Abstract
Melanoma progression is generally associated with increased transcriptional activity mediated by the Yes‐associated protein (YAP). Mechanical signals from the extracellular matrix are sensed by YAP, which then activates the expression of proliferative genes, promoting melanoma progression and drug resistance. Which extracellular signals induce mechanotransduction, and how this is mediated, is not completely understood. Here, using secretome analyses, we reveal the extracellular accumulation of amyloidogenic proteins, i.e. premelanosome protein (PMEL), in metastatic melanoma, together with proteins that assist amyloid maturation into fibrils. We also confirm the accumulation of amyloid‐like aggregates, similar to those detected in Alzheimer disease, in metastatic cell lines, as well as in human melanoma biopsies. Mechanistically, beta‐secretase 2 (BACE2) regulates the maturation of these aggregates, which in turn induce YAP activity. We also demonstrate that recombinant PMEL fibrils are sufficient to induce mechanotransduction, triggering YAP signaling. Finally, we demonstrate that BACE inhibition affects cell proliferation and increases drug sensitivity, highlighting the importance of amyloids for melanoma survival, and the use of beta‐secretase inhibitors as potential therapeutic approach for metastatic melanoma.
Keywords: amyloid, BACE2, mechanosignaling, metastasis, secretome
Subject Categories: Cancer, Molecular Biology of Disease, Signal Transduction
The accumulation of PMEL fibrils in the metastatic melanoma microenvironment modulates YAP activity. Preventing aggregate formation reduces metastatic cell proliferation and chemosensitivity, suggesting beta‐secretase inhibition as treatment for metastatic melanoma.

Introduction
Melanoma is the most aggressive cutaneous cancer, resulting from the transformation and proliferation of skin melanocytes (Shain & Bastian, 2016). While melanoma only accounts for 1% of skin cancers, it is responsible for the majority of skin cancer deaths with an incidence in rapid increase over the past 30 years. In Europe, melanoma accounts for about 150,000 new cases and 27,147 deaths/year (Globocan 2018, https://gco.iarc.fr/today/fact-sheets-populations). Approximately 85% of melanomas are diagnosed at early stages when the tumor is thin and surgery is curative in > 95% of cases (American Cancer Society, www.cancer.org). For advanced disease, either unresectable or already metastatic, the therapeutic landscape has benefitted since few years from an unprecedented number of new drugs (e.g. immune checkpoint inhibitors and small‐molecule targeted drugs) which have significantly improved the prognosis of advanced patient which is otherwise dismal. That notwithstanding, < 30% of these cases reaches the 5‐year landmark disease free, clearly indicating that a deeper insight into the biology of melanomas is an unmet clinical need (Guy et al, 2015; Pasquali et al, 2018). The main cause of death is widespread metastases, which commonly develop in regional lymph nodes or in distant organs. Melanoma cells travel along external vessel lattices by regulating adhesion molecules, matrix metalloproteases, chemokines, and growth factors. After steadied in the metastatic sites, melanoma cells develop mechanisms that protect them against the attack of the immune system (Zbytek et al, 2008).
Progression to metastatic melanoma is accompanied by increased cell stiffness and the acquisition of higher plasticity by tumor cells, due to their ability to control stiffness in response to diverse adhesion conditions (Weder et al, 2014). During melanoma development, tumor cells are exposed to various types of extracellular matrix (ECM) such as tenascin‐C, fibronectin (Frey et al, 2011), and collagen fibers which led to an overall more rigid tumor microenvironment (Yu et al, 2011). Stiffness was suggested to control phenotypic states and to contribute to the acquisition of a malignant phenotype. Indeed, in epithelial cancers, an extracellular environment characterized by softer matrix enables differentiation, while a stiffer matrix increases proliferation (Lee et al, 2017). Increased ECM rigidity might also serve as “safe haven” for melanoma cells, protecting them from the effects of chemotherapy; as such, these drug‐induced biomechanical niches foster tumor growth and residual disease favoring melanoma resistance (Hirata et al, 2015). It was observed that BRAF inhibitors do not only act on tumor cells but also on the neighboring tumor fibroblasts, paradoxically activating them to produce a stiff, collagen‐rich ECM. Melanoma cells fast respond to this new microenvironment by increasing ECM attachment, and reactivating MAPK signaling in a BRAF‐independent manner. Understanding how cancer cell‐derived ECM is regulated, and how it participates in tumor microenvironment remodeling and signaling is critical for developing novel cancer treatment strategies. In this context, secretome studies from tumor and stromal cells provide novel insights in the understanding of the cross‐talk between cells within the tumor microenvironment, since they are very sensitive in revealing the key effectors required for the establishment of pre‐metastatic niches (Kaplan et al, 2005; Karagiannis et al, 2010; Blanco et al, 2012).
We therefore sought to explore tumor melanoma microenvironment by secretome analysis, investigating the molecular mechanism behind malignant matrix stiffening.
Results
In vitro model of metastatic and primitive melanoma
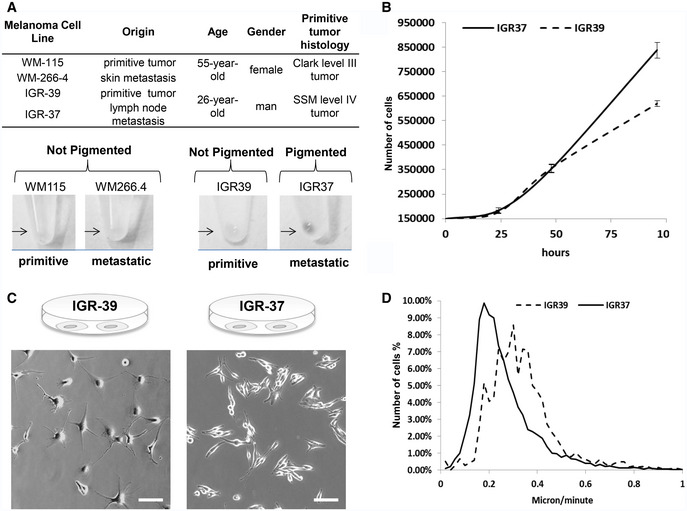
With the aim to understand the functional pathways that differentiate tumor microenvironment of metastatic and primitive phenotype, we investigated two pairs of matched melanoma cell lines. In particular, IGR39 and IGR37 were derived from primitive tumor and lymph node metastasis, respectively, collected from the same 26‐year‐old male patient. Similarly, WM115 and WM266.4 matched cell lines were derived from cutaneous primitive tumor and skin metastasis, respectively, from the same 55‐year‐old female patient (Fig 1A). Despite their common origin, these cell lines display different phenotypes. In both cases, metastasis‐derived cell lines showed a faster growth rate and increased ability to undergo rapid division compared to the matched primitive tumor‐derived cell line (Figs 1B and EV1A). Differences in morphology were also denoted between the two matched cell lines: Metastasis‐derived IGR37 appeared with a short, elongated shape with a spontaneous predisposition to form clusters, while primitive tumor‐derived IGR39 remained commonly isolated, displaying higher number of branches and branch elongations (Fig 1C). On the other hand, IGR39 had higher mobility compared with IGR37 when monitored live using time‐lapse microscopy (Fig 1D, Movie EV1 and EV2). All these data suggest that cells isolated from metastatic tumors grow faster, but move slower than primitive tumors, symptomatic of a proliferative phenotype (Hoek et al, 2008) for IGR37 and an invasive phenotype for IGR39.
Figure 1. Analysis of a cellular system for primitive and metastatic melanoma.
-
ACharacteristics of IGR39/IGR37 and WM115/WM266.4 melanoma cell lines (Upper Panel). Pigmentation of melanoma cells pellets (lower panel).
-
BGrowth curve of primitive IGR39 and metastatic IGR37 cell line (N = 3 biological replicates). Data are mean ± SD.
-
CPhase‐contrast images of IGR39 and IGR37 cells. Scale bar is 100 μm.
-
DAnalysis of IGR39 and IGR37 speed of migration by time‐lapse microscopy.
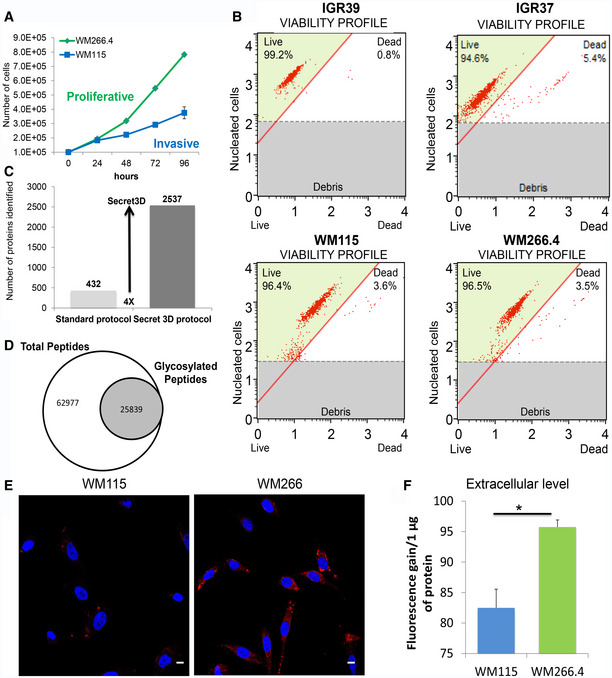
Figure EV1. (Related to Figs 1 and 2) Analysis of a model system for primitive and metastatic melanoma, Secret3D and Proteostat analysis.
-
AGrowth curve of primitive WM115 and metastatic WM266.4 cell line (N = 3 biological replicates) measured as number of cells/time.
-
BViability profile of IGR and WM cell lines after 24‐h starvation, detected by measuring cell confluence (%) and number of dead and alive cells using Muse™ Cell Analyzer.
-
CNumber of proteins identified in the secretome by Secret3D compared to a standard protocol.
-
DNumber of total and glycosylated peptides identified by Secret3D.
-
EConfocal immunofluorescence images of Proteostat (1:2,000, red spots) and DAPI staining (blue) for WM115 and WM266.4 cells. Scale bar is 10 μm.
-
FQuantitation of aggregates in the extracellular space in WMs. Fluorescence gain of proteins present in the supernatant treated with Proteostat reagent. (T‐test, *P < 0.05) N = 4 biological replicates. Data are mean ± SD.
Global analysis of secreted proteins reveals specific signatures of tumor microenvironment
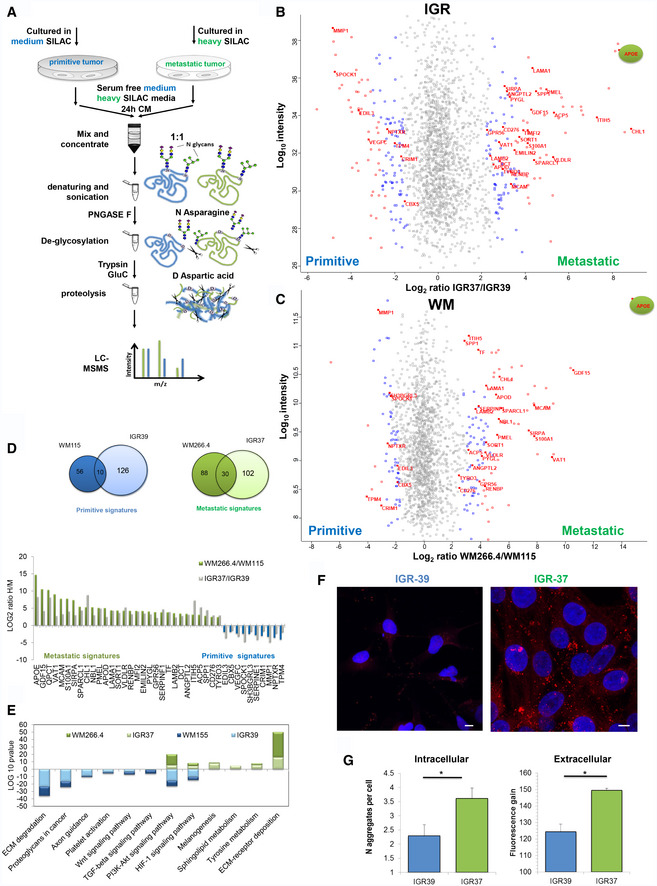
To investigate the molecular composition of melanoma secretome, we performed a global analysis of the proteins secreted by metastasis‐derived (IGR37 and WM266.4) and primitive tumor‐derived (IGR39 and WM115) cells lines. To differentiate between proteins that were secreted versus the ones present in the serum from cultured conditions, a triple SILAC was performed. We labeled the proteins coming from primitive and metastatic cell line, respectively, with medium and heavy amino acids (Fig 2A). For each sample, we analyzed the conditioned medium (CM) after 24 h of serum deprivation to avoid contamination of high abundant proteins as albumin. We checked for the absence of proteins derived from dead cells by measuring the viability of the cell lines upon starvation, and we confirmed that none of them suffered that condition (viability > 95%, Fig EV1B). As far as secreted proteins are highly glycosylated and this modification might mask proteolytic sites hampering protein digestion, we set up a novel method, named Secret3D (Secretome De‐glycosylation Double Digestion protocol), where a de‐glycosylation step (PNGase) was added prior to protein digestion performed with double proteolysis to increase protein coverage. Our method enables the unambiguous identification of secreted proteins with high efficiency and quantitative accuracy (Dataset EV1 and EV2). As reported in Fig 2A, by examining an equivalent of 500,000 cells, we identified 2,356 proteins in the SILAC IGR37/IGR39, and 2,157 proteins in the SILAC WM266.4/WM115, increasing four times the yield compared to digestion without de‐glycosylation (Fig EV1C, Dataset EV3 and EV4, Peptide Atlas repository; glycosylated peptides represent about one‐fourth of the entire dataset, Fig EV1D), and improving the proteome coverage if compared to existing methods (Liberato et al, 2018). All proteomics analyses were done in biological duplicate, and for each biological replicate two technical replicates were performed. By statistical analysis, 270 proteins were found to be differentially secreted in IGR39 versus IGR37. A parallel analysis was conducted in WM115 versus WM 266.4 cells where the number of differentially secreted protein was 185 (Fig 2B and C, Dataset EV1 and EV2). Results were clustered in order to highlight secretome commonalities. Despite showing only a partial overlap of the differentially secreted proteins (Fig 2D), both primitive tumor‐derived cell lines specifically secrete proteins belonging to ECM matrix degradation (MMP1), Wnt signaling pathway (WNT5a), TGFB signaling pathway (TPM4), proteoglycan degradation (SPOCK1), and platelet activation (VEGFC, SERPINE1, EDIL3). These proteins are in agreement with their invasive phenotype, as primitive tumor cells are able to move and invade through the basement membrane or through the vessels walls (Fig 1D).
