Abstract
No other tissue in the body depends more on the composition and organization of the extracellular matrix (ECM) for normal structure and function than the corneal stroma. The precise arrangement and orientation of collagen fibrils, lamellae and keratocytes that occurs during development and is needed in adults to maintain stromal function is dependent on the regulated interaction of multiple ECM components that contribute to attain the unique properties of the cornea: transparency, shape, mechanical strength, and avascularity. This review summarizes the contribution of different ECM components, their structure, regulation and function in modulating the properties of the corneal stroma. Fibril forming collagens (I, III, V), fibril associated collagens with interrupted triple helices (XII and XIV), network forming collagens (IV, VI and VIII) as well as small leucine-rich proteoglycans (SLRP) expressed in the stroma: decorin, biglycan, lumican, keratocan, and fibromodulin are some of the ECM components reviewed in this manuscript. There are spatial and temporal differences in the expression of these ECM components, as well as interactions among them that contribute to stromal function. Unique regions within the stroma like Bowman’s layer and Descemet’s layer are discussed. To define the complexity of corneal stroma composition and structure as well as the relationship to function is a daunting task. Our knowledge is expanding, and we expect that this review provides a comprehensive overview of current knowledge, definition of gaps and suggests future research directions.
Keywords: cornea, stroma, collagens, proteoglycans, collagen fibril, structure, composition
1. Introduction
The cornea is the major refractive structure of the eye and transparency is its major attribute. In addition, the cornea provides mechanical stability and is a protective barrier for the eye. The cornea is avascular and is composed of three layers. The corneal epithelial layer is the most anterior region while the corneal endothelium is the posterior layer. Between these layers rests the corneal stroma that makes up approximately 90% of the corneal thickness. The stroma extends from the corneal epithelial basement membrane to the endothelial basement membrane. The corneal stroma is the focus of this review.
2. Corneal stroma
The corneal stroma has 3 distinct regions. Bowman’s layer is the most anterior region providing an interface with the epithelial basement membrane. The stroma proper makes up the bulk of the corneal stroma. Descemet’s Membrane is the most posterior region separating the stroma proper from the corneal endothelial layer. Each of these structures have unique structural, compositional and functional characteristics.
2.1. Bowman’s layer
This is the most anterior region of the corneal stroma. It is often considered a separate corneal region; however, it can be considered a specialized region of the anterior stroma mediating interactions with the corneal epithelium. This smooth layer is approximately 15–18 μm thick in humans as determined by live imaging and avoiding chemical tissue fixation (Kermani et al., 2008; Li et al., 1997). It is believed to have a mechanical role in maintaining corneal shape (Tong et al., 2019). Bowman’s layer is present in humans, but not in all mammals. In humans, Bowman’s layer thins with aging (Germundsson et al., 2013). This region is composed of a network of very small diameter collagen fibrils around 18 – 22 nm (Birk et al., 1986; Gordon et al., 1994; Linsenmayer et al., 1998). Bowman’s layer is acellular and the network of thin collagen fibrils and their non-lamellar organization is distinct from that seen in the stroma proper. This interfacial ECM integrates the corneal epithelial basement membrane and attached epithelial cells with the corneal stroma proper. In addition to the collagen fibrillar networks, Bowman’s layer contains anchoring complexes composed of collagen VII-containing anchoring fibrils and collagen IV-containing anchoring plaques. These complexes intertwine with the fibrillar network of Bowman’s layer to anchor the basement membrane to the underlying stroma proper (Gipson et al., 1987; Tisdale et al., 1988). Species without a structurally distinct Bowman’s layer may have some of the compositional properties characteristic of the region providing unique properties to this interfacial region and is an area requiring further investigation. It is well known that the absence of Bowman’s layer, as seen following photorefractive keratectomy, does not seem to affect corneal function, and that once it is disrupted, it will not regenerate (Wilson, 2020; Wilson and Hong, 2000). However, in recent years, a technique for harvesting Bowman’s layer from a cadaveric donor and transplantation into recipients with progressive corneal ectasia and significant anterior corneal scars has been reported with success (Tong et al., 2019).
The collagen composition in Bowman’s layer is unique and differs from that of the corneal stroma proper both in collagen content and fibril structure. This was originally defined by analyses of avian corneas and is consistent in all species studied including human. In corneas reacted with antibodies directed against the triple helical domain of collagen V strong reactivity is observed in Bowman’s layer with little to no reactivity in the stroma proper. However, after disruption of fibril structure there was strong reactivity for collagen V in the stroma proper as well as Bowman’s layer. These results suggest differences in the collagen fibril organization in Bowman’s layer and the stroma proper (Birk et al., 1986; Fitch et al., 1984; Gordon et al., 1994; Linsenmayer et al., 1998). A higher ratio of collagen V to collagen I is present during development at the stromal – epithelial interface due to higher Col5a1 expression by epithelial cells compared to keratocytes. These data indicate both epithelial and keratocyte contributions to Bowman’s layer, a key interfacial matrix. The higher availability of collagen V is one explaination for the smaller collagen fibrils present in Bowman’s layer (Gordon et al., 1994; Linsenmayer et al., 1998; Linsenmayer et al., 1993). However, the epithelial cells had high expression of the Col5a1 gene, but not the Col5a2 gene. There are multiple collagen V isoforms with the a1(V)2a2(V) form being the predominant form in the stroma proper. However, the a1(V)3 isoform has been found in other tissues and organized into smaller fibrils organized as networks (Bonod-Bidaud et al., 2012; Chanut-Delalande et al., 2004). It is likely that increased Col5a1 expression would favor epithelial homotrimer formation whereas the keratocytes would assemble the heterotrimer (Roulet et al., 2010). Therefore, differences in collagen V isoforms in Bowman’s layer would determine its unique structure. However, this has not been demonstrated. It is expected that differences in collagen composition, fibril size and structure as well as fibril organization in Bowman’s layer create functional differences between Bowman’s layer and the corneal stroma proper.
2.2. Stroma proper
The stroma proper makes up ~90% of the corneal structure and provides unique properties necessary for function; including transparency, avascularity, as well as its mechanical properties necessary for strength, and the maintenance of shape. The stroma is composed of ECM molecules, water and a communicating network of neural crest derived keratocytes that synthesize the stromal extracellular matrix (Chen et al., 2015; Hassell and Birk, 2010; Linsenmayer et al., 1998; Massoudi et al., 2016; Quantock and Young, 2008). The function of the stroma proper relies on the tightly controlled assembly of collagen I into fibrils and higher ordered structures (Hassell and Birk, 2010; Linsenmayer et al., 1998; Maurice, 1957). The stroma proper contains homogeneous, small diameter collagen fibrils that are characteristic of the corneal stroma. The fibrils are highly organized with a rigid packing pattern. Interactions of different fibrillar collagens, fibril-associated collagens (FACIT), small leucine rich proteoglycans (SLRP) and glycoproteins have all been implicated in the regulation of corneal fibril structure and packing (Birk and Trelstad, 1984; Chen et al., 2015; Hassell and Birk, 2010; Linsenmayer et al., 1998; Mienaltowski and Birk, 2014; Quantock and Young, 2008). Corneal collagen fibrils are then organized as fibers that form lamellae. Adjacent lamellae are oriented ~90° from each other with an approximate orthogonal arrangement (Maurice, 1957; Meek and Knupp, 2015). Lamellae are structures of variable thickness, in humans typically of 0.5 to 250 μm in width and up to 2.5 μm in thickness (Komai and Ushiki, 1991). Rigid control at all levels of hierarchal structure are essential for stromal function and dysfunction at any level may contribute to ocular pathologies. Alterations in hierarchical organization are hypothesized to be an important contributor to keratoconus. Alterations in lamellae organization have been demonstrated in X-ray scattering studies, and possible inter lamellae slippage proposed as the main etiology of corneal ectasias (Hayes et al., 2007). What causes or induces lamellar slippage is unknown, but oxidative stress is suspected in some subgroups of patients (Shinde et al., 2020).
The structure and composition of the stroma differs in the anterior and posterior stroma. The interfibrillar spacing is increased in the posterior stroma compared to the anterior stroma, (Freund et al., 1995) and in mouse corneas, extracellular matrix components are not homogeneously distributed e.g., lumican is localized mainly to the adult posterior stroma (Chakravarti et al., 2000). These structural differences translate to functional differences including in light scattering and propensity to swell (Freund et al., 1995; Kikkawa and Hirayama, 1970; Meek et al., 2003). There are differences in the refractive index between the anterior and posterior stroma with the anterior stroma having a higher refractive index (Patel et al., 1995).
Differences in interfibrillar spacing are more noticeable during corneal edema and in this clinical situation, increases in interfibrillar spacing are more significant in the posterior stroma compared to the anterior stroma (Meek et al., 2003; Patel et al., 1995). These differences in structure and function are noted in the propensity for corneal edema to occur first in the posterior stroma, a clinical sign known as Descemet folds (Freund et al., 1995; Kikkawa and Hirayama, 1970). The limbal area is a unique interfacial region of the stroma at the corneal and scleral junction. This region has a different organization of the fibrillar extracellular matrix distinct from the stroma proper and functions to integrate these 2 adjacent tissues. At the limbus, a variable circumcorneal annulus of collagen fibrils is present as revealed by synchroton X-ray diffraction. The scleral and corneal fibrils have different orientations, and bend at this annulus to run circumferentially at the limbus. It is hypothesized that unique mechanical properties in the limbal area may regulate corneal curvature and are essential to resist increased circumferential tension (Kamma-Lorger et al., 2010; Newton and Meek, 1998a, b).
Imaging of the stromal extracellular matrix and its hierarchical organization can be supplemented with new technologies or new uses of old technologies that provide new insight or make previously unaccessible studies accessible. Imaging approaches have been central to analyses of corneal structure since the pioneering work of Jakus (Jakus, 1954, 1956, 1962). Conventional transmission electron microscopy still plays a central role, however, a broadening of its use has enhanced the ability to analyze structure at different levels. Initially, a three-dimensional electron imaging study of the cornea was done by stacking serial electron microscopic images taken sequentially after tissue was removed using an ion beam in the microscope (Bushby et al., 2011). Currently, serial block face scanning electron microscopy allows for reconstruction of tissues and their individual elements at the ultrastuctural level. A serial, aligned, stack of images is obtained that can be reconstructed in three dimensions for analysis (Denk and Horstmann, 2004; Kremer et al., 2015). Additionally, tomography using transmission electron microscope can generate high resolution three-dimensional images for analysis of three-dimensional structure (Kremer et al., 2015; Midgley and Dunin-Borkowski, 2009; Quantock et al., 2015). This technology can be used for determining molecular structures as well as the three-dimensional ultrastructure of organelles and macromolecular interactions such as fibril-SLRP relationships (Kremer et al., 2015; Midgley and Dunin-Borkowski, 2009). Recently, this technique was used to demonstrate endothelial projections into the stroma during development in mouse corneas (Feneck et al., 2020a). Light and confocal microscopy are central to the study of corneal structure and localization of matrix components. Second harmonic generation microscopy (SHG) is well suited to analyze structures built of fibrillar collagens in tendon, bone, blood vessels and cartilage (Zipfel et al., 2003). In the cornea, SHG imaging is a powerful approach in evaluating organization of collagen fibers and lamellae as well as fibrosis after wound healing in different species including mice and humans (Farid et al., 2008; Kivanany et al., 2018; Morishige et al., 2006). There are limitations, this approach can image fibril bundles, but in the cornea, stromal fibrils can not be detected due to their small size (Quantock et al., 2015). Synchrotron x-ray diffraction approaches provide another useful way to characterize the structure and organization of tissues at the nano level. X-ray diffraction was used originally to define the structure of collagen fibrils. This technique uses the scattering of a beam of X-rays to provide objective measurements. Wide-angle scattering provides data on molecules within fibrils while small-angle scattering provides data on fibrils within lamellae (Meek et al., 1991; Meek and Quantock, 2001; Quantock et al., 2015; Sayers et al., 1982). It has been applied in numerous studies addressing stromal function, aging, ocular pathologies, and to evaluate the function of different matrix components. For example, the effects of aging on interfibrillar spacing and fibril diameter in aging human corneas have been studied using this technique (Daxer et al., 1998). Corneal buttons obtained from patients with keratoconus, macular corneal dystrophy and Morquio syndrome also have been characterized (Fullwood et al., 1992; Palka et al., 2010; Rawe et al., 1997). In addition, mouse models deficient in mimecan and lumican have been studied to evaluate the function of theses matrix components in stromal structure and function (Beecher et al., 2005; Quantock et al., 2001).
2.3. Descemet’s membrane
This layer is an interfacial matrix integrating the posterior stroma with the corneal endothelial basement membrane. Descemet’s membrane is formed in fetal life and the endothelial cells continuously add a material making the Descemet’s membrane thicker with aging. It is an acellular extracellular matrix composed of hexagonal collagen VIII networks, as well as associated collagens IV and XII (Fitch et al., 1990; Hemmavanh et al., 2013; Ljubimov et al., 1996; Marchant et al., 2002; Sawada et al., 1990). It has not been characterized, but it is likely that the unique structural and compositional properties of Descemet’s membrane are involved in the integration of the corneal endothelial layer with the stroma proper. Corneal endothelial processes project through the endothelial basement membrane, and Descemet’s membrane into the stroma (Cintron et al., 1988; Feneck et al., 2020a; Hemmavanh et al., 2013; Jeang et al., 2020). It has recently been demonstrated that these processes allow functional communication between the corneal endothelium and posterior stroma keratocytes (Jeang et al., 2020).
3. Cellular types in the corneal stroma
3.1. Keratocytes
The predominant cell type in the corneal stroma is the keratocyte. Keratocytes are a unique population of neural crest-derived cells that populate the developing corneal stroma and synthesize the stromal extracellular matrix components. Keratocytes are essential for the development of the stroma and for the maintenance of its unique properties (Hassell and Birk, 2010; Jester et al., 1994). During avian stromal development, neural crest cells migrate into a hyauronate-rich environment between the developing corneal epithelium and endothelium (Johnston et al., 1979; Linsenmayer et al., 1998; Trelstad and Coulombre, 1971). These neural crest derivatives differentiate into active keratocytes (keratoblasts), that proliferate in this space and synthesize components of the stromal matrix, i.e., collagens and keratan sulfate as well as dermatan sulfate proteoglycans that replace the hyaluronan/water-rich extracellular matrix with the densely packed collagen fibrillar extracellular matrix seen in adult corneas (Cintron et al., 1983; Funderburgh et al., 1986). A similar sequence of events occurs in all species, but a distinct primary stroma is nor presnt in all species, including human.
Keratocytes regulate and direct the deposition of collagen fibrils and organize lamellae during development (Birk and Trelstad, 1984; Young et al., 2014). Keratocytes define extracellular domains, during stromal extracellular matrix assembly, where the sequential steps in stromal collagen fibril assembly can be partitioned and regulated. This is the case in the stroma and an array of other connective tissues (Birk and Trelstad, 1986; Canty and Kadler, 2002; Ploetz et al., 1991). Fibril assembly begins in deep recesses or channels in the keratocyte surface (Birk and Trelstad, 1984). The extracellular channels form during secretion as specialized post-Golgi secretory compartments. The compartments then fuse with the keratocyte surface and because of slow membrane recycling due to the presence of newly assembled fibrils (protofibrils) the channels are transiently maintained. Three-dimensional electron microscopy studies point to actin rich cytoplasmic protrusions in the keratocytes, named keratopodia, as regulators of cell-cell and cell-matrix interactions that regulate fibril deposition during stromal development (Young et al., 2014). The channels and/or keratopodia are reorganized and fibrils are deposited into a second domain where fibrils are collected into small fibers (Birk and Trelstad, 1984, 1986). As stromal matrix assembly continues the fibers are collected in a larger domain where lamellae assembly takes place. This hierarchy of compartments represents a series of spatially restricted sites where specific interactions of extracellular molecules involved in the regulation of stromal matrix assembly are compartmentalized. Keratocyte-directed matrix deposition seems to be the leading process of collagen and matrix deposition because the axes of the extensive network of keratocyte cell processes is well aligned with the alignment of stromal fibrils (Birk and Trelstad, 1984; Young et al., 2014). The fact that the dendritic keratocytes are organized into a communicating network throughout the stroma provides the means to coordinate the long-range assembly of the stromal hierarchal organization. In contrast, collagen deposition and organization in early development, in the acellular primary stroma in avian embryos, before keratocytes invade the primary stroma could be self-directed (Young et al., 2019).
