Abstract
Tobacco cigarette (TC) smoking has never been lower in the United States, but electronic cigarette (EC) vaping has reached epidemic proportions among our youth. Endothelial dysfunction, as measured by flow-mediated vasodilation (FMD) is a predictor of future atherosclerosis and adverse cardiovascular events and is impaired in young TC smokers, but whether FMD is also reduced in young EC vapers is uncertain. The aim of this study in otherwise healthy young people was to compare the effects of acute and chronic tobacco cigarette (TC) smoking and electronic cigarette (EC) vaping on FMD. FMD was compared in 47 nonsmokers (NS), 49 chronic EC vapers, and 40 chronic TC smokers at baseline and then after EC vapers (n = 31) and nonsmokers (n = 47) acutely used an EC with nicotine (ECN), EC without nicotine (EC0), and nicotine inhaler (NI) at ~4-wk intervals and after TC smokers (n = 33) acutely smoked a TC, compared with sham control. Mean age (NS, 26.3 ± 5.2 vs. EC, 27.4 ± 5.45 vs. TC, 27.1 ± 5.51 yr, P = 0.53) was similar among the groups, but there were more female nonsmokers. Baseline FMD was not different among the groups (NS, 7.7 ± 4.5 vs. EC:6.6 ± 3.6 vs. TC, 7.9 ± 3.7%∆, P = 0.35), even when compared by group and sex. Acute TC smoking versus control impaired FMD (FMD pre-/postsmoking, −2.52 ± 0.92 vs. 0.65 ± 0.93%∆, P = 0.02). Although the increase in plasma nicotine was similar after EC vapers used the ECN versus TC smokers smoked the TC (5.75 ± 0.74 vs. 5.88 ± 0.69 ng/mL, P = 0.47), acute EC vaping did not impair FMD. In otherwise healthy young people who regularly smoke TCs or ECs, impaired FMD compared with that in nonsmokers was not present at baseline. However, FMD was significantly impaired after smoking one TC, but not after vaping an equivalent “dose” (estimated by change in plasma nicotine) of an EC, consistent with the notion that non-nicotine constituents in TC smoke mediate the impairment. Although it is reassuring that acute EC vaping did not acutely impair FMD, it would be dangerous and premature to conclude that ECs do not lead to atherosclerosis.
NEW & NOTEWORTHY In our study of otherwise healthy young people, baseline flow-mediated dilation (FMD), a predictor of atherosclerosis and increased cardiovascular risk, was not different among tobacco cigarette (TC) smokers or electronic cigarette (EC) vapers who had refrained from smoking, compared with nonsmokers. However, acutely smoking one TC impaired FMD in smokers, whereas vaping a similar EC “dose” (as estimated by change in plasma nicotine levels) did not. Finally, although it is reassuring that acute EC vaping did not acutely impair FMD, it would be premature and dangerous to conclude that ECs do not lead to atherosclerosis or increase cardiovascular risk.
Keywords: electronic cigarettes, endothelial function, flow-mediated dilation, nicotine, tobacco cigarettes
INTRODUCTION
The vast majority of people who smoke tobacco cigarettes (TCs) begin smoking in their teens or early twenties, but TC-related diseases, including cardiovascular diseases, are insidious, presenting only after decades of TC smoking (44). Each puff of TC smoke contains 1015 free radicals and over 7,000 different chemicals, several of which are known toxicants that have prooxidant effects on endogenous pathways (7, 10, 33). Oxidative stress plays a critical role in inflammation and is now recognized to be a pivotal early component in the development of atherosclerosis (2, 9, 39).
TC smoking initiates and propagates this excessive oxidative stress in the vasculature, uncoupling endothelial nitric oxide (NO) synthase and decreasing bioavailability of NO (2, 10, 22). NO underlies a number of important functions of the healthy endothelium, including vasodilation, as well as anti-thrombotic and anti-inflammatory functions (8, 10, 23). Endothelial dysfunction can be noninvasively detected by impaired brachial-artery flow-mediated dilation (FMD) in response to an ischemic stimulus, such as inflation of a sphygmomanometric cuff to suprasystolic levels on the forearm (43). Upon cuff deflation, blood flow in the brachial artery increases in response to this acute ischemia, thereby increasing shear stress on endothelial cells. Healthy endothelial cells then release vasodilating factors, including NO, which mediate smooth muscle relaxation and acute vasodilation. Impaired NO bioavailability, which can be caused by excessive oxidative stress, contributes to impaired FMD (28).
Brachial-artery endothelial dysfunction as measured by impaired FMD correlates with coronary artery endothelial dysfunction (3) and is the earliest marker of future coronary atherosclerosis. Importantly, impaired FMD is associated with increased risk for future adverse cardiovascular events (18, 38). Reduced FMD has been reported in TC smokers and those exposed to secondhand smoke and is directly associated with smoking burden (5, 6). Both regular or “light” cigarettes are associated with reduced FMD, but FMD can be improved following smoking cessation or with antioxidant therapy (1, 14, 15, 20). Oxidative stress induced by TC smoking has been implicated as a major contributor underlying reduced FMD (23, 28). Surprisingly, pharmaceutical grade nicotine spray, without the combusted constituents present in TC smoke, has also been reported to acutely impair endothelial dysfunction, although to a lesser extent than smoking a TC with similar nicotine yield (34).
TC smoking prevalence has never been lower in otherwise healthy young people, but electronic cigarette (EC) vaping, introduced in 2007, is reaching epidemic proportions (29). In 2019, almost one in three high school seniors reported vaping a nicotine-containing EC in the previous month (29). ECs are not cigarettes at all; in fact, only the first generation “cigalikes” even simulated the appearance of a tobacco cigarette. ECs are battery-powered, handheld devices that are available in many shapes, including the shape of a flash drive. When the heating element is activated by puffing on the mouthpiece, a heated aerosol composed of solvents, flavorings, and usually nicotine is released into the user’s mouth. While ECs are generally believed to be less harmful than TC smoking, the effect of acute and chronic EC vaping on vascular health in otherwise healthy young people is largely unknown. The aim of the current study in otherwise healthy young people was to compare the effects of acute and chronic TC smoking and EC vaping on endothelial function as measured by brachial-artery FMD, a predictor of future atherosclerosis and adverse cardiovascular events.