Figure 2. Proteomic analysis of the secretome from primitive and metastatic melanoma cells.
-
AMS workflow of Secret3D: Secretome De‐glycosylation Double Digestion protocol.
-
BScatter plot of identified and quantified proteins in the secretome of primitive IGR39 and metastatic IGR37. Red dots represent proteins that were significant with FDR < 0.05, and blue dots represent proteins with P < 0.05.
-
CScatter plot of identified and quantified proteins in the secretome of primitive WM115 and metastatic WM266.4. Red dots represent proteins that were significant with FDR < 0.05, and blue dots represent proteins with P < 0.05.
-
D(Left) Venn diagram of the significant proteins shared by both IGR and WM cell lines. (Right) Histograms representing the metastatic and primitive signatures H/M ratios.
-
EKEGG pathway enrichment analysis of the significant proteins.
-
FConfocal fluorescence images of Proteostat (1:2,000, red spots) and DAPI staining (blue), scale bar is 10 μm.
-
GQuantitation of aggregates/cell in IGRs cell lines by immunofluorescence analysis, left panel; fluorescence gain of soluble proteins treated with Proteostat reagent, right panel. (T‐test analysis, *P < 0.05, N = 3 biological replicates, data are mean ± SD).
Conversely, metastasis‐derived cell lines secrete specific proteins belonging to ECM deposition (LAMA1, LAMB2, SPP1), cell adhesion molecule (MCAM, CD276, EMILIN2), lipid transporters (APOE, APOD, PLTP, VLDLR), and melanogenesis‐related proteins (DCT, KIT1, PMEL; Fig 2D and E). Interestingly, APOE is the most secreted proteins in both metastatic cell lines. APOE is a lipoprotein whose primary function is transporting cholesterol, but it also controls the formation of protein aggregates in Alzheimer disease (AD) through the regulation of amyloid‐β (Aβ) metabolism, aggregation, and deposition. Together with APOE, in metastatic secretome, we found enriched SORT1 and QPCT, proteins known to have a role in facilitating Aβ metabolism (Gunn et al, 2010; Morawski et al, 2014). Notably, in pigmented cells, PMEL maturation into fibrils is also mediated by APOE and shares similarities with amyloid‐β maturation (Van Niel et al, 2015). PMEL amyloid fibrils are known to serve as scaffold for the polymerization of melanin within melanosomes. We found all the above proteins, PMEL included, enriched in the extracellular space highlighting the possibility of fibril formation extracellularly, i.e. plaques.
To test this hypothesis, we used a protein aggregation detection dye (Proteostat). Both pairs of IRGs and WMs matched cell lines were explored: Proteostat staining highlighted an enrichment of aggregated proteins in the metastasis‐ versus primitive tumor‐derived cell lines both intracellularly and in the extracellular space (Figs 2F and G, EV1E and F). To note, amyloid fibrils, together with adhesive proteins, may contribute to the formation of a highly fibrotic extracellular environment specifically in the metastatic cell lines.
Proliferative and invasive protein signatures are conserved across melanoma cell lines
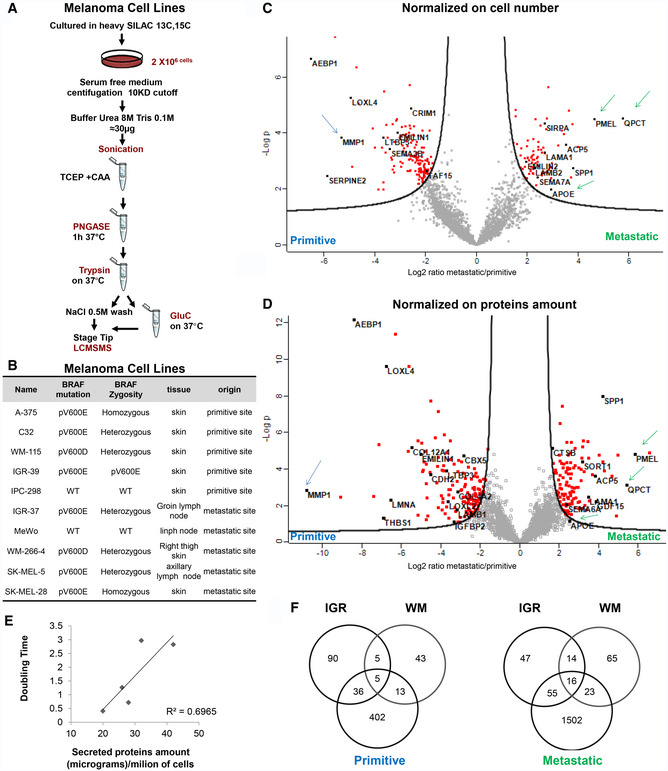
The secretome signature that distinguishes primitive versus metastatic melanoma was further validated on another cohort of cell lines derived from different patients (Fig EV2A). The secretion rate of all the cell lines analyzed (Fig EV2B) differs greatly from each other and inversely correlates with growth rate (Fig EV2C). Analysis was performed using a mixed model based on merged results from dataset normalized either by total number of cells or total protein content. We selected the proteins that were statistically significantly regulated with both approaches. Despite different melanoma cells have different doubling times, different shapes, and different protein secretion rates, we observed highly conserved metastatic and primitive signatures (Fig EV2D and E, [Link], [Link], [Link], [Link], Peptide Atlas repository). Among the differentially secreted proteins, five were conserved in all the primitive cell lines analyzed: MMP1, CBX5, NPTXR, CRIM1, and TPM4, and sixteen proteins were present in all the metastatic cell lines. In accordance with our previous data, APOE, PMEL, QPCT, and SORT1 were specifically secreted in all metastatic tumor cell lines together with proteins involved in ECM deposition and adhesive proteins supporting the hypothesis of amyloid fibril deposition (Fig EV2F).
Figure EV2. (Related to Fig 2) Proteomic analysis of the secretome from primitive and metastatic melanoma cells.
-
AMS workflow of Secret3D applied on melanoma cell lines described in (B).
-
BCell lines used in this study. Their origin and BRAF mutation status are reported.
-
C, DSecretome analysis of A375, C32, IPC298, SKMEL5, SKMEL28, MEWO melanoma cell lines described in (B). Volcano plot of the quantified proteins with both normalization strategies discussed in the main text. Green arrows indicate proteins overexpressed in metastatic cells, and blue arrows indicate proteins overexpressed in primary cells.
-
EScatter plot of secreted proteins versus doubling time of the cell lines analyzed.
-
FVenn diagram of the significant proteins shared by all melanoma cell lines.
By interrogating the same melanoma cell lines analyzed above in Broad‐Novartis Cancer Cell Line Encyclopedia (CCLE), the proteins involved in amyloid fibrils maturation found enriched in the metastatic cell lines were found also enriched at the transcriptomic level (Fig EV3A). Together with the conserved secretome profile, we found other proteins involved in protein aggregation, such as APP, APLP2, and APLP1, secreted by melanoma in a cell line‐specific manner. Formation of protein aggregates in the metastatic cell lines was visualized with Proteostat (Fig EV3B).
Figure EV3. (Related to Figs 3 and 4) Gene expression and protein aggregates level in primitive vs. metastatic melanoma cell lines. Mosaic acquisitions of melanoma metastases. Expression profiling by cancer type and overall survival analysis for BACE2 in melanoma patients. Cell viability in IGRs upon BACE inhibition.
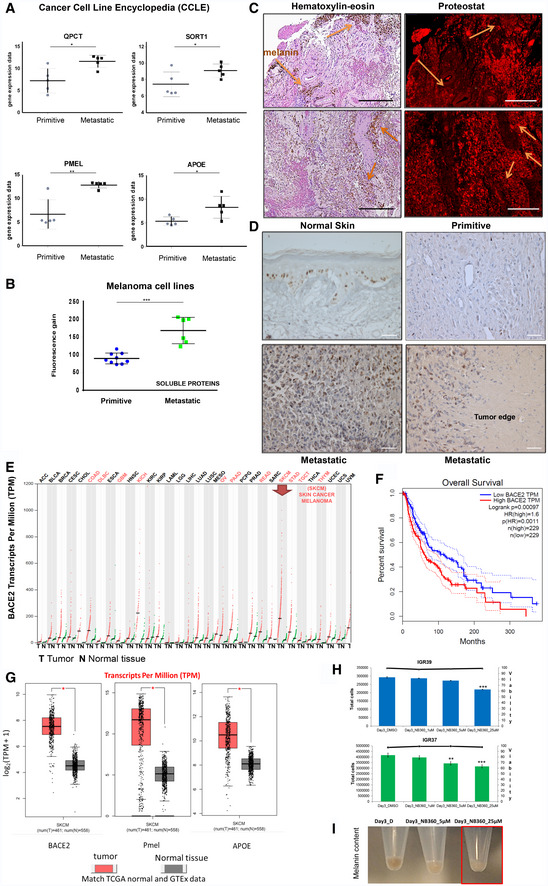
-
AGene expression data from the Cancer Cell Line Encyclopedia (CCLE). Gene expression level for the selected genes was searched in the same melanoma cell lines used for proteomic analysis. Primitive melanoma cell lines were WM115, IGR39, A375, C32, and IPC298, while metastatic melanoma cell lines were WM266.4, IGR37, SKMEL5, SKMEL28, and MEWO. T‐test, *P < 0.05, **P < 0.001. Data are expressed as mean ± SD.
-
BAnalysis of protein aggregates present in the secretome of 10 different melanoma cell lines. Fluorescence gain of proteins present in the supernatant treated with Proteostat reagent is reported. T‐test analysis, ***P < 0.001. Data are mean ± SD.
-
CMosaic acquisition of melanoma metastases in human brain stained with hematoxylin–eosin or with Proteostat. The arrows point at melanin signals. Scale bar is 300 μm.
-
DKI‐67 staining (brown) in healthy tissue, primitive melanoma and metastatic melanoma as indicated. Scale bar is 50 μm.
-
EBACE2 expression profiling by cancer type in TCGA normal and GTEx dataset by using GEPIA software (http://gepia.cancer-pku.cn/). The gene expression profile across all tumor samples (red dots) and paired normal tissues (green dots). Each dot represents expression of samples.
-
FOverall survival analyses performed using the GEPIA online platform for melanoma dataset. The solid line represents the survival curve, and the dotted line represents the 95% confidence interval. Log‐rank P < 0.05 was considered to indicate a statistically significant difference. Patients with expression above the median are indicated by red lines, and patients with expression below the median are indicated by blue lines. BACE2 expression level (Transcript Per Million, TPM) is negatively associated with the overall survival of melanoma patients.
-
GGene expression level (Transcript Per Million, TPM) in cancer/normal tissue for the selected genes extracted from TCGA normal and GTEx data in melanoma dataset contained in GEPIA software. T‐test: *P < 0.05.
-
HCell viability of IGR cell lines treated with NB‐360 at different concentrations and time of incubation as reported. N = 3, T‐test analysis. **P < 0.01; ***P < 0.001. The histogram represents total cell number. The horizontal line represents % of alive cells.
-
IPigmentation of IGR37 upon incubation with NB‐360. Red box shows depigmentation after NB‐360 administration.