Keratocytes are mitotically quiescent, exhibit a dendritic morphology with extensive intercellular contacts and gap junctions (Jester et al., 1994; Poole et al., 1993; Watsky, 1995). Keratocytes are organized as networks between the stromal lamellae with compact small cell bodies that minimize light scattering. There is no single specific marker that defines the keratocyte phenotype. However, panels of markers can be used to define the keratocyte phenotype and could include keratocan, (Liu et al., 2003; Liu et al., 1998), crystallins (Jester, 2008; Jester et al., 1995) and CD34 (Espana et al., 2004; Joseph et al., 2003; Toti et al., 2002) among others. When a scar forms during corneal wound healing, keratocyte dendritic morphology is lost and there is a down-regulation of keratocan, associated with phenotypic conversion into activated fibroblasts with loss of keratocan, CD-34 and crystallin expression (Espana et al., 2003; Jester, 2008; Jester et al., 2012; Kawakita et al., 2005a; Toti et al., 2002). Fibroblasts can continue to activate and phenotypically transition into highly contractile myofibroblasts by developing muscle-like features, including formation of actin-myosin bundles (Hinz, 2016; Hinz and Lagares, 2020).
Keratocytes synthesize and store crystallins in their cytoplasm at high concentrations. This accumulation of crystallins within the keratocyte, is throght to contribute to stromal transparency, in a manner similar to cells in the natural lens (Jester, 2008; Jester et al., 1999). Keratocytes occupy a significant volume of the stroma, up to 15%, (Huang and Meek, 1999) and the presence of crystallins in the keratocyte cytoplasm is believed to minimize light scattering. Crystallins also function to absorb UV-light in the lens and cornea, but they also may play a role in matching the refractive index of the cell cytoplasm to that of the surrounding extracellular matrix and decrease or eliminate scattering (Chen et al., 2013b). In the mammalian cornea, the most likely candidate crystallins are aldehyde dehydrogenases, particularly ALDH3A1. Members of the ALDH class 1 and ALDH3A1 are well conserved in mammals, showing ~90% homology in amino acid sequence among human, rabbit, mouse and rat (Chen et al., 2013b; Manzer et al., 2003). ALDH3A1 directly absorbs UV energy, and reduces damage to inner ocular tissues. Interestingly, ALDH3a1-deficient mice have normal clear and transparent corneas (Nees et al., 2002). Another mouse model with genetic mutations in the ALDH3a1 gene has no evident stromal changes (Downes et al., 1994). These results suggest further studies on the roles of corneal crystallins are required to fully elucidate their role(s) in corneal biology.
3.2. Stem cells
Adult stem cells are present in the corneal stroma and are located in the limbal stromal region (Chen et al., 2011b; Du et al., 2005; Kawakita et al., 2005b; Pinnamaneni and Funderburgh, 2012). Characteristic of stem cells, these cells can differentiate into different cell lineages when properly stimulated including chondrocytes and glial fibrillar acidic protein expressing cells (Du et al., 2005). These cells also can be expanded as keratocytes that are able to recreate stromal tissue under specific culture conditions (Du et al., 2005; Funderburgh et al., 2016). Mesenchymal “niche cells” previously described by Tseng’s laboratory who isolated them from the limbal stroma with collagenase after removing an intact epithelial sheet seem to have similar characteristics (Chen et al., 2011b; Xie et al., 2012). Clinically, the existence of stromal “progenitor cells” was suspected decades ago based on the analysis of sex-mismatched human full thickness corneal transplants, by fluorescence in situ hybridization of the sex-chromosomes, that showed host invasion and repopulation by keratocytes into the donor tissue. These finding suggested that keratocytes can replicate and that progenitor stromal cells exist in vivo (Wollensak and Green, 1999). Although the exact function of these cells is unknown, it is believed that, in vivo, adult corneal stromal stem cells provide a biological support system for maintenance of the epithelial stem cells in the limbal niche where both cell types interact (Dziasko and Daniels, 2016; Yamada et al., 2015). Stem cells isolated from limbal biopsies and expanded in vitro have shown promise in the regeneration of the corneal stroma following injury in mice (Basu et al., 2014; Ghoubay et al., 2020). Clinically, the success of the technique of Simple Limbal Epithelial Transplantation where pieces of epithelial and stromal limbal tissue are transplanted to damaged corneas to restore functionality suggests that stromal stem cells are functional and have the potential for significant translational use (Sangwan et al., 2012).
3.3. Dendritic cells
Dendritic cells are a subset of phagocytic tissue-specific antigen-presenting cells that regulate the immune response (Davies et al., 2013; Ginhoux and Guilliams, 2016; Liddiard et al., 2011). Dendritic cells are considered the most efficient antigen presenting cells in processing and presenting exogenous antigens on both MHC I and MHC II molecules to T cells, and initiate the adaptive immune response. Dendritic cells are a heterogeneous population of bone-marrow-derived cells that are present in all tissues including the cornea (Hattori et al., 2016). Once dendritic cells phagocytose an antigen in the cornea, they traffic to the lymph node and present antigens to initiate an immune response (Banchereau and Steinman, 1998; Steinman, 2012). In the cornea, as in other organs, dendritic cells are key players in the pathogenesis of ocular allergy, (Liu et al., 2014; Saban, 2014) response to infection,(Hamrah et al., 2009; Hu et al., 2015) and even wound healing (Gao et al., 2011). Dendritic cells seem to be essential for dynamic and functional interaction with corneal nerves (Hamrah et al., 2016). The term neuroimmunology synapse or cross-talk is used to explain the interactions between the immune and nervous systems (Hamrah et al., 2016). A classic example of such interaction in the cornea is the increased infiltration of dendritic cells around the nerve fibers with subsequent damage of the sub-basal nerve plexus noted in diabetics (Leppin et al., 2014). Dendritic cells are required for proper epithelial wound healing in the cornea and depletion of dendritic cells is associated with decreased healing (Gao et al., 2011).
4. Extracellular matrix of the corneal stroma
The precise structural organization of the stroma is a requisite for corneal transparency. Corneal transparency relies on the regular packing of homogeneous, small diameter collagen fibrils (Hassell and Birk, 2010; Linsenmayer et al., 1998; Maurice, 1957). Fibril diameter varies based on species, fibril diameters range from 23–35 nm (Bard and Bansal, 1987; Meek and Leonard, 1993). The fibrils are regularly packed with equidistance spacing (Massoudi et al., 2016; Meek and Leonard, 1993). Finally, the fibrils are organized into layers or lamellae that are approximately perpendicular to one another (Birk and Trelstad, 1984; Linsenmayer et al., 1998). The structure and organization of collagen fibrils are the most distinctive features of the corneal stroma. The complexity of the stromal hierarchical organization is clearly noted in the evolutionary changes observed between species. Species specific differences in stromal structure have been identified (Koudouna et al., 2018). The angle between parallel fibers that are rotated relative to each other is less than 90° angles in mammals, but over 100– 200° in nonmamalian corneas. Second, the length of continuous lamellae varies with lamellae extending from limbus to limbus in nonmammalian corneas. Finally, an increased complexity of the corneal lamellae structure is observed from from reptiles to birds.
The predominant fibril-forming collagen type in the corneal stroma is collagen I. However, corneal stroma structure and extracellular matrix structure, in general, is not determined by the collagen type present. Collagen I is the predominant collagen in striated collagen fibrils that have a vast array of tissue-specific structures and organizations. A good example being the corneal stroma and the sclera, both composed predominantly of collagen I, but with markedly different fibril structures, organizations and hierarchal structure (Birk, 2011; Chakravarti et al., 2003; Komai and Ushiki, 1991; Meek and Fullwood, 2001). A likely explanation is that collagen fibrils in different tissues, at different developmental stages, or in response to injury have different macromolecular compositions that include other collagen types as well as non-collagenous components. Complex interactions with collagen I – containing fibrils include networks of collagenous structures in the interfibrillar spaces and associated with cellular interfaces; non-fibrillar collagens and proteoglycans; networks of fibrillin; and a variety of glycoproteins. These additional macromolecules may vary widely in amount, however, invariably the tissue-specific composite structure of collagen fibrils is a major determinant of architecture and function. These stromal extracellular matrix components have a functional role in determining stromal structure, however, many have additional functions that are not fully defined. Work remains to fully elucidate the importance of the components in the structure and function of the cornea.
4.1. Collagens
4.1a. Fibril-forming collagens
The structural unit of the corneal stroma is the collagen fibril. The fibril-forming collagen subfamily includes collagens I, II, III, V, and XI. These collagens have a uninterrupted triple helical domain of about 300 nm (Birk, 2011). Collagen I is most abundant protein in the corneal stroma and comprises the bulk of collagen fibrils. Collagens V and XI are quantitatively minor collagens found co-assembled with collagens I, II and III in different tissues (Birk, 2011; Smith and Birk, 2012). In the corneal stroma collagen V makes up 10–20% of the fibril-forming collagens (McLaughlin et al., 1989; Poschl and von der Mark, 1980; Tseng et al., 1982) and collagen XI may be present in trace amounts in development, as is the case in other collagen I containing tissues. The collagen V/XI subclass has been termed regulatory fibril-forming collagens (Birk, 2011; Chen et al., 2015; Smith and Birk, 2012). The regulatory fibril-forming collagens retain portions of the N-terminal propeptide and are involved in the regulation of fibril assembly (Fichard et al., 1995; Smith and Birk, 2012). The α1(V)2α2(V)1 isoform of collagen V is present in the stroma proper. It is a quantitatively minor regulatory fibril-forming collagen and is the primary determinant responsible for control of collagen fibril diameter in the corneal stroma with diameters of ~25–35 nm, compared to fibril diameter in other tissues, for example, the sclera, that has minimal collagen V content and contains collagen fibrils ranging from 25 – 250 nm (Komai and Ushiki, 1991).
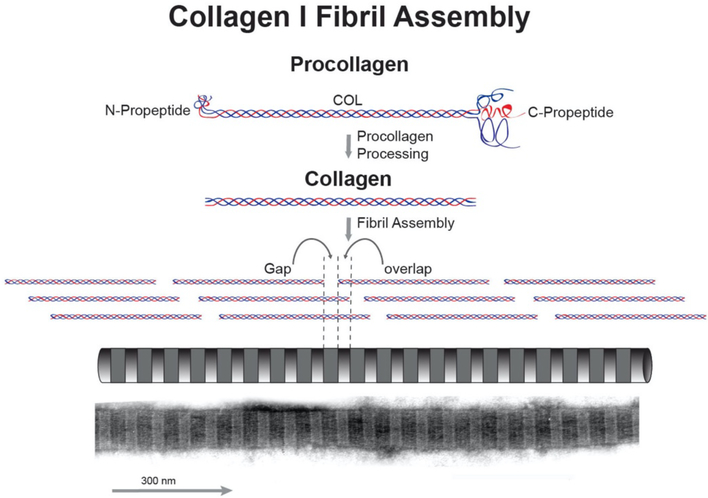
The fibril-forming collagens are synthesized and secreted as procollagens. Procollagens contain a non-collagenous C-terminal propeptide and an N-terminal propeptide. The N-propeptide is composed of several non-collagenous domains and a short collagenous domain. The presence of the propeptides prevents premature assembly of collagen molecules into fibrils (Hulmes, 2002; Kadler, 2017). The initial assembly of collagen into fibrils is regulated by the processing of the propeptides and processing involves a number of enzymes. The C-propeptides are processed by BMP-1/tolloid proteinases or furin (Hopkins et al., 2007). The processing of the N-propeptides involves ADAMTS 2, 3 and 14 as well as BMP-1 (Colige et al., 2005; Hopkins et al., 2007). These processing enzymes have specificity for different collagen types (Kadler et al., 2007). In the corneal stroma, propeptide processing may be complete, i.e., collagen I, leaving a collagen molecule with one large central triple helical domain and terminal, short noncollagenous sequences termed the telopeptides. Processing also can be incomplete, i.e., in collagens V and XI, leaving a C-telopeptide and a partially processed N-propeptide domain. The N terminal domains of collagens V and XI both have been implicated in the regulation of fibrillogenesis (Birk, 2011; Smith and Birk, 2012; Wenstrup et al., 2011). After processing of the propeptides, collagen molecules self-assemble to form striated fibrils with a periodicity of 67 nm. Within the fibril, the collagen molecules are arranged in longitudinally staggered arrays with a gap occurs between the ends of neighboring molecules. This generates a gap-overlap structure in all collagen fibrils with a D-periodic banding pattern (Fig. 1). This basic collagen fibril structure was worked out in simple well ordered tissues such as the rat tail tendon which is primarily collagen I with little proteoglycan content. Attempts have been made to correlate periodicity with the genetic collagen type without success (Eikenberry et al., 1980). However, tissue-specific difference in composition of collagens, e.g. heterotypic fibrils, and other matrix macromolecules such as proteoglycans can influence fibril structure. This is demonstrated in the stroma where the fibril periodicity is 65nm (Meek, 2009).
Fig. 1. Fibril-Forming Collagens: Fibrils.
Collagen I is the major stromal protein. This fibril-forming collagen is synthesized as procollagen. Procollagens have a central collagen (COL) domain with flanking N-and C-terminal propeptides. Extracellularly, the propeptides are processed and the resulting collagen molecules assemble to form striated fibrils. The collagen I molecule is approximately 300 nm in length and 1.5 nm in diameter. During fibril assembly, the collagen molecules are staggered N to C and this staggered pattern of collagen molecules gives rise to a 67nm repeat. An electron micrograph of a negative stained fibril is shown at the bottom of the panel. This fibril has the characteristic alternating light/dark pattern representing the gap (dark) and overlap (light) regions of the fibril.
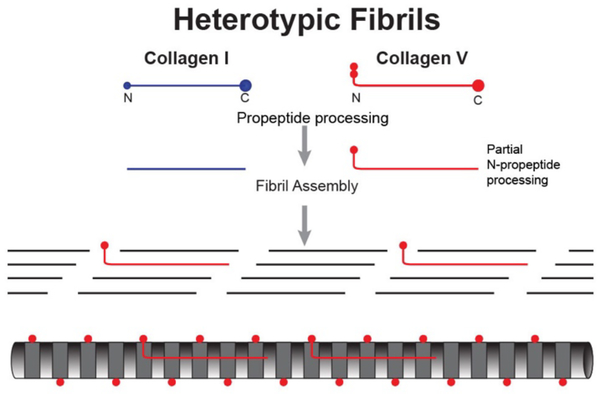
Collagen fibrils are heterotypic, assembled of 2 or more fibril-forming collagen types. The corneal stroma contains collagen I, the quantitatively major fibril-forming collagen, as well as minor amounts of collagens V and XI. These quantitatively minor regulatory fibril-forming collagens V and XI are characterized by a partial processing of the N-propeptide domain. The N-propeptides have a flexible, hinge domain between the triple helical domain and a short triple helical domain. The N-terminal domain is composed of variable and PARP domains. Processing involves specific cleavage of the PARP domain with retention of the hinge, COL2 and variable domains (Gregory et al., 2000; Hoffman et al., 2010; Linsenmayer et al., 1993). These regulatory fibril-forming collagens co-assemble with the major fibril-forming collagens to form a heterotypic fibril. The N-terminal domain of the regulatory fibril forming collagens cannot be integrated into the staggered packing of the helical domains. The hinge region (NC2) is flexible so that the rigid COL2 domain can project toward the fibril surface in the gap region and the variable domain is present in the gap and on the fibril surface (Fig. 2).
Fig. 2. Corneal Stroma Heterotypic Fibrils.
Corneal stromal collagen fibrils are heterotypic, co-assembled from quantitatively a major fibril forming collagen, e.g., collagen I and regulatory fibril-forming collagen, e.g., collagen V. Regulatory fibril-forming collagens have a partially processed N-terminal propeptide, retaining a non-collagenous domain that must be in/on the gap region/fibril surface. This is the major regulatory domain.
