Abstract
Objective
To estimate temporal trends in total and out-of-pocket (OOP) expenditures for ophthalmic prescription medications among adults in the United States.
Design
A retrospective longitudinal cohort study.
Participants
Participants in the 2007–2016 Medical Expenditure Panel Survey (MEPS), age 18 years or older. The MEPS is a nationally representative survey of the noninstitutionalized, civilian US population.
Methods
We estimated trends in national and per capita annual ophthalmic prescription expenditures by pooling data into 2-year cycles and using weighted linear regressions. We also identified characteristics associated with greater total or OOP expenditure with multivariable weighted linear regression. Costs were adjusted to 2016 US dollars using the Gross Domestic Product Price Index.
Main Outcome Measures
Trends in total and OOP annual expenditures for ophthalmic medications from 2007–2016 as well as factors associated with greater expenditure.
Results
From 2007–2016, 9,989 (4.2%) MEPS participants reported ophthalmic medication prescription use. Annual ophthalmic medication utilization increased from 10.0 to 12.2 million individuals from 2007–2008 to 2015–2016. In this same time period, national expenditures for ophthalmic medications increased from $3.39 billion to $6.08 billion and OOP expenditures decreased from $1.34 to $1.18 billion. While the average number of ophthalmic prescriptions filled did not change over the study period (4.2, p=0.10), the average expenditure per prescription increased significantly from $72.30 to $116.42 (p<0.001). Per capita expenditure increased from $338.72 to $499.42 (p<0.001) and per capita OOP expenditure decreased from $133.48 to $96.67 (p<0.001) from 2007–2008 to 2015–2016 respectively. In 2015–2016, dry eye (29.5%) and glaucoma (42.7%) medications accounted for 72.2% of all ophthalmic medication expenditures. Patients who were older than 65 (p<0.001), uninsured (p<0.001), and visually impaired (p<0.001) were significantly more likely to have greater OOP spending on ophthalmic medications.
Conclusion
Total ophthalmic medication expenditure in the United States increased significantly over the last decade while OOP expenses decreased. Increases in coverage, copayment assistance and utilization of expensive brand drugs may be contributing to these trends. Policy makers and physicians should be aware that rising overall drug expenditures may ultimately increase indirect costs to the patient and offset a decline in OOP prescription drug spending.
Introduction
Greater utilization of prescription drugs and rising drug prices have rapidly increased medication expenditures in the United States.1,2 Growing out-of-pocket (OOP) spending for prescription medications is of particular concern for policymakers as well as consumers and has contributed to substantial legislation and research.2–6 However, despite recent policy efforts, cost-sharing charges are still a significant proportion of pharmaceutical expenditures. In 2017, 14% of all US prescription expenditures were in the form of OOP payments—over $46 billion in total.7
In ophthalmology, OOP spending is a highly topical issue as a large proportion of its patient base are older adults, who face significantly higher OOP expenditures despite Medicare’s near universal coverage.3,8 Visual impairment and eye disease have also been well demonstrated to add substantial economic burden.9,10 Furthermore, certain ophthalmic medications can be expensive, with growing prices over time.11,12 This is of particular concern in the context of recent findings that eye care professionals generated the highest percentage of brand name medication Medicare Part D claims among all prescribers in 2013.13 Rising OOP expenses are not only economically taxing, they also have implications for clinical care: higher prescription drug prices have been associated with lower ophthalmic medication adherence.14,15
While previous research has examined expenditure trends for specific ophthalmic conditions including dry eye syndrome and glaucoma,16,17 these studies examined ophthalmic expenditure data only until 2006. A more recent study reported total costs of Medicare Part D prescriptions for ophthalmic medications but did not include OOP costs or examine trends over time.13 Substantial developments in ophthalmic drug utilization—including the growing use of brand medications for dry eye syndrome—as well as significant changes in insurance coverage highlight the need for more recent data.
The objective of this study was to examine trends in expenditure for ophthalmic medications from 2007–2016 in the United States. Specifically, we describe temporal trends in national and per capita, total and out-of-pocket spending for ophthalmic medications. We also identify individual factors associated with greater expenditure.
Methods
This study was a cross-sectional retrospective analysis of total and OOP expenditures for ophthalmic prescription medications from 2007 to 2016 in the United States using data from the Medical Expenditure Panel Survey (MEPS). Data utilized in this study was publicly available from the Agency for Healthcare Research and Quality (AHRQ) and was exempted by the Yale University Institutional Review Board. This study was conducted in adherence to the tenets of the Declaration of Helsinki.
Data Source
The MEPS is a nationally representative survey of non-institutionalized individuals in the United States that includes participants drawn from the prior year’s National Health Interview Survey.18 Panels consist of approximately 15,000 households annually who are followed for two and a half years with 5 rounds of interviews administered by telephone. At the end of each panel, participants are assigned person-weights which include calibrations to nonresponse. To calculate accurate health care use and expenditures, additional information from medical providers, hospitals and pharmacies were also gathered to supplement individual response data. The MEPS has been utilized by multiple previous analyses to assess longitudinal trends in medication use, expenditure and economic burden in ophthalmology and other conditions.4,10,16,17,19,20
For this study, we utilized the MEPS Household Component’s Full-Year Consolidated Data files and the Prescribed Medicine files from 2007–2016. Response rates in the MEPS Household Component ranged from 46.0–59.3% during this study period.21 To smooth analyses of trends, we pooled annual data into 2-year periods beginning with 2007–2008 through 2015–2016. Person weights were accordingly adjusted to represent annual estimates. We excluded participants younger than 18 years of age and included only those with positive person survey weights, to better represent the cross-sectional national population.
Ophthalmic Medication Utilization and Expenditure
During MEPS interviews, participants were asked to provide the name of any prescribed medicines that they or anyone in their family had purchased or otherwise obtained. Respondents reporting prescriptions were subsequently asked for consent for the MEPS to obtain additional drug detail information including the medicine name, fill date, as well as sources of payment and associated payment amounts from their pharmacy. In 2011, 69.6% of participants signed pharmacy permission forms and 73.3% of contacted pharmacies provided prescription data.22 The definition of a prescription in this study was any prescription of an individual medication with varying days of supply. For example, if a patient filled latanoprost 4 times in a year they would have had 4 prescriptions that given year.
