Summary
Background
Increasing numbers of neonates are undergoing painful procedures in low-income and middle-income countries, with adequate analgesia seldom used. In collaboration with a multi-disciplinary team in Kenya, we aimed to establish the first evidence-based guidelines for the management of routine procedure-related neonatal pain that consider low-resource hospital settings.
Methods
We did a systematic review by searching MEDLINE, Embase, CINAHL, and CENTRAL databases for studies published from Jan 1, 1953, to March 31, 2019. We included data from randomised controlled trials using heart rate, oxygen saturation (SpO2), premature infant pain profile (PIPP) score, neonatal infant pain scale (NIPS) score, neonatal facial coding system score, and douleur aiguë du nouveau-né scale score as pain outcome measures. We excluded studies in which neonates were undergoing circumcision or were intubated, studies from which data were unextractable, or when pain was scored by non-trained individuals. We did a narrative synthesis of all studies, and meta-analysis when data were available from multiple studies comparing the same analgesics and controls and using the same outcome measures. 17 Kenyan health-care professionals formed our clinical guideline development panel, and we used the Grading of Recommendations, Assessment, Development and Evaluation framework and the panel's knowledge of the local health-care context to guide the guideline development process. This study is registered with PROSPERO, CRD42019126620.
Findings
Of 2782 studies assessed for eligibility, data from 149 (5%) were analysed, with 80 (3%) of these further contributing to our meta-analysis. We found a high level of certainty for the superiority of breastfeeding over placebo or no intervention (standardised mean differences [SMDs] were −1·40 [95% CI −1·96 to −0·84] in PIPP score and −2·20 [–2·91 to −1·48] in NIPS score), and the superiority of oral sugar solutions over placebo or no intervention (SMDs were −0·38 [–0·61 to −0·16] in heart rate and 0·23 [0·04 to 0·42] in SpO2). We found a moderate level of certainty for the superiority for expressed breastmilk over placebo or no intervention (SMDs were −0·46 [95% CI −0·87 to −0·05] in heart rate and 0·48 [0·20 to 0·75] in SpO2). Therefore, the panel recommended that breastfeeding should be given as first-line analgesic treatment, initiated at least 2 min pre-procedure. Given contextual factors, for neonates who are unable to breastfeed, 1–2 mL of expressed breastmilk should be given as first-line analgesic, or 1–2 mL of oral sugar (≥10% concentration) as second-line analgesic. The panel also recommended parental presence during procedures with adjunctive provision of skin-to-skin care, or non-nutritive sucking when possible.
Interpretation
We have generated Kenya's first neonatal analgesic guidelines for routine procedures, which have been adopted by the Kenyan Ministry of Health, and have shown a framework for clinical guideline development that is applicable to other low-income and middle-income health-care settings.
Funding
Wellcome Trust Research Programme, and the Africa-Oxford Initiative.
Introduction
Global efforts to reduce neonatal mortality have led to substantial increases in the numbers of neonates being treated as inpatients. Observational studies have shown that neonates often undergo more than a dozen painful procedures per day while on neonatal intensive care units.1 Furthermore, untreated pain is associated with significant neurophysiological and developmental consequences.2 Ethics boards, the neonatology community, and parents have emphasised the need to minimise pain,3 but the paucity of clear guidelines, busy clinical environments, and difficulty in reliably measuring pain in neonates have resulted in an ongoing and substantial burden of unaddressed neonatal pain.4
In resource-poor settings, periprocedural analgesia is rarely administered. A national cross-sectional survey done in Kenya found that over a single day, no neonate received analgesia for any of the 404 routine procedures that were done.5 Untreated neonatal pain, therefore, represents a huge global source of short-term and potentially long-term clinical morbidity. In view of increasing attention paid to patients' health-care experiences, especially in vulnerable groups and their families, inadequate treatment of pain is also an important quality-of-care concern.6
Research in context.
Evidence before this study
Generic global guidance for low-income and middle-income countries (LMICs) on neonatal (aged ≤28 days) pain management is lacking. Local practice or guidance varies substantially and might not be based on the best available evidence, and recommendations for high-income countries are not suitable for all health-care settings. In Kenya specifically, no evidence-based neonatal analgesic guidelines for non-tertiary hospitals exist, which probably contributes substantially to the observed paucity of provision of any form of analgesia during routine, painful procedures. We searched MEDLINE, Embase, CINAHL, and the Cochrane Central Register of Controlled Trials for studies or guidelines published between Jan 1, 1953, and March 31, 2019, with search terms relevant to a range of analgesics and procedures commonly seen in the non-tertiary LMIC setting. This search did not identify any analgesic guidelines specifically for neonates undergoing routine procedures in the LMIC setting.
Added value of this study
This study describes specific recommendations generated by a local panel of neonatal experts for the management of routine procedure-related pain, which have subsequently been adopted nationally in Kenya. These recommendations were generated after discussion of the findings of a rigorous systematic review and meta-analysis, application of the Grading of Recommendations, Assessment, Development and Evaluation framework to this evidence, and local context-specific considerations. We have shown a framework for clinical guideline development, which can be applied to other clinical contexts in LMICs.
Implications of all the available evidence
Based on our findings, when possible, breastfeeding should be promoted above all other analgesic strategies during routine procedures in district and county hospitals. For neonates who are unable to breastfeed, expressed breastmilk is preferred, but oral sugar solutions are an adequate alternative. Periprocedural parental presence should be encouraged, and adjunctive provision of skin-to-skin care or non-nutritive sucking should be provided to all neonates when appropriate. Clinical care in LMICs can be improved by efficient use of high-quality systematic reviews linked to structured decision making processes to generate recommendations, which place local context at the heart of the process.
Previous work has mostly been studies of single, discrete interventions,7, 8, 9 and many existing clinical guidelines do not account sufficiently for variation in local resources and patient demographics.10, 11 Clinicians, therefore, face difficulties in making good, evidence-based choices for particular analgesic strategies in specific patient populations.
We did a systematic review and meta-analysis of the evidence relating to a range of analgesics and procedures appropriate to populations at the first-referral hospital level prioritised by WHO.12 This non-tertiary health-care setting is represented by the county hospitals in Kenya, and by the district hospitals in other low-income and middle-income countries. We aimed to translate the existing evidence and generate new national guidelines for managing neonatal pain during routine procedures in Kenya.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, a group consisting of experts in global neonatal and paediatric care determined the inclusion and exclusion criteria, and the various population, intervention, control, outcome (PICO) questions under consideration. Procedures and analgesics included those that are most widely seen in our target setting of non-tertiary Kenyan hospitals (panel 1).
Panel 1. Procedures, analgesics, and outcome measures included in the systematic review.
Procedures
-
•
Heel prick
-
•
Intramuscular or subcutaneous injections
-
•
Venepuncture or venous cannulation
-
•
Arterial puncture
-
•
Continuous positive airway pressure prongs insertion
-
•
Lumbar puncture
-
•
Urinary catheterisation
Analgesics
-
•
Breastfeeding
-
•
Expressed breastmilk
-
•
Oral sugar solutions (sucrose, glucose, dextrose, sweetener, or fructose)
-
•
Skin-to-skin care (including kangaroo mother care)
-
•
Non-nutritive sucking
-
•
Swaddling
-
•
Music
-
•
Topical local anaesthetic
-
•
Paracetamol
-
•
Ibuprofen
-
•
Morphine
-
•
Ketamine
Outcome measures
-
•
Heart rate
-
•
Transcutaneous oxygen saturation
-
•
Premature infant pain profile score
-
•
Neonatal infant pain scale score
-
•
Neonatal facial coding system score
-
•
Douleur aiguë du nouveau-né scale score
We searched MEDLINE, Embase, CINAHL, and the Cochrane Central Register of Controlled Trials databases for articles published between Jan 1, 1953, and March 31, 2019 (appendix p 1), without any language restrictions. We identified additional studies by screening references found in systematic reviews identified in our original search. Following the literature search, we retrospectively excluded studies on neonates undergoing circumcision because circumcision was felt to represent a severe pain stimulus over a prolonged time period, and risked confounding results when combined with procedures inducing more acute pain.
