Abstract
Each day, ~1.7 kg of NaCl and 180 liters of water are reabsorbed by nephron segments in humans, with urinary excretion fine tuned to meet homeostatic requirements. These tasks are coordinated by a spectrum of renal Na+ transporters and channels. The goal of the present study was to investigate the extent to which inhibitors of transepithelial Na+ transport (TNa) along the nephron alter urinary solute excretion and how those effects may vary between male and female subjects. To accomplish that goal, we developed sex-specific multinephron models that represent detailed transcellular and paracellular transport processes along the nephrons of male and female rat kidneys. We simulated inhibition of Na+/H+ exchanger 3 (NHE3), bumetanide-sensitive Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and amiloride-sensitive epithelial Na+ channel (ENaC). NHE3 inhibition simulations predicted a substantially reduced proximal tubule TNa, and NKCC2 inhibition substantially reduced thick ascending limb TNa. Both gave rise to diuresis, natriuresis, and kaliuresis, with those effects stronger in female rats. While NCC inhibition was predicted to have only minor impact on renal TNa, it nonetheless had a notable effect of enhancing excretion of Na+, K+, and Cl−, particularly in female rats. Inhibition of ENaC was predicted to have opposite effects on the excretion of Na+ (increased) and K+ (decreased) and to have only a minor impact on whole kidney TNa. Unlike inhibition of other transporters, ENaC inhibition induced stronger natriuresis and diuresis in male rats than female rats. Overall, model predictions agreed well with measured changes in Na+ and K+ excretion in response to diuretics and Na+ transporter mutations.
Keywords: diuresis, epithelial Na+ channel, Na+-Cl− cotransporter, Na+/H+ exchanger 3, Na+-K+-2Cl− cotransporter, natriuresis
INTRODUCTION
Hypertension is a growing epidemic in the contemporary Western world, affecting ~25% of the adult population worldwide. Its prevalence is predicted to increase by 60% by the year 2025, when a total of 1.56 billion patients are expected to be affected (36, 74). A major determinant of extracellular fluid volume and, thus, arterial blood pressure is total body Na+ (52). At steady state, the amount of Na+ excreted in the urine roughly matches Na+ intake. As such, the kidneys, and, in particular, the transport mechanisms that mediate Na+ reabsorption, play a crucial role in long-term blood pressure control.
Substantial experimental efforts during the past decades have contributed to our understanding of how ~180 liters of water and 1.5 kg of salt are reabsorbed daily by nephron segments in humans and how urinary excretion is fine tuned to match output to input to maintain homeostasis (59). The proximal tubule receives the glomerular filtrate and reabsorbs ~60−70% of the filtered Na+ and water. The essential components of reabsorption include basolateral Na+-K+-ATPase driving Na+ transport (TNa) via apical Na+/H+ exchanger (NHE)3, paracellular claudin 2, and water reabsorption by aquaporin 1 (AQP1) (49, 59, 71). The next major site of TNa is the thick ascending limb of the loop of Henle, which reabsorbs 25−30% of the filtered NaCl via Na+-K+-2Cl− cotransporter (NKCC2), also driven by basolateral Na+-K+-ATPase and dependent on apical K+ secretion by the renal outer medullary K+ channel (ROMK) and basolateral Cl− reabsorption by ClC-Kb (23, 25). Regulation of Na+ reabsorption along the proximal tubule and thick ascending limb is affected by numerous factors, including hormones [such as angiotensin (ANG) II and parathyroid hormone], sympathetic innervation, and autocrine and paracrine factors, including endothelin, nitric oxide, dopamine, and arachidonic acid metabolites (23, 51). An additional 5−10% of the filtered Na+ load is reabsorbed past the macula densa, mediated by the distal convoluted tubule apical Na+-Cl− cotransporter (NCC), driven by basolateral Na+-K+-ATPase and activated by low interstitial K+ concentration, which turns on a kinase cascade that phosphorylates and traffics NCC to the apical membrane, sympathetic innervation, and vasopressin (32, 81). The collecting duct principal cell epithelial Na+ channel (ENaC) mediates the final fine tuning of TNa regulated by aldosterone and ANG II as well as paracrine and autocrine agents, including ATP, endothelin, nitric oxide, and arachidonic acid metabolites (60, 90). Loss- or gain-of-function mutations in these transporters, channels, and kinase cascades can produce chronic hypotension or hypertension, illustrating their importance in regulating the effective circulating volume (64); for example, loss-of-function mutations in NKCC2 (Bartter syndrome) or NCC (Gitelman syndrome) are characterized by salt wasting, low blood pressure, hypokalemia, and metabolic alkalosis (14).
Across ethinicities, men have higher blood pressure than women throughout much of life (80). This sex difference has also been reported across species, including dogs (87), rats, mice, and chickens (65), as well as in animal models of hypertension (68, 83). Given the essential role of the kidney in blood pressure control, sex differences in hypertension may be attributable, in part, to differences in kidney structure and function. Here, we focused on sex differences in renal electrolyte transporters, channels, and claudins (which we collectively refer to as transporters). Veiras et al. (88) reported sexual dimorphism in transporter abundance patterns in rodents. Their findings revealed markedly different transport capacity in the proximal tubule of male and female rat nephrons. Additionally, the study demonstrated that, compared with male rat nephrons, female rat nephrons exhibit, in the proximal tubule, greater NHE3 phosphorylation and redistribution to the base of the microvilli, where activity is lower (6), as well as lower abundance of Na+-Pi cotransporter 2 (NaPi2), AQP1, and claudin-2. Compared with male rats, in female rats, the proximal tubule reabsorbs a substantially lower fraction of filtered Na+ (88). In contrast, past the macula densa, female rats exhibit higher abundance and phosphorylation of NCC and more abundant ENaC and claudin-7 (88).
The central roles of NKCC2, NCC, and ENaC in Na+ balance and blood pressure regulation are underscored by the common use of their pharmacological inhibitors, such as loop diuretics, thiazide diuretics, and K+-sparing diuretics, such as amiloride, respectively, to treat hypertension (26). In addition to pharmacological inhibition, the activity of these transporters and of NHE3 may be also physiologically adjusted by endogenous agents, such as dopamine, endothelin, parathyroid hormone, ANG II, aldosterone, adenosine, and ATP (4, 22, 30, 62, 73, 84, 85). In the present study, we developed and applied sex-specific computational models of solute and water transport along the nephrons to investigate the extent to which inhibitors of Na+ transporters alter transepithelial solute transport and excretion and how these effects vary between the sexes.
METHODS
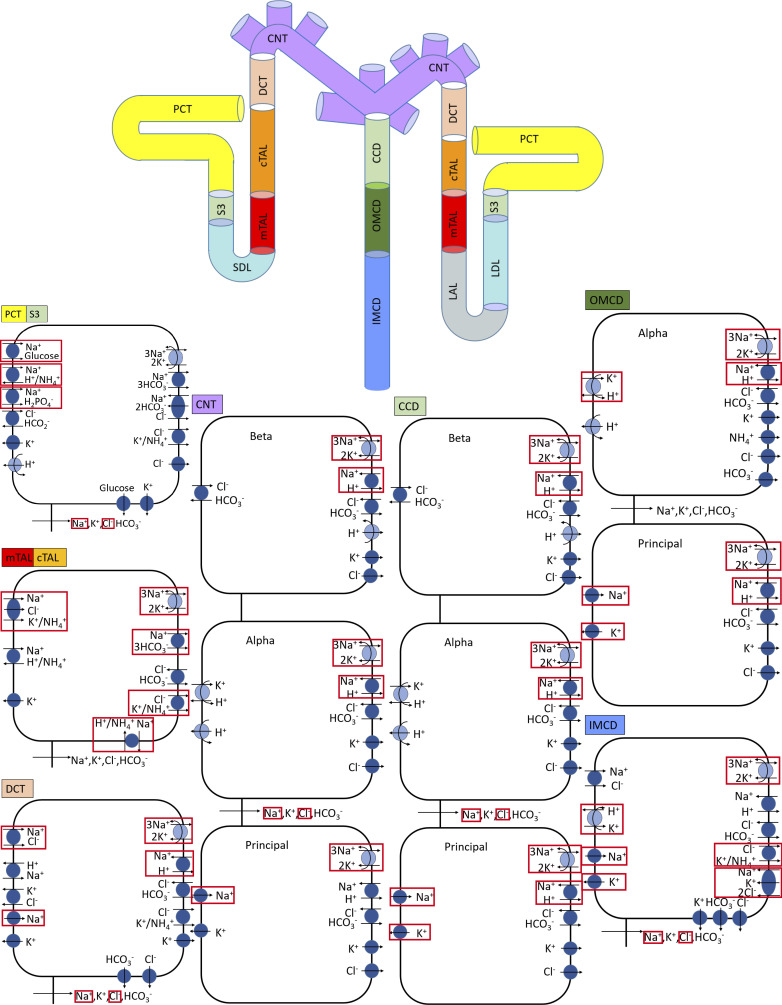
We previously developed an epithelial cell-based model of solute transport along different nephron populations of a rat kidney in a male rat (34). The model represents six classes of nephrons: a superficial nephron and five juxtamedullary nephrons. The superficial nephrons account for two-thirds of the nephron population, and they extend from Bowman's capsule to the papillary tip. The remaining one-third are juxtamedullary nephrons, which possess loops of Henle that reach to different depths in the inner medulla; most of the long loops turn within the upper inner medulla. Baseline single nephron glomerular filtration rate (SNGFR) is set to ∼30 and ∼45 nL/min for the superficial and juxtamedullary nephrons, respectively, in male rats and to ~24 and ~36 nL/min for the superficial and juxtamedullary nephrons, respectively, in female rats (discussed below). The transport capacity of the juxtamedullary proximal tubule is assumed to be 75% higher than that of the superficial proximal tubule, such that fluid flow into the S3 segment is predicted to be between 11 mL/min (for the superficial nephron) and 13 nL/min. The model accounts for the following 15 solutes: Na+, K+, Cl−, , H2CO3, CO2, NH3, , , , H+, , H2CO2, urea, and glucose. The model is formulated for the steady state and predicts luminal fluid flow, hydrostatic pressure, luminal fluid solute concentrations, and, with the exception of the descending limb segment, cytosolic solute concentrations, membrane potential, and transcellular and paracellular fluxes. A schematic diagram for the model is shown in Fig. 1.
Fig. 1.
Schematic diagram of the nephron system (not to scale). The model includes one representative superficial nephron and five representative juxtamedullary nephrons, each scaled by the appropriate population ratio. Only the superficial nephron and one juxtamedullary nephron are shown. Along each nephron, the model accounts for the transport of water and 15 solutes (see text). The distal tubule is divided into the early and late portions of the distal convoluted tubule (DCT). The diagram displays only the main Na+, K+, and Cl− transporters. Red boxes highlight model membrane transporters whose expressions differ between male and female subjects. PCT, proximal convoluted tubule; SDL, short or outer medullary descending limb; mTAL, medullary thick ascending limb; cTAL, cortical thick ascending limb; CNT, connecting tubule; CCD, cortical collecting duct; OMCD, outer-medullary collecting duct; IMCD, inner medullary collecting duct; LDL, thin descending limb; LAL, thin ascending limb.
In the present study, we developed an analogous multinephron model for the female rat. We followed the approach implemented in our recent studies (34, 47a), in which we formulated sex-specific epithelial cell-based models of solute transport along a single superficial nephron of the male and female rat kidney. The male and female multinephron models presented here account for sex differences in the expression levels of apical and basolateral transporters (67, 88), in SNGFR (55, 61), and in tubular dimensions (66).
The model is implemented in Python and can be accessed via GitHub.
Tubuloglomerular feedback.
