Abstract
Objective:
The goal of this study was to review relevant studies in order to determine the efficacy of decompression with fusion versus decompression in the treatment of lumbar spinal stenosis.
Methods:
Using appropriate keywords, we identified relevant studies using PubMed, the Cochrane library, and Embase. Key pertinent sources in the literature were also reviewed, and all articles published through October 2019 were considered for inclusion. For each study, we used odds ratios, mean difference (MD), and 95% confidence interval (95% CI) to assess and synthesize outcomes.
Results:
We found 13 studies that were consistent with this meta-analysis with a total of 29066 patients. Compared with decompression, decompression with fusion significantly increased the incidence of complications (RR: 1.41, 95%CI: 1.26–1.57), the length of hospital stay (WMD: 1.868, 95%CI: 1.394–2.343), operative time (WMD: 80.399, 95%CI: 44.397–116.401), estimated blood loss (WMD: 309.356, 95%CI: 98.008–520.704) and Zurich claudication questionnaire in symptom severity (WMD: 0.200, 95%CI: 0.006–0.394). The reoperation rate was lower in the decompression with fusion group than the decompression group but without significant difference (RR: 0.91, 95%CI: 0.82–1.00). There was no significant difference between 2 groups in visual analog scale (leg pain and back pain), ODI, Short Form 36 Health Survey physical component summary, Short Form 36 Health Survey mental component summary, and Zurich claudication questionnaire physical function.
Conclusion:
Decompression with fusion has no significant clinical advantages in treatment of lumbar spinal stenosis when compared with decompression.
Keywords: decompression, fusion, lumbar spinal stenosis, meta-analysis
1. Introduction
Lumbar spinal stenosis (LSS) is a group of syndromes due to the stenosis of the central, lateral recess and intervertebral foramen of the lumbar spinal canal, which causes nerve compression and blood circulation disorder. LSS patients present with the symptoms of lower limb pain, neurogenic intermittent claudication or back pain. LSS is a common and frequently occurring orthopedic disease. With the aging of the population, social life and work are increasingly tense, and its incidence rate gradually increases, which not only seriously affects the life and work of patients, but also causes great economic losses to the society. In terms of etiology, LSS has roughly 3 etiologies: congenital, degenerative and other causes. Because a series of symptoms including intermittent claudication, sciatica, horsetail, and so on, LSS often causes great trouble on the lives of patients, and seriously influences the patient's quality of life.[1–4]
Currently, the treatment of LSS includes non-surgical and surgical treatment. Non-operative treatment is suitable for patients with mild and moderate symptoms. Conservative treatments commonly include manipulation, treatments, drug therapy, nerve block therapy, lumbar back exercise, waist protection, and other treatments such as hyperthermia, ice therapy, ultrasound, and massage, electrical stimulation and traction. Surgical treatment is 1 of the effective methods when the patient's quality of life is reduced and pain is intolerable and the conservative treatment is ineffective, the symptoms are recurrent and the nerve root symptoms are obvious. LSS surgery can be divided into lumbar laminectomy and decompression, pedicle screw internal fixation and bone graft fusion.[5–9] The aim of this study was to perform a meta-analysis of all available literature to obtain updated evidence about the efficacy of decompression with fusion versus decompression in the treatment of LSS.
2. Methods
The ethical approval was not provided because this study was performed by including the published studies. The data that support the findings of this meta-analysis will be available from the corresponding author on reasonable requests.
2.1. Search strategy
To identify studies pertaining to the efficacy of decompression with fusion versus decompression in the treatment of LSS, we reviewed the Cochrane library, PubMed, and Embase databases for relevant articles published through October 2019. We also reviewed the references of all identified articles to identify additional studies. Search terms were as follows: LSS, lumbar stenosis, LSS, decompression, micro decompression, and endoscopy decompression, fusion. These terms were used in combination with “AND” or “OR”. This literature review was performed independently by 2 investigators, with a third resolving any disputes as needed.
