Abstract
Anhedonia or inability to experience pleasure not only is a core symptom of major depressive disorder (MDD), but also is identified as an important component of the positive valence system in the NIMH Research Domain Criteria. The Snaith–Hamilton Pleasure Scale (SHAPS) has been developed for the assessment of hedonic experience or positive valence, but has not been well-studied in depressed outpatient populations. The current study examined the reliability and validity of the SHAPS using a sample of adult outpatients with treatment resistant MDD. Data for the current study were obtained from 122 adult outpatients with a diagnosis of MDD and non-response to adequate treatment with an SSRI and who participated in Project TReatment with Exercise Augmentation for Depression (TREAD). A Principal Components Analysis was used to define the dimensionality of the SHAPS. Convergent and discriminant validity were evaluated via correlations of the SHAPS total score with “gold standard” measures of depression severity and quality of life. The SHAPS was found to have high internal consistency (Cronbach’s coefficient α = .82). A Principal Components Analysis suggests that the SHAPS is mainly “unidimensional” and limited to hedonic experience among adult outpatients with MDD. Convergent and discriminant validity were assessed by examining the Spearman rank-order correlation coefficient between the SHAPS total score and the HRSD17 (rs = 0.22, p<.03), IDS-C30 (rs = 0.26, p<.01), IDS-SR30 (rs = 0.23, p<.02), QIDS-C16 (rs = 0.22, p<.03), QIDS-SR16 (rs = 0.17, p<.10), QLES-Q (rs = −0.32, p<.002), and the pleasure/enjoyment item (sub-item 21) of the IDS-C (rs = 0.44, p<.0001) and IDS-SR (rs = 0.38, p<.0002). The self-administered SHAPS showed modest sensitivity (76%) and specificity (54%) with the self-administered pleasure/enjoyment single item (sub-item 21) of IDS-SR30. The current study shows that the SHAPS is a reliable and valid instrument to assess hedonic experience or positive valence in adult outpatients with MDD and provides a broader assessment of this important domain.
Keywords: Major depressive disorder, hedonic experience, Snaith-Hamilton, anhedonia
1. Introduction
Low hedonic experience or anhedonia is considered a core symptom in the psychopathology of major depressive disorder (APA, DSM IV, 2000; ICD, WHO, 1992). Among the various extant anhedonia scales (e.g., Chapman et al. 1976; Fawcett et al., 1983; Gard et al., 2006; Snaith et al., 1995), most of which are clinician-rated, is the 14-item, self-administered, Snaith–Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995), which was developed for the assessment of hedonic experience or positive valence, but the evaluation of its validity and reliability has received little research attention in patients with MDD or other Axis I disorders. Three validation studies that previously examined the psychometric properties of the SHAPS in a clinical setting used the French (Loas et al., 1997), German (Franz et al., 1998), or Dutch (Franken et al., 2007) version of the SHAPS and used small clinical samples of outpatients with MDD or inpatients with schizophrenia (sample sizes ranged from 20 to 103 inpatients). Nakonezny et al. (2010) is the only validation study to date (to our knowledge) that examined the psychometric properties of the English version of the SHAPS using a large sample of 461 adult outpatients with MDD and, taken together with the three previous validation studies of the non-english versions of the SHAPS (Franken et al., 2007; Franz et al., 1998; Loas et al., 1997), provide some of the necessary foundation for this study.
The purpose of the current study was to replicate our previous study (Nakonezny et al., 2010), which evaluated the psychometric properties of the original (English) version of the SHAPS (Snaith et al., 1995), in the context of using a sample of adult outpatients who were (on average at study exit) less depressed than the sample of adult outpatients used in the Nakonezny et al. (2010) study (sample obtained from Project IMPACTS; Trivedi et al., 2007). As in our previous study (Nakonezny et al., 2010), we used classical test theory analysis to examine the internal consistency, scale dimensionality, and convergent and discriminant validity of the SHAPS. Also, in this study, we examined the sensitivity and specificity of the SHAPS as well as the relationship of the SHAPS scores with participant demographic and clinical characteristics.
2. Methods
2.1. Study and Participants
The current study was conducted using data from the TREAD (TReatment with Exercise Augmentation for Depression) project. The procedures and methods of the TREAD study have been detailed elsewhere (Trivedi et al., 2011). Briefly, Project TREAD examined the efficacy of two doses of aerobic exercise as an augmentation treatment for MDD patients. Participants were 122 adults outpatients with nonpsychotic MDD that, at the time of study entry, had non-response (17-item Hamilton Rating Scale for Depression score of ≥ 14 or greater) to SSRI monotherapy of adequate dosage and duration (Trivedi et al., 2011). The Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) was used to verify the entry diagnosis of MDD.
2.2. Measures
The current study used three separate clinician-rated and two self-administered instruments to measure depression severity, a self-administered instrument to measure hedonic experience, and a self-administered instrument to measure quality of life, enjoyment, and satisfaction. Clinical raters were blind to treatment assignment throughout the course of the study.
