Abstract
Background:
Drinking water contamination related to the use of aqueous film-forming foam (AFFF) has been documented at hundreds of military bases, airports, and firefighter training facilities. AFFF has historically contained high levels of long-chain per- and polyfluoroalkyl substances (PFAS), which pose serious health concerns. However, the composition and toxicity of legacy AFFF mixtures are unknown, presenting great uncertainties in risk assessment and affected communities.
Objectives:
This study aimed to determine the fluorinated and nonfluorinated chemical composition of a legacy AFFF sample and its toxicity in zebrafish embryos.
Methods:
A sample of legacy AFFF (3% application formulation, manufactured before 2001) was provided by the Massachusetts Department of Environmental Protection. High resolution mass spectrometry (HRMS) was used to identify PFAS and nonfluorinated compounds, and a commercial laboratory measured 24 PFAS by a modified U.S. EPA Method 537.1. AFFF toxicity was assessed in zebrafish embryos in comparison with four major constituents: perfluorooctanesulfonic acid (PFOS); perfluorohexanesulfonic acid (PFHxS); sodium dodecyl sulfate (SDS); and sodium tetradecyl sulfate (TDS). End points included values, and sublethal effects on growth, yolk utilization, and pancreas and liver development.
Results:
We identified more than 100 PFAS. Of the PFAS detected, PFOS was measured at the highest concentration () followed by PFHxS (). Fourteen nonfluorinated compounds were identified with dodecyl sulfate and tetradecyl sulfate the most abundant at 547.8 and , respectively. An of AFFF was calculated, representing a dilution of the 3% formulation. TDS was the most toxic of the constituents tested but could not predict the AFFF phenotype in larval zebrafish. PFOS exposure recapitulated the reduction in length but could not predict effects on development of the liver, which was the tissue most sensitive to AFFF.
Discussion:
To our knowledge, this research is the first characterization of the chemical composition and toxicity of legacy AFFF, which has important implications for regulatory toxicology. https://doi.org/10.1289/EHP6470
Introduction
Drinking-water contamination from activities related to the use of aqueous film–forming foam (AFFF) has been documented at hundreds of military bases, airports, and firefighter training facilities across the United States and abroad (Kishi and Arai 2008; Sullivan 2018). AFFF has historically contained high levels of long-chain per- and polyfluoroalkyl substances (PFAS). These anthropogenic surfactant and nonstick compounds present serious human and environmental health concerns because of their long half-lives (Li et al. 2018) and associated adverse outcomes (Sunderland et al. 2019).
Toxicity assessments of PFAS have been largely conducted on an individual chemical-by-chemical basis; however, the proprietary AFFF foams contain a multitude of unspecified PFAS congeners and other ingredients. The toxicity of the point source AFFF mixture is unknown, presenting great uncertainties to risk assessors and affected communities. Long-chain PFAS, such as perfluorooctanesulfonic acid (PFOS), have been voluntarily phased out of general use in the United States (U.S. EPA 2014), but these compounds are resistant to degradation and persist in the environment (Jian et al. 2017). In sites of historical AFFF use, PFAS contamination has been detected in groundwater, waterways (Høisæter et al. 2019; Houtz et al. 2018), and biota (Kannan et al. 2005; Lanza et al. 2017; Munoz et al. 2017; Oakes et al. 2010) even a decade after discontinued PFOS-based AFFF use (Filipovic et al. 2015). According to the U.S. Department of Defense, 60.7% of tested groundwater wells near installations where these foams were used were above the U.S. Environmental Protection Agency (U.S. EPA) Lifetime Health Advisory of combined 70 parts per trillion (ppt) for PFOS and perfluorooctanoic acid (PFOA) (Sullivan 2018).
PFOS is one of the most widely studied PFAS and has been associated with adverse outcomes in humans (Fleisch et al. 2017; Halldorsson et al. 2012; Høyer et al. 2015) and toxicity in animal models, including zebrafish (Menger et al. 2020; Sharpe et al. 2010; Xu et al. 2016). These outcomes include liver toxicity, thyroid disruption, neurotoxicity, immunotoxicity, cardiovascular toxicity, renal toxicity, and effects on the reproductive system (reviewed in Saikat et al. 2013; Zeng et al. 2019). Additionally, PFOS exposure has been demonstrated to be a developmental toxicant (ATSDR 2019).
Few studies have examined mixture toxicity of PFAS compounds. PFAS target multiple receptors, including the nuclear receptor peroxisome proliferator–activated receptor α ( and cellular transporters (Rosen et al. 2013; Zhao et al. 2017). The chain length and terminal moiety of the PFAS congeners lead to differing affinities of each molecule to target receptors (Weaver et al. 2010; Wolf et al. 2014; Zhang et al. 2014; Zhao et al. 2017) and plausibly an additive-type response on these receptors in PFAS mixture scenarios. However, in vivo studies have revealed that interactions are more complicated. In zebrafish, single mixtures of PFOS and PFOA were shown to have both additive and synergistic effects on toxicity, depending on the ratio of PFOS:PFOA in each mixture (Ding et al. 2013), and similarly reduced potencies were observed with complex PFAS mixtures in zebrafish behavioral responses (Menger et al. 2020). Therefore, it is critical to assess the toxicity of the PFAS mixtures present in complex AFFF samples.
The zebrafish is an important model for identifying developmental toxicants (Bambino and Chu 2017). The developing zebrafish is sensitive to PFAS toxicity (Chen et al. 2014; Dasgupta et al. 2020; Hagenaars et al. 2014; Jantzen et al. 2016; Menger et al. 2020; Zheng et al. 2011). PFAS have high water solubility, and waterborne concentrations can cross the protective chorion as well as enter the fish through oral, dermal, or gill absorption (Wang et al. 2015). However, the toxicokinetics can vary across PFAS congeners (Menger et al. 2020; Vogs et al. 2019). We have previously shown that nominal waterborne and PFOS exposures during development caused a reduction in larval growth and decreased yolk utilization (Sant et al. 2017); other groups have reported similar effects in zebrafish following developmental PFOS exposures (Chen et al. 2014; Hagenaars et al. 2014; Jantzen et al. 2016; Zheng et al. 2011). Our group has also identified pancreas-specific effects, including reduced exocrine pancreas length, decreased pancreatic endocrine beta cell islet area, and increased incidence of aberrant islet morphology (Sant et al. 2017).
This study identifies the chemical composition and tests the toxicity of a legacy PFOS-based AFFF formulation provided by the Massachusetts Department of Environmental Protection (MA-DEP), collected from within the state. To characterize the fluorinated and nonfluorinated chemical composition of the mixture, we used liquid chromatography–tandem mass spectrometry (LC-MS/MS) and high-resolution MS (HRMS). Toxicity was assessed in vivo using the zebrafish embryo model (Danio rerio), which has been shown to be sensitive to toxicants during developmental exposures and predictive of human health effects (Bambino and Chu 2017). Toxicity was assessed by identifying the and sublethal morphometric alterations in embryonic and larval zebrafish. Developmental lethality was compared with levels of the most prevalent PFAS and non-PFAS compounds found present in the AFFF sample.
Methods
Chemicals
A legacy AFFF, a 3% application formula, was acquired through the MA-DEP. The formulation and manufacturer of this mixture were unknown. A serial dilution of 0.003% AFFF was prepared for quantification analyses, and a serial dilution, starting at 0.00352% AFFF was prepared for toxicity testing. Perfluorooctanesulfonic acid (PFOS) (Sigma; Product 77282, Lot: BCBX5798) was prepared as a stock in 100% dimethylsulfoxide (DMSO). Perfluorohexanesulfonic acid (PFHxS) (Sigma; Product 50929, Lot: BCBX0925) was prepared as a stock. Additionally, a PFOS/PFHxS mixture was prepared containing PFOS and PFHxS. Sodium dodecyl sulfate (SDS) (Fisher Scientific, Product BP166-100, Lot: 136458) and sodium tetradecyl sulfate (TDS) (Alfa Aesar, Product B21941, Lot: U20F005) were prepared as stocks in deionized water.
Orbitrap Fusion™ Tribrid™ Mass Spectrometer
The nontarget screening of PFAS was carried out using an Orbitrap Fusion™ Tribrid™ Mass Spectrometer equipped with UltiMate™ 3000 UHPLC (Thermo Fisher Scientific), in negative ionization mode. The mass range was set at m/z 100–1,000 with the Orbitrap resolution power of 30,000. For ionization, a spray voltage of and an ion transfer tube temperature of 300°C were used. Chromatographic separation was performed on a Waters ACQUITY BEH C18 column ( and particle size). The column oven temperature was set at 40°C. The mobile phase consisted of a) ammonium acetate; and b) acetonitrile. The mobile phase flow rate was , and the following gradient program was used: initial conditions were 5% B held for , then increased to 95% B over where it was held for . The ratio of B was restored to 5% over and maintained for . A total analytical running time was , and the injection volume was .
The Xcalibur™ (version 4.1; Thermo Fisher Scientific) with Qualbrowser was used for the peak detection, and Compound Discoverer software (version 3.0.0; Thermo Fisher Scientific) with mzCloud was used for the nontarget screening of PFAS compounds. Reported in Table 1 are tentative identities of PFAS compounds beyond the 24 tested using a modified U.S. EPA Method 537.1. Relative peak intensities are provided for non-PFAS compounds in Table 2.
Table 1.
