Stealthy intracellular bacterial pathogens are known to establish persistent and sometimes lifelong infections. Some of these pathogens also have a tropism for the reproductive system, thereby increasing the risk of reproductive disease and infertility. To date, the pathogenic mechanism involved remains poorly understood. Here, we demonstrate that Brucella abortus, a notorious reproductive pathogen, has the ability to infect the nonpregnant uterus, sustain infection, and induce inflammatory changes during both acute and chronic stages of infection.
KEYWORDS: antagonism, antibody, Brucella, regulatory T cells, TNFR2, tropism
ABSTRACT
Stealthy intracellular bacterial pathogens are known to establish persistent and sometimes lifelong infections. Some of these pathogens also have a tropism for the reproductive system, thereby increasing the risk of reproductive disease and infertility. To date, the pathogenic mechanism involved remains poorly understood. Here, we demonstrate that Brucella abortus, a notorious reproductive pathogen, has the ability to infect the nonpregnant uterus, sustain infection, and induce inflammatory changes during both acute and chronic stages of infection. In addition, we demonstrated that chronically infected mice had a significantly reduced number of pregnancies compared to naive controls. To investigate the immunologic mechanism responsible for uterine tropism, we explored the role of regulatory T cells (Tregs) in the pathogenesis of Brucella abortus infection. We show that highly suppressive CD4+FOXP3+TNFR2+ Tregs contribute to the persistence of Brucella abortus infection and that inactivation of Tregs with tumor necrosis factor receptor II (TNFR2) antagonistic antibody protected mice by significantly reducing bacterial burden both systemically and within reproductive tissues. These findings support a critical role of Tregs in the pathogenesis of persistence induced by intracellular bacterial pathogens, including B. abortus. Results from this study indicate that adverse reproductive outcomes can occur as sequelae of chronic infection in nonpregnant animals and that fine-tuning Treg activity may provide novel immunotherapeutic and prevention strategies against intracellular bacterial infections such as brucellosis.
INTRODUCTION
Despite the presence of functional host defenses, some intracellular bacterial pathogens develop various survival mechanisms to cause persistent and occasionally lifelong infections. A number of these pathogens are known to have tropism for the pregnant reproductive system, thereby increasing the risk of complications, including spontaneous abortion and stillbirth. To begin to understand the underlying mechanisms that culminate in the observed adverse reproductive complications, this study focused on Brucella abortus, an intracellular bacterial pathogen primarily known to cause reproductive diseases, including infertility and severe pregnancy complications, particularly in livestock, and most recently described in humans (1–3). Brucella pathogenesis has been extensively studied using mouse and other natural host animal models (4). Several of these studies have shown the survival and replication of Brucella spp. in various organs of infected animals (5, 6). Of particular emphasis is the tropism of Brucella for the pregnant uterus. For example, up to 1013 CFU of Brucella have been recovered in the utero-placental unit of infected animal models (mice, cattle, and goats) that had classic pregnancy complications, such as spontaneous abortion (1, 6, 7). However, until this study, investigation of the pathogenic mechanisms of Brucella colonization and survival in the female reproductive system has revolved around pregnant animal models (1, 6–10), with little or no information about the pathogenic effects of these organisms on the nongravid reproductive system. Hence, the impact of Brucella infection on the reproductive tract of nonpregnant animals is unknown.
Therefore, the objectives of this study were to (i) determine if Brucella can colonize the nonpregnant uterus and sustain a chronic infection, (ii) determine if Brucella can induce persistent pathology composed of highly suppressive host regulatory T cells (Tregs) expressing tumor necrosis factor receptor II (TNFR2) that hamper infection elimination and contribute to infertility, and (iii) identify the possible therapeutic benefit of selective host Treg cell elimination through TNFR2 antagonistic antibody monitored by pathogen elimination. Specifically, we investigated the contribution of Tregs to the pathogenesis of Brucella infection in nonpregnant mice based on the increasing evidence that Treg-mediated immune suppression perpetuates chronic infections, including a possible role of the most suppressive TNFR2 Tregs (11–14). The findings of this study contribute to our understanding of the cellular mechanisms of Brucella-induced reproductive disease in animals and humans, as well as the sequelae of chronic infection.
RESULTS
Brucella abortus persists in the uterus of nonpregnant mice during acute infection.
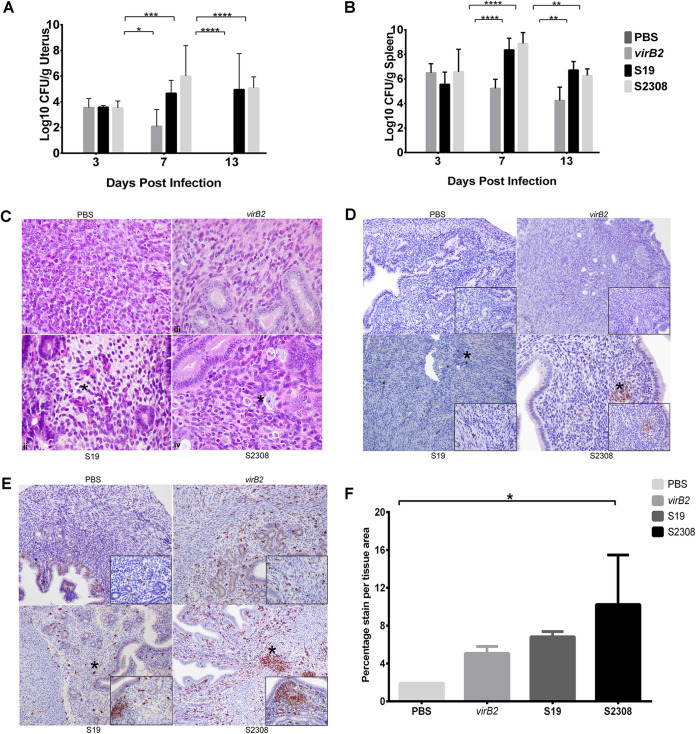
To assess bacterial colonization and to characterize the gross and histopathological lesions induced by virulent B. abortus strain 2308, live-attenuated vaccine strain S19, and attenuated mutant strain 2308ΔvirB2 (virB2), four groups of five mice each were inoculated intraperitoneally with 1 × 106 CFU or PBS. Uterine colonization was evaluated at 3, 7, and 13 days postinfection (dpi). By 13 dpi, both S19 and S2308 persisted in the spleen and uterus, maintaining the infection, while virB2 was completely cleared (Fig. 1A and B). Interestingly, all the uteri were grossly unremarkable with no evidence of lesions, regardless of the treatment group or time postinoculation. However, despite the unremarkable gross appearance of the uterus, both S19 and S2308 induced infiltration of inflammatory cells in which the severity was highly correlated with the virulence of the challenge strain; S2308 induced a more severe macrophage infiltration than S19 (Fig. 1C).