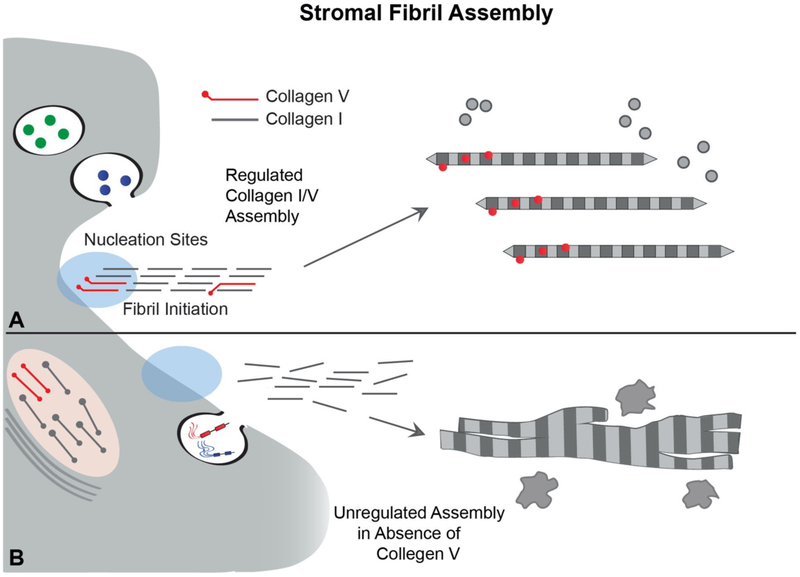
It is clear that interaction between fibrillar collagens act as key regulators of the collagen organization in the fibril, resulting in tissue-specific fibril differences. Absence of collagen V is lethal during development, but corneal stroma-specific deletion of collagen V expression in mouse models results in an abnormal matrix with abnormal large diameter fibrils, aberrant fibril structure, disorganized fibrils, corneal thinning due primarily to a reduced number of fibrils and loss of corneal transparency (Sun et al., 2011). Patients with classic Ehlers - Danlos syndrome, deficient in collagen V, present with limbus to limbus corneal thinning, (Segev et al., 2006) and to our knowledge there is no reported increased incidence of corneal ectasia or spontaneous corneal peroration in humans. A heterozygous mouse model for collagen V replicates corneal thinning suggesting a role for collagen V in corneal thickness (Segev et al., 2006). Figure 3 presents a model for regulated control of initial fibril assembly, diameter and fibril number via collagen V/I interactions. Collagen V acts as a nucleator associated with the keratocyte surface. It interacts with collagen I and initiates fibril assembly. Diameter is regulated by increasing or decreasing the number of nucleation sites to decrease and increase fibril diameter respectively. This assumes a constant expression of collagen I. This mechanism also determines the number of fibrils assembled. Since the collagen V is associated with the keratocyte surface it provides a mechanism whereby keratocytes can influence fibril deposition and organization.
Fig. 3. Heterotypic Collagen I/V Interactions Regulate Initial Fibril Assembly.
Stromal fibril assembly involves a sequence of regulatory interactions. (A) Initially, collagen V interacts with collagen I to nucleate collagen assembly into fibrils at the keratocyte surface. This results in the regulated assembly of immature, small diameter, short fibrils termed protofibrils. This initial step in fibril assembly is cell-directed involving interactions with organizers at the keratocyte surface, e.g., integrins and syndecans either directly or through intermediate interactions, e.g. fibronectin (indicated by shaded blue region). This permits kertocyte control over the initial assembly steps and allows for positioning of newly assembled fibrils into the stromal matrix. (B) In the absence of collagen V regulation of collagen I assembly and positioning of assembled fibrils is lost. This results in formation of fewer, larger and heterogenoeus fibril diameters, structurally abberent fibrils as well as disrupted fibril organization. This unregulated assembly is not consistent with stromal transparency.
Collagen II, a homotrimeric protein, and is the major fibril-forming collagen in cartilage and vitreous where it assembles fibrils with collagen XI as a nucleator. In the cornea, collagen II is found in the developing avian primary stroma (Chen et al., 1993; Linsenmayer et al., 1990). There are several alternatively spliced forms of collagen II and collagen IIA is expressed in a variety of developing tissues including mouse cornea (Cheah et al., 1991). Also, expression of collagen II can be induced in isolated rat keratocytes with dexamethasone and TGF-β3 (Greene et al., 2016). The collagen II gene also undergoes alternative splicing to generate the α3(XI) chain of collagen XI. So the possibility for miss identification in tissues exists. Overall, the specific function(s) of collagen II in developing tissues, including the corneal stroma, remain to be defined.
The presence and role of collagen III in the corneal stroma is controversial. Collagen III is widely distributed in multiple organs, including blood vessels, skin, uterus and bowel (Laurent et al., 1981; Pope et al., 1975). In humans, the vascular type of Ehlers-Danlos disease is secondary to a deficiency of collagen III (Kontusaari et al., 1990; Pope et al., 1975; Prockop and Kivirikko, 1984). In the cornea, the expression is contradictory. Some authors report no expression found in the adult human stroma, (White et al., 1997) while other found collagen III in human corneal tissue (Newsome et al., 1982). Collagen III was observed in the adult rabbit cornea and during development, as well as in Descemet’s membrane, and it was suggested that collagen III is secreted by endothelial cells (Cintron et al., 1988). In bovine stroma, the expression of collagen III is present during development, but decreases with aging (Schmut, 1977). The decrease in expression with maturation is present in tissue culture models of bovine keratocytes where higher expression is found in cultures from younger tissue (Kane et al., 2009). In contrast, while the presence of collagen III in normal, uninjured corneal tissue is controversial, its presence during the injury response is much stronger. Collagen III gene expression is suggested as a marker of scarring in mouse injury models following trauma or induced stromal injury (Ghoubay et al., 2020; Gupta et al., 2018; Marino et al., 2017; Shojaati et al., 2019). The processing of procollagen III is unique in that the N-propeptide is processes slowly compared to procollagen I (Fessler et al., 1981). The slow processing of the N-prppepide has been suggested to confer transient regulator properties similar to those seen with collagen V. Further analysis of the role(s) of collagen III in the normal stroma as well as in the injury response is needed.
4.1b. Fibril-associated collagens with interrupted triple helices (FACIT)
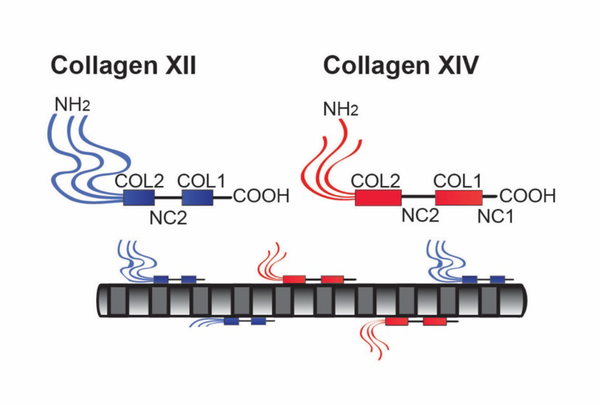
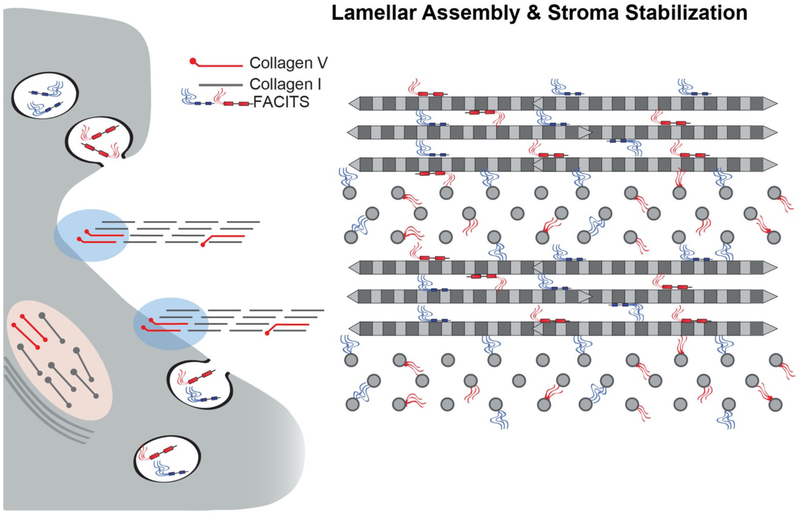
FACIT collagens do not form fibrils but interact with collagen fibrils (Fig. 4). In addition, these collagens interact with basement membranes and cell interfaces. FACIT collagens have been implicated in regulation of matrix organization and cell behavior (Birk, 2011; Chiquet et al., 2014; Gordon and Hahn, 2010; Linsenmayer et al., 1998). This group of collagens includes collagens IX, XII, XIV, XIX, XX, XXI, and XXII (Ricard-Blum, 2011). The two most studied FACITs expressed in the stroma are collagens XII and XIV. FACIT collagens are localized on the surface of the fibrils and in the interfibrillar space (Fig. 2). The changes in fibril properties afforded by these interactions generate diversity in the properties of stromal collagen fibrils, fibers and lamellae. Their function is not completely understood, but they are implicated in the regulation of tissue structure and function as well as cell organization(Birk, 2011; Chiquet et al., 2014; Gordon and Hahn, 2010; Izu et al., 2011; Sun et al., 2020). In addition, their expression is influenced by mechanical forces.(Birk, 2011; Kadler et al., 2007; Linsenmayer et al., 1998; Massoudi et al., 2016)
Fig. 4. FACIT Collagens.
Collagens XII and XIV are fibril-associated in the stroma. Their domain structures are illustrated. All FACITs have alternativly spliced variants and collagen XII can have glycosaminoglycan chains covalently attached. The FACIT collagens have 2–3 collagen (COL) domains and 3–4 non-collagenous (NC) domains. Characteristic of this collagen type is a large N-terminal NC domain that projects into the inter-fibrillar space. The FACIT collagens all associate with the surface of collagen fibrils and this is illustrated, including N-truncated isoforms due to alternative splicing in collagen XII. Collagen XII is capable of non-fibrillar interactions (not shown), it is not known if this is true for collagen XIV.
Collagen XII is expressed in the human, mouse, chicken and other corneal stromas (Font et al., 1996; Gordon et al., 1987; Marchant et al., 2002; Massoudi et al., 2012). This FACIT collagen has the highest stromal expression. It is a homotrimeric molecule composed of two collagenous domains, COL1–COL2, and three noncollagenous domains, NC1 to NC3 (Shaw and Olsen, 1991). Alternatively spliced variants at the NC1 and NC3 terminals are believed to confer tissue specific properties (Kania et al., 1999). Human stroma and sclera contain the long variant form as the predominant form of collagen XII (Wessel et al., 1997). Collagen XII is expressed in tissue regions of high mechanical stress, and its expression is up-regulated by mechanical stimulation (Arai et al., 2008). Collagen XII plays a major role in establishing hierarchical organization of the stroma as demonstrated by collagen XII upregulation at times of fibril formation and organization and disruption of keratocyte and lamellar organization in a mouse model null for collagen XII (Sun et al., 2020). Figure 5 is a schematic illustrating how FACIT – fibril interactions regulate and stabilize lamellae formation. A lack of collagen XII expression in null mouse models is associated with disruption of stromal structure at the fibril, lamellar and tissue level (Sun et al., 2020). In human and mouse corneas, collagen XII is upregulated during stromal injury and is present in scarred stromas suggesting a role in stromal remodeling and regeneration (Massoudi et al., 2012). In addition, in zebrafish models of spinal injury, Wnt/β-catenin signaling induces a specific axon regrowth permissive matrix with collagen XII in the lesion site, suggesting that recapitulation of collagen XII expression occurs with regeneration (Wehner et al., 2017). Collagen XII is overexpressed in the subepithelial area in patients with bullous keratopathy (Ljubimov et al., 1996). In keratoconus corneas, proteomic analysis demonstrated decreased collagen XII, as well as decreased proteoglycans and collagen I levels (Chaerkady et al., 2013).
Fig. 5. FACIT Regulation of Stromal Hierarchal Assembly.
FACIT collagens bind to fibril surfaces. In addition, they can interact with cells and a number of other matrix components. These properties are required for integration of different stromal components. FACIT collagens XII and XIV are associated with stromal fibrils. These interactions, along with SLRPs (see Fig. 9) can stabilize fibril diameter and spacing. In the absence of collagen XII, stromal lamellae fail to develop properly and there is a general disorganization of lammelar and stromal architecture. This indicates a critical role in integration of the fibrillar components necessary for stable lamellae formation. There is also evidence that collagen XII is enriched at cell and basement membrane interfaces (not shown) and may facilitate cell-matrix integration.
Collagen XIV is a homotrimer that contains two collagen triple-helical domains (COL1 and COL2) and three non-collagenous domains (NC1, NC2, and NC3).The triple-helical domains interact with and adhere to the surface of fibrillar collagens (Ansorge et al., 2009; Birk, 2011; Gordon et al., 1996). The collagen XIV COL1 and NC1 domains interact with collagen I. The NC3 domain is a large amino terminal globular domain that extends away from the fibril into the interfibrillar space. The NC3 subdomain has structural homology to von Willebrand factor A domains and fibronectin type III repeats.
Collagen XIV is implicated in the regulation of corneal stromal compaction (Gordon et al., 1996) and promotes collagen gel contraction by fibroblasts in vitro (Nishiyama et al., 1994). In vivo studies of chicken tendon during embryogenesis and early post-hatching stages showed high collagen XIV expression during development that decreases during tissue maturation (Young et al., 2002). Similar findings were reported in mouse tendons and heart during development (Ansorge et al., 2009; Tao et al., 2012). These studies suggest that collagen XIV plays an important role during early development, but its expression, regulation and specific roles in adult tissue and during wound healing remain to be elucidated.
4.1c. Network forming collagens
These collagens do not form fibrils, but rather form networks with different spatial localizations and functions within the corneal stroma. It is unlikely that the network-forming collagens have a direct influence on regulation of fibril assembly and organization. Rather they are involved in integrating cells and matrix structures and/or integration of different matrix structures such as basement membranes and the stroma. These collagens are differentially expressed in Bowman’s layer, the stroma proper and Descemet’s Membrane and play vital roles in stromal structure and function.
Collagen IV networks form the backbone of the corneal basement membrane. The collagen IV network also interacts with basement membrane proteoglycans and glycoproteins that determine its function (Khoshnoodi et al., 2008; Pozzi et al., 2017; Yurchenco, 2011). Interactions with the components of basement membranes are important in integrating epithelia and the supporting stroma. Collagen IV is present in the corneal epithelial and endothelial basement membranes (Kabosova et al., 2007; Medeiros et al., 2018; Saikia et al., 2018). Collagen IV also is present in Bowman’s Layer as a part of the anchoring complex (Gipson et al., 1983; Gipson et al., 1987; Keene et al., 1987) and extends into Descemet’s membrane (Fitch et al., 1990). However, for the purposes of this review we are excluding further discussion of the basement membranes.
Collagen VI is a ubiquitous component of connective tissues and is widely and abundantly expressed in the corneal stroma. It is found as an extensive network of beaded filaments associated with collagen fibrils and is often enriched in pericellular regions. In addition to beaded microfibrils, there are other tissue forms, i.e., hexagonal networks and broad banded structures (Bruns et al., 1986; Linsenmayer et al., 1986; von der Mark et al., 1984). Collagen VI binds a large number of extracellular molecules including: collagens I, II, IV, XIV, microfibril-associated glycoprotein (MAGP-1), perlecan, decorin, biglycan, hyaluronan, heparin and fibronectin as well as integrins and the cell-surface proteoglycan NG2 (Bonnemann, 2011; Cescon et al., 2015; Lamande and Bateman, 2018). Since collagen VI is able to bind to multiple matrix and cell surface proteins it has the capacity to integrate the surrounding connective tissue with cells and organize the three-dimensional tissue architecture (Cescon et al., 2015; Lamande and Bateman, 2018). In addition, collagen VI may influence cell migration, differentiation and apoptosis/proliferation. It also promotes adhesion and spreading of keratocytes (Doane et al., 1996; Doane et al., 1992).This indicates a role(s) in the development of tissue-specific extracellular matrices, repair processes and in the maintenance of tissue homeostasis.
The best characterized form of collagen VI is a heterotrimer composed of α1(VI), α2(VI) and α3(VI) chains (Chu et al., 1987; Kielty et al., 1990).This form is present in the corneal stroma (Takahashi et al., 1993). The collagen VI monomer has a short,105 nm triple helical domain with flanking N- and C-terminal globular domains. The N–terminal domain is approximately twice the size of the C-terminal domain and is primarily derived from the α3(VI) chain. In addition, alternative splicing of the α3(VI) N–terminal domain introduces structural heterogeneity. There are multiple isoforms of collagen VI with 3 additional α chains, α4(VI), α5(VI), α6(VI) able to form different combinations (Fitzgerald et al., 2008; Gara et al., 2008). These chains have high homology with the α3(VI) chain and may form additional isoforms.
