Abstract
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of adult and elderly individuals with insomnia. Some current pharmacologic treatments for insomnia cause respiratory depression, a serious safety concern, particularly for individuals with obstructive sleep apnea (OSA). This phase 1, randomized, double‐blind, placebo‐controlled, two‐period crossover study examined respiratory safety parameters in individuals with mild OSA following treatment with lemborexant. Participants (n = 39) were randomized to one of two treatment sequences, including placebo and lemborexant 10 mg. Each treatment period lasted 8 days and was separated by a washout of at least 14 days. Following single or multiple doses, there were no significant differences in mean apnea–hypopnea index for lemborexant 10 mg versus placebo (least squares mean [LSM] difference [95% confidence interval {CI}]: day 1, −0.03 [−2.22, 2.17]; day 8, −0.06 [−1.95, 1.83]) or peripheral capillary oxygen saturation during sleep (LSM difference [95% CI]: day 1, 0.07 [−0.31, 0.46]; day 8, 0.25 [−0.11, 0.61]). There were no significant differences versus placebo for the percentage of total sleep time during which peripheral capillary oxygen saturation was <80% (LSM difference [95% CI]: day 1, 0.002 [−0.019, 0.023]; day 8, 0.006 [−0.015, 0.026]), <85% (LSM difference [95% CI]: day 1, 0.067 [−0.124, 0.258]; day 8, 0.056 [−0.117, 0.228]) or <90% (LSM difference [95% CI]: day 1, 0.312 [−0.558, 1.181]; day 8, 0.088 [−0.431, 0.607]). The incidence of treatment‐emergent adverse events was low and similar for lemborexant and placebo. Lemborexant demonstrated respiratory safety in this study population and was well tolerated.
Keywords: clinical trial, pharmacodynamics, pharmacotherapy
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder in which breathing is impaired during sleep, with either a decrease or cessation of airflow, often causing disrupted sleep and daytime sleepiness (Semelka, Wilson, & Floyd, 2016). OSA severity can be classified using the apnea–hypopnea index (AHI), which measures rate of respiratory events during sleep (Kapur et al., 2017). In addition to the use of mechanical devices and surgery to treat OSA, sedatives are sometimes prescribed to persons with OSA who experience poor sleep (Jordan, O'Donoghue, Cori, & Trinder, 2017).
Sedative‐hypnotic drugs, including benzodiazepines and the non‐benzodiazepine “Z” drugs, are the most commonly prescribed pharmacologic treatments for insomnia (Sateia, Buysse, Krystal, Neubauer, & Heald, 2017). However, sedative hypnotics are associated with central respiratory depression, which is an important safety consideration for all patients and especially those with OSA (Kripke, 2018). Concerns have been raised that inhibiting arousal during sleep could cause an increase in respiratory events or oxygen desaturation in persons with OSA (Jordan et al., 2017; Mason, Cates, & Smith, 2015). Indeed, earlier small studies found that zolpidem (Cirignotta et al., 1988) and triazolam (Berry, Kouchi, Bower, Prosise, & Light, 1995) decreased peripheral capillary oxygen saturation (SpO2) in participants with OSA (Mason et al., 2015). Prescribing information for hypnotic agents often advises caution in patients with compromised respiratory function (Merck & Co. Inc, 2018; Sanofi‐Aventis U.S. Llc., 2019; Sunovion Pharmaceuticals Inc., 2014), particularly those with OSA (Sanofi‐Aventis U.S. Llc., 2019). It is therefore important to investigate potential effects of insomnia agents on respiratory function in persons with OSA.
