Abstract
Dysmenorrhea is highly prevalent and is the leading cause of work and school absences among women of reproductive age. However, self-management of dysmenorrhea is not well understood in the US, and little research has explored factors that influence dysmenorrhea self-management. Guided by the Common Sense Model, we examined women’s representations of dysmenorrhea (beliefs about causes, symptoms, consequences, timeline, controllability, coherence, and emotional responses), described their dysmenorrhea self-management behaviors, and investigated the relationship between representations and self-management behaviors. We conducted a cross-sectional, web-based survey of 762 adult women who had dysmenorrhea symptoms in the last six months. Participants described various causes of their dysmenorrhea symptoms which were perceived as a normal part of life. Dysmenorrhea symptoms were reported as moderately severe, with consequences that moderately affected daily life. Women believed they understood their symptoms moderately well, and perceived them as moderately controllable, but expected their timeline to continue through menopause. Most women did not seek professional care but rather used a variety of pharmacologic and complementary health approaches. Care seeking and use of self-management strategies were associated with common sense beliefs about dysmenorrhea cause, consequences, timeline, and controllability. The findings may inform development and testing of self-management interventions that address dysmenorrhea representations and facilitate evidence-based management.
Keywords: dysmenorrhea, self-management, Common Sense Model, beliefs
Dysmenorrhea is characterized by pelvic pain occurring just before or during menstruation (International Association for the Study of Pain, 2011). In addition, women with dysmenorrhea may experience pain radiating toward the lower back or upper legs and gastrointestinal symptoms such as nausea and vomiting (International Association for the Study of Pain, 2011). Approximately 16%−91% women of reproductive age experience dysmenorrhea, 2%−29% of whom report the pain as severe or excruciating (Ju, Jones, & Mishra, 2014b). As the leading cause of absence from school and work among women, dysmenorrhea poses a great socioeconomic burden on society as a whole (Dawood, 1988).
Recent evidence suggests connections between dysmenorrhea and other co-occurring chronic pain conditions, such as migraines, fibromyalgia, irritable bowel syndrome, temporomandibular disorders, painful bladder syndrome, and abdominal musculoskeletal pain (Altman et al., 2006; Berkley, 2013; Shaver, Wilbur, Robinson, Wang, & Buntin, 2006). Dysmenorrhea can also aggravate other painful conditions (Altman et al., 2006; Giamberardino, 2008) and may increase women’s risk for future pain (Olafsdottir, Gudjonsson, Jonsdottir, Björnsson, & Thjodleifsson, 2012; Vincent et al., 2011).
Self-management involves both seeking care from health care providers, as well as independent activities to treat a condition and promote health (Miller, Lasiter, Ellis, & Buelow, 2015). A few US studies have documented the strategies that women use to self-manage dysmenorrhea symptoms, such as taking medications, resting, applying heat, using distraction, and ingesting special food or drink (Banikarim, Chacko, & Kelder, 2000; Jarrett, Heitkemper, & Shaver, 1995; Johnson, 1988; O’Connell, Davis, & Westhoff, 2006; Voge, 1996), but these studies have several limitations. Few were theory-based, and most used small (e.g., N=27) (Jarrett et al., 1995) or highly selective samples (e.g., Hispanic adolescents, adolescents participating in a contraceptive clinical trial, or military women) (Banikarim et al., 2000; O’Connell et al., 2006; Voge, 1996). Most studies were conducted in the 1980s or early 1990s (Jarrett et al., 1995; Johnson, 1988; Klein & Lift, 1981; White & Wildman, 1986), before nonsteroidal anti-inflammatory drugs (NSAIDs) were available over-the-counter, when fewer types of hormonal contraceptives were available, and before the use of complementary health approaches became mainstream (Consumer Healthcare Products Association, 2014; Guttmacher Institute, 2014; National Center for Complementary and Alternative Medicine, 2014).
Leventhal’s Common Sense Model (CSM) offers a systematic way to understand individuals’ health-related behaviors including self-management behaviors (Hagger & Orbell, 2003). The CSM and an extension proposed by Horne (1997, 2003) postulate that individuals construct common sense (“lay”) beliefs about their health problems, known as cognitive representations, to manage their health conditions (Leventhal et al., 2012; Leventhal, Nerenz, & Steele, 1984). Representations are beliefs about the etiology of the health condition (cause), its symptoms (identity), the impact on life functions (consequences), the expected duration (timeline), the expectations of how the person, health care provider, or treatment can influence or control the outcome of the health condition (control), the degree of overall understanding of the condition (coherence) (Leventhal et al., 2012; Moss-Morris et al., 2002; Weinman, Petrie, Moss-Morris, & Horne, 1996), and emotional responses to the health condition (Leventhal et al., 2012). According to the CSM, these cognitive and emotional representations influence which coping procedures individuals select including self-management behaviors (Hagger & Orbell, 2003; Leventhal et al., 2012).
Limited research in the US has systematically described women’s beliefs about dysmenorrhea or tested the relationship between beliefs about dysmenorrhea and self-management behaviors. One study showed that perceived symptomatology (i.e., identity) was a predictor of care seeking among women with dysmenorrhea (White & Wildman, 1986). Symptomatology, however, only explained a very small portion (2%) of the variance in care seeking in their study. In another study, women with dysmenorrhea were asked for the reasons behind not seeking care. Perceiving symptoms as not severe, and perceiving care-seeking as not helpful were cited as common reasons for not seeking care (Johnson, 1988).
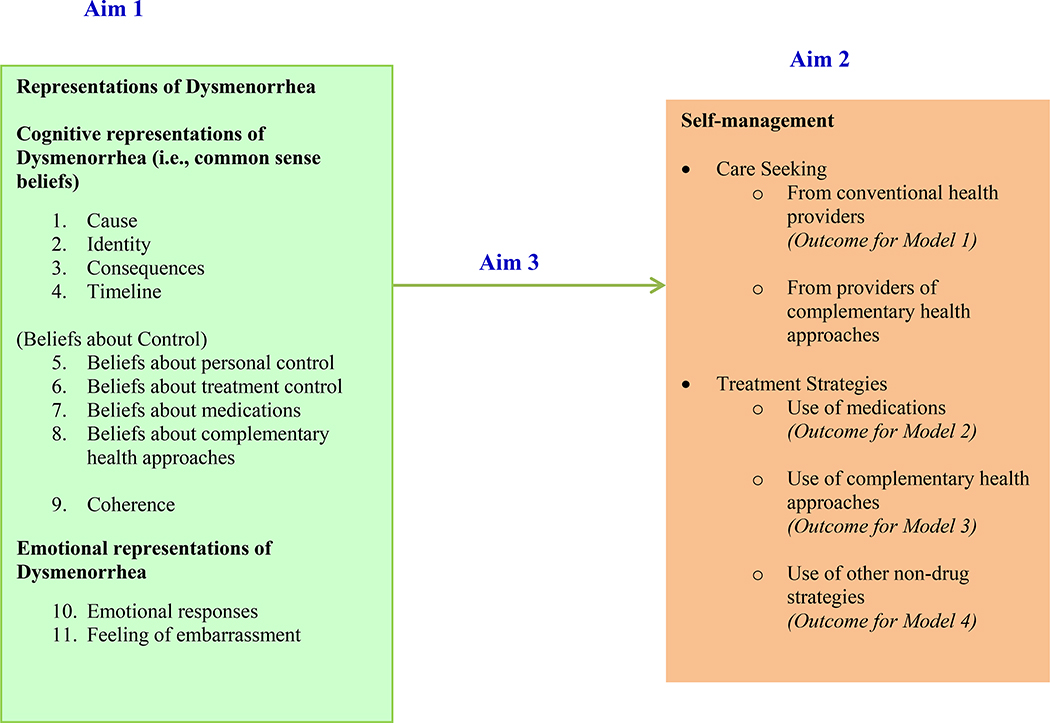
The purpose of this study was to understand women’s representations of dysmenorrhea and to investigate the association between representations and self-management of dysmenorrhea. Figure 1 shows the extended CSM applied to dysmenorrhea, as used in the current study. Self-management includes both seeking care from providers and use of self-management strategies. Complementary health approaches refer to practices and products whose origins are outside of mainstream medicine, including such products and practices as herbal supplements, other dietary supplements, meditation, and acupuncture (National Center for Complementary and Integrative Medicine (NCCIM), 2015). “Other non-drug strategies” refers to self-management strategies that do not fit the definition of complementary health approaches (e.g., rest and heat). The specific aims of this study were (1) to describe women’s representations of dysmenorrhea (including beliefs about causes, symptoms, consequences, timeline, controllability, the degree of overall understanding of dysmenorrhea / coherence, and emotional responses to dysmenorrhea); (2) to describe women’s self-management of dysmenorrhea, including seeking care from conventional health providers and complementary health providers, as well as use of medications, complementary health approaches, and other non-drug strategies; and (3) to investigate the association between representations and self-management of dysmenorrhea (seeking care, use of medications, use of complementary health approaches, and use of other non-drug strategies).