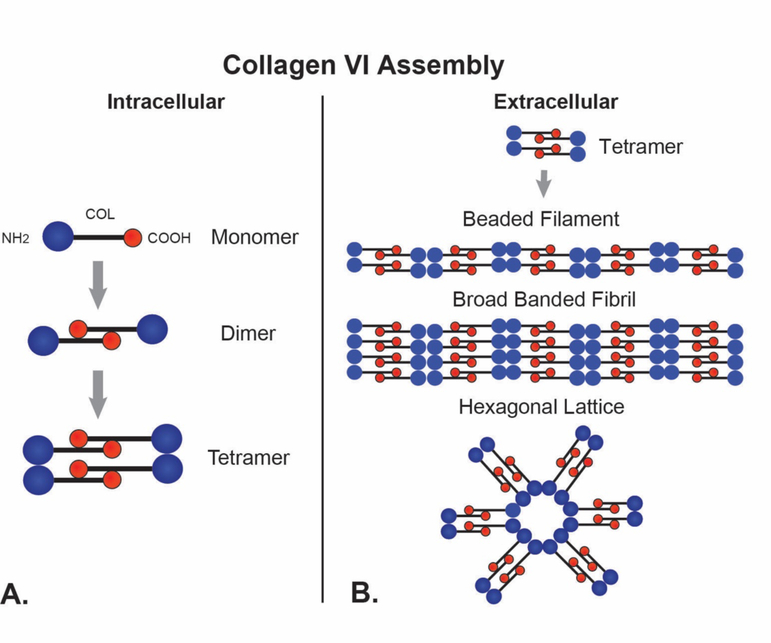
The assembly of collagen VI into beaded filaments begins intracellularly (Fig. 6). First, a dimer is formed via lateral, anti-parallel association of two monomers. The monomers are staggered by 30nm with the C-terminal domains interacting with the helical domains. This overlap generates a central 75nm helical domain flanked by a non-overlapped region with the N– and C-globular domains, each about 30 nm. These interactions are stabilized by disulfide bonds (Ball et al., 2003). This is followed by tetramer formation where two dimers align with the ends in register. Finally, tetramers are secreted and are the building blocks used to assemble beaded filaments. Extracellularly, tetramers associate end-to-end to form to thin, beaded filaments (3–10nm) with a periodicity of approximately 100nm. These beaded filaments laterally associate, forming beaded microfibrils (Baldock et al., 2003; Bruns et al., 1986; Furthmayr et al., 1983). In addition to beaded microfibrils, other collagen VI -containing supramolecular structures are found in the ECM including hexagonal lattices; and broad banded fibrils with a 100nm periodicity. The broad banded fibrils represent continued lateral growth of beaded microfibrils and/or lateral association of preformed beaded microfibrils. In contrast, hexagonal lattices are formed via end-to-end interactions of tetramers in a non-linear fashion (Wiberg et al., 2002).
Fig. 6. Collagen VI.
Collagen VI forms networks of beaded filaments in the corneal stroma. (A) Collagen VI monomers have a C-terminal non-collagenous (NC) domain, a central triple helical domain and an N-terminal NC domain. Intracellularly, monomers assemble N-C to form dimmers. Tetramers are assembled from two dimmers aligned in register. (B) The tetramers are secreted, and extracellularly they form the building blocks of 3 different collagen VI Assemblies. These include beaded filaments, broad banded fibrils and hexagonal lattices form via end-to-end interactions of tetramers and varying degrees of lateral association.
Comparable to fibrillar collagen, supramolecular assemblies of collagen VI are composite structures with other associated molecules modulating the functional properties of these assemblies. For example, biglycan interactions with tetramers induced hexagonal lattice formation rather than beaded microfibrils which was dependent on the glycosaminoglycan chains. In contrast, decorin, which binds to the same site, was less effective in inducing hexagonal lattice formation (Wiberg et al., 2002). The interaction of small leucine-rich proteoglycans with collagen VI influences the structure of the tissue aggregate and therefore its function. This provides a mechanism to assemble different collagen VI structures in adjacent regions or tissues with different functions.
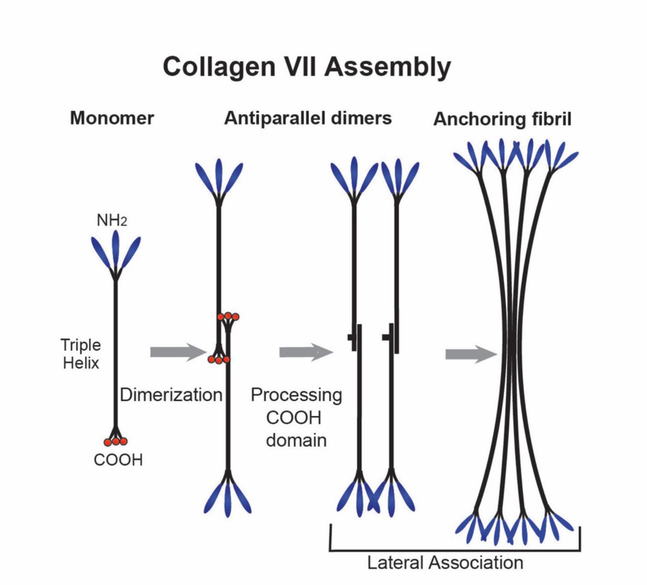
Collagen VII is a large collagen that is assembled into anchoring fibrils (termed fibrils, but ‘anchoring fibrils’ are structurally distinct from ‘collagen fibrils’) that form networks tethering epithelial basement membranes to the underlying connective tissue (Burgeson and Christiano, 1997). As a homotrimer, the large central collagenous domain of collagen VII is flanked by N- and C-terminal non-collagenous domains (Bruckner-Tuderman et al., 1999). Unlike the fibril-forming collagens, the collagenous domain contains numerous interruptions that provide flexibility to this collagen. Collagen VII is secreted into the ECM where it forms antiparallel tail-to-tail dimers with a central C-terminal overlap and with the N-termini pointing outwards. There is a proteolytic processing of a portion of the NC2 domain that permits lateral association. Subsequently, the processed dimers aggregate laterally in a non-staggered manner into the anchoring fibrils (Fig. 7). Mature anchoring fibrils are stabilized by transglutaminase cross-links.
Fig. 7. Collagen VII.
Collagen VII forms a network of anchoring fibrils that adhere to the epithelial basement membrane and form loops entrapping the collagen fibrils in Bowman’s layer. This integrates the epithelial basement membrane with the underlying stroma. Collagen VII has a long central triple-helical collagenous (COL) domain containing numerous interruptions conferring flexibility to the domain. The COL domain is flanked by non-collagenous N- (NC-1) and C-terminal (NC-2) domains. Two monomers interact to form an anti-parallel dimer with a central C-terminal overlap and the NC-1 domains pointing out. Processing occurs, with a cleavage of the NC-2 propeptide and covalent stabilization of the dimer. At this point, a non-staggered lateral association of dimers occurs that produces anchoring fibrils.
In the corneal stroma, anchoring fibrils extend from the epithelial basement membrane into Bowman’s layer, thus integrating the corneal epithelium with the stroma. The NC1 domains of collagen VII at both ends of the anchoring fibrils bind to macromolecules in the basement membrane including collagen IV and laminin. This forms a network of loops that entrap collagen fibrils. It has been shown that collagen VII has poor affinity to most collagens, including collagen I (Brittingham et al., 2006). However, in the dermis, anchoring fibrils bind tightly to cross-striated collagen fibrils containing, among other types, collagen I. This indicates that there are binding determinants that exist only at the level of supramolecular aggregates and these sites are important for dermo-epidermal cohesion (Villone et al., 2008) and are compromised in patients with dystrophic epidermolysis bullosa, a severe heritable skin blistering disease (Bruckner-Tuderman, 2010). This bullous disorder of the skin and mucous membranes, is frequently associated with ocular complication. Eyelid ulcerations, chronic conjunctivitis, diffuse subepithelial corneal scarring, recurrent corneal ulcerations and sclerocornea are described in these patients (Destro et al., 1987; Sharkey et al., 1992).
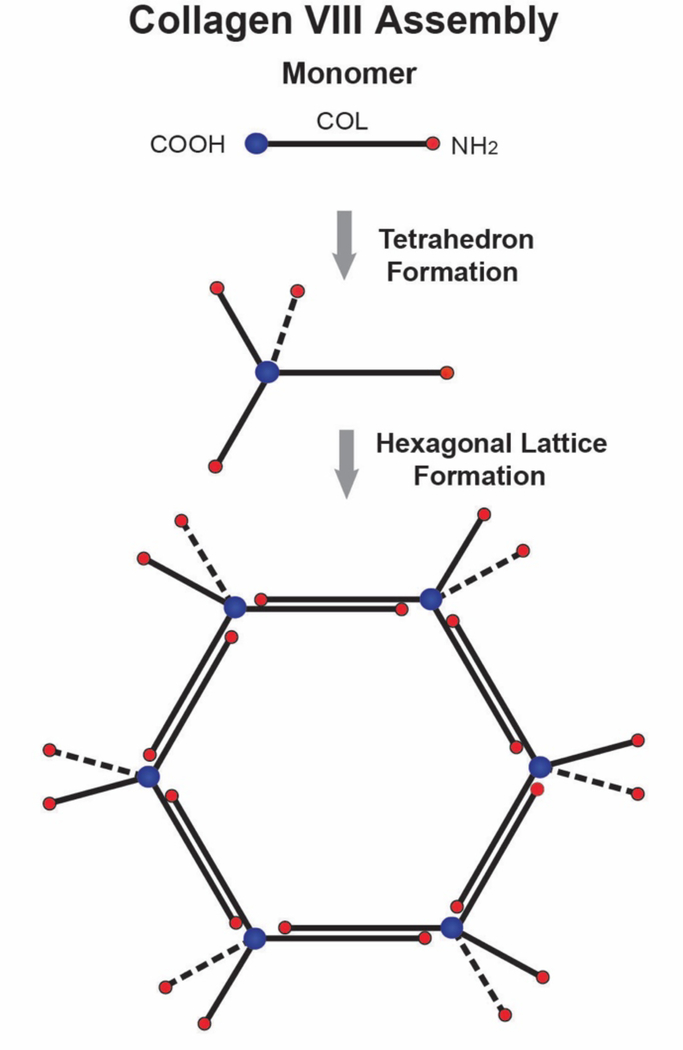
Collagen VIII is a short chain collagen found as a major component in Descemet’s membrane (Sawada et al., 1990; Shuttleworth, 1997). Descemet’s membrane is composed of layers of hexagonal lattices (Jakus, 1956). These lattices contain collagen VIII (Sawada et al., 1990). Collagen VIII is a homo- or hetero-trimer of α1(VIII) and α2(VIII) chains and both homotrimers and the α1(VIII)2α2(VIII) heterotrimer exist in tissues (Illidge et al., 1998, 2001). The collagen VIII monomer has a short central COL domain and is flanked by N- and C-terminal NC domains. The monomers form lattices in vitro comparable to those in tissues (Stephan et al., 2004). It was proposed that collagen VIII monomers form a tetrahedron through the interaction of 4 molecules. This structure serves as the building block that assembles into three-dimensional hexagonal lattices. The assembly of a layered hexagonal lattice could involve interaction of the N-terminal non-collagenous domains or anti-parallel interactions involving both helical and terminal domains (Fig. 8). Collagen VIII expression in Descemet’s membrane has been the object of different studies due to its association with Fuchs endothelial dystrophy. Missense mutations in the gene encoding the α2(VIII) subunit of collagen VIII are associated with an early-onset form of Fuchs dystrophy (Biswas et al., 2001; Gottsch et al., 2005; Mok et al., 2009). In addition, two different mouse models with Col8a2 mutations, recapitulate some of the findings in endothelial cells and Descemet’s membrane found in humans (Jun et al., 2012; Meng et al., 2013).
Fig. 8. Collagen VIII Forms Hexagonal Lattices In Descemet’s. Membrane.
Collagen VIII is a short chain collagen with a central collagenous (COL) domain and flanking N- and C-terminal non-collagenous (NC) domains. The C-terminal NC domains of 4 collagen VIII molecules interact to form tetrahedrons. Tetrahedrons assemble further to form hexagonal lattices. A planar hexagonal lattice is illustrated. In Descemet’s membrane continued assembly, involving interactions of the N-terminal NC domains or anti-parallel interactions involving both helical and terminal domains (not shown) generates the layered hexagonal lattice characteristic of Descemet’s membrane (not shown).
4.2. Proteoglycans
Small leucine-rich proteoglycans (SLRP) consist of a protein core with a central leucine-rich repeat domain that is flanked by N- and C-terminal cysteine-rich domains. Covalently linked to the protein core are 1 or 2 glycosaminoglycan (GAG) chains. The GAGs are chondroitin sulfate/dermatan sulfate in the Class I SLRPs, keratan sulfate in the class II corneal SLRPs and class III osteoglycin. Class I SLRPs includes decorin and biglycan. Class II includes: fibromodulin, lumican, and keratocan. Six SLRPs are expressed in the stroma: decorin, biglycan, lumican, keratocan, fibromodulin, and osteoglycin. These proteoglycans act as tissue organizers, influence cell growth, modulate and tether growth-factors, bind water through their GAG chains maintaining stromal hydration, and regulate collagen fibrillogenesis. Decorin, biglycan and fibromodulin bind TGF-β, (Brown et al., 2002; Hildebrand et al., 1994) to reduce its bioavailability and compete with the signaling receptors to attenuate signal transduction (Droguett et al., 2006; Frikeche et al., 2016). The importance of the GAG chains covalently attached to the protein core to from a proteoglycan is evident in macular corneal dystrophy. In macular corneal dystrophy, mutations in the sulfotransferase 6 gene creates deficiencies in the sulfation of keratan sulfate GAG chains with unsulfated keratan sulfate proteoglycans being deposited in the stroma with subsequent stromal opacification (Akama et al., 2000; Lewis et al., 2019). Sulfation of keratan sulfate GAG chains is blocked in a mouse model deficient in the enzyme N-acetyl-glucosamine-6-O-sulfotransferase. These mice have transparent corneas. However, the stroma is thin compared to wild type controls with abnormal collagen fibril organization and packing with increased inter-fibrillar spacing (Hayashida et al., 2006). These data clearly illustrate the importance of GAG chains in stromal structure and function.
4.2a. Decorin and Biglycan
Expression of both class I SLRPs is homogenous across the corneal stroma. Biglycan expression is low in the mature stroma while decorin expression is higher (Doane et al., 1996; Mohan et al., 2011; Rada et al., 1993; Zhang et al., 2009). Biglycan expression is increased during wound healing (Basu et al., 2014). Secreted biglycan interacts via its core protein or GAG chains with numerous components of the stromal extracellular matrix including collagens I, II, III and becomes integrated into the matrix (Douglas et al., 2006; Hunzelmann et al., 1996; Schonherr et al., 1995). These SLRPs play key roles in the regulation of stromal fibril structure and organization. The absence of decorin in mutant mice models results in a severe disruption of fibril structure and stromal organization; however, the absence of biglycan has little or no effect on corneal stromal structure (Zhang et al., 2009). In decorin null stromas biglycan was upregulated masking most of the decorin effect. This indicates a potentially modulatory role biglycan in the corneal stroma depending on development stage and/or in the injury response.
Two important decorin related processes are of translational relevance in the cornea. Autosomal-dominant congenital stromal corneal dystrophy, a rare human genetic disease is linked to mutations in decorin (Rodahl et al., 2006). The mutations result in a truncation of the decorin core altering the ability to bind collagen fibrils. This results in a disruption of matrix organization and opacity (Bredrup et al., 2010; Chen et al., 2011a; Kamma-Lorger et al., 2016). A novel animal model that recapitulated this corneal dystrophy was generated in mutant mice. Corneal opacities were found with increased severity toward the posterior stroma. The architecture of the lamellae was disrupted with relatively normal lamellae separated by regions of abnormal fibril organization (Chen et al., 2011a). While the exact(s) mechanisms underlieing this congenital disorder are not fully understood abnormal decorin has a central role (Chen et al., 2013a; Mellgren et al., 2015). Second, studies indicate that decorin is an effective candidate for diminishing TGF-β bioavailability and therefore down-regulation of haze and corneal scarring. Through formation of complexes with TGF-β, decorin neutralizes TGF-β, and by competitive inhibition, decorin inhibits the binding of TGF-β to its receptor reducing scar formation (Harper et al., 1994; Zhang et al., 2018).
4.2b. Lumican and keratocan
Lumican and keratocan are the predominant class II SLRPs in the corneal stroma. While not totally corneal-specific together these SLRPs are excellent biomarkers for the tissue. They are expressed homogeneously across the cornea stroma at birth, but the expression of lumican is restricted to the posterior stroma in the adult. The role of lumican in regulating stromal function has been studied in lumican null mouse models. In lumican deficient corneas, an initially clear cornea becomes cloudy, postnatally, with a majority of the light backscattering located in the posterior stroma where lumican expression is located in adult corneas (Chakravarti et al., 2000). This suggest a role for lumican in regulating corneal transparency in post-natal maturation (Chakravarti et al., 1998). In lumican deficient corneas, analysis of hierarchical structure shows that lamellar structure was disrupted across the stroma with disorganized fibrils, and altered fibril packing in the posterior stroma, indicating that lumican is a regulator of fibrillogenesis with changing expression patterns providing spatially restricted regulation. There are no well characterized human conditions associated with mutations in lumican. Mutant mice deficient for keratocan expression have transparent, but thin corneal stromas with a mild increase in collagen fibril diameter with no further abnormalities (Liu et al., 2003). In humans, mutations in the keratocan gene are associated with a flattened cornea in a rare condition known as cornea plana (Pellegata et al., 2000). The relative specificity of keratocan to corneal keratocytes has been useful for tissue-specific targeting for genetic manipulation of the corneal stroma (Sun et al., 2011; Zhang et al., 2017).
