Abstract
Conventional electric stimuli of micro- and millisecond duration excite or activate cells at voltages 10–100 times below the electroporation threshold. This ratio is remarkably different for nanosecond electric pulses (nsEP), which caused excitation and activation only at or above the electroporation threshold in diverse cell lines, primary cardiomyocytes, neurons, and chromaffin cells. Depolarization to the excitation threshold often results from (or is assisted by) the loss of the resting membrane potential due to ion leaks across the membrane permeabilized by nsEP. Slow membrane resealing and the build-up of electroporation damages prevent repetitive excitation by nsEP. However, peripheral nerves and muscles are exempt from this rule and withstand multiple cycles of excitation by nsEP without the loss of function or signs of electroporation. We show that the damage-free excitation by nsEP may be enabled by the membrane charging time constant sufficiently large to (1) cap the peak transmembrane voltage during nsEP below the electroporation threshold, and (2) extend the post-nsEP depolarization long enough to activate voltage-gated ion channels. The low excitatory efficacy of nsEP compared to longer pulses makes them advantageous for medical applications where the neuromuscular excitation is an unwanted side effect, such as electroporation-based cancer and tissue ablation.
Keywords: Nanosecond pulses, Electroporation, Electropermeabilization, nsPEF, Nanosecond pulse stimulation, Electrostimulation
1. Introduction: What is wrong with the nanosecond pulse stimulation?
Electrical stimulation leads to excitation or cell activation when the resting membrane potential is depolarized by 10–20 mV, to the threshold for opening of voltage-gated sodium and/or calcium channels (VGSC and VGCC). When electrical stimuli are made strong enough to increase the membrane potential to 200–500 mV of either de- or hyperpolarization, they damage the membrane by a process known as electroporation or electropermeabilization[1–5]. The increased membrane permeability to usually impermeable ions and solutes results in the loss of the resting membrane potential and inactivation of VG channels, eventually rendering cells unexcitable.
The large “safety gap” between the excitatory and damaging membrane potentials enables multiple excitation cycles without membrane disruption and is the basis of countless applications of electrical stimulation in research and medicine. These applications typically employ electric pulses from milliseconds down to tens of microseconds in duration. Cell membrane charging by an externally applied electric pulse takes time, so the shorter pulses need to impose a stronger electric field to reach a certain threshold. This “strength-duration” (SD) rule, known for over a century[6–8], predicts that electroporation thresholds should increase proportionally to excitation thresholds as pulses get shorter, and the “safety gap” should be preserved. However, recent experiments with nanosecond pulse stimulation (NPS) came in a stark contrast with this prediction, with electroporation often observed at or even below the excitation threshold. The NPS-induced action potentials (APs) could be a secondary effect of the loss of the resting membrane potential caused by membrane electroporation, rather than a direct effect of depolarization by the imposed electric field (Schematic 1). This review will comprehensively summarize published experimental findings on cell activation and electroporation by NPS in diverse excitable systems, introduce an explanation for the paradoxical excitation/electroporation balance, and discuss its implications for research and medical use of NPS.
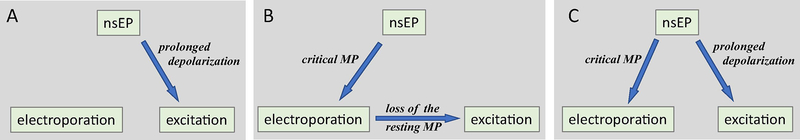
Schematic 1.
Different scenarios of excitation by nanosecond electric pulses (nsEP). A: nsEP causes a small depolarization of the cell membrane that lasts sufficiently long to open voltage-gated channels (same as conventional electrostimulation). B: The membrane potential (MP) induced by nsEP exceeds the critical value for electroporation. Ionic leaks through the damaged membrane depolarize the resting MP to the threshold of excitation. C: nsEP meets the conditions for both electroporation and excitation, and the loss of the resting MP after electroporation is not sufficient (e.g., too slow) to open channels. This loss can either assist excitation (by depolarizing the membrane towards the excitation threshold) or impede it (by inactivating voltage-gated channels and by facilitating the membrane discharge; see text).
2. How nanosecond electric pulses stimulate cells and tissues
A study that pioneered excitation by sub-microsecond pulses was performed in a classic isolated preparation of a frog gastrocnemius muscle[9]. The authors performed an exhaustive quantitative study of excitation thresholds for pulse durations from 100 ms down to about 1 ns. The S-D curves on log-log plots had a classic linear appearance from the shortest pulses up to about 1 ms. Even the shortest pulses evoked muscle contraction, at the electric field threshold of 24 kV/cm for 1.8-ns nominal pulse duration (the actual pulse had a complex shape, with ~1-ns peak followed by an 8–12 ns “tail”). For 1.8-ns pulses only, the voltage and current excitation thresholds fell about 2-fold below the values predicted by Blair fit[6]; this discrepancy was attributed to the complex pulse shape. In order to fit the data, the authors estimated the charging time constant (t) being about 300 μs, with a large span from 37 to 1261 μs when using different calculation approaches. This range of t values corresponded to the excitation of nerve terminals within the muscle[8]. The linear appearance of the S-D curves and the stability of excitation thresholds during 4–5 h of experimentation suggested that excitation was not accompanied by electroporation or any type of cell damage, and that mechanisms of excitation by NPS were the same as with longer pulses.
Likewise, no indications of damage were observed when APs were induced in an isolated frog sciatic nerve by 12-ns pulses[10]; 200-, 300-, and 700-ns pulses[11]; or 340-ns pulses[12]. Nerves responded consistently to repetitive stimuli (up to tens of thousands of excitation cycles) showing just slow (hours) rundown due to in vitro conditions, but not associated with NPS. The stability of AP amplitude, shape, and latency over multiple cycles of even high-rate NPS evidenced for VGSC activation without electroporation.