SNGFR is determined, in part, by the tubuloglomerular feedback (TGF) response, which adjusts afferent arteriolar smooth muscle tone and, hence, SNGFR (7, 72). The TGF signal is based on the luminal fluid Cl− concentration near the macula densa, which corresponds to the model’s cortical thick ascending limb outflow Cl− concentration (denoted CMD). Specifically, the model assumes the following:
| (1) |
For each nephron, the reference SNGFR0 is initially taken to be its baseline SNGFR (see above). ΔQ is set to 10 nL/min; the operating point Cop is taken to be 40 and 80 mM, respectively, for the superficial and juxtamedullary nephrons in the male rat and to be 30 and 60 mM, respectively, for the superficial and juxtamedullary nephrons in the female rat. Note that the TGF operating points are assumed to have reset to lower values in female rats to account for the relatively lower Cl− delivery to the macula densa. The parameter m is taken to be 20 and 40 mM for the superficial and juxtamedullary nephrons, respectively, in both sexes.
Simulating NHE3 inhibition.
In two separate simulations, expression of NHE3 was inhibited by 50% and 80%. Our choice to simulate 80% rather than full inhibition of NHE3 is motivated by the presence of other amiloride-sensitive NHEs in the proximal tubule that may contribute significantly to TNa (2, 12), whereas NHE3 is the only NHE represented in the model. In addition to NHE3, NHE2 has been shown to be expressed in the apical membrane of the cortical and medullary thick ascending limbs (9, 82). Thus, in the 50% and 80% NHE3 inhibition simulations, we also inhibited NHE expression along the thick ascending limbs by 25% and 40%, respectively.
We assume that NHE3 was inhibited for a period of time long enough for washout and changes in interstitial osmolarity to occur but before the manifestation of compensatory mechanisms. The baseline parameters correspond to a rat kidney in a mild antidiuretic state, such that urine osmolality is 771 mosmol/(kg H2O). When NHE3 is inhibited, fluid delivery to the loops of Henle and collecting ducts increases (see NHE3 inhibition). The resulting significantly higher osmotic load presented to the concentrating mechanism is expected to overwhelm and impede the concentrating effect. Given these observations, we lowered the medullary interstitial concentrations of selected solutes in the NHE3 inhibition simulations; cortical interstitial concentration profiles were assumed to remain unaffected. For details, see Ref. 42.
Simulating NKCC2 inhibition.
NKCC2 is expressed on the apical membrane of the thick ascending limbs of the loops of Henle. We followed the procedures in Ref. 42 to simulate NKCC2 inhibition. We assumed that the NKCC2 inhibitor was administrated for long enough to significantly impair the kidney's ability to generate an axial osmolality gradient. Thus, as in the NHE3 inhibition simulations, we lowered the interstitial fluid concentrations of selected solutes; for details, see Ref. 42. In addition to the thick ascending limb cells, NKCC2 is also expressed in the apical membrane of macula densa cells. Indeed, NKCC2-mediated transport constitutes the initial step in the signaling pathway between the macula densa cells and afferent arteriole smooth muscle cells. Targeted deletion of NKCC2 significantly attenuates the TGF response (35, 58, 93). Thus, in our NKCC2 inhibition simulations, we assumed that SNGFR remained at baseline values, consistent with an experimental study in the rat (63).
Simulating NCC inhibition.
NCC is expressed on the apical membrane of the distal convoluted tubule. In the NCC inhibition simulations, baseline interstitial concentration profiles were used. All model parameters other than NCC activities remained at baseline values.
Simulating ENaC inhibition.
ENaC is expressed on the apical membrane of the last third of the distal convoluted tubules as well as the full length of the connecting tubules and collecting ducts. As in the NCC inhibition simulations, baseline interstitial concentration profiles and non-ENaC parameters were used.
RESULTS
Baseline results.
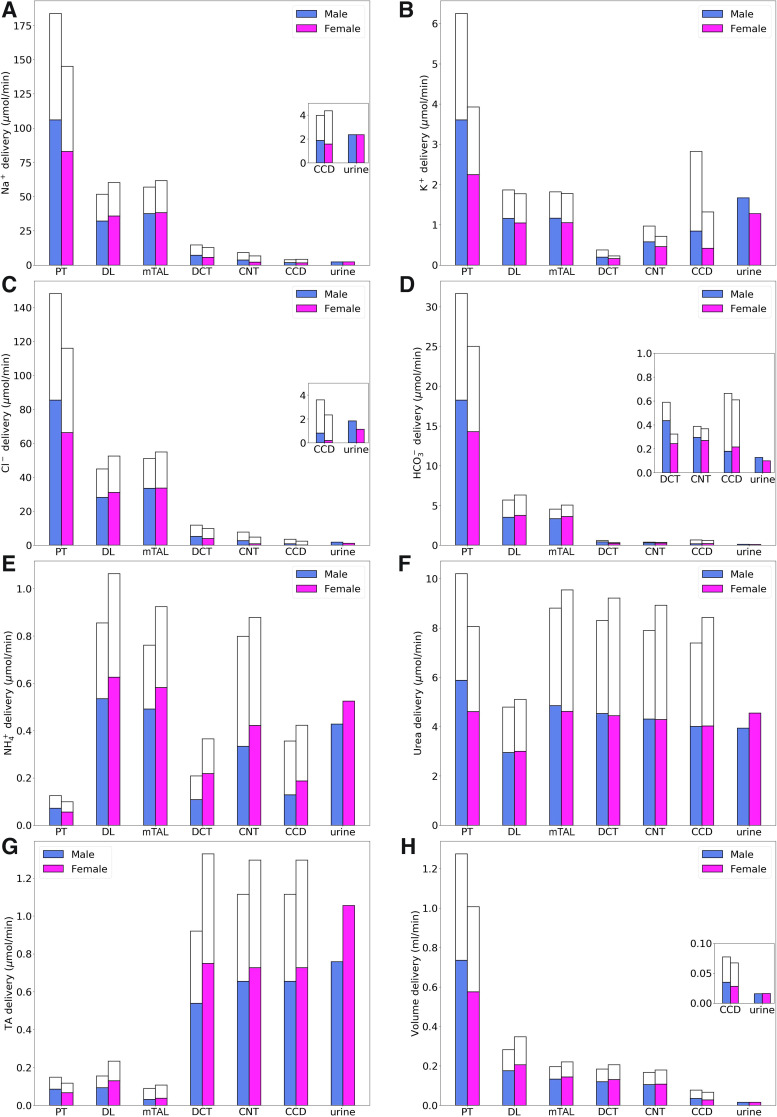
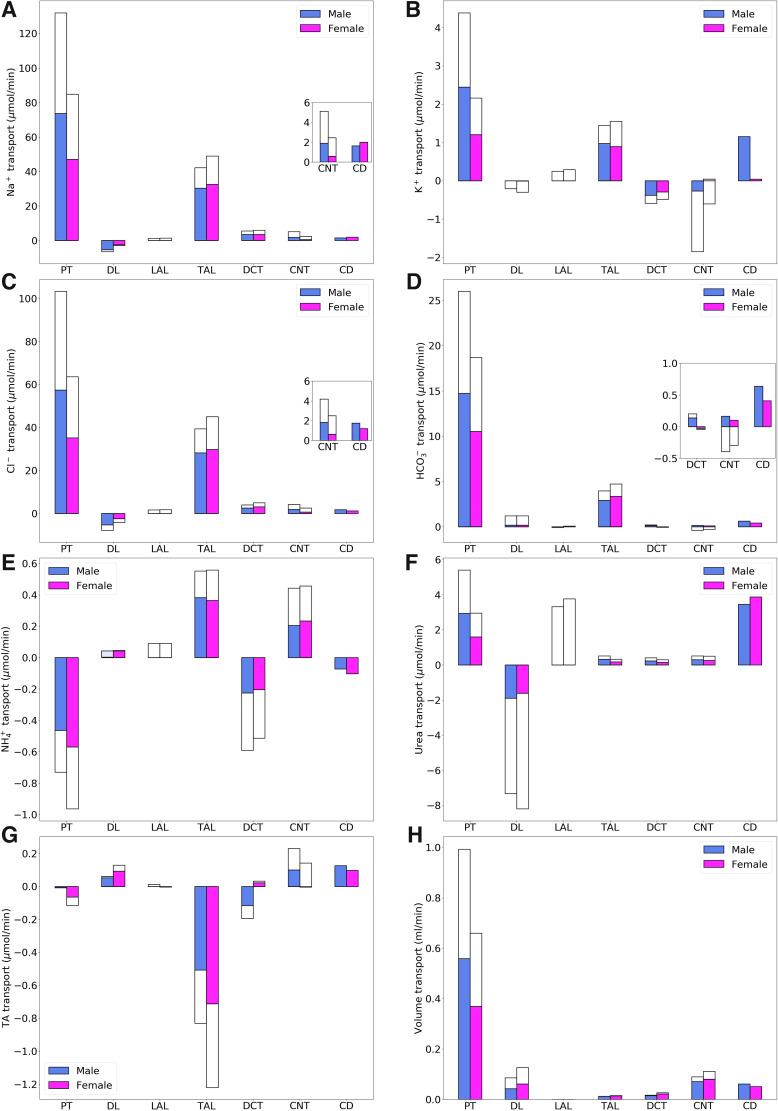
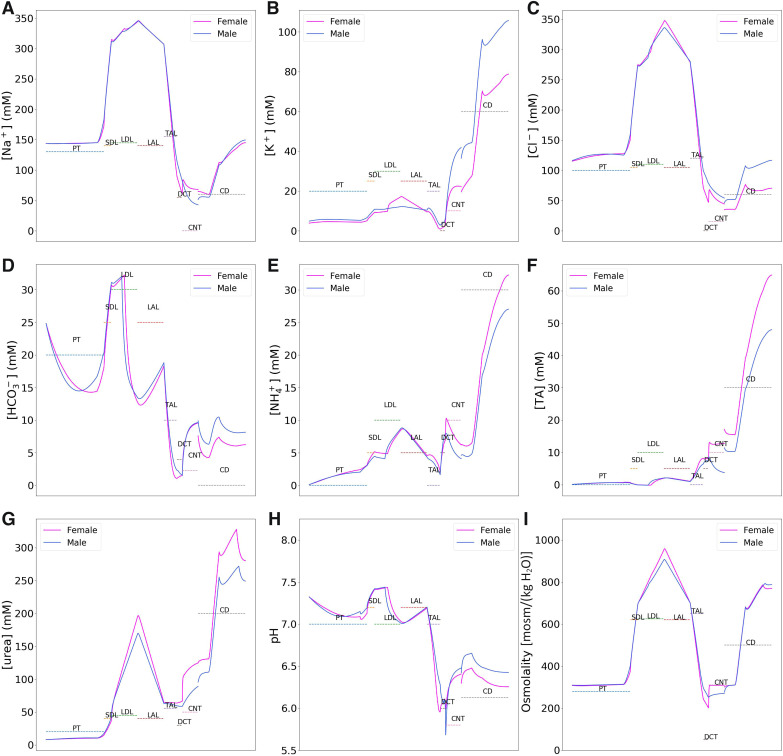
Using baseline parameters, we computed the model’s luminal fluid flow, luminal fluid solute concentrations, cytosolic solute concentrations, membrane potential, and fluxes. Figure 2 shows the predicted delivery of key solutes [Na+, K+, Cl−, , , urea, and titratable acid (TA)] and fluid to the inlets of individual superficial and juxtamedullary nephron segments, expressed per kidney, in the male and female models. TA concentration is computed from concentration and concentration. Recall that SNGFR in the female nephron is assumed to be 80% of that in the corresponding male nephron (24 vs. 30 nL/min for superficial nephrons and 36 vs. 45 nL/min for juxtamedullary nephrons); thus, solute delivery into the proximal tubules is lower in female rats. Recall also that there are twice as many superficial than juxtamedullary nephrons; thus, the smaller juxtamedullary contribution per kidney despite the higher SNGFR in the juxtamedullary nephrons. Solute and fluid transport rates along individual superficial and juxtamedullary nephron segments are shown in Fig. 3. Tubular fluid solute concentrations are shown in Fig. 4. Luminal fluid flow is shown in Fig. 5.