Figure 1.
The extended common sense model applied to dysmenorrhea self-management
Methods
Design and Sample
This was a cross-sectional descriptive study. We recruited participants from a list of survey panel registrants maintained by a survey provider (Qualtrics, Provo, UT). Eligibility criteria were adult women (≥18 years old), self-identifying as having experienced symptoms of dysmenorrhea in the last 6 months, and able to read and write in English.
The target sample size was at least 733 participants. This value was determined by the rule of ten events per explanatory variable (EPV) rule, where event denotes the cases belonging to the less frequent category in the outcome variable (Peduzzi, Concato, Kemper, Holford, & Feinstein, 1996). We used the formula N = 10 k / p (where p is the proportion of cases belonging to the less frequent category in the outcome variable and k is the number of explanatory variables) (Peduzzi, Concato, Kemper, Holford, & Feinstein, 1996). We had k =11 explanatory variables and estimated the proportion of cases belonging to the less frequent category (i.e., seeking professional care) to be p = 15%, based on the literature (Banikarim et al., 2000; Johnson, 1988). Thus, our minimal goal for sample size was N =10 * 11/.15 = 733.
Instruments
Representations of Dysmenorrhea
We measured representations of dysmenorrhea using the Brief Illness Perception Questionnaire (Brief IPQ) (Broadbent, Petrie, Main, & Weinman, 2006), the Beliefs about Medicines Questionnaire (BMQ)® (Horne, Weinman, & Hankins, 1999), and the Complementary and Alternative Medicine Beliefs Inventory (CAMBI) (Bishop, Yardley, & Lewith, 2005). All of these measures were developed based on the extended CSM, and have acceptable reliability and validity (Table 1) (Bishop et al., 2005; Broadbent et al., 2006; Broadbent, et al., 2015; Horne et al., 1999). Additional items were developed specifically for this study as noted in several sections below.
Table 1.
Summary of explanatory variables for logistic regressions
| Variable Name | Survey items | Scale score | Potential Range | Psychometrics |
|---|---|---|---|---|
| Cause-Normalcy | Two items designed for this study | Average of the item score | 1–5 High scores indicate strong normalcy belief |
Cronbach’s alpha = .85 for the current study |
| Identity/Beliefs about symptoms | Single item from the Brief-IPQ. | Single item score | 0–10 High scores indicate strong beliefs about symptom severity |
Test-retest reliability of this single-item scale over three weeks was 65 (Broadbent et al., 2006). Concurrent, predictive, and discriminant validity were supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Beliefs about consequences | Single item from the Brief-IPQ. | Single item score | 0–10 High scores indicate strong beliefs about negative consequences of dysmenorrhea symptoms |
Test-retest reliability of this single-item scale over three weeks was .70 (Broadbent et al., 2006). Concurrent, validity, and discriminant validity were supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Beliefs about timeline | Single item from the Brief-IPQ. | Single item score | 0–10 High scores indicate strong beliefs about the chronicity of symptoms until menopause |
Test-retest reliability of this single-item scale over three weeks was .67 (Broadbent et al., 2006). Concurrent, predictive, and discriminant validity were supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Beliefs about personal control | Single item from the Brief-IPQ. | Single item score | 0–10 High scores indicate positive beliefs about personal control of dysmenorrhea symptoms |
Test-retest reliability of the “personal control” item over three weeks was .63 (Broadbent et al., 2006). Concurrent, predictive, and discriminant validity were supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Beliefs about treatment control | Single item from the Brief-IPQ. | Single item score | 0–10 scale (High scores indicate positive beliefs about treatment control of dysmenorrhea symptoms) | Test-retest reliability of the “personal control” item over three weeks was .55 (Broadbent et al., 2006). Concurrent, predictive, and discriminant validity were supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Beliefs about medications | Eight items from the BMQ | Average of the item scores | 1–5 High scores indicate negative beliefs about medications. |
The BMQ measure has demonstrated adequate validity in previous research with people with a variety of health conditions (Horne et al., 1999). The Cronbach’s alpha for the current study was .85. |
| Beliefs about complementary health approaches | Twelve items from the CAMBI | Average of the item scores | 1–5 High scores indicate pro-complementary alternative medicine beliefs |
The reliability and validity of CAMBI were supported (Bishop et al., 2005). The Cronbach’s alpha for the current study was .85. |
| Beliefs about coherence | Single item from the Brief-IPQ. | Single item score | 0–10 High scores indicate positive beliefs about self-understanding of dysmenorrhea symptoms |
Test-retest reliability of this single-item scale over three weeks was .66 (Broadbent et al., 2006). Concurrent, predictive, and discriminant validity was supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Emotional representation | Single item from the Brief-IPQ. | Average of the item scores | 0–10 High scores indicate strong emotional responses to dysmenorrhea symptoms |
Test-retest reliability of this single-item scale over three weeks was .65. (Broadbent et al., 2006). Concurrent validity, predictive validity, discriminant validity was supported (Broadbent et al., 2006; Broadbent et al., 2015). |
| Emotional representation- feeling of embarrassment | Two items designed for the study | Single item score | 1–5 High scores indicate strong feelings of embarrassment about discussing dysmenorrhea symptoms |
The Cronbach’s alpha for the current study was .78. |
Cause
Causal attribution was measured using the single Brief-IPQ cause item. We asked the participants to list the leading factor that contributed to their dysmenorrhea symptoms. We categorized responses using codes previously generated by researchers who studied causal beliefs (Moss-Morris et al., 2002; Weinman et al., 1996). For any response that could not be categorized with the initial coding scheme, a new code was generated. All responses were coded independently by the lead author, and a subsample (20%) was also coded by a second researcher. Inter-rater agreement was satisfactory (Cohen’s Kappa =0.79).
Cause-normalcy
We designed two items for this study to measure normalcy beliefs. Participants were asked to rate their agreement with two statements on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree): “Dysmenorrhea symptoms are a normal part of the menstrual cycle,” and “Dysmenorrhea symptoms are part of being a woman.”
Identity
Beliefs about severity of dysmenorrhea symptoms were measured with the single Brief-IPQ identity item. The question asked participants to rate symptom severity on an 11-point scale, from 0 (not severe at all) to 10 (extremely severe).
Consequences
Beliefs about consequences of dysmenorrhea were measured with the single Brief-IPQ consequences item. The item asked participants to rate how much their dysmenorrhea symptoms affected their lives on an 11-point scale, from 0 (no effect at all) to 10 (severely affects my life).
Timeline
The Brief IPQ timeline question was adapted and asked participants to rate how long they thought their dysmenorrhea symptoms would continue on an 11-point scale. To better suit the condition of interest (i.e., symptoms of dysmenorrhea), the original 10-point anchor “forever” was changed to “until menopause.”
Control
According to our theoretical model, control beliefs encompass 1) beliefs about “personal control,” 2) beliefs about “treatment control,” 3) beliefs about medications, and 4) beliefs about complementary health approaches. We measured beliefs about “personal control” using the Brief-IPQ personal control item, which asked participants to rate how much control they had over dysmenorrhea symptoms from 0 (absolutely no control) to 10 (extreme amount of control). Beliefs about “treatment control” were measured with the Brief-IPQ treatment control item, which asked participants to rate how much they thought the strategies they used could help dysmenorrhea symptoms from 0 (not at all) to 10 (completely). Beliefs about medications were measured using the 8-item BMQ-General scale. The items asked participants to rate their agreement with statements about the dangers of medications (i.e., medications are harmful and overused). All items were rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Beliefs about complementary health approaches were measured using two subscales from the CAMBI. Six items asked participants to rate their agreement with positive statements about holistic health, and six items asked participants to rate their agreement with positive statements about natural treatments. Participants rated the extent to which they agreed with each statement using a 5-point scale from 1 (strongly disagree) to 5 (strongly agree).
