Abstract
Background:
This meta-analysis aimed to identify the prognostic role of Ki-67 in patients with nasopharyngeal carcinoma (NPC).
Methods:
Relevant studies were retrieved in the PubMed, Embase, Web of Science, and Cochrane Library databases up to November 2019. The pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to estimate the association between Ki-67 expression and survival outcomes. Combined odds ratios (ORs) and 95% CIs were measured as effect size on the association between Ki-67 expression and clinical factors.
Results:
A total of eight studies involving 936 patients with NPC were included in this meta-analysis. The pooled HR indicated that Ki-67 expression was significantly associated with poor overall survival (HR = 2.86, 95% CI = 1.91–4.27, p < 0.001), progression-free survival (HR = 1.78, 95% CI = 1.15–2.74, p = 0.009), and distant metastasis-free survival (HR = 1.65, 95% CI = 1.15–2.36, p = 0.007). However, there was no significant correlation between Ki-67 expression and local recurrence-free survival (HR = 1.07, 95% CI = 0.54–2.14, p = 0.843). Ki-67 overexpression was associated with higher T stage (OR = 1.48, 95% CI = 1.00–2.20, p = 0.052), and the relationship between Ki-67 expression and advanced stage was nearly significant (OR = 2.25, 95% CI = 0.99–5.14, p = 0.054). However, high Ki-67 expression was not significantly correlated with sex, age, N stage, or histological type.
Conclusion:
This meta-analysis demonstrated that Ki-67 overexpression was a significant marker for poor prognosis in patients with NPC. Ki-67 should be recommended as a useful index for prognostication in patients with NPC.
Keywords: Ki-67, management, meta-analysis, nasopharyngeal carcinoma, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) has distinct characteristics from other head and neck cancers and accounts for only 0.7% of all cancer cases diagnosed in 2018 globally.1,2 More than 70% of new NPC cases were reported in East and Southeast Asia.1 There were approximately 42,100 new cases of NPC in China in 2013, accounting for 1.14% of all new cancer cases in China.3 The primary treatment for early-stage NPC is intensity-modulated radiotherapy, alone or in combination with chemotherapy.4,5 However, approximately 8–10% of these patients have recurrent or distant metastasis, and the prognosis is poor.6 To date, various biological markers, including Epstein-Barr virus (EBV) infection,7 C-reactive protein level,8 and neutrophil-to-lymphocyte ratio (NLR),9 have been reported to be associated with the prognosis of NPC. However, the prognostic efficacy and accuracy are still unsatisfactory; therefore, the identification of effective and prognostic markers is important for the optimization of treatment strategies.
Ki-67 is a nuclear marker expressed in actively proliferating cells.10 Ki-67 exists in almost all phases of the cell cycle, including G1, S, G2, and M stages, except for the G0 stage.11 The expression level of Ki-67 detected by immunohistochemical (IHC) staining is usually considered as an index that reflects the activity of cell proliferation.12 Many studies have also reported the prognostic value of Ki-67 in a variety of solid tumors, such as non-small-cell lung cancer,13 colorectal cancer,14 bladder cancer,15 and renal cell carcinoma.11 Previous studies also indicated an association between Ki-67 expression and prognosis of patients with NPC, but the results were conflicting.16–23 Some studies showed the significant predictive role of Ki-67 for worse prognosis,16,18 while other studies presented high Ki-67 expression as an indicator of favorable prognosis.23 Therefore, this meta-analysis aimed to more accurately identify the prognostic and clinicopathological significance of Ki-67 in patients with NPC.
Materials and methods
Search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.24 Ethical approval and patient consent were not required because the data were extracted from previously published studies. The PubMed, Embase, Web of Science, and Cochrane Library databases were searched up to November 2019. The keywords and MeSH terms were as follows: (Ki67 or Ki-67 or MIB-1 or MIB1) and (nasopharyngeal carcinoma or NPC or nasopharyngeal cancer or nasopharyngeal neoplasm or nasopharyngeal or carcinoma of the nasopharynx). The search details in PubMed are shown in Supplemental File S1. The references of relevant studies were screened for potential inclusions.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: (a) the diagnosis of NPC was histopathologically confirmed; (b) the Ki-67 expression in tissues was detected by IHC analysis; (c) studies reported at least one survival outcome, including overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) with hazard ratio (HR) with 95% confidence interval (CI); (d) studies were published in English. The exclusion criteria were: (a) reviews, conference abstracts, letters, and case reports; (b) duplicate studies; and (c) studies that did not provide necessary data.