MATERIAL AND METHODS
Study Population
The study population consisted of healthy male and female subjects between the ages 21–45 yr, who were 1) chronic (>12 mo) EC vapers who did not smoke TCs (no dual users), 2) chronic (>12 mo) TC smokers, or 3) nonsmokers. All groups were required to meet the following criteria: 1) nonobese, i.e., <30 kg/m2 body mass index (BMI); 2) not pregnant; 3) no known health problems, including asthma, hypertension, heart disease, diabetes, or hyperlipidemia; 4) alcoholic intake < 2 drinks/day and no regular illicit drug use determined through screening questionnaire, confirmed at each visit with a urine toxicology test; and 5) not taking prescription medications regularly (oral contraceptives were allowed); and 6) not competitive (intercollegiate) athletes. Chronic EC vapers and nonsmokers who were former TC smokers were eligible for the study if they had quit smoking >1 yr before the study. End-tidal CO was measured in EC vapers and nonsmokers at each visit to detect those who were surreptitiously smoking TCs, if the CO was >10 parts/million, it was presumed the participant had smoked a combustible tobacco product, leading to elimination from the study. A urine toxicology test was performed at the beginning of each visit to exclude surreptitious marijuana use. On the day of the written, informed consent, before the day of the first experimental session, all subjects were familiarized and acclimated to the experimental setup. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written, informed consent was obtained from each participant. This study is registered at ClinicalTrials.gov NCT02740595 and NCT03072628.
Acute EC vaping.
In this open-label, randomized crossover study, chronic EC vapers and nonsmokers participated in up to four 30-min acute exposure sessions in random order separated by 4 wk: 1) sham vaping, a control session consisting of puffing on an empty EC; 2) EC with nicotine (ECN); 3) EC without nicotine (EC0); and 4) nicotine inhaler (NI), a “clean” source of nicotine, with inactive menthol flavoring and no solvents.
Acute tobacco cigarette smoking.
Chronic TC smokers, but not nonsmokers, participated in up to two acute smoking sessions in random order separated by 4 wk: 1) sham smoking, a control session consisting of puffing on an empty straw, and 2) smoking one TC (own brand).
Smoking Topography
Electronic cigarette and nicotine inhaler.
EC topography was standardized: participants were verbally cued every 30 s with a recording: “Ready, set” (place EC in mouth), “go, 2, 3” (inhale 3 s), “hold, 2, 3” (hold aerosol in), then exhale. Participants used the EC for up to 30 min (60 puffs), since we have reported that this topography was tolerable and sufficient to increase plasma nicotine levels (30). According to the package insert and company literature, using this same topography the nicotine inhaler was expected to achieve very similar plasma nicotine levels seen with our second-generation EC device (30).
Tobacco cigarette.
Subjects puffed on an empty straw or smoked one TC in 7 min, a typical time interval to smoke one TC.
EC Device
A second‐generation “pen-like” EC device (1.0 Ω, eGo‐One by Joyetech, Irvine, CA), was used with strawberry-flavored VG/PG liquid, since fruit-flavored e-liquids were widely used (40), with 1) 1.2% nicotine, 2) 0% nicotine, or 3) empty (control). In 2019, it was recognized that the JUUL was the most popular vaping device, and thus we switched to this device. A total of 10 EC vapers used the JUUL with mint-flavored pods [the most widely used flavor (21), 5% nicotine, and without nicotine (Cyclone)].
Nicotine and Cotinine Plasma Levels
Before and after EC or TC exposures, blood was drawn from the opposite arm used for FMD according to laboratory specifications and sent to the UCLA Clinical Laboratories for nicotine (half-life, 1 to 2 h) and cotinine (half-life, 16–20 h) levels. The assay for plasma nicotine and cotinine was run by the commercial laboratory, Quest Laboratories, with a limit of quantitation of 2 ng/mL for both plasma nicotine and cotinine.
Measurement of brachial-artery FMD.
High-resolution ultrasound (Logic 7, General Electric, Inc.) measurement of brachial-artery FMD and endothelium-independent dilation in response to 0.15 mg sublingual nitroglycerin was performed by the same investigator (K.P.H.) according to current guidelines (42, 43). Assessments were done with a 7.5-MHz linear array transducer ultrasound system in spectral Doppler mode. A sphygmomanometric cuff was placed just below the antecubital fossa. The brachial artery was imaged with assistance from a probe holder between 5 to 8 cm above the antecubital crease. Image was optimized in B-mode, and landmarks were noted and were also marked on the arm to ensure matching images pre-/postexposure. Vascular imager software with automated edge detector was used for recording and analysis (Vascular Analysis Tools, Medical Imaging Applications, LLC). After baseline diameter was recorded for 30 s, a sphygmomanometric cuff was inflated to 250 mmHg for 5 min (43). The image was recorded 30 s before cuff deflation and continued for 2 min after release. FMD calculations were expressed as the absolute change (mm∆) and relative change (%∆) in poststimulus diameter in relation to the baseline diameter. Mean blood velocity was measured with an insonation angle of 60°. The shear stress stimulus was evaluated by calculating peak shear rate (velocity/diameter) and integrated shear rate (42, 43). To account for the potential differences in shear rate stimulus, FMD is also normalized for shear stress (area under the curve) (43). To test endothelium-independent vasodilation, sublingual nitroglycerin (0.15 mg) was then administered. Two minutes later the image was recorded for 7 min. To assess microvascular function, peak velocity during reactive hyperemia (VHR) and shear stress during reactive hyperemia (SSRH) were compared. SSRH was calculated according to the following formula: SSHR (dyn/cm2) = 8 × 0.035 (dyn·s/cm2) × [VRH/(baseline diameter/10)] (17, 25, 35–37).
Blood pressure.
Systolic blood pressure (SBP), diastolic BP (DBP), mean BP (MBP), and heart rate (HR) were measured after a 10-min rest period in the supine position at baseline and after a 5-min rest period following each exposure, with a noninvasive BP monitor (Casmed 740, Avante Health Solutions) according to American Heart Association guidelines (32).
Experimental session.
To avoid the potential influence of circadian rhythm on FMD, subjects were studied midday (usually between 10:00 am and 2:00 pm). Studies were separated by ~4-wk intervals, and women were studied in the early follicular phase or during the placebo phase of oral contraceptive use. Subjects were instructed not to use over-the-counter medications, including vitamins for 24 h before the study session. After abstaining from smoking, caffeine, and exercise for at least 12 h, fasting participants were placed in a supine position in a quiet, temperature-controlled (21°C) room in the Human Physiology Laboratory located in the UCLA Clinical and Translational Research Center. No cell phones or digital stimuli were allowed, and during data acquisition, talking was minimized. The participant was instrumented, blood was drawn, and after a 10-min rest period, blood pressure and heart rate were measured, and the FMD was measured. The participant then underwent an assigned exposure: ECN, EC0, NI, or sham vaping control for EC users and nonsmokers, and TC or sham smoking control for TC smokers. After repositioning, and a 5-min rest period, blood pressure and heart rate were measured, and FMD was measured. In a subset of subjects (n = 86), 0.15 mg nitroglycerin was placed under the tongue, and brachial-artery diameter was again measured. Blood was then drawn, and the study was concluded.
Statistical Analysis
The primary outcome was baseline FMD in the three study groups and then the change in FMD from baseline following each exposure. Secondary outcomes were SBP, DBP, mean BP (MBP), heart rate (HR), VRH, and SSRH and the change in these outcomes with each acute exposure.
