Abstract
Mitochondria are eukaryotic organelles known best for their roles in energy production and metabolism. While often thought of as simply the ‘powerhouse of the cell,’ these organelles participate in a variety of critical cellular processes including reactive oxygen species (ROS) production, regulation of programmed cell death, modulation of inter- and intracellular nutrient signaling pathways, and maintenance of cellular proteostasis. Disrupted mitochondrial function is a hallmark of eukaryotic aging, and mitochondrial dysfunction has been reported to play a role in many aging-related diseases. While mitochondria are major players in human diseases, significant questions remain regarding their precise mechanistic role. In this review, we detail mechanisms by which mitochondrial dysfunction participate in disease and aging based on findings from model organisms and human genetics studies.
Introduction
The importance of mitochondria in human health is underscored by the fact that neurodegeneration, cardiomyopathies, and musculoskeletal disorders can all result from mutations in mitochondrial or mitonuclear genes or environmental toxins that target mitochondria. Severe genetic defects causing mitochondrial dysfunction lead to mitochondrial diseases; these include Leigh Syndrome (LS), Mitochondrial Encephalopmyopathy with Lactic Acidosis and Stroke-like episodes (MELAS), Leber’s Hereditary Optic Neuropathy (LHON), and many others (1, 2). Among human age-related diseases, Parkinson’s Disease (PD), Alzheimer’s Disease (AD), Amyotrophic Lateral Sclerosis (ALS), metabolic disease, certain cancers, and age-related cardiovascular diseases (CVD) have all been directly associated with genetic variation in genes encoding mitochondrial proteins (3, 4).
In addition to driving severe monogenic pathologies, mitochondrial dysfunction has been implicated in many chronic diseases in humans, particularly those associated with aging. Pathologies associated with aging are driven by dysregulation and dysfunction of tissue, cell, and molecular homeostatic pathways (5). Across species, aging is associated with a decreased capacity to combat stress, an increased risk of multiple diseases, and increased frailty. Interventions targeting the underlying processes of aging are expected to have a significant impact on human health, as aging is the primary risk factor for many chronic diseases (6, 7). Studies in model organisms have defined a number of mechanisms by which aging and age-related disease are modulated by mitochondrial function (5). Mitochondria associated pathways of age-related disease include modulation of signaling through the mechanistic Target of Rapamycin (mTOR), insulin/insulin-like growth factor (IGF), AMP-activated protein kinase (AMPK), and sirtuin pathways; mitochondrial proteostatic mechanisms such as the mitochondrial unfolded protein response (mtUPR); mitochondrial retrograde signaling; energetics and ROS production; and redox balance, which influences nutrient signaling and stress responses (8–10).
In this review, we will discuss mitochondrial structure, genetics, and functions in the context of human disease.
Mitochondrial structure and organization
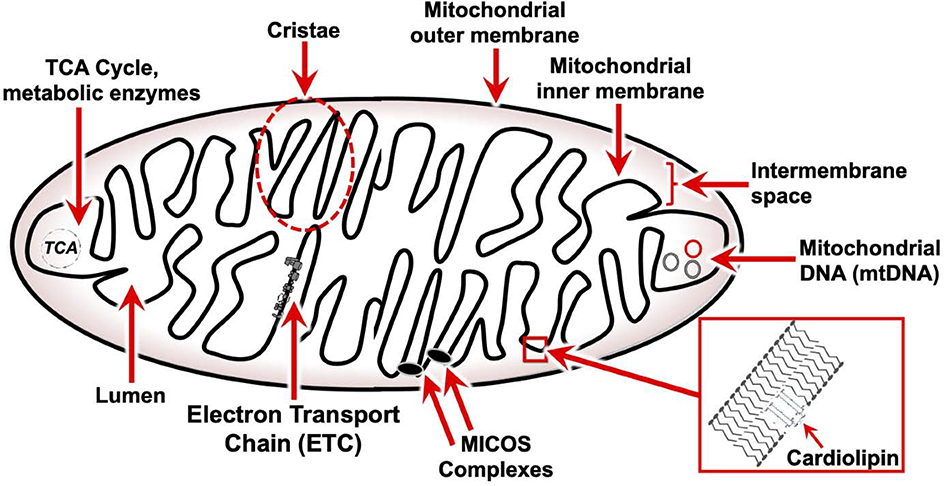
Mitochondria are complex double-membrane organelles with functionally and compositionally distinct outer and inner membranes (Fig. 1) (11). The mitochondrial outer membrane (MOM) is similar in composition to the eukaryotic plasma membrane, separating the organelle from the cytoplasm and sequestering mitochondrial proapoptotic proteins, such as cytochrome c (Cyt C), a heme protein that carries electrons between complexes III and IV of the electron transport chain (see Mitochondrial electron transport chain). Various channels and pores make the MOM largely permeable to ions and uncharged small molecules. Multiprotein translocase and chaperone structures, including the translocase of the outer membrane (TOM), translocase of the inner membrane (TIM), and prohibitin complexes enable import, folding, and assembly of nuclear encoded mitochondrial proteins.
Figure 1 – Structure and organization of mitochondria.
Generalized structure and organization of the mitochondria. Mitochondria have two phospholipid bilayer membranes. The outer membrane defines the organelle and separates protein components but is permeable to ions and uncharged small molecules. The inner membrane is impermeable and has a distinct lipid composition, including the specialized phospholipid cardiolipin. Cardiolipin promotes membrane invaginations called cristae, where the large multiprotein electron transport chain complexes localize. Mitochondrial contact site and cristae organizing system (MICOS) complexes regulate the separation of individual cristae. The matrix harbors enzymes of the tricarboxylic acid (TCA) cycle and other metabolic pathways and houses the mitochondrial genome and mitochondrial genetic machinery. Multiple copies of the mitochondrial genome co-exist in each mitochondria.
The space between outer and inner membranes is referred to as the intermembrane space (IMS). Components of the IMS participate in various functions including protein folding and degradation, transport of metabolites between the cytoplasm and inner membrane, proapoptotic factor release, and reactive oxygen species (ROS) signaling (12).
In order to maintain the transmembrane gradient required for respiratory metabolism the mitochondrial inner membrane (MIM), unlike the MOM, is largely impermeable. The MIM has a distinct lipid composition, including the specialized phospholipid cardiolipin which physically contributes to formation of the characteristic membrane invaginations called cristae. The large multiprotein complexes responsible for respiratory metabolism and ATP synthesis, collectively referred to the electron transport chain (ETC), are localized almost entirely to the lateral surfaces of these cristae (13). MICOS complexes (mitochondrial contact site and cristae organizing system) regulate the electrical sequestration of individual cristae so that many energetic sub-domains of intermembrane space exist within the same mitochondria (14).
Finally, the matrix, or lumen, is the space contained within the inner membrane. The matrix harbors the enzymes of the tricarboxylic acid (TCA) cycle and other metabolic pathways, and houses the mitochondrial genome and mitochondrial genetic machinery (13).
Mitochondrial genetics
Mitochondrial genome
The prevailing view of the evolution of mitochondria is that mitochondria arose early in eukaryotic evolution as a result of endosymbiosis of a primordial eukaryote with free-living eubacteria (15, 16). Over the course of eukaryotic evolution, the majority of genes in the primitive mitochondrial genome translocated to the nuclear genome (17). In fact, over 99% of genes encoding mitochondrial proteins are located in the nuclear genome (3). Accordingly, mitochondria in eukaryotes are dependent on the nuclear genome for even basic functions such as the replication of mtDNA and aerobic respiration (10). Mitochondrial DNA exists in multiple copies per mitochondria as a circular molecule, as in its prokaryotic ancestor (18). In humans, mtDNA is 16.5 kilobase-pairs in length and encodes mitochondrial ribosomal RNAs (mt-rRNAs), mitochondrial transfer RNAs (mt-tRNAs), and 13 mitochondrial proteins, all of which are subunits of the ETC, which is discussed at length below. The mitochondrial genome has also been found to encode a series of mitochondrial peptides; in humans, these include humanin, MOTS-c, and the short humanin-like peptides (SHLP’s) (19). These mitochondria-derived peptides are involved in retrograde signaling and nuclear gene regulation, especially in response to energetic status, as well as both exocrine and paracrine signaling (19–22).
Inheritance patterns
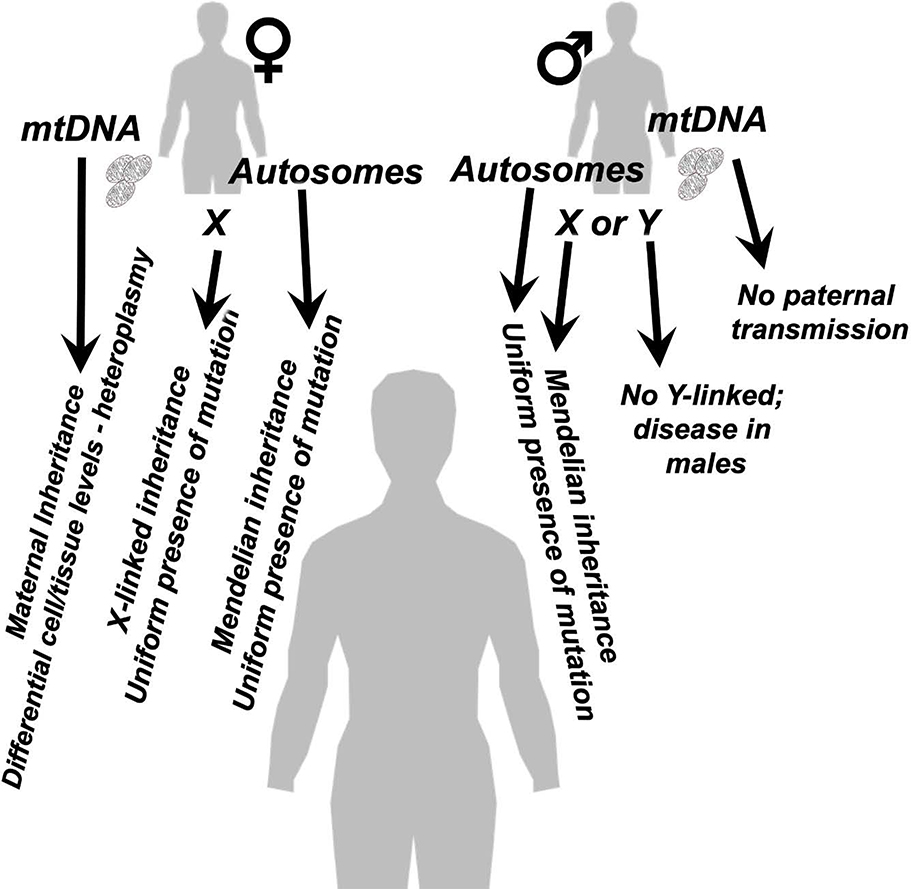
Mitochondrial dysfunction and genetic mitochondrial diseases can arise from defects in either the nuclear or mitochondrial genome, and through a variety of possible inheritance patterns (Fig. 2) (23). Mitochondria are generally inherited through ovum and not sperm (though the possibility of some rare paternal inheritance has very recently been revisited) (24, 25). As a result, mitochondrial diseases arising from defects in mtDNA are maternally inherited. Mitochondrial defects arising from nuclear genes encoding mitochondrial proteins (mitonuclear genes) follow a Mendelian inheritance pattern when the defective gene is located on an autosome, while X-linked genes follow sex-linked inheritance pattern. Y-linked mitochondrial diseases have not been reported. In addition to these classic inheritance patterns, mitochondrial diseases can arise from complex heterozygosity in mitochondrial protein encoding genes, and nuclear genome/mitochondrial DNA genetic interactions appear to greatly impact the clinical presentation of mitochondrial dysfunction (26, 27).
