Abstract
B cells are recognized as the main effector cells of humoral immunity which suppress tumor progression by secreting immunoglobulins, promoting T cell response, and killing cancer cells directly. Given these properties, their anti-tumor immune response in the tumor micro-environment (TME) is of great interest. Although T cell-related immune responses have become a therapeutic target with the introduction of immune checkpoint inhibitors, not all patients benefit from these treatments. B cell and B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis) play key roles in activating immune response through humoral immunity and local immune activation via tertiary lymphoid structure (TLS) formation. However they have some protumorigenic works in the TME. Thus, a better understanding of B cell and B cell-related pathways is necessary to develop effective cancer control. In this review, we summarize recent evidences regarding the roles of B cell and B cell-related pathways in the TME and immune response and discuss their potential roles for novel cancer treatment strategies.
Keywords: B cells; Tfh cells; TLS; CCL19, -21/CCR7 axis; CXCL13/CXCR5 axis; cancer
Introduction
Host immune system is one of the key factors for antitumor function. The contribution of recruited immune cells to solid tumors is now a widely accepted mechanism of cancer pathogenesis [1], which is gaining momentum in clinical oncology. Most of tumor-infiltrating immune cells are composed of T and B cells, and the remaining are composed of dendritic cells (DCs), tumor-associated macrophages (TAMs), and natural killer (NK) cells, etc [1]. Existing evidences have showed that high numbers of tumor-infiltrating lymphocytes (TILs) are associated with anti-tumor response and patient outcome [2, 3]. In addition, the role of T cell-related immune responses has been utilized to develop therapeutic advancements such as immune checkpoint inhibitors (anti-PD-1, anti-PD-L1, and anti-CTLA-4) [4] and CAR-T cell therapies [5]. Recent studies showed that the combination of immune checkpoint inhibitor and chemotherapy significantly improved progression-free survival relative to conventional chemotherapy in patients with first-line advanced non-small cell lung cancer (NSCLC) [6], and the combination of immune checkpoint inhibitors also provided improved efficacy relative to immune checkpoint inhibitor monotherapy in previously treated patients with microsatellite instability–high metastatic colorectal cancer (CRC) [7]. However, since not all patients benefit from these treatments, a new immunologic treatment strategy is necessary. B cells, being majority of tumor-infiltrating immune cells, may be an immune-related therapeutic target, leading to a next “breakthrough”.
B cell has various functions for immune response. Tumor-infiltrating B lymphocytes (TIBs) can be observed in various solid tumors. Existing evidences show that TIBs suppress tumor progression by secreting immunoglobulins, promoting T cell response, and killing cancer cells directly [8] (Figure 1). TIBs and B cell-related pathways also maintain the structure and function of tertiary lymphoid structure (TLS). TLSs are transient ectopic lymphoid aggregates which resemble the structural organization and functionality of secondary lymphoid organ (SLO) [9], and consist of T-cell-rich and B-cell-rich areas that are sites for the differentiation of effector and memory T cells and B cells [10]. TLSs induce cytotoxic T lymphocyte (CTL) infiltration into the tumor [10], contributing to potent anti-tumor responses and better patient outcomes [9, 11]. On the other hand, regulatory B cells (Bregs) reportedly induce tumor activity through immunosuppressive factors, such as IL10 and/or TGF-β [12] (Figure 1).
Figure 1.
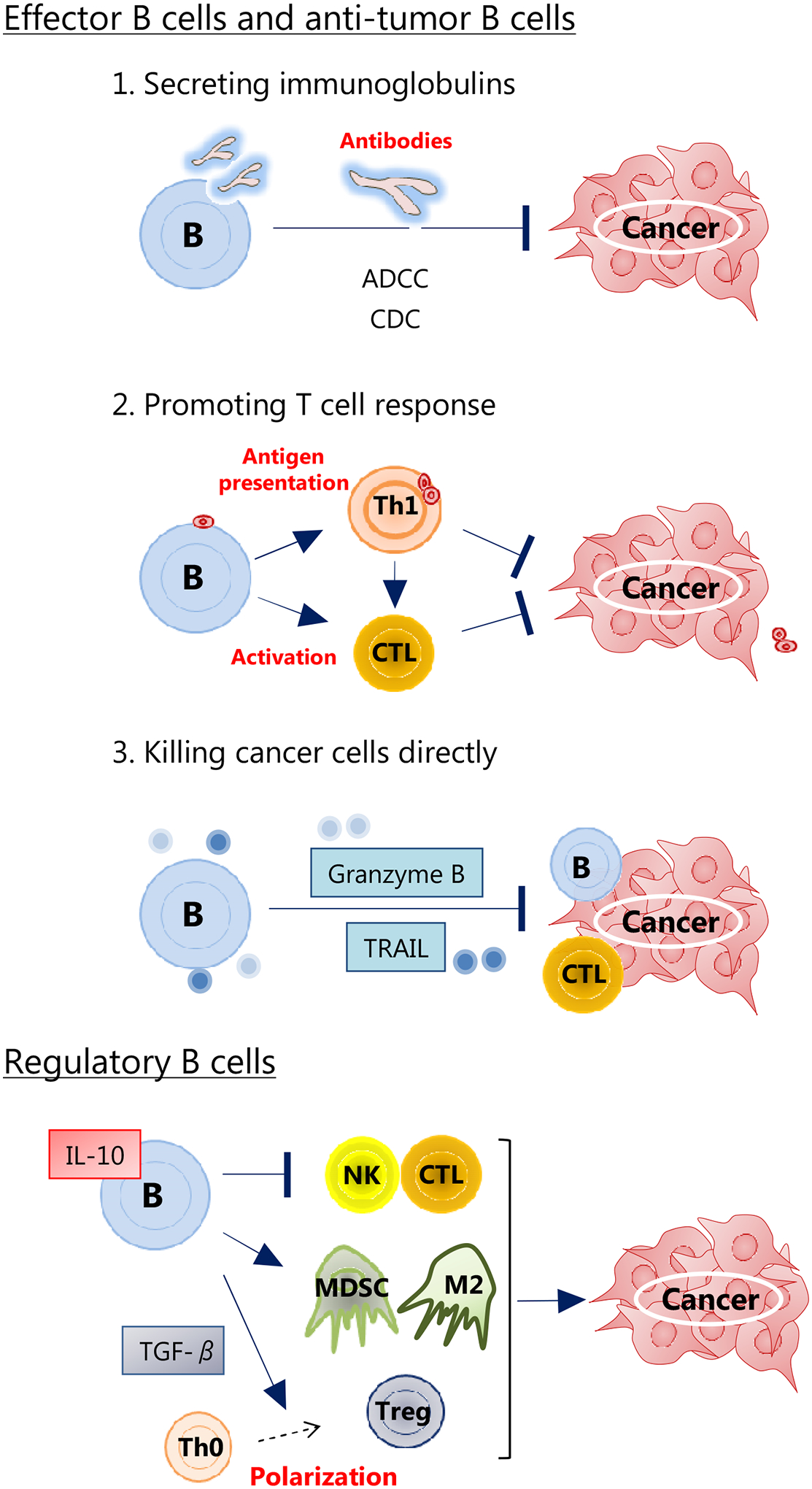
The roles of B cells in the tumor micro-environment.
