Abstract
Background
Postpartum depression (PPD) is a common mental disease happens in perinatal period. Ketamine as an anesthesia and analgesia drug has been used for a long time. In recent years, ketamine is proved to have an antidepression effect with a single administration. We hypothesized that intraoperative ketamine can reduce postpartum depressive symptoms after cesarean delivery.
Methods
In a randomized, double‐blind, placebo‐controlled study trail, healthy women scheduled for cesarean delivery were randomly assigned to receive intravenous ketamine (0.25 mg/kg diluted to 5 ml with 0.9% saline) or placebo (5 ml of 0.9% saline) within 5 min following clamping of the neonatal umbilical cord. The primary outcome was the degree of postpartum depressive symptoms, which was evaluated by Edinburgh Postnatal Depression Scale (EPDS, a threshold of 9/10 was used) at 1 week, 2 weeks, and 1 month after delivery. The secondary outcome was the numerical rating scale (NRS) score of pain at 2 days postpartum. This trail is registered in the Chinese Clinical Trial Registry, number ChiCTR1900022464.
Results
Between 26 January 2019 and 15 July 2019, 502 subjects were screened and 330 were randomly allocated: 165 (50%) to the ketamine group and 165 (50%) to the placebo group. There were significant differences in the degree of postpartum depressive symptoms between subjects in the ketamine group and the placebo group at 1 week postpartum (13.1% vs. 22.6%, respectively; p = .029). However, no difference was found between subjects in the two groups at 2 weeks (11.8% vs. 16.8%, respectively; p = .209) and 1 month postpartum (10.5% vs. 14.2%, respectively; p = .319). The NRS score of wound pain (3.0 ± 0.9 vs. 4.0 ± 1.0, respectively; p < .001) and uterine contraction pain (3.0 ± 0.9 vs. 4.1 ± 0.9, respectively; p < .001) was lower in the ketamine group at 2 days postpartum compared with placebo group. The prevalence of headache, hallucination, and dizziness was higher in the ketamine group than the placebo group during the operation.
Conclusions
Operative intravenous ketamine (0.25 mg/kg) can reduce the postpartum depressive symptoms for 1 week. The long‐time effect is remained to be seen.
Keywords: cesarean, depression, ketamine, pain, postpartum
Operative intravenous ketamine (0.25 mg/kg) can prevent postpartum depressive symptoms for 1 week. The long‐time effect is remained to be seen.
1. INTRODUCTION
Postpartum depression (PPD) is a major depressive episode “with peripartum onset of mood symptoms occurring during pregnancy or within 4 weeks following delivery” included in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM‐5). Symptoms of postpartum depression often include sleep disturbance, anxiety, irritability, a feeling of being overwhelmed, and an obsessional preoccupation with the baby's health and feeding. What's more, 70% of new mothers have mild depressive symptoms called “baby blues,” which peak between 2 and 5 days after delivery and typically, include weepiness, sadness, mood lability, irritability, and anxiety. “Blues” do not seriously impair functioning or include psychotic symptoms; typically, these symptoms begin to abate spontaneously within 2 weeks, although some cases will progress to postpartum depression (Wisner, Parry, & Piontek, 2002). PPD can have a negative impact on family harmony (Ngai & Ngu, 2015), couple relationship, maternal health, and infant health and development (Paulson, Dauber, & Leiferman, 2006).
The estimated prevalence of postpartum depression ranges from 6.5% to 12.9% or even higher in lower‐income and middle‐income countries (Gaynes et al., 2005; Munk‐Olsen, Laursen, Pedersen, Mors, & Mortensen, 2006). Before the 1980s, PPD was seldom reported in China. In recent years, the reported rates of PPD from studies enrolling Chinese populations have ranged from 10% to 20% (Hansotte, Payne, & Babich, 2017; Meltzer‐Brody et al., 2018; Xie et al., 2007). Many factors are found to increase the likelihood of getting PPD, included cesarean delivery (Xu, Ding, Ma, Xin, & Zhang, 2017; Yang, Shen, Ping, Wang, & Chien, 2011). Cesarean might induce adverse physiological outcomes, such as infection, postpartum hemorrhage, injury to the ureter and bladder, uterine rupture, chronic pelvic pain, and gastrointestinal dysfunction (Murphy, Liebling, Verity, Swingler, & Patel, 2001). These adverse outcomes and surgical trauma might enhance stress, which might affect the psychological function and increase the risk of PPD for mothers (Desborough, 2000). Compared with women who delivered vaginally, women who had a cesarean delivery are more likely to have PPD.
Ketamine, an N‐methyl‐D‐aspartate (NMDA) antagonist, has been used as an anesthesia drug for more than half a century. When used in subanesthetic doses, has analgesic properties that have been used for treatment of acute and chronic pain. Two meta‐analyses revealed that low‐dose ketamine administrated during the perioperative period can reduce postoperative pain intensity (Brinck et al., 2018) and prevent chronic pain (Chaparro, Smith, Moore, Wiffen, & Gilron, 2013), which may be related to reduction in central sensitization and hyperalgesia via inhibition of NMDA receptors. Over the past decade, it has been provoked a single administration of ketamine elicits fast (in as little as half an hour) and sustained antidepressant effects both in human (Berman et al., 2000; Zarate et al., 2006)and animal models of depression. There are some potential mechanisms of antidepressant actions of ketamine. MK‐801, a noncompetitive NMDA receptor antagonist, produced antidepressant‐like actions in the animal model of depression (Trullas & Skolnick, 1990). Ketamine can also increase hippocampal brain‐derived neurotrophic factor levels, which may be important for producing a rapid onset of antidepressant action (Garcia et al., 2008) A recent study found ketamine could quickly elevate mood by blocking NMDAR receptor‐dependent bursting activity of the lateral habenula neurons to disinhibit downstream monoaminergic reward centers and provide a framework for developing new rapid‐acting antidepressants (Yang et al., 2018).