FIG 1.
Brucella abortus colonized the uterus and induced endometritis in nonpregnant ICR mice. Mice were inoculated intraperitoneally with 1 × 106 CFU of PBS, B. abortus mutant 2308ΔvirB2 (virB2), live-attenuated vaccine strain S19, or wild-type Brucella abortus S2308. Uterine samples were collected at 3, 7, and 13 days postinfection (dpi), and bacteria were enumerated via culture. (A and B) Bar graphs representing the average (n = 5) with the standard deviation (SD). Statistical differences between the groups were determined using two-way ANOVA and Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (C) Representative micrographs (hematoxylin and eosin stain) of normal uterus from control and infected nonpregnant mice at 13 dpi, respectively (×60 magnification). Mild to moderate macrophage infiltration of the endometrium and expansion of the endometrial tissue with clear space (edema) was evident. Asterisks represent edema and inflammatory cell infiltration. (D and E) Brucella antigen distribution (D) and positive immunolabelling of macrophages (E) in the uterus of the respective mice at 13 dpi. Asterisks indicate Brucella antigens or Iba-1-positive macrophages, respectively. (F) Percentage stain area in the uterus of nonpregnant mice. Estimation of stain area intensity was performed with Image J analysis. Significance was estimated using one-way ANOVA and Tukey’s multiple-comparison post hoc test. *, P < 0.05.
The presence of Brucella antigen in the uterine tissues of infected mice was also evaluated. At all time points, macrophages in the endometrium and myometrium of mice infected with S19 and S2308 demonstrated strong intracytoplasmic immunolabeling for Brucella-specific antigen (Fig. 1D). However, mice infected with virB2 had less antigen at 3 dpi and 7 dpi, and by 13 dpi, no immunolabeled signal was observed in the uterus of these mice (Fig. 1D). Mice in the phosphate-buffered saline (PBS) group were negative for Brucella antigen at all time points. Strong positive Iba-1 immunolabelling (a marker for activated macrophages) was also detected in the uterus of all groups (Fig. 1E) but significantly higher in uterus samples of animals infected with the virulent strain, S2308 (Fig. 1F).
To demonstrate consistent and typical infection by these Brucella strains, spleens were also collected from the animals. Remarkable levels of colonization were present in the spleen of all infected mice at all time points (Fig. 1B). In accordance with previous studies (15, 16), by 2 weeks postinfection, all the strains persisted in the spleen, but virB2 was maintained at a significantly lower level than S19 and S2308 due to its attenuation.
Virulent B. abortus S2308 induced inflammatory cell infiltration in the uterus of nonpregnant mice.
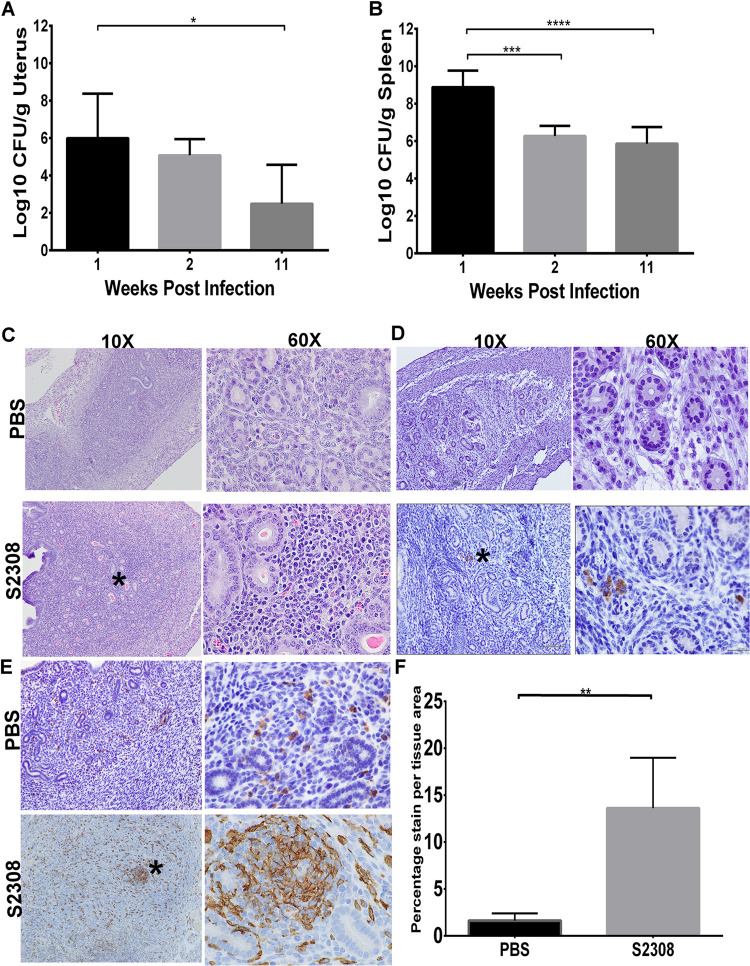
The pathogenesis of adverse reproductive outcomes during Brucella infection is unclear but may be due to a range of factors, including sustained inflammation of the uterus of infected animals. Previous results from this study demonstrated that B. abortus can colonize the uterus and induce acute endometritis in nonpregnant mice (Fig. 1). However, it is not known whether Brucella can persist in the uterus of nonpregnant mice for long periods and if persistence is associated with reduced fertility or adverse pregnancy events. Interestingly, infection with virulent B. abortus strain S2308 persisted in the uterus of all infected mice at 11 weeks postinfection and induced a long-lasting inflammatory cellular infiltrate (Fig. 2A, C to F). Although no observable gross lesions were detected, histopathological evaluation of the uterine samples revealed multifocal areas of cellular infiltration (Fig. 2C). Positive immunostaining of Brucella-specific antigen (Fig. 2D) and Iba-1-positive macrophages within the endometrium were also observed (Fig. 2E and F). These results demonstrate that B. abortus not only persisted for long periods in the uterus of nonpregnant mice but also induced a long-lasting inflammatory cell infiltrate.
FIG 2.
(A and B) Bacterial (log10 CFU/g) recovery in the uterus (A) and spleen (B) of nonpregnant mice (n = 9/group) infected with B. abortus S2308 and uninfected controls inoculated with PBS. Bar graphs represent the average with SD. The black, light gray, and dark gray indicate 1, 2, and 11 weeks postinfection, respectively. Statistical differences between the groups were determined using one-way ANOVA and Tukey’s multiple-comparison test. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001. (C) Inflammatory cell infiltrates composed of macrophages and lymphocytes in the endometrium of infected nonpregnant mice. (D and E) Positive immunolabelling of Brucella antigen (D) and Iba1-specific activated macrophages (E) in the endometrium of infected nonpregnant mice at 13 dpi. (F) Percentage stain per tissue area of macrophages in the endometrium of the respective mice. Estimation of stain area intensity was performed with Image J analysis. Significance was estimated using the Mann-Whitney test. **, P < 0.01. Asterisks on the images indicate inflammatory cell infiltrates (C), Brucella antigens (D), or Iba-1-positive macrophages (E).