Coherence
Understanding of dysmenorrhea was measured with the Brief-IPQ coherence item. Participants were asked to rate how well they understood their dysmenorrhea symptoms on an 11-point scale from 0 (don’t understand at all) to 10 (understand very clearly).
Emotional Representation- Emotional Responses
The Brief-IPQ item that measures emotional representations was used. The question asked participants to rate how much dysmenorrhea symptoms negatively affected them emotionally on an 11-point scale from 0 (not at all affected emotionally) to 10 (extremely affected emotionally).
Emotional Representation- Feeling of Embarrassment
Based on a review of the literature (Ortiz, Rangel-Flores, Carrillo-Alarcón, & Veras-Godoy, 2009; Wong, 2011), we added two items that assessed women’s embarrassment to communicate about their dysmenorrhea symptoms. Participants were asked to rate the extent to which they agreed with the statements, “I find it embarrassing to talk about menstrual symptoms with others” and “Menstrual symptoms should not be discussed socially.” The items were rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree).
Self-management of Dysmenorrhea
Self-management behaviors were measured with items designed for the current study. Participants were asked if they sought care for dysmenorrhea with reference time frames of “ever” and “in the last 6 months”. If they sought care, we asked what type of providers they saw (a checklist of provider types was given). Participants were categorized as seeking/not seeking care from conventional health providers, and seeking/ not seeking care from providers of complementary health approaches. To measure the use of treatment strategies, checklists of medications, complementary health approaches (including natural products, relaxation exercises, acupressure, yoga, following a special diet, nerve stimulation, and massage from a massage therapist) and other non-drug strategies ( including self-massage, rest, heat, and distraction) were provided. Open-ended questions asked participants to list any other medications or modalities that they used. Participants who used any of the medications were categorized as medication users; otherwise, they were categorized as medication non-users. Similarly, persons who used any of the complementary health approaches or other non-drug approaches were categorized as complementary health approach users or other non-drug approach users, respectively. Prior to launching the survey, we had piloted the survey among 26 individual women and two focus groups of community members from diverse racial, socioeconomic, and educational backgrounds to identify possible sources of difficulty/confusion and revised the survey questions accordingly.
Procedure
The local Institutional Review Board approved this study and data were collected in January and February, 2015. The survey provider (Qualtrics) used demographic data on file to send an email invitation to potentially eligible women. Women who were interested in participating clicked the survey hyperlink embedded in the message. A statement explaining consent appeared as the first page of the survey. A screening question followed, which further identified women who had experienced dysmenorrhea symptoms in the last 6 months. The definition of dysmenorrhea was given to participants as “abdominal cramps and other symptoms occurring just before or during a menstrual period (e.g., low back pain, nausea, vomiting, change in the number and type of bowel movements, change in appetite)”. Only women who passed the screening question were asked to complete the survey. Participants were allowed to skip questions that they prefered not to answer in order to reduce risk of distress or harm, and to prevent having a high drop-out rate (Dillman, 2007). Qualtrics reimbursed each participant <$3 for completion the survey.
Data Analysis
For Aims 1 and 2, we used descriptive statistics to summarize domains of dysmenorrhea representations and self-management behaviors. For Aim 3, we used multiple logistic regressions with maximum likelihood estimation to evaluate the relationships between the explanatory variables (domains of representations) and the outcome variables (self-management behaviors) (See Figure 1). Table 1 summarizes the explanatory variables for the logistic regressions and provides descriptions of corresponding scales. Four logistic regression models were created, one for each of four dichotomous (yes/no) outcome variables: (1) seeking care from conventional health providers, (2) using conventional medications, (3) using complementary health approaches, and (4) using other non-drug strategies. To prevent a serious violation of the 10 EPV rule for logistic regression, we did not perform the regression analysis for seeking care from providers of complementary health approaches as the proportion of cases was very small (< 3%); However, we did perform the regression analyses for seeking care from conventional health providers and using other non-drug approaches, where the violations were not as great (proportion of less frequent cases 12.6% and 11.8%, respectively). The type I error rate was set at .05 for each test. Data missing completely at random were imputed using expectation maximization algorithms (Little, 1988). Statistical analyses were performed using IBM SPSS Package V22.0 and R software.
Results
Sample Characteristics
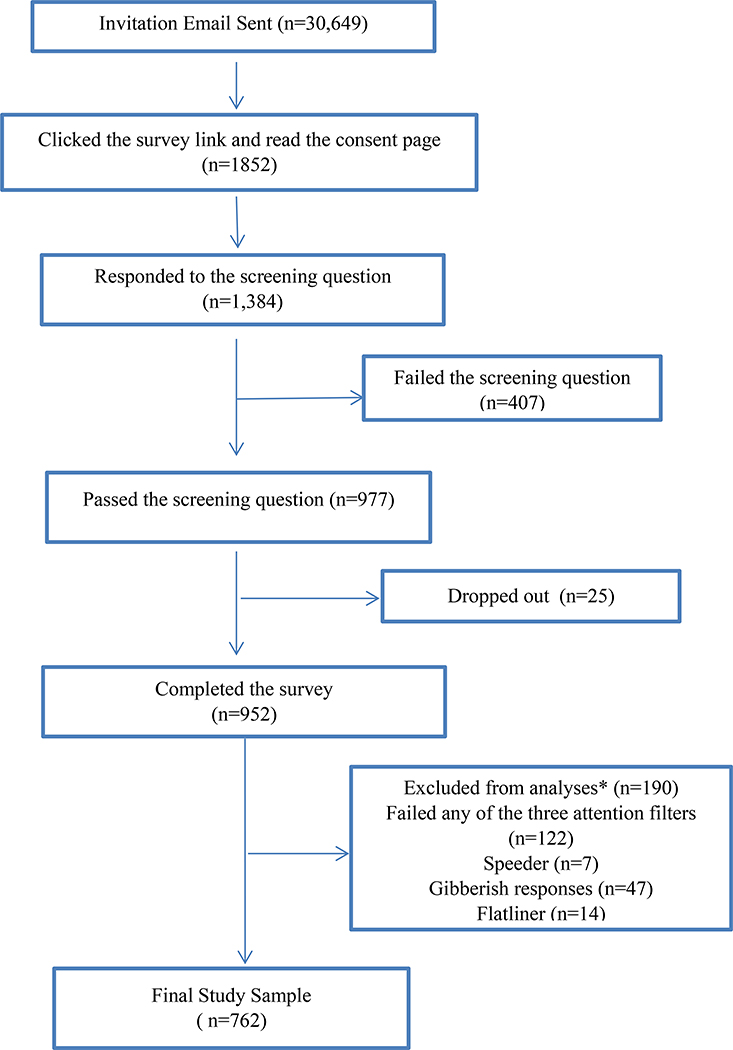
Figure 2 illustrates the flow of participants through the study. We analyzed responses from 762 participants. Demographic and clinical characteristics are reported in Table 2. We had a relatively diverse sample in terms of age (M=34, SD=7, range: 18–59,), race (75.6% Caucasian), education level (39% bachelor’s degree or above), and insurance status (85.6% having insurance). A small percentage of participants (18%) reported a history of a condition related to secondary dysmenorrhea (e.g., uterine fibroids, pelvic inflammatory disease, ovarian cysts and endometriosis). A majority of participants (69.7%) reported having another chronic pain condition (e.g., back pain, migraine headaches, non-migraine headaches, and irritable bowel syndrome).
Figure 2.
Flow of the participants
* We used several strategies to ensure the quality of survey responses. Three attention filters (“trap” questions) were used to flag respondents who were not paying close attention to directions. “Speeders” were the respondents who took less than 1/3 of the median time to complete the survey. “Flat-liners” were those who rushed through the survey and selected the same answer every time). We also dropped responses from people who put nonsense in the text entry questions.
Table 2.