Lemborexant, a dual orexin receptor antagonist (Murphy et al., 2017), was recently approved for the treatment of adults and elderly individuals (aged 65 years and older) with insomnia by the United States Food and Drug Administration and Japan Pharmaceuticals and Medical Devices Agency at doses up to 10 mg (Eisai Inc., 2019). Lemborexant is also being investigated in irregular sleep–wake rhythm disorder. In two phase 3 pivotal studies for insomnia, SUNRISE 1 (NCT02783729; E2006‐G000‐304) (Rosenberg et al., 2019) and SUNRISE 2 (NCT02952820; E2006‐G000‐303) (Yardley et al., 2019), lemborexant treatment led to significantly greater benefits on sleep onset and sleep maintenance outcomes compared with placebo (both studies) (Rosenberg et al., 2019; Yardley et al., 2019) and with zolpidem tartrate extended release (SUNRISE 1) (Rosenberg et al., 2019). In both studies, adverse events (AEs) were generally mild to moderate in severity (Rosenberg et al., 2019; Yardley et al., 2019). Although cognitive behavioural therapy for insomnia (CBT‐I) is considered the first‐line treatment option for insomnia disorder and has demonstrated benefit in persons with OSA (Sweetman, Lack, Lambert, Gradisar, & Harris, 2017), CBT‐I is not always effective or accessible to all patients; in these cases, a pharmacologic treatment may be considered (Qaseem, Kansagara, Forciea, Cooke, & Denberg, 2016).
The current study was a two‐part phase 1 study (NCT03471871; E2006‐A001‐102) examining the respiratory safety of lemborexant. We report results from Part 2, which examined pharmacodynamic respiratory safety parameters in patients with mild OSA following lemborexant treatment. Part 1 examined the respiratory safety of lemborexant among healthy adult and elderly volunteers (reported separately).
The primary objective of this study was to determine whether lemborexant increased the AHI during total sleep time (TST) after multiple doses in adult and elderly participants with mild OSA versus placebo. Secondary objectives were to determine whether the AHI increased following a single dose of lemborexant, assess whether SpO2 during TST decreased on average or below defined thresholds following single or multiple doses of lemborexant, determine the proportion of participants with one or more incidents of SpO2 <90% for ≥30 s during TST, and evaluate the safety and tolerability of lemborexant.
2. MATERIALS AND METHODS
This multicentre, randomized, double‐blind, placebo‐controlled, two‐period crossover study was conducted at eight sites in the United States. The study received approval from an institutional review board and followed the principles of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the Declaration of Helsinki. Written informed consent was obtained from all participants prior to any screening procedures.
2.1. Participants
Participants were males and females aged 18 to 90 years old who reported habitually sleeping ≥5.5 hr/night with a habitual bedtime between 21:00 and 01:00 hours. Inclusion criteria included SpO2 ≥94%, body mass index ≤40 kg/m2 and AHI ≥5 and <15 (mild OSA) (Epstein et al., 2009) at screening, and diagnosis of OSA according to the International Classification of Sleep Disorders, Third Edition (Sateia, 2014). Polysomnography was conducted during screening to confirm the diagnosis and severity of OSA prior to treatment. Exclusion criteria included: SpO2 <80% for ≥5% of TST at screening; use of a continuous positive airway pressure device or dental appliance; evidence of active respiratory disorder other than OSA or other clinically significant disease; diagnosis or symptoms of restless legs syndrome, periodic limb movement disorder, circadian rhythm sleep disorder, narcolepsy or parasomnia; drug or alcohol use disorder or current excessive alcohol intake; excessive caffeine intake; nocturia; use of prohibited medication; or breastfeeding or pregnant. Participants with a medical history of insomnia disorder or sleep difficulties were otherwise eligible. A complete list of enrolment criteria is available as supplementary information (Appendix S1).
2.2. Study design and treatment
After a screening period of ≤21 days, participants were randomized 1:1 using a computer‐generated randomization scheme to one of two treatment sequences, with randomization stratified by age (<65 vs. ≥65 years). Sequence A consisted of placebo then lemborexant 10 mg, each for one treatment period. Sequence B consisted of lemborexant 10 mg then placebo. In the phase 3 trials (Rosenberg et al., 2019; Yardley et al., 2019), lemborexant 10 mg was the maximum dose administered. Participants and all study personnel were blinded to treatment.
