Abstract
Background
Vagus nerve (VN) stimulation is currently evaluated as a novel approach to treat immune‐mediated disorders. The optimal stimulation parameters, however, largely depend on the VN composition potentially impacting on its clinical translation. Hence, we evaluated whether morphological differences exist between the cervical and abdominal VNs across different species.
Materials and methods
The cervical and abdominal VNs of mouse, pig, and humans were stained for major basic protein and neurofilament F to identify the percentage and size of myelinated and non‐myelinated fibers.
Results
The percentage of myelinated fibers was comparable between species, but was higher in the cervical VN compared with the abdominal VN. The cervical VN contained 54 ± 4%, 47 ± 7%, and 54 ± 7% myelinated fibers in mouse, pig, and humans, respectively. The myelinated fibers consisted of small‐diameter (mouse: 71%, pig: 80%, and humans: 63%), medium‐diameter (mouse: 21%, pig: 18%, and humans: 33%), and large‐diameter fibers (mouse: 7%, pig: 2%, and humans: 4%). The abdominal VN predominantly contained unmyelinated fibers (mouse: 93%, pig: 90%, and humans: 94%). The myelinated fibers mainly consisted of small‐diameter fibers (mouse: 99%, pig: 85%, and humans: 74%) and fewer medium‐diameter (mouse: 1%, pig: 13%, and humans: 23%) and large‐diameter fibers (mouse: 0%, pig: 2%, and humans: 3%).
Conclusion
The VN composition was largely similar with respect to myelinated and unmyelinated fibers in the species studied. Human and porcine VNs had a comparable diameter and similar amounts of fibrous tissue and contained multiple fascicles, implying that the porcine VN may be suitable to optimize stimulation parameters for clinical trials.
Keywords: histology, human, mouse, pig, vagus nerve
The cervical and abdominal vagus nerve have a similar fiber composition across the studied species. However, the cervical vagus nerve generally possess a higher level of myelination compared to its abdominal counterpart, suggesting that higher stimulation parameters are required to activate the abdominal vagus nerve.
![]()
Key Points.
To date, vagus nerve stimulation is evaluated as a novel treatment for immune‐mediated disorders. Since its activation threshold significantly differs depending on the type of vagal fiber targeted, new insights in the vagal composition across different species may be key in determining optimal stimulation parameters to be used in clinical trials.
We found that the unmyelinated and myelinated nerve fiber composition of the vagus nerve is largely similar across the studied species. However, the human and porcine vagus nerves contain multiple fascicles surrounded by fibrous tissue in contrast to the murine vagus nerve.
The porcine vagus nerve may be more relevant to optimize stimulation parameters for a clinical setting than the murine vagus nerve.
1. INTRODUCTION
More than two decades ago, the therapeutic potential of cervical vagus nerve stimulation (VNS) was reported for epilepsy, migraine, and depression. 1 Recently, the discovery of the cholinergic anti‐inflammatory pathway has broadened the application of VNS to chronic immune‐mediated disorders such as rheumatoid arthritis 2 and Crohn's disease 3 . VNS was indeed shown to exert an anti‐inflammatory effect in a model of sepsis by modulation of macrophages via the α7 nicotinic acetylcholine receptor (α7nAChR). 4 , 5 Also in the gastrointestinal (GI) tract, we and others demonstrated that cervical VNS dampens macrophage activation in experimental colitis, 6 , 7 food allergy, 8 and postoperative ileus. 9 , 10 Interestingly, our group recently showed that electrical stimulation of both the subdiaphragmatic posterior and anterior vagal branches possesses similar anti‐inflammatory properties as cervical VNS in mice 11 , 12 and thus could be used as an alternative approach for cervical VNS.
To date, it, however, remains to be elucidated whether the therapeutic effect of VNS is explained by afferent or efferent signaling of the VN. In early studies, cervical VNS was always considered to control peripheral inflammation via the efferent vagal pathway. 13 , 14 Recent studies have, however, indicated that, in addition to this efferent pathway, VNS can also trigger afferent signaling to the brain modulating the immune system via vagovagal, vagosympathetic, or vagoadrenal reflexes. 15 , 16 , 17 , 18 , 19 Especially as the stimulation threshold varies significantly according to the type of nerve fiber targeted, knowledge of the composition of the VN and the type of nerve fibers involved may be of great interest in view of the optimal stimulation parameters to be used. Moreover, if the VN composition would significantly differ from that of humans, one may question to what extent data on optimal stimulation parameters in preclinical models can be translated to the human situation.
