Abstract
Cell division without growth results in progressive cell size reductions during early embryonic development. How do the sizes of intracellular structures and organelles scale with cell size and what are the functional implications of such scaling relationships? Model organisms, in particular Caenorhabditis elegans worms, Drosophila melanogaster flies, Xenopus laevis frogs, and Mus musculus mice, have provided insights into developmental size scaling of the nucleus, mitotic spindle, and chromosomes. Nuclear size is regulated by nucleocytoplasmic transport, nuclear envelope proteins, and the cytoskeleton. Regulators of microtubule dynamics and chromatin compaction modulate spindle and mitotic chromosome size scaling, respectively. Developmental scaling relationships for membrane-bound organelles, like the endoplasmic reticulum, Golgi, mitochondria, and lysosomes, have been less studied, although new imaging approaches promise to rectify this deficiency. While models that invoke limiting components and dynamic regulation of assembly and disassembly can account for some size scaling relationships in early embryos, it will be exciting to investigate the contribution of newer concepts in cell biology such as phase separation and interorganellar contacts. With a growing understanding of the underlying mechanisms of organelle size scaling, future studies promise to uncover the significance of proper scaling for cell function and embryonic development, as well as how aberrant scaling contributes to disease.
Graphical/Visual Abstract and Caption
How do the sizes of intracellular organelles adapt to reductions in cell size that occur during early embryogenesis? We address mechanisms of developmental organelle size scaling in a variety of model organisms and, if known, the functional significance of scaling.
Introduction
Early development is generally characterized by rapid cell divisions with little to no cell growth. As cell number increases, cell size decreases during early embryogenesis in a wide variety of organisms, including model species such as zebrafish (Danio rerio), frogs (Xenopus laevis), worms (Caenorhabditis elegans), and mice (Mus musculus) (Tadros & Lipshitz, 2009). A fundamental cell biological question concerns how these reductions in cell size influence the size and function of intracellular structures. From a physical perspective, it reasons that the sizes of certain organelles must become smaller as cell size decreases. For instance, the nucleus of an early stage X. laevis embryo would barely fit within the confines of a later stage blastomere (Jevtic & Levy, 2015) (Fig. 1A). Beyond physical considerations, scaling of organelle size with cell size might also be critical to ensure that organelle function is appropriately matched to cell size. In this review, we will present an overview of which organelles and intracellular structures are known to scale with cell size during early development in a variety of different model organisms, focusing on the nucleus, mitotic spindle, and chromosomes, and touching on cilia, endoplasmic reticulum (ER), Golgi, mitochondria, and lysosomes/vacuoles. Where it is known, we will discuss the mechanistic basis for these scaling relationships and the functional consequences of disrupted organellar scaling. The advantages of different model organisms have provided unique insights into organelle scaling, for example genetics in worms and flies, biochemistry in frogs, and imaging in zebrafish and sea urchins. Where appropriate, we will briefly discuss organelle scaling in single cell model organisms like yeast and Tetrahymena as well as in the processes of cell differentiation and aging, as mechanisms identified in these systems may be relevant to developmental organelle size scaling. We conclude with what we view as important outstanding questions and potential approaches to addressing these questions in the future.
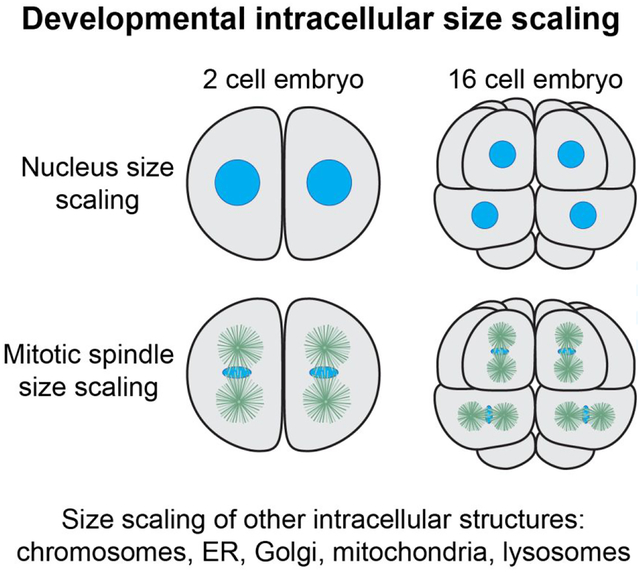
Figure 1: Developmental size regulation of intracellular structures in Xenopus.
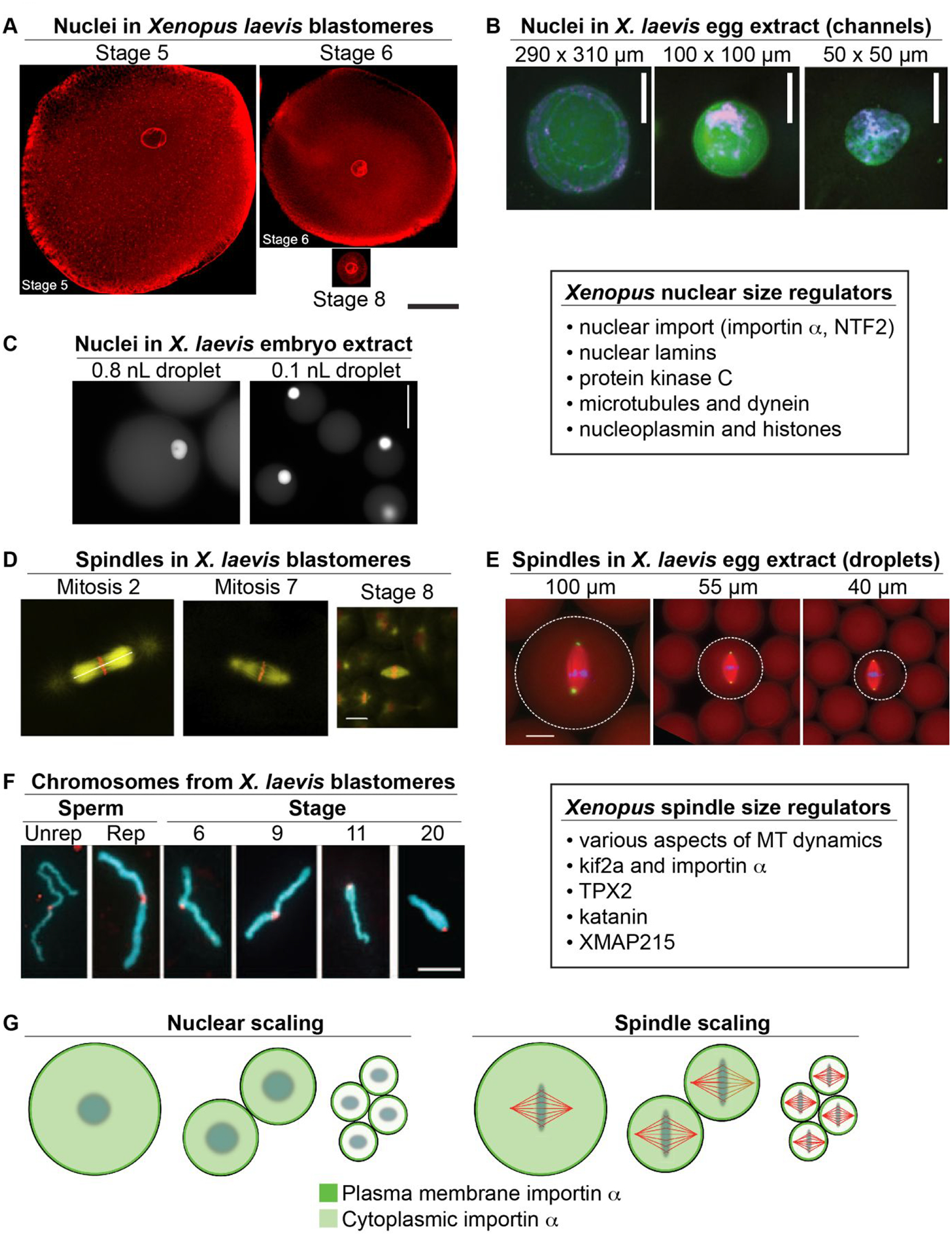
(A) Blastomeres were isolated from different stage X. laevis embryos and stained for the NPC. Scale bar, 50 μm. Adapted with permission from (Jevtic & Levy, 2015). (B) Nuclei were assembled in X. laevis egg extract in microfluidic channels of varying dimensions. Channel dimensions are indicated as height × width. Membranes, DNA, and incorporated dUTP are labeled green, blue, and red, respectively. Scale bar, 20 μm. Adapted with permission from (Hara & Merten, 2015). (C) Nuclei and cytoplasm from stage 10 X. laevis embryos were encapsulated in droplets of varying volume and allowed to reach steady-state sizes. Droplet volumes are indicated above each panel. Nuclei are visualized by import of GFP-NLS. Scale bar, 50 μm. Adapted with permission from (P. Chen et al., 2019). (D) Different stage X. laevis embryos were fixed and stained for tubulin (yellow) and DNA (red). Scale bar, 20 μm. Adapted with permission from (Wuhr et al., 2008). (E) Spindles were assembled in different volumes of X. laevis egg extract. Tubulin, DNA, and NuMA are labeled red, blue, and green, respectively. Droplet diameters are indicated above each panel. Scale bar, 25 μm. Adapted with permission from (Hazel et al., 2013). (F) Condensed mitotic chromosomes from different stage X. laevis embryos are shown. For comparison, unreplicated (Unrep) and replicated (Rep) sperm chromosomes were incubated in X. laevis egg extract. DNA and kinetochores are labeled blue and red, respectively. Scale bar, 5 μm. Adapted with permission from (Kieserman & Heald, 2011). (G) As cells become smaller during early Xenopus development, palmitoylated importin α is increasingly partitioned to the plasma membrane. In the case of the nucleus, this reduces nuclear import and size. In the case of the spindle, reduced kif2a inhibition promotes MT depolymerization and spindle shortening. Adapted with permission from (Brownlee & Heald, 2019).
As already mentioned, reductions in cell size over early development are driven by rapid cell divisions without cell growth, so cell cycle timing is a key determinant of cell size in the early embryo. There is also evidence that cell size and cytoplasmic volume determine cell division timing, with cell size reductions leading to increased cell cycle lengths (Arata & Takagi, 2019; Guan et al., 2018; Masui & Wang, 1998). Characteristic of many early developmental programs, asymmetric cell divisions give rise to different sized cells within the embryo (Cadart, Zlotek-Zlotkiewicz, Le Berre, Piel, & Matthews, 2014). Positioning of the interphase nucleus and mitotic spindle often determine the division site. Such positioning is dictated by both length-dependent microtubule (MT) forces and polarity domains at the cell cortex that alter such forces (Hasley, Chavez, Danilchik, Wuhr, & Pelegri, 2017; Minc & Piel, 2012; Pierre, Salle, Wuhr, & Minc, 2016; Xiao, Tong, Yang, & Wu, 2017). Other general regulators of cell size include nutrient availability (Leitao & Kellogg, 2017; Lucena et al., 2018; Vadia et al., 2017), signalling (Acebron, Karaulanov, Berger, Huang, & Niehrs, 2014; Luo, Liu, & Nassel, 2013), mechanical force (Perez Gonzalez et al., 2018), pressure (Pham et al., 2019), and expression and localization of cell cycle regulators (Keifenheim et al., 2017; Pan, Saunders, Flor-Parra, Howard, & Chang, 2014; Patterson, Rees, & Nurse, 2019; Schmoller & Skotheim, 2015; Zapata et al., 2014). Open questions remain about how these factors might regulate cell size in the early embryo. Maintenance of proper cell size is clearly important for intracellular function, for instance inappropriate cell enlargement can lead to cytoplasm dilution if nucleic acids and protein biosynthesis do not scale proportionately with cell size, potentially contributing to cellular senescence (Neurohr et al., 2019).
