Summary
The Chinese ginseng Panax notoginseng is a domesticated herb with significant medicinal and economic value. Here we report a chromosome-level P. notoginseng genome assembly with a high (∼79%) repetitive sequence content. The juxtaposition with the widely distributed, closely related Korean ginseng (Panax ginseng) genome revealed contraction of plant defense genes (in particular R-genes) in the P. notoginseng genome. We also investigated the reasons for the larger genome size of Panax species, revealing contributions from two Panax-specific whole-genome duplication events and transposable element expansion. Transcriptome data and comparative genome analysis revealed the candidate genes involved in the ginsenoside synthesis pathway. We also performed a genome-wide association study on 240 cultivated P. notoginseng individuals and identified the associated genes with dry root weight (63 genes) and stem thickness (168 genes). The P. notoginseng genome represents a critical step toward harnessing the full potential of an economically important and enigmatic plant.
Subject Areas: Biological Sciences, Plant Biology, Genomics, Transcriptomics
Graphical Abstract
Highlights
-
•
A chromosome-level P. notoginseng genome assembly is provided
-
•
Panax-specific WGD events and TE expansion contribute to the larger genome size
-
•
Candidate genes involved in the ginsenoside synthesis pathway are revealed
-
•
The associated genes with agronomic traits were identified using GWAS data
Biological Sciences; Plant Biology; Genomics; Transcriptomics
Introduction
The genus Panax in the flowering plant family Araliaceae contains several medicinally and economically important ginseng species, including Panax ginseng (Korean ginseng), Panax quinquefolius (American ginseng), and Panax notoginseng (Chinese ginseng; sānqī in Chinese) (Briskin, 2000). Unlike P. ginseng and P. quinquefolius, which are widely distributed in several countries in the northern hemisphere (including the United States, Canada, China, and the Koreas), P. notoginseng is restricted to several mountain areas in Southern China (e.g., Wenshan Prefecture in Yunnan Province, Guo et al., 2010). P. notoginseng is susceptible to a wide range of pathogens, and identification of the genes conferring disease resistance is a major focus of research (Ou et al., 2011). The cultivation of P. notoginseng faces several challenges, including diseases that decrease production quality and yield (Baeg and So, 2013), and a potentially sparse repertoire of disease resistance genes. The major active components in ginseng genus Panax (Leung and Wong, 2010; Yang et al., 2014) are ginsenosides. Ginsenosides are steroid-like compounds with diverse pharmacological properties in addition to a role in plant defense. These include hepatoprotection, renoprotection, estrogen-like activities, and protection against cerebrocardiovascular ischemia and dyslipidemia (Li et al., 2009; Ng, 2006; Son et al., 2009; Xiang et al., 2011; Yang et al., 2010). For example, the Danshen dripping pill (which comprises P. notoginseng, Salvia miltiorrhiza [the Chinese herbal plant dānshēn], and synthetic borneol) is undergoing phase III clinical trials in the United States as a potential treatment for cardiovascular disease (Luo et al., 2015).
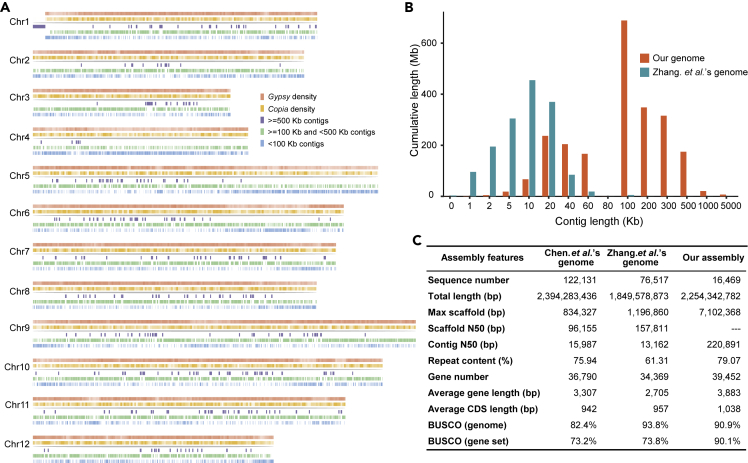
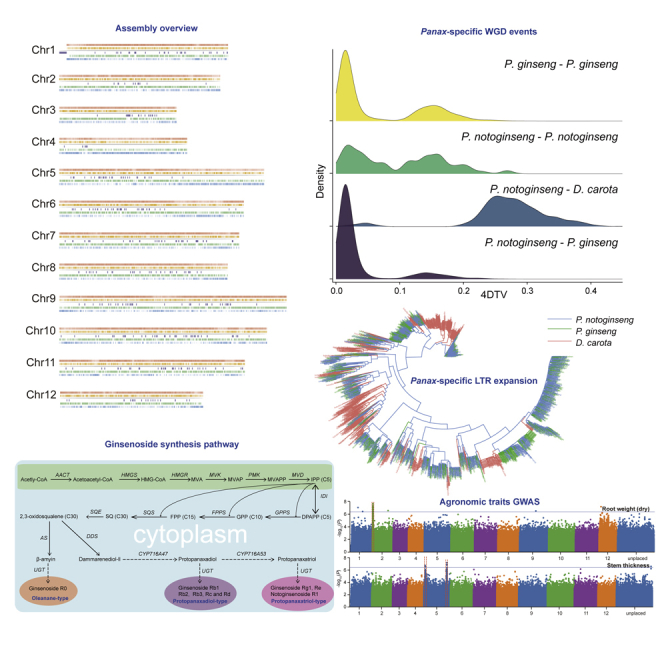
Two P. notoginseng genomes were published in 2017. Chen and colleagues' genome assembly (Chen et al., 2017a) is ∼2.39 Gb (contig N50 of 16 kb and scaffold N50 of 96 kb), and Zhang and colleagues' assembly (Zhang et al., 2017) is ∼1.85 Gb (scaffold N50 of 158 kb and contig N50 of 13.2 kb) (Figure 1C). Surprisingly, there is a notable difference in assembly size (∼540 Mb) between the two versions (the estimated genome size using flow cytometry analysis is about 2.31 Gb). The Chen et al. assembly has ∼75.94% repetitive sequences (∼1.71 Gb), whereas the Zhang et al. assembly has 61.31% repetitive sequences (∼1.13 Gb), suggesting that P. notoginseng has a large number of repetitive sequences. Repetitive elements are abundant in many plant species and pose a significant challenge to genome sequencing and assembly (Jiao et al., 2017). Here, we report on a third, more continuous and chromosome-level genome assembly of P. notoginseng.