PFAS identified in the AFFF mixture using Orbitrap HRMS.
| No. | Compound name | Abbreviation | CAS RN® | Compound discoverer™ | MDL(ng/L) | PFAS in 3% AFFF (mg/L) | Relative % contribution to total PFAS content | Relative % contribution to total AFFF content | |
|---|---|---|---|---|---|---|---|---|---|
| Found | mzCloud match (%)a | ||||||||
| 1 | Perfluorooctyl sulfonate | PFOS | 1763-23-1 | Yes | 99 | 30,500 | 9,410 | 79.2 | 31.4 |
| 2 | Perfluorohexyl sulfonate | PFHxS | 355-46-4 | Yes | 99.8 | 20,000 | 1,500 | 12.6 | 5.0 |
| 3 | Perfluoropentane sulfonate | PFPeS | 2706-91-4 | Yes | 99.5 | 7,500 | 223 | 1.9 | 0.7 |
| 4 | Perfluorobutyl sulfonate | PFBS | 375-73-5 | Yes | 100 | 12,300 | 220 | 1.9 | 0.7 |
| 5 | Perfluoroheptane sulfonate | PFHpS | 375-92-8 | Yes | 98.6 | 23,800 | 157 | 1.3 | 0.5 |
| 6 | Perfluorohexanoic acid | PFHxA | 307-24-4 | Yes | 55.4 | 19,000 | 130 | 1.1 | 0.4 |
| 7 | Perfluorooctanoic acid | PFOA | 335-67-1 | Yes | 97.3 | 15,800 | 108 | 0.9 | 0.4 |
| 8 | Perfluoropentanoic acid | PFPeA | 2706-90-3 | Yes | 99.5 | 15,800 | 44.9 | 0.4 | 0.1 |
| 9 | Perfluoroheptanoic acid | PFHpA | 375-85-9 | Yes | ND | 22,800 | 43.6 | 0.4 | 0.1 |
| 10 | Perfluorobutanoic acid | PFBA | 375-22-4 | Yes | 98.2 | 25,000 | 38.9 | 0.3 | 0.1 |
| 11 | Perfluorononane sulfonate | PFNS | 68259-12-1 | Yes | 99.1 | 20,000 | ND | — | — |
| 12 | Perfluorodecane sulfonate | PFDS | 335-77-3 | Yes | 98.9 | 22,500 | ND | — | — |
| 13 | Perfluorooctane sulfonamide | FOSA | 754-91-6 | No | — | 2,50,000 | ND | — | — |
| 14 | Perfluorononanoic acid | PFNA | 375-95-1 | No | — | 6,750 | ND | — | — |
| 15 | Perfluorodecanoic acid | PFDA | 335-76-2 | No | — | 19,300 | ND | — | — |
| 16 | Perfluoroundecanoic acid | PFUnA | 2058-94-8 | No | — | 13,300 | ND | — | — |
| 17 | Perfluorododecanoic acid | PFDoA | 307-55-1 | No | — | 14,800 | ND | — | — |
| 18 | Perfluorotridecanoic acid | PFTriA | 72629-94-8 | No | — | 15,000 | ND | — | — |
| 19 | Perfluorotetradecanoic acid | PFTeA | 376-06-7 | No | — | 23,300 | ND | — | — |
| 20 | N-methylperfluorooctane sulfonamidoacetic acid | NMeFOSAA | 2355-31-9 | No | ND | 42,500 | ND | — | — |
| 21 | N-ethylperfluorooctane sulfonamidoacetic acid | NEtFOSAA | 2991-50-6 | No | ND | 37,500 | ND | — | — |
| 22 | 4:2 FTS | — | 757124-72-4 | No | ND | 1,30,000 | ND | — | — |
| 23 | 6:2 FTS | — | 27619-97-2 | No | ND | 1,15,000 | ND | — | — |
| 24 | 8:2 FTS | — | 39108-34-4 | No | ND | 72500 | ND | — | — |
| 25 | Perfluoro-1-hexanesulfonamide | — | 41997-13-1 | Yes | 99.7 | — | NA | — | — |
| 26 | N-(3-(Dimethylamino) propyl)tridecafluoro hexanesulphonamide | — | 50598-28-2 | Yes | 81 | — | NA | — | — |
Note: Concentrations of the compounds were analyzed with a modified U.S. EPA Method 537.1 by a commercial laboratory. —, no data; AFFF, aqueous film-forming foam; HRMS, high-resolution mass spectrometry; MDL, method detection limit; NA, not analyzed; ND, not detected; PFAS, per- and polyfluoroalkyl substances.
mzCloud match is percent match to compounds in the mzCloud mass spectral database.
Table 2.
Non-PFAS compounds detected in AFFF mixture using Orbitrap HRMS.
| No. | Name | CAS RN® | Molecular weight (g/mol) | RT (min) | mzCloud best match (%)a | Relative peak intensity | Concentration (mg/L) | Industrial uses |
|---|---|---|---|---|---|---|---|---|
| 1 | Tetradecyl sulfate | 1191-50-0 | 294.18642 | 8.363 | 100 | 6950653 | 496.4 | Wetting agent, emulsifier |
| 2 | Dodecyl sulfate | 151-41-7 | 266.15529 | 7.133 | 100 | 6229696 | 574.8 | Wetting agent, emulsifier |
| 3 | Octyl gallate | 1034-01-1 | 282.1500 | 6.85 | 88.6 | 3848182 | NA | Antioxidant |
| 4 | Lauric acid | 143-07-7 | 200.17761 | 7.340 | 99.9 | 649775 | NA | Soap production |
| 5 | Decanoic acid | 334-48-5 | 172.14613 | 6.531 | 99.9 | 236499 | NA | Lubricant |
| 6 | Oleic acid | 112-80-1 | 282.25596 | 9.386 | 99.7 | 240571 | NA | Soap emulsifier |
| 7 | 3,4-Dihydroxyphenyl propionic acid | 71693-95-3 | 182.0612 | 5.088 | 99.2 | 218594 | NA | Antioxidant |
| 8 | Pentadecanoic acid | 1002-84-2 | 242.22646 | 8.522 | 100 | 224956 | NA | Corrosion inhibitor, water repellant, plastic production |
| 9 | Nonanoic acid | 112-05-0 | 158.13043 | 6.111 | 99.9 | 198824 | NA | Plasticizer production |
| 10 | Disperse orange 3 | 730-40-5 | 242.08246 | 4.813 | 94 | 142701 | NA | Indicator dye (Type I aviation deicing fluid) |
| 11 | 4-Dodecylbenzene sulfonic acid | 121-65-3 | 326.1916 | 7.50 | 86.2 | 575934 | NA | Wetting agent, emulsifier |
| 12 | 2825-68-5 | 254.22479 | 8.362 | 99.8 | 68528 | NA | Pesticide production | |
| 13 | 4-methyl benzotriazole_2 | 29878-31-7 | 133.06364 | 4.331 | 9.79 | 34981 | NA | Corrosion inhibitor |
| 14 | 4-methyl benzotriazole_1 | 29878-31-7 | 133.06364 | 4.708 | 98.5 | 30637 | NA | Corrosion inhibitor |
Note: AFFF, aqueous film-forming foam; HRMS, high-resolution mass spectrometry; min, minutes; NA, not analyzed; non-PFAS, non–per- and polyfluoroalkyl substances; RT, retention time.
mzCloud match is percent match to compounds in the mzCloud mass spectral database.
PFAS Quantification
A sample of AFFF (1:1,000 serial dilution of the 3% sample) was sent to Eurofins TestAmerica, a commercial laboratory, for quantification of PFAS compounds in the AFFF formulation. Eurofins provides PFAS testing in water through a propriety modified method based on U.S. EPA Method 537.1. Table 1 contains the 26 PFAS and other fluorinated alkyl substances, with their method detection limits (MDL), that were identified using the Orbitrap Fusion™ Tribrid™ Mass Spectrometer.
Non-PFAS Surfactant Quantification
The surfactants SDS and TDS were prepared in stocks in water and acetonitrile, respectively. These concentrations were based on the active ingredient, excluding the sodium ion. One milliliter of the AFFF sample was added to a volume of water/acetonitrile solution (1:1, v/v). Serial dilutions of the solution were prepared to reach a final AFFF sample dilution of 1:10,000. Analysis was completed on a Waters ACQUITY UPLC H-class system equipped with Waters Xevo TQD Triple Quadrupole Mass Spectrometer having an electrospray ionization source. The quantitation of target compounds was performed in the negative ionization mode with the optimized multiple reaction monitoring transitions. Chromatographic separation was performed on a Phenomenex Kinetex PFP column ( and particle size). The mobile phase consisted of a) 0.1% formic acid in water and b) 0.1% formic acid in acetonitrile. The mobile phase flow rate was , and the following gradient program was used: initial conditions were 5% B held for , then increased to 95% B over , where it was held for . The ratio of B was restored to 5% over and maintained for . A total analytical running time was , and the injection volume was . These analyses were repeated in triplicate.
Zebrafish Husbandry
Adult zebrafish, Danio rerio, were housed in Aquaneering stand-alone systems. In the colony, fish were maintained in tanks containing 30–40 male and female fish. Fish were maintained on a 14:10h light:dark cycle and fed twice daily a diet of GEMMA Micro 300 (Skretting). Water quality was monitored daily to ensure pH was within pH 7.3–7.4, conductivity was within , and temperature was held at . Glass containers with mesh lids were placed into tanks with decreased water levels to encourage breeding. Breeding occurs roughly as the lights turn on in the facility, and embryos were collected from the containers at approximately 1 h post fertilization (hpf). Embryos were cleaned to remove dead embryos and debris. Mortality and morphometric assessments were conducted in the wildtype AB strain obtained from Zebrafish International Resource Center (ZIRC). Liver morphology was assessed in the Tg(gut:GFP) strain obtained from University of Massachusetts Medical School (UMass Medical), which fluoresce in the liver through the ef1a promoter (Field et al. 2003). Pancreas morphology was examined in Tg(ins:GFP), which fluoresce in the insulin-producing beta cells of the endocrine pancreas, and Tg(ptf1a:GFP), which fluoresce in the exocrine pancreas, also obtained from UMass Medical (diIorio et al. 2002; Godinho et al. 2005). All experiments were conducted following protocols of the University of Massachusetts Amherst Institutional Animal Care and Use Committee (A3551-01).