APOE and PMEL proteins are overrepresented in the secretome of metastatic melanoma
As discussed before, APOE is the most abundant protein in metastatic secretome. APOE is known to be regulated by liverX receptor (LXR) which is activated by 24‐ and 25‐hydroxycholesterol. In order to verify the involvement of APOE and of cholesterol metabolites in metastatic melanoma, we measured the level of oxysterols in melanoma cells. Indeed, both 24 and 25‐hydroxycholesterol were more abundant in metastatic melanoma cells than in primitive tumor cells, thus possibly explaining the proteomic data in matched cell lines (Fig 3A). Importantly, these data were confirmed also in the other cohort of melanoma cells, where 24‐hydroxycholesterol showed the best correlation with APOE levels (Fig 3B), indicating a specific cholesterol metabolism activation in metastatic melanoma. These evidences sustain the activation of LXR receptor in the regulation of APOE expression in metastatic melanoma, similarly to what reported in astrocytes by Abildayeva and coworkers (Abildayeva et al, 2006), thus enhancing the maturation of PMEL into amyloid fibrils.
Figure 3. Oxysterol quantification in primitive and metastatic melanoma cells and PMEL expression in IGR37 and IGR39 melanoma cell line.
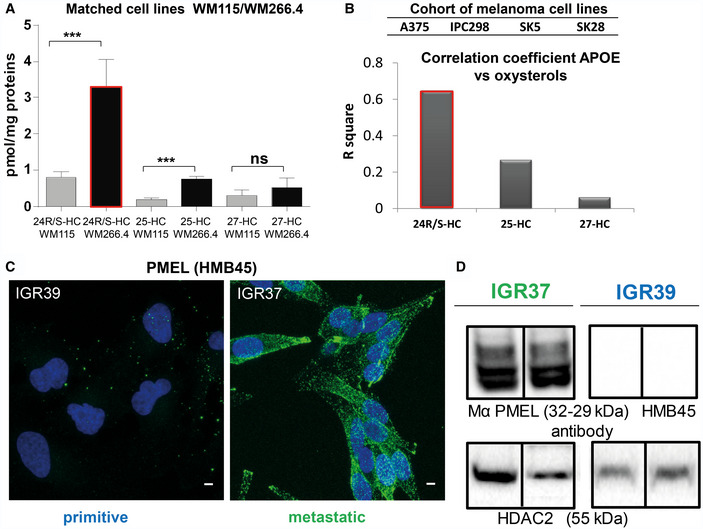
-
AAbsolute quantitation of 24‐, 25‐ and 27‐hydroxycholesterol in primitive and metastatic melanoma cells as indicated. (T‐test analysis, ***P < 0.001, N = 3 biological replicates, data are mean ± SD).
-
BHistogram representing R square of correlation analysis between absolute quantitation of 24‐, 25‐ and 27‐hydroxycholesterol and label free quantitation of APOE in a cohort of primitive (A375, IPC298) and metastatic (SKMEL5, SKMEL28) melanoma cell lines.
-
CConfocal fluorescence images of anti‐HMB45 PMEL antibody signal (green) and DAPI staining (blue) in IGR37 and IGR39. Scale bar is 10 μm.
-
DWestern blot on IGR37 and 39 cellular lysates probed with anti‐HMB45 PMEL antibody and anti HDAC2 to check the loading of similar amount of total lysates.
Source data are available online for this figure.
We then verified the presence of PMEL amyloidogenic fragments in metastatic melanoma cells by Western blot analysis using an antibody that recognizes the mature form of the protein. As reported in Fig 3C, PMEL is exclusively expressed in metastatic cells and not in their primitive counterparts and the molecular weight corresponds to the mature form.
Amyloid‐like aggregated proteins accumulate in metastatic lesions of melanoma patients
Starting from the observation that amyloid‐like aggregated proteins were found enriched particularly in the secretome of metastatic melanoma cell lines, we explored if protein aggregates are present also in melanoma patients’ tissues. To this aim, we examined samples deriving from primitive tumors and metastases. Archival formalin‐fixed paraffin‐embedded (FFPE) specimens collected from skin primitive tumors and differently localized metastatic sites (i.e. skin, brain, and lung) were stained with Proteostat and analyzed by high‐resolution large‐scale mosaic/confocal imaging. In both primitive and metastatic tumor samples, we detected a weak or absent signal of protein aggregates localized in the healthy region surrounding tumor tissue (Fig 4A). Conversely, in primitive tumors, protein aggregates were found spreading along the tissues in small isolated regions inside the tumor area (Fig 4B). Interestingly, a much higher representation of protein aggregates was detected in metastatic melanoma tissues, without any difference of metastases localization (lung, brain, and subcutaneous skin) (Fig 4C and D). These data support the hypothesis that progression from primitive to metastatic melanoma is accompanied by increased proteins aggregation.
Figure 4. Protein aggregates accumulate in human metastatic melanoma.
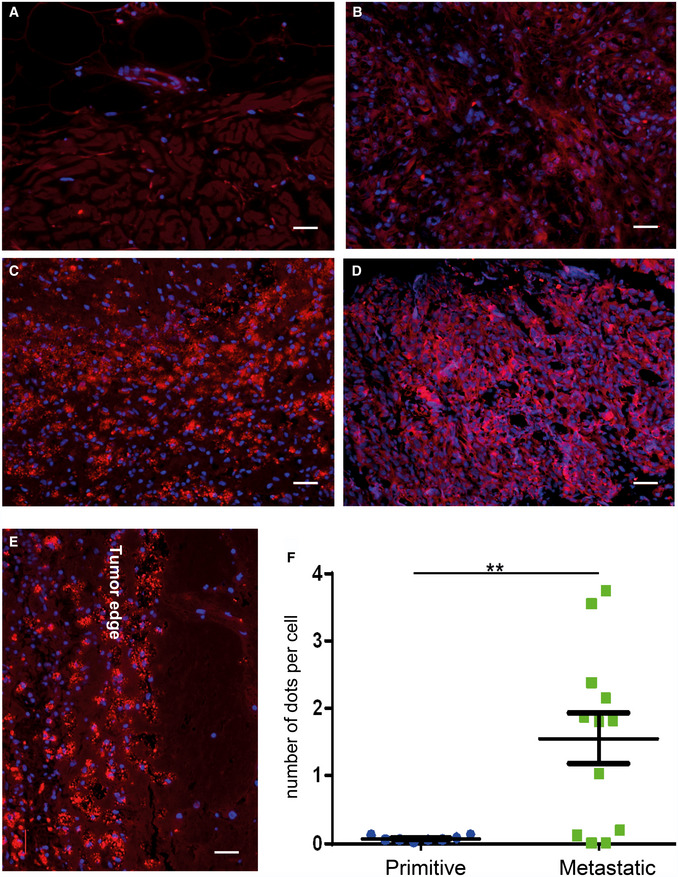
-
A–DImmunofluorescence images with Proteostat (red) and DAPI (blue) staining on (A) human normal skin, (B) samples of primitive melanomas, (C) melanoma metastases in brain and (D) melanoma metastases in lung. Scale bar is 30 μm.
-
EDetails of brain metastases. Scale bar is 30 μm.
-
FQuantitation of Proteostat‐positive dots in primitive vs metastatic melanoma tissues: 6 tissues from metastatic lesions and 6 from primitive melanoma lesions were analyzed. For each tissue, two sections were quantified. T‐test analysis was applied. T‐test analysis, **P < 0.01 (N = 6, data are mean ± SEM).
In details, we observed that protein aggregates appear as dot‐like structure on tumor tissue, clearly defining tumor edge (Fig 4E). Moreover, the number of protein aggregates, quantitated by counting the number of dots per cell, was significantly enriched in metastatic lesions compared to primitive tumors (Fig 4F). Interestingly, the presence of protein aggregates is not related to pigmentation, as there is no correlation between melanin (hematoxylin–eosin) and Proteostat staining (Fig EV3C); on the other hand, a remarkable correlation with cell proliferation (Ki‐67 staining) can be observed (Fig EV3D), symptomatic of a more proliferative phenotype for the metastatic tissues (Hoek et al, 2008). These results are in accordance with the proliferative phenotype observed in metastatic versus primitive cell lines (Figs 1 and EV1A).
BACE inhibition impairs the amyloidogenic machinery in metastatic melanoma cell lines
After demonstrating the enrichment of protein aggregates in metastatic tissues, we wondered if it would be possible to interfere with their production and affect metastasis behavior. The beta‐secretase (BACE 1 and 2) enzymes are known to be involved in the formation of protein amyloids. Indeed, PMEL and APP are cleaved by BACE 2 and 1, respectively, and are able to form mature amyloid fibrils through an APOE‐mediated process (Rochin et al, 2013). Notably, by interrogating gene expression profiling in TCGA and GTEx dataset, we found that BACE 2 is overexpressed in melanoma more than in any other cancer type (Fig EV3E) and correlates with a poor prognosis (Fig EV3F). Moreover, melanoma is also characterized by higher mRNA levels of APOE and PMEL with respect to healthy donors (Fig EV3G). We therefore choose to pharmacologically inhibit BACE to test if it is actually involved in the formation of the protein aggregates that we observed in melanoma metastasis. NB‐360 is a dual BACE inhibitor, known to impair the maturation into fibrils of both APP, in the central nervous system, and PMEL, in normal melanocytes (Neumann et al, 2015). We decided to use a dual BACE inhibitor as it has been observed that BACE1 is able to compensate for the function of BACE2 and vice‐versa (Shimshek et al, 2016). Matched melanoma cell lines, i.e. IGRs, were treated with NB‐360 at a concentration which is not cytotoxic. As shown in Fig EV3H (solid horizontal lines), cell viability is not affected upon NB360 treatment while the number of alive cells is decreased in both IGRs (Fig EV3H, histogram). Moreover, NB360 is able to decrease the amount of melanin (Fig EV3I), indicating an impairment of PMEL amyloidogenic fragment formation (Shimshek et al, 2016). Successively, the secretome was analyzed by Secret3D (Dataset EV9, Peptide Atlas repository). Notably, primitive and metastatic secretome clustered separately, displaying a different profile which is coherent with the observation of a different phenotype; moreover, NB360 treatment affects both primitive and metastatic cells by targeting different proteins (Fig 5A, Dataset EV6). In particular, the amount of secreted PMEL decreased upon treatment in metastatic cells together with other amyloidogenic proteins and known BACE targets (Fig 5B). The overall impact of the drug was analyzed by performing pathway enrichment analysis. Upon treatment, the downregulated pathways were found to be linked to endocytosis, cell adhesion regulation, and ECM (Fig 5C). Among these pathways, we found that the majority of proteins affected by the treatment belong to the metastatic signature identified in the secretome (Fig 5B). Indeed, even if in both primitive and metastatic cells, the same pathways appear to be perturbed, we observed a stronger impact on the metastatic phenotype (Fig 5C). In particular, confocal microscopy analysis of Proteostat labeled cells after NB360 treatment showed a significant decrease of protein aggregates demonstrating that BACE is involved in their maturation into fibrils (Fig 5D and E).
Figure 5. Secretome analysis of IGRs upon BACE inhibition.
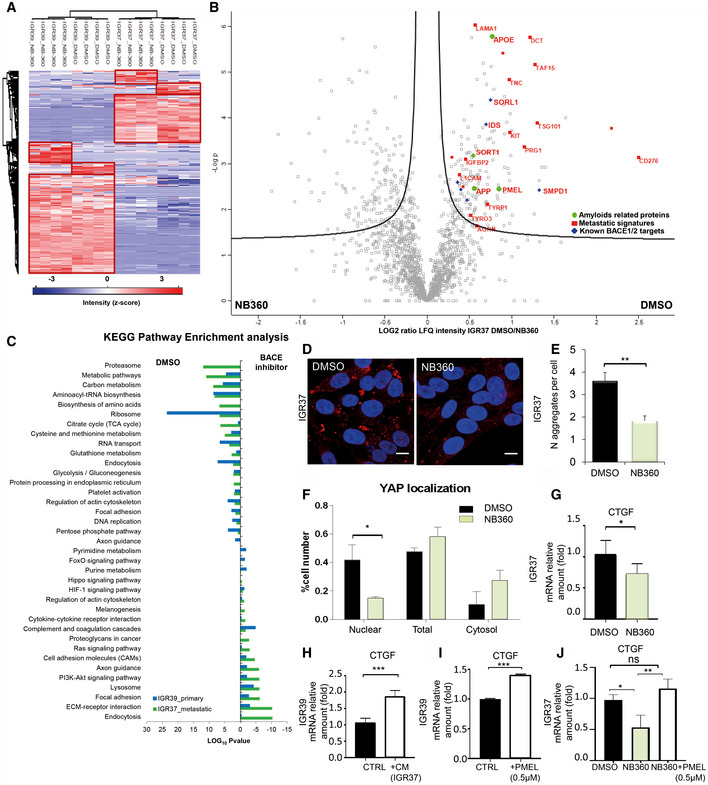
-
AUnsupervised hierarchical clustering of the proteins identified and quantified in IGR37 and IGR39 upon treatment with DMSO or NB‐360.
-
BVolcano plot of the proteins secreted by IGR37 cells treated with DMSO or NB‐360.
-
CKEGG enrichment pathway analysis of the significantly regulated proteins upon BACE inhibition in both IGRs.
-
DConfocal fluorescence images of Proteostat signal (1:2,000, red spots) and DAPI staining (blue), scale bar is 10 μm.