Demographic and Clinical Characteristics (N=762)
| n | (%) | Mean (SD) | Demographic composition of the US population (%)* | |
|---|---|---|---|---|
| Age (years old) | ||||
| Race and Ethnicity | ||||
| African-American | 108 | (14.2) | 34.1 (6.6) | (13.2) |
| Asian | 45 | (5.9) | (5.4) | |
| Caucasian | 576 | (75.6) | (77.4) | |
| Native American | 15 | (2.0) | (1.2) | |
| Hispanic /Latino | 78 | (10.2) | (17.4) | |
| Education | ||||
| High school or below | 137 | (18.0) | ||
| Some College or Associate Degree | 328 | (43.0) | ||
| Bachelor’s and above | 297 | (39.0) | (29.3) | |
| Growing up in the US | 732 | (96.1) | ||
| Reported having insurance | 652 | (85.6) | ||
| Reported another chronic pain condition | 531 | (69.7) | ||
| History of conditions related to secondary dysmenorrhea | 137 | (18.0) |
Sources: U.S. Census Bureau (2015). QuickFacts, Retrieved from http://www.census.gov/quickfacts/table/PST045214/00
Representations of Dysmenorrhea
Table 3 summarizes the representations of dysmenorrhea. Commonly endorsed causes of dysmenorrhea were stress (14.3%), physiology / menstruation (13%), hormonal changes (12.6%), and diet / eating habits (9.6%), unknown / I don’t know (8.9%), heredity (6.7%), aging (4.6%), diseases or disorders (4.6%), and overweight (3.1%). Most participants (71.5%) agreed agreed that dysmenorrhea was a normal part of women’s lives. On average, participants believed that their symptoms were moderately severe with consequences that moderately affected daily life, and that the symptoms would continue until close to menopause. Participants believed that they had a moderate degree of personal control and treatment control over their symptoms. On average, participants had neutral beliefs about the dangers of medications and about the value of complementary health approaches. In terms of coherence, participants believed that they had a moderately clear understanding of dysmenorrhea symptoms. Participants reported they were moderately affected emotionally (e.g., upset or depressed) by their dysmenorrhea. Twenty percent of participants agreed that they felt embarrassed to talk about dysmenorrhea symptoms.
Table 3.
Representations of Dysmenorrhea (N = 762)
| Domains of Representation | Scale | Mean | SD |
|---|---|---|---|
| Cause-Normalcy | 1–5 scale (High scores indicate strong normalcy belief) | 3.65 | .94 |
| Identity/Beliefs about symptoms | 0–10 scale (High scores indicate strong beliefs about symptom severity) | 6.24 | 2.14 |
| Consequences | 0–10 scale (High scores indicate strong beliefs about negative consequences) | 5.86 | 2.36 |
| Timeline | 0–10 scale (High scores indicate strong beliefs about the chronicity of symptoms till menopause) | 8.63 | 2.05 |
| Personal control | 0–10 scale (High scores indicate positive beliefs about personal control of dysmenorrhea symptoms) | 4.11 | 2.51 |
| Treatment control | 0–10 scale (High scores indicate positive beliefs about treatment control of dysmenorrhea symptoms) | 5.45 | 2.10 |
| Beliefs about medications (harm and overuse) | 1–5 scale (High scores indicate negative beliefs about medications) | 3.11 | .76 |
| Beliefs about complementary health approaches | 1–5 scale (High scores indicate pro-complementary alternative medicine beliefs) | 3.87 | .51 |
| Coherence | 0–10 scale (High scores indicate positive beliefs about self-understanding of dysmenorrhea symptoms) | 5.72 | 2.69 |
| Emotional representation | 0–10 scale (High scores indicate strong emotional responses to dysmenorrhea symptoms) | 5.87 | 2.70 |
| Emotional representation- feeling of embarrassment | 1–5 scale (High scores indicate strong embarrassment about discussing dysmenorrhea symptoms) | 2.31 | 1.00 |
Self-management of dysmenorrhea symptoms
Care-Seeking
A small percentage of participants (12.6%) had sought care for dysmenorrhea in the last six months, while 33.2% had ever sought care for dysmenorrhea. For those who sought care at any point, gynecologists (20.9%), and general practitioners (16.8%) were the most commonly seen providers. Only seventeen (2.2%) participants had ever sought care from providers of complementary health approaches.
Self-management Strategies
Table 4 illustrates the strategies that participants had used to manage dysmenorrhea symptoms. A small number of participants used prescription pain medications, including NSAIDS (7.1%), opioids (6.3%) and acetaminophen (2%). Over-the-counter NSAIDs (41.2%), acetaminophen with caffeine (20.9%), and acetaminophen (14.3%) were commonly used. Hormonal contraceptives were commonly used to control dysmenorrhea symptoms (40.2%), with oral contraceptive pills as the most common form (20.9%). About one third (32.2%) of the participants had used medications to control gastrointestinal symptoms associated with dysmenorrhea. Complementary health approaches were used by most participants (73.2%), including relaxation (57.5%), natural products (34%), yoga (27.6%), and special diet (25.6%). Almost all participants (95.7%) had used other non-drug strategies, including rest (91.6%), heat (83.9%), distraction (77.3%), and self-massage (61.3%).
Table 4.
Treatment strategies used to manage dysmenorrhea symptoms (N= 762)
| Ever Used | Used in the last 6 months | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Medications | 604 | (79.3) | 557 | (73.1) |
| Prescription Pain Medications | 156 | (20.5) | 82 | (10.8) |
| NSAIDs | 54 | (7.1) | 32 | (4.2) |
| Opioids | 48 | (6.3) | 23 | (3.0) |
| Acetaminophen | 15 | (2.0) | 7 | (.9) |
| Others | 13 | (1.7) | 11 | (1.4) |
| Over-the-counter Pain Medications | 578 | (75.9) | 511 | (67.1) |
| NSAIDs | 314 | (41.2) | 290 | (38.1) |
| Acetaminophen and Caffeine | 159 | (20.9) | 140 | (18.4) |
| Acetaminophen | 109 | (14.3) | 103 | (13.5) |
| Others | 9 | (1.2) | 8 | (1.0) |
| Hormonal Contraceptives | 306 | (40.2) | 133 | (17.5) |
| Oral Contraceptive Pills | 159 | (20.9) | 85 | (63.9) |
| Hormonal IUDs | 23 | (3.0) | 20 | (15.0) |
| DMPA | 22 | (2.9) | 7 | (5.3) |
| Medications for gastrointestinal symptoms | 245 | (32.2) | 148 | (19.4) |
| Complementary health approaches | 558 | (73.2) | 381 | (50.0) |
| Relaxation | 428 | (57.5) | 263 | (34.5) |
| Natural products/ dietary supplements | 259 | (34.0) | 160 | (21.0) |
| Special diet (add/avoid certain food) | 195 | (25.6) | 109 | (14.3) |
| Nerve stimulation (e.g., TENS) | 73 | (8.3) | 28 | (3.7) |
| Yoga | 210 | (27.6) | 84 | (11.0) |
| Acupressure | 54 | (7.1) | 16 | (2.1) |
| Massage from a therapist | 133 | (17.5) | 44 | (5.8) |
| Other non-drug strategies | 729 | (95.7) | 672 | (88.2) |
| Rest | 698 | (91.6) | 609 | (79.9) |
| Heat | 639 | (83.9) | 511 | (67.1) |
| Distraction (e.g., watching television) | 589 | (77.3) | 481 | (63.1) |
| Self-massage (rubbing abdomen) | 467 | (61.3) | 331 | (43.4) |
NSAIDs: Nonsteroidal anti-inflammatory drug
IUD: Intrauterine Device
DMPA: Depot medroxyprogesterone acetate (i.e., Depo injection)
TENS: Transcutaneous electrical nerve stimulation
Associations Between Representations and Self-management Behaviors
In testing the associations between representation and self-management behaviors we used only recent (i.e., last 6 months) self-management behaviors as the outcome variable, to reduce the likelihood of using current beliefs to explain past behaviors. The item-level missing data rates ranged from 0%−7.8%, which is acceptable (Bennett, 2001; Fox-Wasylyshyn, & El-Masri, 2005). Because data were missing completely at random (MCAR), we imputed missing data using expectation maximization algorithms (Fox-Wasylyshyn, & El-Masri, 2005; Little, 1988). Sensitivity analyses indicated similar conclusions with or without imputing data. Variance inflation factors were all below three indicating that multicollinearity between the explanatory variables was not a concern. Hosmer and Lemeshow tests (Hosmer, 2013) showed that goodness of fit was acceptable for all four models (p>.05).
