Abstract
Orexin is a neuropeptide secreted from lateral hypothalamus and pre-frontal cortex concerned in the wakefulness and excitement. This study aimed to review the possible neurobiological effect of orexin. A diversity of search strategies was adopted and assumed which included electronic database searches of Medline and PubMed using MeSH terms, keywords, and title words during the search. Orexin plays a vital role in activation of learning, memory acquisition, and consolidation through activation of monoaminergic system, which affect cognitive flexibility and cognitive function. Orexin stimulates adrenocorticotropin and corticosteroid secretions via activation of central corticotropin-releasing hormone. Cerebrospinal fluid (CSF) and serum orexin serum levels are reduced in depression, schizophrenia, and narcolepsy. However, high orexin serum levels are revealed in drug addictions. Regarding neurodegenerative brain diseases, CSF and serum orexin serum levels are reduced Parkinson disease, Alzheimer dementia, Huntington's disease, amyotrphic lateral sclerosis, and multiple sclerosis. Orexin antagonist leads to significant reduction of sympathetic over-activity during withdrawal syndrome. As well, orexin antagonist improves sleep pattern. Orexinergic system is involved in the different psychiatric and neurological disorders; therefore, targeting of this system could be possible novel pathway in the management of these disorders. In addition, measurement of CSF and serum orexin levels might predict the relapse and withdrawal of addict patients.
Keywords: Addiction, depression, learning, memory, orexin, schizophrenia, sleep disorders
Introduction
Orexigenic system
Orexin is a neuropeptide secreted from orexigenic neurons at hypothalamus and prefrontal cortex in about 10,000–20,000 neurons.[1] Orexin is responsible for the regulation of weak fullness and arousal, which was discovered in rat brain in 1998 by two researcher groups, one name it hypocretin, since it produced from hypothalamus, and the second group called it orexin from the orexin Greek word which means appetite.[2]
Two distinct types of orexin, orexin-A and orexin-B, were identified with 50%, similarity, they act on specific receptors which are orexin receptor type 1 (OX1R) and orexin receptor type 2 (OX2R) receptors. Orexin-A activates these equally, while orexin-B has a higher affinity to (OX2R) than (OX1R) [Figure 1].[3]
Figure 1.
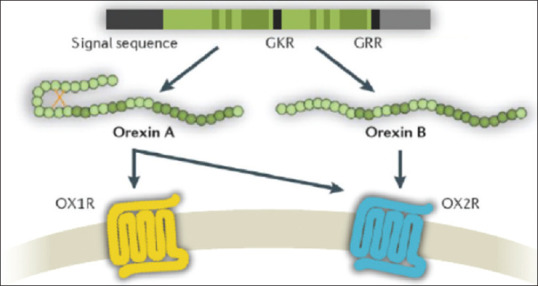
Schematic representation of orexin system
Orexin receptors are distributed mainly in the lateral hypothalamus and adjacent areas; their nerve fibers project to multiple brain regions. Orexinergic neurons in the lateral hypothalamus group are closely associated with reward related functions. These neurons preferentially innervate the ventral tegmental area and the ventromedial prefrontal cortex (mPFC). These neurons project inter-hypothalamically, as well as to the brainstem, where the release of orexin modulates various autonomic processes. Indeed, accumulating evidence shows that the orexin/receptor system is ectopically expressed in several neurological disorders, suggesting that it plays an important role in the incidence and pathogenesis of different neurological diseases.[4]
It has been verified that hypothalamic orexigenic neurons are involved in reward functions, while prefrontal orexigenic neurons are linked in the regulation of autonomic and arousal functions. Moreover, orexin provokes and stimulates food intake via inhibition of autonomic digestive feedbacks. Orexigenic neurons are inhibited by leptin and food intake, and stimulated by hypoglycemia and ghrelin. Amino acid and high protein diets paradoxically block glucose-induced orexigenic neuron activations.[5] Animal model studies have shown that orexin link sleep with the body metabolism, since sleep deprivation leads to higher food intake and induction of catabolism.[6]
Moreover, orexin stimulate different neurotransmitters which are linked to the activation of central nervous system, including acetylcholine, histamine, noradrenaline, and dopamine. Therefore, mutations of orexin receptors lead to sleep disorders. Mice with orexin knockout are subjected to narcolepsy and excessive daytime sleepiness.[7] Alizamini et al.'s study showed that central administration of orexin leads to stimulation of locomotion, psychomotor performance, body temperature, and energy expenditure. Furthermore, mice with orexin deficient are subjected to obesity due to reduction of basal metabolic and energy expenditure rates. Beside, orexin knockout out mice is characterized by a reduction in brown adipose tissue thermogenesis with poor differentiation of preadipocyte into adipocytes in the adipose tissue.[8] Central and peripheral effects of orexin are illustrate in Figure 2.
Figure 2.
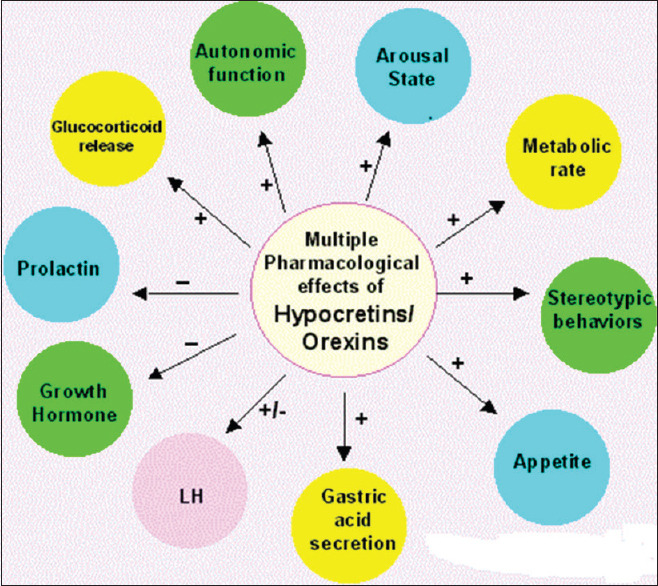
Central and peripheral effects of orexin
The aim of this study article was to provide a narrative review of the neurobiological effect of orexin system and to examine the association between orexin neurotransmission and different psychoneurological disorders, including depression, schizophrenia, addiction, Parkinson disease (PD), and dementia. Evidence from experimental, preclinical, and clinical studies is evaluated for the relationships between orexin neurobiology and psychoneurological disorders.