In contrast, excitation of isolated adult rat cardiomyocytes by 4-ns, 10–80 kV/cm pulses involved nanoelectroporation [13]. Cells were loaded with a rhod-2 Ca2+ indicator and subjected to either NPS or conventional stimulation (0.5–2.4 kV/cm, 1 ms). A single nsEP usually induced Ca2+ transients similar to those triggered by conventional pulses, but it could also induce anodally-initiated Ca2+ waves and impair the recovery of the diastolic Ca2+ level. Delivering repeated nsEP stimuli generated consecutive Ca2+ waves leading to Ca2+ destabilization. Excitation by NPS was not consistent with the all-or-none pattern and was regarded rather as a dose-dependent or a cumulative response. NPS effects were resistant to the effects of verapamil (a VGCC blocker) and could overcome VGSC inhibition by tetrodotoxin. These and some other features distinguished NPS from the conventional stimulation and suggested nonselective ion channel transport via sarcolemmal nanopores as a triggering mechanism. The authors speculated that 4-ns pulses could be too brief to activate VGSC, and suggested that AP generation by NPS involved nanopore opening, which caused nonselective entry of Ca2+ and Na+, depolarization, and secondary activation of VGSC (Schematic 1B).
NPS of individual cardiac cells and myocardium has gained further attention as a promising new modality for more efficient but safer defibrillation [14, 15]. In rat embryonic cardiomyocytes, a single 10-ns pulse at 36 kV/cm elicited full-amplitude, all-or-none Ca2+ transients similarly to conventional 4-ms stimuli. However, repetitive NPS was not tested in this study and a modest electroporative damage could have remained undetected.
In a later study[16], enzymatically isolated murine, rabbit, and swine adult ventricular cardiomyocytes (VCM) were loaded with a Ca2+ indicator Fluo-4 or Fluo-5N and subjected to stimuli of increasing amplitude until a Ca2+ transient was optically detected. Tested pulse durations ranged from 200 ns to 4 ms. When Ca2+ transients were evoked by a single pulse just above the threshold, they were the same for all pulse durations, matching the earlier reports [13, 17]. The single stimulus data provided no indication of differences in the opening of VG channels or Ca2+ mobilization and handling by NPS and conventional stimuli. The S-D curves in the nanosecond range continued the same pattern as with longer pulses, suggesting the similarity of excitation mechanisms and matching the earlier conclusions [9].
These conclusions, however, were challenged by observations with repetitive NPS (5 stimuli with 1-s intervals). In most cells which responded with normal transients to conventional stimuli, repetitive NPS caused abnormal responses already at the excitation threshold. Cells either failed to generate one or several transients, or the shape of transients was distorted, or cytosolic Ca2+ did not return to its base level. Such poor performance with repetitive NPS was a sign of nanoelectroporation of the sarcolemma and/or the sarcoplasmic reticulum[18–20], or of the inhibition of VG ion channels[21–23].
The next study was specifically intended to separate NPS impact on cell excitation from downstream effects on Ca2+ handling[24]. Fluorescent imaging of optical APs and Ca2+ transients evoked by 200-ns pulses in isolated VCM revealed abnormal membrane effects such as slow sustained depolarization (SSD) even at nsEP amplitudes below the AP threshold. Already at the threshold, APs were typically followed by abnormal afterdepolarization waves. The authors concluded that APs were not induced by NPS directly, but resulted from the SSD-associated Ca2+ entry, which caused depolarization, which eventually culminated in an AP. This Ca2+ entry could bypass VGCC and was likely caused by the electroporative damage, which was regarded as the mechanism of excitation.
While these findings were discouraging for the development of NPS-based defibrillation, the trials of defibrillation by 300-ns shocks in Langendorff-perfused rabbit hearts were successful at much lower energies than conventional defibrillation[14]. NPS caused no baseline shift of the membrane potential (that could be indicative of electroporation damage), no changes in the AP duration, and only briefly changed the diastolic interval, for one beat after the shock. Histology showed no tissue death or electroporation anywhere in the heart. The discrepancy between the findings in the whole heart and in isolated cardiomyocytes was acknowledged but not resolved[15].
A series of studies in electrically excitable neurosecretory chromaffin cells with 4- and 5-ns stimuli[23, 25, 26] and with 150-, 200-, and 400-ns stimuli[27, 28] found that Ca2+ activation by NPS is always accompanied or mediated by nanoelectroporation (or perhaps by the opening of unidentified non-selective cation channels). With 4- and 5-ns pulses, all Ca2+ entry was through VGCC, but the opening of VGCC was not a direct effect of the applied field. Instead, it was caused by cell depolarization due to the nsEP-induced, tetrodotoxin (TTX)-independent Na+ entry. The activation of an inward Na+ current with characteristics typical for nanopores was demonstrated in chromaffin cells directly by patch clamp[29]. With the longer nsEP, Ca2+ entered cells both through VGCC and through a pathway insensitive to VGCC inhibitors (presumably through nanopores). Similarly to findings in VCM, there was no activation without electroporative disruption of the cell membrane.
Human epithelial kidney (HEK) cells typically do not express VGCC, and the activation of cytosolic Ca2+ by 300-ns pulses presumably results from nanoelectroporation. A successful transfection of HEK cells with CaV1.3 L-type VGCC did not lower the threshold for Ca2+ activation. Thus, the nanoporation threshold was lower or equal to the VGCC activation threshold, and VGCC activation might be caused by the loss of the resting membrane potential in permeabilized cells[30]. At the same time, the expression of VGCC might affect the membrane sensitivity to the electrical stress, which was also noted in studies with sub-nanoseconds pulses[31, 32].
Ca2+ activation by nsEP (30–600 ns) in NG108 neuroblastoma cells and in mouse primary hippocampal neurons was not inhibited by either TTX or Cd2+ (a VGCC blocker)[33]. The authors concluded that Ca2+ activation was caused by nanoporation of the cell plasma membrane.
In contrast, Cooper and co-authors reported extraordinarily low thresholds for NPS of nociceptor neurons in vitro [34, 35]. They combined whole-cell patch clamp and optical recording of CaL+ transients by loading Fluo-4 dye into cells through the recording pipette. APs were elicited by a single 350- or 12-ns pulse at the electric field of only 0.129 or 0.403 kV/cm, respectively. Excitation was confirmed by detecting Ca2+ transients and their inhibition by Cd2+ and TTX. Electroporation by 12-ns pulses was evaluated by the uptake of propidium and required a much stronger field of 3–4 kV/cm. Applying 12-ns stimuli in 25-ms bursts further dropped the excitation threshold to 0.024 and 0.016 kV/cm for 1 and 4 kHz pulse repetition rates, respectively. Current clamp recording showed a clear stepwise voltage build-up on the membrane even with 1-kHz bursts, with roughly a 30% discharge during 1-ms intervals between the pulses. Such performance corresponds to the membrane electrical time constant of about 1 ms, a value which is unusually large for mammalian cells and could be influenced by the patch clamp pipette attached to the cell.