Typically, the VN is composed of 3 fiber types: large‐diameter myelinated fibers with a amplitude duration threshold ranging between 0.02 and 0.2 mA, small‐ and medium‐diameter myelinated fibers with a higher excitation threshold between 0.04 and 0.6 mA, and finally small unmyelinated fibers that require stimulation currents higher than 2.0 mA. 1 Comparative morphological studies on the cervical and abdominal VNs are, however, scarce in both studied animals and humans. 20 , 21 , 22 , 23 Data are available from rabbit, 24 rat, 25 , 26 , 27 , 28 and cat, 29 but apart from rats, these animals are not used to evaluate the effect of VNS. Instead, most VNS preclinical studies are performed in murine, rat, and porcine models. 30 , 31 , 32 , 33 Hence, the main objective of this study was to study the composition of the cervical and abdominal VNs in these species and to compare it with that of the human VN.
2. METHODS
2.1. Species
Nine‐week‐old wild‐type mice (C57BL/6JOlaHsd) were purchased from Envigo (The Netherlands). All mice were housed at the University of Leuven animal facility under specific pathogen‐free (SPF) conditions on a 12:12‐h light‐dark cycle and fed with commercially available chow (ssniff R/M‐H; ssniff Spezialdiäten GmbH). Mice were anesthetized by intraperitoneal (i.p.) injection of a mixture of ketamine (Ketalar 100 mg/kg; Pfizer) and xylazine (Rompun 10 mg/kg; Bayer). Cervical right VN and subdiaphragmatic posterior abdominal VN were excised from the mice at the VNS implant level as previously described. 10 , 12 After this procedure, the mice were sacrificed with a CO2 overdose.
Five 12‐ to 14‐week‐old Landrace pigs weighing 30 ± 3 kg were kept at the KU Leuven animal facility under SPF conditions. Anesthetized pigs (0.1 ml/kg Zoletil 100 and Xylazine mixture, intramuscular) were intubated and connected to a respirator for ventilation. Anesthesia was maintained with 2.5% of isoflurane. During the surgical procedure, pigs were positioned on a heating pad (32°C) in reversed Trendelenburg position. The cervical right VN was excised at the level where the VNS electrode is normally placed. 12 , 34 To obtain the posterior abdominal VN, the left liver lobes were retracted. After identification of the esophagogastric junction, the lesser omentum was opened, the right diaphragmatic hiatus was identified, and the posterior VN was dissected free from the esophagus just distally from diaphragmatic hiatus. After this procedure, the pigs were sacrificed with an intravenous injection of T61® (MSD, AH, embutramide 200 mg/ml, mebenzonium iodide 50 mg/ml, tetracaine hydrochloride 5 mg/ml). All experimental procedures were approved by the Animal Care and Animal Experiments Committee of the Medical Faculty of the KU Leuven (Leuven, Belgium).
Six human abdominal VNs (age: 35‐74 years) were harvested from brain‐dead organ donors at the level of the abdominal VNS implantation site as described previously. 12 Since we could not obtain the cervical VN during this organ harvest, 6 human cervical VNs (age: 54‐90 years; 5 right VNs and 1 left VN) were obtained from formalin‐fixed (femoral infusion of ~ 10‐liter fixative followed by 4 weeks of fixation in a formalin bath) human corpses as previously described. 35 No previous surgical interventions of head and neck had been performed. All procedures were approved by the Ethical Committee of University Hospital of Leuven (Leuven, Belgium) or Ethical Committee of University of Maastricht (Maastricht, The Netherlands).
2.2. Tissue processing
After collection, the VN was washed with cold phosphate‐buffered saline (PBS). The porcine VNs and human abdominal VNs were fixed overnight in 4% paraformaldehyde (PFA) at 4°C, while the murine VNs were fixed for 30 minutes in 4% PFA at room temperature. After fixation, the specimens were washed with cold PBS, incubated overnight with a 30% sucrose solution + 0.01% sodium azide (in PBS), and embedded in Tissue‐Tek OCT Compound (Sakura Finetek Europe, Alphen aan de Rijn, The Netherlands). Thin sections (5 µm) were cut and stored at − 80°C for further processing.
2.3. Immunofluorescence
The sections were hydrated with PBS and blocked with 1% bovine serum albumin (BSA) for 2 hours at room temperature. The sections were incubated with a chicken antineurofilament 200 kD (NeuF; 1:5000; Abcam, Cambridge, UK) and mouse antimyelin basic protein (MBP; Santa Cruz, Dallas, USA) antibody diluted in 1% BSA + 0.5% Triton X‐100 overnight at 4°C. Subsequently, sections were incubated for 2 hours at room temperature with a secondary Cy5‐conjugated donkey anti‐chicken and Cy3‐conjugated donkey anti‐mouse antibody (1:800, all Jackson ImmunoResearch, West Grove, USA) in 1% BSA + 0.5% Triton X‐100. Thereafter, the sections were washed three times with PBS for 5 min. A negative control (ie, no primary antibody) was included to ascertain the specificity of the secondary antibodies.