2.2.1. Hedonic Experience
The 14-item Snaith–Hamilton Pleasure Scale (SHAPS; Snaith, 1993) is a self-administered instrument that was used to measure hedonic experience or positive valence. Each of the items has a set of four response categories--Strongly Agree, Agree, Disagree, and Strongly Disagree, with either of the Agree responses receiving a score of 0 and either of the Disagree responses receiving a score of 1. Total scores ranged from 0 to 14 (Snaith, 1993), with higher scores indicating a lower level of hedonic experience or a higher level of anhedonia.
2.2.2. Quality of Life, Enjoyment, and Satisfaction
The Quality of Life, Enjoyment, and Satisfaction Questionnaire (QLES-Q short form; Endicott et al., 1993) is a 16-item self-administered questionnaire designed to measure degree of enjoyment and satisfaction in general activities. The QLES-Q measured each of the items using a 5-point Likert-type scale. The QLES-Q was first scored as the sum of the first 14 items so that total scores ranged from 14 to 70 and then the sum was converted into a percentage of the maximum possible score. Higher QLES-Q scores reflected greater enjoyment and satisfaction with general activities. The QLES-Q has previously been found to have acceptable reliability and validity (Endicott et al., 1993).
2.2.3. Depressive Symptom Severity
The 17-item, clinician-administered, Hamilton Rating Scale for Depression (HRSD17; Hamilton, 1960) was used to assess depressive severity. Total score on the HRSD17 ranges from 0 to 52, with higher scores representing greater severity of depressive symptoms. The HRSD17 has established psychometric properties (Hamilton, 1960; Schwab et al., 1967).
The 30-item Inventory of Depressive Symptomatology (IDS-C30 and IDS-SR30; Rush et al., 1996; Trivedi et al., 2004) was administered. The IDS-C30, the clinician-administered version, and the IDS-SR30, the self-report version, each includes the 16 items making up the nine domain symptoms of the DSM IV diagnosis of MDD and contained on the QIDS16, as well as 14 additional items designed to assess melancholic, atypical, and anxious features. Total score on the IDS-C30 and IDS-SR30 ranges from 0 to 84, with higher scores representing greater severity of depressive symptoms. The IDS-C/IDS-SR item 21 assesses pleasure/enjoyment using a 4-point scale ranging from 0 (derives usual sense of enjoyment from pleasurable activities) to 3 (unable to register any sense of pleasure/enjoyment from anything). The psychometric properties of the IDS-C30 and IDS-SR30 have been previously evaluated (Rush et al., 2003, 1996; Trivedi et al., 2004).
The 16-item Quick Inventory of Depressive Symptomatology (QIDS-C16 and QIDS-SR16; Rush et al., 2003; Trivedi et al., 2004) were derived from the IDS-C30 and IDS-SR30, respectively Total score on the QIDS-C16 and QIDS-SR16 ranges from 0 to 27, with higher scores representing greater severity of depressive symptoms. The psychometric properties of the QIDS-C16 and QIDS-SR16 have been extensively evaluated (Rush et al., 2003, 1996; Trivedi et al., 2004).
2.3. Statistical Analysis
The Spearman rank-order/point-biserial partial correlation coefficient (controlling for QIDS-C16 at exit) was used to assess the relationship between SHAPS total score (at exit) and participant demographic and clinical characteristics. For the point-biserial correlation analysis, sex, race, and first versus recurrent episode of depression were binary indicators; sex coded as male = 0 and female = 1, race coded as Caucasian = 0 and non-Caucasian = 1, and first versus recurrent episode of depression coded as first = 0 and recurrent = 1.
Classical Test Theory (CTT) analysis was used to generate the mean, standard deviation, and item/total correlation (rit) for each SHAPS item as well as the overall scale mean and scale standard deviation. CTT analysis also evaluated the internal consistency of the SHAPS using Cronbach’s coefficient alpha (α).
A Principal Components Analysis, with Varimax rotation, and a Parallel analysis (Bernstein et al., 2009; Horn, 1965; Humphreys and Montanelli, 1975) were used to define the dimensionality of the SHAPS. In a unidimensional scale, the observed eigenvalue of the first principal component should exceed the eigenvalue of the first randomly generated principal component (using Parallel analysis), but the reverse should be the case of all subsequent eigenvalues.
Convergent and discriminant validity were evaluated via correlations of the SHAPS with “gold standard” measures of depression severity and quality of life. In particular, we examined the Spearman rank-order correlation coefficient (rs) between the SHAPS total score and the pleasure/enjoyment item (sub-item 21) of the IDS-C30/IDS-SR30, and the QLES-Q, HRSD17, IDS-C30, IDS-SR30, QIDS-C16, and QIDS-SR16 total scores respectively. Further, the one-way Welch’s ANOVA (Welch, 1951) was used to examine, on the SHAPS total score, those who were “severely-to-very severely depressed” (≥ 37 IDS-C30/IDS-SR30 total score) versus those who were “mild-to-moderately depressed” (< 37 IDS-C30/IDS-SR30 total score). Also, the pleasure/enjoyment item of the IDS-C30/IDS-SR30 (sub-item 21) was categorized into three groups (those who scored 0 vs. 1 vs. 2 or 3) and then a one-way Welch’s ANOVA was used to evaluate the relationship between the three groups of the IDS-C30/IDS-SR30 sub-item 21 and the SHAPS total score. The Tukey-Kramer procedure was used to examine the post hoc pairwise comparisons among the three groups of the IDS-C30/IDS-SR30 sub-item 21 on the SHAPS total score.
