Abstract
The trend in our society to delay procreation increases the difficulty to conceive spontaneously. Thus, there is a growing need to use assisted reproduction technologies (ART) to form a family. With advanced maternal age, ovaries not only produce a lower number of oocytes after ovarian stimulation but also a lower quality—mainly aneuploidies—requiring further complex analysis to avoid complications during implantation and pregnancy. Although there are different options to have a child at advanced maternal age (like donor eggs), this is not the preferred choice for most patients. Unless women had cryopreserved their eggs at a younger age, reproductive medicine should try to optimize their opportunities to become pregnant with their own oocytes, when chances of success are reasonable. Aging has many causes, but telomere attrition is ultimately one of the main pathways involved in this process. Several reports link telomere biology and reproduction, but the molecular reasons for the rapid loss of ovarian function at middle age are still elusive. This review will focus on the knowledge acquired during the last years about ovarian aging and disease, both in mouse models of reproductive senescence and in humans with ovarian failure, and the implication of telomeres in this process. In addition, the review will discuss recent results on ovarian rejuvenation, achieved with stem cell therapies that are currently under study, or ovarian reactivation by tissue fragmentation and the attempts to generate oocytes in vitro.
Keywords: Telomeres, reproduction, ovary, stem cells, iPS cells
Introduction
Aging can be described as a degenerative process leading to the accumulation of senescent cells [1] and poor tissue homeostasis, accompanied with the decline of organ function [2]. In fact, the removal of senescent cells of tissues increases lifespan [3]. Ovaries are different to other organs in that they have a limited window of time for full function, which ends at middle age in humans, usually around 50 years, when menopause ensues. Accordingly, the concept of ovarian aging is different, and usually understood as a decline in the number and quality of oocytes produced with age [4] until cessation of menses, but during all this time, ovarian somatic cells such as granulosa and cumulus cells will develop their function normally. Among all the pathways involved in aging, telomere attrition is a primary cause of aging. Telomere function has a crosstalk with other bona fide aging pathways such as stem cell exhaustion, genomic instability, epigenetic alterations, mitochondrial dysfunction, and cellular senescence [5]. Mutations in telomerase lead to poor organ regeneration and diseases, both in mice and humans [6]. Ovarian dysfunction, caused by normal aging or diseases (idiopathic or not) is a topic of intense research, but many questions remain open, such as the possibility of oocyte renewal in the adult ovary, the choice of reactivating the ovaries to rescue dormant follicles, or the likelihood of developing in vitro functional oocytes.
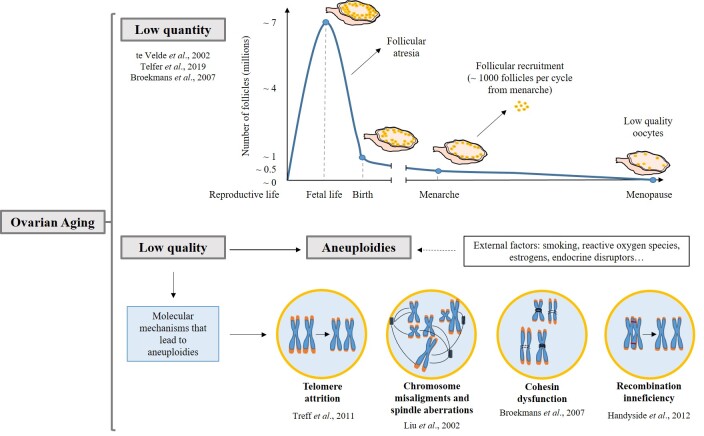
Ovarian Aging and Telomeres