Figure 1.
Comparison of the P. notoginseng Genome Assembly with Previous Assemblies
(A) The characters of each chromosome of P. notoginseng.
(B) Comparison of the contig length of our assembly with the published assembly.
(C) Comparison of the assembly assessment values among the three assembly versions.
Results
Sequencing and Genome Assembly
We generated 178.2-Gb-long Nanopore reads (74.25×, with an average length of 11.49 kb) and 13.0-Gb-long PacBio reads (6×, with an average read length of 9 kb) (Table S1 and Figures S1 and S2). Using these long reads and 75.86-fold massively parallel sequencing data (Table S2), we obtained a genome assembly spanning 2.24 Gb—with a contig N50 of 220.89 kb and a 90.90% BUSCO (Seppey et al., 2019) completeness score (Tables S3 and S4 and Figures 1A and S3). This assembly represents a 10-fold increase in N50 compared with the two previous assemblies (Chen et al., 2017a; Zhang et al., 2017) (Figures 1B and 1C). Moreover, to meet the requirement of a chromosome-level reference genome for genome-wide association study (GWAS) analysis, we constructed a Hi-C library and sequenced 295.32-Gb data (123.05×) on the DNBSEQ sequencing platform (Table S5 and Figures S4 and S5). We anchored 2.0 Gb of scaffold sequences (88.89% of the total assembly) into 12 P. notoginseng chromosomes (Table S6), generating a final integrated P. notoginseng assembly. We annotated 1.78-Gb repetitive sequences spanning 79.07% of the genome (Table S7 and Figure 1C). We predicted 39,452 protein-coding genes with a BUSCO completeness score of 90.1% (Table S8 and Figure S6), an increase from the two previous assemblies (Chen assembly: 36,790 genes and 73.2% completeness; Zhang assembly: 34,369 genes and 73.8% completeness). It is also worth mentioning that the genome size and gene number of P. notoginseng are both notably lower than those of P. ginseng (genome assembly: 2.98 Gb; gene: 59,352) (Kim et al., 2018).
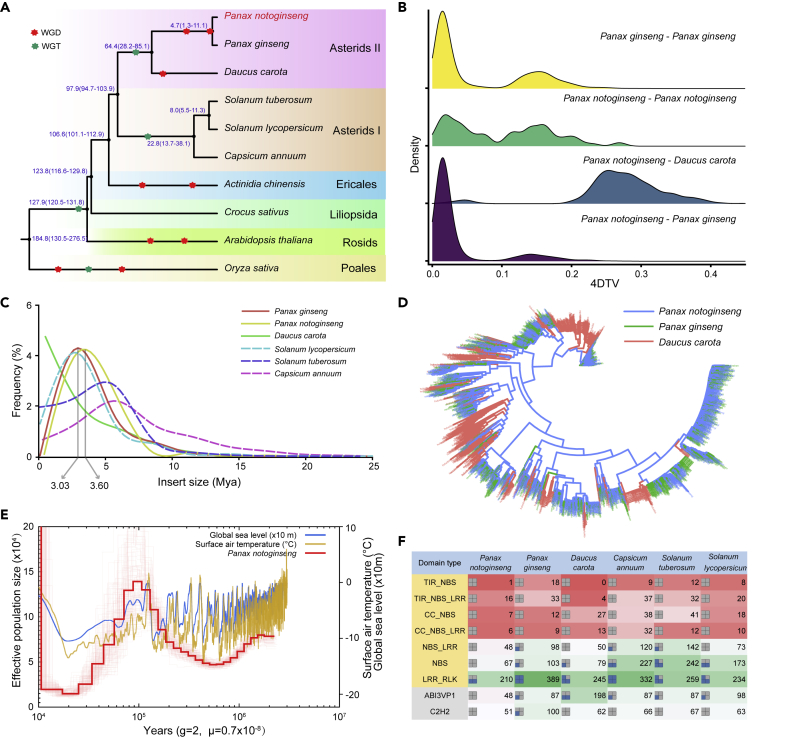
Genome Evolution of Ginseng Species
The phylogenetic tree showed that P. notoginseng and P. ginseng diverged approximately 4.7 mya and Panax species diverged with carrot (Daucus carota) ∼64.4 mya (Figure 2A). We noticed that the genome size of P. notoginseng and P. ginseng were considerably larger than that of carrot (421.5 Mb) (Table S9). We next investigated the possible drivers for the genome size variation. Fourfold degenerate synonymous site (4DTv) comparisons on gene synteny blocks (Wang et al., 2012) revealed two recent Panax-specific whole-genome duplication (WGD) events (Figure 2B). The time of the most recent WGD event closely matches the divergence time of these two species, suggesting that it occurred before their speciation (Figure 2B). We also observed a higher proportion of transposable elements (TEs) in P. notoginseng (∼79.07%) and P. ginseng (∼79.52%) compared with carrot (∼46.37%) (Table S9). A similar pattern was observed in their sister clades: pepper (81% of 3.48 Gb genome) versus the potato (∼52.69% of 840 Mb genome) and tomato (53.81% of 950 Mb genome). We calculated the insert time of long terminal repeats (LTRs) (the major TEs of these species), revealing that P. notoginseng and P. ginseng experienced an LTR expansion ∼3.03–3.6 mya (Figure 2C). In agreement, a phylogenetic tree of representative LTRs showed that P. ginseng and P. notoginseng share all LTR subtypes, indicating the Panax-specific LTR expansion occurred before the split of P. ginseng and P. notoginseng (Figure 2D). The LTR expansion is not observed in the carrot, suggesting that it contributes to the relatively larger genome sizes of genus Panax.