Search strategy
A diversity of search strategies including; electronic database searches of Medline and Pubmed using MeSH terms, keywords and title words during the search. The terms used for these searches were as follows: (orexin OR hypocretin) AND (cognitive function OR vigilance OR depression OR schizophrenia OR addiction OR Alzheimer dementia OR stroke OR sleep disorders). (suvorexant OR orexin antagonists) AND (sleep disorders OR vigilance OR depression OR schizophrenia OR addiction). Reference lists of notorious articles were reviewed. Besides, only English articles were considered and case reports were not involved in the review. The key features of recognized relevant search studies were considered, and the conclusions summarized in a narrative review.
Role of orexin in vigilance and cognitive function
Orexin regulates behavioral and neuro-endocrine response during stressful conditions as these events lead to the impairment of cognitive flexibility and cognitive function. As well, patients with psychiatric disorders such as panic disorder are associated with significant reduction of hypothalamic orexin activations.[9]
It has been shown, stress improves male cognitive flexibility, but it worsens female cognitive flexibility due to gender differences in stress-induced orexin neuropeptide activations. Women are twice as likely as men to suffer from stress-related psychiatric disorders, such as posttraumatic stress disorder and major depressive disorder; however, the biological basis of these sex differences is not fully understood. Interestingly, orexins are known to be dysregulated in these disorders. Both preclinical and clinical studies have reported higher orexin system expression in females, which, contributes to exaggerated neuroendocrine and behavioral responses to stress. Therefore, orexins may be important in the etiology of stress-related psychiatric disorders that present differently in men and women.[10] Piantadosi et al. illustrated that stimulation of prefrontal cholinergic neurons lead to the release of orexin from hypothamic neurons, which play an important role in cognitive activation, since high orexin activates the arousal state and executive functions via activation of cortical cholinergic neurons.[11] Chieffi et al.'s study reported the beneficial effects of exercise in stimulation of orexin release due to enhancement of hippocampal activity as exercise attenuates hippocampal deterioration and depressive symptoms in elderly persons through regulation of orexin release.[12]
As well, cognitive impairment is the main feature of neurological and neuropsychiatric disorders as in dementia and narcolepsy. Therefore, intranasal orexin peptide may be an effective agent for cognitive dysfunction.[13] Astonishingly, orexin plays a crucial role in activation of learning and memory, as orexin-A provokes memory acquisition and consolidation through activation of monoaminergic system. Consequently, orexin antagonist leads to significant memory dysfunction in the experimental rats.[14] Kim et al.'s study revealed that orexin is an important key factor of hippocampal neurogenesis as orexin-A participates in the hippocampal neuronal proliferation and neuroprotection following stroke; thus, orexin agonist participates in the prevention of negative stroke outcomes.[15] On the other hand, Uslaner et al. exhibited that dual orexin receptor antagonists-22 is an effective sedative agent, with less cognitive disability compared with GABA allosteric modulators, which cause significant cognitive dysfunctions.[16,17]
Endocrine effects of orexin
Orexin is involved in the regulation of central and peripheral signals to regulate metabolic homeostasis. Alongside, orexin stimulates adrenocorticotropin (ACTH) and corticosteroid secretions via activation of central corticotropin-releasing hormone, and vasopressin. Therefore, orexin through OX2R receptor controls hypothalamic-pituitary axis (HPA).[18] Malendowicz et al. illustrated that a chronic orexin administration led to dose-dependent increased in cortisol and aldosterone plasma levels independent of ACTH levels, indicating a direct stimulating effect of orexin on the adrenal cortex.[19] But, in spite of these findings, Patel et al.'s study confirmed insignificant effect of orexin antagonists on ACTH and cortisol serum levels as well as on the markers of sympathetic nervous system.[20]
It has been reported that orexin administration leads to significant suppression of the hypothalamic prolactin release, which is not upturns by dopamine receptor antagonists like metoclopramide suggesting a novel pathway in controlling of prolactin secretion. The mechanism of prolactin inhibition may be through inhibition of prolactin releasing factor or stimulation of prolactin inhibiting factor. But, previous study illustrated insignificant effect of orexin antagonist on prolactin plasma levels.[21,22]
Many studies showed that the body metabolism, mainly glucose is regulated by central orexin through regulation of hepatic glucose production, skeletal glucose consumption, and thermogenesis. High orexin or dys-rhythmic in orexin secretion is linked with the development of obesity and insulin resistance.[23,24] Thus, suvorexant and other orexin antagonists are effective in the management of obesity and insulin resistance via amelioration of body adiposity and augmentation of energy expenditure that improve glucose metabolism. Moreover, orexin-A has important roles in the regulation of pancreatic islet biology through activation of insulin secretion and prolongation of pancreatic islets life span.[25]
Tsuneki et al.'s study illustrated that suvorexant improves glucose tolerance through inhibition of hepatic gluconeogenic factors, when administrated at resting time. However, administration of suvorexant at awaking time illustrates insignificant effect on glucose tolerance due to differential effects on the orexin sleep/wake operating system.[26]
Flores et al.'s study illustrated an interaction between endocannabiniod and orexigenic neurons as there is a similarity between Ox1R and CB1 receptors with diffuse overlapping in the anatomical distribution of these neurons. Therefore, the pharmacological effect of cannabinoid may be through orexigenic receptors.[27]
Role of Orexin in Psychiatric Disorders
Depression
Among important etiological factors involved in the pathophysiology of depression, disturbances of monoamines and HPA are the main mechanistic pathways leading to functional disorders of neuroplasticity, which is regarded as a cardinal step in the onset of depression.[28]
It has been reported that orexin level is significantly decreased in patients with depression in comparison with healthy subjects.[29] But, paradoxical high orexin serum levels are seen in some depressed patients, which normalized by selective serotonin reuptake inhibitors. Since, orexin-A cerebrospinal fluid (CSF) levels negatively correlated with depressive symptoms.[30]
Long-term antidepressant agents improve orexin serum levels regardless the type of anti-depressant medications.[31] Nevertheless, there are different findings concerning orexin levels in depression. Feng et al. reported that depression is linked to reduction of serotonergic neuronal activity which responsible for modulation of orexinergic activity.[32] Thus, reduction of serotonergic neuronal activity leads to activation of orexin neuroactivity leading to depression. However, orexin levels are significantly reduced in depression compared with healthy control.[33]
The initial animal model study observed reduction in the orexinergic neurons by 18% with diminution in size of these neurons in comparison with normal rats. As well, prepro-orexin messenger RNA (mRNA) expression and orexin-A were reduced compared with control.[34]
Previous preclinical study revealed a strong connection between low orexin and risk of depression which are inconsistence with previous studies that illustrated hypo-activity of orexinergic neurons in patients with depression, since short-term anti-depressant therapy improves sleep pattern through increasing and decreasing the expression of mRNA of orexin-A and orexin-B respectively.[35]
Ito et al. showed that administration of orexin-A leads to significant reduction of despair behavior in depression with important hippocampal neurogenesis via up-regulation of neuropeptide Y. These changes are inhibited by co-administration of orexin-A antagonist.[36]
Therefore, orexin levels are different according to the pathophysiology of depression. Low orexin in depressed patients is associated with hypersomnia whereas; high orexin in depressed patients is associated with insomnia and interrupted sleep.[17] Ji et al. illustrated that orexinergic neurons have direct connection to the ventral pallidum (VP) which is concerned with stress response and rewarding system. Orexin stimulates VP and prevents depressive behavior. Therefore, high orexin in VP is associated with elevated serum corticosterone serum levels during acute stress, which perse prevent a depressive reaction against stressful events through improvement of stress resilience.[37]
Schizophrenia
The association between orexin and schizophrenia had not previously explored precisely.[38] Clinical and preclinical findings proposed that orexin and orexin agonist are of great value and useful in treating cognitive deficit in schizophrenia.[39] There are widespread connection and interaction between orexin and dopaminergic neurons in midbrain, thalamo-cortical and amygdale suggesting the potential role of orexinergic neurons in schizophrenia.[40]
Modafinil is an atypical dopamine reuptake inhibitor used in the treatment of narcolepsy and anti-psychotic drug-induced sleep disorder [Figure 3].[41] Modafinil has been revealed as a complement of drugs in therapy of schizophrenia; it reduced negative symptoms with no effect on the positive symptoms. Modafinil improves locomotors and psychomotor performances through activation of orexinergic neurons.[42]
Figure 3.
