Abstract
Our understanding of cerebellar involvement in brain disorders has evolved from motor processing to high-level cognitive and affective processing. Recent neuroscience progress has highlighted hierarchy as a fundamental principle for the brain organization. Despite substantial research on cerebellar dysfunction in schizophrenia, there is a need to establish a neurobiological framework to better understand the co-occurrence and interaction of low- and high-level functional abnormalities of cerebellum in schizophrenia. To help to establish such a framework, we investigated the abnormalities in the distribution of sensorimotor-supramodal hierarchical processing topography in the cerebellum and cerebellar-cerebral circuits in schizophrenia using a novel gradient-based resting-state functional connectivity (FC) analysis (96 patients with schizophrenia vs 120 healthy controls). We found schizophrenia patients showed a compression of the principal motor-to-supramodal gradient. Specifically, there were increased gradient values in sensorimotor regions and decreased gradient values in supramodal regions, resulting in a shorter distance (compression) between the sensorimotor and supramodal poles of this gradient. This pattern was observed in intra-cerebellar, cerebellar-cerebral, and cerebral-cerebellar FC. Further investigation revealed hyper-connectivity between sensorimotor and cognition areas within cerebellum, between cerebellar sensorimotor and cerebral cognition areas, and between cerebellar cognition and cerebral sensorimotor areas, possibly contributing to the observed compressed pattern. These findings present a novel mechanism that may underlie the co-occurrence and interaction of low- and high-level functional abnormalities of cerebellar and cerebro-cerebellar circuits in schizophrenia. Within this framework of abnormal motor-to-supramodal organization, a cascade of impairments stemming from disrupted low-level sensorimotor system may in part account for high-level cognitive cerebellar dysfunction in schizophrenia.
Keywords: schizophrenia, sensorimotor, cerebellum, cerebellar-cerebral circuits, resting-state functional connectivity, functional gradient
Introduction
The role of the dysfunctional cerebellum in the pathogenesis of schizophrenia has been highlighted by the “dysmetria of thought” theory (also referred to as “cognitive dysmetria” theory). These models state that a common neurological impairment (dysmetria) underlies impaired sequencing and coordination of both sensorimotor and mental processes in cerebellar dysfunction, and thus provide a mechanistic hypothesis to explain how cerebellar abnormalities may underlie dys-coordinated and disordered thought and behavior in schizophrenia.1–4 Multiple empirical studies further support the role of cerebellum in the pathogenesis of schizophrenia.5 For instance, the development of cerebellar and cerebello-cortical connectivity may in part determine the onset and severity of childhood psychiatric diseases,6 including schizophrenia. Reduced cerebellar volume has been reported in schizophrenia, and is significantly correlated with a broad risk for psychopathology.7–10 Moreover, studies of clinical high-risk subjects indicate that dysconnectivity of cerebellar-cortical circuits can serve as a state-independent neural signature for psychosis prediction and characterization,11 and recent evidence indicates that cerebellar circuits alterations reflect a primary neuropathology that participates causally in schizophrenia.12
Our understanding of the contributions of the cerebellum to neurological function has evolved from a traditional view that focused on motor coordination, to a modern understanding that implicates the cerebellum in a broad range of high-level cognitive and attentional processes.3,13 Correspondingly, our understanding of cerebellar involvement in schizophrenia has also extended from abnormal motor and sensory processing to a broader contribution to motor as well as high-level cognitive deficits.14–20 Notably, dysfunctional connectivity of cerebellum has been recognized as a key feature of the psychopathology of schizophrenia.11,12,21 Previous connectivity analyses mainly took the form of regions of interest (ROIs) analyses to reveal alterations in the cerebellar motor-sensory and high-level cognitive circuits.22–29 However, this focus has so far precluded an integrative view on intrinsic cerebellar functional network anomalies in schizophrenia. Gradient-based analyses can provide a more integrated vision by capturing continuous spatial patterns of connectivity beyond segregated networks in the human brain.30–34 Therefore, this gradient-based approach can capture additional key characteristics of how these cerebellar functional networks (eg, motor-sensory and high-level cognitive control networks) assemble together and their relationship with cerebral cortical networks interact in schizophrenia, namely as topographical or hierarchical processing gradients.34
In contrast to the common practice of partitioning brain regions into discrete communities with sharp boundaries,35,36 gradient-based approach, which is a nonlinear decomposition of high-dimensional resting-state functional connectivity (rsFC), can identify brain functional hierarchies by representing brain connectivity in a continuous, low-dimensional space. This data-driven analysis results in connectivity gradients that provide a description of the connectome where each voxel is located along a gradient according to its connectivity pattern. Voxels with similar connectivity patterns are located close to one another along a given connectivity gradient.34 This gradient-based decomposition method thus provides a novel framework for describing different functional modules in a continuous manner, reflecting the hierarchical organization of brain function.33,34,37,38 Furthermore, evidence has indicated that there is reduced functional connectivity (FC) differentiation between neighboring areas (eg, anterior and posterior insula) in schizophrenia,39 which highlights the need to adopt continuous approaches to characterize cerebellar FC in schizophrenia. A recent study using gradient-based analyses revealed a continuous gradient of rsFC within cerebral cortex from primary to transmodal processing areas.33 The principal gradient of cerebellar functional organization followed a similar graded organization from sensorimotor to cognitive processing,3,32 ie, a sensorimotor-supramodal hierarchical organization. However, whether abnormalities exist in the principal functional gradient of the cerebellum in schizophrenia remains unknown. To address this knowledge gap, we characterized the central axis of macroscale functional topography in the cerebellum with the objective to establish a neurobiological framework that relates to the co-occurrence and interaction of low-level sensorimotor and high-level cognitive functional abnormalities of cerebellar circuits in schizophrenia.
Taken together, this study aims to investigate alterations in the principal gradient of cerebellar FC in schizophrenia using recently developed gradient-based analyses of rsFC. Given the importance of cerebellar-cortical circuits to understand the pathogenesis of schizophrenia,11,12 and the fact that a dense and reciprocal network of connections are formed between the cerebellar cortex and the cerebral cortex,3,13,40 we analyzed intra-cerebellar FC gradients, as well as cerebellar-cerebral and cerebral-cerebellar FC gradients. Based on previous seed-based rsFC analyses showing abnormal connectivity within and between motor and cognitive networks in schizophrenia,22–29 we hypothesized that schizophrenia patients would show altered sensorimotor-supramodal cognition gradients in intra-cerebellar and cerebello-cerebral cortical circuits relative to healthy controls. Finally, exploratory analyses examined network-based FC, as well as possible correlations between gradient values and clinical variables.
Methods
Participants
The present study used the same dataset from our previous study.41 Patients were diagnosed with schizophrenia according to the structured clinical interview for DSM-IV Axis I disorders—clinical version (SCID-I-CV). Ninety-six schizophrenia patients and 120 healthy controls were included in the final analysis. See supplementary methods for details about participant recruitment. All patients received treatment with antipsychotics. The average illness duration was 15 years. The average severity of symptoms was 13.44 for positive symptoms, 20.73 for negative symptoms, 28.22 for general psychopathology symptoms based on assessment of Positive and Negative Syndrome Scale. Written informed consent was obtained from all healthy participants and from the patients or guardian of patients. This study was approved by the Ethics Committee of the Clinical Hospital of Chengdu Brain Science Institute in accordance with the Declaration of 1975, as revised in 1983. Demographic and clinical information of all participants is shown in table 1. The two groups did not show statistically significant differences in age, sex, and education (table 1)
Table 1.