Figure 2.
Genome Evolution and Disease Resistance
(A) Phylogenetic tree and divergence time of two Panax species. The events of WGD and WGT were represented by red and green asterisks, respectively.
(B) Detection of whole-genome duplication events of two Panax species by 4-fold degenerate synonymous sites (4DTv) comparisons.
(C) Calculated LTR insertion time of two Panax species compared with other related species.
(D) The maximum likelihood tree constructed using LTR copia. For simplicity, 1,000 LTR sequences were randomly selected for each species. Blue, green, and red colors represent P. notoginseng, P. ginseng, and D. carota, respectively.
(E) Pairwise sequential Markovian coalescent (PSMC) analysis of the historical effective population size of P. notoginseng. Global sea level and surface air temperatures are shown in blue and yellow lines, respectively.
(F) Comparison of number of R-genes and two transcription factor genes among six species.
Reduced Plant Defense Gene Repertoire in P. notoginseng
Pairwise sequentially Markovian coalescent (PSMC) analysis (Li and Durbin, 2011) revealed a sharp decline in the effective population size of the mountain-dwelling P. notoginseng from 100,000 to 10,000 years ago consistent with the reduction of the atmospheric surface air temperature during this time (Figure 2E). Despite the relatively recent divergence time of extant ginseng species, P. notoginseng and P. ginseng have profoundly different disease resistance capabilities (Chen et al., 2017b; Lee et al., 2015; Mao et al., 2013; Ryu et al., 2014) and contemporary effective population sizes (Jang et al., 2020; Li et al., 2011b; Pan et al., 2016). Disease resistance genes (R-genes) serve to detect and respond to plant pathogens (Gururani et al., 2012). Most R-genes encode proteins with nucleotide-binding site and leucine-rich-repeat (NBS-LRR) domains. We identified 356 R-genes in P. notoginseng. This is notably fewer than that in P. ginseng (662), and also less than those of five other related species (418 in carrot, 796 in pepper, 741 in potato, and 537 in tomato) (Figure 2F). Moreover, we compared the R-gene subfamilies of the two Panax species and found that the NBS-LRR and NBS subfamilies have contracted in P. notoginseng (Figures S7–S10). In addition, transcription factor gene families involved in stress responses (Kielbowicz-Matuk, 2012; Zhuang et al., 2011) and correlated with the disease-resistant phenotype (Li et al., 2011a) have contracted in P. notoginseng. These include genes of the ABI3/VP1 family (48 in P. notoginseng, 87 in P. ginseng, 198 in carrot, 87 in pepper, 87 in potato, and 98 in tomato) and the C2H2-type zinc finger transcript factor family (51 in P. notoginseng, 100 in P. ginseng, 62L in carrot, 66 in pepper, 67 in potato, and 63 in tomato) (Figure 2F).
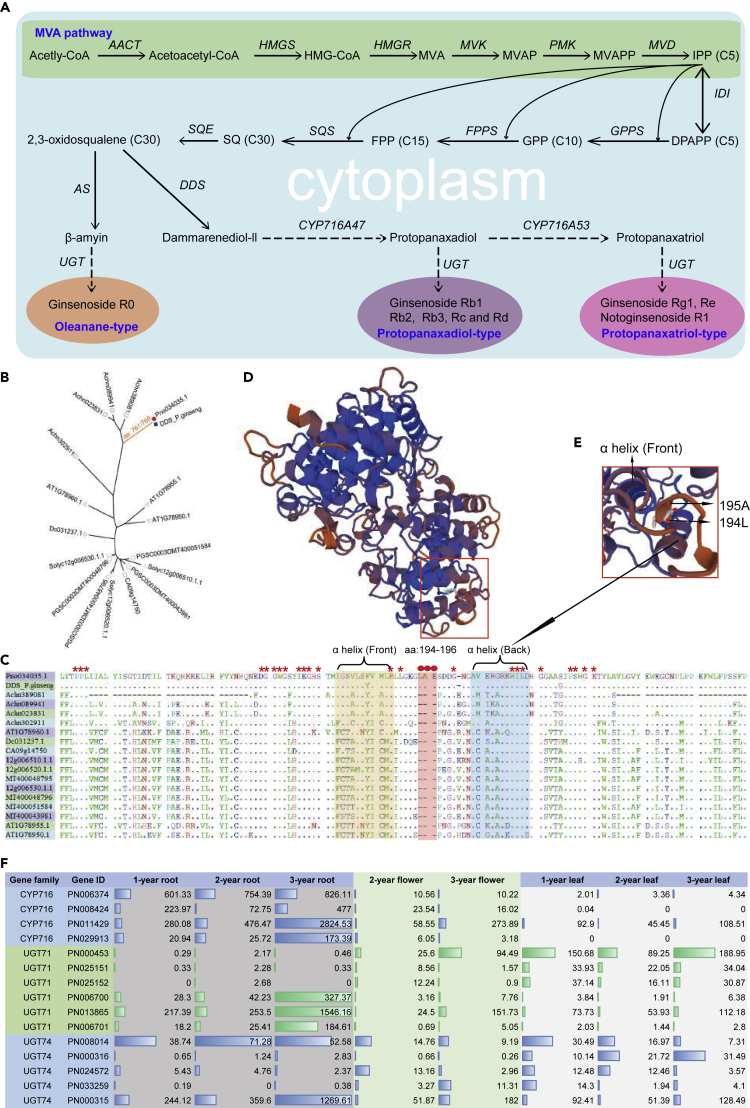
Identification of Genes in Ginsenoside Biosynthesis Pathway