Two authors (CW and JSF) independently screened titles, abstracts, and full texts for eligibility. Any discrepancies were resolved through consensus discussion with an additional third author. We included only randomised and quasi-randomised controlled trials done on neonates (mean postnatal age 0–28 days). Our study focused on the physiological and behavioural measures of neonatal pain most commonly used in the experimental setting. These measures were heart rate, oxygen saturation (SpO2), premature infant pain profile (PIPP) score,13 neonatal infant pain scale (NIPS) score,14 neonatal facial coding system (NFCS),15 and douleur aiguë du nouveau-né (DAN) scale.16 In the absence of clear evidence of the superiority of any of these measures over the others, all six were deemed critical outcomes from a Grading of Recommendations, Assessment, Development and Evaluation (GRADE)17 perspective. These six measurements could not be combined into a single outcome measure of pain and were therefore analysed separately. The exclusion of other potential measures of neonatal pain, such as respiratory rate and crying duration, was based upon inferior evidence of the validity of these measures, and their less frequent use precluding meaningful meta-analysis.6, 14 Additional exclusion criteria were studies in which neonates were intubated, studies from which data were unextractable, or when pain was scored by non-trained individuals. We combined no intervention and placebo groups for the purposes of our analysis, having considered that the well described placebo analgesic effect seen in adults is significantly less pronounced in neonates, and on the basis of sensitivity analyses of our own data (appendix pp 50–55).18, 19
Data were extracted in duplicate from eligible studies by two authors (CW and JSF) using Covidence systematic review software. When data were presented only graphically, the two authors discussed and generated a consensus estimate, excluding studies when representation was ambiguous. Two authors (EQ and AtWN) independently assessed the quality of the included studies and the potential risk of bias using the Cochrane risk of bias tool.20 Any discrepancies between authors in data extraction or quality assessment were resolved through consensus discussion with an additional third author. The protocol is registered with PROSPERO, CRD42019126620.
Data analysis
Pre-piloted documents developed by the authors were used to summarise the data from every included study within a given PICO question. The data encapsulated within these documents enabled the review group to compose a narrative synthesis of the qualitative information captured by all studies matching eligibility criteria in our review, and to supplement meta-analysis findings.
Meta-analysis could only be done when data were available from multiple studies comparing the same analgesics and controls and using the same outcome measures (appendix p 2). For each study, mean, SD, and sample size were extracted or calculated for each comparison group's pain outcome measure. We used the DerSimonian and Laird random-effects model21 to compute standardised mean difference (SMD; Hedges' g) and pool estimates for each exposure comparison and outcome. We assessed heterogeneity between the studies included in the analysis using the I2 statistic. For studies included in the meta-analysis, we used funnel plot and Egger's regression asymmetry test to assess publication bias and small-study effects for comparisons with ten or more studies for each outcome.
Clinical guideline development
17 Kenyan health-care professionals formed the Neonatal Pain Guideline Group (NPGG), which constituted our clinical guideline development panel. This group included four neonatologists, seven paediatricians, two pharmacists, two neonatal nurses, one lecturer in neonatology, and one representative from the Kenyan Clinical Officers Council. NPGG members were selected for their multi-disciplinary representation of important stakeholder groups, on the basis of the members' expertise in non-tertiary Kenyan neonatal care and engagement in previous clinical guideline development processes. NPGG members declared no competing interests. We were unable to include representatives of patients' families or do formal cost-effectiveness analyses.
Based on the findings of the systematic review, the key clinical question that the NPGG sought to answer was: in neonates, which analgesics should be recommended to reduce pain while undergoing routine procedures? This broad question was handled by considering individual PICO questions within each analgesic category using the GRADE framework. This is a transparent framework for guideline developers to make specific clinical recommendations on the basis of certainty in the available evidence. Elements of the GRADE approach include risk of bias of studies, inconsistency of findings, indirectness of the evidence, imprecision of the effect estimate, and publication bias. Several NPGG members were familiar with the GRADE process, and the NPGG meeting began with a presentation on use of the framework. Following this, detailed findings of the meta-analysis and narrative synthesis were presented by CW and discussed by the NPGG. This process was repeated, with the NPGG reaching consensus on whether certainty in the evidence of the observed effect was high, moderate, low, or very low for each specific PICO question to produce the final GRADE summary of findings tables.
With these tables as the starting point, the NPGG discussed how these should inform recommendations for context-appropriate clinical guidelines. Discussions considered the balance of benefits and harms of each intervention, local feasibility, compatibility with broader policy agendas, and likely staff and family preferences. At the end of the 1-day NPGG meeting, consensus recommendations were agreed. These recommendations were circulated to all NPGG members in written form for further clarification and review over a period of 3 weeks. All NPGG members approved the final recommendations.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
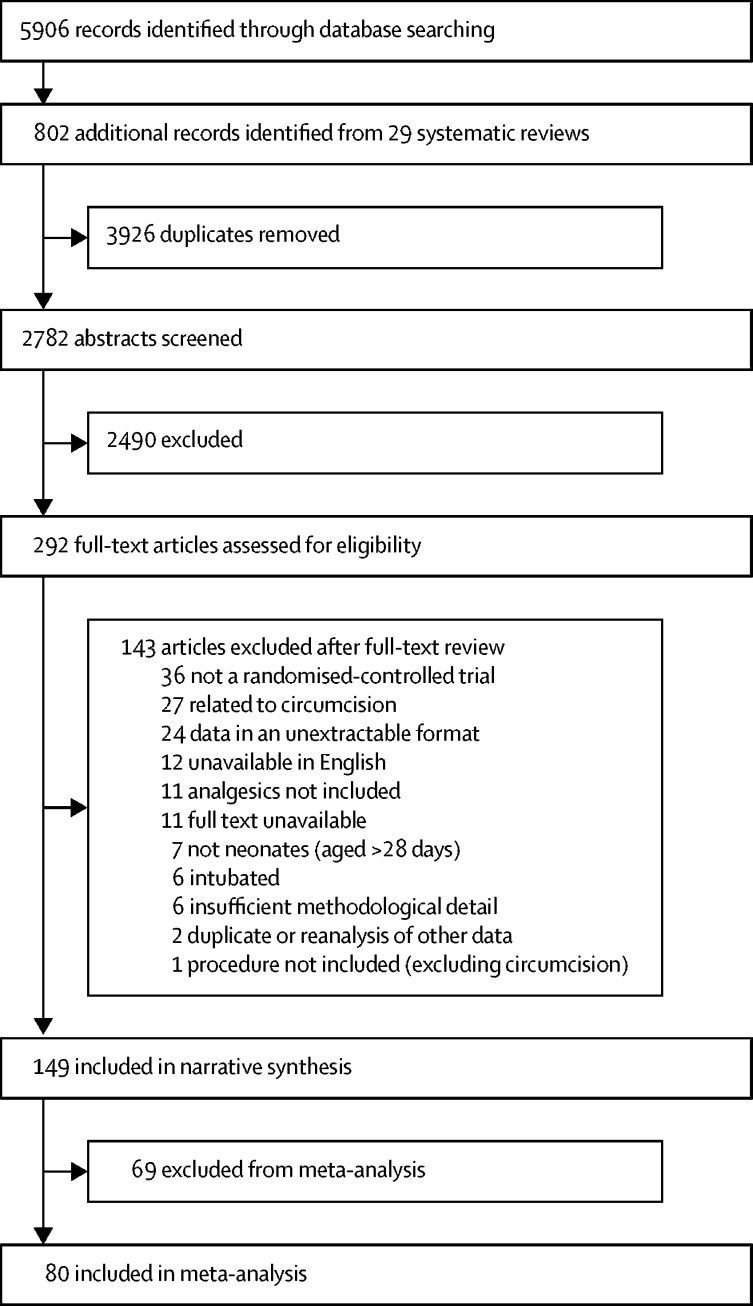
We retrieved 5906 records, with 802 additionally found through screening systematic reviews identified in our original search. After removal of duplicates, 2782 (41%) abstracts were screened, with 292 (10%) deemed eligible for full-text screening. Of these, 149 (5%) studies were included in the narrative synthesis (n=13 169), and 80 (54%) of these 149 in the meta-analysis (n=5869; figure 1; appendix pp 3–6).
Figure 1.
Study selection
PICO question comparisons that were prioritised by the NPGG in their discussions were those involving breastfeeding, oral sugar, expressed breastmilk, skin-to-skin care, and non-nutritive sucking.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122 Complete results from meta-analysis relevant to these PICO questions involving breastfeeding, oral sugar, expressed breastmilk, skin-to-skin care, and non-nutritive sucking22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122 are presented in figure 2 and in the appendix (pp 12–64).