Table 5 summarizes the results for logistic regressions. Seeking care from conventional health providers was positively associated with beliefs that dysmenorrhea negatively impacts daily life (consequences) (OR=1.32, 95% CI [1.09,1.62]) and beliefs of having a clear understanding of dysmenorrhea (coherence) (OR=1.21, 95% CI [1.08,1.35]), but negatively associated with beliefs that dysmenorrhea symptoms would continue until menopause (timeline) (OR=.85, 95% CI [.74,.96]) and dysmenorrhea symptoms are normal (OR=.66, 95% CI [.52,.84]). Use of medications was negatively associated with beliefs about dangers of medications (OR=.56, 95% CI [.43,.74]. Use of complementary health approaches was positively associated with strong beliefs about negative consequences of dysmenorrhea (OR=1.29, 95% CI [1.15, 1.45]), strong beliefs about treatment control (OR=1.11, 95% CI [1.01–1.22]), and strong beliefs about the value of complementary health approaches (OR=2.20, 95% CI [1.54, 3.15]), but negatively associated with beliefs that dysmenorrhea symptoms are normal (OR=.76, 95% CI [.64-.91]). Use of other non-drug approaches was positively associated with strong chronicity (timeline) beliefs (OR=1.17, 95% CI [1.06, 1.29]), and strong beliefs about treatment control (OR=1.24, 95% CI [1.08–1.42]). We conducted post-hoc analyses controlling demographic variables (age, race, ethnicity, educational level, insurance status, access to care, and whether one was raised in the US) in each of the regression models. In these analysis, associations between beliefs and self-management behaviors were unchanged, and none of the demographic variables was a significant explanatory variable.
Table 5.
Variables explaining care seeking, use of medications, complementary health approaches, and other non-drug strategies in the last 6 months (N = 762)
| B | S.E. | Sig. | Odds Ratio | |
|---|---|---|---|---|
| Care Seeking from Conventional Health Provider | ||||
| Cause (Normalcy Belief) | −0.41 | 0.12 | 0.001 | *0.66 |
| Identity/ Beliefs about symptoms | 0.14 | 0.10 | 0.15 | 1.16 |
| Consequences | 0.28 | 0.10 | 0.006 | *1.32 |
| Timeline | −0.17 | 0.07 | 0.012 | *0.85 |
| Personal Control | 0.09 | 0.06 | 0.11 | 1.10 |
| Treatment Control | 0.08 | 0.07 | 0.28 | 1.08 |
| Beliefs about Medications (harm and overuse) | −0.18 | 0.18 | 0.31 | 0.83 |
| Beliefs about Complementary Alternative Medicine | −0.44 | 0.28 | 0.11 | 0.65 |
| Coherence | 0.19 | 0.06 | 0.001 | *1.21 |
| Emotional Representation | 0.12 | 0.07 | 0.08 | 1.13 |
| Feeling of Embarrassment | −0.05 | 0.13 | 0.67 | 0.95 |
| Constant | −2.40 | 1.22 | 0.05 | 0.09 |
| Mckelvey & Zavoina’s R2 = .38, Nagelkerke R2 =.27, McFadden R2 = .21 | ||||
| Use of Medication | ||||
| Cause (Normalcy Belief) | −.02 | .10 | .88 | .98 |
| Identity/ Beliefs about symptoms | .12 | .06 | .06 | 1.13 |
| Consequences | .04 | .06 | .54 | 1.04 |
| Timeline | .06 | .04 | .19 | 1.06 |
| Personal Control | −.03 | .05 | .52 | .97 |
| Treatment Control | .09 | .05 | .09 | 1.09 |
| Beliefs about Medications (harm and overuse) | −.58 | .14 | .000 | *.56 |
| Beliefs about Complementary Alternative Medicine | .08 | .20 | .68 | 1.09 |
| Coherence | .07 | .04 | .08 | 1.07 |
| Emotional Representation | .05 | .04 | .25 | 1.05 |
| Feeling of Embarrassment | −.04 | .09 | .69 | .97 |
| Constant | .23 | .87 | .80 | 1.25 |
| Mckelvey & Zavoina’s R2 = .13, Nagelkerke R2 =.13, McFadden R2 = .07 | ||||
| Use of Complementary Health Approaches | ||||
| Cause (Normalcy Belief) | −.27 | .09 | .003 | *.76 |
| Identity/ Beliefs about symptoms | −.10 | .06 | .09 | .90 |
| Consequences | .26 | .06 | .000 | *1.29 |
| Timeline | .02 | .04 | .56 | 1.02 |
| Personal Control | .05 | .04 | .26 | 1.05 |
| Treatment Control | .10 | .05 | .04 | *1.11 |
| Beliefs about Medications (harm and overuse) | −.08 | .12 | .52 | .93 |
| Beliefs about Complementary Alternative Medicine | .79 | .18 | .000 | *2.20 |
| Emotional Representation | .03 | .04 | .39 | 1.03 |
| Feeling of Embarrassment | −.05 | .08 | .55 | .95 |
| Constant | −4.3 | .83 | .00 | .01 |
| Mckelvey & Zavoina’s R2 = .17, Nagelkerke R2 =.17, McFadden R2 = .10 | ||||
| Use of Other Non-drug Strategies | ||||
| Cause (Normalcy Belief) | −.02 | .14 | .89 | .98 |
| Identity/ Beliefs about symptoms | .04 | .08 | .60 | 1.05 |
| Consequences | .13 | .08 | .13 | 1.14 |
| Timeline | .16 | .05 | .002 | *1.17 |
| Personal Control | −.08 | .06 | .19 | .92 |
| Treatment Control | .21 | .07 | .003 | *1.24 |
| Beliefs about Medications (harm and overuse) | −.06 | .19 | .73 | .94 |
| Beliefs about Complementary Alternative Medicine | −.15 | .27 | .57 | .86 |
| Coherence | −.01 | .06 | .89 | .99 |
| Emotional Representation | .08 | .06 | .17 | 1.08 |
| Feeling of Embarrassment | .10 | .12 | .41 | 1.11 |
| Constant | −.27 | 1.16 | .82 | .76 |
| Mckelvey & Zavoina’s R2 = .16, Nagelkerke R2 =.14, McFadden R2 = .10 | ||||
Discussion
Women have lay beliefs about dysmenorrhea that may or may not fit a biomedical model, but these beliefs can influence their self-management behaviors. Medically speaking, symptoms of dysmenorrhea are mainly caused by increased prostanoid production during endometrial sloughing (Dawood, 2006). Epidemiological evidence suggests that a positive family history, high stress, and extreme body mass index may also contribute to dysmenorrhea (Ju et al., 2014b; Ju, Jones, & Mishra, 2015). About half of our participants accurately attributed the cause and risk factors of dysmenorrhea symptoms to hormonal changes, menstruation, stress, heredity, and being overweight. Others (14.2%) endorsed causes such as diet/eating habits and aging, where evidence is still unclear (Ju, Jones, & Mishra, 2014a; Ju et al., 2014b). Nine percent of participants responded that they did not know the cause of dysmenorrhea. Educating women about the causes of dysmenorrhea may help them make effective treatment choices (e.g., using NSAIDs which blocks prostaglandin synthesis in the uterus instead of using acetaminophen which does not).
Women, in general, endorsed a strong normalcy belief about dysmenorrhea. This finding is consistent with Woods et al. (1992) who reported that many women perceived menstrual symptoms as a normal part of their lives. The current study also demonstrates that strong normalcy beliefs were a barrier to seeking care. This finding is consistent with research on endometriosis which reports that normalization of dysmenorrhea symptoms significantly delayed care seeking, diagnosis, and treatment (Hudelist et al., 2012) by an average of 11.7 years (Hadfiled et al., 1996). While dysmenorrhea symptoms do not always suggest underlying pathologies (e.g., endometriosis or pelvic inflammatory diseases), seeking professional care may help women whose symptoms are not well controlled and may reduce diagnostic and treatment delays for those with secondary dysmenorrhea. This study highlights the importance of reassuring women that dysmenorrhea is a legitimate reason to seek treatment. In addition, because many women believe that dysmenorrhea symptoms are normal, they may not disclose their experience during clinical encounters. Thus, it is necessary to screen women for dysmenorrhea symptoms in routine clinic visits and recommend effective treatment, if needed.
Our participants generally endorsed a chronic timeline of dysmenorrhea symptoms (i.e., they would continue until menopause). Research suggests that dysmenorrhea symptoms improve with increasing age (Ju et al., 2014b) and after childbirth (Ju et al., 2014b; Juang et als., 20006), but such improvement is not universal (Ju et al., 2014a). Interestingly, a strong chronicity belief was negatively associated with seeking care from providers but was positively associated with using non-drug strategies. Perhaps women’s expectations that dysmenorrhea will not abate until menopause leave them with a sense of needing to tolerate the symptoms on their own. It is important to educate women that there are effective treatments available for dysmenorrhea and they do not have to tolerate the symptoms until menopause.