Accordingly, we hypothesized that intraoperative ketamine can reduce the postpartum depressive symptoms after cesarean delivery.
2. METHODS
2.1. Study design
In this double‐blind, randomized prospective trial, we explored the severity of postpartum depressive symptoms in pregnant woman who use ketamine or not any intravenous analgesic during cesarean delivery. The study protocol was approved by the Shengjing Hospital of China Medical University, Shenyang, Liaoning, and written informed consent was obtained from all subjects. The study was registered in the Chinese Clinical Trial Registry, number ChiCTR1900022464.
The inpatient of obstetrics from 26 June 2019 to 15 July 2019 in Shengjing hospital was enrolled. Demographic and basic information were collected for all patients, including age, body mass index, employment, partner employment, number of births, gestational age, and obstetric complications. Eligibility criteria included the following: (a) undergoing cesarean delivery in Shengjing hospital, (b) the age of the pregnant is ≥20 and ≤40, (c) the birth age is ≥37 and ≤42 weeks, and (d)the birth weight is ≥2,500 and ≤4,000 g. Exclusion criteria included the following: (a) The pregnant has experienced depression and has been diagnosed as depression by a psychiatrist before, (b) the pregnant has experienced domestic violence before, (c) the pregnant with a serious obstetric complication or serious foundational diseases, (d) the new born has serious genetic or congenital diseases, (e) multiple pregnancy, and (f) the maternal weight is <50 or >100 kg, and the maternal height is <150 or >180 cm.
2.2. Randomization and masking
Randomization assignments were from a computer‐generated random number table. The group assignment and the investigational drug protocol were concealed in sequentially numbered, sealed, opaque envelopes. On the day of caesarean delivery, the assistant, who was not blinded to the study protocol opened the envelopes in the order of subject enrolment, reconstituted the investigational drug into a 5‐mL syringe and then labeled the syringe with the assigned subject ID. The subjects, anesthesiologists, and follow‐up investigators were all blinded to the subjects’ group assignments. This information was concealed until the subject recruitment and follow‐up had been completed.
2.3. Procedures
All patients received a spinal anesthesia at the level of L3–L4 or L2‐L3. The spinal block solution was bupivacaine 7.5 mg (1.5 ml 0.5% bupivacaine in saline) given at 1 ml/5 s to achieve a block level up to T4–T6. The operating table was tilted 30°to the left when the pregnant turned to a supine position. The patient's vital signs were closely monitored, and phenylephrine (iv) was administered when necessary. Within 5 min after clamping the neonatal umbilical cord, ketamine (0.25 mg/kg diluted to 5 ml with 0.9% saline) or placebo (5 ml of 0.9% saline) was slowly injected (iv) by the anesthesiologist who was blinded to the study protocol. The prepregnant weights of the subjects were recorded for the dose calculation. Five and fifteen minutes after the administration of the study drugs, and before the patient leaving the operation room, the anesthesiologist asked the subject if she had headache, vomiting, dizziness, or hallucination. At the end of surgery, epidural injection of 0.5 mg/ml morphine 4 ml was used for postoperative analgesia for each subject.
2.4. Outcome measures
The primary outcome was the degree of postpartum depressive symptoms which is assessed by the Edinburgh Postnatal Depression Scale (EPDS) score at 1 week, 2 weeks, and 1 month postpartum assessed by phone. EPDS is a questionnaire which is recommended by both the American College of Obstetricians and Gynecologists (Sit et al., 2015) and the American Academy of Pediatrics (Committee on Psychosocial Aspects of Child & Family Health, 2001) as a method of identifying possible postpartum depression. As a screening questionnaire for PPD, the positive findings from it should lead to a comprehensive clinical interview to ascertain a diagnosis (Antenatal & Postnatal Mental Health, 2014). It has been translated into Chinese assessing Chinese patients. When the threshold of 9/10 is used as the diagnostic criterion, the sensitivity and specificity are 82% and 86%, respectively (Lee et al., 1998), which can reduce the failed detection of cases than the threshold score of 12/13. The threshold of 9/10 may be appreciate if the scale is considered for routine use by primary care workers (Cox, Holden, & Sagovsky, 1987). Here, we used an EPDS score >9 as the cutoff value to screen postpartum depression. We defined the subjects whose EPDS scores were >9 had severer depressive symptoms and higher risk of PPD, whom we recommended to go to the psychiatric clinic to ascertain diagnoses. The secondary outcome was the pain intensity, which was assessed by study personnel using numerical rating scale (NRS) pain scores at 2 days postpartum. We also measured the sleep duration at 2 days postpartum.
2.5. Statistical analysis
The prevalence of PPD varied from country to country; in lower‐income and middle‐income countries, it can be higher (Howard et al., 2014). In China, the estimated prevalence of PPD is 22%. What is more, cesarean section delivery as an independent risk factor can increase the risk of PPD (Xu, Ding, et al., 2017). We aimed that the incidence rate of postpartum depression can be reduced from 20% to 10% by the use of ketamine. Group sample size of 150 achieved 80% power to detect this difference. The significance level of the test was targeted at 0.05. In order to account for dropouts, the study randomized 165 patients to each group. The primary outcome measure, the degree of postpartum depressive symptoms which was evaluated by the EPDS, was compared between groups using the chi‐square test. The score of EPDS was performed by analysis of variance (AVONA). The NRS pain score was compared between groups using the Mann–Whitney U test. A p value <.05 was required to reject the null hypothesis.