Data extraction and quality assessment
Two independent investigators (Z.S. and W.J.) reviewed the eligible studies and extracted basic information; any disagreements were settled by discussion. The following data were extracted: first author, publication year, country/region, sample size, sex, age, study duration, detection method, follow-up, disease stage, study type, and HRs and 95% CIs of OS, PFS, DMFS, and LRFS. If HRs and 95% CIs were not reported directly, they were calculated using Parmar’s method from survival curves.25 The quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS) for cohort studies.26 NOS score ranges from 0 to 9 and estimates studies from three aspects: selection, comparability, and outcome. Studies with scores ⩾6 were regarded as high-quality studies.
Statistical analysis
All data analyses were conducted using Stata SE12.0 (Stata Corp., College Station, TX, USA). The pooled HRs and 95% CIs were calculated to quantify the correlation between Ki-67 expression and survival outcomes. Combined odds ratios (ORs) and 95% CIs were measured as effect size on the association between Ki-67 expression and clinical factors in NPC. The heterogeneity among studies was quantified using Cochran’s Q statistic and I2 statistic. In the presence of significant heterogeneity (I2 > 50% or p < 0.10), the random-effects model was applied; otherwise, a fixed-effects model was used. Sensitivity analysis was performed to assess the stability of the results. Begg’s test was used to assess potential publication bias. A p-value < 0.05 was considered statistically significant.
Results
Search results and study characteristics
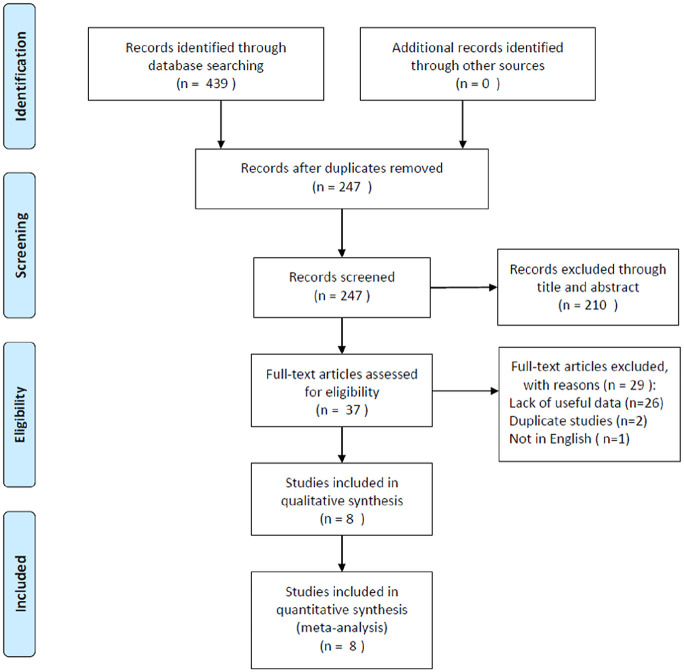
A flow chart of the article retrieval process is shown in Figure 1. A total of 439 records were identified through an initial search. After duplicate records were removed, 247 studies were screened by title and abstract. Subsequently, 210 studies were excluded, and 37 full-text articles were evaluated for eligibility. Then, 29 studies were excluded for different reasons: 26 studies lacked necessary data, two studies used overlapped patients, and one study was not published in English. Finally, eight studies16–23 were included in this meta-analysis. The detailed characteristics of the included studies are shown in Table 1. Seven studies were conducted in China16–18,20–23 and one was performed in Taiwan.19 The sample sizes ranged from 45 to 334, and the total number was 936. All eight studies used IHC detection methods, seven studies had a retrospective design,16–22 and one study had a prospective design.23 The cutoff values for Ki-67 ranged from 10% to 77.5%. Three studies reported the histological types of NPC. The NOS scores ranged from 6 to 8, with a median value of 7.16,18,19
Figure 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of the included studies.