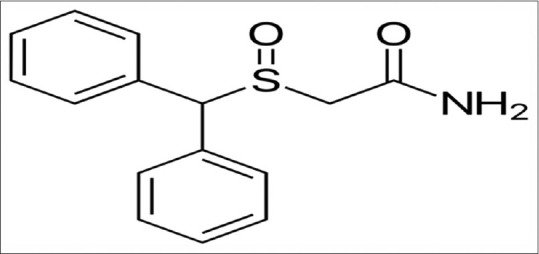
Chemical structure of modafinil
Therefore, activations of orexinergic neurons by modafinil may be an imperative step for future antipsychotic medications. These findings document that dopaminergic agonists mainly at D1 and D2 receptors modify orexinergic neurotransmissions.[43] As well, dopamine antagonists that cause weight gain lead to activation of orexin pathway, but; dopamine antagonists, which not cause weight gain not activate orexin pathway.[44] Nevertheless, amphetamine which indirectly activates dopamine leads to activation of orexinergic neurotransmission despite of induction of weight loss. Moreover, clozapine activates only orexinergic neurons in prefrontal cortex.[45] Similarly, orexin antagonists abolish olanzapine and haloperidol effect on midbrain dopaminergic neurons, suggesting that orexin is an important neurotransmitter mediates the action of antipsychotic drugs.[46] As well, Chen et al. illustrated that orexin-A is stimulated and upregulated by nonobesegenic antipsychotic drugs.[47] Also, the high orexin level in patients with schizophrenia treated with antipsychotic drugs is regarded as a protective factor against the development and risk of drug-induced metabolic syndrome.[48] Furthermore, orexin agonist like modafinil ameliorates cognitive function, attention, and antipsychotic-induced sedation.
Addiction
Orexinergic system has broad projections and connections to different brain area which are concerned with drug-induced neuroadaptation, including midbrain dopaminergic neurons, ventral tegmental area (VTA), nucleus accumbens (NA), amygdale and mPFC. Drug abuse leads to augmentation of dopaminergic activity in NA through activation of orexinergic neurons at mesocorticolimbic pathway.[49] Correspondingly, experimental studies illustrated that OX1R and OX2R are highly expressed in the NA leading to inhibitory effect instead of excitatory effects seen on VTA, amygdale and mPFC. Therefore, a differential effect of orexin is receptor type dependent.[50]
Acute administration of methamphetamine, nicotine and amphetamine leads to activation of orexinergic neurons at lateral hypothalamus. However, acute administration of cocaine and morphine not affect orexinergic neurons. Besides, chronic administration of abusing drugs causing activation of orexinergic neurons mainly at OX2R receptors, but; chronic rising dose of abusing drugs leads to down-regulation of orexinergic receptors.[51] Carr and Kalivas reported that orexin is an important mediator enables the cocaine to induce addiction-like behavior in rats due to dopaminergic neuronal changes.[52] As well, James et al. verified that orexinergic neurons at lateral hypothalamus play a vital role in expression of addiction-like phenotype.[53] Thus, orexinergic system is regarded as an important novel target for drug therapies to treat addiction.
Orexin serum level in chronic smoker subjects is related to craving and the phase of abstinence, since it increased during addiction phase and reduced during the withdrawal phase. This reduction leads to increased in the craving and risk of relapse.[54] Therefore; orexin serum level is regarded as potential biomarker predicts time and risk of smoking relapse.
Tsai and Huang reported that the orexin serum level is increased in heroin addicts shifted on methadone maintenance therapy compared with controls suggesting that methadone increases orexin serum levels.[55] Similarly, orexin serum level is increased in chronic alcoholism, which is positively correlated with the severity of alcohol withdrawal. Alleviation of alcohol withdrawal syndrome is linked with reduction of the orexin serum level, which monitors the status of alcoholic patients during the abstinence period.[56]
Sleep disorders
Narcolepsy is an excessive daytime sleepiness or an intractable urge to sleep in, which duration of rapid eye movement sleeps (REM) is reduced. Cataplexy is a sudden reduction in muscle tones with preserved consciousness. Narcolepsy is commonly associated with cataplexy, which triggers by emotional stimuli.[57] Methylphenidate, modafinil and other psychostimulants are effective in the management of these sleep disorders.[58] Dysregulation of NREM sleep leads to narcolepsy only, whereas; Dys-regulation of REM sleep leads combined narcolepsy with cataplexy.[59] It has been reported that orexin increases vigilance through increases awaking time and decreases REM and NREM sleep periods. Both OX1R and OX2R are involved in the maintenance of arousal state directly or indirectly through the activation of monoaminergic neurons (noradrenalin, dopamine, histamine and serotonin). As well, orexin activates cholinergic neurons in basal forebrain, which also important for arousal statues.[60] Yamanaka et al.'s study illustrated that activation of OX2R by orexin leads wakefulness which is mediated by histamine neurotransmitter, since antihistamine blocks the excitatory effect of orexin. While, activation of OX1R by orexin leads wakefulness, which is mediated by noradrenalin neurotransmitter.[61] Reduction of orexin level in the cerebrospinal fluid was documented in patients with narcolepsy and nowadays is regarded as one of the diagnostic criteria in the diagnosis of narcolepsy. Likewise, human postmortem study found that orexin peptide and prepro-orexin mRNA are deficient in the pons and cerebral cortex.[62] Therefore, these findings unveil that orexin is an important neuropeptide in the regulation of sleep and consolidated wakefulness, Figure 4.
