Abstract
Nicotinamide riboside (NR) is a vitamin B3 precursor of NAD+ that blunts diabetic and chemotherapy-induced peripheral neuropathy in preclinical models. This study examined whether NR also blunts the loss of intraepidermal nerve fibers (IENF) induced by paclitaxel, which is associated with peripheral neuropathy. The work was conducted in female rats with N-methyl-nitrosourea (MNU)-induced tumors of the mammary gland to increase its translational relevance, and to assess the interaction of NR with paclitaxel and NR’s effect on tumor growth. Once daily oral administration of 200 mg/kg NR p.o. beginning with the first of three i.v. injections of 6.6 mg/kg paclitaxel to tumor-bearing rats significantly decreased paclitaxel-induced hypersensitivity to tactile and cool stimuli, as well as place-escape avoidance behaviors. It also blunted the loss of IENF in tumor-bearing rats, as well as a separate cohort of tumor-naïve rats. Unexpectedly, concomitant administration of NR during paclitaxel treatment further decreased tumor growth; thereafter, tumor growth resumed at the same rate as vehicle-treated controls. Administration of NR also decreased the percentage of Ki67-positive tumor cells in these rats. Once daily administration of NR did not appear to alter tumor growth or the percentage of Ki67-positive tumor cells in rats that were not treated with paclitaxel and followed for three months. These results further support the ability of NR to play a protective role following nerve injury. They also suggest that NR may not only alleviate peripheral neuropathy in patients receiving taxane chemotherapy, but also offer an added benefit by possibly enhancing its tumor-suppressing effects.
In tumor-bearing rats, nicotinamide riboside blunts the allodynia and loss of intraepidermal nerve fibers induced by paclitaxel, and unexpectedly enhances paclitaxel’s suppression of tumor growth.
INTRODUCTION
The relief of chemotherapy-induced peripheral neuropathy (CIPN) remains a significant unmet need in healthcare [28] with nearly 65% of survivors reporting neuropathies in the month after the end of therapy [42; 44]. The paucity of effective treatments is not due to a lack of research effort, as much as it reflects a ‘failure’ of preclinical findings to translate into efficacious treatments in the clinic [23]. Contributing factors may include (1) animal models in which the dose, frequency and route of chemotherapeutic agent do not mirror that in the clinic, (2) dependent measures whose application varies among laboratories, (3) measures that cannot replicate those made in the clinic [46], and (4) overestimation of effect size [9; 14; 20]. Interestingly, the use of tumor-naïve animals is rarely mentioned as a possible disconnect. The argument is made that many patients receive chemotherapy after removal of the tumor [19]. However, surgery does not guarantee complete removal, many patients receive chemotherapy prior to surgery, and some tumors are not resectable.
Evidence continues to accrue that cancer cells and nerve fibers interact in the tumor microenvironment in a feed-forward manner with each releasing substances that can promote the growth or infiltration of the other [1; 3; 13; 32]. Further, cancer cells and associated stromal cells release substances that sensitize or activate sensory afferents [38]. This interaction likely extends beyond the microenvironment given that tumors secrete extracellular vesicles [39], cytokines, and chemokines, as well as cells into the circulation [54]. Other issues that cannot be assessed in tumor-naïve animals include determination that the drug of interest does not interfere with the efficacy of the chemotherapeutic agent or itself promote tumorigenesis.
We recently reported that nicotinamide riboside (NR), a vitamin B3 precursor of NAD+, suppresses the tactile hypersensitivity and aversive dimensions of paclitaxel-induced peripheral neuropathy in tumor-naïve rats [26]. Although its mechanism is unclear, NR-induced increases in NAD+ may rectify one or more of the many candidate mechanisms implicated in CIPN [18; 37; 52; 58]. Of these, mitochondrial dysfunction or peripheral nerve degeneration may be most relevant. For example, NR delays mitochondrial myopathy in a mouse model of mitochondrial respiratory chain disease [30]. NR protects against noise-induced hearing loss and death of spiral ganglion neurons in mice [8], and as well as deficits in nerve conduction, heat hypoalgesia, and loss of corneal and intraepidermal nerve fibers (IENF) in diabetic or prediabetic mice [51]. The principal goals of this study were to re-evaluate the ability of NR to relieve CIPN in tumor-bearing rats, and to determine whether it could prevent the loss of IENF. Secondary goals were to establish that NR neither interfered with paclitaxel’s suppression of tumor growth nor facilitated tumor growth. The studies were conducted in female rats in which tumors of the mammary gland were induced by administration of the environmental carcinogen, 1-methyl-1-nitrosourea (MNU) [50]. Its advantages include de novo tumor growth, recapitulation of the multi-stage development of cancer, organ specificity, predictive responsiveness to therapeutic and chemo-preventive agents, and its autochthonous nature [2; 17; 22].
METHODS AND MATERIALS
Animals and Models
These studies were approved by the University of Iowa Animal Care and Use Committee (protocols # 4101188 and 7091188) and were conducted in accordance with guidelines of the National Institutes of Health and the International Association for the Study of Pain. For studies in which MNU was administered, female Sprague Dawley rats at 14 days of age with dams (Charles River Laboratories, Raleigh, NC; Crl:SD) were purchased. For studies using naïve rats, female Sprague-Dawley rats weighing 150–175 g were purchased from the same supplier. Estrous cycle was not monitored in line with recommendations of a workgroup of the International Association for the Study of Pain [24] and because vaginal lavage was a stressor to be avoided [50; see below]. Rats were housed on a 12:12 hr light/dark cycle with ab libitum access to food and water. Table 1 lists the number of rats in each experiment, as well as reasons for exclusion or loss to study.
Table 1.
Allocation and Disposition of Subjects
| Test | Treatment | No. Entered | No. Used | No. Excluded and Reason |
|---|---|---|---|---|
| Von Frey and Colda | Vehicle | 15 | 10 | 3 euthanized for tumor load before behavior was complete; 2 died after paclitaxel injection |
| NR | 12 | 12 | ||
| PEAPa | Vehicle | 15 | 8 | As above, plus 2 euthanized for tumor load before PEAP could be conducted |
| NR | 12 | 12 | ||
| Locomotor Activitya,b | Tumor Only | 8 | 8 | |
| Tumor + Pac/Vehicle | 6 | 6 | ||
| Tumor + Pac/NR | 9 | 7 | 2 excluded due to freezing behavior | |
| Naïve | 8 | 8 | ||
| Naïve i.v. PAC | 6 | 6 | ||
| Naïve i.v. KES | 6 | 6 | ||
| NORc | Naïve | 8 | 7 | 1 rat did not fulfill test criteria |
| MNU+PAC | 7 | 5 | 2 rats did not meet test criteria | |
| MNU+KES | 6 | 6 | ||
| MNU only (no tumor) | 7 | 6 | 1 rat did not fulfill test criteria | |
| Natural Progressiond | Vehicle | 14 | 12 | 2 excluded; 1 for keratin cyst and 1 for benign tumor |
| NR | 14 | 14 | ||
| PAC Treatment of Tumorse | Vehicle | 16 | 13 | 1 excluded; very large tumor at start of study; 2 died after paclitaxel injection |
| NR | 12 | 12 | ||
| IENF in Tumor Naivef | Naïve | 5 | 5 | |
| PAC + Vehicle | 5 | 5 | ||
| PAC + NR | 5 | 5 | ||
| IENF in Tumor Bearingg | Naïve | 7 | 7 | |
| PAC + Vehicle | 7 | 7 | ||
| PAC + NR | 7 | 7 | ||
Common superscripts indicate the same cohorts of rats were used for these studies. For the studies of Ki67 expression, tumors were randomly chosen from rats in the treatment groups in the natural progression and the PAC + tumor growth experiments.
To induce tumors, rats were injected with 50 mg/kg i.p. MNU (MRIGlobal, Kansas City, MO) at 21 days of age and weaned at 25 days of age. MNU was dissolved in saline in small quantities and used immediately because it is unstable in solution. As noted [50], tumor development was variable and was influenced by environmental stressors. Rats were housed in isolation bays or in small rooms that were not shared with many other investigators to minimize foot-traffic. They were undisturbed in the vivarium until day 42 when tumor development began to be monitored. The thoracic and abdominal mammary chains were palpated twice weekly while the rat was lightly sedated with isoflurane. Tumor number and location were recorded. Tumor size (length, wide and height) was measured using calipers, and volume was estimated by the formula of (4/3)*3.141516*length*width*height. Euthanasia was mandated when a tumor exceeded 3 cm in one dimension or threatened to necrotize through the skin. Excised tumors were routinely processed, embedded in paraffin, sectioned at 4 μm and subsequently stained with hematoxylin and eosin for characterization of tumor pathology.