For prescriptions without pharmacy data, prescription and payment information was imputed using a proprietary matching program based on matching variables including a participant’s prior prescriptions, a prescription’s National Drug Code (NDC), active ingredients, dosage form and strength. Imputation also utilized data from other respondents based upon matching prescription or patient characteristics including age, sex, health conditions and health status. Previous research comparing drug use between the MEPS and Medicare Part D claims found an agreement rate of 0.97 indicating “substantial” agreement.23 Drug expenditure was also similar between the two databases, differing on a per capita basis by only 4%, with Lin’s concordance of 0.78, affirming the validity of MEPS prescription data.23 Data prior to 2007 and after 2016 was available but was not utilized for longitudinal analysis due to changes in the editing and imputation process of expenditure data.18 In brief, the AHRQ implemented methodological changes in 2007 regarding the identification of expenditure outliers based upon a report benchmarking the distribution of prices in the MEPS to private claims data. In 2016, the AHRQ began allowing higher imputed pricing to account for the rising prices of specialty drugs. More information about these changes as well as the editing and imputation process are available elsewhere.18,22
We identified all ophthalmic prescriptions in the MEPS database based on Multum Lexicon Therapeutic Class Codes for ophthalmic medications and “ophthalmic preparations” across the entire study period and cross-referenced brand and generic names of medications on this list against all other medications in each annual file to identify potentially miscategorized medications.18 Ophthalmic medications were categorized into 7 sub-classes: glaucoma, steroids, lubricants, antihistamines/decongestants, dry eyes, non-steroidal anti-inflammatory drugs (NSAIDS) and anti-infectives. During this study period, the only medication in the dry eye sub-class was topical cyclosporine 0.05% (Restasis; Allergan, Irvine CA). Ophthalmic steroids with anti-infectives were categorized as steroids.
In this study, we utilized the AHRQ definition of total expenditure as the sum of payments from all sources (including the participant, insurance, Worker’s Compensation and other federal or state sources) for a given prescription. OOP expenditures were a respondent’s self-paid amount for a given prescription after manufacturer discounts or rebates if applicable. We computed national expenditures, which was the sum of all individual expenditures, as well as per capita expenditures. For drug sub-class analysis, per capita expenditures for a sub-class were calculated among individuals reporting use of any medication of that sub-class. All expenditures were adjusted for inflation with the Gross Domestic Product Price Index to 2016 US dollars.
Demographic Covariates
We also collected demographic factors including age, sex, race/ethnicity, primary insurance, family income and region of residence. Participants were split by age into three categories: 18–39, 40–64 and 65 years and older. Race/ethnicity was participant reported and categorized into mutually exclusive groups including non-Hispanic Black, non-Hispanic White, non-Hispanic Asian, Hispanic and other. We simplified primary insurance status into 5 categories: uninsured, privately insured, insured by Medicaid, any Medicare coverage (including Medicare advantage) and other. Family income level was categorized as proportions of the federal poverty level (FPL): <100%, 100 to <125%, 125 to <200%, 200 to <400% and 400% or greater. We also included visual impairment status which was assessed with the survey question, “Is anyone in the family blind or does anyone have serious difficulty seeing, even when wearing glasses?"
Statistical Analysis
We describe cohort demographics and expenditures with mean and standard error (SE) for continuous variables and proportion for categorical variables. Differences between MEPS participants who did and did not report use of any ophthalmic prescription medications as well as differences in participant demographics between periods were assessed with Pearson χ2 and student’s t-tests. We assessed trends in per capita expenditures and utilization using weighted linear regression with individual-level data and the cycle as the independent covariate. Trends in national expenditure were calculated by linear regression with year as the independent covariate—the sample size for this analysis was substantially smaller as analysis was conducted on a year- rather than individual-level. We identified predictors of ophthalmic medication expenditure with weighted linear multivariable regressions. P-values in multivariable regression analyses were corrected for multiple comparisons (2) with the Bonferroni correction. In all analyses, a 2-sided p-value <0.05 was considered statistically significant.
All statistical analyses were conducted using final person-weights and variance estimations with StataSE 15 (StataCorp, College Station, Texas). Graphpad Prism 8 (Graphpad Software, San Diego, CA) was used for all graphical depictions.
Results
From 2007 to 2016, a total of 237,363 MEPS participants were eligible for analysis of which 9,989 (4.2%) reported use of any ophthalmic medication (Table 1). From 2007–2008 to 2015–2016 the number of individuals using ophthalmic medications increased from 10.0 to 12.2 million annually. Among ophthalmic medication users, the average age was 61.5 (SE 0.3), 59.7% were female and 73.5% were non-Hispanic White. There was a significantly greater proportion of wealthy (p<0.001), female (p<0.001), white (p<0.001), Medicare insured (p<0.001) and visually impaired participants (p<0.001) as well as those over 65 years of age (p<0.001) who reported ophthalmic medication prescriptions compared to the total MEPS population. Characteristics of MEPS responders using any ophthalmic medication over time are reported in Table S1 (available at http://www.aaojournal.org). From 2007–2008 to 2015–2016, the proportion of participants using ophthalmic medications with Medicare (50.0 to 57.4%, p<0.001) increased while the proportion who were uninsured decreased (3.6% to 1.9%, p<0.001). The average number of ophthalmic prescriptions filled per individual using any ophthalmic medications was 4.2 (0.9) and did not change over time by linear regression (p=0.10). However, the average expenditure per prescription increased significantly through the study period $72.30 to $116.42 (p<0.001).
Table 1.
Socioeconomic and demographic characteristics of adult MEPS participants from 2007–2016 by usage of ophthalmic medications.