Similar to previous studies in humans, 23 fiber populations at cervical and abdominal levels were divided into different categories based on size, namely small‐diameter (0‐3 µm), medium‐diameter (3‐10 µm), and large‐diameter (>10 µm) myelinated fibers for the higher‐order species (ie, pig and humans). In mice, the VNs were divided into small‐diameter (0‐1.5 µm), medium‐diameter (1.5‐5 µm), and large‐diameter (>5 µm) myelinated fibers. Immunohistochemical preparations were visualized using the Olympus BX4 microscope (Olympus America, Center Valley, USA) with specific filter cubes (EX/DM/EM in nm). In the case of murine VN, each preparation was entirely screened, while 20%‐30% of the total cross‐sectional area of the porcine and human VNs was counted in one slide per individual nerve as previously described. 29
2.4. Statistical analysis
To compare two independent groups and a single variable, the unpaired t test was performed. Probability level of P < .05 was considered statistically significant. Results are shown as mean ± standard deviation (SD). Graph Pad Prism V.5.01 software was used to perform statistical analysis.
3. RESULTS
3.1. Gross composition of the vagus nerve
The diameter of the cervical and abdominal VNs was 183 ± 16 µm and 112 ± 9 µm in mice (n = 4) and 2927 ± 777 µm and 2085 ± 274 µm in pigs (n = 5), respectively. The human cervical and abdominal VNs (n = 6) had a diameter of 2017 ± 347 µm and 1916 ± 472 µm, respectively.
Transverse vagal sections typically consisted of connective tissue, fat, vessels, and multiple fascicles except in mice. In more detail, the murine VNs were composed of a single fascicle, while the porcine VN contained 46 ± 10 and 43 ± 8 bundles at cervical and abdominal levels, respectively (Figures 1, 2, 3). The human cervical and abdominal VNs consisted of 7 ± 3 and 16 ± 6 fascicles, respectively (Figures 1 and 4).
Figure 1.
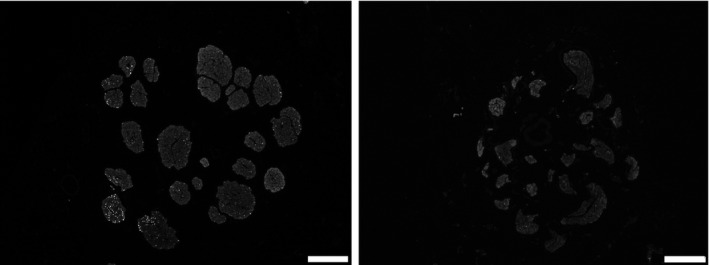
Representative image of porcine (left panel) and human (right panel) abdominal VNs to illustrate the diameter and the surface area in‐between nerves. Scale bars are 250 µm
Figure 2.
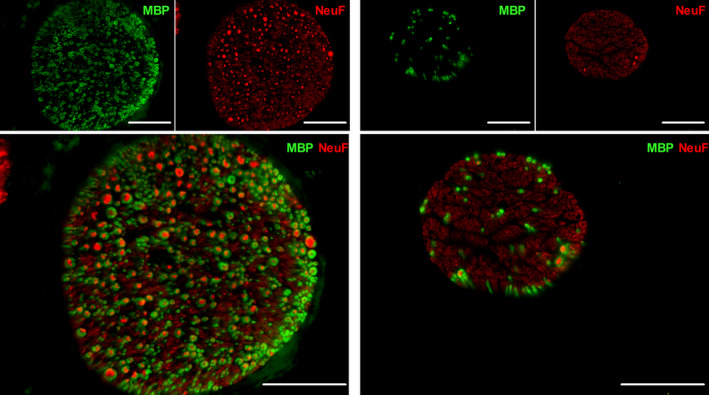
Representative image of a murine cervical (left panel) and abdominal (right panel) VNs. The nerve fibers (NeuF) are depicted in red, and the myelin (MBP) is illustrated in green. Scale bars are 50 µm. NeuF: neurofilament F, MBP: major basic protein
Figure 3.