Following the PICOS (Participants, Interventions, Comparisons, Outcomes and Study design) principle, the key search terms included (P) patients with LSS; (I) patients were treated by decompression with fusion or decompression; (C/O) the clinical efficacy of decompression with fusion versus decompression, the outcomes including length of hospital stay, operative time, estimated blood loss, complication, reoperation, the score of Oswestry disability index (ODI score), visual analog scale (VAS), the Short Form (36) Health Survey, and Zurich claudication questionnaire (ZCQ). (S) randomized controlled trial, case-control or cohort study.
2.2. Study selection criteria
Included studies met the following criteria:
-
(1)
randomized controlled trials, case-control or cohort studies;
-
(2)
the inventions were decompression with fusion or decompression;
-
(3)
the subjects were patients with LSS;
-
(4)
4) the publications were in English and Chinese.
Studies were excluded for meeting the following criteria:
-
(1)
duplicate articles or results;
-
(2)
clear data errors;
-
(3)
case reports, case-control studies, theoretical research, conference reports, systematic reviews, meta-analyses, and other forms of research or comment not designed in a randomized controlled manner;
-
(4)
lack of clinical outcomes of interest;
-
(5)
lack of a control group.
Two investigators independently determined whether studies met the inclusion criteria, with a third resolving any disputes as needed.
2.3. Data extraction and quality assessment
For each included study, 2 categories of information were extracted: basic information and primary study outcomes. Basic information relevant to this meta-analysis included: author names, year of publication, sample size, mean age, gender, and surgery strategy. Primary clinical outcomes relevant to this analysis included length of hospital stay, operative time, estimated blood loss, complication, reoperation, the score of ODI score, VAS score of leg pain and back pain, the Short Form (36) Health Survey (SF-36) score of physical component summary and mental component summary, ZCQ score of symptom severity and physical function. Data extraction was performed independently by 2 investigators, with a third resolving any disputes as needed.
2.4. Statistical analysis
STATA v10.0 (TX) was used for all analyses. Heterogeneity in study results was assessed using chi-squared and I2 tests and appropriate analysis models (fixed-effects or random-effects) were determined. A chi-squared P ≤.05 and an I2 > 50% indicated high heterogeneity and the random-effects model was used in this case. A chi-squared P > .05 and an I2≤50% indicated acceptable heterogeneity and the fixed-effects model was instead used. Continuous variables were given as mean ± standard deviation and compared on the basis of mean difference (MD), while categorical data were given as percentages and compared based on relative risk (RR)/odds ratios. MD and 95% confidence interval (CI) was used to analyze all the indexes except complications and reoperation.
3. Results
3.1. Overview of the included studies
We reviewed a total of 733 articles identified by our initial keyword search, of which 666 were excluded following title/abstract review. The remaining 67 articles were subject to a complete full-text assessment, leading to 54 articles being excluded for failing to meet the study inclusion criteria. Reasons for exclusion of these studies included theoretical research (8), lack of clinical outcomes (29), duplicate articles (2), and case report (15). We ultimately identified a total of 13 randomized controlled trials[10–22] that met the inclusion criteria for this meta-analysis, including 29066 patients. The study selection process is outlined in Figure 1.
Figure 1.
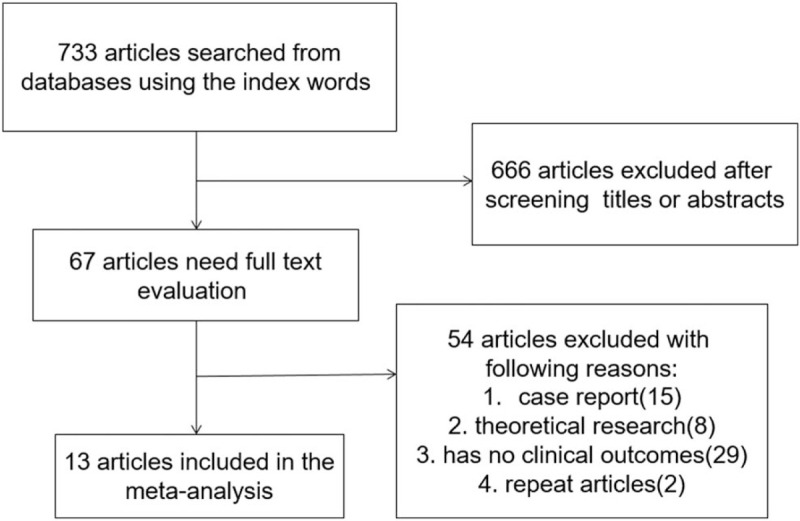
Literature search and selection strategy.
