Summary
The role of lipid metabolism in human pluripotent stem cells (hPSCs) is poorly understood. We have used large-scale targeted proteomics to demonstrate that undifferentiated hPSCs express different fatty acid (FA) biosynthesis-related enzymes, including ATP citrate lyase and FA synthase (FASN), than those expressed in hPSC-derived cardiomyocytes (hPSC-CMs). Detailed lipid profiling revealed that inhibition of FASN resulted in significant reduction of sphingolipids and phosphatidylcholine (PC); moreover, we found that PC was the key metabolite for cell survival in hPSCs. Inhibition of FASN induced cell death in undifferentiated hPSCs via mitochondria-mediated apoptosis; however, it did not affect cell survival in hPSC-CMs, neurons, or hepatocytes as there was no significant reduction of PC. Furthermore, we did not observe tumor formation following transplantation of FASN inhibitor-treated cells. Our findings demonstrate the importance of de novo FA synthesis in the survival of undifferentiated hPSCs and suggest applications for FASN inhibition in regenerative medicine.
Subject Areas: Biological Sciences, Cell Biology, Stem Cells Research, Proteomics, Metabolomics, Metabolic Flux Analysis
Graphical Abstract
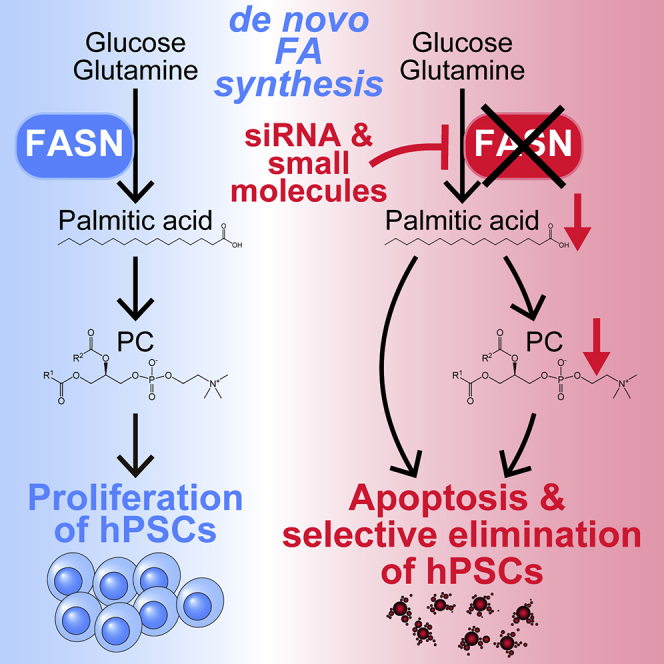
Highlights
-
•
Undifferentiated hPSCs upregulate de novo FA synthesis-related enzymes
-
•
Inhibition of de novo FA synthesis induces cell death in undifferentiated hPSCs
-
•
Phosphatidylcholine is the key metabolite required for hPSC survival
-
•
FASN inhibition eliminates undifferentiated hPSCs from hPSC derivatives
Biological Sciences; Cell Biology; Stem Cells Research; Proteomics; Metabolomics; Metabolic Flux Analysis
Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), are capable of self-renewing, proliferating, and differentiating into cells of the three germ layers (Takahashi et al., 2007; Thomson et al., 1998). Regenerative therapy using hPSCs holds promise for many diseases resistant to conventional medical therapies, including progressive heart failure, retinal diseases, and central nervous system diseases (Mandai et al., 2017; Nakamura and Okano, 2013; Yoshida and Yamanaka, 2017). However, to provide safe and effective regenerative therapy, it is essential to obtain highly qualified target cells from hPSCs.
As metabolism plays a key role in the pluripotency, self-renewal, and survival of hPSCs, metabolic regulation enables several efficient and cost-effective techniques, including generation of hiPSCs, differentiation into required cell types, and elimination of residual undifferentiated stem cells (Ben-David et al., 2013; Cornacchia et al., 2019; Shiraki et al., 2014; Sone et al., 2017; Tohyama et al., 2013, 2016; Yanes et al., 2010). Similar to cancer cells, hPSCs show activated glycolysis even under sufficiently aerobic conditions, a phenomenon referred to as the Warburg effect. However, recent studies have raised questions on this classical dogma in cancer cells, in that specific amino acids and fatty acids (FAs) are essential for maintenance of cancer stemness and progression. In hPSCs, the importance of amino acids (e.g., glutamine, methionine, and kynurenine) has also been reported (Shiraki et al., 2014; Tohyama et al., 2016; Yamamoto et al., 2019). In contrast to glucose and amino acid metabolism, little is known about the role of lipid metabolism in hPSCs.
We screened expression profiles of metabolic enzymes in hiPSCs and hiPSC-derived differentiated cardiomyocytes (hiPSC-CMs) by large-scale targeted proteomics, the results from which demonstrated that FA synthase (FASN) is expressed at the highest levels in hiPSCs but not in hiPSC-CMs. FASN is the final enzyme in the de novo FA synthesis pathway and is upregulated in cancer cells that originate in multiple tissues, including mammary glands, prostate, and lungs (Ali et al., 2018; De Schrijver et al., 2003; Pizer et al., 2000).
Lipids play a wide variety of roles in cell physiology, as energy sources, signaling molecules, protein modification, and cellular components, although these roles vary between cell types. Specifically in hESCs, a mixture of lipids stimulates their self-renewal (Garcia-Gonzalo and Izpisua Belmonte, 2008). Also, it has been reported that FA synthesis activation is critical for the maintenance of pluripotency by promoting mitochondrial fission (Wang et al., 2017). Meanwhile, during the reprogramming of embryonic fibroblasts into induced pluripotent stem cells, the gene expression profiles as well as the metabolic profiles become dramatically altered (Folmes et al., 2011; Meissen et al., 2012; Wu et al., 2019). In regard to lipids, the cytidine diphosphate-ethanolamine pathway contributes to reprogramming mouse embryonic fibroblasts into mouse induced pluripotent stem cells (Wu et al., 2019). A recent report also showed that de novo FA synthesis skewed hPSCs toward a naive-to-primed intermediate state by inhibiting endogenous ERK (Cornacchia et al., 2019). Despite the importance of FA synthesis, detailed lipid profiles and their roles in hPSC survival remain unknown. Therefore, in this study, we determined metabolic features, lipid profiles, and the role of FA synthesis in hPSCs relative to their derivatives. We then applied our findings to the improvement of regenerative medicine.