A variety of different, non-mutually exclusive models might account for the scaling of organelle size with cell size (Marshall, 2015, 2016). Limiting component models posit that the early embryo is preloaded with enough organelle building blocks to sustain a certain number of cell divisions (Fig. 2A). As components limiting for organelle size are partitioned into greater numbers of smaller cells, organelle size becomes smaller (Goehring & Hyman, 2012). In a related model, enzymes responsible for organelle assembly might also become limiting. An intriguing new area in cell biology is the notion of phase separation, in which specific proteins separate from the bulk cytoplasm and coalesce into membraneless organelles (Shin & Brangwynne, 2017). Reductions in cytoplasmic volume over development might affect the size and behavior of such phase-separated organelles (Brangwynne, 2013). Changes in cytoplasmic composition, for instance changes in ribosome concentrations, can influence the phase separation properties of membraneless organelles (Delarue et al., 2018). Ruler models implicate the size of constituent components or templates in determining final organelle size, and reductions in component or template size over development could account for organelle size scaling (Fig. 2B). Given that most subcellular structures are orders of magnitude larger than their protein constituents, the ruler model prompts the question of how component number is regulated to determine the final size of the structure. In more dynamic models, organelle growth and disassembly rates determine steady-state size (Fig. 2C). Developmentally-regulated reductions in growth and/or increases in disassembly could therefore give rise to smaller organelles. A related model is one where organelle functional output could feedback on organelle growth and/or disassembly. Given that many organelles are connected through contiguous membrane systems, it is easy to imagine how active mechanisms regulating the size of one organelle might passively influence the size of other interconnected organelles (Fig. 2D). Throughout this review, we will refer to these various general organelle size-scaling models in cases where sizing mechanisms are known.
Figure 2: Models for developmental organelle size scaling.
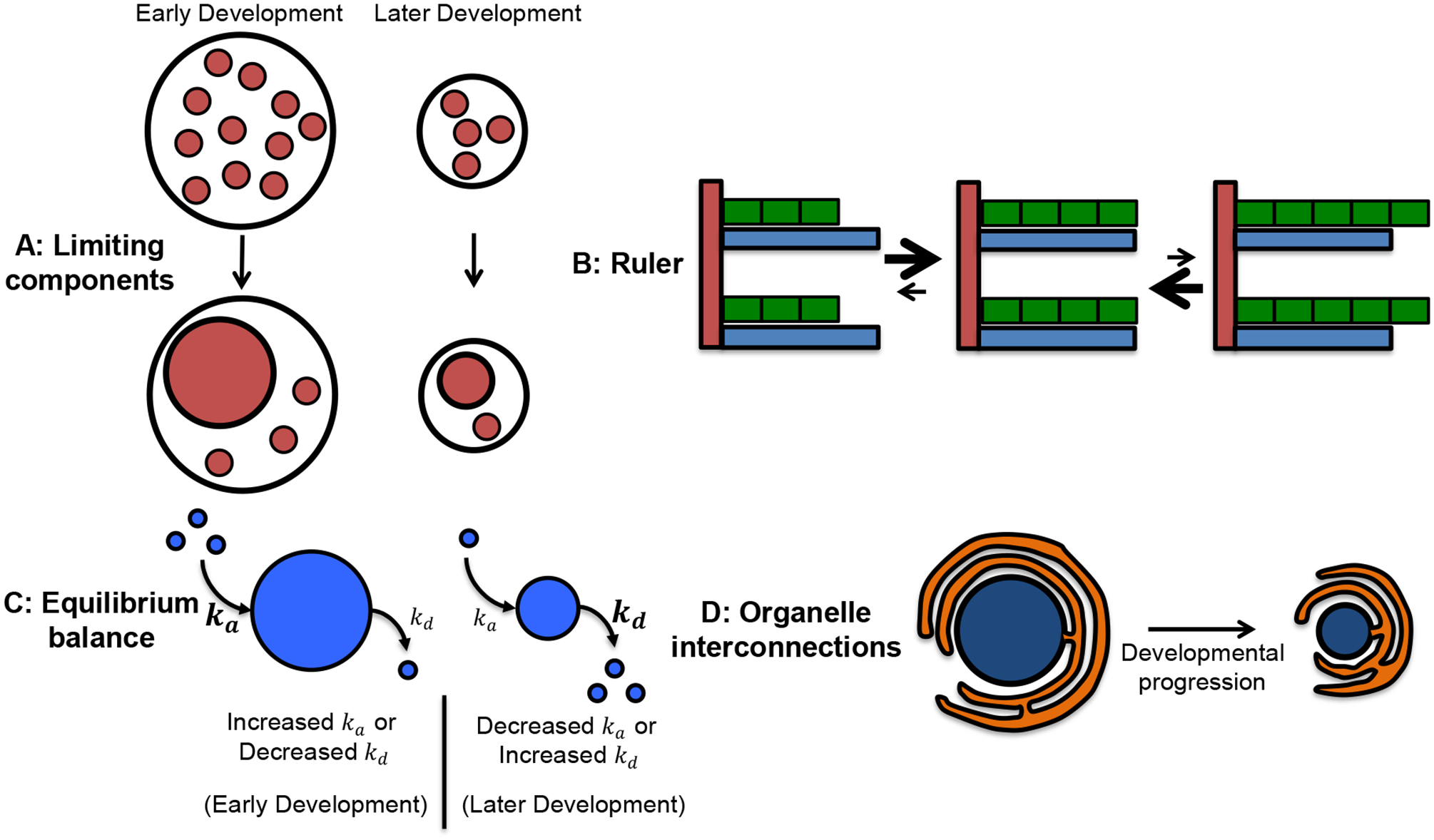
(A) Small red circles represent components that limit assembly and/or growth of the larger red organelle. Over development, limiting components are partitioned into greater numbers of smaller cells, such that the absolute amounts of limiting components per cell decrease leading to smaller organelle size. (B) Green rectangles assemble to form a linear structure whose length is dictated by the blue ruler. Developmental changes in the size of the ruler or building blocks might lead to reductions in the overall size of the structure. (C) A balance of assembly and disassembly sets steady-state organelle size. Developmental changes in assembly and disassembly rates would lead to organelle size scaling. (D) The orange and blue organelles are connected through membranes. Developmental reductions in the size of one organelle might lead to concomitant size scaling of the interconnected organelle. Another possible scenario is that total membrane amount remains constant but membrane distribution changes such that one organelle increases in size and the other becomes smaller.
Nucleus
Perhaps one of the most well documented examples of organelle size scaling during development is nuclear scaling. It has been known for over a century that nuclear size generally scales proportionately to cell size (Conklin, 1912; Wilson, 1925). While DNA amount correlates with nuclear size across species and likely sets a minimum nuclear size, experimentally manipulating DNA content in yeast and Xenopus did not lead to large changes in nuclear size, suggesting that DNA amount may not directly contribute to nuclear size determination (Jevtic & Levy, 2017; Levy & Heald, 2010; Neumann & Nurse, 2007). Consistent with these findings, nuclear size generally scales smaller during early development, while nuclear DNA content remains constant. In Xenopus, the first few cell divisions occur synchronously and are symmetric, after which asymmetric divisions divide the embryo into larger vegetal pole cells packed with yolk and smaller animal pole cells. The Drosophila early embryo is not cellularized, consisting instead of a syncytium of nuclei. After 14 nuclear divisions, nuclei migrate to the periphery of the embryo and form the cellular blastoderm, at which time damaged nuclei are eliminated (Iampietro et al., 2014). Cell divisions in C. elegans are neither synchronous nor symmetric during early developmental stages and short cell migrations reorganize the embryo. In zebrafish, embryonic development starts with largely symmetric, synchronous cell divisions that become progressively less synchronous, eventually resulting in smaller animal pole cells (Kimmel, Ballard, Kimmel, Ullmann, & Schilling, 1995). In spite of these variations in cell division patterns across these organisms, reductions in nuclear size during embryonic development are almost always observed. In Xenopus, reductions in nuclear size over development are accompanied by an increasing nuclear-to-cytoplasmic (N/C) volume ratio because cell size decreases more rapidly than nuclear size (Jevtic & Levy, 2015) (Fig. 1A). In zebrafish, nuclear size scales with developmental progression and thus cell size (Reisser et al., 2018). In C. elegans, nuclear and cell sizes scale from the 2-cell stage onward (Arata, Takagi, Sako, & Sawa, 2014; Hara, Iwabuchi, Ohsumi, & Kimura, 2013) (Fig. 3A), and nuclear size scales hypoallometrically with cell size in developing intestinal cells (Uppaluri, Weber, & Brangwynne, 2016). Though direct measurements have not been reported, Drosophila nuclear size decreases with each replication cycle during embryogenesis (Kotadia, Crest, Tram, Riggs, & Sullivan, 2010) (Fig. 4A), and during cellularization the farnesylated inner nuclear membrane protein Kugelkern induces nuclear lengthening (Brandt et al., 2006). Nuclear volume decreases over ten-fold in mouse embryos from the 1-cell stage to blastocyst with concomitant increases in the N/C ratio (Tsichlaki & FitzHarris, 2016) (Fig. 4B). While absolute N/C volume ratios differ in different species, the general trend is an increase in the N/C ratio during developmental progression (Fig. 5). Past early embryonic stages, N/C volume ratios tend to stabilize, and it has been proposed that maintenance of proper N/C ratios is important for cell function (Walters, Bommakanti, & Cohen-Fix, 2012).
Figure 3: Developmental size regulation of intracellular structures in C. elegans.