The major active ingredient of ginseng is ginsenosides (tetracyclic triterpenoid saponins). Ginsenosides are synthesized from dammarenediol-II after hydroxylation via cytochrome P450 (CYP450) (Shibuya et al., 2006) and glycosylation by UDP-glycosyltransferases (UGTs) (Choi et al., 2005). There are several gene families involved in ginseng biosynthesis, including dammarenediol synthase (DDS), CYP716, and UGTs (Figure 3A). We identified 320 and 532 CYP450 genes in 31 subfamilies in P. notoginseng (8 CYP716) and P. ginseng (15 CYP716), respectively (Figure S11). We also identified 185 and 308 UGT genes in 12 subfamilies in P. notoginseng (12 UGT71 and 17 UGT74) and P. ginseng (29 UGT71 and 37 UGT74), respectively (Figure S12). As expected, the gene numbers of ginsenoside biosynthesis gene families is smaller in P. notoginseng compared with P. ginseng. A notable exception is the DDS family, where a single copy is present in both species. DDS is highly conserved in ginseng species (98% similarity), differing by only eight amino acids (Figure 3B). Interestingly, we identified three Panax-specific amino acid residue insertions (L194, A195, and E196) in P. notoginseng and P. ginseng DDS (Han et al., 2006) (Figure 3C). This insertion is located in the cyclase-N domain of the protein (Figures 3D and 3E). We speculate that this variant is critical for the synthesis of ginsenosides.
Figure 3.
The Pathway of Ginsenoside Biosynthesis and Several Key Gene Families
(A) The ginsenoside synthesis pathway from glycolysis involves terpenoid backbone biosynthesis.
(B) Gene tree of DDS genes of two Panax species and other species.
(C) Protein sequencing: multiple comparisons of DDS among P. notoginseng, P. ginseng, and other representative plants. Stars represent amino acids conserved in all protein sequences; red circles represent amino acids that are present in P. notoginseng and P. ginseng, but missing in all other plants.
(D) The protein 3D structure of DDS of P. notoginseng constructed by the SWISS-MODEL.
(E) The partial enlarged view of the protein 3D structure of DDS, and arrows indicate two amino acids specific to P. notoginseng and P. ginseng.
(F) Differentially expressed genes (CYP716, UGT71, and UGT74) among the different tissues and living years.
P. notoginseng produces several different ginsenosides. These include protopanaxadiol-type (e.g., Rb1, Rb2, Rc, and Rd) and protopanaxatriol-type (e.g., Re, Rf, and Rg1) ginsenosides found in different tissues and developmental stages throughout the plant lifespan. Protopanaxatriol-type ginsenosides are primarily found in roots, whereas protopanaxadiol-type ginsenosides are predominant in aerial parts (leaves and flowers). To identify the candidate genes involved in the ginsenoside biosynthesis pathway, we interrogated transcriptome data from different tissues and life stages (Table S10). Four CYP716 genes (PN006374, PN008424, PN011429, and PN029913), three UGT71 genes (PN006700, PN006701, and PN013865), and two UGT74 genes (PN000315 and PN008014) were expressed at a higher level in the roots and tissues of older plants (Figure 3F) (p < 0.01). These genes may be involved in protopanaxatriol-type ginsenoside biosynthesis. Three UGT71 genes (PN000453, PN025151, and PN025152) and three UGT74 genes (PN000316, PN024572, and PN033259) showed higher expression in aerial tissues and may thus be associated with protopanaxadiol-type ginsenoside biosynthesis (Figure 3F).