Ovarian oocytes are generated during development and will remain arrested at meiosis prophase I for many years. Only upon follicle growth, starting at menarche, meiosis can continue, and oocytes can be fertilized [7]. At birth, ovaries contain over 1 million follicles (Figure 1), but most of them will decay, thus, at menarche only 300,000 to 400,000 follicles will be operative in the ovary [8]. Endocrine and paracrine factors such as Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) are decisive for follicle growth and survival [9]. About 1000 follicles are activated each month, but most of them undergo atresia, and those ovulated, have lower quality [10]. Indeed, advanced age correlates with the loss of sister-chromatid cohesion which may cause spindle instability and chromosome segregation errors, leading to aneuploidies (Figure 1) [8]. Analysis of blastocyst biopsies [11] showed that between 26 and 30 years, the incidence of aneuploidies is the lowest, with a constant increase up to 43 years [12,13]. At menopause, very few primordial follicles remain in the ovary [8] and the levels of FSH and LH are elevated while AMH levels are low. AMH is a bona fide marker of ovarian reserve [9]. Therefore, at advanced age, the ovarian reserve decreases (diminished ovarian reserve, DOR) leading to female infertility [14]. In fact, childbearing delay is one of the most important reasons for female infertility and the request for ART (14% of couples considering female and male factors). Apart from natural ovarian aging, some women have a rapid decay of follicles, known as Premature Ovarian insufficiency (POI), which can evolve to Premature Ovarian Failure (POF), when none or some residual follicles remain in the ovary before the age of 40 years. It can be due to autoimmune disease, ovarian surgery, cancer therapies, and can have genetic causes (Figure 2) [15,16]. Natural ovarian aging as well as POI and POF increase the risk of aging associated diseases, such as cardiovascular, osteoporosis, and neurodegenerative disorders [17,18].
Figure 1.
Ovarian aging. Molecular mechanisms that lead to aneuploidy. The top panel represents the follicular decay in the ovary during aging. At 16/20 weeks of fetal development, follicular quantity is maximum. Due to atresia, the number of follicles falls from 7 million to 1 million at birth. At the time of menarche, women have 300,000 to 400,000 follicles, which are recruited cyclically until menopause (1,000 follicles). The lower panel depicts the molecular mechanisms that lead to aneuploidies in the aged ovary. External factors can aggravate molecular alterations promoting aneuploidies. Chromosomes are represented in blue, telomeres in orange, spindles in dark blue and chiasmas are drawn in red. Cohesins are represented in black line and altered cohesins in broken lines.
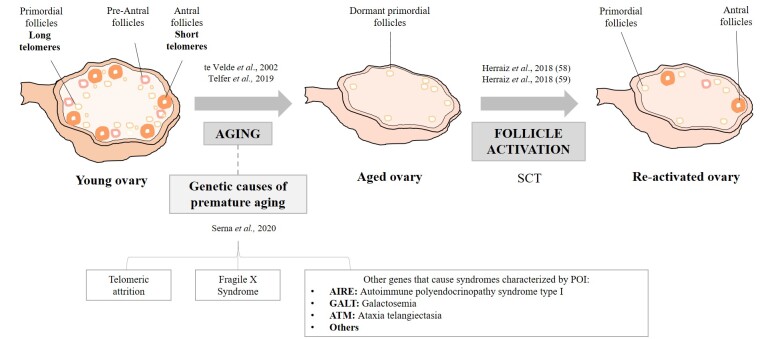
Figure 2.
Telomeres and follicle dynamics in the ovary. Telomere length decreases as primordial follicles mature to ovulatory follicles. The aged ovary (also prematurely aged) presents a decrease in the total number of follicles. Different genetic causes can be involved in premature ovarian aging. Primordial follicles that remain in the menopause ovaries do not show the ability to resume their development, but upon stem cell therapy (SCT), they are reactivated for maturation and ovulation.