Chronic infection with virulent B. abortus reduced the number of successful pregnancies in mice.
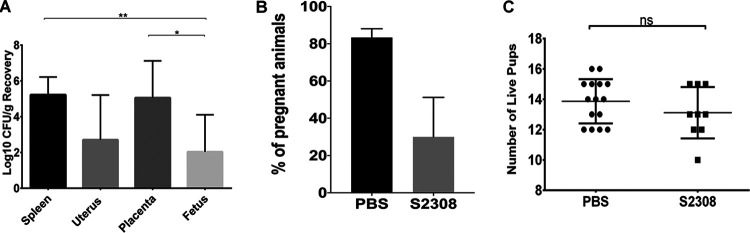
The adverse effect of chronic experimental Brucella infection on reproductive success in mice has not yet been established. In this study, we sought to determine if chronic brucellosis, particularly with the virulent strain S2308, negatively impacts pregnancy success. Nonpregnant mice were either challenged with the virulent strain S2308 or inoculated with PBS as negative controls. After 8 weeks of infection, all mice were exposed to male bedding for 3 to 5 days to synchronize estrus and were subsequently mated with age-matched, stud males of the same strain. Vaginal plugs were observed after overnight breeding to confirm mating and estimate the start of the gestation period. At gestation day 18, the number of chronically infected mice with successful pregnancies was significantly lower than that of uninfected control animals (Fig. 3B). This finding suggests that S2308 persisted in the uterus of infected mice, resulting in persistent infection and inflammatory cellular infiltrates that contributed to a significantly reduced rate of pregnancies. Interestingly, the chronically infected pregnant mice had a similar number of live pups as the uninfected controls, despite the fact that Brucella was recovered from the reproductive and gestational tissues (Fig. 2A and C). The persistent infection and inflammatory lesions seemed to decrease the probability of the pregnancy. Therefore, adverse reproductive outcomes resulted as sequelae of chronic infection, and chronicity of infection negatively impacted reproductive performance in these animals.
FIG 3.
Brucella abortus S2308 persisted in the uterus of chronically infected nonpregnant mice, reducing the number of successful pregnancies. (A) Bacterial recovery in the chronically infected mice (animals were infected for 8 weeks prior to breeding, and tissues were evaluated on gestational day 18) that had successful pregnancies (n = 9). Data are presented as the mean ± SD. (B) Percentage of animals that had successful pregnancies in the PBS or S2308-challenged groups. Fisher’s exact test (PBS, n = 9/20; S2308, n = 13/15). (C) Number of live pups in those chronically infected mice that had successful pregnancies. Mann-Whitney test. Error bars indicate the standard deviation. *, P < 0.05; **, P < 0.01; ns, not significant.
Regulatory T cells expand and promote the persistence of Brucella abortus in mice.
There is increasing evidence that Tregs enhance susceptibility to and persistence of various infectious diseases, including brucellosis, tuberculosis, and listeriosis, among others (11–14, 17). Therefore, to identify immunologic mechanisms that contribute to the tropism and persistence of Brucella in the uterus, we investigated the role of regulatory Tregs in the pathogenesis of brucellosis (Fig. 4A). Irrespective of the virulence of the infecting Brucella strain, CD4+FOXP3+ Tregs were expanded significantly in the spleen of all infected mice at all time points compared to uninfected controls (Fig. 4B and C). Given that an expansion of Tregs induced immunosuppression and increased susceptibility to infection, we examined bacterial burden in the uterus and spleen of infected mice. Overall, Brucella significantly stimulated a systemic expansion of Tregs, which was associated with increased bacterial burdens in the spleen and uterus of infected animals (Fig. 1A and B).
FIG 4.
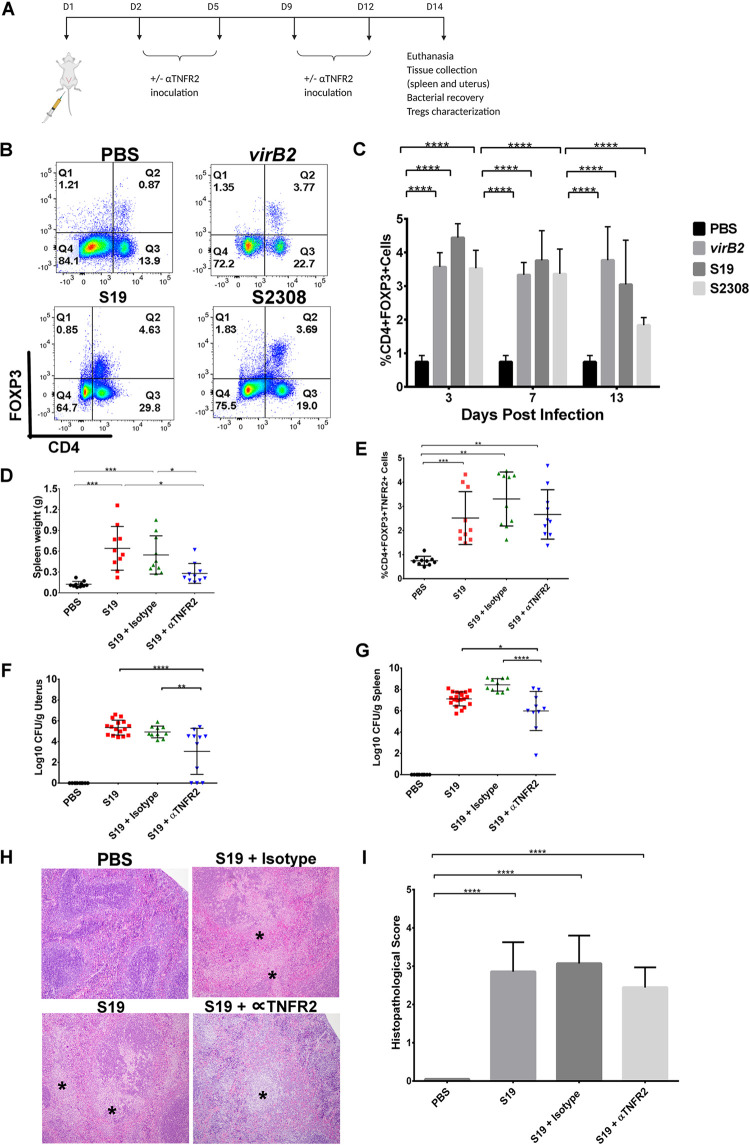
A systemic expansion of CD4+FOXP3+ regulatory T cells is associated with increased bacterial burden in Brucella-infected mice. (A) Nonpregnant mice were inoculated with Brucella abortus S19 via intraperitoneal (IP) inoculation on day 1 and were subsequently given biweekly doses of anti-TNFR2 antibody or isotype control as detailed in Materials and Methods. Animals were sacrificed at 14 days postinfection, and the spleen and uterus were collected and evaluated for bacterial recovery, histopathology, and Treg characterization. (B and C) Percentage of FOXP3+ T cells among CD4+ splenocytes in nonpregnant mice infected with Brucella abortus ΔvirB2, S19, or S2308. Bar graphs represent an average with standard deviation (n = 10). (D to G) Spleen weight (D), percentage of CD4+FOXP3+TNFR2+ Tregs in the spleen (E), and CFU/g recovery in the uterus (F) and spleen (G) of the animals at 14 days postinfection (dpi). Each dot represents one animal. (H and I) TNFR2 antagonistic antibodies marginally diminished splenic pathology in mice infected with B. abortus S19 (n = 10) (14 dpi). Statistical differences between all groups were analyzed using two-way (B) or one-way (C, D, E, F, and H) ANOVA followed by Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. The statistical software used was GraphPad Prism (La Jolla, CA, USA).