Treatment periods were eight nights in duration and were separated by a washout interval of ≥14 days (Figure 1). On day 1 of each treatment period, participants remained overnight in the sleep laboratory. The study drug was administered 5 min before lights out. Median habitual bedtime for each participant was calculated from the most recent 5 days of the participant's sleep diary. On days 2 to 7, participants were provided with the study drug and instructed to take the medication within 5 min of bedtime. On day 8, participants again remained overnight in the laboratory and underwent the same procedures as on day 1. Treatment period 2 followed the same schedule and procedures as treatment period 1 and was followed ~14 days later by a safety follow‐up visit.
Figure 1.
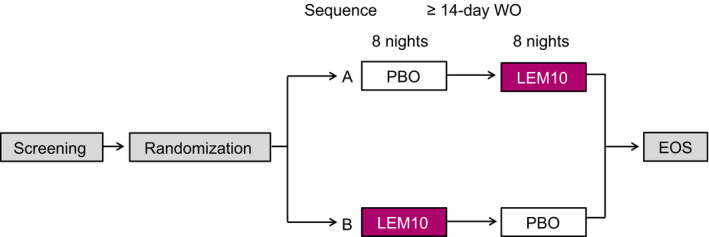
Overview of study design. EOS, end of study; LEM10, lemborexant 10 mg; PBO, placebo; WO, washout
Compliance with study medication was assessed as the number of tablets dispensed less the number of tablets returned or lost, divided by the number of tablets expected to be taken, and multiplied by 100. During the study, participants were prohibited from taking strong cytochrome P450 Family 3A (CYP3A) inhibitors, all CYP3A inducers, and any pharmacologic treatment for insomnia disorder or OSA. Participants were required to abstain from alcohol on days 1 and 8 and to limit alcohol intake to two or fewer alcohol‐containing drinks/day (female participants) or three or fewer drinks/day (male participants) throughout the study. Caffeine was permitted in limited quantities.
2.3. Assessments
On days 1 and 8 of each treatment period, polysomnography began at lights out and continued for 8 hr. Polysomnography records were scored in 30‐s epochs by trained polysomnography scorers according to standard criteria (Berry, Albertario, & Harding, 2018).
The AHI was defined as the number of apneas and hypopneas (Berry et al., 2018) (combined) divided by TST (in minutes) and multiplied by 60; that is, the mean number of apneas and hypopneas per hour of sleep.
SpO2 during TST was monitored using transmissive pulse oximetry; the sensor device was placed on the fingertip. Thresholds were defined as percentage of TST during which SpO2 was <90%, <85% and <80%.
2.4. Safety
Treatment‐emergent adverse events (TEAEs) were recorded and assessed for severity. Serious TEAEs were recorded. Routine physical and clinical laboratory evaluations included measurement of vital signs and weight and administration of electrocardiograms.
2.5. Statistical analyses
An assumed sample size of 30 participants (20 adult, 10 elderly) completing the study and an assumed true between‐treatment difference of ≤1.5 in AHI on day 8 provided 82% power that the upper bound of the 90% confidence interval (CI) for the treatment difference in the AHI on day 8 would be <5, a difference considered clinically meaningful in studies of sleep agents in OSA (Kryger, Wang‐Weigand, & Roth, 2007; Sun et al., 2016).
Differences between placebo and lemborexant in mean AHI, mean SpO2 and percentage of TST with SpO2 below defined thresholds were analysed on days 1 and 8 using an analysis of covariance (ANCOVA) mixed‐effect model, which included sequence, period and treatment as fixed effects and a random effect for participant within sequence. A two‐sided 90% CI was equivalent to a one‐sided upper 95% CI for the true mean difference (lemborexant − placebo) in the AHI and p‐value. For the AHI, if the upper bound of the one‐sided 95% CI of the treatment difference of the AHI was <5, this provided evidence that the given dose of lemborexant does not result in a clinically significant increase in the AHI with mild OSA compared with placebo. For SpO2, if the lower bound of the one‐sided 95% CI of the treatment difference of SpO2 was > –5, the dose of lemborexant did not result in a clinically significant decrease in SpO2 compared with placebo. All statistical tests were based on a significance level of 5% (two‐sided).
