Abstract
Productivity of tropical lowland moist forests is often limited by availability and functional allocation of phosphorus (P) that drives competition among tree species and becomes a key factor in determining forestall community diversity. We used non-target 31P-NMR metabolic profiling to study the foliar P-metabolism of trees of a French Guiana rainforest. The objective was to test the hypotheses that P-use is species-specific, and that species diversity relates to species P-use and concentrations of P-containing compounds, including inorganic phosphates, orthophosphate monoesters and diesters, phosphonates and organic polyphosphates. We found that tree species explained the 59% of variance in 31P-NMR metabolite profiling of leaves. A principal component analysis showed that tree species were separated along PC 1 and PC 2 of detected P-containing compounds, which represented a continuum going from high concentrations of metabolites related to non-active P and P-storage, low total P concentrations and high N:P ratios, to high concentrations of P-containing metabolites related to energy and anabolic metabolism, high total P concentrations and low N:P ratios. These results highlight the species-specific use of P and the existence of species-specific P-use niches that are driven by the distinct species-specific position in a continuum in the P-allocation from P-storage compounds to P-containing molecules related to energy and anabolic metabolism.
Keywords: 31P-NMR metabolic profiling, Iceland, tropical lowland, P-containing compounds, species-specific P-use niches
1. Introduction
Nitrogen (N) and phosphorus (P) are essential nutrients for photosynthetic carbon assimilation and represent the most common net primary productivity limiting nutrients in terrestrial ecosystems. Nitrogen availability constrains plant productivity in many temperate and boreal forests by limiting leaf initiation and expansion [1] and synthesis of Rubisco and other photosynthetic proteins [2]. However, bioavailability of P often also limits productivity of terrestrial and aquatic ecosystems [3], including in wet tropical forests [4]. Stocks, chemical forms and availability of P change with ecosystem development and succession processes determining ecosystem properties along spatial and temporal gradients [5,6,7,8]. Lowland tropical forest trees tend to experience long-term low P bioavailability, so it is likely that species have evolved responding and adapting to P-limitation and thus was reflected in tree community composition. Indeed, a recent study in a Panamanian tropical forest showed that P-limitation of plant growth is species-specific, and growth of species adapted to low P availability is rapid [9].
Tropical forests are characterized by high levels of biodiversity and aboveground biomass at a range of spatial scales; however, the spatial distribution of plant species and soils is highly variable within these forests, despite the tropical rainforest presented very often general characteristics, such as high productivity, rapid nutrient turnover, highly weathered soils and low soil pH [10]. Variations in climate, soil traits and gradients, and topography are drivers of diverse plant communities and soil processes that interact to produce high levels of biodiversity [11]. Factors, such as geological history [12], soil type [13,14] and trait [15,16], soil nutrient availability gradients [14,17,18,19], micro-site singularities [20], topography [21] and slope differences [22], disturbance and regeneration regimes [23] and species-specific responses to herbivory [24] are key drivers of species coexistence and tree diversity in the tropics.
Understanding the allocation of P to different biologic functions, for example, growth, energy transfer and storage, in sympatric tree species in a P-limited ecosystem will clarify strategies and mechanisms of species niche segregation and intra-species avoidance of competition. This can provide new essential clues that drives the high levels of tree diversity in tropical forests.
Shifting leaf allocation of P is a key response mechanism to low soil P availability [25]. Foliar P is functionally divided into four major fractions, comprising metabolic P—which includes low-molecular-weight phosphate esters (ADP, ATP and sugar phosphates)—and inorganic phosphate (Pi); nucleic acid P, most of which is contained in ribosomal RNA; structural P in membrane phospholipids; and, residual P in phosphorylated proteins and unidentified residues [25,26,27]. Of these, metabolic P is important in the study of P-limitation, due to the key roles of P-containing metabolites in the Calvin–Benson cycle, where insufficient metabolic P may limit maximum photosynthetic rates [28]. Nucleic acid P, which generally represents 40%–60% of the organic P pool in leaves [29], is mostly (>85%) contained in RNA, particularly ribosomal RNA (rRNA) in which high P-allocations sustain rapid protein synthesis that is required for growth and photosynthesis [25]; thus, there tends to be a positive correlation between rRNA and protein content, as well as with growth rate, over a range of taxa [30]. Structural P accounts for 10%–20% of all foliar P [31] and is mainly contained in phospholipids that are an essential component of plasmalemma and organelle membranes. The fourth fraction, residual P, may represent 20% of total foliar P in tropical trees [25] and is probably mostly contained in phosphorylated proteins. Concentration of residual P tends to be relatively constant, because phosphorylated proteins are essential for many metabolic processes; however, under extreme P-limited conditions, phosphatases may trigger dephosphorylation of proteins [31] that leads to a reduction in residual P concentrations.
31P nuclear magnetic resonance (NMR)-based metabolic profiling allows the quantitative and qualitative analysis of organic-P from molecules involved in biologic functioning in plants and may demonstrate species differences in proportional P-use among plant functions. This methodology has been particularly used for molecular-level characterization of leaf organic P [32,33,34,35].
Here, we used 31P-NMR-based metabolic profiling to study species-specific P-allocation to different biologic functions in contrasting plant communities along soil–topographic gradients to understand drivers and mechanisms of niche differentiation related with species-specific P-use. Specifically, we tested the hypotheses that (1) distribution and proportions of P-molecular compounds are species-specific, and (2) there is a link between concentration and ratios of different P-metabolite groups and plant functional traits.
2. Materials and Methods
2.1. Study Area
French Guiana lies between 2°10′ and 5°45′ N and 51°40′ and 54°30′ W, where 97% of the region is covered by lowland wet tropical forest [36]. The dry season, which extends from September to November, is associated with the displacement of the inter-tropical convergence zone. Mean annual temperature is 25.8 ± 2 °C, with mean daily variations of 7 and 10 °C in the rainy and dry seasons, respectively [37,38]. Study sites were in two old-growth rainforests, one at the Paracou Research Station (5°18′ N, 52°53′ W) and the other at Nouragues Research Station (4°05′ N, 52°40′ W), where mean annual rainfall is 3160 and 2990 mm, respectively [37,38]. Three topographic positions were selected at each study, comprising hilltop (top), mid-slope at an intermediate elevation (slope) and lower slope at low elevation, just above the creek (bottom).