| Total Population | No Ophthalmic Use | Ophthalmic Use | P-value† | |
|---|---|---|---|---|
| No. of MEPS participants | 237,363 | 227,374 | 9,989 | |
| No. of Weighted Individuals, millions | 2,357.2 | 2,248.5 | 108.7 | |
| Parameter, % | ||||
| Gender | <0.001 | |||
| Female | 51.7 | 51.3 | 59.7 | |
| Male | 48.3 | 48.7 | 40.3 | |
| Age (SE), y | 46.8 (0.2) | 46.0 (0.2) | 61.5 (0.3) | <0.001 |
| Age category | <0.001 | |||
| 18–39 | 38.7 | 39.9 | 14.6 | |
| 40–64 | 43.1 | 43.5 | 34.6 | |
| 65 and over | 18.2 | 16.6 | 50.9 | |
| Race/ethnicity | <0.001 | |||
| Non-Hispanic White | 66.1 | 65.8 | 73.5 | |
| Non-Hispanic Black | 11.6 | 11.6 | 10.9 | |
| Non-Hispanic Asian | 5.2 | 5.2 | 4.3 | |
| Hispanic | 14.8 | 15.0 | 9.7 | |
| Other | 2.4 | 2.4 | 1.7 | |
| Insurance status | <0.001 | |||
| Uninsured | 13.6 | 14.1 | 3.4 | |
| Private | 56.3 | 57.2 | 37.3 | |
| Medicaid | 7.3 | 7.5 | 3.8 | |
| Any Medicare | 20.7 | 19.1 | 54.2 | |
| Other (public/private) | 2.2 | 2.2 | 1.3 | |
| Family income level | <0.001 | |||
| <100% FPL | 12.0 | 12.1 | 9.6 | |
| 100–124% FPL | 4.3 | 4.2 | 5.1 | |
| 125%-199% FPL | 13.3 | 13.3 | 13.2 | |
| 200–399% FPL | 29.8 | 29.9 | 28.1 | |
| >400% FPL | 40.7 | 40.5 | 44.0 | |
| Region | 0.006 | |||
| Northeast | 18.2 | 18.1 | 20.4 | |
| Midwest | 21.5 | 21.4 | 22.7 | |
| South | 37.0 | 37.1 | 35.5 | |
| West | 23.3 | 23.4 | 21.4 | |
| Visually impaired | <0.001 | |||
| No | 94.9 | 95.4 | 85.7 | |
| Yes | 5.1 | 4.6 | 14.3 | |
Denotes comparison between non-ophthalmic use and ophthalmic use population by Pearson χ2 for categorical variables and student’s t-test for mean age.
Abbreviations: MEPS, Medical Expenditure Panel Survey; SE, standard error; FPL, federal poverty line
Trends in National Ophthalmic Expenditures
Annual total expenditures for ophthalmic medications nearly doubled from $3.39 billion to $6.08 billion from 2007–2008 to 2015–2016 (p=0.016). In contrast, the annual OOP decreased from $1.34 billion to $1.18 billion in the same period (p=0.15). Trends in total and OOP expenditure by ophthalmic sub-class are shown in Table S2 (available at http://www.aaojournal.org). Glaucoma medications comprised the majority of expenditures in the beginning of the period, totaling $2.28 billion annually (67.3% of all ophthalmic medication expenditures). By 2015–2016, this proportion had decreased to 42.7%. In contrast, total expenditures and spending as a proportion of all ophthalmic expenditures increased for dry eye medications from $310.8 million (9.2%) in 2007–2008 to $1.79 billion (29.5%) by 2015–2016. Similar trends were observed in contribution by sub-class to national OOP expenditures. National spending on ophthalmic medications by participant demographic are reported in Table S3 (available at http://www.aaojournal.org).
Trends in Per Capita Ophthalmic Expenditures
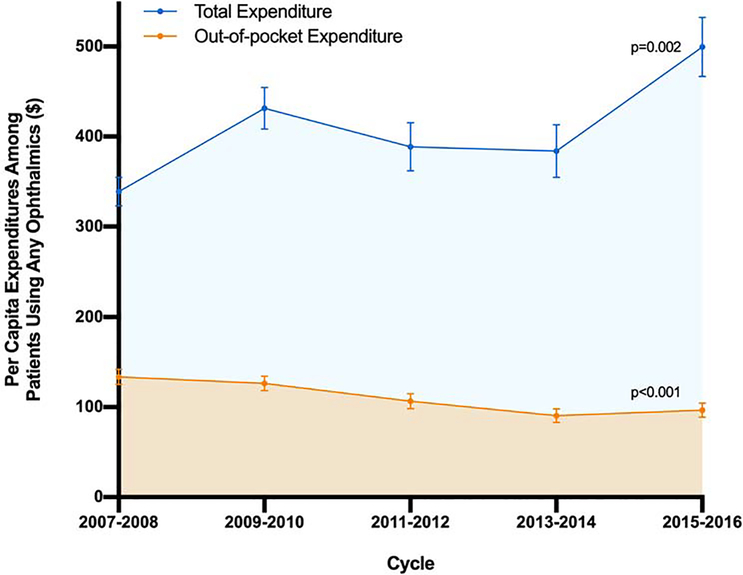
Per capita expenditure for ophthalmic prescription medications among participants using any ophthalmic medications increased from $338.72 to $499.42 (p=0.002) from 2007–2008 to 2015–2016 while per capita OOP expenditure decreased steadily from $133.48 to $96.67 (p<0.001, Figure 1). Among individuals using any ophthalmic medications, the percentage of their total annual prescription medication expenditure spent on ophthalmic medication increased from 29.6% to 32.2% from 2007–2008 to 2015–2016, this increase was not significant (p=0.08). Ophthalmics composed a smaller proportion of total OOP expenditure and there was a significant decrease in this percentage over this study period from 15.5% to 12.7% (p=0.004). We also reported trends in per capita total and OOP ophthalmic expenditure by participant demographic among those reporting any ophthalmic prescription in Table S4 and S5 (available at http://www.aaojournal.org). Of note, individuals who were female (p<0.001), over the age of 65 (p<0.001), white (p=0.006), and had Medicare insurance (p<0.001) experienced significant decreases over time in OOP expenditure. In contrast, non-Hispanic black individuals (p=0.005) and those reporting visual impairment (p=0.001) observed a significant increase.
Figure 1.
Per capita expenditures of MEPS participants 18 years of age and older on ophthalmic medications from 2007–2016 by total and out-of-pocket expenditure. Total expenditures were the sum of payments from all payers (including the participant, insurance and other sources) for a given prescription. Out-of-pocket expenditures were a respondent’s self-paid amount for a given prescription after manufacturer discounts or rebates if applicable. P-values indicate the association between per capita expenditure and year by weighted linear regression.