Demographic Characteristics of Schizophrenia Patients and Controls
| Patients (N = 96) | Healthy Controls (N=120) | ||||
|---|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD | P Value |
| Age (y) | 39.78 | 11.48 | 38.12 | 14.51 | .36 |
| Sex (Male: Female) | 66: 30 | 81: 39 | .84a | ||
| Handedness (Right: Left) | 93: 3 | 119: 1 | .33a | ||
| Education (y)b | 11.64 | 2.94 | 11.07 | 3.22 | .22 |
| Chlorpromazine equivalents (mg/d)c | 332.95 | 165.06 | |||
| Duration of illness (y)d | 15.10 | 10.33 | - | - | |
| PANSS-positivee | 13.44 | 5.88 | - | - | |
| PANSS-negativee | 20.73 | 6.00 | - | - | |
| PANSS-generale | 28.22 | 5.81 | - | - | |
| PANSS-totale | 62.39 | 13.11 | - | - | |
| FD | 0.049 | 0.038 | 0.046 | 0.027 | .42 |
Note: FD, Framewise Displacements; PANSS, Positive and Negative Syndrome Scale.
a x 2 test.
bData of 76 patients and 111 controls available.
cData of 72 patients available.
dData of 88 patients available.
eData of 64 patients available.
Data Acquisition and Image Preprocessing
Structural and resting-state functional MRI data were acquired on a 3-T GE Discovery MR 750 scanner at the MRI Center of University of Electronic Science and Technology of China. See supplementary methods for details.
All preprocessing steps were consistent with our previous study,41 see figure 1 and supplementary methods for details. Importantly, we found that the mean frame-to-frame motion quantified framewise displacements (FD) was not associated with altered gradient scores (all P values in this analysis were >.1), indicating that gradient group differences are unlikely to be driven by head motion.
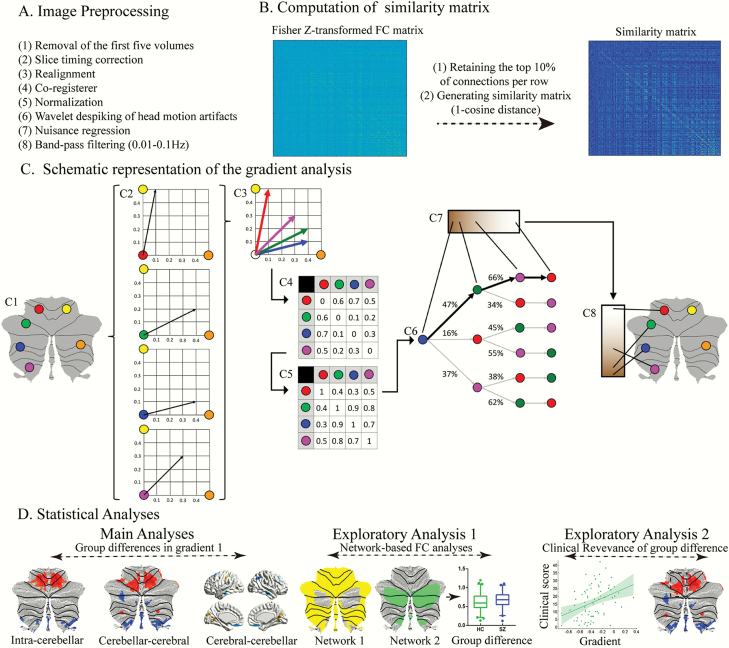
Fig. 1.
Summary of workflow. (A) The steps for image preprocessing. (B and C) Summary of the steps for gradient analysis. Figure 1C adapted from Guell et al32. This example (C1) presents a schematic representation of gradient analysis. It illustrates the calculation of the principal functional gradient of 4 cerebellar voxels (red, green, blue, magenta) based on their functional connectivity with 2 target cerebellar voxels (yellow, orange). (C2) Connectivity from each cerebellar voxel (red, green, blue, magenta) to the 2 target cerebellar voxels (yellow, orange) is represented as a 2-dimensional vector. (C3) All vectors can be represented in the same 2-dimensional space. (C4) Cosine distance between each pair of vectors is calculated, and (C5) an affinity matrix is constructed as (1-cosine distance) for each pair of vectors. This affinity matrix represents the similarity of the connectivity patterns of each pair of voxels. (C6) A Markov chain is constructed using information from the affinity matrix. Information from the affinity matrix is thus used to represent the probability of transition between each pair of vectors. In this way, there will be higher transition probability between pairs of voxels with similar connectivity patterns. This probability of transition between each pair of vectors can be analyzed as a symmetric transformation matrix, thus allowing the calculation of eigenvectors. (C7) Eigenvectors derived from this transformation matrix represent the principal orthogonal directions of transition between all pairs of voxels. Here we illustrate the first resulting component of this analysis – the principal functional gradient of our 4 cerebellar voxels (red, green, blue, magenta) based on their connectivity with our 2 target cerebellar voxels (yellow, orange) progresses from the blue, to the green, to the magenta, to the red voxel. (C8) This order is mapped back into our cerebellum map, allowing us to generate functional neuroanatomical descriptions. Of note, cerebellar functional gradients were calculated using functional connectivity values of each cerebellar voxel with the rest of cerebellar voxels, or each cerebellar voxel with each cerebral cortical voxel, or each cerebral cortical voxel with each cerebellar voxel (rather than between 4 voxels and only 2 target cerebellar voxels, as in this example). Vectors in our analysis thus possessed many more than just two dimensions, but cosine distance can also be calculated between pairs of high-dimensional vectors. (D) Statistical analyses.
Connectivity Gradient Analyses and Group Contrast
We used diffusion map embedding42 to identify a low-dimensional embedding from a high-dimensional connectivity matrix. This methodological strategy has been able to successfully identify relevant aspects of functional organization in cerebral cortex and cerebellum in previous studies.32,33 The result of diffusion embedding is not one single mosaic of discrete networks, but multiple, continuous maps (gradients), which capture the similarity of each voxel’s functional connections along a continuous space. We analyzed intra-cerebellar FC gradients, as well as cerebellar-cerebral and cerebral-cerebellar FC gradients. See figure 1, and supplementary methods for details. Gradient values represent information about the spatial pattern in the embedding space—shifts in value are not meaningful in terms of “higher” or “lower” scores, but rather reflect changes in relative similarity within a latent dimension, ie, the similarity of functional connectivity patterns along each dimension (“gradient”). The gradient values are a scalar, and for this reason significant gradient value alterations in schizophrenia reflect the extent to which the patient group deviates from the HC group.
To compare the SZ and HC groups, we used an average connectivity matrix calculated from all patients and controls to produce a group-level gradient component template. We then performed Procrustes rotation to align gradients of each participant to this template, as in previous analyses.43,44 To maximize reliability, reproducibility, and interpretability, we only used the first gradient component in our analyses. The first gradient (or principal gradient) explains as much of the variance in the data as possible (supplementary figure S1). It represents a well-understood sensorimotor-to-supramodal organizational principle in the cerebellar and cerebro-cerebral connections.32 It has been shown to be reproducible at the single-subject level in the cerebellum (Guell et al32; note that gradient 2 could not be reproduced as successfully as the principal gradient at the single-subject level). The explained variance of principle gradient (29%) was similar to recent reports using diffusion map embedding analyses in functional connectivity.32,33,38,44
To determine gradient score differences between SZ and HC, 2-sample t-tests were performed on individual Z-normalized gradient maps, with age, sex, handedness and mean FD regressed out. Among these 3 main analyses, results are reported at a voxel-based threshold corrected for false discovery rate of FDR P < .05/3. A threshold of 0.05/3 rather than 0.05 was used to account for the fact that group comparisons were calculated for 3 different gradients. We also imposed a minimum cluster extent of 10 voxels (only for the purposes of clear data visualization).