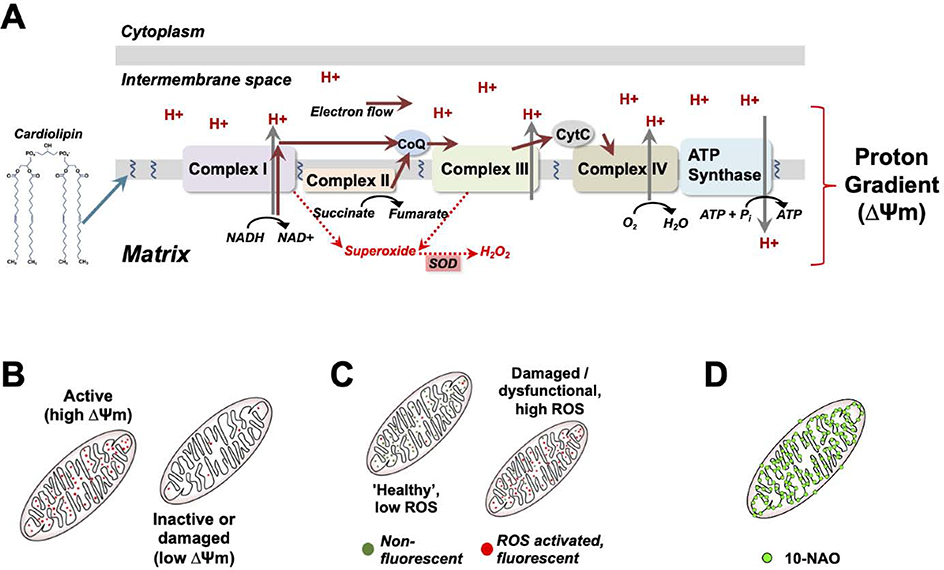
Figure 2 – The electron transport chain and proton gradient.
(A) overview of the mitochondrial electron transport chain (ETC). ETC localization and assembly are aided by cardiolipin, which also promotes inner membrane cristae structures. The ETC converts chemical energy into a proton electrochemical gradient, which drives ATP synthase to produce ATP, the primary energy currency of the cell. Reactive oxygen species (ROS) are produced at multiple steps of the ETC. Anti-oxidant enzymes, such as the superoxide dismutates (SOD’s), protect cellular macromolecular components by neutralizing ROS species. (B-C) Membrane permeable fluorescent compounds differentially accumulate in the mitochondria based on the membrane potential and are used to assess overall health, such as the highly membrane potential sensitive dyes TMRE and TMRM (B), or mitochondrial ROS production, using dyes which are charged and localize to mitochondria but only become fluorescent upon reaction with ROS. (D) Mitochondria can also be detected using 10-NAO, a fluorescent dye which binds to cardiolipin.
Polymorphisms and heteroplasmy
Each mitochondrion contains many copies of the mitochondrial genome with varying genetic sequences. Heteroplasmy, the presence of multiple distinct sequences of mtDNA in the same organism, plays an important role in driving the clinical heterogeneity associated with diseases arising from defects in the mitochondrial genome (28). Many mtDNA polymorphisms are benign, and low-heteroplasmy levels of deleterious mutations typically do not cause overt symptoms. The presence of deleterious point mutations or deletions at sufficient levels can result in severe genetic disorders, such as the typically adult-onset mitochondrial disorder Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like episodes (MELAS), or the severe pediatric subacute necrotizing encephalomyelopathy associated with Leigh Syndrome (LS) (29, 30). Mitochondrial DNA heteroplasmy exists both at the inter- and intra-cellular levels, and the mtDNA complement can vary widely between cells and tissues in the same individual. The penetrance and overall clinical presentation of mtDNA mutation related diseases are a product of the heteroplasmy and the tissue distribution of variants (31, 32). Benign and/or pathogenic variants may co-exist at different levels of heteroplasmy, and the impact of individual mtDNA variants is often unclear; causal relationships between novel variants and disease are difficult to establish, and dozens of variants of unknown significance have been identified (33).
Overall mitochondrial DNA heteroplasmy, including the accumulation of new sequence variation, changes as a function of chronological and biological age, and is influenced by environmental exposures and disease processes, such as inflammation. Furthermore, high mtDNA mutation load has been implicated in age-related diseases and in the process of aging itself (28, 34–36). In agreement, mitochondrial DNA polymerase gamma (PolG) mutant mice, which accumulate mtDNA mutations, display multiple phenotypes of premature aging; patients harboring POLG mutations are pre-disposed to at least some age-related disorders (37–40).
Environmental and age-related changes in heteroplasmy
Age-related changes in overall mtDNA lesion load (mutations and deletions) adds a progressive stochastic layer of mitochondrial heteroplasmy in an age-dependent fashion, as do environmental exposures which influence mitochondrial function such as anti-retroviral therapy in AIDS patients (41–44) or chemotherapy in cancer patients (45–47)). The accumulation of mtDNA mutations as a function of age or pathology has been found to drive ETC dysfunction, increased ROS production at the ETC, and, as a result, induce further mtDNA lesions, a so-called ‘vicious cycle’ of self-induced mitochondrial damage (10, 48). Assessing the role of mtDNA mutations in pathology is complicated by these factors.
Mitochondrial/nuclear compatibility
The function of mitochondrial macromolecular machinery, including multi-protein complexes and tRNA machinery, requires that the gene products produced in mitochondria are compatible with the gene products arising from nuclear encoded genes. Accordingly, the overall fitness of an organism depends on the successful cooperation of these two genomes, and mitochondrial-nuclear genome epistatic interactions co-evolve with species and within distinct populations of the same species. So-called ‘mitochondrial mismatch’ or ‘mitochondrial-nuclear incompatibility’ can arise when mitochondrial and nuclear genetic variants that have co-evolved in distinct populations are mixed and result in sub-optimal mito-nuclear pairing.
Mito-nuclear mismatch has been reported to substantially affect aging in Drosophila (49–51) and influence cognitive aging and physical performance in mice (52, 53). A discrete example of mito-nuclear incompatibility arises from Drosophila simulans and Drosophila melanogaster, which harbor divergent mutations in the mtDNA encoded tyrosine transfer RNA and nuclear encoded tyrosyl-tRNA synthase (54). These variants were found to have little effect individually but result in a severe mitochondrial defect, which could be rescued by transgenic expression of the ‘correct’ nuclear variant, when combined (55). Mitochondrial/nuclear DNA mismatching has also been reported to improve longevity in mice, possibly through an ROS mediated stress-response mechanism (56). This discrepancy may be the result of the extent or nature of the specific ‘mismatch’, or, alternatively, an uncharacterized harmful variant may have been eliminated in this pairing. Human population genetics suggests that significant mitochondrial-nuclear linkage disequilibrium is present throughout the genome, but observed associations do not appear to be linked to nuclear encoded mitochondrial genes (57). The role, if any, of mitonuclear mismatch in human disease or aging remains unclear.
Mitochondrial metabolism
The electron transport chain
Respiratory metabolism, the aerobic catabolism of nutrients such as carbohydrates, fats, and proteins for energy production, takes place in the mitochondria. Complete catabolism of glucose, an important molecule for energy storage and transport, occurs in stages which are spatially separated between the cytoplasm and mitochondria. Glycolysis, which converts glucose into pyruvate, occurs in the cytoplasm, while the TCA cycle and oxidative phosphorylation (via the ETC) occur in the mitochondria. The TCA cycle and ETC function to produce adenosine triphosphate, ATP, the primary energy currency for enzymatic reactions.
Embedded in the mitochondrial inner membrane, the ETC in higher eukaryotes is comprised of five multiprotein complexes (Fig. 3). These complexes couple transfer of electrons to pumping of protons across the inner membrane, generating an electrochemical proton gradient between the inner membrane and matrix with a net negative charge in the matrix. In brief, electron transport begins as complex I (CI) oxidizes the cofactor NADH (which is generated by the TCA cycle and glycolysis) to NAD+. Electrons also enter the ETC at Complex II (CII) through oxidation of succinate to fumarate, which is also a step in the TCA cycle. Electron pairs generated by both CI and CII are transferred to the coenzyme factor ubiquinone (also called Coenzyme Q, or Q), which allows for electron passing to Cyt C via complex III (CIII). Cyt C shuttles electrons to Complex IV (CIV), where oxygen is the final electron acceptor. During electron transfer, protons are pumped by CI, CIII, and CIV. The proton gradient generated by ETC CI-IV powers ATP production by complex V (13). Complex V, also called ATP synthase, functions as a molecular rotor which generates ATP from ADP and phosphate, powered by the proton gradient. Recent structural studies of the ETC have revealed the elegance of the multiprotein complexes involved (58, 59).
Figure 3 – Mitochondrial genetics.