Exposure Paradigm for Lethality and Morphological Assessments
Determination of values and examination of morphological effects were accomplished using embryos of wildtype AB strain of fish. Embryos were screened under light microscopy, and healthy embryos staged at 3 hpf, the 1,000-cell stage, were selected for treatment. Fifteen embryos were assigned at random to each treatment or control group, with each embryo placed into an individual glass vial containing solution. Exposure solutions were prepared in Danieau’s solution, NaCl, KCl, , , HEPES buffer, pH 7.2 (Westerfield 2000). The 3% AFFF formulation was diluted so that the relative PFOS concentration of the dilution matched PFOS concentrations previously examined by our lab, PFOS (Sant et al. 2017). However, embryos were not viable at these AFFF concentrations, necessitating additional dilutions. Serial dilutions of the AFFF sample were prepared so that 8 concentrations from to AFFF, defined in Table 3, were examined for mortality assessment. Each vial contained total volume, and solutions were prepared and refreshed daily. Embryos were reared at , and embryonic health was monitored daily through 96 hpf, as outlined in OECD 236, Fish Embryo Acute Toxicity Test (OECD 2013). End points such as craniofacial malformations, spinal curvature, yolk malformations, swim bladder inflation, and hatch rate were noted. There was health and survival in the control group. This experiment was replicated a total of 4 times ( larvae per treatment group).
Table 3.
AFFF dilutions examined in the present study for morphometric assessment and concentrations of the two most abundant fluorinated, PFOS and PFHxS, and nonfluorinated SDS and TDS in these AFFF dilutions.
| AFFF dilution (%) | PFOS (mg/L) | PFHxS (mg/L) | SDS (mg/L) | TDS (mg/L) | |
|---|---|---|---|---|---|
| Stock | 3 | 9,410 () | 1,500 | 574.8 | 496.4 |
| Doses examined | 1.38 () | 0.22 | 0.084 | 0.073 | |
| 0.69 () | 0.11 | 0.042 | 0.036 | ||
| 0.14 () | 0.022 | 0.008 | 0.007 | ||
| 0.069 () | 0.011 | 0.004 | 0.004 |
Note: AFFF, aqueous film-forming foam; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; SDS, sodium dodecyl sulfate; TDS, sodium tetradecyl sulfate.
To provide comparison with the most prevalent PFAS, PFOS and PFHxS, individual compound exposures and coexposures were completed following the same paradigm. Stock concentrations of PFOS, PFHxS, and a PFOS/PFHxS mixture were prepared in DMSO as described above. Therefore, control embryos received a 0.01% DMSO control exposure. Dilutions were prepared in 0.3× Danieau’s solution to mimic PFOS and PFHxS doses present in the AFFF sample, and therefore, PFOS/PFHxS mixture held at a ratio of 6.27:1 PFOS:PFHxS. The dilutions are summarized in Table 4. These experiments were run at the same time and replicated thrice ( embryos in total).
Table 4.
Exposures to a PFOS and PFHxS mixture were prepared to replicate the contribution of these compounds in AFFF dilutions.
| Combined PFOS and PFHxS (mg/L) | PFOS contribution (mg/L) | PFHxS contribution (mg/L) | Relative % AFFF | |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 5.11 | 4.41 | 0.70 | 0.0014 | |
| 10.23 | 8.82 | 1.41 | 0.0028 | |
| 20.46 | 17.64 | 2.81 | 0.0056 | |
| 40.91 | 35.29 | 5.63 | 0.0113 | |
| 81.83 | 70.58 | 11.25 | 0.0225 | |
| 163.65 | 141.15 | 22.50 | 0.0450 | |
| 327.30 | 282.30 | 45.00 | 0.0900 | |
Note: These dilutions were made keeping the PFOS:PFHxS ratio (6.27:1) as defined in the legacy 3% AFFF sample. Single exposures to PFOS and PFHxS at these defined concentrations were completed alongside the mixture exposures. AFFF, aqueous film-forming foam; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid.
The use of DMSO was necessary in stock preparations of the PFAS compounds, therefore necessitating the use of DMSO as a solvent control in the toxicity testing of the individual PFAS compounds but not the AFFF sample. Dilutions of the stock were prepared such that DMSO concentrations were at 0.01% DMSO, which is previously reported to have no impact on zebrafish morphological development (Hallare et al. 2006; Turner et al. 2012; Kais et al. 2013).
This exposure paradigm was replicated for the surfactants, SDS and TDS. Stocks of each surfactant were prepared in distilled water at concentrations of . Stocks were diluted in 0.3× Danieau’s solution to reach test dilutions of 32, 16, 8, 4, 2, 1, and . An additional dose of was added to the TDS exposures. Each experiment was replicated thrice ( embryos in total).
Exposure Paradigms for Target Organ Assessments
A smaller range of AFFF concentrations (2.20 to AFFF) were examined in transgenic strains to assess impacts of exposure on development of endoderm-derived tissues and in wildtype fish with exposures carried to 120 hpf to examine swim bladder inflation. The transgenic strains examined for target organ assessments included the Tg(gut:GFP) for liver development, Tg(ins:GFP) for endocrine pancreatic beta cell islet development, and Tg(ptf1a:GFP) for exocrine pancreas development. The wildtype strain AB was replicated to examine swim bladder inflation at 120 hpf. For these target organ assessments, 10 embryos at 3 hpf were group housed in glass scintillation vials containing of AFFF dilutions. Solutions were prepared and refreshed daily. Transgenic experiments were carried until 96 hpf, and the swim bladder inflation was assessed at 120 hpf. Three vials were prepared to test each AFFF dilution, and each experiment was replicated 2–3 times. Average measurements are reported for each vial ( vials).
Our lab has previously reported that developmental exposure to PFOS reduced the growth of both the exocrine and endocrine pancreas in 96 hpf larvae (Sant et al. 2017). In the present study, the same a paradigm was followed to assess liver morphology in the Tg(gut:GFP) strain. Because the PFOS stocks were prepared in DMSO, an 0.01% DMSO control exposure was necessary. Embryo were exposed from 3 hpf to 96 hpf to 16 or PFOS, or 0.01% v/v DMSO. Solutions were prepared and refreshed daily. This experiment was replicated thrice ().
Microscopy and Image Analysis
Following exposures, larvae were thoroughly washed and imaged live. Larvae were anesthetized using 2% v/v MS-222, tricaine mesylate, and positioned in 3% methyl cellulose. Trans images for the morphological assessment of the nontransgenic larvae were captured using a Zeiss Axiozoom v16 microscope. Fluorescent tissues of the transgenic larvae were imaged using a customized Olympus upright microscope by Kramer Scientific and equipped with an Axiocam 503 camera (Carl Zeiss Inc.) and an 89 North® PhotoFluor® II light source. Images were analyzed using ZEN2 software, and larval length and yolk sac area were measured for all larvae.
Liver morphology was assessed through measurements of dorsal-ventral organ length which was normalized to individual body length. Similarly, exocrine pancreas anterior caudal length, which was measured from the depressed fluorescence of the endocrine beta cell islet to the pancreas tail, was normalized to individual body length. Stunted and severely stunted pancreatic phenotypes were determined using the thresholds of 10th and 1st percentiles of control pancreas lengths, respectively, as defined in Sant et al. (2019). The area of the primary endocrine pancreatic beta cell islets was measured, and qualitative assessment of islet structure was completed to stratify normal, tight structure from fragmented islets. The percentage of normal and fragmented morphologies was determined for the population of larvae in each vial, and averages of these are reported. All endoderm measurements and assessments were completed on blinded images.
Statistical Analyses
Data are presented as , and figures were produced using either Excel or GraphPad Prism 8.2.1 software. Statistical analyses were completed using JMP14 software (SAS Institute Inc.), and was defined as significant. values and 95% confidence intervals (CI) were determined through Probit analysis. Mortality curves were compared using parallelism tests of the Probit analysis. Statistical significance for developmental measurements was determined through one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc test. A Fisher’s Exact test was used to determine significance of aberrant pancreas morphologies.
Results
Analytical Chemistry
Of the 24 perfluoroalkyl compounds assessed using a modified EPA Method 537.1, 10 compounds were detected at quantifiable levels (Table 1). PFOS made up 79.2% of the detected PFAS, followed by PFHxS (12.6%) for a subtotal of 91.8% between these two compounds. Perfluorobutyl sulfonate (PFBS) and perfluoropentane sulfonate (PFPeS) (both at 1.9%), perfluoroheptane sulfonate (PFHpS; 1.3%), perfluorohexanoic acid (PFHxA; 1.1%) PFOA (0.9%), perfluoropentanoic acid (PFPeA; 0.4%), perfluoroheptanoic acid (PFHpA; 0.4%), and perfluorobutanoic acid (PFBA; 0.3%) made up the remaining 8.1% of the total quantified PFAS content. These 10 PFAS were also identified using the nontargeted Orbitrap HRMS method (Table 1). PFNS and PFDS were identified by the Orbitrap HRMS method but were not quantified by the modified EPA method because they were below the MDL. The dilution of the AFFF sample analyzed by Eurofins was too great to quantitate PFNS and PFDS because a 1:1,000 dilution was necessary to bring the PFOS and PFHxS concentrations within the working range of the instrument (Table 1). Perfluoro-1-hexanesulfonamide and N-(3-(dimethylamino)propyl)tridecafluorohexanesulfonamide were also identified by the Orbitrap HRMS method. None of the remaining 13 compounds of the 24 PFAS listed in the EPA method were detected by either approach.
An additional 100 PFAS with relative intensity percent values greater or equal to 0.1% of PFOS were detected using exact mass by Orbitrap HRMS (Table S1). Of these 100 PFAS, there were 21 unidentified fluorinated compounds with relative intensity percent values greater or equal to 1.0% of PFOS, representing the first 21 compounds in Table S1. However, the compounds were not assigned structures because the mzCloud software was not able to detect fragmentation patterns of known compounds in the library.