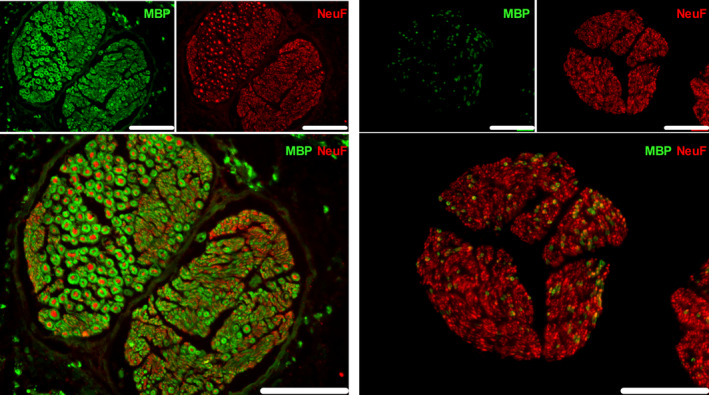
Representative image of a porcine nerve fascicle(s) in the cervical (left panel) and abdominal (right panel) VNs. The nerve fibers (NeuF) are depicted in red, and the myelin (MBP) is illustrated in green. Scale bars are 50 µm. NeuF: neurofilament F, MBP: major basic protein
Figure 4.
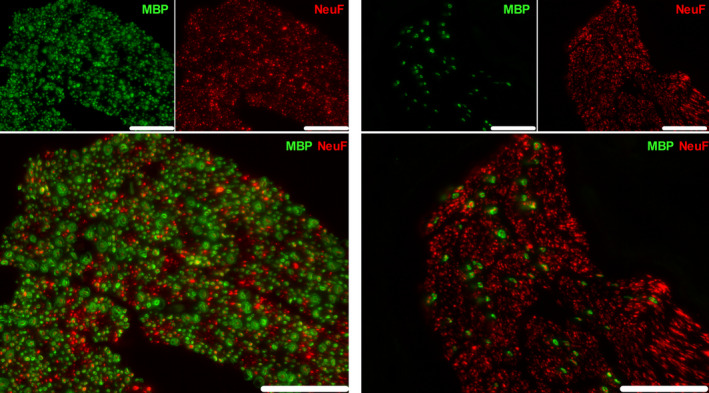
Representative image of a human nerve fascicle in the cervical (left panel) and abdominal (right panel) VNs. The nerve fibers (NeuF) are depicted in red, and the myelin (MBP) is illustrated in green. Scale bars are 50 µm. NeuF: neurofilament F, MBP: myelin basic protein
The number of fibers in the cervical VN was 1426 ± 31 in mouse, 62 235 ± 11 688 in pig, and 51 288 ± 10 224 in humans (Figures 2, 3, 4 and Table 1), while the abdominal VN consisted of 1189 ± 390 fibers in mouse, 41 153 ± 12 267 fibers in pig, and 28 714 ± 15 996 fibers in humans, respectively (Figures 2, 3, 4 and Table 2). The myelination level of the cervical VN was higher (54 ± 4% in mouse, 47 ± 7% in pig, and 54 ± 7 in humans) compared with the abdominal VN (7 ± 2% in mouse, 10 ± 2% in pig, and 6 ± 2% in humans) (Tables 1 and 2), but it was comparable between species.
Table 1.
Characteristics of cervical vagus nerve of mice, pigs, and humans
| Mouse | Pig | Human | |
|---|---|---|---|
| Diameter (µm) | 183 ± 16 | 2927 ± 777 | 2017 ± 347 |
| Fascicles | 1 | 46 ± 10 | 7 ± 3 |
| Fibers (number) | 1426 ± 31 | 62 235 ± 11 688 | 51 288 ± 10 225 |
| Myelination (%) | 54 ± 4 | 47 ± 7 | 54 ± 7 |
Table 2.
Characteristics of abdominal vagus nerve of mice, pigs, and humans
| Mouse | Pig | Human | |
|---|---|---|---|
| Diameter (µm) | 112 ± 9 | 2085 ± 274 | 1916 ± 472 |
| Fascicles | 1 | 43 ± 8 | 16 ± 6 |
| Fibers (number) | 1189 ± 390 | 41 153 ± 12 267 | 28 714 ± 15 996 |
| Myelination (%) | 7 ± 2% | 10 ± 2 | 6 ± 2 |
3.2. Fiber‐type composition
3.2.1. Cervical VN
As small‐ to medium‐diameter myelinated fibers have been implicated in relaying the “inflammatory reflex,” 18 the myelinated fibers of the cervical and abdominal VNs were further studied to determine whether their composition (ie, small‐diameter, medium‐diameter, and large‐diameter fibers) differed across species. 18 , 36 The myelinated fraction of the porcine and murine cervical VNs was composed of the three different populations. In more detail, the murine cervical VN contained 7.7% large‐diameter, 20.9% medium‐diameter, and 71.4% small‐diameter myelinated fibers. In the pig, the cervical VN consisted of 1.7% large‐diameter, 17.8% medium‐diameter, and 80.5% small‐diameter myelinated fibers compared with 3.7% large‐diameter, 33.6% medium‐diameter, and 62.6% small‐diameter myelinated fibers in humans.