Figure 4.
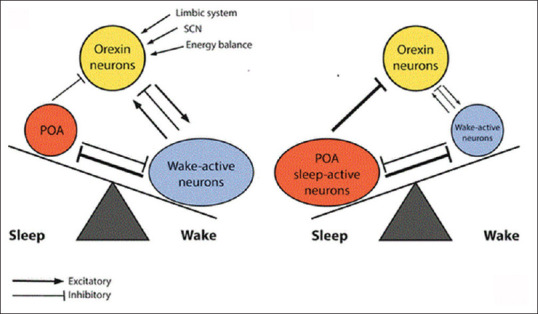
Role of orexin in sleep disorders
Role of Orexin in Neurodegenerative Diseases
Parkinson disease
Orexinergic neurons are severely affected in PD; previously Fronczek et al. confirmed that orexinerigic neurons density was reduced in the prefrontal cortex by 40% with significant reduction in CSF orexin levels in PD patients compared to the healthy control.[63]
Furthermore, animal model study illustrated that 15% damage to the orexinergic neurons did not affect CSF orexin, while damage more than 70% leads to 50% decline in the CSF orexin.[64] These findings may explain the association for narcolepsy in the PD, since both dopamine and orexin are interplay in the regulation of sleep pattern through activation of midbrain and thalamo-cortical pathway.[65] Feng et al. illustrated that in PD, there is a deficiency in hypoxia inducible factor 1 alpha (HIF-α) due to mitochondrial dysfunction, and the administration of orexin A leads to significant neuroprotective effect on the dopaminergic neurons through activation of HIF-α.[66]
Moreover, orexin A improves dopaminergic neurons in PD through attenuation the reduction of tyrosine hydroxylase and activation of brain derived neurotrophic factor (BDNF) in the substantia nigra.[44] Therefore, orexin antagonist may increase risk of PD due to reduce the neuroprotective and stimulating effects on the dopaminergic neurons at substantia nigra.[67] Sheng et al. found that orexin play important roles in activation of the subthalamic nucleus which may give a new evidence for the participation of the subthalamic orexinergic system in PD. Importantly, orexin-A increased the protein level of BDNF in dopaminergic neurons of the substantia nigra. The upregulation of BDNF is mainly via OX1R.[68] Long-term therapy with ropinirole in PD leads to significant reduction in the orexin activity which might explain the adverse effect of ropinirole-induced sleep disorder through inhibition of glutamaterigic excitatory effect on the orexinergic neurons. Therefore, pharmacotherapy of PD should be re-evaluated in this context.[69]
Alzheimer disease
Alzheimer disease (AD) is a neurodegerative disease affecting different brain areas characterized by cognitive deficit and progressive memory loss.[70] AD also affects hypothalamic orexinergic neurons, leading to excessive daytime sleepiness, which correlated with low orexin CSF levels, as reduction 40% of the brain cell number is linked with a 14% reduction in orexin CSF levels.[71] Normally, orexin regulates cholinergic and monoamiergic neurons firing during sleep and wakefulness. In AD, a reduction in the cholinergic pathway leads to disturbance in the sleep patterns leading to daytime sleepiness and insomnia at night which are a hallmark of sleep rhythm in AD.[72] Besides, reduction of cholinergic activity causes over-activity of orexinergic neurons, which causing abnormal sleep and cognitive functions. These changes lead to an elevation of the orexin CSF level, which is linked with reduced REM sleep.[73]
Dementia with Lewy bodies characterized by an elevation in α-synuclien level, which accumulated in the orexin containing neurons at hypothalamus causing interference in orexin axonal transport. This effect leads to a reduction in the activity of the orexinergic system in dementia with Lewy bodies but not in AD.[74] Therefore, there are complexities in the orexinergic system according to the clinical presentation and sleep pattern in patients with AD.
Huntington's disease
Huntington's disease (HD) is a hereditary neurodegenerative disorder characterized by personality changes, motor disturbances, cognitive decline and weight loss.[75] HD is caused by a defect in the gene encoding huntingtin, a protein with unclear function, which is essential for cell survival during development and in adult life.[76] In HD, there are neurodegeneration involving neostriatum and cerebral cortex, with the manifestation of intra-neuronal aggregates of misfolded huntingtin. Moreover, in patients with end-stage HD, there is about 90% of neuronal loss in the tuber nucleus of the lateral hypothalamus. Orexin A and B are synthesized from the same precursor gene and are expressed in the same neurons with their cell bodies concentrated to the lateral hypothalamus.[77] Preclinical and clinical studies observed that orexin serum and CSF levels are decreased by 72% in HD. In healthy subjects, orexin CSF level is >200 pg/ml but in HD and narcolepsy this level is decreased below 110 pg/ml, due to degeneration of orexinergic neurons in the lateral hypothalamus. Therefore, CSF orexin level is regarded as a biomarker to evaluate the disease progression and usefulness of therapeutic intervention in patients with HD.[78,79] However, Meier et al. illustrated that CSF and serum orexin levels are of no diagnostic value in prediction and follow up of HD.[80]
Cabanas et al. observed that orexin in HD has aberrant effect leads to abnormal sleep pattern, and thus orexin antagonist suvorexant may be of great value in restoring normal sleep and behavioral disturbance in HD.[81] in addition, despite reduction of orexinergic density in HD, these neurons remain functional and illustrate paradoxical effect, it become more modifiable and affect by serotonine and noradrenaline, and less sensitive to the effect of suprachiasmatic nucleus (the master clock of the brain) causing abnormal biological circadian rhythm.[81,82]
Multiple sclerosis
Multiple sclerosis (MS) is a demyelinating disease of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, blindness in one eye, muscle weakness and trouble with sensation or coordination. MS takes several forms, with new symptoms either occurring in isolated attacks (relapsing forms) or building up over time (progressive forms). Between attacks, symptoms may disappear completely; however, permanent neurological problems often remain, especially with the advancement of the disease.[83,84]
The three main characteristics of MS are the formation of lesions in the central nervous system, inflammation, and the destruction of myelin sheaths of neurons. These features interact in a complex and not yet fully understood manner to produce the breakdown of nerve tissue and in turn the signs and symptoms of the disease. Cholesterol crystals are believed to both impair myelin repair and aggravate inflammation. MS is believed to be an immune-mediated disorder that develops from an interaction of the individual's genetics and as yet unidentified environmental causes. Damage is believed to be caused, at least in part, by attack on the nervous system by a person's own immune system.[85]