There were also 14 nonfluorinated compounds identified in the AFFF sample using the Orbitrap HRMS method (Table 2). SDS and TDS were the most abundant in the AFFF mixture.
Concentrations of the two most prevalent non-PFAS compounds found in the nontarget analysis, SDS and TDS, were determined through HRMS. SDS was present in the AFFF sample at a concentration of , and TDS was present at a concentration of .
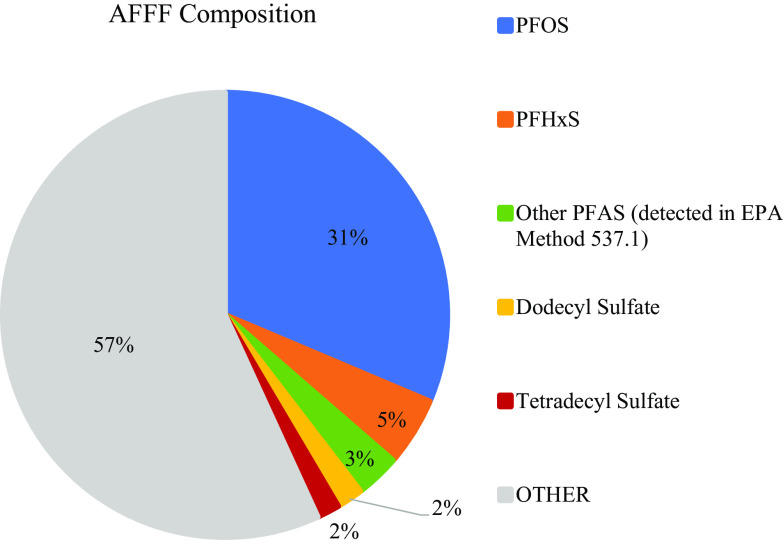
Figure 1 depicts a breakdown of the composition of the 3% AFFF sample, assuming that the sample represents an accurate preparation of solids per water. PFOS was the most abundant compound at (31% of all the analytes measured in the AFFF). PFHxS was the second most abundant PFAS at (5% of all the analytes measured in the AFFF). Eight other PFAS were quantified through the EPA Method 537.1, together at a concentration of (3% of all the analytes measured in the AFFF). Assessment of the relative peaks of the compounds detected in the nontarget analysis revealed SDS and TDS to be abundant non-PFAS compounds in the AFFF sample. These were quantified at SDS and TDS. Each surfactant comprised roughly 2% of the AFFF sample. The remaining 57% of the AFFF sample consisted of other PFAS and non-PFAS compounds identified, but not quantified, through nontarget analysis, as well as other compounds not detected in our methods.
Figure 1.
Composition of the legacy 3% aqueous film-forming foam (AFFF) sample. Ten PFAS were quantified through U.S. EPA Method 537.1: PFOS (, 31% of sample), PFHxS (, 5% of sample), and other PFAS (together , 3% of sample). Two non-PFAS compounds discovered through the nontarget analysis were quantified: SDS (, 2% of sample) and TDS (, 2% of sample). The remaining “Other” is composed of PFAS and non-PFAS compounds identified through the nontarget analysis yet not quantified, as well as compounds not detected through these methods. Note: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid.
Developmental Lethality Assessment
The of the AFFF mixture at 96 hpf was calculated to be AFFF (Figure 2A). The 95% CI were to AFFF. The relative concentrations of each compound in the AFFF are summarized in Table 5.
Figure 2.
Larval mortality at 96 h post fertilization (hpf) with varying concentrations of AFFF) and individual constituents of the AFFF sample. Exposures in wildtype embryos began at 3 hpf and were refreshed daily until 96 h post fertilization (hpf). Each experiment consisted of 15 individually exposed embryos per dose. AFFF exposures were replicated 4 times (), whereas all other compounds were replicated 3 times (). (A) AFFF mortality curve. The was AFFF, and 95% CI: to AFFF. (B) Mortality curves for single exposures to PFOS, PFHxS, and PFOS/PFHxS mixture, maintained at a ratio of these congeners equal to that determined in that AFFF sample. (C) Mortality curves of the non-PFAS surfactants, SDS and TDS. Note: AFFF, aqueous film-forming foam; PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; SDS, sodium dodecyl sulfate; TDS, sodium tetradecyl sulfate.
Table 5.
Comparison of in 96 hpf larvae following exposure to AFFF and its main constituents.
| PFOS (mg/L) | PFHxS (mg/L) | PFAS mixture (total PFOS & PFHxS mg/L) | SDS (mg/L) | TDS (mg/L) | |
|---|---|---|---|---|---|
| Amount of each in AFFF : AFFF | 2.32 | 0.37 | 2.69 | 0.14 | 0.12 |
| per compound | 31.03 | N.D. | 29.63 | 3.67 | 0.66 |
| 95% Confidence interval | (23.15, 42.99) | (24.18, 36.14) | (3.09, 4.38) | (0.59, 0.74) |
Note: The first row lists the concentrations of perfluorooctanesulfonic acid (PFOS), perfluorohexanesulfonic acid (PFHxS), PFOS/PFHxS in combination, dodecyl sulfate and tetradecyl sulfate found in the of AFFF ( AFFF) are listed. The second and third rows list the and 95% confidence intervals, determined in the present study, of each compound. AFFF, aqueous film-forming foam; ND, not determined in this study; PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; SDS, sodium dodecyl sulfate; TDS, sodium tetradecyl sulfate.
From exposures to PFOS alone, an of PFOS was calculated (95% CI: , Figure 2B). Test concentrations of PFHxS were too low to derive an value. An value of PFAS was determined for the PFOS/PFHxS mixture (95% CI: ; Figure 2B). Although this value was slightly lower than the of the mixture, the CI of the PFAS mixture and PFOS alone overlapped. However, exposures to PFOS and the PFOS/PFHxS mixture produced mortality curves that were statistically different from the AFFF mortality curve (PFOS: for growth rate, inflection point, and upper asymptote; PFOS/PFHxS mixture for growth rate, for inflection point, and for upper asymptote).
Developmental toxicity was also assessed for the non-PFAS surfactants, TDS and SDS, (Figure 2C). The of TDS was (95% CI: ), and the of SDS was (95% CI: ). Neither compound produced a mortality curve statistically different from the mortality curve of AFFF ( for growth rate, inflection point and upper/lower asymptote). These values are summarized in Table 5. Taken together, the AFFF mixture was observed to be more toxic to the developing zebrafish than were exposures to any of the four individual constituents found in the highest concentrations in the AFFF sample.
Morphological Assessment
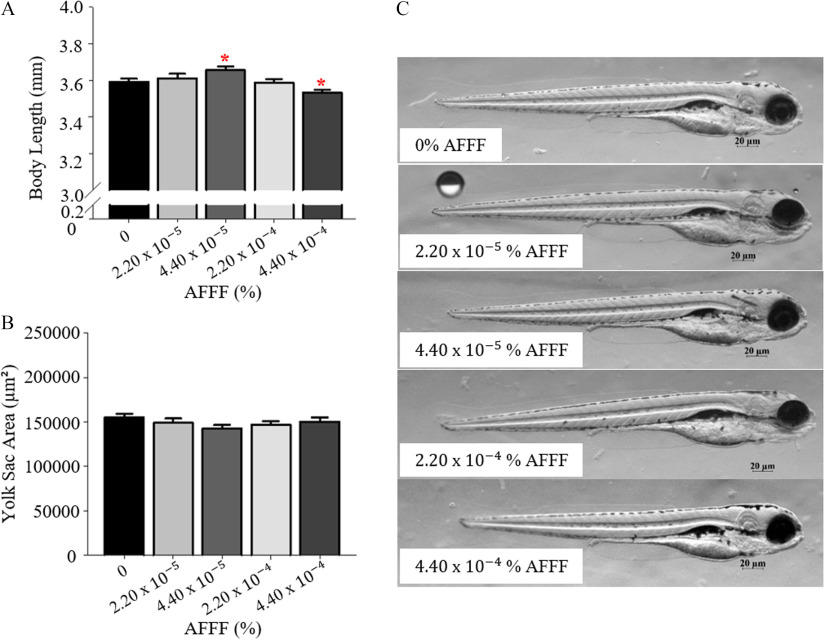
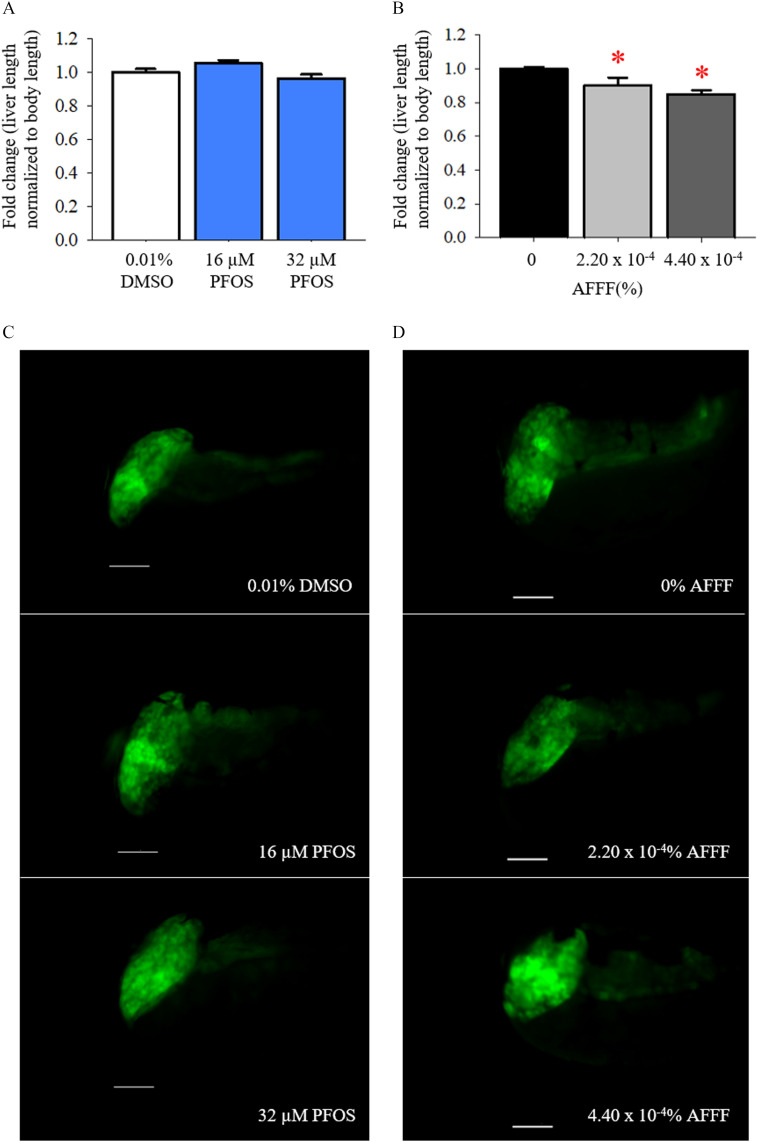
Exposure to AFFF affected the development of the wildtype larvae, specifically in larval length (Figure 3). Larvae exposed to the highest concentration of AFFF had significantly reduced body lengths compared to control larvae (). It is interesting to note that larvae exposed to the concentration of AFFF were significantly longer in body length than were control larvae (). There was no significant difference in yolk sac area following AFFF exposure (Figure 3B). Additional exposures to AFFF were completed to 120 hpf to examine whether AFFF exposure impacted swim bladder inflation, yet no significant effects were observed (Figure S1).