| Author | Country/region | No. of patients | Gender (M/F) |
Age (years) Median (range) |
Stage | Duration | Follow-up (month) Median (range) |
Study design | NOS score | Survival analysis | Resource of HR and 95% CI | Cut-off value for Ki-67 | Histological type | WHO subtype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al.16 | China | 45 | 31/14 | 47 (27–79) | I–IV | 2013–2015 | 41 (15–56) | Retrospective | 7 | OS, PFS | Reported | 77.5% | Non-keratinizing undifferentiated: 45 | WHO III |
| Zhao et al.17 | China | 108 | 75/33 | 47 (17–82) | III–IVa | 2008–2012 | 51.5 (2–91) | Retrospective | 7 | PFS | Reported | 30% | NA | NA |
| Lu et al.18 | China | 334 | 244/90 | 48 (17–72) | I–IV | 2011–2014 | 48.7 | Retrospective | 7 | OS, PFS, DMFS, LRFS | Calculated | 25% | Non-keratinizing carcinoma Differentiated: 17 Undifferentiated: 317 |
WHO II and WHO III |
| Chang et al.19 | Taiwan | 124 | 95/29 | 48.6 (20–83) | I–IV | 1993–2002 | 71 (1–141) | Retrospective | 8 | PFS, DMFS, LRFS | Calculated | H-score ⩾median |
Keratinizing: 5 Non-keratinizing: 42 Undifferentiated: 77 |
WHO I, WHOII and WHO III |
| Zhang et al.20 | China | 59 | 43/16 | 48.4 (15–80) | I–IV | 2000–2008 | 68 (5–84) | Retrospective | 7 | OS | Calculated | 25% | NA | NA |
| You et al.21 | China | 118 | 76/42 | NA | I–IV | NA | NA | Retrospective | 6 | OS | Reported | 50% | NA | NA |
| Guan et al.22 | China | 58 | 42/16 | 45 (24–74) | I–IV | 2001–2011 | 96 | Retrospective | 7 | OS, PFS | Reported | 10% | NA | NA |
| Hui et al.23 | China | 90 | 74/16 | 47 (29–75) | I–IV | 1998–1999 | NA | Prospective | 8 | OS, PFS, DMFS, LRFS | Reported | 26.1% | NA | NA |
CI, confidence interval; DMFS, distant metastasis-free survival; F, female; HR, hazard ratio; IHC, immunohistochemistry; LRFS, local recurrence-free survival; M, male; NA, not available; NOS, Newcastle Ottawa Scale; OS, overall survival; PFS, progression-free survival.
Association between Ki-67 expression and OS, PFS, DMFS, and LRFS
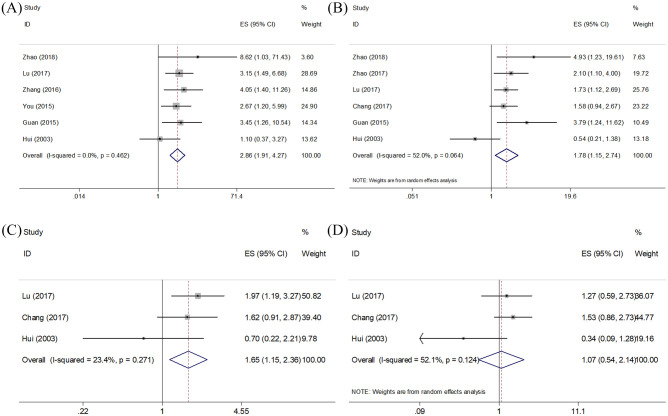
Six studies reported the prognostic value of Ki-67 in OS.16,18,20–23 As shown in Figure 2 and Table 2, a fixed-effects model was used because of nonsignificant heterogeneity (I2 = 0, p = 0.462). The pooled data were as follows: HR = 2.86, 95% CI = 1.91–4.27, p < 0.001, indicating that high Ki-67 expression predicted poor OS. Similarly, the combined HRs and 95% CIs from six studies,16–19,22,23 and three studies,18,19,23 showed that Ki-67 overexpression was also correlated with worse PFS (HR = 1.78, 95% CI = 1.15–2.74, p = 0.009; I2 = 52%, p = 0.064) and unfavorable DMFS (HR = 1.65, 95% CI = 1.15–2.36, p = 0.007; I2 = 23.4%, p = 0.271) (Figure 2, Table 2). However, no significant correlation was found between high Ki-67 expression and LRFS (HR = 1.07, 95% CI = 0.54–2.14, p = 0.843; I2 = 52.1%, p = 0.124) based on the data of three studies.18,19,23
Figure 2.
Forest plot of OS, PFS, DMFS, and LRFS. Note. (A) Meta-analysis of Ki-67 expression and OS. (B) Meta-analysis of Ki-67 expression and PFS. (C) Meta-analysis of Ki-67 expression and DMFS. (D) Meta-analysis of Ki-67 expression and LRFS.
DMFS, distant metastasis-free survival; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
Table 2.