A supplementary exploratory analysis tested group differences using the second gradient component in our analysis. Further analyses based on the second gradient were outside of the scope and beyond the statistical power of the present study (these analyses would require correction for multiple comparisons), but a supplementary exploratory analysis using the second gradient was performed to evaluate for the possibility of additional differences between patients diagnosed with schizophrenia and controls using alternative dimensions of cerebellar and cerebellar-cerebral cortical functional anatomy.
Gradient calculations as employed here in the single-group control and schizophrenia sample analyses are based on descriptive statistics. In the analyses calculating functional gradients for each individual group, no null hypothesis is tested, similar to the case of describing a dataset by calculating mean and standard deviation values. In contrast, null hypothesis testing and the related concepts of type I and type II error apply to the group contrasts (controls vs schizophrenia)—in our study, a voxel-based threshold of FDR P < .05/3 was used for each of the 3 contrasts analyzed.
Correlations Between Altered Gradient Scores and Clinical Variables
In this exploratory analysis, to examine the relationship between altered gradient scores and clinical features, we calculated partial correlations between gradient scores and multiple clinical measures (PANSS positive, negative, general psychopathology subscales and overall scores; illness duration) in the patients’ group. Both disease duration and medications (measured by chlorpromazine equivalents) were included as regressors of no interest in correlation analyses. We corrected for multiple comparisons using an FDR P < .05 threshold. To examine confounding effects of medication, we calculated Pearson correlations between gradient scores and chlorpromazine equivalents.
Network-Based FC Analyses
Based on the observation of compression between the sensorimotor and supramodal poles of gradients (below), it is possible that increased functional connectivity, and more specifically, increased functional connectivity between sensorimotor and cognitive networks, may underlie the compressed spatial gradient pattern. In an exploratory analysis, to test this hypothesis, we first used K-mean clustering analyses45 and 1-sample t-test to obtain a parcellation (ROIs) of the cerebellar and cerebral cortex. See supplementary methods and supplementary figure S2 for details. We then computed Fisher Z-transformed Pearsons correlation between the mean BOLD time courses of these networks as a measure of FC. We obtained the following 11 measures of FC: 3 for ROIs based on intra-cerebellar functional gradient (FC within sensorimotor network, FC within cognition network, and FC between sensorimotor - cognition network); 4 for ROIs based on cerebellar-cortical functional gradient (FC between cerebellar sensorimotor - cerebral sensorimotor, cerebellar cognition - cerebral cognition, cerebellar sensorimotor - cerebral cognition, and cerebellar cognition - cerebral sensorimotor networks); and 4 for ROIs based on cortical-cerebellar functional gradient (FC between cerebral sensorimotor - cerebellar sensorimotor, cerebral cognition - cerebellar cognition, cerebral sensorimotor - cerebellar cognition, and cerebral cognition - cerebellar sensorimotor networks). We corrected for multiple comparisons using an FDR P < .05 threshold.
Code Availability
All code used in the present study and main result files have been released in Github (https://github.com/DeboDong/cerebellum_gradients_schizophrenia).
Results
Intra-Cerebellar Functional Gradient in Schizophrenia
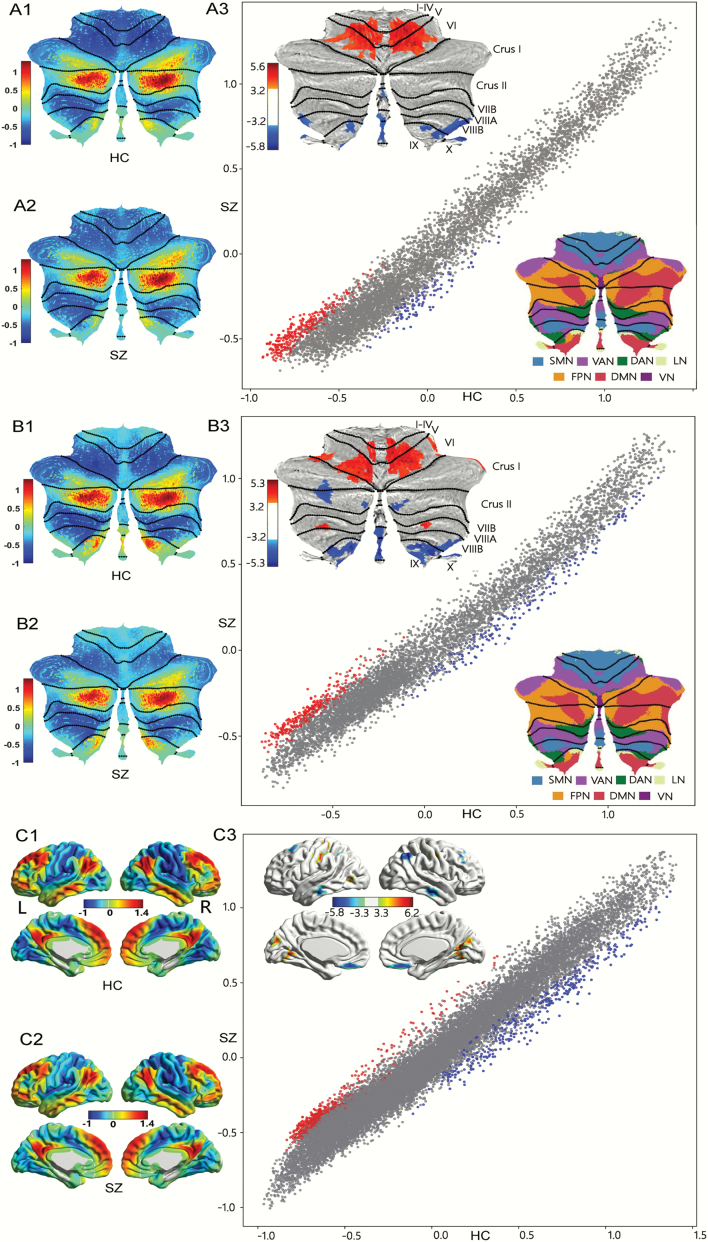
The principal gradient of intra-cerebellar FC showed a similar spatial distribution in SZ and HC (figure 2A1 and 2 A2), which was similar to previous reports in healthy humans.32 See supplementary methods for details. As shown in supplementary table S1 and figure 2A3 increased gradient values were found in bilateral cerebellar lobules IV/V/VI, corresponding to sensorimotor network of cerebellum.40 Decreased gradient values were found in cerebellar default-mode network (DMN) and dorsal attention network (DAN), including bilateral lobules VIII/IX/X. Of note, cerebellar network allocations are based on Buckner et al.40 As indicated in the scatterplot of figure 2A3, higher gradient values for SZ were localized in the lowest pole of the principal gradient (ie, motor territories), and lower gradient values were localized in middle aspects of the principal gradient (corresponding to frontalparietal network (FPN))] as well as DMN non-motor regions).
Fig. 2.