2.2. Study Plots
We established four 20 × 20 m plots at each topographic position; distance between plots, which were in the vicinity of undisturbed long-term (30 years) monitoring plots, was 10–200 m (both in Paracou and Nouragues stations) (Figure 1). Sand content of soils was higher, and clay content was lower at the lower slope plots than in the hilltop and mid-slope plots [38].
Figure 1.
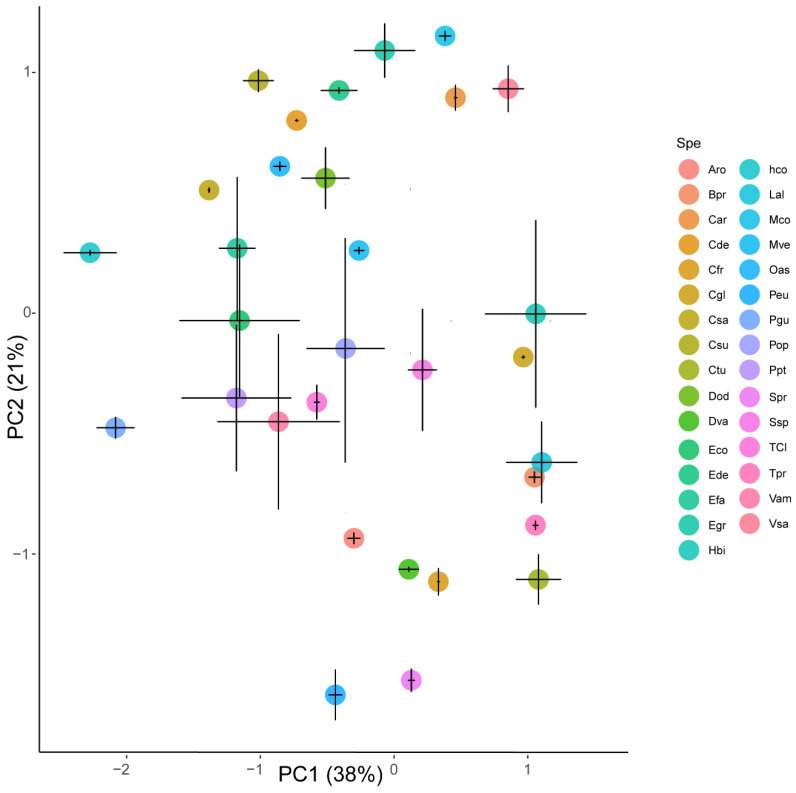
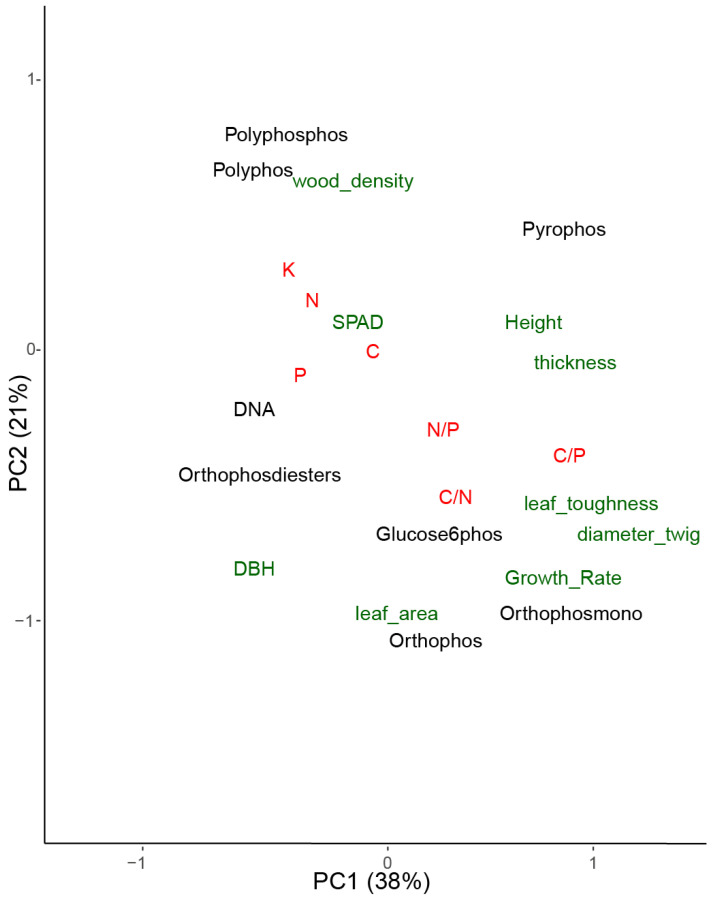
Principal components analysis (PCA) of P organic compounds and elements in leaf material among (A) species and (B) variables. In panel B, metabolomic families are in black text; ecophysiological variables are in green text; and stoichiometric variables are in red text. Polyphos—polyphosphate; pyrophos—pyrophosphate; polyphosphos—polyphosphonate; orthophosphate (orthophos), orthophosphate monosester (orthomono), orthophos—orthophosphate-diester; glucose6phos—glucose-6phosphates; SPAD—chlorophyll content; C—carbon; N—nitrogen; K—potassium; P—phosphorus. the closest tree species to each sampling point with the corresponding S.D. (B). The abbreviation of the legend refer to the closest tree species: Aro—Aniba rosaeodora; Bpr—Bocoa prouacensis; Car—Chrysophyllum argenteum—Cde—Capiro decorticans; Cfr—Catostemma fragrans; Cgl—Caryocar glabrum; Csa—Chrysophyllum sanguinolentum; Csu—Carapa surimensis; Ctu—Chimarrhis turbita; Dod—Dipteryx odorata; Dva—Drypetes variabilis; Eco—Eschweilera coriacea; Ede—Eschweilera decolorans; Efa—Eperua falcata; Egr—Eperua grandiflora; Hbi—Hirtella bicornis; hco—Hymanea courbaril; Lal—Licania alba; Mco—Moronobea coccinea; Mve—Micropholis venulosa; Oas—Oxandra asbeckii; Peu—Pouteria eugeniifolia; Pgu—Paloue guianensis; Pop—Protium opacum; Ppt—Pradosia ptychandra; Spr—Sterculia pruriens; Ssp—Sterculia speciose; TCl—Tovomita clusiaceae; Tpr—Talisia praealta; Vam Vouacapoua america; Vsa—Vochysia sabatieri.