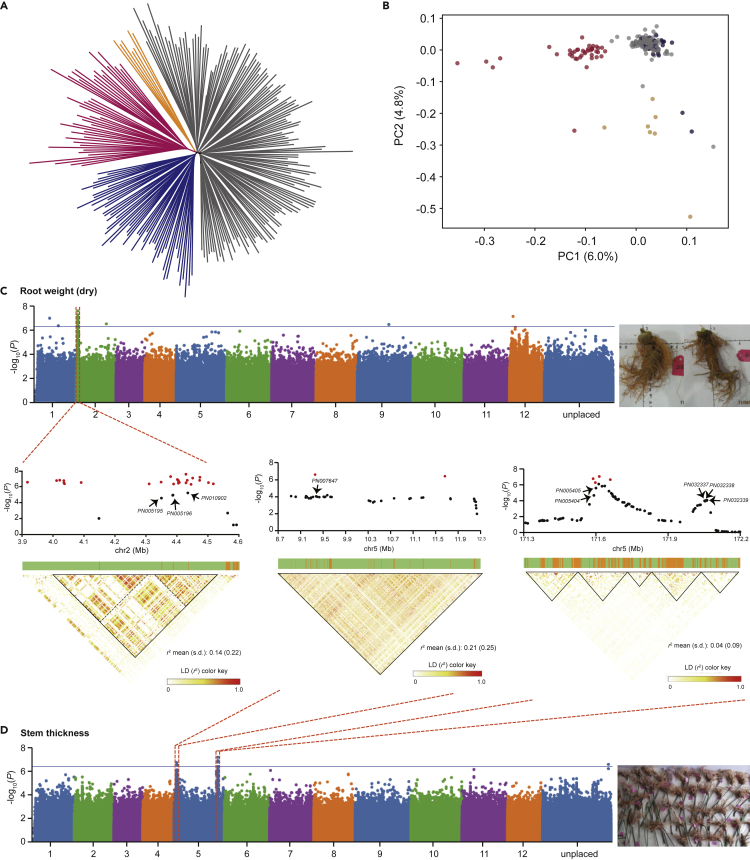
P. notoginseng Population Structure and Association Mapping of Agronomic Traits
To study the population structure and identify SNP markers possibly associated with agronomic traits, we collected and sequenced 240 individuals of P. notoginseng. An average of ∼27.02 Gb of data was obtained (∼11× sequencing depth) (Table S11), providing a good foundation for variation calling. Using the SNP data, we reconstructed the P. notoginseng population structure, revealing four distinct sub-populations (Figures S13 and S14). This structure was supported by phylogenetic tree and principal-component analysis (Figures 4A and 4B). The population structure revealed evidence of frequent gene flow between these subpopulations, probably due to their extensive horticulture. We filtered the SNP data using individual-level filter criteria, retaining ∼11.8 M SNPs for GWAS analysis. The phenotypic (trait) data were normally distributed and not skewed (Figure S15). In the GWAS analysis, we considered the population structure (top 10 principal components) and the kinship (relatedness matrix) of seven phenotypic traits (Figures S16–S23). Two phenotypic traits (dry root weight and stem thickness) had a significant signal (Figure 4C). We detected 91 loci and 63 genes located on chromosome 2 associated with root weight (Figures 4D and S24). These included three genes encoding cysteine/histidine-rich C1 domain proteins (PN005195, PN005196, and PN010902) closest to the SNP peak of the Manhattan plot. Such genes mediate plant growth and root development and are required for plant cell death and pathogen defense in pepper (Hwang et al., 2014). A large number (128) of genes were associated with stem thickness (Figures 4D and S25). These included the nine genes closest to the SNP peak of the Manhattan plot. Among them, APC6 (PN038243) gene was the most prominent. APC6 controls the overall number of lateral roots and root elongation in the legume Medicago truncatula (Bangham and McMichael, 1990) and is also involved in the amount of vascular tissue in Arabidopsis thaliana (Marrocco et al., 2009). WRKY71 (PN005405) accelerates flowering by regulating FLOWERING LOCUS T, and LEAFY, and also has pivotal roles in shoot branching by regulating the transcription of RAX genes and auxin pathways (Guo et al., 2015; Yu et al., 2016). We speculate that WRKY71 might be of pivotal importance in the developmental plasticity and stem thickness of P. notoginseng. Similarly, Reduced Wall Acetylation 3 (RWA3; P. notoginseng gene PN005404) protein has a role in plant cell wall acetylation, revealing the importance of this process for plant growth and development (Manabe et al., 2013). Finally, plant ubox 17 (PUB17, PN032338) has E3 ubiquitin ligase activity. Ubiquitination regulates plant growth and development, including flowering and responses to abiotic and biotic stresses (Sharma et al., 2016). For the disease resistance trait, we identified 33 genes. Likely because disease resistance is a complex trait, the associated SNPs were separated into four chromosomes with indistinct signals (Figures S26 and S27). We assessed the association of these candidate genes using fastBAT (Bakshi et al., 2016), a gene set-based association test method (Figure S28). The disease resistance trait was significantly enriched for the RIG-I-like receptor signaling pathway, which functions to recognize different pathogens (Mayor et al., 2007) (Figure S29). Genes driving this enrichment included LRR receptor-like serine/threonine-protein kinases (e.g., PN033297 and PN010029), members of large gene families with critical role in defense (Afzal et al., 2008).
Figure 4.
Population Structure and GWAS Analysis of 240 Individuals
(A) The SNP tree of 240 individuals.
(B) Principal-component analysis results of 240 individuals.
(C) The results of GWAS analysis and linkage disequilibrium blocks for the root weight based on SNP data.
(D) The results of GWAS analysis and linkage disequilibrium blocks for the stem thickness based on SNP data.
Discussion
We here report a chromosome-level genome assembly of P. notoginseng. This high-quality genome provides insights into ginseng (genus Panax) evolution, including the timing of WGD events and TE expansion in Panax. Moreover, we identify candidate genes associated with functional diversification in Panax. The reduced pathogen resistance in P. notoginseng may be attributed to its comparatively smaller disease resistance gene repertoire. A compensatory role for P. notoginseng ginsenoside in pathogen defense cannot be ruled out, however. Phenolics, alkaloids, and terpenoids are three major classes of chemicals involved in plant defenses (Freeman and Beattie, 2008). Ginsenosides have antimicrobial and antifungal actions, as shown in numerous laboratory studies (Bernards et al., 2006; Nicol et al., 2002). The addition of methyl jasmonate (a signaling molecule specifically expressed by plants in response to insect and pathogenic attacks) enhances overall ginsenoside production and conversion of PPD-type ginsenosides to PPT-type ginsenosides (Palazón et al., 2003). Similarly, pepper and tomato (two species phylogenetically close to Panax) produce alkaloids (capsaicinoids and tomatine, respectively), which function as deterrents against pathogens (Friedman, 2002; Kim et al., 2014). We also identify several key genes in the ginsenoside synthesis pathway and show a unique 3-amino acid insertion in Panax DDS, an enzyme in ginsenoside biosynthesis. Transcriptome data revealed ginsenoside biosynthesis pathway genes associated with distinct tissues and life stages. Population structure analysis of 240 individuals revealed four distinct populations likely marked by agriculture-enhanced gene flow.