Activated Tregs suppress the proliferation, activation, and effector functions of other immune cells (18, 19). In mice, activated Tregs express tumor necrosis factor receptor II (TNFR2), and these Treg subsets have been shown to inhibit the proliferation of effector T cells (18, 20–22). Additionally, TNFR2 signaling causes either an expansion or contraction of Tregs, depending on the initiating signal (22, 23). We therefore hypothesized that counteracting TNFR2 expression on Tregs would inactivate these cells and their functions. Treatment of mice with TNFR2 antagonistic antibody resulted in marginal reduction of TNFR2+ Tregs (Fig. 4E) and a significant reduction in bacterial burden in the uterus and spleen compared to untreated mice (Fig. 4F and G). In addition, splenomegaly, one of the classic lesions of Brucella infection (15, 16, 24), was significantly reduced in treated animals at 14 dpi (Fig. 4D). Histopathological evaluation also revealed a mild obliteration of the splenic parenchyma in animals treated with TNFR2 antagonistic antibody, even though inflammation was not significantly reduced (Fig. 4H and I). In summary, the specific effects of the TNFR2 antagonistic antibody on Treg inactivation were confirmed using an isotype antibody control, which showed results similar to those observed in mice infected with S19 only. Therefore, these data highlight the crucial role of Tregs in the persistence of Brucella both systemically and in reproductive tissues.
The current study aimed to understand the role of immunosuppressive Tregs in the pathogenesis of brucellosis. The specific mechanism (for example, the inhibition of proinflammatory cytokines) of Treg-induced immunosuppression that facilitates the colonization of Brucella abortus and the pathological manifestations in nonpregnant mice was also explored.
Since the findings of the current study demonstrated a role of Tregs in the persistence of Brucella abortus in mice, we next investigated the specific mechanism involved. Several mechanisms of Treg-induced immunosuppression have been characterized, including the modulation of dendritic cell maturation and function, cytolysis, metabolic disruption, or secretion of inhibitory cytokines such as transforming growth factor β (TGFβ), interleukin 10 (IL-10), and IL-35. We hypothesized that an expanded population of Tregs favors the persistence of B. abortus through the induction of inhibitory cytokines (TGFβαγ and IL-10). Therefore, treatment with TNFR2 antagonistic antibody, which inactivates Tregs, should lead to a reduction of inhibitory cytokines and an elevation of proinflammatory cytokines needed to initiate pathogen clearance. To test this hypothesis, groups of mice were inoculated with B. abortus vaccine strain S19 and subsequently treated with TNFR2 antagonistic antibody, isotype control, or PBS as previously described. At 14 dpi, mice were euthanized, the spleens were processed to obtain a single cell suspension, and cytokine expression was quantified using flow cytometry analyses.
In corroboration with previous studies, we found that CD4+ T cells were induced to a significantly higher percentage in the spleen of all infected animals at 14 dpi (see Fig. S2 in the supplemental material). Unexpectedly, Treg-induced persistence of B. abortus was not associated with the induction of inhibitory cytokines, because inactivation of Tregs by TNFR2 antagonistic antibody only resulted in a marginal reduction in the level of inhibitory cytokines (see Fig. S2).
DISCUSSION
Historically, Brucella spp. are known to have an exceptional tropism to the reproductive tissues of livestock, with consequential adverse pregnancy outcomes. Hence, brucellosis is considered a significant problem in pregnant animals. Several of the experimental studies delineating the implications of Brucella infection on the female reproductive system have investigated pregnant animal models (e.g., ruminants and rodents) (6, 7, 9, 25). However, in a review of the literature, little or no data were found on the pathogenic effects of Brucella on the nonpregnant reproductive system. Therefore, one of the objectives of this study was to assess whether Brucella abortus can infect, sustain infection, cause persistent inflammation of the nonpregnant uterus, and subsequently impact successful pregnancy in infected animals. For the initial acute infection study, mice were infected with B. abortus strain 19 (live-attenuated vaccine strain), B. abortus S2308 (virulent strain), and as a control, B. abortus 2308ΔvirB2 (an attenuated strain with a defect in intracellular replication and persistence in tissues) (26, 27). Both S19 and S2308 are smooth strains that are also known to colonize reproductive tissues, causing spontaneous abortion in their target species (cattle), with the rate of abortion being significantly higher with S2308 than S19.
Results from this study demonstrate that Brucella abortus has a tropism for the reproductive organs in mice that is not exclusively associated with pregnancy despite the historical assumption that brucellosis is mainly a significant problem during pregnancy. These findings provide new evidence that pregnancy is not a prerequisite for significant reproductive complications associated with Brucella infection. Another important finding was that colonization and tropism of all the strains (2308ΔvirB2, S19, and S2308) to the uterus of infected nonpregnant animals were dependent on virulence (Fig. 1A), because S19 and S2308 persisted and sustained the infection, which also indicated that even though S19 is an attenuated vaccine strain, it has the capability to invade and colonize the uterus of nonpregnant mice almost at the same level as the virulent strain, S2308 (Fig. 1A). Previous studies demonstrated that despite S19 being an attenuated strain, it can still invade the uterus and placenta of pregnant animals, resulting in spontaneous abortion, particularly in livestock (6, 28, 29). The results from the current study and others confirm not only the undesired side effect of S19 as a vaccine, but also that the tropism of B. abortus S19 to the uterus of mice is independent of reproductive status. In contrast, 2308ΔvirB2, which is attenuated both in vitro and in vivo, did not survive and was completely cleared in the uterus of nonpregnant mice by 2 weeks postinfection. The result of this study corroborates previous experimental studies using virB mutants of Brucella, which demonstrated the lack of survival and replication of these mutants in both in vitro and in vivo models of infection (26, 27).