-
EQuantitation of protein aggregates in IGRs by immunofluorescence analysis using Fiji software. (T‐test analysis, **P < 0.01, N = 3 biological replicates, data are mean ± SD).
-
FQuantitation, by immunofluorescence analysis, of YAP in different cellular compartments (T‐test analysis, *P < 0.05, N = 3 biological replicates, data are mean ± SD). Images were quantified by subdividing cells into mostly cytosolic YAP (Cytosol), mostly nuclear YAP (Nuclear), or equal distribution (Total) from three biological replicates.
-
GmRNA levels of CTGF measured by real‐time PCR in IGR37 treated with DMSO or NB‐360 (T‐test analysis, *P < 0.05, N = 3 biological replicates, data are mean ± SD).
-
HCTGF mRNA level measured by real‐time PCR in IGR39 treated with IGR39 conditioned medium (CTRL) or with IGR37 conditioned medium (CM), N = 4 biological replicates. T‐test analysis, ***P < 0.001, data are mean ± SD.
-
ICTGF mRNA level measured by real‐time PCR in IGR39 supplemented with recombinant PMEL amyloid fibrils (0.5 μM), N = 3 biological replicates. T‐test analysis: ***P < 0.001, data are mean ± SD.
-
JCTGF mRNA level measured by real‐time PCR in IGR37 treated with DMSO, NB‐360 or NB‐360 plus recombinant PMEL amyloid fibrils (0.5 μM), N = 3 biological replicates. T‐test analysis *P < 0.05, **P < 0.01. Data are mean ± SD.
BACE2 inhibition impairs mechanotransduction in metastatic melanoma
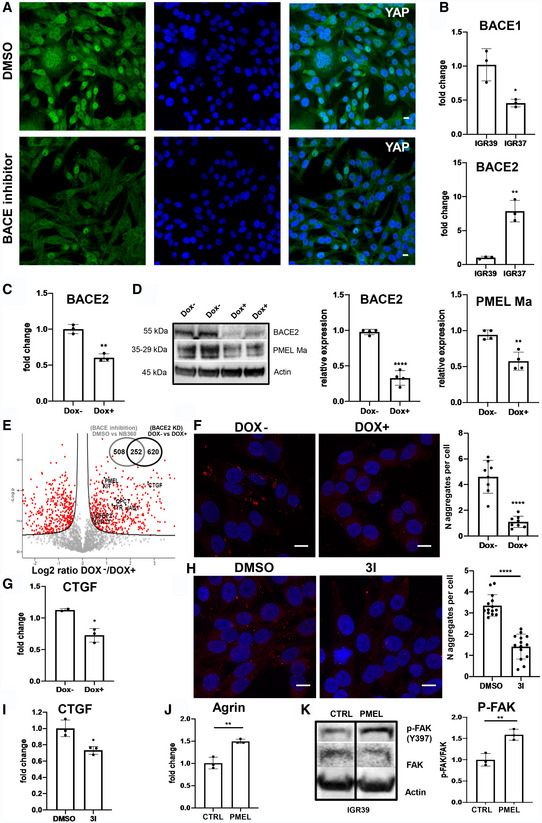
Notably, among BACE‐downregulated proteins, we identified Agrin (Dataset EV10). Agrin is a key protein that senses the extracellular stiffness and activates signaling events to induce the translocation of Yes‐associated protein (YAP) into the nucleus (Chakraborty et al, 2017). YAP is a transcription factor that plays an important role in mechanotransduction along with the transcriptional co‐activator with PDZ‐binding motif (TAZ) (Dupont et al, 2011; Lamar et al, 2012). We postulated that protein aggregates in the extracellular space might activate mechanosignaling leading to YAP‐mediated transcription. Endorsing our hypothesis, YAP nuclear localization was decreased in response to NB‐360 treatment (Figs 5F and EV4A) and YAP target genes, e.g. CTGF, TFGBR2, IGBP4 and FZD1, were found to be downregulated by the drug in the metastatic secretome (Dataset EV6). YAP target gene, i.e. CTGF, is downregulated also at mRNA level upon NB360 treatment, attesting that YAP transcriptional activity is actually inhibited by the drug (Fig 5G).
Figure EV4. (Related to Fig 5) Effect of BACE1/2 inhibition and BACE2 KD in IGR37 metastatic melanoma cells and of PMEL administration in IGR39 primitive cells.
-
AConfocal fluorescence images of anti‐YAP antibody (green) and DAPI staining (blue) in IGR37 upon treatment with DMSO or BACE1/2 inhibitor. Scale bar is 10 μm.
-
BBACE1 and BACE2 mRNA levels measured by real‐time PCR in IGR37 and IGR39. N = 3 biological replicates. T‐test, *P < 0.05, **P < 0.001. Data are mean ± SD.
-
CBACE2 mRNA levels measured by real‐time PCR in iBACE2 KD IGR37 treated or not with doxycycline. N = 4 biological replicates. T‐test analysis, **P < 0.001. Data are mean ± SD.
-
DWestern blot and densitometric analysis of BACE2 and PMEL Ma relative expression in iBACE2 KD IGR37 treated or not with doxycycline. Actin was used as loading control. N = 4 biological replicates. T‐test, **P < 0.01, ****P < 0.0001. Data are mean ± SD.
-
EVolcano plot of the proteins secreted by iBACE2 KD IGR37 cells treated or not with doxycycline (Secret3D workflow). N = 4 biological replicates. Proteins downregulated upon BACE2 silencing are reported in the right part of the plot. Venn diagram of statistically significant proteins common between NB‐360 treatment and BACE KD in IGR37 (right upper corner).
-
FConfocal fluorescence images of Proteostat signal (1:2,000, red) and DAPI staining (blue), scale bar is 10 μm, and quantitation of protein aggregates in iBACE2 KD IGR37 cell lines treated or not with doxycycline by immunofluorescence analysis using Fiji software. N = 8 biological replicates. T‐test, ****P < 0.0001. Data are mean ± SD.
-
GmRNA levels of CTGF measured by real‐time PCR in iBACE2 KD IGR37 treated or not with doxycycline. N = 3 biological replicates. T‐test, *P < 0.05. Data are mean ± SD.
-
HConfocal fluorescence images of Proteostat signal (1:2,000, red) and DAPI staining (blue), scale bar is 10 μm, and quantitation of protein aggregates in IGR cell lines treated or not with 3I inhibitor by immunofluorescence analysis using Fiji software. N = 14 biological replicates. T‐test, ****P < 0.0001. Data are mean ± SD.
-
ImRNA levels of CTGF measured by real‐time PCR in IGR37 treated or not with 3I inhibitor. N = 3 biological replicates. T‐test analysis, *P < 0.05. Data are mean ± SD.
-
JmRNA levels of Agrin measured by real‐time PCR in IGR39 treated with recombinant PMEL. N = 3 biological replicates. T‐test, **P < 0.01. Data are mean ± SD.
-
KWestern blot and densitometric analysis of pFAK(Y397) in IGR39 treated with recombinant PMEL. Actin was used as loading control. N = 3 biological replicates. T‐test, **P < 0.01. Data are mean ± SD.
Source data are available online for this figure.
Knowing that BACE1 and BACE2 are both involved in amyloid processing, we hypothesized that BACE2 should be mainly responsible for amyloid maturation in metastatic melanoma as it is more expressed in metastatic compared to primitive cells, while BACE1 shows an opposite behavior (Fig EV4B). Thus, we silenced BACE2 in metastatic IGR37 melanoma cells (iBACE2 KD IGR37), by using a pLKO‐TET‐on BACE2 shRNA (Fig EV4C). After doxycycline administration, BACE2 expression was reduced together with PMEL maturation into fibrils (Fig EV4D). Consequently, PMEL secretion was impaired together with other known BACE2 targets, i.e. SORT1 (Fig EV4E, Dataset EV11). Indeed, we found that more than 250 significantly regulated proteins were in common between BACE2 KD and NB360 treatment (Venn diagram in Fig EV4E) attesting that the pharmacological and the genetic approach shares a similar behavior. Moreover, we detected less protein aggregates by Proteostat staining (Fig EV4F) and we also confirmed that the YAP target CTGF was downregulated (Fig EV4G). Therefore, we can conclude that BACE2 KD effectively diminishes PMEL amyloid‐like structures in the extracellular space and leads to YAP inactivation in metastatic melanoma cells. These findings were further confirmed by the specific BACE2 inhibitor 3I (BACE2 Ki = 1.6 nM; BACE1 Ki = 815.1 nM; Ghosh et al, 2019). Actually, 3I treatment of metastatic cells abrogates protein aggregates formation (Fig EV4H) and affects YAP transcriptional activity as measured by CTGF expression (Fig EV4I).
PMEL amyloid fibrils induce mechanotransduction triggering the Agrin‐pFAK‐YAP axis
To get insights into the mechanism that links BACE2‐dependent protein aggregates and YAP, we supplemented primary melanoma IGR39 cells with metastatic IGR37 conditioned medium and we measured the CTGF expression as exemplary of YAP target genes. As reported in Fig 5H, we detected an increased level of CTGF proving that the metastatic secretome is indeed able to modulate YAP activity. Furthermore, to investigate if the extracellular amyloidogenic proteins act as “mechanotransducers” and are sufficient to activate YAP signaling, we exogenously added recombinant PMEL amyloid fibrils to the primary melanoma IGR39 cells. We choose PMEL because it is the most abundant amyloidogenic protein found in the secretome of metastatic melanoma cells (Fig 2E). Interestingly, PMEL fibrils alone increase CTGF expression (Fig 5I) thus demonstrating that amyloids impinge on a signaling pathway able to activate YAP.
These data were further strengthened by a rescue experiment demonstrating that the administration of PMEL fibrils to NB360 treated cells is able to restore the expression of CTGF (Fig 5J). This experiment also indicates that, despite the broad effect of the dual BACE inhibitor, PMEL fibrils alone impact on YAP transcriptional activity similarly to what happens when YAP is activated by canonical mechanotransduction signals. Furthermore, we also observed that PMEL fibrils promote Agrin expression and increase focal adhesion kinase (FAK) phosphorylation (Fig EV4J and K), indicating that extracellular amyloids are linked to mechanotransduction via FAK phosphorylation thus promoting the nuclear translocation and activation of YAP (Chakraborty et al, 2017).
BACE inhibition impacts on proliferation and enhances chemo‐sensitivity in melanoma cells
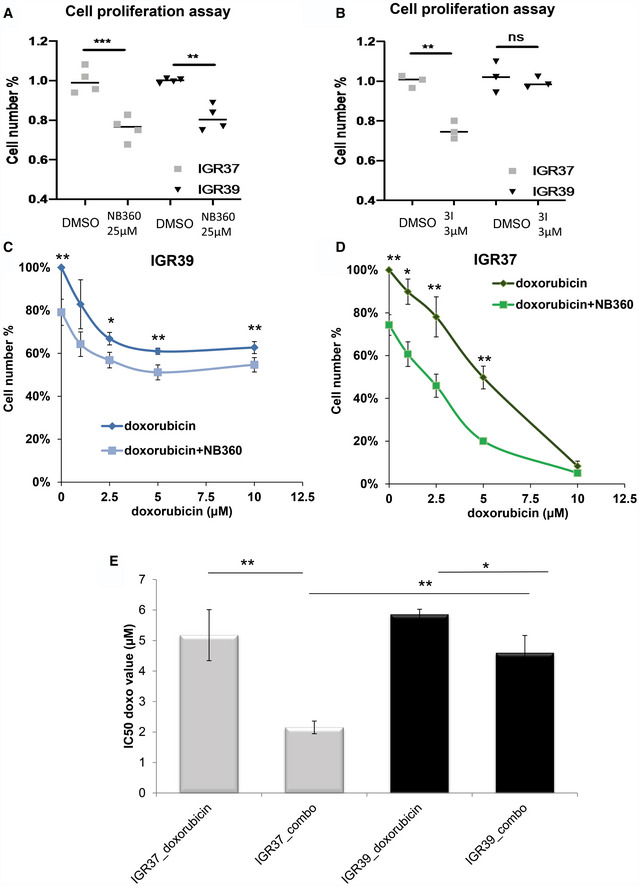
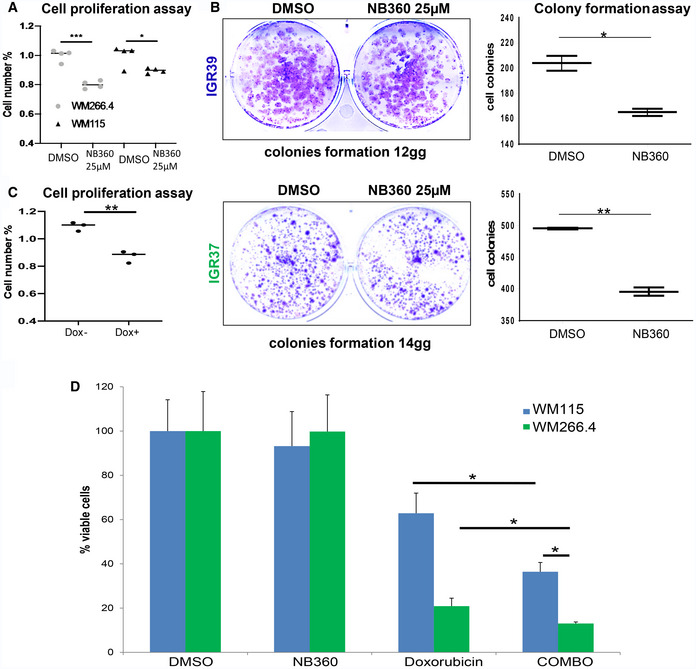
Convincing evidences indicate that mechanotransduction, through YAP activation, is able to affect cell proliferation and confer drug resistance to different chemical compounds (Oku et al, 2015).Therefore, we wondered if NB‐360 might impact on metastatic proliferation and chemo‐sensitivity. We evaluated the clonogenic activity of IGR melanoma cells upon treatment with NB360, and we found a diminished formation of new colonies and a decreased proliferation rate (Figs 6A and EV5A and B). This effect was similar in both primitive and metastatic cell lines. To address a preferential role of BACE2 in the metastatic phenotype, we used the selective BACE2 inhibitor 3I. Notably, 3I treatment impairs proliferation of metastatic cells while it has no effect on primary cells (Fig 6B). A similar behavior was also observed when BACE2 was genetically knocked down excluding off‐target effects of the drug and demonstrating that the impairment of BACE2 expression directly impacts on cell proliferation in metastatic melanoma cells (Fig EV5C).