3.2.2. Abdominal VN
The myelinated fraction of the abdominal murine VN was mainly composed of small‐diameter myelinated fibers. More specifically, the murine abdominal VN consisted of 99% small‐diameter myelinated fibers and 1% medium‐diameter myelinated fibers. Of note, large‐diameter myelinated fibers were absent in the abdominal VN of this species. The myelinated fraction of the porcine and human abdominal VNs mostly contained small‐diameter myelinated fibers (ie, 85.4% in pig and 73.8% in humans). Medium‐diameter myelinated fibers represented 13% and 23.3% in pig and humans, respectively. Interestingly, 1.7% and 2.9% of the myelinated fibers in the porcine and human abdominal VNs had a large diameter (> 10 µm), respectively.
4. DISCUSSION
Cervical and abdominal VNS has been successfully studied as a treatment of immune‐mediated diseases such as postoperative ileus, colitis, and arthritis in preclinical models. 6 , 10 , 37 Yet, the exact mechanism of how VNS dampens immune cell activation should be further studied. In addition, the optimum stimulation parameters of VNS still require further investigation as it remains to be further elucidated which type(s) of vagal fibers are actually responsible for the cholinergic anti‐inflammatory pathway.
The VN is typically composed of 3 fiber types including A, B, and C fibers. Their excitation threshold following VNS depends on several factors: (a) fibrous tissue surrounding the nerve fascicles increases resistance, altering the electric field and resulting in increased voltage requirements for fiber excitation; (b) fiber myelination; (c) fiber diameter and (d) the electrode design (eg, cuff vs hook and possible inclusion of recording electrodes); and (e) implantation position. 38 , 39 In general, C fibers are small, unmyelinated, and thus have a higher excitation threshold than A and B fibers which are larger and myelinated. As the excitation threshold significantly differs depending on the type of vagal fibers targeted, knowledge about the VN composition would be of great interest as it might determine the stimulation parameters to be used in preclinical and clinical studies. Notably, in a previous study the B fibers were implicated in transmitting the anti‐inflammatory effect of VNS. 18 In the present study, we therefore investigated the composition of the cervical and abdominal VNs in 3 different mammalian species, that is, mice, pig, and humans.
We observed that the increased size of the VN was associated with a higher number of nerve fascicles and more fibrous tissue surrounding the fascicles in the porcine and human VNs compared with the murine VN. Previous studies have shown that the number of fascicles and amount of fibrous tissue within the VN enhances the excitation threshold of large‐diameter and medium‐diameter myelinated fibers following VNS, indicating that a higher‐intensity stimulation is required to activate the porcine and human fibers. 39 Consequently, caution is warranted to use rodent models to optimize stimulation parameters for the human situation. Notably, however, the composition of both the cervical and abdominal VNs with respect to myelination and fiber diameter was quite similar between the studied species, arguing that the anti‐inflammatory effect of VNS could still act via the same vagal fiber type across species. Of importance, similar to the human cervical VN, we found that its porcine counterpart is also sparsely innervated with tyrosine hydroxylase‐positive (ie, sympathetic) fibers implying that VNS may have a possible sympathetic component that could contribute to its anti‐inflammatory properties. 35
Currently, only few clinical data are available concerning the anti‐inflammatory properties of VNS in patients with inflammatory disorders. Bonaz et al (2016) and Koopman et al (2016) recently demonstrated that chronic stimulation of the cervical VN (10 Hz, 0.25‐0.5 ms, and 0.25‐2 mA) reduced inflammation and alleviated disease severity in patients with Crohn's disease and rheumatoid arthritis, respectively. 2 , 3 Electrophysiological studies of the porcine and human cervical VNs showed that these stimulation parameters (current: 0.25‐2 mA, frequency: 20 Hz) activate small‐, medium‐, and large‐diameter myelinated fibers. 40 , 41 , 42 Indeed, it is generally accepted that the excitation threshold of large‐diameter myelinated ranges between 0.02 and 0.2 mA, while small‐ and medium‐diameter fibers have an activation threshold between 0.04 and 0.6 mA. 1 Of interest, the aforementioned electrophysiological studies in patients showed that the activation of unmyelinated fibers within the cervical VN could only be evoked with stimulation intensities above 3.5 mA. Similarly, in pigs, only supramaximal intensity currents (ie, >9 mA) were able to excite unmyelinated fibers of the cervical VN. 30 , 43 This is further supported by observations in rodents showing that the anti‐inflammatory pathway of cervical VNS is mediated through myelinated fibers 18 , 36 and is independent of unmyelinated fibers. 44