Taking into account that patch clamp measurements may be prone to nsEP pick-up, we opted for membrane potential imaging with FluoVolt dye to analyze effects of 200-ns pulses in cultured hippocampal neurons[36]. Electroporation depolarized cells in <1 ms, with the threshold at 1.5–1.9 kV/cm, whereas APs could only be evoked by a stronger electric field of 1.9–4 kV/cm. AP induction by electric fields below the electroporation threshold was not observed. At the same time, VGSC opening could already be detected in 0.5 ms after nsEP, when the loss of the resting membrane potential due to the electroporation (as measured in TTX-blocked neurons) was yet too small for VGSC activation (Schematic 1C). Therefore, the overlap of electroporation and AP thresholds did not necessarily mean that APs were caused by electroporation but evidenced for a low potency of nsEP for AP induction. These results did not support Cooper's group findings of neuronal excitation at 0.129 kV/cm for nsEP of comparable duration, 350 ns[35].
To summarize all these studies, NPS is different from the conventional electrostimulation in that it typically cannot elicit APs or activate Ca2+ without concurrent membrane disruption[12, 13, 16, 20, 23–25, 27–33, 36]. This disruption is often regarded as nanoelectroporation, although its exact nature is not fully known and may vary for different cell types and treatment conditions. The membrane disruption by NPS impairs cell recovery after excitation and prevents repetitive excitation. However, studies in peripheral nerves[10–12], muscle[9], and in nociceptor neurons[34, 35] make a notable exception from this rule and prove that, in principle, excitation can be achieved by NPS without membrane damage.
3. A delay between the electroporation and excitation and different membrane charging time constants (τ) may be a key to the controversy
Molecular dynamics simulations[37–39], computational models[40, 41], and experimental measurements[42] suggest that electroporation occurs immediately (within nanoseconds) after a critical transmembrane potential is reached. In contrast, the process of opening of VG channels is not instant, which was already reflected in the Hodgkin-Huxley model of channel opening[43]. It is the actual translocation of a large segment of a protein molecule and conformational changes, which take a certain minimum time no matter how high the electric driving force is. Long timescale molecular dynamics simulations found that the channel opening process takes multiple conformation change steps over the course of several microseconds[44, 45]. The process of opening of VG channels can be revealed electrophysiologically as gating currents[46–48] which can last milliseconds, although a faster component with τ=12 μs was also reported[48–50]. Gating currents are thought to result from the integration of jump-like openings of many individual ion channels at various times after the critical depolarization is reached[48, 51], but the minimal duration of each jump-like event has not been measured. One can presume that depolarization of the cell membrane culminates in channel opening only if it lasts long enough to drive the channel protein through all the conformation change steps. If the depolarization does not last long enough, there is no more driving force to move the voltage sensor through the remaining steps towards opening, and the opening will probably fail.
Recently, we were able to estimate the minimum depolarization time for opening of the critical number of VGSC needed to initiate a propagating AP in a frog sciatic nerve preparation[11]. This was accomplished by delivering paired nsEP of the opposite polarity with different intervals, which limited the maximum depolarization time. The initiation of a propagating AP by the 2nd nsEP in the pair was prevented by ligating the nerve between the two stimulating electrodes. This study found that APs could not be generated when the total duration of depolarization was less than 11 μs, regardless of how strong the electric field applied was. Although opening of isolated individual VGSC in a shorter time frame could not be ruled out, excitation of the propagating AP always required depolarization for 11 μs at least. This time interval may be different in warm-blooded animals, and for different types of VGSC and in VGCC. However, 11 μs is the only value available to date, and we will use it here as an example to test when nsEP may or may not elicit AP without electroporation.
The interplay of electroporation and delayed excitation can already be demonstrated with basic capacitor equations, considering cell membrane a simple capacitor:
| (1) |
and
| (2) |
where PD is the charging pulse duration (ns), t is the time from the onset of the pulse (ns),?V is the induced transmembrane potential (V), and Δ1Vmax is ΔV reached by the end of the charging pulse. We intentionally leave out any complex and dynamic features that might obscure the main message of this section, which is the key role of t in determining the mechanism of excitation by nsEP. Such features (including the highest ΔV that would not destroy the membrane; the reduction of τ by electroporation; the superposition of ΔV with the cell resting membrane potential; etc.) are not necessarily reflected in figures but will be considered in the analysis. The reported ΔV thresholds of electroporation for varied experimental conditions range from about 0.2 V[4, 52–54] to 0.5 V[55, 56] and even 1 V[2, 56]. For the illustration of the interplay between electroporation and excitation, below we will utilize ΔV values of 0.5 V and 20 mV as examples of electroporation and excitation thresholds, respectively.
With Eq. 1 and for PD >> τ, cell membrane will be charged to 1 V (in the absence of electroporation or any active responses). The external electric field (E, V/m) needed to induce the transmembrane potentials of +1V and −1V at the electrode-facing opposite poles of a round cell with radius R (m) can be obtained with a simplified steady-state Schwan’s equation [3, 57]:
| (3) |
which yields 0.66 kV/cm for a cell with 10-μm radius. For any t, cell size, and pulse duration, a stronger electric field (e.g., 3x, 5x, 15x stronger) will charge the membrane to a proportionally higher ΔVmax.
The initiation of an AP requires that the membrane remains depolarized above the assumed 20-mV threshold for 11 μs or longer. In other words, excitation never happens during nsEP (which, by definition, is a pulse shorter than 1 μs); instead, it is the lasting depolarization after nsEP that may culminate in the excitation.
There are only three principal scenarios how charging a cell membrane by nsEP can lead to excitation: (1) by means of electroporation, which will cause a lasting loss of the resting membrane potential and eventually evoke an AP (Schematic 1B); (2) without electroporation, if the discharge after nsEP is slow enough to keep ΔV > 20 mV for 11 μs (Schematic 1A), and (3) in parallel with electroporation, which occurs before excitation but is not the major cause of it (Schematic 1C).