Data from pen-like ECs and JUULs were analyzed as a single EC group, distinguished only by liquid with and without nicotine. Baseline mean comparisons were made via an analysis of variance model. Mean postexposure minus baseline differences were compared across ECN, EC0, NI, and control using a crossover repeated measure (mixed) analysis of variance model adjusting for session and order. Normal quantile plots (not shown) were examined, and the Shapiro-Wilk statistic computed to confirm that the model residual errors followed the normal distribution on the appropriate original or log scale. Means ± SE for baseline to postexposure changes were adjusted by session and order effects.
Associations between two continuous variables were assessed using the nonparametric Spearman correlation (rs) since the relation was monotone but not necessarily linear. Differences or associations were considered statistically significant when P ≤ 0.05.
Sample size calculation.
Sample size was based on end points of FMD. In preliminary studies conducted in nonsmokers in which mean FMD ± SD was 7.6 ± 3.3%, it was calculated that 22 participants per group (nonsmokers, EC vapers, and TC smokers) would permit detection of a Δ of 1.47%, and 44 participants per group would permit detection of a Δ of 1.03% between groups. Even fewer participants would be necessary to detect a mean difference in baseline versus exposure in a paired comparison, assuming similar standard deviations with exposures for 80% power using a two-sided α = 0.05. Our final analysis included at least 40 participants per group.
RESULTS
Study Population
Of 148 participants, 12 were excluded: 4 urine positive for marijuana, 3 nonsmokers with positive plasma cotinine consistent with current tobacco product use, 3 with poor (uninterpretable) brachial-artery ultrasound image, 1 EC vaper with carbon monoxide > 10 parts/million consistent with surreptitious TC use, and 1 illness, leaving 136 participants, including 47 nonsmokers, 49 chronic EC vapers, and 40 chronic TC smokers who were enrolled in this study. Baseline characteristics of the three groups are displayed in Table 1. The groups had similar characteristics including age, race, and body mass index (BMI), but there were more women in the nonsmoking group. Baseline plasma cotinine level was not different in the EC vapers and TC smokers, indicative of similar smoking burden. Nine EC users and 9 TC smokers did not completely abstain from smoking before the study, as indicated by detectable plasma nicotine levels > 3 ng/mL. An analysis was performed without these participants, and results were unchanged (data not shown).
Table 1.
Baseline characteristics
| Nonsmokers | EC Vapers | TC Smokers | P Value | |
|---|---|---|---|---|
| n | 47 | 49 | 40 | |
| Mean age, yr | 26.3 ± 5.20 | 27.4 ± 5.45 | 27.1 ± 5.51 | 0.53 |
| Sex, men/women | 22/25 | 36/13 | 26/14 | 0.02 |
| Mean BMI, kg/m2 | 23.5 ± 2.91 | 24.2 ± 3.58 | 24.7 ± 3.92 | 0.47 |
| Race, n | 0.60 | |||
| African American | 4 | 2 | 5 | |
| Caucasian | 26 | 29 | 25 | |
| Asian | 9 | 13 | 8 | |
| Hispanic | 5 | 5 | 2 | |
| Hawaiian | 2 | 0 | 0 | |
| Unknown | 1 | 0 | 0 | |
| Base cotinine, ng/mL* | 0 | 83.2 (17.6, 141.5) | 82.0 (34.6, 160.5) | 0.68† |
| Former TC smoker, n (%) | 2 (4.3) | 28 (57.1) | N/A | |
| SBP, mmHg | 118.2 ± 13.1 | 120.8 ± 11.0 | 118.0 ± 10.4 | 0.37 |
| DBP, mmHg | 74.7 ± 11.3 | 76.1 ± 10.9 | 73.6 ± 8.3 | 0.66 |
| MBP, mmHg | 88.3 ± 11.1 | 89.6 ± 9.9 | 86.9 ± 8.3 | 0.56 |
| HR, beats/min | 66.7 ± 9.5 | 63.9 ± 9.5 | 63.7 ± 8.5 | 0.23 |
| Peak shear rate, s−1* | 78,437 (58,340, 108,744) |
86,427 (50,760, 116,471) |
91,680 (965,953, 124,978) |
0.51 |
| Artery diameter, mm | 3.44 ± 0.47 | 3.74 ± 0.51 | 3.64 ± 0.57 | 0.008 |
Values are means ± SD. BMI, body mass index; DBP, diastolic blood pressure; EC, electronic cigarette; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure; TC, tobacco cigarette; N/A, not applicable.
Median, Q1–Q3.
P value, EC vapers vs. TC smokers.
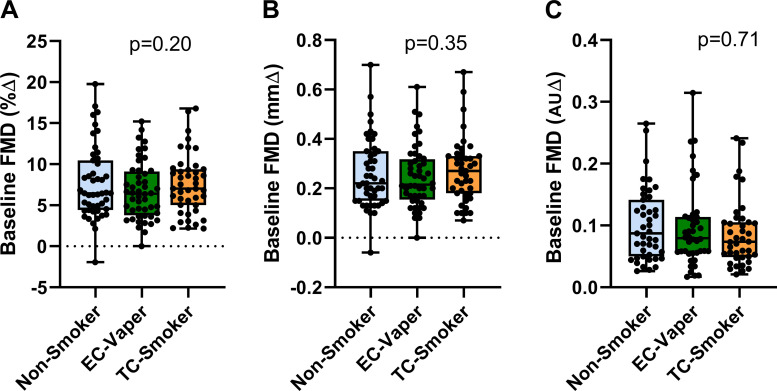
Baseline FMD
Baseline brachial-artery diameter was smaller in the nonsmokers compared with the other groups (Table 1). Shear rate stimulus was not different among the groups (Table 1). Baseline FMD, unadjusted (Fig. 1) or adjusted for baseline artery diameter, was not different among the three groups, nonsmoker versus EC vaper versus TC smoker, whether measured by percent change (adjusted %∆, 7.2 ± 0.59 vs. 6.9 ± 0.56 vs. 8.0 ± 0.60, respectively; P = 0.22), absolute change (adjusted mm∆, 0.24 ± 0.02 vs. 0.25 ± 0.02 vs. 0.28 ± 0.02, respectivel;, P = 0.44), or normalized for shear stress (adjusted AU∆, 0.086 ± 0.02 vs. 0.081 ± 0.02 vs. 0.085 ± 0.02, respectively; P = 0.84). This was true when primary outcomes were compared by group and sex as well (%∆: group, P = 0.59; sex, P = 0.71; group × sex, P = 0.73; mm∆: group, P = 0.80; sex, P = 0.12; group × sex, P = 0.68; or AU∆: group, P = 0.68; sex, P = 0.19; group × sex, P = 0.73) Sublingual nitroglycerin, which evokes endothelium-independent vasodilation, caused dilation in all groups (nonsmokers, 21.3 ± 5.4%; EC vapers, 19.9 ± 6.4%; and TC smokers, 23.2 ± 8.6%).
Fig. 1.