2.3. Sample Collection
We collected leaves from 199 trees of 31 species in the wet season (June) of 2015. The trees were representative of the whole rainforest of the French Guiana and of the two studied sites, Paracou and Nouragues. We collected leaves always in the same position and always of similar age (mature middle-age leaves) with the help of professional climbers. Samples used in metabolomic analyses require rapid processing and appropriate storage [39,40,41,42], so we placed about 2 g of leaf tissue per sample immediately into a paper container that was then frozen in liquid nitrogen and transported to the laboratory.
Frozen leaves were lyophilized and stored in paper containers at −80 °C; then samples were ground to a fine powder using a ball mill at 1500 rpm for 3 minutes and stored at −80 °C prior to analysis extraction.
2.4. Nutrient Pools
Leaf subsamples were pulverized in a ball mill (MM400, Retsch, Haan, Germany) for the analysis of elemental composition. Between 0.15 and 0.2 g of leaf was weighed with a microbalance (MX5 Mettler Toledo, Columbus, OH, USA) for the determination of C and N contents by combustion coupled to an isotope ratio mass spectrometer at the Stable isotopes facility (UC Davis, Davis, CA, USA). P and K contents were determined by diluting 0.25 g of soil with an acid mixture of HNO3 (60%) and H2O2 (30% p/v) and digested in a microwave oven (MARS Xpress, CEM Corporation, Matthews, NC, USA). The digested solutions were then diluted to a final volume of 50 mL with ultrapure water and 1% HNO3. Blank solutions (5 mL of HNO3 with 2 mL of H2O2 with no sample biomass) were regularly analyzed. The content of each element was determined using inductively coupled plasma/optical emission spectrometry (ICP-OES Optima 4300DV, PerkinElmer, Wellesley, MA, USA). We used the standard certified biomass NIST 1573a to assess the accuracy of the biomass digestion and analytical procedures.
2.5. One Dimensional 31P-NMR Analysis
Standard 1D 31P-NMR was used to quantify concentrations of the main organic and inorganic P classes, comprising as DNA, total diesters and monoesters, phosphonates, pyrophosphate and polyphosphate. Phosphorus was extracted by shaking 1.5 g of dry and ground leaf from each composite leaf sample in 30 mL of a solution containing 250 mM NaOH and 50 mM Na2EDTA (ethylenediaminetetraacetate) for 4 h [43,44,45]. Then, extracts were centrifuged (30 min, 14,000× g), and 23 mL of resulting supernatant was frozen at −80 °C overnight and lyophilized. Lyophilization yielded 750 ± 50 mg of material, 80 mg of which was redissolved in 640 μL (1:8 w/v ratio) of a solution containing 530 μL of D2O, 10 μL of 14.2 M NaOD and 50 μL of 16 mM methylene diphosphonic acid (MDPA) trisodium salt (CH3O6P2Na3, Sigma-Aldrich, St. Louis, MO, USA, product number M1886). The MDPA was a reference for the quantification of individual P compounds, where each 50 μL spike contained 50 μg of P. The redissolved solution was vortexed for 2 min and subsequently centrifuged for 5 min at 10,000 rpm; then 560 μL of the solution was transferred to a 5-mm NMR tube for analysis. During the extraction, we continuously maintained the pH at 8. We used all the samples at the same pH to compare all the samples in the same condition. Subsequent organic phosphorus extraction in NaOH–EDTA improved spectral resolution in solution 31P-NMR spectroscopy. Spectra were obtained using a Bruker Avance III 600 MHz spectrometer (Bruker, Germany) operating at 161.76 MHz for 31P; NaOH–EDTA extracts were analyzed using zgpg30 pulse sequence, with a relaxation delay of 2.0 s, an acquisition time of 0.9 s, broadband proton decoupling, 8k scans were acquired per sample and using 64 K time domain data points, lasting each run an overall time of 4 h 40 min. Spectra were processed with a line broadening of 2 Hz, and chemical shifts were determined in parts per million (ppm) relative to an external standard of 85% orthophosphoric acid (H3PO4). Identification of the 5target P classes was based on chemical shifts and previous reported data [32,36]. Spectral processing was done using TopSpin 2.0 software. After a peak picking process, peak areas were calculated by deconvolution and integration of individual peaks. Concentration of P-containing compounds (mg P kg−1 air dried leaf) were calculated using the known concentration of spiked MDPA.
31P-NMR is an NMR technique that does not a require isotope labeling and highly sensitive with NMR detection. Intensity in 31P spectra signals was assigned to P types by integrating across the broad chemical shift regions of −21.5 to −18.5 ppm for non-terminal polyphosphate (poly-P), −5.3 to −4.8 ppm for pyrophosphate (pyro P), −4.8 to −4.0 for terminal poly P, which is mainly assigned to inorganic pyrophosphate and polyphosphates, −1.5 to 2.5 for diester-P and 2.5 to 7 ppm for orthophosphate (ortho-P and monoester-P). Deconvolution, which was then used to determine the intensity of up to 16 resonances in the ortho-P and monoester-P region, was initiated by manually identifying the chemical shift of peaks and shoulders. Chemical shifts of corresponding resonances varied only slightly between samples, and the largest variation was for orthophosphate resonance (range 5.56–5.74 ppm). This large variation most likely reflects sensitivity of orthophosphate chemical shifts to slight differences in pH between samples [46].
2.6. Statistical Analyses
Differences in leaf P-compounds between species tested using PERMANOVA [47] of the NMR data for each tree from which a leaf was collected, using Euclidean distance of species as a fixed factor. The number of permutations was set at 2000. We scaled normalized areas of metabolite peaks and then characterized and visualized differences among species and P compounds using principal components analysis (PCA), partial least squares discriminant analysis (PLSDA) and Pearson’s correlation were used to test the degree of correspondence between the 1D NMR measurements and the relationship between elements and P organic compounds.
Statistical procedures were performed using R v2.12 (www.r-project.org) Core software using the SEQKNN, DOBY, PHEATMAP, VEGAN, FACTOEXTRA, FACTOMINER, DPLYR and MIXOMICS packages. Presented data are means ± SEM (Standard error of the mean), unless otherwise stated.