Trends in Per Capita Expenditures by Ophthalmic Drug Class
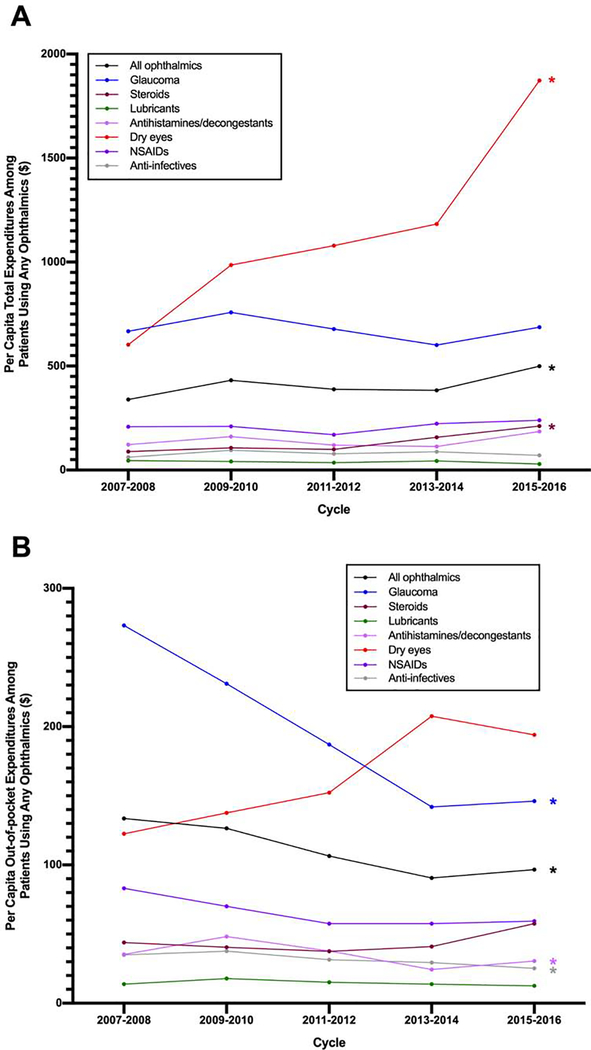
When examining annual per capita total expenditure by ophthalmic drug sub-classes, several subclasses exhibited significant trends over the study period (Figure 2A, Table 2). Annual dry eye medication (Restasis) per capita expenditures increased significantly from $602.40 to $1872.80 over the last decade (p<0.001) with an increase of $287.67 per 2-year cycle by linear regression. Per capita expenditure of ophthalmic steroids also grew from $88.86 to $211.75 (p<0.001).
Figure 2.
Per capita total (A) and out-of-pocket (B) expenditures of MEPS participants 18 years of age and older by users of different ophthalmic drug classes from 2007–2016. Total expenditures were the sum of payments from all payers (including the participant, insurance and other sources) for a given prescription. Out-of-pocket expenditures were a respondent’s self-paid amount for a given prescription after manufacturer discounts or rebates if applicable. Asterisks indicate significant trends on weighted linear regression (p<0.05). Abbreviations: MEPS, Medical Expenditure Panel Survey; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 2.
Per capita ophthalmic medication expenditure of adult MEPS participants using any ophthalmic medication by ophthalmic medication class from 2007–2016 (adjusted for inflation to 2016 GDP Price Index).
|
Total expenditure, $ (SE) |
|||||||
| 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 | Slope | P value† | |
| All ophthalmic medications | 339.15 (15.97) | 431.47 (23.21) | 388.51 (26.69) | 383.78 (29.39) | 499.42 (32.94) | 27.72 | 0.002 |
| Dry eyes | 602.40 (75.43) | 985.78 (96.60) | 1,078.70 (140.89) | 1,183.27 (156.10) | 1,872.80 (197.92) | 287.67 | <0.001 |
| Glaucoma | 667.09 (33.44) | 758.55 (42.06) | 677.84 (62.41) | 600.85 (72.20) | 686.38 (69.46) | −12.40 | 0.55 |
| Steroids | 88.86 (5.02) | 106.79 (11.72) | 99.40 (9.70) | 157.83 (11.83) | 211.75 (18.08) | 30.64 | <0.001 |
| Lubricants | 45.85 (10.60) | 41.09 (6.60) | 35.38 (3.67) | 44.15 (5.71) | 29.01 (2.30) | −3.41 | 0.09 |
| Antihistamines/decongestants | 122.65 (13.55) | 160.68 (16.08) | 119.51 (15.72) | 113.64 (14.20) | 185.76 (27.28) | 7.47 | 0.30 |
| NSAIDs | 208.17 (22.77) | 209.94 (40.78) | 169.78 (28.59) | 222.90 (27.88) | 239.58 (28.01) | 9.26 | 0.39 |
| Anti-infectives | 62.07 (4.31) | 95.04 (8.22) | 78.52 (8.12) | 87.90 (7.70) | 70.73 (5.65) | 0.72 | 0.71 |
| Other | 34.75 (6.40) | 42.74 (16.04) | 78.01 (31.17) | 127.91 (41.05) | 135.98 (24.19) | 28.79 | <0.001 |
|
Out-of-pocket expenditure, $ (SE) |
|||||||
| 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 | Slope | P value† | |
| All ophthalmic medications | 133.63 (8.54) | 126.48 (8.29) | 106.50 (8.61) | 90.60 (7.51) | 96.67 (7.84) | −10.82 | <0.001 |
| Dry eyes | 122.51 (25.27) | 137.68 (19.06) | 152.24 (27.75) | 207.61 (45.85) | 194.00 (54.30) | 20.52 | 0.18 |
| Glaucoma | 273.21 (20.93) | 230.99 (19.26) | 187.02 (23.94) | 141.94 (17.25) | 146.15 (15.05) | −34.11 | <0.001 |
| Steroids | 43.91 (3.44) | 40.48 (3.97) | 37.53 (4.57) | 41.03 (4.62) | 57.56 (6.35) | 3.07 | 0.08 |
| Lubricants | 13.76 (3.52) | 17.88 (3.65) | 15.17 (3.00) | 13.85 (2.60) | 12.64 (1.26) | −0.78 | 0.32 |
| Antihistamines/decongestants | 35.26 (3.32) | 48.28 (6.04) | 37.72 (5.36) | 24.37 (3.91) | 30.51 (5.20) | −3.60 | 0.02 |
| NSAIDs | 83.10 (10.69) | 70.11 (11.42) | 57.59 (8.05) | 57.60 (9.55) | 59.41 (8.66) | −5.48 | 0.11 |
| Anti-infectives | 34.89 (2.99) | 37.61 (3.14) | 31.48 (2.59) | 29.41 (2.98) | 25.22 (3.01) | −2.79 | 0.004 |
| Other | 20.32 (3.53) | 33.51 (16.70) | 72.80 (30.36) | 41.51 (12.48) | 50.32 (12.59) | 6.16 | 0.09 |
Significance denotes association between drug-class expenditure and year effect by linear regression
Abbreviations: MEPS, Medical Expenditure Panel Survey; SE, standard error; NSAIDs, non-steroidal anti-inflammatory drugs; GDP, Gross Domestic Product
OOP per capita expenditures for glaucoma medications significantly decreased during the study period from $273.21 to $146.15 (p<0.001) (Table 2). In post hoc analysis of glaucoma medication utilization, we identified that the average number of glaucoma prescriptions per individual decreased over time from 7.4 to 6.8 (p=0.05) from 2007–2008 to 2015–2016 while the average expenditure per prescription did not change significantly over time ($99.64, p=0.41). Per capita OOP also significantly decreased for antihistamines/decongestants and anti-infectives from $35.26 to $30.51 (p=0.02) and $34.89 to $25.22 (p=0.004) respectively from the 2007–2008 to 2015–2016. Per capita OOP expenditure for dry eye prescriptions started at $122.51 in 2007–2008 and peaked in 2013–2014 at $207.61 although the association between time and OOP expenditure was not significant (p=0.18).