As we recently proposed VNS of the subdiaphragmatic posterior VN as an alternative approach to treat inflammatory intestinal disorders, 12 we also examined the fiber composition of the subdiaphragmatic posterior VN. We chose not to study the subdiaphragmatic anterior vagal branch, as our previous study showed that stimulation of both the anterior and posterior VNs reduced TNF‐α production in a model of sepsis to a similar degree. 12 Moreover, the posterior VN is also more appealing for VNS applications in the GI tract, as it innervates the GI tract until the splenic flexure of the large intestine, while the anterior vagal branches only reach as far as the proximal duodenum. 28
We found that the abdominal VN mostly contained unmyelinated fibers and small‐diameter myelinated fibers in all studied species. 22 , 24 , 29 An electrophysiological study of isolated human abdominal VNs showed that these small‐myelinated fibers had a rather low activation threshold of 5‐10 V (ie, comparable to a current intensity of 1‐2 mA based on the resistance of our previous study 12 ), while the unmyelinated fibers were only activated with supramaximal intensities (ie,> 50 V comparable to a current intensity of > 10 mA 12 ). Thus, it seems unlikely that unmyelinated fibers are involved in the anti‐inflammatory effect of abdominal VNS. 1 , 45 Indeed, in our recent pilot study, abdominal VNS was performed in patients undergoing abdominal surgery to activate the vagal anti‐inflammatory pathway in an attempt to reduce intestinal inflammation and prevent postoperative ileus. We used stimulation parameters (20 Hz, 1 ms, and 2.5 mA for 2 min at the beginning and end of the surgery) above the excitation threshold (ie, 0.04‐0.6 mA) of small‐ and medium‐diameter myelinated fibers, but below that of unmyelinated fibers (ie,> 9 mA). Abdominal VNS during surgery decreased endotoxin‐induced IL‐8 and IL‐6 production by whole blood. 12 Of note, abdominal VNS did not lead to significant antidromic conductance interfering with cardiac function. 12 , 34 As a result, these data indirectly suggest that small‐ or medium‐diameter myelinated fibers are most likely responsible for the transmission of vagal anti‐inflammatory effect to the gut. If further studies confirm the anti‐inflammatory properties of abdominal VNS, it would be of great interest to further develop a chronic abdominal VN stimulator to treat macrophage‐mediated disorders in the GI tract. This approach may have fewer side effects than cervical VNS, in particular as the site of stimulation avoids stimulation of the larynx and minimizes the risk to interfere with cardiopulmonary function. 12 , 34
In conclusion, there is a large similarity in composition of the VN with respect to myelinated and unmyelinated fibers in the three species studied. Human and porcine VNs, but not murine VN, have a comparable diameter, contain similar amounts of fibrous tissue, and are composed of multiple fascicles, implying that the porcine VN rather than murine VN may be used to optimize stimulation parameters to be used in clinical trials. Moreover, as the abdominal VN mostly consisted of unmyelinated and small‐ and medium‐diameter myelinated fibers and given the high stimulation threshold of unmyelinated nerve fibers, our data suggest that most likely small‐diameter myelinated fibers or perhaps medium‐diameter myelinated fibers are responsible for the vagal anti‐inflammatory input to the gut.
CONFLICT OF INTEREST
The authors declare to have no conflict of interest.
AUTHOR CONTRIBUTION
NS, PJGP, GM, and GEB planned and designed the experiments. NS, PJGP, TJMV, AMW, AD, RF, PH, and GM performed or supervised the experiments. NS and GEB reviewed the data and wrote the manuscript. All other authors corrected and approved the final version of the manuscript.