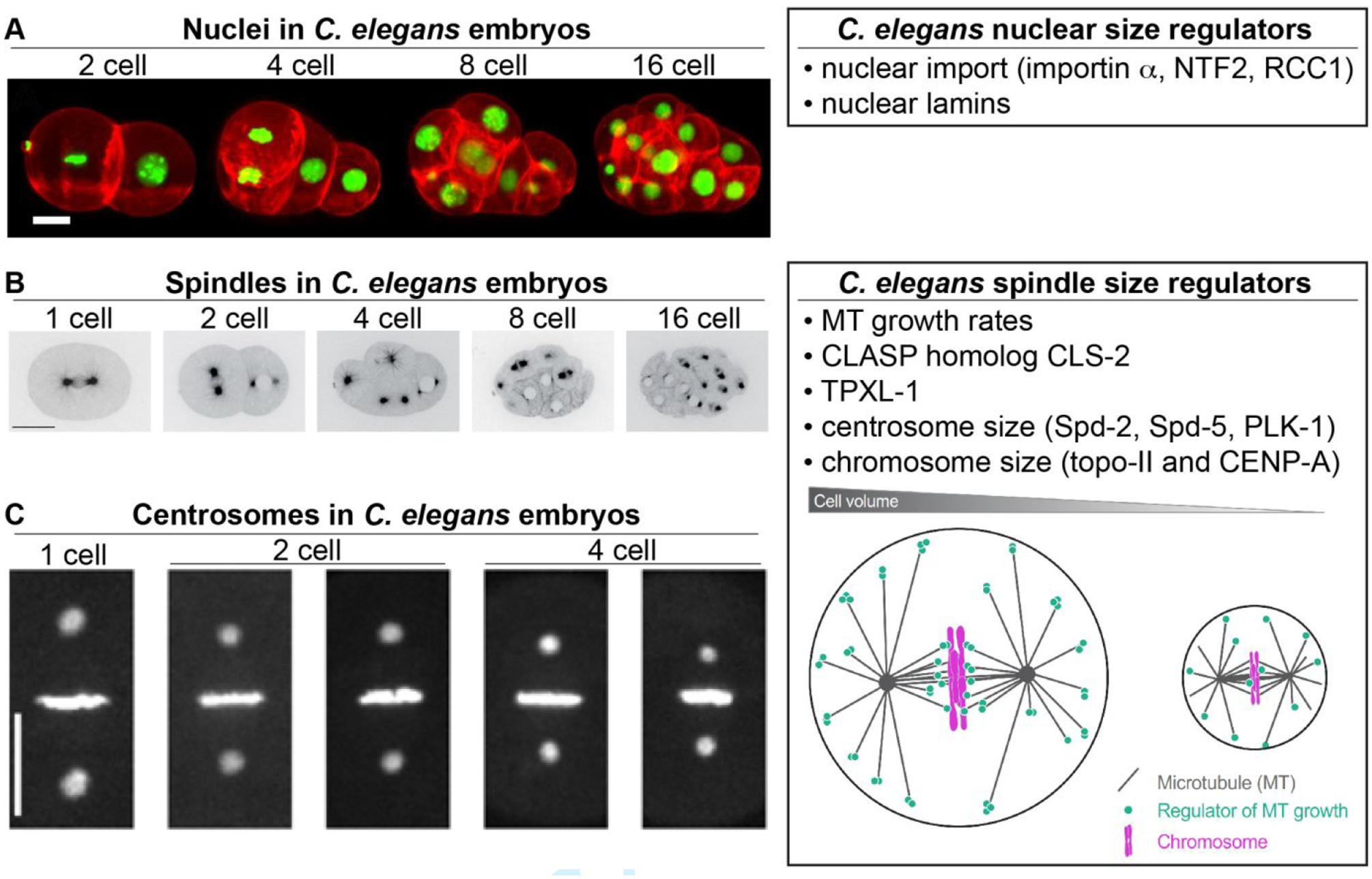
(A) Different stage C. elegans embryos with nuclei labeled green for H2B and plasma membrane labeled red. Scale bar, 10 μm. Images adapted from (Fickentscher & Weiss, 2017) and made available under a Creative Commons Attribution 4.0 International License. (B) Different stage C. elegans embryos expressing GFP-tagged β-tubulin. Scale bar, 20 μm. Adapted with permission from (Lacroix et al., 2018). (C) Different stage C. elegans embryos expressing GFP-tagged γ-tubulin and H2B. Scale bar, 10 μm. Adapted with permission from (Greenan et al., 2010). The cartoon model in the “C. elegans spindle size regulators box” was adapted with permission from (Lacroix et al., 2018).
Figure 4: Developmental size regulation of intracellular structures in other model organisms.
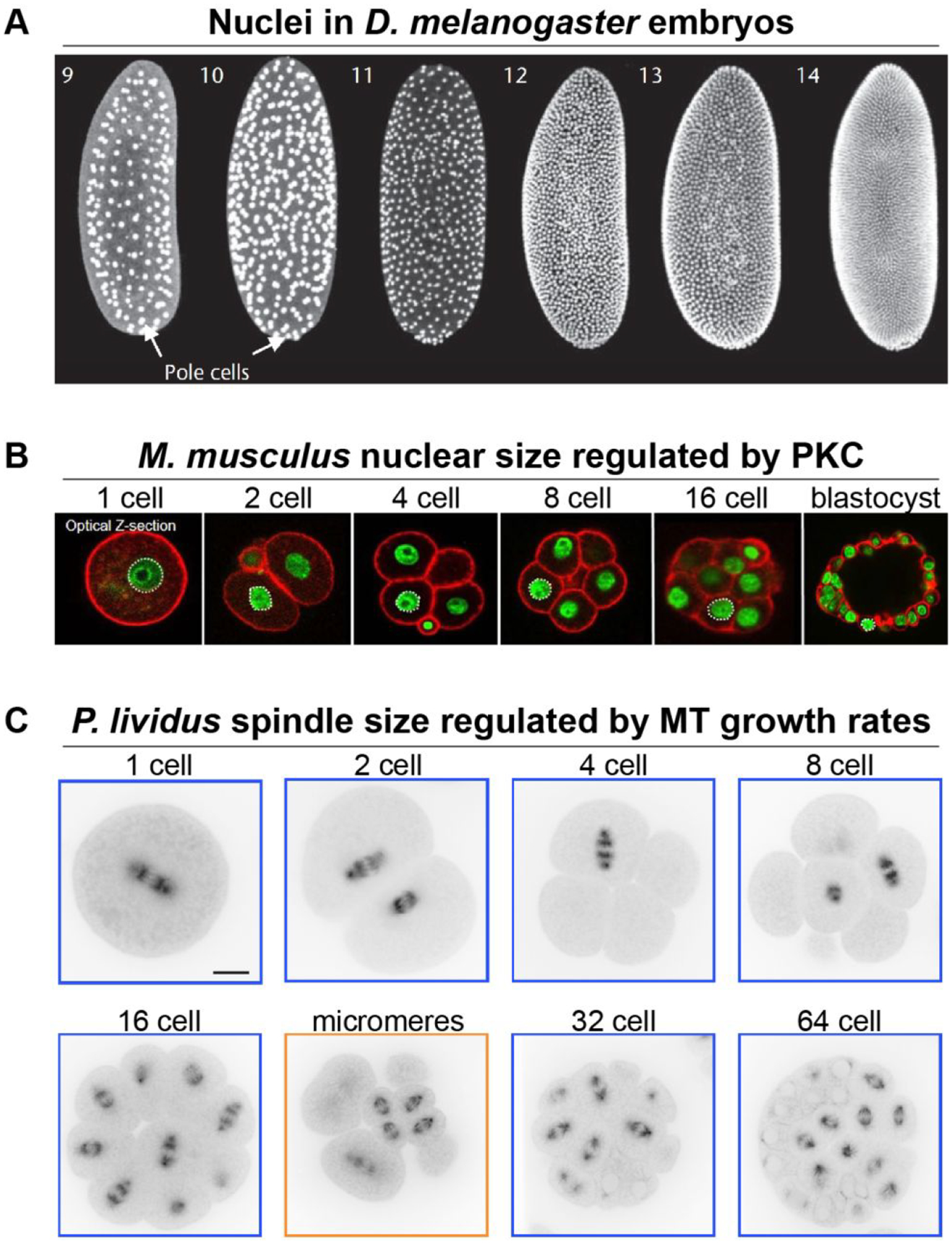
(A) Different stage D. melanogaster embryos showing labeled nuclei. Numbers denote the nuclear cycle. Note that nuclei are present in a syncytium until cellularization at nuclear cycle 14. Images adapted with permission from (Kotadia et al., 2010). (B) Different stage M. musculus embryos with DNA labeled green and cell cortex labeled red with phalloidin. Images adapted from (Tsichlaki & FitzHarris, 2016) and made available under a Creative Commons Attribution 4.0 International License. (C) Different stage P. lividus sea urchin embryos microinjected with ATTO 565-labelled tubulin. Scale bar, 20 μm. Adapted with permission from (Lacroix et al., 2018).
Figure 5: N/C volume ratios generally increase during early development in different species.
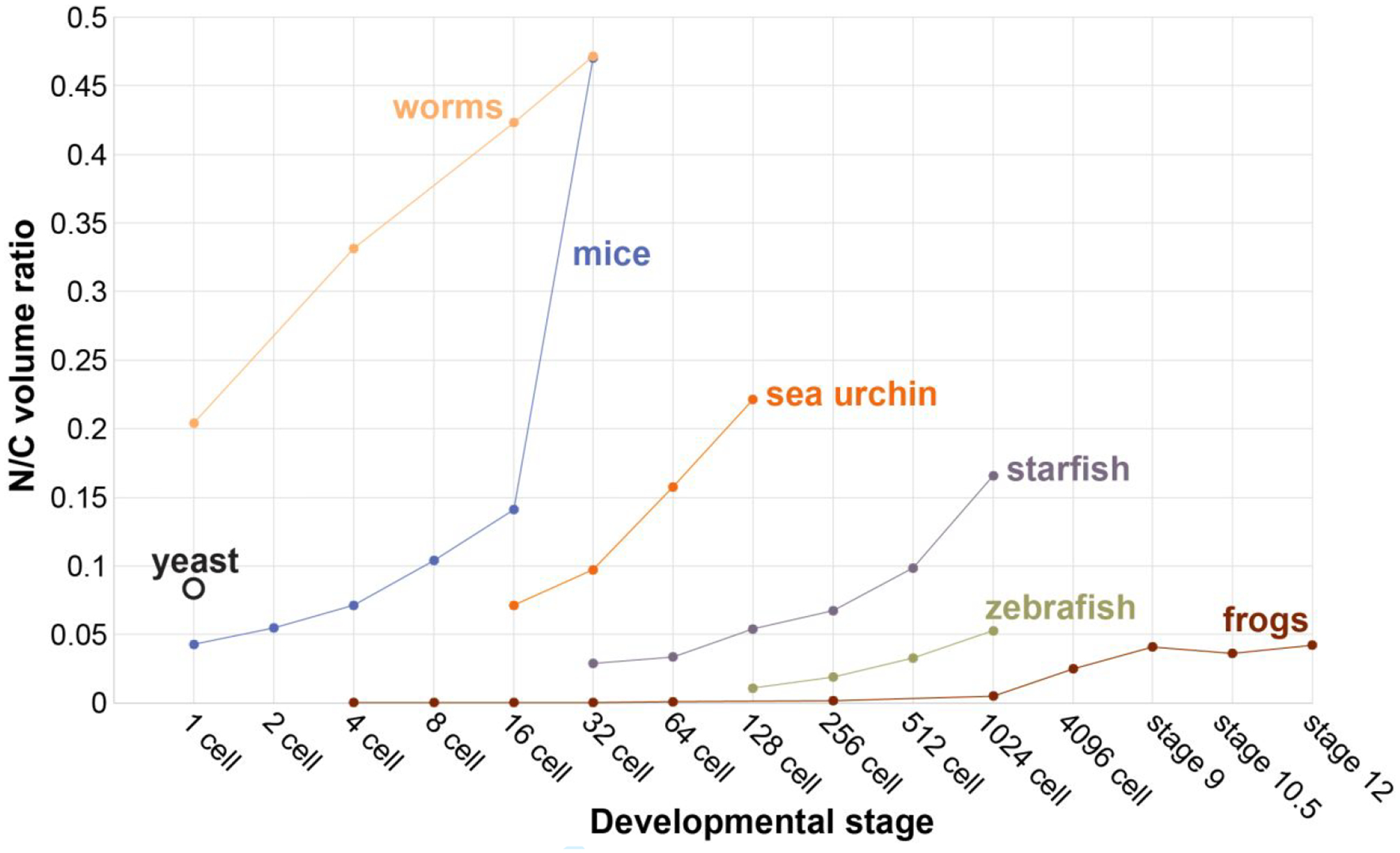
C. elegans worm data are from (Hara & Kimura, 2013). Fission yeast data are from (Neumann & Nurse, 2007). M. musculus data are from (Tsichlaki & FitzHarris, 2016). H. pulcherrimus sea urchin data are from (Masui & Kominami, 2001). A. pectinifera starfish data are from (Masui, Yoneda, & Kominami, 2001). D. rerio zebrafish data are from (Joseph et al., 2017). X. laevis frog data are from (Jevtic & Levy, 2015).