The genetic basis of mitochondrial dysfunction is complex. Mitochondrial defects arising from defects in mtDNA are maternally inherited, whereas mitochondrial defects arising from mitonuclear genes can follow a Mendelian inheritance pattern, when on an autosome, or an X-linked pattern. Y-linked mitochondrial diseases have not been reported. Primary mitochondrial diseases can also arise from complex heterozygosity in mitonuclear genes. Penetrance and presentation of defects related to mtDNA variants is regulated by heteroplasmy levels. Differential cell and tissue levels of mtDNA variants also effect the presentation of mitochondrial diseases.
Mitochondrial membrane potential
The proton gradient generated by the ETC creates a membrane potential across the mitochondrial inner membrane (mitochondrial membrane potential, ΔΨm). In addition to providing the energetic driving force to power ATP synthase, the membrane potential generated by the ETC provides a signal for mitochondrial energetic status which is sensed by the mitophagy machinery (see Mitochondrial Quality Control). Mitochondrial uncoupling proteins (UCP’s), or chemical uncoupling agents such as 2,4-DNP or CCCP, deplete the mitochondrial membrane potential, uncoupling ATP generation from the ETC; this increases heat production (as in brown adipose tissue), and can lead to targeting of mitochondria for removal by cellular quality control machinery (60–62).
UCP’s are thought to play an important role in metabolic homeostasis (63, 64). Human genetic studies have identified associations between UCP polymorphisms and metabolic traits including diabetes and obesity (65, 66) (67). In mice, overexpression of UCP’s has been found to reduce adipose mass, plasma glucose, and insulin levels, prompting investigation of uncoupling as a therapeutic intervention for metabolic disorders (68, 69). Recently, one uncoupler known historically for its use as a diet drug in the 1930’s, 2,4-Dinitrophenol (DNP), has been revisited as a potential diabetic treatment (62, 70). In recent years, some of the mechanisms underlying the beneficial metabolic effects of mitochondrial uncoupling have been delineated. Disruption of ATP synthesis has been shown to induce a cellular response which mimics that of caloric restriction (71). In response, nutrient and energetic sensing pathways, particularly protein kinase C (PKC) and AMPK signaling, are activated (72, 73). In mouse models of diabetes, 2,4-DNP was shown to decrease circulating glucose levels, and even reverse disease at low doses (74).
Assessing function through membrane potential
Due to the net negative charge in the matrix, positively charged lipophilic chemicals accumulate in active mitochondria, and charged lipophilic fluorescent compounds can be used to measure membrane potential (see Fig. 3). Dyes relatively insensitive (or less sensitive) to membrane potential, such as the cardiolipin binding 10-n-nonyl acridine orange (10-NAO) allow for assessment of relative membrane potential in relation to mitochondrial mass. ROS activated dyes further exploit the mitochondrial membrane potential, providing tools for detection of mitochondrial ROS production. While each of these approaches has various limitations and caveats, they are frequently used for visualization of mitochondria or general assessments of mitochondrial function (75).
TCA cycle dysfunction
Both ETC and TCA cycle dysfunction have been implicated in age-related disease. The TCA cycle is the major producer of NADH equivalents used by the ETC to power the proton gradient, thus powering ATP production. Two TCA enzymes, aconitase and α-ketoglutarate (α-KG) dehydrogenase, have been shown to have decreased activity with age in mice and flies (76, 77). The consequences of reduced TCA activity are largely unknown, but could impede NADH homeostasis (see Complex I and NADH) and lead to a depression of respiratory metabolism. In addition, dysfunction of these enzymes may produce an accumulation of two TCA intermediates, α-KG and citrate. α-KG and citrate are crucial activators of DNA demethylases and histone acetyltransferases, respectively, and citrate is a potent inhibitor of fatty acid oxidation (76, 78). Increased DNA demethylation and histone acetylation driven by TCA cycle dysfunction have been found to correlate with aging and age-related pathology (79–81), but the precise role of the TCA cycle in age-related pathologies remains unclear.
A complicated relationship between respiration and age-related disease
Decreased mitochondrial respiratory metabolism has been shown to occur with aging in various model systems, including cultured cells, worms, flies, rats, and monkeys (82–84). Accumulation of mtDNA mutations, particularly in the 13 mtDNA encoded ETC subunits, is thought to contribute to decreased respiratory function with age (85) (see “Polymorphisms and Heteroplasmy”). Consistent with this view, the mtDNA ‘mutator mouse,’ which harbors an exonuclease-deficient mtDNA polymerase, shows increased mtDNA mutation accumulation, decreased ETC enzyme activity, increased oxidative damage, and accelerated signs of aging (85–87).
Impairment of normal mitochondrial function may directly drive senescence and age-relate pathology. Pharmacologic inhibition of ETC complexes has been reported to induce oxidative stress in cells and in vivo, and to induce premature senescence (88, 89). While reduced mitochondrial function can drive pathology in some settings, reduced mitochondrial function has been shown to increase lifespan and prevent disease in other paradigms. In mice, COX deficiency following SURF1 knock out extended lifespan, as did heterozygous knock out of MCLK1, a gene necessary for ubiquinone synthesis (90, 91). Similar lifespan extensions have been observed in studies of yeast, fly, and nematode models with genetic or pharmacologic disruption of the ETC, often through ROS dependent mechanisms (92–94). In addition, mitochondrial uncoupling has been shown to attenuate disease in various systems, as discussed (61, 62, 95, 96).
A number of theories have been proposed to reconcile these contradictory findings regarding ETC function. For example, a study comparing progressive ETC inhibition by RNA interference (RNAi) in C. elegans found that the impact on lifespan is dose-dependent, suggesting that modest ETC inhibition is beneficial, while ETC reduction that surpasses a certain threshold drives pathology and shortens lifespan (97). A second theory suggests that the interplay between oxidative damage and ETC reduction determines whether an organism will experience lifespan extension or shortening (98). Notably, the increased lifespan of the C. elegans isp-1 mutant, with defective CIII, has been found to be completely separable from the increased resistance to oxidative stress in this line, which was found to be mediated by increased expression of antioxidant enzymes (99). While this study did not determine the mechanism of lifespan increase, the uncoupling of oxidative stress resistance and longevity in this model argue against ROS and antioxidant responses playing a role in the longevity of mitochondrial mutant lines.
Mitochondrial ROS
The concept that mitochondria are key regulators of lifespan was first proposed in a cohesive model via the mitochondrial free radical theory of aging (100, 101). In this theory, aging is primarily driven by oxidative damage resulting from ROS that are generated as a by-product of oxidative phosphorylation performed by the ETC. Oxidative damage to cellular components impairs macromolecular function, and damage to mitochondrial components (including the ETC) can result in increased ROS production, a so-called ‘vicious cycle’(10) of increasing damage.
Consistent with this theory, macromolecular products of oxidative damage generally increase with age in multiple organisms, while genetic and dietary interventions which increase lifespan tend to increase expression of antioxidant enzymes. However, while ROS do appear to play a role in many age-related pathologies and overall frailty with aging, the free radical theory of aging is now generally considered to assign an overly simple role to ROS. Substantial evidence now argues against ROS as a primary driver of normal aging (102, 103). Exogenous antioxidants and overexpression of antioxidant enzymes generally fail to delay age-related pathologies, which are also generally not accelerated by reduction of antioxidant enzymes through genetic manipulation (104–106). In addition, comparative studies examining oxidative damage, metabolic rate, and ROS production across species show no clear correlation between free radical production, neutralization capacity, mitochondrial function, or even oxidative damage accumulation with lifespan (107, 108). Oxidative stress can, of course, modify age-related disease and healthspan, and drive pathologies beyond age-related disease (109).
Complex I and NADH/NAD+
ETC CI may plays a unique role in regulating disease related to mitochondrial function through its NADH dehydrogenase activity which can be at least partly uncoupled from its energetic and ROS roles. Decreased CI activity leads to an increase in the ratio of NADH and NAD+. NAD+ is a crucial cofactor for enzymes which regulate a wide range of cellular processes, such as DNA repair, cytoplasmic and mitochondrial metabolism, and protein acetylation. NAD+-dependent enzymes such as the sirtuins, poly(ADP-ribose) polymerase-1 (PARP-1), and glucose dehydrogenase have been implicated in age and age-related diseases (110–114). Sirtuins are protein deacetylases whose activity are influenced by redox status of NADH/NAD+ and regulate target protein functions through removal of the post-translational acetyl moieties. By responding to NADH/NAD+ levels, sirtuins act as sensors and effectors of intracellular energetic sensing and responses. Sirtuins that have been linked to longevity in organisms from yeast to mammals, though the specific mechanistic role of sirtuins varies; Sir2 regulates longevity in yeast via extrachromosomal ribosomal DNA circle regulation, which is specific to yeast, while Sirt3 and Sirt6 impact specific age-related pathologies in mammals through regulation of mitochondrial acetylation or DNA damage, respectively (115–117).
Recent animal intervention studies have found that NAD+ precursor supplementation reproducibly provides health span benefits, and some data suggests a lifespan benefit in mice, though these findings have since been questioned (118–121). NAD+ precursors have recently been found to modestly attenuate disease and extend survival in the Ndufs4(KO) mouse model of ETC CI deficiency and human Leigh syndrome, suggesting that NADH/NAD+ status directly drives some of the pathology in this disease (122). A very recent study expressing a yeast NADH dehydrogenase (Ndi1) in the Ndufs4(KO) background, which was shown to rescue NADH status in this model, reported a dramatic extension of survival but no rescue of ataxia, strongly indicating that NADH levels mediate some, but not all, aspects of ETC CI related disease (123). Altered redox status of NADH/NAD+ and the impact on NAD+ dependent enzymes may account for the therapeutic benefits observed, but the precise relationships between CI activity, NADH/NAD+, and individual pathologies remains unclear.
Mitochondrial dynamics and quality control
Within individual cells, mitochondria form a highly dynamic network, constantly undergoing fission, fusion, biogenesis, and degradation through the process of mitophagy, the removal of mitochondria via autophagy (reviewed extensively in (124–126)) (Fig. 4). Fission and fusion are coordinated processes which regulate the morphology, size, and number of mitochondria in a cell (127–129). Mitochondrial fission contributes to the removal of damaged/dysfunctional mitochondrial content by fragmenting the mitochondrial network, which allows for removal of poorly functioning mitochondrion by degradation through mitophagy. In contrast, fusion is a process which merges individual mitochondria and can alleviate mitochondrial defects by enabling an exchange of protein, lipid, and nucleic acid components.