3. Results
3.1. Phosphorus Compounds Identification
On average, 31P-NMR spectra of NaOH–EDTA extracts (and relative proportions,%) from the leaf samples were characterized thus: monoester at 3.4 to 5.4 ppm (c. 25%), unhydrolyzed diesters at −1 to 2.3 ppm (c. 20%), DNA at −0.3 ppm (c. 20%), polyphosphate at −5.6 to −3.8 (c. 10%), inorganic orthophosphate (hereafter called ‘phosphate’ at 5.7 to 6.5 (c. 10%), pyrophosphate (pyrophos) inorganic form of P at 0.5 to 0.6. ppm (c. 5%) and glucose-6phosphates (glucose6phos) at 5.3 to 5.4 ppm (c. 5%). However, there were three main resonance areas in the spectra of acid-insoluble compounds: P in monoesters, phospholipids (orthophosphate diester) and DNA. Signals from nucleic acids (DNA −0.37 ppm) and phospholipids were differentiated in the orthophosphate diester (orthophosdiester) region and identified in a leaf sample. Inorganic (polyphos) and organic polyphosphates were differentiated by the presence of a signal at −9 ppm from the α phosphate of organic polyphosphates (polyphosphos). Some orthophosphate monoesters, which were mainly represented by RNA-derived mononucleotides and phosphatidyl choline, degraded rapidly to orthophosphate diesters in NaOH–EDTA; DNA and other phospholipids were more stable [32,36].
3.2. Phosphorus Compounds and Elements Among Species
There were species differences in P organic compounds profiles (pseudo-F = 3.08; R2 = 0.67, p < 0.001), where 67% of the variability was explained by species (Table 1).
Table 1.
Test for species differences in phosphorus (P) organic compound content in leaf material.
| Source | df | SS | MS | F | R 2 | p |
|---|---|---|---|---|---|---|
| Species | 33 | 10206396 | 309,285 | 3.08 | 0.67 | 0.001 |
| Residuals | 49 | 4914344 | 100,293 | 0.33 | ||
| Total | 82 | 15120740 | 1 |
Concentrations of P in the tree leaf material varied from 0.73 to 2.10 mg kg−1 (Table 2), where NaOH–EDTA extracted P represented 65%–90% of total P; 15%–40% was inorganic P that was almost completely lost during NaOH–EDTA extraction and loss of Porg did not exceed 15%–20%. Proportions of monoester and diester-P varied among tree species (Table 2). The highest proportion of diesters (55%) was recorded in Calostemma fragrans, Sterculia pruriens, S. speciosa, Protium opacum, Aniba rosaeodora and Drypetes variabilis, while the lowest (10%) was found in Chrysophyllum argenteum, Tovomita clusiacege, Eschweilera decolorans, Capirona decorticans, Paloue guianensis, Dipteryx odorata and Talisia praealta. The proportion of phospholipid-P exceeded 30% in extracts from Tovomita clusiacege and was 10% in extracts from the other species (Table 2).
Table 2.
Profile of P-compounds in leaf material of tree species. 31P NMR spectra of NaOH–EDTA extracts (and relative proportions, %) from the leaf samples were characterized.
| Species | Total P (%, D.W.) |
Total C (%, D.W.) |
Total N (%, D.W.) |
C:N | C:P | N:P | K (%, D.W.) |
Phosphorus Species Distribution, % of Total P in Extract | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphos | Orthophos | Glucose-6phos | Orthophos-mono | Orthophos-diesters | DNA | Pyrophos | Poly-P | ||||||||
| Licania alba | 0.04 | 48.59 | 1.44 | 34.7 | 1151.91 | 33.17 | 0.35 | 3.55 | 7.11 | 0 | 14.12 | 28.57 | 16.57 | 17.30 | 12.79 |
| Vouacapoua americana | 0.07 | 50.2 | 1.99 | 25.6 | 687.46 | 26.84 | 0.67 | 1.67 | 8.57 | 24.17 | 22.41 | 15.52 | 6.75 | 13.92 | 6.99 |
| Chrysophyllum argenteum | 0.03 | 49.7 | 1.34 | 37.2 | 1435.60 | 38.59 | 0.40 | 40.25 | 0 | 0 | 11.77 | 11.57 | 16.82 | 0 | 19.60 |
| Oxandra asbeckii | 0.05 | 48.1 | 1.94 | 24.8 | 1042.83 | 42.13 | 1.69 | 24.25 | 63.24 | 0 | 0.65 | 1.30 | 10.04 | 0 | 0.52 |
| Hirtella bicornis | 0.04 | 47.1 | 1.32 | 35.9 | 1215.23 | 33.94 | 0.75 | 0 | 0 | 0 | 26.38 | 13.88 | 9.03 | 25.13 | 25.58 |
| Tovomita sp. | 0.07 | 49.3 | 1.61 | 30.6 | 670.28 | 21.91 | 0.72 | 0 | 6.65 | 16.55 | 6.99 | 16.12 | 22.00 | 0 | 31.70 |
| Moronobea coccinea | 0.03 | 48.8 | 1.28 | 38.4 | 1469.22 | 38.49 | 0.27 | 5.88 | 0 | 0 | 1.82 | 46.14 | 23.60 | 0 | 22.55 |
| Eschweilera coriacea | 0.07 | 50.0 | 1.71 | 30.7 | 975.27 | 30.72 | 0.50 | 1.60 | 16.35 | 10.46 | 17.44 | 22.40 | 16.29 | 8.91 | 6.56 |
| Hymanea courbaril | 0.12 | 48.5 | 1.91 | 25.3 | 412.80 | 16.29 | 0.87 | 42.19 | 38.05 | 3.09 | 0 | 0 | 11.35 | 5.31 | 0 |
| Eschweilera decolorans | 0.05 | 50.0 | 1.77 | 28.2 | 1103.39 | 39.14 | 0.58 | 32.27 | 0 | 0 | 9.36 | 27.51 | 6.00 | 0 | 24.85 |
| Capirona decorticans | 0.05 | 48.7 | 2.08 | 23.4 | 1077.96 | 46.08 | 0.41 | 0 | 0 | 0 | 11.37 | 53.25 | 32.62 | 0 | 2.77 |
| Pouteria eugeniifolia | 0.08 | 48.6 | 2.89 | 16.9 | 650.54 | 38.58 | 0.69 | 0 | 35.00 | 0 | 15.72 | 19.61 | 19.61 | 0 | 10.06 |
| Eperua falcata | 0.08 | 49.6 | 1.70 | 30.1 | 687.85 | 23.49 | 0.63 | 7.37 | 0 | 6.41 | 25.85 | 43.73 | 10.22 | 0 | 6.41 |
| Catostemma fragrans | 0.03 | 25.2 | 0.90 | 14.0 | 455.01 | 16.29 | 0.28 | 0 | 49.45 | 2.50 | 28.06 | 0 | 20 | 0 | 0 |