Figure 6. BACE inhibition affects proliferation and chemo‐sensitivity in melanoma cells.
-
AMTT assay for IGRs treated with DMSO or NB‐360 (25 μM). N = 4 biological replicates. T‐test analysis **P < 0.01, ***P < 0.001. Data are mean ± SD.
-
BMTT assay for IGRs cells treated with DMSO or inhibitor 3I (3 μM). N = 3 biological replicates. T‐test analysis **P < 0.01. Data are mean ± SD.
-
C, DMTT assay of IGRs treated with NB‐360 (25 μM) and different concentration of doxorubicin as indicated. N = 4 biological replicates. T‐test analysis: 0.01 < *P < 0.05, **P < 0.01. Data are mean ± SD.
-
ET‐test analysis of IC50 values for doxorubicin used alone or in combination with NB‐360 (combo). Data are mean ± SD. T‐test analysis: 0.01 < *P < 0.05; 0.001 < **P < 0.01.
Figure EV5. (Related to Fig 6) Effect of BACE inhibition and BACE2 KD on colony formation, cell proliferation, and chemo‐sensitivity.
-
AMTT assay for WMs treated with DMSO or NB‐360 (25 μM). N = 4 biological replicates. T‐test analysis, *P < 0.05, ***P < 0.001. Data are mean ± SD.
-
BColony formation assay and relative quantitation on the right panels, for IGR39 and IGR37 cells treated with DMSO or NB‐360. N = 3 biological replicates. T‐test analysis, *P < 0.05, **P < 0.01. Data are mean ± SD.
-
CMTT assay of iBACE2 KD IGR37 cells treated or not with doxycycline. N = 3 biological replicates. T‐test analysis, **P < 0.01. Data are mean ± SD.
-
DViability of WM melanoma cell lines treated with DMSO, doxorubicin 10 μM, NB‐360 25 μM, and the combination of doxorubicin and NB‐360 measured as % of viable cells. N = 3 biological replicates. T‐test analysis, *P < 0.05. Data are mean ± SD.
We then decided to test if BACE inhibition is also able to enhance the effect of conventional chemotherapy agents such as doxorubicin. Interestingly, the combination of the two drugs makes metastatic cells more sensitive to treatment (Fig 6C and D). Indeed, by evaluating the IC50, we observed that the combined therapy of doxorubicin and NB360 is more efficient compared to doxorubicin alone (Fig 6E). We also tested if the combined therapy performed equally in WMs melanoma cell lines and we confirmed that, also in this case, the response to the combinatorial treatment had a more pronounced effect than treatment with doxorubicin alone (Fig EV5D).
Discussion
Cross‐talk between tumor cells and the microenvironment has recently gained increasing attention as it actively contributes to cancer progression and metastasis (Wang et al, 2017b). By performing system‐level analysis on cellular models of primitive and metastatic phenotypes, we found the presence of protein aggregates in metastatic cells, both at cellular and extracellular levels. Secretome analysis revealed that proteins involved in amyloid deposition are enriched in the metastatic microenvironment together with proteins involved in ECM scaffolding. Altered ECM is frequently observed in various cancers (Lampi & Reinhart‐King, 2018) including melanoma (Miskolczi et al, 2018), where stiffening precedes disease development driving its progression through specific mechanical signaling (Pickup et al, 2014). We hypothesize that amyloidogenic proteins in the extracellular space might aggregate and that the deposition of such highly rigid material (Fitzpatrick et al, 2013) might activate signaling pathways in melanoma microenvironment. In accordance with our hypothesis, we found APOE as the most secreted protein in the metastatic cell lines. APOE is a lipoprotein, whose primitive function is transporting cholesterol, but it is also involved in the stabilization of amyloid‐β fibrils in AD and of PMEL fibrils in melanocyte maturation (Bissig et al, 2016). Recently, APOE variants were found to be involved in melanoma progression and survival (Ostendorf et al, 2020). APOE expression is regulated by the nuclear LXR activation. In agreement, we showed higher level of the endogenous LXR agonist, i.e. 24‐hydroxycholesterol in metastatic versus primitive cells. LXR activation was observed also in AD (Abildayeva et al, 2006), and recently, the involvement of oxysterols in tumor progression was reported also in melanoma (Ortiz et al, 2019). In our study, APOE was found associated with the secretion of proteins such as SORT1 (Carlo et al, 2013) and QPCT both involved in amyloid‐β fibril stabilization (Morawski et al, 2014). Moreover, by using a specific dye, we observed the presence of protein aggregates both at cellular and extracellular levels. All these observations sustain our hypothesis that in metastatic melanoma extracellular environment there is an overproduction of amyloid‐like structures. Despite melanoma progression is accompanied by cellular pigmentation (Kirkpatrick et al, 2006; Sarna et al, 2014), we found that also metastatic unpigmented cells, i.e. WM cell lines, actively secrete protein aggregates.
By analyzing tissues from melanoma patients, we highlighted the presence of protein aggregates also in vivo. According to our proteomic data, we observed amyloid‐like protein aggregation enriched in metastatic lesions compared to primitive tumor tissues. Protein aggregates are hallmark of neurodegenerative disease such as AD, but their involvement in cancer progression is still poorly understood (Xu et al, 2011).
With the aim of understanding the biological relevance of these protein aggregates, we interfered with their production by targeting BACE2, the enzyme that assists the maturation and release of amyloidogenic peptides (Rochin et al, 2013). Interestingly, by interrogating TCGA and GTEX data, we found that BACE2 is highly expressed in melanoma patients compared to healthy donors, and its level of expression correlates with poor prognosis. Moreover, in IGRs cell lines we found that BACE2 was more expressed in metastatic compared to primitive cells. By using a BACE inhibitor or by genetically impairing BACE2 expression, we reduced the formation of protein aggregates, impaired PMEL shedding, but also affected the secretion of APOE, SORT1, and proteins of the extracellular matrix, as Agrin. In AD, Agrin co‐localizes with amyloid plaques and stabilizes amyloid‐β fibrils (Cotman et al, 2000). Agrin is also a mechanical sensor that transduces ECM rigidity signals by inducing Yes‐associated protein (YAP) activation (Chakraborty et al, 2017). Over the past decade, YAP has emerged as important driver of cancer development (Lamar et al, 2012). It has been postulated that YAP contributes to both invasive and metastatic behavior in melanoma (Nallet‐Staub et al, 2014), encouraging researchers to target its activity for anti‐cancer therapy (Johnson & Halder, 2014). In the present work, we demonstrated that amyloid fibrils in metastatic melanoma induce YAP nuclear localization and its transcriptional activity. Moreover, we also demonstrated that PMEL fibrils are sufficient to promote YAP activation and to increase Agrin expression and FAK phosphorylation, which are known and well‐recognized markers of mechanotransduction (Chakraborty et al, 2017). Mechanistically, we propose a model where BACE2 assists the maturation of protein aggregates, which accumulate in the extracellular space where they could be sensed by Agrin leading to YAP activation (Fig 7). We cannot exclude that amyloid fibrils have also other effects but we clearly show that they impinge on the mechanotransduction pathway. Further work is necessary to clarify if this is a direct link or if and which other molecules are required.
Figure 7. BACE as a new regulator of YAP in metastatic melanoma cells.
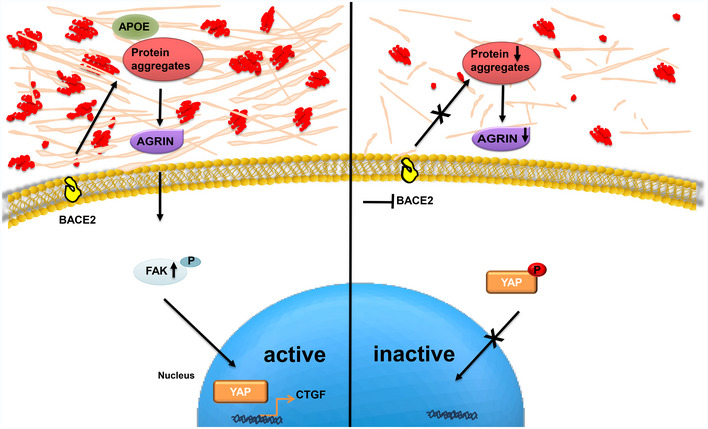
In metastatic melanoma, BACE2 activity assists the secretion of protein aggregates into the extracellular space. The presence of these aggregates might be sensed by Agrin, known to activate the YAP signaling cascade, and is able to induce YAP‐mediated CTGF transcription. In turn, melanoma cells treated with BACE inhibitors produce fewer protein aggregates and show a lower YAP transcriptional activity.
In melanoma, YAP overexpression confers resistance to BRAF inhibitor, whereas YAP depletion increases drug sensitivity (Kim et al, 2016). Consistently, we observed that melanoma cells treated with BACE inhibitor become less proliferative and more sensitive to chemotherapy. Our data are consistent with a recent study where BACE inhibition was able to impair melanoma brain metastatic proliferation in vivo (preprint: Kleffman et al, 2019). Indeed, we found that a combined treatment of doxorubicin and NB360 is more effective than doxorubicin alone. Actually, protein aggregates rigidity might contribute in the formation of the “safe haven”, favoring tumor growth and melanoma resistance (Hirata et al, 2015). Supporting this hypothesis, rigid tumor microenvironment was often associated with the formation of a physical barrier affecting drug uptake (Holle et al, 2016). Moreover, coherently with our model, Wang et al reported that PMEL silencing affects mouse melanoma cells’ proliferation similarly to what we observed by BACE inhibition (Wang et al, 2017a), while PMEL expression is associated with resistance to chemotherapy in melanoma (Johansson et al, 2013). In particular, siRNA inhibition of PMEL in the MNT‐1 melanoma cells sensitized the cells to both paclitaxel and cisplatin in line with the increased chemo‐sensitivity induced by BACE inhibition described in our work.
Melanoma recent therapies are remarkably efficient in a subpopulation of patients; for those who do not respond though, melanoma remains a devastating disease raising the need of alternative therapies. Here, we found a potential new druggable target, i.e. BACE, able to affect melanoma microenvironment. Targeting BACE in combination with chemotherapy might open new ways to counteract metastatic melanoma. Moreover, this therapy might also interfere with the mechanosignaling pathway that can promote metastatic growth and survival (Lamar et al, 2012). In our work, we have underlined a cell autonomous effect of protein aggregates deposition, but it would be interesting to explore the effect of the presence of amyloid‐like structures also on neighboring cells. It has been recently demonstrated that in vivo ECM production is mostly fibroblastic dependent, while ECM remodeling is both tumor cell and fibroblastic cell dependent (Lamar et al, 2012). Here, we provided evidences that amyloid aggregates are secreted by melanoma cells and might contribute to ECM remodeling. Moreover, as amyloidogenic protein overexpression has been reported also in other tumor types, such as breast (Danish Rizvi et al, 2018) and pancreas (Westermark et al, 2017), it is attractive to think that the same mechanism could be exploited also in other diseases.