Finally, using the binary cut-off scoring of ≤ 2 (“normal) and > 2 (“abnormal”) of “normal/abnormal” hedonic experience for the SHAPS (Snaith et al., 1995), we also assessed the sensitivity and specificity of the self-administered SHAPS in relation to the pleasure/enjoyment item (sub-item 21) of the self-report IDS-SR30 (coded as binary indicators: 0 vs. 1 or 2 or 3 for this sensitivity/specificity analysis).
All analyses were carried out using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC). Patients who did not have any post-baseline data were not included in the analysis. The level of significance for all tests was set at α = .05 (two-tailed).
3. Results
3.1. Participant Characteristics
The study sample included 22 males (18.0%) and 100 females (82.0%), with an average age of 47.0 years (SD=9.9; age range: 22–67 years). Participants included 105 (86.1 %) Caucasians, 14 (11.5 %) African-Americans, 1 (0.80%) Hispanic, and 2 (1.6%) other (not specified). Approximately 30% of the participants were in their first episode of depression. The average length of illness was 19.8 years (SD=11.9), and the average duration of the current depressive episode was 6.7 years (SD=7.9). Demographic and clinical characteristics (baseline and exit) of the participants are reported in Table 1.
Table 1.
Participant Characteristics
| Participant Characteristic | Patient Sample (N = 122) |
|---|---|
| Demographics | |
| Age in years, M (SD) | 47.0 (9.9) |
| Education in years, M (SD) | 14.2 (4.6) |
| Race, N (%) | |
| Caucasian | 105 (86.1) |
| African American | 14 (11.5) |
| Hispanic | 1 (0.80) |
| Other (not specified) | 2 (1.6) |
| Sex, N (%) | |
| Female | 100 (82.0) |
| Male | 22 (18.0) |
| Clinical Characteristics | |
| First MDD Episode, N (%) | 36 (29.5) |
| Current MDD Episode Length in years, M (SD) | 6.7 (7.9) |
| Age of MDD Onset in years, M (SD) | 27.4 (11.3) |
| Length of Illness in years, M (SD) | 19.8 (11.9) |
| BMI at baseline, M (SD) | 30.8 (6.2) |
| BMI at exit, M (SD) | 30.4 (5.9) |
| SHAPS total at baseline, M (SD) | 3.1 (3.0) |
| SHAPS total at exit, M (SD) | 1.7 (2.5) |
| HRSD17 total at baseline, M (SD) | 18.0 (3.8) |
| HRSD17 total at exit, M (SD) | 11.9 (5.9) |
| IDS-C30 at baseline, M (SD) | 34.0 (7.4) |
| IDS-C30 at exit, M (SD) | 21.9 (11.9) |
| IDS-SR30 at baseline, M (SD) | 32.4 (9.6) |
| IDS-SR30 at exit, M (SD) | 20.6 (11.4) |
| QIDS-C16 at baseline, M (SD) | 13.9 (2.6) |
| QIDS-C16 at exit, M (SD) | 9.4 (4.7) |
| QIDS-SR16 at baseline, M (SD) | 12.6 (4.0) |
| QIDS-SR16 at exit, M (SD) | 8.1 (4.4) |
| IDS-C30 sub-item 21 at baseline, M (SD) | 1.1 (0.91) |
| IDS-C30 sub-item 21 at exit, M (SD) | 0.54 (0.85) |
| IDS-SR30 sub-item 21 at baseline, M (SD) | 1.2 (0.71) |
| IDS-SR30 sub-item 21 at exit, M (SD) | 0.62 (0.69) |
| QLES-Q at baseline, M (SD) | 59.8 (10.7) |
| QLES-Q at exit, M (SD) | 70.0 (12.5) |
Note: M = Mean; SD = Standard Deviation; N = sample size at baseline (n=92 at exit).
3.2. SHAPS and Participant Characteristics
The Spearman partial correlations (controlling for QIDS-C16 at exit) between the SHAPS total score at exit and participant characteristics, including measures of depressive severity and quality of life, are reported in Table 2.
Table 2.
