Over the past two decades, Acinetobacter baumannii has emerged as a leading cause of nosocomial infections worldwide. Of particular concern are panresistant strains, leading the World Health Organization (WHO) to designate carbapenem-resistant A. baumannii as a priority 1 (critical) pathogen for research and development of new antibiotics. A key component in supporting this effort is accessibility to diverse and clinically relevant strains for testing. Here, we describe a panel of 100 diverse A. baumannii strains for use in this endeavor.
KEYWORDS: Acinetobacter baumannii, antimicrobial resistance, drug development
ABSTRACT
Over the past two decades, Acinetobacter baumannii has emerged as a leading cause of nosocomial infections worldwide. Of particular concern are panresistant strains, leading the World Health Organization (WHO) to designate carbapenem-resistant A. baumannii as a priority 1 (critical) pathogen for research and development of new antibiotics. A key component in supporting this effort is accessibility to diverse and clinically relevant strains for testing. Here, we describe a panel of 100 diverse A. baumannii strains for use in this endeavor. Whole-genome sequencing was performed on 3,505 A. baumannii isolates housed at the Multidrug-Resistant Organism Repository and Surveillance Network. Isolates were cultured from clinical samples at health care facilities around the world between 2001 and 2017. Core-genome multilocus sequence typing and high-resolution single nucleotide polymorphism (SNP)-based phylogenetic analyses were used to select a final panel of 100 strains that captured the genetic diversity of the collection. Comprehensive antibiotic susceptibility testing was also performed on all 100 isolates using 14 clinically relevant antibiotics. The final 100-strain diversity panel contained representative strains from 70 different traditional Pasteur scheme multilocus sequence types, including major epidemic clones. This diversity was also reflected in antibiotic susceptibility and antimicrobial resistance (AMR) gene content, with phenotypes ranging from pansensitive to panresistant, and over 100 distinct AMR gene alleles identified from 32 gene families. This panel provides the most diverse and comprehensive set of A. baumannii strains for use in developing solutions for combating antibiotic resistance. The panel and all available metadata, including genome sequences, will be available to industry and academic institutions and federal and other laboratories free of charge.
INTRODUCTION
Acinetobacter baumannii, once considered to be a low-virulence organism, is today a prominent member of the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE) group of pathogens, a clique of microorganisms with reduced antibiotic susceptibility and a prominent role in hospital-acquired infections (HAIs) (1, 2). A. baumannii is primarily associated with wound and burn infections and ventilator-associated pneumonia but is also an important cause of urinary tract infections and nosocomial bacteremia (3, 4). Due to both intrinsic and acquired antimicrobial resistance, these infections regularly result in increased morbidity and mortality, particularly among immunocompromised patients (5).
The rapid rise in the clinical relevance of A. baumannii was precipitated by hard-to-treat and increasingly antibiotic-resistant lineages that emerged from an ancestral susceptible pool (6). In particular, members of clonal complex I and II have been extremely successful, although acquisition of carbapenemases may have played an important role in their selection (7). While global epidemiology studies have focused on the major clonal lineages (7, 8), A. baumannii remains a heterogeneous species with over 1,380 different sequence types (STs) identified by traditional Pasteur scheme multilocus sequence typing (MLST). Furthermore, several studies have shown that A. baumannii gene repertoires are very diverse, with fewer than half of the genes being part of the species core genome (9–12). This diversity also reflects the dynamism of this species, as new strains harboring epidemic potential emerge (13, 14).
A key component in the success of A. baumannii has been its remarkable ability to develop antibiotic resistance through mutation (15, 16), gene amplification (17), or horizontal acquisition of resistance genes (18, 19). Of particular concern is the emergence of carbapenem-resistant A. baumannii (CRAB), resulting in infections treatable only with “last-line” antibiotics (4, 7, 20, 21). For example, notwithstanding its nephrotoxicity, colistin (polymyxin E) is considered the last-line drug for treating infections caused by CRAB, but colistin-resistant strains have been isolated (22–24). Although many factors have contributed to this predicament, the absence of new antibiotics can be directly traced to the near-complete withdrawal of pharmaceutical companies from antibiotic development in the late 1980s (25, 26). Today, as the ramifications of this disengagement have become apparent, a concerted effort to develop new treatments is gaining traction among government, academic, and industry organizations (27–29). Recently, the World Health Organization (WHO) listed CRAB as a priority 1 (critical) pathogen for research and development of new treatments, solidifying its status as one of the most important clinical pathogens (30).