Meta-analysis of pooled HRs and 95% CIs for the association between Ki-67 and OS, PFS, DMFS, and LRFS in NPC patients.
| Outcome | No. of studies | HR (95% CI) | p | Heterogeneity | Effects model | |
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| OS | 6 | 2.86 (1.91–4.27) | <0.001 | 0 | 0.462 | Fixed |
| PFS | 6 | 1.78 (1.15–2.74) | 0.009 | 52 | 0.064 | Random |
| DMFS | 3 | 1.65 (1.15–2.36) | 0.007 | 23.4 | 0.271 | Fixed |
| LRFS | 3 | 1.07 (0.54–2.14) | 0.843 | 52.1 | 0.124 | Random |
CI, confidence interval; DMFS, distant metastasis-free survival; HR, hazard ratio; LRFS, local recurrence-free survival; NPC, nasopharyngeal carcinoma; OS, overall survival; PFS, progression-free survival.
Correlation between Ki-67 expression and clinicopathological factors
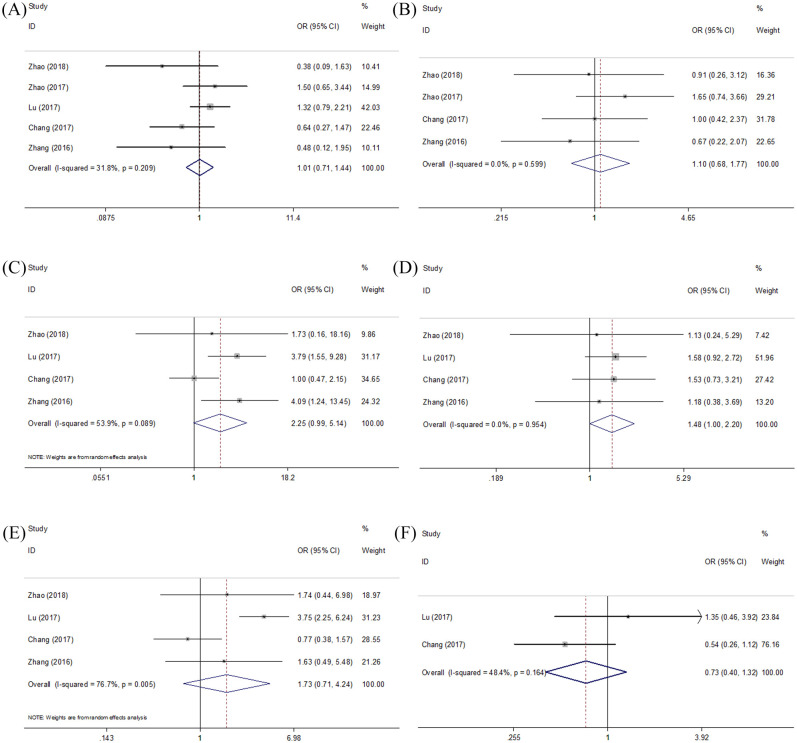
We investigated the relationship between Ki-67 expression and various clinicopathological characteristics, including sex, age, clinical stage, T stage, N stage, and histological type. As shown in Figure 3 and Table 3, the merged results demonstrated that Ki-67 overexpression was associated with higher T stage (OR = 1.48, 95% CI = 1.00–2.20, p = 0.052). In addition, the association between Ki-67 expression and advanced stage was nearly significant (OR = 2.25, 95% CI = 0.99–5.14, p = 0.054). However, high Ki-67 expression is not significantly correlated with sex (OR = 1.01, 95% CI = 0.71 to –1.44, p = 0.963), age (OR = 1.10, 95% CI = 0.68–1.77, p = 0.699), N stage (OR = 1.73, 95% CI = 0.71–4.24, p = 0.231), or histological type (OR = 0.74, 95% CI = 0.40–1.32, p = 0.295).
Figure 3.
Meta-analysis of Ki-67 expression and clinical factors: (A) gender, (B) age, (C) clinical stage, (D) T stage, (E) N stage, and (F) histological type.
Table 3.
Meta-analysis of the association between high Ki-67 expression and clinicopathological features of NPC.
| Variables | No. of studies | OR (95% CI) | p | Heterogeneity | Effects model | |
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| Gender (male versus female) | 5 | 1.01 (0.71–1.44) | 0.963 | 31.8 | 0.209 | Fixed |
| Age (years) (⩾45 versus <45) | 4 | 1.10 (0.68–1.77) | 0.699 | 0 | 0.599 | Fixed |
| Clinical stage (III+IV versus I+II) | 4 | 2.25 (0.99–5.14) | 0.054 | 53.9 | 0.089 | Random |
| T stage (T3+T4 versus T1+T2) | 4 | 1.48 (1.00–2.20) | 0.052 | 0 | 0.954 | Fixed |
| N stage (N2+N3 versus N0+N1) | 4 | 1.73 (0.71–4.24) | 0.231 | 76.7 | 0.005 | Random |
| Histological type (undifferentiated versus keratinizing/non-keratinizing) | 2 | 0.74 (0.40–1.32) | 0.295 | 48.4 | 0.164 | Fixed |
CI, confidence interval; NPC, nasopharyngeal carcinoma; OR, odds ratio.