The pharmacodynamic analysis set, used for analyses of primary and secondary endpoints, included participants who received one or more doses of study drug during each treatment period and had sufficient data to derive one or more pharmacodynamic parameters. The safety analysis set included participants who received the study drug and had one or more post‐dose safety assessments.
The proportion of participants with one or more incidents of SpO2 <90% for ≥30 s was summarized using descriptive statistics. Safety data were also summarized with descriptive statistics.
3. RESULTS
3.1. Participants
Of 107 individuals screened, 39 (36.4%) were randomized into the study. Of 68 screen failures, 56 participants did not meet the inclusion criteria, four withdrew consent, one had an AE, one was lost to follow‐up, and six were excluded for other reasons.
Of 39 randomized participants, 19 were randomized to sequence A and 20 to sequence B. Two participants in sequence A and one in sequence B discontinued (forgot study medication, positive for tricyclic antidepressants, and could not return to the clinic within the window) and 36 participants (92.3%) completed the study. The pharmacodynamic analysis set included 37 participants and the safety analysis set included 39 participants. One participant for each treatment did not receive placebo or lemborexant, resulting in 38 participants in the analysis set for each treatment.
Baseline characteristics, including mean baseline AHI and SpO2, were similar between treatment sequences (Table 1). One‐third of participants were ≥65 years (elderly). The mean baseline AHI was 9.0.
Table 1.
Demographic and baseline characteristics (safety analysis set)
|
Sequence A (n = 19) |
Sequence B (n = 20) |
Overall (n = 39) |
|
|---|---|---|---|
| Age, yearsa | |||
| Mean (SD) | 55.9 (13.6) | 58.4 (12.9) | 57.2 (13.1) |
| Median (range) | 58.0 (31–82) | 58.0 (28–77) | 58.0 (28–82) |
| ≥18 to <65, n (%) | 13 (68.4) | 13 (65.0) | 26 (66.7) |
| ≥65, n (%) | 6 (31.6) | 7 (35.0) | 13 (33.3) |
| Sex, n (%) | |||
| Male | 8 (42.1) | 7 (35.0) | 15 (38.5) |
| Female | 11 (57.9) | 13 (65.0) | 24 (61.5) |
| Race, n (%) | |||
| White | 17 (89.5) | 18 (90.0) | 35 (89.7) |
| Black or African American | 1 (5.3) | 2 (10.0) | 3 (7.7) |
| Native Hawaiian/Pacific Islander | 1 (5.3) | 0 | 1 (2.6) |
| Hispanic/Latino ethnicity, n (%) | 9 (47.4) | 7 (35.0) | 16 (41.0) |
| BMI, mean (SD), kg/m2 | 29.3 (4.7) | 29.0 (4.5) | 29.1 (4.6) |
| % SpO2 during TST, mean (SD) | 94.71 (1.35) | 94.91 (1.52) | 94.81 (1.42) |
| AHI events/hr during TST, mean (SD) | 9.15 (3.09) | 8.86 (3.18) | 9.00 (3.10) |
Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; SD, standard deviation; SpO2, peripheral capillary oxygen saturation; TST, total sleep time.
Age was calculated at date of informed consent.
Participants were dispensed nine tablets for the eight‐night study; the majority of participants were compliant with the prescribed dosing of the study drug. Of 38 participants treated, 33 (86.8%) were 80%–100% compliant with lemborexant and 36 (94.7%) were compliant with placebo. Some participants (2 for placebo and 5 for lemborexant) took the study drug for 9 days, resulting in a mean treatment compliance rate of 100.1% (range, 88%–113%) for lemborexant and placebo.