The significant genetic diversity of A. baumannii poses a challenge to the design and testing of new treatments, and access to strains that span this diversity is crucial for developing effective therapies. Over the past 10 years, the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) has collected over 3,500 A. baumannii strains from around the world. In this paper, we describe how this collection was used to construct a reference panel of 100 strains that captures the genetic diversity of A. baumannii and maximizes phylogenetic distance and pangenome diversity. Furthermore, this panel has diverse antibiotic susceptibility profiles and antimicrobial resistance (AMR) gene content, with phenotypes ranging from pansusceptible to panresistant. In addition to being a resource for those interested in many aspects of clinical A. baumannii diversity, this panel will greatly aid the design and development of novel antimicrobial agents and diagnostics.
RESULTS
Strain diversity and initial isolate selection.
Of the initial 3,505 A. baumannii genomes, 3,056 (cultured from 2,084 patients) passed the 90% gene threshold stipulated by core-genome MLST (cgMLST). These isolates were then used to generate a minimum spanning tree to better visualize the overall diversity (Fig. 1A). Notably, despite the 13% reduction in isolates, the remaining isolates retained the temporal and clinical diversity observed with the original, except that the number of isolates with no culture type recorded decreased from 18.7% to 6%, with a concomitant increase in isolates from blood (10.7%), respiratory swabs (20.3%), surveillance swabs (5.4%), and wound (39%) samples.
FIG 1.
Genetic diversity of A. baumannii strains. (A) cgMLST minimum spanning tree of the 3,056 A. baumannii genomes used for initial panel selection. cgMLST allelic profiles are represented by circles, and circle sizes are proportional to the number of isolates sharing that profile. The line length connecting the isolates represents the number of allelic differences between each profile. Isolates with 10 or less allelic differences are highlighted with gray shading around the lines. The most prominent traditional MLST groups (Pasteur scheme) are indicated. The 226 isolates initially selected to represent the breadth of diversity (see the text for details) are shown in purple and red with purple also representing the 100 strains in the final panel. (B) Core-genome SNP-based phylogenetic tree of the 226 A. baumannii isolates initially selected to represent the breadth of diversity (see the text for details). The 100 isolates selected for the final panel are indicated with purple triangles. Inset: a zoomed-in view showing one clade from the accessory genome-based phylogenetic tree. The isolates selected for the final panel are indicated with purple triangles.
When analyzed using traditional MLST, 106 distinct ST were identified. However, 74% (2,271 isolates) of the isolates were assigned to just seven STs (ST-1, 2, 3, 25, 32, 81, and 94), reflecting both the clonal expansion of specific strains during Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) (unpublished data) and the global dissemination of these epidemic clones (7). However, even though many isolates from the same ST were genetically indistinguishable (Fig. 1A, larger circles), the high-resolution provided by cgMLST revealed substantial genetic diversity within each ST. For example, up to 807 allelic differences were identified between strains assigned to ST-2.
Selection of a nonredundant, genetically diverse panel of A. baumannii.
Based on the cgMLST analysis, an initial subset of 226 isolates was selected to represent the breadth of genetic diversity across the isolates and to minimize clonal redundancy (Fig. 1A). Although this subset encompassed just 7.3% of the starting number of isolates, 87 of the original 106 traditional Pasteur STs were still represented, confirming that the more streamlined strain set retained a high level of genetic diversity. However, working with 226 isolates would be cumbersome and attempting to provide a panel of this size for use by the broader scientific community would be impractical. As a consequence, the core genomes of the 226 isolates were compared at the highest resolution using a single nucleotide polymorphism (SNP)-based phylogenetic tree (Fig. 1B). As illustrated, this phylogenetic tree contains multiple diverse isolates on long branches as well as clusters of more related isolates centered on the more common STs. This analysis was then used to select strains that best encapsulated the genetic diversity of this group, culminating in a final panel of 100 strains that maximized diversity (Fig. 1B).
The final panel contains strains that have been isolated from a wide range of clinical samples across 4 continents between 2003 and 2017 (see Table S2 in the supplemental material), a time frame that captures the global dissemination of resistant lineages. The panel contains representative strains from 70 different STs and includes genetically distinct isolates within the most globally prevalent clones, such as ST-2 (Table S2). The panel also contains 49 different capsular polysaccharide (K) loci, including 9 that may represent novel loci (“match confidence” of “none”) and 10 of the 12 different outer core biosynthesis loci (OCL) curated by Wyres and colleagues (31). When combined, 73 different K locus (KL)/OCL combinations are present in the 100 strains.
This substantial diversity was also reflected in the gene content, where just 2,119 genes were shared by all 100 isolates as part of the core genome, while >16,000 distinct genes were present in the pangenome (Fig. 2). In addition, the size of the pangenome never plateaus, as each additional genome contained previously unsampled genes, highlighting the large amount of gene diversity present in the isolates.
FIG 2.
Core and pan genome sizes. Number of genes found in the core (left) and pan (right) genomes for the 100 strains of the diversity panel. Core genome is defined as the number of genes found in 99% of the genomes. Boxplots indicate the variation in the number of genomes used for the calculation at each position.