Results
FASN Is Upregulated in Undifferentiated hPSCs
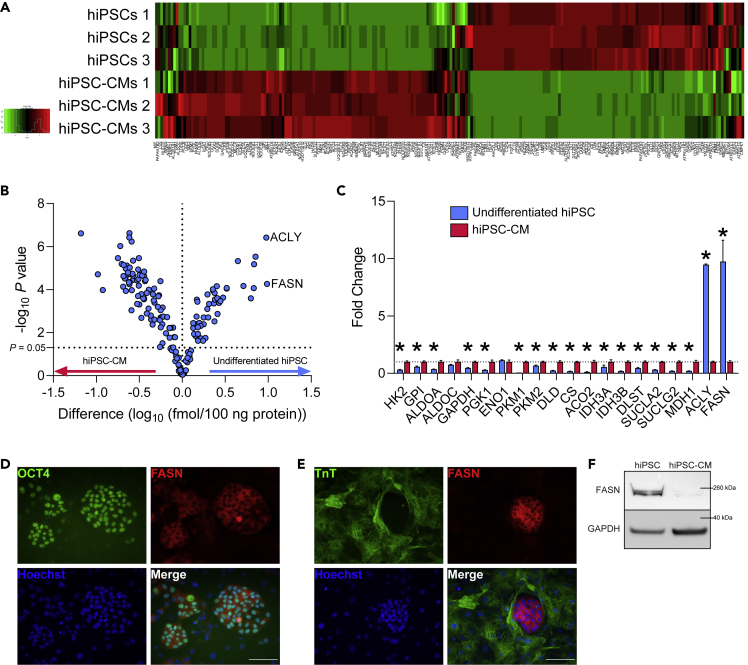
To identify unique metabolic features of hPSCs, we performed large-scale targeted proteomics with a focus on metabolism-related enzymes in hiPSCs and hiPSC-CMs as representative differentiated cells. The proteomic data revealed distinct differences between the two cell types (Figures 1A and 1B). The differentially expressed proteins included ATP citrate lyase (ACLY) and FASN, both involved in de novo FA synthesis (Figures 1B and 1C). Moreover, undifferentiated hiPSCs showed higher levels of enzymes involved in the pentose phosphate and serine synthesis pathways (Figures S1A and S1B); meanwhile, hiPSC-CMs had higher concentrations of enzymes involved in the tricarboxylic acid (TCA) cycle, FA oxidation, and oxidative phosphorylation (Figures 1C and S1C–S1E).
Figure 1.
FASN Is Highly Expressed in Undifferentiated hPSCs
(A) Heatmap of proteomic analysis of undifferentiated hiPSCs (253G4) and hiPSC (253G4)-CMs (n = 3).
(B) Volcano plot of proteomic analysis of undifferentiated hiPSCs (253G4) and hiPSC (253G4)-CMs. Protein levels were transformed to log10 scale. Student's t test was performed for each protein (n = 3).
(C) Relative abundance of enzymes involved in glycolysis, TCA cycle, and de novo FA synthesis compared with hiPSC (253G4)-CMs in proteomic analysis. Student's t test was performed for each protein (n = 3).
(D and E) (D) and (E) Representative image of immunocytochemistry of cocultured hiPSCs (253G4) and hiPSC (253G4)-CMs. Scale bars, 100 μm.
(F) Representative image of western blot of hiPSCs (253G4) and hiPSC (253G4)-CMs.
Data are presented as means ± SD; ∗p < 0.05.
These differences suggest that hiPSC-CMs are highly catabolic, whereas undifferentiated hiPSCs are highly anabolic, likely reflecting the differences between non-proliferating and proliferating states, respectively. We also confirmed the difference in FASN expression by immunocytochemistry and western blotting (Figures 1D–1F). These data suggest that undifferentiated hiPSCs express higher amounts of enzymes in anabolic pathways, particularly those involved in de novo FA synthesis, compared with hiPSC-CMs. Therefore, we focused on FASN, which generates palmitic acid (Pal) from acetyl-CoA, malonyl-CoA, and NADPH, and sought to investigate its significance in hPSCs.
Pal Is Required for the Survival of Undifferentiated hPSCs
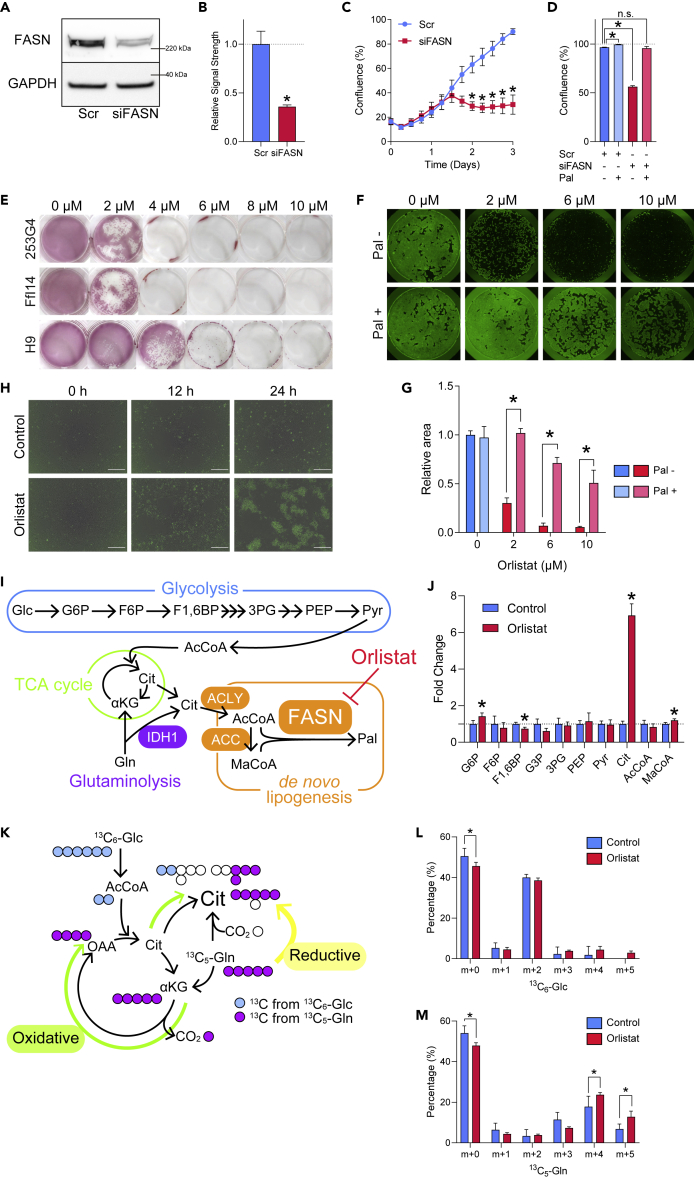
To investigate the role of FASN in hPSC proliferation and survival, FASN expression was knocked down using small interfering RNA (siRNA) (Figures 2A and 2B). The knockdown significantly inhibited hiPSC proliferation, whereas supplementation with Pal, the product of FASN, restored proliferation (Figures 2C and 2D).