3.2. AHI and SpO2 during TST
There were no significant differences in mean AHI for lemborexant versus placebo following a single dose (day 1) or multiple doses (day 8) of the study drug based on the ANCOVA analysis (Table 2). Least squares mean (LSM) estimates of the AHI were 10.19 with lemborexant and 10.22 with placebo on day 1 and 9.93 with lemborexant and 10.00 with placebo on day 8. In addition, although mean AHI was higher during rapid eye movement (REM) sleep than during non‐REM sleep, which is a common phenomenon, especially in persons with OSA (Alzoubaidi & Mokhlesi, 2016), similar numerical values were observed with placebo and lemborexant on day 1 and day 8 for either sleep stage (Table S1).
Table 2.
AHI and mean SpO2 during TST on day 1 and day 8 (pharmacodynamic analysis set)
|
PBO (n = 37) |
LEM10 (n = 37) |
|
|---|---|---|
| AHI during TST | ||
| Day 1a | ||
| Mean (SD) | 10.24 (7.09) | 10.29 (6.68) |
| LSM estimate (95% CI)b | 10.22 (7.93, 12.50) | 10.19 (7.89, 12.50) |
| LSM difference versus PBO (95% CI)b | −0.03 (−2.22, 2.17) | |
| p‐valueb | .979 | |
| Day 8c | ||
| Mean (SD) | 10.03 (6.80) | 9.99 (5.88) |
| LSM estimate (95% CI)b | 10.00 (7.94, 12.05) | 9.93 (7.91, 11.95) |
| LSM difference versus PBO (95% CI)b | −0.06 (−1.95, 1.83) | |
| p‐valueb | .948 | |
| Mean SpO2 during TST | ||
| Day 1d | ||
| Mean (SD) | 94.53 (1.62) | 94.54 (1.47) |
| LSM estimate (95% CI)e | 94.54 (94.02, 95.06) | 94.62 (94.09, 95.14) |
| LSM difference versus PBO (95% CI)e | 0.07 (−0.31, 0.46) | |
| p‐valuee | .699 | |
| Day 8f | ||
| Mean (SD) | 94.46 (1.32) | 94.65 (1.54) |
| LSM estimate (95% CI)e | 94.39 (93.92, 94.87) | 94.65 (94.17, 95.12) |
| LSM difference versus PBO (95% CI)e | 0.25 (−0.11, 0.61) | |
| p‐valuee | .169 | |
Abbreviations: AHI, apnea–hypopnea index; CI, confidence interval; LEM10, lemborexant 10 mg; LSM, least squares mean; PBO, placebo; SD, standard deviation; SpO2, peripheral capillary oxygen saturation; TST, total sleep time.
n = 37 in PBO; n = 36 in LEM10.
AHI was analysed using a mixed‐effect model that included fixed effects for sequence, period and treatment, and a random effect for participant within sequence.
n = 35 in PBO; n = 37 in LEM10.
n = 37 in PBO; n = 36 in LEM10.
SpO2 was analysed using a mixed‐effect model that included fixed effects for sequence, period and treatment, and a random effect for participant within sequence.
n = 35 in PBO; n = 37 in LEM10.
No significant differences in mean SpO2 for lemborexant versus placebo were observed on days 1 or 8 (Table 2). LSM estimates of SpO2 were 94.62 on day 1 and 94.65 on day 8 for lemborexant and 94.54 and 94.39, respectively, for placebo. Mean SpO2 values were similar with placebo and lemborexant during REM and non‐REM sleep on both days (Table S1).
No significant differences in percentage of TST during which SpO2 was <90%, <85% or <80% for lemborexant versus placebo were observed on days 1 or 8 (Table 3).
Table 3.