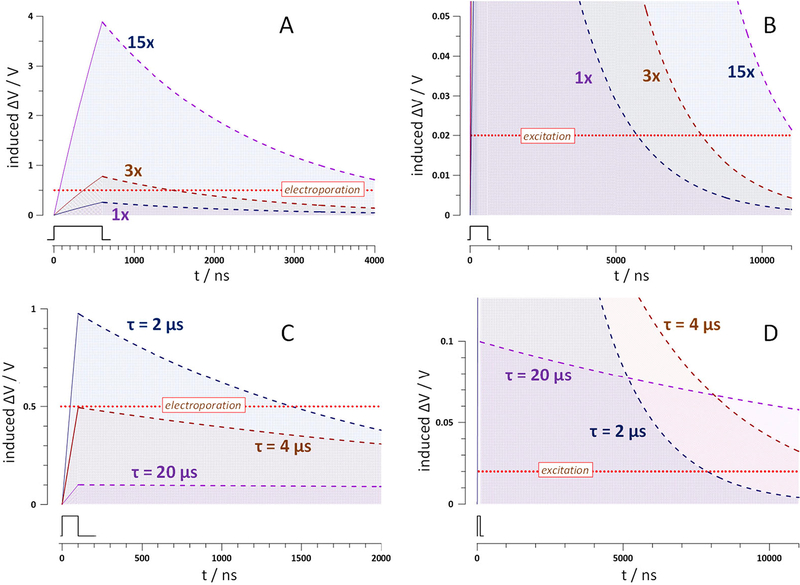
Fig. 1A,B explores if excitation without electroporation is achievable for a relatively long 600-ns pulse in a cell with τ=2 μs (which is a typical τ value for many mammalian cells[4]). For a fixed τ, ΔV at any time after the pulse is determined solely by the ΔV at the onset of the discharge, i.e., by ΔVmax. It is only a 15x stronger nsEP that charges the membrane high enough (to ~ 4V) to maintain ΔV>20 mV for 11 μs. In reality, the membrane will be destroyed long before reaching ΔV =4V. Thus, for a cell with τ=2 μs, achieving excitation by a 600-ns pulse without electroporation is not possible, regardless of the pulse amplitude: The discharge after “weak” pulses is too brief, whereas “strong” pulses inevitably cause electroporation.
Fig. 1.
The effect of pulse amplitude (A,B) and charging time constant τ (C,D) on the calculated time course of the induced membrane potential ΔV during and after nsEP. Panels B and D present the same curves as A and C, respectively, but on different time and amplitude scales. Dotted horizontal lines designate the assumed 20-mV excitation and 0.5-V electroporation thresholds, as labeled; excitation would only occur if its threshold is exceeded for 11 μs (see text). In A and B, charging and discharging curves were obtained with Eqs. 1 and 2 for t=2 μs and a 600-ns pulse (inset) at 1x, 3x, and 15x amplitude. In B and C, same calculations were performed for a 100-ns pulse (inset) of a fixed 1x amplitude, for t of 2, 4, and 20 μs. Note that excitation without electroporation is prohibited at τ=2 μs regardless of the pulse amplitude (A,B) whereas a larger t may enable it even for a shorter pulse (C,D).
The only conceivable way to meet these two mutually exclusive requirements for direct excitation by nsEP is an increase of τ. Fig. 1C,D shows that a larger t favors direct excitation in two complementary ways, namely, by reducing ΔVmax and thereby preventing electroporation, and by extending the discharge time. With the charging pulse amplitude kept constant at 1x, an increase of t from 2 to 4 μs enables excitation while avoiding electroporation (albeit just barely). With still larger τ = 20 μs, the excitation conditions are easily met while keeping the depolarization all the time at least 5 times below the electroporation threshold.
Of note, the initial charging time from 0 to 20 mV reduces the actual time at ΔV>20 mV in the interval from 0 to 11 μs. This reduction is negligible for short nsEP (such as in Fig. 1C,D), but becomes significant for pulses longer than 0.5–1 μs at large τ. To keep this calculation error small (<2% of 11 μs), all plots in the next Fig. 2 and excitation threshold plots in Fig. 3 were limited to the region where charging to 20 mV takes less than 200 ns.
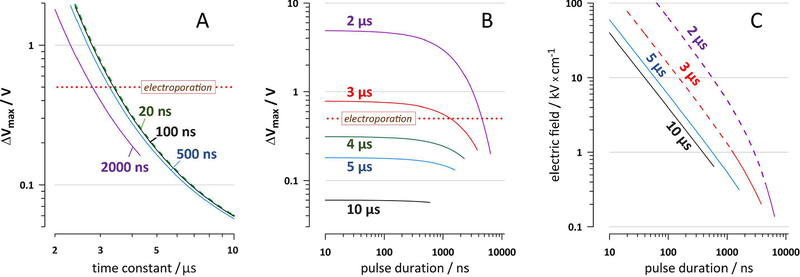
Fig. 2.
Membrane charging time constant t determines whether the excitation by nsEP is possible without electroporation. A and B: The effect of the charging pulse duration and t on ΔVmax that would discharge to 20 mV by 11 μs after nsEP onset. The required ΔVmax is plotted as a threshold for excitation. The plots continue only through the region where the time to charge to 20 mV is small (< 200 ns, i.e., <2% of 11 μs; see text). The resting membrane potential and the decrease of τ after electroporation are not considered. Labels in the plots are pulse durations (A), in ns, and t values (B), in μs. In A, curves for 20-ns pulses (green dashes) and 100-ns pulses (black solid line) are nearly identical. Dotted horizontal lines designate the assumed electroporation threshold of 0.5 V. Panel C shows the same dependence as B, but ΔVmax thresholds are re-calculated into the external electric field strength needed to reach these thresholds by the end of the charging pulse. The calculations are for a cathode-facing pole of a round cell with a 10-μm radius (see text). Dashed portions of the curves for 2- and 3-μs time constants correspond to the conditions when the electroporation threshold is exceeded and excitation without membrane damage is not possible. See text and Fig. 1 for more detail.
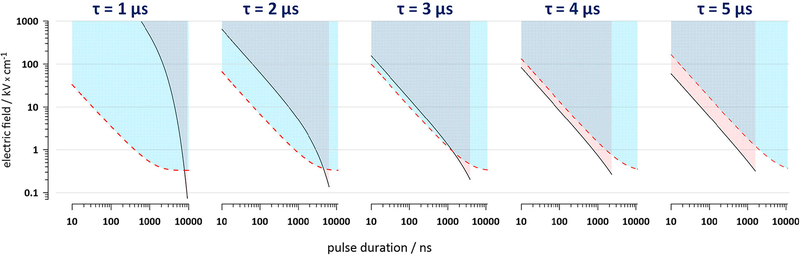
Fig. 3.