To induce CIPN, rats were lightly anesthetized with isoflurane for each of three i.v. injections of 6.6 mg/kg paclitaxel (Lot E046865AA, Hospira, Inc. Lake Forest, IL) given over five days [26]. Control rats received i.v. injections of the Kolliphor:ethanol:saline vehicle (KES) for paclitaxel. Rats were monitored daily.
Experimental Design for Behavioral Studies
The ability of NR to suppress paclitaxel-induced hypersensitivity to tactile and cooling stimuli, as well as place-escape avoidance behaviors was assessed in tumor-bearing rats. Paclitaxel treatment was initiated within three days of tumor appearance. The median time to onset of tumors was 55 days in both vehicle- and NR-treated groups (range: 43 to 74 days). After assessing baseline paw withdrawal threshold to tactile and cooling stimuli, the rats received the first injection of paclitaxel and were randomized to receive 200 mg/kg NR or vehicle by gavage once daily for the remaining 28 days of the study or until tumor burden necessitated euthanasia. Thresholds to tactile stimuli were redetermined at 14 and 21 days, while thresholds to cooling stimuli were tested at 15 and 22 days (Fig. 1A). Place escape avoidance behaviors were assessed on day 24. Locomotor activity was additionally measured on day 28 in the second and third replicates of this experiment. At study end, the rats were euthanized by CO2 and tumors excised for pathology. Rats were assigned a new temporary identifier on each test occasion to keep experimenters blinded to treatment condition.
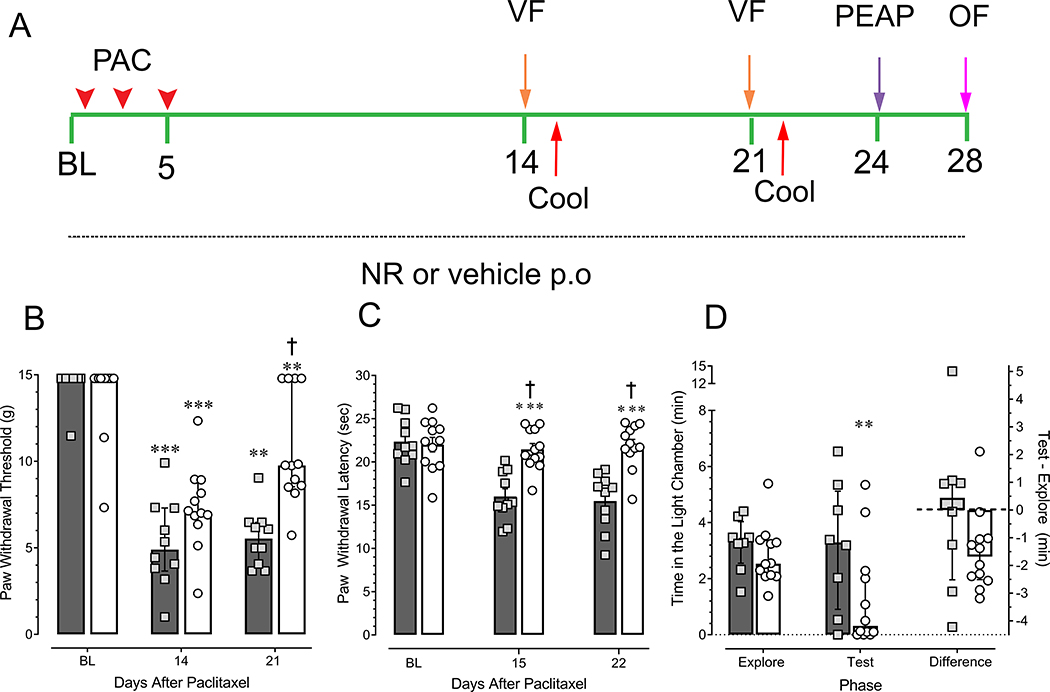
Figure 1.
Concomitant daily treatment of tumor-bearing female rats with NR blunts paclitaxel-induced hypersensitivity to both tactile and cool stimuli, as well as place escape avoidance behaviors. (A) schema of experimental design. OF: open field. Mammary gland tumors were induced by administration of 50 mg/kg i.p. MNU at 21 days of age. Upon appearance of tumors rats received the first of three injections of 6.6 mg/kg i.v. paclitaxel (PAC, arrowheads) and treatment with either 200 mg/kg p.o. NR or vehicle began. Numbers are the days from the start of the experiment (baseline, BL). (B)Tactile hypersensitivity, median with interquartile range. (C) Cool hypersensitivity, mean ± SEM. (D) Place escape avoidance behavior, median with interquartile range. In all panels, shaded bars and squares depict vehicle-treated rats whereas open bars and circles depict NR-treated rats. NR-treated group N = 12 for all panels. Vehicle-treated group N is 10, 10, and 8 in panels B, C, and D, respectively. **P < 0.01; ***P < 0.001 compared to baseline values for that group. † P < 0.001 compared to vehicle-treated rats at the corresponding time point.
Measures of Behavior
Tactile hypersensitivity
Rats were acclimated to the behavior testing room for 30 min and to the testing chambers for an additional 15 min. Withdrawal threshold to tactile stimulation of the hind paw was determined using the Up-and-Down method as previously described [4]. Given the smaller size of the rats at study outset, a different set of filaments was used (1, 1.4, 2, 4, 6, 8, 10, and 15 g); testing began with the 2 g filament. The highest filament passively lifted the hind paw of rats of this weight. Rats that did not respond to the 15 g filament were assigned this value. The threshold of each paw was averaged to yield a single value for the rat. Paw withdrawal threshold values are non-parametric and were not normally distributed. Therefore, the Friedman repeated measure analysis on ranks test was used for within treatment group comparisons over time, and a Mann-Whitney test was used for between treatment comparisons at each time point. A P < 0.05 or less was accepted for this and all other statistical analyses.
Cold Hypersensitivity
Rats were acclimated to the behavior testing room for 30 min and to the testing chambers for an additional 15 min. To determine cold hypersensitivity, rats were placed on an elevated glass surface. A 3-cc syringe, from which the luer tip had been removed, was packed with pulverized dry ice and applied under the glass surface on which the hind paw rested [7]. The surface area of the ice was 78 mm2 and it cooled the glass to 18°C within 25 sec. If a withdrawal response did not occur within 30 s, the test ended and the rat was assigned this latency. The time to withdrawal of the hind paw was recorded for each paw and averaged to generate a single value for that rat. Data were analyzed by two-way repeated measures ANOVA in which treatment was one factor and time was the repeated factor. Holm-Sidak’s test was used for post-hoc comparisons among group means.
Modified place escape avoidance paradigm
The affective/aversive nature of paclitaxel-induced peripheral neuropathy was assessed with a modification of the place escape avoidance paradigm (PEAP) [26]. After a 15 min period during which the rats were free to explore the brightly-lit chamber and the dark chamber (explore phase), a 15 min test phase began during which the plantar surface of each hind paw was repetitively stimulated with a 10 g von Frey filament each time that the rat entered the dark chamber. Stimuli were delivered at 15 sec intervals to alternate hind paws until the rat exited the dark chamber for the brightly lit chamber. Stimulation of the hind paws ceased when the rat entered the brightly lit chamber. The chambers were washed thoroughly between rats to minimize any olfactory cues. The amount of time spent in each chamber was recorded. Data were expressed as median and interquartile range. The Mann-Whitney test was used for between treatment group comparisons and the Wilcoxon Rank Sum Test was used for within treatment group comparisons. Differences scores were compared by a Student’s t-test.
Locomotor Activity
Locomotor activity in an open field [26] was assessed after completion of the von Frey, cold, and PEAP tests in tumor-bearing rats being treated with vehicle or NR. In addition, a cohort of naïve rats and a cohort of untreated tumor-bearing rats were also tested; these latter two groups did not undergo von Frey, cold, or PEAP testing. Rats were placed in a 40 cm by 40 cm open field on which a 3 × 3 grid was superimposed by the software program. Total distance traveled and number of grid crossing during two 15 min epochs were remotely tracked and analyzed off-line using Limelight2 software data offline (Colbourn Instruments; Holliston, MA). The field was cleaned between each rat. Total distance traveled and number of grid crossings during two 15 min epochs were remotely tracked and analyzed off-line. The data were analyzed by two-way repeated measures ANOVA in which treatment was one factor and time was the repeated factor followed by Holm-Sidak’s test.