Group patterns and differences in principal gradient. (A) Cerebellar principal functional gradient calculated based on intra-cerebellar functional connectivity. (A1) Gradient pattern in HC. (A2) Gradient pattern in SZ. (A3) Group differences between SZ and HC. Scatterplot represents cerebellar gradient of SZ (y axis) vs cerebellar gradient of HC (x axis). Scatterplot colors correspond to group differences map as shown in top-left corner of figure 2 (A3): higher gradient value in SZ (red), and lower gradient value in SZ (blue) compared to HC. (B) Cerebellar principal functional gradient calculated based on FC between cerebellum and cerebral cortex. (B1) Gradient pattern in HC. (B2) Gradient pattern in SZ. (B3) Group differences between SZ and HC. Scatterplot represents cerebellar gradient of SZ (y axis) vs cerebellar gradient of HC (x axis). Scatterplot colors correspond to group differences map as shown in top left corner of figure 2 (B3): higher gradient value in SZ (red), and lower gradient value in SZ (blue) compared to HC. (C) Cerebral principal functional gradient calculated based on FC between the cerebral cortex and cerebellum. (C1) Gradient pattern in HC. (C2) Gradient pattern in SZ. (C3) Group differences between SZ and HC. Scatterplot represents cerebellar gradient of SZ (y axis) vs cerebral gradient of HC (x axis). Scatterplot colors correspond to group differences map as shown in top left corner of figure 2 (C3): higher gradient value in SZ (red), and lower gradient value in SZ (blue) compared to HC. All results for each gradient are shown after FDR correction (P < .05/3). Cerebellar representations of cerebral cortical resting-state networks40 are shown in bottom right corner of figure 2 (A3 and B3) based on Buckner et al.40
Cerebellar-Cerebral Cortex Functional Gradient in Schizophrenia
Functional gradients calculated using FC from the cerebellum to the cerebral cortex revealed a remarkably similar spatial distribution from sensorimotor network to DMN regions of the cerebellum when compared to gradients calculated from intra-cerebellar connectivity across patient and controls group (figure 2B1 and 2 B2). Two-sample t-test indicated that patients with schizophrenia exhibited significantly increased gradient values in bilateral cerebellum lobules IV/V/VI/VII, which are widely distributed across sensorimotor network and ventral attention network of cerebellum; and decreased gradient values in bilateral cerebellum lobules IX, left Crus I/II, and right VIII, which are mainly located in DMN, FPN, and DAN40 (supplementary table S1 and figure 2B3). Accordingly, as shown in the scatterplot of figure 2B3, functional gradient abnormalities extended across the whole principal gradient spectrum. More specifically, significantly higher gradient values in the SZ group were localized in the lowest pole of the principal gradient (which corresponds to sensorimotor network), and significantly lower values in the SZ group extended from the middle aspects to the highest poles of the principal gradient (FPN and DMN, respectively).
Cerebral Cortical-Cerebellar Functional Gradient in Schizophrenia
When using FC from the cerebral cortex to the cerebellum, the principal functional gradient demonstrated a similar sensorimotor-to-supramodal gradient of cortical organization in HC and SZ (figure 2C1 and 2 C2). As shown in supplementary table S1 and figure 2C3, schizophrenia patients showed increased gradient values in regions of sensorimotor network and visual network, such as bilateral post/precentral gyrus, middle occipital gyrus and lingual gyrus; and decreased gradient values in some supramodal regions belonging to DMN and FPN, ie, bilateral medial frontal gyrus, middle frontal gyrus, angular gyrus, and middle temporal gyrus. Similarly, functional gradient abnormalities, in this case, extended across the whole principal gradient spectrum. More specifically, significantly higher gradient values in the SZ group were localized in the lowest pole of the principal gradient (which corresponds to primary motor and visual processing areas), and significantly lower values in the SZ group extended from the medium aspects to the highest pole of the principal gradient (FPN and DMN, respectively).
Correlations Between Altered Gradient Scores and Clinical Variables
Severity of negative symptoms was related to decreased cerebral cortical-cerebellar functional gradient values in right middle temporal gyrus and medial frontal cortex (supplementary figure S3a). There was a positive correlation between cerebellar-cortical functional gradient scores in lobule VI and illness duration (left of supplementary figure S3b), and a negative correlation between cortical-cerebellar functional gradient values in left MTG and illness duration (right of supplementary figure S3b). Given the multicollinearity between age and illness duration, we regressed out the age effect and found that these 3 correlations remained significant (P < .05). In addition, chlorpromazine equivalents were not associated with altered gradient scores (all P > .1).
Compressed Gradient Pattern and its Underlying Altered FC Architecture
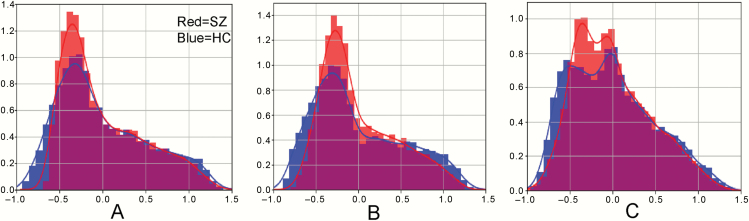
To better characterize the altered distribution of sensorimotor-supramodal hierarchical gradient, global histogram analyses were performed. This analysis revealed that there is a prominent compression of the lowest portion of the principal functional gradient, and a less prominent compression of the highest portion of the principal functional gradient, across intra-cerebellar, cerebellar-cerebral cortical, and cerebral cortical-cerebellar functional gradients (figure 3). To quantitatively demonstrate the compression of intra-cerebellar, cerebellar-cerebral cortical, and cerebral cortical-cerebellar functional gradients, we tested whether there was a linear correlation between X and (Y-absolute(X)) per corresponding voxel (X = SZ group gradient values, ie, red histogram of figure 3, Y = HC group gradient values, ie, blue histogram of figure 3). We found there was a significant correlation between X and (Y-absolute(X)) (r(intra-cerebellar) = .82, r(cerebellar-cerebral cortical) = .84, r(cerebral cortical-cerebellar) = .82, P < .001), which supports and quantifies the gradient value compression in the SZ group compared to the HC group.
Fig. 3.
Compressed gradient pattern in SZ shown in density histograms. We observed a prominent compression of the lowest portion of the principal gradient, and a less prominent compression of the highest portion of the principal gradient across intra-cerebellar functional gradient (A), cerebellar-cerebral cortical gradient (B), and cerebral cortical-cerebellar gradient (C).