The interplay of electroporation (the area above the dashed line threshold) and excitation (the area above the solid line threshold) for different pulse durations (10 ns-10 μs) and charging time constants (1–5 μs). The thresholds were calculated for a cathode-facing pole of a round cell with a 10-μm radius using Eq. 3. The excitation thresholds are plotted only to the limit where the time for charging to 20 mV is under 200 ns. See text and Fig. 2 for details.
Considering the membrane discharge as a passive process, as described by the Eq. 2, the residual depolarization by 11 μs after nsEP onset is a function of τ and ΔVmax (Fig. 2). The exact duration of pulses in the nanosecond range has relatively small impact since the nsEP “occupies” only a small portion of the 11-μs interval (Fig. 2A,B). For a 500-ns pulse, excitation without electroporation is only possible at τ > 3.2 μs, and increasing the pulse duration beyond the nanosecond range, to 2 μs, decreases the critical τ value only slightly, to about 2.7 μs (Fig. 2A). For relatively small τ values of 2 and 3 μs, the minimal pulse duration enabling excitation without electroporation is 4.4 and 1.4 μs, respectively (Fig. 2B). However, with a small increase in the time constant, τ > 4 μs, even the shortest nsEP should cause excitation without electroporation (Fig. 2B), as long as the electric field during the pulse is strong enough to reach the needed ΔVmax by the end of the pulse. Sample calculations of the applied electric field needed to excite a round cell with a 10-μm radius are illustrated in Fig. 2C. The electric field threshold expectedly increases for shorter pulses and decreases for larger τ. Dashed segments of the curves for the time constants of 2 and 3 μs in Fig. 2C indicate that excitation without electroporation actually would not be possible, because the electroporation threshold ΔVmax = 0.5 V is already exceeded by the end of the pulse.
In Fig. 3, we used the same size hypothetical cell to illustrate the interplay of electroporation and excitation thresholds for the externally applied electric field. The resting membrane potential was disregarded, and both the electroporation and excitation were assumed to occur at the cathode-facing (depolarized) pole of the cell. The thresholds for electroporation (dashed lines) and for excitation without electroporation (solid lines) were plotted against the pulse duration for the membrane charging time constants from 1 to 5 μs. For any pulse duration in Fig. 3, higher t reduces the excitation threshold while increasing the electroporation threshold. The thresholds for electroporation and excitation may go almost parallel throughout the entire nanosecond range without crossing (compare t of 3 and 4 μs). This behavior suggests that tuning nsEP duration should not affect the mechanism of excitation for a particular exposure setup and cell type. For example, if the electroporation threshold for a 20-ns pulse happens to be lower than the direct excitation threshold, then increasing pulse duration to 100, 400, or 900 ns will not change it (and vice versa, if a 900-ns pulse excites without electroporation, then a 20-ns pulse should do it as well).
4. Modeling versus experimental findings: implications and limitations
Above we showed that different threshold conditions for electroporation and activation of VG channels might be responsible for the peculiarities of excitation by NPS. The electroporation occurs immediately once a certain high threshold of ΔV is reached. The excitation occurs at a much lower threshold, but only if it is maintained for a certain minimum time, which is in the microsecond range. This difference makes the membrane charging time constant, τ, the most important parameter that determines which mechanism prevails. Increasing τ shifts the balance from electroporation towards excitation, with little relevance to other nsEP parameters. Of note, the membrane charging time constant considered in our paper should not be confused with a similarly termed “passive membrane time constant” of neurons[58]. Even though it is called “passive”, it refers to the temporal summation of postsynaptic events and is determined by activation and inactivation of ion channels, as well as by the membrane electrical properties. This neuronal time constant is usually in the multi-millisecond range, whereas the charging events discussed here are orders of magnitude faster and refer solely to the membrane charging as a capacitor.
The key role of t provides a simple and possibly a sufficient explanation to the controversies reported by the studies reviewed in Section 2. Nerve bundles within the frog gastrocnemius muscle preparation had an estimated τ=300 μs, with the lowest boundary measured at 37 μs [9]. Either way, this τ was large enough to ensure the damage-free excitation. For isolated frog sciatic nerve stimulation, τ can be derived from the repetitive stimulation threshold, which starts to decrease when the interpulse interval is at 3–5 t [12]. This interval was about 200 μs (Fig. 1A in [12]), yielding t of 40–70 μs. This value is also consistent with the electroporation-free excitation as reported in [10–12] for pulses from 12- to 700-ns long. The only two remaining studies which reported excitation without electroporation are those in nociceptor neurons using patch clamp [34, 35]. As noted above, patch clamp traces used for the illustration of the temporal summation in these neurons (Fig. 5D,E in [34]) correspond to a large t of about 1 ms. Whether it was the actual t characteristic for these neurons (e.g., because of a large and branched dendritic tree) or an artifact from charging the recording pipette, the damage-free excitation is consistent with our analysis.
Published estimates of the charging time constant for mammalian cells vary from about 2 μs [4] down to 0.1–1 μs [59, 60]. These values support the observations that in diverse types of cultured and primary cells the excitation was mediated or accompanied by the electroporative membrane disruption [12, 13, 16, 20, 23–25, 27–33, 36]. Moreover, multiple studies in diverse cells consistently reported the electroporation threshold of 1–2 kV/cm for 200- to 600-ns pulses [21, 24, 33, 36, 61–63], which is exactly the value expected for a time constant of 1–2 μs (Fig. 3).
While this is a surprisingly good match for such a simple model, there are also many limitations to consider. The time course of the membrane charging and discharging is not necessarily a single-exponential process, and the time constant(s) are affected by the environment, intra- and extracellular electrical conductivity, cell shape, electric field gradient, the proximity to organelles and to the opposite side of the cell, etc. For the shortest nanosecond and sub-nanosecond pulses, the membrane charging by ion currents is gradually replaced by the dielectric stacking mechanism [32, 38], and the induced ΔV will exist approximately for the duration of the pulse only. In the transition zone between the two coupling mechanisms, the charging time constant will effectively become smaller (or there will be two constants), which would prohibit excitation without the formation of conductive membrane defects as an intermediate step.