Interestingly, in the absence of gross lesions, S19- and S2308-infected mice demonstrated histologic evidence of an increased inflammatory response evident at 7 dpi (compared to those at 3 dpi or uninfected controls) and increased by 13 dpi. It is important to highlight that physiologic cellular infiltration of the mouse uterus occurs during the estrous cycle (which consists of the diestrus, estrus, metestrus, and proestrus stages) (30, 31). The quantity and composition of cells infiltrating the lamina propria of the uterus vary considerably with estrous stages (30, 31). Unfortunately, the stage of estrus was not synchronized in this study, so it was a challenge to determine how much of the neutrophilic infiltration of the endometrium was related to Brucella-induced inflammatory change versus the stage of estrus. In contrast to studies that have investigated neutrophil fluctuations with estrous cycle, little has been reported on the presence of or changes in macrophage numbers by estrous stage. This leads to dual hypotheses: (i) Brucella induces macrophage infiltration into the uterus, or (ii) resident macrophages phagocytose Brucella. Based on the relative increase in macrophage number in the uterus of mice infected with wild-type strain S2308, we favor the former hypothesis that Brucella induces an inflammatory reaction within the uterus. It can also be hypothesized that positive immunolabeling of Brucella antigens in the uterine macrophages (Fig. 2D to F) of infected animals is a result of phagocytosis by those cells that are normally present during the various stages of the estrous cycle. However, the moderate numbers of macrophages in the uterine sections, as estimated via immunohistochemistry and quantitative Image J analysis, signify an inflammatory response to Brucella infection in these mice (Fig. 1F and 2F). In addition, one of the first lines of innate defense against Brucella is the migration and accumulation of macrophages, which phagocytose the bacteria. It is important to note that the effect of Brucella infection on the estrous cycle or how the estrous cycle impacts the severity of Brucella infection in nonpregnant mice is beyond the scope of this study. Hence, future studies exploring the influence of various estrous stages on the susceptibility of mice to Brucella infection are required.
Immunohistochemical staining was used to detect Brucella-specific antigen in the uterus of infected animals. Strong intracytoplasmic immunolabelling of Brucella antigen was observed inside uterine macrophages of animals infected with S19 and S2308 (Fig. 1C and D, 2D). The antigen distribution was consistently associated with the areas of inflammation present in these animals. The antigen was also present extracellularly within uterine tissues (which usually occurs when intracellular bacteria are released after cell death), similar to what is observed in other infected tissues. Interestingly, there was no significant difference in the distribution of Brucella antigens in the uterus of animals infected with either the live-attenuated vaccine strain (S19) or the virulent strain (S2308), indicating that S19 has similar capability as the virulent strain (S2308) to colonize and persist in the uterus of nonpregnant mice. As expected, Brucella antigen was observed only at early time points (3 and 7 dpi) but not at 13 dpi in 2308ΔvirB2-infected mice, indicating that the bacteria cannot sustain infection like the wild type, which is consistent with its attenuated phenotype (Fig. 1). The findings of the current study may therefore support the hypothesis that S19 and S2308 enhance inflammation, particularly accumulation of macrophages, in the uterus of nonpregnant mice.
To establish the biological relevance of the survival and persistence of B. abortus in the uterus of nonpregnant mice, we conducted further experiments to demonstrate that B. abortus not only persisted for long periods, but also induced chronic inflammation. When chronically infected mice were exposed to stud males, an adverse reproductive outcome was observed, which was characterized by a significantly reduced number of successful pregnancies compared to uninfected control animals. The persistent macrophage infiltration of the endometrium may have contributed to the impaired reproductive performance in these animals. In addition, previous studies have shown that various factors associated with chronic endometritis, including persistent inflammatory processes, induction of immune mediators, and hormonal imbalance, interfere with gamete or embryo physiology, reduce endometrial receptivity, and cause implantation failure (32–34). Therefore, further studies are required to explore the cellular and molecular mechanisms of Brucella-induced endometritis and characterize the specific reproductive consequences.
Another objective of the current study was to identify the immunologic mechanism that drives susceptibility to and persistence of Brucella infection in nonpregnant mice. One of the many functions of regulatory T cells (Tregs) is to control the activity of the immune system by downregulating excessive immune response to infection (11–14, 17, 21, 35–37). Hence, Tregs function as a double-edged sword, having both protective and detrimental effects on host immune defense against invading pathogens. Activated Tregs suppress the proliferation, activation, and effector functions of other immune cells (18, 19), and these Tregs have been shown to express tumor necrosis factor receptor II (TNFR2) (18, 20–22). Some myeloid-derived stem cells, endothelial cells, and differentiating neurons also express TNFR2. However, expression of this receptor is restricted to highly suppressive Tregs among other lymphoid cells, and TNFR2 signaling causes either an expansion or contraction of Tregs, depending on the initiating signal (22, 23). We investigated the critical involvement of regulatory T cells (Tregs) in Brucella pathogenesis, based on the evidence that Tregs play a role in the regulation of immunity to infection (11–14, 38, 45, 46). We hypothesized that Brucella takes advantage of the Treg cell-mediated immunosuppression for unrestricted colonization and survival. Interestingly, our results showed that irrespective of the virulence of the infecting strain, Brucella abortus induced a significant systemic expansion of Tregs that was associated with increased bacterial burden in the spleen and uterus, particularly through a bystander suppressive effect (21, 44). A possible mechanism by which Brucella drives the expansion of Tregs could be through the induction of tumor necrosis factor (TNF) on antigen-presenting cells. TNF is a ligand of TNFR2, and the interaction between these two molecules results in the proliferation of Tregs (23). Previous studies have shown that expanded Tregs can enhance the progression and chronicity of diseases primarily by limiting the effector functions of immune cells that are critical in the clearance of pathogens (11–14, 17). This observation led us to conclude that a systemic expansion of activated Tregs by Brucella is one of the various mechanisms by which Brucella exploits the host immune system to enhance its colonization and survival. We confirmed that the immunosuppressive Tregs induced during Brucella infection were activated and suppressive based on their expression of TNF receptor type II (TNFR2), a member of the TNF receptor superfamily (TNFRSF) (20, 21). Previous studies have shown that TNFR2 is expressed by a unique subset of mouse splenic Tregs that have an activated phenotype with potent suppressive activity (18). Hence, we hypothesized that blocking TNFR2 expression on Tregs will inactivate these cells and allow the proliferation of effector T cells, which will reduce Brucella colonization and survival in tissues. The TNFR2 antagonistic antibody used in this study was developed in-house and has been shown to suppress Treg proliferation while enabling the expansion of effector T cells (22).