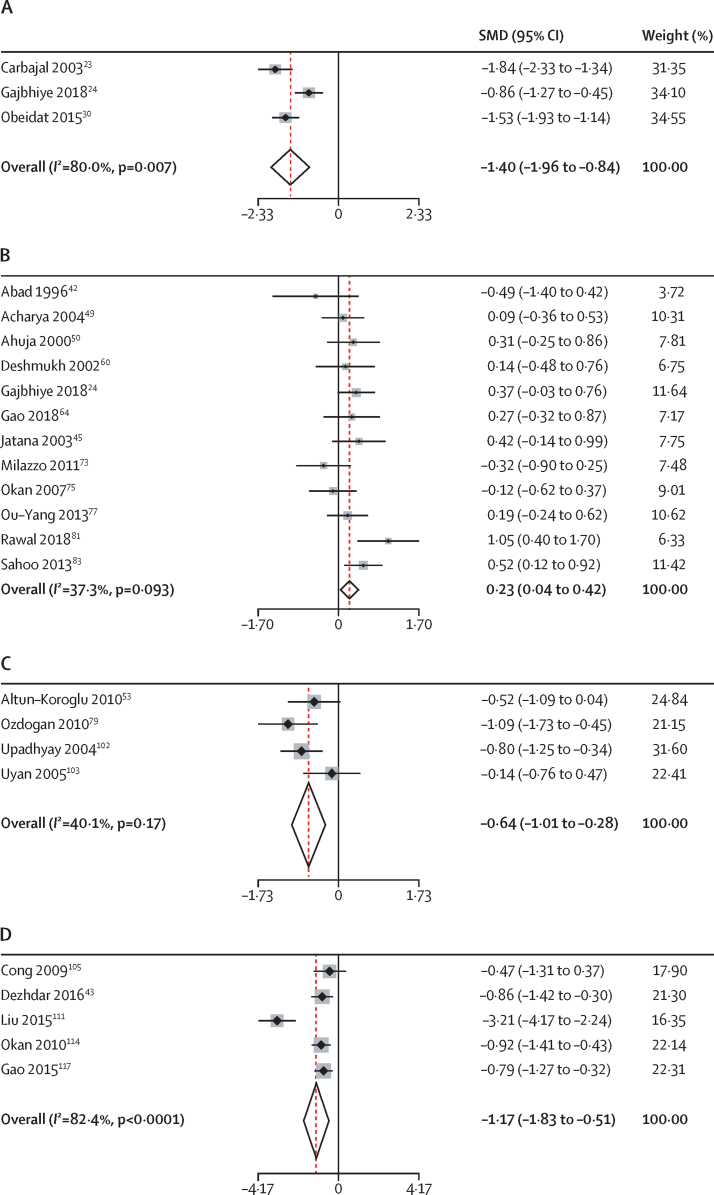
Figure 2.
Selected random-effects meta-analyses of main findings
(A) Breastfeeding vs placebo or no intervention for premature infant pain profile score. (B) Oral sugar vs placebo or no intervention for oxygen saturation. (C) Expressed breastmilk vs placebo or no intervention for neonatal facial coding system score. (D) Skin-to-skin care vs placebo or no intervention for heart rate. SMD=standardised mean difference.
The NPGG's GRADE certainty in the evidence for these prioritised comparisons is shown in the appendix (p 7). The appendix (p 8) presents a summary of the narrative synthesis described here and the associated references for each PICO question. GRADE summaries for other PICO questions considered by the NPGG but not discussed here due to the NPGG deprioritising them on the basis of local feasibility and analgesic efficacy are shown in the appendix (pp 9, 68–69). Within the 149 studies, heel prick was the most commonly observed procedure (88 [59%] studies), oral sugar the most commonly used analgesic (78 [52%] studies), and heart rate the most commonly used pain outcome measure (76 [51%] studies). Full tallies are presented in the appendix (p 10).
12 studies (n=991) compared breastfeeding with placebo or no intervention.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Meta-analysis showed evidence for a reduction in PIPP score (SMD −1·40 [95% CI −1·96 to −0·84]; n=327) and NIPS score (−2·20 [–2·91 to −1·48]; n=225; figure 2A; appendix p 14), although not for heart rate (−1·49 [–3·44 to 0·46]; n=210) or SpO2 (0·59 [–1·26 to 2·43]; n=150; appendix pp 12, 13). Narrative synthesis further supported this finding with all 12 studies finding a superiority of breastfeeding compared with placebo or no intervention (appendix p 8). Therefore, the NPGG had a high level of certainty in the superiority of breastfeeding over placebo or no intervention when initiated at least 2 min before the painful procedure (appendix p 7). However, the NPGG had a low certainty in the superiority of breastfeeding over oral sugar (appendix p 7). Narrative synthesis (n=670) revealed inconsistencies, with five (63%) of eight studies with direct comparisons suggesting breastfeeding was superior to oral sugar,24, 34, 35, 36, 37 two (25%) showing equivalence,38, 39 and one (13%) inferiority to oral sugar (appendix p 8).40 Meta-analysis suggested no difference using PIPP score (−0·21 [–0·78 to 0·35]; n=346), but superiority of breastfeeding using NIPS score (−1·51 [–2·48 to −0·53]; n=182; appendix pp 15, 16). Based upon results from three studies (n=136), the NPGG had a moderate certainty in the superiority of breastfeeding over giving expressed breastmilk (appendix p 7), with two studies showing superiority with a large effect size,27, 34 and one showing inferiority (appendix p 8).39 Finally, the NPGG found a moderate certainty in the superiority of breastfeeding over skin-to-skin care (appendix p 7), with both studies (n=160) showing breastfeeding's superiority for NIPS score (−1·52 [–2·82 to −0·22]; n=160; appendix p 17).37, 41
Oral sugar solutions ranged in volume from 0·05 mL to 5 mL, concentration from 5% to 50% (median 25%), and solution type included sucrose (52 studies), glucose (33 studies), dextrose (eight studies), sweetener (two studies), and fructose (one study). When considering the six studies (n=453) directly comparing the efficacy of various concentrations of oral sugar, the NPGG had a moderate certainty in the superiority of solutions with a concentration of 24% or more over solutions with a concentration below 24% (appendix p 7).42, 43, 44, 45, 46, 47 However, the NPGG found insufficient evidence to draw any conclusions regarding whether increasing the solution concentration above 24% showed an analgesic benefit (appendix p 7). Based on indirect comparisons across studies, the NPGG had a moderate certainty that increasing the volume of oral sugar solutions above 2 mL showed no benefit (appendix p 7). When sufficient numbers of studies for a given PICO question allowed, sensitivity analyses did not suggest any difference in the analgesic efficacy of various types of sugar solutions for measures of heart rate (p=0·28) or PIPP score (p=0·33) but suggested possible superiority of dextrose for SpO2 (p=0·03; appendix pp 41–49). On the basis of these findings, subsequent analysis of the overall efficacy of oral sugar considered all solutions together irrespective of solution type, concentration, or volume.
58 studies (n=3948) directly compared oral sugar solutions with placebo or no intervention.24, 42, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 50 (86%) studies showed sugar to be superior in narrative synthesis,24, 42, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 and eight (14%) showed equivalence (appendix p 8).92, 93, 94, 95, 96, 97, 98, 99 This finding was supported by meta-analysis showing superiority of sugar for all outcome measures: heart rate (SMD=–0·38 [95% CI −0·61 to −0·16]; n=813); SpO2 (0·23 [0·04 to 0·42]; n=650); PIPP score (−1·00 [–1·58 to −0·41]; n=835); NIPS score (−1·01 [–1·69 to −0·32]; n=623); NFCS score (−0·77 [–1·36 to −0·17]; n=559), and DAN score (−0·96 [–1·42 to −0·50]; n=307; figure 2B; appendix pp 18–22). Therefore, the NPGG had a high certainty in this estimate of effect (appendix p 7). 14 studies (n=920) compared oral sugar directly with expressed breastmilk. We found some variation in the narrative synthesis, with nine (64%) studies suggesting the superiority of oral sugar,45, 46, 76, 77, 79, 81, 83, 91, 100 and five (36%) suggesting equivalence (appendix p 8).34, 39, 53, 72, 99 Meta-analysis did not show a significant difference between oral sugar and expressed breastmilk for heart rate (0·16 [–0·07 to 0·39]; n=293) SpO2, (−0·11 [–0·48 to 0·27]; n=283); PIPP score (0·55 [–0·03 to 1·12]; n=309), and NFCS score (0·93 [–0·29 to 2·15]; n=93; appendix pp 23–26). Therefore, the NPGG had a moderate certainty of the superiority of oral sugar over expressed breastmilk (appendix p 7). 11 studies (n=645) compared oral sugar with non-nutritive sucking, with two studies (18%) suggesting superiority of oral sugar,91, 101 six (55%) equivalence,61, 64, 70, 88, 92, 98 and three (27%) inferiority (appendix p 8).58, 71, 72 Meta-analysis revealed no difference between non-nutritive sucking and oral sugar for heart rate (−0·16 [–0·76 to 0·45]; n=151), SpO2 (−0·01 [–0·36 to 0·35]; n=122), NIPS score (−0·37 [–2·29 to 1·54]; n=188), or DAN score (−0·21 [–0·65 to 0·24]; n=77; appendix pp 27–30). Therefore, the NPGG had a low certainty in the superiority of oral sugar over non-nutritive sucking (appendix p 7).