3. RESULT
Between 26 June 2019 and 15 July 2019, 502 subjects were screened and 330 were randomly allocated: 165 (50%) to the ketamine group and 165 (50%) to the placebo group. 12 subject were lost to follow‐up in the ketamine group, and 10 subjects were lost to follow‐up in the placebo group. In the end, data from 153 subjects in the ketamine group and 155 in the placebo group were included in the analysis (Figure 1). The total number of patients with discontinuations was 194 (Figure 2). Subjects’ characteristics or the intraoperative maternal and neonatal general parameters (Table 1) did not differ between groups.
FIGURE 1.
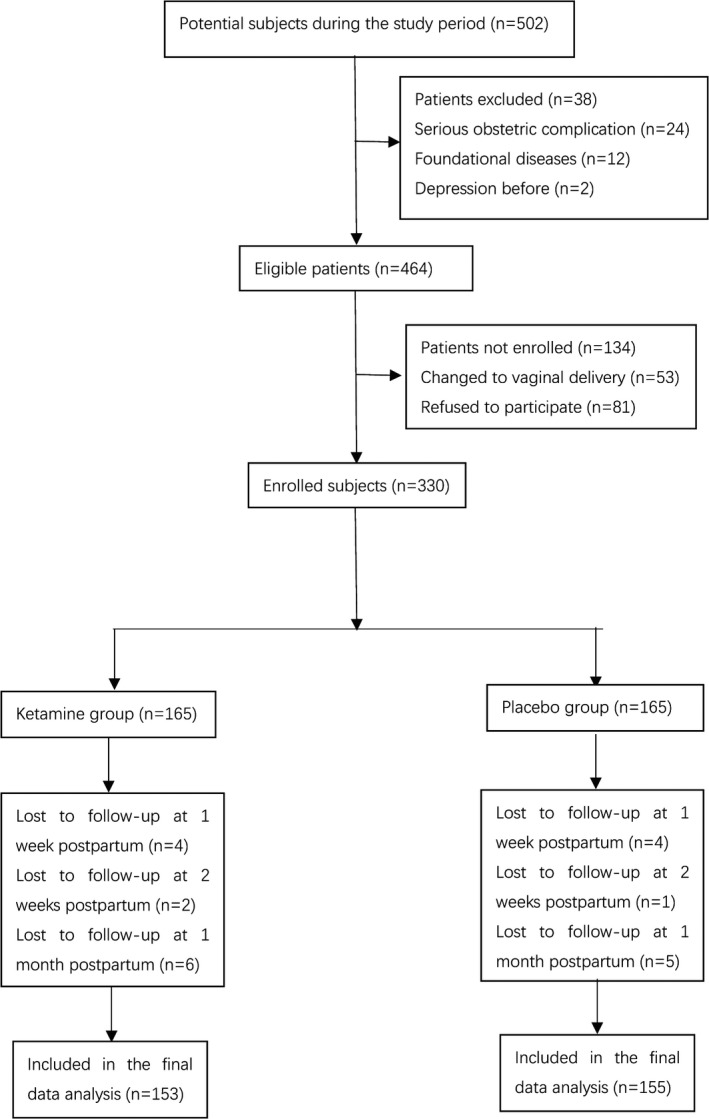
Patient flow
FIGURE 2.
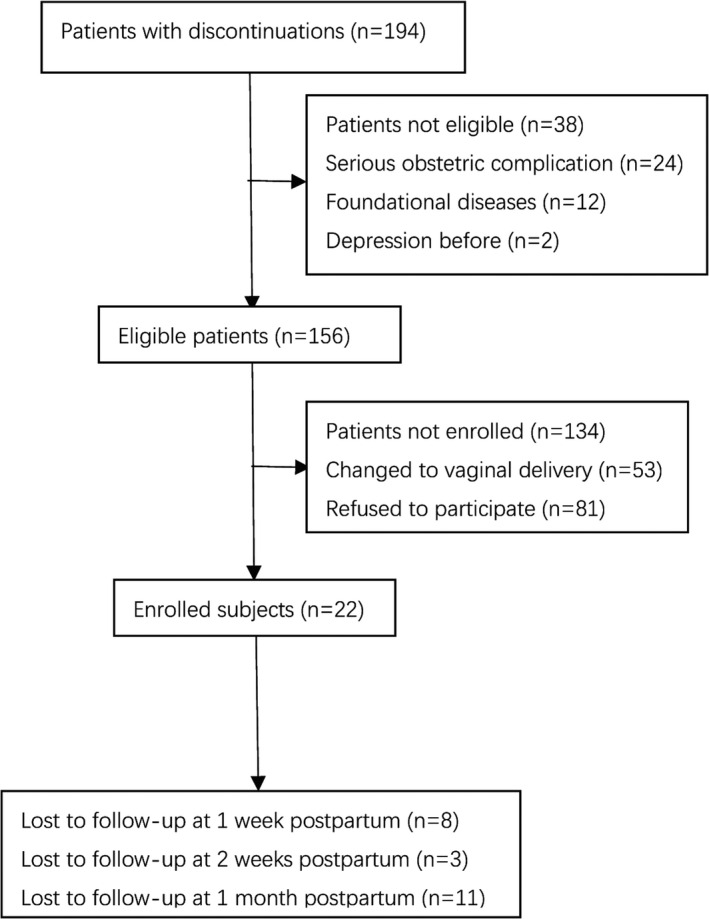
Patient flow with discontinuation
TABLE 1.