We previously showed that the live-attenuated vaccine strain B. abortus S19 induced colonization, histopathologic changes, and expanded Tregs similar to those of the virulent strain S2308 (Fig. 1 and 3). Therefore, S19 was used as a surrogate infection agent for the Treg antagonism study. It is interesting to note that the significantly reduced bacterial burden and tissue inflammation in infected mice that were treated with TNFR2 antagonistic antibody was due to the inactivation of Tregs by this antibody, because mice treated with isotype control had results comparable to S19-infected mice (Fig. 4F and G). This finding was in corroboration with previous studies in other disease models that showed that ablation of Tregs reversed susceptibility to infection (11–14). Taken together, these findings demonstrate that Tregs have a crucial role in immune suppression during Brucella infection and suggest the escape of Brucella from host immune surveillance. There are still many unanswered questions about the mechanisms of Brucella-mediated Treg immunosuppression. An important issue for future research will be to identify and characterize both the systemic and local T cell effector response in the presence or absence of anti-TNFR2 treatment.
Tregs mediate their suppressive activities via various mechanisms, including direct (e.g., cell-to-cell contact) and indirect (induction of inhibitory cytokines) mechanisms. Hence, we investigated the potential indirect mechanism (inhibition of proinflammatory cytokines) of Treg-induced immunosuppression that facilitates the colonization of Brucella abortus in mice. The proinflammatory cytokines (TNF-α and interferon-γ [IFN-γ]) evaluated in this study were based on several studies indicating the crucial role of these cytokines in controlling Brucella infection (9, 37, 39–41), while the anti-inflammatory cytokines (IL-10 and TGFβ) were based on indirect immunosuppressive mechanisms of Tregs. Contrary to expectations, results from this study were unable to demonstrate that inactivation of Tregs with TNFR2 antagonistic antibody either reduced anti-inflammatory or increased proinflammatory cytokine levels in the spleen (see Fig. S2 in the supplemental material). Some possible explanations for this finding might include the dose and timing of the TNFR2 antagonistic antibody treatment or a redundancy of cytokine production by other immune cells. Although the potent Treg-mediating suppression was eliminated with TNFR2 antagonistic antibodies (20, 21), the desired activation of cytotoxic effector cells for pathogen elimination might still be expected to maintain inflammation for effective function. One of the limitations of the current study is the lack of characterization of Tregs in the uterus of infected mice and how treatment of chronically infected animals with anti-TNFR2Ab ameliorates deleterious reproductive outcomes induced by Brucella. Another limitation is that while previous studies showed that TNFR2 expression is strictly limited to subpopulations of the lymphoid system, particularly a subset of highly potent FOXP3+ Tregs, this molecule has also been observed on endothelial cells and certain neurons during normal growth. Therefore, the anti-TNFR2 antagonistic antibody used in this study may potentially impact other cell types and should be a subject of future investigation.
Nevertheless, the current study has provided some insights into the mechanisms involved in the persistence and pathogenesis of Brucella in nonpregnant mice. In particular, the findings herein demonstrated for the first time not only that Brucella is a pathogen that is of reproductive significance during pregnancy, but also that adverse reproductive outcomes can occur as sequelae of chronic infection in nonpregnant animals. In addition, we showed a critical involvement of potent TNFR2+-expressing Tregs in the persistence of Brucella infection in nonpregnant mice. Future investigations are required to identify the mechanism by which Brucella specifically activates Tregs and the potential implications during pregnancy. Overall, the insights from the findings of this study will help to establish immunotherapeutic strategies and approaches to control intracellular bacterial infections with known predilection for the uterus, including brucellosis.
MATERIALS AND METHODS
Ethics statement.
This study was conducted according to the recommendations in the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (47). The Texas A&M University Institutional Animal Care and Use Committee (IACUC) approved this research under the Animal Use Protocol IACUC 2018-0046.
Animals.
ICR female mice, aged 6 to 8 weeks, were purchased from the Texas Institute of Genomic Medicine (College Station, TX, USA) and acclimatized in the Texas A&M University (TAMU) Laboratory Animal Resources and Research biosafety level 2 or TAMU animal biosafety level 3 facilities (College Station, TX, USA) for a week prior to experimental infection. For the breeding study, stud ICR male mice with proven breeding performance were used.
Bacterial strains.
The strains used in this study were the virulent strain B. abortus S2308 (National Animal Disease Center, U.S. Department of Agriculture [USDA] Agricultural Research Service [ARS], USA), live-attenuated vaccine strain B. abortus S19 (National Veterinary Sciences Laboratories, Ames, IA, USA), and B. abortus 2308ΔvirB2 (25). To prepare inoculums, these strains were grown in tryptic soy agar (TSA; BD Biosciences, NJ, USA) at 37°C for 3 to 5 days. Inoculum was prepared according to published studies (5, 10), and doses of inoculum were confirmed retrospectively by counts of distinct colonies of the inoculum residue.
Experimental infection.
To assess bacterial colonization and to characterize the gross and histopathological lesions induced by these strains of B. abortus in nonpregnant mice, four groups of animals were inoculated intraperitoneally with 1 × 106 CFU of S2308, S19, or 2308ΔvirB2 or with PBS. Mice were monitored daily for signs of disease. At 3, 7, and 13 days postinfection, mice were euthanized by CO2 asphyxiation followed by cervical dislocation, and a full necropsy was performed. At each time point, uterine and splenic samples were aseptically collected and processed for bacterial isolation, histopathology, and immunohistochemical analyses. For the chronic infection study, female ICR mice at 6 to 8 weeks of age were inoculated intraperitoneally with 106 CFU of the virulent strain S2308. Eight weeks after infection, mice were housed in cages containing male mouse bedding to synchronize estrus and subsequently set up with stud males of a similar strain. The presence of vaginal plugs confirmed mating. At gestation day 18, mice were euthanized, pregnancy outcome was assessed, and tissues were collected for bacterial recovery.
TNFR2/Treg antagonism.
Nonpregnant ICR mice were inoculated intraperitoneally (IP) with 1 × 106 CFU of S19, and treated with four doses of 100 μg of hamster anti-mouse TNFR2 antagonistic antibody, an isotype IgG control, or PBS via intraperitoneal (IP) inoculation. At 14 days postinfection, mice were euthanized and tissues were collected for flow cytometry analysis, bacterial enumeration, and histopathology.
Bacterial colonization.
To determine the degree of colonization of B. abortus S2308, S19, and 2308ΔvirB2 in the uterus compared to the spleen of nonpregnant mice, tissues harvested at 3, 7, and 13 days postinfection were weighed, homogenized, and serially diluted in sterile PBS. Dilutions were plated on Farrell’s medium and incubated for 3 to 5 days at 37°C. Plates were monitored daily for growth, and Brucella was identified based on morphological characteristics. The recoverable bacteria were enumerated as log10 CFU/g of uterine and splenic tissues.