Baseline flow-mediated dilation (FMD) in the 3 groups. Among the 3 groups, including nonsmokers (n = 47), chronic electronic cigarette (EC) vapers (n = 49), and chronic tobacco cigarette (TC) smokers (n = 40), baseline FMD was not different unadjusted or adjusted for artery diameter, whether reported as percent change (%∆; A), absolute change (mm∆; B), or normalized for shear stress [arbitrary units ∆ (AU∆); C]. Unadjusted means compared between groups using a repeated-measure (mixed) analysis of variance model and displayed as mean (25–75%) with whiskers to minimum to maximum of the data.
Baseline Hemodynamics
Baseline hemodynamics (Table 1), including SBP, DBP, MBP, and HR, were not different among nonsmokers, chronic EC vapers, and chronic TC smokers.
Acute Exposures
We then assessed the acute effects of TC smoking in 33 chronic TC smokers and the acute effects of EC vaping in 47 nonsmokers and 31 chronic EC vapers. Baseline characteristics of the three groups did not differ in age, sex, BMI, or race (Table 2).
Table 2.
Baseline characteristics: acute exposure
| Nonsmokers | EC Vapers | TC Smokers | P Value | |
|---|---|---|---|---|
| n | 47 | 31 | 33 | |
| Mean age, yr | 26.3 ± 5.2 | 27.2 ± 5.7 | 26.9 ± 4.9 | 0.69 |
| Sex, men/women | 22/25 | 21/10 | 20/13 | 0.17 |
| Mean BMI, kg/m2 | 23.5 ± 2.9 | 23.8 ± 3.3 | 23.9 ± 2.8 | 0.90 |
| Race, n | 0.88 | |||
| African American | 4 | 1 | 4 | |
| Caucasian | 26 | 18 | 19 | |
| Asian | 9 | 8 | 8 | |
| Hispanic | 5 | 4 | 2 | |
| Hawaiian | 2 | 0 | 0 | |
| Unknown | 1 | 0 | 0 |
Values are means ± SD. BMI, body mass index; EC, electronic cigarette; TC, tobacco cigarette.
TC Smokers
Acute changes in FMD following acute TC smoking.
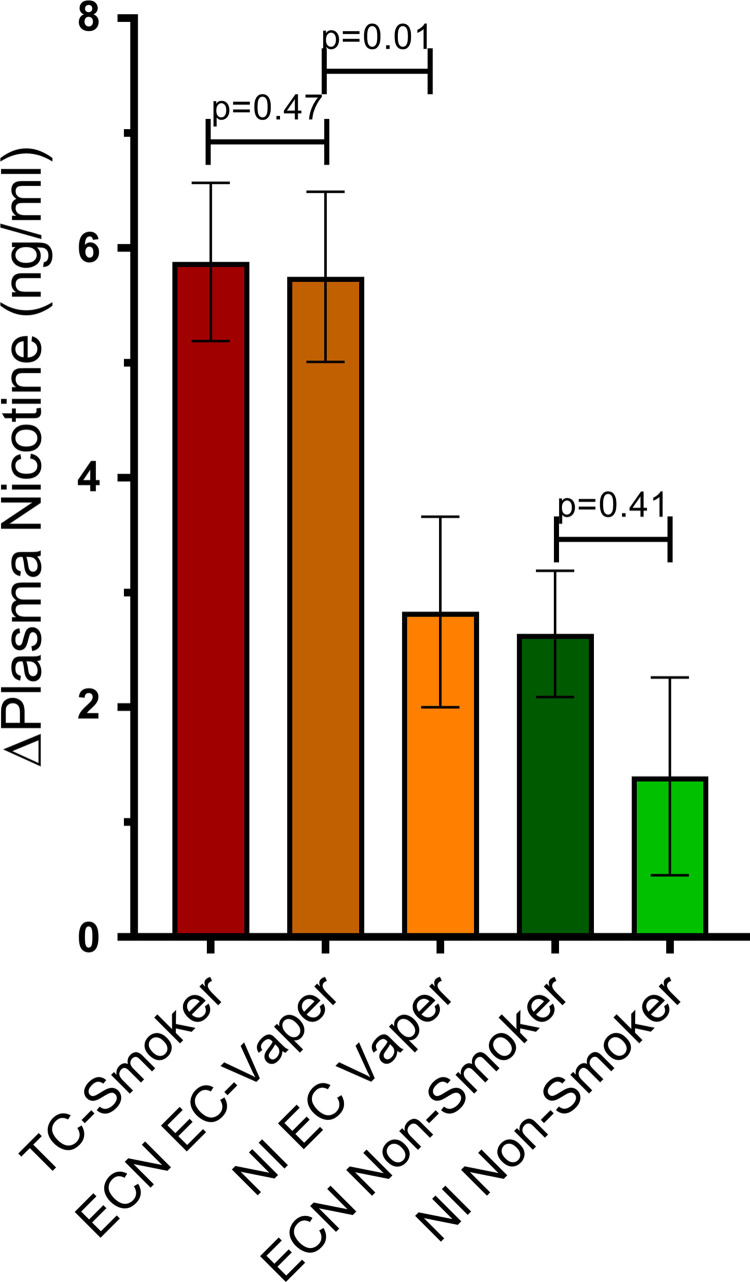
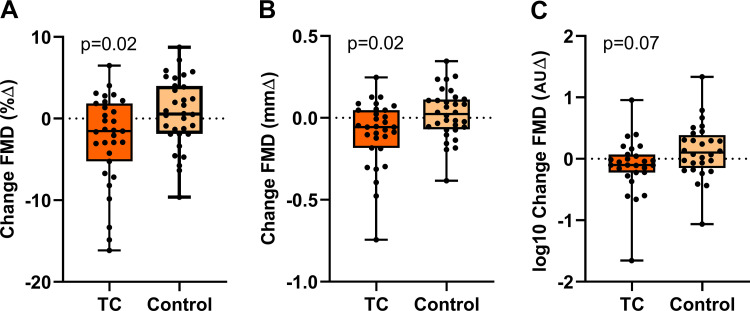
TC smoking increased plasma nicotine levels by 5.88 ± 0.69 ng/mL (Fig. 2). Brachial-artery diameter was not different on the TC smoking versus straw control day (3.59 ± 0.11 vs. 3.59 ± 0.11 mm; P = 0.94). TC smoking compared with straw control significantly decreased FMD, reported as percent change (−2.52 ± 0.92 vs. 0.65 ± 0.93%, respectively; P = 0.02), absolute change (−0.091 ± 0.033 vs. 0.023 ± 0.034 mm, respectively; P = 0.02), and tended to decrease FMD when normalized for shear stress, although this did not reach significance (−0.11 ± 0.09 vs. 0.13 ± 0.09 AU, respectively; P = 0.07; Fig. 3). The decrease in FMD was not correlated with the increase in plasma nicotine levels (Table 3).
Fig. 2.