We identified genes associated with target traits by GWAS analysis. Recently, there have been a number of studies related to the agricultural trait for the success of plant GWAS projects, for examples, GWAS for improving grain yield, stress resilience, and quality of bread wheat (Juliana et al., 2019); GWAS for NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice (Tang et al., 2019); and GWAS of 12 agronomic traits in peach (Cao et al., 2016). Identifying molecular genetic marker and major effect QTL associated with important agricultural traits is of great interest to breeders. Root weight and stem thickness of P. notoginseng are two of many traits for the highly valued commercial markets. Some of these may be useful in ginseng breeding programs, for example, in concert with the development of transgenic ginseng lines using methods such as CRISPR (Schreiber et al., 2018), a method now possible with the availability of a high-quality ginseng genome. As a result, the findings in our GWAS study represent the valuable resources of P. notoginseng, providing new opportunities and foundation for geneticists and breeders to collectively explore the genetics underlying a wide array of agricultural traits.
Limitations of the Study
We sequenced the genome, transcriptome data, and population data and detected the candidate genes associated with different traits. The reference genome and the variations were used for the GWAS analysis mainly focused on these 12 chromosome sequences, which are about 88.98% of the whole genome sequences, suggesting that our work lacks part of GWAS results on the remaining 11.02% sequences. Besides, the candidate genes associated with agronomic traits were only detected by sequencing data with no experimental validation, and the function of these candidate SNP and gene markers should be further validated using experiment results or other technologies.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xin Liu (liuxin@genomics.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The sequencing reads and genome assembly of P. notoginseng have been deposited in the CNGBdb under accession number CNP0001042, the link is http://ftp.cngb.org/pub/CNSA/data2/CNP0001042/CNS0223752/(for the whole-genome sequencing data, the accessions are from CNX0187192 to CNX0187217; for the RNA sequencing data, the accessions are from CNX0205085 to CNX0205093; for the genome assembly and annotations, the accession is CNA0013972). Meanwhile, all the sequencing data of P. notoginseng have been deposited in the NCBI under Bioproject number PRJNA656117 (for the whole-genome sequencing data, the accessions are from SRR11794023 to SRR11794041, for the RNA sequencing data, the accessions are from SRR12506286 to SRR12506294) and the genome assembly is available in the NCBI with the accession number JACBWS000000000.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was funded by The Science and Technology Development Fund, Macau SAR (File nos. 0058/2019/A1 and 0016/2019/AKP) Fund, Macau SAR and the Ministry of Science and Technology of China (MOST) joint funding scheme (File. no. FDCT 017/2015/AMJ), and University of Macau (MYRG2017-00112-ICMS, MYRG2019-00105-ICMS and MYRG2016-00056-FST) and the Guangdong Provincial Academician Workstation of BGI Synthetic Genomics (No. 2017B090904014) and the Guangdong Provincial Key Laboratory of Genome Read and Write (No. 2017B030301011). The data that support the findings of this study have been deposited in the CNSA (https://db.cngb.org/cnsa/) of CNGBdb.
Author's Contributions
S.M.-Y.L., X.X., H.Y., and X.L. designed the project. B.Y., X.S., and G.Z. prepared the samples and conducted the experiments. X.L., Y.F., and J.W. performed the genome assembly and annotation. C.S., Y.Z., A.K.C.W., D.Z., and H.Z. performed genome evolution analysis. S.S., X.D., K.H., and L.S. performed the population analysis. G.F., Y.Z., S.M.-Y.L., I.S., and S. K-W.T wrote and revised the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101538.
Contributor Information
Stephen Kwok-Wing Tsui, Email: kwtsui@cuhk.edu.hk.
Xin Liu, Email: liuxin@genomics.cn.
Simon Ming-Yue Lee, Email: simonlee@umac.mo.