Spearman rank-order correlation coefficients between the SHAPS and various other scales at exit and participant demographic and clinical characteristics
| HRSD17 | IDS-C30 | IDS-SR30 | QIDS-C16 | QIDS-SR16 | IDS-C21 | IDS-SR21 | QLES-Q | Age | Sex | Race | Education | First/Recurrent Episode | Current Epidose Length | Length of Illness | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHAPS Total (p value) | 0.22 (0.0383) | 0.26 (0.0120) | 0.23 (0.0272) | 0.22 (0.0335) | 0.17 (0.1040) | 0.44 (<.0001) | 0.38 (0.0002) | −0.32 (0.0022) | 0.02a (0.8562) | 0.04a (0.7386) | 0.09a (0.3799) | −0.11a (0.3033) | −0.05a (0.6574) | 0.09a (0.4016) | 0.05a (0.6226) |
Note. For the point-biserial correlation analysis, sex, race, and first versus recurrent episode of depression were binary indicators; sex coded as male = 0 and female = 1, race coded as Caucasian = 0 and non-Caucasian = 1, and first versus recurrent episode of depression coded as first = 0 and recurrent = 1. Education, current episode length, and length of illness are all measured in years.
IDS-C21 & IDS-SR21 = the pleasure/enjoyment item of the IDS-C30 & IDS-SR30, respectively (sub-item 21).
HRSD17, 17-item Hamilton Rating Scale for Depression; IDS-C30, 30-item Inventory of Depressive Symptomatology – Clinician-rating; IDS-SR30, 30-item Inventory of Depressive Symptomatology – Self Report-rating; QIDS-C16, 16-item Quick Inventory of Depressive Symptomatology—Clinician-rating; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology—Self Report-rating; QLES-Q, Quality of Life, Enjoyment, and Satisfaction Questionnaire—General Activities sub-scale; SHAPS, Snaith–Hamilton Pleasure Scale.
Correlation coefficient adjusted for QIDS-C16 at exit.
3.3. Internal Consistency and Scale Dimensionality
The internal consistency (Cronbach’s coefficient alpha) of the SHAPS was 0.82. The mean item-total correlation was 0.44 (ranging from 0.16 to 0.67). Table 3 summarizes the classical test theory results for the SHAPS. Cronbach’s coefficient alpha for each of the depression scales (at exit) used in the current study was as follows: HRSD17 (α = 0.77), IDS-C30 (α = 0.87), IDS-SR30 (α = 0.88), QIDS-C16 (α = 0.79), and QIDS-SR16 (α = 0.78).
Table 3.
Item means, item/total correlations, scale internal consistency (α), scale mean, scale standard deviation, and factor loadings for the SHAPS-items at Exit (n = 92)
| Rotated Factor Loadingsa | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Scale Items | Mean | SD | rit | Factor 1 | Factor 2 | Factor 3 | |
| 1. I would enjoy my favorite television or radio program | 0.141 | 0.350 | 0.458 | 0.598 | 0.283 | −0.089 | |
| 2. I would enjoy being with family or close friends | 0.097 | 0.298 | 0.168 | −0.068 | −0.007 | 0.761 | |
| 3. I would find pleasure in my hobbies and pastimes | 0.250 | 0.435 | 0.678 | 0.706 | 0.267 | 0.290 | |
| 4. I would be able to enjoy my favorite meal | 0.141 | 0.350 | 0.547 | 0.209 | 0.399 | 0.702 | |
| 5. I would enjoy a warm bath or refreshing shower | 0.065 | 0.248 | 0.211 | −0.003 | 0.125 | 0.531 | |
| 6. I would find pleasure in the scent of flowers or the smell of a fresh sea breeze or freshly baked bread | 0.163 | 0.371 | 0.381 | 0.661 | 0.089 | −0.128 | |
| 7. I would enjoy seeing other people’s smiling faces | 0.043 | 0.205 | 0.373 | −0.026 | 0.789 | 0.058 | |
| 8. I would enjoy looking smart when I have made an effort with my appearance | 0.108 | 0.312 | 0.433 | 0.581 | 0.296 | −0.142 | |
| 9. I would enjoy reading a book, magazine or newspaper | 0.119 | 0.326 | 0.649 | 0.344 | 0.724 | 0.210 | |
| 10. I would enjoy a cup of tea or coffee or my favorite drink | 0.097 | 0.298 | 0.478 | 0.648 | −0.113 | 0.453 | |
| 11. I would find pleasure in small things; e.g., bright sunny day, a telephone call from a friend | 0.086 | 0.283 | 0.622 | 0.707 | 0.142 | 0.332 | |
| 12. I would be able to enjoy a beautiful landscape or view | 0.119 | 0.326 | 0.409 | 0.069 | 0.687 | 0.181 | |
| 13. I would get pleasure from helping others | 0.065 | 0.248 | 0.345 | 0.749 | −0.186 | 0.001 | |
| 14. I would feel pleasure when I receive praise from other people | 0.163 | 0.371 | 0.450 | 0.125 | 0.793 | 0.035 | |
| Variance Explained by each Factor | 23.6% | 19.5% | 13.4% | ||||
| Cronbach’s Coefficient α | 0.818 | ||||||
| Scale Mean | 1.663 | ||||||
| Scale Standard Deviation | 2.455 | ||||||
| Mean Item-Total Correlation | 0.443 | ||||||
Varimax rotation from a Principal Components Analysis.