Changes in plasma nicotine. The increase in plasma nicotine levels was not different in chronic tobacco cigarette (TC) smokers (n = 31) after smoking 1 TC and in chronic electronic cigarette (EC) vapers (n = 22) after using the EC with nicotine (ECN). When EC vapers used the ECN, the increase in nicotine was significantly greater compared with the nicotine inhaler (NI, n = 19). When nonsmokers used the ECN (n = 41), the increase in plasma nicotine was not different compared with the NI (n = 17). Mean postexposure minus baseline differences were compared across TC, ECN, and NI using a crossover repeated-measure (mixed) analysis of variance model, and results are displayed as means ± SE.
Fig. 3.
Change in flow-mediated dilation (FMD) in tobacco cigarette (TC) smokers after TC smoking. In TC smokers, FMD was significantly impaired pre-/postacute TC smoking (n = 31) compared with pre-/post-sham control (n = 32), whether reported as percent change (%∆; A), absolute change (mm∆; B), or normalized for shear stress [arbitrary units ∆ (AU∆); C]. Mean postexposure minus baseline differences were compared across TC and sham control using a t-tests and displayed as mean (25–75%) with whiskers to minimum to maximum of the data.
Table 3.
Spearman correlation with increase in plasma nicotine
| Nonsmokers |
EC Vapers |
TC Smokers |
||||
|---|---|---|---|---|---|---|
| Correlation | P value | Correlation | P value | Correlation | P value | |
| FMD, %∆ | 0.134 | 0.12 | 0.154 | 0.15 | −0.068 | 0.60 |
| FMD, mm∆ | 0.105 | 0.23 | 0.146 | 0.17 | −0.083 | 0.52 |
| SBP, mmHg | 0.371 | 0.00001 | 0.410 | 0.0001 | 0.407 | 0.002 |
| DBP, mmHg | 0.274 | 0.001 | 0.300 | 0.005 | 0.321 | 0.01 |
| MBP, mmHg | 0.388 | 0.00001 | 0.373 | 0.0003 | 0.418 | 0.002 |
| HR, beats/min | 0.238 | 0.006 | 0.515 | 0.00001 | 0.687 | 0.00001 |
DBP, diastolic blood pressure; EC, electronic cigarette; FMD, flow-mediated dilation; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure; TC, tobacco cigarette.
Acute changes in hemodynamics following acute TC smoking.
After smoking the TC compared with straw control, all hemodynamic outcomes (SBP, DBP, MBP, and HR) were significantly increased (Table 4). The increase in all hemodynamic outcomes were moderately to strongly correlated with the increase in plasma nicotine levels (Table 3).
Table 4.
Changes in hemodynamics
| ∆SBP, mmHg | ∆DBP, mmHg | ∆MBP, mmHg | ∆HR, beats/min | |
|---|---|---|---|---|
| TC Smokers | ||||
| TC | 14.6 ± 2.1 | 8.7 ± 1.7 | 9.8 ± 1.8 | 13.7 ± 1.7 |
| Control | 8.0 ± 2.1 | 3.3 ± 1.7 | 3.7 ± 1.8 | −1.0 ± 1.7 |
| P value | 0.04 | 0.03 | 0.03 | 0.00001 |
| EC Vapers | ||||
| ECN | 4.53 ± 1.84 | 2.67 ± 1.99 | 3.88 ± 1.77 | 15.83 ± 2.1 |
| EC0 | −4.03 ± 1.76 | −3.38 ± 1.89 | −2.73 ± 1.69 | 4.49 ± 2.01 |
| NI | 1.65 ± 2.14 | 1.96 ± 2.3 | 1.57 ± 2.05 | 5.06 ± 2.45 |
| Control | −5.58 ± 4.17 | −4.19 ± 4.49 | −3.77 ± 4.01 | 1.42 ± 4.78 |
| P value | 0.001 | 0.03 | 0.01 | 0.0001 |
| Nonsmokers | ||||
| ECN | 9.5 ± 1.41 | 3.69 ± 1.38 | 5.97 ± 1.32 | 9.79 ± 1.47 |
| EC0 | −1.73 ± 1.41 | −1.87 ± 1.38 | −0.89 ± 1.32 | 4.45 ± 1.47 |
| NI | 2.41 ± 2.11 | 5.03 ± 2.08 | 5.60 ± 1.98 | 5.54 ± 2.22 |
| Control | −0.53 ± 1.92 | 1.55 ± 1.88 | 0.37 ± 1.80 | 3.11 ± 2.01 |
| P value | 0.00001 | 0.007 | 0.0001 | 0.002 |
Values are means ± SD. DBP, diastolic blood pressure; EC, electronic cigarette; ECN, electronic cigarette with nicotine; EC0, electronic cigarette without nicotine; HR, heart rate; MBP, mean blood pressure; NI, nicotine inhaler; SBP, systolic blood pressure; TC, tobacco cigarette.
EC Vapers
Acute changes in FMD following acute EC vaping.
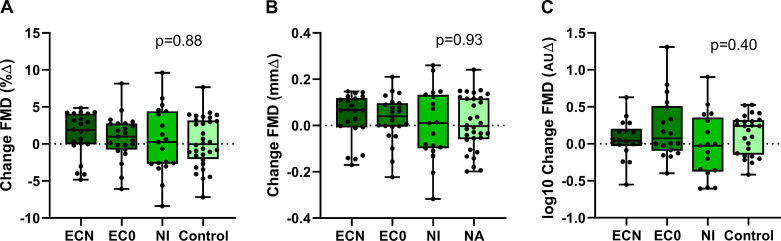
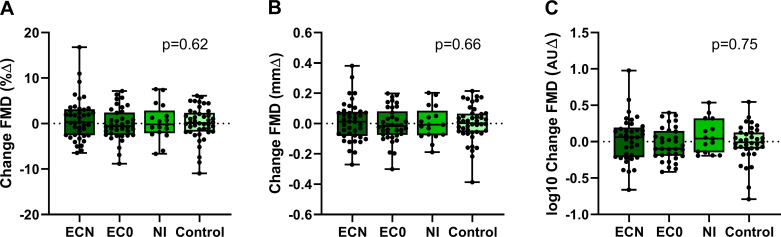
The change in plasma nicotine level when analyzed by EC device type was not different in EC vapers (pen-like vs. JUUL, 7.80 ± 2.14 vs. 5.00 ± 1.17 ng/mL; overall, P = 0.25), thus the EC data were grouped as a single EC device, distinguished only by liquid with and without nicotine. The increase in plasma nicotine was similar after using the EC with nicotine compared with the TC (5.75 + 0.74 vs. 5.88 + 0.69 ng/mL, P = 0.47, respectively), and significantly greater than the NI (2.83 ± 0.83 ng/mL, P = 0.01; Fig. 2). Brachial-artery diameter was not different on the ECN, EC0, NI, or straw control days (3.87 ± 0.10 vs. 3.82 ± 0.10 vs. 3.78 ± 0.10 vs. 3.77 ± 0.26 mm, respectively; P = 0.49). None of the exposures, including the ECN, EC0, or NI, produced a significant change in FMD compared with the sham control, reported as percent change (1.29 ± 0.84 vs. 0.87 ± 0.81 vs. 0.39 ± 1.01 vs. 0.26 ± 1.98%, respectively; P = 0.88), absolute change (0.037 ± 0.029 vs. 0.030 ± 0.028 vs. 0.004 ± 0.035 vs. 0.010 ± 0.068 mm, respectively; P = 0.93), or normalized for shear stress (0.061 ± 0.10 vs. 0.21 ± 0.10 vs. −0.031 ± 0.11 vs. 0.073 ± 0.20 AU, respectively; P = 0.40; Fig. 4).