14 studies (n=863) compared giving expressed breastmilk with placebo or no intervention, with nine (64%) showing superiority,45, 53, 76, 77, 81, 83, 91, 99, 102 and five (36%) equivalence (appendix p 8).27, 46, 72, 79, 103 We found evidence for a reduction in pain for heart rate (SMD=–0·46 [95% CI −0·87 to −0·05]; n=431), SpO2 (0·48 [0·20 to 0·75]; n=421); PIPP score (−1·47 [–2·48 to −0·46]; n=172), and NFCS score (−0·64 [–1·01 to −0·28]; n=217), but not DAN scores (−2·24 [–5·82 to 1·35]; n=132; figure 2C; appendix pp 31–34). Therefore, the NPGG had a moderate certainty in the superiority of expressed breastmilk over placebo or no intervention (appendix p 7).
The NPGG had a high certainty in the superiority of skin-to-skin care over placebo or no intervention (appendix p 7), with 15 (94%) studies suggesting the superiority of skin-to-skin care,43, 94, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 and one (6%) suggesting equivalence (n=1054; appendix p 8).116 This finding was supported by comparisons of heart rate (SMD=–1·17 [95% CI −1·83 to −0·51]; n=264), although not SpO2 (0·41 [–0·06 to 0·88]; n=233) or NFCS score (−0·57 [–1·50 to 0·36]; n=392; figure 2D; appendix pp 35, 36).
Of the 16 studies (n=932) assessing non-nutritive sucking versus placebo or no intervention, 15 (94%) suggested the superiority of non-nutritive sucking,29, 56, 58, 61, 64, 70, 71, 72, 88, 91, 98, 118, 119, 120, 121 and one (6%) suggested equivalence (appendix p 8).122 Meta-analysis revealed evidence of pain reduction for PIPP score (SMD=–1·06 [95% CI −2·03 to −0·08]; n=137) and DAN score (−2·33 [–4·46 to −0·20]; n=124), but not for heart rate (−0·23 [–0·71 to 0·26]; n=306) or SpO2 (0·59 [–0·02 to 1·19]; n=104; appendix pp 37–40). Therefore, the NPGG found a moderate certainty in the superiority of non-nutritive sucking over placebo or no intervention (appendix p 7).
We found a high degree of heterogeneity (I2≥75%) for 22 (67%) of the 33 meta-analyses we were able to do for the analgesics outlined in panel 1 that the NPGG had prioritised (figure 2; appendix pp 12–40). Sensitivity analysis of oral sugar versus no intervention compared with versus placebo did not reveal a contribution to this observed heterogeneity. We found no significant interactions between oral sugar versus no intervention and oral sugar versus placebo for heart rate (p=0·36), SpO2, (p=0·72), and PIPP score (p=0·76; appendix pp 50–55). These findings also supported our having combined placebo and no intervention control groups for analysis. Further sensitivity analysis of oral sugar versus placebo or no intervention comparisons did not find that the timing of measuring pain outcomes during experiments contributed to heterogeneity. We found no significant interactions between pain scores measured 1 min or less and more than 1 min after the procedure commenced for heart rate (p=0·47), SpO2 (p=0·09), and PIPP (p=0·63; appendix pp 56–61). Prematurity (birth at <37 weeks' gestational age) might have contributed to heterogeneity in these comparisons with significant interaction between premature and full-term neonates for heart rate (p=0·002), but not SpO2 (p=0·65) or PIPP score (p=0·48; appendix pp 62–64). We also did sensitivity analyses according to the type of sugar solution used (appendix pp 41–49). Further investigations of sources of heterogeneity were limited by the requirement of a sufficient number of studies using the same comparisons and outcome measures to do these analyses.
Only comparisons of oral sugar versus placebo or no intervention could be assessed for publication bias because these groups were the only ones with ten or more studies for each outcome. We did not find evidence of publication bias or small-study effects in studies that reported differences in heart rate (p=0·13) or SpO2, (p=0·46; appendix pp 65, 66). However, we found possible publication bias or small-study effects for this comparison using PIPP scores (p=0·03; appendix p 67). We found that a substantial number of studies had a high overall risk of bias. 69 (46%) were deemed to have a low overall risk of bias, compared with 80 (54%) having a high overall risk of bias (appendix p 11).
After discussion of the findings of the narrative synthesis, meta-analysis, the final GRADE summary of findings tables, and the local health-care context to which these guidelines would be applied, the NPGG made the consensus recommendations detailed in panel 2. Despite low certainty of its superiority over giving oral sugar, breastfeeding was promoted as first-line analgesic because of an absent side-effect profile, and specific contextual factors such as free cost to the state, the promotion of maternal participation in care, and strong policy preference for exclusive breastfeeding in Kenya. Despite having a moderate certainty that expressed breastmilk was inferior to oral sugar, the group had concerns that use of oral sugar by health-care professionals might inadvertently be viewed as supporting the undesirable practice of offering glucose-water to newborn babies at home.123 Further concerns raised included the possible infection risk from reuse or shared use of bottles of sugar solution, and that neonates undergoing multiple procedures would receive large amounts of daily sugar. The NPGG were also concerned that recommending the potentially more effective sugar solutions with a concentration of 24% or more might result in health-care workers having to prepare this from the more readily available 10% and 50% solutions, and the potential harm caused from diluting with unsterile water. Therefore, for neonates who are unable to breastfeed, the NPGG recommended expressed breastmilk administered via syringe into the mouth as first-line analgesic, and oral sugar as second-line. In recommending the adjunctive provision of skin-to-skin care, or encouraging non-nutritive sucking in all possible cases, the NPGG considered the evidence of an enhanced analgesic effect from the combination of these analgesics compared with their use in isolation. The group was particularly keen to formally recommend these options because doing so would further encourage periprocedural parental involvement, something Kenyan health-care professionals have been aiming to promote.124
Panel 2. Final recommendations for analgesia in neonates undergoing routine procedures in non-tertiary hospitals, made by the Neonatal Pain Guideline Group.
If able to breastfeed
-
•
Breastfeeding should be the first-line analgesic, initiated at least 2 min before the procedure and, when possible, continued throughout
-
•
When possible, the neonate should be held skin-to-skin with the mother
-
•
Second-line and third-line analgesics should follow recommendations for neonates who are unable to breastfeed
If unable to breastfeed
-
•
Expressed breastmilk is the first-line analgesic, with oral sugar a second-line option
-
•
1–2 mL of expressed breastmilk should be given via syringe into the mouth at least 2 min before the procedure, or 1–2 mL of any oral sugar solution (≥10% concentration) via syringe into the mouth at least 2 min before the procedure
-
•
If dilution is required to reach the target concentration, then do so with sterile water
-
•
When possible, position the neonate to prevent choking and stop administration if choking occurs
-
•
Nasogastric or orogastric tubes should not be used for administration of these solutions for analgesic purposes; syringing of solutions into the mouth is still an option in most of these cases
-
•
Caution should be taken not to administer too much sugar over a 24-h period when neonates are undergoing multiple procedures
-
•
Skin-to-skin care or non-nutritive sucking of a neonate's own fist or a parent's cleaned finger after administration of expressed breastmilk or oral sugar should be promoted when appropriate
General considerations specified by the Neonatal Pain Guideline Group
-
•
Emphasis should be placed on predicting the need for a procedure ahead of time and encouraging periprocedural parental presence
-
•
Health-care professionals should make efforts to routinely measure neonatal pain around procedures by use of a validated pain measure score
-
•
Pacifiers or dummies are generally not recommended for use for non-nutritive sucking
An updated literature search of the MEDLINE database (from April 1, 2019, to May 15, 2020) yielded a further 11 studies that would have met inclusion criteria. Having reviewed these studies, we do not feel that they reveal any unexpected new findings in the field beyond those already described in our original review. These studies support the superiority of breastfeeding, oral sugar, and non-nutritive sucking over other interventions, and further suggest that a combination of these interventions enhances analgesic efficacy in some instances. Therefore, we feel that the inclusion of these further studies was unlikely to have significantly altered the guideline decisions made by the NPGG.
Discussion
We found that breastfeeding was the preferred analgesic option for neonates undergoing routine procedures in non-tertiary health-care settings in Kenya. Second-line options include expressed breastmilk and oral sugar, and the adjunctive provision of skin-to-skin care and non-nutritive sucking is also recommended. Our study focused upon the routine procedures and analgesics that are most widely seen in the non-tertiary Kenyan setting, and therefore we are cautious about the applicability of our recommendations to the higher-income setting. Moreover, we had important methodological concerns for many of our included studies. The high risk of bias in many of our included studies reflects a general need for more rigorous experimental standards in neonatal pain studies. Furthermore, the neonatal pain research community is increasingly aware of the limitations of the highly subjective pain scoring systems used in experimental and clinical settings; whether these tools better quantify distress in neonates as a distinct entity to nociception is increasingly being discussed.6, 125 Nevertheless, a reduction in neonatal distress is of obvious intrinsic value. The NPGG therefore regarded data showing meaningful improvements in these experimental measures of pain to also be of clinical significance.