Materials and Methods
Reagents and Tools table
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal HMB‐45 | Thermo Fisher | Catalog # MA5‐13232 |
| Mouse monoclonal anti‐YAP | Santa Cruz | sc‐101199 |
| Rabbit polyclonal anti‐HDAC2 | Abcam | Ab16032 |
| Mouse monoclonal anti‐FAK | Santa Cruz | sc‐271195 |
| Mouse monoclonal anti‐pFAK (Y397) | Santa Cruz | sc‐81493 |
| Mouse monoclonal anti‐Actin | Santa Cruz | sc‐47778 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 25‐hydroxycholesterol (D6) | Avanti Polar Lipids | LM‐4110 |
| 24(R/S)‐hydroxycholesterol (D6) | Avanti Polar Lipids | LM‐4110 |
| 27‐hydroxycholesterol (D6) | Avanti Polar Lipids | LM‐4114 |
| NB‐360 | Novartis | N/A |
| Doxorubicin | Selleck Chemicals | #S1208 |
| ECL | Amersham | RPN2232 |
| DAPI | Sigma‐Aldrich | CAS Number 28718‐90‐3 |
| MUSE | Millipore | Catalogue Number MCH100102 |
| Proteostat | Enzo Life science | ENZ‐51023 |
| DMSO | Euroclone | EMR385258 |
| Critical Commercial Assays+40:64 | ||
| Bradford | Applied Biosystems | Cat#4368814 |
| LightCycler® 480 SYBR Green I Master | Roche | Product No. 04707516001 |
| SuperScript™ VILO™ cDNA Synthesis Kit | Invitrogen | Cat. No. 11754‐050 and 11754‐250 |
| Ni‐NTA Agarose | Qiagen | Cat No./ID: 30230 |
| Experimental Models: Melanoma Cell Lines | ||
| IGR37 | DSMZ | ID ACC 237 |
| IGR39 | DSMZ | ID ACC 239 |
| WM115 | IZSBS | ID BS‐TCL74 |
| WM266.4 | ATCC | ID CRL‐1676 |
| A‐375 | IZSBS | ID BS‐TCL88 |
| C32 | IZSBS | ID BS‐TCL150 |
| IPC‐298 | DSMZ | ID ACC 251 |
| SK‐MEL‐5 | NCI‐60 | ID 0507403 |
| MeWo | ICLC | ID HTL97019 |
| Sk‐MEL‐28 | NCI‐60 | ID 0507398 |
| HEK293T | ICLC | ID HTL04001 |
| Software and Algorithms | ||
| GraphPad Prism software | GraphPad Software | http://www.graphpad.com |
| Microsoft Excel 2010 | Microsoft Office | N/A |
| Fiji | ImageJ Software | https://imagej.net/Fiji |
| MaxQuant | N/A | http://www.coxdocs.org/doku.php?id=maxquant:common:download_and_installation |
| Perseus | N/A | http://www.coxdocs.org/doku.php?id=perseus:start |
| Venny | N/A | http://bioinfogp.cnb.csic.es/tools/venny/ |
| Deposited data | ||
| .RAW files of the proteomic data were deposited together with all peptides identified and parameters used for the analysis | Peptide Atlas repository | PASS01358 |
Methods and Protocols
Cell culture
Human melanoma cell lines such as IGR37, IGR39, and IPC‐298 were purchased from DSMZ; MEWO cell line was purchased from ICLC; SK‐MEL‐5 and SK‐MEL‐28 were purchased from NCI‐60; WM266.4 was purchased from ATCC; WM115, A‐375 and C32 were purchased from IZSBS; and HEK293T cells were purchased from ICLC.
All the cells were cultured in Dulbecco modified Eagle's medium (DMEM)+ 10% FBS S.A.+ 2 mM l‐Glutamine except for IPC‐298 that was cultured in RPMI‐1640+ 10% FBS S.A.+ 2 mM l‐Glutamine. Cell lines were tested for mycoplasma by mycoplasma PCR Test Kit.
Analysis of human biopsies
Formalin‐fixed paraffin‐embedded tissues were sliced into serial 8‐μm‐thick sections and collected for immunohistochemical (IHC) staining. Human paraffin samples were stained for Haematoxylin/Eosin (Diapath) to assess histological features, according to standard protocol. For Ki67 immunoanalysis, paraffin was removed with xylene and sections were rehydrated in graded alcohol. Tissue slides were incubated in 10% peroxidase solution for 1 h at 65°C to remove melanin pigments, and then, antigen retrieval was carried out using preheated target retrieval solution for 45 min at 95°C. Tissue sections were blocked with FBS serum in PBS for 60 min and incubated overnight with primary antibody (Thermo Scientific, 1:50). The antibody binding was detected using a polymer detection kit (GAR‐HRP, Microtech) followed by a diaminobenzidine chromogen reaction (Peroxidase substrate kit, DAB, SK‐4100; Vector Lab). All sections were counterstained with Mayer's hematoxylin, mounted in Eukitt (Bio‐Optica), and then visualized with an Olympus BX51 or an Olympus BX63 upright widefield microscope using NIS‐Elements (Nikon, Tokyo, Japan) or MetaMorph 7.8 software (Molecular Devices, San Jose, CA, USA), respectively. For Proteostat aggresome detection, deparaffinized and rehydrated slides were fixed in 4% PFA for 15 min, incubated in Proteostat solution (1:2,000, Proteostat Aggresome Detection Kit, Enzo) for 3 min, and then destained in 1% acetic acid for 20 min at room temperature. To visualize the cell nuclei, human slides were counterstained with 4,6‐diamidino‐2‐phenylindole (DAPI, Sigma‐Aldrich), mounted with a phosphate‐buffered saline/glycerol solution, and examined with confocal or widefield microscopy. Confocal microscopy was performed on a Leica TCS SP5 confocal laser scanning based on a Leica DMI 6000B inverted motorized microscope. The images were acquired with a HC FLUOTAR L 25X/NA0.95 VISIR water immersion objective using the 405 nm and the 488 nm laser lines. The software used for all acquisitions was Leica LAS AF. Widefield microscopy was performed on an Olympus BX63 upright microscope equipped with a motorized stage for mosaic acquisitions and with both Hamamatsu ORCA‐AG black and white camera and Leica DFC450C color camera. The mosaic images were acquired using a UPlanSApo 4X/NA0.16 dry objective with MetaMorph 7.8 software (Molecular Devices). Quantitative analysis of stained signals was performed using ImageJ software. Both protein aggregates (dots) and nuclei were analyzed. Dots were normalized on nuclei, and T‐test statistical analysis was performed to estimate the differences between primary (N = 6) and metastatic tissues (N = 6). All the analysis was done in technical duplicates.
Time‐lapse microscopy
For live cell imaging experiments, melanoma cells were cultured in six‐well plates (5 × 103 cells per plate). Cultures were transferred to a live cell imaging workstation composed by an Olympus IX81 inverted microscope equipped with motorized stage and a Hamamatsu ORCA‐Flash4.0 camera. The images were collected every 5 min for a total recording time of 72 h for each dish using a LUC Plan FLN 20X/NA0.45 Ph dry objective with CellSens software (Olympus). The analysis was done, in biological triplicate, by using trackmate (Fiji).
Secretome preparation from cell cultures and SILAC labeling
All melanoma cells were grown in a DMEM except for IPC‐298 which was grown in a RPMI medium, complemented with essential amino acids Arg and Lys, containing naturally occurring atoms (Sigma) (the light medium) or two of their stable‐isotope counterparts (the medium and heavy media) (Cambridge Isotope Laboratories, Inc.; CIL). The medium culture contained arginine (l‐Arg 13C6‐14N4) and lysine (l‐Lys 13C6‐15N2), and the heavy culture contained arginine (l‐Arg 13C6‐15N4) and lysine (l‐Lys 13C6‐15N2) amino acids. After five cell divisions to obtain full incorporation of the labeled amino acids into the proteome, cells were counted and equal numbers of cells were split to 15‐cm dishes at roughly 50% confluence. Once cell lines reached ~70% confluence, one 15‐cm dish of each cell line was washed 3 × with PBS and 3 × with serum‐free media. Cells were starved in serum‐free media for 18 h, and the conditioned media (CM) were centrifuged (800 g, 3 min), filtered (0.22 μm) to remove detached cells, and concentrated via centrifugation at 4,500 g in 10 kDa molecular weight cutoff concentrating columns. Then, 500 μl of concentrated medium was filtered by microcon filters with 10 kDa cutoff (Millipore) and buffer was exchanged with 8 M Urea 100 mM Tris or PBS.
Protein aggregates detection
Aggregates Fluorescence measurement was performed following manufacturer's instructions (http://www.enzolifesciences.com/fileadmin/files/manual/ENZ-51023_insert.pdf). Briefly, 2 μl of the diluted PROTEOSTAT®Detection Reagent was added into the bottom of each well of a 96‐well microplate. 98 μl of the secreted proteins in PBS was added to each well. Protein concentration was 10 μg/ml. The final concentration of the PROTEOSTAT detection dye was 1,000‐fold dilution in the assay. We run control samples as well as 1× Assay Buffer alone (no protein), as a blank sample. The microplate containing samples was incubated in the dark for 15 min at room temperature. Generated signals were read with a fluorescence Microplate Reader (Tecan Infinite 200) using an excitation setting of about 550 nm and an emission filter of about 600 nm.
Secretome analysis
Proteins secreted by 2 × 106 cells were replaced in 8 M Urea 100 mM Tris pH 8 and sonicated with BIORUPTOR (3 cycles: 30 s on/30 s off). By using microcon filters with 10 kDa cutoff (Millipore), cysteine reduction and alkylation were performed adding 10 mM TCEP (Thermo scientific) and 40 mM 2‐Chloroacetamide (Sigma‐Aldrich) in 8 M Urea 100 mM Tris pH 8 for 30 min at room temperature, as described for the FASP protocol. Buffer was exchanged by centrifugation at 9,300 g for 10 min, and PNGase F (New England Biolabs) (1:100 = enzyme: secreted proteins) was added for 1 h at room temperature following manufacturer's instruction. Buffer was again exchanged by centrifugation at 9,300 g for 10 min with 50 mM ammonium bicarbonate, and proteins were in solution digested with trypsin (Trypsin, Sequencing Grade, modified from ROCHE) (1:50 = enzyme: secreted proteins) overnight at 37°C. Peptides were recovered on the bottom of the microcon filters by centrifugation at 9,300 g for 10 min and on the top, adding two consecutive wash of 50 μl of 0.5 M NaCl. The undigested polypeptides on the top of the filters were further digested with GluC (Endoproteinase Glu‐C Sequencing Grade ROCHE) (1:50 = enzyme: secreted proteins) overnight at 37°C upon buffer exchange with phosphate buffer (pH 7.8). Eluted peptides were purified on a C18 StageTip. 1 μg of digested sample was injected onto a quadrupole Orbitrap Q‐exactive HF mass spectrometer (Thermo Scientific). Peptide separation was achieved on a linear gradient from 95% solvent A (2% ACN, 0.1% formic acid) to 55% solvent B (80% acetonitrile, 0.1% formic acid) over 75 min and from 55% to 100% solvent B in 3 min at a constant flow rate of 0.25 μl/min on UHPLC Easy‐nLC 1000 (Thermo Scientific) where the LC system was connected to a 23‐cm fused‐silica emitter of 75 μm inner diameter (New Objective, Inc. Woburn, MA, USA), packed in‐house with ReproSil‐Pur C18‐AQ 1.9 μm beads (Dr Maisch Gmbh, Ammerbuch, Germany) using a high‐pressure bomb loader (Proxeon, Odense, Denmark).
The mass spectrometer was operated in DDA mode as described previously (Matafora et al, 2017): dynamic exclusion enabled (exclusion duration = 15 s), MS1 resolution = 70,000, MS1 automatic gain control target = 3 × 106, MS1 maximum fill time = 60 ms, MS2 resolution = 17,500, MS2 automatic gain control target = 1 × 105, MS2 maximum fill time = 60 ms, and MS2 normalized collision energy = 25. For each cycle, one full MS1 scan range = 300–1,650 m/z was followed by 12 MS2 scans using an isolation window of 2.0 m/z.
All the proteomic data as raw files, total proteins, and peptides identified with relative intensities and search parameters have been loaded into Peptide Atlas repository (PASS01358).
MS analysis and database search
MS analysis was performed as reported previously (Matafora et al, 2015). Raw MS files were processed with MaxQuant software (1.5.2.8), making use of the Andromeda search engine (Cox et al, 2011). MS/MS peak lists were searched against the UniProtKB Human complete proteome database (uniprot_cp_human_2015_03) in which trypsin and GluC specificity was used with up to two missed cleavages allowed. Searches were performed selecting alkylation of cysteine by carbamidomethylation as fixed modification, and oxidation of methionine, N‐terminal acetylation and N‐Deamination as variable modifications. Mass tolerance was set to 5 ppm and 10 ppm for parent and fragment ions, respectively. A reverse decoy database was generated within Andromeda, and the false discovery rate (FDR) was set to < 0.01 for peptide spectrum matches (PSMs). For identification, at least two peptide identifications per protein were required, of which at least one peptide had to be unique to the protein group.
Quantification and statistical analysis
SILAC and Label free from DDA.raw files were analyzed by MaxQuant software for protein quantitation, and depending from the experiment, SILAC Ratio or LFQ intensities were used. Statistical analysis was performed by using Perseus software (version 1.5.6.0) included in MaxQuant package. T‐test and ANOVA statistical analysis were performed applying FDR < 0.05 or P < 0.05 as reported. KEGG enrichment pathway analysis was performed via EnrichR (http://amp.pharm.mssm.edu/Enrichr), using the Gene ID of the identified proteins.
