Abstract
Distinct abnormalities in kynurenine pathway (KP) metabolism have been reported in various psychiatric disorders, including schizophrenia (SZ). Kynurenic acid (KYNA), a neuroactive metabolite of the KP, is elevated in individuals diagnosed with SZ and has been linked to cognitive impairments seen in the disorder. To further understand the role of KYNA in SZ etiology, we developed a prenatal insult model where kynurenine (100 mg/day) is fed to pregnant Wistar rats from embryonic day (ED) 15 to ED 22. As sex differences in the prevalence and severity of SZ have been observed, we presently investigated the impact of prenatal kynurenine exposure on KP metabolism and spatial learning and memory in male and female offspring. Specifically, brain tissue and plasma from offspring (control: ECon; kynurenine-treated: EKyn) in prepuberty (postnatal day (PD) 21), adolescence (PD 32–35), and adulthood (PD 56–85) were collected. Separate cohorts of adult offspring were tested in the Barnes maze to assess hippocampus- and prefrontal cortex-mediated learning and memory. Plasma tryptophan, kynurenine, and KYNA were unchanged between ECon and EKyn offspring across all three ages. Hippocampal and frontal cortex KYNA was elevated in male EKyn offspring only in adulthood, compared to ECon, while brain KYNA levels were unchanged in adult females. Male EKyn offspring were significantly impaired during acquisition of the Barnes maze and during reversal learning in the task. In female EKyn offspring, learning and memory remained relatively intact. Taken together, our data demonstrate that exposure to elevated kynurenine during the last week of gestation results in intriguing sex differences and further support the EKyn model as an attractive tool to study the pathophysiology of schizophrenia.
Keywords: kynurenic acid, hippocampus, prefrontal cortex, tryptophan, Barnes maze, learning and memory
1. Introduction
Cognitive symptoms are a major feature of the neurodevelopmental disorder schizophrenia (SZ), yet current treatments do not adequately alleviate these aspects of the disorder. Observed cognitive changes include impairments in hippocampus-mediated learning and memory, which are associated with structural abnormalities and volumetric reductions in this area (Arnold et al., 2015; Bobilev et al., 2019; Heckers et al., 1998; Li et al., 2015; Steen et al., 2006; Tamminga et al., 2010). Sex differences in cognitive domains, including spatial learning and memory (Jimenez et al., 2010; Joseph et al., 2013), have been observed in individuals with SZ, and several animal models of the disorder have defined behavioral differences between male and female subjects (Leger and Neill, 2016; Mendrek and Mancini-Marie, 2016). While the occurrence of SZ does not differ significantly between men and women, the growing number of studies suggesting sex differences in the clinical characteristics and course of the illness highlights the need to better understand sex differences and the mechanisms that mediate them.
Developmental animal models of SZ mimic brain insults that are sustained early in life, but not fully aggravated until early adulthood (Jones et al., 2011). As the clinical onset of SZ typically occurs after puberty, the long delay between the presumed neurodevelopmental insult and the adult brain manifestation of illness is a key characteristic of SZ (Castle et al., 1998; DeLisi, 2008; Kinney et al., 2010; Lieberman et al., 2001; Meyer and Feldon, 2010). Risk factors associated with SZ, including stress and infections during prenatal development (Brown and Derkits, 2010; Meyer and Feldon, 2010; van Os and Selten, 1998), result in the activation of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase and metabolism of the essential amino acid tryptophan into kynurenine (Leklem, 1971), the premier metabolite of the kynurenine pathway (KP)(Schwarcz et al., 2012). Kynurenine enters the brain from the circulation and is then irreversibly transaminated to kynurenic acid (KYNA) in astrocytes by kynurenine aminotransferase II (KAT II) (Gal and Sherman, 1978; Guidetti et al., 2007; Guidetti et al., 1997). Upon its release into the extracellular space, it acts as a neuromodulator by antagonizing N-methyl-D-aspartate (NMDA) and α7 nicotinic acetylcholine (α7nACh) receptors, which are both prevalent in the hippocampus and have been causally related to cognitive deficits in patients with SZ (Ben-Ari et al., 1997; Dwyer et al., 2009; Timofeeva and Levin, 2011). Elevations in brain KYNA and its immediate precursor kynurenine are observed in postmortem brain tissue and cerebrospinal fluid of individuals with SZ (Erhardt et al., 2001; Linderholm et al., 2012; Miller et al., 2006; Nilsson et al., 2005; Sathyasaikumar et al., 2011; Schwarcz et al., 2001), thereby implicating the KP in the central nervous system pathology. Furthermore, preclinical studies acutely elevating brain KYNA levels support the notion that increased KYNA may contribute to cognitive dysfunction and clinical relevance in SZ (Chess et al., 2009; Chess et al., 2007; Erhardt et al., 2004; Pocivavsek et al., 2017; Pocivavsek et al., 2011; Shepard et al., 2003).
In an effort to evaluate the role of KYNA during prenatal development, we have established a rodent embryonic kynurenine (EKyn) model (Alexander et al., 2013; Hahn et al., 2018; Pershing et al., 2015; Pershing et al., 2016; Pocivavsek et al., 2019; Pocivavsek et al., 2014). In the EKyn rat model, KYNA is increased during the last week of gestation, from embryonic day (ED) 15 to ED 22, by adding kynurenine to chow fed to dams. Our previous studies have focused exclusively on adult male offspring from EKyn mothers and characterized extensive biochemical alterations and cognitive deficits in the EKyn males, including contextual learning and memory (Pershing et al., 2016; Pocivavsek et al., 2019; Pocivavsek et al., 2014), reversal learning in a set shifting task (Alexander et al., 2013; Pershing et al., 2015), and attentional broad monitoring in a five-choice paradigm (Hahn et al., 2018). We presently investigate sex differences for the first time in the EKyn model and explore the trajectory of KP biochemical changes in both sexes of offspring during pre-adolescence, adolescence and adulthood. Tryptophan, kynurenine and KYNA were measured in the plasma and KYNA was measured in both the hippocampus and frontal cortex. A male-specific increase in brain KYNA was determined only in adult EKyn animals, thus we assessed sex differences in spatial learning and memory across training days and reversal learning in the Barnes maze (Barnes, 1979) in adult offspring. Our findings demonstrate that prenatal kynurenine elevation induces intriguing sex-specific disruptions in learning and memory.
2. Materials and Methods
2.1. Animals
Adult, pregnant Wistar rats (gestational age: 2 days) were obtained from Charles River Laboratories. All experimental animals were housed in a temperature control facility that is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at the Maryland Psychiatric Research Center. The rats had constant and unlimited access to food and water and were kept on a 12 hour light-12 hour dark cycle. All protocols adhered to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine.
2.2. Prenatal kynurenine treatment and experimental groups
Rodent chow was finely ground in a blender, and each dam was fed 30 grams of food each day. Kynurenine (100 mg) was administered to EKyn dams each day from embryonic day (ED) 15 to ED 22, as previously described (Pocivavsek et al., 2014)(Figure 1). The kynurenine was thoroughly mixed into the dry powdered rodent chow and then combined with water to produce a wet mash. The control (ECon) treatment dams received wet mash alone. Both dams given kynurenine-treated mash and regular wet mash consistently ate all of the food, demonstrating no difference in potential nutrition received during the last week of gestation. After giving birth, each dam was given normal rodent chow pellets ad libitum on postnatal day (PD) 0. Special attention was given to ensure that prenatal treatment did not disturb maternal behavior (Pocivavsek et al., 2014; Pocivavsek et al., 2019). Male and female offspring were used in biochemical and behavioral experiments. Cohorts of offspring were euthanized at PD 21, PD 32 – 35, and PD 56 – 85 for biochemical experiments and separate cohorts were used in behavioral experiments. Due to the large number of experimental outcomes (i.e. biochemical assessments at three ages and behavioral testing in adulthood), the number males and females per litter was not always sufficient to fully balance littermates across all endpoints. The distribution of offspring from a single ECon or EKyn litter was 1 – 2 rats per sex for biochemical assays and 2 – 3 rats per sex for behavior. When necessary, additional ECon or EKyn litters were added to yield a minimum of N = 6 litters per condition for biochemical experiments at each age and N = 16 offspring, from 9 – 10 litters, for behavioral experiments. Supplemental Table 1 indicates the number of samples per experimental assay.
Figure 1.