Fig. 4.
Change in flow-mediated dilation (FMD) in electronic cigarette (EC) vapers after EC vaping or nicotine inhaler (NI). In EC vapers, FMD was unchanged pre-/postacute use of an EC with nicotine (n = 22), EC without nicotine (n = 23), or NI (n = 19) compared with pre-/post-sham control (n = 31), whether reported as percent change (%∆; A), absolute change (mm∆; B), or normalized for shear stress [arbitrary units ∆ (AU∆); C]. Mean postexposure minus baseline differences were compared across electronic cigarette with nicotine (ECN), electronic cigarette without nicotine (EC0), NI, and sham control using a crossover repeated-measure (mixed) analysis of variance model and displayed as mean (25–75%) with whiskers to minimum to maximum of the data.
Acute changes in hemodynamics following acute EC vaping.
After the use of the ECN, but not EC0 or NI, all hemodynamic outcomes (SBP, DBP, MBP, and HR) were increased compared with the sham control (Table 4). The increase in all hemodynamic outcomes were strongly correlated with the increase in plasma nicotine levels (Table 3).
Nonsmokers
Acute changes in FMD following acute EC vaping.
The change in plasma nicotine level when analyzed by EC device type was not different in nonsmokers (pen-like vs. JUUL, 2.08 ± 0.06 vs. 1.55 ± 2.03 ng/mL; overall, P = 0.80), thus the EC data were grouped as a single EC device, distinguished only by liquid with and without nicotine. The increase in plasma nicotine when nonsmokers used the ECN or the NI was not significantly different (2.64 ± 0.55 vs. 1.40 ± 0.86 ng/mL; P = 0.41; Fig. 2). The increase in plasma nicotine when nonsmokers used the ECN or NI was significantly lower compared with when chronic EC vapers used the ECN or when chronic TC smokers smoked a TC (Fig. 2). Brachial-artery diameter was not different on the ECN, EC0, NI, or straw control days (3.50 ± 0.08 vs. 3.42 ± 0.08 vs. 3.45 ± 0.09 vs. 3.46 ± 0.08 AU, respectively; P = 0.46). None of the exposures, including the ECN, EC0, or NI, produced a significant change in FMD compared with the sham control, reported as percent change (0.94 ± 0.67 vs. −0.14 ± 0.70 vs. 0.31 ± 1.04 vs. 0.05 ± 0.94%, respectively; P = 0.62), absolute change (0.028 ± 0.022 vs. −0.007 ± 0.023 vs. 0.002 ± 0.034 vs. 0.000 ± 0.031 mm, respectively; P = 0.65), or normalized for shear stress (−0.006 ± 0.049 vs. −0.035 ± 0.053 vs. 0.073 ± 0.078 vs. −0.023 ± 0.073 AU, respectively; P = 0.75; Fig. 5).
Fig. 5.
Change in flow-mediated dilation (FMD) in nonsmokers after electronic cigarette (EC) vaping or nicotine inhaler (NI). In nonsmokers, FMD was unchanged pre-/postacute use of an EC with nicotine (n = 41), EC without nicotine (n = 39), or NI (n = 17) compared with pre-/post-sham control (n = 44), whether reported as percent change (%∆; A), absolute change (mm∆; B), or normalized for shear stress [arbitrary units ∆ (AU∆); C]. Mean postexposure minus baseline differences were compared across electronic cigarette with nicotine (ECN), electronic cigarette without nicotine (EC0), NI, and sham control using a crossover repeated-measure (mixed) analysis of variance model and displayed as mean (25–75%) with whiskers to minimum to maximum of the data.
Acute changes in hemodynamics following acute EC vaping.
After the use of the ECN, but not EC0 or NI, the SBP, MBP, and HR were increased compared with those levels in the sham control (Table 4) and were correlated with changes in nicotine levels (Table 3).
Microvascular Function
Velocity reactive hyperemia (VHR) and shear stress reactive hyperemia (SSRH).
Microvascular function, as estimated by VHR or SSRH, was not different among the three groups at baseline (NS vs. EC vs. TC: VHR, 125.3 ± 26.5 vs. 129 ± 31.9 vs. 133.7 ± 28.3 cm/s respectively; P = 0.27; and SSHR, 104.1 ± 29.5 vs. 99.5 ± 31.3 vs. 105.4 ± 31.3 dyn/cm2, respectively; P = 0.60). Furthermore, none of the exposures, including TC in smokers or ECN, EC0, or NI in EC vapers and nonsmokers, produced a significant change in VHR or SSRH compared with straw control (Table 5).
Table 5.
Microvascular function
| ∆VHR, cm/s | ∆SSHR, dyn/cm2 | |
|---|---|---|
| TC Smokers | ||
| TC | 7.03 ± 7.57 | 5.87 ± 5.88 |
| Control | −1.60 ± 7.36 | 0.71 ± 5.72 |
| P value | 0.36 | 0.47 |
| EC Vapers | ||
| ECN | −5.69 ± 8.12 | 0.00 ± 6.26 |
| EC0 | −11.47 ± 8.26 | −10.11 ± 6.36 |
| NI | −14.34 ± 9.68 | −11.64 ± 7.44 |
| Control | −4.04 ± 17.41 | −4.41 ± 13.45 |
| P value | 0.76 | 0.79 |
| Nonsmokers | ||
| ECN | −0.97 ± 5.20 | 2.71 ± 4.40 |
| EC0 | 4.10 ± 5.65 | 2.40 ± 4.79 |
| NI | −17.65 ± 8.40 | −12.94 ± 7.12 |
| Control | −6.89 ± 7.82 | −6.42 ± 6.63 |
| P value | 0.18 | 0.21 |
Values are means ± SD. EC, electronic cigarette; ECN, electronic cigarette with nicotine; EC0, electronic cigarette without nicotine; NI, nicotine inhaler; SSHR, shear stress reactive hyperemia; TC, tobacco cigarette; VHR, velocity reactive hyperemia.