Figure 4 – Mitochondrial dynamics.
Mitochondria form dynamic networks within individual cells regulated by mitochondrial fission, fusion, and removal through mitophagy. Fusion allows for mixing of mitochondrial components, whereas fission allows mitochondrial regions containing dysfunctional ETC machinery to be targeted for degradation. Compromised mitochondrial membrane potential in dysfunctional mitochondria leads to stabilization and phosphorylation of Pink1. Pink1 recruits and activates Parkin, which ubiquitinates adapter proteins such as optineurin. These ubiquitinated substrates are recongnized by the autophagy machinery, leading to engulfment and degradation of targeted mitochondria. A number of proteins involved in mitochondrial dynamics have been causally linked to human disease.
The number, biomass, structure, and organization of mitochondria is cell type and context dependent, and influenced by energetic and metabolic status, including the ratio of ATP:ADP and NADH:NAD (130, 131). Cell and tissue specific differences in mitochondrial content, energetic demands, and mitochondrial morphology and organization are all thought to contribute to the overall clinical manifestation of mitochondrial dysfunction, including primary genetic disorders, toxicology related, and age-related pathologies (127, 132). Various components of fission, fusion, and mitophagy (degradation of damaged mitochondria) have been associated with disease, as detailed below.
Fission and fusion
Dysregulation of either fission or fusion alone or the net rate of mitochondrial fissing and fusing drives pathology, including age-related pathologies (133). In drosophila, genetic manipulations impacting fission and fusion in model organisms generally suggest that increased fission is protective against disease; upregulation of the fission factor Drp1 was reported to increase lifespan in Drosophila through an increased clearance of defective mitochondria resulting from increased network fragmentation (134), and overexpression of Parkin (see “Variants of Strong Effects”) has been shown to increase lifespan through increased targeting of mitochondria for removal, as well as depletion of mitofusins (proteins involved in mitochondrial fusion) (135). While the net increase in mitophagy through fission factor overexpression appears to drive mitochondrial flux and promote health, fusion is also necessary for mixing of mitochondrial contents and adaptations to metabolic demands, and proper balance between fission and fusion is necessary for health and longevity assurance (136).
Mitophagy
Removal of damaged or dysfunctional mitochondria through macroautophagy is a critical pathway for mitochondrial quality control known as mitophagy. The process of, and factors involved in, mitophagy are thoroughly reviewed by Pickles et al, 2018 (137) (see also (126, 138, 139), Fig. 4). Briefly, during mitophagy, dysfunctional mitochondria are targeted by the autophagic machinery for degradation in the lysosome. Multiple pathways target dysfunctional mitochondria for degradation, the best characterized mechanism involves PINK1 (PTEN-induced putative kinase 1) and Parkin, which are well-known for their association with Parkinson’s disease (see Human Genetics section). In functional mitochondria, PINK1 is imported through the translocase of the outer mitochondrial membrane (TOM) and translocase of the inner membrane (TIM23) complexes to eventually span the inner membrane. Here it is cleaved by the protease PARL (PINK1/PGAM5-Associated Rhomboid-Like protease) and the released cleavage product is degraded in the cytoplasm. Transit through the TIM23 complex is membrane potential dependent (140, 141); under conditions where mitochondrial membrane potential is compromised, PINK1 fails to transit through TIM23, and is stabilized on the outer mitochondrial membrane by TOM. Here, PINK1 autophosphorylation leads to recruitment and activation of Parkin1, which ubiquitinates outer mitochondrial membrane protein substrate proteins that then act as adaptors for the autophagosome maturation protein ULK1. Two such adaptors have been shown to be necessary, but redundant, for mitophagy in mammals: NDP52 and optineurin (OPTN) (142).
Impairment of autophagy/mitophagy is thought to impact overall mitochondrial energetics as damaged or defective organelles accumulate. Model organism studies have reported a correlation between autophagic flux and longevity and healthspan (143–145). Human age-related diseases, particularly neurodegenerative diseases, have also been directly associated with defective autophagic processes (see Human Genetic Evidence section). Mitochondrial quality control through mitophagy is an important longevity assurance mechanism, but whether activation of mitophagy is sufficient for improvement in lifespan and healthspan under normal conditions is unclear. Only in Drosophila has activation of mitophagy, through overexpression of Parkin, been shown to increase lifespan (135, 146). Conversely, while evidence directly linking mitophagy to improved longevity is limited, defective mitophagy is clearly linked to a variety of diseases, mostly age-related or age-associated. For example, OPTN defects are linked to ALS, glaucoma, and other neurodegenerative disorders (147, 148). Parkinson’s disease is another notable example, where multiple causal genes directly regulate mitophagy, but mitochondrial turnover is also important in cardiovascular, skeletal, and non-parkinsonian neurological aging (149–152).
Hormesis and retrograde signaling
ROS are now understood to play important roles in intracellular and cell to cell signaling, and there is growing evidence that ROS can promote longevity under some conditions (153). For example, low levels of mitochondrial stress upregulate quality control mechanisms that confer stress resistance and enhance survival, a concept known as hormesis or, when specifically referring to mitochondria, mitohormesis (154). Various genetic and pharmacological methods for increasing lifespan in C. elegans have been shown to be dependent on ROS, including ETC component mutations and pro-oxidant compounds (155, 156). In these settings, ROS mediate lifespan extension, at least partly, through feedback regulation of the nutrient and oxygen sensing factors mTOR, AMPK, and HIF-1 (93, 157).
In addition to ROS, several other forms of mitochondria regulated intracellular retrograde responses (i.e. mitochondria driven signaling to the nucleus) have been characterized, including the mitochondrial unfolded protein response (mtUPR or UPRmt, see below), and the intrinsic apoptotic pathway (158). One of the best described retrograde responses is in yeast, where mitochondrial dysfunction leads to a decreased ATP:ADP ratio or lowered membrane potential. These changes activate specific transcription factors that regulate nuclear-encoded mitochondrial protein expression and modify nitrogen and carbon metabolism (159, 160). In yeast, the transcription factor PHO84 was shown to be both necessary and sufficient for lifespan extension resulting from this retrograde response (161). In nematodes, a p38/MAPK dependent pathway was recently reported to underlie energetic deficit driven retrograde signaling in C. elegans that mediates longevity (162). Various mammalian retrograde signaling pathways have also been identified (163–165).
Mitochondrial unfolded protein response
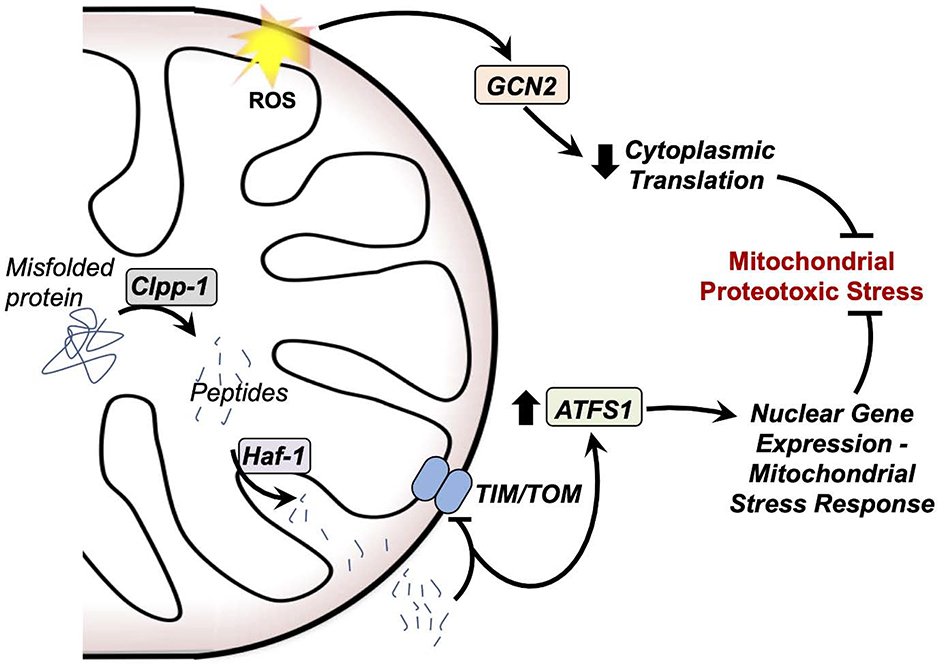
The mtUPR is a set of proteostasis regulating retrograde signaling responses; detection of unfolded mitochondrial proteins leads to the induction of nuclear-encoded mitochondrial chaperones and proteases (166). While the mtUPR was first described in mammalian cells and has been demonstrated in yeast (167), this pathway has been best described in C. elegans (168, 169) (Fig. 5). Briefly, nematode mtUPR is initiated by cleavage of unfolded mitochondrial proteins into peptides by the mitochondrial protease CLPP-1, and export of the peptides into the cytoplasm by the peptide transporter HAF-1. Cytoplasmic mitochondria-derived peptide accumulation inhibits the TIM/TOM complex, resulting in extra-mitochondrial accumulation of the transcription factor ATFS-1, which is normally targeted to mitochondria and degraded. Increased ATFS-1 levels coordinate induction of mitochondrial stress response genes (170).
Figure 5 – Mitochondrial unfolded protein responses.
Mitochondria detect and respond to proteotoxic stress through at least two distinct pathways. In one pathway, mitochondrial ROS activate the kinase GCN2, which reduces cytoplasmic translation through inhibition of the eukaryotic transcription initiation factor. In another, misfolded proteins in the mitochondria are cleaved by mitochondrial proteases, including Clpp-1, and exported through the peptide transporter Haf-1. These peptides inhibit the TIM and TOM transport machinery, leading to a cytoplasmic accumulation of the transcription factor ATFS1. ATFS1 upregulates expression of proteins involved in the mtUPR. Together, these two pathways reduce proteotoxic stress at the mitochondria.
In a parallel pathway, mitochondrial ROS-mediated activation of the kinase GCN-2 leads to inhibition of the eukaryotic transcription initiation factor 2a (eIF2a), which suppresses protein translation, aiding in relieving proteotoxic stress. Many of these factors have been found to be conserved to mammals (171, 172).