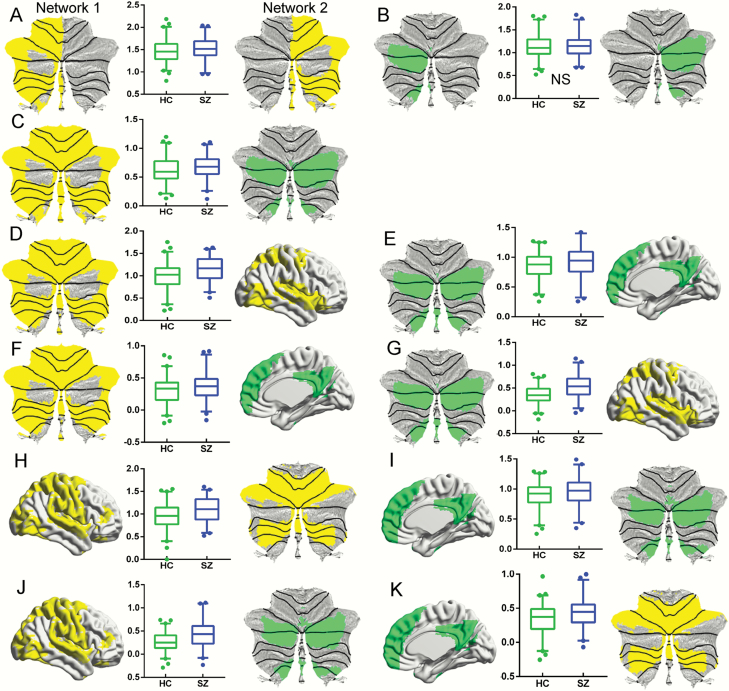
To test whether increased between- or within-network connectivity underlaid the observed compression of functional gradients, we analyzed FC using ROI-based analyses (see the Methods section). Consistent with our hypotheses, for intra-cerebellar FC, schizophrenia patients showed increased FC within cerebellar sensorimotor network. We also found increased FC between cerebellar sensorimotor and cognition network. However, we did not observe any significant changes within cerebellar cognition network (supplementary table S2 and figure 4A–C). When analyzing cerebellar-cerebral functional connectivity, we found increased FC between cerebellar sensorimotor - cerebral sensorimotor, cerebellar cognition - cerebral cognition, cerebellar sensorimotor - cerebral cognition, and cerebellar cognition - cerebral sensorimotor networks in schizophrenia relative to HC (supplementary table S2 and figure 4D–G). Likewise, when analyzing cerebral-cerebellar FC (supplementary table S2 and figure 4H–K), patients showed increased FC between cerebral sensorimotor - cerebellar sensorimotor, cerebral cognition - cerebellar cognition, cerebral sensorimotor - cerebellar cognition, and cerebral cognition - cerebellar sensorimotor networks.
Fig. 4.
Group differences in FC within or between network(s). (A) Increased FC within sensorimotor network (border 1) of intra-cerebellar functional gradient in SZ, measured by FC between the Right and Left sections. (B) Nonsignificant (NS) FC alteration within cognition network (border 2) of intra-cerebellar functional gradient in SZ, measured by FC between the Right and Left sections. (C) Increased FC between sensorimotor (border 1) - cognition network (border 2) of intra-cerebellar functional gradient in SZ. (D) Increased FC between cerebellar sensorimotor (border 1 of cerebellar-cerebral cortical gradient, left panel) - cerebral sensorimotor networks (right panel) in SZ. (E) Increased FC between cerebellar cognition (border 2 of cerebellar-cerebral cortical gradient, left panel) - cerebral cognition networks (right panel) in SZ. (F) Increased FC between cerebellar sensorimotor (border 1 of cerebellar-cerebral cortical gradient, left panel) - cerebral cognition networks (right panel) in SZ. (G) Increased FC between cerebellar cognition (border 2 cerebellar-cerebral cortical gradient, left panel) - cerebral sensorimotor networks (right panel) in SZ. (H) Increased FC between cerebral sensorimotor (border 1 of cerebral cortical-cerebellar gradient, left panel) - cerebellar sensorimotor networks (right panel) in SZ. (I) Increased FC between cerebral cognition (border 2 of cerebral cortical-cerebellar gradient, left panel) - cerebellar cognition networks (right panel) in SZ. (J) Increased FC between cerebral sensorimotor network (border 1 of cerebral cortical-cerebellar gradient, left panel) - cerebellar cognition networks (right panel) in SZ. (K) Increased FC between cerebral cognition network (border 2 of cerebral cortical-cerebellar gradient, left panel) - cerebellar sensorimotor networks (right panel) in SZ. Network shown on the right panel was calculated using a threshold T = 23 in a 1-sample t-test of all subjects’ FC maps calculated from the corresponding network shown in the left panel. Dots in boxplots reflect values below 2.5% or above 97.5%. All results are shown after FDR corrected (P < .05); note that results in (b) are not significant.
Discussion
We identified a compression of the principal sensorimotor-to-supramodal gradient of cerebellar FC in schizophrenia. A prominent compression of the sensorimotor portion of the gradient and a less prominent compression of the FPN and DMN portion of the gradient were consistently observed in intra-cerebellar, cerebellar-cerebral, and cerebral-cerebellar rsFC. Hyper-connectivity between and within sensorimotor network and cognitive network could be a potential driving mechanism underlying this compression of functional gradients. We thus report a neurobiological framework that might improve our understanding of the co-occurrence and interaction of sensorimotor and supramodal cognitive functional abnormalities of cerebellar and cerebellar-cerebral cortical circuits in schizophrenia. These findings indicate that there might be a cascade of impairments stemming from disrupted low-level sensorimotor systems that may, in part, account for high-level cognitive functions in schizophrenia. This framework encourages in this way a more sensory and motor oriented focus to investigate the pathophysiology of this disorder.
Prominent Compression of the Sensorimotor Portion of the Cerebellar Gradient
Abnormalities of cerebellar motor-sensory and high-level cognitive circuits in schizophrenia have been increasingly recognized.7,14–20 No previous study has examined cerebellar functional gradients in psychosis. Here, we provided novel evidence to show an alteration of the principal axis of macroscale functional organization of the cerebellum, as indexed by gradient-based FC analyses. These abnormalities in intra-cerebellar and cerebellar-cerebral cortical interactions were located most prominently at the sensorimotor network of cerebellum (lobules IV/V/VI), and also in the cerebellar DMN and FPN networks (posterior Crus I/II and IX). Most of these locations were in line with previous meta-analyses of morphometry,7,19 task activation,18 and resting-state brain activity.19 Functional gradients based on FC between cerebellar and cerebral cortex revealed similar alterations, but with a wider spectrum of abnormality in supramodal cognition regions, ie, cerebellar crus I/II and lobules IX. This is coherent with the understanding of schizophrenia as a brain-wide disorder that includes cerebellum but that also affects many other brain structures.11,46 Perhaps as a consequence, more abnormalities were detected when including not only cerebellar cortex but also cerebral cortex in our analyses.
For the first time, we constructed the principal gradient of functional organization of the cerebral cortex using cerebral cortical-cerebellar FC. The results of this analysis were similar to a previous study analyzing intra-cerebral cortical (rather than cerebral cortical-cerebellar) FC.33 In that study and also in our analysis, the principal functional gradient in the cerebral cortex extended from primary cortices (visual, somatosensory/motor, and auditory) to regions corresponding to the DMN. Adding into a recent study,32 this observation supports that the cerebellum and cerebral cortex share a similar macroscale principle of organization, namely, that both structures share a hierarchical organization that gradually progresses from unimodal to supramodal streams of information processing. It is thus not surprising that we also observed group differences in both ends of the cerebral cortical - cerebellar functional gradient, ie, regions in the sensorimotor and visual network, as well as DMN and FPN. The“cognitive dysmetria” theory has posited that disruption to the cerebello-cortical circuit leads to difficulties of schizophrenia in synchronizing and integrating neural computations and processing in order to generate orderly and meaningful motor and cognitive processes.1 In this circuit, the cerebellar node plays a primary coordinative role.14 Increasing clinical and experimental evidence indicates that the cerebellum regulates not only movement but also a wide range of cognitive functions, and that these contributions are based on anatomical connections that link the cerebellum to cerebral areas relevant for a broad spectrum of neural function.3,47 Recent evidence shows that cerebellar interactions with cerebral cortical areas involved in cognitive functions involve temporal coordination of neuronal oscillations, providing possible mechanistic explanations that may subserve cerebellar modulation of cerebral activity.48,49 Of note, a recent study found that patients with schizophrenia have diminished temporal coordination of cerebral motor activity by cerebellum during the continuation tapping portion of sensorimotor synchronization.50 Our findings further contribute to this expanding body of evidence showing aberrant interactions between cerebellum and cerebrum in schizophrenia.