With regard to control beliefs, participants, on average, believed that they had a moderate degree of personal and treatment control over dysmenorrhea symptoms, and neutral beliefs about dangers of medications and the value of complementary health approaches. Consistent with research in other populations, negative beliefs about medications (i.e., medications are harmful and overused) are barriers to medication use in dysmenorrhea, while pro-complementary alternative medicine beliefs (positive beliefs about holistic health and natural treatments) are facilitators of using complementary health approaches (Bishop et al., 2005; Horne et al., 1999). Such findings suggest the need to elicit women’s treatment beliefs and preferences, and address their concerns, particularly those that may prevent women from using effective treatments (e.g., misconceptions about pain medications and hormonal contraceptives) (Jensen & Speroff, 2000; Monsivais & McNeill, 2007).
In terms of coherence, participants, on average, believed that they had a moderately clear understanding of their dysmenorrhea symptoms. Coherence was positively associated with seeking care from conventional care providers. Because representations are dynamic in nature and can be influenced by interactions with medical professionals (Leventhal et al., 2012), it is possible that women’s knowledge of dysmenorrhea increased as a result of seeking care; however, this interpretation needs to be confirmed in future research using a longitudinal design.
Participants, on average, believed that their dysmenorrhea symptoms were moderate-to-severe (identity) and moderately affected their daily life (consequence). Care seeking from a health provider as well as use of complementary health approaches were positively associated with beliefs about negative consequences, but not associated with beliefs about symptom severity (i.e., identity). Findings from a Turkish study reported that perceived symptom severity was associated with the use of complementary health approaches (Midilli, Yasar, & Baysal, 2015), but a US study reports that professional care seeking and use of medications did not differ based on menstrual pain severity (moderate vs. severe) (O’Connell et al., 2006). In addition, an earlier study reported that perceived symptom severity accounted for only 2% of the variance in seeking care (White & Wildman, 1986). Based on our data, the perceived impact of dysmenorrhea on daily life may play a more important role in care seeking and self-management than do beliefs about symptomatology.
Regarding emotional representations, only a small percentage of participants (20%) agreed that they felt embarrassed to talk about dysmenorrhea symptoms. This finding is in contrast with another report in which 41% of female students at a US university endorsed the belief that menstruation is a taboo subject and should not be discussed in public (Berkley, 2013). Our study participants are likely to be older and more demographically diverse than Berkley’s sample. Future research should explore factors that may influence the embarrassment representation of dysmenorrhea.
The data show that women had used a wide variety of strategies to self-manage dysmenorrhea symptoms. Some of strategies have good evidence (e.g., NSAIDs, heat), while others have little evidence (e.g., acetaminophen, opioids) or inconclusive evidence (e.g., natural products and relaxation exercises).
Consistent with other reports (Banikarim et al., 2000; Johnson, 1988; O’Connell et al., 2006; Woods et al., 1992), prescription and over-the-counter pain medications were commonly used for dysmenorrhea. Among pain medications, NSAIDs are recommended as evidence-based treatment by practice guidelines (Marjoribanks, Proctor, Farquhar, & Derks, 2010). However, we noted that many women in our sample used some medications that are not evidence-based. While acetaminophen is not as effective for dysmenorrhea as NSAIDs (Marjoribanks, Proctor, Farquhar, & Derks, 2010), more than one third of our participants reported the use of acetaminophen alone or combination with caffeine. This wide use may be partially due to marketing advertisements for dysmenorrhea management products that contain acetaminophen. Also worth noting is that some women used opioids (6.3%) to control their dysmenorrhea symptoms, which is not recommended by clinical guidelines (Smith & Kaunitz, 2014). Consistent with guideline recommendations (Smith & Kaunitz, 2014), participants used hormonal contraceptives to manage their dysmenorrhea symptoms including oral contraceptive pills and other forms of hormonal contraceptives (e.g., hormonal intrauterine devices, Depo injection).
Almost all participants used some form of complementary health approaches and other non-drug strategies. Consistent with other studies (Banikarim et al., 2000; Jarrett et al., 1995; O’Connell et al., 2006; Woods et al., 1992), women commonly use rest, heat, distraction, relaxation, and special diet to manage dysmenorrhea symptoms. In addition to previously reported strategies, our study indicates that women use natural products / dietary supplements, yoga, and massage for dysmenorrhea. These findings coincide with the trend of increasing use of complementary health approaches in the US population (National Center for Complementary and Alternative Medicine, 2014).
Although a large percentage of women used complementary health and other non-drug approaches to manage dysmenorrhea, there is limited evidence to support or refute their efficacy (Smith & Kaunitz, 2014). One exception is heat, the efficacy of which is supported by randomized trials (Smith & Kaunitz, 2014). Another exception is transcutaneous electrical nerve stimulation (TENS). Based on a systematic review, high-frequency TENS is an effective treatment for primary dysmenorrhea (Proctor, Smith, Farquhar, & Stones, 2002); however, TENS was not widely used in the current sample. Limited evidence suggests that certain natural products, Chinese herbal medicine, acupuncture, acupressure, massage, and behavioral interventions (e.g., relaxation and guided imagery) may be effective; however, due to poor methodological quality of previous studies, no firm conclusions can be drawn (Dawood, 2006; Proctor & Murphy, 2001; Proctor, Murphy, Pattison, Suckling, & Farquhar, 2007; Zhu, Proctor, Bensoussan, Smith, & Wu, 2007). In addition, the efficacy of yoga, distraction, and massage for dysmenorrhea is not clear (Osayande, & Mehulic, 2014). More than 90% of participants used complementary health and non-drug approaches with limited or unclear evidence. This study highlights a strong need to study the efficacy of complementary health and non-drug approaches for dysmenorrhea given their high prevalence of use.
This study has several strengths. First, we studied self-management of dysmenorrhea using a current, diverse, and relatively large US sample. Our study demonstrates that dysmenorrhea is relevant not only to adolescents, but also to women across the reproductive age span and across racial and ethnic groups. Second, the current study collected detailed information about dysmenorrhea self-management, and identified areas for future research and development of dysmenorrhea self-management interventions. Third, the study systematically addressed women’s beliefs and emotional responses to dysmenorrhea using an established theoretical model. To the best of our knowledge, this is the first time that the Common Sense Model has been used to study dysmenorrhea. Our findings can provide insights to develop interventions tailored to women’s dysmenorrhea beliefs.
This study has limitations. First, the participants were self-selected individuals, who chose to sign up for the survey panel and who had Internet access. Due to coverage bias and self-selection bias, this is not a random sample. Because the sample was non-random, no estimates of sample error could be calculated. Second, the cross-sectional design prevents conclusions about causality, and it is possible that beliefs reflect the effect of using self-management strategies rather than beliefs causing self-management behavior. Third, due to the limited number of cases (2.2%), we could not explore factors influencing care seeking from complementary health providers. Interestingly, 5.8% of participants reported getting a massage from a massage therapist in the last six months, indicating that at least some participants did not interpret “providers of complementary health approaches” as we had intended. Fourth, two of our logistic models did not strictly meet the 10 EPV rule, with the proportions of cases lower than the number (15%) we used to estimate the sample size (12.6% for seeking care and 11.8% for not using other non-drug approaches in the last six months, which translates to 8.7 EPV and 8.2 EPV respectively). This may lead to slightly decreased power and biased model estimates (Peduzzi et al., 1996) Fifth, although multi-item scales are usually more reliable, we measured several domains of representations using single items from the Brief-IPQ (Broadbent et al., 2006). During the pilot testing of the survey instrument, respondents perceived the full length IPQ (over 80 items) (Moss-Morris et al., 2012) as too long and some items to be redundant. Based on their feedback, the Brief-IPQ was chosen over the IPQ to reduce survey length, response burden, and risk for incurring more missing data. Sixth, our models explained less than 13–38% variance in the outcome variables. Exploring the roles of other factors in dysmenorrhea self-management is warranted.