4.2c. Fibromodulin
Fibromodulin is not considered to be a major corneal SLRP. Fibromodulin and lumican show 50% identity in primary sequence and bind the same site on collagen I (Kalamajski and Oldberg, 2009). In mouse models deficient in fibromodulin, lumican compensates for the absence of fibromodulin, suggesting that they can function as a coordinated pair. Fibromodulin is expressed in scleral tissue, but its expression in the corneal stroma of adult mice is weak (Chakravarti et al., 2003). However, during development, fibromodulin is found in the peripheral cornea and the posterior stroma. In adult corneas, stromal expression has regressed and is localized to the scleral and limbal stroma, with minimal posterior peripheral stroma expression (Chen et al., 2010). The data suggest a key role in regulation eye growth in emmetropization as well as corneal-stromal integration.
4.2d. Osteoglycin
Osteoglycin also known as mimecan is a class III SLRP. Corneal development in osteoglycinnull mice appeared normal and changes in corneal clarity were not detected (Tasheva et al., 2002). There were minor effects on corneal collagen fibril diameter and interfibrillar spacing, observed in these mice. Immuno-localization of osteoglycin in the mouse cornea showed localization primarily to the epithelium and epithelial basement membrane with minor stromal localization. In the stroma, osteoglycin was primarily localized to the keratocytes (Chakravarti et al., 2006). Therefore, the stromal role of this SLRP is ambiguous.
4.2e. SLRPs in regulation of stromal collagen fibrillogenesis
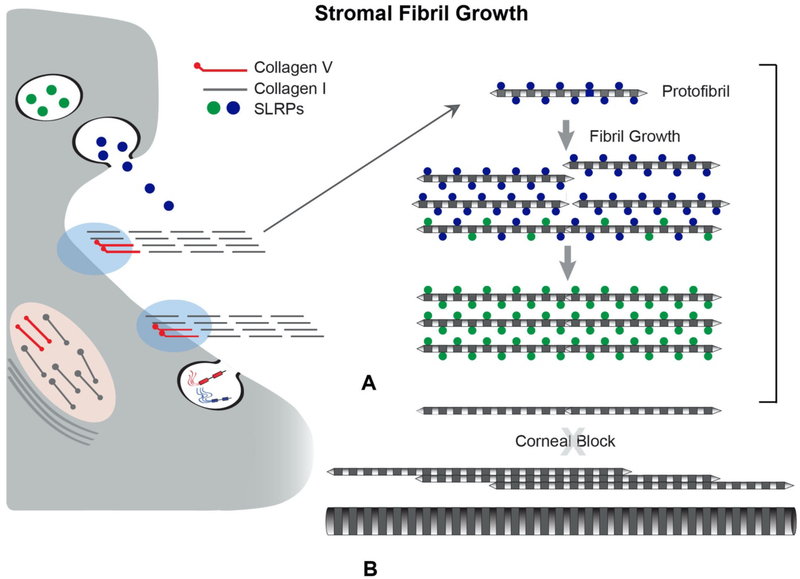
SLRPs are regulators of collagen fibril assembly. They have been shown to be critical in regulating linear and lateral growth of protofibrils into mature fibrils in the corneal stroma as well as other tissues (Chakravarti et al., 1998; Chakravarti et al., 2003; Chakravarti et al., 2006; Chen and Birk, 2013; Chen et al., 2010; Ezura et al., 2000; Mienaltowski and Birk, 2014; Zhang et al., 2009). Figure 9 presents a model illustrating SLRP and collagen fibril interactions in the regulation of stromal fibril growth. Mouse models deficient for decorin, biglycan, lumican, and keratocan have been particularly informative in the corneal stroma. Interestingly, null mice deficient in decorin or biglycan have a mild stromal phenotype. However, deficiency in both decorin and biglycan results in a severe phenotype. These compound null stromas have an increase in large diameter fibrils, a very heterogeneous diameter distribution, and irregular, fibril contours in both the anterior and the posterior stroma. Biglycan is up-regulated in the absence of decorin and it functionally compensate for the loss of the class I SLRP decorin in both in vivo and in vitro studies (Chen et al., 2015; Zhang et al., 2009). Lumican-null mice demonstrate a progressive corneal opacity with age. This opacity results from irregularly packed, and large diameter fibrils with irregular contours in the posterior stroma. The altered collagen fibril properties seen in the absence of decorin and lumican are related to a dysfunctional stromal regulation of lateral fibril growth. Since stromal transparency requires a homogeneous population of small diameter fibrils, the observed abnormal fibril phenotypes results in increased light scattering and opacity(Chakravarti et al., 2000; Song et al., 2003).
Fig 9. SLRPs Regulate Lateral Fibril Growth.
SLRPs regulate linear and lateral stromal collagen fibril growth by binding to fibril surfaces. (A) Newly assembled stromal fibrils (protofibrils) are deposited into the matrix where they are stabilized via interactions with SLRPs. After deposition into the stromal matrix, protofibrils mature by a process of fibril growth. Bound SLRPs mediate and controlled fibrillar interactions resulting in coordinated growth of mature stromal fibrils. In the stroma there is a a tissue-specific regulation of lateral fibril growth. Diameter and packing are rigidly regulated for corneal transparency. (B) In most tissues there is a robust lateral growth resulting in heterogeneous populations of large diameter fibrils this is blocked in the stroma. Changes in fibril stabilization necessary for growth can result from processing, turnover, and/or displacement of SLRPs. SLRPs affect fibril diameter and spacing in the corneal stroma. When SLRPs are absent, as in null mice or in gene mutations in human patients, fibril structure, organization and spacing are impacted, compromising transparency. Also, the block to lateral fibril growth is lost in the stroma when SLPR expression is altered.
Keratocan or fibromodulin null mice do not have changes in stromal fibril structure. Keratocan null-mice have thinner corneal stromas and narrower cornea-iris angles suggesting an involvement in stromal hydration and shape during development (Liu et al., 2003). Fibromodulin is expressed in a narrow window during stromal development. The data suggests that it contributes to cornea-sclera integration (Chen et al., 2010). Interestingly, a deficiency of lumican in the stroma alters expression of both keratocan and fibromodulin (Shao et al., 2011). Additionally, SLRP expression can be altered by the presence or absence of other class members. Moreover, regulatory interactions across classes also have been demonstrated by an increased severity of the corneal stromal fibril structural phenotype in the absence of both biglycan and lumican, compared to either one alone (Chen et al., 2014). This is evidence of inter-class cooperation in the regulation of fibril growth. While decorin and lumican appear to be the major SLRPs regulating stromal lateral fibril growth, others SLRPs have the ability to modulate their effects. Defining the network of regulatory SLRP interactions is essential to elucidate the roles in development, maintenance and regeneration of tissue-specific structures and functions.
The mechanisms regulating linear fibril growth within the corneal stroma are less understood. Prior to compaction of the stroma the newly formed protofibrils may be spaced so that the frequency of end to end interaction is inhibited. Ex vivo studies demonstrated that when end to end interaction of fibrils are increased linear fibril growth results (Graham et al., 2000). It has been suggested that the end-to-end interactions increase in development as the charge densities of GAG chains on stromal SLRPs increase that would induce order to the inter-fibrillar environment. In addition, the tapered ends of stromal protofibrils may have binding properties that stabilize the ends, with the normal turnover of SLRPs and/or other bound molecules providing increased opportunities for controlled interactions.
4.3. Fibrillin/elastic fiber associated macromolecules
Elastic fibers are extracellular macromolecules that allow tissue deformability and recoil (Kielty et al., 2002). Elastic fibers are complex structures composed of numerous molecules. Simplified, an elastic fiber consists of an elastin core surrounded associated with fibrillin microfibrils (Hanlon et al., 2015; Kielty et al., 2002). Although there is no clear evidence of elastic fibers in the adult stroma, (Bruns et al., 1987; Carlson and Waring, 1988; Hanlon et al., 2015) microfibrillar structures are present in the stroma of some species, (Feneck et al., 2020b; Hanlon et al., 2015) and recently elastin tissue expression has been demonstrated in human Descemet’s membrane (Lewis et al., 2019; Mohammed et al., 2018). Microfibrils are found widely distributed in the entire stroma as orthogonal arrays of parallel bundles in different species, including young and adult organisms (Bruns et al., 1987; Carlson and Waring, 1988; Hanlon et al., 2015). Transmission electron microscopy has shown 10–15 nm microfibrils arranged in quasi-parallel bundles within or between orthogonally arranged stromal collagen lamellae and some structures crossing Descemet’s membrane are suspected to be of elastic origin (Bruns et al., 1987; Carlson and Waring, 1988; Mohammed et al., 2018). In the family of the microfibrils, fibrillin 1 is the most common of the 3 proteins (Jensen et al., 2012). Marfan syndrome is an autosomal dominant disease of connective tissue in humans caused by mutations in the fibrillin 1 gene encoding the fibrillin-1 protein and it is characterized by a thinner cornea (Sultan et al., 2002).Studies in a mouse model deficient in fibrillin 1, that recapitulates Marfan’s syndrome, concluded that the function of this elastic tissue is to provide the cornea with mechanical strength/elastic recoil, and maintain corneal curvature (White et al., 2017). Fibrillin 1 binds different extracellular matrix proteins including decorin, collagen VI and latent transforming growth factor-β binding protein (Kielty et al., 2002). By binding latent transforming growth factor-β binding protein, fibrillin-1 may have a mechanotransduction role and affect transforming growth factor- β signaling (Buscemi et al., 2011; Robertson et al., 2015). A fibrillin 1 deficient mouse model is accompanied by a thinner cornea with decreased collagen I production, abnormalities in transforming growth factor-β as well as decreased decorin content in the cornea (Feneck et al., 2020b). While the fibrillin microfibrils have not been observed in the human cornea this deserves further evaluation to address its potential presence in an alternative structural form and their role in pathological conditions like keratoconus.
4.4. Stromal glycoproteins
4.4a. Fibronectin
Fibronectin is a well-studied large and structurally ubiquitous complex glycoprotein that binds to (Hsia and Schwarzbauer, 2005)multiple matrix structures, various proteoglycans, and growth factors. Fibronectin occurs in two principal forms, the soluble plasma fibronectin circulating in the blood and the cellular fibronectin, which polymerizes into insoluble fibers in the matrix of tissues (Pankov and Yamada, 2002; Zollinger and Smith, 2017). Fibronectin fibril assembly involves interactions between its RGD sequence and corresponding binding sites within cell surface receptors such as integrins (Labat-Robert, 2012; Singh et al., 2010; Zollinger and Smith, 2017). Fibronectin is also essential to the process of collagen matrix deposition and is highly expressed in the corneal stroma during wound healing since fibronectin forms a temporary scaffold in the early stages of injury (Barker and Engler, 2017; Kadler et al., 2008). During wound healing, an early provisional matrix matures in later phases, the early provisional fibrin-rich matrix, is replaced primarily by fibronectin and proteoglycans (Barker and Engler, 2017; Kadler et al., 2008). Inhibition of fibronectin matrix assembly inhibited deposition of collagens I and III (McDonald et al., 1982). Fibronectin repeats form a promiscuous binding domain that interacts with multiple growth factors: platelet-derived growth factor, vascular endothelial growth factor, and some of the transforming growth factor-β family (Martino and Hubbell, 2010; Zhu and Clark, 2014). Fibronectin recruits and stores latent transforming growth factor-β binding protein and therefore regulates transforming growth factor-β. The extra domain A fibronectin variant is expressed in embryos, but only during wound healing in adults (Ffrench-Constant et al., 1989). Due to its pro-fibrotic effect, extracellular availability, and high expression in fibrosis, this alternatively spliced variant is proposed as an anti-fibrotic target (Walraven and Hinz, 2018). Keratocytes are known to express fibronectin during homeostasis (Jester et al., 1994). Fibronectin is upregulated in the stroma during wound healing in different injury models including stromal or epithelial debridement (Basu et al., 2014; Fujikawa et al., 1984; Nickeleit et al., 1996; Tervo et al., 1991).
4.4b. Matricellular proteins
This is a group of secreted glycoproteins that facilitate cellular control over their surrounding extracellular matrix and influence structure and function in multiple tissues. The matricellular family includes among others: thrombospondins 1 and 2, SPARC (secreted protein, acidic and rich in cysteine), and osteopontin (Murphy-Ullrich and Sage, 2014).
Thrombospondins comprise a family of extracellular, multidomain, calcium-binding glycoproteins. The family consists of thrombospondins 1 to 5. Thrombospondin 5 is also known as COMP. Thrombospondins interact with cell surfaces, growth factors, cytokines, and other components of the extracellular matrix. Thrombospondin-1 (TSP-1) is a large (450 kDa) trimeric extracellular glycoprotein released by platelets, and epithelial and mesenchymal cells in response to wound healing, angiogenesis, tumor cell migration, and platelet aggregation. It is abundant in platelet granules, and its expression is increased dramatically during the woundhealing process. Absence of TSP-1 leads to prolonged inflammation, delayed wound healing, and delayed scab loss (Agah et al., 2002).
In the human and bovine cornea, TSP-1 localizes to the epithelial basement membrane and posterior Descemet’s membrane. No stromal or keratocyte expression is reported (Hiscott et al., 1997). In murine corneas, minimal expression is found on epithelial and endothelial cells with no keratocyte expression (Matsuba et al., 2011). However, TSP-1 expression increases significantly 48 hours after creating a full thickness corneal laceration. TSP-1 expression in the wound increases daily and peaks by day 14, and upregulation in expression is present in the entire wound from epithelium to endothelium. In a TSP-1 deficient mouse model, stromal, as well as endothelial healing, are severely impaired. TSP-1 deficient corneas rapidly become opaque after an incision wound and never appeared to heal after a 1 month follow-up (Blanco-Mezquita et al., 2013). TSP-1 is believed to act by activation of TGF-β during wound healing. TSP-1 binds to the latent complex and converts latent TGF-β to its biologically active form through a non-proteolytic mechanism. These data suggest that TSP is a potent physiologic regulator of TGF-β activation (Schultz-Cherry and Murphy-Ullrich, 1993). The expression and role of Thrombospondins 2–5 in the stroma are mostly unknown (Armstrong et al., 2003; Si et al., 2003).
SPARC is a 32 kDa calcium-binding matricellular protein also known as osteonectin or basement membrane protein 40. Initially found in bone, expression of SPARC in adult tissues is frequently associated with excessive deposition of collagen (Bradshaw, 2009). A lack of SPARC expression has been shown to diminish mature collagen accumulation in the matrix of many connective tissues (Murphy-Ullrich and Sage, 2014; Rosset and Bradshaw, 2016). For example, SPARC-null mice have decreased total collagen I content in bone, and other tissues (Bradshaw, 2009; Murphy-Ullrich and Sage, 2014). The increased in SPARC during wound healing and pathological fibrosis is a topic of interest. In the corneal stroma, SPARC is upregulated during the response to injury and during stimulation of cultured corneal fibroblasts with TGF-β (Berryhill et al., 2003). A potential role for SPARC in the etiology of corneal ectasias has been explored (De Bonis et al., 2011).
Osteopontin (OPN) is a matrix and structural glycophosphoprotein that is abundantly expressed in tissues during inflammation and repair. OPN has different functions, including in matrix remodeling and repair, inflammatory response, cell death, and survival (Giachelli and Steitz, 2000; Icer and Gezmen-Karadag, 2018; Liaw et al., 1998). In a mouse model deficient in OPN, healing of a corneal incision injury was delayed, with fewer myofibroblasts and decreased TGF-β1 expression, as well as a higher incidence of corneal perforation (Miyazaki et al., 2008).
4.4c Tenascins are a family of large oligomeric extracellular matrix glycoproteins that consists of 4 members: tenascin C, R, X, and W (Hsia and Schwarzbauer, 2005; Midwood et al., 2016). They have similar structures, but their expression patterns vary in different tissues. Tenascin-C knock out mouse models have a normal phenotype, but an impaired response to stress and injury (Mackie and Tucker, 1999). There is no expression of tenascin C in the normal adult uninjured cornea (Maseruka et al., 1997). However, tenascin C expression is upregulated following corneal injury and in scarred corneas (Maseruka et al., 1997). During healing of a perforating injury wound model, only weak induction of tenascin-C was seen in corneas from wild type mice, and healing in tenascin-C null mice was indistinguishable from that of wild type mice. In a sutured wound model, fibronectin expression was absent in the tenacin-C knockout model, but not in wild type wounds. Tenascin-C seemed to regulate the expression of fibronectin or its retention in the extracellular matrix (Matsuda et al., 1999). More work is needed to explore the roles of tenascins in the corneal stroma during development and in pathological conditions.