Closer inspection of altered gradient distribution demonstrated a similar compressed pattern across cerebellar and cerebral cortex gradients. We found prominent compression at the lowest portion of the gradient (which corresponds to sensorimotor network in cerebellar cortex, and sensorimotor as well as visual in cerebral cortex), and a less prominent compression at the middle or highest portion of the gradient (which corresponds to task-positive or default-mode networks, respectively, in both cerebellar and cerebral cortex). This compressed architecture may suggest the disruption of the inherent separation between sensorimotor systems involved in immediate environment and supramodal cognitive systems that support complex cognitive inferences in schizophrenia.51 The human brain is organized by hierarchical modularity, and such a functional organization facilitates segregated processing of specialized function domains, while also enabling a dynamic configuration and cross-communication of networks for more complex and integrated mental activity.52 Cerebellar and cerebro-cerebellar circuits supporting sensorimotor functions are segregated from those mediating cognitive functions.53,54 Increased within-network and increased between-network connectivity in SZ may both explain a compressed principle gradient. Increased between-network connectivity means that each network is less different than the other networks, and therefore the range of principle gradient is decreased (principle gradient is compressed). Increased within-network connectivity means that there is less variability within each network, and therefore the range of principle gradient is decreased (principle gradient is compressed). A recent structural study reported abnormalities in the modular architecture of cerebellum in schizophrenia.55 Combined with this evidence, our finding of a compressed cerebellar and cerebello-cerebral cortical functional gradient suggest that a less differentiated global hierarchy is a relevant feature of macroscale functional organization as indexed by resting-state fMRI in schizophrenia. These findings provide converging evidence for abnormal hierarchy organization as a system-level substrate of schizophrenia and highlight the relevance of the cerebellum in this abnormal macroscale organization.
The observation of the prominent compression of the sensorimotor portion across 3 kinds of gradients in the present study is in contrast with recent studies linking psychotic symptoms to reduced volume of cerebellar cognitive areas, such as cerebellar crus I/II.7–9 However, the present study characterized the architecture of information flow from sensorimotor portion to supramodal cognitive portion using the gradient-based rsFC analysis, not possible using morphometry-based analysis. Meanwhile, closer inspection of previous neuroimaging studies suggests that abnormalities in sensory and perceptual systems are often reported in psychosis,41,56,57 but not emphasized within prevailing psychiatric models.
Recent studies have found instability of information processing in sensorimotor and visual systems in schizophrenia, as evidenced by increased temporal dynamics.41,58 This increased temporal dynamic may make it possible for sensory information to erroneously leak into other systems, generating “information pollution” in schizophrenia. This possibility is supported by a recent study that showed sensorimotor and visual systems in schizophrenia over-actively participating in multiple brain dynamic states59 - there was dysconnectivity of sensorimotor and visual systems with other systems across multiple brain dynamic states, while dysconnectivity of high-order networks (DMN and FPN) occurred only in a few states. These findings could explain why the most prominent compression was observed here in the lowest pole of functional gradients (sensorimotor network in cerebellar cortex, and sensorimotor and visual network in cerebral cortex). The marked compression of this functional gradient in sensorimotor network territories may reflect an impaired ability to decode information from sensorimotor systems, which might generate a cascade of impairments stemming from disrupted lower-level sensory and motor systems that in part accounts for high-order cognitive dysfunction.57,60 Of note, the term high-order cognitive functions used here refers to all aspects of cognitive/associative processing (ie, temporal processing, learning, memory, executive function, decision making), as opposed to primary processing functions such as primary motor or sensory processing.
Functional gradient differences presented in figure 2 could represent either pathological abnormalities or a compensatory reorganization changes in patients diagnosed with schizophrenia compared to controls. The results presented in supplementary figure S3 support the former possibility (pathological changes rather than compensatory reorganization changes), because pathological changes are expected to be more prominent in individuals with higher symptom scores, and to become more predominant at later compared to earlier stages of disease. This conclusion is supported by the fact that the directions of differences shown in figure 2 are accentuated in those patients with higher symptom scores or longer disease progression. More specifically, patients with schizophrenia showed lower functional gradient values in right MTG, MFG, and left MTG, and higher functional gradient values in left cerebellum VI (figure 2). In supplementary figure S3 we show that these differences are more accentuated in those patients with higher symptom scores or disease duration (ie, right MTG and MFG functional gradient values are lower in patients with more severe symptoms, left MTG values are lower in patients with higher disease duration, and left cerebellum VI values are higher in patients with more severe symptoms). However, this interpretation is not definitive as it is also reasonable to consider that compensatory reorganization changes may be more present in those subjects with the highest amount of clinical impairment or time of disease progression – a distinction between pathological or compensatory changes cannot be definitively established based on our correlational analyses.
A large body of evidence has shown that the cerebellum is relevant for a wide range of motor, cognitive, and affective functions.3 There are anatomical connections linking the cerebellum to not only motor but also transmodal association areas.61 Isolated cerebellar injury or degeneration is sufficient to generate not only motor but also cognitive and affective symptoms.4,62–64 Neuroimaging data shows cerebellar task and functional connectivity in numerous domains of motor, cognitive, and affective processing.65 An overarching theoretical framework that characterizes the nature of cerebellar cognition in health and disease includes the universal cerebellar transform and dysmetria of thought theories, that state that there is a uniform cytoarchitecture and thus a uniform computation underlying all cerebellar functions, and thus a uniform impairment (dysmetria) that underlies cerebellar dysfunction in the motor, cognitive, and affective spheres.2,66 Within this context of a large and expanding body of literature on cerebellar cognition, our study provides additional evidence supporting a link between cerebellar function and motor and non-motor processing, here in the particular case of abnormal cerebellar hierarchy organization in schizophrenia.
Hyper-Connectivity Between Sensorimotor Network and Cognitive Network
Our ROI-based FC analyses further hinted at a potential driving mechanism underlying this compression of functional gradients. Schizophrenia patients showed hyper-connectivity between the cerebellar sensorimotor – cerebellar cognition, cerebellar sensorimotor - cerebral cognition, and cerebellar cognition - cerebral sensorimotor networks. These results are consistent with a recent seed-based study investigating cerebellar-cerebral cortical FC,27 and resonate with the hypothesis that there is decreased modularity, or “dysmodularity,” 67 in schizophrenia.
The observed hyper-connectivity may also resonate with the concept of an excitation / inhibition (E/I) imbalance, as suggested by the NMDA receptor hypo-function model.68,69 A hypofunction of the NMDA receptor bearing on inhibitory GABAergic interneurons would lead to reduced GABAergic inhibitory tone of the cerebellum in schizophrenia,70 contributing to cognitive impairments in this disorder.71 The cell density of GABAergic Purkinje inhibitory neurons in cerebellum has been reported to be decreased in psychosis patients.72 As the principal output of the cerebellum is inhibitory,73 deficient cerebellar inhibitory activity in schizophrenia may result in aberrant cerebellar modulation of cerebral cortical information.74 A recent computational neuroscience study showed that an elevated E/I ratio could predict widespread elevated FC in the cerebral cortex in schizophrenia.75 Future computational model studies directly linking compressed macroscale anomalies in functional hierarchy of cerebellar and cerebellar-cerebral FC to imbalanced E/I microcircuit properties may enrich our understanding of cerebellar dysfunction in schizophrenia.