Fig. 2.
Delivery of key solutes (A–G) and fluid (H) to the beginning of individual nephron segments in male and female rats. Colored bars denote superficial nephron values; white bars denote juxtamedullary values, computed as weighed totals of the five representative model juxtamedullary nephrons. The model assumes a superficial-to-juxtamedullary nephron ratio of 2:1, thus, the superficial delivery values are generally higher. In each graph, the two bars for “urine” are identical since the superficial and juxtamedullary nephrons have merged at the cortical collecting duct (CCD) entrance. PT, proximal tubule; DL, descending limb; mTAL, medullary thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; TA, titratable acid. Insets: reproductions of distal segment values.
Fig. 3.
Net transport of key solutes (A–G) and fluid (H) along individual nephron segments in male and female rats. Colored bars denote superficial nephron values; white bars denote juxtamedullary values, computed as weighed totals of the five representative model juxtamedullary nephrons. Transport is taken to be positive out of a nephron segment. Insets: reproductions of distal segment values. PT, proximal tubule; DL, descending limb; LAL, thin ascending limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; TA, titratable acid.
Fig. 4.
Tubular fluid solute concentrations (A–G), pH (H), and osmolality (I) along the superficial nephrons of male and female rat models. Tubular fluid solute concentrations are similar in the two sexes along the proximal segments but diverge downstream of the thick ascending limbs. Concentration profiles along the juxtamedullary nephrons are similar to the corresponding superficial nephrons. PT, proximal tubule; SDL, short descending limb; LDL, thin descending limb; LAL, thin ascending limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; TA, titratable acid. Brackets denote concentration.
Fig. 5.
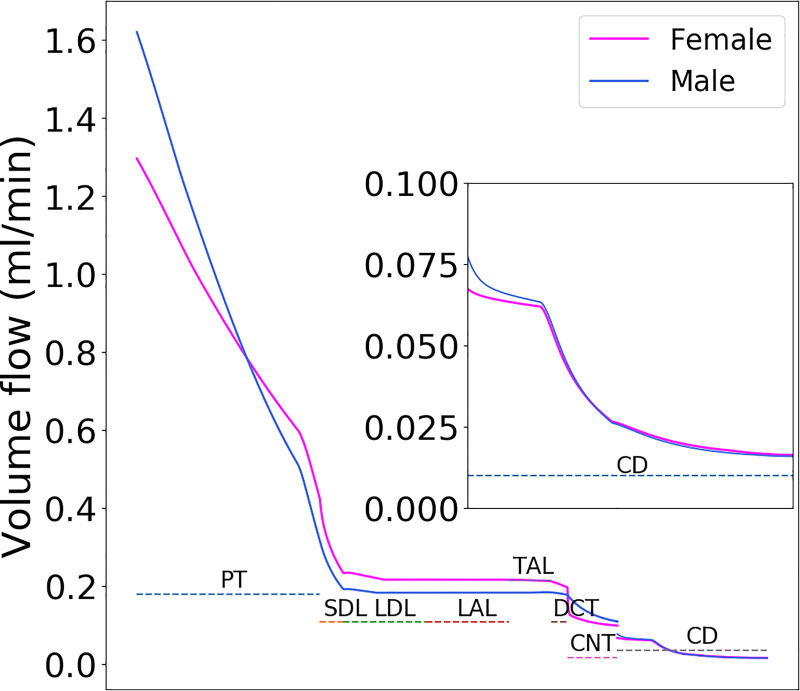
Tubular fluid flow along the superficial nephrons of male and female rat models. Flow profiles along the juxtamedullary nephrons are similar to the corresponding superficial nephrons. The single nephron glomerular filtration rate in the female rat is 80% that of the male rat. However, proximal tubular outflow is slightly higher in female rats because of its lower water reabsorption. That excess fluid is reabsorbed along the distal segments, so that urine output is similar in the two sexes. PT, proximal tubule; SDL, short descending limb; LDL, thin descending limb; LAL, thin ascending limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct.
The models predicted that Na+ and Cl− reabsorption is substantially lower in the proximal tubules of the female kidney model, which amounted to ~63% of the Na+ and Cl− reabsorbed in male kidney model (Fig. 3, A and C). This difference can be attributed to the smaller transport area and lower NHE3 activity assumed in both the superficial and juxtamedullary proximal tubules in the kidneys of female rats. In terms of the filtered loads, the model predicted that proximal tubules of male and female rats reabsorb 72% and 58%, respectively, of filtered Na+, primarily via NHE3 and Na+-K+-ATPase, and similar fractions of filtered Cl−. This reduced transport yields higher Na+ and Cl− flows at the proximal tubule outlet in female rats (Fig. 2, A and C). Thus, to yield a similar urinary excretion rate, downstream nephron segments in female rats must compensate with enhanced transport despite their smaller transport areas. For instance, although the thick ascending limbs of female rats have smaller transport areas, they are assumed to exhibit higher NKCC2, K+-Cl− cotransporter (KCC), and Na+-K+-ATPase activities (Table 1). Consequently, Na+ and Cl− reabsorption was predicted to be ~15% higher in the female thick ascending limb model compared with the male model (Figs. 4, A and C), with the increase in transport higher in the cortical segments. Urine excretion fractions were predicted to be 1.3% for Na+ and 1.2% for Cl− in the male kidney model and 1.6% for Na+ and 1% for Cl− in the female model. Urine Na+ excretion is comparable in the kidneys of male and female rats; Na+ excretion fraction differs between the sexes because of the lower filtration rate in female rats.
Table 1.
Key parameter differences between male and female rat nephron models
| Female-to-Male Ratio |
Female-to-Male Ratio |
||||
|---|---|---|---|---|---|
| Parameter | Superficial | Juxtamedullary | Parameter | Superficial | Juxtamedullary |
| PCT | S3 | ||||
| NHE3 activity | 0.83 | 0.83 | NHE3 activity | 0.83 | 0.83 |
| NaPi2 | 0.75 | 0.75 | NaPi2 | 0.75 | 0.75 |
| PNa, PCl (paracellular) | 0.4 | 0.4 | PNa, PCl (paracellular) | 0.4 | 0.4 |
| Pf (transcellular) | 0.64 | 0.64 | Pf (transcellular) | 0.64 | 0.64 |
| SGLT2 | 2.5 | 2.5 | SGLT2 | ||
| SDL | LDL | ||||
| PNa, PCl (transcellular) | 0.5 | PNa, PCl (transcellular) | 0.5 | ||
| Pf (transcellular) | 2 | Pf (transcellular) | 2 | ||
| xNaCl | 1.25 | ||||
| LAL | |||||
| PNa, PCl (transcellular) | 2 | ||||
| mTAL | cTAL | ||||
| NKCC activity | 2 | 2.5 | NKCC activity | 2 | 2.5 |
| KCC activity | 1.5 | 2.25 | KCC activity | 1.5 | 2.25 |
| Na+-K+-ATPase activity | 2 | 2 | Na+-K+-ATPase activity | 2 | 2 |
| Na+/H+ exchanger | 0.8 | 0.8 | Na+/H+ exchanger | 0.8 | 0.8 |
| Na+- cotransporter | 0.8 | 0.8 | Na+- cotransporter | 0.8 | 0.8 |
| Na+- cotransporter | 0.8 | 0.8 | Na+- cotransporter | 0.8 | 0.8 |
| PNa, PCl (paracellular) | 0.9 | 0.9 | PNa, PCl (paracellular) | ||
| DCT | CNT | ||||
| NCC activity | 1.6 | 1.6 | NCC activity | ||
| Na+-K+-ATPase activity | 2 | 2 | Na+-K+-ATPase activity | 2 | 2 |
| Na+/H+ exchanger | 0.85 | 0.85 | Na+/H+ exchanger | 0.9 | 0.9 |
| Na+- cotransporter | 0.85 | 0.85 | Na+- cotransporter | 0.9 | 0.9 |
| ENaC activity | 2 | 2 | ENaC activity | 1.3 | 1.3 |
| PNa, PCl (paracellular) | 1.4 | 1.4 | PNa, PCl (paracellular) | 1.4 | 1.4 |
| Pf (transcellular) | 2 | 2 | Pf (transcellular) | 1.5 | 1.5 |
| CCD | OMCD | ||||
| Na+-K+-ATPase activity | 1.5 | Na+-K+-ATPase activity | 1.1 | ||
| H+-K+-ATPase activity | 1 | H+-K+-ATPase activity | 1.5 | ||
| Na+/H+ exchanger | 0.9 | Na+/H+ exchanger | 0.9 | ||
| Na+- cotransporter | 0.9 | Na+- cotransporter | 0.9 | ||
| ENaC activity | 1.5 | ENaC activity | 1.2 | ||
| PNa, PCl (paracellular) | 1.4 | PNa, PCl (paracellular) | 1 | ||
| PK (transcellular) | 0.7 | PK (transcellular) | 1.2 | ||
| Pf (transcellular) | 2 | Pf (transcellular) | 2 | ||
| IMCD | Morphology | Superficial | Juxtamedullary | ||
| Na+-K+-ATPase activity | 1.2 | PCT, S3 | |||
| H+-K+-ATPase activity | 1.5 | Length | 0.8 | 0.8 | |
| KCC | 1.2 | Diameter | 0.8 | 0.8 | |
| NKCC1 activity | 0.6 | Distal segments | |||
| PNa, PCl (paracellular) | 0.5 | Length | 0.85 | 0.85 | |
| PK (transcellular) | 2 | Diameter | 0.85 | 0.85 | |
| PNa (transcellular) | 0.5 | ||||
| Pf (transcellular) | 2 | Hemodynamics | |||
| xurea | 0.89 | SNGFR | 0.8 | 0.8 | |
PCT, proximal convoluted tubule; NHE3, Na+/H+ exchanger 3; NaPi2, Na+-Pi cotransporter 2; PNa, Na+ permeability; PCl, Cl− permeability; Pf, fluid permeability; xNaCl, fractional length of the NaCl-permeable initial LDL outer medullary segment; SGLT2, Na+-glucose cotransporter 2; SDL, short or outer medullary descending limb; LAL, thin ascending limb; mTAL, medullary thick ascending limb; NKCC, Na+-K+-2Cl− cotransporter; KCC, K+-Cl− cotransporter; DCT, distal convoluted tubule; NCC, Na+-Cl− cotransporter; ENaC, epithelial Na+ channel; CCD, cortical collecting duct; IMCD, inner medullary collecting duct; PK, K+ permability; Purea; urea permeability; xurea, fractional length of initial IMCD segment with low urea permeability; LDL, thin descending limb; cTAL, cortical thick ascending limb; CNT, connecting tubule; OMCD, outer medullary collecting duct; SNGFR, single nephron glomerular filtration rate.
Filtered K+ in the kidneys of female rats is only 63% that of males due to the female rat’s lower plasma K+ concentration (3.9 vs. 4.9 mM) and SNGFR. The model predicted that the amount of K+ reabsorbed by the female proximal tubules is only 49% of male proximal tubules (Fig. 4B). In terms of filtered K+ loads, the model predicted that in male rats, 70% of the filtered K+ is reabsorbed along the proximal tubules compared with 55% in female rats; the majority of the remaining K+ is reabsorbed along the thick ascending limb. Downstream of Henle’s loop, the connecting tubules in this model were assumed to vigorously secrete K+. The connecting tubules in the kidneys of the male rat model were predicted to secrete twice as much K+ compared with the female rat model. This can be attributed, in part, to the higher Na+ flow in the connecting tubules in male rats, which results in a higher Na+ reabsorption rate and creates a more favorable transmembrane electrochemical gradient to drive K+ secretion. Because of their longer length, juxtamedullary connecting tubules secrete substantially more K+ than superficial segments. Eventually, 27% of the filtered load of K+ in male rats and 33% in female rats is excreted.