Considering the multiplicity of symptoms associated with MS, there is possibility that hypocretin system function might be involved in the pathogenesis of the disease. Papuć et al. showed that orexin CSF level did not in patients with MS as compared with healthy controls, but it positively correlated with fatigue level, suggesting a compensatory mechanism for the production of orexin in MS.[86] On the other hand, Nozaki et al. illustrated that orexin CSF level is reduced and correlated with symmetrical hypothalamic lesion and spinal cord damage in MS. Therefore, low orexin level implicated in the pathogenesis of hypersomnia and cognitive deficit in patients with MS.[87] Recently, Pallais et al. confirmed that orexin has a neuroprotective effect in MS through inhibition of inflammatory and proinflammatory mediators mainly matrixmetaloproteinase (MMP-3, MMP-9) which are involved in damage of neuronal matrix proteins. Consequently, low CSF orexin level indicate underlying active disease.[88]
Therefore, CSF orexin level is valuable biomarker in the diagnosis and prediction of the severity of MS.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease is a disease that leads the death of neurons controlling voluntary muscles. The underlying mechanism involves damage to both upper and lower motor neurons. ALS is characterized by stiff muscles, muscle twitching, and muscle weakness. The cause of ALS is not known in 90% of cases, but is believed to involve both genetic and environmental factors. The remaining 10% of cases is inherited.[89] Previously, Van Rooij et al. illustrated that CSF orexin level was normal in patients with ALS and not correlated with age and gender. However, a disturbance in the orexinergic system is involved in the pathogenesis of ALS.[90] Moreover, the pathogenesis of ALS is associated of lateral hypothalamic lesions, a site of orexinergic system leading to sleep disturbances and hypersomnia.[91]
Amide different and large body of literature survey little is known about CSF orexin levels, in clinical and preclinical studies in ALS.
Orexin antagonists and neurobiology
Regarding orexin antagonists, suvorexant is a dual orexin receptor antagonist was approved by Food and Drug Administration on 13 August 2014.[92] Other orexin antagonists are almorexant, lemborexant and filorexant, which are used in the management of insomnia and other sleep disorders. Also, these drugs may be of great value in the control of depressive disorders and peripheral diabetic neuropathy.[93]
Suvorexant [Figure 5] is the first orexin antagonists approved in the United State for treatment of insomnia, which is effective in reduction time to sleep onset and increasing of total sleeping time.[94] Moreover, administration of SB-33867 which is an orexin antagonist leads to significant reduction of sympathetic tone causing a reduction in blood pressure, heart rate and plasma noradrenalin. These findings suggest that orexin through OX1 receptor regulates sympathetic tone, since intravenous administration of orexin leads to parallel increases in noradrenalin plasma levels.[95]
Figure 5.
Chemical structure of suvorexant
Hatta et al.'s study confirmed the significant effect of suvorexant in the management of delirium in elderly patients in acute care units. The anti-delirium effect is due to regulation of circadian biology.[96] Delirium is proposed to be related to disturbances and disorders in sleep pattern in critically ill patients in the intensive care unit. Also, attention disorders are caused by disturbances in the ascending reticular activating system (ARAS) which is responsible for maintenance of human arousal. Normally, the arousal state is regulated and stimulated by ARAS neurotransmitters and by hypothalamic orexin.[97] Therefore, orexin receptor antagonists may play important role in the regulation of hypothalamic and brain stem stress during acute injury. Moreover, a recent study by Kawada et al.'s study illustrated that suvorexant add on therapy to ralmeton in the management of sleep disorders in patients with acute stroke is more effective than when combined with benzodiazepines.[98]
It has been verified that prolong alcohol consumption is associated with sleep disturbance which is a powerful factor for relapse and set-back to alcohol use. Suvorexant reduces the motivation properties of alcohol so; it plays a crucial role in the prevention of alcoholism.[99]
Gentile et al.'s study revealed the possible role of suvorexant in reduction of motor impulsitivity of cocaine-induced psycho-stimulant effects. Thus, suvorexant may be effective in attenuation of cocaine withdrawal syndrome.[100]
As well, suvorexant had placebo like effect on EEG in comparison with zolpidem which has a significant reduction in the spectral density of REM and non-REM sleep pattern.[101]
In spite of the wide uses of suvorexant in the management of sleep disorders and controlling insomnia it did not reduce the psychomotor performances as documented by Vermeeren et al.'s study.[102]
Orexin A is involved in regulation of feeding; it stimulates nocturnal feeding through OX1 receptor. Therefore, OX1 receptor antagonist regulates feeding and reduced nocturnal feeding, thus, orexin antagonist could be useful in the treatment of obesity.[103] Orexin A is implicated in the pathogenesis of obesity; it promotes hyperphagia through central activation of cannabinoid receptors and inhibition of melanocyte stimulating hormone.[104] Both orexin-A and endocannabinoid increases glucose response of neuronal excitability in arcuate nucleus leading to induction of feeding and obesity.[105]
Therefore, more research is required to reinforce the extant information on the importance of the limited number of factors studied to date and provide data on additional potentially relevant effects. Similarly, rubric for such research should shift from preclinical and animal model studies to clinical studies to illustrate disease progression and treatment effects in relation to orexin neurobiology. This study suggests that orexin system is a future target in the management of different pschyo-neurological disorders after delineating the specific role of orexin receptor agonists and antagonists. Moreover, measurement of orexin serum level which is an easy method may be of great value in evaluation and assessment of different neurological disorders. As well, ratio of orexin serum level: CSF orexin level may reflect the activity of endogenous orexinergic system.
Conclusion
Orexinergic system is involved in the different psychiatric and neurological disorders, therefore targeting of this system could be possible novel pathway in the management of these disorders. In addition measurement of CSF and serum orexin levels might predict the relapse and withdrawal of addict patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors express deep thanks for all enrolled patients and volunteers.