Histopathology.
To evaluate the histological changes associated with B. abortus S2308, S19, and 2308ΔvirB2 infection in the uterus of nonpregnant mice, sections of uterus from all mice were fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin wax. Uterine sections of 5 μm thickness were stained with hematoxylin and eosin (H&E) and examined via light microscopy by a board-certified veterinary anatomic pathologist in a blinded fashion (see Table S1 in the supplemental material). Uterine and splenic samples from the noninfected group were used as negative controls.
Immunohistochemistry.
The presence of Brucella antigen in the uterine tissues of infected mice was evaluated in formalin-fixed paraffin-embedded tissues via immunohistochemistry. Briefly, uterine sections of 5 μm thickness were deparaffinized and rehydrated. Antigen retrieval was performed using the 2100 antigen retriever system according to the manufacturer’s instructions (Aptum Biologics Ltd., Southampton, United Kingdom), followed by blocking of endogenous peroxidase activity with Bloxall blocking solution (Vector Laboratories, Inc., Burlingame, CA, USA). Sections were subsequently incubated in 2% normal goat serum to reduce nonspecific binding and background staining. Primary antibodies (rabbit anti-Brucella polyclonal IgG; 1:400; Biocompare, South San Francisco, CA, USA or microglia/macrophage-specific protein Iba-1; 1:300; Invitrogen, Carlsbad, CA, USA) and an IgG control (negative control) were incubated overnight at 4°C. Secondary antibody (biotinylated anti-rabbit IgG) was used for 30 min at room temperature. The avidin-biotin peroxidase system was used for detection (Vectastain Elite ABC HRP kit; Vector Laboratories Inc., Burlingame, CA, USA), and visualization was done using 3,3′-diaminobenzidine (DAB) substrate (Betazoid DAB chromogen kit, Biocare Medical, Pacheco, CA, USA). Uterine sections were finally counterstained with hematoxylin, dehydrated, mounted, and examined using light microscopy.
Quantification of inflammatory response.
The percentage stain area of macrophages in the uterus of naive and infected nonpregnant mice was estimated using the image processing software Image J (https://imagej.nih.gov/ij/). Briefly, multiple images (at least five) from each uterine section were obtained at ×60 magnification, and Iba-1 expression was evaluated as percentage positively stained macrophages.
Antibodies and flow cytometry.
The spleen of each animal was processed using previously published protocols and analyzed with flow cytometry (17). Briefly, the spleen was processed by mechanical disruption using a Miltenyi tissue dissociator (Miltenyi Biotec) to obtain a single cell suspension. Cell suspensions were then stained for flow cytometry analysis using fluorophore-conjugated mouse antibodies against surface and intracellular Tregs or cytokines (efluor450 anti-mouse CD4 clone GK1.5, phycoerythrin cyanine 5 PECy5 anti-mouse FOXP3 clone: FJK16s, and phycoerythrin PE hamster anti-mouse TNFR2-CD120b clone TR75-54.7; IL-10 fluorescein isothiocyanate [FITC], TGFβ PE-Cy7; TNF-α PE, and IFN-γ FITC) (see Fig. S1 in the supplemental material). Cells were activated for cytokine production using a cell stimulation cocktail with protein transport inhibitors (which consisted of phorbol myristate acetate [PMA], ionomycin, brefeldin A, and monensin) and incubated at 37°C for 5 h, fixed, and stained intracellularly, and expression was analyzed using flow cytometry.
Prior to intracellular staining of FOXP3, cells were fixed and permeabilized with the FOXP3 transcription factor buffer set by BD Biosciences according to the manufacturer’s instructions (BD Biosciences, San Jose, California, USA). BD Biosciences LSR Fortessa X-20 was used to collect and compensate data, and approximately 1 × 106 cells were acquired. Flowjo software was used to analyze the data. Cells infected with the virulent strain S2308 were inactivated using an in-house-validated protocol before analysis on the flow cytometer.
Statistical analysis.
The recoverable log10 bacterial CFU/g of uterine and splenic tissues and the Treg population between mice of all treatment groups as well as macrophage staining intensity on uterine tissues were analyzed using the Mann-Whitney test, two-way or one-way analysis of variance (ANOVA), and Tukey’s multiple-comparison test as appropriate. GraphPad Prism software (La Jolla, CA, USA) was used for the analyses.
Data availability.
The bacterial strains and other data sets presented in this study are available with no restrictions from the corresponding author.
Supplementary Material
ACKNOWLEDGMENTS
A.M.A.-G., S.A.A., D.L.F., and L.G.A. conceived and designed the experiments. S.A.A., O.H.K., and D.G.G.-G. performed the experiments. M.E.H. evaluated and analyzed the histology results. S.A.A., M.E.H., and A.M.A.-G. wrote the manuscript. A.M.A.-G., S.A.A., D.L.F., and L.G.A. reviewed and edited the manuscript. All authors reviewed the manuscript.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Poester FP, Samartino LE, Santos RL. 2013. Pathogenesis and pathobiology of brucellosis in livestock. Rev Sci Tech 32:105–115. doi: 10.20506/rst.32.1.2193. [DOI] [PubMed] [Google Scholar]
- 2.Arenas-Gamboa AM, Rossetti CA, Chaki SP, Garcia-Gonzalez DG, Adams LG, Ficht TA. 2016. Human brucellosis and adverse pregnancy outcomes. Curr Trop Med Rep 3:164–172. doi: 10.1007/s40475-016-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect Dis 7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 4.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. 2015. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol 185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenas-Gamboa AM, Rice-Ficht AC, Fan Y, Kahl-McDonagh MM, Ficht TA. 2012. Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16MΔvjbR and S19ΔvjbR in the immunocompromised IRF-1−/− mouse model. Clin Vaccine Immunol 19:249–260. doi: 10.1128/CVI.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosseray N. 1980. Colonization of mouse placentas by Brucella abortus inoculated during pregnancy. Br J Exp Pathol 61:361–368. [PMC free article] [PubMed] [Google Scholar]
- 7.Tobias L, Schurig GG, Cordes DO. 1992. Comparative behaviour of Brucella abortus strains 19 and RB51 in the pregnant mouse. Res Vet Sci 53:179–183. doi: 10.1016/0034-5288(92)90107-D. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Wang SS, Wang GL, Wu TL, Lv YL, Wu QM. 2014. A pregnant mouse model for the vertical transmission of Brucella melitensis. Vet J 200:116–121. doi: 10.1016/j.tvjl.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Lee DS, Watanabe K, Furuoka H, Suzuki H, Watarai M. 2005. Interferon-γ promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol 5:22. doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias L, Cordes DO, Schurig GG. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol 30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 12.Kursar M, Koch M, Mittrücker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. 2007. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol 178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 13.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. 2009. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J Immunol 161:2636–2641. [PubMed] [Google Scholar]