Early data suggested that activation of the mtUPR could extend lifespan, but more recent studies have led to a re-evaluation of those findings. While the mtUPR plays an undisputed role in survival and longevity assurance by mediating cellular stress responses, and dysfunction of the mtUPR may play an important role in age-related pathology, it is not clear whether activation of the mtUPR is either sufficient for extension of lifespan in model organisms on its own or necessary for lifespan extension in conditions where mitochondrial function is modulated, including caloric restriction (158, 173, 174). The mtUPR has, however, been shown to be necessary for enhanced survival in response to certain mitotoxic stresses (170).
Human genetics
Variants of strong effects
Rare genetic variants with strong effect can provide insight into biological processes that influence age-related pathologies. In particular, progeroid syndrome and inherited forms of classical age-related pathologies have revealed ‘longevity assurance’ pathways. Disruption of these pathways results in pathologic changes reminiscent of normal aging. While various forms of mitochondrial dysfunction have been associated with pathologies of aging, mutations in the mitochondrial genome or in nuclear genes encoding mitochondrial proteins do not generally seem to cause multi-system progeroid phenotypes (with the exception of the so-called ‘mutator mouse’, see above). Rather, genetic variants with strong deleterious effects tend to cause a variety of distinct clinical syndromes, which are reviewed elsewhere (1, 18).
Mitonuclear genetic variants with functional effects lesser than those causing pediatric disorders, yet still strong enough to follow classic single-gene inheritance patterns, have been directly linked to adult onset mitochondrial diseases as well as certain age-related pathologies. For example, genes leading to familial Parkinson’s disease, accounting for ~10–15 percent of all cases, include multiple factors involved in mitophagy, such as PARK2 (parkin) and VPS13C (involved in pink1/parkin mediated autophagy) (175, 176). ApoE mutations are associated with a dramatically increased risk of Alzheimer’s disease, as well as cardiovascular disease (177, 178). SOD1 mutations lead to familial amyotrophic lateral sclerosis (ALS). Though ALS pathology is distinct from classical age-related neurodegeneration, ALS is age-associated, and the Sod1/ALS link demonstrates a link between oxidative stress pathways and neurodegenerative pathologies as a function of age.
Common variants - genome-wide association studies (GWAS)
While the number of individual mitonuclear genes directly implicated in age-related pathologies through strong genetic associations is small, variants with subtler effects can also influence the function of the encoded target. If the impact of subtler variants in a gene are strong enough, the gene can be identified as trait-associated through genome-wide association studies (GWAS). GWAS associate human traits with genetic variants, often single-nucleotide polymorphisms (SNP’s), spread throughout the genome, providing a powerful tool for identifying genes responsible for human phenotypes, including complex human disease. GWAS identify genetic regions, rather than genes per se, implicating adjacent genes but not demonstrating causality or definitively pointing to individual local genetic features. Furthermore, SNP’s themselves may be functional, but are more often silent markers that simply co-segregate with functional variants elsewhere in the region. GWAS findings are also strongly influenced by the genetic background of the population studies and suffer from various statistical limitations.
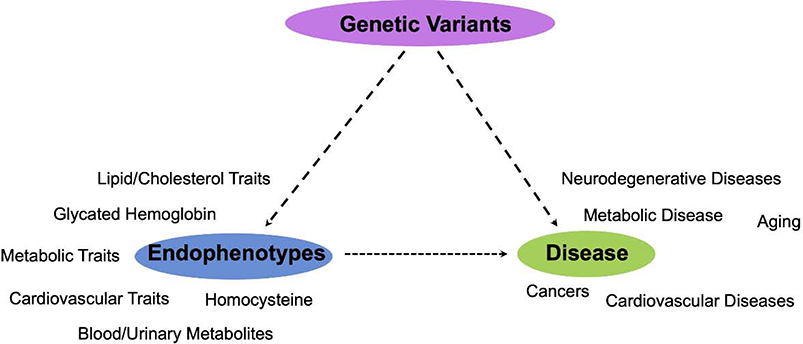
The catalog of all published Genome-Wide Association Studies is kept up-to-date and publicly available through the National Human Genome Research Initiative (NHGRI) (https://www.ebi.ac.uk/gwas/) (179). This catalog contains all variant-gene associations reaching a suggestive association threshold of p≤10–5. Here, we provide a brief review GWAS data linking genes encoding mitochondrial proteins (see (3) for criteria defining mitonuclear genes) to disease and endophenotypes, tractable discrete physiologic endpoints that are related to complex phenotypes (Fig. 6, Tables 1 and 2).
Figure 6 – Direct and indirect associations between mitonuclear genes and human disease.
Genetic association studies have identified gene regions directly associated with the risk of some complex human diseases (Table 1). In addition, GWAS studies have identified associations between mitonuclear genetic regions and endophenotypes which are associated with disease states.
Table 1 –
GWAS variants in mitonuclear genes associating with disease (see GWAS catalog (182) for references to primary literature)
| Gene | Disease | SNP Risk Allele | Context | p-value |
|---|---|---|---|---|
| ROS/Redox Regulation | ||||
| SOD1 | Amyotrophic lateral sclerosis | rs13048019-T | Intron | 3.00E-08 |
| Proteostasis / Turnover | ||||
| KIF1B | Hepatocellular carcinoma | rs17401966-A | Intron | 2.00E-18 |
| PARK2* | Aging* | rs16892673 | Intron | 5.00E-06 |
| ZNF746 | Amyotrophic lateral sclerosis | rs855913-A | Intergenic | 4.00E-08 |
| PPARG | Type 2 diabetes | rs1801282-C | Missense | 6.00E-10 |
| Metabolism | ||||
| ACAD10 | Coronary heart disease | rs11066015-A | Intron | 5.00E-11 |
| ACYP2 | Sudden cardiac arrest | rs1559040 | Intron | 4.00E-08 |
| ATP5G1 | Coronary heart disease | rs46522-T | Intron | 2.00E-08 |
| COX11 | Breast cancer | rs6504950-G | Intron | 2.00E-13 |
| CPS1 | Chronic kidney disease | rs7422339-A | Missense | 1.00E-15 |
| CYP24A1 | Multiple sclerosis | rs2248359-G | Near 5′UTR | 3.00E-11 |
| CYP27B1 | Multiple sclerosis | rs703842-A | 3′UTR | 5.00E-11 |
| GCK | Metabolic syndrome | rs3757840-A | Intergenic | 4.00E-13 |
| LIPC | Age-related macular degeneration | rs10468017 | Intron | 3.00E-12 |
| Age-related macular degeneration | rs920915-C | Intron | 3.00E-11 | |
| Age-related macular degeneration | rs10468017 | Intron | 1.00E-08 | |
| Metabolic syndrome | rs1532085-G | Intron | 5.00E-24 | |
| Metabolic syndrome (bivariate traits) | rs2043085-A | |||
| PEMT | Coronary heart disease | rs12936587-G | Intergenic | 4.00E-10 |
| PFKP | Amyotrophic lateral sclerosis (sporadic) | rs9329300 | Intergenic | 2.00E-09 |
| PPAP2B | Coronary artery disease | rs17114036-G | Intron | 1.00E-08 |
| Ischemic Stroke | rs17114036-G | Intron | 3.00E-08 | |
| Large artery stroke | rs17114036-G | Intron | 2.00E-08 | |
| Coronary heart disease | rs17114036-A | Intron | 4.00E-19 | |
| SUCLA2* | Aging* (time to event) | rs8001976-T | Intergenic | 3.0E-06 |
Table 2 –
GWAS variants in mitonuclear genes associated with disease-related endophenotypes (see GWAS catalog (182) for references to primary literature).
| Gene | Endophenotype | SNP Risk Allele |
|---|---|---|
| Redox | ||
| CYBA | Glycated hemoglobin levels | rs9933309 |
| MSRA | Adiposity | rs7826222 |
| Proteostasis | ||
| MRPL33 | Fasting glucose-related traits | rs3736594 |
| TOMM20 | Total cholesterol, LDL cholesterol | rs514230, rs514230 |
| TUFM | Body mass index | rs7359397 |
| PHB | Diastolic blood pressure | rs16948048 |
| Metabolic | ||
| ACAD11 | HDL cholesterol, LDL cholesterol | rs17404153 |
| ACYP2 | Telomere length | rs11125529 |
| ALOX5 | Cholesterol, total; HDL cholesterol | rs970548 |
| CERS2 | LDL cholesterol | rs267733 |
| CPS1 | HDL cholesterol | rs1047891 |
| Homocysteine levels | rs7422339, rs1047891 | |
| CPT1A | Lipid metabolism phenotypes (LA/PUFA) | rs17610395 |
| FADS1 | Fasting glucose-related traits (FPG, HOMA-B, interaction with BMI) | rs174550 |
| Heart rate | rs174549, rs174547 | |
| Plasma EPA, ALA, DPA levels | rs174550, rs174547, rs174547 | |
| Total cholesterol, HDL cholesterol, LDL cholesterol | rs174546, rs174547, rs174548 | |
| Lipid metabolism phenotypes (LA/PUFA) | rs174547 | |
| Plasma omega-6 polyunsaturated fatty acid levels | rs174550, rs2727270, rs174547, rs174555 | |
| Triglycerides | rs174546, rs174547, rs174548 | |
| GCK | Fasting glucose-related traits (HOMA-B, FPG, BMI interaction, glucose) | rs4607517 |
| Glycated hemoglobin levels | rs1799884, rs730497 | |
| Glycemic traits (1-hPG, 2-hPG, FPG) | rs1799884 | |
| GPAM | Total cholesterol, LDL cholesterol | rs2255141 |
| HK1 | Glycated hemoglobin levels | rs16926246, rs7072268 |
| HMGCR | Cholesterol, total; LDL cholesterol | rs12916, rs10045497, rs3846662; rs12654264, rs3846663, rs12916, rs3846662, rs7703051, rs10045497, rs12654264 |
| Body mass index | rs2112347 | |
| LIPC | Cholesterol, total; HDL cholesterol | rs1532085; rs10468017, rs1532085, rs1800588, rs10468017, rs261334, rs4775041, rs1077835, rs1532085, rs1077834 |
| Lipid metabolism phenotypes | rs1532085, rs1532085, rs35853021, rs1532085, rs2043085, rs588136 | |
| Phospholipid levels (plasma) | rs10468017 | |
| Triglycerides | rs1532085, rs1532085, rs4775041 | |
| SPTLC3 | LDL cholesterol | rs364585 |
GWAS evidence linking mitochondrial pathways to human disease
Mitonuclear genes found to associate with human cardiovascular diseases at a GWAS suggestive significance level are largely involved in lipid metabolism: ACAD10, involved in beta-oxidation; ATP5G1, a subunit of ATP synthase of the ETC; LIPC, a lipase involved in triglyceride metabolism and lipoprotein uptake; PPAP2B, involved in de novo synthesis of glycerolipids and signal transduction through phospholipase D. ACYP2, a muscle-specific acylphosphatase, and PEMT, an enzyme involved in plasma membrane lipid synthesis, have also been associated with cardiovascular disease by GWAS.