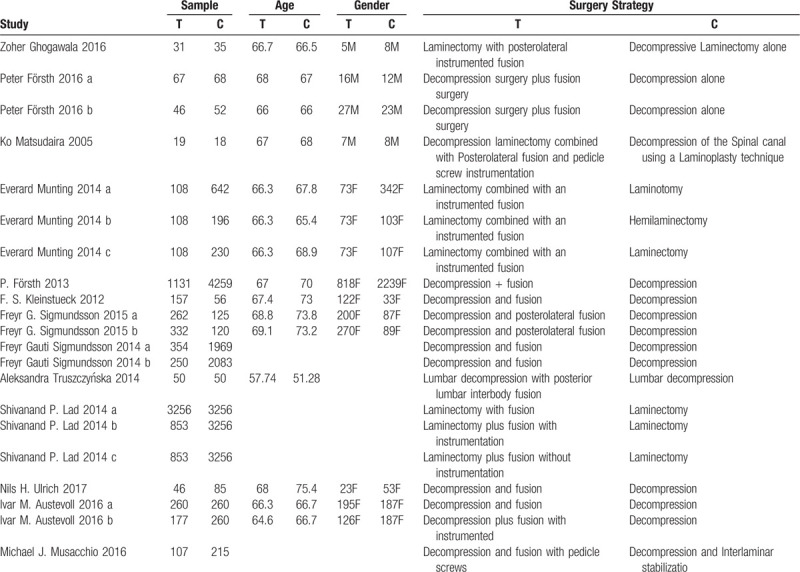
Table 1 summarizes the basic information of each study, including author names, year of publication, sample size, mean age, gender, and surgical strategy.
Table 1.
The basic characteristics description of included studies.
3.2. Complications
In total 7 studies were included, including 5887 patients in the decompression with fusion group and 11487 patients in the decompression group. Based on a chi-squared P=.552 and an I2 = 0.0%, the fixed-effects model was chosen to assess complications. The incidence of complications was significantly higher in the decompression with fusion group than the decompression group (RR: 1.41, 95%CI: 1.26–1.57).
The results are presented in Figure 2.
Figure 2.
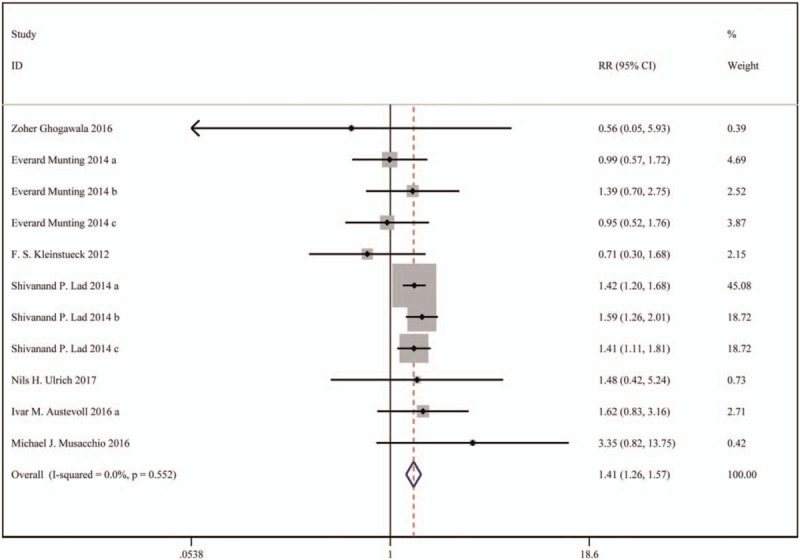
Forest plot for complications.
