.F., Aaronson J.S., Rosinski J., Coppola G., Horvath S., et al. MicroRNA signatures of endogenous Huntingtin CAG repeat expansion in mice. PLoS ONE. 2018;13:e0190550. doi: 10.1371/journal.pone.0190550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li X., Standley C., Sapp E., Valencia A., Qin Z.-H., Kegel K.B., Yoder J., Comer-Tierney L.A., Esteves M., Chase K., et al. Mutant huntingtin impairs vesicle formation from recycling endosomes by interfering with Rab11 activity. Mol. Cell. Biol. 2009;29:6106–6116. doi: 10.1128/MCB.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoss A.G., Labadorf A., Latourelle J.C., Kartha V.K., Hadzi T.C., Gusella J.F., MacDonald M.E., Chen J.-F., Akbarian S., Weng Z., et al. miR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genom. 2015;8:10. doi: 10.1186/s12920-015-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee S.-T., Chu K., Im W.-S., Yoon H.-J., Im J.-Y., Park J.-E., Park K.-H., Jung K.-H., Lee S.K., Kim M., et al. Altered microRNA regulation in Huntington’s disease models. Exp. Neurol. 2011;227:172–179. doi: 10.1016/j.expneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 156.Miska E.A., Alvarez-Saavedra E., Townsend M., Yoshii A., Sestan N., Rakic P., Constantine-Paton M., Horvitz H.R. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McGleenon B.M., Dynan K.B., Passmore A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease: Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 2001;48:471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee S.T., Im W., Ban J.J., Lee M., Jung K.H., Lee S.K., Chu K., Kim M. Exosome-Based Delivery of miR-124 in a Huntington’s Disease Model. JMD. 2017;10:45–52. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reed E.R., Latourelle J.C., Bockholt J.H., Bregu J., Smock J., Paulsen J.S., Myers R.H., PREDICT-HD CSF ancillary study investigators MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology. 2018;90:e264–e272. doi: 10.1212/WNL.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chia R., Chiò A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ajroud-Driss S., Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim. Biophys. Acta Mol. Basis Dis. 2015;1852:679–684. doi: 10.1016/j.bbadis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 162.Kawahara Y., Mieda-Sato A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc. Natl. Acad. Sci. USA. 2012;109:3347–3352. doi: 10.1073/pnas.1112427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Buratti E., De Conti L., Stuani C., Romano M., Baralle M., Baralle F. Nuclear factor TDP-43 can affect selected microRNA levels: TDP-43 and miRNA regulation. FEBS J. 2010;277:2268–2281. doi: 10.1111/j.1742-4658.2010.07643.x. [DOI] [PubMed] [Google Scholar]
- 164.Morgan S., Orrell R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016;119:87–98. doi: 10.1093/bmb/ldw026. [DOI] [PubMed] [Google Scholar]
- 165.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 166.Hamzeiy H., Suluyayla R., Brinkrolf C., Janowski S.J., Hofestädt R., Allmer J. Visualization and Analysis of miRNAs Implicated in Amyotrophic Lateral Sclerosis Within Gene Regulatory Pathways. Stud. Health Technol. Inform. 2018;253:183–187. [PubMed] [Google Scholar]
- 167.Capitano F., Camon J., Licursi V., Ferretti V., Maggi L., Scianni M., Del Vecchio G., Rinaldi A., Mannironi C., Limatola C., et al. MicroRNA-335-5p modulates spatial memory and hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2017;139:63–68. doi: 10.1016/j.nlm.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 168.Freischmidt A., Müller K., Zondler L., Weydt P., Mayer B., von Arnim C.A.F., Hübers A., Dorst J., Otto M., Holzmann K., et al. Serum microRNAs in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging. 2015;36:2660.e15–2660.e20. doi: 10.1016/j.neurobiolaging.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 169.Xu Z., Henderson R.D., David M., McCombe P.A. Neurofilaments as Biomarkers for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0164625. doi: 10.1371/journal.pone.0164625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Freischmidt A., Müller K., Ludolph A.C., Weishaupt J.H. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013;1:42. doi: 10.1186/2051-5960-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]