Genes associating with neurodegenerative disease are generally related to redox and metabolism. They include SOD1, which is associated with familial ALS (see above); the zinc-finger transcriptional factor encoding gene ZNF746, which represses peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor which regulates fatty acid and glucose metabolism; PFKP, encoding the glycolysis enzyme phosphofructokinase; CYP24A1, cytochrome P450 monooxygenase, involved in metabolism of cholesterol, steroids, and lipids; and CYP27B1, an inner mitochondrial membrane enzyme that is involved in the synthesis of the active form of vitamin D3.
Two mitonuclear genes associate with metabolic disease: PPARγ and GCK, encoding glucokinse. Glucokinase is a hexokinase isozyme expressed in liver and pancreas and is involved in mammalian regulation of systemic glucose metabolism. Unlike the other hexokinases, glucokinase is not product inhibited, providing liver and pancreas specific supply side driven regulation of glucose metabolism.
Cancer traits show two GWAS associations with candidate mitonuclear aging gene: KIF1B, a kinesin motor protein that transports mitochondria, and COX11, which encodes a subunit of cytochrome c oxidase (complex IV of the ETC).
Endophenotypes
Many genes encoding mitochondrial proteins have been associated by GWAS with relevant endophenotypes, tractable traits linked to the risk or presentation of age-related pathologies (see Table 2). These include traits like blood cholesterol levels, body mass index, heart rate, and telomere length. The majority of GWAS age-related disease endophenotypes relate to blood metabolite levels, and many of the GWAS loci are in genetic regions which encode metabolic proteins, as might be expected. Four genes are involved in mitochondrial proteostasis (MRPL33, TOMM20, TUFM, and PHB) and three in ROS/redox (CYBA, MRSA). These associate with age-related endophenotypes including blood pressure, blood cholesterol levels, adiposity, and glycated hemoglobin levels (Table 2, Fig. 6). 10 out of 19 of the genes identified are related to circulating cholesterol levels, including HDL, LDL, and total cholesterol, and six are linked to circulating glucose or glycated hemoglobin, a marker of overall glucose levels. Fat metabolites and overall body mass index are linked to six genes.
Additional evidence from GWAS
GWAS are powerful tools for providing initial evidence linking genetic loci (and associated genes) to traits, but they do not demonstrate causality, provide directionality, are susceptible to technical and statistical errors, and are influenced by underlying differences in the studied populations. GWAS meta-analyses, studies pooling the findings from multiple GWAS, can enhance the confidence with which individual gene-regions are associated with traits by compiling data from multiple platforms, populations, and research groups to assess the overall confidence of GWAS associations. A 2015 meta-analysis of all age-related GWAS disease traits found that cholesterol metabolism genes are enriched among multiple age-related diseases (180). Those findings suggest that lipoprotein/cholesterol metabolism is a crucial modulator of normal human aging and age-related disease, and that this may be the most important mitochondrial pathway of aging at the population level.
Newer approaches to examining genome-wide association data push the boundaries of what these data can reveal concerning complex disease, including the role of mitochondria in human diseases. A 2016 network-analysis based assessment of GWAS associations arising from mitonuclear genes found that each of five major age-related disease categories (cardiovascular disease, cancer, neurodegenerative disease, metabolic disease, and inflammatory diseases) is associated distinct functional mitochondrial pathways (181). Cancer traits were significantly enriched for associations with genes and protein network interactions involved in mitochondrial intrinsic apoptosis, for example, whereas cardiovascular disease associated mitonuclear genes are largely involved in apolipoprotein metabolism. Tissue expression quantitative trait loci (eQTL) assessment suggested that GWAS identified genes show differential patterns of expression in human tissues, stratified by disease. Neurodegenerative disease-associated mitonuclear genes are highly expressed only in the brain, while other disease associated genes show widespread expression.
Discussion
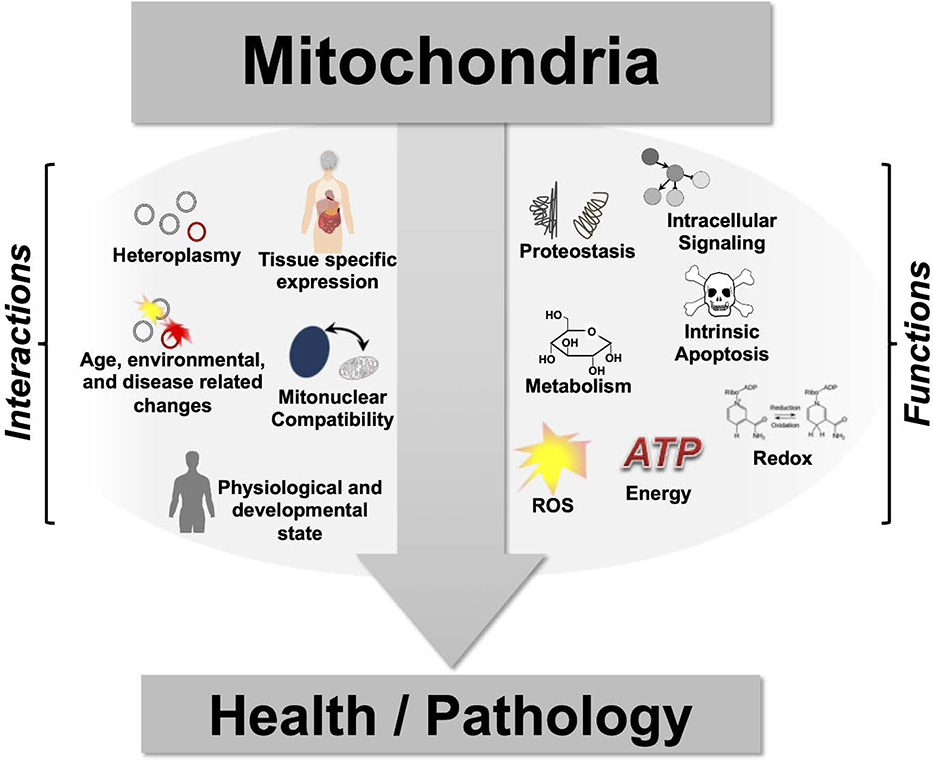
It has become clear in recent years that mitochondria play a much more complex and nuanced role in human disease than simply acting as the cellular powerhouse, or as producers of toxin reactive oxygen species. Mitochondria modulate energy sensing and nutrient signaling, redox status, cellular proteostasis, intrinsic apoptosis, and a diversity of metabolic processes (Fig. 7). Given this wide array of biological functions, it is perhaps unsurprising that mitochondria have been genetically and mechanistically linked to a wide range of pathogenic conditions, such as cancer, cardiovascular disease, immune function, and neurodegeneration (181). Elucidating the precise function of mitochondria in a given condition is, however, greatly complicated by the nature of the architecture of genes encoding mitochondrial transcripts. Mitochondria are influenced by complex interactions between the nuclear and mitochondrial genomes, the complement of heteroplasmy within a given cell, mitochondria, or organism, and epigenetic variation in the nuclear genome.
Figure 7 – The role of mitochondria in human disease.
Determining the precise role of mitochondrial in disease is complicated by both the diverse biological functions mitochondria play and the complex nature of mitochondrial form and function. In addition, various primary causes can trigger mitochondrial dysfunction, including nuclear genetic defects, mitochondrial heteroplasmy, mitonuclear interactions, environmental insults, and age-related macromolecular damage accumulation. Data indicates that presentation of mitochondrial dysfunction is influenced by function impacted and tissue expression of impacted genes, but the mechanisms underlying presentation of any specific defect are often unknown. A systems approach considering mitochondrial biology, cellular interactions, tissue expression, and organism physiology, will be necessary to fully define the role of mitochondria in human health and disease.
Mitochondria-associated pathways of age-related disease, as defined by model organism studies or genetic associations, should be carefully considered in the context of human tissue expression data – a lifespan regulating pathway in C. elegans may only be important to a single tissue in mammals. Pharmacological targeting of pathways which show tissue specificity will likely be limited by tissue delivery methods, such as strategies to cross the blood-brain barrier in CNS specific pathways. Systemic manipulation of processes with tissue specific roles in aging and age-related disease may result in unanticipated effects outside of the relevant tissue. Nuanced approaches may be required in order to translate model organism or genetic findings related to mitochondria to human disease or biogerontology. Systems approaches to assessing mitochondrial pathways in mammalian disease may bridge the gap between basic research and therapeutic interventions.
Highlights.