Baseline characteristics of subjects
| Ketamine (n = 153) | Saline (n = 155) | p | |
|---|---|---|---|
| Age (years) | 30 ± 4 | 30 ± 3 | .749 |
| Body mass index (kg/m2) | 29 ± 3 | 28 ± 3 | .346 |
| Employment | 121 (79.1%) | 113 (72.9%) | .284 |
| Partner employment | 141 (92.2%) | 147 (94.8%) | .365 |
| Fetus | |||
| First‐born | 111 (72.6%) | 117 (75.5%) | .557 |
| Second‐born | 42 (27.4%) | 38 (24.5%) | .557 |
| Gestational age (day) | 270 ± 9 | 269 ± 11 | .525 |
| Pregnancy with obstetric disease | 41 (26.5%) | 45 (29%) | .662 |
| Neonatal gender | |||
| Male | 67 (43.8%) | 82 (52.9%) | .110 |
| Female | 86 (56.2%) | 73 (47.1%) | .110 |
| Neonatal body weight (g) | 3,312 ± 433 | 3,371 ± 329 | .179 |
For the primary outcome of our study, there were significant differences in the degree of postpartum depressive symptoms between the ketamine group and the placebo group at 1 week postpartum (13.1% vs. 22.6%, respectively; p = .029). However, no significant differences were found between two groups at 2 weeks (11.8% vs. 16.8%, respectively; p = .209) and 1 month (10.5% vs. 14.2%, respectively; p = .319) postpartum. For the secondary outcome, The NRS score of wound pain (3.0 ± 0.9 vs. 4.0 ± 1.0, respectively; p < .001) and uterine contraction pain (3.0 ± 0.9 vs. 4.1 ± 0.9, respectively; p < .001) was lower in the ketamine group at 2 days postpartum compared with placebo group. The sleep duration at 2 days did not differ between groups (4.8 ± 2.3 vs. 4.4 ± 2.0, respectively; p = .115) (Table 2). The incidence of headache was higher in the ketamine group at 5 min after dosing and that of hallucinations and dizziness were higher at both 5 and 15 min after dosing (Table 3).
TABLE 2.
Outcome measures of subject
| Ketamine (n = 153) | Saline (n = 155) | P | |
|---|---|---|---|
| 2 days postpartum | |||
| NRS score | |||
| Wound pain | 3.0 ± 0.9 | 4.0 ± 1.0 | <.001 |
| Contraction pain | 3.0 ± 0.9 | 4.1 ± 0.9 | <.001 |
| Sleep duration | 4.8 ± 2.0.3 | 4.4 ± 2.0 | .115 |
| 1 week postpartum | |||
| EPDS score | 7.5 ± 2.2 | 8.2 ± 2.0 | <.001 |
| Occurrence of postpartum depression | 20 (13.1%) | 35 (22.6%) | .029 |
| 2 weeks postpartum | |||
| EPDS score | 7.4 ± 2.0 | 7.8 ± 2.1 | .155 |
| Occurrence of postpartum depression | 18 (11.8%) | 26 (16.8%) | .209 |
| 1 month postpartum | |||
| EPDS score | 7.3 ± 2.1 | 7.5 ± 2.2 | .366 |
| Occurrence of postpartum depression | 16 (10.5%) | 22 (14.2%) | .319 |
TABLE 3.
Side effects after administration during the operation
| Ketamine (n = 153) | Saline (n = 155) | p | |
|---|---|---|---|
| Vomiting 5 min | 4 (2.6%) | 2 (1.3%) | .668 |
| 15 min | 3 (2.0%) | 1 (0.6%) | .606 |
| Out of operation room | 1 (0.7%) | 0 (0%) | .497 |
| Headache 5 min | 15 (9.8%) | 3 (1.9%) | .003 |
| 15 min | 6 (3.9%) | 1 (0.6%) | .122 |
| Out of operation room | 2 (1.3%) | 1 (0.6%) | .991 |
| Hallucination 5 min | 24 (15.7%) | 0 (0%) | <.001 |
| 15 min | 6 (3.9%) | 0 (0%) | .014 |
| Out of operation room | 1 (0.7%) | 0 (0%) | .497 |
| Dizziness 5 min | 56 (36.6%) | 0 (0%) | <.001 |
| 15 min | 34 (22.2%) | 2 (1.3%) | <.001 |
| Out of operation room | 2 (1.3%) | 1 (0.6%) | .991 |
4. DISCUSSION
The important finding of this study was that operative intravenous ketamine (0.25 mg/kg) could reduce the depressive symptoms for 1 week.
The rate of PPD is higher among developing countries like China. What's more, as the implementation of the second child policy, the number of elderly women attempting to bear children has increased. The quality of woman's eggs and a man's sperm declined dramatically with increasing age, leading to an increased risk of pregnancy‐related complications and rate of cesarean delivery among older women, which may lead to the increased incidence of PPD (Li & Deng, 2017). They may have some of the following concerns related to their pregnancy: the loss of some occupation‐promotion, opportunities, worries about the risk of health problems, during pregnancy and the neonatal period, the pressure of social and economic factors, and depression and other negative emotions that may increase the incidence of postpartum depression. PPD is also a strong predictor of suicidality in the postpartum period (Do, Hu, Otto, & Rohrbeck, 2013). The risk factors of suicidal ideation of new mothers are higher self‐esteem, lower social support and severe depression. Moreover, suicidal ideation after delivery is significantly correlated with thoughts of hurting baby (Babu, Subbakrishna, & Chandra, 2008). As a result, it is important to screen PPD and treat it at an early time.