Many insights into the mechanisms of developmental nuclear size scaling have been gleaned from Xenopus experiments, in particular using Xenopus egg extracts that support de novo assembly of interphase nuclei and mitotic spindles (P. Chen & Levy, 2018; Field & Mitchison, 2018). One such mechanism involves the balance between nuclear import and export. Two nuclear transport factors, importin α and NTF2, contribute to differences in nuclear size between two Xenopus species (Levy & Heald, 2010; Vukovic, Jevtic, Zhang, Stohr, & Levy, 2016). In early stage embryos, while a fraction of palmitoylated importin α is associated with the plasma membrane, a large pool of importin α is cytoplasmic and available to drive nuclear growth through import. As cell size decreases over development and the blastomere surface area-to-volume ratio increases, an increasingly larger fraction of palmitoylated importin α is partitioned to the plasma membrane. With less available cytoplasmic importin α, import rates and nuclear growth both decrease, and increasing the levels of cytoplasmic importin α is sufficient to increase nuclear size (Brownlee & Heald, 2019; Levy & Heald, 2010; Wilbur & Heald, 2013) (Fig. 1G). While reductions in nuclear import contribute to nuclear size scaling in early Xenopus embryos, nuclear export appears to not play a major role (Edens & Levy, 2014), although in other systems reducing nuclear export can increase nuclear size (Jevtic, Schibler, et al., 2019; Kume et al., 2017; Neumann & Nurse, 2007). In Tetrahymena thermophila, macronucleus and micronucleus sizes are determined by nucleus-specific nuclear pore complex (NPC) components, importins, and histones (Iwamoto et al., 2009; Iwamoto et al., 2017; Malone et al., 2008; Shen, Yu, Weir, & Gorovsky, 1995). How does nuclear import regulate size? Nuclear growth is known to depend on importin α-mediated import of nuclear lamins (Jenkins et al., 1993; Newport, Wilson, & Dunphy, 1990), intermediate filament proteins that are part of the nuclear lamina underlying the inner nuclear membrane (Dittmer & Misteli, 2011). Lamins are one nuclear imported cargo that contributes to nuclear size scaling (Jevtic et al., 2015; Levy & Heald, 2010), and it is likely that other cargos are involved. Nuclear import and lamins also control nuclear size in C. elegans (Ladouceur, Dorn, & Maddox, 2015; Meyerzon et al., 2009). Given that NPCs and lamins exhibit age-dependent deterioration, changes in import capacity could contribute to altered nuclear morphology in aging cells (H. Chen, Zheng, & Zheng, 2014; D’Angelo, Raices, Panowski, & Hetzer, 2009; Flint Brodsly et al., 2019; Frost, Bardai, & Feany, 2016; Toyama et al., 2019).
While nuclear import represents a mechanism that drives nuclear growth, are there balanced nuclear disassembly activities that determine steady-state nuclear size? Using Xenopus embryo extracts, it was shown that late stage embryonic cytoplasm possesses an activity that can cause large nuclei to shrink in size. This activity is dependent on conventional protein kinase C (PKC), and altering PKC activity in vitro and in vivo affects nuclear size. Consistent with PKC being a developmental nuclear size regulator, PKC activity and nuclear localization increase during Xenopus development, and this leads to changes in the phosphorylation and dynamic nuclear envelope (NE) association of lamin B3 (Edens, Dilsaver, & Levy, 2017; Edens & Levy, 2014). PKC has also been implicated as a regulator of nuclear morphology in mouse embryos and neurons (Nishimura et al., 2014; Tsichlaki & FitzHarris, 2016). The fate of nuclear membrane during nuclear shrinking is unknown, however it could stream back into the ER, fold into invaginations, be degraded by nucleophagy (Mochida et al., 2015), or perhaps give rise to nuclear lipid droplets recently described in yeast (Romanauska & Kohler, 2018).
There is also evidence that changes in cytoplasmic volume during development scale nuclear size through titration of limiting components. When nuclei assembled in X. laevis egg extract were confined in microfluidic channels, nuclei reached different steady-state sizes depending on the dimensions of the channel. Nuclei in small channels exposed to less cytoplasm were smaller than nuclei exposed to larger cytoplasmic volumes in larger channel (Fig. 1B). The explanation for this was that channel size dictates the size of microtubule (MT) asters that form next to the nuclei. Small asters lead to less dynein-mediated accumulation of membrane around the nuclei and less nuclear growth, while large asters in larger channels allow for greater accumulation of perinuclear membrane and more nuclear growth (Hara & Merten, 2015), consistent with work showing that a proper tuning of MT polymerization is required for normal nuclear assembly and size (Xue, Woo, Postow, Chait, & Funabiki, 2013). Consistent with perinuclear membrane playing a role, altering the ER tubule-to-sheet ratio and membrane flow can alter nuclear size (Anderson & Hetzer, 2007, 2008; Jevtic & Levy, 2015; Kume, Cantwell, Burrell, & Nurse, 2019). Experiments in which X. laevis embryonic extract and nuclei were encapsulated in microfluidic droplets of defined volume also support a limiting component model. Nuclei grew more in larger droplets (Fig. 1C), and biochemical fractionation identified the histone chaperone nucleoplasmin as one factor that limits nuclear growth (P. Chen et al., 2019). During development, absolute nucleoplasmin amounts per cell decrease, correlating with reduced nuclear histone levels and chromatin compaction and consistent with the idea that nuclear volume is limited by nucleoplasmic factors rather than NE availability (Walters et al., 2019). The proposed mechanism is that increased nuclear import of histones by nucleoplasmin leads to increased chromatin compaction, and stiffer chromatin imparts intranuclear pushing forces on the NE that promote nuclear growth. While extreme chromatin decondensation can increase nuclear size (Bustin & Misteli, 2016), more subtle physiological increases in chromatin compaction might also drive nuclear growth. An open question is the relative contributions of DNA amount and chromatin compaction to nuclear size determination. Future studies will address whether these varied mechanisms of developmental nuclear size scaling are generally conserved in other organisms and in differentiation of various tissues.
An issue related to nuclear morphology and unique to early development in many organisms concerns the formation of a single nucleus. In many embryos, early cell divisions are so rapid that nuclear assembly initiates around individual chromatin masses, forming small nuclei called karyomeres. This allows DNA replication to begin quickly and complete before the next mitosis. In zebrafish, the protein brambleberry ensures that these karyomeres fuse into a single nucleus (Abrams et al., 2012). In C. elegans embryos, a paired nuclei phenotype results when Polo-like kinase PLK-1 or the lipin activator CNEP-1 is inhibited (Bahmanyar et al., 2014; Rahman et al., 2015), bilobed nuclei form in yeast secretion mutants (Walters et al., 2019), and in human cells BAF and KIF18A are important for the formation of a single nucleus after mitosis (Fonseca et al., 2019; Samwer et al., 2017). Failure of these mechanisms can have dire consequences, for instance many cancers exhibit micronuclei in which massive genomic rearrangements called chromothripsis can drive cancer progression (Crasta et al., 2012; Hatch, Fischer, Deerinck, & Hetzer, 2013; C. Z. Zhang et al., 2015). Another unique developmental change in nuclear morphology occurs in Xenopus tail fin cells that become extremely branched through an actin- and lamin-dependent process (Arbach, Harland-Dunaway, Chang, & Wills, 2018).
Recent studies on nuclear structure and function promise to inform novel mechanisms of developmental nuclear size scaling. One such area is nuclear and chromatin mechanics (Cho, Irianto, & Discher, 2017; Heo et al., 2016; Lele, Dickinson, & Gundersen, 2018; Shimamoto, Tamura, Masumoto, & Maeshima, 2017; Stephens, Banigan, & Marko, 2019). For instance, embryonic heart stiffening during development coincides with nuclear lamin A accumulation to prevent nuclear rupture (Cho et al., 2019), stretching forces can lead to NPC opening and increased nuclear import (Elosegui-Artola et al., 2017), and shear force can induce nuclear shrinking (Jetta, Gottlieb, Verma, Sachs, & Hua, 2019). How changes in intranuclear pressure over development might contribute to size scaling is largely unexplored (T. J. Mitchison, 2019). Studies in cultured cells and mouse embryos suggest that intranuclear f-actin contributes to nuclear growth (Baarlink et al., 2017), and whether a similar mechanism scales nuclear size during development is an open question, although nuclear actin levels are known to change during Drosophila oogenesis (Kelpsch, Groen, Fagan, Sudhir, & Tootle, 2016) and early Xenopus development (H. Oda, Shirai, Ura, Ohsumi, & Iwabuchi, 2017). Linker of nucleoskeleton and cytoskeleton (LINC) complexes should be explored with respect to developmental nuclear size scaling (Cantwell & Nurse, 2019; D’Alessandro et al., 2015; Lu et al., 2012; Sun et al., 2019; Tan et al., 2018), as should lipid synthesis at the NE (Barbosa et al., 2019; Haider et al., 2018). In early Drosophila embryos, pre-assembled NPCs are incorporated into nuclei, and developmental regulation of this process could potentially contribute to scaling of nuclear import and size (Hampoelz et al., 2016).
Does nuclear size scaling influence cell function and embryonic development? Specific to early embryogenesis in many species, the midblastula transition (MBT) is characterized by upregulated zygotic gene expression and altered cell cycle kinetics (Blythe & Wieschaus, 2015; Jukam, Shariati, & Skotheim, 2017; Schulz & Harrison, 2019; Vastenhouw, Cao, & Lipshitz, 2019). Altering the N/C ratio in Xenopus embryos changed the timing of MBT onset with concomitant effects on later development (Jevtic & Levy, 2015, 2017; Jevtic, Mukherjee, Chen, & Levy, 2019), and the timing of zygotic genome activation (ZGA) is strongly correlated with cell size (H. Chen, Einstein, Little, & Good, 2019). In Drosophila multinucleate muscle cell development, global scaling of nuclear size with cell size is important for muscle function, while individual nuclear size is spatially regulated (Windner, Manhart, Brown, Mogilner, & Baylies, 2019), as has also been observed in a multinucleate fungus (Dundon et al., 2016). Lamin levels influence nuclear morphology and embryonic heart and retina development (Razafsky et al., 2016; Tran, Zheng, & Zheng, 2016). Increasing ploidy in various amphibians leads to reduced numbers of larger cells such that animal size and the morphology of internal structures are relatively unchanged. For example, higher ploidy and larger cells mean that kidney cell morphology is more drastically altered to ensure proper dimension tubules, while lens epithelial cells in the eye become more flattened to reach the correct thickness (Fankhauser, 1939, 1945a, 1945b; Fankhauser & Watson, 1942). Similar observations have been reported in plants (Robinson et al., 2018). There are also fascinating correlations between cell size and brain morphology in amphibians (G. Roth & Walkowiak, 2015), and polyploidization-induced cell size increases contribute to would healing in Drosophila (Losick, Fox, & Spradling, 2013).
Some nuclei must adopt unique morphologies appropriate for cell function. A classic example is the multi-lobulated neutrophil nucleus that imparts nuclear flexibility to facilitate migration through narrow constrictions (Manley, Keightley, & Lieschke, 2018), regulated in part through altered expression of nuclear lamina proteins and genome organization (Rowat et al., 2013; Zhu et al., 2017). Changes in nuclear shape and mechanics are also important for constricted cell migration during development and cancer metastasis (Bone, Chang, Cain, Murphy, & Starr, 2016; Denais et al., 2016; Jayo et al., 2016; Pfeifer et al., 2018; Raab et al., 2016; Smith, Irianto, Xia, Pfeifer, & Discher, 2019). At the extreme, erythrocytes in some small amphibian species undergo partial or complete enucleation to allow for their circulation through narrow vessels (Mueller, 2015; Mueller, Gregory, Gregory, Hsieh, & Boore, 2008), and lens fiber cells must degrade their nuclei (Chaffee et al., 2014). Many questions remain about the relationship between nuclear size and function, in the context of both embryonic development and disease, particularly laminopathies where altered nuclear lamina composition contributes to a varying array of pathologies (Dobrzynska, Gonzalo, Shanahan, & Askjaer, 2016; Osmanagic-Myers & Foisner, 2019; Worman & Schirmer, 2015) and cancers with enlarged nuclei (Chow, Factor, & Ullman, 2012; Jevtic & Levy, 2014; Zink, Fischer, & Nickerson, 2004).