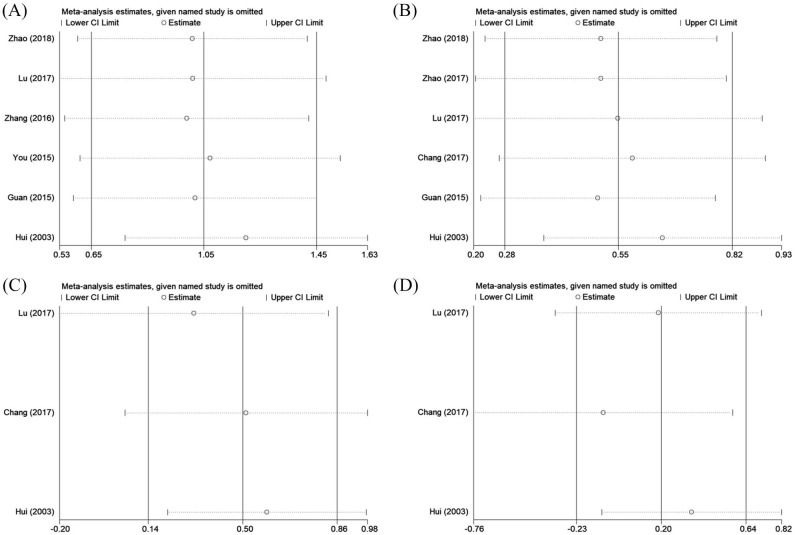
Sensitivity analyses
To evaluate the stability of the results, a sensitivity analysis by sequential deletion of each single study was conducted. As shown in Figure 4, the results showed that no separate study significantly affected the total HRs, which suggested that this meta-analysis presented reliable results.
Figure 4.
Sensitivity analysis for (A) OS, (B) PFS, (C) DMFS, and (D) LRFS.
DMFS, distant metastasis-free survival; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
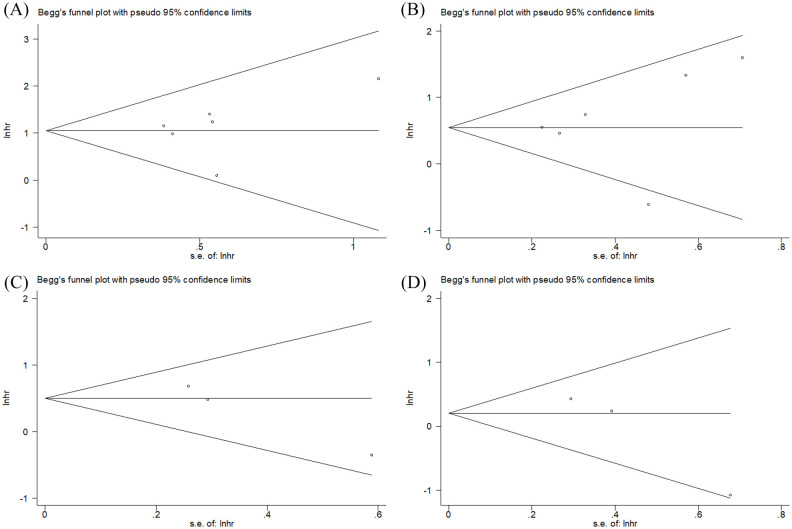
Publication bias
Publication bias was evaluated using Begg’s test. As shown in Figure 5, the funnel plot did not indicate any evidence of publication bias for OS (p = 0.707), PFS (p = 0.260), DMFS (p = 0.296), and LRFS (p = 0.296).
Figure 5.
Funnel plots for publication bias of (A) OS, (B) PFS, (C) DMFS, and (D) LRFS.
DMFS, distant metastasis-free survival; LRFS, local recurrence-free survival; OS, overall survival; PFS, progression-free survival.