Supplemental Information
References
- Afzal A.J., Wood A.J., Lightfoot D.A. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol. Plant Microbe Interact. 2008;21:507–517. doi: 10.1094/MPMI-21-5-0507. [DOI] [PubMed] [Google Scholar]
- Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J. Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi A., Zhu Z., Vinkhuyzen A.A., Hill W.D., McRae A.F., Visscher P.M., Yang J. Fast set-based association analysis using summary data from GWAS identifies novel gene loci for human complex traits. Sci. Rep. 2016;6:32894. doi: 10.1038/srep32894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C.R., McMichael A.J. HIV infection. Why the long latent period? Nature. 1990;348:388. doi: 10.1038/348388a0. [DOI] [PubMed] [Google Scholar]
- Bernards M.A., Yoesef L.F., Nicol R.W. Netherlands: Springer; 2006. The Allelopathic Potential of Ginsenosides. [Google Scholar]
- Briskin D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124:507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K., Zhou Z., Wang Q., Guo J., Zhao P., Zhu G., Fang W., Chen C., Wang X., Wang X. Genome-wide association study of 12 agronomic traits in peach. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Kui L., Zhang G., Zhu S., Zhang J., Wang X., Yang M., Huang H., Liu Y., Wang Y. Whole-genome sequencing and analysis of the Chinese herbal plant Panax notoginseng. Mol. Plant. 2017;10:899–902. doi: 10.1016/j.molp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Chen Z.J., Ma X.H., Dong L.L., Zhang L.J., Wei G.F., Xiao L.N., Wang Y., Wei F.G., Liu W.L., Yu Y.Q. DNA marker-assisted selection of medicinal plants (III)Evaluation of disease resistance of "Miaoxiang Kangqi 1" --a new cultivar of Panax notoginseng. Zhongguo Zhong Yao Za Zhi. 2017;42:2046–2051. doi: 10.19540/j.cnki.cjcmm.20170519.001. [DOI] [PubMed] [Google Scholar]
- Choi D.W., Jung J., Ha Y.I., Park H.W., Dong S.I., Chung H.J., Liu J.R. Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep. 2005;23:557–566. doi: 10.1007/s00299-004-0845-4. [DOI] [PubMed] [Google Scholar]
- Freeman B.C., Beattie G.A. The Plant Health Instructor; 2008. An Overview of Plant Defenses against Pathogens and Herbivores. [Google Scholar]
- Friedman M. Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 2002;50:5751–5780. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- Guo D., Zhang J., Wang X., Han X., Wei B., Wang J., Li B., Yu H., Huang Q., Gu H. The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis. Plant Cell. 2015;27:3112–3127. doi: 10.1105/tpc.15.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Cui X., An N., Cai G. Sanchi ginseng (Panax notoginseng (Burkill) F. H. Chen) in China: distribution, cultivation and variations. Genet. Resour. Crop Evol. 2010;57:453–460. [Google Scholar]
- Gururani M.A., Venkatesh J., Upadhyaya C.P., Nookaraju A., Pandey S.K., Park S.W. Plant disease resistance genes: current status and future directions. Physiol. Mol. Plant Pathol. 2012;78:51–65. [Google Scholar]
- Han J.Y., Kwon Y.S., Yang D.C., Jung Y.R., Choi Y.E. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006;47:1653–1662. doi: 10.1093/pcp/pcl032. [DOI] [PubMed] [Google Scholar]
- Hwang I.S., Choi D.S., Kim N.H., Kim D.S., Hwang B.K. The pepper cysteine/histidine-rich DC1 domain protein CaDC1 binds both RNA and DNA and is required for plant cell death and defense response. New Phytol. 2014;201:518–530. doi: 10.1111/nph.12521. [DOI] [PubMed] [Google Scholar]
- Jang W., Jang Y., Kim N.-H., Waminal N.E., Kim Y.C., Lee J.W., Yang T.-J. Genetic diversity among cultivated and wild Panax ginseng populations revealed by high-resolution microsatellite markers. J. Ginseng Res. 2020;44:637–643. doi: 10.1016/j.jgr.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Peluso P., Shi J., Liang T., Stitzer M.C., Wang B., Campbell M.S., Stein J.C., Wei X., Chin C.S. Improved maize reference genome with single-molecule technologies. Nature. 2017;546:524–527. doi: 10.1038/nature22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana P., Poland J., Huerta-Espino J., Shrestha S., Crossa J., Crespo-Herrera L., Toledo F.H., Govindan V., Mondal S., Kumar U. Improving grain yield, stress resilience and quality of bread wheat using large-scale genomics. Nat. Genet. 2019;51:1530–1539. doi: 10.1038/s41588-019-0496-6. [DOI] [PubMed] [Google Scholar]
- Kielbowicz-Matuk A. Involvement of plant C(2)H(2)-type zinc finger transcription factors in stress responses. Plant Sci. 2012;185-186:78–85. doi: 10.1016/j.plantsci.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kim N.H., Jayakodi M., Lee S.C., Choi B.S., Jang W., Lee J., Kim H.H., Waminal N.E., Lakshmanan M., van Nguyen B. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 2018;16:1904–1917. doi: 10.1111/pbi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Park M., Yeom S.I., Kim Y.M., Lee J.M., Lee H.A., Seo E., Choi J., Cheong K., Kim K.T. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014;46:270–278. doi: 10.1038/ng.2877. [DOI] [PubMed] [Google Scholar]
- Lee B.D., Dutta S., Ryu H., Yoo S.J., Suh D.S., Park K. Induction of systemic resistance in Panax ginseng against Phytophthora cactorum by native Bacillus amyloliquefaciens HK34. J. Ginseng Res. 2015;39:213–220. doi: 10.1016/j.jgr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin. Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.W., Su R.C., Cheng C.P., Sanjaya, You S.J., Hsieh T.H., Chao T.C., Chan M.T. Tomato RAV transcription factor is a pivotal modulator involved in the AP2/EREBP-mediated defense pathway. Plant Physiol. 2011;156:213–227. doi: 10.1104/pp.111.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Deng C.Q., Chen B.Y., Zhang S.P., Liang Y., Luo X.G. Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. ethnopharmacol. 2009;121:412–418. doi: 10.1016/j.jep.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Li J., Yang X.-L., Cheng Z., Zhang W.-J. Genetic diversity and differentiation of cultivated ginseng (Panax ginseng CA Meyer) populations in North-east China revealed by inter-simple sequence repeat (ISSR) markers. Genet. Resour. Crop Evol. 2011;58:815–824. [Google Scholar]