Predictors of Greater Per Capita Expenditure
On multivariable-adjusted analyses, adults 65 years and over ($221.63; 95% confidence interval [CI], 114.70 to 328.56; p<0.001) and age 40–64 ($178.84; 95% CI, 131.59 to 226.08; p<0.001) expended significantly more than those 18–39 years of age (Table 3). Individuals with private insurance ($117.77; 95% CI, 61.28 to 174.27; p<0.001) and Medicare ($252.76; 95% CI, 154.16 to 351.36; p<0.001) were significantly more likely to have greater ophthalmic prescription expenditure than uninsured participants. Regional differences also existed with residence in the Northeast associated with significantly greater expenditure than all other regions (all p<0.05). Lastly, individuals who were visually impaired were significantly more likely to have greater expenditure ($208.24; 95% CI, 59.15 to 357.34; p=0.01) independent of other demographic factors.
Table 3.
Multivariable weighted linear regression of factors associated with total and OOP per capita expenditure for ophthalmic medications among adult MEPS participants using any ophthalmic medications.
| Total Expenditure | Out-of-pocket Expenditure | |||
|---|---|---|---|---|
| Multivariable analysis β ($) (95% CI) | P-value | Multivariable analysis β ($) (95% CI) | P-value | |
| Cycle | 23.55 (5.02 to 42.08) | 0.03 | −10.84 (−16.50 to −5.18) | <0.001 |
| Gender | ||||
| Male | 1 [Reference] | 1 [Reference] | ||
| Female | 31.96 (−22.32 to 86.24) | 0.50 | 1.35 (−15.49 to 18.19) | 0.99 |
| Age category | ||||
| 18–39 | 1 [Reference] | 1 [Reference] | ||
| 40–64 | 178.84 (131.59 to 226.08) | <0.001 | 47.55 (33.48 to 61.62) | <0.001 |
| 65 and over | 221.63 (114.70 to 328.56) | <0.001 | 101.41 (75.20 to 127.63) | <0.001 |
| Race/ethnicity | ||||
| Non-Hispanic White | 1 [Reference] | 1 [Reference] | ||
| Non-Hispanic Black | 36.25 (−20.99 to 93.49) | 0.42 | −5.24 (−21.85 to 11.37) | 0.99 |
| Non-Hispanic Asian | 58.93 (−37.90 to 15.77) | 0.46 | −18.42 (−40.31 to 3.47) | 0.28 |
| Hispanic | 55.51 (−26.09 to 137.11) | 0.36 | −23.89 (−39.16 to −8.62) | 0.004 |
| Other | 181.52 (−38.85 to 401.89) | 0.21 | 11.76 (−39.90 to 63.42) | 0.99 |
| Insurance status | ||||
| Uninsured | 1 [Reference] | 1 [Reference] | ||
| Private | 117.77 (61.28 to 174.27) | <0.001 | −25.35 (−51.86 to −1.18) | 0.10 |
| Medicaid | 28.67 (−39.85 to 97.20) | 0.82 | −50.89 (−79.51 to −22.28) | <0.001 |
| Any Medicare | 252.76 (154.16 to 351.36) | <0.001 | −34.14 (−64.59 to −3.68) | 0.05 |
| Other (public/private) | 78.89 (−124.95 to 282.73) | 0.90 | −34.26 (−78.18 to 9.65) | 0.25 |
| Family income level | ||||
| <100% FPL | 1 [Reference] | 1 [Reference] | ||
| 100–124% FPL | −57.19 (−150.70 to 36.31) | 0.46 | 4.00 (−20.60 to 28.60) | 0.99 |
| 125%–199% FPL | −41.85 (−129.82 to 46.13) | 0.70 | 40.10 (13.21 to 66.99) | 0.02 |
| 200–399% FPL | −53.19 (−129.50 to 23.12) | 0.34 | 29.81 (12.66 to 46.95) | 0.002 |
| >400% FPL | 16.60 (−74.92 to 108.12) | 0.99 | 49.52 (29.35 to 69.69) | <0.001 |
| Region | ||||
| Northeast | 1 [Reference] | 1 [Reference] | ||
| Midwest | −141.84 (−262.53 to −21.16) | 0.04 | −40.73 (−78.27 to −2.01) | 0.08 |
| South | −152.56 (−268.14 to −36.44) | 0.02 | −47.19 (−84.59 to −11.93) | 0.02 |
| West | −177.35 (−301.64 to −53.06) | 0.01 | −50.10 (−87.63 to −12.56) | 0.02 |
| Visually impaired | ||||
| No | 1 [Reference] | 1 [Reference] | ||
| Yes | 208.24 (59.15 to 357.34) | 0.01 | 91.79 (51.44 to 132.13) | <0.001 |
Abbreviations: MEPS, Medical Expenditure Panel Survey; OOP, out-of-pocket, CI, confidence interval; FPL, federal poverty line
With regard to OOP spending, participants 65 and over ($101.41; 95% CI, 75.20 to 127.63; p<0.001), and age 40–64 ($47.55; 95% CI, 33.48 to 61.62; p<0.001) were significantly more likely to have greater OOP expenditure compared to those 18–39 years of age (Table 3). Hispanic participants were significantly more likely to have less OOP compared to non-Hispanic Whites (−$23.89; 95% CI, −39.16 to −8.62; p=0.004). Additionally, uninsured participants had significantly greater OOP expenditures than those with Medicaid (−$50.89; 95% CI, −79.51 to −22.28; p<0.001) and Medicare (−$34.14; 95% CI, − 64.59 to −3.68; p=0.05). OOP insurance expenditure increased with family wealth with those above 400% of the FPL, 200–399% of the FPL and 125–199% of the FPL expending $49.52 (95% CI, 29.35 to 69.69; p<0.001), $29.81 (95% CI, 12.66 to 46.95; p=0.002) and $40.10 (95% CI, 13.21 to 66.99; p=0.02) more than those below 100% of the FPL respectively. Regional variations also existed with residence in the Northeast associated with significantly greater OOP expenditure by approximately $50 per capita compared to the South (p=0.02) and West (p=0.02) regions. Lastly, participants who were visually impaired were significantly more likely to have greater expenditure ($91.79; 95% CI, 51.44 to 132.13; p<0.001).