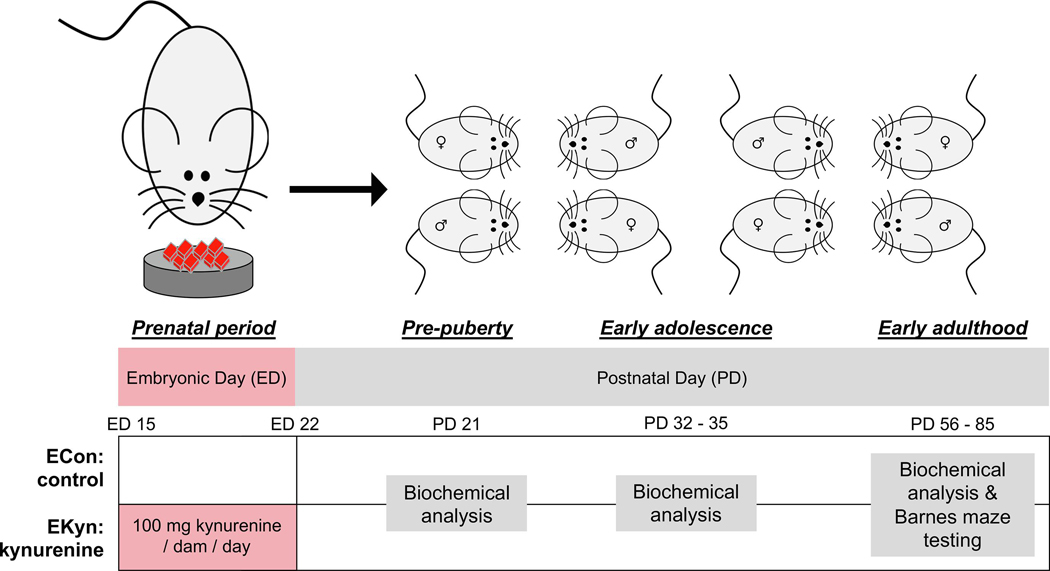
Schematic representation of experimental design and timeline. Pregnant rats were fed a control (ECon) or kynurenine-laced (EKyn) chow with 100 mg kynurenine per dam per day from embryonic day (ED) 15 until ED 22. Upon birth, normal rodent chow was fed to all male and female offspring. Biochemical analysis was conducted at prepuberty (postnatal day [PD] 21), in early adolescence (PD 32 – 35), and early adulthood (PD 56 – 85). Behavioral testing in the Barnes maze was performed in adult animals.
2.3. Sample collection
Brain and plasma were collected at PD 21, 32 – 35 and 56 – 85 during the light phase (between zeitgeber time 3 and 9). Animals were euthanized by CO2 asphyxiation, whole blood was collected in tubes containing 25 μl K3-EDTA (0.15%) as an anticoagulant and the brain was rapidly removed and frozen on dry ice. Blood was then centrifuged (300 × g, 10 min) and the supernatant plasma was transferred to new tubes, frozen on dry ice and all samples were stored at −80°C until biochemical analysis.
2.4. Plasma tryptophan, kynurenine, and KYNA
Plasma samples were thawed and diluted in ultrapure water (tryptophan- 1:1000, kynurenine- 1:2, KYNA- 1:10). Twenty-five μL of 6% perchloric acid were added to 100 μL of each diluted preparation to acidify the sample. The precipitate was separated by centrifugation (12,000 × g, 10 min) and the supernatant was analyzed by high performance liquid chromatography (HPLC). Twenty μL of supernatant were injected into a ReproSil-Pur C18 column (4 × 150 mm; Dr. MaischGmbh, Ammerbuch, Germany) using a mobile phase comprising 50 mM sodium acetate and 5% acetonitrile (pH adjusted to 6.2 with glacial acetic acid). The samples were run at a flow rate of 0.5 mL/min and detected using 500 mM zinc acetate delivered after the column at a flow rate of 0.1 mL/min. Tryptophan (excitation/emission wavelength of 285/365nm), kynurenine (excitation/emission wavelength of 365nm/480nm), and KYNA (excitation/emission wavelength of 344nm/398nm) were detected in the eluate fluorometrically (Waters Alliance, 2475 fluorescence detector, Bedford, MA) at retention times of 11, 6 and 11 minutes respectively.
2.5. Brain KYNA
On the day of the assays, the frontal cortex and hippocampus were dissected on wet ice, weighed and sonicated in ultrapure water (1:10, w/v for all brain areas). Twenty-five μL of 25% perchloric acid were added to 100 μL of each diluted preparation. The precipitate was separated by centrifugation (12,000 × g, 10 min) and 30 μL of the supernatant were analyzed using HPLC as described above to detect KYNA.
2.6. Barnes maze
Behavioral analysis was performed in a separate cohorts of adult, PD 56 – 85, offspring. A Barnes maze acquisition paradigm was used to assess hippocampus-dependent spatial learning and memory. The maze consists of an elevated circular platform (122 cm) with twenty holes around its edge (10 cm hole). Nineteen of these holes are false bottomed, while only one leads to an actual escape box (Rosenfeld and Ferguson, 2014). The maze was surrounded by a white curtain and extra-maze visual cues. All rats were habituated to the Barnes maze 3 days before the start of the training trials. During the habituation trials, a rat was placed in the escape box for 2 minutes to acclimate to the box, and then the rat was given time to explore the maze until it entered the escape box or 5 minutes had elapsed; if the rat did not enter the escape after 5 minutes, it was gently guided into the box. After spending an additional 15 seconds there, the rat was returned to its home cage. The training trials of the Barnes maze consisted of 2 trials per day for 3 consecutive days with an inter-trial interval of 4 hours each day. Animals were tested at the same times of day across the 3 acquisition days. First a rat was placed in the center of the maze and given up to 5 minutes to locate the escape box. If the animal did not enter within the 5-minute period, it was gently guided to the escape box and kept there for 15 seconds. On the fourth day, animals were tested in two reversal trials, to engage frontal cortex function, separated by a 4 h inter-trial interval, wherein the escape box was moved approximately 180 degrees. All behavior was recorded using Any-Maze Behavioral Tracking software. For acquisition trials, escape latency, errors committed, mean speed, distance traveled, and search strategy were analyzed. For the reversal trials, the number of entries into the previous escape box location was also recorded. Acquired data were averaged across the 2 trials for each respective testing day. Errors were defined as looking into, sniffing, or walking across a false-bottomed hole. Search strategy was categorized into 3 categories: direct, serial and random (Betancourt et al., 2017; Locklear and Kritzer, 2014; Rosenfeld and Ferguson, 2014), with direct being the most efficient strategy and random being the least efficient (Table 1).
Table 1.
Search strategy definitions.
| Strategy Measure | Description |
|---|---|
| Direct | Make less than 3 errors within 2 holes from the escape OR make less than 3 errors followed by course correction directly toward escape location |
| Serial | Make at least 3 errors (>50 % are sequential) with less than 2 changes of direction |
| Random | Make at least 3 errors (<50% are sequential) OR make at least 3 errors with 2 or more changes of direction |
2.7. Statistical Analysis
Biochemical experiments:
For assessment of biochemical data (plasma tryptophan, plasma kynurenine, plasma KYNA, hippocampal KYNA, frontal cortex KYNA), comparisons were made using three-way analysis of variance (ANOVA). For each analysis, age, sex, and prenatal condition and possible interactions were assessed. Three-way ANOVA was conducted first, then analyses were followed up with the appropriate two-way interactions. Further analyses were focused on the main effects of age, sex, and prenatal condition. Where appropriate, significant effects were followed up with the Bonferroni post hoc test.
Behavioral experiments:
Barnes maze acquisition training data (escape latency, distance traveled, errors, and speed) were analyzed using a three-way repeated measures ANOVA with prenatal treatment group and sex as between-subject factors and testing day as a within-subject factor. Within treatment groups, significant main effect of day was followed up with Bonferroni post hoc test. Between treatment groups on different days, post hoc analysis was assessed with uncorrected Fisher’s LSD. Reversal day data (entries into previous escape location, escape latency, distance traveled, errors, speed) were analyzed using a two-way ANOVA with prenatal treatment group and sex as between-subject factors. Where appropriate, significant effects were followed up with the Bonferroni post hoc test. Search strategy differences in the Barnes maze were analyzed using a Chi-square distribution test. In all analyses, statistical significance was defined as P < 0.05. All statistical functions were completed using GraphPad Prism 8.0.
3. Results
3.1. Prenatal kynurenine treatment does not impact postnatal peripheral KP metabolism
Tissues from male and female offspring of EKyn and ECon dams were tested for tryptophan, kynurenine and KYNA at prepuberty (PD 21), early adolescence (PD 32 – 35), and during early adulthood (PD 56 – 85) to determine the impact of prenatal kynurenine treatment on KP metabolism across postnatal development. Tryptophan in the plasma was not impacted by three-way age X sex X prenatal condition interaction (F2,90 = 0.7, P = 0.49) (Figure 2A). In addition, no significant two-way interactions were determined for plasma tryptophan (age X sex: F2,90 = 1.2, P = 0.29; age X prenatal treatment: F2,90 = 0.7, P = 0.50; sex X prenatal treatment: F1,90 = 0.2, P = 0.70). Levels of tryptophan in the plasma were however significantly impacted by age (F2,90 = 50.2, P < 0.0001) and sex (F1,90 = 4.8, P < 0.05) alone, but not by ECon vs EKyn prenatal treatment (F1,90 = 1.0, P = 0.32).