DISCUSSION
Traditional cardiovascular risk factors, such as age, hypertension, diabetes mellitus, hyperlipidemia, and, importantly, TC smoking, are all associated with endothelial dysfunction as detected by impaired FMD, the earliest marker of future atherosclerosis and a predictor of adverse cardiovascular events (10, 43). Impaired FMD is indicative of decreased endothelial NO bioavailability, as well as the presence of excessive oxidative stress that promotes atherosclerosis by oxidizing lipids and activating proinflammatory monocytes (28). Impaired FMD is predictive of future adverse cardiovascular events in those with and without known cardiovascular disease (18, 38, 43). Impaired FMD is not static and can be reversed when risk factors are treated, and this reversal is associated with improved cardiovascular prognosis (38).
To our knowledge, this is the first study to compare baseline FMD in a large cohort of otherwise healthy young EC vapers and TC smokers to nonsmokers and to compare acute EC vaping in EC vapers to acute TC smoking in TC smokers. There are two major new findings from this study: 1) baseline endothelial function is not different among the three groups of otherwise healthy young people, including nonsmokers, EC vapers, and TC smokers, and 2) TC smoking but not EC vaping acutely and markedly impairs endothelium-dependent vasodilation as measured by FMD.
It is perhaps surprising that these chronic TC smokers do not have impaired endothelial function as assessed by brachial-artery FMD. Evidence in preclinical and clinical studies supports the notion that endothelial dysfunction is an early and sensitive indicator of uncompensated oxidative stress in humans (7, 10, 16, 33). Since TC smoking is a well-known source of oxidative stress, the lack of impairment in FMD in our smokers is unexpected. In fact, even nonsmokers exposed to secondhand smoke have been shown to have impaired endothelial function measured by FMD (5). There are several potential explanations for our findings.
First of all, it should be clarified that unfiltered secondhand smoke has up to 10-fold the toxicants as mainstream, filtered smoke (31, 44), so it is deceptive to think that since a nonsmoker is “only” inhaling secondhand smoke that her exposure to prooxidants is necessarily less than that of the TC smoker. Second, our otherwise healthy, young TC smokers were overall light smokers as suggested by their relatively low-plasma cotinine levels, a metabolite of nicotine. Importantly, impaired FMD in smokers is directly related to smoking burden (6). Third, our protocol specified that TC smokers refrain from smoking 12 h before the baseline study. This is in stark contrast to the protocol followed by Celermajer et al. (6), which mandated that TC smokers must smoke at least one TC within 12 h of the FMD measurement. Finally, in contrast to the demographics of TC smokers in prior reports, all of our TC smokers were young, nonobese, without comorbidities and did not use recreational drugs, including marijuana. In short, with the exception of their TC smoking, they apparently engaged in relatively healthy lifestyles.
A similar line of reasoning could explain why endothelium-dependent vasodilation was not attenuated in chronic EC vapers compared with nonsmokers. Cotinine levels in EC vapers were not different from those in TC smokers, indicative of relatively light vaping habits. These were similarly otherwise healthy, nonobese, young people who did not regularly use drugs. Of course, this finding should not be interpreted as TC smoking or EC vaping is not harmful when one is young. The development of atherosclerosis is an insidious, slow process, and the lack of abnormal FMD may just reflect the sensitivity of the test rather than the true absence of pathology (45).
The second novel finding in our study was that when chronic TC smokers acutely smoked one TC, endothelium-dependent vasodilation was significantly impaired, whereas when chronic EC vapers vaped a similar EC dose as measured by the increase in plasma nicotine pre-/postexposure, endothelium-dependent vasodilation was not impaired. This is consistent with the notion that EC vaping imposes less of an oxidative stress burden compared with TC smoking. The non-nicotine, prooxidative toxicants in TC smoke such as volatile free radicals, aldehydes, and acrolein, which interrupt cellular enzymatic pathways leading to excessive oxidative stress, are in greater abundance in TC smoke compared with EC emissions (7, 10, 13, 27). Although the dose of nicotine may be the same, the dose of toxicants was not. Additionally, the impairment in FMD was not correlated with the change in plasma nicotine levels in TC smokers in these studies.
Interestingly, Neunteufl et al. (34) found that nicotine alone, as delivered by nicotine nasal spray, which is free of non-nicotine toxicants, significantly attenuated FMD in chronic TC smokers, albeit to a significantly lesser degree than acute TC smoking. The explanation for this finding in humans is uncertain; evidence of oxidative stress in plasma biomarkers was not uncovered, although the study may have been underpowered (34). This finding contrasts with preclinical studies, in which nicotine alone, at doses present in TC smokers, has no effect, or only minimal effects, on endothelial function (2, 24, 41). This finding also is at odds with our finding that acute EC vaping did not attenuate FMD, despite a similar increase in nicotine as acute TC smoking. Additionally, the impairment in FMD with acute TC smoking in our study was not correlated with the change in plasma nicotine levels. Finally, this finding also contrasts with the finding of George et al. (12), who showed that switching from TCs to ECs with or without nicotine, significantly increased endothelial function at one month. In George’s study (12), endothelial function was not different between those that switched to the ECs with nicotine compared with those who switched to ECs without nicotine.
In contrast to our study and to George’s study (12), Carnevale et al. (4) reported that chronic TC smokers had similar acute impairment in FMD after smoking a TC compared with vaping nine puffs from an early generation EC. Unfortunately, acute changes in plasma nicotine were not measured, so it is unknown if these exposures were equivalent. Surprisingly, despite similar impairments in FMD, the impact of acute EC vaping on plasma markers of oxidative stress were less than acute TC smoking. One explanation for these findings of similar impairment in FMD after EC vaping or TC smoking is that in Carnevale’s study, chronic TC smokers used the EC, whereas in our study, chronic EC vapers (non-TC smokers) used the EC. It is possible that TC smokers have less vascular reserve; that is, they are more vulnerable to stressors of endothelial function compared with EC vapers who do not smoke TCs.
Study Limitations
These are studies in humans, who are heterogeneous; thus, the groups may have differed in cofounders for which we did not account. We relied on self-report for past medical history and use of tobacco products and drugs. However, we also performed confirmation testing. Specifically, we measured exhaled CO to detect surreptitious TC smoking in EC vapers and nonsmokers and tested urine for marijuana. The JUUL, used in only a small number of our acute studies, delivers alveolar nicotine, similar to TC smoking. Although the acute increase in plasma nicotine in TC smokers and EC vapers was not different in our study, the pharmacokinetics of the increase were likely different. Future studies using the JUUL or another pod-EC device would be of interest. The NI contained menthol flavoring described as “inactive,” but we cannot rule out a vasodilatory effect of the menthol flavoring in our participants. We did not simultaneously measure plasma markers of oxidative stress in TC smokers and EC vapers. However, the purpose of our study was not to determine mechanisms for endothelial dysfunction in TC smokers, but to detect its presence. After all, there is already a large body of animal and human data supporting the notion that excessive oxidative stress underlies endothelial dysfunction and abnormal FMD (7, 10, 33, 43). Oxidative stress degrades tetrahydrobiopterin, the cofactor for endothelial NO synthase, thereby uncoupling NO synthase, which leads to greater generation of oxidative stress in the form of superoxide anion and less NO bioavailability (23, 26).