Figure 3.
Larval developmental measurements at 96 h post fertilization (hpf) with AFFF exposures. Larval body length (A) and yolk sac area (B) are graphed. (C) Representative images from AFFF treatment groups. Bars represent . Asterisk (*) indicate compared with 0% AFFF, one-way ANOVA, Tukey’s HSD post hoc test. larvae per treatment group. Note: AFFF, aqueous film-forming foam; ANOVA, analysis of variance.
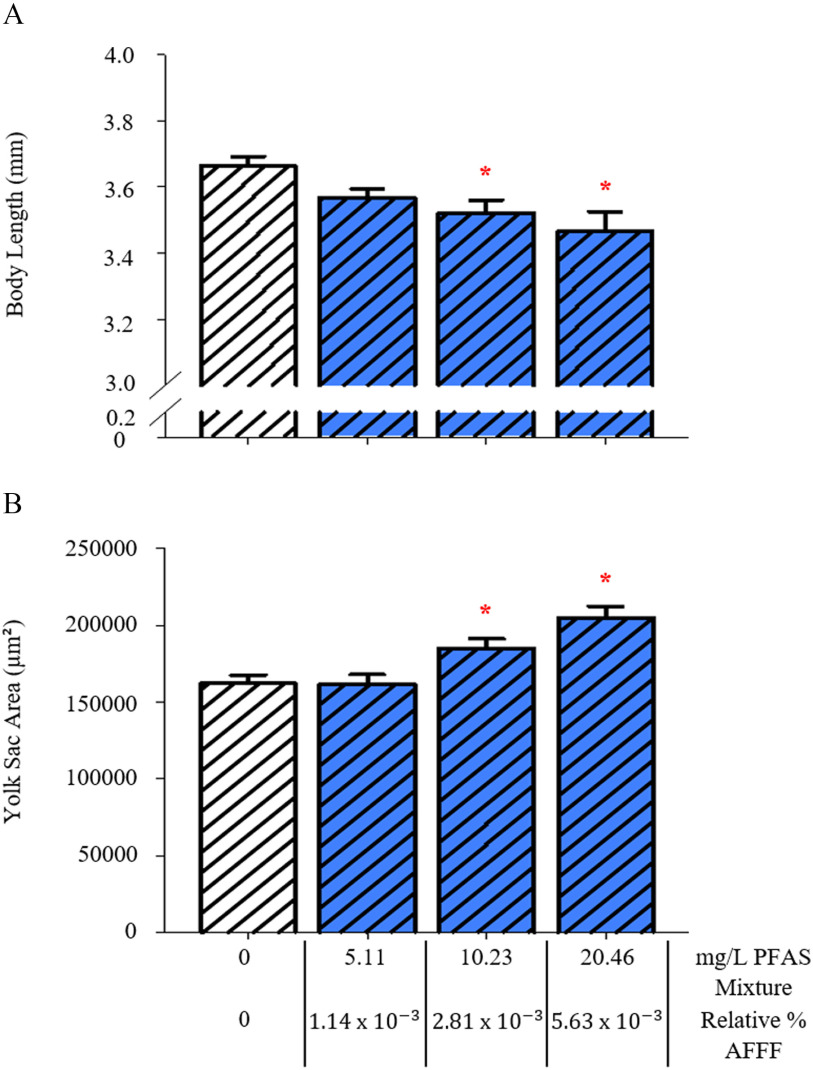
Larvae exposed to the PFOS/PFHxS mixture had a significantly shorter body length at 96 hpf after exposure to PFAS (equivalent to the amount of combined PFOS and PFHxS in AFFF, ) and PFAS (equivalent to AFFF, ) (Figure 4A and Figure S2). These larvae also had a significantly larger yolk sac area after exposure to PFAS () and PFAS () (Figure 4B).
Figure 4.
Measurements of larval development with exposure to a perfluorooctanesulfonic acid/perfluorohexanesulfonic acid (PFOS/PFHxS) mixture, at a ratio consistent with the legacy AFFF sample (6.21:1, PFOS:PFHxS). Larval body length (A) and yolk sac area (B) are reported as total PFAS concentration of the mixture and the relative % AFFF those mixtures represent. Control larvae were exposed to a 0.01% v/v DMSO solution. Bars represent . Asterisk (*) indicate compared with PFAS mixture, one-way ANOVA, Tukey’s post hoc test, larvae per treatment group. Note: AFFF, aqueous film-forming foam; ANOVA, analysis of variance; PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid.
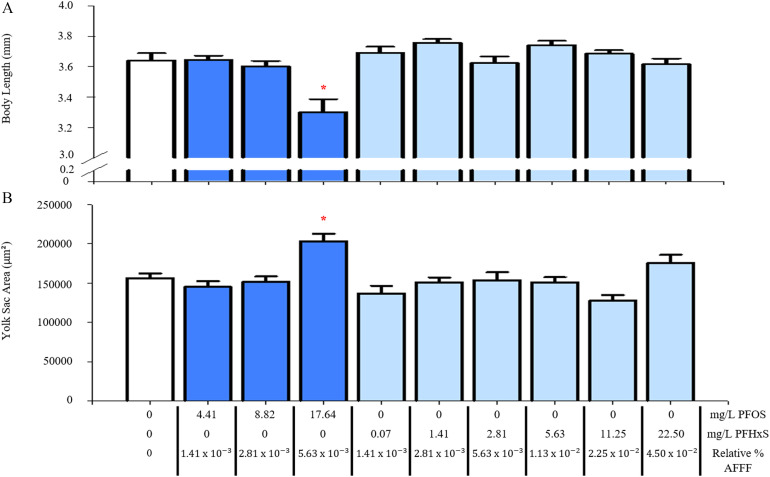
Single exposures to PFOS and PFHxS were also completed to test their relative contribution to toxicity. Exposure to PFOS alone at (equivalent to the amount of PFOS in AFFF) resulted in significantly smaller larval length () and larger yolk sac area () (Figure 5 and S2). However, PFHxS up to (equivalent to the amount of PFHxS found in AFFF) was insufficient to affect either morphometric end point.
Figure 5.
Measurements of larval development with single exposures to PFOS or PFHxS. Larval body length (A) and yolk sac area (B) are reported as PFOS or PFHxS concentration and the relative % AFFF those concentrations represent. Control larvae were exposed to a 0.01% v/v DMSO solution. Bars represent . Asterisk (*) indicate compared with PFOS and PFHxS, one-way ANOVA, Tukey’s post hoc test, larvae per treatment group. Note: AFFF, aqueous film-forming foam; ANOVA, analysis of variance; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid.
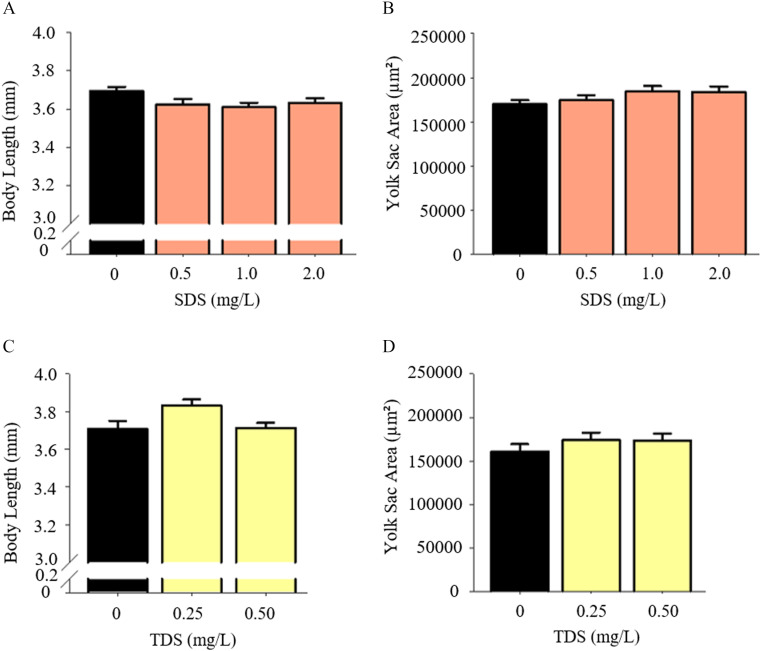
Larvae exposed to SDS and TDS, doses below their respective , appeared to not have different body lengths or yolk sac areas compared with control larvae (Figure 6 and Figure S3).