Novel Object Recognition (NOR)
Rats were acclimated to the room environment and empty testing chambers for three days. On the fourth day, after the acclimation phase, rats were transferred to the 40 cm x 40 cm testing chamber in which two identical objects were situated each 4 cm in from each side wall and 20 cm from the back wall. The objects were either Dr. Wacko’s Mad Lab Green Goo or amber glass wide mouth bottles (100 ml volume). Preliminary trials indicated the rats did not prefer one of these objects over the other but could readily distinguish the two objects. The choice of object for the sampling period was counterbalanced. Rats were permitted to sample the chamber and objects for five min and then returned to their home cage. Ninety min later, the rat was returned to the same chamber in which one of the objects had been switched out for the other object and permitted to explore the novel object for three min. Exploratory activity was videotaped and analyzed offline. Exploring was defined as a rat having its nose oriented toward and within 2 cm of the object, and exhibiting biting, scratching, or sniffing behavior. Rats that did not explore both objects (spending a minimum of 2 sec on a single object) for at least 20 sec during the sampling phase or 15 sec during the test phase were excluded. Those occasions when the rat placed its paws on the object with its head and nose oriented away from or above the object were excluded from analysis. Data were expressed as a discrimination index [(time spent exploring novel object- time spent exploring familiar object)/(time spent exploring familiar object+ time spent exploring novel object)]. Data were analyzed by one-way analysis of variance.
Experimental Design for Studies of IENF
The effect of NR treatment on loss of IENF induced by paclitaxel was examined in a separate cohort of tumor-bearing rats, as well as in adult tumor naïve rats. Tumor-naïve were randomized to either receive no treatment (untreated), i.v. paclitaxel and 200 mg/kg p.o. NR, or i.v. paclitaxel and vehicle p.o.. Tumor-bearing rats were similarly randomized into the three treatment groups on the first appearance of a tumor. Treatment with NR was initiated with the first dose of paclitaxel and continued for another 14 days, at which time the rats were euthanized and a 2 mm by 2 mm full skin-depth biopsy was obtained from the plantar surface of the hind paw behind the tori.
Tissue was placed in 4% paraformaldehyde, 5% picric acid in phosphate buffered for 24 hrs after which it was transferred to 30% sucrose in phosphate buffer. Five to six 20-micron sections through each biopsy were obtained with a cryostat and stained with antibody to PGP 9.5 (1 μg/ml; MCA-BH7, Lot# 120718, RRID: AB_2572394, EnCor Biotechnology Inc., Gainesville, CA) and to collagen IV (160 μg/ml; 1340–01, Lot# H2915-YC18, RRID: AB_2721907, SouthernBiotech, Birmingham, AL); the latter served to demarcate the dermis from epidermis. Sections were rinsed four times in 0.1 M PBS and then incubated for 1 hr in 2% normal donkey serum (Lampire, Pipersville, PA) with 0.3% Triton X-100 prepared in 0.1 M PBS pH 7.4, which was also used as the diluent for all antibody solutions. The sections were then incubated in primary antibody solutions overnight at 4˚C. After four washes in 0.1 M PBS, the sections were incubated in secondary antibody solutions (1.88 μg/ml) for 1 hr at room temperature. The secondary antibodies were donkey anti-mouse Cy3 (715–165-151, lot 132845) and donkey anti-goat Cy2 (705–225-174, lot 1333888). They were purchased from Jackson ImmunoResearch (West Grove, PA) and were highly cross absorbed for minimal species cross-reactivity. The sections were then washed three times with 0.1 M phosphate buffer and allowed to dry overnight at room temperature. Sections were cleared in xylenes for 1 min and coverslipped with DPX mounting media.
To ensure unbiased counts, StereoInvestigator (MBF Biosciences; Villiston, VT) was used to randomly place counting frames (150 by 40 microns, width x height) along the epidermal-dermal border for the length of each section. Fibers that crossed the border into the epidermis were counted in each frame using a 40X objective, summed, and expressed as total number of fibers per mm for that section. Values from the five to six sections obtained from each biopsy (technical replicate) were averaged to yield a single value for each rat. Data were analyzed by two-way analysis of variance in which factors were tumor status and treatment, followed by Holm-Sidak’s test. The experimenter was blinded to the treatment assignment.
Experimental Design for Tumorigenesis Studies
NR and Natural Progression
To determine whether NR treatment alone altered tumor development, a separate group of rats received MNU. On day 47, irrespective of tumor status, the rats were randomized to receive once daily treatment with vehicle or 200 mg/kg p.o. NR. Treatment with NR continued until tumor load necessitated euthanasia or day 82, whichever occurred earlier. The appearance of a tumor was considered a new incidence. The incidence of tumor development over time was analyzed using the Kolmogorov-Smirnov test. Tumor dimensions were determined twice weekly. If a rat was euthanized due to tumor burden before the planned end of the study, the data for tumor volume were not carried forward for analysis. Data were log10-transformed for analysis in line with recommendations [16; 22]. Treatment effects at each time point were compared by a Mann-Whitney test. Kaplan-Meier estimates of survival, as well as tumor-free survival were compared using the Mantel-Cox test.
NR Interaction with Paclitaxel
This experiment was conducted with the same rats in which the ability of NR to suppress nociceptive behaviors was tested. Tumor size and location were recorded twice a week for the duration of the study. On day 28 (or earlier if tumor burden necessitated), rats were euthanized by CO2 exposure, each tumor was excised, assigned an identifier, and submitted for pathology. If a rat was euthanized before day 28, its tumor volume was not carried forward for analysis. Data were log10-transformed and analyzed as above. Post-hoc comparisons were made by Mann-Whitney test. Kaplan-Meier analyses were performed and compared using the Mantel-Cox test.
Ki-67 Staining
Rats were randomly selected from each treatment group in the natural progression and paclitaxel interaction experiments. If the rat had more than one tumor, one was randomly selected for analysis. Sections from each tumor were deparaffinized, rehydrated, and then subjected to antigen retrieval using pH 6.0 citrate buffer at 110°C for 15 min. Sections were twice washed in Dako buffer (Agilent; Santa Clara, CA), after which endogenous peroxidase was quenched by incubation in 3% H2O2 in 100% methanol for 8 min. Sections were again twice washed in buffer, and then incubated in Dako background buster for 30 min. Sections were then incubated for 60 min at room temperature in monoclonal rabbit anti-KI67 (Abcam #Ab16667, RRID:AB_302459) diluted 1:400 in Dako buffer. After further washes, sections were processed with the Dako DAB Plus kit (Vector Laboratories, Santa Clara, CA). After further washes, the sections were counterstained with hematoxylin, dehydrated through graded alcohols, cleared with xylenes, and mounted with coverslip medium. One section was selected by the pathologist, who was blind to treatment assignment, for further analysis. A low power image of the section was displayed on a monitor and overlaid with a grid of numbered squares (1.4 mm2). To limit bias, a random number generator was used to select which squares (totaling 10% of the tumor area) were then scanned at 40X using an Aperio Ariol Slide Scanner (Leica Biosystems, Buffalo Grove, IL). The number of Ki67 positive cells and the total number of cells were counted in each square and summed to yield a single value for that rat. The data were expressed as the percentage of cells that were Ki67 positive. Treatment groups were compared by Student’s t-test.
RESULTS
NR prevents paclitaxel-induced hypersensitivity in tumor-bearing rats
In vehicle-treated rats, i.v. administration of paclitaxel produced hypersensitivity to both tactile (Fig. 1B) and cooling stimuli (Fig. 1C) within 14 days that was sustained through 21 days. Concomitant treatment with NR beginning at day 0 significantly blunted tactile hypersensitivity at 21 days as compared to vehicle-treated rats (P < 0.001) as well as to its baseline values (P < 0.001). The effect at day 14 did not differ from vehicle (P = 0.081). Treatment with NR prevented the development of hypersensitivity to cooling at both 14 and 21 days as compared to values in vehicle-treated rats at the corresponding time points (P < 0.001 each time point), and to its own baseline values (P < 0.001) (Fig. 1C).