Summary of SpO2 during TST below defined thresholds on day 1 and day 8 (pharmacodynamic analysis set)
| Day 1 | Day 8 | |||
|---|---|---|---|---|
| PBO (n = 37) | LEM10 (n = 37) | PBO (n = 37) | LEM10 (n = 37) | |
| % TST with SpO2 < 90%a | ||||
| Mean (SD) | 1.044 (1.885) | 1.362 (2.617) | 1.011 (1.421) | 1.102 (1.547) |
| LSM estimate (95% CI)b | 1.037 (0.281, 1.793) | 1.349 (0.584, 2.113) | 1.014 (0.508, 1.521) | 1.102 (0.606, 1.598) |
| LSM difference versus PBO (95% CI)b | 0.312 (−0.558, 1.181) | 0.088 (−0.431, 0.607) | ||
| p‐valueb | .472 | .733 | ||
| Participants with ≥ 1 incident of SpO2 < 90% for ≥ 30 s, n (%) | 25 (67.6) | 28 (75.7) | 28 (75.7) | 31 (83.8) |
| % TST with SpO2 < 85%a | ||||
| Mean (SD) | 0.104 (0.304) | 0.170 (0.513) | 0.109 (0.297) | 0.162 (0.435) |
| LSM estimate (95% CI)b | 0.102 (−0.035, 0.240) | 0.170 (0.030, 0.309) | 0.108 (−0.020, 0.236) | 0.164 (0.039, 0.288) |
| LSM difference versus PBO (95% CI)b | 0.067 (−0.124, 0.258) | 0.056 (−0.117, 0.228) | ||
| p‐valueb | .479 | .518 | ||
| % TST with SpO2 < 80%a | ||||
| Mean (SD) | 0.012 (0.048) | 0.014 (0.043) | 0.009 (0.037) | 0.015 (0.060) |
| LSM estimate (95% CI)b | 0.012 (−0.003, 0.027) | 0.014 (−0.001, 0.029) | 0.009 (−0.008, 0.026) | 0.015 (−0.002, 0.032) |
| LSM difference versus PBO (95% CI)b | 0.002 (−0.019, 0.023) | 0.006 (−0.015, 0.026) | ||
| p‐valueb | .852 | .576 | ||
Abbreviations: CI, confidence interval; LEM10, lemborexant 10 mg; LSM, least squares mean; PBO, placebo; SD, standard deviation; SpO2, peripheral capillary oxygen saturation; TST, total sleep time.
Day 1: n = 37 in PBO; n = 36 in LEM10; Day 8: n = 35 in PBO; n = 37 in LEM10.
SpO2 was analysed using a mixed‐effect model that included fixed effects for sequence, period and treatment, and a random effect for participant within sequence.
A similar proportion of participants receiving placebo versus lemborexant had ≥1 incident of SpO2 <90% for ≥30 s following a single dose (day 1) or multiple doses (day 8) (Table 3); no statistical comparisons were performed.
3.3. Safety
A similar percentage of participants experienced TEAEs while receiving lemborexant (6/38 [15.8%]) versus placebo (5/38 [13.2%]). The most common TEAE was somnolence, reported by two participants receiving lemborexant. No other TEAE was observed in more than one participant with either placebo or lemborexant. There were no severe TEAEs. No serious TEAEs, deaths, study drug discontinuations or interruptions due to a TEAE, or significant laboratory abnormalities were reported during the study.
4. DISCUSSION
In this study of participants with mild OSA, lemborexant 10 mg, the highest dose administered in phase 3 insomnia studies (Rosenberg et al., 2019; Yardley et al., 2019) and the highest dose approved for the treatment of adults and elderly individuals with insomnia, did not have negative effects on the AHI or SpO2. The AHI did not increase between day 1 (single dose) and day 8 (multiple doses), and there were no differences between treatments. Mean SpO2 did not change over 8 days of treatment and there were no differences between treatments. Levels of SpO2 below predefined thresholds (<90%, <85% or <80%) were observed rarely in this study, and there were no between‐treatment differences in mean percentage of TST below each threshold.
Despite concerns about the respiratory safety of sedative‐hypnotic agents among persons with OSA (Jordan et al., 2017; Kripke, 2018; Mason et al., 2015), lemborexant did not negatively affect AHI, a measure of OSA severity, and SpO2, a key measure of respiratory function, in these participants with mild OSA.
The incidence of TEAEs in this study was low (<16% of participants) and most TEAEs were mild. There were no serious TEAEs.
Limitations of this study include the relatively small number of participants and study participant selection criteria, which may limit the generalizability of results. The study did not assess the variables of respiratory safety in participants with moderate or severe OSA. Additional studies are required in these populations, as clinically meaningful respiratory effects of lemborexant cannot be excluded in those patients based on the present study. However, these results suggest that lemborexant may be used in patients with undiagnosed mild OSA.