Figure 2.
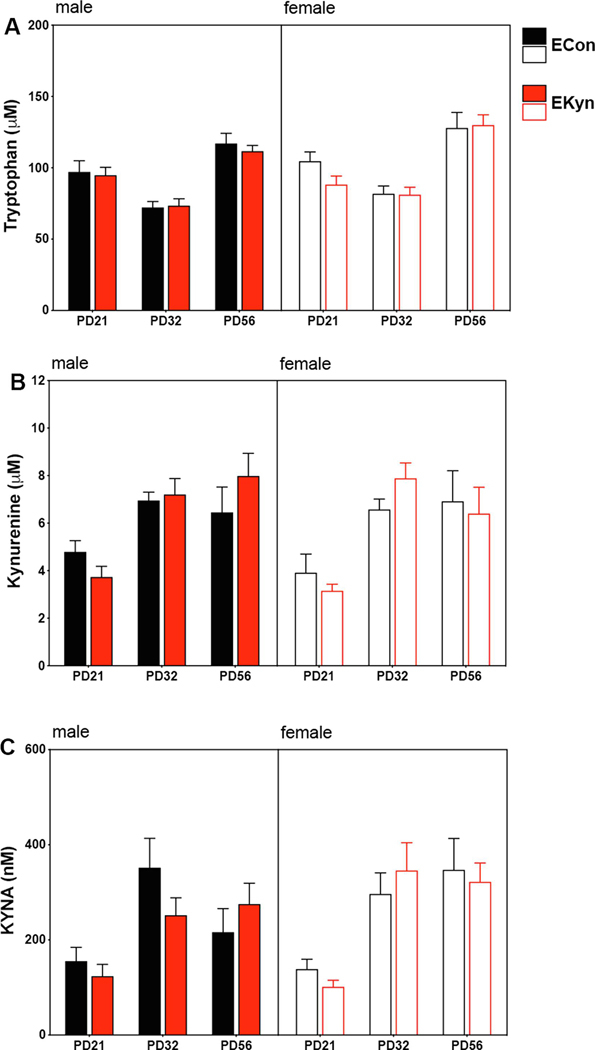
Plasma levels of tryptophan, kynurenine and KYNA in pre-pubertal (PD21), adolescent (PD 32 – 35), and adult (PD 56 – 85) offspring are not altered by prenatal exposure to kynurenine (EKyn). (A) Plasma tryptophan: no significant three-way age x sex x prenatal condition interaction; significant main effect of age and sex, but not prenatal treatment. (B) Plasma kynurenine: no significant three-way age x sex x prenatal condition interaction; significant main effect of age, but not sex or prenatal treatment. (C) Plasma KYNA: no significant three-way age x sex x prenatal condition interaction; significant main effect of age, but not sex or prenatal treatment. All data are mean ± SEM. N = 6 – 12 litters per group.
Plasma kynurenine analysis revealed no three-way age X sex X prenatal condition interaction (F2,97 = 1.0, P = 0.36)(Figure 2B). Levels of kynurenine were also not impacted by any two-way interaction (age X sex: (F2,97 = 0.3, P = 0.72); age X prenatal treatment: (F2,97 = 1.2, P = 0.30); sex X prenatal treatment: (F1,97 = 0.06, P = 0.81)). A main effect of age (F2,97 = 19.5, P < 0.0001), but not sex (F1,97 = 0.7, P = 0.42) or prenatal treatment (F1,97 = 0.07, P = 0.79) was found for plasma kynurenine.
Similarly, plasma KYNA was not impacted by a three-way age X sex X prenatal condition interaction (F2,98 = 1.6, P = 0.20)(Figure 2C). No significant two-way interactions were observed for plasma KYNA levels (age X sex: (F2,98 = 1.3, P = 0.27); age X prenatal treatment: (F2,98 = 0.3, P = 0.71); sex X prenatal treatment: (F1,98 = 0.1, P = 0.72)). Plasma KYNA was impacted by age (F2,98 = 16.4, P < 0.0001), but not sex (F1,98 = 1.1, P = 0.29) or prenatal treatment (F1,98 = 0.3, P = 0.60). There were no significant post-hoc findings.
3.2. Brain KYNA levels are altered in EKyn offspring in a sex- and age-dependent manner
Levels of KYNA were assessed in the hippocampus and frontal cortex to determine the impact of prenatal kynurenine treatment on brain KP metabolism. In the hippocampus, a significant three-way age X sex X prenatal treatment interaction was observed (F2,101 = 3.6, P < 0.05)(Figure 3A). In addition, two-way analysis revealed a significant sex X prenatal treatment interaction (F1,101 = 4.4, P < 0.05), but no impact of age X sex (F2,101 = 2.8, P = 0.06) or age X prenatal treatment (F2,101 = 2.4, P = 0.10). A main effect of age on hippocampal KYNA (F2,101 = 16.9, P < 0.0001) was found, but not of sex (F1,101 = 2.5, P = 0.12) or prenatal treatment (F1,101 = 0.3, P = 0.57). Post-hoc analysis revealed a significant difference at PD 56 between ECon and EKyn males (P < 0.01), with KYNA levels elevated 1.9-fold in the hippocampus of EKyn male offspring.
Figure 3.
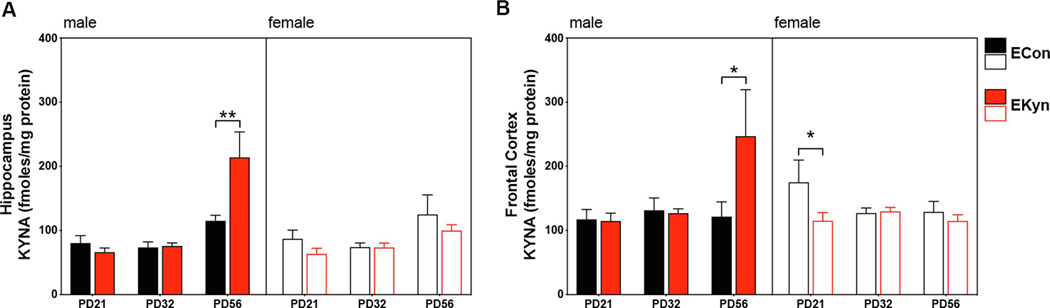
KYNA is elevated in the hippocampus and frontal cortex of adult male, but not female EKyn offspring. (A) Hippocampal KYNA: Significant three-way age x sex x prenatal condition interaction, significant sex x prenatal condition interaction; significant main effect of age, but not sex or prenatal treatment. (B) Frontal cortex KYNA: no significant age x sex x prenatal condition interaction; significant age x sex, age x prenatal treatment, sex x prenatal treatment interactions. All data are mean ± SEM. Post-hoc comparisons indicate *P < 0.05, **P < 0.01. N = 6 – 12 litters per group.
We also assessed KYNA levels in the frontal cortex of ECon and EKyn offspring across the lifespan. No three-way interaction between age X sex X prenatal treatment was determined (F2,98 = 2.8, P = 0.06) (Figure 3B). Significant age X sex (F2,98 = 4.4, P < 0.05), age X prenatal treatment (F2,98 = 4.0, P < 0.05) and sex X prenatal treatment (F1,98 = 6.0, P < 0.05) interactions were observed, but there was no main effect of age (F2,98 = 1.5, P = 0.22), sex (F1,98 = 0.8, P = 0.38) or prenatal treatment (F1,98 = 0.4, P = 0.55). At PD 21, female, but not male, EKyn offspring had significantly lower levels of KYNA compared to controls (P < 0.05). In contrast, KYNA levels in the frontal cortex of male EKyn offspring were elevated 2.0-fold compared to controls at PD 56 (P < 0.05). This finding was not observed in female offspring, indicating sexually dimorphic developmental trajectories following prenatal kynurenine treatment.