In both sexes, the proximal tubule is a major site of secretion via substitution of H+ in the NHE3 transporter and secretion of NH3. In contrast, a substantial fraction of is reabsorbed along the thick ascending limbs by substituting for K+ in NKCC2. Along the connecting tubules and collecting ducts, parallel secretion of ammonia and H+ increases, without significant transmembrane transport of . excretion is predicted to be 3.4-fold of its filtered load in male rats versus 5.3-fold in female rats (Fig. 2E).
The model predicted that 78% of the filtered volume is reabsorbed along the proximal tubules in male kidneys and 66% in female kidneys (Fig. 3H). Water reabsorption along the proximal tubules also drives paracellular urea reabsorption in that segment. More water is reabsorbed downstream, along the descending thin limbs, connecting tubules, and collecting ducts, albeit at a slower rate. Urea is reabsorbed along downstream segments as well, except the descending limbs of the loops of Henle, where urea enters as a result of the presumed higher interstitial urea concentration. Taken together, 48% of the filtered urea is reabsorbed along the collecting ducts in female kidneys compared with 34% in male kidneys (Fig. 3F).
NHE3 inhibition.
We examined the effects of NHE3 inhibition on tubular transport. The baseline models predicted that NHE3 mediates ∼35% of renal TNa in both sexes. In two separate sets of simulations, NHE3 activity was inhibited by 50% and 80%.
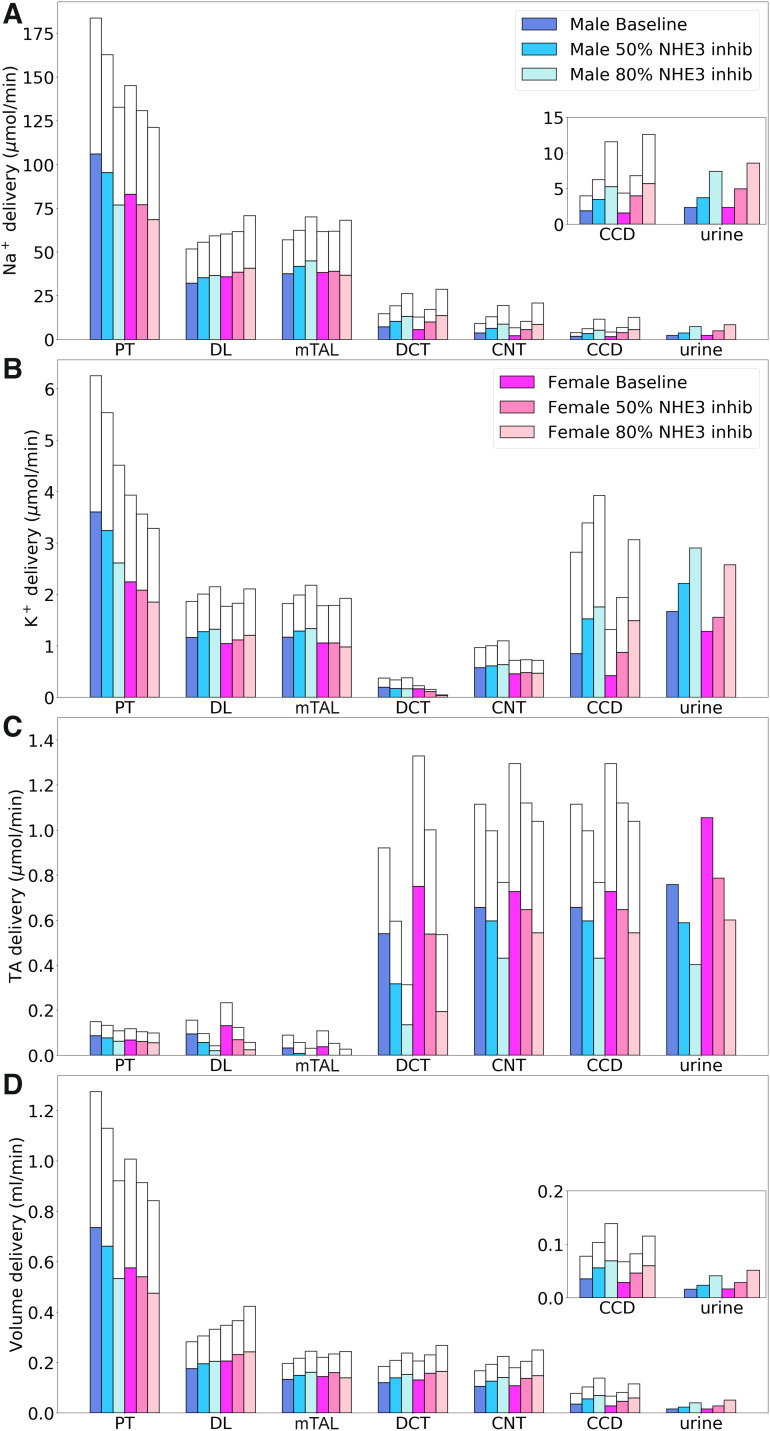
The predicted segmental deliveries of Na+, K+, TA, and fluid are shown in Fig. 6. The effects of inhibiting NHE3 in the kidney of male rats have been described in Ref. 42 and are summarized below. NHE3 inhibition resulted in a significant decrease in TNa and in Cl− and water reabsorption along the proximal tubules. In the baseline male kidney, proximal convoluted tubule fractional Na+ reabsorption was predicted to be 65%. That dropped to 59% with 50% NHE3 inhibition and to 48% with 80% inhibition. Similar decreases in Cl− and water reabsorption were predicted. The resulting larger salt load on the thick ascending limbs increased macula densa Cl− concentration, thereby activating TGF and lowering SNGFR. Despite the reduction in SNGFR, the delivery of Na+, K+, Cl−, and water to distal nephron segments generally increased as NHE3 was progressively inhibited. The competing effects of elevated Na+ load and reduced NHE3 expression in the thick ascending limbs resulted in minimal changes in their TNa. The higher fluid flow along the connecting tubule increased K+ secretion, resulting in substantially higher K+ delivery to the cortical collecting duct. Consequently, kaliuresis was predicted with the inhibition of NHE3. As NHE3 was progressively inhibited, TA delivery to all nephron segments, as well as net acid excretion, decreased, resulting in a slight alkalization of urine.
Fig. 6.
Comparison of solute delivery (A–C) and fluid delivery (D) obtained for the base case and for 50% and 80% inhibition of Na+/H+ exchanger 3 (NHE3). Colored bars show superficial nephron values; white bars show juxtamedullary values. Delivery values are computed per kidney. Insets: reproductions of distal segment values. PT, proximal tubule; DL, descending limb; mTAL, medullary thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; TA, titratable acid.
NHE3 inhibition induces a stronger natriuretic and diuretic response in the kidneys of female rats. We assume that the TGF response (feedback gain; Eq. 1) is the same in male and female rats; consequently, both models predicted a similar proportional reduction in SNGFR as NHE3 was progressively inhibited. Because of the lower baseline TNa capacity in female rats, the further reduction in NHE3-mediated transport along the proximal tubule and thick ascending limb overwhelms Na+ reabsorption more so in female rats compared with male rats. That difference, which can be seen in Na+ delivery to the distal convoluted tubule, is almost imperceptible compared with the filtered load (Fig. 6A). Nonetheless, because urinary Na+ excretion is ~1% of filtered load, the difference in proximal transport manifests as a 112% increase in Na+ excretion in females rats versus 57% increase in male rats above baseline when NHE3 is inhibited by 50% and a 263% increase in female rats and 213% increase in male rats at 80% NHE3 inhibition. Analogous results were predicted for urine output (Figs. 6D).
NKCC2 inhibition.
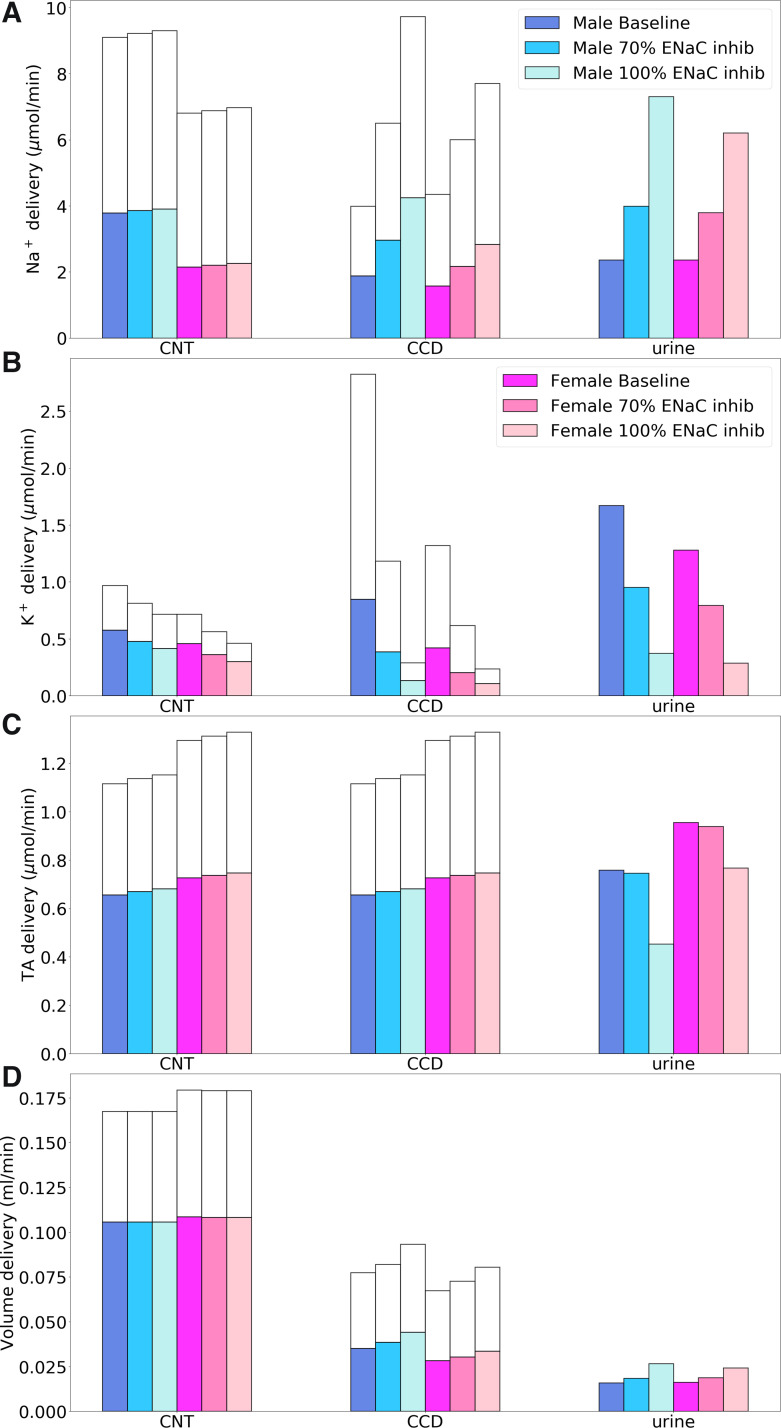
Next, we simulate 70% and 100% inhibition of NKCC2, expressed in the apical membrane of the thick ascending limbs of the loops of Henle. Under baseline conditions, NKCC2 was predicted to mediate 30% of renal TNa in female rats compared with 22% in male rats. The resulting segmental deliveries of Na+, K+, TA, and fluid are shown in Fig. 7.
Fig. 7.