Oxysterol quantification
Oxysterols were prepared from melanoma cell lines using a modified version of the protocol described by Griffiths et al (2013), consisting in an alcoholic extraction and a double round of reverse‐phase (RP) solid‐phase extraction (SPE; Soncini et al, 2016). Briefly, melanoma cell pellets (2 × 106 cells) were sonicated for 5 min by adding 1.0 ml of ethanol supplemented with + 20 pmol of each deuterated standard. 400 μl H2O was added and sonicated for 5 min (final volume 1.5 ml of 70% ethanol). Upon centrifugation at 14,000 g, 4 °C for 30 min, the supernatant was collected. The extract was applied to a preconditioned Sep‐Pak tC18 cartridge (Waters). The oxysterol‐containing flow‐through was collected, together with the first 70% (vol/vol) ethanol wash. The collected oxysterols were vacuum‐evaporated and reconstituted in 100% (vol/vol) isopropanol, diluted in 50 mM phosphate buffer, and oxidized by cholesterol oxidase addition. The reaction was stopped by methanol. Reactive oxysterols were then derivatized by Girard P reagent (TCI Chemical) and further purified by reverse‐phase chromatography using a Sep‐Pak tC18 cartridge to eliminate the excess of GirardP reagent. Purified oxysterols were diluted in 60% (vol/vol) methanol and 0.1% formic acid. Eight μl of sample was resolved by on a nano‐HPLC system connected to a 15‐cm fused‐silica emitter of 75 μm inner diameter (New Objective, Inc. Woburn, MA, USA), packed in‐house with ReproSil‐Pur C18‐AQ 1.9 μm beads (Dr Maisch Gmbh, Ammerbuch, Germany) using a high‐pressure bomb loader (Proxeon, Odense, Denmark). It was used a 12‐min gradient from 20% to 100% of solvent B [63.3% (vol/vol) methanol, 31.7% (vol/vol) acetonitrile, and 0.1% formic acid], where solvent A is composed of 33.3% methanol, 16.7% acetonitrile, and 0.1% formic acid. Eluting oxysterols were acquired on a quadrupole Orbitrap Q‐Exactive HF mass spectrometer (Thermo Scientific), where the survey spectrum was recorded at high resolution (R = 140,000 at 200 m/z) and the five most intense peaks were further fragmented. The identification of the oxysterols species was made by comparing the retention times of the analytes with those of the synthetic, deuterated standards previously run on the same system in the same chromatographic conditions. The quantification was achieved by means of stable‐isotope dilution MS using internal standards. The total ion current for derivatized oxysterols was extracted for each acquisition, areas of the peaks were integrated manually using Xcalibur software, and the absolute amount of oxysterols was determined by comparing their areas with those of the internal standards, using the following equation:
Protein quantification
Protein quantification was performed using Bradford assay (Bio‐Rad). For each sample, the absorbance was measured by a spectrophotometer at a wavelength of 595 nm. Sample protein concentration was determined based on a bovine serum albumin (BSA) standard curve.
Western blot assays
For Western blot analyses, proteins were extracted in buffer containing 8 M Urea, 100 mM Tris–HCl pH 8. Briefly, cell lysates (50 μg) were separated by SDS–PAGE using a precast polyacrylamide gel with a 4% to 12% gradient (Invitrogen). After the electrophoretic run, proteins were transferred onto a 0.22 μm nitrocellulose membrane (Amersham Protran, GE Healthcare) in wet conditions. The assembled sandwich was loaded in a Trans‐Blot Cell (Bio‐Rad) and immersed in 1× cold Tris‐Glycine transfer buffer with the addition of 20% methanol. The transfer was allowed overnight at constant voltage (30 V). Correct protein transfer was verified staining the membrane with Ponceau red (Sigma‐Aldrich) for few seconds. After washing the membrane with Tris‐buffered Saline‐Tween 20 (TBST, 1× TBS with 0.1% Tween‐20), non‐specific binding of antibodies was blocked by adding 5% low‐fat dry milk in TBST for 1 h at room temperature. Murine Anti‐human Pmel17 (HMB45 Thermo scientific) primary monoclonal antibody was diluted in the same blocking solution to a final concentration of 1:100, mouse anti‐FAK(1:500) (Santa Cruz), mouse anti‐pFAK Y397 (1:300) (Santa Cruz) The anti‐HDAC2 antibody (Cell Signalling) and anti‐Actin antibody (Santa Cruz) were used to normalize the amount of proteins loaded onto the gel. Anti‐murine IgG1 secondary antibody conjugated with the enzyme horseradish peroxidase (HRP) was used to a final concentration of 1:2,000 in 5% milk‐TBST.
Immunofluorescence
Cells were fixed and permeabilized as describe previously (Matafora et al, 2009). After treatment, cells were fixed with 4% (wt/vol) paraformaldehyde, blocked with PBS‐BSA (1% wt/vol), made permeable with Triton X‐100 0.2% (Sigma‐Aldrich) for 3 min, and incubated with Proteostat (1:1,000) or specific antibodies diluted in 0.2% bovine serum albumin in PBS. Cells were then washed three times with PBS and stained with DAPI (Sigma‐Aldrich). Cells were observed by confocal microscopy performed on a Leica TCS SP5 or a Leica TCS SP2 AOBS confocal laser scanning. The confocal systems were, respectively, based on a Leica DMI 6000B or a DM IRE2 inverted microscope equipped with motorized stage. The images were acquired with an HCX PL APO 63X/NA1.4 oil immersion objective using the 405 nm, 488 nm, or 561 nm laser lines. The software used for all acquisitions was Leica LAS AF (on TCS SP5 system) or Leica Confocal Software (on TCS SP2 AOBS System).
Recombinant PMEL (rMα) expression and purification
The luminal fragment of PMEL, rMα, consisting of amino acids 25–467 was subcloned from PGEX vector into a pET28a vector, in order to have 6xHis tag at the N‐terminus, and expressed in BL21‐DE3 Escherichia coli. Shaken cultures were grown at 37°C to OD600 = 0.5 in the presence of kanamycin and then induced with 1 mM IPTG for 4 h. Cells were collected via centrifugation at 4°C, resuspended in TBS (Tris‐buffered saline: 150 mM NaCl, 50 mM Tris–HCl, pH 7.6), and frozen at −80°C. The resuspended pellet was thawed, and the cells were lysed by probe sonication. rMα formed inclusion bodies that were collected by centrifugation after three washings in 1.5 M NaCl, 100 mM Tris–HCl pH 7.4, 1% Triton X‐100 buffer and two in TBS (Fowler et al, 2006). The inclusion bodies were dissolved in 9 M Urea, 100 mM NaH2PO4, 10 mM Tris–HCl pH 8.0 and then filtered through a 0.22 μm cellulose acetate filter and stored at room temperature. The protein was purified using Ni‐NTA agarose beads (Qiagen, Germany) under denaturing condition. Briefly, 2 ml 50% slurry of Ni‐NTA agarose beads were equilibrated with binding buffer (9 M Urea, 100 mM NaH2PO4, 10 mM Tris–HCl pH 8.0) before adding the sample. After binding for 1 h and 30 min, two washes were performed with 9 M Urea, 100 mM NaH2PO4, 10 mM Tris–HCl pH 6.5; elution was obtained in 9 M Urea, 100 mM NaH2PO4, 10 mM Tris–HCl pH 4.5.
PMEL aggregates refolding and administration to cells
Recombinant PMEL aggregates refolding was obtained by slightly modifying a previously described protocol (Fowler et al, 2006). In particular, we did sequential dilutions form denaturing to native condition by performing first gel filtration and then buffer exchange. Briefly, after Ni‐NTA purification, recombinant PMEL was subjected to gel filtration in mild denaturating buffer (4 M Urea, 100 mM Tris–HCl pH 8.0) on a Superdex 200 16/60 column (GE Healthcare Life Sciences, USA), in order to allow partial refolding and avoid the elution of the protein in the void volume. The fractions corresponding to PMEL elution were pulled together and concentrated by using Amicon Ultra centrifugal tubes with 10 kDa cutoff (Millipore, USA). To allow a complete refolding and cell culture treatment, the buffer was exchanged with PBS. Recombinant PMEL aggregates were administered to cells in culture media at a final concentration of 0.5 μM.
IGR39 treatment with IGR37 conditioned medium
IGR39 were seeded at the concentration of 100,000 cells/well. At the same time, IGR37 and IGR39 were seeded at 60% confluency in a 10‐cm petri dish. After 24 h, the media deriving from IGR37 and from IGR39 were filtered on a 0.22 μm cellulose acetate filter, in order to remove dead cells and cells debris, and administered to IGR39. After 24 h, cells were harvested and RNA extraction was performed.
RNA extraction, RT–PCR and real‐time PCR
Total RNA was extracted using Maxwell RSC simply RNA (Promega, USA) according to manufacturer's instructions, and RNA was quantified by nanodrop. 1 μg of total RNA was used for retro‐transcription using SuperScript™ VILO™cDNA Synthesis Kit (Invitrogen, USA). cDNA was diluted 1:10, and qPCR was performed using LightCycler® 480 SYBR Green I Master (Roche, Switzerland). The primer sequences are provided below. Expression data were normalized to the geometric mean of the housekeeping gene RPLP0 to control the variability in expression levels and were analyzed using the 2‐ΔΔCT method. Primers for qPCR: CTGF‐Forward primer: GGGAAATGCTGCGAGGAGT, CTGF‐Reverse primer: GCCAAACTGTCTTCCAGTC; AGRN‐Forward primer: TTGTCGAGTACCTCAACGCT, AGRN‐Reverse primer: CAGGCTCAGTTCAAAGTCGT; RPLP0‐Forward primer: GTTGCTGGCCA ATAAGGTG, RPLP0‐ Reverse primer: GGGCTGGCACAGTGACTT.
Cell viability assays
Melanoma cell lines were seeded into 6‐well plates. MUSE reagent was added to detached cells, and cell viability was assessed according to the manufacturer's instructions (http://www.merckmillipore.com/IT/it/product/Muse-Count-Viability-Assay-Kit-100-Tests,MM_NF-MCH100102#anchor_UG). Viability was accessed by measuring cell confluence (%) and number of dead and alive cells by using Muse™ Cell Analyzer.
MTT cell viability assay
To perform 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; Sigma) cell viability assay, melanoma cells were seeded in 96‐well plates (5 × 103 cells/well) and were treated with 3I, doxorubicin or/and NB360 as indicated in the text. At the end of the experiments, the cell cultures were supplemented with 150 μl of 0.5 mg/ml MTT assay and incubated for an additional 4 h. Then, equal volume of solubilizing solution (dimethyl sulfoxide 40%, SDS 10% and acetic acid 2%) was added to the cell culture to dissolve the formazan crystals and incubated for 10 min at room temperature. The absorbance rate of the cell culture was detected at 570 nm by using a Microplate Reader (Bio‐Rad, Hercules, CA, USA). Each experiment was performed as biological quadruplicate.
Clonogenic assay
Melanoma cells (2,000 cells/well) were seeded into 6‐well plates, and following cell attachment, they were treated with DMSO or NB360 as indicated. Then, the plates were incubated at 37°C with 5% CO2, until cells formed colonies (12–15 days). Colonies were fixed with 75% methanol and stained with 0.5% crystal violet, then rinsed with PBS, dried, and counted using the ImageJ software.
Plasmid construction and generation
For the generation of the sh‐BACE2 plasmid, annealed oligonucleotides were designed according to the RNA Consortium's recommendation (http://www.broadinstitute.org/rnai/trc) targeting GCACTCCTACATAGACACGTA in the coding region of BACE2 and were cloned into pLKO‐Tet‐On by Age1 and EcoR1 sites to produce pLKO‐Tet‐On‐shBACE2. The construct was confirmed through DNA sequencing.
IGR37 infection and selection
To generate lentiviral particles, HEK293T cells were seeded in 10‐cm dishes and transfected with 10 μg of pLKO‐Tet‐On‐shBACE2, 2.8 μg of ENV (VSV‐G) (Addgene, USA), 5 μg of pMDL (gag&pol) (Addgene, USA), and 2.5 μg of REV (Addgene, USA) adding CaCl2 (Carlo Erba, Italy). After 72 h, the viral supernatant was recovered and filtered on a 0.45 μm filter (Millipore, USA). IGR37 cells cultured in 6 wells‐cell culture plate (Corning, USA) were infected adding 1 ml of filtered viral supernatant in the presence of polybrene (8 μg/ml; Millipore, USA) by centrifugation for 20 min at 650 g. The infected cells were selected with 500 μg/μl of Neomycin G418 (Life Technologies, USA) added to the culture medium.
Single‐cell cloning for iBACE2 KD IGR37
After selection, iBACE2 KD IGR37 cells were sorted by FACS Aria (Becton Dickinson), and a single cell was seeded in a 96‐well plate containing 250 μl of DMEM supplemented with 10% FBS S.A., 1% l‐Glu, and 500 μg/μl of Neomycin G418 + 250 μl of conditioned medium. Cells were incubated at 37°C and 5% CO2 until 70% of confluency and then moved to a bigger plate, up to 100‐mm dish. shRNA knockdown was induced by adding 1 μg/ml of doxycycline.
Study approval
Informed consent was obtained from all study participants. Study approval was given by the Institutional Review Board of the Grande Ospedale Metropolitano Niguarda. All cases of melanoma cancer were pathologically confirmed.