Figure 2.
Palmitic Acid Is Essential for the Survival of Undifferentiated hPSCs
(A) Representative western blot image for FASN and GAPDH of hiPSCs (253G4) after treatment with Scr or FASN siRNAs.
(B) Quantification of relative FASN content compared with GAPDH by western blot. Student's t test, n = 3.
(C) Growth of hiPSCs (253G4) treated with Scr RNA or FASN siRNAs. Holm-Sidak test was performed for each time point (n = 3).
(D) Confluence at 72 h after introduction of either Scr RNA or FASN siRNA to hiPSCs (253G4) with or without 50 μM Pal 24 h after introduction of RNAs. The same concentration of BSA (8.3 μM) was used as control. One-way ANOVA with Dunnett's test was performed with Scr RNA without Pal as control (n = 3).
(E) Alkaline phosphatase (AP) staining of hESCs (H9) and hiPSCs (253G4 and FfI14) treated with orlistat of the indicated concentrations for 72 h.
(F) Representative images of hiPSCs (253G4) cultured in 12-well plates stained with calcein AM after treatment with orlistat at the indicated concentrations for 24 h with or without 50 μM Pal. The same concentration of BSA (8.3 μM) was used as control for Pal.
(G) Quantification of hiPSC (253G4) relative cell area after treatment with orlistat at the indicated concentrations for 24 h, with or without 50 μM Pal. The same concentration of BSA (8.3 μM) was used as a control for Pal. Student's t test was performed to compare the presence and absence of Pal at each concentration of orlistat (n = 3).
(H) Detection of apoptosis by IncuCyte Caspase-3/7 Green Reagent staining following orlistat treatment. Scale bar, 200 μm.
(I) Metabolic pathway from glucose to Pal via glycolysis, glutaminolysis, TCA cycle, and de novo lipogenesis.
(J) CE-MS of intermediate metabolites in de novo FA synthesis from glucose after 3 h of 6 μM orlistat treatment. Data are normalized to controls. Student's t test was performed for each metabolite (n = 3). G6P, glucose 6-phosphate; F6P, fructose 6-phosphate, F1,6BP, fructose 1,6-bisphosphate; G3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; Pyr, pyruvate; Gln, glutamine, αKG, α-ketoglutarate; AcCoA, acetyl-CoA; MaCoA, malonyl-CoA; ACLY, ATP citrate lyase; ACC, acetyl-CoA carboxylase; IDH1, isocitrate dehydrogenase 1.
(K) Metabolic pathway from glucose and glutamine to citrate via glycolysis, TCA cycle, and glutaminolysis. The major labeled form of citrate produced from 13C6-glucose, 13C5-glutamine via oxidative pathway, and glutamine via reductive pathway are m+2, m+4, and m+5, respectively.
(L) Percentage of labeled citrate formed after 1 h of orlistat treatment with 13C6-glucose. Student's t test was performed for each labeled form of citrate (n = 3).
(M) Percentage of labeled citrate formed after 1 h of orlistat treatment with 13C5-glutamine. Student's t test was performed for each labeled form of citrate (n = 3).
Data are presented as means ± SD; ∗p < 0.05.
In addition to knockdown by siRNA, we also utilized a pharmacological approach to inhibit FASN. Orlistat, an anti-obesity drug approved by the US Food and Drug Administration in the United States and other countries, inhibits FASN and suppresses the proliferation of cancer cells expressing high levels of FASN (Kridel et al., 2004). Expectedly, orlistat induced cell death in hESCs and hiPSCs (Figure 2E). This effect was greatly attenuated by Pal supplementation, suggesting that the cytotoxic effect was mediated by a decrease in Pal, and not by any off-target effect (Figures 2F and 2G). Furthermore, two FASN inhibitors, FASN-IN-2 and FT-113, were found to induce cell death in hiPSCs (Figure S2A). Similarly, an ACLY inhibitor, SB204990, induced cell death of undifferentiated hiPSCs (Figure S2B). However, although the effect of the FASN inhibitors were attenuated by supplementation of external Pal, that of the ACLY inhibitor was not. This difference likely arose from the wide range of applications assigned to acetyl-CoA, as it is used not only for de novo FA synthesis but also for other reactions, including acetylation of various proteins (e.g., histones) (Wellen et al., 2009).
Next, to determine the mechanism of cell death induced by orlistat, we performed time-lapse imaging of orlistat-treated cells and observed activation of caspase 3/7 within 12 h of initiation of treatment, suggesting apoptosis (Figure 2H). As FASN links glycolysis, TCA cycle, and glutaminolysis with lipid metabolism, its inhibition would theoretically cause dramatic alterations in these processes (Figure 2I). Hence, to determine the effects of pharmacological FASN inhibition on metabolism, we performed capillary electrophoresis and mass spectrometry (CE-MS)-based metabolomics. Inhibition of FASN by orlistat resulted in significant accumulation of citrate and malonyl-CoA, which is one of the upstream metabolites in de novo FA synthesis, confirming successful inhibition of FASN (Figure 2J). However, the increase observed for citrate was dramatically higher than that for malonyl-CoA. Hence, to exclude the possibility of alteration in activities of ACLY and ACC under orlistat treatment, we performed western blotting, which did not show significant change in phosphorylation of ACLY or ACC, suggesting activation and inhibition, respectively (Figure S2C). Citrate can be synthesized from glucose and glutamine. Glutamine-derived citrate is synthesized via two pathways: oxidative TCA cycle (forward) and reductive carboxylation via cytosolic isocitrate dehydrogenase 1 (IDH1) (reverse), which produces citrate from glutamine by consuming NADPH (Figure 2K). To evaluate the pathway from which the observed increase in citrate was derived, we performed metabolic flux analysis with either [U-13C]-glucose (13C6 Glc) or [U-13C]-glutamine (13C5 Gln) in orlistat-untreated or orlistat-treated hiPSCs (Figure 2K). A dramatic increase was observed in citrate derived from both glucose and glutamine (Figures 2L, 2M, and S2D). Focusing on the citrate derived from 13C6 Glc, a large proportion of the labeled form was determined to be m+2, which is derived from glycolytic acetyl-CoA and oxaloacetate (Figures 2L and S2D). Meanwhile, the labeled form of citrate derived from 13C5 Gln was m+4 citrate, which is derived from glutamine via the forward TCA cycle and glycolytic acetyl-CoA (Figures 2M and S2D). Cumulatively, these data indicate that the major contributor to the observed citrate increase was glucose-derived acetyl-CoA and forward TCA cycle-derived oxaloacetate, which are converted to citrate in the mitochondrial matrix. Therefore, although we cannot distinguish the localization of citrate in our CE-MS-based metabolome analysis, considering that citrate must be transported from the mitochondrial matrix to the cytosol for de novo FA synthesis, the dramatic increase in citrate may suggest accumulation of citrate in the mitochondrial matrix. Hence, as the biosynthesis of acetyl-CoA and malonyl-CoA takes place in the cytosol, we postulated that this is the mechanism responsible for the observed discrepancy in the increase in citrate and malonyl-CoA.