6. Summary
The stroma has unique properties: transparency, tissue strength, avascularity and precise curvature. Early in corneal development, the precise temporal and spatial regulation of stromal extracellular matrix assembly, determining the structural and functional properties of the stroma, is dependent on cell-matrix and matrix-matrix interactions. Therefore, the keratocytes and the extracellular matrix molecules they secrete are critical determinants of stromal properties. The major structural building blocks of the stroma are the collagen fibrils. These fibrils are tissue specific to the stroma, and with a distinct hierarchal organization that define stromal structure, and therefore, function. The regulation of collagen fibrillogenesis and stromal hierarchical organization in the cornea involves interactions of fibril-forming collagens I and V. However, these regulatory interactions are fine-tuned by interactions with fibril-associated molecules including; FACIT collagens, and SLRPs (decorin, lumican, keratocan, and biglycan) that regulate lateral association of collagen molecules during fibril growth and regulate hierarchical organization. Superimposed of this are other extracellular matrix networks that add critical elements to the development and maintenance of stromal structure and function. For instance, collagen VII form a network of anchoring fibrils that integrate the epithelial basement membrane with the stroma; collagen VIII assembles a hexagonal lattice network in Descemet’s membrane and serves as an interfacial matrix connecting the posterior stroma with the endothelium; and collagen VI networks bind to cells and numerous matrix molecules to integrate the different cell and matrix components within the stroma proper. In addition, there are networks of elastin associated molecules, e.g. fibrillins that may contribute to the mechanical properties of the stroma. In addition, fibronectin and matricellular proteins that are present in the stroma may have structural roles or be involved in mediating matrix interaction; they have major role(s) influencing cell behavior and biosynthetic profiles. These and other components of the stromal extracellular matrix have poorly defined roles that might regulate stromal function and may be essential for wound repair and in the pathogenesis of stromal diseases. Future research in this area will continue to expand our understanding of the roles of different matrix components, cells and cytokines in the regulation of stromal structure and function during development and in pathological conditions. The purpose of this review was to summarize our current knowledge and spark enthusiasm for the understanding of the regulation of matrix components essential for the function of this unique tissue.
Highlights.
The unique stromal structure and function is regulated by different extracellular matrix components.
Different structures within the stroma, including Bowman’s and Descemet’s layers, have unique ECM composition and function.
Not only collagens are essential for regulation of fibril formation, other ECM components like Small Leucine Rich Proteoglycans regulate fibrillogenesis and have other functions including modulation of the availability of growth factors and stromal hydration.
Regulation of ECM components play a major role in wound healing
Acknowledgments
Supported by NIH/NEI grant EY029395.
Footnotes
Proprietary Interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agah A, Kyriakides TR, Lawler J, Bornstein P, 2002. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama TO, Nishida K, Nakayama J, Watanabe H, Ozaki K, Nakamura T, Dota A, Kawasaki S, Inoue Y, Maeda N, Yamamoto S, Fujiwara T, Thonar EJ, Shimomura Y, Kinoshita S, Tanigami A, Fukuda MN, 2000. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet 26, 237–241. [DOI] [PubMed] [Google Scholar]
- Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE, 2009. Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. J Biol Chem 284, 8427–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Nagashima Y, Takemoto T, Nishiyama T, 2008. Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells. Cell Struct Funct 33, 203–210. [DOI] [PubMed] [Google Scholar]
- Armstrong DJ, Hiscott P, Batterbury M, Kaye S, 2003. Keratocyte matrix interactions and thrombospondin 2. Mol Vis 9, 74–79. [PubMed] [Google Scholar]
- Baldock C, Sherratt MJ, Shuttleworth CA, Kielty CM, 2003. The supramolecular organization of collagen VI microfibrils. J Mol Biol 330, 297–307. [DOI] [PubMed] [Google Scholar]
- Ball S, Bella J, Kielty C, Shuttleworth A, 2003. Structural basis of type VI collagen dimer formation. J Biol Chem 278, 15326–15332. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM, 1998. Dendritic cells and the control of immunity. Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- Bard JB, Bansal MK, 1987. The morphogenesis of the chick primary corneal stroma. I. New observations on collagen organization in vivo help explain stromal deposition and growth. Development 100, 135–145. [DOI] [PubMed] [Google Scholar]
- Barker TH, Engler AJ, 2017. The provisional matrix: setting the stage for tissue repair outcomes. Matrix Biol 60–61, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL, 2014. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 6, 266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher N, Carlson C, Allen BR, Kipchumba R, Conrad GW, Meek KM, Quantock AJ, 2005. An x-ray diffraction study of corneal structure in mimecan-deficient mice. Invest Ophthalmol Vis Sci 46, 4046–4049. [DOI] [PubMed] [Google Scholar]
- Berryhill BL, Kane B, Stramer BM, Fini ME, Hassell JR, 2003. Increased SPARC accumulation during corneal repair. Exp Eye Res 77, 85–92. [DOI] [PubMed] [Google Scholar]
- Birk DE, Bruckner P, 2011. Collagens, Suprastructures, and Collagen Fibril Assembly, in: RP M (Ed.), The Extracellular Matrix. Springer-Verlag, Berlin, pp. 77–116. [Google Scholar]
- Birk DE, Fitch JM, Linsenmayer TF, 1986. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci 27, 1470–1477.3531080 [Google Scholar]
- Birk DE, Trelstad RL, 1984. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol 99, 2024–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL, 1986. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol 103, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Munier FL, Yardley J, Hart-Holden N, Perveen R, Cousin P, Sutphin JE, Noble B, Batterbury M, Kielty C, Hackett A, Bonshek R, Ridgway A, McLeod D, Sheffield VC, Stone EM, Schorderet DF, Black GC, 2001. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet 10, 2415–2423. [DOI] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AE, Zieske JD, 2013. Role of thrombospondin-1 in repair of penetrating corneal wounds. Invest Ophthalmol Vis Sci 54, 6262–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemann CG, 2011. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol 7, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonod-Bidaud C, Roulet M, Hansen U, Elsheikh A, Malbouyres M, Ricard-Blum S, Faye C, Vaganay E, Rousselle P, Ruggiero F, 2012. In vivo evidence for a bridging role of a collagen V subtype at the epidermis-dermis interface. J Invest Dermatol 132, 1841–1849. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, 2009. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredrup C, Stang E, Bruland O, Palka BP, Young RD, Haavik J, Knappskog PM, Rodahl E, 2010. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Invest Ophthalmol Vis Sci 51, 5578–5582. [DOI] [PubMed] [Google Scholar]
- Brittingham R, Uitto J, Fertala A, 2006. High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun 343, 692–699. [DOI] [PubMed] [Google Scholar]
- Brown CT, Lin P, Walsh MT, Gantz D, Nugent MA, Trinkaus-Randall V, 2002. Extraction and purification of decorin from corneal stroma retain structure and biological activity. Protein Expr Purif 25, 389–399. [DOI] [PubMed] [Google Scholar]
- Bruckner-Tuderman L, 2010. Systemic therapy for a genetic skin disease. N Engl J Med 363, 680–682. [DOI] [PubMed] [Google Scholar]
- Bruckner-Tuderman L, Hopfner B, Hammami-Hauasli N, 1999. Biology of anchoring fibrils: lessons from dystrophic epidermolysis bullosa. Matrix Biol 18, 43–54. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Press W, Engvall E, Timpl R, Gross J, 1986. Type VI collagen in extracellular, 100-nm periodic filaments and fibrils: identification by immunoelectron microscopy. J Cell Biol 103, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Press W, Gross J, 1987. A large-scale, orthogonal network of microfibril bundles in the corneal stroma. Invest Ophthalmol Vis Sci 28, 1939–1946. [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM, 1997. The dermal-epidermal junction. Curr Opin Cell Biol 9, 651–658. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B, 2011. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol 21, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Bushby AJ, P’Ng K M, Young RD, Pinali C, Knupp C, Quantock AJ, 2011. Imaging three-dimensional tissue architectures by focused ion beam scanning electron microscopy. Nat Protoc 6, 845–858. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE, 2002. Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol A Mol Integr Physiol 133, 979–985. [DOI] [PubMed] [Google Scholar]
- Carlson EC, Waring GO 3rd, 1988. Ultrastructural analyses of enzyme-treated microfibrils in rabbit corneal stroma. Invest Ophthalmol Vis Sci 29, 578–585. [PubMed] [Google Scholar]
- Cescon M, Gattazzo F, Chen P, Bonaldo P, 2015. Collagen VI at a glance. J Cell Sci 128, 3525–3531. [DOI] [PubMed] [Google Scholar]
- Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S, 2013. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J Proteomics 87, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H, 1998. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol 141, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE, 2003. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci 44, 2422–2432. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE, 2000. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci 41, 3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE, 2006. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn 235, 2493–2506. [DOI] [PubMed] [Google Scholar]
- Chanut-Delalande H, Bonod-Bidaud C, Cogne S, Malbouyres M, Ramirez F, Fichard A, Ruggiero F, 2004. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Mol Cell Biol 24, 6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah KS, Lau ET, Au PK, Tam PP, 1991. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development 111, 945–953. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fitch JM, Gibney E, Linsenmayer TF, 1993. Type II collagen during cartilage and corneal development: immunohistochemical analysis with an anti-telopeptide antibody. Dev Dyn 196, 47–53. [DOI] [PubMed] [Google Scholar]
- Chen S, Birk DE, 2013. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 280, 2120–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Mienaltowski MJ, Birk DE, 2015. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res 133, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Oldberg A, Chakravarti S, Birk DE, 2010. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dyn 239, 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sun M, Iozzo RV, Kao WW, Birk DE, 2013a. Intracellularly-retained decorin lacking the C-terminal ear repeat causes ER stress: a cell-based etiological mechanism for congenital stromal corneal dystrophy. Am J Pathol 183, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sun M, Meng X, Iozzo RV, Kao WW, Birk DE, 2011a. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy: C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol 179, 2409–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Young MF, Chakravarti S, Birk DE, 2014. Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix Biol 35, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC, 2011b. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods 17, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Thompson DC, Koppaka V, Jester JV, Vasiliou V, 2013b. Ocular aldehyde dehydrogenases: protection against ultraviolet damage and maintenance of transparency for vision. Prog Retin Eye Res 33, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Birk DE, Bonnemann CG, Koch M, 2014. Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol 53, 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Mann K, Deutzmann R, Pribula-Conway D, Hsu-Chen CC, Bernard MP, Timpl R, 1987. Characterization of three constituent chains of collagen type VI by peptide sequences and cDNA clones. Eur J Biochem 168, 309–317. [DOI] [PubMed] [Google Scholar]
- Cintron C, Covington H, Kublin CL, 1983. Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci 24, 543–556. [PubMed] [Google Scholar]
- Cintron C, Covington HI, Kublin CL, 1988. Morphogenesis of rabbit corneal endothelium. Curr Eye Res 7, 913–929. [DOI] [PubMed] [Google Scholar]
- Colige A, Ruggiero F, Vandenberghe I, Dubail J, Kesteloot F, Van Beeumen J, Beschin A, Brys L, Lapiere CM, Nusgens B, 2005. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. J Biol Chem 280, 34397–34408. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR, 2013. Tissue-resident macrophages. Nat Immunol 14, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxer A, Misof K, Grabner B, Ettl A, Fratzl P, 1998. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci 39, 644–648. [PubMed] [Google Scholar]
- De Bonis P, Laborante A, Pizzicoli C, Stallone R, Barbano R, Longo C, Mazzilli E, Zelante L, Bisceglia L, 2011. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis 17, 2482–2494. [PMC free article] [PubMed] [Google Scholar]
- Denk W, Horstmann H, 2004. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2, e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destro M, Wallow IH, Brightbill FS, 1987. Recessive dystrophic epidermolysis bullosa. Arch Ophthalmol 105, 1248–1252. [DOI] [PubMed] [Google Scholar]
- Doane KJ, Ting WH, McLaughlin JS, Birk DE, 1996. Spatial and temporal variations in extracellular matrix of periocular and corneal regions during corneal stromal development. Exp Eye Res 62, 271–283. [DOI] [PubMed] [Google Scholar]
- Doane KJ, Yang G, Birk DE, 1992. Corneal cell-matrix interactions: type VI collagen promotes adhesion and spreading of corneal fibroblasts. Exp Cell Res 200, 490–499. [DOI] [PubMed] [Google Scholar]
- Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H, 2006. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 7, 2388–2393. [DOI] [PubMed] [Google Scholar]
- Downes JE, Swann PG, Holmes RS, 1994. Differential corneal sensitivity to ultraviolet light among inbred strains of mice. Correlation of ultraviolet B sensitivity with aldehyde dehydrogenase deficiency. Cornea 13, 67–72. [DOI] [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E, 2006. Extracellular proteoglycans modify TGF-beta bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol 25, 332–341. [DOI] [PubMed] [Google Scholar]
- Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL, 2005. Multipotent stem cells in human corneal stroma. Stem Cells 23, 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziasko MA, Daniels JT, 2016. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul Surf 14, 322–330. [DOI] [PubMed] [Google Scholar]
- Eikenberry E, Brodsky B, Cassidy K, 1980. Does the genetic type of collagen determine fibril structure? Biophys J 32, 221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SC, 2003. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci 44, 5136–5141. [DOI] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Liu CY, Tseng SC, 2004. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci 45, 2985–2991. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE, 2000. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol 151, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid M, Morishige N, Lam L, Wahlert A, Steinert RF, Jester JV, 2008. Detection of corneal fibrosis by imaging second harmonic-generated signals in rabbit corneas treated with mitomycin C after excimer laser surface ablation. Invest Ophthalmol Vis Sci 49, 4377–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feneck EM, Lewis PN, Meek KM, 2020a. Identification of a Primary Stroma and Novel Endothelial Cell Projections in the Developing Human Cornea. Invest Ophthalmol Vis Sci 61, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feneck EM, Souza RB, Lewis PN, Hayes S, Pereira LV, Meek KM, 2020b. Developmental abnormalities in the cornea of a mouse model for Marfan syndrome. Exp Eye Res 194, 108001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler LI, Timpl R, Fessler JH, 1981. Assembly and processing of procollagen type III in chick embryo blood vessels. J Biol Chem 256, 2531–2537. [PubMed] [Google Scholar]
- Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO, 1989. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 109, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichard A, Kleman JP, Ruggiero F, 1995. Another look at collagen V and XI molecules. Matrix Biol 14, 515–531. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Birk DE, Linsenmayer C, Linsenmayer TF, 1990. The spatial organization of Descemet’s membrane-associated type IV collagen in the avian cornea. J Cell Biol 110, 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch JM, Gross J, Mayne R, Johnson-Wint B, Linsenmayer TF, 1984. Organization of collagen types I and V in the embryonic chicken cornea: monoclonal antibody studies. Proc Natl Acad Sci U S A 81, 2791–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J, Rich C, Zhou FH, Hansen U, 2008. Three novel collagen VI chains, alpha4(VI), alpha5(VI), and alpha6(VI). J Biol Chem 283, 20170–20180. [DOI] [PubMed] [Google Scholar]
- Font B, Eichenberger D, Rosenberg LM, van der Rest M, 1996. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol 15, 341–348. [DOI] [PubMed] [Google Scholar]
- Freund DE, McCally RL, Farrell RA, Cristol SM, L’Hernault NL, Edelhauser HF, 1995. Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Relation to transparency. Invest Ophthalmol Vis Sci 36, 1508–1523. [PubMed] [Google Scholar]
- Frikeche J, Maiti G, Chakravarti S, 2016. Small leucine-rich repeat proteoglycans in corneal inflammation and wound healing. Exp Eye Res 151, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa LS, Foster CS, Gipson IK, Colvin RB, 1984. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Biol 98, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood NJ, Tuft SJ, Malik NS, Meek KM, Ridgway AE, Harrison RJ, 1992. Synchrotron x-ray diffraction studies of keratoconus corneal stroma. Invest Ophthalmol Vis Sci 33, 1734–1741. [PubMed] [Google Scholar]