In recent years, ketamine has been found to produce rapid amelioration of major depressive disorder (Han et al., 2016). A study reported that ketamine anesthesia provided faster response and remission after electroconvulsive therapy (ECT) compared with propofol anesthesia. However, there was limited evidence said ketamine could treat unipolar or bipolar depression (McCloud et al., 2015). More high‐quality RCTs are needed to determine the different efficacy of ketamine for unipolar depression and bipolar depression. In psychiatry, the existing antidepressant medications show limited effectiveness and delayed clinical response (I). So, it is of much interest to see whether the rapid efficacy of ketamine can bring more benefits to patients. Studies found ketamine could rapidly (within 1 day) and significantly reduced suicidal ideation and its effect continued for up to 1 week (Wilkinson et al., 2018). Adjunctive ketamine demonstrated a greater reduction in clinically significant suicidal ideation in depressed patients within 24 hr compared with midazolam, partially independently of antidepressant effect (Grunebaum et al., 2018). The rapid efficacy of ketamine can make up for the delayed clinical response of traditional antidepressants.
Ketamine had also been explored for improving postoperative depressive state. In depressed patients undergoing surgeries, small‐dose ketamine improved the postoperative depressive state and relieved postoperative pain (Kudoh, Takahira, Katagai, & Takazawa, 2002). They believed patients with symptoms of depression have increased postoperative pain. As a result, pain relief might contribute to an improvement in the depressive state. The founding that Ketamine had a rapid antidepression effect raised the possibility intraoperative administration of ketamine might improve postoperative mood and enhance resilience in the setting of surgical stress. A randomized trial of healthy (ASA physical status 1 or 2) surgical patients receiving subanesthetic ketamine during induction of anesthesia found positive effects on postoperative depressive symptoms (Jiang et al., 2016). The intraoperative application of ketamine was associated with improved scores for depressed mood and increased serum brain‐derived neurotrophic factor levels. However, one study had a contrast conclusion. When using older adults who were undergoing major surgery, the administration of ketamine during prolonged general anesthesia created unfavorable neural conditions for antidepressant effects at both cortical and subcortical levels (Mashour et al., 2018). In our study, we used ketamine among “healthy” subjects who had not diagnosed as depression. As an anesthetic and analgesic drug, ketamine had been widely used for the prevention of postoperative pain (Porter et al., 2015). Another study found intravenous low‐dose ketamine (0.15 mg/kg) combined with intrathecal bupivacaine for cesarean delivery provides longer postoperative analgesia and lower postoperative analgesic consumption than bupivacaine alone (Sen, Ozmert, Aydin, Baran, & Caliskan, 2005). Instead of preventing depressive symptoms, using ketamine can provide better analgesia which is commonly used in the realm of anesthesia. That is the reason why we use it among “healthy” subjects.
We chose 1 week as the timing to evaluate the degree of postpartum depressive symptoms for it was the duration that ketamine could be effective. A randomized, double‐blind, clinical trial found single bolus low dose of ketamine did not prevent postpartum depression (Xu, Li, et al., 2017). No significant differences were found in the prevalence of postpartum depression at 3 days and 6 weeks after delivery. The timing when ketamine could provide the best efficiency is still remained to be seen. Postpartum depressive symptoms which was measured 1 week postpartum were neither too short to be influenced by the postoperative changes nor too long. When the time passed on, the influence of a single dose of ketamine was getting smaller. The other factors, like economic status and the employment status of the spouse, health problems in the newborn, problems with family and spouse, reduced social support, might play more important roles in the happening of postpartum depressive symptoms (Ozcan, Boyacioglu, & Dinc, 2017). Because of these social and economic confounders, the benefits of ketamine might be covered. That might be also the reason why we did not find any difference in the degree of postpartum depressive symptoms between two group at 2 weeks and 1 month postpartum.
However, the effect of ketamine during the short time postpartum still do much benefits to new mothers. As we mentioned before, PPD is a strong predictor of suicidality in the postpartum period (Do et al., 2013). Screening positive for depression at 1–2 days postpartum is significantly correlated with suicidal ideation after delivery (Bodnar‐Deren, Klipstein, Fersh, Shemesh, & Howell, 2016). What's more, compared with before delivery, there is a trend of lower depression level, but higher suicidal ideation at early postpartum stage. As a result, ketamine used at an early time postpartum might can reduce the suicidal rates of new mothers with serious PPD.
A huge number of clinical trials explored the use of ketamine for the management and prevention of postoperative pain (Elia & Tramer, 2005). It was believed that low‐dose ketamine (0.1–0.5 mg/kg) might relieve postoperative pain management when used as an adjunct to local anesthetic, opioids, or other analgesic agents (Kohrs & Durieux, 1998; Schmid, Sandler, & Katz, 1999). We observed that postoperative analgesic consumption was significantly lower in ketamine group at 2 days after delivery, compared to saline group. The finding was similar to some previous studies (Kudoh et al., 2002; Nitta, Goyagi, & Nishikawa, 2013). Another study found no additional postoperative analgesic benefit of low‐dose ketamine during cesarean delivery in patients who received intrathecal morphine and intravenous ketorolac, but the subjects who received ketamine reported lower pain scores 2 weeks postpartum (Bauchat et al., 2011). They found no evidence of the relationship between ketamine and analgesic efficacy in a near time after operate. However, they indicated that ketamine might relieve chronic pain. What made the differences may because we just used loxoprofen sodium tablets for breakthrough pain without continuous analgesia, making the small analgesic benefit of one dose ketamine easier to be observed.