Antibiotic susceptibility and AMR gene content of the final panel.
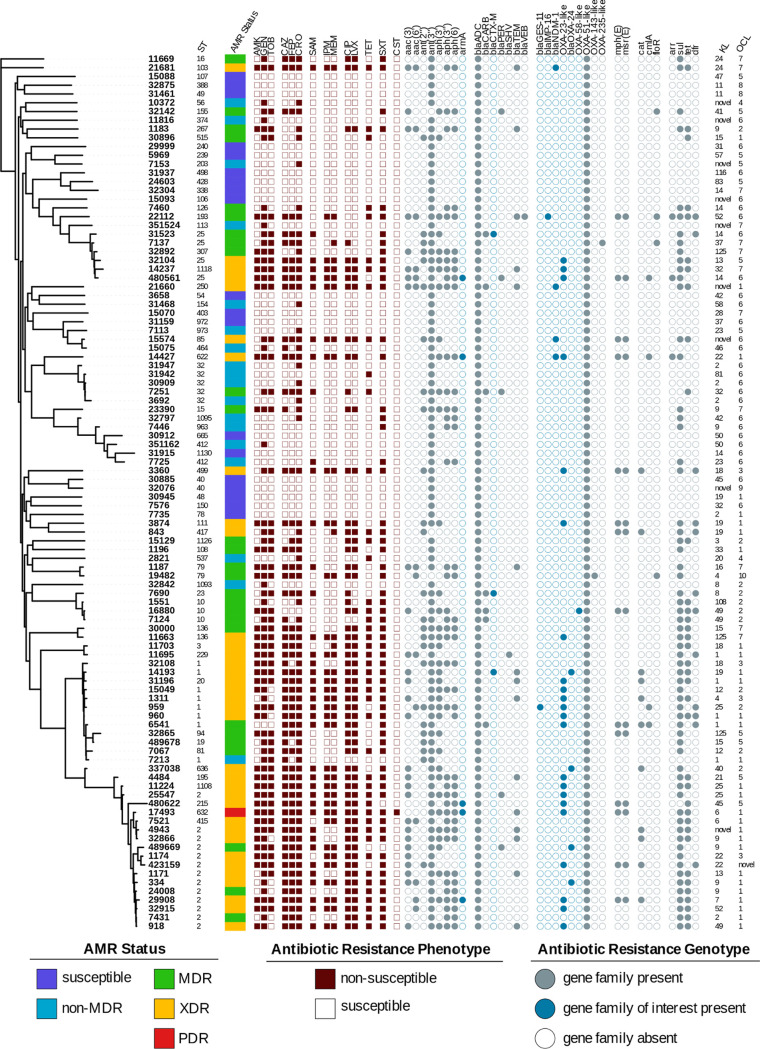
Given the starting collection and isolate selection methods, it is no surprise that the final isolate panel has a diverse range of susceptibilities (Table S2). Nineteen isolates were pansusceptible, 1 isolate (MRSN 17493) was resistant to all 14 antibiotics tested (pandrug resistant [PDR]), and the remaining isolates were classified as either non-multidrug resistant (non-MDR; n = 18), MDR (n = 27), or extensively drug resistant (XDR; n = 35) based on the criteria developed by Magiorakos and colleagues (32). Overall, 52 distinct antibiotic susceptibility profiles were observed, with 34 strains displaying carbapenem resistance and 35 displaying resistance to all tested aminoglycosides (Fig. 3; Table S2).
FIG 3.
Characteristics of the A. baumannii diversity panel. Core genome SNP-based phylogenetic tree of the 100 strains in the final diversity panel. Strain sequence type (ST) is indicated next to the strain number. The assigned antimicrobial resistance (AMR) phenotype (see the text for details) is provided, and the dark red-brown squares indicate a result of nonsusceptible (filled) or susceptible (open) to the tested antibiotic. The gray circles indicate the presence of a known resistance gene in that family, with the blue-gray color highlighting those gene families of particular interest. The capsular polysaccharide locus (KL) and outer core biosynthesis locus (OCL) types are indicated. The figure was produced using the iTOL tool (63).