3.3. Reoperation
In total 6 studies were included, including 6601 patients in the decompression with fusion group and 15430 patients in the decompression group. Based on a chi-squared P = .046 and an I2 = 47.5%, the fixed-effects model was chosen to assess reoperation. The reoperation rate was lower in the decompression with fusion group than the decompression group but without significant difference (RR: 0.91, 95%CI: 0.82∼1.00).
The results are presented in Figure 3.
Figure 3.
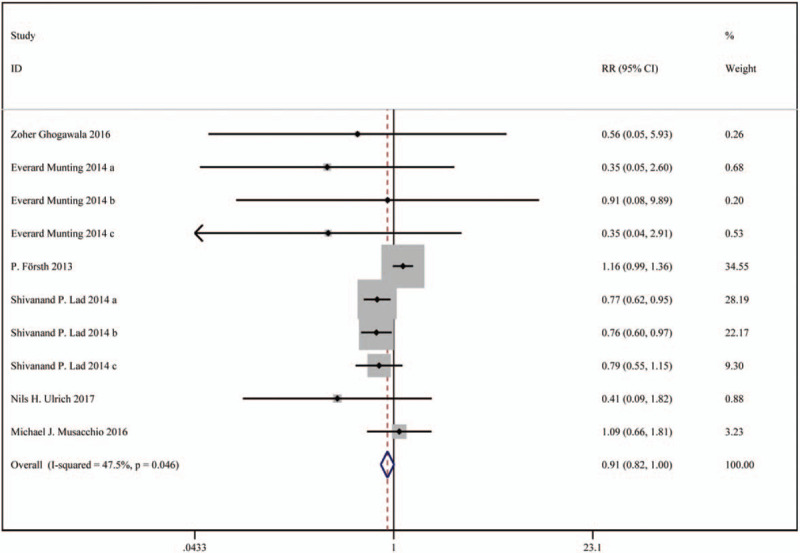
Forest plot for reoperation.
3.4. VAS
In total 6 studies were included, including 3203 patients in the decompression with fusion group and 10264 patients in the decompression group. Based on a chi-squared P < .001 and an I2 = 86.7%, the random-effects model was chosen to assess VAS. There was no significant difference in VAS (leg pain) scores (WMD: 0.78, 95%CI: -0.26 –1.82), and VAS (back pain) scores (WMD: -0.75, 95%CI: -2.10 – 0.59) between the 2 groups.
The results are presented in Figures 4 and 5.
Figure 4.
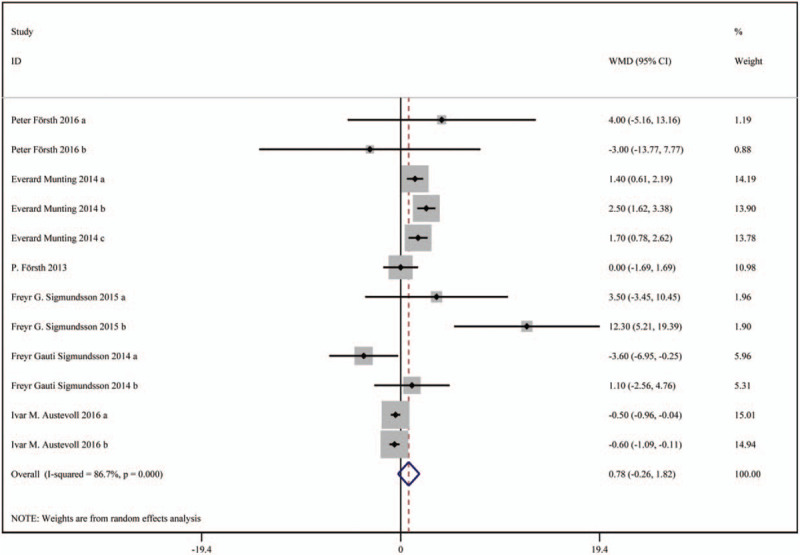
Forest plot for VAS scores (leg pain). VAS = visual analog scale.