In our study, we observed a higher incidence of hallucination and dizziness in the ketamine group, but it did not last for long time. The most common acute psychiatric side effect of ketamine is anxiety; the others include agitation or irritability, euphoria or mood elevation, delusions or unusual thoughts, panic, and apathy. The most common psychotomimetic side effect reported was dissociation, followed by perceptual disturbance, odd or abnormal sensation, derealization, hallucinations, feeling strange, weird, bizarre, or unreal, and depersonalization. No long‐term psychotomimetic side effects were reported. However, those short‐term effects still reminded us of the safety when we use ketamine. It was necessary to recognized the major gaps that remain in our knowledge about the safety of ketamine. Future research is needed to address these unanswered questions and concerns (Sanacora et al., 2017).
There are several limitations in our study. First, we did not test the score of EPDS before delivery. PPD can also happen before the birth of child. From previous studies, ketamine might improve mood better in patients who are depressed than patients who are not. It will be more specific if we use ketamine in patients who have PPD and make it easier to see whether ketamine can help to improve the state of PPD. Second, besides the personal information of the subjects we recorded, like the perinatal complications and depression before, the social and family factors including low social support, marital difficulties, and negative life events can raise the risk of getting PPD, as well. What's more, the unwanted pregnancy or unwanted infant sex can also make an influence (Shitu, Geda, & Dheresa, 2019). We studied the employment of the couples which has no differences between groups, but failed to compare other factors among our groups, which might lead to some unknown bias. Third, we excluded the subjects with diagnosed depression before, who are considered as at higher risks of suffering from PPD. Because there would be more psychiatric confounders among that people than those who had never experienced depression before. Considering the limited sample size of our study, we excluded them. However, it might be of greater clinical significance to study the prevention of PPD among these people. Further related researchers may be done in the future. Fourth, although we recommended the subjects whose EPDS scores were higher than threshold to go to the psychiatric clinic to ascertain the diagnoses of PPD, we failed to continue follow‐up. As a result, we did not know the prevalence of PPD between groups.
In conclusion, we found a subanesthetic dose of ketamine (0.25 mg/kg) administered in cesarean delivery can reduce postpartum depressive symptoms at 1 week postpartum. We were not sure about the antidepressant effect of ketamine on woman diagnosed as PPD. Studies are needed to assess the safety and the efficacy of ketamine in women with high risks of getting PPD and its antidepressant effect on women have PPD.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Yao J: Carried out the study and wrote the manuscript. Song T: Helped patient recruitment and data collection. Zhang Y and Guo N: Helped data collection. Zhao P: Designed and supported this study.
ACKNOWLEDGMENT
This work was supported by the National Nature Science Foundation of China (No. 81870838, No. 81671311), the Key Research and Development Program of Liaoning Province (No. 2018225004), Liaoning Province Distinguished Professor Support Program (No. XLYC1802096), and the Outstanding Scientific Fund of Shengjing Hospital (No. 201708). The authors thank Prof. Ping Zhao for valuable technical advices, and the others of the group for their excellent operation and helpful discussion.
Yao J, Song T, Zhang Y, Guo N, Zhao P. Intraoperative ketamine for reduction in postpartum depressive symptoms after cesarean delivery: A double‐blind, randomized clinical trial. Brain Behav. 2020;10:e01715 10.1002/brb3.1715
The peer review history for this article is available at https://publons.com/publon/10.1111/brb3.1715
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
REFERENCES
- Antenatal and Postnatal Mental Health (2014). Clinical management and service guidance: Updated edition. Leicester, UK. [PubMed] [Google Scholar]
- Babu, G. N. , Subbakrishna, D. K. , & Chandra, P. S. (2008). Prevalence and correlates of suicidality among Indian women with post‐partum psychosis in an inpatient setting. Australian and New Zealand Journal of Psychiatry, 42(11), 976–980. 10.1080/00048670802415384 [DOI] [PubMed] [Google Scholar]
- Bauchat, J. R. , Higgins, N. , Wojciechowski, K. G. , McCarthy, R. J. , Toledo, P. , & Wong, C. A. (2011). Low‐dose ketamine with multimodal postcesarean delivery analgesia: A randomized controlled trial. International Journal of Obstetric Anesthesia, 20(1), 3–9. 10.1016/j.ijoa.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Berman, R. M. , Cappiello, A. , Anand, A. , Oren, D. A. , Heninger, G. R. , Charney, D. S. , & Krystal, J. H. (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47(4), 351–354. 10.1016/S0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Bodnar‐Deren, S. , Klipstein, K. , Fersh, M. , Shemesh, E. , & Howell, E. A. (2016). Suicidal ideation during the postpartum period. Journal of Women's Health (Larchmt), 25(12), 1219–1224. 10.1089/jwh.2015.5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinck, E. C. , Tiippana, E. , Heesen, M. , Bell, R. F. , Straube, S. , Moore, R. A. , & Kontinen, V. (2018). Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Systematic Review, 12, CD012033 10.1002/14651858.CD012033.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro, L. E. , Smith, S. A. , Moore, R. A. , Wiffen, P. J. , & Gilron, I. (2013). Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews, 24(7), CD008307 10.1002/14651858.CD008307.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Psychosocial Aspects of Child and Family Health (2001). American Academy of Pediatrics. The new morbidity revisited: a renewed commitment to the psychosocial aspects of pediatric care. Committee on Psychosocial Aspects of Child and Family Health. Pediatrics, 108(5), 1227–1230. [DOI] [PubMed] [Google Scholar]