Figure 6.
Larval growth measurements with exposure to non-PFAS surfactants. Larval body length (A) and yolk sac area (B) were examined at concentrations up to SDS. Larval body length (C) and yolk sac area (D) were examined at concentrations up to sodium TDS. Bars represent . larvae per treatment group. . Note: non-PFAS, non–per- and polyfluoroalkyl substances; SDS, sodium dodecyl sulfate; TDS, sodium tetradecyl sulfate.
Liver Development
In the present study, developmental PFOS exposures were completed in Tg(gut:GFP) embryos through 96 hpf to provide a comparison with the morphological effects with AFFF exposure on liver development. Liver lengths were normalized to body length to account for the changes in growth observed in PFOS- and AFFF-exposed larvae. Developmental exposure to PFOS did not appear to affect the elongation of the liver (Figure 7A). In contrast, larvae exposed to AFFF had significantly smaller liver lengths at doses of AFFF () and AFFF () in comparison with control larvae (Figure 7C). The liver length measurements were consistent with liver area measurements, where mean liver areas normalized to body length were 0.027, 0.026, and for 0, , and AFFF, respectively.
Figure 7.
The impacts of liver elongation with developmental PFOS and AFFF exposures in 96 h post fertilization (hpf) larvae after developmental PFOS exposures, (A) liver length normalized to larval body length are graphed and (C) representative image are depicted. Scale bars represent in all images. Following developmental AFFF exposures, (B) liver length normalized to larval body length are graphed and (D) representative image are depicted. Bars represent . Asterisk (*) indicate compared with 0% AFFF, one-way ANOVA, Tukey’s HSD post hoc test. PFOS exposures: . AFFF exposures: . Note: AFFF, aqueous film-forming foam; ANOVA, analysis of variance; PFOS, perfluorooctanesulfonic acid.
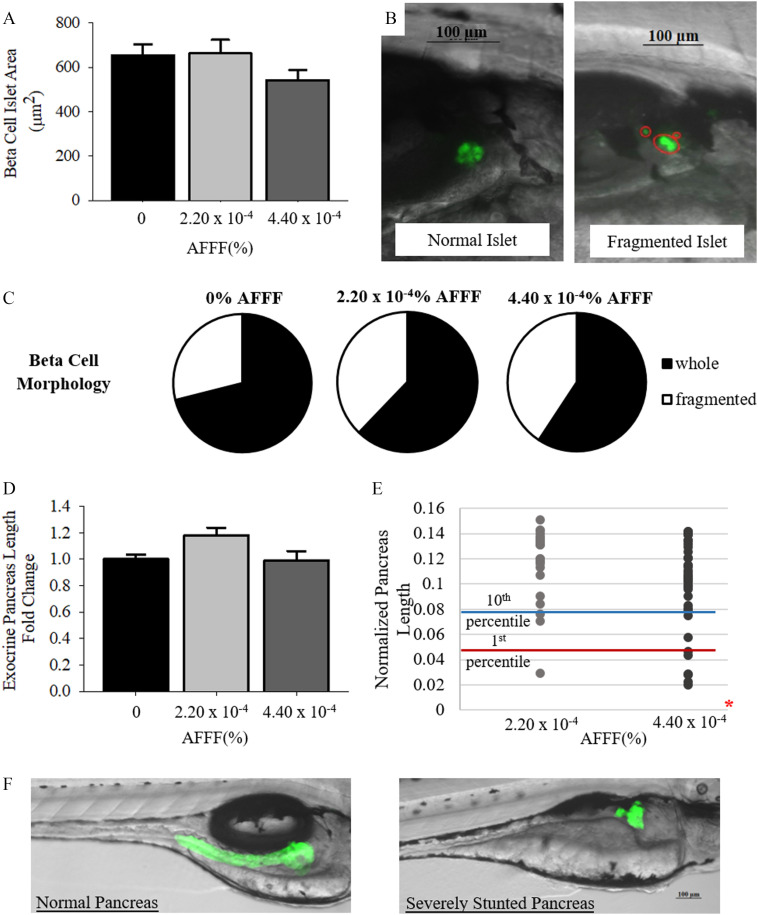
Endocrine and Exocrine Pancreas Development
Morphological assessment of the exocrine pancreas and endocrine beta cell islets was completed in Tg(ptf1a:GFP) and Tg(ins:GFP) larvae, respectively, at 96 hpf. AFFF exposure did not result in any significant differences in beta cell islet area () or the morphology of the islet () (Figure 8A–C). Raw values are located in Table S2. Additionally, mean exocrine pancreas length did not differ with AFFF exposure in comparison with control larvae () (Figure 8D). However, at AFFF, there was a significant increase in the incidence of stunted () and severely stunted () exocrine pancreas phenotypes (, Figure 8E–F).
Figure 8.
Exocrine pancreas and endocrine pancreatic beta cell development at 96 h post fertilization (hpf) larvae with AFFF exposure. (A) The area of endocrine pancreatic beta cell islets is graphed. (B) Representative images of normal and fragmented islet morphology in 96 hpf larvae. Separate islet fragments are circled. (C) Pie charts depict the percentage of whole and fragmented beta cell islet morphologies observed. (D) The length of the exocrine pancreas normalized to larval body length is graphed. (E) Individual pancreas lengths are plotted for each treatment group. The 10th (stunted) and 1st (severely stunted) percentile marks are set by control pancreas measurements. (F) Representative images depict a normal exocrine pancreas and severely stunted pancreas in a larva exposed to AFFF. Bars represent . Asterisk (*) indicate compared with 0% AFFF, one-way ANOVA, Tukey’s HSD post hoc test or Fisher’s Exact Test for qualitative assessments. vials per treatment group. Note: AFFF, aqueous film-forming foam; ANOVA, analysis of variance.
Discussion
PFAS are ubiquitous in the environment, yet little is known of the toxicity of PFAS mixtures or AFFF, which historically contained long-chain PFAS that have since been phased out of use. In the current study, the composition of a legacy 3% AFFF formulation was evaluated and its toxicity assessed through a fish embryo toxicity test. Toxicity was then compared with the most abundant PFAS, PFOS and PFHxS, and nonfluorinated compounds, SDS and TDS.
Orbitrap HRMS was used to identify PFAS and non-PFAS compounds in this mixture. Additionally, a sample was sent to a commercial laboratory, which determined concentrations for 24 PFAS compounds using a modified U.S. EPA Method 537.1. Of the 24 compounds measured, 10 PFAS were detected at quantifiable levels (Table 1). The most abundant PFAS was PFOS detected at , comprising 31% of the AFFF sample (Figure 1). The high abundance of PFOS in the AFFF formulation is consistent with a legacy formulation produced prior to the voluntary phase-out of PFOS and PFOA in the 2000s (U.S. EPA 2014). The voluntary phase-out of long-chain PFAS has led to the use of short-chain PFAS, like PFBS and other alternatives in new AFFF formulations (Wang et al. 2015).
Nontargeted analysis also revealed the presence of nonfluorinated surfactants in the AFFF formulation; the most prevalent were SDS and TDS. These compounds were quantified through MS analysis at SDS and TDS, both of which equate to 2% of the AFFF sample (Figure 1). The remaining 57% of the AFFF sample was composed of the PFAS and nonfluorinated compounds identified through nontarget analysis, as well as additional compounds not detected by these methods. This remaining composition may include very polar compounds that would be removed with the extraction process used in this study.
Dilutions of 3% AFFF working stock formulation underwent toxicological assessment in the developing zebrafish model. The AFFF mixture was found to have an of AFFF at 96 hpf (Figure 2A). One study examining bioconcentration of AFFF in juvenile trout demonstrated no mortality at a dilution of 3% FC-203CF light water AFFF produced by 3M Corporation (Yeung and Mabury 2013). Notably, the composition of quantifiable PFAS for the FC-203CF light water AFFF formulation examined in the Yeung and Mabury study was 80% PFOS and 11% PFHxS, which is similar to the chemical assessment of our AFFF sample. Aside from the toxicokinetic study discussed above, few studies have examined AFFF in biological systems, and no studies have yet identified the toxicity profiles following exposure of AFFF formulations.
The primary PFAS component, PFOS, has been extensively researched, including embryo toxicity tests in the zebrafish model. In the present study, the of PFOS in 96 hpf larvae was (Table 5). PFOS have been reported as in 72 hpf zebrafish larvae (Zheng et al. 2011) and in 96 hpf larvae (Hagenaars et al. 2011). Based on our analysis of PFOS in the legacy AFFF, the dilution representing the value, AFFF, is equivalent to PFOS (Table 5). This mortality curve of AFFF was also statistically different from that produced with exposure to PFOS alone. Together these suggest that PFOS is less toxic than AFFF. These values exceed the mean serum PFOS levels, , reported in U.S. populations (Olsen et al. 2017). To test whether the addition of PFHxS was sufficient to account for the increase in developmental toxicity of the AFFF sample, exposures to a PFOS/PFHxS mixture were examined. The ratio of PFOS:PFHxS was maintained at 6.27:1 to replicate that found in the AFFF sample (Table 4). The of the PFOS/PFHxS mixture was , which is not beyond the CI from the PFOS alone exposures. Therefore, the most abundant PFAS, PFOS and PFHxS, which together comprise 36% of the AFFF formulation, were not the sole drivers of developmental lethality.
Lethality of the most abundant nonfluorinated compounds, SDS and TDS, was similarly assessed in the developmental zebrafish assay. The of SDS was found to be , and the of TDS was (Table 5). Exposure to neither compound produced a mortality curve significantly different from AFFF exposure. Additionally, the of TDS () was similar to that of the concentration of this compound in the AFFF sample at its the of AFFF ( AFFF, TDS). This finding was consistent with levels reported in the literature; a range of is reported in OECD guideline testing in 96 hpf zebrafish (OECD 2012). These suggest that the surfactants, particularly TDS, determines lethality of the developmental AFFF exposure.