3.3. Hippocampus-dependent learning deficits on the Barnes maze in prenatal kynurenine-treated rats
To assess hippocampus-dependent learning and memory, adult ECon and EKyn animals were trained in the Barnes maze task for 3 consecutive days. Maze acquisition was assessed with escape latency, distance traveled, false escape entry errors, and mean speed. The latency to entering the escape box was not impacted by a three-way day X sex X prenatal treatment interaction (F2,128 = 0.2, P = 0.8) or by subsequent two-way analyses including day X sex (F2,128 = 0.03, P = 0.98), day X prenatal treatment (F2,128 = 1.6, P = 0.2) and sex X prenatal treatment interactions (F1,64 = 0.4, P = 0.52) (Figure 4A). Sex did not significantly impact escape latency during acquisition trials in the Barnes maze (F1,64 = 3.6, P = 0.06), however there was a main effect of training day (F2,128 = 30.5, P < 0.0001) and prenatal treatment (F1,64 = 10.6, P < 0.01). Post-hoc analyses revealed both male and female EKyn rats had longer escape latency times on trial days 2 and 3 (P < 0.05) compared to ECon controls. Additionally, both male and female ECon animals showed significantly lower escape latencies by day 2 compared to day 1 (P < 0.01), while EKyn animals did not have significantly lower escape latencies on either day 2 or 3 compared to day 1 of acquisition training.
Figure 4.
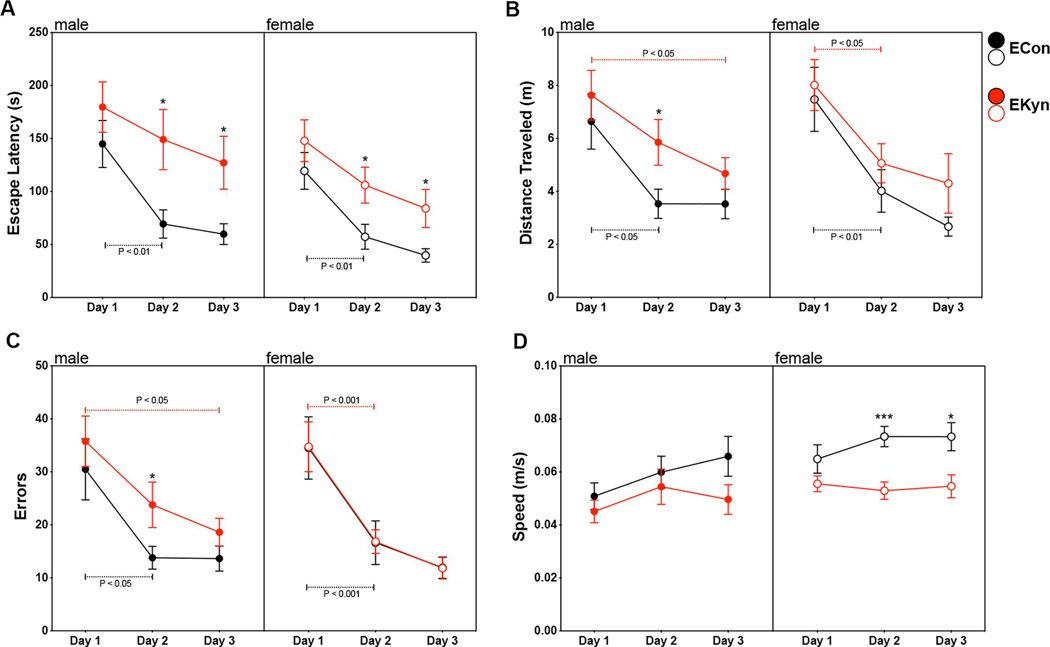
Spatial learning and memory deficits in adult EKyn rats. Animals were tested in the Barnes maze during adulthood (PD 56 – 85). Acquisition testing occurred across 3 days with the mean ± SEM of 2 trials per day shown. (A) Escape latency: main effect of training day and prenatal condition, but not sex. (B) Distance traveled: main effect of testing day, but not sex or prenatal treatment. (C) Errors committed: main effect of testing day, but not sex or prenatal condition (D) Average speed: main effect of testing day and prenatal condition, but not sex. All data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. N = 16 – 20 per group.
Analysis of the distance traveled during Barnes maze acquisition revealed a main effect of testing day (F1,64 = 32.17, P < 0.0001), but not of sex (F1,64 = 0.01, P = 0.94) or prenatal treatment (F1,64 = 3.6, P = 0.06) (Figure 4B). There were no significant interactions between day X sex (F2,128 = 0.8), P = 0.44), day X prenatal treatment (F2,128 = 0.5, P = 0.62), sex X prenatal treatment (F1,64 = 0.1, P = 0.76) or a three-way day X sex X prenatal treatment (F2,128 = 0.4, P = 0.66). Male EKyn animals traveled a greater distance compared to ECon on day 2 (P < 0.05), however there were no significant differences between female ECon and EKyn rats. Distance traveled was lower by day 2 compared to day 1 for both male (P < 0.05) and female (P < 0.01) ECon rats. Female EKyn rats (P < 0.05) also traveled a significantly shorter distance by the second day of testing, while male EKyn animals did not have significant improvements in the total distance traveled until day 3 (P < 0.05).
Barnes maze performance was also assessed by the number of errors or entries into false escape options before entering the actual escape box. We determined a significant main effect of day (F2,128 = 45.2, P < 0.0001) but not of sex (F1,64 = 0.3, P = 0.57) or prenatal treatment (F1,64 = 1.5, P = 0.43) (Figure 4C). No significant interactions between day X sex (F2,128 = 0.9, P = 0.43), day X prenatal treatment (F2,128 = 0.2, P = 0.81), sex X prenatal treatment (F1,64 = 1.4, P = 0.24) or day X sex X prenatal treatment (F2,128 = 0.2, P = 0.83) were found. Total errors were lower by day 2 compared to day 1 for male ECon (P < 0.05), female ECon (P < 0.01) and female EKyn rats (P < 0.05), while male EKyn rats did not have significantly fewer errors in the acquisition trials of the Barnes maze until day 3 (P < 0.05).
The mean speed at which rats completed acquisition trials in the Barnes maze was significantly impacted by day (F2,128 = 4.7, P < 0.05) and prenatal treatment (F1,64 = 9.0, P < 0.01) but not sex (F1,64 = 3.7, P = 0.06) (Figure 4D). There were no significant interactions between day X sex (F2,128 = 1.1, P =0.34), day X prenatal treatment (F2,128 = 2.1, P = 0.12), sex x prenatal treatment (F1,64 = 0.7, P = 0.41) or day X sex X prenatal treatment (F2,128 = 1.0, P = 0.36). Of note, it was the female ECon animals that were significantly faster than female EKyn animals on day 2 (P < 0.001) and day 3 (P < 0.05) while there were no significant differences between male ECon and EKyn animals.
3.4. EKyn impairments and sex differences on Barnes maze reversal trials
Reversal learning in the Barnes maze was tested on the fourth day, after 3 consecutive acquisition days. For this protocol, we also assessed the number of attempted entries into the previous escape location. A main effect of prenatal treatment on number of entries into the previous escape location was observed (F1,64 = 12.7, P < 0.01) but there were no significant effects of sex (F1,64 = 4.5, P = 0.06) or sex X prenatal treatment interaction (F1,64 = 0.1, P = 0.77) (Figure 5A). Male EKyn offspring entered the previous escape more often than the male ECon offspring (P < 0.05) while the female EKyn and ECon offspring were not significantly different. Escape latency during the reversal trials was significantly affected by sex (F1,61 = 4.8, P < 0.05) and prenatal treatment (F1,61 = 11.1, P < 0.01), but no significant interaction of sex X prenatal treatment was observed (F1,61 = 0.36, P = 0.55) (Figure 5B). Male EKyn animals had significantly higher escape latencies compared to male ECon animals (P < 0.05), while female EKyn animals were not significantly different from female ECon animals.
Figure 5.