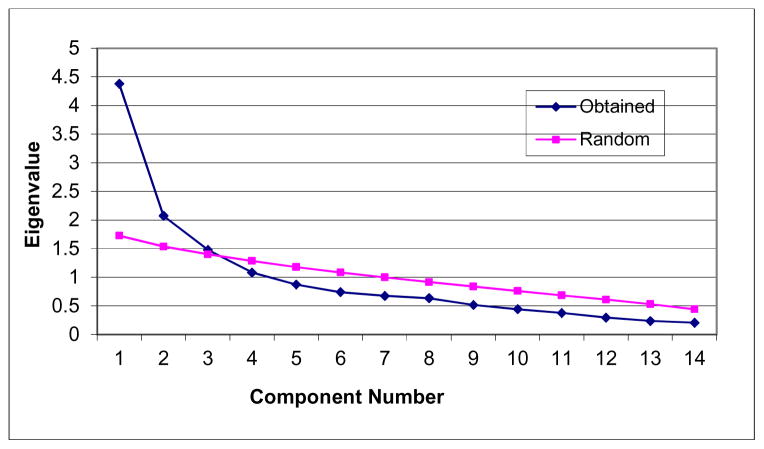
A principal components analysis as well as a parallel analysis both suggests perhaps a two- or three-factor solution (i.e., observed eigenvalues of the first three components were 4.37, 2.07, and 1.47; Figure 1). However, examination of the rotated factor pattern indicated that these components do not differ with regard to content (i.e., a distinct interpretation of each of the three components is not clear; Table 3). Thus, this result suggests that a unidimensional-factor structure yields the most adequate solution for the SHAPS in the current study.
Figure 1.
Scree Plot of the SHAPS at exit (n=92)
3.4. Convergent and Discriminant Validity
When the pleasure/enjoyment item of the IDS Clinician-rated scale (IDS-C30 sub-item 21) was categorized into three groups (those who scored: 0 vs.1 vs. 2 or 3), the one-way Welch’s ANOVA revealed a significant between-subjects group effect on the SHAPS total score (F = 10.25, df = 2, 19.33, r = 0.49, p< .0009). The means for SHAPS total scores were lower for those who scored “0” on IDS-C30 sub-item 21 (“derives usual sense of enjoyment from pleasurable activities,” M = 0.95, SD = 1.59, n=61) than those who scored “1” on IDS-C30 sub-item 21 (“does not feel usual enjoyment from pleasurable activities,” M = 2.31, SD = 3.18, n=19) or those who scored “2 or 3” on IDS-C30 sub-item 21 (“rarely derives pleasure from any activities” or “unable to register any sense of pleasure/enjoyment from anything,” M = 4.63, SD = 2.73, n=11). The Tukey-Kramer pairwise comparisons of the three groups (0, 1, 2 or 3) on the SHAPS total score indicated a significant difference between those who scored “2 or 3” and those who scored “1” (p< .01), between those who scored “2 or 3” and those who scored “0” (p< .0001), and between those who scored “0” and those who scored “1” (p< .04).
When the pleasure/enjoyment item of the IDS Self-Report scale (IDS-SR30 sub-item 21) was also categorized into three groups (those who scored 0 vs.1 vs. 2 or 3), the one-way Welch’s ANOVA revealed a significant between-subjects group effect on the SHAPS total score (F = 5.35, df = 2, 15.16, r = 0.43, p<.017). The means for SHAPS total scores were lower for those who scored “0” on IDS-SR30 sub-item 21 (“derives usual sense of enjoyment from pleasurable activities,” M = 0.84, SD = 1.71, n=45) than those who scored “1” on IDS-SR30 sub-item 21 (“does not feel usual enjoyment from pleasurable activities,” M = 1.97, SD = 2.33, n=39) or those who scored “2 or 3” on IDS-SR30 sub-item 21 (“rarely derives pleasure from any activities” or “unable to register any sense of pleasure/enjoyment from anything,” M = 4.71, SD = 4.15, n=07). The Tukey-Kramer post-hoc analysis of the three coded IDS-SR30 sub-item 21 groups revealed that the mean rating on the SHAPS total score for those who scored “2 or 3” was significantly greater than those who scored “1” (p< .01) and those who scored “0” (p< .0001), respectively. Those who scored “0” and those who scored “1” were also significantly different from each other on the SHAPS total score (p< .05). Further, the SHAPS total score was negatively correlated with the QLES-Q (rs = −0.32, p< .002).