B cells have various functions for immune response. B cells suppress tumor progression by secreting immunoglobulins, promoting T cell response, and killing cancer cells directly. On the contrary, regulatory B cells increase tumor activity via regulating immune cells.
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CDC, complement dependent cytotoxicity; CTL, cytotoxic T lymphocyte; MDSC, myeloid derived suppressor cell; M2, M2-polarized macrophage; NK, natural killer cell; Th0, naive T cell; Th1, T helper 1 cell; TRAIL, tumor necrosis factor-related apoptosis inducing ligand; Treg, regulatory T cell.
In humoral immunity, B cell and B cell-related pathways also play the leading part through germinal center (GC) reaction. Simply summarized for GC reaction (Figure 2), DCs with a chemokine receptor CCR7 activated by NK cells migrate to the T cell zone of SLOs through lymphoid vessels via chemokines CCL19 and CCL21. In the similar way, naive T cells and B cells with CCR7 migrate to T cell zone through high endothelial venules (HEVs) via CCL19 and CCL21 [13]. DCs make the antigen presentation to naive T cells, which promote differentiation from naive T cells into T follicular helper cells (Tfh cells) [14]. Tfh cells gradually increase a chemokine receptor, CXCR5, expression along with decreasing CCR7 expression, and migrate to B cell zone by the concentration gradient of chemokine CXCL13 produced by stromal cells in B cell zone [15]. The interaction between Tfh cells and B cells with follicular DCs promotes GC reaction for immune activation, which results in B cell differentiation into memory B cells and long-term surviving plasma cells. However, there are some reports showing that CCR7 and CXCR5 are expressed in cancer cells, and CCL19. -21/CCR7 axis and CXCL13/CXCR5 axis promote tumor development respectively [16, 17] (Figure 2). Therefore, the potential of B cell and B cell-related pathways as a new immune-related therapeutic target is still controversial and warrants further discussion.
Figure 2.
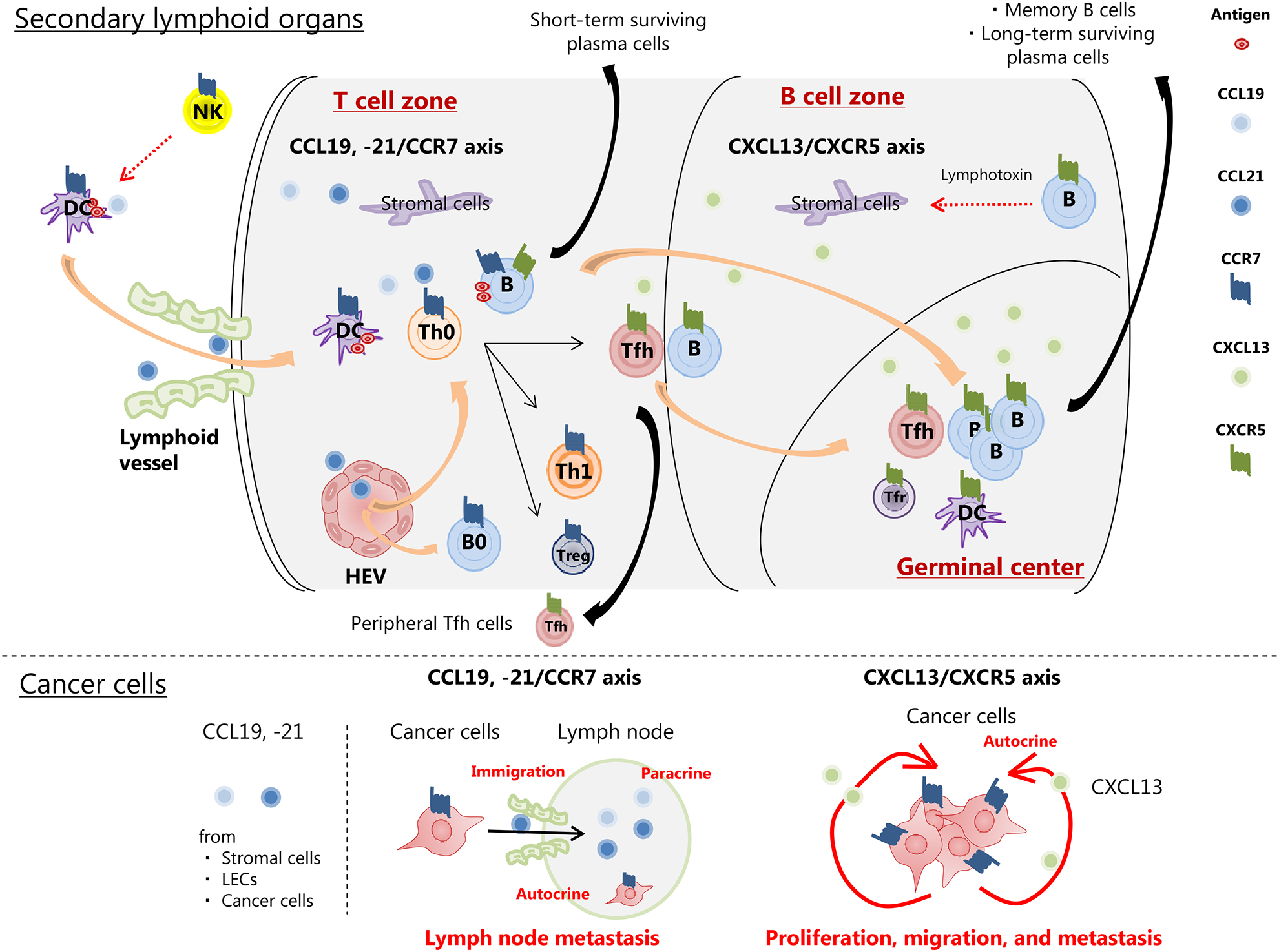
The role of B cell-related pathways in secondary lymphoid organs and cancer cells.
B cell/Tfh cell interaction in SLOs is the basis of adaptive immune response. CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis play the important role in immune cells migration to SLOs or tumor sites to activate immune response. On the contrary, CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis in cancer cells also play the role of creating a protumorigenic micro-environment.
Abbreviations: B0, naive B cell; DC, dendritic cell; HEV, endothelial venule; LECs, endothelial cells; NK, natural killer cell; SLOs, secondary lymphoid organs; Tfh, T follicular helper cell; Tfr, T follicular regulatory cell; Th0, naive T cell; Th1, T helper 1 cell; Treg, regulatory T cell.
In this review, we discuss the accumulating evidence about the roles of B cell and B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis) in tumor micro-environment (TME) and immune response. The adoption of more extensive immunogenetic profiling of B cell and B cell-related pathways might pave the way toward understanding the integrated tumoral immune system as well as help to discover novel cancer treatment strategies.