ACKNOWLEDGMENT
This work was supported by the European Research Council (ERC) Advanced Grant (ERC‐2013‐Adg: 340101 Cholstim) to GEB. GEB is also supported by Flanders Fund for Innovation by Science and Technology (IWT‐TBM; 110699), and Research Foundation—Flanders (FWO; G.0566.12N and G.0890.18N). NS is supported by a postdoctoral research fellowship of FWO (1509020N and 12V3619N). GM is supported by an FWO grant (G.0D83.17N) and by KU Leuven grants (ZKD2906‐C14/17/097 and ZKC9531‐C12/15/016)
Stakenborg N, Gomez‐Pinilla PJ, Verlinden TJM, et al. Comparison between the cervical and abdominal vagus nerves in mice, pigs, and humans. Neurogastroenterology & Motility. 2020;32:e13889 10.1111/nmo.13889
REFERENCES
- 1. Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493‐500. 10.1016/j.neubiorev.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 2. Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016; 113(29):8284‐8289. 10.1073/pnas.1605635113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonaz B, Sinniger V, Hoffmann D, et al. Chronic vagus nerve stimulation in Crohn's disease: a 6‐month follow‐up pilot study. Neurogastroenterol Motil. 2016; 28(6):948‐953. 10.1111/nmo.12792 [DOI] [PubMed] [Google Scholar]
- 4. Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384‐388. 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- 5. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458‐462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 6. Meregnani J, Clarençon D, Vivier M, et al. Anti‐inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82‐89. 10.1016/j.autneu.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 7. Meroni E, Stakenborg N, Gomez‐Pinilla PJ, et al. Functional characterization of oxazolone‐induced colitis and survival improvement by vagus nerve stimulation. PLoS ONE. 2018;13:e0197487 10.1371/journal.pone.0197487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bosmans G, Appeltans I, Stakenborg N, et al. Vagus Nerve Stimulation dampens intestinal inflammation in a murine model of experimental Food Allergy. Allergy. 2019;74(9):1748‐1759. 10.1111/all.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2‐STAT3 signaling pathway. Nat Immunol. 2005;6(8):844‐851, doi:10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 10. Stakenborg N, Labeeuw E, Gomez‐Pinilla PJ, et al. Preoperative administration of the 5‐HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68:1406‐1416. 10.1136/gutjnl-2018-317263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolthuis AM, Bislenghi G, Fieuws S, et al. Incidence of prolonged postoperative ileus after colorectal surgery: A systematic review and meta‐analysis. Colorectal Dis. 2016;18:O1‐9. 10.1111/codi.13210 [DOI] [PubMed] [Google Scholar]
- 12. Stakenborg N, Wolthuis AM, Gomez‐Pinilla PJ, et al. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol Motil. 2017;29(9):e13075 10.1111/nmo.13075 [DOI] [PubMed] [Google Scholar]
- 13. Borovikova LV, Ivanova S, Nardi D, et al. Role of vagus nerve signaling in CNI‐1493‐mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141‐147. 10.1016/S1566-0702(00)00233-2 [DOI] [PubMed] [Google Scholar]
- 14. Matteoli G,Gomez‐Pinilla PJ, Nemethova A, et al. A distinct vagal anti‐inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2013;gutjnl‐2013‐304676;63(6):938–48. [DOI] [PubMed] [Google Scholar]
- 15. Bratton BO, Martelli D, McKinley MJ, et al. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180‐1185. 10.1113/expphysiol.2011.061531 [DOI] [PubMed] [Google Scholar]
- 16. Inoue T, Abe C, Sung S‐SJ, et al. Vagus nerve stimulation mediates protection from kidney ischemia‐reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126:1939‐1952. 10.1172/JCI83658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vida G, Pena G, Deitch EA, Ulloa L. alpha7‐cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186:4340‐4346. 10.4049/jimmunol.1003722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olofsson PS, Levine YA, Caravaca A, et al. Single‐pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron Med. 2015;2:37‐42. [Google Scholar]
- 19. Bassi GS, Dias DPM, Franchin M, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330‐343. 10.1016/j.bbi.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keen JA. A note of the fibre composition of the vagus nerve in man. S. Afr. Med. J. 1966;40:981‐984. [PubMed] [Google Scholar]
- 21. Hammer N, Löffler S, Cakmak YO, et al. Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation ‐ A cadaveric study. Sci Rep. 2018;8:7997 10.1038/s41598-018-26135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffman HH, Schnitzlein HN. The numbers of nerve fibers in the vagus nerve of man. The Anatomical record. 1961;139:429‐435. [DOI] [PubMed] [Google Scholar]