Figure 5.
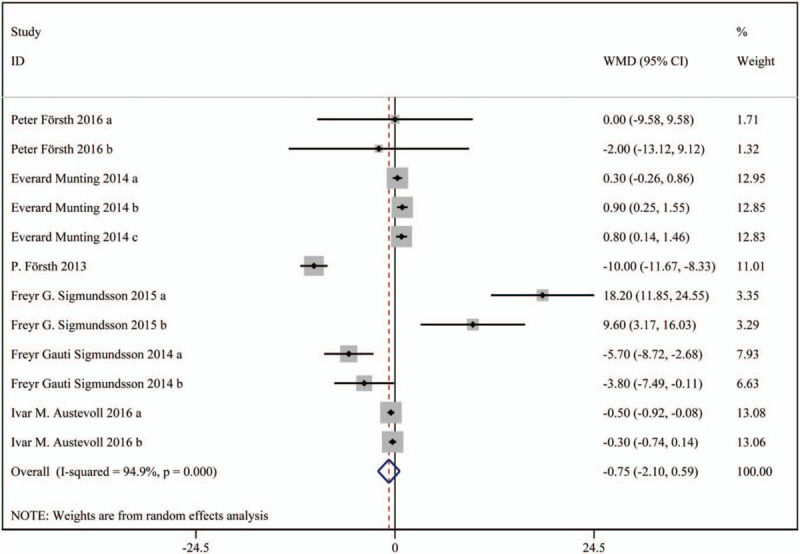
Forest plot for VAS scores (back pain). VAS = visual analog scale.
3.5. Other results
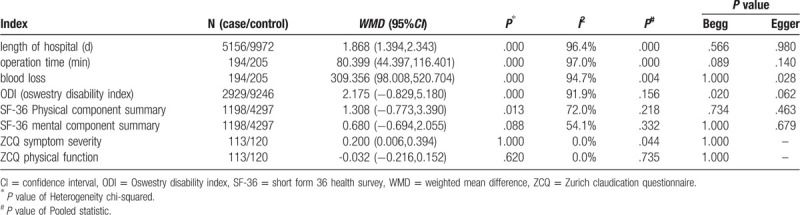
Compared with decompression, decompression with fusion significantly increased the length of hospital stay (WMD: 1.868, 95%CI: 1.394 –2.343), operation time (WMD: 80.399, 95%CI: 44.397 –116.401), estimated blood loss (WMD: 309.356, 95%CI: 98.008 –520.704) and ZCQ in symptom severity (WMD: 0.200, 95%CI: 0.006 –0.394). Besides, there eas no significant difference between the 2 groups in ODI (WMD: 2.175, 95%CI: -0.829 –5.180), SF-36 physical component summary (WMD: 1.308, 95%CI: -0.773 –3.390), SF-36 mental component summary (WMD: 0.680, 95%CI: -0.694 –2.055), and ZCQ physical function (WMD: -0.032, 95%CI: -0.216 –0.152).
The results are presented in Table 2.
Table 2.
The results of meta-analysis on included studies.
3.6. Quality and bias assessment
An assessment of study quality and risk of bias was performed using multiple complementary methods including: funnel plots, Begg and Mazumdar rank test, and Egger test. There was clear symmetry in the log WMD funnel plot for VAS for these studies, suggesting a low publication bias risk (Fig. 6). The results of Begg and Mazumdar rank test (Z = 0.48, P = .631) and Egger test (P = .252) both suggested that there was not any significant risk of bias among the study results.
Figure 6.