In the metabolic flux analysis using 13C5 Gln, we found that the percentage of m+5 citrate was significantly increased, suggesting that the reductive pathway was also activated (Figures 2M and S2D). Citrate generated from glutamine via reductive carboxylation can be utilized for de novo FA synthesis depending on the cell type and culture conditions (Jiang et al., 2016; Metallo et al., 2011; Yoo et al., 2008). The increase in the reductive pathway may suggest a possible role for IDH1-mediated citrate biosynthesis as an NADP+ recycling mechanism to compensate for the reduced NADPH consumption caused by FASN inhibition. Meanwhile, in rapidly growing cancer cells with defective mitochondria, this reductive pathway contributes to de novo FA synthesis (Mullen et al., 2011). Therefore, activation of the reductive pathway may suggest that FASN inhibition causes mitochondrial dysfunction.
Next, considering that citrate reportedly inhibits tumor growth (Newsholme et al., 1985; Ren et al., 2017), to exclude the possibility of citrate-induced growth inhibition and cell death, we supplemented external citrate and quantified the intracellular citrate level. We then confirmed that the increase in intracellular citrate was not associated with cell proliferation or survival in hPSCs (Figures S2E and S2F). We also quantified the intracellular citrate level when Pal was supplemented with orlistat and confirmed that the citrate level remained elevated despite Pal supplementation (Figure S2G). These data suggest that accumulation of citrate is not the mechanism responsible for inducing cell death following FASN inhibition.
Regarding the relationship between metabolism, epigenetics, and pluripotency in hPSCs, Moussaieff et al. reported that glucose-derived cytosolic acetyl-CoA supports the maintenance of pluripotent status through histone acetylation, whereas decreased acetyl-CoA levels induce differentiation (Moussaieff et al., 2015). To evaluate the acetylation status of orlistat-treated hPSCs, we examined the acetylation of histone H3 lysine 27 by western blotting and confirmed that it was not significantly changed (Figure S2H). In addition, no significant change was observed in the expression of pluripotent markers OCT4, NANOG, and TRA1-60, as assessed by immunocytochemistry and flow cytometry (Figures S2I and S2J). Cumulatively, these results indicate that orlistat does not affect the pluripotent status of hPSCs.
Previous studies suggested that inhibition of carnitine palmitoyl transferase 1, via accumulation of malonyl-CoA, is the mechanism by which orlistat induces cell death (Thupari et al., 2001). To determine whether hiPSCs utilize FA oxidation, we supplemented the hPSC maintenance medium with albumin-bound [U-13C]-labeled palmitic acid (13C16-Pal) for 72 h and quantified 13C16-Pal-derived metabolites by CE-MS. We did not detect significant levels of 13C in TCA cycle metabolites irrespective of orlistat treatment, indicating that hiPSCs did not utilize FA oxidation (data not shown), consistent with a previous report (Zhang et al., 2016). These data suggest that Pal is an indispensable metabolite for the proliferation and survival of hiPSCs, in a manner that is independent of its utilization as a fuel source.
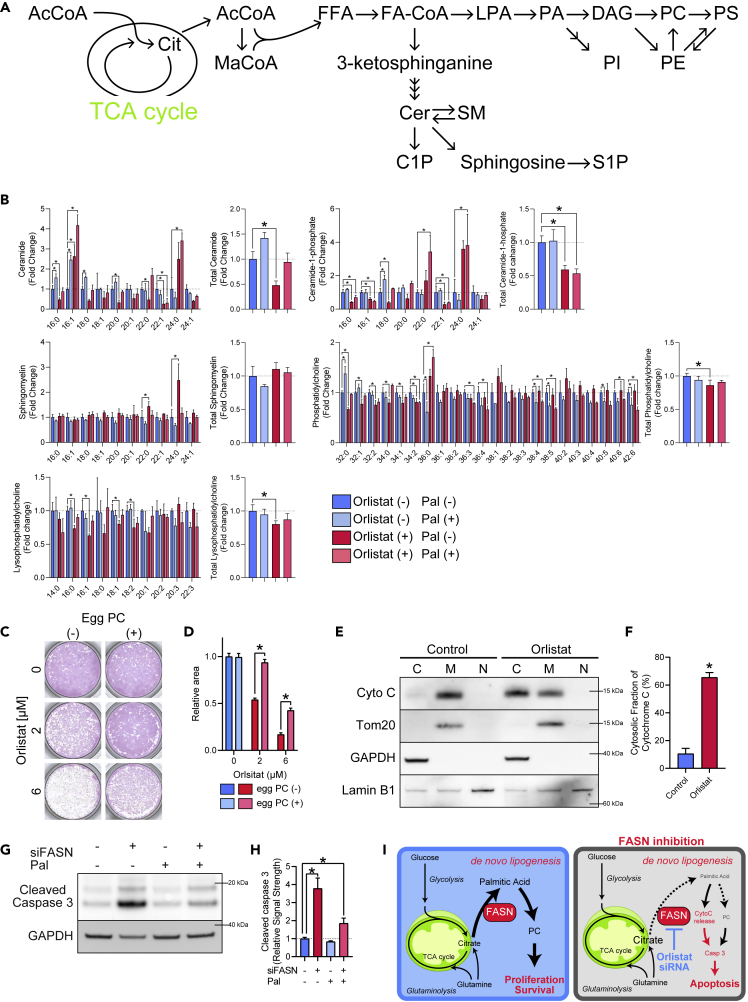
Inhibition of FASN Leads to Decreased Phosphatidylcholine
As Pal is utilized in a broad range of lipid metabolism, we performed liquid chromatography-MS-based lipidomics to evaluate changes in downstream metabolites caused by FASN inhibition (Figures 3A and 3B). Previous studies reported that the accumulation of ceramides (Cers), which are regarded as proapoptotic lipids in some cell lines, following FASN inhibition contributes to cell death, because inhibition of FASN could be rescued by coadministration of the Cer synthase inhibitor fumonisin B1 (FB1) or the serine palmitoyltransferase inhibitor myriocin (Alwarawrah et al., 2016; Bandyopadhyay et al., 2006; Obeid et al., 1993; Pizer et al., 2000; Thupari et al., 2001). Surprisingly, we did not observe accumulation of Cers, rather we observed significant reduction in their levels (Figure 3B). Ceramide-1-phosphate (Cer1P), formed from Cer by ceramide kinase, is associated with survival and proliferation and was found to be concomitantly decreased (Figure 3B). To ascertain the significance of the decrease in these two sphingolipids (SLs), we supplemented the medium with them. However, SL supplementation did not attenuate the effect of orlistat (Figure S3A). In addition, inhibition of de novo Cer synthesis by FB1 alone did not induce cell death in hiPSCs (Figure S3B). Thus, we concluded that the decrease in Cer or Cer1P does not mediate cell death caused by FASN inhibition.