The Spearman correlations revealed a positive relationship between the SHAPS total score and the total scores on the HRSD17 (rs = 0.22, p<.03), IDS-C30 (rs = 0.26, p<.01), IDS-SR30 (rs = 0.23, p<.02), QIDS-C16 (rs = 0.22, p<.03), and QIDS-SR16 (rs = 0.17, p<.10). The direction and magnitude of these correlation coefficients suggested that persons with greater levels of depression severity had relatively higher levels of anhedonia (or lower levels of hedonic experience; Table 2). Indeed, patients who were “severely-to-very severely depressed” at exit on the IDS Clinician-rated scale (≥ 37 IDS-C30 total score, n=12) had higher mean SHAPS total scores (higher levels of anhedonia) than those who were “mild-to-moderately depressed” (< 37 IDS-C30 total score, n=80), M=3.41 (SD=3.47) versus M=1.40 (SD=2.17), F = 3.82, df = 1, 12.32, p<.07, R2 = .08, r = .28. Patients who were “severely-to-very severely depressed” at exit on the IDS Self-Report scale (≥ 37 IDS-SR30 total score, n=9) also had higher mean SHAPS total scores (higher levels of anhedonia) than those who were “mild-to-moderately depressed” (< 37 IDS-SR30 total score, n=83), M=3.55 (SD=3.64) versus M=1.46 (SD=2.23), F = 2.87, df = 1, 8.66, p<.12, R2 = .07, r = .26.
3.5. Sensitivity and Specificity
Based on the binary cut-off scoring of ≤ 2 (“normal) and > 2 (“abnormal”) of “normal/abnormal” hedonic experience for the SHAPS (Snaith et al., 1995), the self-administered SHAPS showed modest sensitivity (76%) and specificity (54%) with the self-report pleasure/enjoyment item (sub-item 21) of IDS-SR30 (coded as binary indicators: 0 vs. 1 or 2 or 3 for this sensitivity/specificity analysis).
4. Discussion
The current study replicated our previous study (Nakonezny et al., 2010), which evaluated the psychometric properties of the original (English) version of the SHAPS (Snaith et al., 1995), in the context of using a sample of adult outpatients who were (on average at study exit) less depressed than the sample of adult outpatients used in the Nakonezny et al. (2010) study (sample obtained from Project IMPACTS; Trivedi et al., 2007).
The internal consistency of the SHAPS in the current study is high (α = 0.82) and is consistent with our previous psychometric evaluation of the SHAPS (Nakonezny et al., 2010) (α = 0.91) and is in line with previous findings in outpatients with MDD, inpatients with schizophrenia (e.g., Franken et al., 2007; Franz et al., 1998; Loas et al., 1997), and depressed and non-depressed patients diagnosed with Parkinson’s disease (Lemke et al., 2006). Unlike our previous psychometric evaluation of the SHAPS (Nakonezny et al., 2010), which found the SHAPS to be clearly unidimensional (one-factor solution), the current study suggests scale dimensionality of perhaps a two- or three-factor solution. Examination of the rotated factor pattern, however, indicates that these components do not differ with regard to content—that is, a distinct interpretation of each of the three components is not clear; thus, we conclude that a “unidimensional-factor structure” most likely yields the most adequate solution for the SHAPS in the current study. Perhaps this difference in finding of scale dimensionality (factor solution), however, is likely a result of the difference in average level of depression severity and hedonic experience between the two outpatient samples (adult outpatients in the current study who were on average at study exit less depressed with a lower level of anhedonia than adult outpatients used in the Nakonezny et al. 2010 study). This finding here, nonetheless, is in line with the Franken et al. (2007) study who also found a three-factor solution in their non-patient, normal control, sample (Study 1), but a one-factor solution in their clinical patient samples (Study 3). Together with results from the few extant psychometric evaluation studies, the SHAPS (English and foreign versions) has high internal consistency and appears to be limited mainly to hedonic experience or positive valence in both patient (Franken et al., 2007; Franz et al., 1998; Lemke et al., 2006; Loas et al., 1997; Nakonezny et al., 2010) and non-patient volunteer populations (Franken et al., 2007; Leventhal et al., 2006).
Convergent and discriminant validity were assessed by evaluating the relationship between the SHAPS total score and five separate measures of depression severity (three clinician-rated and two self-administered), the pleasure/enjoyment item (sub-item 21) of the IDS-C30/IDS-SR30, and a measure of Quality of Life, Enjoyment, and Satisfaction. The pattern of the SHAPS mean scores is consistent with the scoring direction on the pleasure/enjoyment item of the IDS-C30/IDS-SR30 (sub-item 21); that is, those who rarely or are unable to derive pleasure from activities (as coded/scored on IDS-C30/IDS-SR30 sub-item 21) also have higher levels of anhedonia or lower levels of hedonic experience (as measured via the SHAPS). The self-administered SHAPS also shows modest sensitivity (76%) and specificity (54%) with the self-report pleasure/enjoyment item (sub-item 21) of IDS-SR30. The SHAPS also correlates negatively with the QLES-Q, suggesting that persons with lower levels of enjoyment and satisfaction with activities (as measured by the QLES-Q) have higher levels of anhedonia (or lower levels of hedonic experience as measured by the SHAPS).