Telomere attrition is one of the main causes of aging [5]. Telomeres are protective caps at the ends of linear chromosomes that prevent chromosome degradation and fusions, to safeguard genome integrity [19]. They consist of several kilobases of tandem repeats of the “TTAGGG” sequence in mammals. Telomeres are bound by a protein complex called Shelterin that prevents the ends of chromosomes from being recognized as double-strand breaks, which must be repaired. Shelterin also controls telomere length and aids telomere replication [20]. In addition, telomeres are epigenetically regulated to create heterochromatic regions, refractory for transcription and unscheduled recombination [21]. When cells divide, the very ends of chromosomes cannot be copied (called the “end replication problem”), leading to telomere shortening. If telomeres reach a critically short length, the DNA Damage Response (DDR) pathways, which detect and repair DNA, are activated, leading to senescence or apoptosis [22]. Telomerase, an enzyme that can add de novo repeats onto chromosome ends, is composed of a protein and an RNA component [23]. In the adult organism telomerase is active in the germ cells. As oocytes mature, telomerase activity decreases [24] and only at the blastocyst stage, telomerase is reactivated, setting the telomere length of the organism [25,26]. Good telomere maintenance in oocytes is critical to ensure correct chromosome segregation. Metaphase II oocytes with short telomeres (4th generation of telomerase-deficient mice), bear misaligned chromosomes, aberrant spindles, and telomeres disconnected from microtubules causing segregation errors [27]. In fact, telomeres are shorter in human aneuploid polar bodies (Figure 1, lower panel) [28]. Interestingly, ovarian somatic cells have telomerase activity [29,30] and lower or null telomerase activity in granulosa cells occur in women with ovarian insufficiency [31,32]. These data together argue that the telomere pathway is critical in reproduction.
Understanding Ovarian Aging in Mice
Because of the ethical concerns to obtain human samples, study embryos, or make fertility treatments, several mouse models have been developed to understand female infertility and the consequences for the offspring. The FSH receptor knockout mice have loss of fertility and aging-associated diseases [33]. The knockout of a subunit of the glutamate cysteine ligase, an enzyme that catalyzes the formation of Glutathione, which is an antioxidant, shows increased follicle decline through excessive follicular activation. In addition, oxidative stress causes the decline of primordial follicles and preimplantation embryo death [34]. OCT4 is expressed during the migration and colonization of primordial germ cells (PGCs) and its conditional deletion in the germline shows its role in germline viability and follicle maturation [35,36]. Cohesin-null mice are infertile because of meiotic errors [37]. Most of these studies show defects of the reproductive system already during development. In order to generate models that show the processes which take place at middle age, the gonadotoxicity of chemotherapy drugs has been tested [38]. Thus, most of the ovarian characteristic and reproductive outcomes of POI and DOR patients have been reproduced, including an increase in LH hormone, ovarian damage, and mild degeneration of oocytes, which are not observed in normal aging [38,39] Telomerase-deficient mice, which at the first generation have normal telomeres, do not show reproductive problems. At later generations, telomeres are shorter and mice show accelerated aging and poor tissue regenerative capacity [40]. Ovaries and uteri of late generations present smaller size, with atrophy in the uterine myometrium. The ovaries produce less follicles, but with a complete spectrum of follicular stages [27]. The number of fertilized oocytes is smaller in telomerase-deficient mice and most of them do not reach the blastocyst stage, producing smaller litters. G6 telomerase-deficient females cannot support exogenous embryo development after zygote implantation. Infertility is caused by poor oocyte production and uterus failure [40]. In addition, telomerase deficiency disturbs meiotic progression only at late generations, suggesting that telomerase knockout per se, does not alter meiosis [41].
Finally, the Senescence Accelerated Mouse Prone 8 (SAMP8) is a spontaneously generated mouse model of the AKT/J strain [42]. SAMP8 mice have a short lifespan and recapitulate all the symptoms of middle-age women [43]. As these mice age, estrous cycles are extended and the levels of LH increased while serum estradiol decreased, suggesting that the function of the hypothalamus-pituitary-gonadal axis is poor [44]. A decrease in fertility at 7 months of age is observed. They ovulate fewer oocytes and present a high incidence of meiotic spindle aberrations and chromosome misalignments [43]. Because telomere shortening has been implicated in aging [5] and in oocyte aneuploidies [27,43], we have hypothesized that telomere attrition could cause the reproductive senescence in SAMP8 mice. We have found that Mouse Embryonic Fibroblasts (MEF) from older mothers show shorter telomeres and an increase in telomere aberrations. These mice also show neurodegenerative defects at advance age [45], that may be caused by the lower levels of estrogen, which seems to protect neurons [44]. These symptoms reflect the increased risk of aging-associated diseases that happen in women when menopause ensues.