NR suppresses the aversive dimension of paclitaxel-induced neuropathy in tumor bearing rats
Rats in both treatment groups preferred the dark chamber during the exploration phase and only spent about three min in the brightly lit chamber (Fig. 1D). Despite repetitive stimulation of the hind paw, NR-treated rats were calm and remained in the dark chamber for a significantly longer time during the test phase compared to the exploration phase (P = 0.009). Unlike tumor-naïve rats [26], vehicle-treated tumor-bearing rats did not escape to the brightly lit chamber when repetitively stimulated in the dark during the test phase (P = 0.94). Rather, these rats became agitated and despite approaching the doorway, many appeared unwilling to exit to the brightly-lit side. Comparison of the time spent in the brightly-lit chamber by NR- and vehicle-treated rats during the test phase did not indicate a significant difference (P = 0.069). When data were expressed as the difference between time spent in the brightly lit chamber during the test and exploration phases (Test-Explore), the two treatment groups did not differ (P = 0.21). The lack of difference is likely due to the large variance in the vehicle-treated group.
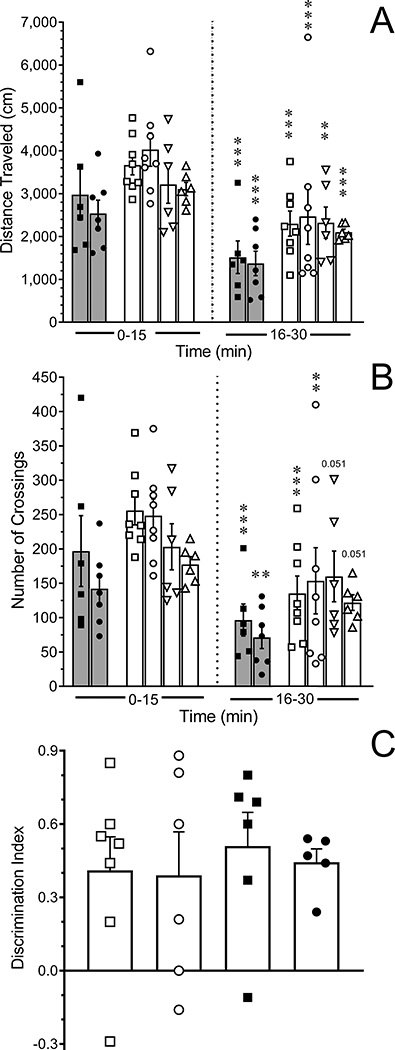
The apparent disinclination of the vehicle-treated tumor-bearing rats to exit to the brightly lit chamber raised several questions. When rats from the second and third cohorts were tested in an open field (Fig. 2A), the two treatment groups did not differ in their exploratory activity during the first 15 min (P = 0.601) and showed a similar decrease in activity in the second 15 min period (P < 0.001). It was thought that the presence of abdominal tumors in conjunction with possible paclitaxel-induced allodynia of the abdomen could contribute to the reluctance of vehicle-treated tumor-bearing rats to exit the dark. Therefore, four additional treatment groups were studied: tumor-naïve rats, untreated tumor-bearing rats, and tumor-naive rats treated only with i.v. paclitaxel or its vehicle. These rats did not undergo extensive handling or somatosensory testing and were therefore analyzed separately. Untreated tumor-bearing rats, as well as tumor-naive rats that received only paclitaxel or its vehicle explored the activity box to a similar extent as tumor-naïve rats during the first 15 min (P = 0.644). Their exploratory activity similarly diminished in the second 15 min period (P < 0.001 for time all groups) (Fig. 2A). Similar conclusions were reached when numbers of grid crossings were analyzed (Fig. 2B). These data indicate that neither the presence of tumors nor paclitaxel treatment interfered with locomotor or exploratory activity.
Figure 2.
Assessment of exploratory activity and novel objection recognition. Scatterplots of (A) the distance traveled and (B) number of grid crossings during sequential 15 min epochs in the open field test. Shaded bars represent tumor-bearing rats treated with paclitaxel and then randomized to receive NR (N=7, solid circles) or vehicle (N=6, solid squares). Open bars collectively represent naïve rats (squares, N=8), untreated tumor-bearing rats (circles, N=8), tumor-naïve rats treated with i.v. KES (N=6, downward triangle), or tumor-naive rats treated with i.v. paclitaxel (N=8, upward triangle). Bars are the mean ± SEM. ***P < 0.001 compared to values in the first 15 min for the respective treatment group. (C) Novel object recognition test. Naïve rats (N=7, open square,), untreated tumor-bearing rats (N=6, solid square), MNU-treated rats without palpable tumors that received i.v. paclitaxel (N=6, open circle,), and tumor-bearing rats that received i.v. paclitaxel (N=5, solid circle) were equally able to discriminate a novel from a familiar object. Bars are the mean ± SEM. Data are expressed as the discrimination index; positive values indicate more time was spent investigating the novel object.
A substantial percentage of patients report cognitive impairment [29; 55]. The novel object recognition test was used to probe the possibility that the failure of the vehicle-treated rats to exit to the brightly-lit chamber behavior reflected a cognitive deficit. Rats that were treated with MNU and did not develop tumors served as a time-matched control group for MNU-treated rats that developed tumors and were randomized to receive i.v. paclitaxel or vehicle. None of these treatment groups exhibited a deficit in their ability to recognize the novel object as compared to untreated tumor-naïve rats (Fig. 2C, P = 0.934).
NR Blunts the Loss of IENF in Paclitaxel-treated Rats
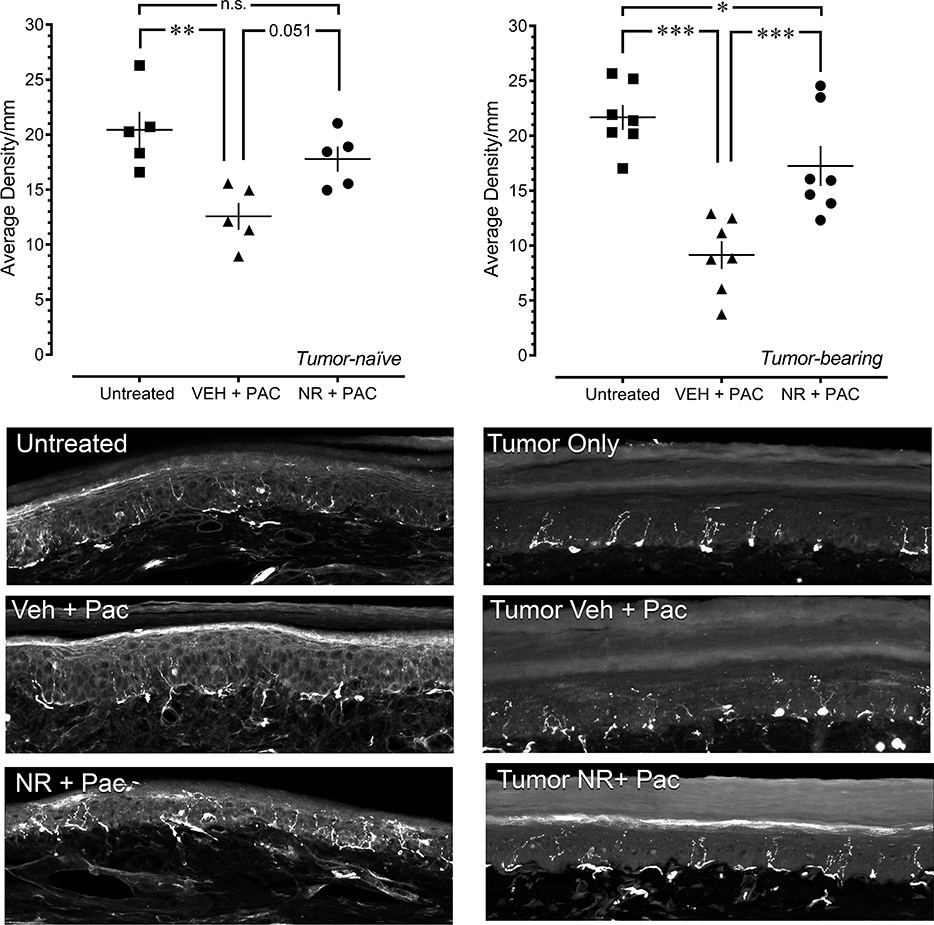
Unbiased assessment of IENF density did not identify any differences between tumor-naïve and tumor-bearing rats in the effects of the different treatments (Fig. 3, P = 0.452 for tumor status). Fourteen days after paclitaxel injection was initiated, the density of IENFs in the plantar surface of the hind paw was decreased by 30–50% in tumor-naïve (P = 0.004) and tumor-bearing (P < 0.001) rats. NR blunted the loss of IENF in tumor-naïve rats (P=0.051 compared to vehicle treatment and P = 0.240 compared to untreated). It also blunted the loss of IENF in tumor-bearing rats; however, it did not cause complete reversal (P < 0.001 compared to vehicle treatment and P = 0.025 compared to untreated). Representative images are presented in Fig. 3.