This phenotypic diversity was also reflected in the AMR gene content, with 100 distinct alleles encompassing 32 families of AMR genes detected in silico (Fig. 3; Table S2). As expected, all strains carried a variant of the intrinsic blaADC and blaOXA-51 genes. Twenty-three strains were nonsusceptible to the third-generation cephalosporins but susceptible to the carbapenems, likely due to the presence of extended-spectrum β-lactamase (ESBL) variants of the blaPER, blaSHV, blaVEB, and blaCTX-M genes or overexpression of the intrinsic blaADC gene. A further 34 strains were resistant to the third- and fourth-generation cephalosporins and the carbapenems, and 31 of these strains carried known carbapenemases, including the class D oxacillinases blaOXA-23 (n = 22), blaOXA-40-like (n = 3), and blaOXA-253 (n = 1) (33) and the class B metallo-β-lactamases blaNDM (n = 4) and blaIMP (n = 1). The remaining three carbapenem-resistant strains had ISAba1 inserted directly upstream of their blaOXA-51-like genes, which has previously been shown to confer carbapenem resistance (34). Notably, MRSN 16880 carried the class D carbapenemase gene blaOXA-58 but was susceptible to all of the carbapenems tested, reflecting the ambiguous carbapenem sensitivity observed in strains carrying this gene (35, 36).
Sixty-nine strains were nonsusceptible to ≥1 aminoglycoside tested, with 35 strains resistant to all 3. Five genetically distinct strains carried the 16S rRNA methyltransferase gene armA, which confers high-level resistance to all clinically relevant aminoglycosides (37). The remaining 30 isolates carried a wide assortment of aminoglycoside modifying enzymes (AMEs), with 19 different AME genes detected (Table S2).
Finally, A. baumannii MRSN 17493 was the only PDR strain, as it was the only colistin-resistant strain in the final panel. Genetic analysis of the pmrCAB locus in this strain identified a guanine-to-adenine mutation in pmrB at position 788 (G788A) that results in the substitution of an arginine for a histidine at amino acid 263 (R263H) in the PmrB kinase that has previously been reported as the only mutation present in a colistin-resistant A. baumannii (38).
DISCUSSION
In an effort to prioritize research and development strategies for new antibiotics, the WHO recently assigned 12 bacterial families that pose the greatest threat to human health into critical, high, and moderate categories (30). Within the critical category, carbapenem-resistant A. baumannii (CRAB) features prominently, reflecting the near-complete absence of therapeutic options in many infections caused by these organisms (39). This categorization has spurred increased interest in developing new therapeutics, and several promising drug candidates, as well as alternate therapeutics, such as lytic phages and monoclonal antibodies, are currently being investigated (40–42).
A critical component in these efforts is access to a comprehensive strain collection to test potential candidates. Unfortunately, it is rare that the precise list of isolates used for testing are known, and even when efforts are made to describe the tested strains, specific names and characterized genetic traits are institute specific or inconsistent, and genomes are rarely available. Furthermore, it is not uncommon for candidate therapeutics to be tested against isolates selected more for convenience (i.e., sourced from local hospitals or based solely on their resistance profiles) than for solid scientific rationale. For example, in a recent study by Isler and colleagues, 3,653 CRAB strains were used across 12 different studies to evaluate the activity of cefiderocol (43). As a result, there is very little consistency in what strains are used for testing, making comparisons across different drug candidates difficult and complicating efforts to reproduce results. This problem has long been recognized, and the 2015 U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria attempted to address this issue by mandating the creation of bacterial repositories to aid “…companies that develop new antibiotics and therapeutics and/or design next-generation tests, diagnostic test developers and regulatory agencies who evaluate these tests, government facilities, academic labs, and pharmaceutical companies that test antibiotics for clinical effectiveness and researchers, regulators, and others who assess the effectiveness of interventions to prevent resistance” (44).
Over the past 3 years, concerted efforts have been made by U.S. federal agencies to develop these repositories. In particular, the Centers for Disease Control and Prevention together with the Food and Drug Administration launched the CDC-FDA Antibiotic Resistance Isolate Bank (https://www.cdc.gov/drugresistance/resistance-bank/index.html). This resource currently consists of 28 distinct panels containing 914 strains representing the major bacterial and fungal pathogenic species. The CDC-FDA A. baumannii panel is composed of 41 strains chosen “to represent a diversity of antimicrobial susceptibility results for drugs that are used to treat infections” (45). Antibiotic susceptibility test results, whole-genome sequencing data, and a list of known AMR genes were provided for the 41 strains. Although this panel is an excellent resource for the scientific community, it has some drawbacks that limit its usefulness for drug testing. For example, the strains lack any metallo-β-lactamase (class B) carbapenemases, and no strain is resistant to colistin. Furthermore, just 7 STs are represented across the 41 genomes, and 36 of the 41 strains belong to either ST-2 or ST-79. This narrow strain set does not represent the true clinical diversity of circulating A. baumannii and does not capture emerging epidemic clones. In 2019, a second Acinetobacter panel was also created by D’Souza and colleagues, but it contains just 8 A. baumannii strains, 3 Acinetobacter pittii strains, and 1 strain of Acinetobacter nosocomialis collected from a single hospital (46). Although the isolates had diverse antimicrobial resistance profiles, none of the eight A. baumannii strains belongs to a successful epidemic clone.