The function of B cell and B cell/Tfh cell interaction in cancer
B cell is recognized as the main effector cell of humoral immunity. Tfh cells are helper T cells that control maturation and activation of B cells. B cell/Tfh cell interaction with follicular DCs in GC is the basis of adaptive immune response (Figure 2). In addition, B cells and Tfh cells themselves can infiltrate into tumors and B cells affect tumor progression through their interaction with Tfh cells [18]. However, the direct efficacy of B cells in cancer treatment is controversial. B cells not only suppress tumor progression by secreting immunoglobulins, promoting T cell response, and killing cancer cells directly but also increase tumor activity via immunosuppressive cytokines [8, 12, 19] (Figure 1). Therefore, immunotherapy based on B cells will require a better understanding of the immunophenotypic characterization and the sub-populations. This section highlights the function of B cell and the B cell/Tfh cell interaction for cancer immunity.
In SLOs, after interacting with Tfh cells, B cells differentiate into short-term surviving plasma cells in B cell zones, or memory B cells and long-term surviving plasma cells through GC reaction. Importantly, CXCL13 secreted by Tfh cells and follicular DCs is responsible for the influx of B cells into TME [8]. Existing evidence shows that distinct B cell subpopulations found in the TME have different roles. First, Katoh et al showed that TIBs produced functional antibodies with growth-suppressive properties, and IgG-dominant B cells were observed in gastric cancer tissues and IgA-dominant B cells in the normal gastric mucosa [20]. Tumor-specific humoral responses by TIBs via affinity maturation can be generated also within TLS in NSCLC [10] and breast cancer [21]. In addition, immunoglobulins secreted by B cells can induce tumor lysis by antibody-dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) [22]. Second, B cells also function as antigen-presenting cells, and facilitate the T cell response in the TME. In NSCLC, high number of T cells localized with high number of B cells is associated with good outcome [23]. Furthermore, helper B Cells, which express CD27 to CD70 presented on CTLs [10], sustain B-cells activation and promote CTLs survival and proliferation independently of antigen presentation [24]. Third, activated B cells may directly lyse tumor cells together with CTL activity, and enhance expression of granzyme B and tumor necrosis factor-related apoptosis inducing ligand (TRAIL), both of which have direct cytotoxicity against cancer cells [25]. On the other hand, B cells are also known to induce carcinogenesis and cancer progression. In melanoma mouse models depleted of B cells, antitumor activity of CTLs and the efficacy of cancer vaccine were increased [26]. Ganti et al. showed that the injection of B cells accumulated in the regional lymph nodes in a mouse with melanoma to induce tumor progression [27]. B cells were also shown to directly inhibit CTL responses in CRC and melanoma mice models [28]. Given these preclinical findings, the existence of Bregs has been clarified. Bregs producing IL-10, an anti-inflammatory cytokine, has been shown to have ability to suppress tumor immunity [29]. This suppression reportedly requires signals from Toll-like receptor (TLR), CD40, and B cell receptor (BCR) [30]. Furthermore, in squamous carcinomas (SCCs) and pancreatic ductal adenocarcinoma (PDAC), Bregs reportedly induce M2 macrophage polarization [31, 32], as well as regulatory T cells (Tregs) [19], that foster CTL suppression.
Tfh cells are essential for B cells to work. Tfh cells are characterized by the expression of CXCR5, inducible T-cell co-stimulator (ICOS), programed death 1 (PD-1), and B cell lymphoma 6 (BCL-6). Bcl-6 is the unique marker of Tfh cells and suppresses the differentiation-inducing gene expression of Th1, Th2, Th17 cells, and Tregs [33]. Tfh cells have a number of functions to help B cells within the GC. ICOS-mediated signals promote the production of IL-21 by inducing the transcription factor c-Maf, and IL-21 induces B cell proliferation and class switching [34]. Tfh cells also express CD40 ligand, a TNF family molecule, which prompts B cell differentiation and class switching [35]. The immunosuppressive molecule PD-1 plays a role in controlling the activation of Tfh cells [36]. In GC, T follicular regulatory (Tfr) cells, which have characteristics of both Tregs and Tfh cells, control the GC size and the amount of antibodies produced [37]. In the peripheral blood, CXCR5 positive helper T cells correspond to memory cells derived from Tfh cells [38]. Furthermore, the memory Tfh cells are fractionated into different cell populations having Th1, Th2, Th17 cell type properties by expression of CXCR3 and CCR6 [39]. In addition, Gu-Trantien et al. showed that tumor-infiltrating CXCL13-producing (CXCR5 negative) Tfh cells induce B cells and are tightly linked with B cell maturation and tumor suppression [3]. These subtypes differ in the ability to induce proliferation and differentiation of B cells.
In consensus with these data, there are various reports about the association between B cells/Tfh cells expression status and cancer patient outcome. In CRC patients, Bindea et al. reported that the number of B cells and Tfh cells in TME increased with tumor stage; but the higher numbers were associated with better prognosis; and patients with CXCL13 depletion or low expressed CXCL13 showed a lower density of B cells, Tfh cells, Th1 cells, and CTLs with worse prognosis [1]. Furthermore, in lung cancer, both tumor-infiltrating plasma cells and Tfh cells are associated with a better outcome [10]. Some evidences also showed that tumors containing both CTLs and B cells are associated with higher survival rates than those containing CTL alone in ovarian cancer [40] or PDAC [41]. Consistently, in lung cancer, higher densitities of CD4+ and CTLs is associated with a high density of B cells within TLSs [11]. On the contrary, high TIBs were reportedly associated with higher stage and tumor recurrence in prostate cancer [42]. The number of B cells in ovarian cancer ascites increased with stage and correlated with poor prognosis [43]. In addition, in SCC and PDAC, there are reports showing that Bregs induce M2 macrophage polarization and CTL suppression, leading to poor prognosis [31, 32]. B cell and Tfh cell responses may be modulated depending on cancer types, cancer progression status, and individual TME.
B cell, a target for cancer treatment
Given the relationship between B cells and patient prognosis, the development of B cell-based immunotherapeutic strategies may be effective. Increasing the activity of anti-tumor B cells (adoptive transfer of stimulated effector B cells or anti-tumor B cell activation) or Bregs inhibition may show anti-tumor effect. We summarize existing preclinical evidences targeting B cells in the TME for cancer treatment (Table 1).
Table 1.
Cancer treatment approaches targeting B cells and TLSs based on pre-clinical models.