Another possible interpretation is that our results reflect a compensatory mechanism in schizophrenia.11 The cerebellum has been proposed to act as a general “forward controller” center in the brain,76 which helps refine sensory, motor, and cognitive information through error-based learning. The observed connectivity abnormalities may represent a compensatory mechanism that responds to excessive error input from the cerebral cortex, requiring schizophrenia patients to employ higher cognitive effort in error processing and adaptive prediction to coordinate their behaviors and thoughts to fit with contextual information.11
Implications for Identifying Neurobiological Abnormalities Across Psychiatric Disorders
Notably, the observed compression pattern of cerebellar functional gradients in the present study is similar to the compressed functional gradient reported recently in cerebral cortex in autism spectrum disorder.44 This similarity resonates with an overlap in clinical presentation (eg, social dysfunction, sensory abnormalities77), genetics,78 and neurobiology79 between these 2 disorders. Recent trends in clinical neuroscience are identifying common neurobiological abnormalities spanning multiple psychiatric disorders.56,80 Because functional gradients analysis provides a very low-dimensional representation of resting-state connectivity to capture the fundamental functional connectome hierarchy, gradient-based analyses as used here might be optimal to detect potential common connectome abnormalities across psychiatric diseases. Exploring functional gradient abnormalities across psychiatric diseases in the future may improve our understanding of common neurobiological mechanisms underlying multiple brain disorders.
Limitations and Future Studies
A limitation of this study is the confounding effect of antipsychotic drugs. Of note, chlorpromazine equivalents were not associated with altered gradient scores. Nonetheless, assessing cerebellar gradient in a medication-naïve population of individuals with schizophrenia or unaffected first-degree relatives would be informative. Another limitation is the correlational nature of our experiment, which cannot allow us to establish evidence for causal relationships between fundamental gradients and the pathogenesis of schizophrenia, though we found some correlational evidence supporting a link between gradient scores and negative psychiatric symptoms (supplementary figure S3a). A recent neurostimulation study provided causal evidence supporting influence of cerebellar dysfunction in the pathogenesis of schizophrenia.12 Future neurostimulation studies might explore causal relationships between altered cerebellar gradients and behavior in neuropsychiatry. Third, although we showed relationships between illness duration and gradient values (supplementary figure S3b), we did not evaluate developmental trajectories. Because altered sensory and motor FC abnormalities have been observed in clinical-high risk, early-stage, and chronic schizophrenia,41,81,82 it is possible that the aberrant pattern of functional gradient compression is present at different stages of the illness, possibly ranging from pre-clinical to early and late stages of the disorder. Future longitudinal studies may evaluate the development of compressed gradient patterns of cerebellar and cerebral cortical-cerebellar circuits in schizophrenia across time. And, future studies may evaluate the possibility of additional differences between patients diagnosed with schizophrenia and controls using alternative dimensions of cerebellar and cerebellar-cerebral cortical functional anatomy. An exploratory analysis in our data showed group differences when using the second gradient component (supplementary figure S4). Further testing of this difference is outside of the scope and beyond the statistical power of the present study (these analyses would require correction for multiple comparisons), but the findings reported here hint at the possibility of cerebellar and cerebellar - cerebral cortical functional gradient abnormalities in schizophrenia beyond the principal gradient of functional connectivity in these structures.
Conclusion
The present study reports a neurobiological framework that informs our understanding of the co-occurrence and interaction of low- and high-level functional abnormalities of cerebellar and cerebellar-cerebral cortical circuits in schizophrenia. We show a compression of the principal axis of cerebellar macroscale organization as indexed by gradient-based analyses of intra-cerebellar, cerebellar-cerebral cortical, and cerebral cortical-cerebellar FC. This compressed pattern was most prominent within somatomotor network, indicating that there may be cascade impairments stemming from disrupted low-level sensorimotor systems that may, in part, account for high-level cognitive functions in schizophrenia. In this way, our results encourage future research to expand beyond traditional cognitive-focused models of schizophrenia and to include sensory and motor domains to investigate low- and high-level dynamic interactions in the pathophysiology of this disorder.
Supplementary Material
Acknowledgments
The authors declare no conflicts of interest. We are grateful to all the participants in this study. Our thanks also go to Dr. Xi Chen (Civil Aviation Flight University of China) and Mr. Xin Chang (University of Electronic Science and Technology of China) for their help to collect the dataset.
Funding
This work was partly supported by the grant from the National Key R&D Program of China (No. 2018YFA0701400), grants from the National Nature Science Foundation of China (No. 61933003, 81771822, 81861128001, and 81771925), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2019-I2M-5-039) and the Project of Science and Technology Department of Sichuan Province (No. 2019YJ0179).
References
- 1. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 2. Guell X, Gabrieli JDE, Schmahmann JD. Embodied cognition and the cerebellum: perspectives from the Dysmetria of Thought and the Universal Cerebellar Transform theories. Cortex. 2018;100:140–148. [DOI] [PubMed] [Google Scholar]
- 3. Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337–364. [DOI] [PubMed] [Google Scholar]
- 4. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 (Pt 4):561–579. [DOI] [PubMed] [Google Scholar]
- 5. Jiang Y, Duan M, Chen X, et al. . Aberrant Prefrontal-Thalamic-Cerebellar circuit in schizophrenia and depression: evidence from a possible causal connectivity. Int J Neural Syst. 2019;29(5):1850032. [DOI] [PubMed] [Google Scholar]
- 6. Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, Gallo V. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci. 2019;20(5):298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moberget T, Doan NT, Alnæs D, et al. ; KaSP Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 2018;23(6):1512–1520. [DOI] [PubMed] [Google Scholar]
- 8. Moberget T, Alnæs D, Kaufmann T, et al. . Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol Psychiatry. 2019; 86(1):65–75. [DOI] [PubMed] [Google Scholar]
- 9. Romer AL, Knodt AR, Houts R, et al. . Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry. 2018;23(4):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Y, Luo C, Li X, et al. . Progressive reduction in gray matter in patients with schizophrenia assessed with mr imaging by using causal network analysis. Radiology. 2018;287(2):729. [DOI] [PubMed] [Google Scholar]
- 11. Cao H, Chén OY, Chung Y, et al. . Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brady RO Jr, Gonsalvez I, Lee I, et al. . Cerebellar-Prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176(7):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–522. [DOI] [PubMed] [Google Scholar]
- 14. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34(1):155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. Cerebellum. 2008;7(4):611–615. [DOI] [PubMed] [Google Scholar]
- 17. Hoppenbrouwers SS, Schutter DJ, Fitzgerald PB, Chen R, Daskalakis ZJ. The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: a review. Brain Res Rev. 2008;59(1):185–200. [DOI] [PubMed] [Google Scholar]
- 18. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3(4):545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding Y, Ou Y, Pan P, et al. . Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging. 2019;283:24–33. [DOI] [PubMed] [Google Scholar]
- 20. He H, Luo C, Luo Y, et al. . Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Hum Brain Mapp. 2019;40(2):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao H, Cannon TD. Cerebellar dysfunction and schizophrenia: from “Cognitive Dysmetria” to a potential therapeutic target. Am J Psychiatry. 2019;176(7):498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen YL, Tu PC, Lee YC, Chen YS, Li CT, Su TP. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res. 2013;149(1–3):26–34. [DOI] [PubMed] [Google Scholar]