In contrast to other panels, active surveillance and collection of MDR bacteria over the past 10 years provided the MRSN with the opportunity to develop a panel of A. baumannii from clinical infections that best represents the genetic diversity of this species. The panel contains 100 strains selected through rigorous phylogenetic analyses to maximize genetic diversity in both the core- and pangenomes. Previous studies have emphasized the high genomic plasticity of A. baumannii and the role it plays in hospital adaptation and pathogenicity (9, 11, 12, 47). This work, and more recent studies analyzing the diversity of thousands of genomes, estimate that the core genome of A. baumannii is comprised of ∼2,200 genes, while its pangenome is projected to be in the region of 19 ,000 distinct genes (48). The fact that comparable numbers are observed with just the 100 isolates in this panel is a testament to the diversity of the MRSN repository. An additional benefit of this panel is the diverse range of susceptibilities to the 14 antibiotics tested, including 1 strain that is panresistant. This is also reflected in the AMR gene content where 100 distinct antibiotic resistance alleles are represented in the panel. Finally, the panel contains extensive diversity in both the capsular polysaccharide (CPS) and outer core biosynthesis loci, as reflected in the 73 different KL/OCL combinations present, making it an attractive resource for phage and virulence testing, particularly in light of the renewed interest in phage therapy for CRAB (43).
In summary, we have leveraged the resources of the MRSN (College of American Pathologists [CAP]-accredited laboratory, core sequencing facility, and unique isolate collection) to develop a panel of 100 A. baumannii strains that encompass (i) the genetic diversity of the species; (ii) both antibiotic-susceptible and -resistant isolates (n = 38 and 62, respectively); and (iii) pandemic, epidemic (isolates from all six identified international clonal lineages [49, 50] are represented), and sporadic clones. We envisage that easy access to both the strains and high-quality draft genomes will foster and facilitate multiple avenues of research into this important human pathogen.
MATERIALS AND METHODS
A. baumannii repository.
The MRSN is the sole entity within the United States Department of Defense (DoD) engaged in real-time surveillance of clinically relevant multidrug-resistant (MDR) organisms across the Military Healthcare System (51). In addition, the MRSN performs similar surveillance across the world in collaboration with Global Emerging Infections Surveillance (GEIS)-affiliated laboratories. Samples are housed in a central repository, which currently contains over 72,000 isolates. Of these, 3,505 are A. baumannii isolates that were cultured from 2,533 patients between 2001 and 2017 (see Fig. S1 in the supplemental material). Approximately half of them were cultured from soldiers injured during Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF), where this pathogen emerged as one of the most highly prevalent infectious agents in wound and blast injuries (52). The remaining isolates vary in their collection location from South and Central America, Europe, Asia, and Africa. Isolates have been cultured from a wide diversity of clinical samples, including blood (9.3%), fluid (1.1%), respiratory (17.7%), surveillance swab (10.6%), tissue (4.7%), wound (34%), urine (3.9%), and unknown (18.7%) specimens.
Antibiotic susceptibility testing.
Antibiotic susceptibility testing (AST) was performed in the MRSN College of American Pathologists (CAP)-accredited clinical lab using three commercial platforms, namely, the Phoenix (panel NMIC/ID304; BD Diagnostics, NJ, USA), the Vitek 2 (card GN AST 71 and GN ID; bioMérieux, NC, USA), and the MicroScan Walkaway (panel NBC47; Beckman Coulter, MD, USA). The final susceptibility profile for each isolate represents the adjudicated value from all three instruments. When discrepancies were noted between instruments, the majority interpretation from the three instruments was used. MICs of colistin were determined in triplicate using broth microdilution (BMD) using Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI 2019).
Whole-genome sequencing.
Isolates were sequenced on a MiSeq or NextSeq benchtop sequencer (Illumina, Inc., San Diego, CA). DNA was extracted using the DNeasy UltraClean microbial kit (Qiagen, Germantown, MD, USA), and libraries were constructed using the Kapa HyperPlus library preparation kit (Roche Diagnostics, Indianapolis, IN, USA). Libraries were quantified using the Kapa library quantification kit Illumina/Bio-Rad iCycler (Roche Diagnostics) on a CFX96 real-time cycler (Bio-Rad, Hercules, CA, USA). For the MiSeq, libraries were normalized to 2 nM, pooled, denatured, and diluted to 20 pM. The pooled samples were further diluted to a final concentration of 14 pM. Samples were sequenced using MiSeq reagent kit v3 (600 cycles; 2 × 300 bp) (Illumina). For the NextSeq, libraries were normalized to 1 nM, pooled, denatured, and diluted to 20 pM. The pooled samples were further diluted to a final concentration of 2.1 pM. Samples were sequenced using NextSeq reagent kit 500/550 v2 (300 cycles; 2 × 150 bp) (Illumina).