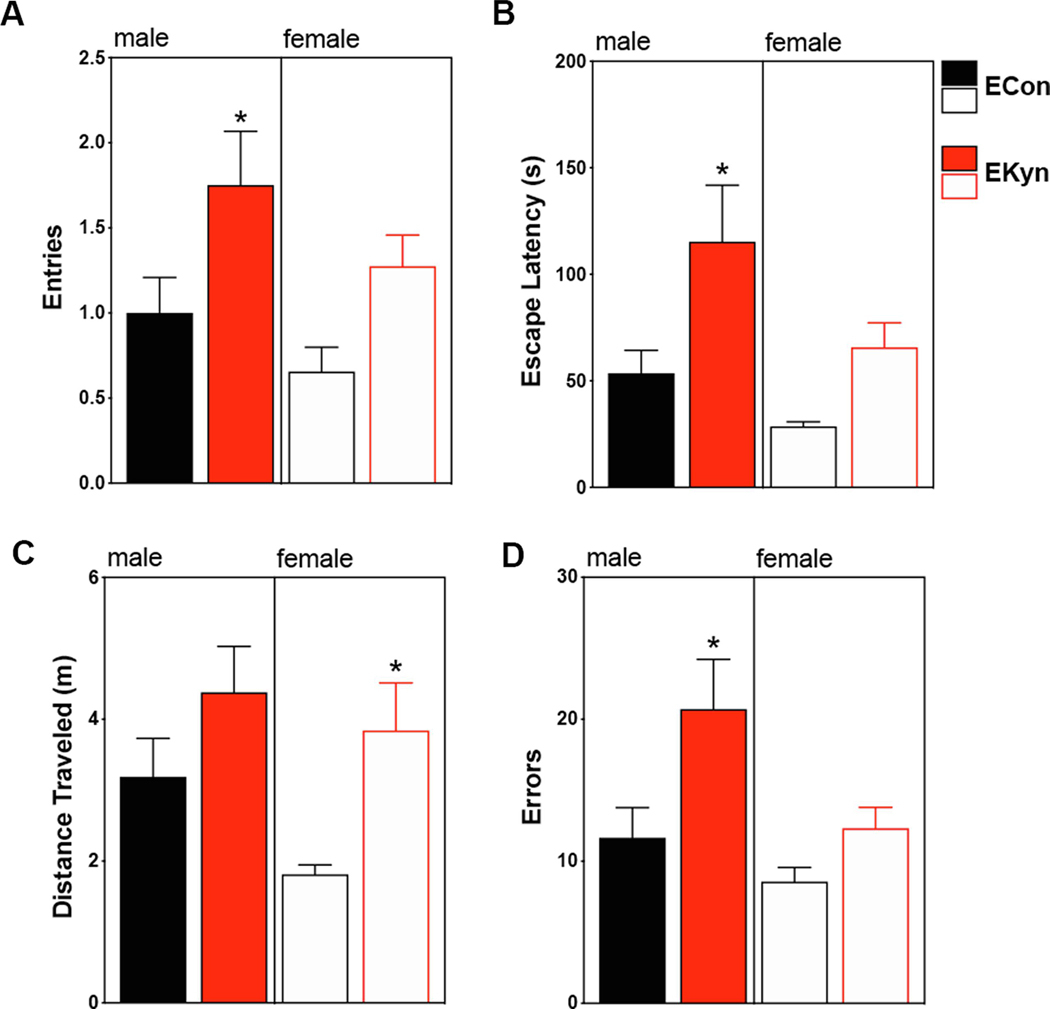
Reversal learning impairments in adult EKyn rats. After three days of acquisition learning in the Barnes maze, the escape location was altered to assess reversal learning. (A) Entries into the previous escape location: main effect of prenatal treatment, but not sex. (B) Escape latency: main effect of both prenatal treatment and sex. (C) Distance traveled: main effect of prenatal treatment, but not sex. (D) Errors committed: main effect of both prenatal treatment and sex. All data are mean ± SEM. *P < 0.05. N = 16 – 20 per group.
During the reversal trials, distance traveled was also significantly impacted by prenatal treatment (F1,64 = 8.0, P < 0.01) but not sex (F1,64 = 2.9, P = 0.1) or sex X prenatal treatment interaction (F1,64 = 0.5, P = 0.46) (Figure 5C). In this case, EKyn females traveled significantly greater distance than ECon females (P < 0.05) while EKyn and ECon males did not differ significantly. For total number of errors during the reversal trials, significant main effects of sex (F1,63 = 6.6, P < 0.05) and prenatal treatment (F1,63 = 8.9, P < 0.01) were found, but there was no significant sex X prenatal treatment interaction (F1,63 = 1.3, P = 0.25) (Figure 5D). Male EKyn rats committed more errors than the ECon males (P < 0.05) whereas the number of errors committed between EKyn and ECon females did not significantly differ.
3.5. EKyn rats continue to utilize random search strategy on the Barnes maze more than ECon rats
Analysis of video track plots obtained during acquisition and reversal trials of the Barnes maze allowed for assessment of the search strategies by which animals located the escape box. Representative track plots of all treatment groups and across days of the Barnes maze are shown in Figure 6. As described in Table 1, animals used either random, serial or direct search strategies to find the escape location. During the first day of training on the Barnes maze, all animals utilized almost exclusively random and serial search strategies to find the escape location (Figure 7). Day 1 search strategy was significantly impacted by prenatal treatment for the male (C2 (2) = 7.4, P < 0.05) but not female rats (C2 (2) = 4.6, P = 0.1), as measured by Chi-squared comparison. On the second and third days of training, both male and female ECon rats abandoned the random strategy in favor of the more efficient serial and direct strategies, but the EKyn rats typically failed to do so. This resulted in a significant effect of prenatal treatment on search strategy for training day 2 in both sexes (Male: C2 (2) = 6.6, P < 0.05, Female: C2 (2) = 7.2, p < 0.05) and training day 3 for males (C2 (2) = 8.1, P < 0.01) but not females (C2 (2) = 5.6, P = 0.06). On the reversal day, most animals utilized a serial strategy to find the new escape location, and there were no significant differences in search strategy between groups.
Figure 6.
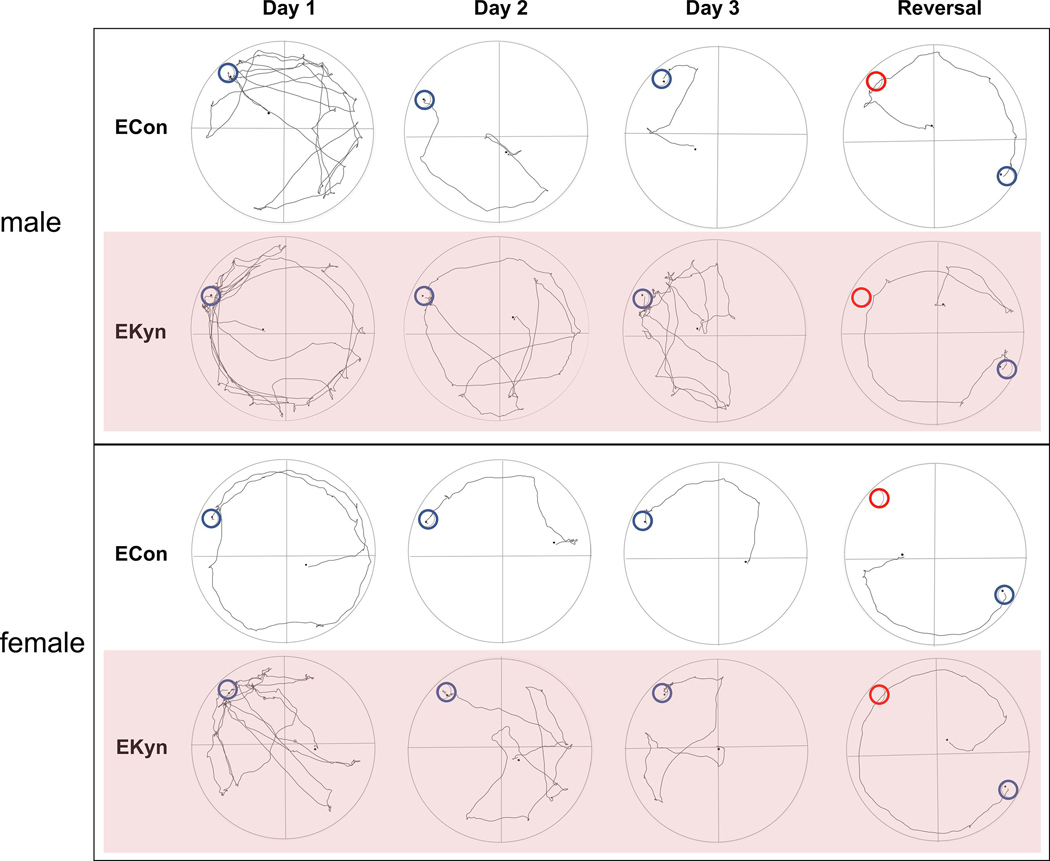
Representative track plots of rats tested in the Barnes maze across the three acquisition days and the reversal learning day. Escape location is circled in blue and, for the reversal learning day, the previous escape location is circled in red.
Figure 7.