Undoubtedly, the mouse work has unveiled the genetic causes involved in ovarian aging, but more work is needed to complete this complex picture. Understanding the molecular mechanisms involved in ovarian aging will help develop therapies targeted to solve the different problems of ovarian failure.
Strategies to Reactivate Ovarian Function
Fertility preservation, either oocyte or ovarian cortex cryopreservation (OCC), functions to delay motherhood. With OCC, recommended for prepubertal girls and cancer-diagnosed women, a 29% of pregnancies are achieved [46]. In many cases, women did not preserve fertility so new strategies must be developed to help couples pass their own genetic material to their offspring.
Mechanical Strategies
After ovarian cortex extraction or OCC, the in vitro activation (IVA), consisting in slicing ovarian cortex into small cubes, changes the cytoskeletal actin dynamics reactivating follicle maturation by interfering with Hippo pathway [47]. This pathway is involved in the regulation of follicular growth through the control of cell proliferation and apoptosis factors [48]. Indeed, IVA using AKT activators, led to two healthy babies from POI women [49,50]. The caveat of IVA is that in cancer patients it could have negative consequences if ovarian cubes contain cancer cells and are introduced in the ovary.
Strategies that Avoid Fibrosis and Inflammation
Fibrosis and inflammation occur with aging, altering tissue homeostasis. Fibrosis, with an increase in collagen fibers [51], the presence of macrophages, and a higher percentage of monocytes, has been reported [51,52] in the ovary. Fibrosis leads to inflammation, which causes alterations in the transcriptome profile contributing to ovarian dysfunction and poor oocyte quality [52]. These observations open the possibility of ovarian rejuvenation through antifibrotic or anti-inflammatory agents [51,53].
Telomerase Reactivation Strategies
Cell rejuvenation using the Yamanaka factors [54] reactivates telomerase, possibly through cMyc and Klf4, which can modulate the expression of telomerase [55,56] causing telomere lengthening to the levels of embryonic stem cells (ESC) [57]. In mouse models, aging can be reversed and health span, extended. When telomerase is reactivated in adult and old mice, both the health span and lifespan of mice are extended without increasing the cancer incidence [58]. Furthermore, reactivation of telomerase in the fourth generation of mice lacking telomerase, restores the number of pups at the levels of control mice [59]. These results suggest that telomerase reactivation could be, from the molecular bases to the organismal function, a plausible option to rejuvenate ovaries.
Ovarian Niche Regeneration with SC
Regeneration of the ovarian niche using stem cells (SC) (Figure 2) create a suitable environment for angiogenesis and dormant follicle reactivation. Indeed, upon ESC transplantation hormone levels are recovered, apoptosis is reduced, and the number of follicles, increased [60]. Mesenchymal SC transplantation also restores ovarian function in POF mice [61]. Bone marrow derived stem cells (BMDSC) have the potential to produce hematopoietic, mesenchymal, and endothelial SC, which enhance the regeneration of the ovaries, increasing vascularization, cell proliferation, and reducing apoptosis [62]. This work is particularly interesting because it shows ovarian niche regeneration in humans for the first time. Later, the “homing” effect of the ovary to attract BMDSC was shown [46]. Infusion of autologous BMDSC, containing hematopoietic and mesenchymal cells into one ovary in poor ovarian responders, improved the biomarkers of ovarian reserve and vascularization and led to three spontaneous live births [48,63]. This method rescues ovarian function, but only in 23% of the cases. In addition, it is not known whether it functions in older women. Importantly, this method allows them to transfer their own genetic material avoiding the use of donated oocytes. Taken together these data indicate that the above methods, alone or in combinations, achieve the activation of dormant follicles and promote live births. However, these methods are experimental, requiring further research.