Another study limitation is that measurement of SpO2 and AHI may not capture the full spectrum of respiratory phenomena and these parameters are influenced by posture and sleep stage (Alzoubaidi & Mokhlesi, 2016; Choi, Park, Yu, Ryu, & Ha, 2016). However, mean AHI was similar with placebo and lemborexant during REM and non‐REM sleep, although AHI values were higher during REM than non‐REM sleep, as expected. Mean SpO2 was also similar with either treatment during these sleep stages.
In summary, lemborexant 10 mg demonstrated respiratory safety after single and multiple doses in adult and elderly participants with mild OSA, as objectively measured by SpO2 and the AHI during TST. Lemborexant was well tolerated in this study population, with a low rate of AEs that were mild to moderate in severity.
CONFLICTS OF INTEREST
JYC, GF, MM, MB and NH are employees of Eisai Inc. GKZ is an employee and shareholder of Clinilabs Drug Development Corporation; has ownership interest in the Sleep Disorders Institute and Home Sleep and Respiratory Care; has served as a consultant for Eisai Inc., Janssen Pharmaceutical, Purdue and Takeda; and has served on the speakers’ bureau for Merck.
AUTHOR CONTRIBUTIONS
Study design: MM, GKZ and GF. Data analysis, interpretation of data and manuscript preparation: JYC, MM, GKZ, GF, MB and NH.
Supporting information
ACKNOWLEDGEMENT
This research was supported by Eisai Inc. The investigators retained full independence in the conduct of this research. Medical writing assistance was provided by Lisa Baker, PhD, of ProScribe – Envision Pharma Group and was funded by Eisai Inc. Envision Pharma Group's services complied with international guidelines for Good Publication Practice (GPP3).
Cheng JY, Filippov G, Moline M, Zammit GK, Bsharat M, Hall N. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: A randomized, double‐blind, placebo‐controlled, crossover study. J Sleep Res. 2020;29:e13021 10.1111/jsr.13021
Funding information
This study was financially supported by Eisai Inc., Woodcliff Lake, New Jersey, USA. Eisai Inc. is the owner and manufacturer of lemborexant.
REFERENCES
- Alzoubaidi, M. , & Mokhlesi, B. (2016). Obstructive sleep apnea during rapid eye movement sleep: Clinical relevance and therapeutic implications. Current Opinion in Pulmonary Medicine, 22, 545–554. 10.1097/MCP.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, R. B. , Albertario, C. L. , & Harding, S. M. (2018). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine. [Google Scholar]
- Berry, R. B. , Kouchi, K. , Bower, J. , Prosise, G. , & Light, R. W. (1995). Triazolam in patients with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 151, 450–454. 10.1164/ajrccm.151.2.7842205 [DOI] [PubMed] [Google Scholar]
- Choi, E. , Park, D. H. , Yu, J. H. , Ryu, S. H. , & Ha, J. H. (2016). The severity of sleep disordered breathing induces different decrease in the oxygen saturation during rapid eye movement and non‐rapid eye movement sleep. Psychiatry Investigation, 13, 652–658. 10.4306/pi.2016.13.6.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirignotta, F. , Mondini, S. , Zucconi, M. , Gerardi, R. , Farolfi, A. , & Lugaresi, E. (1988). Zolpidem‐polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacology, Biochemistry, and Behavior, 29, 807–809. 10.1016/0091-3057(88)90212-2 [DOI] [PubMed] [Google Scholar]
- Eisai Inc. (2019). Dayvigo [package insert]. Eisai Inc., Woodcliff Lake, NJ. [Google Scholar]
- Epstein, L. J. , Kristo, D. , Strollo, P. J. Jr , Friedman, N. , Malhotra, A. , Patil, S. P. , … Weinstein, M. D. (2009). Clinical guideline for the evaluation, management and long‐term care of obstructive sleep apnea in adults. Journal of Clinical Sleep Medicine, 5, 263–276. [PMC free article] [PubMed] [Google Scholar]