- Cox, J. L. , Holden, J. M. , & Sagovsky, R. (1987). Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Desborough, J. P. (2000). The stress response to trauma and surgery. British Journal of Anaesthesia, 85(1), 109–117. 10.1093/bja/85.1.109 [DOI] [PubMed] [Google Scholar]
- Do, T. , Hu, Z. , Otto, J. , & Rohrbeck, P. (2013). Depression and suicidality during the postpartum period after first time deliveries, active component service women and dependent spouses, U.S. Armed Forces, 2007–2012. MSMR, 20(9), 2–7. [PubMed] [Google Scholar]
- Elia, N. , & Tramer, M. R. (2005). Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain, 113(1–2), 61–70. 10.1016/j.pain.2004.09.036 [DOI] [PubMed] [Google Scholar]
- Garcia, L. S. B. , Comim, C. M. , Valvassori, S. S. , Réus, G. Z. , Barbosa, L. M. , Andreazza, A. C. , … Quevedo, J. (2008). Acute administration of ketamine induces antidepressant‐like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in Neuro‐psychopharmacology & Biological Psychiatry, 32(1), 140–144. 10.1016/j.pnpbp.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Gaynes, B. N. , Gavin, N. , Meltzer‐Brody, S. , Lohr, K. N. , Swinson, T. , Gartlehner, G. , … Miller, W. C. (2005). Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ), 119, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum, M. F. , Galfalvy, H. C. , Choo, T.‐H. , Keilp, J. G. , Moitra, V. K. , Parris, M. S. , … Mann, J. J. (2018). Ketamine for rapid reduction of suicidal thoughts in major depression: A midazolam‐controlled randomized clinical trial. American Journal of Psychiatry, 175(4), 327–335. 10.1176/appi.ajp.2017.17060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Chen, J. , Zou, D. , Zheng, P. , Li, Q. , Wang, H. , … Xie, P. (2016). Efficacy of ketamine in the rapid treatment of major depressive disorder: A meta‐analysis of randomized, double‐blind, placebo‐controlled studies. Neuropsychiatric Disease and Treatment, 12, 2859–2867. 10.2147/NDT.S117146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansotte, E. , Payne, S. I. , & Babich, S. M. (2017). Positive postpartum depression screening practices and subsequent mental health treatment for low‐income women in Western countries: A systematic literature review. Public Health Reviews, 38, 3 10.1186/s40985-017-0050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, L. M. , Molyneaux, E. , Dennis, C. L. , Rochat, T. , Stein, A. , & Milgrom, J. (2014). Non‐psychotic mental disorders in the perinatal period. Lancet, 384(9956), 1775–1788. 10.1016/S0140-6736(14)61276-9 [DOI] [PubMed] [Google Scholar]
- Jiang, M. , Wang, M.‐H. , Wang, X.‐B. , Liu, L. I. , Wu, J.‐L. , Yang, X.‐L. , … Zhang, C.‐X. (2016). Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. Journal of Anesthesia, 30(2), 232–237. 10.1007/s00540-015-2096-7 [DOI] [PubMed] [Google Scholar]
- Kohrs, R. , & Durieux, M. E. (1998). Ketamine: Teaching an old drug new tricks. Anesthesia and Analgesia, 87(5), 1186–1193. [DOI] [PubMed] [Google Scholar]
- Kudoh, A. , Takahira, Y. , Katagai, H. , & Takazawa, T. (2002). Small‐dose ketamine improves the postoperative state of depressed patients. Anesthesia & Analgesia, 95(1), 114–118. 10.1097/00000539-200207000-00020 [DOI] [PubMed] [Google Scholar]
- Lee, D. T. S. , Yip, S. K. , Chiu, H. F. K. , Leung, T. Y. S. , Chan, K. P. M. , Chau, I. O. L. , … Chung, T. K. H. (1998). Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 172, 433–437. 10.1192/bjp.172.5.433 [DOI] [PubMed] [Google Scholar]
- Li, Q. , & Deng, D. (2017). New medical risks affecting obstetrics after implementation of the two‐child policy in China. Frontiers of Medicine, 11(4), 570–575. 10.1007/s11684-017-0552-5 [DOI] [PubMed] [Google Scholar]
- Mashour, G. A. , Ben Abdallah, A. , Pryor, K. O. , El‐Gabalawy, R. , Vlisides, P. E. , Jacobsohn, E. , … PODCAST Research Group (2018). Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: An international, multicentre, double‐blind, randomised clinical trial. British Journal of Anaesthesia, 121(5), 1075–1083. 10.1016/j.bja.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud, T. L. , Caddy, C. , Jochim, J. , Rendell, J. M. , Diamond, P. R. , Shuttleworth, C. , … Cipriani, A. (2015). Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database of Systematic Reviews, 29(9), CD011611 10.1002/14651858.CD011611.pub2 [DOI] [PubMed] [Google Scholar]
- Meltzer‐Brody, S. , Howard, L. M. , Bergink, V. , Vigod, S. , Jones, I. , Munk‐Olsen, T. , … Milgrom, J. (2018). Postpartum psychiatric disorders. Nature Reviews Disease Primers, 4, 18022. 10.1038/nrdp.2018.22 [DOI] [PubMed] [Google Scholar]
- Munk‐Olsen, T. , Laursen, T. M. , Pedersen, C. B. , Mors, O. , & Mortensen, P. B. (2006). New parents and mental disorders: A population‐based register study. JAMA, 296(21), 2582–2589. 10.1001/jama.296.21.2582 [DOI] [PubMed] [Google Scholar]