Figure 3.
Metabolomic Analysis under FASN Inhibition and Mechanism of Cell Death
(A) Metabolic pathway from TCA cycle to various lipids. FFA, free fatty acid; FA-CoA, fatty acyl-CoA; LPA, lysophosphatidic acid; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin; S1P, sphingosine-1-phosphate.
(B) Liquid chromatography-MS of glycerolipids and SLs after 3 h of 6 μM orlistat treatment with or without 50 μM Pal. Data are normalized to controls without orlistat or Pal. Numbers indicate carbon chain lengths followed by degrees of desaturation. One-way ANOVA with Dunnett's test was performed with orlistat (−) Pal (−) as control (n = 3).
(C) Representative image of AP staining of hiPSCs (253G4) after 24 h of orlistat and chicken egg PC treatment.
(D) Quantification of relative AP-positive cell area in hiPSCs (253G4) after 24 h of orlistat and chicken egg PC treatment. Student's t test was performed for each concentration of orlistat (n = 3).
(E) Representative western blot image of cytochrome c subcellular localization in hiPSCs (253G4) 12 h after orlistat treatment. Cyto C, cytochrome c.
(F) Cytosolic fraction of cytochrome c of hiPSCs (253G4) 12 h after orlistat treatment quantified by western blotting. Student's t test, n = 3.
(G) Representative western blotting image of hiPSCs (253G4) 48 h after transfection with either Scr RNA or FASN siRNA. Pal (50 μM) was supplemented 24 h after transfection.
(H) Relative amount of cleaved caspase 3 in hiPSCs (253G4) 48 h after transfection with either Scr RNA or FASN siRNA quantified by western blotting. Pal (50 μM) was supplemented 24 h after transfection. Signal strengths were standardized using those of GAPDH. One-way ANOVA with Dunnett's test was performed with Scr RNA and without Pal as a control (n = 3).
(I) Schematic of metabolic features of undifferentiated hPSCs and consequences of FASN inhibition.
Data are presented as means ± SD; ∗p < 0.05.
Among glycerophospholipids, we observed a significant decrease in phosphatidylcholine (PC) and lysophosphatidylcholine (Figure 3B). In contrast, the levels of phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine were not significantly changed (Figure S3C). PC is a major component of lipid bilayers, including cellular membranes; its production via de novo FA synthesis increases during cytokinesis (Scaglia et al., 2014). It is worth noting that 3 h of FASN inhibition caused a significant decrease in PC, because cells contain abundant PC. To determine the physiological significance of this distinct decrease in PC among glycerophospholipids, we supplemented PC with orlistat and confirmed that exogenous PC attenuated the effect of FASN inhibition (Figures 3C and 3D), suggesting that the decrease in PC plays a key role in cell death induced by FASN inhibition. Moreover, PC with relatively short fatty acyl chains were decreased by orlistat treatment, presumably reflecting a decreased flow of FAs synthesized de novo to PC metabolism. Concordant with this hypothesis, unlike the lipid profiles of hiPSCs, PC was not significantly changed in hiPSC-CMs following 3 h of orlistat treatment, suggesting that its reduction in undifferentiated hPSCs is due to its dependency on de novo FA synthesis for proliferation (Figure S3D). This finding is consistent with the previous study described earlier that demonstrated the importance of PC production via de novo FA synthesis in cytokinesis (Scaglia et al., 2014).
FASN Inhibition Induces Cytochrome c Release and Caspase-3 Activation
The initiation of apoptosis is primarily regulated by intrinsic and extrinsic pathways. To identify the pathway critical in the cell death induced following FASN inhibition, we measured the levels of cytochrome c and observed significant increases in cytoplasmic fractions (Figures 3E, 3F, and S3E). Next, moving upstream from cytochrome c, we measured levels of Noxa and Bid. We found that Noxa was increased, whereas Bid was not changed by orlistat treatment (Figures S3F and S3G). Expectedly, FASN knockdown increased cleaved caspase-3 levels in hPSCs, which were greatly attenuated by both Pal and PC supplementation (Figures 3G, 3H, and S3H). These data indicate that the mitochondrial intrinsic pathway plays a primary role in FASN inhibition-induced apoptosis. This is consistent with our CE-MS analysis and a previous study that reported that during mitochondrial dysfunction, reductive carboxylation of glutamine supports de novo FA synthesis, as described previously (Figure 2M) (Mullen et al., 2011). Among the upstream components involved in the intrinsic pathway activation, we did not observe any activation of the endoplasmic reticulum (ER) stress response or accumulation of p53 (Figures S3I and S3J). Taken together, our findings indicate that whereas undifferentiated hPSCs upregulate de novo lipid synthesis for proliferation, FASN inhibition results in caspase 3-mediated apoptosis via cytochrome c release and decrease in PC level (Figure 3I).