- Jordan, A. S. , O'Donoghue, F. J. , Cori, J. M. , & Trinder, J. (2017). Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. American Journal of Respiratory and Critical Care Medicine, 196, 814–821. 10.1164/rccm.201612-2511PP [DOI] [PubMed] [Google Scholar]
- Kapur, V. K. , Auckley, D. H. , Chowdhuri, S. , Kuhlmann, D. C. , Mehra, R. , Ramar, K. , & Harrod, C. G. (2017). Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 13, 479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke, D. F. (2018). Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Research, 5, 918 10.12688/f1000research.8729.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger, M. , Wang‐Weigand, S. , & Roth, T. (2007). Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep & Breathing, 11, 159–164. 10.1007/s11325-006-0096-4 [DOI] [PubMed] [Google Scholar]
- Mason, M. , Cates, C. J. , & Smith, I. (2015). Effects of opioid, hypnotic and sedating medications on sleep‐disordered breathing in adults with obstructive sleep apnoea. Cochrane Database of Systematic Reviews, 10.1002/14651858.CD011090.pub2 [DOI] [PubMed] [Google Scholar]
- Merck & Co., Inc. (2018). Belsomra [package insert]. Merck & Co., Whitehouse Station, NJ. [Google Scholar]
- Murphy, P. , Moline, M. , Mayleben, D. , Rosenberg, R. , Zammit, G. , Pinner, K. , … Satlin, A. (2017). Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: Results from a Bayesian, adaptive, randomized, double‐blind, placebo‐controlled study. Journal of Clinical Sleep Medicine, 13, 1289–1299. 10.5664/jcsm.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem, A. , Kansagara, D. , Forciea, M. A. , Cooke, M. , Denberg, T. D. , & Clinical Guidelines Committee of the American College of Physicians (2016). Management of chronic insomnia disorder in adults: A Clinical Practice Guideline from the American College of Physicians. Annals of Internal Medicine, 165, 125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- Rosenberg, R. , Murphy, P. , Zammit, G. , Mayleben, D. , Kumar, D. , Dhadda, S. , … Moline, M. (2019). Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: A phase 3 randomized clinical trial. JAMA Network Open, 2, e1918254 10.1001/jamanetworkopen.2019.18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi‐Aventis U.S. Llc. (2019). Ambien [package insert]. Sanofi-Aventis, U.S. Llc, Bridgewater, NJ. [Google Scholar]
- Sateia, M. J. (2014). International classification of sleep disorders‐third edition: Highlights and modifications. Chest, 146, 1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- Sateia, M. J. , Buysse, D. J. , Krystal, A. D. , Neubauer, D. N. , & Heald, J. L. (2017). Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 13, 307–349. 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semelka, M. , Wilson, J. , & Floyd, R. (2016). Diagnosis and treatment of obstructive sleep apnea in adults. American Family Physician, 94, 355–360. [PubMed] [Google Scholar]
- Sun, H. , Palcza, J. , Card, D. , Gipson, A. , Rosenberg, R. , Kryger, M. , … Troyer, M. D. (2016). Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. Journal of Clinical Sleep Medicine, 12, 9–17. 10.5664/jcsm.5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunovion Pharmaceuticals Inc (2014). Lunesta [package insert]. Sunovion Pharmaceuticals Inc., Marlborough, MA. [Google Scholar]
- Sweetman, A. , Lack, L. , Lambert, S. , Gradisar, M. , & Harris, J. (2017). Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Medicine, 39, 38–46. 10.1016/j.sleep.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Yardley, J. , Pinner, K. , Murphy, P. , Filippov, G. , Zammit, G. , & Moline, M. (2019). Efficacy of lemborexant compared with placebo in adult and elderly subjects with insomnia: results from a phase 3 study (SUNRISE‐2). Paper presented at: Advances in Sleep and Circadian Science/Sleep Research Society. Clearwater Beach, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