ESC and iPSC as Starting Point to Develop Oocytes and Follicles
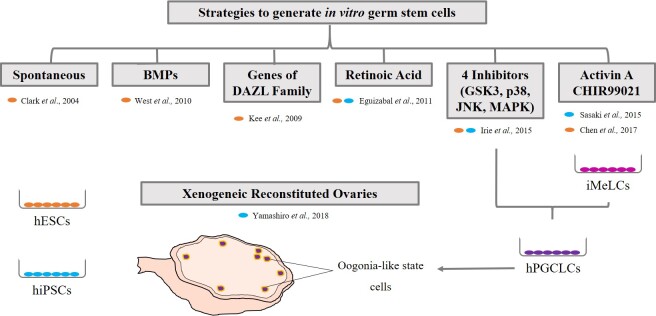
Germ cells in the mammalian ovary are thought to originate during fetal life, therefore at birth the number of oocytes is fixed. However, recent studies have described putative cells that could produce germ cell renewal. This topic is under intense investigation [7]. A strategy to solve the problem of oocyte exhaustion in the ovary, is the in vitro differentiation of oocytes from pluripotent stem cells, which can give rise to any cell type. However, this strategy involves legal and ethical concerns [64]. With the aim of generating PGCs alike and develop competent gametes that can give rise to healthy offspring, several approaches have been used. The first studies were done in animal models [65,66]. In humans, derivation of germ cells is challenging, because the in vivo process is not fully understood [54]. Thus, the knowledge from animal research is being applied to humans. Oocytes can be generated from both embryonic stem cells (ESC) derived from the inner cell mass of the blastocyst or induced pluripotent stem cells (iPSC), derived from somatic cells, using the Yamanaka factors [54]. The strategies (Figure 3) include random [67] or direct differentiation with bone morphogenetic proteins in the medium [68], overexpression of genes from the DAZL family [69], which promote entry in meiosis and the use of compounds such as retinoic acid [70]. However, the induction process is very inefficient. In 2015, two studies succeeded in inducing human PGC like cells, with similar characteristics to those observed at 7 weeks of development [71,72] and another one succeeded using the method of Sasaki and colleagues (2015), but with human ESC [73]. Yamashiro and colleagues (2018) have generated xenogeneic reconstituted ovaries adding human PGC like cells within fetal ovarian somatic cells from mouse embryos [74]. The oogonia-like cells obtained showed characteristics of epigenetic reprogramming.
Figure 3.
Methods for the in vitro production of germ cells. Several strategies, from spontaneous differentiation to the supplementation of the culture media, have been used to generate PGC like cells. Yamashiro and colleagues have made a further step to build xenogeneic ovaries. Orange dots indicates studies carried out with hESC, and blue dots specify studies with hiPSC. BMPs: Bone Morphogenetic Proteins; DAZL: Deleted in Azoospermia-like; GSK3: Glycogen Synthase Kinase 3; JNK: c-Jun N-terminal Kinase; MAPK: Mitogen-Activated Protein Kinase iMeLCs: incipient Mesoderm-Like Cells; hPGCLCs: human Primordial Germ Cell like Cells.
In view of all the work and recent studies, it seems likely that in vitro human gametes will be generated in the future. However, their efficacy and safety will have to be tested before they are used with patients. Because embryonic stem cells lengthen their telomeres as they are expanded in vitro, without manipulating the telomerase gene [26], and longer telomeres seem to give tissue-functional advantages to the organism [75], it would be interesting to expand ESC or iPSC in vitro before differentiation into PGC like cells, so that newly-formed oocytes can have longer telomeres.
Conclusions and Outlook
There has been intense research in the reproductive field to find answers for the problem of female infertility, which not only affects reproduction, but also decreases the chances of healthy aging. Currently, the concepts of ovarian aging and different aspects of several pathologies associated with infertility are better understood. There are new tools, such as several animal models to further study these infertility situations. Importantly, several strategies have been developed to either restore normal ovarian function or produce PGC in vitro. However, the strategies used to reactivate ovarian function need more development so they can be applied to other types of infertility and the in vitro gametogenesis yielding PGC-like cells should be tested, but this has ethical limitations. Therefore, new approaches should be developed, including reactivation of telomerase in the ovary.
Acknowledgments
The Telomeres in Reproduction Group is founded by Forward Grant, Ferring, CDTI and by IVI Foundation (projects 1707-FIVI-084-MV and 1711-FIVI-111-MV).