References
- 1.Burdakov D. Reactive and predictive homeostasis: Roles of orexin/hypocretin neurons. Neuropharmacology. 2019;154:61–7. doi: 10.1016/j.neuropharm.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Airapetov MI, Sekste EA, Eresko SO, Bychkov ER, Lebedev AA, Shabanov PD. Chronic alcoholism influences the mRNA level of the orexin receptor type 1 (OX1R) in emotiogenic structures of the rat brain. Biomed Khim. 2018;64:451–4. doi: 10.18097/PBMC20186405451. [DOI] [PubMed] [Google Scholar]
- 3.Lu GL, Lee MT, Chiou LC. Orexin-mediated restoration of hippocampal synaptic potentiation in mice with established cocaine-conditioned place preference. Addict Biol. 2019;24:1153–66. doi: 10.1111/adb.12672. [DOI] [PubMed] [Google Scholar]
- 4.Wei Q, Krolewski DM, Moore S, Kumar V, Li F, Martin B, et al. Uneven balance of power between hypothalamic peptidergic neurons in the control of feeding. Proc Natl Acad Sci U S A. 2018;115:E9489–98. doi: 10.1073/pnas.1802237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomba L, Silvestri C, Imperatore R, Morello G, Piscitelli F, Martella A, et al. Negative regulation of leptin-induced reactive oxygen species (ROS) formation by cannabinoid CB1 receptor activation in hypothalamic neurons. J Biol Chem. 2015;290:13669–77. doi: 10.1074/jbc.M115.646885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneko K, Yoshikawa M, Ohinata K. Novel orexigenic pathway prostaglandin D2-NPY system-involvement in orally active orexigenic δ opioid peptide. Neuropeptides. 2012;46:353–7. doi: 10.1016/j.npep.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kuraishy HM, Al-Gareeb AI. Central beneficial effects of trimetazidine on psychomotor performance in normal healthy volunteers. Advanced Biomedical Res. 2017:623–9. doi: 10.4103/2277-9175.190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizamini MM, Kavianpour M, Karimi-Haghighi S, Fatahi Z, Haghparast A. Intra-hippocampal administration of orexin receptor antagonists dose-dependently attenuates reinstatement of morphine seeking behavior in extinguished rats. Peptides. 2018;110:40–6. doi: 10.1016/j.peptides.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Al-Kuraishy HM, Al-Gareeb AI, Naji MT, Al-Mamorry F. Role of vinpocetine in ischemic stroke and poststroke outcomes: A critical review. Brain Circulation. 2020;6:1–9. doi: 10.4103/bc.bc_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: Correlation with improved attention in rat. J Neurosci. 2005;25:5225–9. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piantadosi PT, Holmes A, Roberts BM, Bailey AM. Orexin receptor activity in the basal forebrain alters performance on an olfactory discrimination task. Brain Res. 2015;1594:215–22. doi: 10.1016/j.brainres.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Chieffi S, Messina G, Villano I, Messina A, Esposito M, Monda V, et al. Exercise influence on hippocampal function: possible involvement of orexin-A. Front Physiol. 2017;8:85. doi: 10.3389/fphys.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calva CB, Fadel JR. Intranasal administration of orexin peptides: Mechanisms and therapeutic potential for age-related cognitive dysfunction. Brain Res. 2020;1731:145921. doi: 10.1016/j.brainres.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J, Kim E, Kim CH, Song HT, Lee JE. The role of orexin in post-stroke inflammation, cognitive decline, and depression. Mol Brain. 2015;8:16. doi: 10.1186/s13041-015-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MK, Park HJ, Kim SR, Choi YK, Shin HK, Jeon JH, et al. Angiogenic role of orexin-A via the activation of extracellular signal-regulated kinase in endothelial cells. Biochem Biophys Res Commun. 2010;403:59–65. doi: 10.1016/j.bbrc.2010.10.115. [DOI] [PubMed] [Google Scholar]
- 16.Uslaner JM, Tye SJ, Eddins DM, Wang X, Fox SV, Savitz AT, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5:179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 17.Alijanpour S, Khakpai F, Ebrahimi-Ghiri M, Zarrindast MR. Co-administration of the low dose of orexin and nitrergic antagonists induces an antidepressant-like effect in mice. Biomed Pharmacother. 2019;109:589–94. doi: 10.1016/j.biopha.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Czerwinska J, Chojnowska K, Kaminski T, Bogacka I, Smolinska N, Kaminska B. Orexin receptor expression in the hypothalamic-pituitary-adrenal and hypothalamic- pituitary-gonadal axes of free-living European beavers (Castor fiber L.) in different periods of the reproductive cycle. Gen Comp Endocrinol. 2017;240:103–13. doi: 10.1016/j.ygcen.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Malendowicz LK, Hochol A, Ziolkowska A, Nowak M, Gottardo L, Nussdorfer GG. Prolonged orexin administration stimulates steroid-hormone secretion, acting directly on the rat adrenal gland. Int J Mol Med. 2001;7:401–4. doi: 10.3892/ijmm.7.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Patel AX, Miller SR, Nathan PJ, Kanakaraj P, Napolitano A, Lawrence P, et al. Neuroendocrine and sympathetic responses to an orexin receptor antagonist, SB-649868, and alprazolam following insulin-induced hypoglycemia in humans. Psychopharmacology (Berl) 2014;231:3817–28. doi: 10.1007/s00213-014-3520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Maiahy TJ, Al-Gareeb AI, Al-kuraishy HM. Prolactin and risk of preeclampsia: A single institution, cross-sectional study. Asian Pacific J Reproduction. 2019;8:112–19. [Google Scholar]
- 22.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/ orexin type 1 receptor in brain: Role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R382–7. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- 23.Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Berberine attenuates olanzapine induced-metabolic syndrome. JPMA. The J Pakistan Medical Association. 2019;69:S88–92.98. [PubMed] [Google Scholar]
- 24.Cigdem Arica P, Kocael A, Tabak O, Taskin M, Zengin K, Uzun H. Plasma ghrelin, leptin, and orexin-A levels and insulin resistance after laparoscopic gastric band applications in morbidly obese patients. Minerva Med. 2013;104:309–16. [PubMed] [Google Scholar]
- 25.Mediavilla C, Risco S. Orexin: Clinical and therapeutic implications. Rev Neurol. 2014;58:117–24. [PubMed] [Google Scholar]
- 26.Tsuneki H, Kon K, Ito H, Yamazaki M, Takahara S, Toyooka N, et al. Timed inhibition of orexin system by suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology. 2016;157:4146–57. doi: 10.1210/en.2016-1404. [DOI] [PubMed] [Google Scholar]
- 27.Flores A, Maldonado R, Berrendero F. Cannabinoid–hypocretin cross-talk in the central nervous system: What we know so far. Front Neurosci. 2013;7:256. doi: 10.3389/fnins.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus C, Castrén E, Kasper S, Lanzenberger R. Serotonin and neuroplasticity-Links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. 2017;77:317–26. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frölich M, et al. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J Clin Endocrinol Metab. 2002;87:805–9. doi: 10.1210/jcem.87.2.8246. [DOI] [PubMed] [Google Scholar]