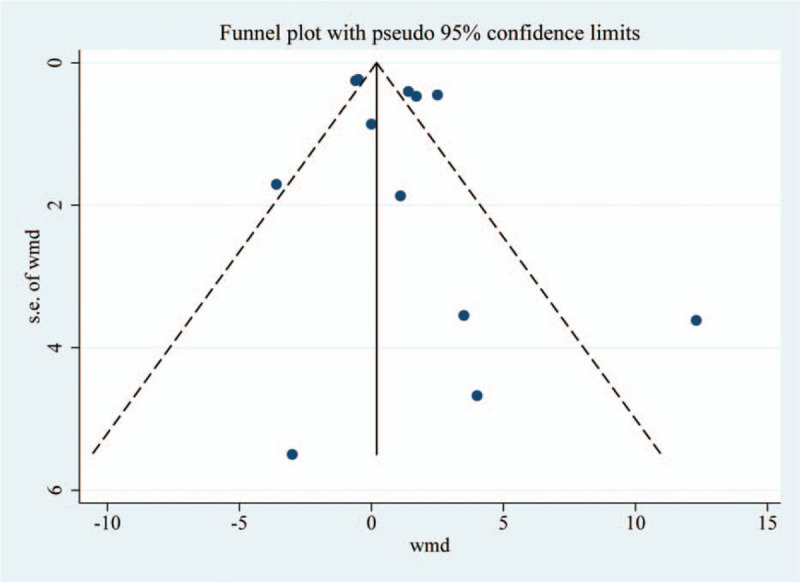
Funnel plot analysis of the included studies.
4. Discussion
The purpose of LSS surgery is not to cure, but to relieve clinical symptoms such as intermittent claudication, lumbago pain and neurological dysfunction, and improve the patients’ quality of life. Most scholars believe that the indications of surgical treatment of LSS mainly include:
-
(1)
moderate and severe nerve root radiation pain or nerve root function damage, with or without back pain;
-
(2)
intermittent claudication, walking distance less than 100 to 200 m or progressive aggravation;
-
(3)
progressive scoliosis and slippage accompanied by corresponding clinical signs and symptoms increase, affecting the function of life activities;
-
(4)
symptoms of cauda equina nerve injury;
-
(5)
patients have no significant relief after conservative treatment for 3 to 6 months; in general, if the paitents can tolerate the operation, they should receive surgical treatment. In recent years, LSS has become a common indication in spinal surgery. LSS surgery can be divided into lumbar laminectomy and decompression, pedicle screw internal fixation and bone graft fusion.
The main purpose of LSS surgery is to decompress, relieve the pressure of dural sac and nerve, and restore the volume of vertebral foramen and spinal canal so as to alleviate the symptoms of lumbago and leg pain and neurological intermittent claudication. However, in order to achieve effective decompression, it is inevitable to destroy the stability of the spine. Therefore, interbody fusion to eliminate segment-instability is very popular among orthopedic surgeons. Decompression and fusion therapy for patients with LSS has become a trend.[23–25] However, it is still controversial whether fusion is needed after decompression in degenerative LSS.
The 2 methods of decompression or decompression plus bone graft fusion and internal fixation have different therapeutic effects. Decompression has the advantages of less trauma, shorter operative time and fewer complications, but the clinical effect is somewhat less than that of decompression with fusion. For patients with degenerative LSS, fusion and internal fixation after decompression can achieve satisfactory results. However, a series of complications may occur, such as unfused graft, pain at the bone removal site, false joint formation, release of the interbody fusion cage and fracture of internal fixation. In addition, intervertebral fusion limits the mobility of the spinal segment, leading to abnormal stress conduction in the vertebra and accelerating the degeneration of adjacent segments at the fusion site. For elderly patients with multiple medical conditions, simultaneous internal fixation and fusion after decompression may increase the risk of fatal complications and death.
In our study, we found that compared with decompression, decompression with fusion significantly increased the incidence of complications (RR: 1.41, 95%CI: 1.26–1.57), the length of hospital stay (WMD: 1.868, 95%CI: 1.394–2.343), operative time (WMD: 80.399, 95%CI: 44.397–116.401), estimated blood loss (WMD: 309.356, 95%CI: 98.008–520.704) and ZCQ in symptom severity (WMD: 0.200, 95%CI: 0.006–0.394). The reoperation rate was lower in the decompression with fusion group than the decompression group but without significant difference (RR: 0.91, 95%CI: 0.82–1.00). More studies are needed to confirm the long-term efficacy of decompression with fusion. There was no significant difference between the 2 groups in VAS (leg pain and back pain), ODI, SF-36 physical component summary, SF-36 mental component summary, and ZCQ physical function.