- Funderburgh JL, Caterson B, Conrad GW, 1986. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev Biol 116, 267–277. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Du Y, 2016. Stem Cells in the Limbal Stroma. Ocul Surf 14, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J, 1983. Electron-microscopical approach to a structural model of intima collagen. Biochem J 211, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Yin J, Yoon GS, Mi QS, Yu FS, 2011. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol 179, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M, Paulsson M, Wagener R, 2008. Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem 283, 10658–10670. [DOI] [PubMed] [Google Scholar]
- Germundsson J, Karanis G, Fagerholm P, Lagali N, 2013. Age-related thinning of Bowman’s layer in the human cornea in vivo. Invest Ophthalmol Vis Sci 54, 6143–6149. [DOI] [PubMed] [Google Scholar]
- Ghoubay D, Borderie M, Grieve K, Martos R, Bocheux R, Nguyen TM, Callard P, Chedotal A, Borderie VM, 2020. Corneal stromal stem cells restore transparency after N2 injury in mice. Stem Cells Transl Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S, 2000. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol 19, 615–622. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M, 2016. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 44, 439–449. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Grill SM, Spurr SJ, Brennan SJ, 1983. Hemidesmosome formation in vitro. J Cell Biol 97, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud SJ, Tisdale AS, 1987. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci 28, 212–220. [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Birk DE, Fitch JM, Linsenmayer TF, 1994. Type V collagen and Bowman’s membrane. Quantitation of mRNA in corneal epithelium and stroma. J Biol Chem 269, 24959–24966. [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Lisenmayer TF, Fitch JM, 1996. Temporal expression of types XII and XIV collagen mRNA and protein during avian corneal development. Dev Dyn 206, 49–58. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Gerecke DR, Olsen BR, 1987. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci U S A 84, 6040–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Hahn RA, 2010. Collagens. Cell Tissue Res 339, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR, 2005. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci 46, 4504–4511. [DOI] [PubMed] [Google Scholar]
- Graham HK, Holmes DF, Watson RB, Kadler KE, 2000. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J Mol Biol 295, 891–902. [DOI] [PubMed] [Google Scholar]
- Greene CA, Green CR, Dickinson ME, Johnson V, Sherwin T, 2016. Keratocytes are induced to produce collagen type II: A new strategy for in vivo corneal matrix regeneration. Exp Cell Res 347, 241–249. [DOI] [PubMed] [Google Scholar]
- Gregory KE, Oxford JT, Chen Y, Gambee JE, Gygi SP, Aebersold R, Neame PJ, Mechling DE, Bachinger HP, Morris NP, 2000. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J Biol Chem 275, 11498–11506. [DOI] [PubMed] [Google Scholar]
- Gupta S, Fink MK, Ghosh A, Tripathi R, Sinha PR, Sharma A, Hesemann NP, Chaurasia SS, Giuliano EA, Mohan RR, 2018. Novel Combination BMP7 and HGF Gene Therapy Instigates Selective Myofibroblast Apoptosis and Reduces Corneal Haze In Vivo. Invest Ophthalmol Vis Sci 59, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Pavan-Langston D, Dana R, 2009. Herpes simplex keratitis and dendritic cells at the crossroads: lessons from the past and a view into the future. Int Ophthalmol Clin 49, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Seyed-Razavi Y, Yamaguchi T, 2016. Translational Immunoimaging and Neuroimaging Demonstrate Corneal Neuroimmune Crosstalk. Cornea 35 Suppl 1, S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SD, Behzad AR, Sakai LY, Burns AR, 2015. Corneal stroma microfibrils. Exp Eye Res 132, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JR, Spiro RC, Gaarde WA, Tamura RN, Pierschbacher MD, Noble NA, Stecker KK, Border WA, 1994. Role of transforming growth factor beta and decorin in controlling fibrosis. Methods Enzymol 245, 241–254. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp Eye Res 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Takahashi H, Dana R, 2016. Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells. Cornea 35 Suppl 1, S49–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ, 2006. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci U S A 103, 13333–13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Boote C, Tuft SJ, Quantock AJ, Meek KM, 2007. A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp Eye Res 84, 423–434. [DOI] [PubMed] [Google Scholar]
- Hemmavanh C, Koch M, Birk DE, Espana EM, 2013. Abnormal corneal endothelial maturation in collagen XII and XIV null mice. Invest Ophthalmol Vis Sci 54, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E, 1994. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302 ( Pt 2), 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, 2016. Myofibroblasts. Exp Eye Res 142, 56–70. [DOI] [PubMed] [Google Scholar]
- Hinz B, Lagares D, 2020. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 16, 11–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott P, Seitz B, Schlotzer-Schrehardt U, Naumann GO, 1997. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res 289, 307–310. [DOI] [PubMed] [Google Scholar]
- Hoffman GG, Branam AM, Huang G, Pelegri F, Cole WG, Wenstrup RM, Greenspan DS, 2010. Characterization of the six zebrafish clade B fibrillar procollagen genes, with evidence for evolutionarily conserved alternative splicing within the pro-alpha1(V) C-propeptide. Matrix Biol 29, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DR, Keles S, Greenspan DS, 2007. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26, 508–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE, 2005. Meet the tenascins: multifunctional and mysterious. J Biol Chem 280, 26641–26644. [DOI] [PubMed] [Google Scholar]
- Hu K, Harris DL, Yamaguchi T, von Andrian UH, Hamrah P, 2015. A Dual Role for Corneal Dendritic Cells in Herpes Simplex Keratitis: Local Suppression of Corneal Damage and Promotion of Systemic Viral Dissemination. PLoS One 10, e0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Meek KM, 1999. Swelling studies on the cornea and sclera: the effects of pH and ionic strength. Biophys J 77, 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJ, 2002. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 137, 2–10. [DOI] [PubMed] [Google Scholar]
- Hunzelmann N, Anders S, Sollberg S, Schonherr E, Krieg T, 1996. Co-ordinate induction of collagen type I and biglycan expression in keloids. Br J Dermatol 135, 394–399. [PubMed] [Google Scholar]
- Icer MA, Gezmen-Karadag M, 2018. The multiple functions and mechanisms of osteopontin. Clin Biochem 59, 17–24. [DOI] [PubMed] [Google Scholar]
- Illidge C, Kielty C, Shuttleworth A, 1998. The alpha1(VIII) and alpha2(VIII) chains of type VIII collagen can form stable homotrimeric molecules. J Biol Chem 273, 22091–22095. [DOI] [PubMed] [Google Scholar]
- Illidge C, Kielty C, Shuttleworth A, 2001. Type VIII collagen: heterotrimeric chain association. Int J Biochem Cell Biol 33, 521–529. [DOI] [PubMed] [Google Scholar]
- Izu Y, Sun M, Zwolanek D, Veit G, Williams V, Cha B, Jepsen KJ, Koch M, Birk DE, 2011. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol 193, 1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus MA, 1954. Studies on the cornea. I. The fine structure of the rat cornea. Am J Ophthalmol 38, 40–53. [PubMed] [Google Scholar]
- Jakus MA, 1956. Studies on the cornea. II. The fine structure of Descement’s membrane. J Biophys Biochem Cytol 2, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus MA, 1962. Further observations on the fine structure of the cornea. Invest Ophthalmol 1, 202–225. [PubMed] [Google Scholar]
- Jeang L, Cha BJ, Birk DE, Espana EM, 2020. Endothelial-Stromal Communication in Murine and Human Corneas. Anat Rec (Hoboken). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SA, Robertson IB, Handford PA, 2012. Dissecting the fibrillin microfibril: structural insights into organization and function. Structure 20, 215–225. [DOI] [PubMed] [Google Scholar]
- Jester JV, 2008. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol 19, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD, 1994. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci 35, 730–743. [PubMed] [Google Scholar]
- Jester JV, Brown D, Pappa A, Vasiliou V, 2012. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Invest Ophthalmol Vis Sci 53, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci 112 ( Pt 5), 613–622. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD, 1995. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci 36, 809–819. [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ, 1979. Origins of avian ocular and periocular tissues. Exp Eye Res 29, 27–43. [DOI] [PubMed] [Google Scholar]
- Joseph A, Hossain P, Jham S, Jones RE, Tighe P, McIntosh RS, Dua HS, 2003. Expression of CD34 and L-selectin on human corneal keratocytes. Invest Ophthalmol Vis Sci 44, 4689–4692. [DOI] [PubMed] [Google Scholar]
- Jun AS, Meng H, Ramanan N, Matthaei M, Chakravarti S, Bonshek R, Black GC, Grebe R, Kimos M, 2012. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet 21, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV, 2007. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48, 4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, 2017. Fell Muir Lecture: Collagen fibril formation in vitro and in vivo. Int J Exp Pathol 98, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Baldock C, Bella J, Boot-Handford RP, 2007. Collagens at a glance. J Cell Sci 120, 1955–1958. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG, 2008. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamajski S, Oldberg A, 2009. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J Biol Chem 284, 534–539. [DOI] [PubMed] [Google Scholar]
- Kamma-Lorger CS, Boote C, Hayes S, Moger J, Burghammer M, Knupp C, Quantock AJ, Sorensen T, Di Cola E, White N, Young RD, Meek KM, 2010. Collagen and mature elastic fibre organisation as a function of depth in the human cornea and limbus. J Struct Biol 169, 424–430. [DOI] [PubMed] [Google Scholar]
- Kamma-Lorger CS, Pinali C, Martinez JC, Harris J, Young RD, Bredrup C, Crosas E, Malfois M, Rodahl E, Meek KM, Knupp C, 2016. Role of Decorin Core Protein in Collagen Organisation in Congenital Stromal Corneal Dystrophy (CSCD). PLoS One 11, e0147948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane BP, Jester JV, Huang J, Wahlert A, Hassell JR, 2009. IGF-II and collagen expression by keratocytes during postnatal development. Exp Eye Res 89, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania AM, Reichenberger E, Baur ST, Karimbux NY, Taylor RW, Olsen BR, Nishimura I, 1999. Structural variation of type XII collagen at its carboxyl-terminal NC1 domain generated by tissue-specific alternative splicing. J Biol Chem 274, 22053–22059. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Hornia A, Yeh LK, Ouyang J, Liu CY, Tseng SC, 2005a. Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling. J Biol Chem 280, 27085–27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC, 2005b. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol 167, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE, 1987. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol 104, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani O, Fabian W, Lubatschowski H, 2008. Real-time optical coherence tomography-guided femtosecond laser sub-Bowman keratomileusis on human donor eyes. Am J Ophthalmol 146, 42–45. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG, 2008. Mammalian collagen IV. Microsc Res Tech 71, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Boot-Handford RP, Ayad S, Shuttleworth CA, Grant ME, 1990. Molecular composition of type VI collagen. Evidence for chain heterogeneity in mammalian tissues and cultured cells. Biochem J 272, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA, 2002. Elastic fibres. J Cell Sci 115, 2817–2828. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Hirayama K, 1970. Uneven swelling of the corneal stroma. Invest Ophthalmol 9, 735–741. [PubMed] [Google Scholar]
- Kivanany PB, Grose KC, Tippani M, Su S, Petroll WM, 2018. Assessment of Corneal Stromal Remodeling and Regeneration after Photorefractive Keratectomy. Sci Rep 8, 12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai Y, Ushiki T, 1991. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci 32, 2244–2258. [PubMed] [Google Scholar]
- Kontusaari S, Tromp G, Kuivaniemi H, Romanic AM, Prockop DJ, 1990. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest 86, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudouna E, Winkler M, Mikula E, Juhasz T, Brown DJ, Jester JV, 2018. Evolution of the vertebrate corneal stroma. Prog Retin Eye Res 64, 65–76. [DOI] [PubMed] [Google Scholar]
- Kremer A, Lippens S, Bartunkova S, Asselbergh B, Blanpain C, Fendrych M, Goossens A, Holt M, Janssens S, Krols M, Larsimont JC, Mc Guire C, Nowack MK, Saelens X, Schertel A, Schepens B, Slezak M, Timmerman V, Theunis C, R, V.A.N.B., Visser Y, Guerin CJ, 2015. Developing 3D SEM in a broad biological context. J Microsc 259, 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Robert J, 2012. Cell-Matrix interactions, the role of fibronectin and integrins. A survey. Pathol Biol (Paris) 60, 15–19. [DOI] [PubMed] [Google Scholar]
- Lamande SR, Bateman JF, 2018. Collagen VI disorders: Insights on form and function in the extracellular matrix and beyond. Matrix Biol 71–72, 348–367. [DOI] [PubMed] [Google Scholar]
- Laurent GJ, Cockerill P, McAnulty RJ, Hastings JR, 1981. A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem 113, 301–312. [DOI] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, Eule JC, Vollmar B, 2014. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci 55, 3603–3615. [DOI] [PubMed] [Google Scholar]
- Lewis PN, White TL, Feneck EM, Young RD, Meek KM, 2019. Elastin Content and Distribution in Endothelial Keratoplasty Tissue Determines Direction of Scrolling. Am J Ophthalmol 197, 181–182. [DOI] [PubMed] [Google Scholar]
- Li HF, Petroll WM, Moller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV, 1997. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF). Curr Eye Res 16, 214–221. [DOI] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL, 1998. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 101, 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR, 2011. Macrophage heterogeneity and acute inflammation. Eur J Immunol 41, 2503–2508. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF, Fitch JM, Gordon MK, Cai CX, Igoe F, Marchant JK, Birk DE, 1998. Development and roles of collagenous matrices in the embryonic avian cornea. Prog Retin Eye Res 17, 231–265. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Gordon MK, Marchant JK, Hayashi M, Fitch JM, 1990. Extracellular matrices of the developing chick retina and cornea. Localization of mRNAs for collagen types II and IX by in situ hybridization. Invest Ophthalmol Vis Sci 31, 1271–1276. [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE, 1993. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol 121, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer TF, Mentzer A, Irwin MH, Waldrep NK, Mayne R, 1986. Avian type VI collagen. Monoclonal antibody production and immunohistochemical identification as a major connective tissue component of cornea and skeletal muscle. Exp Cell Res 165, 518–529. [DOI] [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW, 2003. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem 278, 21672–21677. [DOI] [PubMed] [Google Scholar]
- Liu CY, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Corpuz LM, Conrad GW, Kao WW, 1998. The cloning of mouse keratocan cDNA and genomic DNA and the characterization of its expression during eye development. J Biol Chem 273, 22584–22588. [DOI] [PubMed] [Google Scholar]
- Liu M, Gao H, Wang T, Wang S, Li S, Shi W, 2014. An essential role for dendritic cells in vernal keratoconjunctivitis: analysis by laser scanning confocal microscopy. Clin Exp Allergy 44, 362–370. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Wu RR, Ninomiya Y, Sado Y, Maguen E, Nesburn AB, Kenney MC, 1996. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci 37, 997–1007. [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP, 1999. The tenascin-C knockout revisited. J Cell Sci 112 ( Pt 22), 3847–3853. [DOI] [PubMed] [Google Scholar]
- Manzer R, Qamar L, Estey T, Pappa A, Petersen DR, Vasiliou V, 2003. Molecular cloning and baculovirus expression of the rabbit corneal aldehyde dehydrogenase (ALDH1A1) cDNA. DNA Cell Biol 22, 329–338. [DOI] [PubMed] [Google Scholar]
- Marchant JK, Zhang G, Birk DE, 2002. Association of type XII collagen with regions of increased stability and keratocyte density in the cornea. Exp Eye Res 75, 683–694. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Torricelli AAM, Wilson SE, 2017. Regeneration of Defective Epithelial Basement Membrane and Restoration of Corneal Transparency After Photorefractive Keratectomy. J Refract Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MM, Hubbell JA, 2010. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J 24, 4711–4721. [DOI] [PubMed] [Google Scholar]
- Maseruka H, Bonshek RE, Tullo AB, 1997. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br J Ophthalmol 81, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoudi D, Malecaze F, Galiacy SD, 2016. Collagens and proteoglycans of the cornea: importance in transparency and visual disorders. Cell Tissue Res 363, 337–349. [DOI] [PubMed] [Google Scholar]
- Massoudi D, Malecaze F, Soler V, Butterworth J, Erraud A, Fournie P, Koch M, Galiacy SD, 2012. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest Ophthalmol Vis Sci 53, 7246–7256. [DOI] [PubMed] [Google Scholar]
- Matsuba M, Hutcheon AE, Zieske JD, 2011. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res 93, 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A, Yoshiki A, Tagawa Y, Matsuda H, Kusakabe M, 1999. Corneal wound healing in tenascin knockout mouse. Invest Ophthalmol Vis Sci 40, 1071–1080. [PubMed] [Google Scholar]