- 30.Grady SP, Nishino S, Czeisler CA, Hepner D, Scammell TE. Diurnal variation in CSF orexin-A in healthy male subjects. Sleep. 2006;29:295–7. doi: 10.1093/sleep/29.3.295. [DOI] [PubMed] [Google Scholar]
- 31.Shariq AS, Rosenblat JD, Alageel A, Mansur RB, Rong C, Ho RC, et al. Evaluating the role of orexins in the pathophysiology and treatment of depression: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:1–7. doi: 10.1016/j.pnpbp.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008;22:784–91. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozsoy S, Olguner Eker O, Abdulrezzak U, Esel E. Relationship between orexin A and childhood maltreatment in female patients with depression and anxiety. Soc Neurosci. 2017;12:330–6. doi: 10.1080/17470919.2016.1169216. [DOI] [PubMed] [Google Scholar]
- 34.Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–5. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Brundin L, Petersén A, Björkqvist M, Träskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 2007;100:259–63. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, et al. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–32. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 37.Ji MJ, Zhang XY, Chen Z, Wang JJ, Zhu JN. Orexin prevents depressive-like behavior by promoting stress resilience. Mol Psychiatry. 2019;24:282–93. doi: 10.1038/s41380-018-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophr Bull. 2007;33:1284–90. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borgland SL, Labouèbe G. Orexin/hypocretin in psychiatric disorders: Present state of knowledge and future potential. Neuropsychopharmacology. 2010;35:353–4. doi: 10.1038/npp.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 41.Andrade C, Kisely S, Monteiro I, Rao S. Antipsychotic augmentation with modafinil or armodafinil for negative symptoms of schizophrenia: Systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2015;60:14–21. doi: 10.1016/j.jpsychires.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–34. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 45.Deutch AY, Bubser M. The orexins/hypocretins and schizophrenia. Schizophr Bull. 2007;33:1277–83. doi: 10.1093/schbul/sbm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: Implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–92. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- 47.Chen PY, Chen CH, Chang CK, Kao CF, Lu ML, Lin SK, et al. Orexin-A levels in relation to the risk of metabolic syndrome in patients with schizophrenia taking antipsychotics. Int J Neuropsychopharmacol. 2019;22:28–36. doi: 10.1093/ijnp/pyy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalal MA, Schuld A, Pollmächer T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol Psychiatry. 2003;8:836–7. doi: 10.1038/sj.mp.4001363. [DOI] [PubMed] [Google Scholar]
- 49.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–8. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 51.Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–7. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- 52.Carr D, Kalivas PW. Orexin: A gatekeeper of addiction. Nat Med. 2006;12:274–6. doi: 10.1038/nm0306-274. [DOI] [PubMed] [Google Scholar]
- 53.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2019;85:925–35. doi: 10.1016/j.biopsych.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.al'Absi M, Lemieux A, Hodges JS, Allen S. Circulating orexin changes during withdrawal are associated with nicotine craving and risk for smoking relapse. Addict Biol. 2019;24:743–53. doi: 10.1111/adb.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai MC, Huang TL. Orexin A in men with heroin use disorder undergoing methadone maintenance treatment. Psychiatry Res. 2018;264:412–5. doi: 10.1016/j.psychres.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Pan JS, Zheng K, Liu JH, Gao ZY, Ye YG, Ye MJ, et al. Orexin might predict status of alcohol dependence. Chin Med J (Engl) 2018;131:2866–7. doi: 10.4103/0366-6999.246068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 58.Hollway JA, Mendoza-Burcham M, Andridge R, Aman MG, Handen B, Arnold LE, et al. Atomoxetine, parent training, and their effects on sleep in youth with autism spectrum disorder and attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2018;28:130–5. doi: 10.1089/cap.2017.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturzenegger C, Baumann CR, Lammers GJ, Kallweit U, van der Zande WL, Bassetti CL. Swiss narcolepsy scale: A simple screening tool for hypocretin-deficient narcolepsy with cataplexy. Clin Transl Neurosci. 2018;2:2514183x18794175. [Google Scholar]
- 60.Almeneessier AS, Alzoghaibi M, BaHammam AA, Ibrahim MG, Olaish AH, Nashwan SZ, et al. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann Thorac Med. 2018;13:48–54. doi: 10.4103/atm.ATM_181_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–45. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 62.Gabelle A, Jaussent I, Hirtz C, Vialaret J, Navucet S, Grasselli C, et al. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging. 2017;53:59–66. doi: 10.1016/j.neurobiolaging.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Fronczek R, Overeem S, Lee SY, Hegeman IM, Van Pelt J, Van Duinen SG, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–85. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 64.Drouot X, Moutereau S, Nguyen JP, Lefaucheur JP, Créange A, Remy P, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61:540–3. doi: 10.1212/01.wnl.0000078194.53210.48. [DOI] [PubMed] [Google Scholar]
- 65.Asai H, Hirano M, Furiya Y, Udaka F, Morikawa M, Kanbayashi T, et al. Cerebrospinal fluid-orexin levels and sleep attacks in four patients with Parkinson's disease. Clin Neurol Neurosurg. 2009;111:341–4. doi: 10.1016/j.clineuro.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Feng Y, Liu T, Li XQ, Liu Y, Zhu XY, Jankovic J, et al. Neuroprotection by Orexin-A via HIF-1α induction in a cellular model of Parkinson's disease. Neurosci Lett. 2014;579:35–40. doi: 10.1016/j.neulet.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 67.Liu MF, Xue Y, Liu C, Liu YH, Diao HL, Wang Y, et al. Orexin-A exerts neuroprotective effects via O×1R in Parkinson's disease. Front Neurosci. 2018;12:835. doi: 10.3389/fnins.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheng Q, Xue Y, Wang Y, Chen AQ, Liu C, Liu YH, et al. The subthalamic neurons are activated by both orexin-A and orexin-B. Neuroscience. 2018;369:97–108. doi: 10.1016/j.neuroscience.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Michinaga S, Hisatsune A, Isohama Y, Katsuki H. An anti-Parkinson drug ropinirole depletes orexin from rat hypothalamic slice culture. Neurosci Res. 2010;68:315–21. doi: 10.1016/j.neures.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Hyman BT. Heterogeneity and complexity in Alzheimer disease. Brain Pathol. 2019;29:185. [Google Scholar]