The magnitude of the correlation between the SHAPS total score and each of the other five measures of depression (three clinician-rated and two self-administered) is modest--and in line with previous findings (e.g., Franken et al., 2007; Franz et al., 1998; Loas et al., 1997; Leventhal et al., 2006; Mazzaa et al., 2009; Nakonezny et al., 2010)--suggesting that hedonic experience or positive valence (as measured by the SHAPS) may either tap a related, but distinct construct from depression, or likely provides a broader assessment of the positive valence system. The SHAPS is also able to discriminate between outpatients who were severely-to-very severely depressed and those who were mild-to-moderately depressed. This pattern held for both the clinician-rated and self-administered instruments that we used to measure depressive severity. The results of the current study in an outpatient sample of adult patients with MDD and those from previous findings (e.g., Franken et al., 2007; Franz et al., 1998; Loas et al., 1997; Leventhal et al., 2006; Mazzaa et al., 2009; Nakonezny et al., 2010) suggest that the SHAPS has adequate construct validity.
Finally, in this study, we examined the relationship of the SHAPS with participant demographic and clinical characteristics. The SHAPS is not influenced by age, sex, race, education, duration of the current depressive episode, length of illness, or first versus recurrent episode of depression. This basic finding is consistent with our previous psychometric evaluation of the SHAPS (Nakonezny et al., 2010) and with the Franken et al. (2007) study and is in line with the supposition that the SHAPS was developed to minimize cultural, sex/gender, and age biases in the evaluation of hedonic experience or positive valence (Snaith et al., 1995).
The current study may be tempered by a key limitation. Because the evaluation of convergent validity of the SHAPS in the current study (and in our previous Nakonezny et al., 2010, study) was limited in scope (and evaluated mainly against the single pleasure/enjoyment sub-item 21 of the IDS-C30/IDS-SR30 and the QLES-Q), the inclusion of a more comprehensive (multi-item) anhedonia scale that measures a broader domain of anhedonia would have further strengthened the assessment of convergent validity of the SHAPS. But the SHAPS was the only multi-item measure of hedonic experience that was collected in both the Project TREAD study (sample used in the current study) and in the Project IMPACTS study (sample used in our previous Nakonezny et al., 2010, study).
Despite this limitation, the current study has strengths, including a fairly large sample of adult outpatients with major depressive disorder. Further, unlike our previous study (Nakonezny et al., 2010), the current study evaluated (validated) the self-administered SHAPS against both self-administered and clinician-rated measures of depression severity.
In conclusion, there are similarities between the results from our previous psychometric evaluation of the SHAPS (Nakonezny et al., 2010) and the current study, which include that the original (English) version of the SHAPS (Snaith et al., 1995) has high internal consistency, with construct validity, and is limited mainly to the trait of hedonic experience or positive valence among adult outpatients with major depressive disorder. The SHAPS is not influenced by participant demographic characteristics. The demonstration and similarity of good psychometric properties of the SHAPS between the two studies, with different mean levels of depression severity and hedonic experience of the two outpatient samples, provides further support for its use in both clinical and research settings of adults with MDD. However, given that anhedonia may reflect a transdiagnostic trait that plays a role in various psychopathologies (e.g., Cohen et al., 2010; Kashdan et al., 2006; Leventhal et al., 2010; Watson and Naragon-Gainey, 2010), future studies are indicated to evaluate the reliability, validity, and utility of the SHAPS across various diagnostic groups and perhaps across various age groups (broad-spanning from adolescents to geriatric patients). Evaluation of the SHAPS across such specific diagnostic groups as bipolar disorder, anxiety disorder, PTSD, schizoaffective disorder, substance use disorder, and recurrent suicidality, for example, may contribute to a more accurate characterization of relevant individual differences in depressive pathology. Nonetheless, the current study provides evidence that the SHAPS could be a useful tool for assessing hedonic experience in adults with MDD.
Highlights.
There are similarities between the results from our previous psychometric evaluation of the SHAPS (Nakonezny et al., 2010) and the current study, which include that the original (English) version of the SHAPS (Snaith et al., 1995) has high internal consistency, with construct validity, and is mainly limited to the trait of hedonic experience among adult outpatients with major depressive disorder.
The SHAPS is not influenced by participant demographic characteristics.
The demonstration and similarity of good psychometric properties of the SHAPS between the two studies, with different mean levels of depression severity and hedonic experience of the two outpatient samples, provides further support for its use in both clinical and research MDD settings.
Acknowledgments
This work was supported by 1-R01-MH067692-01(TReatment with Exercise Augmentation for Depression) awarded through the National Institute of Mental Health, Madhukar H. Trivedi, M.D., Principal Investigator.
Role of Funding Source
Funding for this study was provided by 1-R01-MH067692-01 (TReatment with Exercise Augmentation for Depression); NIMH had no further role in study design; or in the collection, analysis and interpretation of data; or in the writing of the manuscript; or in the decision to submit the paper for publication.
Footnotes
Contributors
Madhukar H. Trivedi designed the study and wrote the protocol. David W. Morris and Tracy Greer managed the literature searches and administered the clinical scales. Thomas J. Carmody managed the database and contributed to the statistical analysis. Ira H. Bernstein contributed to the statistical analysis and proof-reading of the manuscript. Bruce Grannemann and Matthew J. Byerly assisted with literature searches and proofreading of the manuscript. Paul A. Nakonezny undertook and managed the statistical analysis and wrote the draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Madhukar H. Trivedi is or has been an advisor/consultant to: Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Cerecor, Concert Pharmaceuticals, Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Inc., Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received research support from: Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health (U01MH092221, T32MH0675-43, HHSN2712011000061, HHSNiHMH2010-24), National Institute on Drug Abuse (5U01DA020024), Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), and Solvay Pharmaceuticals, Inc.