- Murphy, D. J. , Liebling, R. E. , Verity, L. , Swingler, R. , & Patel, R. (2001). Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: A cohort study. Lancet, 358(9289), 1203–1207. 10.1016/S0140-6736(01)06341-3 [DOI] [PubMed] [Google Scholar]
- Ngai, F. W. , & Ngu, S. F. (2015). Predictors of maternal and paternal depressive symptoms at postpartum. Journal of Psychosomatic Research, 78(2), 156–161. 10.1016/j.jpsychores.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Nitta, R. , Goyagi, T. , & Nishikawa, T. (2013). Combination of oral clonidine and intravenous low‐dose ketamine reduces the consumption of postoperative patient‐controlled analgesia morphine after spine surgery. Acta Anaesthesiologica Taiwanica, 51(1), 14–17. 10.1016/j.aat.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Ozcan, N. K. , Boyacioglu, N. E. , & Dinc, H. (2017). Postpartum depression prevalence and risk factors in Turkey: A systematic review and meta‐analysis. Archives of Psychiatric Nursing, 31(4), 420–428. 10.1016/j.apnu.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Paulson, J. F. , Dauber, S. , & Leiferman, J. A. (2006). Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics, 118(2), 659–668. 10.1542/peds.2005-2948 [DOI] [PubMed] [Google Scholar]
- Porter, S. B. , McClain, R. L. , Howe, B. L. , Ardon, A. E. , Mazer, L. S. , Knestrick, B. M. , & Clendenen, A. M. (2015). Perioperative ketamine for acute postoperative analgesia: The Mayo Clinic‐Florida experience. Journal of PeriAnesthesia Nursing, 30(3), 189–195. 10.1016/j.jopan.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Sanacora, G. , Frye, M. A. , McDonald, W. , Mathew, S. J. , Turner, M. S. , Schatzberg, A. F. , … Nemeroff, C. B. (2017). A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry, 74(4), 399–405. 10.1001/jamapsychiatry.2017.0080 [DOI] [PubMed] [Google Scholar]
- Schmid, R. L. , Sandler, A. N. , & Katz, J. (1999). Use and efficacy of low‐dose ketamine in the management of acute postoperative pain: A review of current techniques and outcomes. Pain, 82(2), 111–125. 10.1016/S0304-3959(99)00044-5 [DOI] [PubMed] [Google Scholar]
- Sen, S. , Ozmert, G. , Aydin, O. N. , Baran, N. , & Caliskan, E. (2005). The persisting analgesic effect of low‐dose intravenous ketamine after spinal anaesthesia for caesarean section. European Journal of Anaesthesiology, 22(7), 518–523. 10.1017/s026502150500089x [DOI] [PubMed] [Google Scholar]
- Shitu, S. , Geda, B. , & Dheresa, M. (2019). Postpartum depression and associated factors among mothers who gave birth in the last twelve months in Ankesha district, Awi zone, North West Ethiopia. BMC Pregnancy Childbirth, 19(1), 435 10.1186/s12884-019-2594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, D. , Luther, J. , Buysse, D. , Dills, J. L. , Eng, H. , Okun, M. , … Wisner, K. L. (2015). Suicidal ideation in depressed postpartum women: Associations with childhood trauma, sleep disturbance and anxiety. Journal of Psychiatric Research, 66, 95–104. 10.1016/j.jpsychires.2015.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas, R. , & Skolnick, P. (1990). Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European Journal of Pharmacology, 185(1), 1–10. 10.1016/0014-2999(90)90204-J [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. T. , Ballard, E. D. , Bloch, M. H. , Mathew, S. J. , Murrough, J. W. , Feder, A. , … Sanacora, G. (2018). The effect of a single dose of intravenous ketamine on suicidal ideation: A systematic review and individual participant data meta‐analysis. American Journal of Psychiatry, 175(2), 150–158. 10.1176/appi.ajp.2017.17040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner, K. L. , Parry, B. L. , & Piontek, C. M. (2002). Clinical practice. Postpartum depression. New England Journal of Medicine, 347(3), 194–199. 10.1056/NEJMcp011542 [DOI] [PubMed] [Google Scholar]
- Xie, R. H. , He, G. , Liu, A. , Bradwejn, J. , Walker, M. , & Wen, S. W. (2007). Fetal gender and postpartum depression in a cohort of Chinese women. Social Science and Medicine, 65(4), 680–684. 10.1016/j.socscimed.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Ding, Y. , Ma, Y. , Xin, X. , & Zhang, D. (2017). Cesarean section and risk of postpartum depression: A meta‐analysis. Journal of Psychosomatic Research, 97, 118–126. 10.1016/j.jpsychores.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Li, Y. , Huang, X. , Chen, D. , She, B. , & Ma, D. (2017). Single bolus low‐dose of ketamine does not prevent postpartum depression: A randomized, double‐blind, placebo‐controlled, prospective clinical trial. Archives of Gynecology and Obstetrics, 295(5), 1167–1174. 10.1007/s00404-017-4334-8 [DOI] [PubMed] [Google Scholar]
- Yang, S. N. , Shen, L. J. , Ping, T. , Wang, Y. C. , & Chien, C. W. (2011). The delivery mode and seasonal variation are associated with the development of postpartum depression. Journal of Affective Disorders, 132(1–2), 158–164. 10.1016/j.jad.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Cui, Y. , Sang, K. , Dong, Y. , Ni, Z. , Ma, S. , & Hu, H. (2018). Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature, 554(7692), 317–322. [DOI] [PubMed] [Google Scholar]
- Zarate, C. A. , Singh, J. B. , Carlson, P. J. , Brutsche, N. E. , Ameli, R. , Luckenbaugh, D. A. , … Manji, H. K. (2006). A randomized trial of an N‐methyl‐D‐aspartate antagonist in treatment‐resistant major depression. Archives of General Psychiatry, 63(8), 856–864. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