Cardiomyocytes are among the largest cells (20–50 μm wide and 100–300 μm long) and are expected to have a larger than average time constant (which is proportional to the cell size). The fact that it was nonetheless not possible to excite them without damage may be indicative of some additional impact of nsEP that has yet to be explored. Alternatively, it may be related to the fact that only a small portion of the cell was subjected to nsEP [16, 24], and the effective time constant was reduced. It may be worth testing whether the application of nsEP to the entire cell using a different pulse delivery setup might trigger APs damage-free. When cardiomyocytes are stimulated in the heart tissue, rather than as isolated individual cells, their charging time constant may be substantially increased, because of the lower extracellular conductivity and because of gap junctions between neighboring cells. The larger time constant of cardiomyocytes in situ may be exactly the difference that enabled defibrillation (= expansive excitation) of the heart without significant injury [14, 15]. Then, findings in isolated cardiomyocytes should not rule out possible utility of nsEP for heart defibrillation and pacing.
The reduced capability of nsEP for neuromuscular excitation is advantageous for applications where such excitation should be avoided, such as cancer and tissue ablation. For conventional irreversible electroporation (IRE) using “long” electric pulses[5, 64, 65], the excitation threshold is 10–50 times below the electroporation threshold and the stimulated tissue volume extends far beyond the ablation volume. Extensive neuromuscular excitation by IRE is a major problem which is only partially addressed by the anesthesia and muscle relaxants. The separation of electroporation and excitation thresholds is much smaller for nsEP (Fig. 3), and the ablated tissue volume should be similar to the stimulation volume. Some advanced ablation protocols already take advantage of the shorter pulse duration and further reduce excitation by switching the pulse polarity[66–68]. One may also anticipate that since the thresholds for excitation and damage go almost parallel throughout the nanosecond range (Fig. 3), shortening pulses from, say, 800 ns to 200 ns will do little to reduce the neuromuscular effects further.
In many nsEP studies and applications, pulse amplitude is tuned high to ensure robust effects, and both the electroporation and excitation thresholds will be exceeded. Since excitation is delayed, regardless of its underlying mechanism, it will unlikely affect electroporation, which would have happened already before the AP. On the contrary, the consequences of electroporation for excitation are complex and may vary. Electroporation will instantly reduce the discharging time constant, thereby diminishing chances for the direct excitation. Concurrently, electroporation will initiate the loss of the resting membrane potential, which may (a) be slow and permit excitation by nsEP as if the membrane remained intact[36], (b) assist excitation by depolarizing the membrane towards to the excitation threshold, (c) increase inactivation of channels and suppress the AP, or (d) promptly depolarize to the excitation threshold and elicit the AP. The exact outcome will depend on the pulse duration and amplitude relative to the thresholds, as well as on the cell physiology.
5. Summary
The requirement of a finite minimum depolarization time to open voltage-gated channels can explain the low efficiency of nsEP for stimulation of excitable cells and tissues. In particular, it resolves the long-standing paradox that excitation by nsEP often occurs only above the electroporation threshold. The membrane charging time constant is perhaps the principal, although not the only parameter that determines if excitation by nsEP can be accomplished or not without the concurrent membrane disruption.
Highlights.
Electroporation by nanosecond pulses often occurs below the excitation threshold
Electroporation may cause, assist, or impede excitation
Nanosecond pulses have low efficiency for cell excitation and activation
Large membrane charging time constant may enable damage-free excitation
Acknowledgements
The study was supported by AFOSR (MURI grant FA9550-15-1-0517), NHLBI (R01HL128381), and Pulse Biosciences.
Footnotes
Conflicts of interest
The authors have stock options in Pulse Bioscience and authored patents on nsEP applications
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Neumann E, Sowers AE, Jordan CA, Electroporation and Electrofusion in Cell Biology, Plenum, New York, 1989. [Google Scholar]
- [2].Zimmermann U, Neil GA, Electromanipulation of cells, CRC Press, Boca Raton, 1996. [Google Scholar]
- [3].Tsong TY, Electroporation of cell membranes, Biophys J, 60 (1991) 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Teissie J, Golzio M, Rols MP, Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge, Biochim Biophys Acta, 1724 (2005) 270–280. [DOI] [PubMed] [Google Scholar]
- [5].Pakhomov AG, Miklavcic D, Markov MS, Advanced Electroporation Techniques in Biology in Medicine, CRC Press, Boca Raton, 2010, pp. 528 [Google Scholar]
- [6].Blair HA, On the Intensity-Time Relations for Stimulation by Electric Currents. I, J Gen Physiol, 15 (1932) 709–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brunel N, van Rossum MCW, Quantitative investigations of electrical nerve excitation treated as polarization, Biological Cybernetics, 97 (2007) 341–349. [DOI] [PubMed] [Google Scholar]
- [8].Reilly JP, Applied Bioelectricity: From Electrical Stimulation to Electropathology, Springer-Verlag New York Inc, New York, 1998. [Google Scholar]
- [9].Rogers WR, Merritt JH, Comeaux JA, Kuhnel CT, Moreland DF, Teltschik DG, Lucas JH, Murphy MR, Strength-duration curve for an electrically excitable tissue extended down to near 1 nanosecond, Ieee Transactions on Plasma Science, 32 (2004) 1587–1599. [Google Scholar]