Mitotic spindles
Mitotic spindles are composed of microtubules (MTs), polymers of repeating α/β tubulin heterodimers. MTs undergo phases of growth and shrinkage known as dynamic instability (T. Mitchison & Kirschner, 1984). A variety of MT binding and regulatory proteins affect MT dynamics and consequently spindle length (Rieckhoff, Ishihara, & Brugues, 2019). Spindles are responsible for equal segregation of chromosomes between daughter cells during cell division. They also interact with the cell cortex, playing a role in the initiation of contractile ring formation and cytokinesis (Akhshi, Wernike, & Piekny, 2014). It is, therefore, important that spindle size scales with cell size to ensure these processes are faithfully executed. As with nuclei, there is evidence that DNA amount has a relatively small impact on spindle size (Brown et al., 2007; Wuhr et al., 2008), although chromatin does modulate MT dynamics and spindle morphology (Carazo-Salas et al., 1999; Dinarina et al., 2009; Hara & Kimura, 2013).
Spindle scaling has been studied extensively during Xenopus, Mus musculus, and C. elegans development. In early Xenopus embryos, reductions in cell size correlate with decreased spindle length in different stage blastomeres (Wuhr et al., 2008) (Fig. 1D). Reaching a plateau in early stages, spindle length scales roughly linearly with cell size beyond the 32-cell stage. This suggests that spindle size is uncoupled from cell size during the early stages of development, with spindle length reaching an upper limit. Similar results are observed in developing C. elegans embryos where spindle size scales in 20 μm-diameter and smaller cells, while such scaling is less evident in larger, earlier stage cells (Hara & Kimura, 2009) (Fig. 3B). In mouse embryos spindle size remains constant during the first three divisions and only scales smaller with cell size after the fourth division (Courtois, Schuh, Ellenberg, & Hiiragi, 2012), although fusion of early stage blastomeres can lead to increased spindle length (Novakova et al., 2016). Cytoplasm removal or embryo fusion showed that spindle scaling is dependent on cytoplasmic volume and not developmental stage (Courtois et al., 2012; Kyogoku & Kitajima, 2017; Lane & Jones, 2017). Interestingly, in sea urchin embryos, spindle length scaling is evident from the first cell division (Lacroix et al., 2018) (Fig. 4C). In asymmetrically dividing C. elegans embryos, cell and spindle sizes correlate at the same developmental stage, providing further support for cytoplasmic volume regulating spindle size (Hara & Kimura, 2009; Lacroix et al., 2018). In addition, the rate of spindle elongation scales with cell size in worms (Hara & Kimura, 2009).
Mechanisms of spindle length scaling have been elucidated using cell-free in vitro approaches that recapitulate spindle assembly. The contribution of cytoplasmic volume to spindle size scaling was explicitly tested by coupling Xenopus egg extracts with microfluidic approaches to encapsulate cytoplasm in droplets of defined size (Oakey & Gatlin, 2018). Similar to results in Xenopus embryos, spindle size scaled with cytoplasmic volume in droplets ranging from 30–80 μm in diameter, with an upper limit to spindle size being reached in larger droplets (Good, Vahey, Skandarajah, Fletcher, & Heald, 2013; Hazel et al., 2013) (Fig. 1E). Because droplet volume and dimensions were both manipulated in these experiments, spindle size might be sensitive to volume and/or boundary sensing. To distinguish these two models, isovolumetric droplets of differing shape were generated. Spindle size was observed to scale with droplet volume and to not be affected by droplet shape (Good et al., 2013; Hazel et al., 2013), supporting the idea that cytoplasmic components may limit spindle size (Goehring & Hyman, 2012).
One obvious candidate limiting component is tubulin itself, since it is the building block of MTs. Indeed, a tubulin-limiting computational model accurately predicted Xenopus developmental spindle scaling data, although supplementing extract droplets with porcine brain tubulin did not affect spindle size (Good et al., 2013). Several lines of evidence suggest that tubulin may not be the primary determinant of spindle length scaling (Lacroix et al., 2018). Another promising candidate is XMAP215, a MT polymerase that binds tubulin and MT plus ends (Reber et al., 2013). Depletion of XMAP215 from Xenopus egg extracts resulted in short spindles, and titrated addition of wild-type XMAP215 led to graded increases in MT growth velocity and spindle length. Addition of XMAP215 mutants with reduced affinity for tubulin revealed a correlation between MT polymerase activity and spindle length (Reber et al., 2013). Consistent with these in vitro data, microinjection of XMAP215 into Xenopus embryos led to increased spindle size beyond the usual upper limit, indicating structural components are not limiting, while a polymerase-deficient mutant decreased spindle length (Milunovic-Jevtic, Jevtic, Levy, & Gatlin, 2018). Taken together, these studies identify XMAP215 as a putative limiting component in developmental spindle size scaling and highlight how factors that affect MT dynamics and growth rates can regulate spindle size, as opposed to spindle structural components.
Following up on these Xenopus studies, developmental changes in MT dynamics were carefully examined in C. elegans embryos (Lacroix et al., 2018). Concomitant with spindle size reductions, MT growth rates also decreased, but no changes in MT shrinkage rates or the frequencies of MT catastrophe and rescue were observed. In addition, the spindle assembly rate was decreased in smaller cells. In mutant 2-cell stage embryos in which cytokinesis was blocked, MT growth rates were similar to 1-cell wild-type embryos, suggesting that MT growth rates and spindle size are dictated by cytoplasmic volume rather than developmental stage or timing. Similar correlations between MT growth rates and cell and spindle sizes were observed during early development of much larger sea urchin embryos, suggesting that scaling of MT growth rates may represent a conserved mechanism of spindle size scaling. Based on computational modelling, the proposed mechanism is that the absolute amounts of positive regulators of MT growth are reduced upon being partitioned into smaller and smaller cells over development, leading to reduced MT growth rates and spindle sizes (Fig. 3). Candidate limiting components for spindle size scaling include Xenopus XMAP215 and the C. elegans CLASP homolog CLS-2 that promotes MT assembly (Lacroix et al., 2018).
There is also evidence that MT depolymerization can contribute to mitotic spindle scaling. Xenopus developmental progression is accompanied by increased spindle recruitment of the MT-depolymerizing kinesin kif2a, leading to increased MT catastrophe frequency and reduced spindle length (Wilbur & Heald, 2013). This developmental change in kif2a localization is regulated by importin α. Kif2a is inhibited by bound importin α, leading to reduced MT depolymerization. As discussed in the context of nuclear scaling, in early embryos there is a large pool of cytoplasmic importin α that acts to inhibit kif2a. As palmitoylated importin α exhibits reduced cargo binding and is partitioned to the plasma membrane over development, there is less cytoplasmic importin α available to inhibit kif2a, so activated kif2a relocalizes to spindles leading to progressive reductions in spindle size (Brownlee & Heald, 2019; Wilbur & Heald, 2013). With a similar importin α-mediated mechanism contributing to developmental nuclear size scaling in Xenopus, this represents a fascinating example of coordinated size regulation of two organelles by a similar mechanism (Fig. 1G). Studies in mouse embryos also suggest that the nuclear-to-cytoplasmic ratio might influence spindle size scaling (Novakova et al., 2016).
In addition to developmental spindle scaling, correlations between embryo, cell, and spindle sizes have been characterized across a wide variety of different species (Crowder et al., 2015; Farhadifar et al., 2015; Valfort, Launay, Semon, & Delattre, 2018). X. laevis spindles are larger than X. tropicalis spindles (Brown et al., 2007). Two regulators that contribute to this difference are the MT-severing protein katanin and TPX2, a MT-associated protein that binds the kinesin-5 motor Eg5. Although katanin levels are similar in the two species, X. tropicalis katanin possesses higher MT severing activity due to the absence of an inhibitory phosphorylation site present in X. laevis katanin (Loughlin, Wilbur, McNally, Nedelec, & Heald, 2011). Higher TPX2 levels in X. tropicalis lead to increased polar recruitment of Eg5, altering MT structure at the poles to reduce spindle length (Helmke & Heald, 2014). Recently described egg extracts from a third Xenopus clawed frog species, X. borealis, promise to provide additional new insights into interspecies organelle size scaling (Kitaoka, Heald, & Gibeaux, 2018).
In summary, spindle size generally scales with cell size during early development. A number of positive and negative regulators of MT dynamics contribute to the phenomenon of spindle size scaling, and it is likely that multiple mechanisms must be invoked to fully account for the magnitude and different regimes of scaling (Brownlee & Heald, 2018). Positive regulators include XMAP215 and CLS-2, while negative regulators include katanin and kif2a. Apart from regulators of MT dynamics, other aspects of cell biology that deserve further consideration with respect to their potential impact on spindle size scaling include: 1) liquid-liquid phase separation that has been shown to enrich MT regulatory factors in acentrosomal spindle assembly (So et al., 2019), possibly akin to the previously described spindle matrix (Jiang et al., 2015; Johansen, Forer, Yao, Girton, & Johansen, 2011), 2) feedback from altered MT dynamics on tubulin expression levels (Gasic, Boswell, & Mitchison, 2019), 3) the influence of actin on MT dynamics (Colin, Singaravelu, Thery, Blanchoin, & Gueroui, 2018; Inoue et al., 2019; Kita et al., 2019; Mogessie & Schuh, 2017), and 4) interphase MT bridges between sister cell pairs in early mammalian embryos (Zenker et al., 2017). Computational models of spindle size regulation may also inform scaling mechanisms (Jiang, 2015; Li & Jiang, 2017). Further investigations into how these and other factors coordinately regulate MT dynamics during development promise to provide a more complete picture of spindle scaling mechanisms. Another exciting area for future research will be to utilize this new mechanistic information to manipulate spindle size scaling in embryos to assess effects on chromosome segregation, cell division site determination, developmental timing, and embryonic morphology and function.
Centrosomes
Centrosomes nucleate the MTs of interphase asters and mitotic spindles. Consisting of a pair of centrioles and a number of proteins referred to as pericentriolar material, nucleus-juxtaposed centrosomes duplicate during interphase to give rise to mitotic spindle poles. In C. elegans, centrosomes begin to mature in interphase, grow during mitosis, and scale smaller with cell size over development (Decker et al., 2011; Greenan et al., 2010) (Fig. 3C). Reducing centrosome size was sufficient to decrease spindle length (Greenan et al., 2010). In the first two cell divisions, wild-type centrosomes continue to grow while a smaller steady-state centrosome size was reached in a small embryo mutant, suggesting centrosome growth rate depends on cell size but not developmental stage. While centrosome growth rates are slower in smaller cells, total centrosome volume remains constant up to the 4-cell stage and in mutants with increased or decreased numbers of centrosomes. These data support a limiting component model where cell size determines the amount of available centrosome components, thereby setting centrosome volume. One putative limiting component is Spd-2, a conserved protein key to centrosome assembly, which when overexpressed increased centrosome growth rate and volume (Decker et al., 2011). Additional factors that may contribute to developmental reductions in centrosome size include Polo-like kinase 1 and Spd-5 (Wueseke et al., 2016), and recent work showing centrosome formation by phase separation may also inform the scaling mechanism (Woodruff et al., 2017).