Figure 3.
NR ameliorates the loss of IENF caused by paclitaxel in both tumor-naïve and tumor-bearing rats. Upper panels are scatterplots of IENF density in individual rats obtained 14 days after initiation of paclitaxel treatment. Bars are the mean ± SEM. * P < 0.05, ** P < 0.01 for indicated comparison. Bottom panels are representative confocal images of PGP.5 immunoreactivity in the plantar skin of the hind paw of untreated, paclitaxel-treated rats that received vehicle, and paclitaxel-treated rats that received NR. Immunostaining for collagen IV is not depicted. Images are the composite maximum intensity projections of ~13 optical sections taken at 1 μm intervals. Imaging parameters and any adjustments for contrast were the same within a treatment condition. Scale bar is 100 microns.
Tumors Induced by MNU
Tumors were palpable beginning about day 42 and onwards and did not develop in a synchronous manner. Rats typically developed one or two tumors, although one vehicle-treated rat and one NR-treated rat respectively developed seven and six tumors in the natural progression experiment. The different types of mammary tumors included tubular, ductal carcinoma in situ, papillary, tubulopapillary, and cribriform. Tumors were not evident elsewhere in the abdominal and/or thoracic cavities.
NR Treatment Does Not Appear to Alter the Natural Progression of Tumors
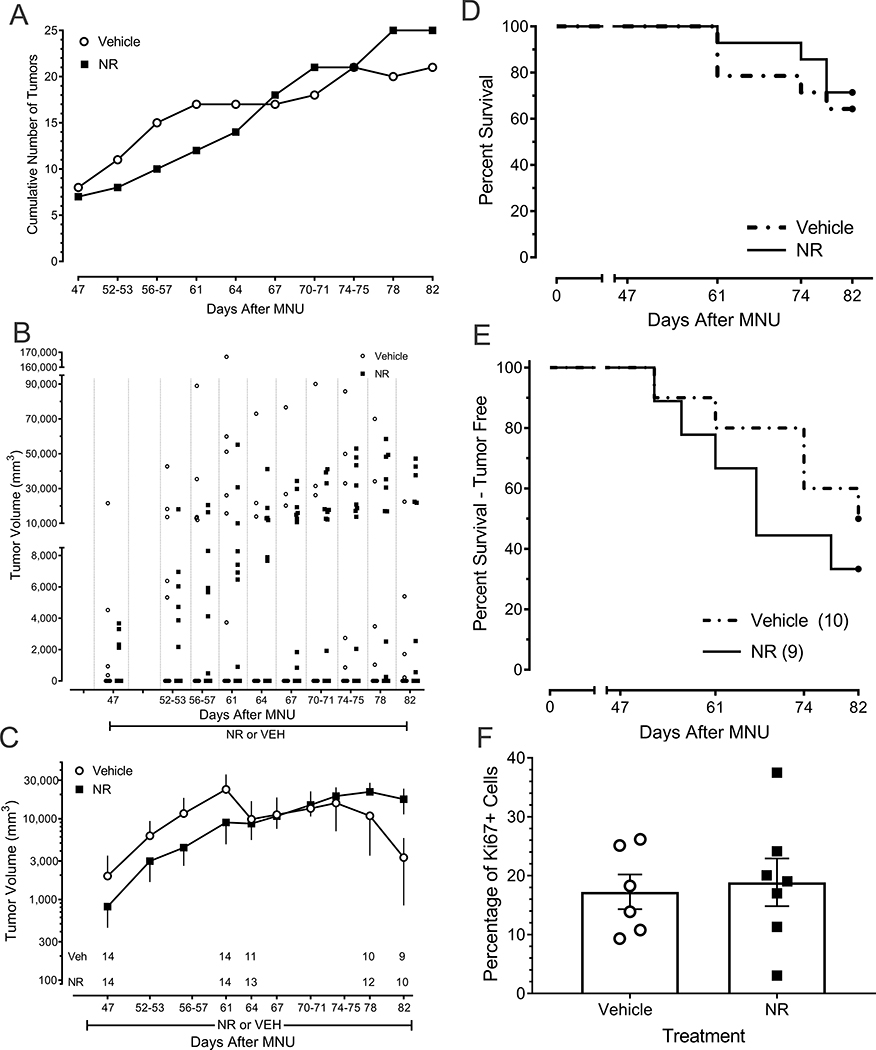
Of the 14 vehicle-treated rats, five did not develop malignant tumors after administration of MNU. Of these five, one developed a benign tumor and another developed a keratin cyst. Of the 14 NR-treated rats, three did not develop malignant tumors after administration of MNU. The percentages did not differ between the two treatment groups (Chi square test, P = 0.678).
Vehicle- and NR-treated rats did not differ in the incidence of tumor development over time (Fig. 4A; Kolmogorov-Smirnov two-sample test, P > 0.1). As the scatterplot in Fig. 4B illustrates, there was significant variability among rats within each treatment group (Fig. 4C). There was no significant difference in tumor growth between the vehicle- and NR-treated rats at any time point (Fig. 4B and C; P > 0.123 for every time point). The survival curve for NR- and vehicle-treated rats did not differ over a period of 82 days (Fig. 4D; Mantel-Cox test, P > 0.254). Similarly, the survival curve of rats that did not have tumors at the outset of the study and that remained tumor-free did not differ between the two treatment groups (Fig. 4E; Mantel-Cox test, P > 0.841). Finally, NR- and vehicle-treated rats did not differ with respect to the percentage of tumor cells that were immunoreactive for Ki67 (Fig. 4F, P = 0.761).
Figure 4.
Lack of effect of NR on the natural progression of MNU-induced mammary gland tumors in female rats. (A) Incidence of tumor development over time (B) Scatterplot of tumor growth for individual rats in each treatment group. (C) Tumor volume in NR- and vehicle-treated rats. Rats that did not develop a tumor were included in this analysis. Data are the mean ± SEM of individual values illustrated in panel B. Numbers indicate number of surviving rats at that time point irrespective of tumor load. Data for rats that were euthanized were not carried forward in panels B and C. (D) The survival curve for NR-treated rats does not differ from that of vehicle-treated rats over a period of 82 days (Kaplan-Meier Survival Analysis, P > 0.6). Data were the day of euthanasia. (E) Tumor-free survival curve. Data were the day of first detectable tumor. (F) Percentage of tumor cells immunoreactive for Ki67. A subset of rats and tumors in each treatment group was selected for this analysis.
NR Treatment Does Not Interfere and May Enhance Suppression of Tumor Growth by Paclitaxel
One of the 25 rats in this study developed tumors that grew very rapidly between identification and randomization to treatment. Paclitaxel neither stabilized or decreased its growth. This rat was considered an outlier and excluded from further analysis, although it is illustrated in Fig. 5A.
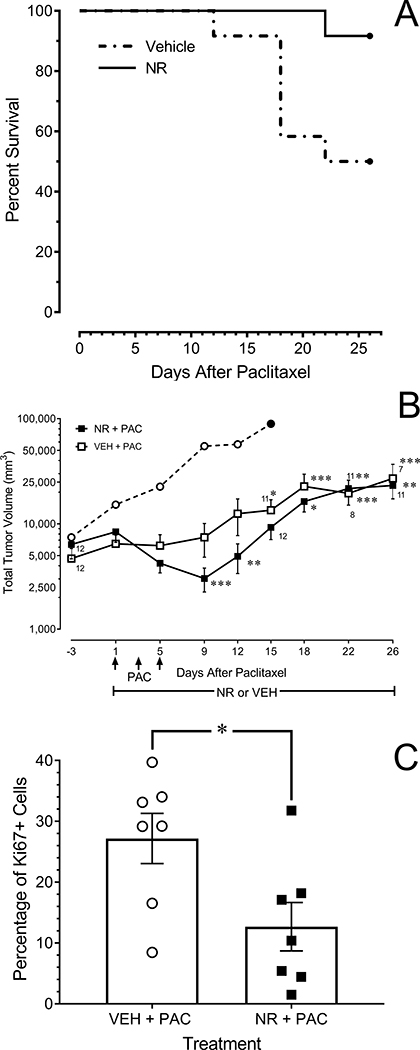
Figure 5.