- Maurice DM, 1957. The structure and transparency of the cornea. J Physiol 136, 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Kelley DG, Broekelmann TJ, 1982. Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol 92, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JS, Linsenmayer TF, Birk DE, 1989. Type V collagen synthesis and deposition by chicken embryo corneal fibroblasts in vitro. J Cell Sci 94 ( Pt 2), 371–379. [DOI] [PubMed] [Google Scholar]
- Medeiros CS, Marino GK, Santhiago MR, Wilson SE, 2018. The Corneal Basement Membranes and Stromal Fibrosis. Invest Ophthalmol Vis Sci 59, 4044–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, 2009. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys Rev 1, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Fullwood NJ, 2001. Corneal and scleral collagens--a microscopist’s perspective. Micron 32, 261–272. [DOI] [PubMed] [Google Scholar]
- Meek KM, Fullwood NJ, Cooke PH, Elliott GF, Maurice DM, Quantock AJ, Wall RS, Worthington CR, 1991. Synchrotron x-ray diffraction studies of the cornea, with implications for stromal hydration. Biophys J 60, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Knupp C, 2015. Corneal structure and transparency. Prog Retin Eye Res 49, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Leonard DW, 1993. Ultrastructure of the corneal stroma: a comparative study. Biophys J 64, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Leonard DW, Connon CJ, Dennis S, Khan S, 2003. Transparency, swelling and scarring in the corneal stroma. Eye (Lond) 17, 927–936. [DOI] [PubMed] [Google Scholar]
- Meek KM, Quantock AJ, 2001. The use of X-ray scattering techniques to determine corneal ultrastructure. Prog Retin Eye Res 20, 95–137. [DOI] [PubMed] [Google Scholar]
- Mellgren AE, Bruland O, Vedeler A, Saraste J, Schonheit J, Bredrup C, Knappskog PM, Rodahl E, 2015. Development of congenital stromal corneal dystrophy is dependent on export and extracellular deposition of truncated decorin. Invest Ophthalmol Vis Sci 56, 2909–2915. [DOI] [PubMed] [Google Scholar]
- Meng H, Matthaei M, Ramanan N, Grebe R, Chakravarti S, Speck CL, Kimos M, Vij N, Eberhart CG, Jun AS, 2013. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Invest Ophthalmol Vis Sci 54, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley PA, Dunin-Borkowski RE, 2009. Electron tomography and holography in materials science. Nat Mater 8, 271–280. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Chiquet M, Tucker RP, Orend G, 2016. Tenascin-C at a glance. J Cell Sci 129, 4321–4327. [DOI] [PubMed] [Google Scholar]
- Mienaltowski MJ, Birk DE, 2014. Structure, physiology, and biochemistry of collagens. Adv Exp Med Biol 802, 5–29. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Okada Y, Yamanaka O, Kitano A, Ikeda K, Kon S, Uede T, Rittling SR, Denhardt DT, Kao WW, Saika S, 2008. Corneal wound healing in an osteopontin-deficient mouse. Invest Ophthalmol Vis Sci 49, 1367–1375. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Ross AR, Britton JO, Said DG, Dua HS, 2018. Elastin Content and Distribution in Endothelial Keratoplasty Tissue Determines Direction of Scrolling. Am J Ophthalmol 194, 16–25. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A, 2011. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med 11, 110–128. [DOI] [PubMed] [Google Scholar]
- Mok JW, Kim HS, Joo CK, 2009. Q455V mutation in COL8A2 is associated with Fuchs’ corneal dystrophy in Korean patients. Eye (Lond) 23, 895–903. [DOI] [PubMed] [Google Scholar]
- Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV, 2006. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg 32, 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Sage EH, 2014. Revisiting the matricellular concept. Matrix Biol 37, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees DW, Wawrousek EF, Robison WG Jr., Piatigorsky J, 2002. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol 22, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome DA, Gross J, Hassell JR, 1982. Human corneal stroma contains three distinct collagens. Invest Ophthalmol Vis Sci 22, 376–381. [PubMed] [Google Scholar]
- Newton RH, Meek KM, 1998a. Circumcorneal annulus of collagen fibrils in the human limbus. Invest Ophthalmol Vis Sci 39, 1125–1134. [PubMed] [Google Scholar]
- Newton RH, Meek KM, 1998b. The integration of the corneal and limbal fibrils in the human eye. Biophys J 75, 2508–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickeleit V, Kaufman AH, Zagachin L, Dutt JE, Foster CS, Colvin RB, 1996. Healing corneas express embryonic fibronectin isoforms in the epithelium, subepithelial stroma, and endothelium. Am J Pathol 149, 549–558. [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, McDonough AM, Bruns RR, Burgeson RE, 1994. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J Biol Chem 269, 28193–28199. [PubMed] [Google Scholar]
- Palka BP, Sotozono C, Tanioka H, Akama TO, Yagi N, Boote C, Young RD, Meek KM, Kinoshita S, Quantock AJ, 2010. Structural collagen alterations in macular corneal dystrophy occur mainly in the posterior stroma. Curr Eye Res 35, 580–586. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM, 2002. Fibronectin at a glance. J Cell Sci 115, 3861–3863. [DOI] [PubMed] [Google Scholar]
- Patel S, Marshall J, Fitzke FW 3rd, 1995. Refractive index of the human corneal epithelium and stroma. J Refract Surg 11, 100–105. [DOI] [PubMed] [Google Scholar]
- Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivela T, Kucherlapati R, Forsius H, de la Chapelle A, 2000. Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genet 25, 91–95. [DOI] [PubMed] [Google Scholar]
- Pinnamaneni N, Funderburgh JL, 2012. Concise review: Stem cells in the corneal stroma. Stem Cells 30, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploetz C, Zycband EI, Birk DE, 1991. Collagen fibril assembly and deposition in the developing dermis: segmental deposition in extracellular compartments. J Struct Biol 106, 73–81. [DOI] [PubMed] [Google Scholar]
- Poole CA, Brookes NH, Clover GM, 1993. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci 106 ( Pt 2), 685–691. [DOI] [PubMed] [Google Scholar]
- Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA, 1975. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A 72, 1314–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl A, von der Mark K, 1980. Synthesis of type V collagen by chick corneal fibroblasts in vivo and in vitro. FEBS Lett 115, 100–104. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Yurchenco PD, Iozzo RV, 2017. The nature and biology of basement membranes. Matrix Biol 57–58, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI, 1984. Heritable diseases of collagen. N Engl J Med 311, 376–386. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Meek KM, Chakravarti S, 2001. An x-ray diffraction investigation of corneal structure in lumican-deficient mice. Invest Ophthalmol Vis Sci 42, 1750–1756. [PubMed] [Google Scholar]
- Quantock AJ, Winkler M, Parfitt GJ, Young RD, Brown DJ, Boote C, Jester JV, 2015. From nano to macro: studying the hierarchical structure of the corneal extracellular matrix. Exp Eye Res 133, 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantock AJ, Young RD, 2008. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn 237, 2607–2621. [DOI] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR, 1993. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res 56, 635–648. [DOI] [PubMed] [Google Scholar]
- Rawe IM, Leonard DW, Meek KM, Zabel RW, 1997. X-ray diffraction and transmission electron microscopy of Morquio syndrome type A cornea: a structural analysis. Cornea 16, 369–376. [PubMed] [Google Scholar]
- Ricard-Blum S, 2011. The collagen family. Cold Spring Harb Perspect Biol 3, a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, 2015. Latent TGF-beta-binding proteins. Matrix Biol 47, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H, 2006. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am J Ophthalmol 142, 520–521. [DOI] [PubMed] [Google Scholar]
- Rosset EM, Bradshaw AD, 2016. SPARC/osteonectin in mineralized tissue. Matrix Biol 52–54, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulet M, Valkkila M, Chanut-Delalande H, Hamalainen ER, Kessler E, Ala-Kokko L, Mannikko M, Bonod-Bidaud C, Ruggiero F, 2010. The collagen V homotrimer [alpha1(V)](3) production is unexpectedly favored over the heterotrimer [alpha1(V)](2)alpha2(V) in recombinant expression systems. J Biomed Biotechnol 2010, 376927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban DR, 2014. The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. Ocul Surf 12, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia P, Medeiros CS, Thangavadivel S, Wilson SE, 2018. Basement membranes in the cornea and other organs that commonly develop fibrosis. Cell Tissue Res 374, 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan VS, Basu S, MacNeil S, Balasubramanian D, 2012. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol 96, 931–934. [DOI] [PubMed] [Google Scholar]
- Sawada H, Konomi H, Hirosawa K, 1990. Characterization of the collagen in the hexagonal lattice of Descemet’s membrane: its relation to type VIII collagen. J Cell Biol 110, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers Z, Koch MH, Whitburn SB, Meek KM, Elliott GF, Harmsen A, 1982. Synchrotron x-ray diffraction study of corneal stroma. J Mol Biol 160, 593–607. [DOI] [PubMed] [Google Scholar]
- Schmut O, 1977. The identification of type III collagen in calf and bovine cornea and sclera. Exp Eye Res 25, 505–509. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H, 1995. Interaction of biglycan with type I collagen. J Biol Chem 270, 2776–2783. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE, 1993. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 122, 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev F, Heon E, Cole WG, Wenstrup RJ, Young F, Slomovic AR, Rootman DS, Whitaker-Menezes D, Chervoneva I, Birk DE, 2006. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci 47, 565–573. [DOI] [PubMed] [Google Scholar]
- Shao H, Chaerkady R, Chen S, Pinto SM, Sharma R, Delanghe B, Birk DE, Pandey A, Chakravarti S, 2011. Proteome profiling of wild type and lumican-deficient mouse corneas. J Proteomics 74, 1895–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey JA, Kervick GN, Jackson AJ, Johnston PB, 1992. Cornea plana and sclerocornea in association with recessive epidermolysis bullosa dystrophica. Case report. Cornea 11, 83–85. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Olsen BR, 1991. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci 16, 191–194. [DOI] [PubMed] [Google Scholar]
- Shinde V, Hu N, Mahale A, Maiti G, Daoud Y, Eberhart CG, Maktabi A, Jun AS, Al-Swailem SA, Chakravarti S, 2020. RNA sequencing of corneas from two keratoconus patient groups identifies potential biomarkers and decreased NRF2-antioxidant responses. Sci Rep 10, 9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, Funderburgh JL, 2019. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl Med 8, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CA, 1997. Type VIII collagen. Int J Biochem Cell Biol 29, 1145–1148. [DOI] [PubMed] [Google Scholar]
- Si Z, Palkama A, Gebhardt BM, Velasquez D, Galeano MJ, Beuerman RW, 2003. Distribution of thrombospondin-4 in the bovine eye. Curr Eye Res 27, 165–173. [DOI] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE, 2010. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26, 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Birk DE, 2012. Focus on molecules: collagens V and XI. Exp Eye Res 98, 105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lee YG, Houston J, Petroll WM, Chakravarti S, Cavanagh HD, Jester JV, 2003. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest Ophthalmol Vis Sci 44, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, 2012. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30, 1–22. [DOI] [PubMed] [Google Scholar]
- Stephan S, Sherratt MJ, Hodson N, Shuttleworth CA, Kielty CM, 2004. Expression and supramolecular assembly of recombinant alpha1(viii) and alpha2(viii) collagen homotrimers. J Biol Chem 279, 21469–21477. [DOI] [PubMed] [Google Scholar]
- Sultan G, Baudouin C, Auzerie O, De Saint Jean M, Goldschild M, Pisella PJ, Marfan Study G, 2002. Cornea in Marfan disease: Orbscan and in vivo confocal microscopy analysis. Invest Ophthalmol Vis Sci 43, 1757–1764. [PubMed] [Google Scholar]
- Sun M, Chen S, Adams SM, Florer JB, Liu H, Kao WW, Wenstrup RJ, Birk DE, 2011. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci 124, 4096–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zafrullah N, Devaux F, Hemmavanh C, Adams S, Ziebarth NM, Koch M, Birk DE, Espana EM, 2020. Collagen XII Is a Regulator of Corneal Stroma Structure and Function. Invest Ophthalmol Vis Sci 61, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Cho HI, Kublin CL, Cintron C, 1993. Keratan sulfate and dermatan sulfate proteoglycans associate with type VI collagen in fetal rabbit cornea. J Histochem Cytochem 41, 1447–1457. [DOI] [PubMed] [Google Scholar]
- Tao G, Levay AK, Peacock JD, Huk DJ, Both SN, Purcell NH, Pinto JR, Galantowicz ML, Koch M, Lucchesi PA, Birk DE, Lincoln J, 2012. Collagen XIV is important for growth and structural integrity of the myocardium. J Mol Cell Cardiol 53, 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW, 2002. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis 8, 407–415. [PubMed] [Google Scholar]
- Tervo K, van Setten GB, Beuerman RW, Virtanen I, Tarkkanen A, Tervo T, 1991. Expression of tenascin and cellular fibronectin in the rabbit cornea after anterior keratectomy. Immunohistochemical study of wound healing dynamics. Invest Ophthalmol Vis Sci 32, 2912–2918. [PubMed] [Google Scholar]
- Tisdale AS, Spurr-Michaud SJ, Rodrigues M, Hackett J, Krachmer J, Gipson IK, 1988. Development of the anchoring structures of the epithelium in rabbit and human fetal corneas. Invest Ophthalmol Vis Sci 29, 727–736. [PubMed] [Google Scholar]
- Tong CM, van Dijk K, Melles GRJ, 2019. Update on Bowman layer transplantation. Curr Opin Ophthalmol 30, 249–255. [DOI] [PubMed] [Google Scholar]
- Toti P, Tosi GM, Traversi C, Schurfeld K, Cardone C, Caporossi A, 2002. CD-34 stromal expression pattern in normal and altered human corneas. Ophthalmology 109, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Coulombre AJ, 1971. Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol 50, 840–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SC, Smuckler D, Stern R, 1982. Comparison of collagen types in adult and fetal bovine corneas. J Biol Chem 257, 2627–2633. [PubMed] [Google Scholar]
- Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P, 2008. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem 283, 24506–24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark H, Aumailley M, Wick G, Fleischmajer R, Timpl R, 1984. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem 142, 493–502. [DOI] [PubMed] [Google Scholar]
- Walraven M, Hinz B, 2018. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol 71–72, 205–224. [DOI] [PubMed] [Google Scholar]
- Watsky MA, 1995. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Invest Ophthalmol Vis Sci 36, 2568–2576. [PubMed] [Google Scholar]
- Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG, 2017. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun 8, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE, 2011. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem 286, 20455–20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N, 1997. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci 38, 2408–2422. [PubMed] [Google Scholar]
- White J, Werkmeister JA, Ramshaw JA, Birk DE, 1997. Organization of fibrillar collagen in the human and bovine cornea: collagen types V and III. Connect Tissue Res 36, 165–174. [DOI] [PubMed] [Google Scholar]
- White TL, Lewis P, Hayes S, Fergusson J, Bell J, Farinha L, White NS, Pereira LV, Meek KM, 2017. The Structural Role of Elastic Fibers in the Cornea Investigated Using a Mouse Model for Marfan Syndrome. Invest Ophthalmol Vis Sci 58, 2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M, 2002. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem 277, 49120–49126. [DOI] [PubMed] [Google Scholar]
- Wilson SE, 2020. Bowman’s layer in the cornea- structure and function and regeneration. Exp Eye Res 195, 108033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Hong JW, 2000. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea 19, 417–420. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Green WR, 1999. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res 68, 341–346. [DOI] [PubMed] [Google Scholar]
- Xie HT, Chen SY, Li GG, Tseng SC, 2012. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci 53, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Young RD, Lewis PN, Shinomiya K, Meek KM, Kinoshita S, Caterson B, Quantock AJ, 2015. Mesenchymal-epithelial cell interactions and proteoglycan matrix composition in the presumptive stem cell niche of the rabbit corneal limbus. Mol Vis 21, 1328–1339. [PMC free article] [PubMed] [Google Scholar]
- Young BB, Zhang G, Koch M, Birk DE, 2002. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem 87, 208–220. [DOI] [PubMed] [Google Scholar]
- Young RD, Knupp C, Koudouna E, Ralphs JR, Ma Y, Lwigale PY, Jester JV, Quantock AJ, 2019. Cell-independent matrix configuration in early corneal development. Exp Eye Res 187, 107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RD, Knupp C, Pinali C, Png KM, Ralphs JR, Bushby AJ, Starborg T, Kadler KE, Quantock AJ, 2014. Three-dimensional aspects of matrix assembly by cells in the developing cornea. Proc Natl Acad Sci U S A 111, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE, 2009. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem 284, 8888–8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L, Zheng J, 2018. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 9, 5480–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kao WW, Hayashi Y, Zhang L, Call M, Dong F, Yuan Y, Zhang J, Wang YC, Yuka O, Shiraishi A, Liu CY, 2017. Generation and Characterization of a Novel Mouse Line, Keratocan-rtTA (KeraRT), for Corneal Stroma and Tendon Research. Invest Ophthalmol Vis Sci 58, 4800–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Clark RAF, 2014. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol 134, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW, 2003. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A 100, 7075–7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger AJ, Smith ML, 2017. Fibronectin, the extracellular glue. Matrix Biol 60–61, 27–37. [DOI] [PubMed] [Google Scholar]