Mitochondrial dysfunction has been reported to play a role in many human diseases and disrupted mitochondrial function is a hallmark of aging
Significant questions remain regarding their precise mechanistic role of mitochondria in individual pathologies
Mitochondria influence a diversity of cellular systems beyond energetics and reactive oxygen species
Studies in model organisms provide mechanistic insight into the role of mitochondrial pathways in disease and longevity
Human genetic diseases and evidence from genome-wide association studies (GWAS) provide additional insight into the role of mitochondria in human health
Acknowledgments
Funding
SCJ was supported by NIH R00 award GM126147. RB was supported by NIH T32GM095421.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorman GS et al. , Mitochondrial diseases. Nat Rev Dis Primers 2, 16080 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW, The genetics and pathology of mitochondrial disease. J Pathol 241, 236–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SC et al. , Network analysis of mitonuclear GWAS reveals functional networks and tissue expression profiles of disease-associated genes. Hum Genet 136, 55–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraja AT et al. , Associations of Mitochondrial and Nuclear Mitochondrial Variants and Genes with Seven Metabolic Traits. Am J Hum Genet 104, 112–138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaeberlein M, Longevity and aging. F1000prime reports 5, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy BK et al. , Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS, Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3, 6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang AB, Jeong DE, Lee SJ, Mitochondria and organismal longevity. Current Genomics 13, 519–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS, Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 16, 1492–1526 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhlbrandt W, Structure and function of mitochondrial membrane protein complexes. BMC Biol 13, 89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann JM, Riemer J, The intermembrane space of mitochondria. Antioxid Redox Signal 13, 1341–1358 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Rich PR, Marechal A, The mitochondrial respiratory chain. Essays Biochem 47, 1–23 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Wolf DM et al. , Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J 38, e101056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagan L, On the origin of mitosing cells. J Theor Biol 14, 255–274 (1967). [DOI] [PubMed] [Google Scholar]
- 16.Lazcano A, Pereto J, On the origin of mitosing cells: A historical appraisal of Lynn Margulis endosymbiotic theory. J Theor Biol 434, 80–87 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bock R, Witnessing Genome Evolution: Experimental Reconstruction of Endosymbiotic and Horizontal Gene Transfer. Annu Rev Genet 51, 1–22 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Wallace DC, Mitochondrial genetic medicine. Nat Genet 50, 1642–1649 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Lee C et al. , The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21, 443–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y et al. , The role of mitochondria-derived peptides in cardiovascular disease: Recent updates. Biomed Pharmacother 117, 109075 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Moreno Ayala MA et al. , Humanin Promotes Tumor Progression in Experimental Triple Negative Breast Cancer. Sci Rep 10, 8542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarate SC, Traetta ME, Codagnone MG, Seilicovich A, Reines AG, Humanin, a Mitochondrial-Derived Peptide Released by Astrocytes, Prevents Synapse Loss in Hippocampal Neurons. Front Aging Neurosci 11, 123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo SE, Mootha VK, The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet 11, 25–44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz-Bonengel S, Parson W, No further evidence for paternal leakage of mitochondrial DNA in humans yet. Proc Natl Acad Sci U S A 116, 1821–1822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo S et al. , Biparental Inheritance of Mitochondrial DNA in Humans. Proc Natl Acad Sci U S A 115, 13039–13044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baertling F et al. , A Heterozygous NDUFV1 Variant Aggravates Mitochondrial Complex I Deficiency in a Family with a Homoplasmic ND1 Variant. J Pediatr 196, 309–313 e303 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Catania A et al. , Compound heterozygous missense and deep intronic variants in NDUFAF6 unraveled by exome sequencing and mRNA analysis. J Hum Genet 63, 563–568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aryaman J, Johnston IG, Jones NS, Mitochondrial Heterogeneity. Front Genet 9, 718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggan RM, Lim A, Taylor RW, McFarland R, Pickett SJ, Resolving complexity in mitochondrial disease: Towards precision medicine. Mol Genet Metab, (2019). [DOI] [PubMed] [Google Scholar]
- 30.Taylor RW, Turnbull DM, Mitochondrial DNA mutations in human disease. Nat Rev Genet 6, 389–402 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan RP et al. , Quantitative Variation in m.3243A > G Mutation Produce Discrete Changes in Energy Metabolism. Sci Rep 9, 5752 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filograna R et al. , Modulation of mtDNA copy number ameliorates the pathological consequences of a heteroplasmic mtDNA mutation in the mouse. Sci Adv 5, eaav9824 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bris C et al. , Bioinformatics Tools and Databases to Assess the Pathogenicity of Mitochondrial DNA Variants in the Field of Next Generation Sequencing. Front Genet 9, 632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn A, Zuryn S, The Cellular Mitochondrial Genome Landscape in Disease. Trends Cell Biol 29, 227–240 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Payne BA et al. , Universal heteroplasmy of human mitochondrial DNA. Hum Mol Genet 22, 384–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart JB, Chinnery PF, The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet 16, 530–542 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Trifunovic A et al. , Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Kujoth GC et al. , Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Bua E et al. , Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 79, 469–480 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graziewicz MA, Bienstock RJ, Copeland WC, The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7,8-dihydro-8-oxo-2’-deoxyguanosine. Hum Mol Genet 16, 2729–2739 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payne BA, Gardner K, Chinnery PF, Mitochondrial DNA mutations in ageing and disease: implications for HIV? Antivir Ther 20, 109–120 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Li M et al. , High frequency of mitochondrial DNA mutations in HIV-infected treatment-experienced individuals. HIV Med 18, 45–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne BA et al. , Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 43, 806–810 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jitratkosol MH et al. , Blood mitochondrial DNA mutations in HIV-infected women and their infants exposed to HAART during pregnancy. AIDS 26, 675–683 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Gorini S et al. , Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid Med Cell Longev 2018, 7582730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carew JS et al. , Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia 17, 1437–1447 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Guigni BA et al. , Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol 315, C744–C756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn A, Zuryn S, Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants (Basel) 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clancy DJ, Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 7, 795–804 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Rand DM, Fry A, Sheldahl L, Nuclear-mitochondrial epistasis and drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172, 329–341 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pichaud N et al. , Age Dependent Dysfunction of Mitochondrial and ROS Metabolism Induced by Mitonuclear Mismatch. Front Genet 10, 130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagao Y et al. , Decreased physical performance of congenic mice with mismatch between the nuclear and the mitochondrial genome. Genes Genet Syst 73, 21–27 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Roubertoux PL et al. , Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet 35, 65–69 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Meiklejohn CD et al. , An Incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet 9, e1003238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmbeck MA, Donner JR, Villa-Cuesta E, Rand DM, A Drosophila model for mito-nuclear diseases generated by an incompatible interaction between tRNA and tRNA synthetase. Dis Model Mech 8, 843–854 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latorre-Pellicer A et al. , Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Sloan DB, Fields PD, Havird JC, Mitonuclear linkage disequilibrium in human populations. Proc Biol Sci 282, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parey K et al. , Cryo-EM structure of respiratory complex I at work. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava AP et al. , High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane. Science 360, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ricquier D, UCP1, the mitochondrial uncoupling protein of brown adipocyte: A personal contribution and a historical perspective. Biochimie 134, 3–8 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Childress ES, Alexopoulos SJ, Hoehn KL, Santos WL, Small Molecule Mitochondrial Uncouplers and Their Therapeutic Potential. J Med Chem 61, 4641–4655 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Geisler JG, 2,4 Dinitrophenol as Medicine. Cells 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pecqueur C, Couplan E, Bouillaud F, Ricquier D, Genetic and physiological analysis of the role of uncoupling proteins in human energy homeostasis. J Mol Med (Berl) 79, 48–56 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Busiello RA, Savarese S, Lombardi A, Mitochondrial uncoupling proteins and energy metabolism. Frontiers in Physiology 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Souza BM et al. , Associations between UCP1 −3826A/G, UCP2 −866G/A, Ala55Val and Ins/Del, and UCP3 −55C/T polymorphisms and susceptibility to type 2 diabetes mellitus: case-control study and meta-analysis. PLoS One 8, e54259–e54259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chathoth S et al. , Association of Uncoupling Protein 1 (UCP1) gene polymorphism with obesity: a case-control study. BMC Med Genet 19, 203–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H-J et al. , Associations between polymorphisms in the mitochondrial uncoupling proteins (UCPs) with T2DM. Clin Chim Acta 398, 27–33 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Clapham JC et al. , Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 406, 415–418 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Choi CS et al. , Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117, 1995–2003 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geisler JG, 2,4 Dinitrophenol as Medicine. Cells 8, 280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liesa M, Shirihai OS, Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17, 491–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khayat ZA et al. , Rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and cPKC. Am J Physiol 275, C1487–C1497 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Pelletier A, Joly E, Prentki M, Coderre L, Adenosine 5’-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology 146, 2285–2294 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Perry RJ, Zhang D, Zhang X-M, Boyer JL, Shulman GI, Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson SC et al. , mTOR inhibitors may benefit kidney transplant recipients with mitochondrial diseases. Kidney Int 95, 455–466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A, Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal 26, 1598–1603 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Yarian CS, Toroser D, Sohal RS, Aconitase is the main functional target of aging in the citric acid cycle of kidney mitochondria from mice. Mech Ageing Dev 127, 79–84 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saha AK et al. , Malonyl-CoA regulation in skeletal muscle: its link to cell citrate and the glucose-fatty acid cycle. Am J Physiol 272, E641–648 (1997). [DOI] [PubMed] [Google Scholar]
- 79.Salminen A, Kauppinen A, Hiltunen M, Kaarniranta K, Krebs cycle intermediates regulate DNA and histone methylation: epigenetic impact on the aging process. Ageing Res Rev 16, 45–65 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Coppieters N et al. , Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging 35, 1334–1344 (2014). [DOI] [PubMed] [Google Scholar]
- 81.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J, Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol 17, 1218–1225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ziegler DV, Wiley CD, Velarde MC, Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell 14, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lesnefsky EJ, Hoppel CL, Oxidative phosphorylation and aging. Ageing Res Rev 5, 402–433 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Sandhu SK, Kaur G, Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology 4, 19–29 (2003). [DOI] [PubMed] [Google Scholar]