- 23. Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao J, Tang X, Wang C, et al. . Aberrant cerebellar neural activity and cerebro-cerebellar functional connectivity involving executive dysfunction in schizophrenia with primary negative symptoms. Brain Imaging Behav. 2019. [DOI] [PubMed] [Google Scholar]
- 25. Guo W, Zhang F, Liu F, et al. . Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res Neuroimaging. 2018;276:73–79. [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34(6):1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shinn AK, Baker JT, Lewandowski KE, Öngür D, Cohen BM. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci. 2015;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L, Zou F, Shao Y, et al. . Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr Res. 2014;160(1–3):67–72. [DOI] [PubMed] [Google Scholar]
- 29. Zhuo C, Wang C, Wang L, et al. . Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging Behav. 2018;12(2):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haak KV, Marquand AF, Beckmann CF. Connectopic mapping with resting-state fMRI. Neuroimage. 2018;170: 83–94. [DOI] [PubMed] [Google Scholar]
- 31. Atasoy S, Donnelly I, Pearson J. Human brain networks function in connectome-specific harmonic waves. Nat Commun. 2016;7:10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guell X, Schmahmann JD, Gabrieli J D E, Ghosh SS. Functional gradients of the cerebellum. Elife. 2018;7:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Margulies DS, Ghosh SS, Goulas A, et al. . Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. 2016;113(44):12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huntenburg JM, Bazin PL, Margulies DS. Large-Scale gradients in human cortical organization. Trends Cogn Sci. 2018;22(1):21–31. [DOI] [PubMed] [Google Scholar]
- 35. Yeo BT, Krienen FM, Sepulcre J, et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Damoiseaux JS, Rombouts SA, Barkhof F, et al. . Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mesulam M. The evolving landscape of human cortical connectivity: facts and inferences. Neuroimage. 2012;62(4):2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bayrak Ş, Khalil AA, Villringer K, Fiebach JB, Margulies DS, Ovadia-Caro S. The impact of ischemic stroke on connectivity gradients. NeuroImage Clin. 2018;24:101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tian Y, Zalesky A, Bousman C, Everall I, Pantelis C. Insula functional connectivity in schizophrenia: subregions, gradients, and symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(4):399–408. [DOI] [PubMed] [Google Scholar]
- 40. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong D, Duan M, Wang Y, et al. . Reconfiguration of dynamic functional connectivity in sensory and perceptual system in schizophrenia. Cereb Cortex. 2018;29(8):3577–3589. [DOI] [PubMed] [Google Scholar]
- 42. Coifman RR, Lafon S, Lee AB, et al. . Geometric diffusions as a tool for harmonic analysis and structure definition of data: multiscale methods. Proc Natl Acad Sci U S A. 2005;102(21):7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Langs G, Golland P, Ghosh SS. Predicting activation across individuals with resting-state functional connectivity based multi-atlas label fusion. Med Image Comput Comput Assist Interv. 2015;9350:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong SJ, Vos de Wael R, Bethlehem RAI, et al. . Atypical functional connectome hierarchy in autism. Nat Commun. 2019;10(1):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pedregosa F, Varoquaux G, Gramfort A, et al. . Scikit-learn: machine Learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 46. Heuvel MP, van den, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24(1):32–48. [DOI] [PubMed] [Google Scholar]
- 47. Deverett B, Koay SA, Oostland M, Wang SSH. Cerebellar involvement in an evidence-accumulation decision-making task. Elife. 2018;7:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McAfee SS, Liu Y, Sillitoe RV, Heck DH. Cerebellar lobulus simplex and crus i differentially represent phase and phase difference of prefrontal cortical and hippocampal oscillations. Cell Rep. 2019;27(8):2328–2334.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parker KL, Kim YC, Kelley RM, et al. . Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry. 2017;22(5):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moussa-Tooks AB, Kim DJ, Bartolomeo LA, et al. . Impaired effective connectivity during a cerebellar-mediated sensorimotor synchronization task in schizophrenia. Schizophr Bull. 2019;45(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy C, Jefferies E, Rueschemeyer SA, et al. . Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage. 2018;171:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meunier D, Lambiotte R, Bullmore ET. Modular and hierarchically modular organization of brain networks. Front Neurosci. 2010;4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salmi J, Pallesen KJ, Neuvonen T, et al. . Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22(11):2663–2676. [DOI] [PubMed] [Google Scholar]
- 54. Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. [DOI] [PubMed] [Google Scholar]
- 55. Kim DJ, Kent JS, Bolbecker AR, et al. . Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull. 2014;40(6):1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elliott ML, Romer A, Knodt AR, Hariri AR. A Connectome-wide functional signature of transdiagnostic risk for mental illness. Biol Psychiatry. 2018;84(6):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kebets V, Holmes AJ, Orban C, et al. . Somatosensory-Motor dysconnectivity spans multiple transdiagnostic dimensions of psychopathology. Biol Psychiatry. 2019;86(10):779–791. [DOI] [PubMed] [Google Scholar]
- 58. Zhang Y, Guo G, Tian Y. Increased temporal dynamics of intrinsic brain activity in sensory and perceptual network of schizophrenia. Front Psychiatry. 2019;10(July):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mennigen E, Jolles DD, Hegarty CE, et al. . State-dependent functional dysconnectivity in youth with psychosis spectrum symptoms. Schizophr Bull. 2020;46(2):408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172(1):17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–198. [DOI] [PubMed] [Google Scholar]
- 62. Guell X, Hoche F, Schmahmann JD. Metalinguistic deficits in patients with cerebellar dysfunction: empirical support for the dysmetria of thought theory. Cerebellum. 2015;14(1):50–58. [DOI] [PubMed] [Google Scholar]
- 63. Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. Cerebellar contribution to social cognition. Cerebellum. 2016;15(6):732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018;141(1):248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guell X, Schmahmann J. Cerebellar functional anatomy: a didactic summary based on human fMRI evidence. Cerebellum. 2020;19(1):1–5. [DOI] [PubMed] [Google Scholar]
- 66. Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–1187. [DOI] [PubMed] [Google Scholar]
- 67. David AS. Dysmodularity: a neurocognitive model for schizophrenia. Schizophr Bull. 1994;20(2):249–255. [DOI] [PubMed] [Google Scholar]
- 68. Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4):215–233. [DOI] [PubMed] [Google Scholar]
- 69. Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38(5):920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yeganeh-Doost P, Gruber O, Falkai P, Schmitt A. The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo). 2011;66(suppl 1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piras F, Piras F, Banaj N, et al. . Cerebellar GABAergic correlates of cognition-mediated verbal fluency in physiology and schizophrenia. Acta Psychiatr Scand. 2019;139(6):582–594. [DOI] [PubMed] [Google Scholar]
- 72. Maloku E, Covelo IR, Hanbauer I, et al. . Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107(9):4407–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3(3):161–169. [DOI] [PubMed] [Google Scholar]
- 74. Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: a preliminary study. Am J Psychiatry. 2005;162(6):1203–1205. [DOI] [PubMed] [Google Scholar]
- 75. Yang GJ, Murray JD, Wang XJ, et al. . Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci U S A. 2016;113(2): E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–313. [DOI] [PubMed] [Google Scholar]
- 77. Konstantareas MM, Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord. 2001;31(1):19–28. [DOI] [PubMed] [Google Scholar]
- 78. Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 2009;1(10):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Foss-Feig JH, Adkinson BD, Ji JL, et al. . Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol Psychiatry. 2017;81(10): 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goodkind M, Eickhoff SB, Oathes DJ, et al. . Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bernard JA, Dean DJ, Kent JS, et al. . Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35(8):4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berman RA, Gotts SJ, McAdams HM, et al. . Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain. 2016;139(Pt 1):276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.