- [10].Casciola M, Xiao S, Pakhomov AG, Damage-free peripheral nerve stimulation by 12-ns pulsed electric field, Sci Rep, 7 (2017) 10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Casciola M, Xiao S, Apollonio F, Paffi A, Liberti M, Muratori C, Pakhomov AG, Cancellation of nerve excitation by the reversal of nanosecond stimulus polarity and its relevance to the gating time of sodium channels, Cell Mol Life Sci, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pakhomov AG, Xiao S, Novickij V, Casciola M, Semenov I, Mangalanathan U, Kim V, Zemlin C, Sozer E, Muratori C, Pakhomova ON, Excitation and electroporation by MHz bursts of nanosecond stimuli, Biochem Biophys Res Commun, 518 (2019) 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang S, Chen J, Chen MT, Vernier PT, Gundersen MA, Valderrabano M, Cardiac myocyte excitation by ultrashort high-field pulses, Biophysical journal, 96 (2009) 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Varghese F, Neuber JU, Xie F, Philpott JM, Pakhomov AG, Zemlin CW, Low-Energy Defibrillation with Nanosecond Electric Shocks, Cardiovasc Res, 113 (2017) 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neuber JU, Varghese F, Pakhomov AG, Zemlin CW, Using Nanosecond Shocks for Cardiac Defibrillation, Bioelectricity, 1 (2019) 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Semenov I, Grigoryev S, Neuber JU, Zemlin CW, Pakhomova ON, Casciola M, Pakhomov AG, Excitation and injury of adult ventricular cardiomyocytes by nano- to millisecond electric shocks, Sci Rep, 8 (2018) 8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Semenov I, Zemlin C, Pakhomova ON, Xiao S, Pakhomov AG, Diffuse, non-polar electropermeabilization and reduced propidium uptake distinguish the effect of nanosecond electric pulses, Biochim Biophys Acta, 1848 (2015) 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Semenov I, Xiao S, Pakhomova ON, Pakhomov AG, Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: the impact of pulse duration, Cell Calcium, 54 (2013) 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Semenov I, Xiao S, Pakhomov AG, Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field, Biochim Biophys Acta, 1828 (2013) 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pakhomov AG, Pakhomova ON, Nanopores: A distinct transmembrane passageway in electroporated cells, in: Pakhomov AG, Miklavcic D, Markov MS (Eds.) Advanced Electroporation Techniques in Biology in Medicine, CRC Press, Boca Raton, 2010, pp. 178–194. [Google Scholar]
- [21].Nesin V, Pakhomov AG, Inhibition of voltage-gated Na(+) current by nanosecond pulsed electric field (nsPEF) is not mediated by Na(+) influx or Ca(2+) signaling, Bioelectromagnetics, 33 (2012) 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nesin V, Bowman AM, Xiao S, Pakhomov AG, Cell permeabilization and inhibition of voltage-gated Ca(2+) and Na(+) channel currents by nanosecond pulsed electric field, Bioelectromagnetics, 33 (2012) 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang L, Craviso GL, Vernier PT, Chatterjee I, Leblanc N, Nanosecond electric pulses differentially affect inward and outward currents in patch clamped adrenal chromaffin cells, PLoS One, 12 (2017) e0181002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Azarov JE, Semenov I, Casciola M, Pakhomov AG, Excitation of murine cardiac myocytes by nanosecond pulsed electric field, J Cardiovasc Electrophysiol, 30 (2019) 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Craviso GL, Choe S, Chatterjee P, Chatterjee I, Vernier PT, Nanosecond electric pulses: a novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels, Cell Mol Neurobiol, 30 (2010) 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vernier PT, Sun Y, Chen MT, Gundersen MA, Craviso GL, Nanosecond electric pulse-induced calcium entry into chromaffin cells, Bioelectrochemistry, 73 (2008) 1–4. [DOI] [PubMed] [Google Scholar]
- [27].Bagalkot TR, Leblanc N, Craviso GL, Stimulation or Cancellation of Ca(2+) Influx by Bipolar Nanosecond Pulsed Electric Fields in Adrenal Chromaffin Cells Can Be Achieved by Tuning Pulse Waveform, Sci Rep, 9 (2019) 11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bagalkot TR, Terhune RC, Leblanc N, Craviso GL, Different Membrane Pathways Mediate Ca2+ Influx in Adrenal Chromaffin Cells Exposed to 150–400 ns Electric Pulses, BioMed Research International, 2018 (2018) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoon J, Leblanc N, Zaklit J, Vernier PT, Chatterjee I, Craviso GL, Enhanced Monitoring of Nanosecond Electric Pulse-Evoked Membrane Conductance Changes in Whole-Cell Patch Clamp Experiments, J Membr Biol, 249 (2016) 633–644. [DOI] [PubMed] [Google Scholar]
- [30].Hristov K, Mangalanathan U, Casciola M, Pakhomova ON, Pakhomov AG, Expression of voltage-gated calcium channels augments cell susceptibility to membrane disruption by nanosecond pulsed electric field, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1860 (2018) 2175–2183. [DOI] [PubMed] [Google Scholar]
- [31].Semenov I, Xiao S, Pakhomov AG, Electroporation by subnanosecond pulses, Biochemistry and biophysics reports, 6 (2016) 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Semenov I, Xiao S, Kang D, Schoenbach KH, Pakhomov AG, Cell stimulation and calcium mobilization by picosecond electric pulses, Bioelectrochemistry, 105 (2015) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roth CC, Tolstykh GP, Payne JA, Kuipers MA, Thompson GL, DeSilva MN, Ibey BL, Nanosecond pulsed electric field thresholds for nanopore formation in neural cells, Journal of biomedical optics, 18 (2013) 035005. [DOI] [PubMed] [Google Scholar]
- [34].Jiang N, Cooper BY, Frequency-dependent interaction of ultrashort E-fields with nociceptor membranes and proteins, Bioelectromagnetics, 32 (2011) 148–163. [DOI] [PubMed] [Google Scholar]
- [35].Nene D, Jiang N, Rau KK, Richardson M, Cooper BY, Nociceptor activation and damage by pulsed E-Fields. - art. no. 621904, Enabling Technologies and Design of Nonlethal Weapons, 6219 (2006) 21904–21904. [Google Scholar]
- [36].Pakhomov AG, Semenov I, Casciola M, Xiao S, Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye, Biochim Biophys Acta, 1859 (2017) 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Levine ZA, Vernier PT, Life cycle of an electropore: field-dependent and field-independent steps in pore creation and annihilation, J Membr Biol, 236 (2010) 27–36. [DOI] [PubMed] [Google Scholar]