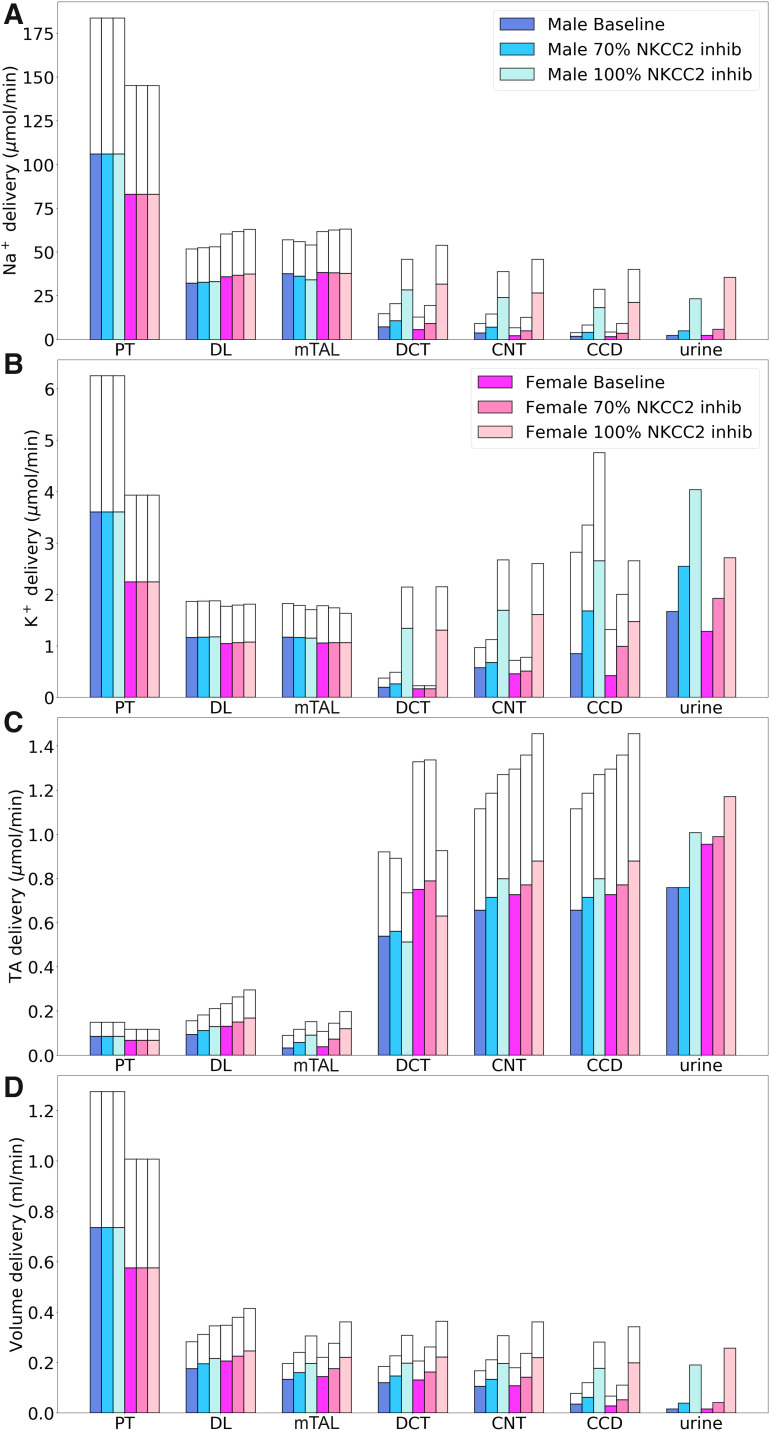
Comparison of solute delivery (A–C) and fluid delivery (D) obtained for the base case and for 70% and 100% inhibition of Na+-K+-2Cl− cotransporter (NKCC2). Colored bars show superficial nephron values; white bars show juxtamedullary values. Delivery values are computed per kidney. PT, proximal tubule; DL, descending limb; mTAL, medullary thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; TA, titratable acid.
Inhibition of NKCC2 had no direct impact on upstream TNa along the proximal tubule. As previously noted, the sensing of NaCl flow by macula densa cells was blocked by NKCC2 inhibition, so TGF was not activated. Thick ascending limb TNa fell similarly in both sexes when NKCC2 was inhibited: by 16% and 12% in male and female rats when NKCC2 was inhibited and by 70% and ~80% in both sexes when NKCC2 was inhibited 100% (Fig. 7). In the 70% inhibition case, an increase in the driving force across NKCC2 (due to a reduction in intracellular concentrations) partly compensated for the decrease in NKCC2 activity. Complete NKCC2 inhibition generated a greater natriuretic effect in female compared with male rats (14- vs. 9-fold increase). In addition, substantially less K+ and Cl− were reabsorbed along the thick ascending limbs in both sexes (see Fig. 7). In particular, inhibition of NKCC2 decreases K+ exit through ROMK. Consequently, with complete NKCC2 inhibition, urinary K+ excretion increased to 2.4-fold in male rats and 2-fold in female rats.
NCC inhibition.
In the next set of simulations, we examined the effects of inhibiting NCC by 70% and 100% on tubular transport. Distal segmental deliveries of Na+, K+, TA, and fluid obtained for differing levels of NCC inhibition are shown in Fig. 8. NCC inhibition has no effect on segments upstream of the distal convoluted tubule. In both sexes, inhibition of NCC had stronger effects on Na+ and K+ reabsorption than on TA along the distal tubular segments, which can be inferred the results shown in Fig. 8, A–C. At full NCC inhibition, distal convoluted tubule TNa decreased 62% in the male model and 77% in the female model. These fractions underestimate the baseline contribution of NCC to the overall distal convoluted tubule TNa, because inhibition of NCC raises distal convoluted tubule Na+ delivery and luminal fluid Na+ concentraiton, thereby increasing the driving force for Na+ reabsorption through other apical Na+ transporters in that segment, namely, NHE2 and ENaC (in the the late portion of the distal convoluted tubule) and Na+-driven Cl−/2 exchanger NDCBE (77). The augmented Na+ flux across ENaC, in turn, generates a more favorable electrochemical gradient for K+ secretion. As a result, K+ flow into the cortical collecting ducts increases by 42% in male rats and 122% in female rats when NCC was fully inhibited. In contrast, the impact on urinary pH was negligible.
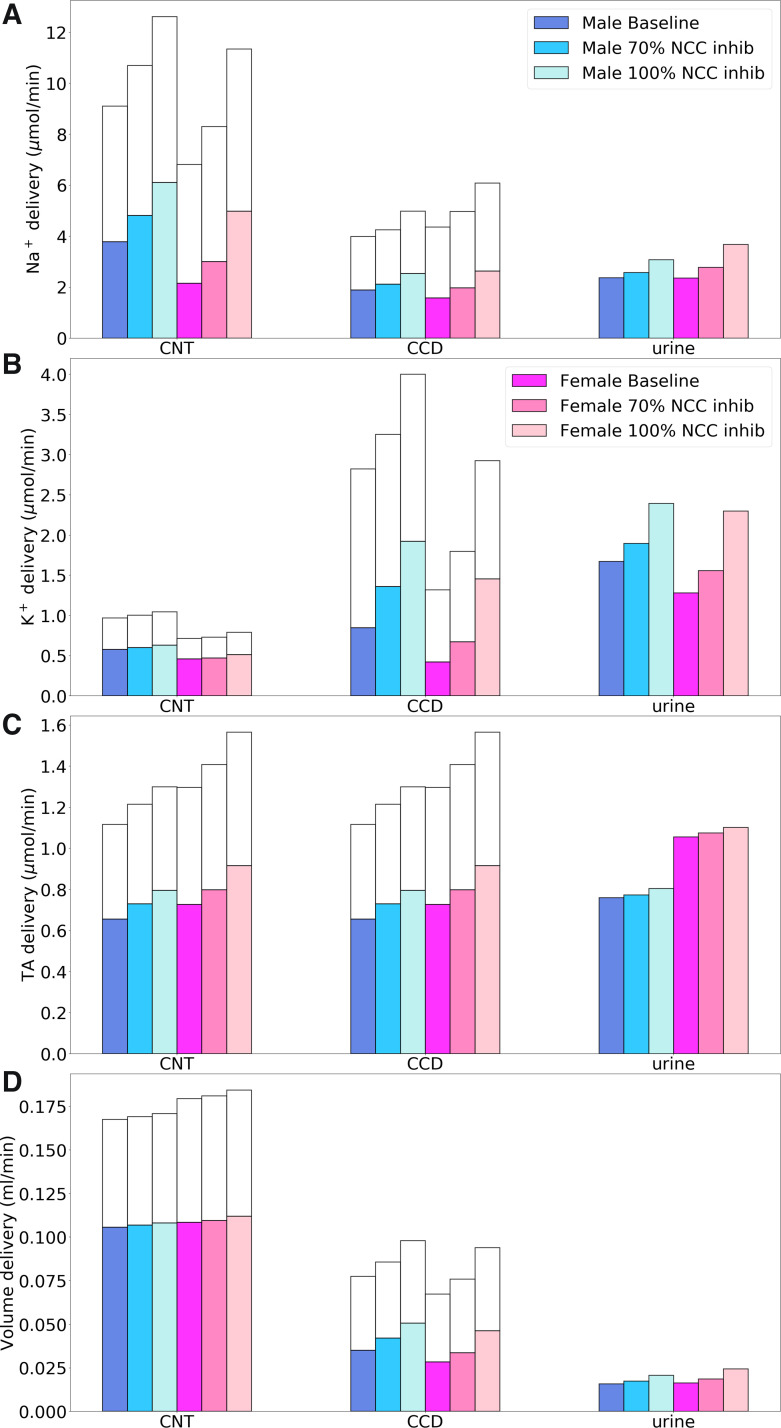
Fig. 8.
Comparison of solute delivery (A–C) and fluid delivery (D) obtained for the base case and for 70% and 100% inhibition of Na+-Cl− cotransporter (NCC). Colored bars show superficial nephron values; white bars show juxtamedullary values. Delivery values are computed per kidney. CNT, connecting tubule; CCD, cortical collecting duct; TA, titratable acid.
The model predicted a stronger natriuretic and diuretic response in female rats than in male rats. Overall, the model predicted that 70% inhibition of NCC increases urinary Na+ excretion by 9% in the male model compared with 18% in the female model, whereas 100% inhibition increases urinary Na+ excretion by 30% in the male model compared with 56% in the female model. It is noteworthy that the impact of NCC inhibition is larger in female subjects, a sex difference that may be attributed to the higher abundance of NCC and the larger fraction of the filtered Na+ being reabsorbed along the distal tubular segments in female subjects compared with male subjects. The effect on TNa is mirrored in Cl− (results not shown) and water transport. At full inhibition, K+ excretion was predicted to increase by 43% in male rats and more markedly by 79% in female rats. Net acid excretion at 100% inhibition increased slightly from the baseline value of 0.76 to 0.8 μmol/min in male rats and from 1.06 to 1.1 μmol/min in female rats.
ENaC inhibition.
ENaC is expressed on the apical membrane of the last third of the distal convoluted tubules as well as the full length of the connecting tubules and collecting ducts. Segmental deliveries of Na+, K+, TA, and fluid with 70 and 100% ENaC inhibition are shown in Fig. 9. Under baseline conditions, ENaC mediated ∼7% and 9% of renal TNa in male and female rats, respectively. Most of the ENaC-mediated TNa occurs along the connecting tubules. But because transcellular Na+ reabsorption is accompanied by substantial paracellular Na+ secretion (70), overall connecting tubule TNa accounted for <3% of renal TNa in both sexes. Similarly, ENaC-mediated TNa along the collecting duct exceeded total TNa along that segment owing to paracellular Na+ secretion. At the whole kidney level, paracellular Na+ secretion into these two segments was predicted to equal ∼5% of filtered Na+. Along the distal convoluted tubule, ENaC-mediated TNa accounted for only a minor fraction (<10%) of segmental transcellular TNa. Because ENaC mediated more TNa in male than female rats due to higher filtered load in male rats, its inhibition increased urinary Na+ excretion more in male (209%) than female rats (163%).
Fig. 9.