| Caryocar glabrum | 0.05 | 52.4 | 1.34 | 39.2 | 1009.50 | 25.75 | 0.36 | 0 | 22.91 | 0 | 20.06 | 20.06 | 32.87 | 3.59 | 0.51 |
| Eperua grandiflora | 0.04 | 50.5 | 1.39 | 37.8 | 1289.63 | 34.47 | 0.34 | 6.39 | 7.22 | 6.46 | 25.13 | 19.73 | 12.13 | 18.70 | 4.25 |
| Paloue guianensis | 0.05 | 52.0 | 1.76 | 29.5 | 1086.24 | 36.77 | 0.22 | 2.21 | 0 | 19.81 | 7.14 | 21.05 | 21.63 | 28.16 | 0 |
| Dipteryx odorata | 0.06 | 49.4 | 1.38 | 35.9 | 891.48 | 24.81 | 0.89 | 0.31 | 12.79 | 8.35 | 4.57 | 16.65 | 3.13 | 0 | 54.19 |
| Protium opacum | 0.06 | 47.7 | 1.25 | 38.5 | 856.32 | 22.39 | 0.67 | 14.49 | 32.93 | 0 | 40.44 | 0.80 | 7.12 | 0 | 4.21 |
| Talisia praealta | 0.04 | 48.9 | 1.23 | 39.9 | 1203.11 | 30.12 | 0.21 | 0 | 0 | 0 | 10.14 | 18.78 | 18.78 | 23.31 | 28.98 |
| Bocoa prouacensis | 0.04 | 48.8 | 1.66 | 31.0 | 1471.96 | 47.02 | 0.35 | 0 | 27.66 | 14.21 | 14.21 | 4.61 | 2.66 | 0.46 | 36.19 |
| Sterculia pruriens | 0.07 | 46.9 | 1.42 | 33.2 | 686.14 | 20.66 | 1.14 | 0 | 19.99 | 22.74 | 44.54 | 0 | 12.73 | 0 | 0 |
| Pradosia ptychandra | 0.08 | 46.7 | 1.79 | 27.1 | 727.94 | 25.16 | 1.57 | 10.66 | 0 | 0 | 28.35 | 17.79 | 10.19 | 0 | 33.01 |
| Aniba rosaeodora | 0.05 | 48.2 | 1.44 | 33.5 | 1019.02 | 30.46 | 0.97 | 0 | 0 | 0 | 50 | 30 | 20 | 0 | 0 |
| Vochysia sabatieri | 0.04 | 47.31 | 1.22 | 39.03 | 1357.56 | 34.89 | 0.23 | 0 | 2.20 | 0 | 30.08 | 41.23 | 14.48 | 2.68 | 9.33 |
| Chrysophyllum sanguinolentum | 0.06 | 48.02 | 2.42 | 19.85 | 816.72 | 41.14 | 1.47 | 0 | 0 | 0 | 18.18 | 59.02 | 13.77 | 0 | 9.03 |
| Sterculia speciosa | 0.05 | 48.63 | 1.35 | 36.10 | 1020.57 | 28.27 | 0.86 | 1.07 | 12.07 | 7.77 | 59.09 | 10 | 10 | 0 | 0 |
| Carapa surinamensis | 0.06 | 48.49 | 1.41 | 34.61 | 816.65 | 23.59 | 0.50 | 30.42 | 0 | 13.52 | 0 | 21.87 | 22.02 | 0 | 12.17 |
| Chimarrhis turbinata | 0.05 | 47.42 | 1.99 | 23.87 | 941.32 | 39.24 | 0.23 | 0 | 15.06 | 22.14 | 15.74 | 17.90 | 4.48 | 24.69 | 0 |
| Drypetes variabilis | 0.05 | 45.13 | 1.45 | 31.15 | 847.58 | 27.14 | 1.03 | 22.84 | 15.94 | 3.22 | 37.99 | 16.00 | 4.00 | 0 | 0 |
| Micropholis venulosa | 0.04 | 47.48 | 1.90 | 25.04 | 1149.58 | 45.91 | 0.65 | 0 | 0 | 0 | 27.64 | 23.10 | 28.69 | 0 | 20.57 |
3.3. Phosphorus Compounds and Elements
PCs 1 and 2 of the PCA of P organic compounds and elements concentration in leaf material among species represented 59% of the variation and showed several separations among species (Figure 1A). The results of the PLS–DA were very similar to the results of PCA (Figure 1). Nutrients were a key driver, as indicated by higher N and P concentrations and lower C:P and C:N ratios along PC1 axis, whereas variation in PC2 was associated with distinct identified P family components, with observed gradient along this axis from P families associated with energy transference and genetic information and protein synthesis to organic P-family (polyphosphates) associated with energy reserves (Figure 1B).
4. Discussion
Our results support our hypothesis that P metabolite profiling differ among species, reflecting niche differentiation in the P-use. We observed that species tended to be characterized by the composition in leaf tissue of main functional groups of metabolic P-containing molecules and elements, indicating species use of different forms of P drive is species-specific diversity in the studied tropical wet forest studied sites. We observed clear species niche differentiation along PC2 (Figure 1B) along the gradient constituted from high-anabolic-energy P-use and low P-storage compounds to low-anabolic-energy P-use and high P-storage compounds; however, there were no clear patterns of this relationship with total P concentration or between total foliar P concentration and the foliar P metabolome. One possible explanation for these contrasting results may be the lack of direct comparison, because the P-metabolome was analyzed using water extracts, whereas P concentrations were determined from leaf structural tissues, where there are species variations in light use or anti-herbivore strategy, may result in species with higher levels of P use for active metabolism, but not necessarily higher levels of total P concentration. Leaves directly exposed to sunlight tend to be sclerophyllous and contain low P concentrations; however, most P is allocated to energy and anabolic metabolism due to the high photosynthetic activity and levels of energy storage.
Our results show that proportional and functional use of P varies with species, indicating niche partitioning. Similarly, previous studies in this tropical forest have observed that general foliar metabolomic profiles show clear species-specific signatures that indicate functional niche separation in dominant tree species [48]. Our results indicate that microsite differences in resource bioavailability create variations in niche conditions that drive multiple species coexistence at small spatial scales.