Glossary
- ART
Assisted Reproduction Techniques
- BMDSC
Bone marrow derived stem cells
- DDR
DNA damage response
- DNA
Deoxyribonucleic acid
- DOR
Diminished ovarian reserve
- ESC
Embryonic stem cells
- FSH
Follicle-Stimulating Hormone
- hESC
Human Embryonic Stem Cell
- hPGC
Human Primordial Germ Cell
- IVA
In vitro activation
- iPSC
Induced pluripotent stem cells
- LH
Luteinizing Hormone
- MEF
Mouse embryonic fibroblasts
- OCC
Ovarian Cortex Cryopreservation
- PGC
Primordial germ cells
- POI
Premature ovarian insufficiency
- POF
Premature ovarian failure
- SC
Stem cells
References
- Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014. July;15(7):482–96. 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010. March;464(7288):504–12. 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- Serrano M. Ageing: tools to eliminate senescent cells. Nature. 2017. May;545(7654):294–6. 10.1038/nature22493 [DOI] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002. Mar-Apr;8(2):141–54. 10.1093/humupd/8.2.141 [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013. June;153(6):1194–217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol. 2017. April;216(4):875–87. 10.1083/jcb.201610111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer EE, Anderson RA. The existence and potential of germline stem cells in the adult mammalian ovary. Climacteric. 2019. February;22(1):22–6. 10.1080/13697137.2018.1543264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007. March;18(2):58–65. 10.1016/j.tem.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res. 1995;50:223–54. 10.1016/b978-0-12-571150-0.50014-7 [DOI] [PubMed] [Google Scholar]
- Knowlton NS, Craig LB, Zavy MT, Hansen KR. Validation of the power model of ovarian nongrowing follicle depletion associated with aging in women. Fertil Steril. 2014. March;101(3):851–6. 10.1016/j.fertnstert.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014. March;101(3):656–663.e1. 10.1016/j.fertnstert.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011. November;26(11):3173–80. 10.1093/humrep/der294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handyside AH, Montag M, Magli MC, Repping S, Harper J, Schmutzler A, et al. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet. 2012. July;20(7):742–7. 10.1038/ejhg.2011.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné S, Cohen J, Sable D. Preimplantation genetic diagnosis for advanced maternal age and other indications. Fertil Steril. 2002. August;78(2):234–6. 10.1016/s0015-0282(02)03239-9 [DOI] [PubMed] [Google Scholar]
- Kovanci E, Schutt AK. Premature ovarian failure: clinical presentation and treatment. Obstet Gynecol Clin North Am. 2015. March;42(1):153–61. 10.1016/j.ogc.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Serna J, Varela E, García-Velasco JA. Genetics of premature ovarian insufficiency. Human Reproductive Genetics. Academic Press; 2020. pp. 173–99. [Google Scholar]
- Podfigurna-Stopa A, Czyzyk A, Grymowicz M, Smolarczyk R, Katulski K, Czajkowski K, et al. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest. 2016. September;39(9):983–90. 10.1007/s40618-016-0467-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008. February;26(5):753–8. 10.1200/JCO.2007.14.1655 [DOI] [PubMed] [Google Scholar]
- Varela E, Blasco MA. 2009 nobel prize in physiology or medicine: telomeres and telomerase. Oncogene. 2010. March;29(11):1561–5. 10.1038/onc.2010.15 [DOI] [PubMed] [Google Scholar]
- Benarroch-Popivker D, Pisano S, Mendez-Bermudez A, Lototska L, Kaur P, Bauwens S, et al. TRF2-Mediated Control of Telomere DNA Topology as a Mechanism for Chromosome-End Protection. Mol Cell. 2016. January;61(2):274–86. 10.1016/j.molcel.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J. 2009. August;28(16):2323–36. 10.1038/emboj.2009.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger RJ. In the end, what’s the problem? Mol Cell. 2014. March;53(6):855–6. 10.1016/j.molcel.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989. January;337(6205):331–7. 10.1038/337331a0 [DOI] [PubMed] [Google Scholar]
- Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod. 2001. October;7(10):947–55. 10.1093/molehr/7.10.947 [DOI] [PubMed] [Google Scholar]