Spinal stenosis is a common and frequently-occurring disease in the elderly. Due to the complexity and diversity of the disease, and elderly patients often have concomitant organ and system diseases, and surgical risk is relatively high. The core of LSS is nerve decompression and spinal stabilization. Physicians should properly handle the relationship between decompression and spinal stabilization, fully evaluate whether appropriate bone graft fusion and internal fixation should be taken at the same time, and select appropriate surgical procedures for different patients. How to choose the operation method becomes the key to treat LSS. If obvious instability and spondylolisthesis of the spine can be excluded in patients with LSS, surgeons should consider the comprehensive situation of the patients (age, severe low back pain and number of decompression segments) in the selection of surgical methods and carefully choose fusion internal fixation surgery.
However, there are certain limitations to the present analysis, which are as follows:
-
(1)
the number of included studies is limited;
-
(2)
the technique levels of operations were varied between studies;
-
(3)
the quality of included studies is limited;
-
(4)
pooled data were analyzed, as individual patient data was not available, precluding more in-depth analyses.
5. Conclusion
Decompression with fusion has no significant clinical advantages in treatment of LSS when compared with decompression. Doctors should comprehensively and objectively analyze the symptoms, signs and imaging data of patients in the perioperative period, actively control other diseases, strictly grasp the surgical indications, and adopt appropriate surgical methods.
Author contributions
Conceptualization: Bo Chen, Yao Lv, Xiu-Cheng Guo.
Data curation: Bo Chen, Yao Lv, Zhi-Cui Wang.
Methodology: Bo Chen, Yao Lv.
Project administration: Xiu-Cheng Guo, Chu-Zhang Chao.
Software: Zhi-Cui Wang.
Writing – original draft: Bo Chen, Yao Lv.
Writing – review & editing: Xiu-Cheng Guo, Chu-Zhang Chao.
Glossary
Abbreviations: CI = confidence interval, LSS = lumbar spinal stenosis, MD = mean difference, ODI = Oswestry disability index, SF-36 = short form 36 health survey, VAS = visual analog scale, ZCQ = Zurich claudication questionnaire.
References
- [1].Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schroeder GD, Kurd MF, Vaccaro AR. Lumbar spinal stenosis: how is it classified? J Am Acad Orthop Surg 2016;24:843–52.. [DOI] [PubMed] [Google Scholar]
- [3].Andresen AK, Ernst C, Andersen MO. Lumbar spinal stenosis. Ugeskr Laeger 2016;178:V04160245. [PubMed] [Google Scholar]
- [4].Arabmotlagh M, Sellei RM, Vinas-Rios JM, et al. Classification and diagnosis of lumbar spinal stenosis. Orthopade 2019;48:816–23.. [DOI] [PubMed] [Google Scholar]
- [5].Rousing R, Jensen RK, Fruensgaard S, et al. Danish national clinical guidelines for surgical and nonsurgical treatment of patients with lumbar spinal stenosis. Eur Spine J 2019;28:1386–96.. [DOI] [PubMed] [Google Scholar]
- [6].Wu L, Cruz R. Lumbar Spinal Stenosis. Treasure Island (FL): StatPearls; 2019. [PubMed] [Google Scholar]
- [7].Mo Z, Zhang R, Chang M, et al. Exercise therapy versus surgery for lumbar spinal stenosis: a systematic review and meta-analysis. Pak J Med Sci 2018;34:879–85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lafian AM, Torralba KD. Lumbar spinal stenosis in older adults. Rheum Dis Clin North Am 2018;44:501–12.. [DOI] [PubMed] [Google Scholar]
- [9].Shi SY, Huang YS, Hao DJ. Therapeutic progress in lumbar spinal stenosis. Zhongguo Gu Shang 2017;30:484–8.. [DOI] [PubMed] [Google Scholar]
- [10].Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 2016;374:1424–34.. [DOI] [PubMed] [Google Scholar]