Comparison of solute delivery (A–C) and fluid delivery (D) obtained for the base case and for 70% and 100% inhibition of epithelial Na+ channel (ENaC). Colored bars show superficial nephron values; white bars show juxtamedullary values. Delivery values are computed per kidney. CNT, connecting tubule; CCD, cortical collecting duct; TA, titratable acid.
ENaC inhibition had a major impact on connecting tubule Na+ transport: when ENaC was inhibited by 70%, its active TNa decreased by 29% in both sexes; when ENaC was fully inhibited, TNa decreased by 67% in male rats and by 60% in female rats (Fig. 9). The fractional reduction in active TNa was significantly lower than the percentage by which ENaC activity was inhibited. The decrease in Na+ reabsorption during 70% ENaC inhibition elevates luminal Na+ concentration and favors its entry into the cell. That is, the increase in the driving force across ENaC partially compensates for the decrease in the activity of the transporter. Another notable effect of higher luminal Na+ concentration is the attenuation of paracellular Na+ secretion. Compared with baseline, when ENaC was fully inhibited, paracellular Na+ secretion decreased by 86% in male rats and 68% in female rats. Analogous results were obtained for the collecting duct.
Additionally, ENaC inhibition lowered K+ secretion in connecting tubules and cortical collecting ducts where Na+ reabsorption generates the driving force (specifically, the apical transmembrane potential difference) that drives K+ secretion. Most notably, total K+ secretion into the connecting tubule lumen decreased from the baseline values of 1.9 and 0.6 μmol/min per kidney in male and female rats, respectively, to a small amount of reabsorption (∼0.4 and 0.2 μmol/min) when ENaC was completely inhibited (Fig. 9). With 100% ENaC inhibition, the urinary K+ excretion rate decreased by 78% in both sexes. The substantially lower luminal K+ concentration, in turn, reduced the activity of H+-K+-ATPase pumps, which decreased H+ secretion. Consequently, at 100% inhibition, urinary pH increased from 6.4 (baseline) to 6.9 and 6.6 in male and female rats, respectively. Net acid excretion decreased from baseline values of 1.1 to 0.02 μmol/min in male rats at 100% inhibition and from 1.4 to 0.63 μmol/min in female rats.
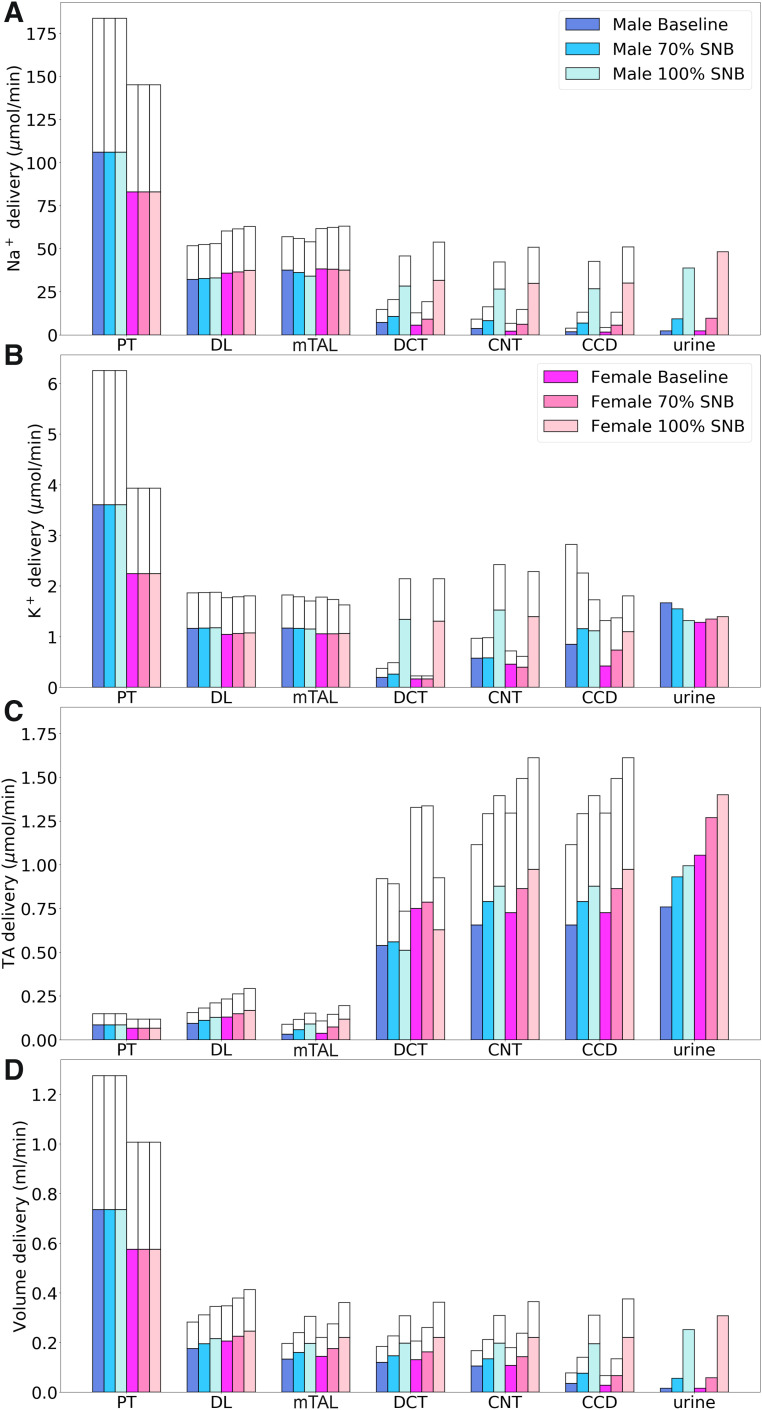
Sequential nephron blockade.
We examined the effects of simultaneously inhibiting NKCC2, NCC, and ENaC by 70% and 100% on tubular transport. Such sequential nephron blockade lowers TNa along the thick ascending limbs, distal convoluted tubules, connecting tubules, and collecting ducts. Segmental deliveries of Na+, K+, TA, and fluid obtained for differing levels of blockade are shown in Fig. 10. In large part, because of NKCC2 inhibition, sequential nephron blockade resulted in substantial increases in Na+ excretion and urine output, especially in female rats. Taken in isolation, inhibition of NKCC2 and NCC increased K+ excretion, whereas ENaC inhibition had the opposite effect. Together, sequential nephron blockade had a negligible effect on K+ excretion. The competing effects on TA excretion of NKCC2 inhibition (increasing) and ENaC inhibition (decreasing) yielded a reduction in urine pH.
Fig. 10.
Comparison of solute delivery (A–C) and fluid delivery (D) obtained for the base case and for 70% and 100% sequential nephron blockade (SNB; simultaneous inhibition of Na+-K+-2Cl− cotransporter, Na+-Cl− cotransporter, and epithelial Na+ channel). Colored bars show superficial nephron values; white bars show juxtamedullary values. Delivery values are computed per kidney. PT, proximal tubule; DL, descending limb; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; TA, titratable acid.
DISCUSSION
What is the physiological implication of the differences in renal transporter distribution between female and rat male nephrons: lower Na+ and water transporters and lower fractional reabsorption in the proximal nephron of female versus male rats, coupled with more abundant transporters in renal tubule segments downstream of the macula densa (88)? Fine control of Na+ excretion is accomplished by the tight regulation of TNa in distal tubular segments (24). Indeed, a decrease in TNa in the proximal nephron segments (i.e., proximal tubule and loop of Henle) can be compensated by changes in transport in the distal nephron, possibly coupled with appropriate adjustments of GFR (49, 57). The model predicted that, together, TNa by the distal convoluted tubule, connecting tubule, and collecting duct amounts to 8.2% of filtered Na+ in male rats and 9.1% in female rats. However, given that ~1% of the filtered Na+ is excreted, distal segmental TNa represents several times the daily Na+ intake (24).
TNa along the distal nephron is exquisitely regulated, and its larger contribution to total renal TNa in female subjects may better prepare them for the fluid retention adaptations required of pregnancy and lactation. Veiras et al. (88) reported that female rats excrete an acute Na+ load faster than male rats. Specifically, given the same saline bolus volume relative to body weight, diuretic and natriuretic responses were more rapid in female rats, with female rats excreting ~30% and male rats <15% of the saline bolus at 3 h. The more robust natriuresis in response to a saline challenge in female rats is consistent with the rightward shift of the pressure-natriuresis curve in male versus female rodents (53).
The lower proximal fractional reabsorption suggests that female rats will maintain salt balance at a lower blood pressure when faced with antinatriuretic stimuli. In a recent study, Veiras et al. (89) compared the effect of chronic ANG II-induced hypertension in male and female rats. Despite differences in transporter profiles and physiological responses at baseline, the relative responses of transporters in female and male rats were similar during ANG II infusion, specifically, increased abundance and activation of cortical NKCC2, NCC, and ENaC, counteracted by reduced abundance of NHE3, NKCC2, and Na+-K+-ATPase along the proximal tubule and medullary thick limb. In addition, the ANG II-provoked increase in blood pressure was also similar between female and male rats, providing no support for a “female advantage.” The authors noted that a lower blood pressure in female versus male rats during ANG II may emerge at lower doses or rates of infusion (89).
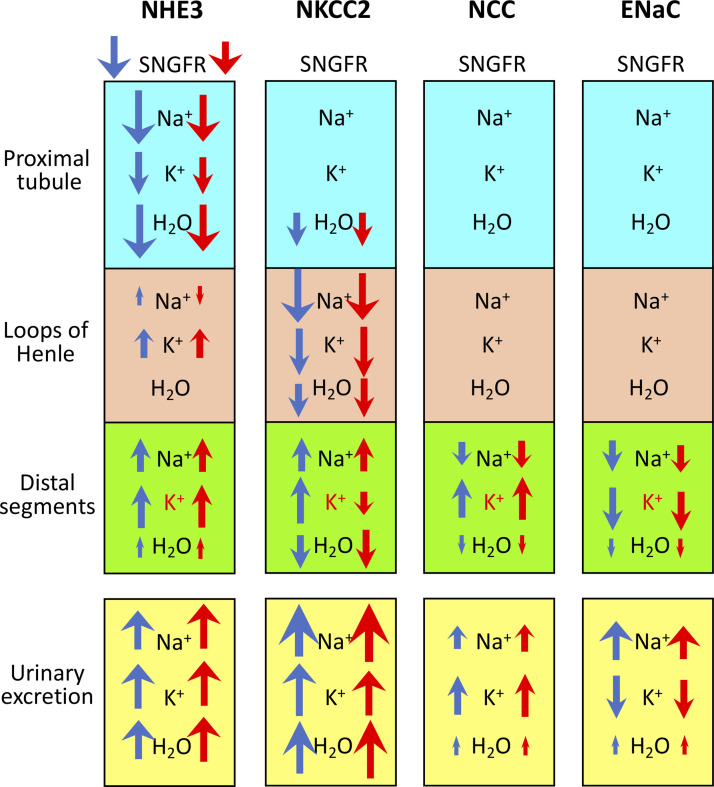
We conducted model simulations to investigate the extent to which inhibitors of transepithelial TNa along the nephron alter tubular transport and urinary solute excretion and how those effects may vary between male and female rats. A summary of the model results is shown in Fig. 11.
Fig. 11.
Effects of inhibiting Na+/H+ exchanger 3 (NHE3), Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and epithelial Na+ channel (ENaC) on single nephron glomerular filtration rate (SNGFR), segmental Na+, K+, and water transport, and urinary excretion. Distal segments include the distal convoluted tubule, connecting tubule, and collecting duct. The size of the arrow roughly corresponds to the relative change from baseline but is not exactly to scale and can be used only to compare excretion or transport of the same solute in the same segment. Up arrows denote increases in reabsorption (which would reduce excretion), except for distal segmental K+ transport, which is in red to denote secretion. Changes in urinary excretion are a result of changes in SNGFR and segmental transport.