The systematic review had some inherent limitations. Our inclusion and exclusion criteria were dictated by our aim of developing guidelines on procedure-related pain in non-tertiary settings in low-income and middle-income countries. These criteria might have therefore resulted in the omission of certain studies relating to particular analgesic strategies, as reflected by some differences in the number of included studies in our and others' reviews.8, 9 Furthermore, only 14 studies assessed pharmacological analgesic options. Given the adverse side-effect profiles of some of these interventions, scarcity of evidence from our review for their benefit, as well as frequent lack of availability in low-income and middle-income countries, the NPGG chose not to recommend these pharmacological analgesics for use during routine procedures. In high-income health-care settings, guidelines might be able to more safely recommend pharmacological analgesics, when increased resources facilitate safer use of such interventions. Additionally, we were limited in our ability to include all studies in our meta-analysis. However, we adhered to a thorough and transparent method of presenting our integrated narrative and quantitative findings to the guideline development panel. We found that certain procedures were done significantly more frequently than others were, with heel prick being the most frequently observed. However, we and others87 contest that the pain stimulus generated by a heel prick is similar to that of our other included procedures, meaning that our findings are generalisable to similar acutely painful procedures. We also detected a high degree of heterogeneity in some meta-analyses. However, we were limited in our ability to fully investigate possible sources of this because of the small number of studies within many comparisons. A final limitation of our review might be an underappreciated publication bias for certain comparisons. Our analyses for small-study effects were limited to comparisons of oral sugar versus placebo or no intervention, in which we identified a possible bias in those studies using PIPP as an outcome measure.
Despite increasing attention devoted to the study of pain in neonates, existing clinical guidelines are rarely employed successfully in clinical practice.126 This disparity might be due to the poor methodological quality of many guidelines, lack of interprofessional collaboration, and insufficient consideration for the context to which the guidelines are being applied.127 We have provided a rigorous systematic review, using both a narrative and a meta-analytical approach, of the evidence relating to a wide range of routine procedures and analgesics, and described the practical translation of the findings into a pragmatic and workable clinical guideline. Effective change to clinical practice in the low-income and middle-income health-care setting requires consideration of local social, financial, and organisational factors.128 We, and others,129 have shown the effectiveness of the GRADE framework in making these context-specific considerations explicit, thereby increasing the transparency of the translation of evidence to clinical recommendations. The NPGG were able to consider the likely values and preferences of Kenyan families and medical staff, local feasibility of interventions, and knowledge of how to obtain meaningful change in service delivery in the local health-care system. This process might lead to different national groups reaching different conclusions that are based on the same evidence, emphasising the importance of global progress in evidence synthesis and translation through nationally led guideline development processes.
The strength of our guideline development process lay in empowering a panel of local experts to make evidence-based recommendations for the local health-care system. The recommendations stemming from our study have since been adopted and included in new national guidelines by the Ministry of Health and partners in Kenya, thereby addressing a national gap in clinical recommendations, and are likely to lead to substantial improvements in neonatal care throughout the country. The efficient use of high-quality systematic reviews linked to structured and transparent processes to develop local guidelines represents an effective model for achieving meaningful improvements in the care of patients in the low-income and middle-income setting.
Acknowledgments
Acknowledgments
Funds from the Wellcome Trust (207522) awarded to ME as a senior fellowship and a grant from the Africa-Oxford Initiative (AfiOX-154) supported this work. We thank the Neonatal Pain Guideline Group for their role in translating the review findings into the guideline adopted by the Ministry of Health and partners in Kenya. This group consisted of Dorothy Agedo, Jalemba Aluvaala, Caren Emadau, Edith Gicheha, Christine Gichuhi, Grace Irimu, Patrick Mburugu, Mercy Mulaku, Florence Murila, Rachel Musoke, Muthoni Ogola, Albert Taigi, Duncan Tumwa, Aggrey Wasunna, Mary Waiyego, and Irene Weru. Thank you also to Tatjana Petrinic of Bodleian Libraries, University of Oxford, Oxford, UK, for her help in designing and conducting the literature searches.
Contributors
All authors contributed to the study design, data interpretation, and revising of the Article. CW had overall responsibility for the work and wrote the Article. CW and JSF screened studies and extracted and analysed data. EQ, LMD, SD, and AtWN contributed to the literature search, data extraction, and analysis. RR led the quantitative analysis. JA, KL, and ME were expert advisers, and coordinated the Neonatal Pain Guideline Group.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur J Pain. 2016;20:489–498. doi: 10.1002/ejp.757. [DOI] [PubMed] [Google Scholar]
- 2.Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: a systematic review. Clin J Pain. 2015;31:355–362. doi: 10.1097/AJP.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJS. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 4.Carbajal R, Rousset A, Danan C. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 5.Kyololo OM, Stevens B, Gastaldo D, Gisore P. Procedural pain in neonatal units in Kenya. Arch Dis Child Fetal Neonatal Ed. 2014;99:F464–F467. doi: 10.1136/archdischild-2014-306003. [DOI] [PubMed] [Google Scholar]
- 6.Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol. 2007;31:283–288. doi: 10.1053/j.semperi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Johnston C, Campbell-Yeo M, Disher T. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev. 2017;2 doi: 10.1002/14651858.CD008435.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit B, Martin-Misener R, Latimer M, Campbell-Yeo M. Breast-feeding analgesia in infants: an update on the current state of evidence. J Perinat Neonatal Nurs. 2017;31:145–159. doi: 10.1097/JPN.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 9.Harrison D, Larocque C, Bueno M. Sweet solutions to reduce procedural pain in neonates: a meta-analysis. Pediatrics. 2017;139 doi: 10.1542/peds.2016-0955. [DOI] [PubMed] [Google Scholar]
- 10.Committee On Fetus And Newborn. Section On Anesthesiology And Pain Medicine Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137 doi: 10.1542/peds.2015-4271. [DOI] [PubMed] [Google Scholar]
- 11.Lee GY, Yamada J, Kyololo O, Shorkey A, Stevens B. Pediatric clinical practice guidelines for acute procedural pain: a systematic review. Pediatrics. 2014;133:500–515. doi: 10.1542/peds.2013-2744. [DOI] [PubMed] [Google Scholar]
- 12.WHO . Second edition, 2013 edition. World Health Organization; Geneva, Switzerland: 2013. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. [PubMed] [Google Scholar]
- 13.Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12:59–66. [PubMed] [Google Scholar]
- 15.Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the neonatal facial coding system in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 16.Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier-Martin M. APN: evaluation behavioral scale of acute pain in newborn infants. Arch Pediatr. 1997;4:623–628. doi: 10.1016/s0929-693x(97)83360-x. [DOI] [PubMed] [Google Scholar]
- 17.Schünemann H, Brożek J, Guyatt G, Oxman A, eds. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html (accessed June 22, 2020).