There are several directions for future research. First, future research should develop dysmenorrhea symptom management interventions that address individuals’ representations. Research on other health conditions has provided evidence for the effectiveness of addressing representations to improve self-management and health outcome (Donovan et al., 2007; McAndrew et al., 2008; Phillips, Leventhal, & Leventhal, 2012). Person-centered interventions that are tailored to individuals’ beliefs and preferences have been shown to be more effective than one-size-fits-all interventions (Lauver et al., 2002). A potential intervention may address normalcy beliefs (by reassuring women that dysmenorrhea is a legitimate reason for seeking treatment), chronicity beliefs (by informing women that dysmenorrhea symptoms can be managed, and that they do not have to be tolerated until menopause), and control beliefs (by addressing misconceptions regarding effective treatments and tailoring treatment recommendations to an individual’s preferences).
Second, women used a variety of complementary health approaches to manage dysmenorrhea symptoms, but many of those approaches have not been well studied. Effectiveness of complementary health approaches for dysmenorrhea should be further tested.
Third, given the unexplained variance in dysmenorrhea self-management, future work could investigate the roles of other factors (e.g., external factors) in dysmenorrhea self-management among US women. In the current study, we explored the role of internal factors (representations) in dysmenorrhea self-management. Research in Asian countries suggests that external factors (e.g, urban vs. rural residents, financial resources, experience with health education in dysmenorrhea) impact dysmenorrhea self-management (Chiu, Wang, Hsu, & Liu, 2013; Wong, 2011; Wong, Ip, Choi, & Lam, 2015), and similar factors should be explored among US women.
Clinicians should support dysmenorrhea management by recommending evidence-based strategies (e.g., NSAIDs, hormonal contraceptives, high-intensity TENS, and heat). In addition, clinicians may be able to provide better care if they are purposefully mindful of and responsive to women’s representations. Understanding individuals’ representations allow clinicians to provide new information in a specific, relevant, and individualized manner (de Ridder, Theunissen, & van Dulmen, 2007; Phillips et al., 2012). Research demonstrates that addressing patients’ lay beliefs has resulted in more discussion about patients’ concerns and preferences has led to an overall improvement in patient-clinician communication and self-management behaviors (de Ridder et al., 2007; Phillips et al., 2012).
In summary, our study shows that women use a variety of self-management strategies for dysmenorrhea, some of which are evidence-based (e.g., NSAIDs, hormonal contraceptives, heat) and others that are not (e.g., opioids, acetaminophen). Some domains of representations (e.g., normalcy beliefs, timeline beliefs, control beliefs) are associated with dysmenorrhea self-management, and may be targeted for future interventions. Clinicians should support evidence-based dysmenorrhea management and tailor self-management recommendations to women’s representations.
References
- Altman G, Cain KC, Motzer S, Jarrett M, Burr R, & Heitkemper M (2006). Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterology Nursing, 29(1), 4–11. doi: 10.1097/00001610-200601000-00002 [DOI] [PubMed] [Google Scholar]
- Banikarim C, Chacko MR, & Kelder SH (2000). Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Archives of Pediatrics and Adolescent Medicine, 154(12), 1226–1229. doi: 10.1001/archpedi.154.12.1226 [DOI] [PubMed] [Google Scholar]
- Bennett DA (2001). How can I deal with missing data in my study? Australian and New Zealand Journal of Public Health, 25(5), 464–469. doi: 10.1111/j.1467-842x.2001.tb00294.x [DOI] [PubMed] [Google Scholar]
- Berkley KJ (2013). Primary dysmenorrhea: an urgent mandate. Pain, 1, 1–8. Retrieved from http://www.belgianpainsociety.org/images/IASP_clinical_update20131.pdf [Google Scholar]
- Bishop F, Yardley L, & Lewith G (2005). Developing a measure of treatment beliefs: the complementary and alternative medicine beliefs inventory. Complementary Therapies in Medicine, 13(2), 144–149. doi: 10.1016/j.ctim.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Broadbent E, Petrie KJ, Main J, & Weinman J (2006). The brief illness perception questionnaire. Journal of Psychosomatic Research, 60(6), 631–637. doi: 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Broadbent E, Wilkes C, Koschwanez H, Weinman J, Norton S, & Petrie KJ (2015). A systematic review and meta-analysis of the brief illness perception questionnaire. Psychology and Health, 30(11), 1361–1385. doi: 10.1080/08870446.2015.1070851 [DOI] [PubMed] [Google Scholar]
- Chiu M, Wang H, Hsu S, & Liu I (2013). Dysmenorrhoea and self-care behaviours among hospital nurses: a questionnaire survey. Journal of Clinical Nursing, 22(21/22), 3130–3140. doi: 10.1111/jocn.12240 [DOI] [PubMed] [Google Scholar]
- Consumer Healthcare Products Association. (2014). Ingredients and dosages transferred from Rx-to-OTC Status (or new OTC approvals) by the Food and Drug Administration since 1975. Retrieved from http://www.chpa.org/SwitchList.aspx
- Dawood M (2006). Primary dysmenorrhea: advances in pathogenesis and management. Obstetrics and Gynecology, 108(2), 428–441. doi: 10.1097/01.aog.0000230214.26638.0c [DOI] [PubMed] [Google Scholar]
- Dawood MY (1988). Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. The American Journal of Medicine, 84(5A), 23–29. doi: 10.1016/0002-9343(88)90473-1 [DOI] [PubMed] [Google Scholar]
- de Ridder DTD, Theunissen NCM, & van Dulmen SM (2007). Does training general practitioners to elicit patients’ illness representations and action plans influence their communication as a whole? Patient Education and Counseling, 66(3), 327–336. doi: 10.1016/j.pec.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Dillman DA (2007). Mail and internet surveys: the tailored design method (2nd ed.), 2007 update with new internet, visual, and mixed-mode guide. Hoboken, N.J.: Wiley. [Google Scholar]
- Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, & Phillips CM (2007). An update on the representational approach to patient education. Journal of Nursing Scholarship, 39(3), 259–265. doi: 10.1111/j.1547-5069.2007.00178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox-Wasylyshyn S, & El-Masri M (2005). Focus on research methods. Handling missing data in self-report measures. Research in Nursing and Health, 28(6), 488–495. doi: 10.1002/nur.20100 [DOI] [PubMed] [Google Scholar]
- Giamberardino MA (2008). Women and visceral pain: Are the reproductive organs the main protagonists? Mini-review at the occasion of the “European Week Against Pain in Women 2007”. European Journal of Pain, 12(3), 257–260. doi: 10.1016/j.ejpain.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Guttmacher Institute. (2014). Contraceptive Use in the United States. Retrieved from http://www.guttmacher.org/pubs/fb_contr_use.html
- Hagger MS, & Orbell S (2003). A meta-analytic review of the common-sense model of illness representations. Psychology and Health, 18(2), 141–184. doi: 10.1080/088704403100081321 [DOI] [Google Scholar]
- Horne R (1997). Representations of medication and treatment: Advances in theory and measurement In Weinman JA (Ed.), Perceptions of health and illness: current research and applications (pp. 155–188). Amsterdam Netherlands: Harwood Academic Publishers. [Google Scholar]
- Horne R (2003). Treatment perceptions and self-regulation In Cameron L & Leventhal H (Eds.), The self-regulation of health and illness behaviour (pp. 138–153). London: Routledge. [Google Scholar]
- Horne R, Weinman J, & Hankins M (1999). The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology and Health, 14(1), 1–24. doi: 10.1080/08870449908407311 [DOI] [Google Scholar]
- Hosmer DW, & Lemeshow S (2000). Applied Logistic Regression. doi: 10.1002/0471722146 [DOI] [Google Scholar]
- Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, … Salzer H (2012). Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Human Reproduction, 27(12), 3412–3416. doi: 10.1093/humrep/des316 [DOI] [PubMed] [Google Scholar]
- International Association for the Study of Pain. (2011). Classification of chronic pain (2nd (Revised). Retrieved from http://www.iasp-pain.org/Content/NavigationMenu/Publications/FreeBooks/Classification_of_Chronic_Pain/default.htm