Dr. Tracy Greer has received research funding from NARSAD and is a paid consultant for H. Lundbeck A/S.
Dr. Ira Bernstein has received grant support from the Joint Research Committee of the National Council of State Boards of Nursing and serves on the advisory board of the Joint Research Committee of the National Council of State Boards of Nursing. Dr. Bernstein receives book royalties from Sage Publications and owns stock in the following companies: Merck & Co Inc, Bristol-Myers Squibb Company, DuPont, EI. de Nemours & CC.
Dr. Matthew Byerly reports receipt of research support and speaker bureau fees from Otsuka.
Drs. Nakonezny, Morris, Carmody and Mr. Grannemann have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington DC: American Psychiatric Press; 2000. [Google Scholar]
- Bernstein IH, Wendt B, Nasr SJ, Rush AJ. Screening for major depression in private practice. J Psychiatr Pract. 2009;15:87–94. doi: 10.1097/01.pra.0000348361.03925.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clinical Psychology Review. 2011;31(3):440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients: The Pleasure Scale. Archives of General Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IVAxis I Disorders, Clinician Version (SCID-1-CV) New York, NY: Biometrics Research Department, New York State Psychiatric Institute, Department of Psychiatry, Columbia University; 1995. [Google Scholar]
- Franken I, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith–Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Franz M, Lemke MR, Meyer T, Ulferts J, Puhl P, Snaith RP. German version of the Snaith-Hamilton-Pleasure Scale (SHAPS-D). Anhedonia in schizophrenic and depressive patients. Fortschr Neurol Psychiatr. 1998;66:407–413. doi: 10.1055/s-2007-995279. [DOI] [PubMed] [Google Scholar]
- Galinowski A, Lehert P. Structural validity of MADRS during antidepressant treatment. International Clinical Psychopharmacology. 1995;10:157–161. doi: 10.1097/00004850-199510030-00004. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and Consummatory Components of the Experience of Pleasure: A Scale Development Study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL. An empirical comparison of various methods for estimating common factor scores. Educ Psychol Meas. 1965;25:313–322. [Google Scholar]
- Humphreys LG, Montanell RG. An investigation of the parallel analysis criterion for determining the number of common factors. Multivariate Behav Res. 1975;10:193–206. [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy. 2006;44(3):457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression Rating Scale, and the Clinical Global Impressions rating scale in antidepressant clinical trials. International Clinical Psychopharmacology. 2002;17:281–285. doi: 10.1097/00004850-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson’s disease. Journal of the Neurological Sciences. 2006;249:266–270. doi: 10.1016/j.jns.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and Clinical Psychopharmacology. 2010;18(6):562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Loas G, Dubal S, Perot P, Tirel F, Nowaczkowski P, Pierson A. Validation of the French version of the Snaith-Hamilton Pleasure Scale (SHAPS, Snaith et al. 1995). Determination of the statistical parameters in 208 normal subjects and 103 hospitalized patients presenting with depression or schizophrenia. Encephale. 1997;23:454–458. [PubMed] [Google Scholar]
- Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P. Effect of aripiprazole on self-reported anhedonia in bipolar depressed patients. Psychiatry Research. 2009;165:193–196. doi: 10.1016/j.psychres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Äsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith–Hamilton Pleasure Scale in adult outpatients with major depressive disorder. International Clinical Psychopharmacology. 2010;25:328–333. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- SAS. Cary, NC: Statistical Analysis System Institute; [Google Scholar]
- Schwab JJ, Bialow MR, Clemmons RS, Holzer CE. Hamilton rating scale for depression with medical in-patients. Br J Psychiatry. 1967;113:83–8. doi: 10.1192/bjp.113.494.83. [DOI] [PubMed] [Google Scholar]
- Snaith RP. Anhedonia: a neglected symptom of psychopathology. Psychological Medicine. 1993;23:957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Medicine. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Claassen CA, Grannemann BD, Kashner TM, Carmody TJ, Daly E, Kern JK. Assessing Physicians’ Use of Treatment Algorithms: Project IMPACTS Study Design and Rationale. Contemporary Clinical Trials. 2007;28:192–212. doi: 10.1016/j.cct.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Dunn AL, Earnest CP, Sunderajan P, Henley SS. Exercise as an augmentation treatment for nonremitted major depressive disorder: A randomized, parallel dose comparison. The Journal of Clinical Psychiatry. 2011;72:677–684. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2010;30(7):839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BL. On the Comparison of Several Mean Values: An Alternative Approach. Biometrika. 1951;38:330–336. [Google Scholar]
- World Health Organization. Clinical Descriptors and Diagnostic Guidelines. Geneva: World Health Organization; 1992. The ICD-10 Classification of Mental and Behavioural Disorders. [Google Scholar]