- Luo J., Song W., Yang G., Xu H., Chen K. Compound Danshen (Salvia miltiorrhiza) dripping pill for coronary heart disease: an overview of systematic reviews. Am. J. Chin. Med. 2015;43:25–43. doi: 10.1142/S0192415X15500020. [DOI] [PubMed] [Google Scholar]
- Manabe Y., Verhertbruggen Y., Gille S., Harholt J., Chong S.L., Pawar P.M., Mellerowicz E.J., Tenkanen M., Cheng K., Pauly M. Reduced Wall Acetylation proteins play vital and distinct roles in cell wall O-acetylation in Arabidopsis. Plant Physiol. 2013;163:1107–1117. doi: 10.1104/pp.113.225193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z.S., Long Y.J., Zhu S.S., Chen Z.J., Wei F.G., Zhu Y.Y. Advances in root rot pathogen of Panax notoginseng research. J. Chin. Med. Mater. 2013;36:2051–2054. [Google Scholar]
- Marrocco K., Thomann A., Parmentier Y., Genschik P., Criqui M.C. The APC/C E3 ligase remains active in most post-mitotic Arabidopsis cells and is required for proper vasculature development and organization. Development. 2009;136:1475–1485. doi: 10.1242/dev.035535. [DOI] [PubMed] [Google Scholar]
- Mayor A., Martinon F., De Smedt T., Petrilli V., Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) J. Pharm. Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Nicol R.W., Traquair J.A., MA B. Ginsenosides as host resistance factors in American ginseng (Panax quinquefolius) Can. J. Bot. 2002;80:557–562. [Google Scholar]
- Ou X., Jin H., Guo L., Yang Y., Cui X., Xiao Y., Liu D. [Status and prospective on nutritional physiology and fertilization of Panax notoginseng] Zhongguo Zhong yao Za Zhi. 2011;36:2620–2624. [PubMed] [Google Scholar]
- Palazón J., Cusidó R.M., Bonfill M., Mallol A., Moyano E., Morales C., Piñol M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol. Biochem. 2003;41:1019–1025. [Google Scholar]
- Pan Y., Wang X., Sun G., Li F., Gong X. Application of RAD sequencing for evaluating the genetic diversity of domesticated Panax notoginseng (Araliaceae) PLoS One. 2016;11:e0166419. doi: 10.1371/journal.pone.0166419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H., Park H., Suh D.S., Jung G.H., Park K., Lee B.D. Biological control of Colletotrichum panacicola on Panax ginseng by Bacillus subtilis HK-CSM-1. J. Ginseng Res. 2014;38:215–219. doi: 10.1016/j.jgr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Stein N., Mascher M. Genomic approaches for studying crop evolution. Genome Biol. 2018;19:140. doi: 10.1186/s13059-018-1528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppey M., Manni M., Zdobnov E.M. Busco: assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019;1962:227–245. doi: 10.1007/978-1-4939-9173-0_14. [DOI] [PubMed] [Google Scholar]
- Sharma B., Joshi D., Yadav P.K., Gupta A.K., Bhatt T.K. Role of ubiquitin-mediated degradation system in plant biology. Front. Plant Sci. 2016;7:806. doi: 10.3389/fpls.2016.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hoshino M., Katsube Y., Hayashi H., Kushiro T., Ebizuka Y. Identification of beta-amyrin and sophoradiol 24-hydroxylase by expressed sequence tag mining and functional expression assay. FEBS J. 2006;273:948–959. doi: 10.1111/j.1742-4658.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- Son H.Y., Han H.S., Jung H.W., Park Y.K. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J. Pharmacol. Sci. 2009;109:368–379. doi: 10.1254/jphs.08197fp. [DOI] [PubMed] [Google Scholar]
- Tang W., Ye J., Yao X., Zhao P., Xuan W., Tian Y., Zhang Y., Xu S., An H., Chen G. Genome-wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-13187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H., Liu Y., Zhang B., Huang J., Li Y., Yang B., Huang Z., Xiang F., Zhang H. The antidepressant effects and mechanism of action of total saponins from the caudexes and leaves of Panax notoginseng in animal models of depression. Phytomedicine. 2011;18:731–738. doi: 10.1016/j.phymed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Yang C.Y., Wang J., Zhao Y., Shen L., Jiang X., Xie Z.G., Liang N., Zhang L., Chen Z.H. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J. ethnopharmacol. 2010;130:231–236. doi: 10.1016/j.jep.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Yu Y., Liu Z., Wang L., Kim S.G., Seo P.J., Qiao M., Wang N., Li S., Cao X., Park C.M. WRKY71 accelerates flowering via the direct activation of flowering locus t and leafy in Arabidopsis thaliana. Plant J. 2016;85:96–106. doi: 10.1111/tpj.13092. [DOI] [PubMed] [Google Scholar]
- Zhang D., Li W., Xia E.H., Zhang Q.J., Liu Y., Zhang Y., Tong Y., Zhao Y., Niu Y.C., Xu J.H. The medicinal herb Panax notoginseng genome provides insights into ginsenoside biosynthesis and genome evolution. Mol. Plant. 2017;10:903–907. doi: 10.1016/j.molp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Sun C.C., Zhou X.R., Xiong A.S., Zhang J. Isolation and characterization of an AP2/ERF-RAV transcription factor BnaRAV-1-HY15 in Brassica napus L. HuYou15. Mol. Biol. Rep. 2011;38:3921–3928. doi: 10.1007/s11033-010-0508-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing reads and genome assembly of P. notoginseng have been deposited in the CNGBdb under accession number CNP0001042, the link is http://ftp.cngb.org/pub/CNSA/data2/CNP0001042/CNS0223752/(for the whole-genome sequencing data, the accessions are from CNX0187192 to CNX0187217; for the RNA sequencing data, the accessions are from CNX0205085 to CNX0205093; for the genome assembly and annotations, the accession is CNA0013972). Meanwhile, all the sequencing data of P. notoginseng have been deposited in the NCBI under Bioproject number PRJNA656117 (for the whole-genome sequencing data, the accessions are from SRR11794023 to SRR11794041, for the RNA sequencing data, the accessions are from SRR12506286 to SRR12506294) and the genome assembly is available in the NCBI with the accession number JACBWS000000000.