- Jarrett M, Heitkemper MM, & Shaver JF (1995). Symptoms and self-care strategies in women with and without dysmenorrhea. Health Care for Women International, 16(2), 167–178. doi: 10.1080/07399339509516167 [DOI] [PubMed] [Google Scholar]
- Jensen JT, & Speroff L (2000). Health benefits of oral contraceptives. Obstetrics and Gynecology Clinics of North America, 27(4), 705–721. doi: 10.1016/s0889-8545(05)70169- [DOI] [PubMed] [Google Scholar]
- Johnson J (1988). Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. Journal of Adolescent Health Care, 9(5), 398–402. doi: 10.1016/0197-0070(88)90036-8 [DOI] [PubMed] [Google Scholar]
- Ju H, Jones M, & Mishra G (2014a). Premenstrual syndrome and dysmenorrhea: symptom trajectories over 13 years in young adults. Maturitas. 78(2), 99–105. doi: 10.1016/j.maturitas.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Ju H, Jones M, & Mishra G (2014b). The prevalence and risk factors of dysmenorrhea. Epidemiologic Reviews, 36(1), 104–113. doi: 10.1093/epirev/mxt009 [DOI] [PubMed] [Google Scholar]
- Ju H, Jones M, & Mishra GD (2015). A U-shaped relationship between body mass index and dysmenorrhea: a longitudinal study. PLoS ONE, 10(7), 1–12. doi: 10.1371/journal.pone.0134187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR, & Lift IF (1981). Epidemiology of adolescent dysmenorrhea. Pediatrics, 68(5), 661. doi: 10.1016/s0002-7138(09)60931-6 [DOI] [PubMed] [Google Scholar]
- Lauver DR, Ward SE, Heidrich SM, Keller ML, Bowers BJ, Brennan PF, … Wells TJ (2002). Patient-centered interventions. Research in Nursing and Health, 25(4), 246–255. doi: 10.1002/nur.10044 [DOI] [PubMed] [Google Scholar]
- Leventhal H, Bodnar-Deren S, Breland JY, Hash-Converse J, Phillips LA, Leventhal EA, & Cameron LD (2012). Modeling health and illness behavior: the approach of the commonsense model In Baum A, Revenson TA, & Singer J (Eds.), Handbook of health psychology (2nd ed.). (pp. 3–35). New York, NY US: Psychology Press. [Google Scholar]
- Leventhal H, Nerenz D, & Steele D (1984). Illness representations and coping with health threats In Handbook of psychology and health. (pp. 221–252). New York: Erlbaum. [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 1198–1202. [Google Scholar]
- Marjoribanks J, Proctor M, Farquhar C, & Derks RS (2010). Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. The Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.cd001751.pub2 [DOI] [PubMed] [Google Scholar]
- McAndrew LM, Musumeci-Szabó TJ, Mora PA, Vileikyte L, Burns E, Halm EA, … Leventhal H (2008). Using the common sense model to design interventions for the prevention and management of chronic illness threats: from description to process. British Journal of Health Psychology, 13(2), 195–204. doi: [DOI] [PubMed] [Google Scholar]
- Midilli T, Yasar E, & Baysal E (2015). Dysmenorrhea characteristics of female students of health school and affecting factors and their knowledge and use of complementary and alternative medicine methods. Holistic Nursing Practice, 29(4), 194–204. doi: 10.1097/hnp.0000000000000091 [DOI] [PubMed] [Google Scholar]
- Miller WR, Lasiter S, Ellis RB, & Buelow JM (2015). Chronic disease self-management: a hybrid concept analysis. Nursing Outlook, 63(2), 154–161. doi: 10.1016/j.outlook.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais D, & McNeill J. (2007). Multicultural influences on pain medication attitudes and beliefs in patients with nonmalignant chronic pain syndromes. Pain Management Nursing, 8(2), 64–71. doi: 10.1016/j.pmn.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, & Buick D (2002). The revised illness perception questionnaire (IPQ-R). Psychology & Health, 17(1), 1–16. [Google Scholar]
- National Center for Complementary and Alternative Medicine. (2014). The Use of Complementary and Alternative Medicine in the United States. Retrieved August 27, 2014, from http://nccam.nih.gov/news/camstats/2007/camsurvey_fs1.htm
- National Center for Complementary and Integrative Medicine (NCCIM). (2015). What is Complementary, Alternative or Integrative Health? Retrieved December 28, 2015, from https://nccih.nih.gov/health/integrative-health
- O’Connell K, Davis AR, & Westhoff C (2006). Self-treatment patterns among adolescent girls with dysmenorrhea. Journal of Pediatric and Adolescent Gynecology, 19(4), 285–289. doi: 10.1016/j.jpag.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Björnsson E, & Thjodleifsson B (2012). Natural history of irritable bowel syndrome in women and dysmenorrhea: a 10-year follow-up study. Gastroenterology Research and Practice, 2012, 1–7. doi: 10.1155/2012/53420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MI, Rangel-Flores E, Carrillo-Alarcón LC, & Veras-Godoy HA (2009). Prevalence and impact of primary dysmenorrhea among Mexican high school students. International Journal of Gynecology and Obstetrics, 107(3), 240–243. doi: 10.1016/j.ijgo.2009.07.03 [DOI] [PubMed] [Google Scholar]
- Osayande AS, & Mehulic S (2014). Diagnosis and initial management of dysmenorrhea. American Family Physician, 89(5), 341–346. [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, & Feinstein AR (1996). A simulation study of the number of events per variable in logistic regression analysis. Journal of Clinical Epidemiology, 49(12), 1373–1379. doi: 10.1016/s0895-4356(96)00236- [DOI] [PubMed] [Google Scholar]
- Phillips LA, Leventhal H, & Leventhal EA (2012). Physicians’ communication of the common-sense self-regulation model results in greater reported adherence than physicians’ use of interpersonal skills. British Journal of Health Psychology, 17(2), 244–257. doi: 10.1111/j.2044-8287.2011.02035.x [DOI] [PubMed] [Google Scholar]
- Proctor ML, & Murphy PA (2001). Herbal and dietary therapies for primary and secondary dysmenorrhoea. The Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.cd002124 [DOI] [PubMed] [Google Scholar]
- Proctor ML, Murphy PA, Pattison HM, Suckling J, & Farquhar CM (2007). Behavioural interventions for primary and secondary dysmenorrhoea. The Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.cd00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor ML, Smith CA, Farquhar CM, & Stones RW (2002). Transcutaneous electrical nerve stimulation and acupuncture for primary dysmenorrhoea. The Cochrane Database of Systematic Reviews doi: 10.1002/14651858.cd002123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver J, Wilbur J, Robinson F, Wang E, & Buntin M (2006). Women’s health issues with fibromyalgia syndrome. Journal of Women’s Health, 15(9), 1035–1045. doi: 10.1089/jwh.2006.15.1035. [DOI] [PubMed] [Google Scholar]
- Smith RP, & Kaunitz AM (2014). Treatment of primary dysmenorrhea in adult women. In Barbieri RL (Ed.), UpToDate. Retrieved from http://www.uptodate.com/home/index.html
- Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, & Tracey I (2011). Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain, 152(9), 1966–1975. doi: 10.1016/j.pain.2011.03.029 [DOI] [PubMed] [Google Scholar]
- Voge VM (1996). Self-reported menstrual concerns of U.S. Air Force and U.S. Army rated women aircrew. Military Medicine, 161(10), 614–615. doi: 10.1097/00043764-199504000-00134 [DOI] [PubMed] [Google Scholar]
- Weinman J, Petrie KJ, Moss-morris R, & Horne R (1996). The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychology and Health, 11(3), 431–445. doi: 10.1080/08870449608400270 [DOI] [Google Scholar]
- White PA, & Wildman BG (1986). Factors related to medical help-seeking in women with menstrual discomfort. Behaviour Research and Therapy, 24(4), 471–474. doi: 10.1016/0005-7967(86)90012-4 [DOI] [PubMed] [Google Scholar]
- Wong CL, Ip WY, Choi KC, & Lam LW (2015). Examining self-care behaviors and their associated factors among adolescent girls with dysmenorrhea: An application of Orem’s self-care deficit nursing theory. Journal of Nursing Scholarship, 47(3), 219–227. doi: 10.1111/jnu.12134 [DOI] [PubMed] [Google Scholar]
- Wong LP (2011). Premenstrual syndrome and dysmenorrhea: urban-rural and multiethnic differences in perception, impacts, and treatment seeking. Journal of Pediatric and Adolescent Gynecology, 24(5), 272–277. doi: 10.1016/j.jpag.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Woods NF, Taylor D, Mitchell ES, & Lentz MJ (1992). Perimenstrual symptoms and health-seeking behavior. Western Journal of Nursing Research, 14(4), 418–443. doi: 10.1177/019394599201400402 [DOI] [PubMed] [Google Scholar]
- Zhu X, Proctor M, Bensoussan A, Smith CA, & Wu E (2007). Chinese herbal medicine for primary dysmenorrhoea. The Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.cd005288.pub3 [DOI] [PubMed] [Google Scholar]