How might centrosome size contribute to spindle length control? One model involves a gradient of centrosome-derived TPXL-1 (C. elegans homolog of TPX2), in which a larger centrosome loads more TPXL-1 onto spindle MTs, producing a wider TPXL-1 gradient and longer spindles (Greenan et al., 2010). Species-specific differences in TPXL-1 and TPX2 may account for their different effects on spindle size in C. elegans and Xenopus (Bird & Hyman, 2008; Helmke & Heald, 2014). Chemical waves emanating from centrosomes have also been proposed to organize MT assemblies in Xenopus (Ishihara et al., 2014), and nuclear size can affect centrosome attachment and separation (Boudreau, Chen, Edwards, Sulaimain, & Maddox, 2019; Meyerzon et al., 2009).
Chromosomes and chromatin
From a physical perspective, it reasons that mitotic chromosome length might scale over development. If chromosome length were to exceed cell length in small cells, this could result in chromosome fragmentation and/or aneuploidy. Indeed, artificially increasing chromosome length in fava bean resulted in growth and developmental defects (Schubert & Oud, 1997), and nuclei in closed yeast mitoses must grow sufficiently to ensure faithful chromosome segregation (Takemoto et al., 2016). In Xenopus, mitotic chromosome length scaling, though not evident in early development, is apparent from the blastula-to-neurula stage (Kieserman & Heald, 2011) (Fig. 1F). Because blastomeres are quite large in the early embryo, it is perhaps expected that chromosome scaling would only be necessary when cells become significantly smaller post-blastula. Mitotic chromosome length also scales with cell size during C. elegans development (Hara et al., 2013; Ladouceur et al., 2015). It is unknown if developmental chromosome length scaling is mediated through active sensing of cell size. Candidate chromosome scaling factors include topoisomerase-II, the histone H3 variant CENP-A, and condensins (Ladouceur et al., 2017; Shintomi & Hirano, 2011). Intriguingly, a new microscopy technique that integrates correlative light and electron microscopy revealed that chromatin constitutes a relatively small amount of the metaphase chromosome mass, begging the question of what other chromatin-associated proteins might contribute to chromosome scaling (Booth et al., 2016).
Another interesting context to consider chromosome scaling is the increase in genome size that occurred during eukaryotic evolution, where the insertion of arginine residues into the N-terminus of histone H2A led to greater chromatin compaction of larger genomes (Macadangdang et al., 2014). Because there is a general correlation between nuclear and chromosome size and because reducing nuclear growth led to smaller mitotic chromosomes (Hara et al., 2013; Ladouceur et al., 2015), one proposal is that nuclear size and/or nucleocytoplasmic transport of chromosome scaling factors during interphase might determine chromosome size (Heald & Gibeaux, 2018). It is tempting to speculate that limiting nuclear import may represent a coordinated mechanism for the control of nuclear and chromosome size as well as MBT timing.
While some mechanisms responsible for mitotic chromosome size scaling have been elucidated, much less is known about interphase chromosome size, in part because quantifying decondensed chromosome length is more difficult. Nonetheless, interphase chromosome size scaling may be functionally relevant. There is evidence in cell culture that cell geometry can influence the 3D positioning of chromosome territories within the nucleus, thereby influencing gene expression (Y. Wang, Nagarajan, Uhler, & Shivashankar, 2017). Perhaps stereotyped changes in chromatin organization and gene expression during embryogenesis might, at least in part, be regulated by cell size and shape (Andrey & Mundlos, 2017; Borsos et al., 2019; Buchwalter, Kaneshiro, & Hetzer, 2019; Flyamer et al., 2017; Perino & Veenstra, 2016; Stadhouders, Filion, & Graf, 2019; Wu et al., 2018). Changes in chromatin architecture, potentially linked to chromosome scaling, are important drivers of early developmental programs. For example, studies in Xenopus, Drosophila, and zebrafish have shown that limiting histone pools and competition for transcription factor binding, whose concentrations can be influenced by nuclear size, are key determinants of ZGA (Amodeo, Jukam, Straight, & Skotheim, 2015; S. H. Chan et al., 2019; Joseph et al., 2017; Reisser et al., 2018; Shindo & Amodeo, 2019; Wilky, Chari, Govindan, & Amodeo, 2019). At least in Drosophila, changes in chromatin architecture associated with ZGA are independent of transcription, suggesting that chromosome re-positioning occurs upstream of, and is required for, ZGA (Hug, Grimaldi, Kruse, & Vaquerizas, 2017). Subsequent to the MBT, cell cycle slowing regulates the establishment of constitutive heterochromatin in Drosophila and C. elegans, through the increased accumulation of histone methyltransferases on the DNA (Mutlu et al., 2018; Seller, Cho, & O’Farrell, 2019). At the extreme, improper chromosome scaling can lead to inviability. For instance, when X. tropicalis eggs are fertilized with X. laevis sperm containing nearly twice the amount of genomic DNA, hybrid embryos die at late blastula stages due to chromosome mis-segregation and unbalanced gene expression (Gibeaux et al., 2018). Although requiring future investigation, chromosome scaling may underlie several key events in early development.
Endoplasmic reticulum and interconnected organelles
The endoplasmic reticulum (ER) is an extensive membrane network composed of tubules that intersect at three-way junctions and ribosome-studded rough ER sheets (N. Wang & Rapoport, 2019; H. Zhang & Hu, 2016). While developmental scaling of ER size and structure have not been studied in great detail, one possibility is that ER is equally partitioned into dividing blastomeres through MT-dependent positioning (S. Wang, Romano, Field, Mitchison, & Rapoport, 2013), resulting in progressive scaling of ER size, although this scaling relationship could be altered if new membrane synthesis occurs. In C. elegans, ER compartmentalization is evident as early as the one-cell stage (Lee, Prouteau, Gotta, & Barral, 2016), consistent with separate ER domains roughly delineating future daughter cells in budding yeast and neural stem cells (Clay et al., 2014; Luedeke et al., 2005; Moore, Pilz, Arauzo-Bravo, Barral, & Jessberger, 2015; Pina, Fleming, Pogliano, & Niwa, 2016). ER structural proteins including REEP3/4 help to clear tubulated ER membrane from metaphase chromatin during mitosis and may facilitate equal partitioning of ER to daughter cells (Kumar, Golchoubian, Belevich, Jokitalo, & Schlaitz, 2019; Schlaitz, Thompson, Wong, Yates, & Heald, 2013). In a subset of cells in gastrulating Drosophila embryos, asymmetric ER partitioning occurs dependent on the conserved ER membrane protein Jagunal (Eritano et al., 2017).
Steady-state size and number of many membrane-bound organelles are determined by balanced fusion and fission that are regulated by diverse activities including lipid signalling, expression of coat and adaptor proteins, and cytoskeletal interactions. This mode of size regulation applies to lysosomes (Saffi & Botelho, 2019), mitochondria (Miettinen & Bjorklund, 2017), and Golgi (Dippold et al., 2009; Sengupta & Linstedt, 2011). In a related model, the functional requirements of an organelle may dictate its size, as is the case for the Golgi where cisternal size is regulated by membrane trafficking kinetics (Bevis, Hammond, Reinke, & Glick, 2002; Bhave et al., 2014). By and large, developmental size regulation of these various organelles has not been thoroughly investigated. Simply determining if it is organelle surface area or volume that scales with cell size may provide clues to the underlying mechanisms. One might imagine surface area to scale for membrane-limiting reactions, for instance protein synthesis in the rough ER, while volume might scale for organelles like lysosomes.
While yeast is not a developmental system per se, mechanisms identified in yeast that regulate the sizes of membrane-bound organelles will undoubtedly inform scaling in higher eukaryotes. For example, ER stress is alleviated by membrane expansion (Schuck, Prinz, Thorn, Voss, & Walter, 2009), while ER-phagy selectively degrades excess ER membrane in the vacuole, the yeast equivalent of the lysosome (Schuck, Gallagher, & Walter, 2014). Mathematical modelling and experimental measurements in yeast showed that stochastic fluctuations in the abundance of Golgi, vacuoles, and peroxisomes must be taken into account when considering size scaling relationships (Mukherji & O’Shea, 2014). SNAREs and membrane trafficking contribute to yeast vacuole size control, and similar mechanisms may regulate lysosome size scaling in higher eukaryotes (Y. H. Chan & Marshall, 2014; Y. H. Chan, Reyes, Sohail, Tran, & Marshall, 2016; D’Agostino, Risselada, Endter, Comte-Miserez, & Mayer, 2018; Desfougeres, Neumann, & Mayer, 2016).
Interesting correlations between mitochondrial size, cell size, and metabolism may inform future scaling studies. Mitochondrial size scales with cell size in yeast, with new mitochondrial accumulation occurring in the bud and reduced mitochondrial content in aging mothers (Jajoo et al., 2016; Rafelski et al., 2012). In addition, the mitochondria-to-cytoplasm ratio remains constant during HeLa cell growth (Posakony, England, & Attardi, 1977). Assuming mitochondrial scaling is similar in yeast and higher eukaryotes, cell size-dependent mitochondrial scaling during development and differentiation might be expected, although future research will be necessary to acquire these measurements and to determine the underlying mechanisms. More is known about the scaling of mitochondrial function with cell size. In cultured Drosophila cells, mitochondrial mass scales with cell size, however mitochondrial function, measured by membrane potential and oxidative phosphorylation, is highest in intermediate sized cells. This optimal cell size also correlates with reduced apoptosis and increased proliferation (Miettinen & Bjorklund, 2016). Consistent with these findings, increasing hepatocyte size downregulates expression of mitochondrial genes and lipogenic transcription factors, and inhibiting mitochondrial function leads to increased cell size (Miettinen et al., 2014). How mitochondrial activity is correlated with cell size is not fully understood, though the mevalonate pathway has been implicated (Miettinen & Bjorklund, 2016). In the developing Drosophila intestine, cell size reductions are accompanied by autophagy-dependent clearance of mitochondria. This mitochondrial scaling is dependent on the ubiquitin binding activity of Vps13D, and large cells and mitochondria in Vps13D mutants are rescued by inhibiting mitochondrial fusion (Anding et al., 2018). In mammalian cells, Largen (gene PRR16) increases translation of mitochondrial proteins, leading to increased mitochondrial size, respiration, and cell size (Yamamoto et al., 2014). Thus multiple mechanisms contribute to the complex scaling relationships between cell size and mitochondrial size and function.
There is evidence that ER morphology controls the size and morphology of other organelles that contact the ER (Gottschling & Nystrom, 2017; H. Zhang & Hu, 2016), including ER-mitochondria contacts that coordinate mitochondrial DNA replication and division (Lewis, Uchiyama, & Nunnari, 2016). Of note, the outer nuclear member is continuous with the ER, and nuclear expansion in early embryos can occur through streaming of ER into the NE as well as by direct incorporation of annulate lamellae (Anderson & Hetzer, 2007; Hampoelz et al., 2016). Manipulations that increase ER tubulation generally decrease nuclear size while increasing ER sheet volume drives nuclear growth, suggesting a tug-of-war between ER and nuclear membranes (Anderson & Hetzer, 2008; Jevtic & Levy, 2015). The ER is also key in the biogenesis of other organelles, such as peroxisomes and lipid droplets (Joshi et al., 2016; Joshi, Zhang, & Prinz, 2017; Sugiura, Mattie, Prudent, & McBride, 2017).