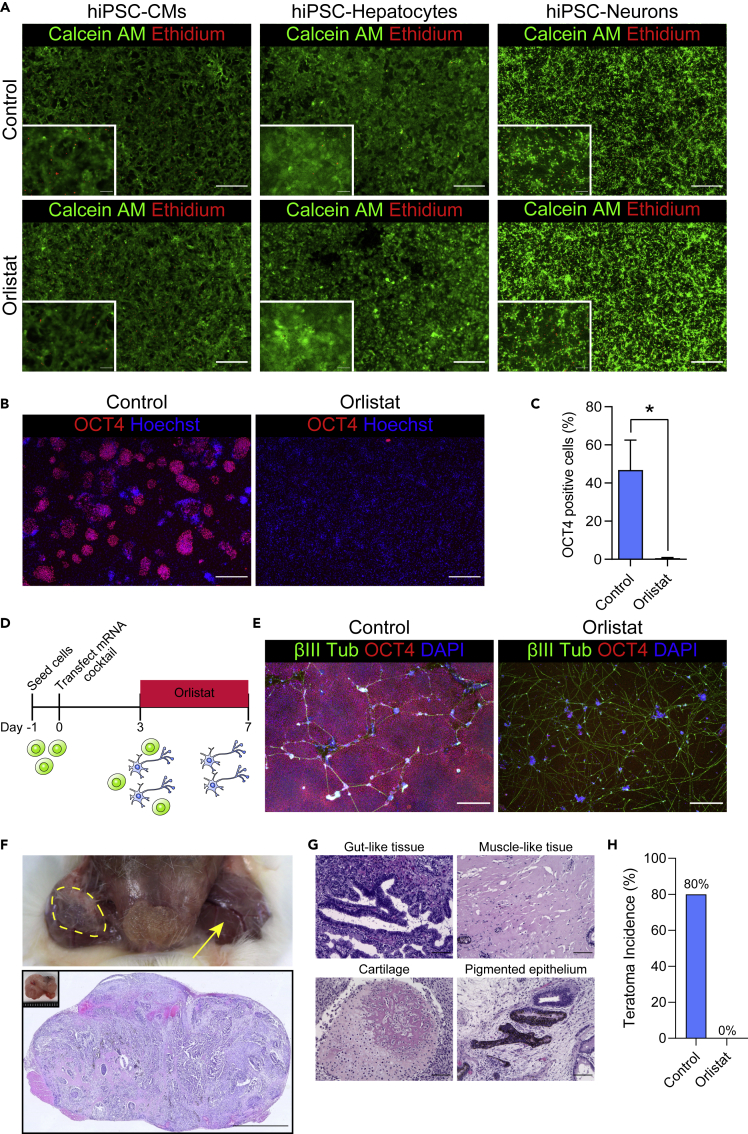
Orlistat Induces Cell Death in Undifferentiated hiPSCs, but Not in hiPSC Derivatives
To determine whether orlistat treatment affects cell survival in hPSC derivatives, including cardiomyocytes, hepatocytes, and neurons, we cultured these cells with orlistat for 72 h. We found that orlistat did not affect cell survival in any of the three differentiated cell types (Figures 4A and S4). Next, to determine whether orlistat treatment could eliminate hPSCs in mixtures with other cell types, we cocultured undifferentiated hiPSCs with hiPSC-CMs with or without orlistat. In contrast to controls, which produced substantial numbers of OCT4-positive colonies, orlistat killed OCT4-positive cells, whereas hiPSC-CMs survived (Figures 4B and 4C). In addition, we administered orlistat to a mixed cell population, composed of neuronal fate-decided cells as well as residual undifferentiated hiPSCs, during neuronal differentiation using synthetic mRNAs. As a result, we succeeded in eliminating OCT4-positive cells and purifying hiPSC-derived neurons (Figures 4D and 4E). These results suggest a possibility for using orlistat to eliminate undifferentiated hPSCs in multiple cellular lineages.
Figure 4.
Orlistat Eliminates Undifferentiated Pluripotent Stem Cells and Prevents Teratoma Formation
(A) Viability of differentiated cells under 6 μM orlistat for 72 h. Live and dead cells were identified using calcein AM and ethidium, respectively. Scale bars, 500 μm and 100 μm.
(B) Selective elimination by orlistat of OCT4-positive undifferentiated hiPSCs (253G4) cocultured with hiPSC (253G4)-CMs. Scale bar, 500 µm.
(C) Quantification of OCT4-positive cells. Student's t test, n = 3.
(D) Schematic of neural differentiation using synthetic mRNAs.
(E) Representative images of hiPSC (201B7)-derived neurons treated with or without orlistat for 96 h. Scale bar, 500 μm.
(F–H) Assay of orlistat treatment using a mouse teratoma model. (F) Macroscopic (top) and microscopic (bottom) overviews of teratomas. Dotted line and yellow arrow indicate tumor formation by control (cells without orlistat treatment) and no tumor formation by orlistat treatment, respectively. Scale bar, 2 mm. (G) Representative images of teratoma histology. Scale bar, 100 μm. (H) Orlistat treatment prevents teratoma incidence (n = 5).
Data are presented as means ± SD; ∗p < 0.05.
Last, we tested whether pretreatment with orlistat prevented tumor formation in immunocompromised, non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice. Undifferentiated hiPSCs were cocultured with human dermal fibroblasts at a ratio of 2:100 with or without orlistat for 72 h and then subcutaneously transplanted into NOD-SCID mice. Pretreatment with orlistat prevented teratoma formation 8 weeks after transplantation (Figures 4F–4H).
Discussion
In this study, we demonstrated that undifferentiated hPSCs express high levels of de novo FA synthesis enzymes relative to hiPSC-CMs. Inhibition of de novo FA synthesis induced apoptotic death of undifferentiated hPSCs via a significant reduction in PC. De novo FA synthesis sustains proliferation by providing building material, whereas it is noteworthy that its inhibition induced cell death, not only cell-cycle arrest, in undifferentiated hPSCs.
Metabolic signatures of hPSCs are known to be similar to those of cancer cells (Zhang et al., 2012). Consistent with this dogma, de novo FA synthesis is activated not only in hPSCs but also in some cancer types, including breast, renal, lung, and prostate (Menendez and Lupu, 2007). Therefore, FA synthesis has been regarded as a drug target for these cancers. In cancer cells, several mechanisms of cell death induced by inhibition of FA synthesis have been proposed, including p53-regulated metabolic stress, Cer accumulation, inhibition of anti-apoptotic proteins, and disturbance of membrane function, but detailed mechanisms remain poorly understood (Menendez and Lupu, 2007). In this study, we demonstrated that the apoptosis induced by inhibition of FASN in hPSCs was not mediated by p53-regulated metabolic stress or Cer accumulation. Sterol regulatory element-binding transcription factor 1 (SREBF1) regulates lipid metabolism including de novo FA synthesis in some cancer cells (Wu and Naar, 2019). However, although SREBF1 is upregulated during somatic cell reprogramming, introduction of Srebf1 did not activate de novo FA synthesis-related genes in mouse induced pluripotent stem cells (Wu et al., 2016). Instead, SREBF1 activated the expression of pluripotent genes by interacting with MYC (Wu et al., 2016). Hence, inhibition of SREBF1 in hPSCs may influence the pluripotent state of the cells instead of cytotoxicity.