- [11].Forsth P, Olafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 2016;374:1413–23.. [DOI] [PubMed] [Google Scholar]
- [12].Matsudaira K, Yamazaki T, Seichi A, et al. Spinal stenosis in grade I degenerative lumbar spondylolisthesis: a comparative study of outcomes following laminoplasty and laminectomy with instrumented spinal fusion. J Orthop Sci 2005;10:270–6.. [DOI] [PubMed] [Google Scholar]
- [13].Munting E, Roder C, Sobottke R, et al. Patient outcomes after laminotomy, hemilaminectomy, laminectomy and laminectomy with instrumented fusion for spinal canal stenosis: a propensity score-based study from the Spine Tango registry. Eur Spine J 2015;24:358–68.. [DOI] [PubMed] [Google Scholar]
- [14].Forsth P, Michaelsson K, Sanden B. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: a two-year follow-up study involving 5390 patients. Bone Joint J 2013;95-B:960–5.. [DOI] [PubMed] [Google Scholar]
- [15].Kleinstueck FS, Fekete TF, Mannion AF, et al. To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J 2012;21:268–75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sigmundsson FG, Jonsson B, Stromqvist B. Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1624 patients. Spine J 2015;15:638–46.. [DOI] [PubMed] [Google Scholar]
- [17].Sigmundsson FG, Jonsson B, Stromqvist B. Preoperative pain pattern predicts surgical outcome more than type of surgery in patients with central spinal stenosis without concomitant spondylolisthesis: a register study of 9051 patients. Spine (Phila Pa 1976) 2014;39:E199–210.. [DOI] [PubMed] [Google Scholar]
- [18].Truszczynska A, Rapala K, Lukawski S, et al. Evaluation of functional outcomes in individuals 10 years after posterior lumbar interbody fusion with corundum implants and decompression: a comparison of 2 surgical techniques. Med Sci Monit 2014;20:1400–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lad SP, Babu R, Ugiliweneza B, et al. Surgery for spinal stenosis: long-term reoperation rates, health care cost, and impact of instrumentation. Spine (Phila Pa 1976) 2014;39:978–87.. [DOI] [PubMed] [Google Scholar]
- [20].Austevoll IM, Gjestad R, Brox JI, et al. The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: a pragmatic comparative non-inferiority observational study from the Norwegian Registry for Spine Surgery. Eur Spine J 2017;26:404–13.. [DOI] [PubMed] [Google Scholar]
- [21].Musacchio MJ, Lauryssen C, Davis RJ, et al. Evaluation of decompression and interlaminar stabilization compared with decompression and fusion for the treatment of lumbar spinal stenosis: 5-year follow-up of a prospective, randomized, controlled trial. Int J Spine Surg 2016;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jang JW, Park JH, Hyun SJ, et al. Clinical outcomes and radiologic changes after microsurgical bilateral decompression by a unilateral approach in patients with lumbar spinal stenosis and grade I degenerative spondylolisthesis with a minimum 3-year follow-up. Clin Spine Surg 2016;29:268–71.. [DOI] [PubMed] [Google Scholar]
- [23].Machado GC, Ferreira PH, Harris IA, et al. Effectiveness of surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PloS One 2015;10:e0122800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim CH, Chung CK, Park CS, et al. Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: a nationwide cohort study. Spine J 2013;13:1230–7.. [DOI] [PubMed] [Google Scholar]
- [25].Farrokhi MR, Yadollahikhales G, Gholami M, et al. Clinical outcomes of posterolateral fusion vs posterior lumbar interbody fusion in patients with lumbar spinal stenosis and degenerative instability. Pain Physician 2018;21:383–406.. [PubMed] [Google Scholar]