Discussion
Using a nationally representative sample of the US adult population, we found that annual national and per capita expenditures for ophthalmic medications significantly grew from 2007 to 2016. Conversely, per capita OOP spending for ophthalmic drugs significantly declined and represented a decreasing proportion of total OOP prescription pharmaceutical costs. Dry eye and glaucoma medications contributed substantially to these trends, accounting for nearly three out of every four dollars spent on ophthalmic drugs. Total expenditures for cyclosporine 0.05% (Restasis) more than tripled over the study period while OOP expenditures for glaucoma medications significantly decreased. Our study also found that despite diminishing overall OOP drug costs, significant inequities in OOP expenditure persist: the elderly, uninsured, and visually impaired all faced higher OOP costs.
While prior research has described the significant economic burden of visual impairment and eye disease,10,24 limited studies have assessed the pharmaceutical costs of ophthalmic prescription drugs. Here, we demonstrate that national expenditures for prescription ophthalmic medications have substantially grown, nearly doubling from 2007–2008 to 2015–2016, continuing the trend of rising expenditures reported by studies examining data from 2001–2006.16,17 Our results indicate that this growth was driven by both an increasing number of individuals utilizing ophthalmic medications as well as greater per capita spending. Furthermore, the average price per prescription grew while the number of prescriptions per individual remained stable, suggesting that rising ophthalmic drug prices and greater use of expensive brand medications may be contributing more to growing expenditure than more frequent utilization. Indeed, a 2018 congressional report demonstrated that the average wholesale acquisition cost of Restasis increased by 92% from 2012–2017, which likely contributed to Restasis comprising nearly 30% of all expenditures by 2015–2016.25 It has been shown that the price of pharmaceuticals and medical services rather than differences in utilization have recently been attributed as the main driver of the disparity in healthcare costs in the US compared to other high-income countries.26 Ophthalmic medications are also comprising an increasing proportion of total prescription expenditures for individuals taking any ophthalmic drugs, implying that rising ophthalmic expenditures are outpacing the growth in overall pharmaceutical costs in this population.
In contrast to total ophthalmic medication expenditure, we found that OOP spending for ophthalmic medications has significantly declined which mirrors industry analysis reporting a small decline in total OOP expenditures in recent years.7,27,28 This may be, in large part, due to coverage gains and closure in coverage gaps through Medicare Part D legislation and the Affordable Care Act.27,29,30 Our study showed that uninsured patients comprised a smaller proportion of individuals reporting any use of ophthalmics over time while the proportion of those with Medicaid and Medicare increased. Additionally, among all insurance groups, we observed the greatest decline in OOP spending and the lowest relative likely amount of OOP spending for Medicare and Medicaid beneficiaries after controlling for patient demographics. Previous studies have shown significantly increased coverage of patients with glaucoma after implementation of Medicare Part D as well as lower frequency of skipping doses or failing to obtain glaucoma prescriptions due to cost.29,31 Decreasing OOP costs in our study were largely driven by declining OOP expenditure for glaucoma medication which corroborates this point.
While it is also possible that increased utilization of generic rather than brand glaucoma medications may be driving this trend, particularly after the approval of generic dorzolamide/timolol maleate (Cosopt) in 2008 and generic latanoprost (Xalatan) in 2011, a substantially increased volume of generic prescriptions would likely have caused a decrease in both total and OOP costs. However, total expenditures for glaucoma medication did not change over our study period. Furthermore, the average expenditure per glaucoma prescription did not significantly decrease over time. These results suggest that other elements may have had a greater impact on limiting OOP spending.
Importantly, we found that certain patient populations, including the elderly, visually impaired and uninsured continued to have significantly greater OOP spending. Higher OOP spending among uninsured patients is unsurprising as coverage gains would not impact this population’s shared-costs for prescription medications. In contrast, greater OOP costs among the elderly and visually impaired likely reflect more frequent utilization of ophthalmic prescription medications rather than lack of coverage.32 Indeed, OOP expenditures for both groups significantly decreased throughout this study period as legislative changes improved prescription drug coverage. However, by the end of the study, adults 65 and over (of which the vast majority were insured by Medicare in this study) continued to have the greatest per capita OOP expenses among all age groups, suggesting that deductibles, copayments and the lack of an OOP maximum among Medicare beneficiaries still contributed to high OOP medication costs.
Several other factors may also be limiting patient cost-sharing but contributing to drug expenditure. For example, manufacturer and charitable patient assistance programs, which provide copayment assistance for certain prescription drugs, have rapidly increased in prevalence and coverage in recent years: copay coupons represented nearly 12% of patient responsibility for retail prescription drug costs in 2016, up from 6% in 2012.27 Although no previous studies have examined the prevalence of these programs for ophthalmic medications, a brief search revealed numerous coupon cards and copay assistance initiatives for glaucoma and dry eye medications which comprised the majority of total and OOP spending throughout this period.33–35 While these programs are essential in helping many patients access affordable medications, they have also received criticism for contributing to rising prescription expenditure. Mechanistically, copayment coupons drive patients towards higher-tier brand-name drugs which often have even lower OOP costs for consumers than generics after manufacturer fee assistance.36 Copayment assistance programs also allow manufacturers to raise drug prices and offer larger copayment coupons to maintain low OOP pricing, which ultimately require insurers to cover greater costs.37–39
Pharmaceutical benefit managers (PBMs) and rebates may also be playing an additional role in limiting OOP spending. PBMs are companies that dictate the insurance formularies and influence drug expenditure and patient costs by negotiating rebates and discounts from drug manufacturers on behalf of insurers. In concept, rebates are passed from PBMs to insurers which ultimately lead to lower consumer copayments. The rising amounts of PBM rebates in recent years—growing from $39.7 billion to 89.5 billion from 2012–2016—in theory have controlled OOP costs;27 However, in response to higher rebates, manufacturers have increased medication list prices.40 Additionally, because PBMs control copayment amounts and tier placements, manufacturers of expensive brand medications can offer more significant rebates to PBMs in exchange for reducing consumer co-payments or placing their medication on a more preferred tier.41 For example, a study of OOP costs for statins found that Lipitor had lower OOP costs than generic atorvastatin after Lipitor’s manufacturer partnered with PBMs.42,43 In ophthalmology specifically, a recent study observed higher drug costs for several generic medications compared to their brand counterparts for Medicare Part D.11 However, we note that the exact extent to which PBMs have influenced ophthalmic medication pricing is unknown due to their confidential nature.