Notably, detailed lipid profiling revealed that not only PC but also Cer and Cer1P were significantly decreased by orlistat treatment. Although previous studies reported accumulation of Cer as the mechanism of cell death upon FASN inhibition, the observed decrease in Cer reported in the present study was reasonable because de novo Cer synthesis requires palmitoyl-CoA as one of the substrates, which is presumably decreased by the inhibition of FASN. We also found that PC supplementation increased the survival of orlistat-treated hPSCs, but SL supplementation did not, suggesting that PC is a key metabolite for survival of hPSCs. As PC is an important factor in the maintenance of membrane function, decreases in PC induced cytochrome c release from mitochondria, leading to apoptotic hPSC death. It is worth noting that that there was a trend that PC with relatively short fatty acyl chains were sensitive to orlistat treatment compared with PC with longer fatty acyl chains. PC with different acyl chains has distinct properties and affects membrane properties including curvature, charge, fluidity, and local architecture (Bi et al., 2019). In fact, PC is dynamically remodeled by lysophosphatidylcholine acyltransferases (LPCAT). In intestinal stem cells, loss of LPCAT3 decreases the abundance of PC with saturated fatty acyl chains, which ultimately results in increased cell proliferation (Wang et al., 2018). Alternatively, gain of LPCAT1 increases saturated PC, leading to cell proliferation via activated epidermal growth factor receptor signaling in non-small cell lung cancer (Bi et al., 2019). Meanwhile, our lipidomics revealed a difference in the degree of susceptibility to FASN inhibition in PC depending on the fatty acyl chains. Although the consequence of altered LPCAT activity seems to be context dependent as described earlier, FASN inhibition may alter membrane properties, and hence signaling pathway activities, that affect cellular viability.
Here, we developed a method to selectively eliminate hPSCs from hPSC derivatives based on differences in lipid metabolism. Although many methods have been reported to reduce hPSC contamination of hPSC derivatives to realize safer regenerative medicine, a metabolic approach has several advantages in terms of scalability, simplicity, and cost. We previously developed a method to eliminate residual undifferentiated hPSC contamination of hPSC-CMs that exploited differences in glucose, lactate, and glutamine metabolism (Tohyama et al., 2013, 2016). However, this method cannot be applied to hepatocytes, neurons, and several other cell types because they cannot survive when depleted of glucose and glutamine even under existence of lactate. In contrast, inhibiting FA synthesis did not affect the viability of differentiated cardiomyocytes, neurons, and hepatocytes, suggesting that this approach can be applied elsewhere. It has been reported that inhibition of stearoyl-CoA desaturase kills hPSCs via ER stress (Ben-David et al., 2013; Li et al., 2017). Theoretically, inhibition of FASN, which acts upstream of stearoyl-CoA desaturase, may also induce cell death by the same pathway. However, we did not observe an activation of the ER stress response (Figure S3I), indicating that inhibition of FASN acts differently than inhibition of stearoyl-CoA desaturase.
Many clinical trials using hPSCs are underway. However, the risk of tumorigenicity is still one of the major barriers to the clinical application of regenerative medicine. To date, glucose and amino acid metabolism of hPSCs and their derivatives have been analyzed in detail and exploited to efficiently eliminate hPSCs from hPSC derivatives (Shiraki et al., 2014; Tohyama et al., 2013, 2016). A deeper understanding of the metabolic differences in lipid metabolism between hPSCs and their derivatives will contribute significantly to the realization of safer regenerative medicine.
Limitations of the Study
Although the activation of reductive carboxylation pathway of glutamine upon FASN inhibition suggests mitochondrial dysfunction, the mechanism linking FASN inhibition and mitochondrial dysfunction remains unclear. Further work unveiling this missing link definitely will advance our knowledge about the role of lipid metabolism in mitochondrial homeostasis. In addition, although we have demonstrated that FASN inhibition can be rescued by supplementation of PC, its effect was modest compared with that of Pal. Hence, we cannot exclude other underlying mechanisms leading to cell death following FASN inhibition. Further studies will be required to uncover the role of de novo synthesized FAs in the wide variety of lipids.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shugo Tohyama, Keio University (shugotohyama@keio.jp).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
There is no dataset and/or code associated with the article.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank the Center for iPSC Research and Application, and Kyoto University, for providing hiPSC lines (253G4, 201B7, and FfI14). The authors thank Hideo Shindou and Takao Shimizu for their assistance in liposome preparation. The present work was mainly supported by Projects for Technological Development, Research Center Network for Realization of Regenerative Medicine by Japan, the Japan Agency for Medical Research and Development (AMED) grant 19bm0404023h0002 to S.T., and partly supported by the Highway Program for Realization of Regenerative Medicine from AMED grant 19bk0104062h0003 to K.F. and JSPS KAKENHI grant 17H05067 to S.T. Infrastructure of metabolomics based on CE-MS was supported by Japan Science and Technology ERATO Suematsu Gas Biology (M.S. was the lead from 2010 to 2015).
Author Contributions
S. Tohyama conceptualized and designed the study; S. Tanosaki performed and analyzed most experiments; S.S., H.N., T.O.-N., T.H., T.A., K.N., Y.K., M.O., Y.M., H.T., Y.S., and H.K. contributed to specific experiments; S. Tanosaki and S. Tohyama wrote the original draft; S. Tohyama, J.F., and K.F. wrote, reviewed, and edited the manuscript; S. Tohyama, J.F., and K.F. acquired funding; S. Tohyama, J.F., M.S.H.K., M.S., and K.F. supervised the study.
Declaration of Interests
S. Tanosaki, S. Tohyama, J.F., and K.F. have a patent pending related to this work. K.F. is a co-founder and CEO of Heartseed, Inc. S. Tohyama is an advisor of Heartseed, Inc. S. Tohyama, J.F., H.K., and K.F. own equity in Heartseed, Inc. The remaining authors have no conflicts of interest to disclose.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101535.
Contributor Information
Shugo Tohyama, Email: shugotohyama@keio.jp.
Jun Fujita, Email: jfujita@keio.jp.