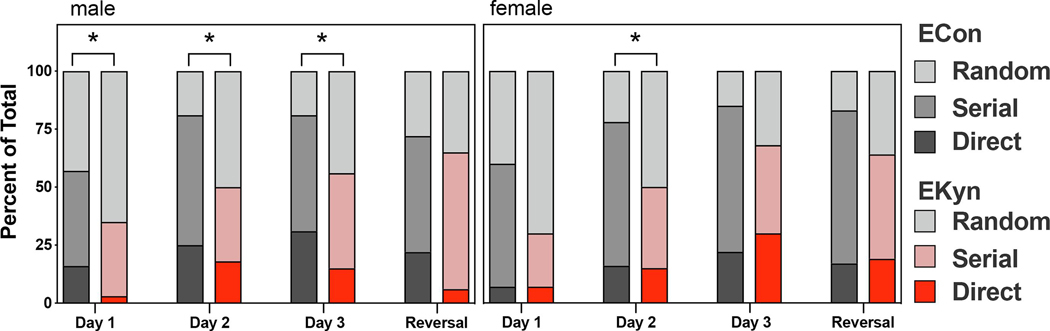
EKyn rats use less efficient search strategies in the Barnes maze. Stacked bar graphs depicting percentages of rats per group that utilized random, serial, or direct search strategies in finding the escape box across the four testing days. Analysis reveals that EKyn rats used the less efficient random strategy more often than the ECon rats. All data are mean ± SEM. *P < 0.05. N = 16 – 20 per group.
4. Discussion
We presently assessed hippocampus-dependent spatial learning and memory, prefrontal cortex-mediated reversal learning, and KP metabolism in both sexes of offspring from mothers that were treated with kynurenine prenatally. Using the Barnes maze, we determined stark acquisition learning and memory impairments in male EKyn offspring and deficiencies in reversal learning in both male and female EKyn animals. Specifically, in the brain, we saw increased KYNA in the hippocampus and frontal cortex of adult male EKyn offspring, but no changes in the EKyn females derived from the same litters. These are our first reported indications of sex differences in the long-term impact of prenatal kynurenine elevation in rats, suggesting a male-specific vulnerability to KYNA elevation and cognitive dysfunction.
The EKyn model was designed to recapitulate the impact of late gestational exposure to insults such as stress (Notarangelo and Schwarcz, 2016), sleep loss (Baratta et al., 2020), maternal immune activation (Clark et al., 2019), and others that induce an increase in KP metabolism and elevate fetal brain KYNA during the last week of gestation (Ortega et al., 2020; Pocivavsek et al., 2014). While it is known that kynurenine can cross both the placental (Goeden et al., 2017) and blood-brain barrier (Fukui et al., 1991) and be converted to KYNA, the exact mechanism by which KYNA accumulates prenatally has not been determined (Notarangelo et al., 2019). Despite prenatal exposure, our present data indicate that this KYNA increase disappears after birth and does not return until adulthood, indicating a post-pubertal change. Our studies with cross-fostered ECon and EKyn offspring demonstrate that maternal behavior does not influence later biochemical and behavioral outcomes (Pocivavsek et al. 2019). However, given that dams have been found to treat male and female offspring differently in the nest, any sex-specific confounds should be further investigated (Hao et al., 2011; Kosten & Nielsen, 2014). Given the male-specific KYNA change seen in adult offspring, we speculate a role of circulating androgens that may leave male animals more susceptible to KP metabolism disruptions (Baratta et al., 2018). Furthermore, the finding that tryptophan and kynurenine levels in the blood were not different in adulthood in EKyn rats indicates that the increased KYNA was a result of changes in KP metabolism within the brain (Gramsbergen et al., 1997). Future studies will investigate this and other possible mechanisms to uncover the cause of this sex-specific, and age-specific, result.
We have previously observed hippocampal learning impairments in EKyn rats on the passive avoidance paradigm and Morris water maze (Notarangelo and Pocivavsek, 2017; Pocivavsek et al., 2019; Pocivavsek et al., 2014). Specifically, in the water maze task, which relies on spatial navigation across multiple acquisition days, we found that male EKyn rats displayed an increased escape latency from the second day of training onwards. The stark similarities in training deficiencies reported presently after the second day of training further support the notion that male EKyn rats have difficulties in spatial learning and memory. The Barnes maze allowed us to challenge the animals with a reversal trial as well, and we determined that both male and female EKyn offspring were impaired compared to controls. These findings provide further support that EKyn offspring are impaired in cognitive flexibility, a prefrontal cortex-dependent function (Pershing et al., 2015). Of note, differences in behavior between EKyn and ECon offspring may also be influenced by non-mnemonic factors that may influence learning and memory, such as broad monitoring deficits and a narrowed attentional focus (Hahn et al. 2018).
We also determined a clear sex differences in Barnes maze performance between EKyn offspring. EKyn females were slower during the trials, but did not commit more errors, suggesting that females have more subtle deficiencies that relate to motor deficits or motivational impairments. Continued reliance on the less efficient random search strategy by EKyn offspring throughout the acquisition trials, rather than the more efficient serial and direct strategies (Betancourt et al., 2017; Rosenfeld and Ferguson, 2014), was also starker in EKyn males compared to EKyn females. While male rodents and humans tend to perform better on spatial memory tasks (Leger and Neill, 2016; Simpson and Kelly, 2012; Yuan et al., 2019), the observed resilience in female rats is consistent with other perinatal insult models (Andersen and Pouzet, 2004; Bath et al., 2017) and further supports our EKyn model as a viable tool to investigate the cognitive effects of perinatal disruptions.
The stark, and consistent, learning and memory impairments in adult male EKyn offspring may be related to the significant elevation in brain KYNA compared to controls. KYNA is an antagonist of NMDA and α7nACh receptors, which are both highly expressed from early development throughout the brain (Ben-Ari et al., 1997; Dwyer et al., 2009; Falk et al., 2002; Jantzie et al., 2015) and have been shown to play an outsized role in learning and memory (Levin et al., 2006; Robbins and Murphy, 2006). Recent studies have attempted to tease apart the individual roles of each receptor in spatial working memory. While knockout of the α7nAch receptor does not lead to spatial memory deficits in rodents (Azzopardi et al., 2013; Paylor et al., 1998), an inducible knockdown model produces spatial memory impairments on the Morris water maze in rats (Curzon et al., 2006), and administering the α7nAChR positive modulator galantamine reverses cognitive flexibility deficits after early life kynurenine exposure (Alexander et al., 2013; Alexander et al., 2012). Additionally, pharmacological inhibition and inflammation-induced loss of the NMDA receptor lead to impairments in spatial memory on the Barnes maze (Malikowska-Racia et al., 2018; Zhang et al., 2017) and the Morris water maze (Bye and McDonald, 2019; Ferretti et al., 2007; Liang et al., 1994), indicating that antagonism of both receptor targets of KYNA lead to impairments in cognitive function. While female animals displayed mild behavioral impairments as well, this was not accompanied by an increase in their brain KYNA. Previous studies have indicated that male EKyn offspring exhibit structural differences in the form of reduced dendritic spine density and deficiencies in glutamate release (Pershing et al., 2015; Pocivavsek et al., 2019). If present in female offspring, these changes may underlie the behavioral deficiencies observed in the absence of altered KYNA levels.
The sex differences observed in this study are intriguing given that they are in agreement with trends seen in SZ cognitive deficits and prevalence (Aleman et al., 2003; Leger and Neill, 2016; Mendrek and Mancini-Marie, 2016; Wickens et al., 2018), but it remains unclear why males are more susceptible to prenatal kynurenine elevation. One possibility is that males are more susceptible to prenatal disruptions in general. Multiple studies observing the effect of prenatal stress or maternal inflammation on offspring have shown male-specific behavioral, biochemical, and morphological alterations (Brunton and Russell, 2010; Carney, 2019; Hunter et al., 2019; Mueller and Bale, 2007; Mueller and Bale, 2008; Roussel et al., 2005; Zuena et al., 2008). Prenatal stress has been found to decrease expression of the X chromosome-linked gene O-linked N-acetylglucosamine transferase, which is normally expressed twice as much in females than males, indicating a possible role of sex-linked genes in male vulnerability (Howerton and Bale, 2014; Howerton et al., 2013). On the other hand, the observed sex differences may be due to inherent differences in KP metabolism (Baratta et al., 2018) or hippocampal neurogenesis (Yagi and Galea, 2019). Future studies are necessary to unravel the true mechanism behind these sexually-dimorphic results.
In an effort to determine if brain KP changes are reflected in the periphery, we also measured levels of tryptophan, kynurenine and KYNA in the plasma. Unlike in the central nervous system, peripheral changes to KP metabolism were not observed. This underscores the fact that peripheral measurements do not reflect brain changes and, to get an accurate picture of what is occurring in the brain, direct measurements need to be taken (Plitman et al., 2017; Sellgren et al., 2019). Special consideration should also be given to the time of day during which samples are collected as we learn more about the impact of circadian phase on the relationship between KYNA and behavior (Lewis et al. 2019; Rentschler et al. 2019).