Classical species niche hypotheses are based on the concept that potential competing sympatric species have varying optimum basic abiotic resource requirements (light, water and nutrients) and contrasting biotic relationships [49]. Previous studies have identified associations between species assemblages and habitat conditions in tropical rainforests [50,51,52,53], such as variations in leaf properties (parental material, drainage), leaf nutrient availability [14] and topography (slope steepness and orientation, margins with water courses), that are consistent with niche theory. Extensions of the classical ecological niche hypothesis, such as the biogeochemical niche hypothesis, claim that species tend to reach an optimal chemical composition that is linked to a singular optimal function (homeostasis) and allows niche occupation [54,55,56,57,58]. Thus, this study provides evidence for the use of a fundamental resource, such as the P, in the characterization of species niche space. This approach may be powerful in sites with limited P, such as in rainforests, where a P-metabolic niche would reflect species-specific functional adaptions along natural gradients that drive bioavailability, total concentration and content of P as consequence of long-term different sympatric species co-evolution that should have optimized community use and conservation of the scarce amounts of P. Tropical trees may occupy finely subdivided niches [59], but there is little direct evidence for a measurable variable that reflects overall functional differences among sympatric species. Our results clearly indicate that species niche specificity may be determined, at least partially, by analyzing variance in the P-metabolic profile among sympatric species, together with other potential sources of environmental variability, such as micro-site conditions and topography. It is likely, therefore, that environmental factors, such as topography and leaf physicochemical properties, could be related to species-specific use of P. That is also consistent with the significant change of P-use and niche differentiation across distinct space situation due slope at microscale spatial level and also to medium spatial distances in this pristine rainforest area in French Guiana. Implies a continuum of strategies form an extreme of high growth rates, high leaf area, low wood density and high P-use in anabolic and energy store and transference to another extreme strategy with low growth rate and leaf area, high wood density and high P-reserve compounds.
5. Conclusions
This study found large differences in P-metabolite profiling among sympatric tree species in French Guianese rainforests. 31P-NMR-based metabolic profiling analysis showed tree species that contained high concentrations of P-organic compounds related to energy, information and protein synthesis had low concentrations of P-organic compounds related to P-storage; in other hand the reverse, were P-organic compounds related to P-storage had higher concentration had low concentration of P-organic compounds related to energy, information and protein synthesis was true.
These results are consistent with the niche theory and demonstrate the usefulness of P-metabolome as an analytical tool to determine niche differences in the functions of plant species in a community under limited nutrient bioavailability. It is likely that metabolomic niches in old, undisturbed tropical forest ecosystems are associated with the optimal use-exploitation of environmental niches (light conditions, water and nutrient availability, and/or specific biotic relationships) acquired through evolutionary processes. In tropical forests on P-poor soils, species exhibit morphologic, physiological, molecular and biochemical adaptions, where P-use forms are related to basic functional traits, such as growth rate, leaf area and wood density.
Described results strongly suggested that existed a continuum of strategies form from the species to relate an extreme of high growth rates, high leaf area, low wood density and high P-use in anabolic and energy store. Another extreme strategy with low growth rate and leaf area, high wood density and high P-reserve compounds.
The results were also consistent with the significant change of P-use and niche differentiation across distinct space situation due to slope at microscale spatial level and to medium spatial distances in this pristine rainforest area in French Guiana.
Author Contributions
A.G.-G. and J.P. designed the study with the help of all co-authors. All authors participated in the field measurements or chemical and statistical analyses. All authors contributed to the writing of the manuscript and the drafting of the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Research Council Synergy grant ERC-2013-SyG-610028 IMBALANCE-P, the European FP7 S-Clima project PIEF-GA-2013-626234, the Spanish Government grant CGL2016-79835, the Catalan Government grant SGR 2017-1005, we thank the staff of the Nouragues station managed by USR mixte LEEISA (CNRS; Cayenne) and the Paracou station managed by UMR Ecofog (CIRAD, INRA; Kourou). Both research stations benefit from “Investissement d’Avenir” grants managed by Agence Nationale de la Recherche (CEBA: ANR-10-LABX-25-01; ANAEE-France: ANR-11-INBS-0001). AGG was supported by the Ministry of Education, Youth and Sports of CR within the project Mobility CzechGlobe, CZ.02.2.69/0.0/0.0/16_027/0008137. AGG and OU were also supported by the project SustES (CZ.02.1.01/0.0/0.0/16_019/0000797).
Conflicts of Interest
The authors declare no competing financial and non-financial interests.
Footnotes
Sample Availability: Samples of the compounds http://globalecology.creaf.cat/ are available from the authors.