Analysis of whole-genome sequencing data.
Kraken2 (53) was used to identify isolate species and check for contamination. Short-read sequencing data were trimmed for adapter sequence content and quality using Btrim64 (54). Overlapping sequence reads were merged using FLASH (55). De novo assembly was performed using Newbler (v2.7). Minimum thresholds for contig size and coverage were set at 200 bp and 49.5×, respectively. In silico multilocus sequence typing (MLST) was performed using the scheme developed by Diancourt and colleagues (Pasteur scheme) (6), which also ensured non-A. baumannii species were excluded from the analysis. Antimicrobial resistance genes were annotated using a combination of Resfinder (56), AMRFinderPlus (57), and ARIBA (58). Capsular polysaccharide K locus (KL) and outer core locus (OCL) types were determined with Kaptive using the database curated by Wyres and colleagues (31). Basic assembly statistics are available (see Table S1 in the supplemental material).
cgMLST analysis.
The draft genomes of all 3,505 A. baumannii isolates were uploaded and analyzed using SeqSphere+ software (Ridom, Germany) using the A. baumannii cgMLST scheme developed by Higgins and colleagues (33). To be included in the analysis, isolates had to contain 90% of the 2,390 genes included in the cgMLST scheme. The resulting minimum spanning tree (MST) was then used to select 226 strains that captured the diversity of the strain collection.
Core-genome SNP and accessory genome analysis.
PanSeq (59) was run with a fragmentation size of 500 bp to find sequences with ≥95% identity in ≥95% of the isolates to generate the core-genome single nucleotide polymorphism (SNP) alignment and accessory genome binary alignment for the selected diversity set of 226 isolates. RAxML (v8.2.11) (60) was used to generate the phylogenetic tree for both the core SNP alignment and the accessory genome. The SNP-based phylogeny was built from a 177-kb variable position alignment found within a 2.2-Mb core-genome alignment using the general time reversible (GTR) GAMMA model and the rapid bootstrapping option for nucleotide sequences, using 100 replicates. The accessory genome dendrogram was based on a presence/absence binary matrix with 26,356 entries using a binary GAMMA model and the rapid bootstrapping option with 100 replicates. Using this approach, 100 strains were selected to represent the final diversity panel.
The final diversity set of 100 isolates was resequenced and reanalyzed to confirm purity. Reads were checked for contamination at the species level using Kraken2 (v2.0.8-beta) (53) and at the strain level using ConFindr (v0.4.8) (61) with parameters bf = 0.05 and q = 30. ConFindr parameters were verified for A. baumannii using 5 isolates that were deemed not contaminated by ConFindr using the default settings. For this verification set, paired reads were randomly selected using seqtk (https://github.com/lh3/seqtk) to a normalized read total of 3 million reads. These isolates were then contaminated in silico with another isolate from a different ribosomal MLST (rMLST) scheme at 0% to 10%, 15%, 20%, 25%, and 50% of the reads. Those isolates with greater than 1% of their reads belonging to a different species as identified by Kraken2, or those with 3 or more single nucleotide variants (SNVs) as identified by ConFindr, were further purified in the laboratory and resequenced. The purity-verified sequences were compared with the original sequences by phylogeny to ensure identity. A pangenome and accessory genome alignment was made using PanSeq, with ≥95% identity in all of the isolates and a fragmentation size of 500 bp. Trees were generated with RAxML as described above.
Genome annotations.
A phylogenetic tree of the 100 isolates was constructed with PanSeq and RAxML as described above except that the core-genome determination was performed with ≥95% identity in all 100 isolates. For these phylogenetic analyses, the core-genome alignment was 1.4 Mb long and contained 93-kb variable positions, while the accessory binary alignment had 23,395 entries. For all 100 isolates included in the panel, genome annotations were performed using NCBI Prokaryotic Genome Annotation Pipeline (v4.8). Core and pangenomes were calculated with Roary (v3.12.0) (62) based on GenBank annotations.
Data availability. The diversity panel will be available for research purposes from BEI Resources. The sequences of the 100 isolates in the diversity panel have been posted to GenBank (BioProject PRJNA545079).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the participation of clinical laboratories across the Military Healthcare System, GEIS-affiliated overseas laboratories, and numerous collaborators for their contributions of bacteria to the MRSN Acinetobacter baumannii repository.