The protocol used here systemically elevated kynurenine, so it is important to consider the alternative arm of the kynurenine pathway when interpreting these results. Kynurenine can also be converted by the enzyme kynurenine-3-monooxygenase (KMO) into 3-hydroxykynurenine and quinolinic acid, both of which have been found to act within the central nervous system (Schwarcz et al., 2012). While it is possible that these metabolites could play a role in the observed results, prenatal administration of a KMO inhibitor, Ro 61–8048, produced behavioral results similar to ours, as well as alterations in NMDA receptor subunit composition and hippocampal synaptic plasticity (Forrest et al., 2013a; Forrest et al., 2013b; Forrest et al., 2015; Khalil et al., 2014), reinforcing the idea that this is a KYNA-driven effect.
In conclusion, this study presents an impairment in hippocampus and prefrontal cortex-dependent memory after prenatal kynurenine elevation, and in male rats, this impairment is greater and coupled with elevations in hippocampus and frontal cortex KYNA. These findings highlight important sex differences in susceptibility to early life insults and confirm a link between KYNA and cognitive deficits. Ongoing investigations are continuing to utilize various pharmacological approaches to attenuate behavioral deficits in EKyn rats by interfering with de novo KYNA synthesis or function (Pocivavsek et al., 2019).
Supplementary Material
Highlights.
Kynurenine-laced food was fed to pregnant rats during the last week of gestation
Male, but not female, offspring from kynurenine fed mothers displayed elevated brain kynurenic acid during adulthood
Male offspring exposed to elevated prenatal kynurenine have significant and distinct impairments in learning and memory compared to females
5. Acknowledgements
The present study was supported in part by the National Institutes of Health [R01 NS102209; P50 MH103222] and a donation from the Clare E. Forbes Trust.
Abbreviations
- α7nACh α7
nicotinic acetylcholine
- ECon
Embryonic control
- ED
Embryonic day
- EKyn
Embryonic kynurenine
- KAT II
Kynurenine aminotransferase II
- KMO
Kynurenine-3-monooxygenase
- KP
Kynurenine pathway
- KYNA
Kynurenic acid
- NMDA
N-methyl-D-aspartate
- PD
Postnatal day
- SZ
Schizophrenia
Footnotes
Declarations of interest: none
Conflict of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Aleman A, Kahn RS, Selten JP, 2003. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 60, 565–571. doi: 10.1001/archpsyc.60.6.565 [DOI] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu H-Q, Pershing ML, Schwarcz R, Bruno JP, 2013. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience 238, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP, 2012. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 220, 627–637. doi: 10.1007/s00213-011-25392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JD, Pouzet B, 2004. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology 29, 1080–1090. doi: 10.1038/sj.npp.1300394 [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Ivleva EI, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, Francis AN, Tandon N, Bidesi AS, Witte B, Poudyal G, Pearlson GD, Sweeney JA, Clementz BA, Keshavan MS, Tamminga CA, 2015. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophr Bull 41, 233–249. doi: 10.1093/schbul/sbu009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi E, Typlt M, Jenkins B, Schmid S, 2013. Sensorimotor gating and spatial learning in alpha7-nicotinic receptor knockout mice. Genes Brain Behav 12, 414–423. doi: 10.1111/gbb.12038 [DOI] [PubMed] [Google Scholar]
- Baratta AM, Buck SA, Buchla AD, Fabian CB, Chen S, Mong JA, Pocivavsek A, 2018. Sex Differences in Hippocampal Memory and Kynurenic Acid Formation Following Acute Sleep Deprivation in Rats. Scientific Reports 8, 6963. doi: 10.1038/s41598-018-25288-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta AM, Kanyuch NR, Cole CA, Valafar H, Deslauriers J, Pocivavsek A, 2020. Acute sleep deprivation during pregnancy in rats: Rapid elevation of placental and fetal inflammation and kynurenic acid. Neurobiology of Stress 12, 100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, 1979. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of comparative and physiological psychology 93, 74. [DOI] [PubMed] [Google Scholar]
- Bath KG, Nitenson AS, Lichtman E, Lopez C, Chen W, Gallo M, Goodwill H, Manzano-Nieves G, 2017. Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiology of stress 7, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL, 1997. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci 20, 523–529. [DOI] [PubMed] [Google Scholar]
- Betancourt E, Wachtel J, Michaelos M, Haggerty M, Conforti J, Kritzer MF, 2017. The impact of biological sex and sex hormones on cognition in a rat model of early, pre-motor Parkinson’s disease. Neuroscience 345, 297–314. doi: 10.1016/j.neuroscience.2016.05.041 [DOI] [PubMed] [Google Scholar]
- Bobilev AM, Perez JM, Tamminga CA, 2019. Molecular alterations in the medial temporal lobe in schizophrenia. Schizophr Res. doi: 10.1016/j.schres.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ, 2010. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167, 261–280. doi: 10.1176/appi.ajp.2009.09030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton P, Russell J, 2010. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: Sex‐specific effects. Journal of neuroendocrinology 22, 258–271. [DOI] [PubMed] [Google Scholar]
- Bye CM, McDonald RJ, 2019. A Specific role of hippocampal NMDA receptors and arc protein in rapid encoding of novel environmental representations and a more general long-term consolidation function. Frontiers in behavioral neuroscience 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RS, 2019. Does Prenatal Exposure to Maternal Inflammation Causes Sex Differences in Schizophrenia-Related Behavioral Outcomes in Adult Rats? eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle D, Sham P, Murray R, 1998. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res 33, 179–183. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ, 2009. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res 201, 325–331. doi: 10.1016/j.bbr.2009.03.013 [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ, 2007. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33, 797–804. doi: 10.1093/schbul/sbl033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Notarangelo FM, Li X, Chen S, Schwarcz R, Tonelli LH, 2019. Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Progress in Neuro-Psychopharmacology and Biological Psychiatry 89, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Anderson DJ, Nikkel AL, Fox GB, Gopalakrishnan M, Decker MW, Bitner RS, 2006. Antisense knockdown of the rat alpha7 nicotinic acetylcholine receptor produces spatial memory impairment. Neurosci Lett 410, 15–19. doi: 10.1016/j.neulet.2006.09.061 [DOI] [PubMed] [Google Scholar]
- DeLisi LE, 2008. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull 34, 312–321. doi: 10.1093/schbul/sbm164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM, 2009. The dynamic effects of nicotine on the developing brain. Pharmacol Ther 122, 125–139. doi: 10.1016/j.pharmthera.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G, 2001. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313, 96–98. doi: 10.1016/s0304-3940(01)02242-x [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M, 2004. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry 56, 255–260. doi: 10.1016/j.biopsych.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellstrom-Lindahl E, 2002. The alpha7 nicotinic receptors in human fetal brain and spinal cord. J Neurochem 80, 457–465. doi: 10.1046/j.0022-3042.2001.00714.x [DOI] [PubMed] [Google Scholar]
- Ferretti V, Sargolini F, Oliverio A, Mele A, Roullet P, 2007. Effects of intra-accumbens NMDA and AMPA receptor antagonists on short-term spatial learning in the Morris water maze task. Behavioural brain research 179, 43–49. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Darlington LG, Stone TW, 2013a. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res 1504, 1–15. doi: 10.1016/j.brainres.2013.01.031 [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, Stone TW, 2013b. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience 254, 241–259. doi: 10.1016/j.neuroscience.2013.09.034 [DOI] [PubMed] [Google Scholar]
- Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW, 2015. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience 310, 91–105. doi: 10.1016/j.neuroscience.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR, 1991. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56, 2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x [DOI] [PubMed] [Google Scholar]
- Gal E, Sherman A, 1978. Synthesis and metabolism of L‐kynurenine in rat brain. Journal of neurochemistry 30, 607–613. [DOI] [PubMed] [Google Scholar]
- Goeden N, Notarangelo FM, Pocivavsek A, Beggiato S, Bonnin A, Schwarcz R, 2017. Prenatal dynamics of kynurenine pathway metabolism in mice: focus on kynurenic acid. Developmental neuroscience 39, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen JBP, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R, 1997. Brain‐specific modulation of kynurenic acid synthesis in the rat. Journal of neurochemistry 69, 290–298. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez‐Ferro M, Albuquerque EX, Schwarcz R, 2007. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 55, 78–92. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Okuno E, Schwarcz R, 1997. Characterization of rat brain kynurenine aminotransferases I and II. Journal of neuroscience research 50, 457–465. [DOI] [PubMed] [Google Scholar]
- Hahn B, Reneski CH, Pocivavsek A, Schwarcz R, 2018. Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood. Psychopharmacology 235, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, & Kosten TA, 2011. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Front Psychiatry, 2, 21. doi: 10.3389/fpsyt.2011.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM, 1998. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1, 318–323. doi: 10.1038/1137 [DOI] [PubMed] [Google Scholar]