NR does not interfere with the ability of paclitaxel to suppress the growth of MNU-induced mammary gland tumors in female rats. (A) Survival curves of paclitaxel-treated rats that received concurrent treatment with NR or vehicle (P =0.005). Data are the day tumor burden required euthanasia. (B) Time course of tumor growth. Data from rats that were euthanized before day 26 were not carried forward in the analysis. NR-treated rats (open squares; N=12). Vehicle-treated rats (solid squares, N=12). The open circle depicts the outlier in the vehicle-treated group, which did not respond to paclitaxel with a decrease in tumor growth. * P < 0.05, ** P < 0.01 compared to vehicle-treated group at corresponding time point. † P < 0.05, ‡ P < 0.01 compared to baseline values at day 1 of treatment. (C) Percentage of tumor cells immunoreactive for Ki67. A subset of rats and tumors in each treatment group was selected for analysis. * P < 0.05.
Tumor growth in both vehicle- and NR-treated rats stabilized or decreased between days one and nine beginning with the first injection of paclitaxel and then resumed. This time course was expected given that the 7 hr half-life of paclitaxel in the rat [33] would predict complete elimination of the last dose of paclitaxel by day nine. Although statistical significance for treatment effect (P = 0.200) or interaction (P = 0.055) was not achieved, a significant effect of time (P < 0.001) was observed. Within-treatment post-hoc comparisons revealed that tumor volume in vehicle-treated rats stabilized during paclitaxel treatment and then resumed growth, exceeding day 1 baseline values as of day 15. In contrast, tumor volume in NR-treated rats decreased compared to baseline on days 9 and 12 and did not exceed baseline values until day 18 (Fig. 5A). Moreover, the probability of survival through 28 days was significantly greater in NR-treated rats (Mantel-Cox test, P = 0.022) (Fig. 5B). Finally, the percentage of Ki-67-positive cells in tumors from NR-treated rats obtained at the conclusion of the experiment was significantly less than in vehicle-treated rats (Fig. 5C; Student’s t-test; P = 0.012). Representative images are provided in Fig. 6A and B.
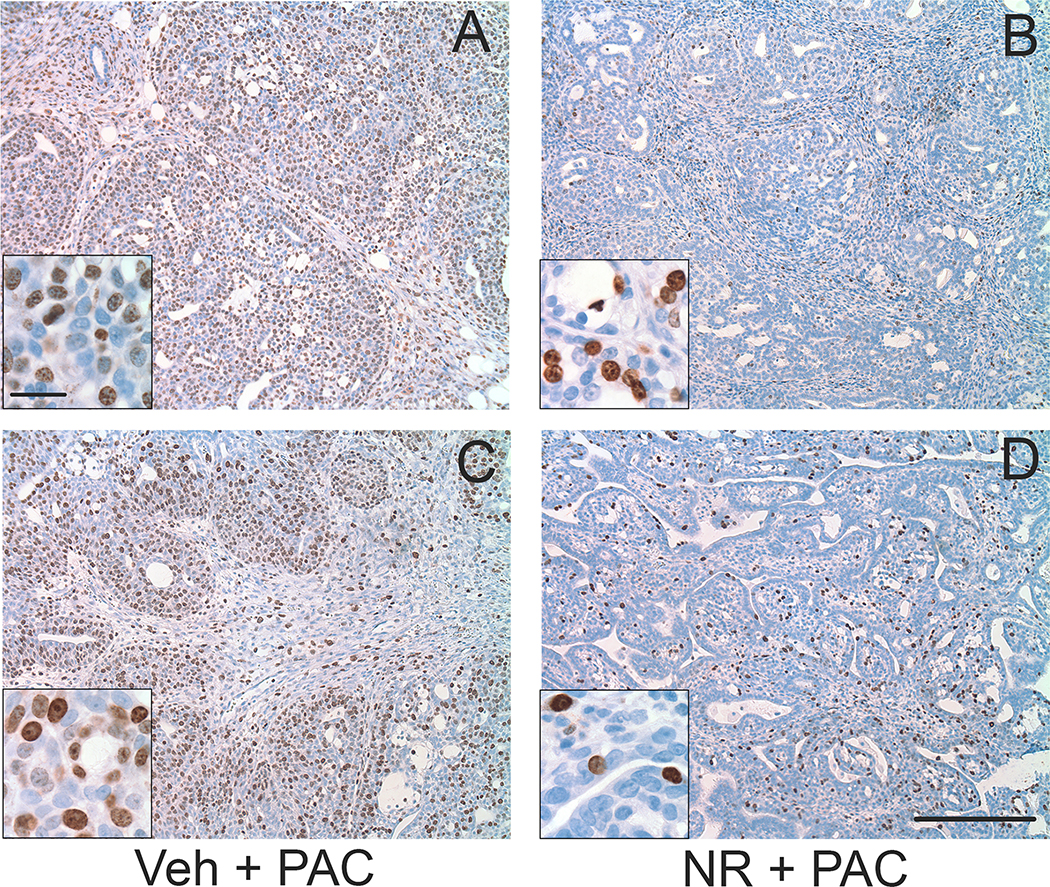
Figure 6.
Representative low-power images of the density of Ki67-positive cells in sections from (A,B) cribiform tumors and (C,D) tubulopapillary tumors. A,C: paclitaxel-treated rats that received vehicle. B,D: paclitaxel-treated rats that received NR. Insets in each panel are higher magnification. Sections were processed for Ki67 immunoreactivity (brown) and counterstained with hematoxylin (blue). Scale bar is 250 microns in low and 25 microns in high magnification images.
DISCUSSION
The principal finding of this study was that concomitant treatment with NR ameliorates paclitaxel-induced tactile and cold hypersensitivity, as well as blunts the loss of IENF in tumor-bearing rats. The dose and route of administration of paclitaxel in this study mirror its clinical effects in terms of (1) scaling to the human dose on a mg/m2 basis, (2) myelosuppression, and (3) suppression of tumor growth [26; this study]. Using rats with carcinogen-induced tumors increased the clinical relevance of the study while enabling an initial assessment of NR’s effect on tumor growth and its possible interaction with paclitaxel. The latter experiment yielded the unexpected finding that tumor growth was further suppressed when NR was administered concomitantly with paclitaxel and that treatment with NR significantly prolonged the survival of these rats.
NR prevents paclitaxel-induced hypersensitivity and aversive behaviors
This study confirmed that NR suppresses tactile hypersensitivity, and additionally extended its efficacy to cold allodynia. The baseline paw withdrawal threshold of tumor-bearing rats in Fig. 1A was lower than the thresholds of tumor-naïve rats previously reported by this laboratory [26]. This difference does not necessarily indicate that tumor-bearing rats are more sensitive to tactile stimuli, but more likely reflects methodological adjustments that included the use of a slightly different set of filaments and limitation of testing to the 15.1 g filament due to the smaller size and younger age of the rats at the outset of the study. Treatment with NR was initiated concomitantly with paclitaxel because the onset of tumor growth was not predictable and continued growth limited the duration of the experiment. Given that the effects of NR do not appear to be fully expressed until seven or more days of administration, it is more appropriate to compare the efficacy of NR in the tumor-bearing rats to the therapeutic intervention paradigm in the tumor-naïve rats [26]. In this respect, NR appears to have similar efficacy in both tumor-naïve and tumor-bearing rats.
NR treatment reduced place escape avoidance behaviors as previously reported [26]. Unexpectedly, tumor-bearing rats treated with paclitaxel in the vehicle group did not exhibit place escape avoidance behaviors. Their failure to exit to the brightly lit chamber on stimulation is unlikely to reflect photophobia, motor impairment, or diminished exploratory behavior. The time (~3 min) that these rats spent in the brightly lit chamber (~4K lux) during exploration was comparable to that of tumor-naïve rats [26]. Also, their locomotor and exploratory activity was similar to that of naïve rats, untreated tumor-bearing rats, or tumor-naïve rats that received either i.v. paclitaxel or KES. Finally, these rats could discriminate a novel object from a familiar object suggesting that a cognitive deficit did not contribute to their failure to exhibit escape avoidance behaviors. Others have reported that low doses of paclitaxel in tumor-naïve rats did not interfere with a general learning task [6] or with source, spatial, or episodic learning, although a transient deficit in reversal learning was identified [41; 47]. A recent study in female tumor-naïve mice similarly reported that paclitaxel did not diminish novel object recognition [34]. Nonetheless, the high incidence of cognitive impairment in patients warrants further study of the effects of clinically-relevant doses of paclitaxel in tumor-bearing rats using more sophisticated models of learning and memory as advocated by Winocur [56].