This study was funded by the U.S. Army Medical Command and the Defense Medical Research and Development Program. The manuscript has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official, or reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 3.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez MS, Bonomo RA, Tolmasky ME. 2020. Carbapenemases: transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules 10:720. doi: 10.3390/biom10050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Rafailidis PI. 2007. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care 11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamidian M, Nigro SJ. 2019. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt K, Kenyon JJ, Hamidian M, Schultz MB, Pickard DJ, Dougan G, Hall R. 2016. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb Genom 2:e000052. doi: 10.1099/mgen.0.000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, MacDonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia DN, Elbourne LD, Hassan KA, Eijkelkamp BA, Tetu SG, Brown MH, Shah BS, Peleg AY, Mabbutt BC, Paulsen IT. 2013. The complete genome and phenome of a community-acquired Acinetobacter baumannii. PLoS One 8:e58628. doi: 10.1371/journal.pone.0058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imperi F, Antunes LC, Blom J, Villa L, Iacono M, Visca P, Carattoli A. 2011. The genomics of Acinetobacter baumannii: insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 63:1068–1074. doi: 10.1002/iub.531. [DOI] [PubMed] [Google Scholar]
- 12.Sahl JWS, Johnson JK, Harris AD, Phillippy AM, Hsiao WW, Thom KA, Rasko DA. 2011. Genomic comparison of multi-drug resistant invasive and colonizing Acinetobacter baumannii isolated from diverse human body sites reveals genomic plasticity. BMC Genomics 12:291. doi: 10.1186/1471-2164-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva KE, Maciel WG, Croda J, Cayo R, Ramos AC, de Sales RO, Kurihara MNL, Vasconcelos NG, Gales AC, Simionatto S. 2018. A high mortality rate associated with multidrug-resistant Acinetobacter baumannii ST79 and ST25 carrying OXA-23 in a Brazilian intensive care unit. PLoS One 13:e0209367. doi: 10.1371/journal.pone.0209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. 2011. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin Microbiol Infect 17:197–201. doi: 10.1111/j.1469-0691.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 15.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48:3298–3304. doi: 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon E-J, Courvalin P, Grillot-Courvalin C. 2013. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother 57:2989–2995. doi: 10.1128/AAC.02556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGann P, Courvalin P, Snesrud E, Clifford RJ, Yoon EJ, Onmus-Leone F, Ong AC, Kwak YI, Grillot-Courvalin C, Lesho E, Waterman PE. 2014. Amplification of aminoglycoside resistance gene aphA1 in Acinetobacter baumannii results in tobramycin therapy failure. mBio 5:e00915. doi: 10.1128/mBio.00915-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel L, Bonnin RA, Nordmann P. 2011. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life 63:1061–1067. doi: 10.1002/iub.532. [DOI] [PubMed] [Google Scholar]
- 20.Butler DA, Biagi M, Tan X, Qasmieh S, Bulman ZP, Wenzler E. 2019. Multidrug resistant Acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep 21:46. doi: 10.1007/s11908-019-0706-5. [DOI] [PubMed] [Google Scholar]
- 21.Evans BA, Hamouda A, Amyes SG. 2013. The rise of carbapenem-resistant Acinetobacter baumannii. Curr Pharm Des 19:223–238. doi: 10.2174/138161213804070285. [DOI] [PubMed] [Google Scholar]
- 22.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 23.Oikonomou O, Sarrou S, Papagiannitsis CC, Georgiadou S, Mantzarlis K, Zakynthinos E, Dalekos GN, Petinaki E. 2015. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: mechanisms of resistance, molecular identification and epidemiological data. BMC Infect Dis 15:559. doi: 10.1186/s12879-015-1297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogue JM, Cohen DA, Marchaim D. 2015. Editorial commentary: polymyxin-resistant Acinetobacter baumannii: urgent action needed. Clin Infect Dis 60:1304–1307. doi: 10.1093/cid/civ044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conly J, Johnston B. 2005. Where are all the new antibiotics? The new antibiotic paradox. Can J Infect Dis Med Microbiol 16:159–160. doi: 10.1155/2005/892058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver LL. 2011. Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 2019. Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 28.Theuretzbacher U, Bush K, Harbarth S, Paul M, Rex JH, Tacconelli E, Thwaites GE. 2020. Critical analysis of antibacterial agents in clinical development. Nat Rev Microbiol 18:286–298. doi: 10.1038/s41579-020-0340-0. [DOI] [PubMed] [Google Scholar]