- Schaetzlein S, Lucas-Hahn A, Lemme E, Kues WA, Dorsch M, Manns MP, et al. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci USA. 2004. May;101(21):8034–8. 10.1073/pnas.0402400101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela E, Schneider RP, Ortega S, Blasco MA. Different telomere-length dynamics at the inner cell mass versus established embryonic stem (ES) cells. Proc Natl Acad Sci USA. 2011. September;108(37):15207–12. 10.1073/pnas.1105414108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002. March;3(3):230–4. 10.1093/embo-reports/kvf055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011. June;7(6):e1002161. 10.1371/journal.pgen.1002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Molina EE, Franasiak JM, Marin D, Tao X, Díaz-Gimeno P, Florensa M, et al. Cumulus cells have longer telomeres than leukocytes in reproductive-age women. Fertil Steril. 2020. January;113(1):217–23. 10.1016/j.fertnstert.2019.08.089 [DOI] [PubMed] [Google Scholar]
- Morin SJ, Tao X, Marin D, Zhan Y, Landis J, Bedard J, et al. DNA methylation-based age prediction and telomere length in white blood cells and cumulus cells of infertile women with normal or poor response to ovarian stimulation. Aging (Albany NY). 2018. December;10(12):3761–73. 10.18632/aging.101670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009. December;94(12):4835–43. 10.1210/jc.2008-2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017. January;32(1):201–7. 10.1093/humrep/dew283 [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998. November;95(23):13612–7. 10.1073/pnas.95.23.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ali S, Liao LS, Nguyen ES, Ortiz L, Reshel S, et al. Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione-deficient mice. Biol Reprod. 2020. April;102(5):1065–79. 10.1093/biolre/ioaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004. November;5(11):1078–83. 10.1038/sj.embor.7400279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng D, Wang Q, Ma X, Tian Y, Xu K, Weng X, et al. Role of OCT4 in the Regulation of FSH-Induced Granulosa Cells Growth in Female Mice. Front Endocrinol (Lausanne). 2020. January;10:915. 10.3389/fendo.2019.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005. June;8(6):949–61. 10.1016/j.devcel.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Zhang T, Yan D, Yang Y, Ma A, Li L, Wang Z, et al. The comparison of animal models for premature ovarian failure established by several different source of inducers. Regul Toxicol Pharmacol. 2016. November;81:223–32. 10.1016/j.yrtph.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Buigues A, Marchante M, Herraiz S, Pellicer A. Diminished Ovarian Reserve Chemotherapy-Induced Mouse Model: A Tool for the Preclinical Assessment of New Therapies for Ovarian Damage. Reprod Sci. 2019. February;1933719119831784:1933719119831784. 10.1177/1933719119831784 [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998. April;392(6676):569–74. 10.1038/33345 [DOI] [PubMed] [Google Scholar]
- Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol. 2002. September;249(1):74–84. 10.1006/dbio.2002.0735 [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp Gerontol. 1997. Jan-Apr;32(1-2):105–9. 10.1016/s0531-5565(96)00036-8 [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Mackenzie AC, Kraemer DC, Morley JE, Farr S, Chaffin CL, et al. Shortened estrous cycle length, increased FSH levels, FSH variance, oocyte spindle aberrations, and early declining fertility in aging senescence-accelerated mouse prone-8 (SAMP8) mice: concomitant characteristics of human midlife female reproductive aging. Endocrinology. 2014. June;155(6):2287–300. 10.1210/en.2013-2153 [DOI] [PubMed] [Google Scholar]
- Yuan M, Wen-Xia Z, Jun-Ping C, Yong-Xiang Z. Age-related changes in the oestrous cycle and reproductive hormones in senescence-accelerated mouse. Reprod Fertil Dev. 2005;17(5):507–12. 10.1071/rd04099 [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des. 2012;18(8):1123–30. 10.2174/138161212799315795 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang H, Zhang Y, Li N, Wen Y, Cao F, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells. 2014. December;37(12):865–72. 10.14348/molcells.2014.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima I, Kawamura K. Regulation of follicle growth through hormonal factors and mechanical cues mediated by Hippo signaling pathway. Syst Biol Reprod Med. 2018. February;64(1):3–11. 10.1080/19396368.2017.1411990 [DOI] [PubMed] [Google Scholar]