- 18.Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, Enck P. Placebo effects in children: a review. Pediatr Res. 2013;74:96–102. doi: 10.1038/pr.2013.66. [DOI] [PubMed] [Google Scholar]
- 19.Janiaud P, Cornu C, Lajoinie A, Djemli A, Cucherat M, Kassai B. Is the perceived placebo effect comparable between adults and children? A meta-regression analysis. Pediatr Res. 2017;81:11–17. doi: 10.1038/pr.2016.181. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Altman DG, Gøtzsche PC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Aydin D, İnal S. Effects of breastfeeding and heel warming on pain levels during heel stick in neonates. Int J Nurs Pract. 2019;25 doi: 10.1111/ijn.12734. [DOI] [PubMed] [Google Scholar]
- 23.Carbajal R, Veerapen S, Couderc S, Jugie M, Ville Y. Analgesic effect of breast feeding in term neonates: randomised controlled trial. BMJ. 2003;326:13. doi: 10.1136/bmj.326.7379.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajbhiye M, Rao S, Singh H. Comparative study between analgesic effect of breast feeding and oral sucrose in full term newborns. J Clin Diagn Res. 2018;12:SC09–SC12. [Google Scholar]
- 25.Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics. 2002;109:590–593. doi: 10.1542/peds.109.4.590. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi F, Taheri L, Ghodsbin F, Pishva N, Vossoughi M. Comparing the effect of swaddling and breastfeeding and their combined effect on the pain induced by BCG vaccination in infants referring to Motahari Hospital, Jahrom, 2010–2011. Appl Nurs Res. 2016;29:217–221. doi: 10.1016/j.apnr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Hatami Bavarsad Z, Hemati K, Sayehmiri K. Effects of breast milk on pain severity during muscular injection of hepatitis B vaccine in neonates in a teaching hospital in Iran. Arch Pediatr. 2018;25:365–370. doi: 10.1016/j.arcped.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Leite AM, Linhares MB, Lander J, Castral TC, dos Santos CB, Silvan Scochi CG. Effects of breastfeeding on pain relief in full-term newborns. Clin J Pain. 2009;25:827–832. doi: 10.1097/AJP.0b013e3181b51191. [DOI] [PubMed] [Google Scholar]
- 29.Lima AH, Hermont AP, Friche AA. Analgesia in newborns: a case-control study of the efficacy of nutritive and non-nutritive sucking stimuli. CoDAS. 2013;25:365–368. doi: 10.1590/s2317-17822013005000002. [DOI] [PubMed] [Google Scholar]
- 30.Obeidat HM, Shuriquie MA. Effect of breast-feeding and maternal holding in relieving painful responses in full-term neonates: a randomized clinical trial. J Perinat Neonatal Nurs. 2015;29:248–254. doi: 10.1097/JPN.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Simalti A, Singh D. Breast feeding as analgesia in neonates: a randomized controlled trial. J Nepal Paediatr Soc. 2017;36:238–242. [Google Scholar]
- 32.Zargham-Boroujeni A, Elsagh A, Mohammadizadeh M. The effects of massage and breastfeeding on response to venipuncture pain among hospitalized neonates. Iran J Nurs Midwifery Res. 2017;22:308–312. doi: 10.4103/ijnmr.IJNMR_119_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Hong-Gu H, Zhou X. Pain relief effect of breast feeding and music therapy during heel lance for healthy-term neonates in China: a randomized controlled trial. Midwifery. 2015;31:365–372. doi: 10.1016/j.midw.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Bembich S, Cont G, Causin E, Paviotti G, Marzari P, Demarini S. Infant analgesia with a combination of breast milk, glucose, or maternal holding. Pediatrics. 2018;142 doi: 10.1542/peds.2017-3416. [DOI] [PubMed] [Google Scholar]
- 35.Chiabi A, Eloundou E, Mah E, Nguefack S, Mekone I, Mbonda E. Evaluation of breastfeeding and 30% glucose solution as analgesic measures in indigenous African term neonates. J Clin Neonatol. 2016;5:46. [Google Scholar]
- 36.Codipietro L, Ceccarelli M, Ponzone A. Breastfeeding or oral sucrose solution in term neonates receiving heel lance: a randomized, controlled trial. Pediatrics. 2008;122:e716–e721. doi: 10.1542/peds.2008-0221. [DOI] [PubMed] [Google Scholar]
- 37.Soltani S, Zohoori D, Adineh M. Comparison the effectiveness of breastfeeding, oral 25% dextrose, kangaroo-mother care method, and EMLA cream on pain score level following heal pick sampling in newborns: a randomized clinical trial. Electron Physician. 2018;10:6741–6748. doi: 10.19082/6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brovedani P, Montico M, Shardlow A, Strajn T, Demarini S. Suckling and sugar for pain reduction in babies. Lancet. 2007;369:1429–1430. doi: 10.1016/S0140-6736(07)60666-7. [DOI] [PubMed] [Google Scholar]
- 39.Simonse E, Mulder PG, van Beek RH. Analgesic effect of breast milk versus sucrose for analgesia during heel lance in late preterm infants. Pediatrics. 2012;129:657–663. doi: 10.1542/peds.2011-2173. [DOI] [PubMed] [Google Scholar]
- 40.Rioualen S, Durier V, Hervé D, Misery L, Sizun J, Roué JM. Cortical pain response of newborn infants to venepuncture: a randomized controlled trial comparing analgesic effects of sucrose versus breastfeeding. Clin J Pain. 2018;34:650–656. doi: 10.1097/AJP.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 41.Fallah R, Naserzadeh N, Ferdosian F, Binesh F. Comparison of effect of kangaroo mother care, breastfeeding and swaddling on Bacillus Calmette-Guerin vaccination pain score in healthy term neonates by a clinical trial. J Matern Fetal Neonatal Med. 2017;30:1147–1150. doi: 10.1080/14767058.2016.1205030. [DOI] [PubMed] [Google Scholar]
- 42.Abad F, Díaz NM, Domenech E, Robayna M, Rico J. Oral sweet solution reduces pain-related behaviour in preterm infants. Acta Paediatr. 1996;85:854–858. doi: 10.1111/j.1651-2227.1996.tb14167.x. [DOI] [PubMed] [Google Scholar]
- 43.Dezhdar S, Jahanpour F, Firouz Bakht S, Ostovar A. The Effects of kangaroo mother care and swaddling on venipuncture pain in premature neonates: a randomized clinical trial. Iran Red Crescent Med J. 2016;18 doi: 10.5812/ircmj.29649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haouari N, Wood C, Griffiths G, Levene M. The analgesic effect of sucrose in full term infants: a randomised controlled trial. BMJ. 1995;310:1498–1500. doi: 10.1136/bmj.310.6993.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jatana SK, Dalal SS, Wilson CG. Analgesic effect of oral glucose in neonates. Med J Armed Forces India. 2003;59:100–104. doi: 10.1016/S0377-1237(03)80048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr. 1997;86:217–220. doi: 10.1111/j.1651-2227.1997.tb08872.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramenghi L, Griffith G, Wood C, Levene M. Effect of non-sucrose sweet tasting solution on neonatal heel prick responses. Arch Dis Child Fetal Neonatal Ed. 1996;74:F129–F131. doi: 10.1136/fn.74.2.f129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abad F, Díaz-Gómez NM, Domenech E, González D, Robayna M, Feria M. Oral sucrose compares favourably with lidocaine-prilocaine cream for pain relief during venepuncture in neonates. Acta Paediatr. 2001;90:160–165. [PubMed] [Google Scholar]
- 49.Acharya AB, Annamali S, Taub NA, Field D. Oral sucrose analgesia for preterm infant venepuncture. Arch Dis Child Fetal Neonatal Ed. 2004;89:F17–F18. doi: 10.1136/fn.89.1.F17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahuja VK, Daga SR, Gosavi DV, Date AM. Non-sucrose sweetener for pain relief in sick newborns. Indian J Pediatr. 2000;67:487–489. doi: 10.1007/BF02760473. [DOI] [PubMed] [Google Scholar]
- 51.Akçam M. Oral fructose solution as an analgesic in the newborn: a randomized, placebo-controlled and masked study. Pediatr Int. 2004;46:459–462. doi: 10.1111/j.1442-200x.2004.01932.x. [DOI] [PubMed] [Google Scholar]
- 52.Akçam M, Örmeci AR. Oral hypertonic glucose spray: a practical alternative for analgesia in the newborn. Acta Paediatr. 2004;93:1330–1333. [PubMed] [Google Scholar]
- 53.Altun-Köroğlu O, Özek E, Bilgen H, Cebeci D. Hindmilk for procedural pain in term neonates. Turk J Pediatr. 2010;52:623–629. [PubMed] [Google Scholar]
- 54.Basnet S, Shrestha L, Shrestha P. Sucrose as an analgesic in relieving procedural pain in neonates. J Neonatal Perinatal Med. 2010;3:325–331. [Google Scholar]
- 55.Bauer K, Ketteler J, Hellwig M, Laurenz M, Versmold H. Oral glucose before venepuncture relieves neonates of pain, but stress is still evidenced by increase in oxygen consumption, energy expenditure, and heart rate. Pediatr Res. 2004;55:695–700. doi: 10.1203/01.PDR.0000113768.50419.CD. [DOI] [PubMed] [Google Scholar]