Discussion
NPC is a rare cancer type, and the prognosis of advanced stage of disease is unsatisfactory.27 In the current study, we performed a meta-analysis to identify the prognostic value of Ki-67 expression in patients with NPC. We found that the Ki-67 index showed significant prognostic efficiency in different survival endpoints, including OS, PFS, and DMFS, suggesting that Ki-67 could be validated as a useful index in the prognostication of patients with NPC. In addition, the pooled data also indicated an association between Ki-67 expression and higher T stage in NPC. Taken together, this is the first study to demonstrate the positive correlation between high Ki-67 expression and worse survival outcomes using the meta-analytic approach.
Ki-67 is present in the active phases of the cell cycle, including G1, S, and G2 phases but is not expressed in quiescent or G0 phases.28 The role of Ki-67 in the cell cycle makes it an optimal marker in the evaluation of the proportion of proliferative cells.12 In patients with cancer, the Ki-67 level determined by IHC analysis is indicative of survival outcomes. Recent studies have also reported the prognostic value of Ki-67 expression in different types of cancer. A meta-analysis including 21 studies showed that high Ki-67 expression in localized prostate cancer was predictive of poor disease-specific survival, biochemical failure-free survival, DFS, and OS.29 Liu et al. reported that Ki-67 overexpression was correlated with poor prognosis in patients with gastric cancer.30 Furthermore, their study also found that Ki-67 positivity was associated with age, lymph node metastasis, and tumor size in patients with gastric cancer.30 Another recent study also revealed the significant relationship between Ki-67 expression and poor OS in patients with ovarian cancer.31 The previous findings of the positive correlations of Ki-67 expression and poor prognosis in various cancers are in accordance with the present study. However, we did not identify an association between high Ki-67 expression and any clinicopathological features in NPC. However, the data of clinical stage (p = 0.054) and T stage (p = 0.052) (Table 3) are extremely close to statistical significance. The negative association may be the result of a limited sample size. Notably, the included studies recruited patients with stage I–IV (seven studies) and stage III–IVa (one study) diseases.16–23 In the survival analysis in each study, the investigators analyzed the prognostic value of Ki-67 and the whole group of patients (stage I–IV or stage III–IVa) but not patients with stage I–II and III–IV diseases. Therefore, the possible difference in the prognostic role of Ki-67 in different disease stages (early versus advanced) could not be determined based on the current evidence. Thus, we suggest that the potential distinct prognostic efficiency of Ki-67 in NPC at different disease stages (early versus advanced) should be explored in further studies.
EBV infection is a risk factor for NPC, and the non-keratinizing subtype of NPC is associated predominantly with EBV infection.1 In the present meta-analysis, the data were insufficient to explore the association between EBV positivity and Ki-67 expression. A previous study involving 19 patients showed that EBV positivity was correlated with highly Ki-67 expression (p = 0.0174) in NPC.32 Another study suggested that the f variant of EBV was correlated with high Ki-67 expression in NPC and therefore indicated the possible role of EBV in nasopharyngeal carcinogenesis.33 This evidence indicates the potential relationship between EBV and proliferation of NPC, which could be measured quantitatively by Ki-67 expression. In the present meta-analysis, the data were insufficient to explore the association between EBV positivity and Ki-67 expression. We suggest that the association between EBV positivity and Ki-67 expression should be explored in large-scale studies.
A variety of prognostic factors for NPC have been explored recently, including programmed cell death ligand-1,34 EBV DNA levels,35 microRNAs,36 NLR,9 and platelet-to-lymphocyte ratio.37 These prognostic indicators showed significant prognostic value in NPC and are associated with the tumor microenvironment, immune responses, and EBV positivity. Compared with these markers, Ki-67 is an index of proliferation and usually measured in pathological tests. Other markers of cell proliferation, including the MCM family, cyclin D1, Bcl-2, CDK family, and p16IN4Ka have also been investigated as prognostic markers in NPC in previous studies.38,39 However, due to insufficient data in the current meta-analysis, direct comparisons between other proliferative markers and Ki-67 could not be performed. We suggest that the distinction between other cell proliferation indexes and Ki-67 in NPC should be explored. Ki-67 is widely available in clinical practice and could provide implications for survival outcomes in the management of patients, as suggested by the current meta-analysis.
The current meta-analysis had several limitations. First, the total sample size was small. Although eight studies were included in the meta-analysis, the total sample size was 936, which may be due to the rarity of NPC. Second, all studies were from China and Taiwan, which restricted the applicability of the results to other regions worldwide. Third, all studies used IHC analysis to detect Ki-67 expression, whereas the criteria to determine high or low Ki-67 expression were different in the included studies. The various cutoff values may contribute to heterogeneity. Fourth, as EBV is associated with nasopharyngeal carcinogenesis, the impact of EBV on Ki-67 expression needs to be investigated. In the current meta-analysis, due to insufficient data, the relationship between EBV infection and Ki-67 expression could not be analyzed. Although previous studies reported the potential association between EBV infection and Ki-67 expression in NPC,32,33 the detailed mechanisms underlying the crosstalk between EBV infection and Ki-67 expression remain to be elucidated in further investigations.