| Target | Approach | Type of cancer | Works for the axis (in TME) | In vitro or in vivo | Outcome | Ref. |
|---|---|---|---|---|---|---|
| B cells | ||||||
| Antibody induction | Natural anti-sulfated glycosaminogly can antibodies | Gastric, PDAC, CRC, HCC, Lung | Targeting sulfated glycosaminoglycan detected as B cell antigen | In vitro | Suppression of the cancer cell proliferations | [20] |
| Intraperitoneal a L-fucose (Fuc)-enriched Reishi polysaccharide fraction | Lung (LLC) | Induction of B cells activating IgM-mediated cytotoxicity | In vivo | Reduction of tumor growth | [45] | |
| Intratumoral recombinant IL-12 injection | Head and neck SCC | Activation of B cells, and induction of immunoglobulin and IFNg production | Phase lb and Phase II study | Good patient prognosis | [46] | |
| Breg | CD20 specific monoclonal antibody | PDAC | Bregs depletion | In vivo | Reduction of tumor growth | [48] |
| CD20 specific monoclonal antibody | SCC | Enhancement of CTL responses via CCR5-dependent mechanisms | In vivo | Reduction of tumor growth, and improving reactivity to platinum- and Taxol-based chemotherapy | [31] | |
| Rituximab | CRC | Reduction of CD20-positive B cells | In human | Reduction of tumor growth | [49] | |
| Total glucoside of paeony | HCC | Reduction of IL-10-producing B cells | In vivo | Reduction of serum ALT, AST, ALP, and AFP | [50] | |
| Lipoxin A4 | HCC | Reduction of Bregs via inactivation of STAT3, and enhancement of CTLs dephosphorylating STAT3 and ERK | In vivo | Reduction of tumor growth | [51] | |
| Resveratrol | Breast | Reduction of Bregs, leading to low TGF-β via inactivation of ST AT3 | In vivo | Inhibition of lung metastasis | [52] | |
| MK886 | Breast | Reduction of Bregs via inactivation of the 5-lipoxygenase/leukotriene/PPARα pathways | In vivo | Inhibition of lung metastasis | [53] | |
| Ibrutinib | PDAC | Reprogramming macrophages toward a Ml phenotype, and restoration in antitumor activity of CTLs | In vivo | Reduction of tumor growth, and improvement of reactivity to gemcitabine | [32] | |
| Combination of ibrutinib and anti-PD-L1 antibody | Lympho ma, breast, and CRC | Enhancement of antitumor T-cell immune responses | In vivo | Reduction of tumor growth, and improvement of reactivity to chemotherapy | [54] | |
| TLS | ||||||
| Vaccine | GVAX in combination with cyclophosphamide | PDAC | Suppression of Tregs pathway, and enhancement of Th17 cells pathway and PD-1-PD-L1 pathway | In human | Induction of TLSs in 33 of 39 patients 2 weeks after vaccine treatment | [59] |
| Vaccination targeting HPV16 E6/E7 antigens | Cervical dysplasia | Induction of CTLs | In human | Induction of TLSs | [60] | |
| GVAX in combination with cyclophosphamide followed by CRS-207 | Metastatic pancreatic adenocarcinoma | Induction of CTLs | Phase II study | Extending survival for patients | [63] | |
| LT | Recombinant antibody-lymph otoxin-alpha fusion protein | Melanoma | Induction of naive T cells | In vivo | Induction of TLSs | [64] |
| Environ mental factor | Inflammatory diet | CRC | - | In human | Reduction of TLS s, and increasing risk of CRC | [65] |
| Corticosteroid intake | Lung SCC | - | In human | Reductions of TLS density and GC formation | [66] | |
| (chemotherapy) | Lung SCC | - | In human | Reduction of GC formation (TLS density was not changed but the prognostic value of TLS was lost.) | [66] | |
NOTE: CRS-207, live-attenuated Listeria monocytogenes-expressing mesothelin; GVAX, granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting vaccine; inflammatory diet, including red and processed meats, refined grains, carbonated beverages, and some vegetables; Ibrutinib, bruton tyrosine kinase inhibitor; rituximab, a humanized monoclonal antibody directed against human CD20; total glucosides of paeony, showing anti-inflammatory, anti-oxidative, and hepato-protective activities; lipoxin A4, an arachidonic acid-derived anti-inflammatory lipid mediator; resveratrol, a plant-derived polyphenol; MK886, inhibiting leukotriene biosynthesis in leukocytes.
Abbreviations: AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, glutamic oxalacetic transaminase; Bregs, regulatory B cells; CRC, colorectal cancer; CTLs, cytotoxic T lymphocytes; GC, germinal center; HCC, hepatocellular carcinoma; LLC, lewis lung cancer; LT, lymphotoxin; PD-1, programmed death-1; PDAC, pancreatic ductal adenocarcinoma; PD-L1, programmed death-ligand 1; SCC, squamous carcinomas; TGF-β, transforming growth factor-β; TLS, tertiary lymphoid structure; TME, tumor microenvironment; Th17 cells, T-helper 17 cells; Tregs, regulatory T cells.
It has been established that TIBs and B cells within TLSs produce anti-tumor antibodies. Katoh Et al. showed that sulfated glycosaminoglycan, which mainly exists on the membrane surface of cancer cells, was a major functional B cell antigen in gastric cancer, and natural anti-sulfated glycosaminoglycan antibodies suppressed tumor growth in various cancers [20]. The clinical relevance of the use of glycosaminoglycan as therapeutic target for cancer is shown in recent report [44]. In lung cancer mouse models, natural compounds, such as Reishi polysaccharide fraction, can suppresse tumor growth with induction of B cells activating IgM-mediated cytotoxicity [45]. Besides, intra-tumoral injection of IL-12 was also shown to activate B cells and induce immunoglobulin and IFN g production, leading to good outcomes in head and neck SCC patients [46].
On the other hand, in PDAC mice models with KRAS-mutations, IL35-producing B cells, which are recruited to TME by CXCL13 from stromal fibroblasts, played a protumorigenic role [47, 48]. Although loss of HIF1α increased CXCL13 expression and induced the Bregs to TME, their protumorigenic role could be inhibited by anti-CXCL13 treatment [47] or CD20 specific monoclonal antibody [48]. Also in SCC, CD20 monoclonal antibodies have shown an improved response to platinum- and taxol-based chemotherapies [31]. In advanced CRC, Rituximab, a humanized monoclonal antibody, directed against human CD20, reduced tumor size [49]. Other agents, such as total glucosides of paeony (showing anti-inflammatory, anti-oxidative, and hepato-protective activities) [50], lipoxin A4 (an arachidonic acid-derived anti-inflammatory lipid mediator) [51], resveratrol (a plant-derived polyphenol) [52], and MK886 (inhibiting leukotriene biosynthesis in leukocytes) [53] also induced an antitumor effect by inhibiting Bregs in various cancers. In addition, Gundersonet et al. showed that B cells promoted bruton tyrosine kinase (BTK), a member of the BCR signaling pathway, and PI3Kγ-dependent M2 macrophage polarization, and led to immune and chemo-sensitivity suppression, which was recovered by BTK or PI3Kγ inhibition [32]. Interestingly, inhibition of BTK may work in synergy with immune checkpoint inhibitors and/or clinical standard chemotherapy, because BTK inhibitors also inhibit interleukin-2-inducible T-cell kinase (ITK), an essential enzyme in Th2 cells, thereby shifting the balance from Th2 to Th1 cells [54]. Currently, two clinical trials are ongoing in patients with PDAC (NCT02436668) and head and neck SCC (NCT02454179) using BTK inhibitor in combination with standard chemotherapy. These studies highlight the possibility of using B cells as novel immunotherapeutic targets and revealed that targeting B cells will have to both promote anti-tumor B cells and inhibit Breg phenotypes.
TLS, a target for cancer treatment
TLSs are transient ectopic lymphoid organizations that can develop in non-lymphoid tissues, in areas with chronic inflammation. TIBs and B cell-related pathways have key roles in forming the TLS and local immune responses which take place in TLSs [18, 55]. Studies have demonstrated that TLS presence in TME is associated with local anti-tumor immune responses and positive patient outcomes [9, 11]. Hence, upregulation of TLS may lead to novel cancer treatments. In addition, B cell and B cell-related pathway might be a promising target through TLS formation.