- 29.Theuretzbacher U, Outterson K, Engel A, Karlen A. 2020. The global preclinical antibacterial pipeline. Nat Rev Microbiol 18:275–285. doi: 10.1038/s41579-019-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 2017. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 31.Wyres KL, Cahill SM, Holt KE, Hall RM, Kenyon JJ. 2020. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom 6:e000339. doi: 10.1099/mgen.0.000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 33.Higgins PG, Prior K, Harmsen D, Seifert H. 2017. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS One 12:e0179228. doi: 10.1371/journal.pone.0179228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 35.Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, Nordmann P. 2005. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 49:202–208. doi: 10.1128/AAC.49.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma V, Testero SA, Amini K, Wei W, Liu J, Balachandran N, Monoharan T, Stynes S, Kotra LP, Golemi-Kotra D. 2011. Hydrolytic mechanism of OXA-58 enzyme, a carbapenem-hydrolyzing class D beta-lactamase from Acinetobacter baumannii. J Biol Chem 286:37292–37303. doi: 10.1074/jbc.M111.280115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi Y, Wachino J-I, Arakawa Y. 2016. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi HJ, Kil MC, Choi JY, Kim SJ, Park KS, Kim YJ, Ko KS. 2017. Characterisation of successive Acinetobacter baumannii isolates from a deceased haemophagocytic lymphohistiocytosis patient. Int J Antimicrob Agents 49:102–106. doi: 10.1016/j.ijantimicag.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Pogue JM, Mann T, Barber KE, Kaye KS. 2013. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti Infect Ther 11:383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 40.Babb R, Pirofski LA. 2017. Help is on the way: monoclonal antibody therapy for multi-drug resistant bacteria. Virulence 8:1055–1058. doi: 10.1080/21505594.2017.1306620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Z, Lin H, Ji X, Yan G, Lei L, Han W, Gu J, Huang J. 2020. Therapeutic applications of lytic phages in human medicine. Microb Pathog 142:104048. doi: 10.1016/j.micpath.2020.104048. [DOI] [PubMed] [Google Scholar]
- 42.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 43.Isler B, Doi Y, Bonomo RA, Paterson DL. 2018. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 63:e01110-18. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The White House. 2015. National action plan for combating antibiotic-resistant bacteria. The White House, Washington, DC. [Google Scholar]
- 45.Lutgring JD, Machado MJ, Benahmed FH, Conville P, Shawar RM, Patel J, Brown AC. 2018. FDA-CDC antimicrobial resistance isolate bank: a publicly available resource to support research, development, and regulatory requirements. J Clin Microbiol 56:e01415-17. doi: 10.1128/JCM.01415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Souza R, Pinto NA, Phuong NL, Higgins PG, Vu TN, Byun JH, Cho YL, Choi JR, Yong D. 2019. Phenotypic and genotypic characterization of Acinetobacter spp. panel strains: a cornerstone to facilitate antimicrobial development. Front Microbiol 10:559. doi: 10.3389/fmicb.2019.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan AP, Sutton G, DePew J, Krishnakumar R, Choi Y, Huang XZ, Beck E, Harkins DM, Kim M, Lesho EP, Nikolich MP, Fouts DE. 2015. A novel method of consensus pan-chromosome assembly and large-scale comparative analysis reveal the highly flexible pan-genome of Acinetobacter baumannii. Genome Biol 16:143. doi: 10.1186/s13059-015-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangas EL, Rubio A, Alvarez-Marin R, Labrador-Herrera G, Pachon J, Pachon-Ibanez ME, Divina F, Perez-Pulido AJ. 2019. Pangenome of Acinetobacter baumannii uncovers two groups of genomes, one of them with genes involved in CRISPR/Cas defence systems associated with the absence of plasmids and exclusive genes for biofilm formation. Microb Genom 5:e000309. doi: 10.1099/mgen.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antunes LC, Visca P, Towner KJ. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 50.Karah N, Sundsfjord A, Towner K, Samuelsen O. 2012. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Updat 15:237–247. doi: 10.1016/j.drup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Waterman P, Kwak Y, Clifford R, Julius M, Onmus-Leone F, Tsurgeon C, Riley M, Black C, McGann P, Lesho E. 2012. A multidrug-resistance surveillance network: 1 year on. Lancet Infect Dis 12:587–588. doi: 10.1016/S1473-3099(12)70149-4. [DOI] [PubMed] [Google Scholar]
- 52.Petersen K, Riddle MS, Danko JR, Blazes DL, Hayden R, Tasker SA, Dunne JR. 2007. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 245:803–811. doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. Genome Biol 20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong Y. 2011. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98:152–153. doi: 10.1016/j.ygeno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu CH, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. 2019. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother 63:e00483-19. doi: 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunt M, Mather AE, Sanchez-Buso L, Page AJ, Parkhill J, Keane JA, Harris SR. 2017. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom 3:e000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laing C, Buchanan C, Taboada EN, Zhang Y, Kropinski A, Villegas A, Thomas JE, Gannon VP. 2010. Pan-genome sequence analysis using Panseq: an online tool for the rapid analysis of core and accessory genomic regions. BMC Bioinformatics 11:461. doi: 10.1186/1471-2105-11-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low AJ, Koziol AG, Manninger PA, Blais B, Carrillo CD. 2019. ConFindr: rapid detection of intraspecies and cross-species contamination in bacterial whole-genome sequence data. PeerJ 7:e6995. doi: 10.7717/peerj.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.