NR blunts the loss of IENF
Administration of NR blunted the loss of IENF induced by paclitaxel in both tumor-naïve and tumor-bearing rats. Two mechanisms that likely contribute to the dying back of peripheral nerve endings include (1) mitochondrial dysfunction and (2) acetylation and polymerization of microtubules. The former produces a bioenergetic deficit whereas the latter interferes with the transport of cargo to the peripheral endings [27; 37]. NR supplementation can ameliorate mitochrondrial dysfunction [30]. Through its ability of increase NAD+ levels, it may also increase or normalize the activity of sirtuin 2, which deacetylates α-tubulin, in the dorsal root ganglia and axons of paclitaxel-treated rats. Sirtuins are NAD+ consumers and sensors whose activity is regulated by NAD+ levels. Of note, NAD+ levels are decreased in the sciatic nerve of paclitaxel-treated rats [36].
NR and Tumor Growth
Cancer cells require high levels of NAD+ to survive as well as to proliferate [15; 45; 59] in part because they shift their metabolic pathway to aerobic glycolysis (lactate production), for which NAD+ is a necessary co-factor. Many cancers demonstrate increased expression of transcripts for enzymes of the de novo or salvage NAD+ biosynthesis [12; 15]. High levels of intracellular NAD+ also increase the activity of PARPs that initiate DNA damage repair, as well as the activity of sirtuins, that regulate many processes involved in tumor initiation and maintenance [15; 45; 59]. Recent data also indicate that NAD+ and the NAD+/NADH balance govern the strength of the proinflammatory secretory response of senescent cells, with increased ratios thought to be tumor promoting [40]. Consequently, drugs that inhibit NAD+ biosynthesis are being actively pursued as chemotherapeutic agents albeit without success to date [57; 59].
Based on the above, there is concern that drugs that increase levels of NAD+ may facilitate the growth of cancer cells. However, recent studies with new tools have revealed that the context (cell type, tumor type, tissue, compartment, cell cycle) in which increases and decreases in NAD+ occur is likely to be a critical determinant of outcome. NAD+ and several of its biosynthetic enzymes are both highly compartmentalized and present in multiple pools within the cell [10; 21]. Regulation of NAD+ levels is a dynamic process that includes competition for shared substrates, exchange between compartments, and also cell-cycle dependency [31; 49; 59]. Moreover, tumor cells and the tissues from which they derive vary greatly with respect to their metabolic dependence on NAD+ and can exhibit preferential if not exclusive reliance on one of the several available NAD+ biosynthetic pathways [12]. The effects of NAD+ precursors on tumor growth are therefore likely to also be context sensitive.
Prolonged treatment with NR did not facilitate tumorigenesis. Thus NR-treated rats did not differ from vehicle treated rats in terms of tumor growth over time, incidence of tumors, or survival. These treatment groups also did not differ in the percentage of Ki67 positive cells or the percentage of rats that remained tumor-free throughout the study. Although heartening, this conclusion should be viewed as preliminary because the subject numbers were comparatively small. However, this finding is concordant with a report that NR supplementation prevented dysplastic lesions, DNA damage, and tumor development in an oncogenic mouse model of hepatocarcinoma [53].
Importantly, prolonged treatment with NR did not interfere with the tumor suppressing effect of paclitaxel. This finding was anticipated because NR did not obtund the myelosuppressive effects of paclitaxel [26]. However, the ability of NR to further suppress tumor growth when administered concomitantly with paclitaxel was unexpected. Rats treated with NR also had a higher probably of survival, and tumors excised from these rats had fewer KI67 positive cells. These findings likely reflect a NR-mediated delay in the resumption of tumor growth. Unfortunately, because tumors were not obtained until the end of the study, it is not known whether the number of Ki67-positive tumors cells was also further reduced during the concomitant administration of paclitaxel and NR.
The mechanism for the cooperative actions of NR and paclitaxel is not yet clear, but several possibilities can be considered. Earlier work revealed that the NAD+/NADH balance of tumor cells regulates tumor growth and metastasis, and that a decrease in this ratio can promote the progression of breast cancer [43]. By increasing intracellular NAD+, NR may normalize this ratio. However, were this the case, one might have expected to see that NR by itself can suppress tumor growth. More likely is the possibility that NR indirectly inhibits PARP via its metabolism to nicotinamide [25; 35], which itself can inhibit PARP [48], or its ability to increase NAD+ and subsequently NADP+. NADP+ was recently determined to be an endogenous inhibitor of PARP and to cooperatively sensitize tumor cells to the tumor suppressing effects of synthetic PARP inhibitors like olaparib [5]. Of note, inhibition of PARP increases intracellular NAD+ [11]. The finding that NR only suppressed tumor growth when given in conjunction with paclitaxel raises the interesting possibility that NR can indirectly function as a PARP inhibitor and synergize with other chemotherapeutic agents.
Conclusion
Transition of this work to tumor-bearing rats reaffirmed the ability of NR to suppress the somatosensory and affective sequelae of paclitaxel while addressing potential concerns of NRs effect on tumor growth. It also revealed an intriguing finding that NR may cooperate with paclitaxel to suppress tumor growth in vivo. While NAD+-dependent processes are attractive possibilities, it should be noted that the exact mechanism by which NR exerts its effects remains to be proven. Nonetheless, these collective findings provide additional support for the neuroprotective effects of NR [8; 51]. While also supportive of its advancement to clinical trial for the relief of CIPN, such studies in the curative population will first require in-depth investigation of the mechanisms by which NR acts in concert with paclitaxel to suppress tumor growth.
Acknowledgement
This work was supported by an Oberley award from the Holden Comprehensive Cancer Center at The University of Iowa and its National Cancer Institute Award P30CA086862, and by R01 AT009705 to DLH. A NIH Shared Instrumentation Grant 1S10 OD014165-01 to DLH enabled purchase of the Leica Aperio Ariol Slide Scanner.
We thank ChromaDex for providing NR free of charge, and Dr. Henry Thompson of the University of Colorado Boulder for helpful advice implementing the breast cancer model. We also thank William Clarke for sharing his protocol for PGP 9.5 staining of IENF, and Sandra Kolker for assistance with image acquisition.
Dr. Hamity, Ms. White, Mr. Blum, and Dr. Gibson-Corley have no potential conflicts to disclose. Dr. Hammond reports non-financial support from ChromaDex in that it has provided nicotinamide riboside free of charge for this study, as well as for two investigator-initiated clinical trials underway (NCT03642990 and NCT04112641). ChromaDex had no role in the design, conduct, analysis, or interpretation of the findings. Dr. Hammond has no financial relationships with ChromaDex.