In cancer immunity, antigens induce chronic inflammation alongside tumor-infiltrating immune cells, where TLSs serve as sites for concentrated and efficient immune responses. TLSs display a similar organization to that observed in SLOs. Like conventional LNs, TLS is composed of T cell-rich and B cell-rich zones with GC, and B cell-rich zone is composed of a mantle of naive B cells, Tfh cells and follicular DCs [10]. The difference between SLOs and TLSs is the absence of NK cells within TLSs [56]. TLS development allows for immune cells maturation, such as B cells (which proliferate and differentiate into plasma cells) and DCs, and induces the infiltration of CTLs to tumor and CD4+ T cell responses [10]. Existing evidences suggest that TLS formation may need three critical factors via B cells and DCs: LT production, lymphoid chemokine production (such as CCL19, CCL21, and CXCL13), and HEVs development [18, 55]. The engagement of lymphotoxin α1β2 (LTα1β2) expressed from CD4+ lymphoid tissue inducer (LTi) cells with lymphotoxin beta receptor (LTβR) on stromal lymphoid tissue organizer (LTo) cells leads to the expression of adhesion molecules (ICAM-1 and VCAM-1), lymphoid chemokines (such as CCL19, CCL21, and CXCL13), cytokines, HEVs, and lymphatic vessels [9]. The local expression of CCL19, CCL21, and CXCL13 triggers the recruitment of T cells and B cells via HEVs and DCs via lymphatic vessels in the TME and the development of TLSs [9, 57]. CXCL13 also promotes LTα1β2 expression from LTi cells, resulting in a positive and stable feedback loop [58].
Several chemotherapeutic and immunotherapeutic agents which induce the development of TLSs have been reported. (Table 1) Immunization with vaccines has been shown to induce TLSs expression secondary to antigen exposure [59, 60]. In PDAC, Lutz et al. showed that the number of TLSs tended to increase when patients were treated with granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting vaccine (GVAX) combined with cyclophosphamide (GVAX/Cy) [59]. This study also clarified that this treatment suppressed the Treg pathway and enhanced Th17 pathway, in accordance with studies showing that Tregs can suppress TLS development [61], and that Th17 cells can promote TLS development [62]. In phase II trial, GVAX/Cy followed by CRS-207, live-attenuated Listeria monocytogenes-expressing mesothelin, extended survival for patients with metastatic pancreatic cancer [63]. In addition, Schrama et al. clarified that LTα promoted TLS development in melanoma mice model [64]. Recent studies have also shown that TLSs can be affected by environmental factors, such as a pro-inflammatory diet (reduction of TLSs) [65] and corticosteroid intake (reduction of TLSs) [66]. Furthermore, in lung cancer patients, neoadjuvant chemotherapy impairs GC formation within TLSs, leading to the loss of prognostic benefit with TLSs expression [66]. Considering the potential role played by B cells via CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis in TLSs induction, targeting B cell and B cell-related pathways may yield beneficial effects through TLS development.
B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis) in immune reaction
CCL19, -21/CCR7 axis works primarily in immune cells, such as T cells, B cells, and DCs, aiding their migration to SLOs or tumor sites to activate host immune response. CXCL13/CXCR5 axis works basically for interaction between B cell and Tfh cell to accelerate GC reaction (Figure 2), and is also involved in the migration of B cells and Tfh cells to the TME [67]. Furthermore, B cells via CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis induce TLSs formation. Therefore, it is critical to understand how these axes are regulated.
CCL19 (also known as EBV-induced molecule 1 ligand chemokine) and CCL21 (also known as secondary lymphoid-tissue chemokine) are both constitutively expressed by stromal cells within the T cell zone of SLOs [68]. CCL19 is expressed by DCs [68, 69] and cancer cells [70]. CCL21 is expressed by HEVs, lymphatic endothelial cells (LECs) [71], and cancer cells [70]. CCR7 (also known as CD197) is mainly expressed in naive T cells, B cells, mature DCs, and cancer cells, and is essential for migration of these cells. CCL19, - 21/ CCR7 axis reportedly mediates immune cells homing to both lymphoid and non-lymphoid tissues [72]. However, naive T cells expressing CCR7 mainly recognize CCL19 and CCL21 produced in T cell zone of SLOs, and migrate to T cell zone through HEVs [71] (Figure 2). In preclinical experiment, plt/plt mice that lack CCL19 and CCL21 in lymphoid organs have defective naive T cell homing into lymph node [73]. In addition, CCR7-deficient mice reduced numbers of naive T cells and DCs in SLOs [74]. CCL19 and CCL21 expression statuses in the TME are mainly correlated with good outcomes in cancer patients: CCL19 expression in lung cancer [75], and CCL21 expression in renal cell cancer [76] and colorectal cancer (CRC) [77].
CXCL13 (also known as B lymphocyte chemoattractant) is constitutively released by stromal cells within B cell zone of SLOs [78] (Figure 2). B cells basically secrete lymphotoxin (LT), which leads to production of CXCL13 from surrounding stromal cells [79]. Indeed, either blockade of LT or depletion of B cells in mice resulted in fewer CXCL13 transcripts [79]. In addition, Tfh-like cells (CXCR5 negative), follicular DCs, and cancer cells produce CXCL13 to induce B cells and Tfh cells to the TME [2, 3, 80]. CXCR5 (also known as CD185) is mainly expressed in B cells, Tfh cells, mature DCs, and cancer cells, and is essential for migration of these cells [1, 3, 17]. Cells with CXCR5 are attracted to B cell zone rich in CXCL13, and B cells are stimulated by Tfh cells and follicular DCs for maturation [15]. In preclinical experiments, CXCR5-deficient mice with CRC accelerated tumor growth and injected recombinant CXCL13 induced tumor rejection [1]. Gu-Trantien et al. showed that CXCL13 expression was associated with TLS expression, higher response to preoperative chemotherapy, and better outcomes in breast cancer patients [2]. Bindea et al. also showed that CXCL13 was a pivotal factor for B cells and Tfh cells infiltrating tumor and associated with good prognosis in CRC patients [1].
On the other hand, CCL19, - 21/ CCR7 axis is also actively involved in trafficking of cancer cells to the T cell zone through chemokine gradients, leading to lymph node metastasis (Figure 2). Current evidences show that cancer cells with CCR7 expression migrate toward LECs that express CCL21 [16], which is also helped by autocrine CCL19, −21 secretions from cancer cells [70]. In addition, CCL21 can recruit myeloid-derived suppressor sells (MDSCs) and Tregs to the TME, and lead to cancer progression [81]. Some studies have shown that the secretion of CCL19 or CCL21 in cancer cells is associated with invasiveness and immune tolerance [81]. Shields et al. knocked down CCL21 in a melanoma model and found that CCL21-deficient tumors grew much slower and induced tumor-specific T cell responses. CCR7 is upregulated in some cancer cells and associated with tumor progression, lymph node metastases, and poor outcome [82]. In PDAC mice model, lack of CCR7 decreased the lymph node metastasis which was enhanced by upregulation of CCL21 [83]. CXCL13/CXCR5 axis in cancer cells is also actively involved in tumor proliferation and metastasis (Figure 2). Current evidences show that elevated CXCL13 via NF-kB pathway mediated migratory and tumorigenic activity in prostate cancer mice model [17]. High CXCL13 expression in tumors is also associated with poor prognosis in some cancers, such as CRC [84], gastric cancer [85], and breast cancer [86]. Similarly, high CXCR5 expression in tumor is also correlated with poor prognosis in CRC [84], prostate cancer [17], and breast cancer [86]. In addition, Biswas et al. clarified that co-expression of CXCL13 and CXCR5 showed a significant association with lymph node metastasis in breast cancer patients [86]. Elevated serum CXCL13 levels have been postulated as a biomarker of good prognosis in hepatocellular carcinoma (HCC) [87], but a poor prognosis in prostate cancer [88] or breast cancer [89].