Because relatively little is known about developmental scaling of membrane-bound organelles, many questions remain about its physiological significance. Still there is abundant evidence that the size and morphology of membrane-bound organelles affect functional output. Cells that synthesize and secrete large amounts of proteins, such as plasma cells and pancreatic acinar cells, are enriched in rough ER sheets. On the other hand, smooth ER tubules are abundant in hepatocytes, adrenal cortical cells, and muscle cells that are specialized for carbohydrate metabolism, steroid hormone synthesis, and calcium signalling, respectively (Black, 1972; Friedman & Voeltz, 2011; Goyal & Blackstone, 2013; Shibata, Voeltz, & Rapoport, 2006; West, Zurek, Hoenger, & Voeltz, 2011). The unfolded protein response and ER stress induced by excess fatty acids lead to expansion of ER sheets, which is energetically more favorable than ER tubule expansion and is thought to provide more space for protein folding (Friedman & Voeltz, 2011; Schuck, 2016; Schuck et al., 2009; Walter & Ron, 2011; Wikstrom et al., 2013). In developing Drosophila photoreceptors, rough ER sheets expand to produce the protein synthetic capacity necessary to build the photosensitive rhabdomere in a process requiring Ire1, a key component of the unfolded protein response (Xu, Chikka, Xia, & Ready, 2016). It is worth noting that changing ER morphology is not sufficient to alter exocytosis (Mukherjee & Levy, 2019), so further studies are required to understand which functional outputs of the ER are sensitive to organelle size and morphology. Disrupting mitotic peroxisome distribution leads to delayed mitosis and altered spindle positioning with concomitant defects in polarized cell division and daughter cell fate determination (Asare, Levorse, & Fuchs, 2017). Golgi and mitochondrial sizes also must scale with cell size and type to ensure the cell’s metabolic requirements are met (Miettinen & Bjorklund, 2017; Sengupta & Linstedt, 2011).
Flagella, cilia, stereocilia, and microvilli
Because flagella and cilia are roughly linear structures (in eukaryotes flagella and cilia refer to the same organelle), they represent somewhat simplified organelles for the study of size regulation. Flagellar length control in particular has been studied in Chlamydomonas and revealed mechanisms involving balanced rates of assembly and disassembly (Hilton, Gunawardane, Kim, Schwarz, & Quarmby, 2013; Ishikawa & Marshall, 2017; Marshall, 2015; Meng & Pan, 2016; Wemmer & Marshall, 2007) as well as molecular rulers (Hendel, Thomson, & Marshall, 2018; T. Oda, Yanagisawa, Kamiya, & Kikkawa, 2014). For instance, NIMA-related kinases have recently been implicated in equilibrium balance models of ciliary length control while the size of the structural FAP59/172 complex influences flagella length. Centrioles are critical for cilia formation, and centriole length varies between different species and cell types (Azimzadeh & Marshall, 2010). In Drosophila embryos, a variety of factors contribute to proper centriole length control, including Polo-like kinase 4 that acts as a homeostatic clock to regulate centriole growth rate (Aydogan et al., 2018) and Asterless that plays independent roles in centriole length control and sperm basal body function (Galletta, Jacobs, Fagerstrom, & Rusan, 2016). In terminally differentiated epithelia of the respiratory tract, centriole amplification produces 100–600 centrioles with a concomitant increase in the number of motile cilia. In airway progenitor cells, ablation of the two parental centrioles did not affect centriole amplification. Rather, by manipulating cell size by varying the extracellular collagen concentration, it was shown that cell surface area scales centriole and cilia number (Nanjundappa et al., 2019). In turn, fluid flow-mediated signalling in primary cilia can regulate epithelial cell volume through an autophagy-based mechanism (Orhon et al., 2016).
Linear actin-based structures that are developmentally regulated include stereocilia and microvilli. Stereocilia that constitute cochlear hair bundles are key to hearing. During hair cell differentiation, changes in the number, length, and organization of stereocilia are developmentally regulated by class III myosins and actin capping proteins, and defects in this process lead to hearing loss in mice (Avenarius et al., 2017; Lelli et al., 2016). During vertebrate oogenesis, oocytes grow larger and synthesize proteins and membranes necessary for early embryogenesis. In Xenopus oocytes, the Ca2+-activated chloride channel Ano1 contributes to this increase in oocyte surface area. Independent of its channel activity, Ano1 regulates microvilli length and scaffolding, leading to stabilization of larger membrane structures (Courjaret et al., 2016). Identifying other cellular systems where the lengths and/or numbers of these various MT- and actin-based linear structures change over development will provide new insights into scaling of these unique organelles.
Other intracellular structures
While not organelles per se, several other intracellular structures exhibit interesting size scaling relationships. The nucleolus is a membraneless organelle within the nucleus that contains rDNA repeats and is therefore key to ribosome biogenesis. In early C. elegans embryos, nucleolar size directly scales with cell and nuclear size. Interestingly, when embryo size is altered by various RNAi treatments, nucleolar size inversely scales with cell size, consistent with a fixed amount of nucleolar material becoming diluted or concentrated in larger or smaller RNAi-treated embryos, respectively (Weber & Brangwynne, 2015). In extremely large Xenopus oocytes, distinct nucleoli are maintained by an F-actin scaffold and disrupting this scaffold causes nucleoli to sediment under the force of gravity and fuse (Feric & Brangwynne, 2013). This fusion behaviour revealed that the nucleolus exhibits liquid-like properties and forms through phase separation, regulating nucleolar size and shape (Brangwynne, Mitchison, & Hyman, 2011). Transcription of rRNA is important for seeding nucleolus assembly and ensuring the proper number of nucleoli form at the correct time and location (Berry, Weber, Vaidya, Haataja, & Brangwynne, 2015; Falahati, Pelham-Webb, Blythe, & Wieschaus, 2016). Reconstitution experiments demonstrated that nucleoli are composed of subcompartments in which distinct RNA processing reactions might occur (Feric et al., 2016). Thus the phase separation properties of the nucleolus are important for both size control and function. It is also worth noting that nucleolar size increases with normal and disease-related aging (Buchwalter & Hetzer, 2017).
The sizes of clathrin-coated endocytic lattices change during cellular differentiation, mediated by altered expression of the AP2μ2 adaptor (Dambournet et al., 2018). Retinal pigment epithelial cells contain melanosomes, melanin-containing structures that reduce backscattered light and neutralize free radicals. Proper regulation of melanosome number and shape are required for normal vision (Burgoyne, O’Connor, Seabra, Cutler, & Futter, 2015). Drosophila embryos possess actin-rich structures called denticles that line the ventral epidermis and may be important for larval locomotion. Denticle number and spacing exhibit MT-dependent scaling with cell length over development (Spencer, Schaumberg, & Zallen, 2017). Acting through a ruler mechanism, the size of the large muscle protein nebulin dictates actin thin filament length and spacing, thereby influencing sarcomere size (Fernandes & Schock, 2014). The size of a unique secreted particle, the Weibel-Palade body, is determined by the size and number of Golgi ministacks, providing an interesting example of how the sizes of two organelles might be interrelated (Ferraro et al., 2014). In Arabidopsis, the speed of myosin-regulated cytoplasmic streaming affects cell and plant size but not cell number (Tominaga et al., 2013). In Plasmodium, sporozoite shape and infectivity are determined by MT number, which is reduced upon knocking down the level of α-tubulin expression (Spreng et al., 2019). Understanding how the sizes of these diverse structures are regulated may inform organelle-scaling mechanisms.
Conclusion
While size scaling of certain organelles with cell size has been known about for over a century, the last decade has witnessed a renewed interest in the phenomenology and mechanisms of organelle size scaling. Many of these recent studies have taken advantage of early developmental systems where reproducible reductions in cell size provide a powerful platform to study size scaling at the intracellular level. While our understanding of the developmental regulation of nuclear and spindle size has advanced, basic questions still remain about how the sizes of many membrane-bound organelles scale over early development, including the ER, Golgi, lysosomes, and mitochondria. Though these organelles exhibit complex morphologies, new microscopy techniques and image analysis approaches promise to facilitate quantification of developmental changes in their sizes and properties (Chung et al., 2013; Ghosh et al., 2019; Guo et al., 2018; Liu et al., 2018; Ou et al., 2017; Stegmaier et al., 2016; Valm et al., 2017). It is also worth considering new models of early development that might be amenable to studying size scaling of these organelles (Behringer, Johnson, & Krumlauf, 2009; Goldstein & King, 2016), as well as regeneration (Stocum, 2017). Developmental scaling processes in human cells might be studied using organoids or micropatterned stem cells (Deglincerti et al., 2016; Lehmann et al., 2019; Morgani, Metzger, Nichols, Siggia, & Hadjantonakis, 2018). Diverse mechanisms can account for organelle scaling in early embryos, including limiting components and dynamic regulation of assembly and disassembly (Fig. 2). It is clear that multiple, non-mutually exclusive mechanisms can contribute to organelle size regulation and more than one model may be required to account for the full extent of organelle size scaling. In that regard, it will be exciting to see how phase separation and interorganellar contacts contribute to developmental control of organelle size. In the way that importin α has been shown to regulate the sizes of both Xenopus spindles and nuclei, another exciting area will be to investigate how the sizes of distinct organelles might be coordinately regulated.
A variety of new techniques and approaches may lend themselves to studies of organelle size. Bottom-up cellular reconstitution allows for precise control of synthetic cell size and shape to address how these properties influence organelle size scaling (Bermudez, Chen, Einstein, & Good, 2017; Gopfrich, Platzman, & Spatz, 2018; Sonnen & Merten, 2019; Vahey & Fletcher, 2014). Identifying the parts list for a given organelle will facilitate studies of size because the presence and levels of individual components can be precisely modulated. In that vein, the tubular ER has been reconstituted in vitro (Powers, Wang, Liu, & Rapoport, 2017). CRISPR approaches now allow for precise genome modification in over 20 different plant and animal species, for minimally disruptive labelling of organelles for size quantification and manipulation of putative size scaling factors (Harrison, Jenkins, O’Connor-Giles, & Wildonger, 2014). Complementary methods allow for more acute, temporally regulated degradation of endogenous proteins (S. Roth, Fulcher, & Sapkota, 2019). Comprehensive transcriptomics and proteomics databases of early development in a variety of organisms provide a rich resource to identify putative developmental regulators of organelle size (Casas-Vila et al., 2017; Lucitt et al., 2008; Peshkin et al., 2015; Xia et al., 2018).
As mechanisms of developmental organelle scaling are elucidated, it will become increasingly feasible to manipulate organelle size in cells and alter normal scaling relationships to address the consequences for cell function and embryonic development. New optogenetic methods might allow for dynamic modulation of organelle size in living embryos (Buckley et al., 2016; Niopek, Wehler, Roensch, Eils, & Di Ventura, 2016). These functional studies will be critical to distinguish whether changes in organelle size over development are merely a secondary consequence of cell size reductions or if organelle size scaling is important for proper spatiotemporal patterning and development of the embryo. As one example, nuclear size may influence gene positioning and expression, and this hypothesis can now be addressed using new approaches to image 3D chromatin organization and transcription in embryos (Bothma, Norstad, Alamos, & Garcia, 2018; Cardozo Gizzi et al., 2019; Y. Chen et al., 2018; Stevens et al., 2017) and to manipulate the positions of specific genomic loci (H. Wang et al., 2018; Wijchers et al., 2016). Functional studies will also inform the aberrant organelle scaling that is often observed in cancer and certain developmental disorders (Chow et al., 2012; Jevtic & Levy, 2014; Zink et al., 2004), and model systems like Xenopus promise to inform disease mechanisms related to altered scaling (Hardwick & Philpott, 2015; Kakebeen & Wills, 2019; Sater & Moody, 2017). With many exciting open questions about the mechanisms and functional significance of developmental organelle size scaling, the next decade is sure to see further advances in this fascinating field.
Acknowledgments
We thank Jérémy Sallé (Institut Jacques Monod) for allowing us to adapt his embryo schematics for the graphical abstract.
Funding Information
Research in the Levy lab is supported by the National Institutes of Health/National Institute of General Medical Sciences (R01GM113028 and P20GM103432) and the American Cancer Society (RSG-15-035-01-DDC).