References
- 1.Vos J., Biemond H. Effects of nitrogen on the development and growth of the potato plant. 1. Leaf appearance, expansion growth, life spans of leaves and stem branching. Ann. Bot. 1992;70:27–35. doi: 10.1093/oxfordjournals.aob.a088435. [DOI] [Google Scholar]
- 2.Evans J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- 3.Elser J.J., Bracken M.E.S., Cleland E.E., Gruner D.S., Harpole W.S., Hillebrand H., Ngai J.T., Seabloom E.W., Shurin J.B., Smith J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanner E.V.J., Vitousek P.M., Cuevas E. experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology. 1998;79:10–22. doi: 10.1890/0012-9658(1998)079[0010:EIONLO]2.0.CO;2. [DOI] [Google Scholar]
- 5.Walker T.W., Syers J.K. The fate of phosphorus during pedogenesis. Geoderma. 1976;15:1–19. doi: 10.1016/0016-7061(76)90066-5. [DOI] [Google Scholar]
- 6.Allison S.D., Vitousek P.M. Rapid nutrient cycling in leaf litter from invasive plants in Hawai. Oecologia. 2004;141:612–619. doi: 10.1007/s00442-004-1679-z. [DOI] [PubMed] [Google Scholar]
- 7.Wardle D.A., Walker L.R., Bardgett R.D. Ecosystem properties and forest decline in contrasting long-term. Science. 2004;305:509–514. doi: 10.1126/science.1098778. [DOI] [PubMed] [Google Scholar]
- 8.Kichenin E., Wardle D.A., Peltzer D.A., Morse C.W., Freschet G.T. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013;27:1254–1261. doi: 10.1111/1365-2435.12116. [DOI] [Google Scholar]
- 9.Turner B.L., Brenes-Arguedas T., Condit R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature. 2018;555:367–370. doi: 10.1038/nature25789. [DOI] [PubMed] [Google Scholar]
- 10.Vitousek P.M., Sanford R.L., Jr. Nutrient cycling in moist tropical forest. Ann. Rev. Ecol. Syst. 1986;17:137–167. doi: 10.1146/annurev.es.17.110186.001033. [DOI] [Google Scholar]
- 11.Vitousek P.M. Nutrient Cycling and Limitation: Hawai‘i as a Model System. Princeton University Press; Princeton, NJ, USA: 2004. [Google Scholar]
- 12.Corlett R.T., Primack R.B. Tropical rainforests and the need for cross-continental comparisons. Trends Ecol. Evol. 2006;21:105–107. doi: 10.1016/j.tree.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Aiba S.I., Sawada Y., Takyu M., Eino T., Kitayama K., Repin R. Structure, floristics and diversity of tropical montane rain forests over ultramafic soils on Mount Kinabalu (Borneo) compared with those on non-ultramafic soils. Aust. J. Bot. 2015;63:191–203. doi: 10.1071/BT14238. [DOI] [Google Scholar]
- 14.John R., Dalling J.W., Harms K.E., Yavitt J.B., Stallard R.F., Mirabello M., Hubbell S.P., Valencia R., Navarrete H., Vallejo M., et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA. 2007;104:864–869. doi: 10.1073/pnas.0604666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennington R.T., Lavin M., Oliveira-Filho A. woody plant diversity, evolution, and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Evol. Syst. 2009;40:437–457. doi: 10.1146/annurev.ecolsys.110308.120327. [DOI] [Google Scholar]
- 16.Matos F.A.R., Magnago L.F.S., Gastauer M., Carreiras J.M.B., Simonelli M., Meira-Neto J.A.A., Edwards D.P. Effects of landscape configuration and composition on phylogenetic diversity of trees in a highly fragmented tropical forest. J. Ecol. 2017;105:265–276. doi: 10.1111/1365-2745.12661. [DOI] [Google Scholar]
- 17.LeBauer D.S., Treseder K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- 18.Fujii K., Shibata M., Kitajima K., Ichie T., Kitayama K., Turner B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2017;33:1–12. doi: 10.1007/s11284-017-1511-y. [DOI] [Google Scholar]
- 19.Xu W., Ci X., Song C., He T., Zhang W., Li Q., Li J. Soil phosphorus heterogeneity promotes tree species diversity and phylogenetic clustering in a tropical seasonal rainforest. Ecol. Evol. 2016;6:8719–8726. doi: 10.1002/ece3.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins K.G., Marques M.C.M., dos Santos E., Marques R. Effects of soil conditions on the diversity of tropical forests across a successional gradient. For. Ecol. Manage. 2015;349:4–11. doi: 10.1016/j.foreco.2015.04.018. [DOI] [Google Scholar]
- 21.Mandl N.A., Kessler M., Robbert Gradstein S. Effects of environmental heterogeneity on species diversity and composition of terrestrial bryophyte assemblages in tropical montane forests of southern Ecuador. Plant. Ecol. Divers. 2009;2:313–321. doi: 10.1080/17550870903341877. [DOI] [Google Scholar]
- 22.Clark D.B., Clark D.A., Read J.M. Edaphic variation and the mesoscale distribution of tree species in a neotropical rain forest. J. Ecol. 1998;86:101–112. doi: 10.1046/j.1365-2745.1998.00238.x. [DOI] [Google Scholar]
- 23.Connell J.H. Diversity in tropical rain forests and coral reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 24.Becerra J.X. On the factors that promote the diversity of herbivorous insects and plants in tropical forests. Proc. Natl. Acad. Sci. USA. 2015;112:6098–6103. doi: 10.1073/pnas.1418643112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidaka A., Kitayama K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011;99:849–857. doi: 10.1111/j.1365-2745.2011.01805.x. [DOI] [Google Scholar]
- 26.Lambers H., Finnegan P.M., Laliberté E., Pearse S.J., Ryan M.H., Shane M.W., Veneklaas E.J. Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant. Physiol. 2011;156:1058–1066. doi: 10.1104/pp.111.174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapin III F.S., Kedrowski R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology. 1983;64:376–391. doi: 10.2307/1937083. [DOI] [Google Scholar]
- 28.Ågren G.I., Wetterstedt J., Billberger M.F.K. Nutrient limitation on terrestrial plant growth--modeling the interaction between nitrogen and phosphorus. New Phytol. 2012;194:953–960. doi: 10.1111/j.1469-8137.2012.04116.x. [DOI] [PubMed] [Google Scholar]
- 29.Veneklaas E.J., Lambers H., Bragg J., Finnegan P.M., Lovelock C.E., Plaxton W.C., Price C.A., Scheible W.-R., Shane M.W., White P.J., et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012;195:306–320. doi: 10.1111/j.1469-8137.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 30.Elser J.J., Brien W.J.O., Dobberfuhl D.R., Dowling T.E. The evolution of ecosystem processes: Growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J. Evol. Biol. 2000;13:845–853. doi: 10.1046/j.1420-9101.2000.00215.x. [DOI] [Google Scholar]