- 85.DeBalsi KL, Hoff KE, Copeland WC, Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res Rev 33, 89–104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stumpf JD, Saneto RP, Copeland WC, Clinical and molecular features of POLG-related mitochondrial disease. Cold Spring Harb Perspect Biol 5, a011395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levin L, Mishmar D, A genetic view of the mitochondrial role in ageing: killing us softly. Adv Exp Med Biol 847, 89–106 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Yoon YS, Byun HO, Cho H, Kim BK, Yoon G, Complex II defect via down-regulation of iron-sulfur subunit induces mitochondrial dysfunction and cell cycle delay in iron chelation-induced senescence-associated growth arrest. J Biol Chem 278, 51577–51586 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Stockl P, Hutter E, Zwerschke W, Jansen-Durr P, Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Exp Gerontol 41, 674–682 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Dell’agnello C et al. , Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet 16, 431–444 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Liu X et al. , Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev 19, 2424–2434 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu C et al. , Genetic inhibition of an ATP synthase subunit extends lifespan in C. elegans. Sci Rep 8, 14836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hwang AB et al. , Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 111, E4458–4467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang HW, Shtessel L, Lee SS, Collaboration between mitochondria and the nucleus is key to long life in Caenorhabditis elegans. Free Radic Biol Med 78, 168–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knorre DA, Severin FF, Uncouplers of Oxidation and Phosphorylation as Antiaging Compounds. Biochemistry (Mosc) 81, 1438–1444 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ, Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7, 552–560 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Rea SL, Ventura N, Johnson TE, Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol 5, e259 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dancy BM, Sedensky MM, Morgan PG, Effects of the mitochondrial respiratory chain on longevity in C. elegans. Exp Gerontol 56, 245–255 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Dues DJ et al. , Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans. Free Radic Biol Med 108, 362–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harman D, Aging: a theory based on free radical and radiation chemistry. Journal of gerontology 11, 298–300 (1956). [DOI] [PubMed] [Google Scholar]
- 101.Harman D, Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci 1067, 10–21 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Vina J, Borras C, Gomez-Cabrera MC, A free radical theory of frailty. Free Radic Biol Med 124, 358–363 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Egea J et al. , European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13, 94–162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perez VI et al. , The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 8, 73–75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jang YC et al. , Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci 64, 1114–1125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ran Q et al. , Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci 62, 932–942 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Andziak B et al. , High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5, 463–471 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Shi Y, Buffenstein R, Pulliam DA, Van Remmen H, Comparative studies of oxidative stress and mitochondrial function in aging. Integr Comp Biol 50, 869–879 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Hamilton RT, Walsh ME, Van Remmen H, Mouse Models of Oxidative Stress Indicate a Role for Modulating Healthy Aging. J Clin Exp Pathol Suppl 4, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nacarelli T et al. , NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 21, 397–407 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Longo VD, Kennedy BK, Sirtuins in aging and age-related disease. Cell 126, 257–268 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Zha S, Li Z, Cao Q, Wang F, Liu F, PARP1 inhibitor (PJ34) improves the function of aging-induced endothelial progenitor cells by preserving intracellular NAD(+) levels and increasing SIRT1 activity. Stem Cell Res Ther 9, 224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J, Wang A, Chen Q, SirT3 and p53 Deacetylation in Aging and Cancer. J Cell Physiol 232, 2308–2311 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Luo XY et al. , SIRT1 in cardiovascular aging. Clin Chim Acta 437, 106–114 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK, Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol 2, E296 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McDonnell E, Peterson BS, Bomze HM, Hirschey MD, SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab 26, 486–492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tasselli L, Zheng W, Chua KF, SIRT6: Novel Mechanisms and Links to Aging and Disease. Trends Endocrinol Metab 28, 168–185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Igarashi M et al. , NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell 18, e12935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rajman L, Chwalek K, Sinclair DA, Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab 27, 529–547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchell SJ et al. , Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab 27, 667–676 e664 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang H et al. , NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Lee CF, Caudal A, Abell L, Nagana Gowda GA, Tian R, Targeting NAD(+) Metabolism as Interventions for Mitochondrial Disease. Sci Rep 9, 3073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McElroy GS et al. , NAD+ Regeneration Rescues Lifespan, but Not Ataxia, in a Mouse Model of Brain Mitochondrial Complex I Dysfunction. Cell Metab, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi R, Guberman M, Kirshenbaum LA, Mitochondrial quality control: The role of mitophagy in aging. Trends Cardiovasc Med 28, 246–260 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Rodger CE, McWilliams TG, Ganley IG, Mammalian mitophagy - from in vitro molecules to in vivo models. FEBS J 285, 1185–1202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tilokani L, Nagashima S, Paupe V, Prudent J, Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62, 341–360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Whitley BN, Engelhart EA, Hoppins S, Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Safiulina D, Kaasik A, Energetic and dynamic: how mitochondria meet neuronal energy demands. PLoS Biol 11, e1001755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zorova LD et al. , Mitochondrial membrane potential. Anal Biochem 552, 50–59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jang SY, Kang HT, Hwang ES, Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem 287, 19304–19314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim B, Song YS, Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic Res, 1–6 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Lombes A, Aure K, Bellanne-Chantelot C, Gilleron M, Jardel C, Unsolved issues related to human mitochondrial diseases. Biochimie 100, 171–176 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Chauhan A, Vera J, Wolkenhauer O, The systems biology of mitochondrial fission and fusion and implications for disease and aging. Biogerontology 15, 1–12 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Rana A et al. , Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat Commun 8, 448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rana A, Rera M, Walker DW, Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A 110, 8638–8643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sebastian D, Palacin M, Zorzano A, Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol Med 23, 201–215 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Pickles S, Vigie P, Youle RJ, Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol 28, R170–R185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bingol B, Sheng M, Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic Biol Med 100, 210–222 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Palikaras K, Lionaki E, Tavernarakis N, Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 20, 1013–1022 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Schendzielorz AB et al. , Two distinct membrane potential-dependent steps drive mitochondrial matrix protein translocation. J Cell Biol 216, 83–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schleyer M, Schmidt B, Neupert W, Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem 125, 109–116 (1982). [DOI] [PubMed] [Google Scholar]
- 142.Lazarou M et al. , The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nalbandian A et al. , In vitro studies in VCP-associated multisystem proteinopathy suggest altered mitochondrial bioenergetics. Mitochondrion 22, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT, Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol 38, 863–876 (2003). [DOI] [PubMed] [Google Scholar]
- 145.Weber TA, Reichert AS, Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol 45, 503–511 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Palikaras K, Lionaki E, Tavernarakis N, Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Slowicka K, Vereecke L, van Loo G, Cellular Functions of Optineurin in Health and Disease. Trends Immunol 37, 621–633 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Ying H, Yue BY, Optineurin: The autophagy connection. Exp Eye Res 144, 73–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J, Aging and Autophagy in the Heart. Circ Res 118, 1563–1576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L et al. , Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci Rep 6, 18725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sarzi E et al. , The human OPA1delTTAG mutation induces premature age-related systemic neurodegeneration in mouse. Brain 135, 3599–3613 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Sebastian D et al. , Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J 35, 1677–1693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hood WR, Zhang Y, Mowry AV, Hyatt HW, Kavazis AN, Life History Trade-offs within the Context of Mitochondrial Hormesis. Integr Comp Biol 58, 567–577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ristow M, Zarse K, How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45, 410–418 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Rauthan M, Ranji P, Abukar R, Pilon M, A Mutation in Caenorhabditis elegans NDUF-7 Activates the Mitochondrial Stress Response and Prolongs Lifespan via ROS and CED-4. G3 (Bethesda) 5, 1639–1648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heidler T, Hartwig K, Daniel H, Wenzel U, Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology 11, 183–195 (2010). [DOI] [PubMed] [Google Scholar]
- 157.Schieber M, Chandel NS, TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep 9, 9–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bennett CF et al. , Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat Commun 5, 3483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Butow RA, Avadhani NG, Mitochondrial signaling: the retrograde response. Mol Cell 14, 1–15 (2004). [DOI] [PubMed] [Google Scholar]
- 160.Jazwinski SM, The retrograde response: when mitochondrial quality control is not enough. Biochim Biophys Acta 1833, 400–409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jiang JC et al. , Identification of the Target of the Retrograde Response That Mediates Replicative Lifespan Extension in Saccharomyces cerevisiae. Genetics, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Munkacsy E et al. , DLK-1, SEK-3 and PMK-3 Are Required for the Life Extension Induced by Mitochondrial Bioenergetic Disruption in C. elegans. PLoS Genet 12, e1006133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cardamone MD et al. , Mitochondrial Retrograde Signaling in Mammals Is Mediated by the Transcriptional Cofactor GPS2 via Direct Mitochondria-to-Nucleus Translocation. Mol Cell 69, 757–772 e757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hunt RJ, Bateman JM, Mitochondrial retrograde signaling in the nervous system. FEBS Lett 592, 663–678 (2018). [DOI] [PubMed] [Google Scholar]
- 165.da Cunha FM, Torelli NQ, Kowaltowski AJ, Mitochondrial Retrograde Signaling: Triggers, Pathways, and Outcomes. Oxid Med Cell Longev 2015, 482582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jovaisaite V, Auwerx J, The mitochondrial unfolded protein response-synchronizing genomes. Curr Opin Cell Biol 33, 74–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schleit J et al. , Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell 12, 1050–1061 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arnould T, Michel S, Renard P, Mitochondria Retrograde Signaling and the UPR mt: Where Are We in Mammals? Int J Mol Sci 16, 18224–18251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shpilka T, Haynes CM, The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol 19, 109–120 (2018). [DOI] [PubMed] [Google Scholar]
- 170.Mao XR, Kaufman DM, Crowder CM, Nicotinamide mononucleotide adenylyltransferase promotes hypoxic survival by activating the mitochondrial unfolded protein response. Cell Death Dis 7, e2113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fiorese CJ et al. , The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr Biol 26, 2037–2043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Al-Furoukh N et al. , ClpX stimulates the mitochondrial unfolded protein response (UPRmt) in mammalian cells. Biochim Biophys Acta 1853, 2580–2591 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Bennett CF, Kaeberlein M, The mitochondrial unfolded protein response and increased longevity: cause, consequence, or correlation? Exp Gerontol 56, 142–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cai H et al. , Life-Span Extension by Axenic Dietary Restriction Is Independent of the Mitochondrial Unfolded Protein Response and Mitohormesis in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 72, 1311–1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tysnes OB, Storstein A, Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124, 901–905 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Piredda R, Desmarais P, Masellis M, Gasca-Salas C, Cognitive and psychiatric symptoms in genetically determined Parkinson’s disease: A systematic review. Eur J Neurol, (2019). [DOI] [PubMed] [Google Scholar]
- 177.Marais AD, Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 51, 165–176 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Yin Y, Wang Z, ApoE and Neurodegenerative Diseases in Aging. Adv Exp Med Biol 1086, 77–92 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Welter D et al. , The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 42, D1001–1006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson SC, Dong X, Vijg J, Suh Y, Genetic evidence for common pathways in human age-related diseases. Aging Cell 14, 809–817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnson SC et al. , Network analysis of mitonuclear GWAS reveals functional networks and tissue expression profiles of disease-associated genes. Human Genetics, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.MacArthur J et al. , The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res 45, D896–D901 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]