The mechanisms by which manufacturer discounts and PBMs influence patient spending are complex, however, they highlight the potential leverage that can be achieved by maintaining low perceived OOP prescription spending. Shielding patients from OOP drug costs can inadvertently remove patient and physician financial disincentives for over-utilization and usage of expensive medications with cheaper therapeutic equivalents. Low OOP costs for ophthalmic medications observed in our study may partly explain a recent analysis that reported that ophthalmologists generate the highest percentage of brand name medication claims among all providers in Medicare which are driven largely by glaucoma and dry eye medications.13 Lower OOP prices can also reduce political incentive to maintain lower drug prices.39
As insurers face rising drug prices, an increasing burden of coverage, and pressure to limit OOP prescription costs, they may be forced to shift costs by increasing premiums or decreasing coverage. For example, while a recent report observed no growth in OOP prescription spending from 2012–2016, both the total value and share of total premiums attributed to retail pharmacy benefits increased substantially.27 Insurer cost-shifting may ultimately increase patient OOP expenditures overall even if prescription OOP spending remains stable. To better characterize insurer cost shifting, future research should examine how OOP costs for patients with eye disorders have changed over time in the context of declining OOP prescription spending.
Limitations
This study has several limitations. First, because data from MEPS is primarily participant-reported, prescription medication use and expenditure data may be inaccurate or incomplete. However, the majority of patients had pharmacy data to verify prescription information and the AHRQ utilized a variety of imputation and editing methods to account for missing data and potential errors.22 Additionally, as mentioned previously, the accuracy of MEPS data has been previously validated in comparison to Medicare Part D claim data.23 Furthermore, underreporting of healthcare utilization within MEPS has been shown to occur similarly across most sociodemographic groups.23 This reduces the impact of underreporting when examining determinants of healthcare use. Second, ophthalmic drugs are more infrequently prescribed compared to other medications, yielding smaller sample sizes than in studies examining more common medications. To address this potential issue, we pooled data into 2-year periods and all analysis groups have significantly greater than 60 unweighted participants which is the MEPS precision standards guideline defined cutoff.44 Only some cycles of ophthalmic medication usage in the “Other” category, which were not analyzed, fell below this threshold. Third, we were unable to account for over-the-counter prescriptions which likely resulted in an underestimation of total ophthalmic expenditures. This effect may disproportionately impact lubricant and antihistamines/decongestant ophthalmic medication classes. Lastly, we were unable to identify the source of out of pocket payments including whether they were derived from copayments, coinsurance payments or deductibles. Future investigation of OOP costs by source will be valuable to identify changes in health plan costs to beneficiaries.
In summary, we found that despite increasing total expenditures for ophthalmic medications, Americans are incurring less out-of-pocket costs for prescription eye drugs. Furthermore, the elderly, uninsured and visually impaired continue to face significantly higher OOP costs. These findings suggest that recent policy efforts to improve prescription drug coverage may be successfully reducing out-of-pocket spending for most Americans, an important step in improving the affordability and accessibility of medications. However, these measures may be inadequate in reducing OOP costs for certain demographic subgroups and addressing total ophthalmic drug expenditure. Continued growth in ophthalmic drug spending may ultimately increase patient costs in the form of increased premiums, decreased coverage of clinical services, and greater OOP spending for clinical care.
Supplementary Material
Acknowledgments
Financial Support: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number T35AG049685 (Recipient: EC). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflicts of Interest(s): None.
From 2007–2016, total expenditures for ophthalmic medications increased while out-of-pocket expenditures decreased. Dry eye and glaucoma medications contributed substantially to these trends, accounting for three of every four dollars spent on ophthalmic drugs by 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could a?ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keehan SP,Cuckler GA, Sisko AM,et al. National health expenditure projections, 2014–24: spending growth faster than recent trends. Health Aff (Millwood) 2015;34(8):1407–1417. [DOI] [PubMed] [Google Scholar]
- 2.Kesselheim AS, Avorn J, Sarpatwari A.The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform. JAMA. 2016;316(8):858–871. [DOI] [PubMed] [Google Scholar]
- 3.Cubanski J,Swoope C, Damico A, Neuman T. How much is enough? Out-of-pocket spending among Medicare beneficiaries: A chartbook Menlo Park, CA: Kaiser Family Foundation;2014. [Google Scholar]
- 4.Goldman AL, Woolhandler S, Himmelstein DU, Bor DH, McCormick D. Out-of-Pocket Spending and Premium Contributions After Implementation of the Affordable Care Act. JAMA Intern Med. 2018;178(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park T, Jung J. The Effect of Medicare Part D on Prescription Drug Spending and Health Care Use: 6 Years of Follow-up, 2007–2012. J Manag Care Spec Pharm. 2017;23(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarna D, Squires D, Bishop S Paying for Prescription Drugs Around the World: Why Is the U.S. an Outlier?: The Commonwealth Fund;2017. [PubMed] [Google Scholar]
- 7.Kamal R, Cox C, McDermott D What are the recent and forecasted trends in prescription drug spending? Peterson-KFF https://www.healthsystemtracker.org/chart-collection/recent-forecasted-trends-prescription-drug-spending/. Published 2019.