Therefore, this meta-analysis demonstrated that Ki-67 overexpression was a significant marker for poor prognosis in patients with NPC. Ki-67 should be recommended as a useful index in the prognostication of patients with NPC after treatment. However, due to some limitations, more large-scale, multicenter prospective studies are still needed to verify our results.
Supplemental Material
Supplemental material, supplumentary_file for Prognostic and clinicopathological value of Ki-67 expression in patients with nasopharyngeal carcinoma: a meta-analysis by Zhaohui Shi, Weihong Jiang, Xiaodong Chen, Min Xu, Xiaocheng Wang and Dingjun Zha in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Z.S., W.J., X.C., and M.X. conceived and designed the study. Z.S., W.J., X.W., and D.Z. performed the analysis, prepared the figures and tables and wrote the main manuscript. All of the authors reviewed the manuscript. All authors read and approved the final manuscript.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dingjun Zha  https://orcid.org/0000-0001-6484-4139
https://orcid.org/0000-0001-6484-4139
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zhaohui Shi, Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, China.
Weihong Jiang, Department of Otolaryngology-Skull Base Surgery, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Xiaodong Chen, Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, China.
Min Xu, Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, China.
Xiaocheng Wang, Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, China.
Dingjun Zha, Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Air Force Medical University, ChangLe West Road 127, Xi’an, Shaanxi 710032, China.
Reference
- 1. Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019; 394: 64–80. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer 2017; 36: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015; 16: 645–655. [DOI] [PubMed] [Google Scholar]
- 5. Daniilidis J, Constantinidis J, Fountzilas G. Combined chemoradiotherapy in locally advanced nasopharyngeal carcinoma. HNO 2001; 49: 732–738. [DOI] [PubMed] [Google Scholar]
- 6. Perri F, Bosso D, Buonerba C, et al. Locally advanced nasopharyngeal carcinoma: current and emerging treatment strategies. World J Clin Oncol 2011; 2: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan CM, Tang YY, Wang JP, et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J Cancer 2018; 9: 2852–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang LQ, Li CF, Chen QY, et al. High-sensitivity C-reactive protein complements plasma Epstein-Barr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. Int J Radiat Oncol Biol Phys 2015; 91: 325–336. [DOI] [PubMed] [Google Scholar]
- 9. Yin J, Qin YA, Luo YK, et al. Prognostic value of neutrophil-to-lymphocyte ratio for nasopharyngeal carcinoma: a meta-analysis. Medicine 2017; 96: e7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y, Hu WQ, Chen P, et al. Ki67 is a biological marker of malignant risk of gastrointestinal stromal tumors: a systematic review and meta-analysis. Medicine 2017; 96: e7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie YP, Chen LY, Ma X, et al. Prognostic and clinicopathological role of high Ki-67 expression in patients with renal cell carcinoma: a systematic review and meta-analysis. Sci Rep 2017; 7: 44281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menon SS, Guruvayoorappan C, Sakthivel KM, et al. Ki-67 protein as a tumour proliferation marker. Clinica Chimica Acta 2019; 491: 39–45. [DOI] [PubMed] [Google Scholar]
- 13. Xu J, Liu PP, Da J, et al. Prognostic value of Ki-67 in stage I non-small-cell lung cancer: a meta-analysis involving 1931 patients. Pathol Res Pract 2019; 215: 855–860. [DOI] [PubMed] [Google Scholar]
- 14. Luo ZW, Zhu MG, Zhang ZQ, et al. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta analysis. BMC Cancer 2019; 19: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He YH, Wang N, Zhou XF, et al. Prognostic value of Ki67 in BCG-treated non-muscle invasive bladder cancer: a meta-analysis and systematic review. BMJ Open 2018; 8: e019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao L, Chen H, Hu B, et al. Prognostic significance of Ki67 expression and the derived neutrophil–lymphocyte ratio in nasopharyngeal carcinoma. Cancer Manag Res 2018; 10: 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao YJ, Shen L, Huang XQ, et al. High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem Biophys Res Commun 2017; 494: 390–396. [DOI] [PubMed] [Google Scholar]