- 56.Bellieni CV, Buonocore G, Nenci A, Franci N, Cordelli DM, Bagnoli F. Sensorial saturation: an effective analgesic tool for heel-prick in preterm infants: a prospective randomized trial. Biol Neonate. 2001;80:15–18. doi: 10.1159/000047113. [DOI] [PubMed] [Google Scholar]
- 57.Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain. 1999;83:611–623. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 58.Carbajal R, Chauvet X, Couderc S, Olivier-Martin M. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ. 1999;319:1393–1397. doi: 10.1136/bmj.319.7222.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carbajal R, Lenclen R, Gajdos V, Jugie M, Paupe A. Crossover trial of analgesic efficacy of glucose and pacifier in very preterm neonates during subcutaneous injections. Pediatrics. 2002;110:389–393. doi: 10.1542/peds.110.2.389. [DOI] [PubMed] [Google Scholar]
- 60.Deshmukh LS, Udani RH. Analgesic effect of oral glucose in preterm infants during venipuncture—a double-blind, randomized, controlled trial. J Trop Pediatr. 2002;48:138–141. doi: 10.1093/tropej/48.3.138. [DOI] [PubMed] [Google Scholar]
- 61.Elserafy FA, Alsaedi SA, Louwrens J, Bin Sadiq B, Mersal AY. Oral sucrose and a pacifier for pain relief during simple procedures in preterm infants: a randomized controlled trial. Ann Saudi Med. 2009;29:184–188. doi: 10.4103/0256-4947.52821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eriksson M, Gradin M, Schollin J. Oral glucose and venepuncture reduce blood sampling pain in newborns. Early Hum Dev. 1999;55:211–218. doi: 10.1016/s0378-3782(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 63.Eriksson M, Finnström O. Can daily repeated doses of orally administered glucose induce tolerance when given for neonatal pain relief? Acta Paediatr. 2004;93:246–249. doi: 10.1080/08035250310008041. [DOI] [PubMed] [Google Scholar]
- 64.Gao H, Li M, Gao H. Effect of non-nutritive sucking and sucrose alone and in combination for repeated procedural pain in preterm infants: A randomized controlled trial. Int J Nurs Stud. 2018;83:25–33. doi: 10.1016/j.ijnurstu.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Gharehbaghi M, Peirovifar A. The effect of oral dextrose on pain relief of newborn infants. Pak J Med Sci. 2007;23:881–884. [Google Scholar]
- 66.Gradin M, Finnström O, Schollin J. Feeding and oral glucose—additive effects on pain reduction in newborns. Early Hum Dev. 2004;77:57–65. doi: 10.1016/j.earlhumdev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Harrison D, Johnston L, Loughnan P. Oral sucrose for procedural pain in sick hospitalized infants: a randomized-controlled trial. J Paediatr Child Health. 2003;39:591–597. doi: 10.1046/j.1440-1754.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 68.Johnston CC, Stremler RL, Stevens BJ, Horton LJ. Effectiveness of oral sucrose and simulated rocking on pain response in preterm neonates. Pain. 1997;72:193–199. doi: 10.1016/s0304-3959(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 69.Johnston CC, Stremler R, Horton L, Friedman A. Effect of repeated doses of sucrose during heel stick procedure in preterm neonates. Biol Neonate. 1999;75:160–166. doi: 10.1159/000014092. [DOI] [PubMed] [Google Scholar]
- 70.Liaw JJ, Zeng WP, Yang L, Yuh YS, Yin T, Yang MH. Nonnutritive sucking and oral sucrose relieve neonatal pain during intramuscular injection of hepatitis vaccine. J Pain Symptom Manage. 2011;42:918–930. doi: 10.1016/j.jpainsymman.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Liu MF, Lin KC, Chou YH, Lee TY. Using non-nutritive sucking and oral glucose solution with neonates to relieve pain: a randomised controlled trial. J Clin Nurs. 2010;19:1604–1611. doi: 10.1111/j.1365-2702.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 72.Mathai S, Natrajan N, Rajalakshmi NR. A comparative study of nonpharmacological methods to reduce pain in neonates. Indian Pediatr. 2006;43:1070–1075. [PubMed] [Google Scholar]
- 73.Milazzo W, Fielder J, Bittel A. Oral sucrose to decrease pain associated with arterial puncture in infants 30 to 36 weeks-gestation. Adv Neonatal Care. 2011;11:406–411. doi: 10.1097/ANC.0b013e318235c1ff. [DOI] [PubMed] [Google Scholar]
- 74.Ogawa S, Ogihara T, Fujiwara E. Venepuncture is preferable to heel lance for blood sampling in term neonates. Arch Dis Child Fetal Neonatal Ed. 2005;90:F432–F436. doi: 10.1136/adc.2004.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okan F, Coban A, Ince Z, Yapici Z, Can G. Analgesia in preterm newborns: the comparative effects of sucrose and glucose. Eur J Pediatr. 2007;166:1017–1024. doi: 10.1007/s00431-006-0373-z. [DOI] [PubMed] [Google Scholar]
- 76.Örs R, Özek E, Baysoy G. Comparison of sucrose and human milk on pain response in newborns. Eur J Pediatr. 1999;158:63–66. doi: 10.1007/s004310051011. [DOI] [PubMed] [Google Scholar]
- 77.Ou-Yang MC, Chen IL, Chen CC, Chung MY, Chen FS, Huang HC. Expressed breast milk for procedural pain in preterm neonates: a randomized, double-blind, placebo-controlled trial. Acta Paediatr. 2013;102:15–21. doi: 10.1111/apa.12045. [DOI] [PubMed] [Google Scholar]
- 78.Overgaard C, Knudsen A. Pain-relieving effect of sucrose in newborns during heel prick. Biol Neonate. 1999;75:279–284. doi: 10.1159/000014105. [DOI] [PubMed] [Google Scholar]
- 79.Ozdogan T, Akman I, Cebeci D, Bilgen H, Ozek E. Comparison of two doses of breast milk and sucrose during neonatal heel prick. Pediatr Int. 2010;52:175–179. doi: 10.1111/j.1442-200X.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 80.Ramenghi LA, Wood CM, Griffith GC, Levene MI. Reduction of pain response in premature infants using intraoral sucrose. Arch Dis Child Fetal Neonatal Ed. 1996;74:F126–F128. doi: 10.1136/fn.74.2.f126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rawal S, Ghai A, Jindal T. Twenty-five percent dextrose and EBM in pain relief during heel lance in late preterm babies using the PIPP score: a randomized controlled trial. J Nematol. 2018;32:43–49. [Google Scholar]
- 82.Rogers AJ, Greenwald MH, Deguzman MA, Kelley ME, Simon HK. A randomized, controlled trial of sucrose analgesia in infants younger than 90 days of age who require bladder catheterization in the pediatric emergency department. Acad Emerg Med. 2006;13:617–622. doi: 10.1197/j.aem.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 83.Sahoo JP, Rao S, Nesargi S, Ranjit T, Ashok C, Bhat S. Expressed breast milk vs 25% dextrose in procedural pain in neonates, a double blind randomized controlled trial. Indian Pediatr. 2013;50:203–207. doi: 10.1007/s13312-013-0067-3. [DOI] [PubMed] [Google Scholar]
- 84.Sajedi F, Kashaninia Z, Rahgozar M, Radrazm L. The efficacy of oral glucose for relieving pain following intramuscular infection in term neonates. Acta Med Iran. 2006;44:316–322. [Google Scholar]
- 85.Slater R, Cornelissen L, Fabrizi L. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. 2010;376:1225–1232. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suhrabi Z, Taghinejad H, Valian K, Sayehmiri K, Taheri S. A comparative study on the efficacy of glucose and sucrose on the vaccination pain: a randomized controlled clinical trial. J Clin Diagn Res. 2014;8:PC01–PC03. doi: 10.7860/JCDR/2014/10057.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taddio A, Shah V, Hancock R. Effectiveness of sucrose analgesia in newborns undergoing painful medical procedures. CMAJ. 2008;179:37–43. doi: 10.1503/cmaj.071734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thakkar P, Arora K, Goyal K. To evaluate and compare the efficacy of combined sucrose and non-nutritive sucking for analgesia in newborns undergoing minor painful procedure: a randomized controlled trial. J Perinatol. 2016;36:67–70. doi: 10.1038/jp.2015.122. [DOI] [PubMed] [Google Scholar]
- 89.Tutag Lehr V, Cortez J, Grever W, Cepeda E, Thomas R, Aranda JV. Randomized placebo-controlled trial of sucrose analgesia on neonatal skin blood flow and pain response during heel lance. Clin J Pain. 2015;31:451–458. doi: 10.1097/AJP.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 90.Uzelli D, Yapucu Güneş Ü. Oral glucose solution to alleviate pain induced by intramuscular injections in preterm infants. J Spec Pediatr Nurs. 2015;20:29–35. doi: 10.1111/jspn.12094. [DOI] [PubMed] [Google Scholar]
- 91.Yilmaz F, Arikan D. The effects of various interventions to newborns on pain and duration of crying. J Clin Nurs. 2011;20:1008–1017. doi: 10.1111/j.1365-2702.2010.03356.x. [DOI] [PubMed] [Google Scholar]