These contradictory findings in CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis may be due to the different TME: the different cancer types, stages of progression, and receptor-expressing cells (immune cells or cancer cells). The paracrine signals of these axis works for B cell/Tfh cell interaction in GC of SLOs and TLSs, and for immune cells (B cells, Tfh cells, and DCs) migration to the TME. On the contrary, the autocrine signal of these axis may play a role in cancer cell proliferation and metastasis.
B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis), targets for cancer treatment
CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis may be an effective target for drug development by activating the paracrine axis (for migration of immune cells) and inhibiting the autocrine axis (for suppression of cancer cells). We summarize treatment strategies targeting these axes. (Table 2)
Table 2.
Cancer treatment approaches targeting CCL19, -21/CCR7 and CXCL13/CXCR5 axes based on pre-clinical models.
| Target | Approach | Type of cancer | Works for the axis (in the TME) | In vitro or in vivo | Outcome | Ref. |
|---|---|---|---|---|---|---|
| CCL19, -21/CCR7 axis | ||||||
| CCL19 | Intratumoral CCL19 transduced with retroviral vector | Breast | (through a mechanism involving NK and CD4+ cells) | In vitro and in vivo | Reduction of tumor growth | [90] |
| CCL21 | Intratumoral recombinant CCL21 | Lung (alveolar carcinoma, LLC) | Induction of CD4+ cells, CTLs, and DCs, also in the draining lymph nodes | In vivo | Reduction of tumor growth | [91] |
| Intratumoral Ad-CCL21-DC | Lung (LLC) | Induction of CD4+ cells, CTLs, and DCs, reduction of Tregs, and upregulation of CXCL9 and CXCL10 | In vivo | Reduction of tumor growth (Depletion of CXCL9 and CXCL10 reduced the antitumor efficacy of Ad-CCL21-DC) | [92] | |
| Intratumoral Ad-CCL21-DC | Myeloma | Induction of CTLs | In vivo | Reduction of tumor growth | [93] | |
| Intratumoral CCL21 nanocapsule | Lung (LLC) | Induction of T cells and DCs, and reduction of MDSCs and Tregs | In vivo | Reduction of tumor growth | [94] | |
| Intratumoral Ad-CCL21-DC | Lung (NSCLC) | Induction of CTLs and tumor PD-L1 expression (and systemic tumor antigen–specific immune responses) | Phase I study | Twenty-five percent (4/16) of patients had stable disease at day 56. | [95] | |
| Anti-CCL21 antibodies | Melanoma | − | In vivo | Reduction of lymph node metastasis | [97] | |
| Inhibition of TGF(3Ip | CRC | Reduction of CCL21 expression in LECs and LEC permeability by inducing the dissociation of VE-cadherin junctions | In vivo | Reduction of tumor lymphangiogenesis and metastasis | [98] | |
| CCR7 | Anti-CCR7 antibody | Breast | Reduction of CCR7 expression in cancer cells | In vivo | Reduction of lymph node metastasis | [96] |
| TAK1 inhibitor, 5Z-O | Breast | Reduction of CCR7 expression in cancer cells | In vivo | Reduction of tumor lymphatic invasion and distant metastasis | [99] | |
| CXCL13/CXCR5 axis | ||||||
| CXCL13 | Intratumoral recombinant CXCL13 | CRC | - | In vivo | Reduction of tumor growth | [1] |
| CXCL13-coupled CpG oligonucleotides (CpG-ODN) | Breast cancer | Stimulation of B cells, inhibition of Bregs expressing low levels of CD20, and induction of CTLs | In vivo | Reduction of lung metastasis | [100] | |
| Anti-CXCL13 inhibitor | PDAC | Inhibitiing the migration of Bregs | In vivo | Reduction of tumor growth | [47] | |
| CXCR5 | CXCL13-KDEL intrakine (suppression of CXCR5) | CRC | Inhibition of CXCR5 function in cancer cells | In vitro and in vivo | Induction of tumor growth arrest in liver metastasis | [101] |
| (depletion of CXCR5) | Prostate | Inhibition of CXCR5 function mediated by NF-kB pathway in cancer cells | In vitro and in vivo | Reduction of tumor growth and metastasis | [17] | |
Note: CXCL13-KDEL intrakine, transfection of CXCL13 extended with a KDEL sequence
Abbreviations: Ad-CCL21-DC, autologous DCs transduced with an adenoviral (Ad) vector expressing the CCL21 gene; Bregs, regulatory B cells; CRC, colorectal cancer; CTLs, cytotoxic T lymphocytes; DCs, dendritic cells; LECS, lymphatic endothelial cells; LLC, Lewis lung cancer; MDSC, myeloid-derived suppressor sells; NSCLC, non-small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; TAK1, TGF-β-activated protein kinase 1; TGFβIp, transforming growth factor-β (TGF-β)-induced protein; TME, tumor microenvironment; Tregs, regulatory T cells; 5Z-O, 5Z-7-oxozeaenol.
Intratumoral agents that augment CCL19 and CCL21 expression may show anti-tumor effect through the infiltration of immune cells to the TME. Although CCR7 inhibition may be simultaneously led into broad immunosuppressive effect, it may be a target molecule for suppressing lymph node metastasis. Intratumoral injection of CCL19 reportedly suppressed tumor development in breast cancer [90]. As DC-based immunotherapy, intratumoral injection of CCL21 has successfully eradicated tumors in lung cancer mice models [91]. Moreover, tumor regression mediated by intratumoral CCL21-expressing DCs [92, 93] or CCL21 nanocapsules [94] was accompanied by a significant increase in tumor-infiltrating CTLs, leading to tumor eradication. In non–small cell lung carcinoma (NSCLC), a phase I study following intratumoral CCL21-expressing DCs was conducted and showed that patients with increased CTLs significantly increased PD-L1 expression [95], suggesting the combination with checkpoint-inhibitor could lead to a better response. On the other hand, the anti-CCR7 antibody significantly suppressed the migration and invasion of breast cancer cells [16], and reduced lymph node metastasis which was induced by CCL21 upregulation in LECs by transforming growth factor-β (TGF-β) 1 in the mice models [96]. Similarly, the anti-CCL21 antibody could block lymph node metastasis in melanoma mice model [97]. A recent experiment has also revealed that inhibition of TGF-β-induced protein (TGFβIp), which increases CCL21 expression from LECs, could reduce CRC lymphangiogenesis and metastasis [98]. Huang et al. showed that inhibition of TGF-β-activated protein kinase 1 (TAK1), which upregulated CCR7 expression, could reduce lymphatic invasion and distant metastasis in breast cancer mice model [99].