- 8.Paez KA, Zhao L, Hwang W. Rising out-of-pocket spending for chronic conditions: a ten-year trend. Health Aff (Millwood) 2009;28(1):15–25. [DOI] [PubMed] [Google Scholar]
- 9.Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries costs are greater for those with progressive vision loss. Ophthalmology. 2007;114(2):238–245. [DOI] [PubMed] [Google Scholar]
- 10.Wittenborn JS, Zhang X, Feagan CW, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013;120(9):1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong D, Chang JS, Barbany M, et al. Comparison of United States and International Ophthalmic Drug Pricing. Ophthalmology. 2019;126(10):1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh R, Feng PW, Tainsh L, et al. Comparison of Ophthalmic Medication Prices Between the United States and Australia. JAMA Ophthalmol. 2019;137(4):358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman-Casey PA, Woodward MA, Niziol LM, Lee PP, De Lott LB. Brand Medications and Medicare Part D: How Eye Care Providers' Prescribing Patterns Influence Costs. Ophthalmology. 2018;125(3):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122(7):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein JD, Shekhawat N, Talwar N, Balkrishnan R. Impact of the introduction of generic latanoprost on glaucoma medication adherence. Ophthalmology. 2015;122(4):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galor A, Zheng DD, Arheart KL, et al. Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea. 2012;31(12):1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam BL, Zheng DD, Davila EP, et al. Trends in glaucoma medication expenditure: Medical Expenditure Panel Survey 2001–2006. Arch Ophthalmol. 2011;129(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. MEPS HC-197A: 2017 Prescribed Medicines Rockville, MD: Agency for Healthcare Research and Quality Center for Financing, Access, and Cost Trends;2019. [Google Scholar]
- 19.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–664. [DOI] [PubMed] [Google Scholar]
- 20.Salami JA, Warraich H, Valero-Elizondo J, et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2(1):56–65. [DOI] [PubMed] [Google Scholar]
- 21.MEPS-HC Response Rates by Panel. Agency for Healthcare Research and Quality. https://meps.ahrq.gov/mepsweb/survey_comp/hc_response_rate.jsp. Published 2018.
- 22.Hill SC, Roemer M, Stagnitti MN Outpatient Prescription Drugs: Data Collection and Editing in the 2011 Medical Expenditure Panel Survey Rockville, MD: Agency for Healthcare Research and Quality;2014. [Google Scholar]
- 23.Hill SC, Zuvekas SH, Zodet MW. Implications of the accuracy of MEPS prescription drug data for health services research. Inquiry 2011;48(3):242–259. [DOI] [PubMed] [Google Scholar]
- 24.Schakel W, van der Aa HPA, Bode C, Hulshof CTJ, van Rens G, van Nispen RMA. The Economic Burden of Visual Impairment and Comorbid Fatigue: A Cost-of-Illness Study (From a Societal Perspective). Invest Ophthalmol Vis Sci. 2018;59(5):1916–1923. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Senate Homeland Security & Governmental Affairs Committee Minority Office. Manufactured Crisis: How Devastating Drug Price Increases Are Harming America’s Seniors. 2018.
- 26.Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024–1039. [DOI] [PubMed] [Google Scholar]
- 27.PEW. The Prescription Drug Landscape, Explored. PEW;2019. [Google Scholar]
- 28.Kaiser Family Foundation. Examining high prescription drug costs for people with employer-sponsored health insurance. Kaiser Family Foundation,;2016. [Google Scholar]
- 29.Blumberg DM, Prager AJ, Liebmann JM. Variation in Prescription Drug Coverage Enrollment Among Vulnerable Beneficiaries With Glaucoma Before and After the Implementation of Medicare Part D. JAMA Ophthalmol. 2016;134(2):212–220. [DOI] [PubMed] [Google Scholar]
- 30.Gotanda H, Jha AK, Kominski GF, Tsugawa Y. Out-of-pocket spending and financial burden among low income adults after Medicaid expansions in the United States: quasi-experimental difference-in-difference study. BMJ. 2020;368:m40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumberg DM, Prager AJ, Liebmann JM, Cioffi GA, De Moraes CG. Cost-Related Medication Nonadherence and Cost-Saving Behaviors Among Patients With Glaucoma Before and After the Implementation of Medicare Part D. JAMA Ophthalmol. 2015;133(9):985–996. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5–ORSF13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MERCK. TRUSOPT https://www.merckhelps.com/TRUSOPT Published 2020.
- 34.NOVARTIS. Patient Assistance Foundation Enrollment. https://www.pharma.us.novartis.com/our-products/patient-assistance/patient-assistance-foundation-enrollment. Published 2020.
- 35.Allergan. Patient Assistance Programs https://www.allergan.com/patient-assistance-programs Published 2020.
- 36.Dusetzina SB, Jazowski S, Cole A, Nguyen J. Sending The Wrong Price Signal: Why Do Some Brand-Name Drugs Cost Medicare Beneficiaries Less Than Generics? Health Aff (Millwood). 2019;38(7):1188–1194. [DOI] [PubMed] [Google Scholar]
- 37.Dafny LS, Ody CJ, Schmitt MA. Undermining Value-Based Purchasing - Lessons from the Pharmaceutical Industry. N Engl J Med. 2016;375(21):2013–2015. [DOI] [PubMed] [Google Scholar]
- 38.Ross JS, Kesselheim AS. Prescription-drug coupons--no such thing as a free lunch. N Engl J Med. 2013;369(13):1188–1189. [DOI] [PubMed] [Google Scholar]
- 39.Ubel PA, Bach PB. Copay Assistance for Expensive Drugs: A Helping Hand That Raises Costs. Ann Intern Med. 2016;165(12):878–879. [DOI] [PubMed] [Google Scholar]
- 40.Sood N, Ribero R, Ryan M, Van Nuys K The Association Between Drug Rebates and List Prices. Leonard D Schaeffer Center for Health Policy & Economics;2020.
- 41.Roy A. Drug Companies, Not 'Middlemen', Are Responsible For High Drug Prices. https://www.forbes.com/sites/theapothecary/2018/10/22/drug-companies-are-responsible-for-high-drug-prices-not-middlemen/#7fe140d34947 Published 2018.
- 42.Warraich HJ, Salami JA, Khera R, Valero-Elizondo J, Okunrintemi V, Nasir K. Trends in Use and Expenditures of Brand-name Atorvastatin After Introduction of Generic Atorvastatin. JAMA Intern Med. 2018;178(5):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson D. Facing Generic Lipitor Rivals, Pfizer Battles to Protect Its Cash Cow. The New York Times; 2011. [Google Scholar]
- 44.Agency for Healthcare Research and Quality. Precision Standards Guidelines for Reporting MEPS-HC Descriptive Statistics. Agency for Healthcare Research and Quality; https://meps.ahrq.gov/survey_comp/precision_guidelines.shtml.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.