NHE3 inhibition.
NHE3 is expressed in the apical membrane of the proximal tubule, where the majority of renal TNa occurs. NHE3 is also critical for TNa in the intestine (71). In part because of the role of NHE3 in the intestine for TNa and volume regulation, whole body NHE3 knockout mice have substantial challenges with Na+ homeostasis and suffer from severe diarrhea, dilation of the intestinal tract, and a high mortality rate subsequent to weaning (71).
In micropuncture experiments performed on adult male Munich-Wistar-Frömter rats, Vallon et al. (86) inhibited NHE3 and observed a dose-dependent inhibition of reabsorption, with a maximum reduction of ∼30% in fractional fluid and Na+ reabsorption along the superficial proximal convoluted tubule. In the baseline scenario of the male model, proximal convoluted tubule fractional Na+ reabsorption was predicted to be 65%, primarily via NHE3 and Na+-K+-ATPase. A 30% reduction would lower TNa to 46% reabsorption, which is close to the 48% reabsorption predicted by 80% inhibition of NHE3 expression (Fig. 6). For the female model, baseline proximal convoluted tubule TNa was 52% of filtered Na+. With 80% inhibition of NHE3, the female model predicted that TNa would decrease to 35%, which corresponds to 57% of baseline TNa (Fig. 6).
Fenton et al. (16) generated renal tubule-specific NHE3 knockout mice (Pax8-Cre), where NHE3 abundance was completely absent in S1, S2, and S3 segments of the proximal tubule and was severely reduced (>85%) in the thick ascending limb. Interestingly, deletion of NHE3 in male NHE3loxloxCre mice results in a tubular Na+ transporter pattern similar to female rats (88), albeit less pronounced, including downregulation of NaPi2, accompanied by upregulation of NCC and ENaC. Fluid intake, urinary flow, urinary Na+/creatinine, and pH are significantly elevated in NHE3loxloxCre mice, whereas urine osmolality and GFR are significantly lower (17).
NKCC2 inhibition.
NKCC2 regulates TNa along the thick ascending limb of Henle's loop and is important in the control of Na+ balance, urine-concentrating ability, and renin release. With the lower TNa capacity in the proximal tubule of the female nephron, fractional Na+ delivery to the thick ascending limb is significantly higher. Veiras et al. (88) did not observe statistically significant higher abundance of NKCC2 in either the medullary or cortical thick ascending limbs. Musselman et al. (56) reported a higher abundance of NKCC2 in intact female compared with male Sprague-Dawley rats, and that difference was suppressed by ovariectomy. In our model simulations, we found it necessary to enhance NKCC2 activity along these segments, to compensate for the lower upstream TNa. That assumption may reflect flow-mediated increase in thick ascending limb TNa or trafficking between apical and subapical pools (1, 54).
Model simulations predicted that complete inhibition of NKCC2 would increase urinary Na+ excretion by nine-fold in male rats (Fig. 7), following a three-fold increase in early distal delivery of Na+. An approximately four-fold increase in early distal Na+ delivery was reported after administration of furosemide in a micropuncture study performed on male Sprague-Dawley rats (33). The present model predicted an even greater furosemide-induced natriuretic effect in female rats, at 14-fold of baseline. That sex difference is consistent with a furosemide study in Wistar rats (5) and reflects the larger role of NKCC2 in Na+ reuptake in female rats. Full NKCC2 inhibition is analogous to type 1 Bartter syndrome in humans (29), which is characterized by markedly reduced or absent salt transport by the thick ascending limbs. Besides severe renal salt wasting and lowered blood pressure, individuals with Bartter syndrome also exhibit hypokalemia and metabolic alkalosis, consistent with the model’s prediction of elevated K+ and net acid excretion (Fig. 7).
NCC inhibition.
In the distal convoluted tubule, Na+, Cl−, and K+ reabsorption is fine tuned, in part, by NCC on the apical membrane, the activity of which is critical for regulation of blood pressure and K+ homeostasis. NCC is the target of the thiazide-type diuretics (13), which are the first-line pharmacological therapy for the management of arterial hypertension. The essential role of NCC activity in the homeostasis of salt, K+, Ca2+, and acid-base is evinced by the diseases that result from NCC dysfunction. Loss-of-function mutations of the SLC12A3 gene (NCC) result in Gitelman syndrome, which is associated with hypotension, hypokalemia, metabolic alkalosis, and hypocalciuria (76). A mirror-image disease is pseudohypoaldosteronism type II, which is a result of NCC activation by mutant kinases (92) or ubiquitin ligases (50), and it is associated with hypertension, hyperkalemia, metabolic acidosis, and hypercalciuria.
Our model predicted that complete inhibition of NCC substantially increases urinary flow, raises urinary excretion of Na+ by about half, approximately doubles Cl− excretion, and substantially increases K+ excretion (~40% in male rats and ~80% in female rats). The increase in salt excretion is consistent with, but lower than, the response measured in Sprague-Dawley rats following the administration of bendroflumethiazide (11) or chlorothiazide (38). That attenuated predicted response may be attributable to the NCC-independent actions of chlorothiazide on carbonic anhydrase in the proximal tubule (38). The model predicted a stronger natriuretic and diuretic response in female subjects than male subjects, consistent with findings in Sprague-Dawley rats (11) and in mice (48). Bendroflumethiazide has been found to decrease the urinary excretion of Ca2+ significantly in female Sprague-Dawley rats, but had no such effect in male Sprague-Dawley rats (11). The hypocalciuric effect of the chronic administration of thiazides to humans is well documented (31). To investigate the sex-specific response that thiazides generate in renal Ca2+ handling, the present model will need to be expanded to track Ca2+ transport along the nephron, as done in Ref. 15.
ENaC inhibition.
ENaC mediates the final step in Na+ reabsorption and is an important contributor to the regulation of Na+ homeostasis and blood pressure (8). In addition to principal cells in the distal nephron, ENaC is also expressed on the apical membrane of a number of epithelial cells, including those in the bladder, lung airway, and distal colon. Model simulations predicted that full ENaC inhibition approximately doubles distal tubular Na+ outflow in the two sexes. Those increases are consistent with but lower than the approximately three-fold increase reported following the administration of amiloride in the micropuncture studies by Hropot et al. (33) and Sonnenber et al. (79). Complete ENaC inhibition was predicted to enhance urine output by 68% in male rats and 49% in female rats and to increase Na+ excretion by 209% in male rats and 163% in female rats. The predicted sex difference is consistent with the greater diuretic and natriuretic response to benzamil reported in male versus female Sprague-Dawley rats (78). ENaC mutations are found in patients with Liddle’s disease (10) or pseudohypoaldosteronism type 1 (10), which, in addition to volume depletion and hypotension, are characterized by metabolic acidosis and hyperkalemia. Similarly, our model predicted that complete inhibition of ENaC reduces net acid excretion and decreases urinary K+ excretion (by 78% in both sexes) (see Fig. 9B).
Model limitations and possible extensions.
A major limitation of the present model is that interstitial fluid composition is assumed to be known a priori. Thus, to simulate a maneuver that substantially affects medullary TNa and the axial osmolality gradient assumed in the baseline interstitial fluid composition, such as the inhibition of NHE3 or NKCC2, we had to estimate, rather than predict, the effects on interstitial fluid composition and adjust interstitial solute concentration profiles accordingly. A sensitivity study previously conducted to assess the impact of variations in interstitial fluid composition on predictions of the male model have pointed to the collecting duct as the nephron segment that is the most sensitive to variations in medullary interstitial fluid composition (42). That sensitivity study predicted that urine concentration values generally vary in the same way that the interstitial fluid concentrations are changed, whereas urine output varies in the opposite direction as the interstitial fluid osmolality. Compared with urine concentrations, solute excretions are less sensitive to variations in interstitial fluid concentration profiles. Instead of prescribing interstitial fluid composition, a more accurate approach would be to capture the interactions among the model nephrons by determining the interstitial fluid concentrations based on solute and water reabsorption from tubular segments. This can be accomplished using the central core formulation (44), where the vasa recta and capillaries are assumed to have infinitely permeable walls and where interstitial fluid is allowed to vary axially but is assumed to be homogeneous at any given medullary level. Alternatively, one may adopt a more complex formulation that includes the vasa recta and a spatially inhomogeneous distribution of the tubules and vasa recta (39–41, 43).
Despite these limitations, the present sex-specific multinephron models can be used as essential components in more comprehensive models of integrated kidney function. For instance, sex differences in prevalence and severity of hypertension have been reported in a number of mammalian and avian species (69). Women tend to have lower rates of hypertension than men before menopause, but higher after menopause (3, 28). Men are at higher risk for cardiovascular and renal diseases (37, 91). Computational models of whole body blood pressure regulation typically include a highly simplified representation of the kidney (e.g., Refs. 27 and 47). Given that the kidney plays an essential role in blood pressure regulation, and that a number of anti-hypertensive medications directly or indirectly target the kidney, incorporating elaborate sex-specific nephron transport models would facilitate detailed drug simulations and analysis of any sex differences in drug efficacy. Another direction of extension is the study of kidney oxygenation. Renal hypoxia is believed to be a major contributor in the pathogenesis of chronic kidney disease (18). Currently, the more sophisticated computational models of renal oxygenation represent blood flow and tubular metabolism (e.g., Refs. 10a, 19–21, 45, and 46), but none considers individual membrane transporters and channels or the sexual dimorphism in renal metabolism. By leveraging the present models, one would be able to conduct a comprehensive in silico study of factors that give rise to the impacts of sex on the progression of chronic kidney disease (44a, 75).
GRANTS
This work was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada, via a Discovery award (to A. T. Layton), and by National Institute of Diabetes and Digestive and Kidney Diseases Grant 2R01DK083785 (to A. A. McDonough).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.L. conceived and designed research; R.H. performed experiments; A.T.L. analyzed data; A.A.M. and A.T.L. interpreted results of experiments; R.H. and A.T.L. prepared figures; A.T.L. drafted manuscript; A.A.M. and A.T.L. edited and revised manuscript; R.H., A.A.M., and A.T.L. approved final version of manuscript.