In wild-type mice with CRC, intratumoral recombinant CXCL13 induced tumor rejection [1]. Bodogai et al showed that B-cell depletion by anti-CD20 was not beneficial in breast cancer, and CXCL13-coupled CpG oligonucleotides (CpG-ODN), which stimulates B cells, could block cancer metastasis by inhibiting Bregs expressing low levels of CD20 [100]. On the other hand, in PDAC mice models with KRAS-mutant, where loss of HIF1α increases the CXCL13 expression and induces the Bregs to the TME, the pro-tumorigenic role could be inhibited by anti-CXCL13 treatment [47]. The suppression of CXCR5 also reduced the tumor growth of CRC in liver metastasis [101]. In addition, the depletion of CXCL13 or CXCR5 in prostate cancer cells impaired their migratory and tumorigenic capacity in nude mice [17].
Concluding Remarks
The current review focuses on exploring the roles of B cell and B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis). They play critical roles in activating immune responses through humoral immunity and local immune activation via TLS formation, thereby influencing cancer treatment efficacy. Based on pre-clinical data, the activation of B cell and B cell-related pathways may lead to new opportunities for more efficient immune therapies, but we should know that they simultaneously have some protumorigenic roles in the TME. Further understanding of the regulation of B cell and B cell-related pathways could pave the way towards novel strategies in the treatment of cancer.
Highlights.
B cells suppress tumor progression by secreting immunoglobulins, promoting T cell response, and killing cancer cells directly.
B cell and B cell-related pathways (CCL19, -21/CCR7 axis and CXCL13/CXCR5 axis) play a key role in activating immune response through local immune activation via tertiary lymphoid structure formation.
In humoral immunity, B cells play the leading part and B cell-related pathways play the important role.
Recent preclinical evidences show the potential role of B cell for novel cancer treatment strategies.
Funding:
R Tokunaga was supported by the Uehara Memorial Foundation. H.-J. Lenz was supported by the National Cancer Institute (grant number P30CA014089), Dhont Family Foundation, San Pedro Peninsula Cancer Guild, and Daniel Butler Research Fund.
Abbreviations
- BCL-6
B cell lymphoma 6
- BCR
B cell receptor
- Bregs
regulatory B cells
- BTK
bruton tyrosine kinase
- CRC
colorectal cancer
- CTL
cytotoxic T lymphocyte
- DCs
dendritic cells
- GC
germinal center
- GVAX
granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting vaccine
- GVAX/Cy
GVAX combined with cyclophosphamide
- HCC
hepatocellular carcinoma
- HEVs
endothelial venules
- ICOS
inducible T-cell co-stimulator
- LECs
endothelial cells
- LT
lymphotoxin
- LTi cells
lymphoid tissue inducer cells
- LTα1β2
lymphotoxin α1β2
- LTβR
lymphotoxin beta receptor
- MDSCs
myeloid-derived suppressor sells
- NK
natural killer
- NSCLC
non-small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PD-1
programed death 1
- SCC
squamous carcinomas
- SLOs
secondary lymphoid organs
- TAMs
tumor-associated macrophages
- Tfh cells
T follicular helper cells
- Tfr cells
T follicular regulatory cells
- TGF-β
transforming growth factor-β
- TIBs
tumor-infiltrating B lymphocytes
- TILs
tumor-infiltrating lymphocytes
- TLS
tertiary lymphoid structure
- TME
tumor environment
- Tregs
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest Statement: All authors have declared no conflicts of interest.
Presentation: We have not presented this review anywhere.
REFERENCES
- [1].Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39: 782–795. [DOI] [PubMed] [Google Scholar]
- [2].Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123: 2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell 2016; 164: 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mata M, Gerken C, Nguyen P, Krenciute G, Spencer DM, Gottschalk S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov 2017; 7: 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borghaei H, Hellmann MD, Paz-Ares LG, Ramalingam SS, Reck M, O’Byrne KJ. Nivolumab (Nivo) plus platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with < 1% tumor PD-L1 expression: Results from CheckMate 227. Journal of Clinical Oncology 2018; 36. [Google Scholar]
- [7].Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. Journal of Clinical Oncology 2018; 36: 773–+. [DOI] [PubMed] [Google Scholar]
- [8].Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014; 14: 447–462. [DOI] [PubMed] [Google Scholar]
- [10].Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189: 832–844. [DOI] [PubMed] [Google Scholar]
- [11].Zhu W, Germain C, Liu Z, Sebastian Y, Devi P, Knockaert S et al. A high density of tertiary lymphoid structure B cells in lung tumors is associated with increased CD4(+) T cell receptor repertoire clonality. Oncoimmunology 2015; 4: e1051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest 2011; 121: 4268–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8: 362–371. [DOI] [PubMed] [Google Scholar]
- [14].Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ et al. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol 2014; 192: 3607–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol 2008; 20: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pan MR, Hou MF, Chang HC, Hung WC. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem 2008; 283: 11155–11163. [DOI] [PubMed] [Google Scholar]
- [17].Garg R, Blando JM, Perez CJ, Abba MC, Benavides F, Kazanietz MG. Protein Kinase C Epsilon Cooperates with PTEN Loss for Prostate Tumorigenesis through the CXCL13-CXCR5 Pathway. Cell Rep 2017; 19: 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kobayashi S, Watanabe T, Suzuki R, Furu M, Ito H, Ito J et al. TGF-beta induces the differentiation of human CXCL13-producing CD4(+) T cells. Eur J Immunol 2016; 46: 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res 2011; 71: 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katoh H, Komura D, Konishi H, Suzuki R, Yamamoto A, Kakiuchi M et al. Immunogenetic Profiling for Gastric Cancers Identifies Sulfated Glycosaminoglycans as Major and Functional B Cell Antigens in Human Malignancies. Cell Rep 2017; 20: 1073–1087. [DOI] [PubMed] [Google Scholar]
- [21].Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res 2003; 63: 3275–3280. [PubMed] [Google Scholar]
- [22].Mizukami M, Hanagiri T, Yasuda M, Kuroda K, Shigematsu Y, Baba T et al. Antitumor effect of antibody against a SEREX-defined antigen (UOEH-LC-1) on lung cancer xenotransplanted into severe combined immunodeficiency mice. Cancer Res 2007; 67: 8351–8357. [DOI] [PubMed] [Google Scholar]
- [23].Kinoshita T, Muramatsu R, Fujita T, Nagumo H, Sakurai T, Noji S et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol 2016; 27: 2117–2123. [DOI] [PubMed] [Google Scholar]
- [24].Deola S, Panelli MC, Maric D, Selleri S, Dmitrieva NI, Voss CY et al. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol 2008; 180: 1362–1372. [DOI] [PubMed] [Google Scholar]
- [25].Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res 2013; 19: 5994–6005. [DOI] [PubMed] [Google Scholar]
- [26].Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res 2006; 66: 7741–7747. [DOI] [PubMed] [Google Scholar]
- [27].Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep 2015; 5: 12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 2010; 29: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang C, Xin H, Zhang W, Yazaki PJ, Zhang Z, Le K et al. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity 2016; 44: 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 2011; 34: 703–714. [DOI] [PubMed] [Google Scholar]
- [31].Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014; 25: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov 2016; 6: 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009; 325: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7–2 in established germinal centers. J Immunol 1995; 155: 556–567. [PubMed] [Google Scholar]
- [36].Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012; 336: 485–489. [DOI] [PubMed] [Google Scholar]
- [37].Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011; 17: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014; 35: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res 2016; 22: 3005–3015. [DOI] [PubMed] [Google Scholar]