- 15.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Gomez G, Rice-Ficht AC. 2009. The Brucella abortus S19 ΔvjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect Immun 77:877–884. doi: 10.1128/IAI.01017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roset MS, Ibanez AE, de Souza Filho JA, Spera JM, Minatel L, Oliveira SC, Giambartolomei GH, Cassataro J, Briones G. 2014. Brucella cyclic β-1, 2-glucan plays a critical role in the induction of splenomegaly in mice. PLoS One 9:e101279. doi: 10.1371/journal.pone.0101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. 2011. Foxp3+ regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Subleski JJ, Kopf H, Howard OZ, Männel DN, Oppenheim JJ. 2008. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+ CD25+ FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol 180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. 2009. Regulatory T cells: how do they suppress immune responses? Int Immunol 21:1105–1011. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Nie Y, Xiao H, Bian Z, Scarzello AJ, Song NY, Trivett AL, Yang D, Oppenheim JJ. 2016. TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci Rep 6:32834. doi: 10.1038/srep32834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Wu X, Zhou Q, Howard OZ, Netea MG, Oppenheim JJ. 2013. TNFR2 is critical for the stabilization of the CD4+ Foxp3+ regulatory T cell phenotype in the inflammatory environment. J Immunol 190:1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, Defusco A, Plager S, Warden S, Huang D, Vanamee E, Foster R, Faustman DL. 2017. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal 10:eaaf8608. doi: 10.1126/scisignal.aaf8608. [DOI] [PubMed] [Google Scholar]
- 23.Faustman DL, Davis M. 2013. TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front Immunol 4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grilló M, Blasco JM, Gorvel JP, Moriyon I, Moreno E. 2012. What have we learned from brucellosis in the mouse model? Vet Res 43:29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis DS, Templeton JW, Ficht TA, Huber JD, Angus RD, Adams LG. 1991. Brucella abortus in bison. II. Evaluation of strain 19 vaccination of pregnant cows. J Wildl Dis 27:258–264. doi: 10.7589/0090-3558-27.2.258. [DOI] [PubMed] [Google Scholar]
- 26.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol 33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 27.Hong PC, Tsolis RM, Ficht TA. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect Immun 68:4102–4107. doi: 10.1128/iai.68.7.4102-4107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zriba S, Garcia-Gonzalez DG, Khalaf OH, Wheeler L, Chaki SP, Rice-Ficht A, Ficht TA, Arenas-Gamboa AM. 2019. Vaccine safety studies of Brucella abortus S19 and S19ΔvjbR in pregnant swine. Vaccine X 3:100041. doi: 10.1016/j.jvacx.2019.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meador VP, Deyoe BL. 1989. Intracellular localization of Brucella abortus in bovine placenta. Vet Pathol 26:513–515. doi: 10.1177/030098588902600609. [DOI] [PubMed] [Google Scholar]
- 30.Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS One 7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caligioni CS. 2009. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 48:A-4I. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park HJ, Kim YS, Yoon TK, Lee WS. 2016. Chronic endometritis and infertility. Clin Exp Reprod Med 43:185–192. doi: 10.5653/cerm.2016.43.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura F, Takebayashi A, Ishida M, Nakamura A, Kitazawa J, Morimune A, Hirata K, Takahashi A, Tsuji S, Takashima A, Amano T, Tsuji S, Ono T, Kaku S, Kasahara K, Moritani S, Kushima R, Murakami T. 2019. Chronic endometritis and its effect on reproduction. J Obstet Gynaecol Res 45:951–960. doi: 10.1111/jog.13937. [DOI] [PubMed] [Google Scholar]
- 34.Wu D, Kimura F, Zheng L, Ishida M, Niwa Y, Hirata K, Takebayashi A, Takashima A, Takahashi K, Kushima R, Zhang G. 2017. Chronic endometritis modifies decidualization in human endometrial stromal cells. Repro Biol Endocrinol 15:16. doi: 10.1186/s12958-017-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belkaid Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol 7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 36.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. 2003. CD4+ CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med 198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman KE, Loriaux PM, Saito M, Tuero I, Villaverde H, Siva T, Gotuzzo E, Gilman RH, Hoffmann A, Vinetz JM. 2013. Ex vivo innate immune cytokine signature of enhanced risk of relapsing brucellosis. PLoS Negl Trop Dis 7:e2424. doi: 10.1371/journal.pntd.0002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasquali P, Thornton AM, Vendetti S, Pistoia C, Petrucci P, Tarantino M, Pesciaroli M, Ruggeri F, Battistoni A, Shevach EM. 2010. CD4+ CD25+ T regulatory cells limit effector T cells and favor the progression of brucellosis in BALB/c mice. Microbes Infect 12:3–10. doi: 10.1016/j.micinf.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Dornand J, Gross A, Lafont V, Liautard J, Oliaro J, Liautard JP. 2002. The innate immune response against Brucella in humans. Vet Microbiol 90:383–394. doi: 10.1016/s0378-1135(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 40.Giambartolomei GH, Scian R, Acosta-Rodríguez E, Fossati CA, Delpino MV. 2012. Brucella abortus-infected macrophages modulate T lymphocytes to promote osteoclastogenesis via IL-17. Am J Pathol 181:887–896. doi: 10.1016/j.ajpath.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Y, Liu Z, Cheers C. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun 64:2782–2786. doi: 10.1128/IAI.64.7.2782-2786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reference deleted.
- 43.Reference deleted.
- 44.Schmidt A, Oberle N, Krammer PH. 2012. Molecular mechanisms of Treg-mediated T cell suppression. Frontiers Immunol 3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasenkrug KJ, Chougnet CA, Dittmer U. 2018. Regulatory T cells in retroviral infections. PLoS Pathog 14: e1006776. doi: 10.1371/journal.ppat.1006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wammes LJ, Wiria AE, Toenhake CG, Hamid F, Liu KY, Suryani H, Kaisar MMM, Verweij JJ, Sartono E, Supali T, Smits HH, Luty AJ, Yazdanbakhsh M. 2013. Asymptomatic plasmodial infection is associated with increased tumor necrosis factor receptor II-expressing regulatory T cells and suppressed type 2 immune responses. J Infect Dis 207:1590–1599. doi: 10.1093/infdis/jit058. [DOI] [PubMed] [Google Scholar]
- 47.Interagency Research Animal Committee. 1985. U.S. government principles for the utilization and care of vertebrate animals used in testing, research, and training. Office of Science and Technology Policy, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bacterial strains and other data sets presented in this study are available with no restrictions from the corresponding author.