REFERENCES
- [1].Allen JK, Armaiz-Pena GN, Nagaraja AS, Sadaoui NC, Ortiz T, Dood R, Ozcan M, Herder DM, Haemmerle M, Gharpure KM, Rupaimoole R, Previs RA, Wu SY, Pradeep S, Xu X, Han HD, Zand B, Dalton HJ, Taylor M, Hu W, Bottsford-Miller J, Moreno-Smith M, Kang Y, Mangala LS, Rodriguez-Aguayo C, Sehgal V, Spaeth EL, Ram PT, Wong STC, Marini FC, Lopez-Berestein G, Cole SW, Lutgendorf SK, De Biasi M, Sood AK. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res 2018;78:3233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvarado A, Faustino-Rocha AI, Colaco B, Oliveira PA. Experimental mammary carcinogenesis - Rat models. Life Sci 2017;173:116–34. [DOI] [PubMed] [Google Scholar]
- [3].Austin M, Elliott L, Nicolaou N, Grabowska A, Hulse RP. Breast cancer induced nociceptor aberrant growth and collateral sensory axonal branching. Oncotarget 2017;8:76606–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balfour DJK. Neural mechanisms underlying nicotine dependence. Addiction 1994;89:1419–23. [DOI] [PubMed] [Google Scholar]
- [5].Bian C, Zhang C, Luo T, Vyas A, Chen SH, Liu C, Kassab MA, Yang Y, Kong M, Yu X. NADP+ is an endogenous PARP inhibitor in DNA damage response and tumor suppression. Nat Commun 2019;10:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boyette-Davis JA, Fuchs PN. Differential effects of paclitaxel treatment on cognitive functioning and mechanical sensitivity. Neurosci Lett 2009;453:170–4. [DOI] [PubMed] [Google Scholar]
- [7].Brenner DS, Golden JP, Gereau RWt. A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012;7:e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 2014;20:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruna J, Alberti P, Calls-Cobos A, Caillaud M, Damaj MI, Navarro X. Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy. Exp Neurol 2020;325:113154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD+. Science 2016;352:1474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Canto C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab 2015;22:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chowdhry S, Zanca C, Rajkumar U, Koga T, Diao Y, Raviram R, Liu F, Turner K, Yang H, Brunk E, Bi J, Furnari F, Bafna V, Ren B, Mischel PS. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling. Nature 2019;569:570–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Currie GL, Angel-Scott HN, Colvin L, Cramond F, Hair K, Khandoker L, Liao J, Macleod M, McCann SK, Morland R, Sherratt N, Stewart R, Tanriver-Ayder E, Thomas J, Wang Q, Wodarski R, Xiong R, Rice ASC, Sena ES. Animal models of chemotherapy-induced peripheral neuropathy: A machine-assisted systematic review and meta-analysis. PLoS Biol 2019;17:e3000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Demarest TG, Babbar M, Okur MN, Dan S, Croteau DL, Fakouri NB, Mattson MP, Bohr VA. NAD+ metabolism in aging and cancer. Ann Rev Cancer Biol 3 2019:105–30. [Google Scholar]
- [16].Demidenko E Three endpoints of in vivo tumour radiobiology and their statistical estimation. Int J Radiat Biol 2010;86:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Faustino-Rocha AI, Ferreira R, Oliveira PA, Gama A, Ginja M. N-Methyl-N-nitrosourea as a mammary carcinogenic agent. Tumour Biol 2015;36:9095–117. [DOI] [PubMed] [Google Scholar]
- [18].Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. Prog Mol Biol Transl Sci 2015;131:471–508. [DOI] [PubMed] [Google Scholar]
- [19].Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth 2017;119:737–49. [DOI] [PubMed] [Google Scholar]
- [20].Gadgil S, Ergun M, van den Heuvel SA, van der Wal SE, Scheffer GJ, Hooijmans CR. A systematic summary and comparison of animal models for chemotherapy induced (peripheral) neuropathy (CIPN). PLoS One 2019;14:e0221787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gaudino F, Manfredonia I, Manago A, Audrito V, Raffaelli N, Vaisitti T, Deaglio S. Subcellular zharacterization of ncotinamide adenine dinucleotide biosynthesis in metastatic melanoma by using organelle-specific biosensors. Antioxid Redox Signal 2019;31:1150–65. [DOI] [PubMed] [Google Scholar]
- [22].Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer 2017;17:751–65. [DOI] [PubMed] [Google Scholar]
- [23].Gewandter JS, Brell J, Cavaletti G, Dougherty PM, Evans S, Howie L, McDermott MP, O’Mara A, Smith AG, Dastros-Pitei D, Gauthier LR, Haroutounian S, Jarpe M, Katz NP, Loprinzi C, Richardson P, Lavoie-Smith EM, Wen PY, Turk DC, Dworkin RH, Freeman R. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology 2018;91:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex G, Pain SIGotI. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gross CJ, Henderson LM. Digestion and absorption of NAD by the small intestine of the rat. J Nutr 1983;113:412–20. [DOI] [PubMed] [Google Scholar]
- [26].Hamity MV, White SR, Walder RY, Schmidt MS, Brenner C, Hammond DL. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain 2017;158:962–72. [DOI] [PubMed] [Google Scholar]
- [27].Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol 2013;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical O. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:1941–67. [DOI] [PubMed] [Google Scholar]
- [29].Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 2017;35:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 2014;6:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kulkarni CA, Brookes PS. Cellular compartmentation and the redox/nonredox functions of NAD+. Antioxid Redox Signal 2019;31:623–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kuol N, Stojanovska L, Apostolopoulos V, Nurgali K. Role of the nervous system in cancer metastasis. J Exp Clin Cancer Res 2018;37:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li J, Tang J, Li Y, Yu J, Zhang B, Yu C. Pharmacokinetic profile of paclitaxel in the plasma, lung, and diaphragm following intravenous or intrapleural administration in rats. Thorac Cancer 2015;6:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liang L, Wei J, Tian L, Padma Nagendra BV, Gao F, Zhang J, Xu L, Wang H, Huo FQ. Paclitaxel induces sex-biased behavioral deficits and changes in gene expression in mouse prefrontal cortex. Neuroscience 2020;426:168–78. [DOI] [PubMed] [Google Scholar]
- [35].Liu L, Su X, Quinn WJ 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 2018;27:1067–80 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP. Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy. Elife 2017;6 e29636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma J, Kavelaars A, Dougherty PM, Heijnen CJ. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018;124:2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 2014;8:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer 2019;18:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma KI, Baur JA, Schug Z, Tang HY, Speicher DW, David G, Zhang R. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 2019;21:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Panoz-Brown D, Carey LM, Smith AE, Gentry M, Sluka CM, Corbin HE, Wu JE, Hohmann AG, Crystal JD. The chemotherapeutic agent paclitaxel selectively impairs reversal learning while sparing prior learning, new learning and episodic memory. Neurobiol Learn Mem 2017;144:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013;63:419–37. [DOI] [PubMed] [Google Scholar]
- [43].Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ, Yagi T, Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 2013;123:1068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014;155:2461–70. [DOI] [PubMed] [Google Scholar]
- [45].Sharif T, Martell E, Dai C, Ghassemi-Rad MS, Kennedy BE, Lee PWK, Gujar S. Regulation of cancer and cancer-related genes via NAD+. Antioxid Redox Signal 2019;30:906–23. [DOI] [PubMed] [Google Scholar]
- [46].Sikandar S, Dickenson AH II. No need for translation when the same language is spoken. Br J Anaesth 2013;111:3–6. [DOI] [PubMed] [Google Scholar]
- [47].Smith AE, Slivicki RA, Hohmann AG, Crystal JD. The chemotherapeutic agent paclitaxel selectively impairs learning while sparing source memory and spatial memory. Behav Brain Res 2017;320:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Surjana D, Halliday GM, Damian DL. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J Nucleic Acids 2010;2010:Article ID 157591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Svoboda P, Krizova E, Sestakova S, Vapenkova K, Knejzlik Z, Rimpelova S, Rayova D, Volfova N, Krizova I, Rumlova M, Sykora D, Kizek R, Haluzik M, Zidek V, Zidkova J, Skop V. Nuclear transport of nicotinamide phosphoribosyltransferase is cell cycle-dependent in mammalian cells, and its inhibition slows cell growth. J Biol Chem 2019;294:8676–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thompson HJ. Mammary Cancer in Rats In: Teicher BA, editor. Tumor Models in Cancer Research, Vol. 10 New York: Humana Press, 2010. pp. 245–58. [Google Scholar]
- [51].Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep 2016;6:26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Trecarichi A, Flatters SJL. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int Rev Neurobiol 2019;145:83–126. [DOI] [PubMed] [Google Scholar]
- [53].Tummala KS, Gomes AL, Yilmaz M, Grana O, Bakiri L, Ruppen I, Ximenez-Embun P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, Wagner EF, Djouder N. Inhibition of de novo NAD+ synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 2014;26:826–39. [DOI] [PubMed] [Google Scholar]
- [54].Vermeer PD. Exosomal induction of tumor innervation. Cancer Res 2019;79:3529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wigmore P The effect of systemic chemotherapy on neurogenesis, plasticity and memory In: Belzumg CV, Wigmore P, editors. Neurogenesis and Neural Plasticity, Vol. 15 New York: Springer, 2013. pp. 211–40. [DOI] [PubMed] [Google Scholar]
- [56].Winocur G Chemotherapy and cognitive impairment: An animal model approach. Can J Exp Psychol 2017;71:265–73. [DOI] [PubMed] [Google Scholar]
- [57].Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol 2018;8:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu Y, Liu J, Park J, Rai P, Zhai RG. Subcellular compartmentalization of NAD+ and its role in cancer: A sereNADe of metabolic melodies. Pharmacol Ther 2019;200:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]