- Gupta S, Lodha P, Karthick MS, Tandulwadkar SR. Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age 45 Year. J Hum Reprod Sci. 2018. Apr-Jun;11(2):125–30. 10.4103/jhrs.JHRS_57_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013. October;110(43):17474–9. 10.1073/pnas.1312830110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015. March;30(3):608–15. 10.1093/humrep/deu353 [DOI] [PubMed] [Google Scholar]
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016. September;152(3):245–60. 10.1530/REP-16-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schlamp F, Huang L, Clark H, Brayboy L. Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction. 2020. March;159(3):325–37. 10.1530/REP-19-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Ling L, Wei T, Wang Y, Xiong Z. Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp Biol Med (Maywood). 2017. April;242(7):683–91. 10.1177/1535370217693323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006. August;126(4):663–76. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998. June;12(12):1769–74. 10.1101/gad.12.12.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Hou PS, Tseng SF, Chien CL, Wu KJ, Chen HF, et al. Krüppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells. 2010. September;28(9):1510–7. 10.1002/stem.477 [DOI] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009. February;4(2):141–54. 10.1016/j.stem.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012. August;4(8):691–704. 10.1002/emmm.201200245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011. January;469(7328):102–6. 10.1038/nature09603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020. January;11(1):3. 10.1186/s13287-019-1508-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8+CD28- T cells. Stem Cell Res Ther. 2020. February;11(1):49. 10.1186/s13287-019-1537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz S, Buigues A, Díaz-García C, Romeu M, Martínez S, Gómez-Seguí I, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018. May;109(5):908–918.e2. 10.1016/j.fertnstert.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, Gómez-Seguí I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018. August;110(3):496–505.e1. 10.1016/j.fertnstert.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Ishii T, Pera RA, Greely HT. Ethical and legal issues arising in research on inducing human germ cells from pluripotent stem cells. Cell Stem Cell. 2013. August;13(2):145–8. 10.1016/j.stem.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Li Y, Ai Z, Kang Y, Shi H, et al. Generation of Cynomolgus Monkey Chimeric Fetuses using Embryonic Stem Cells. Cell Stem Cell. 2015. July;17(1):116–24. 10.1016/j.stem.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nakamura T, Okamoto I, Yabuta Y, Iwatani C, Tsuchiya H, et al. The Germ Cell Fate of Cynomolgus Monkeys Is Specified in the Nascent Amnion. Dev Cell. 2016. October;39(2):169–85. 10.1016/j.devcel.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004. April;13(7):727–39. 10.1093/hmg/ddh088 [DOI] [PubMed] [Google Scholar]
- West FD, Roche-Rios MI, Abraham S, Rao RR, Natrajan MS, Bacanamwo M, et al. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum Reprod. 2010. January;25(1):168–78. 10.1093/humrep/dep338 [DOI] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009. November;462(7270):222–5. 10.1038/nature08562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, et al. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011. August;29(8):1186–95. 10.1002/stem.672 [DOI] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015. January;160(1-2):253–68. 10.1016/j.cell.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell. 2015. August;17(2):178–94. 10.1016/j.stem.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Chen D, Liu W, Lukianchikov A, Hancock GV, Zimmerman J, Lowe MG, et al. Germline competency of human embryonic stem cells depends on eomesodermin. Biol Reprod. 2017. January;97(6):850–61. 10.1093/biolre/iox138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. 2018. October;362(6412):356–60. 10.1126/science.aat1674 [DOI] [PubMed] [Google Scholar]
- Varela E, Muñoz-Lorente MA, Tejera AM, Ortega S, Blasco MA. Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations. Nat Commun. 2016. June;7(1):11739. 10.1038/ncomms11739 [DOI] [PMC free article] [PubMed] [Google Scholar]