- 71.Osorio RS, Ducca EL, Wohlleber ME, Tanzi EB, Gumb T, Twumasi A, et al. Orexin-A is associated with increases in cerebrospinal fluid phosphorylated-tau in cognitively normal elderly subjects. Sleep. 2016;39:1253–60. doi: 10.5665/sleep.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Triaca V, Calissano P. Impairment of the nerve growth factor pathway driving amyloid accumulation in cholinergic neurons: The incipit of the Alzheimer's disease story? Neural Regen Res. 2016;11:1553–6. doi: 10.4103/1673-5374.193224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 74.Lessig S, Ubhi K, Galasko D, Adame A, Pham E, Remidios K, et al. Reduced hypocretin (orexin) levels in dementia with Lewy bodies. Neuroreport. 2010;21:756–60. doi: 10.1097/WNR.0b013e32833bfb7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheong RY, Gabery S, Petersén Š. the role of hypothalamic pathology for non-motor features of Huntington's disease. J Huntingtons Dis. 2019;8:375–91. doi: 10.3233/JHD-190372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duarte AI, Sjögren M, Santos MS, Oliveira CR, Moreira PI, Björkqvist M. Dual therapy with liraglutide and ghrelin promotes brain and peripheral energy metabolism in the R6/2 mouse model of Huntington's disease. Sci Rep. 2018;8:8961. doi: 10.1038/s41598-018-27121-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petersén A, Gil J, Maat-Schieman ML, Björkqvist M, Tanila H, Araújo IM, et al. Orexin loss in Huntington's disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- 78.Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 79.Duffy CM, Hofmeister JJ, Nixon JP, Butterick TA. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem. 2019;157:41–7. doi: 10.1016/j.nlm.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meier A, Mollenhauer B, Cohrs S, Rodenbeck A, Jordan W, Meller J, et al. Normal hypocretin-1 (orexin-A) levels in the cerebrospinal fluid of patients with Huntington's disease. Brain Res. 2005;1063:201–3. doi: 10.1016/j.brainres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 81.Cabanas M, Pistono C, Puygrenier L, Rakesh D, Jeantet Y, Garret M, et al. Neurophysiological and behavioral effects of anti-orexinergic treatments in a mouse model of Huntington's disease. Neurotherapeutics. 2019;16:784–96. doi: 10.1007/s13311-019-00726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams RH, Morton AJ, Burdakov D. Paradoxical function of orexin/hypocretin circuits in a mouse model of Huntington's disease. Neurobiol Dis. 2011;42:438–45. doi: 10.1016/j.nbd.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 84.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–20. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 85.Fleischer V, Radetz A, Ciolac D, Muthuraman M, Gonzalez-Escamilla G, Zipp F, et al. Graph theoretical framework of brain networks in multiple sclerosis: A review of concepts. Neuroscience. 2019;403:35–53. doi: 10.1016/j.neuroscience.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 86.Papuć E, Stelmasiak Z, Grieb P, Paweł G, Rejdak K. CSF hypocretin-1 concentrations correlate with the level of fatigue in multiple sclerosis patients. Neurosci Lett. 2010;474:9–12. doi: 10.1016/j.neulet.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 87.Nozaki H, Shimohata T, Kanbayashi T, Sagawa Y, Katada S, Satoh M, et al. A patient with anti-aquaporin 4 antibody who presented with recurrent hypersomnia, reduced orexin (hypocretin) level, and symmetrical hypothalamic lesions. Sleep Med. 2009;10:253–5. doi: 10.1016/j.sleep.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 88.Pallais JP, Kotz CM, Stanojlovic M. Orexin/hypocretinin in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen Res. 2020;15:1039–40. doi: 10.4103/1673-5374.270310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takata M, Tanaka H, Kimura M, Nagahara Y, Tanaka K, Kawasaki K, et al. Fasudil, a rho kinase inhibitor, limits motor neuron loss in experimental models of amyotrophic lateral sclerosis. Br J Pharmacol. 2013;170:341–51. doi: 10.1111/bph.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Rooij FG, Schelhaas HJ, Lammers GJ, Verbeek MM, Overeem S. CSF hypocretin-1 levels are normal in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:487–9. doi: 10.3109/17482960802315024. [DOI] [PubMed] [Google Scholar]
- 91.Panda S, Gourie-Devi M, Sharma A. Sleep disorders in amyotrophic lateral sclerosis: A questionnaire-based study from India. Neurol India. 2018;66:700–8. doi: 10.4103/0028-3886.232327. [DOI] [PubMed] [Google Scholar]
- 92.Herring WJ, Roth T, Krystal AD, Michelson D. Orexin receptor antagonists for the treatment of insomnia and potential treatment of other neuropsychiatric indications. J Sleep Res. 2019;28:e12782. doi: 10.1111/jsr.12782. [DOI] [PubMed] [Google Scholar]
- 93.Perrey DA, Zhang Y. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. 2020;1731:145922. doi: 10.1016/j.brainres.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with insomnia: Results from two 3-month randomized controlled clinical Trials. Biol Psychiatry. 2016;79:136–48. doi: 10.1016/j.biopsych.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–48. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hatta K, Kishi Y, Wada K, Takeuchi T, Ito S, Kurata A, et al. Preventive effects of suvorexant on delirium: A randomized placebo-controlled trial. J Clin Psychiatry. 2017;78:e970–9. doi: 10.4088/JCP.16m11194. [DOI] [PubMed] [Google Scholar]
- 97.De Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front Pharmacol. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawada K, Ohta T, Tanaka K, Miyamura M, Tanaka S. Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients. J Stroke Cerebrovasc Dis. 2019;28:142–8. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 99.Campbell EJ, Marchant NJ, Lawrence AJ. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res. 2020;1731:145902. doi: 10.1016/j.brainres.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, et al. Effects of suvorexant, a dual orexin/hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology. 2018;43:1001–9. doi: 10.1038/npp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Struyk A, Gargano C, Drexel M, Stoch SA, Svetnik V, Ma J, et al. Pharmacodynamic effects of suvorexant and zolpidem on EEG during sleep in healthy subjects. Eur Neuropsychopharmacol. 2016;26:1649–56. doi: 10.1016/j.euroneuro.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Vermeeren A, Vets E, Vuurman EF, Van Oers AC, Jongen S, Laethem T, et al. On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30mg in healthy elderly. Psychopharmacology (Berl) 2016;233:3341–51. doi: 10.1007/s00213-016-4375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA. Sleep disorders, obesity, and aging: The role of orexin. Ageing Res Rev. 2015;20:63–73. doi: 10.1016/j.arr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morello G, Imperatore R, Palomba L, Finelli C, Labruna G, Pasanisi F, et al. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proc Natl Acad Sci U S A. 2016;113:4759–64. doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morello G, Imperatore R, Palomba L, Finelli C, Labruna G, Pasanisi F, et al. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proc Natl Acad Sci U S A. 2016;113:4759–64. doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]