- Howerton CL, Bale TL, 2014. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci U S A 111, 9639–9644. doi: 10.1073/pnas.1401203111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL, 2013. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci U S A 110, 5169–5174. doi: 10.1073/pnas.1300065110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Hoffman MC, D’Alessandro A, Noonan K, Wyrwa A, Freedman R, Law AJ, 2019. Male fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychological Medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE, 2015. Developmental expression of N-methyl-D-aspartate (NMDA) receptor subunits in human white and gray matter: potential mechanism of increased vulnerability in the immature brain. Cereb Cortex 25, 482–495. doi: 10.1093/cercor/bht246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JA, Mancini-Marie A, Lakis N, Rinaldi M, Mendrek A, 2010. Disturbed sexual dimorphism of brain activation during mental rotation in schizophrenia. Schizophr Res 122, 53–62. doi: 10.1016/j.schres.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC, 2011. Animal models of schizophrenia. Br J Pharmacol 164, 1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Bae G, Silverstein SM, 2013. Sex, symptom, and premorbid social functioning associated with perceptual organization dysfunction in schizophrenia. Front Psychol 4, 547. doi: 10.3389/fpsyg.2013.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil OS, Pisar M, Forrest CM, Vincenten MC, Darlington LG, Stone TW, 2014. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur J Neurosci 39, 1558–1571. doi: 10.1111/ejn.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Hintz K, Shearer EM, Barch DH, Riffin C, Whitley K, Butler R, 2010. A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med Hypotheses 74, 555–563. doi: 10.1016/j.mehy.2009.09.040 [DOI] [PubMed] [Google Scholar]
- Kosten TA, & Nielsen DA, 2014. Litter and sex effects on maternal behavior and DNA methylation of the Nr3c1 exon 17 promoter gene in hippocampus and cerebellum. Int J Dev Neurosci, 36, 5–12. doi: 10.1016/j.ijdevneu.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Neill JC, 2016. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev 68, 979–1000. doi: 10.1016/j.neubiorev.2016.06.029 [DOI] [PubMed] [Google Scholar]
- Leklem JE, 1971. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr 24, 659–672. doi: 10.1093/ajcn/24.6.659 [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH, 2006. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184, 523–539. doi: 10.1007/s00213-005-0164-7 [DOI] [PubMed] [Google Scholar]
- Lewis AM, Wagner NTJ, Rentschler KM, Buck SA, Baratta AM, Beggiato S, Mong JA, Pocivavsek A Differential impact of light or dark phase kynurenine challenge in male and female rats: Biochemical alterations and disruptions in sleep-wake behavior. Program No. 502.09 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019. Online. [Google Scholar]
- Li W, Ghose S, Gleason K, Begovic A, Perez J, Bartko J, Russo S, Wagner AD, Selemon L, Tamminga CA, 2015. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am J Psychiatry 172, 373–382. doi: 10.1176/appi.ajp.2014.14010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Hon W, Tyan Y-M, Liao W-L, 1994. Involvement of Hippocampal NMDA and AMPA Receptors in Acquisition, Formation and Retrieval. Chinese Journal of Physiology 37, 201–212. [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R, 2001. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 49, 487–499. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S, 2012. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull 38, 426–432. doi: 10.1093/schbul/sbq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF, 2014. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav 66, 298–308. doi: 10.1016/j.yhbeh.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikowska-Racia N, Podkowa A, Salat K, 2018. Phencyclidine and Scopolamine for Modeling Amnesia in Rodents: Direct Comparison with the Use of Barnes Maze Test and Contextual Fear Conditioning Test in Mice. Neurotox Res 34, 431–441. doi: 10.1007/s12640-018-9901-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marie A, 2016. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev 67, 57–78. doi: 10.1016/j.neubiorev.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, 2010. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 90, 285–326. doi: 10.1016/j.pneurobio.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S, 2006. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res 1073–1074, 25–37. doi: 10.1016/j.brainres.2005.12.056 [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL, 2007. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 91, 55–65. doi: 10.1016/j.physbeh.2007.01.017 [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL, 2008. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience 28, 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, Nordin C, Karanti A, Persson P, Erhardt S, 2005. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res 80, 315–322. doi: 10.1016/j.schres.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Notarangelo FM, Beggiato S, Schwarcz R, 2019. Assessment of Prenatal Kynurenine Metabolism Using Tissue Slices: Focus on the Neosynthesis of Kynurenic Acid in Mice. Developmental neuroscience, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Pocivavsek A, 2017. Elevated kynurenine pathway metabolism during neurodevelopment: implications for brain and behavior. Neuropharmacology 112, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Schwarcz R, 2016. Restraint stress during pregnancy rapidly raises kynurenic acid levels in mouse placenta and fetal brain. Developmental neuroscience 38, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega DR, Rodríguez PO, Pineda B, Esquivel DFG, Chávez LAR, Cervantes GIV, Roldán GR, de la Cruz GP, Ruiz AD, Armenta MM, 2020. Kynurenine Pathway as a New Target of Cognitive Impairment Induced by Lead Toxicity During the Lactation. Scientific Reports 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A, 1998. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem 5, 302–316. [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jørgensen CV, Vunck SA, Leuner B, Schwarcz R, Bruno JP, 2015. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology 90, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Phenis D, Valentini V, Pocivavsek A, Lindquist DH, Schwarcz R, Bruno JP, 2016. Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology 233, 3725–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, Kim J, Takeuchi H, Chakravarty MM, Remington G, 2017. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophrenia bulletin 43, 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Baratta AM, Mong JA, Viechweg SS, 2017. Acute Kynurenine Challenge Disrupts Sleep-Wake Architecture and Impairs Contextual Memory in Adult Rats. Sleep 40. doi: 10.1093/sleep/zsx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Elmer GI, Schwarcz R, 2019. Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus 29, 73–77. doi: 10.1002/hipo.23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R, 2014. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology 231, 2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R, 2011. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36, 2357–2367. doi: 10.1038/npp.2011.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler K, Baratta AM, Ditty AL, Lewis AM, Wagner NTJ, Wright CJ, Mong JA, Pocivavsek A An experimental system of prenatal kynurenine elevations: Distinct sex-dependent alterations in brain kynurenic acid and sleep disturbances in adulthood. Program No. 502.10 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019. Online. [Google Scholar]
- Robbins TW, Murphy ER, 2006. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci 27, 141–148. doi: 10.1016/j.tips.2006.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Ferguson SA, 2014. Barnes maze testing strategies with small and large rodent models. J Vis Exp, e51194. doi: 10.3791/51194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel S, Boissy A, Montigny D, Hemsworth P, Duvaux-Ponter C, 2005. Genderspecific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Hormones and behavior 47, 256–266. [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R, 2011. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 37, 1147–1156. doi: 10.1093/schbul/sbq112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nature Reviews Neuroscience 13, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC, 2001. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50, 521–530. [DOI] [PubMed] [Google Scholar]
- Sellgren CM, Gracias J, Jungholm O, Perlis RH, Engberg G, Schwieler L, Landen M, Erhardt S, 2019. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Translational psychiatry 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R, 2003. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology 28, 1454–1462. doi: 10.1038/sj.npp.1300188 [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP, 2012. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res 229, 289300. doi: 10.1016/j.bbr.2011.12.036 [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA, 2006. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 188, 510–518. doi: 10.1192/bjp.188.6.510 [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD, 2010. The hippocampal formation in schizophrenia. Am J Psychiatry 167, 1178–1193. doi: 10.1176/appi.ajp.2010.09081187 [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Levin ED, 2011. Glutamate and nicotinic receptor interactions in working memory: importance for the cognitive impairment of schizophrenia. Neuroscience 195, 21–36. doi: 10.1016/j.neuroscience.2011.08.038 [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP, 1998. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry 172, 324–326. [DOI] [PubMed] [Google Scholar]
- Wickens MM, Bangasser DA, Briand LA, 2018. Sex differences in psychiatric disease: a focus on the glutamate system. Frontiers in molecular neuroscience 11, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Galea LA, 2019. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Kong F, Luo Y, Zeng S, Lan J, You X, 2019. Gender Differences in LargeScale and Small-Scale Spatial Ability: A Systematic Review Based on Behavioral and Neuroimaging Research. Front Behav Neurosci 13, 128. doi: 10.3389/fnbeh.2019.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang X, Ai S, Ouyang W, Le Y, Tong J, 2017. Sepsis-induced selective loss of NMDA receptors modulates hippocampal neuropathology in surviving septic mice. PLoS One 12, e0188273. doi: 10.1371/journal.pone.0188273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, 2008. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PloS one 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