Author contributions
Methods development, VM, UR, and GM; Validation and formal analysis, VM; Investigation, VM, GM, FF, UR, ST, CB, FP, AS, and FC; Resources, AB, EB, SM, and LL; Writing original draft, VM, GM, FF, and AB; Supervision, project administration, and funding acquisition, AB.
Conflict of interest
The authors declare that no conflict of interest exists.
Supporting information
Expanded View Figures PDF
DatasetEV1
DatasetEV2
DatasetEV3
DatasetEV4
DatasetEV5
DatasetEV6
DatasetEV7
DatasetEV8
DatasetEV9
DatasetEV10
DatasetEV11
Movie EV1
Movie EV2
Source Data for Expanded View
Review Process File
Source Data for Figure 3
Acknowledgments
We thank the IFOM Functional Proteomics group and the Proteomics facility for critical comments and suggestions. We thank Luca Azzolin and Stefano Piccolo for YAP antibody and for their comments on our work. We acknowledge the Imaging, Mass Spectrometry and the Histopathology units at IFOM for their precious work. We thank Giannino Del Sal for discussion. We acknowledge Giuseppe Ossolengo for technical advice for gel filtration. We thank Jeffry W. Kelly for providing PMEL amyloidogenic fragment plasmid. We thank Marco Cirò from IFOM for providing pLKO‐TET‐on plasmid. Francesco Farris is a PhD student within the European School of Molecular Medicine (SEMM). We also thank Dr. Ulf Neumann from Novartis for kindly providing NB360 according to MTA agreement. This work has been supported by AIRC IG 18607 and IG 14578 to Angela Bachi.
EMBO Reports (2020) 21: e50446
Data availability
Proteomic datasets produced in this study are available in the following databases:
Proteomics Identification database: PeptideAtlas PASS01358 (http://www.peptideatlas.org/PASS/PASS01358)
References
- Abildayeva K, Jansen PJ, Hirsch‐Reinshagen V, Bloks VW, Bakker AH, Ramaekers FC, De Vente J, Groen AK, Wellington CL, Kuipers F (2006) 24 (S)‐hydroxycholesterol participates in a liver X receptor‐controlled pathway in astrocytes that regulates apolipoprotein E‐mediated cholesterol efflux. J Biol Chem 281: 12799–12808 [DOI] [PubMed] [Google Scholar]
- Bissig C, Rochin L, van Niel G (2016) PMEL amyloid fibril formation: the bright steps of pigmentation. Int J Mol Sci 17: 1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MA, LeRoy G, Khan Z, Alečković M, Zee BM, Garcia BA, Kang Y (2012) Global secretome analysis identifies novel mediators of bone metastasis. Cell Res 22: 1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo A‐S, Gustafsen C, Mastrobuoni G, Nielsen MS, Burgert T, Hartl D, Rohe M, Nykjaer A, Herz J, Heeren J (2013) The pro‐neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid‐β peptide in the brain. J Neurosci 33: 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, Tergaonkar V, Lim CT, Hong W (2017) Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep 18: 2464–2479 [DOI] [PubMed] [Google Scholar]
- Cotman SL, Halfter W, Cole GJ (2000) Agrin binds to β‐amyloid (Aβ), accelerates Aβ fibril formation, and is localized to Aβ deposits in Alzheimer's disease brain. Mol Cell Neurosci 15: 183–198 [DOI] [PubMed] [Google Scholar]
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 10: 1794–1805 [DOI] [PubMed] [Google Scholar]
- Danish Rizvi SM, Hussain T, Subaiea GM, Shakil S, Ahmad A (2018) Therapeutic targeting of amyloid precursor protein and its processing enzymes for breast cancer treatment. Curr Protein Pept Sci 19: 841–849 [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AW, Park ST, Zewail AH (2013) Exceptional rigidity and biomechanics of amyloid revealed by 4D electron microscopy. Proc Natl Acad Sci 110: 10976–10981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory‐Jost C, Marks MS, Balch WE, Kelly JW (2006) Functional amyloid formation within mammalian tissue. PLoS Biol 4: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey K, Fiechter M, Schwager K, Belloni B, Barysch MJ, Neri D, Dummer R (2011) Different patterns of fibronectin and tenascin‐C splice variants expression in primary and metastatic melanoma lesions. Exp Dermatol 20: 685–688 [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Brindisi M, Yen YC, Lendy EK, Kovela S, Cardenas EL, Reddy BS, Rao KV, Downs D, Huang X et al (2019) Highly selective and potent human beta‐secretase 2 (BACE2) inhibitors against type 2 diabetes: design, synthesis, X‐ray structure and structure‐activity relationship studies. ChemMedChem 14: 545–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths WJ, Crick PJ, Wang Y, Ogundare M, Tuschl K, Morris AA, Bigger BW, Clayton PT, Wang Y (2013) Analytical strategies for characterization of oxysterol lipidomes: liver X receptor ligands in plasma. Free Radic Biol Med 59: 69–84 [DOI] [PubMed] [Google Scholar]
- Gunn AP, Masters CL, Cherny RA (2010) Pyroglutamate‐Aβ: role in the natural history of Alzheimer's disease. Int J Biochem Cell Biol 42: 1915–1918 [DOI] [PubMed] [Google Scholar]
- Guy GP Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC (2015) Vital signs: melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb Mortal Wkly Rep 64: 591 [PMC free article] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer‐Dene B, Matsuda M, Larkin J, Marais R, Sahai E (2015) Intravital imaging reveals how BRAF inhibition generates drug‐tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 27: 574–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS, Eichhoff OM, Schlegel NC, Döbbeling U, Kobert N, Schaerer L, Hemmi S, Dummer R (2008) In vivo switching of human melanoma cells between proliferative and invasive states. Can Res 68: 650–656 [DOI] [PubMed] [Google Scholar]
- Holle AW, Young JL, Spatz JP (2016) In vitro cancer cell–ECM interactions inform in vivo cancer treatment. Adv Drug Deliv Rev 97: 270–279 [DOI] [PubMed] [Google Scholar]
- Johansson CH, Azimi A, Stolt MF, Shojaee S, Wiberg H, Grafström E, Hansson J, Brage SE (2013) Association of MITF and other melanosome‐related proteins with chemoresistance in melanoma tumors and cell lines. Melanoma Res 23: 360–365 [DOI] [PubMed] [Google Scholar]
- Johnson R, Halder G (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discovery 13: 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA (2005) VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature 438: 820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis GS, Pavlou MP, Diamandis EP (2010) Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol 4: 496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Kim J, Hong H, Lee SH, Lee JK, Jung E, Kim J (2016) Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J 35: 462–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick SJ, Wang RK, Duncan DD, Kulesz‐Martin M, Lee K (2006) Imaging the mechanical stiffness of skin lesions by in vivo acousto‐optical elastography. Opt Express 14: 9770–9779 [DOI] [PubMed] [Google Scholar]
- Kleffman K, Levinson G, Wong E, Galán‐Echevarría F, Von‐Itter R, Rose I, Blumenberg L, Floristán A, Tranos J, Argibay D et al (2019) Melanoma‐secreted amyloid beta suppresses neuroinflammation and promotes brain metastasis. BioRxiv 10.1101/854885 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, Schindler JW, Jiang Z‐G, Hynes RO (2012) The Hippo pathway target, YAP, promotes metastasis through its TEAD‐interaction domain. Proc Natl Acad Sci 109: E2441–E2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi MC, Reinhart‐King CA (2018) Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci Transl Med 10: eaao0475 [DOI] [PubMed] [Google Scholar]
- Lee CI, Chen LE, Elmore JG (2017) Risk‐based breast cancer screening: implications of breast density. Med Clin North Am 101: 725–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberato T, Pessotti DS, Fukushima I, Kitano ES, Serrano SM, Zelanis A (2018) Signatures of protein expression revealed by secretome analyses of cancer associated fibroblasts and melanoma cell lines. J Proteomics 174: 1–8 [DOI] [PubMed] [Google Scholar]
- Matafora V, D'Amato A, Mori S, Blasi F, Bachi A (2009) Proteomics analysis of nucleolar SUMO‐1 target proteins upon proteasome inhibition. Mol Cell Proteomics 8: 2243–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matafora V, Cuccurullo M, Beneduci A, Petrazzuolo O, Simeone A, Anastasio P, Mignani R, Feriozzi S, Pisani A, Comotti C (2015) Early markers of Fabry disease revealed by proteomics. Mol BioSyst 11: 1543–1551 [DOI] [PubMed] [Google Scholar]
- Matafora V, Corno A, Ciliberto A, Bachi A (2017) Missing value monitoring enhances the robustness in proteomics quantitation. J Proteome Res 16: 1719–1727 [DOI] [PubMed] [Google Scholar]
- Miskolczi Z, Smith MP, Rowling EJ, Ferguson J, Barriuso J, Wellbrock C (2018) Collagen abundance controls melanoma phenotypes through lineage‐specific microenvironment sensing. Oncogene 37: 3166–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawski M, Schilling S, Kreuzberger M, Waniek A, Jäger C, Koch B, Cynis H, Kehlen A, Arendt T, Hartlage‐Rübsamen M (2014) Glutaminyl cyclase in human cortex: correlation with (pGlu)‐amyloid‐β load and cognitive decline in Alzheimer's disease. J Alzheimers Dis 39: 385–400 [DOI] [PubMed] [Google Scholar]
- Nallet‐Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, Sudol M, Herlyn M, Mauviel A (2014) Pro‐invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J Invest Dermatol 134: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann U, Rueeger H, Machauer R, Veenstra SJ, Lueoend RM, Tintelnot‐Blomley M, Laue G, Beltz K, Vogg B, Schmid P (2015) A novel BACE inhibitor NB‐360 shows a superior pharmacological profile and robust reduction of amyloid‐β and neuroinflammation in APP transgenic mice. Mol Neurodegener 10: 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Nishiya N, Shito T, Yamamoto R, Yamamoto Y, Oyama C, Uehara Y (2015) Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio 5: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, Carbone CJ, Yu Q, Katlinski KV, Katlinskaya YV (2019) An interferon‐driven oxysterol‐based defense against tumor‐derived extracellular vesicles. Cancer Cell 35: 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, Tavazoie SF (2020) Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med 26: 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S (2018) Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2: CD011123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, Marks MS, De Strooper B, Raposo G, van Niel G (2013) BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci 110: 10658–10663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna M, Zadlo A, Hermanowicz P, Madeja Z, Burda K, Sarna T (2014) Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp Dermatol 23: 813–818 [DOI] [PubMed] [Google Scholar]
- Shain AH, Bastian BC (2016) From melanocytes to melanomas. nature reviews. Cancer 16: 345 [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Jacobson LH, Kolly C, Zamurovic N, Balavenkatraman KK, Morawiec L, Kreutzer R, Schelle J, Jucker M, Bertschi B (2016) Pharmacological BACE1 and BACE2 inhibition induces hair depigmentation by inhibiting PMEL17 processing in mice. Sci Rep 6: 21917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncini M, Corna G, Moresco M, Coltella N, Restuccia U, Maggioni D, Raccosta L, Lin C‐Y, Invernizzi F, Crocchiolo R (2016) 24‐Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc Natl Acad Sci 113: E6219–E6227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Niel G, Bergam P, Di Cicco A, Hurbain I, Cicero AL, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers LJ (2015) Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Rep 13: 43–51 [DOI] [PubMed] [Google Scholar]
- Wang JJ, Li ZF, Li XJ, Han Z, Zhang L, Liu ZJ (2017a) Effects of microRNA‐136 on melanoma cell proliferation, apoptosis, and epithelial‐mesenchymal transition by targetting PMEL through the Wnt signaling pathway. Biosci Rep 37: 5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K (2017b) Role of tumor microenvironment in tumorigenesis. J Cancer 8: 761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder G, Hendriks‐Balk MC, Smajda R, Rimoldi D, Liley M, Heinzelmann H, Meister A, Mariotti A (2014) Increased plasticity of the stiffness of melanoma cells correlates with their acquisition of metastatic properties. Nanomedicine 10: 141–148 [DOI] [PubMed] [Google Scholar]
- Westermark GT, Krogvold L, Dahl‐Jorgensen K, Ludvigsson J (2017) Islet amyloid in recent‐onset type 1 diabetes‐the DiViD study. Upsala J Med Sci 122: 201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, Cornelis A, Rozenski J, Zwolinska A, Marine J‐C (2011) Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol 7: 285 [DOI] [PubMed] [Google Scholar]
- Yu H, Mouw JK, Weaver VM (2011) Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Carlson JA, Granese J, Ross J, Mihm M, Slominski A (2008) Current concepts of metastasis in melanoma. Expert Rev Dermatol 3: 569–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
DatasetEV1
DatasetEV2
DatasetEV3
DatasetEV4
DatasetEV5
DatasetEV6
DatasetEV7
DatasetEV8
DatasetEV9
DatasetEV10
DatasetEV11
Movie EV1
Movie EV2
Source Data for Expanded View
Review Process File
Source Data for Figure 3
Data Availability Statement
Proteomic datasets produced in this study are available in the following databases:
Proteomics Identification database: PeptideAtlas PASS01358 (http://www.peptideatlas.org/PASS/PASS01358)