Developmental AFFF exposure caused an apparent reduction in larval growth at AFFF, but no effects on yolk sac use, swim bladder inflation, or other gross deformities were observed (Figures 3 and S1). The reduction in larval length can represent a developmental delay or persistent effect on growth, and for example, the latter has been observed because zebrafish developmentally exposed to PFOS had significant reductions in length and weight observed at adulthood (Cheng et al. 2016). Single exposures to PFOS and PFAS, of the PFOS/PFHxS mixture, produced similarly smaller larval lengths (Figures 4 and 5). This contrasts TDS and SDS. Exposure to both at concentrations below their resulted in no apparent effects on morphometric measurements (Figure 6). These data, summarized in Table 6, suggest the PFAS component of the AFFF mixture is driving the sublethal effects observed in the larvae.
Table 6.
Summary comparison of LOAEL in larval growth and yolk sac area.
| AFFF | PFOS | PFHxS | PFOS/ PFHxS mixture | SDS | TDS | |
|---|---|---|---|---|---|---|
| Effect on length | Decrease | Decrease | None | Decrease | None | None |
| LOAEL | — | — | — | |||
| Relative AFFF dilution (% AFFF) | — | — | — | — | ||
| Effect on yolk sac area | None | Increase | None | Increase | None | None |
| LOAEL | — | — | — | — | ||
| Relative AFFF dilution (% AFFF) | — | — | — | — |
Note: Embryos were exposed to AFFF, PFOS, PFHxS, PFOS/PFHxS mixture, SDS, and TDS. LOAELs are reported. —, no data; AFFF, aqueous film-forming foam; LOAELs, lowest observable adverse effect levels; PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; SDS, sodium dodecyl sulfate; TDS, sodium tetradecyl sulfate.
We sought to identify sensitive targets of AFFF toxicity beyond the morphometric assessment in larval zebrafish. Because PFOS was the most abundant compound in the AFFF formulation, it was predicted that there would be similarity in phenotypes of larvae developmentally exposed to PFOS or AFFF. Our lab has identified the pancreas, both the exocrine pancreas and the endocrine beta cell islet, as tissues that are sensitive to PFOS exposures. Previous studies have shown developmental exposure of , or , PFOS disrupted the normal morphology of the exocrine pancreas (Sant et al. 2017). Similarly, exposure to AFFF (containing PFOS) in the present study yielded a significant increase in stunted ( controls) and severely stunted ( controls) pancreata (Figure 8). However, growth of beta cell islets of the endocrine pancreas was affected, following exposure to PFOS alone but not following exposure to the AFFF mixture. Developmental exposures to , or , PFOS were previously shown to cause a reduction in beta cell cluster area and increased aberrant islet morphology at 48 and 168 hpf (Sant et al. 2017). Here, AFFF exposure up to AFFF (containing PFOS) yielded no significant impact on beta cell morphology or overall size of the structure (Figure 8).
In the present study, we aimed to examine the effect of toxicant exposure on the development of another endoderm-derived tissue, the liver. Hepatotoxicity is a well-reported outcome of PFAS toxicity, and liver enlargement and steatosis have been reported in adult zebrafish following chronic PFOS exposures (Chen et al. 2016; Cui et al. 2017). Formation of the liver structure and hepatocyte aggregation occur during the budding phase between 24 and 48 hpf, and the liver extends and thickens until liver growth arrests between 72 and 120 hpf (Ober et al. 2003). The larvae in the present study were examined at 96 hpf, during the elongation phase of liver development, yet developmental exposure to PFOS did not appear to affect liver elongation (Figure 7). Exposure to AFFF during liver development resulted in larvae with smaller liver lengths; an effect that was dose-dependent. This was observed at AFFF a dose at which no significant effect on larval growth were observed. Not only was the liver the most sensitive organ to AFFF exposure identified in this study, but the liver was also differentially affected by AFFF and PFOS alone. Table 7 summarizes these findings.
Table 7.
Summary comparison of developmental impacts in larvae with exposure to a legacy AFFF mixture and PFOS alone.
| AFFF (%) | PFOS () | |||
|---|---|---|---|---|
| 16 | 32 | |||
| ( PFOS) | ( PFOS) | () | () | |
| Liver length | Decrease | Decrease | — | — |
| Beta cell islet area | — | — | — | Decrease |
| Beta cell aberrant morphology | — | — | — | — |
| Exocrine pancreas length | — | — | — | Decrease |
| Stunted exocrine pancreas morphology | — | Increase | NT | NT |
Note: All AFFF data and PFOS liver data are from the present study. PFOS pancreatic data are summarized from Sant et al. 2017, 2019. —, no effect; AFFF, aqueous film-forming foam; NT, not tested; PFOS, perfluorooctanesulfonic acid.
The phenotype of PFOS exposure and AFFF also differed with regards to additional end points. Other studies have shown that PFOS exposure disrupted swim bladder inflation (Chen et al. 2014; Hagenaars et al. 2014). In the present study, fish were reared in AFFF solution until 120 hpf, yet swim bladder inflation occurred properly in doses up to AFFF (Figure S1). Finally, PFOS exposure caused a significant increase in yolk sac area where no effect was observed with AFFF exposure (Figures 3 and 5). These studies demonstrate a unique toxicity profile for the legacy AFFF used in the present study in comparison with that of PFOS.
The unique toxicity profile of AFFF may be determined by a mixture effect of all components. There were 10 PFAS in this mixture detected at quantifiable levels and an additional 100 PFAS detected through nontargeted Orbitrap HRMS analysis (Table 1 and Table S1). Few studies have examined the toxicity of PFAS mixtures, so the combination of PFAS could be driving the observed effects (Ding et al. 2013; Menger et al. 2020). Analysis of the AFFF also revealed 14 nonfluorinated compounds, including fatty acids, organic surfactants, detergents, dyes, and preservatives (Table 2). The findings in this study suggest that it is the critical mixture of these different compounds that drives AFFF toxicity.
With regard to regulation of these complex mixtures in environmental settings, it may be beneficial to consider only the PFAS compounds. The present study found the surfactants to be critical in lethality of the AFFF mixture; however, these compounds will readily disperse in the environment (Ivankovic and Hrenovic, 2010). The PFAS do not readily degrade and have long half-lives in the environment (Buck et al. 2011). Therefore, it may be critical in mixture assessment of AFFF formulations to build mixtures reflective of the PFAS congeners present.
Although many studies have focused on the toxicity and adverse outcomes associated with PFOS and other individual PFAS, fewer have examined the mixture of PFAS in products such as AFFF that represent environmental exposure sources. In the present study we report a list of PFAS and non-PFAS compounds discovered in the legacy AFFF sample. Toxicity assessment of the AFFF mixture and the most abundant PFAS and non-PFAS compounds revealed that the zebrafish larvae were more sensitive to this complex mixture. Additionally, one component could not solely predict toxicity. The nonfluorinated surfactants appeared to drive the lethality of the mixture, particularly TDS. PFOS appeared to drive the morphological effects observed in the AFFF-exposed larvae, specifically the effects on larval length, but this compound cannot fully recapitulate the AFFF phenotype. We further categorized the phenotype in larval zebrafish after developmental AFFF exposure, identifying the liver as a sensitive target organ and determining the LOAEL of AFFF exposure. These findings stress the importance of assessing mixture toxicity in areas of known AFFF contamination.
Supplementary Material
Acknowledgments
Funding for this work was provided in part by the National Institutes of Health (grant numbers R01ES025748 to ART-L and F31ES030975 to MAR) and a predoctoral fellowship to M.A.R. from the University of Massachusetts Amherst as part of the Biotechnology Training Program (National Research Service Award T32 GM108556). W.L. was supported through R25ES031498 and by the National Science Foundation–UMASS STEM Ambassadors Program (REU award number 1726808). The authors also acknowledge the efforts of members of the Timme-Laragy laboratory for fish facility maintenance.