- 31.Schlüter U., Colmsee C., Scholz U., Bräutigam A., Weber A.P.M., Zellerhoff N., Bucher M., Fahnenstich H., Sonnewald U. Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genom. 2013;14:442. doi: 10.1186/1471-2164-14-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner B.L., Mahieu N., Condron L.M. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci. Soc. Am. J. 2003;67:497–510. doi: 10.2136/sssaj2003.4970. [DOI] [Google Scholar]
- 33.Cade-Menun B.J., Liu C.W., Nunlist R., McColl J.G. Soil and litter Phosphorus-31 nuclear magnetic resonance spectroscopy. J. Environ. Qual. 2002;31:457–465. [PubMed] [Google Scholar]
- 34.Vincent A.G., Turner B.L., Tanner E.V.J. Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur. J. Soil Sci. 2010;61:48–57. doi: 10.1111/j.1365-2389.2009.01200.x. [DOI] [Google Scholar]
- 35.Vestergren J., Vincent A.G., Jansson M., Persson P., Ilstedt U., Giesler R. High-resolution characterization of organic phosphorus in soil extracts using 2D 1 H−31 P NMR correlation spectroscopy. Environ. Sci. Technol. 2012;46:3950–3956. doi: 10.1021/es204016h. [DOI] [PubMed] [Google Scholar]
- 36.Me Chave J.R., Ra B.R., Dubois M.-A. Estimation of biomass in a neotropical forest of French Guiana: Spatial and temporal variability. J. Trop. Ecol. 2001;17:79–96. doi: 10.1017/S0266467401001055. [DOI] [Google Scholar]
- 37.Gourlet-Fleury S., Guehl J.-M., Laroussinie O. Ecology and Management of a Neotropical Rainforest: Lessons Drawn from Paracou, a Long-Term Experimental Research Site in French Guiana. Elseiver; Paris, France: 2004. [Google Scholar]
- 38.Courtois E.A., Stahl C., Van den Berge J., Bréchet L., Van Langenhove L., Richter A., Urbina I., Soong J.L., Peñuelas J., Janssens I.A. Spatial variation of soil CO2, CH4 and N2O fluxes across topographical positions in tropical forests of the guiana shield. Ecosystems. 2018;21:1445–1458. doi: 10.1007/s10021-018-0232-6. [DOI] [Google Scholar]
- 39.Sardans J., Peñuelas J., Rivas-Ubach A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology. 2011;21:191–225. doi: 10.1007/s00049-011-0083-5. [DOI] [Google Scholar]
- 40.Rivas-Ubach A., Pérez-Trujillo M., Sardans J., Gargallo-Garriga A., Parella T., Peñuelas J. Ecometabolomics: Optimized NMR-based method. Methods Ecol. Evol. 2013;4:464–473. doi: 10.1111/2041-210X.12028. [DOI] [Google Scholar]
- 41.Deborde C., Moing A., Roch L., Jacob D., Rolin D., Giraudeau P. Plant metabolism as studied by NMR Spectrosc. Prog. Nucl. Magn. Reson. Spectrosc. 2017;102–103:61–97. doi: 10.1016/j.pnmrs.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Wilson I.D., Plumb R., Granger J., Major H., Williams R., Lenz E.M. HPLC-MS-based methods for the study of metabonomics. J. Chromatogr. B. 2005;817:67–76. doi: 10.1016/j.jchromb.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Cade-Menun B.J., Preston C.M. A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci. 1996;161:770–785. doi: 10.1097/00010694-199611000-00006. [DOI] [Google Scholar]
- 44.Makarov M.I., Haumaier L., Zech W., Marfenina O.E., Lysak L.V. Can 31P NMR spectroscopy be used to indicate the origins of soil organic phosphates? Soil Biol. Biochem. 2005;37:15–25. doi: 10.1016/j.soilbio.2004.07.022. [DOI] [Google Scholar]
- 45.Turner B.L. Soil organic phosphorus in tropical forests: An assessment of the NaOH-EDTA extraction procedure for quantitative analysis by solution 31P NMR spectroscopy. Eur. J. Soil Sci. 2008;59:453–466. doi: 10.1111/j.1365-2389.2007.00994.x. [DOI] [Google Scholar]
- 46.Smernik R.J., Doolette A.L., Marschner P., Bu E.K., Stonor R., Wakelin S.A., Mcneill A.M. Soil biology & biochemistry forms of phosphorus in bacteria and fungi isolated from two Australian Soils. Soil Biol. Biochem. 2008;40:1908–1915. [Google Scholar]
- 47.Anderson M.J., Gorley R.N., Clarke K.R. PERMANOVA + for PRI-MER: Guide to Software and Statistical Methods. Primer-E; Plymouth, UK: 2008. [Google Scholar]
- 48.Wright S.J. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia. 2002;130:1–14. doi: 10.1007/s004420100809. [DOI] [PubMed] [Google Scholar]
- 49.Williams W.T., Lance G.N., Webb L.J., Tracey J.G., Connell J.H. Studies in the numerical analysis of complex rain-forest communities: IV. A method for the elucidation of small-scale forest pattern. J. Ecol. 1969;57:635–654. doi: 10.2307/2258489. [DOI] [Google Scholar]
- 50.Whitmore T.C. Tropical Rain Forests of the Far East. Clarendon; Oxford, UK: 1975. [Google Scholar]
- 51.Richards P.W. The Tropical Rain Forest; An Ecological Study. Cambridge Univ. Press; New York, NY, USA: 1952. [Google Scholar]
- 52.Janzen D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970;104:501–528. doi: 10.1086/282687. [DOI] [Google Scholar]
- 53.Peñuelas J., Sardans J., Ogaya R., Estiarte M. Nutrient stoichiometric relations and biogeochemical niche in coexisting plant species: Effect of simulated climate change. Pol. J. Ecol. 2008;56:613–622. [Google Scholar]
- 54.Gargallo-Garriga A., Sardans J., Granda V., Llusià J., Peguero G., Asensio D., Ogaya R., Urbina I., Van Langenhove L., Verryckt L.T., et al. Different “metabolomic niches” of the highly diverse tree species of the French Guiana rainforests. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-63891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penuelas J., Sardans J., Llusià J., Owen S.M., Carnicer J., Giambelluca T.W., Rezende E.L., Waite M., Niinemets Ü. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob. Chang. Biol. 2010;16:2171–2185. doi: 10.1111/j.1365-2486.2009.02054.x. [DOI] [Google Scholar]
- 56.Peñuelas J., Fernández-Martinez M., Ciais P., Jou D., Piao S., Obersteiner M., Vicca S., Janssens I.A., Sardans J. The bioelements, the elementome, and the biogeochemical niche. Ecology. 2019;100:e02652. doi: 10.1002/ecy.2652. [DOI] [PubMed] [Google Scholar]
- 57.Sardans J., Janssens I.A., Alonso R., Veresoglou S.D., Rillig M.C., Sanders T.G., Carnicer J., Filella I., Farré-Armengol G., Peñuelas J. Foliar elemental composition of European forest tree species associated with evolutionary traits and present environmental and competitive conditions. Glob. Ecol. Biogeogr. 2015;24:240–255. doi: 10.1111/geb.12253. [DOI] [Google Scholar]
- 58.Urbina I., Sardans J., Grau O., Beierkuhnlein C., Jentsch A., Kreyling J., Peñuelas J. Plant community composition affects the species biogeochemical niche. Ecosphere. 2017;8:e01801. doi: 10.1002/ecs2.1801. [DOI] [Google Scholar]
- 59.Ricklefs R.E. Environmental heterogeneity and plant species diversity: A hypothesis. Am. Nat. 1977;111:376–381. doi: 10.1086/283169. [DOI] [Google Scholar]