Supplemental Information
References
- Ali A., Levantini E., Teo J.T., Goggi J., Clohessy J.G., Wu C.S., Chen L., Yang H., Krishnan I., Kocher O. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 2018;10:e8313. doi: 10.15252/emmm.201708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwarawrah Y., Hughes P., Loiselle D., Carlson D.A., Darr D.B., Jordan J.L., Xiong J., Hunter L.M., Dubois L.G., Thompson J.W. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-Neu model of HER2(+) breast cancer. Cell Chem. Biol. 2016;23:678–688. doi: 10.1016/j.chembiol.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Zhan R., Wang Y., Pai S.K., Hirota S., Hosobe S., Takano Y., Saito K., Furuta E., Iiizumi M. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006;66:5934–5940. doi: 10.1158/0008-5472.CAN-05-3197. [DOI] [PubMed] [Google Scholar]
- Ben-David U., Gan Q.F., Golan-Lev T., Arora P., Yanuka O., Oren Y.S., Leikin-Frenkel A., Graf M., Garippa R., Boehringer M. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Bi J., Ichu T.A., Zanca C., Yang H., Zhang W., Gu Y., Chowdhry S., Reed A., Ikegami S., Turner K.M. Oncogene amplification in growth factor signaling pathways renders cancers dependent on membrane lipid remodeling. Cell Metab. 2019;30:525–538. doi: 10.1016/j.cmet.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchia D., Zhang C., Zimmer B., Chung S.Y., Fan Y., Soliman M.A., Tchieu J., Chambers S.M., Shah H., Paull D. Lipid deprivation induces a stable, naive-to-primed intermediate state of pluripotency in human PSCs. Cell Stem Cell. 2019;25:120–136. doi: 10.1016/j.stem.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schrijver E., Brusselmans K., Heyns W., Verhoeven G., Swinnen J.V. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- Folmes C.D., Nelson T.J., Martinez-Fernandez A., Arrell D.K., Lindor J.Z., Dzeja P.P., Ikeda Y., Perez-Terzic C., Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F.R., Izpisua Belmonte J.C. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Shestov A.A., Swain P., Yang C., Parker S.J., Wang Q.A., Terada L.S., Adams N.D., McCabe M.T., Pietrak B. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridel S.J., Axelrod F., Rozenkrantz N., Smith J.W. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- Li J., Condello S., Thomes-Pepin J., Ma X., Xia Y., Hurley T.D., Matei D., Cheng J.X. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20:303–314. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Meissen J.K., Yuen B.T., Kind T., Riggs J.W., Barupal D.K., Knoepfler P.S., Fiehn O. Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. PLoS One. 2012;7:e46770. doi: 10.1371/journal.pone.0046770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D., Nemirovski A., Shen-Orr S., Laevsky I., Amit M. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Mullen A.R., Wheaton W.W., Jin E.S., Chen P.H., Sullivan L.B., Cheng T., Yang Y., Linehan W.M., Chandel N.S., DeBerardinis R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013;23:70–80. doi: 10.1038/cr.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E.A., Crabtree B., Ardawi M.S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci. Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Obeid L.M., Linardic C.M., Karolak L.A., Hannun Y.A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Pizer E.S., Thupari J., Han W.F., Pinn M.L., Chrest F.J., Frehywot G.L., Townsend C.A., Kuhajda F.P. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- Ren J.G., Seth P., Ye H., Guo K., Hanai J.I., Husain Z., Sukhatme V.P. Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci. Rep. 2017;7:4537. doi: 10.1038/s41598-017-04626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia N., Tyekucheva S., Zadra G., Photopoulos C., Loda M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle. 2014;13:859–868. doi: 10.4161/cc.27767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N., Shiraki Y., Tsuyama T., Obata F., Miura M., Nagae G., Aburatani H., Kume K., Endo F., Kume S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014;19:780–794. doi: 10.1016/j.cmet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Sone M., Morone N., Nakamura T., Tanaka A., Okita K., Woltjen K., Nakagawa M., Heuser J.E., Yamada Y., Yamanaka S. Hybrid cellular metabolism coordinated by Zic3 and Esrrb synergistically enhances induction of naive pluripotency. Cell Metab. 2017;25:1103–1117. doi: 10.1016/j.cmet.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thupari J.N., Pinn M.L., Kuhajda F.P. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 2001;285:217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- Tohyama S., Fujita J., Hishiki T., Matsuura T., Hattori F., Ohno R., Kanazawa H., Seki T., Nakajima K., Kishino Y. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016;23:663–674. doi: 10.1016/j.cmet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Wang B., Rong X., Palladino E.N.D., Wang J., Fogelman A.M., Martin M.G., Alrefai W.A., Ford D.A., Tontonoz P. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell. 2018;22:206–220. doi: 10.1016/j.stem.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang T., Wang L., Cai Y., Zhong X., He X., Hu L., Tian S., Wu M., Hui L. Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J. 2017;36:1330–1347. doi: 10.15252/embj.201695417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E., Hatzivassiliou G., Sachdeva U.M., Bui T.V., Cross J.R., Thompson C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Naar A.M. SREBP1-dependent de novo fatty acid synthesis gene expression is elevated in malignant melanoma and represents a cellular survival trait. Sci. Rep. 2019;9:10369. doi: 10.1038/s41598-019-46594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen K., Liu X., Huang L., Zhao D., Li L., Gao M., Pei D., Wang C., Liu X. Srebp-1 interacts with c-myc to enhance somatic cell reprogramming. Stem Cells. 2016;34:83–92. doi: 10.1002/stem.2209. [DOI] [PubMed] [Google Scholar]
- Wu Y., Chen K., Xing G., Li L., Ma B., Hu Z., Duan L., Liu X. Phospholipid remodeling is critical for stem cell pluripotency by facilitating mesenchymal-to-epithelial transition. Sci. Adv. 2019;5:eaax7525. doi: 10.1126/sciadv.aax7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hatabayashi K., Arita M., Yajima N., Takenaka C., Suzuki T., Takahashi M., Oshima Y., Hara K., Kagawa K. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal. 2019;12:eaaw3306. doi: 10.1126/scisignal.aaw3306. [DOI] [PubMed] [Google Scholar]
- Yanes O., Clark J., Wong D.M., Patti G.J., Sanchez-Ruiz A., Benton H.P., Trauger S.A., Desponts C., Ding S., Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H., Antoniewicz M.R., Stephanopoulos G., Kelleher J.K. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J. Biol. Chem. 2008;283:20621–20627. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Yamanaka S. Induced pluripotent stem cells 10 years later: for cardiac applications. Circ. Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- Zhang H., Badur M.G., Divakaruni A.S., Parker S.J., Jager C., Hiller K., Murphy A.N., Metallo C.M. Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep. 2016;16:1536–1547. doi: 10.1016/j.celrep.2016.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Nuebel E., Daley G.Q., Koehler C.M., Teitell M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There is no dataset and/or code associated with the article.