- 23. Schnitzlein HN, Rowe LC, Hoffman HH. The myelinated component of the vagus nerves in man. The Anatomical record. 1958;131:649‐667. [Google Scholar]
- 24. Evans DH, Murray JG. Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat. 1954;88:320‐337. [PMC free article] [PubMed] [Google Scholar]
- 25. Gabella G, Pease HL. Number of axons in the abdominal vagus of the rat. Brain Res. 1973;58:465‐469. [DOI] [PubMed] [Google Scholar]
- 26. Soltanpour N, Santer RM. Preservation of the cervical vagus nerve in aged rats: morphometric and enzyme histochemical evidence. J Auton Nerv Syst. 1996;60:93‐101. 10.1016/0165-1838(96)00038-0 [DOI] [PubMed] [Google Scholar]
- 27. Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol. 1990;181:101‐115. 10.1007/bf00198950 [DOI] [PubMed] [Google Scholar]
- 28. Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. The American journal of physiology. 1991;260:R200‐207. [DOI] [PubMed] [Google Scholar]
- 29. Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. The Journal of physiology. 1957;135:182‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tosato M, Yoshida K, Toft E, Nekrasas V, Struijk JJ. Closed‐loop control of the heart rate by electrical stimulation of the vagus nerve. Med Biol Eng Compu. 2006;44:161‐169. 10.1007/s11517-006-0037-1 [DOI] [PubMed] [Google Scholar]
- 31. Stakenborg N, Di Giovangiulio M, Boeckxstaens GE, Matteoli G. The versatile role of the vagus neve in the gastrointestinal tract. EMJ Gastroenterol. 2013;1:106‐114. [Google Scholar]
- 32. Czura CJ, Schultz A, Kaipel M, et al. Vagus nerve stimulation regulates hemostasis in swine. Shock. 2010;33:608‐613. 10.1097/SHK.0b013e3181cc0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tubbs RS, Wellons JC 3rd, Blount JP, Oakes WJ. Left‐sided vagus nerve stimulation decreases intracranial pressure without resultant bradycardia in the pig: a potential therapeutic modality for humans. Childs Nerv Syst. 2004;20:309‐312. 10.1007/s00381-004-0947-x [DOI] [PubMed] [Google Scholar]
- 34. Wolthuis AM, Stakenborg N, D'Hoore A, Boeckxstaens GE. The pig as preclinical model for laparoscopic vagus nerve stimulation. Int J Colorectal Dis. 2016;31:211‐215. 10.1007/s00384-015-2435-z [DOI] [PubMed] [Google Scholar]
- 35. Verlinden TJ, Rijkers K, Hoogland G, Herrler A. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol Scand. 2016;133:173‐182. 10.1111/ane.12462 [DOI] [PubMed] [Google Scholar]
- 36. Huston JM, Gallowitsch‐Puerta M, Ochani M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762‐2768. 10.1097/01.CCM.0000288102.15975.BA [DOI] [PubMed] [Google Scholar]
- 37. Levine YA, Koopman FA, Faltys M, et al. Neurostimulation of the cholinergic anti‐inflammatory pathway ameliorates disease in rat collagen‐induced arthritis. PLoS ONE. 2014;9:e104530 10.1371/journal.pone.0104530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noller CM, Levine YA, Urakov TM, Aronson JP, Nash MS. Vagus Nerve stimulation in rodent models: An overview of technical considerations. Front Neurosci. 2019;13:911 10.3389/fnins.2019.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Helmers SL, Begnaud J, Cowley A, et al. Application of a computational model of vagus nerve stimulation. Acta Neurol Scand. 2012;126:336‐343. 10.1111/j.1600-0404.2012.01656.x [DOI] [PubMed] [Google Scholar]
- 40. Koo B, Ham SD, Sood S, Tarver B. Human vagus nerve electrophysiology: a guide to vagus nerve stimulation parameters. J Clin Neurophysiol. 2001;18:429‐433. [DOI] [PubMed] [Google Scholar]
- 41. Evans MS, Verma‐Ahuja S, Naritoku DK, Espinosa JA. Intraoperative human vagus nerve compound action potentials. Acta Neurol Scand. 2004;110:232‐238. 10.1111/j.1600-0404.2004.00309.x [DOI] [PubMed] [Google Scholar]
- 42. Kurstjens G. in 15th Nordic‐Baltic Conference on Biomedical Engineering and Medical Physics (NBC 2011). 261‐263, Springer.
- 43. Ordelman SC, Kornet L, Cornelussen R, Buschman HP, Veltink PH. An indirect component in the evoked compound action potential of the vagal nerve. J Neural Eng. 2010;7:066001 10.1088/1741-2560/7/6/066001 [DOI] [PubMed] [Google Scholar]
- 44. Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C‐fibers does not alter subsequent vagus nerve stimulation‐induced seizure suppression in rats. Epilepsia. 2001;42:586‐589. [DOI] [PubMed] [Google Scholar]
- 45. Andrews PL, Taylor TV. An electrophysiological study of the posterior abdominal vagus nerve in man. Clin Sci. 1982;63:169‐173. [DOI] [PubMed] [Google Scholar]