- 18. Lu Y, Huang H, Kang M, et al. Combined Ki67 and ERCC1 for prognosis in non-keratinizing nasopharyngeal carcinoma underwent chemoradiotherapy. Oncotarget 2017; 8: 88552–88562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang SL, Chan TC, Chen TJ, et al. HOXC6 overexpression is associated with Ki-67 expression and poor survival in NPC patients. J Cancer 2017; 8: 1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang JP, Liu YY, Deng Y, et al. Ki67 and nm23 are potential prognostic markers in patients with nasopharyngeal carcinoma. Int J Clin Exp Pathol 2016; 9: 6350–6356. [Google Scholar]
- 21. You B, Shan Y, Shi S, et al. Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma. Cancer Sci 2015; 106: 1506–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan GF, Zhang DJ, Wen LJ, et al. Prognostic value of TROP2 in human nasopharyngeal carcinoma. Int J Clin Exp Pathol 2015; 8: 10995–11004. [PMC free article] [PubMed] [Google Scholar]
- 23. Hui EP, Poon TCW, Teo PML, et al. A prospective study of pre-treatment cell kinetics and clinical outcome in nasopharyngeal carcinoma. Radiother Oncol 2003; 69: 53–62. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 25. Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 27. Wu L, Li CR, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med 2018; 15: 3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sobecki M, Mrouj K, Colinge J, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res 2017; 77: 2722–2734. [DOI] [PubMed] [Google Scholar]
- 29. Berlin A, Castro-Mesta JF, Rodriguez-Romo L, et al. Prognostic role of Ki-67 score in localized prostate cancer: a systematic review and meta-analysis. Urol Oncol 2017; 35: 499–506. [DOI] [PubMed] [Google Scholar]
- 30. Liu G, Xiong DS, Zeng JJ, et al. Clinicopathological and prognostic significance of Ki-67 immunohistochemical expression in gastric cancer: a systematic review and meta-analysis. Onco Targets Ther 2017; 10: 4321–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu DM, Cai WQ, Zhang ZQ, et al. High Ki-67 expression is significantly associated with poor prognosis of ovarian cancer patients: evidence from a meta-analysis. Arch Gynecol Obstet 2019; 299: 1415–1427. [DOI] [PubMed] [Google Scholar]
- 32. Kijima T, Kinukawa N, Gooding WE, et al. Association of Epstein-Barr virus with tumor cell proliferation: clinical implication in nasopharyngeal carcinoma. Int J Oncol 2001; 18: 479–485. [DOI] [PubMed] [Google Scholar]
- 33. Liu QY, Han AJ, You SY, et al. The association of genomic variation of Epstein-Barr virus BamHI F fragment with the proliferation of nasopharyngeal carcinoma. APMIS 2010; 118: 657–664. [DOI] [PubMed] [Google Scholar]
- 34. Liu XF, Shan CG, Song YL, et al. Prognostic value of programmed cell death ligand-1 expression in nasopharyngeal carcinoma: a meta-analysis of 1,315 patients. Front Oncol 2019; 9: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qu HL, Huang YL, Zhao SF, et al. Prognostic value of Epstein-Barr virus DNA level for nasopharyngeal carcinoma: a meta-analysis of 8128 cases. Eur Arch Otorhinolaryngol 2020; 277: 9–18. [DOI] [PubMed] [Google Scholar]
- 36. Sabarimurugan S, Kumarasamy C, Baxi S, et al. Systematic review and meta-analysis of prognostic microRNA biomarkers for survival outcome in nasopharyngeal carcinoma. PLoS One 2019; 14: e0209760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang JK, Feng WN, Ye ZH, et al. Prognostic significance of platelet-to-lymphocyte ratio in patients with nasopharyngeal carcinoma: a meta-analysis. Future Oncol 2020; 16: 117–127. [DOI] [PubMed] [Google Scholar]
- 38. Yang GD, Wang ZC, Chen QY, et al. p53, latent membrane protein 1, bcl-2, and prognosis in nasopharyngeal carcinoma: a meta-analysis. Histol Histopathol 2019; 34: 103–110. [DOI] [PubMed] [Google Scholar]
- 39. Gioacchini FM, Alicandri-Ciufelli M, Kaleci S, et al. The prognostic value of cyclin D1 expression in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2016; 273: 801–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplumentary_file for Prognostic and clinicopathological value of Ki-67 expression in patients with nasopharyngeal carcinoma: a meta-analysis by Zhaohui Shi, Weihong Jiang, Xiaodong Chen, Min Xu, Xiaocheng Wang and Dingjun Zha in Therapeutic Advances in Medical Oncology