- [38].Vernier PT, Levine ZA, Ho MC, Xiao S, Semenov I, Pakhomov AG, Picosecond and Terahertz Perturbation of Interfacial Water and Electropermeabilization of Biological Membranes, J Membr Biol, 248 (2015) 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu Q, Viswanadham S, Joshi RP, Schoenbach KH, Beebe SJ, Blackmore PF, Simulations of Transient Membrane Behavior in Cells Subjected to a High-Intensity, Ultra-Short Electric Pulse, Phys. Rev., E 71 (2005) 031914. [DOI] [PubMed] [Google Scholar]
- [40].Vasilkoski Z, Esser AT, Gowrishankar TR, Weaver JC, Membrane electroporation: The absolute rate equation and nanosecond time scale pore creation, Physical Review E 74 (2006) 021904. [DOI] [PubMed] [Google Scholar]
- [41].Smith KC, Gowrishankar TR, Esser AT, Stewart DA, Weaver JC, Spatially distributed, dynamic transmembrane voltages of organelle and cell membranes due to 10 ns pulses: predictions of meshed and unmeshed transpoort network models, IEEE Transactions on Plasma Science 34 (2006) 1394–1404. [Google Scholar]
- [42].Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nuccitelli R, Beebe SJ, Schoenbach KH, Kolb JF, Plasma membrane voltage changes during nanosecond pulsed electric field exposure, Biophys J, 90 (2006) 3608–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hille B, Ionic Channels of Excitable Membranes, 3 ed., Sinauer Associates, Sunderland, MA, 2001. [Google Scholar]
- [44].Delemotte L, Kasimova MA, Klein ML, Tarek M, Carnevale V, Free-energy landscape of ion-channel voltage-sensor-domain activation, Proc Natl Acad Sci U S A, 112 (2015) 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Delemotte L, Tarek M, Klein ML, Amaral C, Treptow W, Intermediate states of the Kv1.2 voltage sensor from atomistic molecular dynamics simulations, Proc Natl Acad Sci U S A, 108 (2011) 6109–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Freites JA, Tobias DJ, Voltage Sensing in Membranes: From Macroscopic Currents to Molecular Motions, J Membr Biol, 248 (2015) 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Catterall WA, Ion channel voltage sensors: structure, function, and pathophysiology, Neuron, 67 (2010) 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bezanilla F, Gating currents, J Gen Physiol, 150 (2018) 911–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bezanilla F, Voltage-gated ion channels, IEEE Trans Nanobioscience, 4 (2005) 34–48. [DOI] [PubMed] [Google Scholar]
- [50].Sigg D, Bezanilla F, Stefani E, Fast gating in the Shaker K+ channel and the energy landscape of activation, Proc Natl Acad Sci U S A, 100 (2003) 7611–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Conti F, Stuhmer W, Quantal charge redistributions accompanying the structural transitions of sodium channels, Eur Biophys J, 17 (1989) 53–59. [DOI] [PubMed] [Google Scholar]
- [52].Gabriel B, Teissie J, Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane, Biophys J, 73 (1997) 2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hibino M, Itoh H, Kinosita K, Time Courses of Cell Electroporation as Revealed by Submicrosecond Imaging of Transmembrane Potential, Biophysical Journal, 64 (1993) 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Teissie J, Rols MP, An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization, Biophys J, 65 (1993) 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bier M, Hammer SM, Canaday DJ, Lee RC, Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells, Bioelectromagnetics, 20 (1999) 194–201. [DOI] [PubMed] [Google Scholar]
- [56].Towhidi L, Kotnik T, Pucihar G, Firoozabadi SM, Mozdarani H, Miklavcic D, Variability of the minimal transmembrane voltage resulting in detectable membrane electroporation, Electromagn Biol Med, 27 (2008) 372–385. [DOI] [PubMed] [Google Scholar]
- [57].Kinosita K, Tsong TY, Voltage-induced pore formation and hemolysis of human erythrocytes, Biochimica et biophysica acta, 471 (1977) 227–242. [DOI] [PubMed] [Google Scholar]
- [58].Koch C, Rapp M, Segev I, A brief history of time (constants), Cereb Cortex, 6 (1996) 93–101. [DOI] [PubMed] [Google Scholar]
- [59].Esser AT, Smith KC, Gowrishankar TR, Vasilkoski Z, Weaver JC, Mechanisms for the intracellular manipulation of organelles by conventional electroporation, Biophys J, 98 (2010) 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schoenbach KS, Hargrave B, Joshi RP, Kolb J, Osgood C, Nuccitelli R, Pakhomov AG, Swanson J, Stacey M, White JA, Xiao S, Zhang J, Beebe SJ, Blackmore PF, Buescher ES, Bioelectric Effects of Nanosecond Pulses, IEEE Transactions on Dielectrics and Electrical Insulation, 14 (2007) 1088–1109. [Google Scholar]
- [61].Bowman AM, Nesin OM, Pakhomova ON, Pakhomov AG, Analysis of plasma membrane integrity by fluorescent detection of Tl(+) uptake, J Membr Biol, 236 (2010) 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ibey BL, Xiao S, Schoenbach KH, Murphy MR, Pakhomov AG, Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose, Bioelectromagnetics, 30 (2009) 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bo W, Silkunas M, Mangalanathan U, Novickij V, Casciola M, Semenov I, Xiao S, Pakhomova ON, Pakhomov AG, Probing Nanoelectroporation and Resealing of the Cell Membrane by the Entry of Ca(2+) and Ba(2+) Ions, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rubinsky B, Irreversible Electroporation, Series in Biomedical Engineering, Springer-Verlag, Berlin Heidelberg, 2010. [Google Scholar]
- [65].Davalos RV, Mir ILM, Rubinsky B, Tissue ablation with irreversible electroporation, Annals of biomedical engineering, 33 (2005) 223–231. [DOI] [PubMed] [Google Scholar]
- [66].Ringel-Scaia VM, Beitel-White N, Lorenzo MF, Brock RM, Huie KE, Coutermarsh-Ott S, Eden K, McDaniel DK, Verbridge SS, Rossmeisl JH, Oestreich KJ, Davalos RV, Allen IC, High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity, Ebiomedicine, 44 (2019) 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].O'Brien TJ, Passeri M, Lorenzo MF, Sulzer JK, Lyman WB, Swet JH, Vrochides D, Baker EH, Iannitti DA, Davalos RV, McKillop IH, Experimental High-Frequency Irreversible Electroporation Using a Single-Needle Delivery Approach for Nonthermal Pancreatic Ablation In Vivo, Journal of Vascular and Interventional Radiology, 30 (2019) 854–862. [DOI] [PubMed] [Google Scholar]
- [68].Arena CB, Sano MB, Rossmeisl JH Jr., Caldwell JL, Garcia PA, Rylander MN, Davalos RV, High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction, Biomed Eng Online, 10 (2011) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]