REFERENCES
- 1.Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 301: F1143–F1159, 2011. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum M, Twombley K, Gattineni J, Joseph C, Wang L, Zhang Q, Dwarakanath V, Moe OW. Proximal tubule Na+/H+ exchanger activity in adult NHE8−/−, NHE3−/−, and NHE3−/−/NHE8−/− mice. Am J Physiol Renal Physiol 303: F1495–F1502, 2012. doi: 10.1152/ajprenal.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics−2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Bobulescu IA, Moe OW. Luminal Na+/H+ exchange in the proximal tubule. Pflügers Arch 458: 5–21, 2009. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandoni A, Villar SR, Torres AM. Gender-related differences in the pharmacodynamics of furosemide in rats. Pharmacology 70: 107–112, 2004. doi: 10.1159/000074675. [DOI] [PubMed] [Google Scholar]
- 6.Brasen JC, Burford JL, McDonough AA, Holstein-Rathlou NH, Peti-Peterdi J. Local pH domains regulate NHE3-mediated Na+ reabsorption in the renal proximal tubule. Am J Physiol Renal Physiol 307: F1249–F1262, 2014. doi: 10.1152/ajprenal.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49: 251–273, 1987. doi: 10.1146/annurev.ph.49.030187.001343. [DOI] [PubMed] [Google Scholar]
- 8.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 9.Chambrey R, Warnock DG, Podevin RA, Bruneval P, Mandet C, Bélair MF, Bariéty J, Paillard M. Immunolocalization of the Na+/H+ exchanger isoform NHE2 in rat kidney. Am J Physiol 275: F379–F386, 1998. doi: 10.1152/ajprenal.1998.275.3.F379. [DOI] [PubMed] [Google Scholar]
- 10.Chang SS, Grunder S, Hanukoglu A, Rösler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 10a.Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol 298: F1369–F1383, 2010. doi: 10.1152/ajprenal.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Vaughn DA, Fanestil DD. Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5: 1112–1119, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest 105: 1141–1146, 2000. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 248: F527–F535, 1985. doi: 10.1152/ajprenal.1985.248.4.F527. [DOI] [PubMed] [Google Scholar]
- 14.Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol 64: 803–843, 2002. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 15.Edwards A. Regulation of calcium reabsorption along the rat nephron: a modeling study. Am J Physiol Renal Physiol 308: F553–F566, 2015. doi: 10.1152/ajprenal.00577.2014. [DOI] [PubMed] [Google Scholar]
- 16.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Busslinger M, Dominguez Rieg JA, Rieg T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol 308: F1409–F1420, 2015. doi: 10.1152/ajprenal.00129.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 19.Fry BC, Edwards A, Layton AT. Impact of nitric-oxide-mediated vasodilation and oxidative stress on renal medullary oxygenation: a modeling study. Am J Physiol Renal Physiol 310: F237–F247, 2016. doi: 10.1152/ajprenal.00334.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry BC, Edwards A, Layton AT. Impacts of nitric oxide and superoxide on renal medullary oxygen transport and urine concentration. Am J Physiol Renal Physiol 308: F967–F980, 2015. doi: 10.1152/ajprenal.00600.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry BC, Edwards A, Sgouralis I, Layton AT. Impact of renal medullary three-dimensional architecture on oxygen transport. Am J Physiol Renal Physiol 307: F263–F272, 2014. doi: 10.1152/ajprenal.00149.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Vicente A, Saez F, Monzon CM, Asirwatham J, Garvin JL. Thick ascending limb sodium transport in the pathogenesis of hypertension. Physiol Rev 99: 235–309, 2019. doi: 10.1152/physrev.00055.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greger R. Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000. doi: 10.1097/00000441-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Greger R, Velázquez H. The cortical thick ascending limb and early distal convoluted tubule in the urinary concentrating mechanism. Kidney Int 31: 590–596, 1987. doi: 10.1038/ki.1987.39. [DOI] [PubMed] [Google Scholar]
- 26.Grossman E, Verdecchia P, Shamiss A, Angeli F, Reboldi G. Diuretic treatment of hypertension. Diabetes Care 34, Suppl 2: S313–S319, 2011. doi: 10.2337/dc11-s246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallow KM, Lo A, Beh J, Rodrigo M, Ermakov S, Friedman S, de Leon H, Sarkar A, Xiong Y, Sarangapani R, Schmidt H, Webb R, Kondic AG. A model-based approach to investigating the pathophysiological mechanisms of hypertension and response to antihypertensive therapies: extending the Guyton model. Am J Physiol Regul Integr Comp Physiol 306: R647–R662, 2014. doi: 10.1152/ajpregu.00039.2013. [DOI] [PubMed] [Google Scholar]
- 28.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 130: 9–18, 2016. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 29.Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens 12: 527–532, 2003. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009. doi: 10.1074/jbc.M804322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins BA, Nassim JR, Collins J, Hilb A. The effect of bendrofluazide on urine calcium excretion. Clin Sci 27: 457–462, 1964. [PubMed] [Google Scholar]
- 32.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100: 321–356, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 28: 477–489, 1985. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- 34.Hu R, McDonough AA, Layton AT. Functional implications of the sex differences in transporter abundance along the rat nephron: modeling and analysis. Am J Physiol Renal Physiol 317: F1462–F1474, 2019. doi: 10.1152/ajprenal.00352.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston PA, Kau ST. The effect of loop of Henle diuretics on the tubuloglomerular feedback mechanism. Methods Find Exp Clin Pharmacol 14: 523–529, 1992. [PubMed] [Google Scholar]
- 36.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 37.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 24: 687–698, 2010. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 38.Kunau RT Jr, Weller DR, Webb HL. Clarification of the site of action of chlorothiazide in the rat nephron. J Clin Invest 56: 401–407, 1975. doi: 10.1172/JCI108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 300: F356–F371, 2011. doi: 10.1152/ajprenal.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. II. Functional implications of three-dimensional architecture. Am J Physiol Renal Physiol 300: F372–F384, 2011. doi: 10.1152/ajprenal.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layton AT, Dantzler WH, Pannabecker TL. Urine concentrating mechanism: impact of vascular and tubular architecture and a proposed descending limb urea-Na+ cotransporter. Am J Physiol Renal Physiol 302: F591–F605, 2012. doi: 10.1152/ajprenal.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 311: F1217–F1229, 2016. doi: 10.1152/ajprenal.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010. doi: 10.1152/ajprenal.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816–F839, 2004. doi: 10.1152/ajprenal.00398.2003. [DOI] [PubMed] [Google Scholar]
- 44a.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 314: F969–F984, 2018. doi: 10.1152/ajprenal.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee C-J, Gardiner BS, Evans RG, Smith DW. A model of oxygen transport in the rat renal medulla. Am J Physiol Renal Physiol 315: F1787–F1811, 2018. doi: 10.1152/ajprenal.00363.2018. [DOI] [PubMed] [Google Scholar]
- 46.Lee C-J, Gardiner BS, Ngo JP, Kar S, Evans RG, Smith DW. Accounting for oxygen in the renal cortex: a computational study of factors that predispose the cortex to hypoxia. Am J Physiol Renal Physiol 313: F218–F236, 2017. doi: 10.1152/ajprenal.00657.2016. [DOI] [PubMed] [Google Scholar]
- 47.Leete J, Layton AT. Sex-specific long-term blood pressure regulation: Modeling and analysis. Comput Biol Med 104: 139–148, 2019. doi: 10.1016/j.compbiomed.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Hatano R, Xu S, Wan L, Yang L, Weinstein AM, Palmer L, Wang T. Gender difference in kidney electrolyte transport. I. Role of AT1a receptor in thiazide-sensitive Na+-Cl− cotransporter activity and expression in male and female mice. Am J Physiol Renal Physiol 313: F505–F513, 2017. doi: 10.1152/ajprenal.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol 277: F447–F453, 1999. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 50.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X; International Consortium for Blood Pressure (ICBP) . KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 51.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 53.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 307: F901–F907, 2014. doi: 10.1152/ajprenal.00288.2014. [DOI] [PubMed] [Google Scholar]
- 54.Morgan T, Berliner RW. A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron 6: 388–405, 1969. doi: 10.1159/000179741. [DOI] [PubMed] [Google Scholar]
- 55.Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol 254: F223–F231, 1988. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- 56.Musselman TM, Zhang Z, Masilamani SM. Differential regulation of the bumetanide-sensitive cotransporter (NKCC2) by ovarian hormones. Steroids 75: 760–765, 2010. doi: 10.1016/j.steroids.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol 18: 440–448, 2007. doi: 10.1681/ASN.2006091070. [DOI] [PubMed] [Google Scholar]
- 59.Palmer LG,, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676–687, 2015. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988. doi: 10.1038/ki.1988.206. [DOI] [PubMed] [Google Scholar]
- 62.Rojas-Vega L, Gamba G. Mini-review: regulation of the renal NaCl cotransporter by hormones. Am J Physiol Renal Physiol 310: F10–F14, 2016. doi: 10.1152/ajprenal.00354.2015. [DOI] [PubMed] [Google Scholar]
- 63.Romano G, Favret G, Federico E, Bartoli E. The site of action of furosemide. Pharmacol Res 37: 409–419, 1998. doi: 10.1006/phrs.1998.0311. [DOI] [PubMed] [Google Scholar]
- 64.Rossier BC, Bochud M, Devuyst O. The hypertension pandemic: an evolutionary perspective. Physiology (Bethesda) 32: 112–125, 2017. doi: 10.1152/physiol.00026.2016. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz-Feria CA, Zhang D, Nishimura H. Age- and sex-dependent changes in pulse pressure in fowl aorta. Comp Biochem Physiol A Mol Integr Physiol 137: 311–320, 2004. doi: 10.1016/j.cbpb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 67.Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sáinz J, Osuna A, Wangensteen R, de Dios Luna J, Rodríguez-Gómez I, Duarte J, Moreno JM, Vargas F. Role of sex, gonadectomy and sex hormones in the development of nitric oxide inhibition-induced hypertension. Exp Physiol 89: 155–162, 2004. doi: 10.1113/expphysiol.2003.002652. [DOI] [PubMed] [Google Scholar]
- 69.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sassi A, Wang Y, Chassot A, Komarynets O, Roth I, Olivier V, Crambert G, Dizin E, Boscardin E, Hummler E, Feraille E. Interaction between epithelial sodium channel γ-subunit and claudin-8 modulates paracellular sodium permeability in renal collecting Duct. J Am Soc Nephrol 31: 1009–1023, 2020. doi: 10.1681/ASN.2019080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 72.Sgouralis I, Layton AT. Theoretical assessment of renal autoregulatory mechanisms. Am J Physiol Renal Physiol 306: F1357–F1371, 2014. doi: 10.1152/ajprenal.00649.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shibata S. 30 Years of the mineralocorticoid receptor: Mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephron. J Endocrinol 234: T35–T47, 2017. doi: 10.1530/JOE-16-0669. [DOI] [PubMed] [Google Scholar]
- 74.Sigmund CD, Carey RM, Appel LJ, Arnett DK, Bosworth HB, Cushman WC, Galis ZS, Green Parker M, Hall JE, Harrison DG, McDonough AA, Nicastro HL, Oparil S, Osborn JW, Raizada MK, Wright JD, Oh YS. Report of the National Heart, Lung, and Blood Institute Working Group on Hypertension: Barriers to Translation. Hypertension 75: 902–917, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 25: 515–533, 1995. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 76.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 77.Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R. Double knockout of the Na+-driven Cl−/ exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017. doi: 10.1681/ASN.2015070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soliman RH, Johnston JG, Gohar EY, Taylor CM, Pollock DM. Greater natriuretic response to ENaC inhibition in male versus female Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 318: R418–R427, 2020. doi: 10.1152/ajpregu.00060.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonnenberg H, Honrath U, Wilson DR. Effects of amiloride in the medullary collecting duct of rat kidney. Kidney Int 31: 1121–1125, 1987. doi: 10.1038/ki.1987.117. [DOI] [PubMed] [Google Scholar]
- 80.Stamler J, Stamler R, Riedlinger WF, Algera G, Roberts RH. Hypertension screening of 1 million Americans. Community Hypertension Evaluation Clinic (CHEC) program, 1973 through 1975. JAMA 235: 2299–2306, 1976. doi: 10.1001/jama.1976.03260470017018. [DOI] [PubMed] [Google Scholar]
- 81.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun AM, Liu Y, Dworkin LD, Tse CM, Donowitz M, Yip KP. Na+/H+ exchanger isoform 2 (NHE2) is expressed in the apical membrane of the medullary thick ascending limb. J Membr Biol 160: 85–90, 1997. doi: 10.1007/s002329900297. [DOI] [PubMed] [Google Scholar]
- 83.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 83: 413–422, 2005. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 84.Vallon V, Mühlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 85.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Liere EJ, Stickney JC, Marsh DF. Sex differences in blood pressure of dogs. Science 109: 489, 1949. doi: 10.1126/science.109.2837.489. [DOI] [PubMed] [Google Scholar]
- 88.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veiras LC, McFarlin BE, Ralph DL, Buncha V, Prescott J, Shirvani BS, McDonough JC, Ha D, Giani J, Gurley SB, Mamenko M, McDonough AA. Electrolyte and transporter responses to angiotensin II induced hypertension in female and male rats and mice. Acta Physiol (Oxf) 229: e13448, 2020. doi: 10.1111/apha.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verouti SN, Boscardin E, Hummler E, Frateschi S. Regulation of blood pressure and renal function by NCC and ENaC: lessons from genetically engineered mice. Curr Opin Pharmacol 21: 60–72, 2015. doi: 10.1016/j.coph.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 92.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard J-M, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 93.Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]