In summary, in healthy young people who smoke TCs or vape ECs, impaired FMD compared with that in nonsmokers was not present at baseline. However, FMD was significantly impaired after smoking one TC, but not after vaping an equivalent “dose” (as estimated by change in plasma nicotine) of an EC. Impaired FMD in TC smokers is most likely attributable to non-nicotine toxicants in TC smoke, since an equivalent increase in plasma nicotine from the EC did not lead to acute impairment in FMD. Although it is reassuring that acute EC vaping did not acutely impair FMD, it would be dangerous and premature to conclude that ECs do not lead to atherosclerosis. However, there is increasing scientific literature (11, 12, 19) that supports the notion that ECs, although not harmless, may be less harmful than TC smoking for cardiovascular risk.
GRANTS
This work was supported by Tobacco-Related Disease Research Program Grants TRDRP 23XT-0006H (to H.R.M.), 25IR-0024H (to H.R.M.), and TRDRP 28IR-0065 (H.R.M.) and by National Institutes of Health, National Center for Advancing Translational Science UCLA CTSI Grant UL1TR001881.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.R.M. conceived and designed research; K.P.H., Y.C., R.S.M., K.H.N., E.U.T., K.L., I.R.R., and J.G. performed experiments; K.P.H., Y.C., I.R.R., J.G., and H.R.M. analyzed data; H.R.M. interpreted results of experiments; H.R.M. prepared figures; H.R.M. drafted manuscript; K.P.H., Y.C., R.S.M., K.H.N., E.U.T., K.L., I.R.R., J.G., and H.R.M. edited and revised manuscript; K.P.H., Y.C., R.S.M., K.H.N., E.U.T., K.L., I.R.R., J.G., and H.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gary L. Pierce, Dept. of Health and Human Physiology, Univ. of Iowa, for generously sharing expertise to train K.P.H. in the technique and analysis of flow-mediated dilation. We are also grateful to Ms. Harshika Chatterjee who enthusiastically helped with the tables.
REFERENCES
- 1.Amato M, Frigerio B, Castelnuovo S, Ravani A, Sansaro D, Tremoli E, Squellerio I, Cavalca V, Veglia F, Sirtori CR, Werba JP, Baldassarre D. Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis 228: 153–160, 2013. doi: 10.1016/j.atherosclerosis.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737, 2004. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park SY, Malenfant S, Gifford JR, Kwon OS, Park SH, Jarrett CL, Shields KL, Hydren JR, Bisconti AV, Owan T, Abraham A, Tandar A, Lui CY, Smith BR, Richardson RS. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 74: 208–215, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 150: 606–612, 2016. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci (Landmark Ed) 14: 3128–3144, 2009. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 10: 219–230, 2013. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 9.El-Kenawi A, Ruffell B. Inflammation, ROS, and Mutagenesis. Cancer Cell 32: 727–729, 2017. doi: 10.1016/j.ccell.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 120: 713–735, 2017. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 11.Garcia PD, Gornbein JA, Middlekauff HR. Cardiovascular autonomic effects of electronic cigarette use: a systematic review. Clin Auton Res. [published online ahead of print, 2020. March 26] doi: 10.1007/s10286-020-00683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, Donnan PT, Khan F, Lang CC. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol 74: 3112–3120, 2019. doi: 10.1016/j.jacc.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med 31: 53–61, 2001. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 15.Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 17.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 19.Ip M, Diamantakos E, Haptonstall K, Choroomi Y, Moheimani RS, Nguyen KH, Tran E, Gornbein J, Middlekauff HR. Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: implication for sudden death risk. Am J Physiol Heart Circ Physiol 318: H1176–H1184, 2020. doi: 10.1152/ajpheart.00738.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 55: 1988–1995, 2010. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leventhal AM, Miech R, Barrington-Trimis J, Johnston LD, O’Malley PM, Patrick ME. Flavors of e-cigarettes used by youths in the United States. JAMA 322: 2132–2134, 2019. doi: 10.1001/jama.2019.17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161–167, 2013. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Barrios V, Buchholz JN, Glenn TC, Duckles SP. Chronic nicotine administration does not affect peripheral vascular reactivity in the rat. J Pharmacol Exp Ther 271: 1135–1142, 1994. [PubMed] [Google Scholar]
- 25.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo S, Lei H, Qin H, Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr Pharm Des 20: 3548–3553, 2014. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 27.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol 29: 1662–1678, 2016. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 28.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34: 509–515, 2014. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 29.Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in adolescent vaping, 2017-2019. N Engl J Med 381: 1490–1491, 2019. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic effects of acute e-cigarette use: role of nicotine and non-nicotine constituents. J Am Heart Assoc 6: e006579, 2017. doi: 10.1161/JAHA.117.006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med 32: 542–543, 2007. doi: 10.1016/j.amepre.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr. Measurement of blood pressure in humans: a scientific statement from the american heart association. Hypertension 73: e35–e66, 2019. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol 70: 212–229, 2017. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, Schmid RW, Maurer G, Stefenelli T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol 39: 251–256, 2002. doi: 10.1016/s0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- 35.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr, Sueta CA, Chang PP, O’Connor CM, Sherwood A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J 178: 108–114, 2016. doi: 10.1016/j.ahj.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philpott A, Anderson TJ. Reactive hyperemia and cardiovascular risk. Arterioscler Thromb Vasc Biol 27: 2065–2067, 2007. doi: 10.1161/ATVBAHA.107.149740. [DOI] [PubMed] [Google Scholar]
- 37.Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol 103: 1610–1615, 2009. doi: 10.1016/j.amjcard.2009.01.376. [DOI] [PubMed] [Google Scholar]
- 38.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 168: 344–351, 2013. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 39.Sack MN, Fyhrquist FY, Saijonmaa OJ, Fuster V, Kovacic JC. Basic biology of oxidative stress and the cardiovascular system: part 1 of a 3-part series. J Am Coll Cardiol 70: 196–211, 2017. doi: 10.1016/j.jacc.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ. Use of flavored electronic cigarette refill liquids among adults and youth in the US-Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014-2015). PLoS One 13: e0202744, 2018. doi: 10.1371/journal.pone.0202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun YP, Zhu BQ, Browne AE, Sievers RE, Bekker JM, Chatterjee K, Parmley WW, Glantz SA. Nicotine does not influence arterial lipid deposits in rabbits exposed to second-hand smoke. Circulation 104: 810–814, 2001. doi: 10.1161/hc3301.092788. [DOI] [PubMed] [Google Scholar]
- 42.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thijssen DH, Bruno RM, van Mil AC, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 44.U. S. Department of Health and Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006. [PubMed] [Google Scholar]
- 45.U. S. Department of Health and Human Services The Health Consequences of Smoking −50 years of Progress: A Report of the Surgeon General. Atlanta: GA US Department of Health and Human Services, Centers for Disease Control and Prevention Coordinating Center for Health Promotion National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health, 2014. [Google Scholar]