References
- ATSDR (Agency for Toxic Substances and Disease Registry). 2019. PFAS: An Overview of the Science and Guidance for Clinicians on Per- and Polyfluoroalkyl Substances (PFAS). Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Bambino K, Chu J. 2017. Zebrafish in toxicology and environmental health. Curr Top Dev Biol 124:331–367, PMID: 28335863, 10.1016/bs.ctdb.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, Voogt VD, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 74(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Tal TL, Gai Z, Ma X, Bai C, et al. 2014. Early life perfluorooctanesulphonic acid (PFOS) exposure impairs zebrafish organogenesis. Aquat Toxicol 150:124–132, PMID: 24667235, 10.1016/j.aquatox.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang X, Ge X, Wang D, Wang T, Zhang L, et al. 2016. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ Pollut 218:702–708, PMID: 27496563, 10.1016/j.envpol.2016.07.064. [DOI] [PubMed] [Google Scholar]
- Cheng J, Lv S, Nie S, Liu J, Tong S, Kang N, et al. 2016. Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquat Toxicol 176:45–52, PMID: 27108203, 10.1016/j.aquatox.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Cui Y, Lv S, Liu J, Nie S, Chen J, Dong Q, et al. 2017. Chronic perfluorooctanesulfonic acid exposure disrupts lipid metabolism in zebrafish. Hum Exp Toxicol 36(3):207–217, PMID: 27193966, 10.1177/0960327116646615. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Reddam A, Liu Z, Liu J, Volz DC. 2020. High-content screening in zebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environ Pollut 256:113550, PMID: 31706782, 10.1016/j.envpol.2019.113550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. 2002. Sonic hedgehog is required early in pancreatic islet development. Dev Biol 244(1):75–84, PMID: 11900460, 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- Ding G, Zhang J, Chen Y, Wang L, Wang M, Xiong D, et al. 2013. Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch Environ Contam Toxicol 64(4):668–675, PMID: 23479250, 10.1007/s00244-012-9864-2. [DOI] [PubMed] [Google Scholar]
- Field HA, Ober EA, Roeser T, Stainier DY. 2003. Formation of the digestive system in zebrafish. I. liver morphogenesis. Dev Biol 253(2):279–290, PMID: 12645931, 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Filipovic M, Woldegiorgis A, Norström K, Bibi M, Lindberg M, Österas AH. 2015. Historical usage of aqueous film forming foam: a case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 129:39–45, PMID: 25262531, 10.1016/j.chemosphere.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. 2017. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect 125(3):481–487, PMID: 27586368, 10.1289/EHP303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, et al. 2005. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development 132(22):5069–5079, PMID: 16258076, 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Stinckens E, Vergauwen L, Bervoets L, Knapen D. 2014. PFOS affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae. Aquat Toxicol 157:225–235, PMID: 25456237, 10.1016/j.aquatox.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Vergauwen L, De Coen W, Knapen D. 2011. Structure–activity relationship assessment of four perfluorinated chemicals using a prolonged zebrafish early life stage test. Chemosphere 82(5):764–772, PMID: 21111445, 10.1016/j.chemosphere.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Hallare A, Nagel K, Köhler HR, Triebskorn R. 2006. Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol Environ Saf 63(3):378–388, PMID: 16125774, 10.1016/j.ecoenv.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 120(5):668–673, PMID: 22306490, 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høisæter Å, Pfaff A, Breedveld GD. 2019. Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. J Contam Hydrol 222:112–122, PMID: 30878240, 10.1016/j.jconhyd.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Houtz E, Wang M, Park JS. 2018. Identification and fate of aqueous film forming foam derived per- and polyfluoroalkyl substances in a wastewater treatment plant. Environ Sci Technol 52(22):13212–13221, PMID: 30339382, 10.1021/acs.est.8b04028. [DOI] [PubMed] [Google Scholar]
- Høyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, et al. 2015. Anthropometry in 5- to 9-year-old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect 123(8):841–846, PMID: 25809098, 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic T, Hrenovic J. 2010. Surfactants in the environment. Arh Hig Rada Toksikol 61:95–110, PMID: 20338873, 10.2478/10004-1254-61-2010-1943. [DOI] [PubMed] [Google Scholar]
- Jantzen CE, Annunziato KA, Bugel SM, Cooper KR. 2016. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat Toxicol 175:160–170, PMID: 27058923, 10.1016/j.aquatox.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J-M, Guo Y, Zeng L, Liang-Ying L, Lu X, Wang F, et al. 2017. Global distribution of perfluorochemicals (PFCs) in potential human exposure source–a review. Environ Int 108:51–62, PMID: 28800414, 10.1016/j.envint.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T, et al. 2013. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquat Toxicol 140–141:229–238, PMID: 23831690, 10.1016/j.aquatox.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP. 2005. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 48(4):559–566, PMID: 15883668, 10.1007/s00244-004-0133-x. [DOI] [PubMed] [Google Scholar]
- Kishi T, Arai M. 2008. Study on the generation of perfluorooctane sulfonate from the aqueous film-forming foam. J Hazard Mater 159(1):81–86, PMID: 18060693, 10.1016/j.jhazmat.2007.09.122. [DOI] [PubMed] [Google Scholar]
- Lanza HA, Cochran RS, Mudge JF, Olson AD, Blackwell BR, Maul JD, et al. 2017. Temporal monitoring of perfluorooctane sulfonate accumulation in aquatic biota downstream of historical aqueous film forming foam use areas. Environ Toxicol Chem 36(8):2022–2029, PMID: 28029183, 10.1002/etc.3726. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger F, Pohl J, Ahrens L, Carlsson G, Örn S. 2020. Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 245:125573, PMID: 31877453, 10.1016/j.chemosphere.2019.125573. [DOI] [PubMed] [Google Scholar]
- Munoz G, Desrosiers M, Duy SV, Labadie P, Budzinski H, Liu J, et al. 2017. Environmental occurrence of perfluoroalkyl acids and novel fluorotelomer surfactants in the freshwater fish Catostomus commersonii and sediments following firefighting foam deployment at the Lac-Mégantic railway accident. Environ Sci Technol 51(3):1231–1240, PMID: 28056502, 10.1021/acs.est.6b05432. [DOI] [PubMed] [Google Scholar]
- Oakes KD, Benskin JP, Martin JW, Ings JS, Heinrichs JY, Dixon DG, et al. 2010. Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam. Aquat Toxicol 98(2):120–129, 10.1016/j.aquatox.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Ober EA, Field HA, Stainier DY. 2003. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev 120(1):5–18, PMID: 12490292, 10.1016/s0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development). 2012. Validation report (Phase 2) for the Zebrafish Embryo Toxicity Test ENV/JM/MONO(2012)25. Paris, France: Organisation for Economic Co-operation and Development. [Google Scholar]
- OECD. 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals. Section 2. Paris, France: Organisation for Economic Co-operation and Development, 10.1787/9789264203709-en. [DOI] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, et al. 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ Res 157:87–95, PMID: 28528142, 10.1016/j.envres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Wood CR, Wolf CJ, Abbott BD, Lau C. 2013. Evaluation of perfluoroalkyl acid activity using primary mouse and human hepatocytes. Toxicology 308:129–137, PMID: 23567314, 10.1016/j.tox.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Saikat S, Kreis I, Davies B, Bridgman S, Kamanyire R. 2013. The impact of PFOS on health in the general population: a review. Environ Sci Process Impacts 15(2):329–335, PMID: 25208696, 10.1039/c2em30698k. [DOI] [PubMed] [Google Scholar]
- Sant KE, Jacobs HM, Borofski KA, Moss JB, Timme-Laragy AR. 2017. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ Pollut 220(pt B):807–817, PMID: 27810111, 10.1016/j.envpol.2016.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, Venezia OL, Sinno PP, Timme-Laragy AR. 2019. Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Danio rerio. Toxicol Sci 167(1):258–268, PMID: 30239974, 10.1093/toxsci/kfy237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RL, Benskin JP, Laarman AH, Macleod SL, Martin JW, Wong CS, et al. 2010. Perfluorooctane sulfonate toxicity, isomer-specific accumulation, and maternal transfer in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 29(9):1957–1966, PMID: 20821653, 10.1002/etc.257. [DOI] [PubMed] [Google Scholar]
- Sullivan M. 2018. Addressing Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Department of Defense FY18 HASC Brief on PFOS-PFOA. https://partner-mcoarchive.s3.amazonaws.com/client_files/1524589484.pdf [accessed 31 August 2017].
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: 30470793, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C, Sawle A, Fenske M, Cossins A. 2012. Implications of the solvent vehicles dimethylformamide and dimethylsulfoxide for establishing transcriptomic endpoints in the zebrafish embryo toxicity test. Environ Toxicol Chem 31(3):593–604, PMID: 22169935, 10.1002/etc.1718. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2014. 2010/2015 PFOA Stewardship Program.
- Vogs C, Johanson G, Näslund M, Wulff S, Sjödin M, Hellstrandh M, et al. 2019. Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio). Environ Sci Technol 53(7):3898–3907, PMID: 30844262, 10.1021/acs.est.8b07188. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K. 2015. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int 75:172–179, PMID: 25461427, 10.1016/j.envint.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. 2010. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci 113(2):305–314, PMID: 19915082, 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. 2000. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed Eugene, OR: University of Oregon Press. [Google Scholar]
- Wolf CJ, Rider CV, Lau C, Abbott BD. 2014. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 316:43–54, PMID: 24374136, 10.1016/j.tox.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL. 2016. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol 290:21–30, PMID: 26548598, 10.1016/j.taap.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung LW, Mabury SA. 2013. Bioconcentration of aqueous film-forming foam (AFFF) in juvenile rainbow trout (Oncorhyncus mykiss). Environ Sci Technol 47(21):12505–12513, PMID: 24060050, 10.1021/es403170f. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Song B, Xiao R, Zeng G, Gong J, Chen M, et al. 2019. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ Int 126:598–610, PMID: 30856447, 10.1016/j.envint.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren XM, Wan B, Guo LH. 2014. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor γ. Toxicol Appl Pharmacol 279(3):275–283, PMID: 24998974, 10.1016/j.taap.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Weaver Y, Ehresman DJ, Chang S-C, Butenhoff JL, et al. 2017. Organic anion transporting polypeptides contribute to the disposition of perfluoroalkyl acids in humans and rats. Toxicol Sci 156(1):84–95, PMID: 28013215, 10.1093/toxsci/kfw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Liu HL, Shi W, Wei S, Giesy JP, Yu HX. 2011. Effects of perfluorinated compounds on development of zebrafish embryos. Environ Sci Pollut Res Int 19(7):2498–2505, PMID: 22828880, 10.1007/s11356-012-0977-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 2A line graph plots mortality (percent), ranging from 0 to 100 in increments of 50 (y-axis) across log [relative percent aqueous film-forming foam], ranging from negative 6 to negative 2 in unit increments (x-axis). Figure 2B is a line graph, plotting mortality (percent), ranging from 0 to 100 in increments of 50 (y-axis) across log [relative percent aqueous film-forming foam], ranging from negative 6 to negative 1 in unit increments (x-axis) for perfluorooctanesulfonic acid or perfluorohexanesulfonic acid mixture, perfluorooctanesulfonic acid, and perfluorohexanesulfonic acid. Figure 2C is a line graph, plotting mortality (percent), ranging from 0 to 100 in increments of 50 (y-axis) across log [relative percent aqueous film-forming foam], ranging from negative 6 to negative 1 in unit increments (x-axis) for Sodium Dodecyl Sulfate and Sodium Tetradecyl Sulfate.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3578/7510953/14f09a15d303/ehp6470_f2.jpg)