- [41].Tewari N, Zaitoun AM, Arora A, Madhusudan S, Ilyas M, Lobo DN. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer 2013; 13: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med 2014; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol 2006; 125: 451–458. [PubMed] [Google Scholar]
- [44].Yip GW, Smollich M, Gotte M. Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther 2006; 5: 2139–2148. [DOI] [PubMed] [Google Scholar]
- [45].Liao SF, Liang CH, Ho MY, Hsu TL, Tsai TI, Hsieh YS et al. Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc Natl Acad Sci U S A 2013; 110: 13809–13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer 2008; 123: 2354–2361. [DOI] [PubMed] [Google Scholar]
- [47].Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB et al. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov 2016; 6: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D et al. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov 2016; 6: 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF Jr., Feng L et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother 2000; 48: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Song SS, Yuan PF, Li PP, Wu HX, Ni WJ, Lu JT et al. Protective Effects of Total Glucosides of Paeony on N-nitrosodiethylamine-induced Hepatocellular Carcinoma in Rats via Down-regulation of Regulatory B Cells. Immunol Invest 2015; 44: 521–535. [DOI] [PubMed] [Google Scholar]
- [51].Wang Z, Cheng Q, Tang K, Sun Y, Zhang K, Zhang Y et al. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Lett 2015; 364: 118–124. [DOI] [PubMed] [Google Scholar]
- [52].Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol 2013; 191: 4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha. J Immunol 2013; 190: 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015; 112: E966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P et al. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011; 71: 5412–5422. [DOI] [PubMed] [Google Scholar]
- [57].de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011; 71: 6391–6399. [DOI] [PubMed] [Google Scholar]
- [58].Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010; 464: 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014; 2: 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 2014; 6: 221ra213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med 2007; 204: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011; 35: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015; 33: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schrama D, thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA et al. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14: 111–121. [DOI] [PubMed] [Google Scholar]
- [65].Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, Zhang X et al. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology 2017; 153: 1517–1530 e1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res 2018; 78: 1308–1320. [DOI] [PubMed] [Google Scholar]
- [67].Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 2000; 12: 471–481. [DOI] [PubMed] [Google Scholar]
- [68].Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A 2000; 97: 12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kaiser A, Donnadieu E, Abastado JP, Trautmann A, Nardin A. CC chemokine ligand 19 secreted by mature dendritic cells increases naive T cell scanning behavior and their response to rare cognate antigen. J Immunol 2005; 175: 2349–2356. [DOI] [PubMed] [Google Scholar]
- [70].Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007; 11: 526–538. [DOI] [PubMed] [Google Scholar]
- [71].Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 1998; 95: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169: 424–433. [DOI] [PubMed] [Google Scholar]
- [73].Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med 2007; 204: 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33. [DOI] [PubMed] [Google Scholar]
- [75].Itakura M, Terashima Y, Shingyoji M, Yokoi S, Ohira M, Kageyama H et al. High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br J Cancer 2013; 109: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xiong Y, Liu L, Wang J, Xi W, Xia Y, Bai Q et al. Low CCL-21 expression associates with unfavorable postoperative prognosis of patients with metastatic renal cell carcinoma. Oncotarget 2017; 8: 25650–25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zou Y, Chen Y, Wu X, Yuan R, Cai Z, He X et al. CCL21 as an independent favorable prognostic factor for stage III/IV colorectal cancer. Oncol Rep 2013; 30: 659–666. [DOI] [PubMed] [Google Scholar]
- [78].Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 1998; 391: 799–803. [DOI] [PubMed] [Google Scholar]
- [79].Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol 2012; 13: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Airoldi I, Cocco C, Morandi F, Prigione I, Pistoia V. CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunol Immunother 2008; 57: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010; 328: 749–752. [DOI] [PubMed] [Google Scholar]
- [82].Xu B, Zhou M, Qiu W, Ye J, Feng Q. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial-mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med 2017; 6: 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sperveslage J, Frank S, Heneweer C, Egberts J, Schniewind B, Buchholz M et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int J Cancer 2012; 131: E371–381. [DOI] [PubMed] [Google Scholar]
- [84].Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D et al. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci 2014; 18: 1916–1924. [PubMed] [Google Scholar]
- [85].Wei Y, Lin C, Li H, Xu Z, Wang J, Li R et al. CXCL13 expression is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Cancer Immunol Immunother 2018; 67: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Biswas S, Sengupta S, Roy Chowdhury S, Jana S, Mandal G, Mandal PK et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat 2014; 143: 265–276. [DOI] [PubMed] [Google Scholar]
- [87].Duan Z, Gao J, Zhang L, Liang H, Huang X, Xu Q et al. Phenotype and function of CXCR5+CD45RA-CD4+ T cells were altered in HBV-related hepatocellular carcinoma and elevated serum CXCL13 predicted better prognosis. Oncotarget 2015; 6: 44239–44253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singh S, Singh R, Sharma PK, Singh UP, Rai SN, Chung LW et al. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett 2009; 283: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Panse J, Friedrichs K, Marx A, Hildebrandt Y, Luetkens T, Barrels K et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer 2008; 99: 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Braun SE, Chen K, Foster RG, Kim CH, Hromas R, Kaplan MH et al. The CC chemokine CK beta-11/MIP-3 beta/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J Immunol 2000; 164: 4025–4031. [DOI] [PubMed] [Google Scholar]
- [91].Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol 2000; 164: 4558–4563. [DOI] [PubMed] [Google Scholar]
- [92].Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res 2004; 10: 2891–2901. [DOI] [PubMed] [Google Scholar]
- [93].Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res 2001; 61: 2062–2070. [PubMed] [Google Scholar]
- [94].Kar UK, Srivastava MK, Andersson A, Baratelli F, Huang M, Kickhoefer VA et al. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS One 2011; 6: e18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE et al. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8(+) T-cell Infiltration. Clin Cancer Res 2017; 23: 4556–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K et al. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016; 35: 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst 2001; 93: 1638–1643. [DOI] [PubMed] [Google Scholar]
- [98].Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene 2016; 35: 196–205. [DOI] [PubMed] [Google Scholar]
- [99].Huang HL, Chiang CH, Hung WC, Hou MF. Targeting of TGF-beta-activated protein kinase 1 inhibits chemokine (C-C motif) receptor 7 expression, tumor growth and metastasis in breast cancer. Oncotarget 2015; 6: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res 2013; 73: 2127–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res 2006; 66: 9576–9582. [DOI] [PubMed] [Google Scholar]