- 92.Bellieni CV, Bagnoli F, Perrone S. Effect of multisensory stimulation on analgesia in term neonates: a randomized controlled trial. Pediatr Res. 2002;51:460–463. doi: 10.1203/00006450-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Cardoso MV, Farias LM, de Melo GM. Music and 25% glucose pain relief for the premature infant: a randomized clinical trial. Rev Lat Am Enfermagem. 2014;22:810–818. doi: 10.1590/0104-1169.0029.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chermont AG, Falcão LF, de Souza Silva EH, de Cássia Xavier Balda R, Guinsburg R. Skin-to-skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics. 2009;124:e1101–e1107. doi: 10.1542/peds.2009-0993. [DOI] [PubMed] [Google Scholar]
- 95.Cook LM, Nichols-Dada J, Damani S. Randomized clinical trial of 24% oral sucrose to decrease pain associated with peripheral intravenous catheter insertion in preterm and term newborns. Adv Neonatal Care. 2017;17:E3–11. doi: 10.1097/ANC.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 96.Golestan M, Akhavan Karbasi S, Modares-Mosadegh M, Sadr-Bafghi M. Analgesic effects of glucose and water in neonates. Acta Med Iran. 2007;45:461–465. [Google Scholar]
- 97.Gormally S, Barr RG, Wertheim L, Alkawaf R, Calinoiu N, Young SN. Contact and nutrient caregiving effects on newborn infant pain responses. Dev Med Child Neurol. 2001;43:28–38. doi: 10.1017/s0012162201000056. [DOI] [PubMed] [Google Scholar]
- 98.Gray L, Lang CW, Porges SW. Warmth is analgesic in healthy newborns. Pain. 2012;153:960–966. doi: 10.1016/j.pain.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsieh KH, Chen SJ, Tsao PC. The analgesic effect of non-pharmacological interventions to reduce procedural pain in preterm neonates. Pediatr Neonatol. 2018;59:71–76. doi: 10.1016/j.pedneo.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Bueno M, Stevens B, de Camargo PP, Toma E, Krebs VL, Kimura AF. Breast milk and glucose for pain relief in preterm infants: a noninferiority randomized controlled trial. Pediatrics. 2012;129:664–670. doi: 10.1542/peds.2011-2024. [DOI] [PubMed] [Google Scholar]
- 101.Lima AG, Santos VS, Nunes MS. Glucose solution is more effective in relieving pain in neonates than non-nutritive sucking: a randomized clinical trial. Eur J Pain. 2017;21:159–165. doi: 10.1002/ejp.912. [DOI] [PubMed] [Google Scholar]
- 102.Upadhyay A, Aggarwal R, Narayan S, Joshi M, Paul VK, Deorari AK. Analgesic effect of expressed breast milk in procedural pain in term neonates: a randomized, placebo-controlled, double-blind trial. Acta Paediatr. 2004;93:518–522. doi: 10.1080/08035250410022792. [DOI] [PubMed] [Google Scholar]
- 103.Uyan ZS, Ozek E, Bilgen H, Cebeci D, Akman I. Effect of foremilk and hindmilk on simple procedural pain in newborns. Pediatr Int. 2005;47:252–257. doi: 10.1111/j.1442-200x.2005.02055.x. [DOI] [PubMed] [Google Scholar]
- 104.Castral TC, Warnock F, Leite AM, Haas VJ, Scochi CG. The effects of skin-to-skin contact during acute pain in preterm newborns. Eur J Pain. 2008;12:464–471. doi: 10.1016/j.ejpain.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Cong X, Ludington-Hoe SM, McCain G, Fu P. Kangaroo care modifies preterm infant heart rate variability in response to heel stick pain: pilot study. Early Hum Dev. 2009;85:561–567. doi: 10.1016/j.earlhumdev.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cong X, Ludington-Hoe SM, Walsh S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol Res Nurs. 2011;13:204–216. doi: 10.1177/1099800410385839. [DOI] [PubMed] [Google Scholar]
- 107.Cong X, Cusson RM, Walsh S, Hussain N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012;13:636–645. doi: 10.1016/j.jpain.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 109.Johnston CC, Stevens B, Pinelli J. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157:1084–1088. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 110.Kashaninia Z, Sajedi F, Rahgozar M, Noghabi FA. The effect of Kangaroo Care on behavioral responses to pain of an intramuscular injection in neonates. J Spec Pediatr Nurs. 2008;13:275–280. doi: 10.1111/j.1744-6155.2008.00165.x. [DOI] [PubMed] [Google Scholar]
- 111.Liu M, Zhao L, Li XF. Effect of skin contact between mother and child in pain relief of full-term newborns during heel blood collection. Clin Exp Obstet Gynecol. 2015;42:304–308. [PubMed] [Google Scholar]
- 112.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (kangaroo care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–387. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nimbalkar SM, Chaudhary NS, Gadhavi KV, Phatak A. Kangaroo mother care in reducing pain in preterm neonates on heel prick. Indian J Pediatr. 2013;80:6–10. doi: 10.1007/s12098-012-0760-6. [DOI] [PubMed] [Google Scholar]
- 114.Okan F, Ozdil A, Bulbul A, Yapici Z, Nuhoglu A. Analgesic effects of skin-to-skin contact and breastfeeding in procedural pain in healthy term neonates. Ann Trop Paediatr. 2010;30:119–128. doi: 10.1179/146532810X12703902516121. [DOI] [PubMed] [Google Scholar]
- 115.Olsson E, Ahlsén G, Eriksson M. Skin-to-skin contact reduces near-infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatr. 2016;105:376–380. doi: 10.1111/apa.13180. [DOI] [PubMed] [Google Scholar]
- 116.Saeidi R, Asnaashari Z, Amirnejad M, Esmaeili H, Robatsangi MG. Use of “kangaroo care” to alleviate the intensity of vaccination pain in newborns. Iran J Pediatr. 2011;21:99–102. [PMC free article] [PubMed] [Google Scholar]
- 117.Gao H, Xu G, Gao H. Effect of repeated kangaroo mother care on repeated procedural pain in preterm infants: a randomized controlled trial. Int J Nurs Stud. 2015;52:1157–1165. doi: 10.1016/j.ijnurstu.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Field T, Goldson E. Pacifying effects of nonnutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics. 1984;74:1012–1015. [PubMed] [Google Scholar]
- 119.Liaw JJ, Yang L, Ti Y, Blackburn ST, Chang YC, Sun LW. Non-nutritive sucking relieves pain for preterm infants during heel stick procedures in Taiwan. J Clin Nurs. 2010;19:2741–2751. doi: 10.1111/j.1365-2702.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 120.Liaw JJ, Yang L, Wang K-WK, Chen CM, Chang YC, Yin T. Non-nutritive sucking and facilitated tucking relieve preterm infant pain during heel-stick procedures: a prospective, randomised controlled crossover trial. Int J Nurs Stud. 2012;49:300–309. doi: 10.1016/j.ijnurstu.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 121.Mirzarahimi M, Mehrnoush N, Shahizadeh S, Samadi N, Amani F. Effect of non-nutritive sucking and leg massage on physiological and behavioral indicators of pain following heel blood sampling in term neonates. Int J Adv Nurs Stud. 2013;2:74–79. [Google Scholar]
- 122.Corbo MG, Mansi G, Stagni A. Nonnutritive sucking during heelstick procedures decreases behavioral distress in the newborn infant. Biol Neonate. 2000;77:162–167. doi: 10.1159/000014211. [DOI] [PubMed] [Google Scholar]
- 123.Shah S, Rollins NC, Bland R. Breastfeeding knowledge among health workers in rural South Africa. J Trop Pediatr. 2005;51:33–38. doi: 10.1093/tropej/fmh071. [DOI] [PubMed] [Google Scholar]
- 124.Kyololo OM, Stevens BJ, Songok J. Mothers' perceptions about pain in hospitalized newborn infants in Kenya. J Pediatr Nurs. 2019;47:51–57. doi: 10.1016/j.pedn.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Slater R. The challenge of distinguishing pain from distress in young children. Lancet Child Adolesc Health. 2019;3:367–368. doi: 10.1016/S2352-4642(19)30118-X. [DOI] [PubMed] [Google Scholar]
- 126.Wilson-Smith EM. Procedural pain management in neonates, infants and children. Rev Pain. 2011;5:4–12. doi: 10.1177/204946371100500303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Balice-Bourgois C, Zumstein-Shaha M, Vanoni F, Jaques C, Newman CJ, Simonetti GD. A systematic review of clinical practice guidelines for acute procedural pain on neonates. Clin J Pain. 2020;36:390–398. doi: 10.1097/AJP.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 128.Kruk ME, Gage AD, Arsenault C. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:e1196–e1252. doi: 10.1016/S2214-109X(18)30386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Agweyu A, Opiyo N, English M. Experience developing national evidence-based clinical guidelines for childhood pneumonia in a low-income setting--making the GRADE? BMC Pediatr. 2012;12:1. doi: 10.1186/1471-2431-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.