Footnotes
Research Resources
No research resources to mention.
No conflicts of interest.
Contributor Information
Chase C. Wesley, Department of Molecular Biology, University of Wyoming.
Sampada Mishra, Department of Molecular Biology, University of Wyoming.
Daniel L. Levy, Department of Molecular Biology, University of Wyoming.
References
- Abrams EW, Zhang H, Marlow FL, Kapp L, Lu S, & Mullins MC (2012). Dynamic assembly of brambleberry mediates nuclear envelope fusion during early development. Cell, 150(3), 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acebron SP, Karaulanov E, Berger BS, Huang YL, & Niehrs C (2014). Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol Cell, 54(4), 663–674. [DOI] [PubMed] [Google Scholar]
- Akhshi TK, Wernike D, & Piekny A (2014). Microtubules and actin crosstalk in cell migration and division. Cytoskeleton (Hoboken), 71(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Amodeo AA, Jukam D, Straight AF, & Skotheim JM (2015). Histone titration against the genome sets the DNA-to-cytoplasm threshold for the Xenopus midblastula transition. Proc Natl Acad Sci U S A, 112(10), E1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, & Hetzer MW (2007). Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol, 9(10), 1160–1166. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, & Hetzer MW (2008). Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J Cell Biol, 182(5), 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding AL, Wang C, Chang TK, Sliter DA, Powers CM, Hofmann K, … Baehrecke EH (2018). Vps13D Encodes a Ubiquitin-Binding Protein that Is Required for the Regulation of Mitochondrial Size and Clearance. Curr Biol, 28(2), 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrey G, & Mundlos S (2017). The three-dimensional genome: regulating gene expression during pluripotency and development. Development, 144(20), 3646–3658. [DOI] [PubMed] [Google Scholar]
- Arata Y, & Takagi H (2019). Quantitative Studies for Cell-Division Cycle Control. Front. Physiol, 10, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata Y, Takagi H, Sako Y, & Sawa H (2014). Power law relationship between cell cycle duration and cell volume in the early embryonic development of Caenorhabditis elegans. Front Physiol, 5, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbach HE, Harland-Dunaway M, Chang JK, & Wills AE (2018). Extreme nuclear branching in healthy epidermal cells of the Xenopus tail fin. J Cell Sci, 131(18):jcs217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare A, Levorse J, & Fuchs E (2017). Coupling organelle inheritance with mitosis to balance growth and differentiation. Science, 355(6324), eaah4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius MR, Krey JF, Dumont RA, Morgan CP, Benson CB, Vijayakumar S, … Barr-Gillespie PG (2017). Heterodimeric capping protein is required for stereocilia length and width regulation. J Cell Biol, 216(11), 3861–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogan MG, Wainman A, Saurya S, Steinacker TL, Caballe A, Novak ZA, … Raff JW (2018). A homeostatic clock sets daughter centriole size in flies. J Cell Biol, 217(4), 1233–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, & Marshall WF (2010). Building the centriole. Curr Biol, 20(18), R816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarlink C, Plessner M, Sherrard A, Morita K, Misu S, Virant D, … Grosse R (2017). A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Nat Cell Biol, 19(12), 1389–1399. [DOI] [PubMed] [Google Scholar]
- Bahmanyar S, Biggs R, Schuh AL, Desai A, Muller-Reichert T, Audhya A, … Oegema K (2014). Spatial control of phospholipid flux restricts endoplasmic reticulum sheet formation to allow nuclear envelope breakdown. Genes Dev, 28(2), 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AD, Lim K, Mari M, Edgar JR, Gal L, Sterk P, … Siniossoglou S (2019). Compartmentalized Synthesis of Triacylglycerol at the Inner Nuclear Membrane Regulates Nuclear Organization. Dev Cell, 50(6), 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Johnson AD, & Krumlauf RE (2009). Emerging Model Organisms: A Laboratory Manual (Vol. 1): Cold Spring Harbor Laboratory Press. [Google Scholar]
- Bermudez JG, Chen H, Einstein LC, & Good MC (2017). Probing the biology of cell boundary conditions through confinement of Xenopus cell-free cytoplasmic extracts. Genesis, 55(1–2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Weber SC, Vaidya N, Haataja M, & Brangwynne CP (2015). RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A, 112(38), E5237–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevis BJ, Hammond AT, Reinke CA, & Glick BS (2002). De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol, 4(10), 750–756. [DOI] [PubMed] [Google Scholar]
- Bhave M, Papanikou E, Iyer P, Pandya K, Jain BK, Ganguly A, … Bhattacharyya D (2014). Golgi enlargement in Arf-depleted yeast cells is due to altered dynamics of cisternal maturation. J Cell Sci, 127(Pt 1), 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AW, & Hyman AA (2008). Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J Cell Biol, 182(2), 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black VH (1972). The development of smooth-surfaced endoplasmic reticulum in adrenal cortical cells of fetal guinea pigs. Am J Anat, 135(3), 381–417. [DOI] [PubMed] [Google Scholar]
- Blythe SA, & Wieschaus EF (2015). Coordinating Cell Cycle Remodeling with Transcriptional Activation at the Drosophila MBT. Curr Top Dev Biol, 113, 113–148. [DOI] [PubMed] [Google Scholar]
- Bone CR, Chang YT, Cain NE, Murphy SP, & Starr DA (2016). Nuclei migrate through constricted spaces using microtubule motors and actin networks in C. elegans hypodermal cells. Development, 143(22), 4193–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DG, Beckett AJ, Molina O, Samejima I, Masumoto H, Kouprina N, … Earnshaw WC (2016). 3D-CLEM Reveals that a Major Portion of Mitotic Chromosomes Is Not Chromatin. Mol Cell, 64(4), 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsos M, Perricone SM, Schauer T, Pontabry J, de Luca KL, de Vries SS, … Kind J (2019). Genome-lamina interactions are established de novo in the early mouse embryo. Nature, 569(7758), 729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothma JP, Norstad MR, Alamos S, & Garcia HG (2018). LlamaTags: A Versatile Tool to Image Transcription Factor Dynamics in Live Embryos. Cell, 173(7), 1810–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau V, Chen R, Edwards A, Sulaimain M, & Maddox PS (2019). PP2A-B55/SUR-6 collaborates with the nuclear lamina for centrosome separation during mitotic entry. Mol Biol Cell, 30(7), 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Papagiannouli F, Wagner N, Wilsch-Brauninger M, Braun M, Furlong EE, … Grosshans J (2006). Developmental control of nuclear size and shape by Kugelkern and Kurzkern. Curr Biol, 16(6), 543–552. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP (2013). Phase transitions and size scaling of membrane-less organelles. J Cell Biol, 203(6), 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, & Hyman AA (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A, 108(11), 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KS, Blower MD, Maresca TJ, Grammer TC, Harland RM, & Heald R (2007). Xenopus tropicalis egg extracts provide insight into scaling of the mitotic spindle. J Cell Biol, 176(6), 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee C, & Heald R (2018). The Incredible Shrinking Spindle. Dev Cell, 45(4), 421–423. [DOI] [PubMed] [Google Scholar]
- Brownlee C, & Heald R (2019). Importin alpha Partitioning to the Plasma Membrane Regulates Intracellular Scaling. Cell, 176(4), 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalter A, & Hetzer MW (2017). Nucleolar expansion and elevated protein translation in premature aging. Nat Commun, 8(1), 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalter A, Kaneshiro JM, & Hetzer MW (2019). Coaching from the sidelines: the nuclear periphery in genome regulation. Nat Rev Genet, 20(1), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CE, Moore RE, Reade A, Goldberg AR, Weiner OD, & Clarke JDW (2016). Reversible Optogenetic Control of Subcellular Protein Localization in a Live Vertebrate Embryo. Dev Cell, 36(1), 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, O’Connor MN, Seabra MC, Cutler DF, & Futter CE (2015). Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J Cell Sci, 128(7), 1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M, & Misteli T (2016). Nongenetic functions of the genome. Science, 352(6286), aad6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadart C, Zlotek-Zlotkiewicz E, Le Berre M, Piel M, & Matthews HK (2014). Exploring the Function of Cell Shape and Size during Mitosis. Dev Cell, 29(2), 159–169. [DOI] [PubMed] [Google Scholar]
- Cantwell H, & Nurse P (2019). A systematic genetic screen identifies essential factors involved in nuclear size control. PLoS Genet, 15(2), e1007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, & Mattaj IW (1999). Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature, 400(6740), 178–181. [DOI] [PubMed] [Google Scholar]
- Cardozo Gizzi AM, Cattoni DI, Fiche JB, Espinola SM, Gurgo J, Messina O, … Nollmann M (2019). Microscopy-Based Chromosome Conformation Capture Enables Simultaneous Visualization of Genome Organization and Transcription in Intact Organisms. Mol Cell, 74(1), 212–222. [DOI] [PubMed] [Google Scholar]
- Casas-Vila N, Bluhm A, Sayols S, Dinges N, Dejung M, Altenhein T, … Butter F (2017). The developmental proteome of Drosophila melanogaster. Genome Res, 27(7), 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BR, Shang F, Chang ML, Clement TM, Eddy EM, Wagner BD, … Taylor A (2014). Nuclear removal during terminal lens fiber cell differentiation requires CDK1 activity: appropriating mitosis-related nuclear disassembly. Development, 141(17), 3388–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Tang Y, Miao L, Darwich-Codore H, Vejnar CE, Beaudoin JD, … Giraldez AJ (2019). Brd4 and P300 Confer Transcriptional Competency during Zygotic Genome Activation. Dev Cell, 49(6), 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YH, & Marshall WF (2014). Organelle size scaling of the budding yeast vacuole is tuned by membrane trafficking rates. Biophys J, 106(9), 1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YH, Reyes L, Sohail SM, Tran NK, & Marshall WF (2016). Organelle Size Scaling of the Budding Yeast Vacuole by Relative Growth and Inheritance. Curr Biol, 26(9), 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Einstein LC, Little SC, & Good MC (2019). Spatiotemporal Patterning of Zygotic Genome Activation in a Model Vertebrate Embryo. Dev Cell, 49(6), 852–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zheng X, & Zheng Y (2014). Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell, 159(4), 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, & Levy DL (2018). Nucleus Assembly and Import in Xenopus laevis Egg Extract. Cold Spring Harb Protoc, doi: 10.1101/pdb.prot097196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Tomschik M, Nelson KM, Oakey J, Gatlin JC, & Levy DL (2019). Nucleoplasmin is a limiting component in the scaling of nuclear size with cytoplasmic volume. J Cell Biol, 218(12), 4063–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Wang Y, Zhang L, Brinkman EK, Adam SA, … Belmont AS (2018). Mapping 3D genome organization relative to nuclear compartments using TSA-Seq as a cytological ruler. J Cell Biol, 217(11), 4025–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Irianto J, & Discher DE (2017). Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol, 216(2), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, … Discher DE (2019). Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev Cell, 49(6), 920–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KH, Factor RE, & Ullman KS (2012). The nuclear envelope environment and its cancer connections. Nat Rev Cancer, 12(3), 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, … Deisseroth K (2013). Structural and molecular interrogation of intact biological systems. Nature, 497(7449), 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay L, Caudron F, Denoth-Lippuner A, Boettcher B, Buvelot Frei S, Snapp EL, & Barral Y (2014). A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. eLife, 3, e01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin A, Singaravelu P, Thery M, Blanchoin L, & Gueroui Z (2018). Actin-Network Architecture Regulates Microtubule Dynamics. Curr Biol, 28(16), 2647–2656. [DOI] [PubMed] [Google Scholar]
- Conklin E (1912). Cell size and nuclear size. J. Exp. Embryol, 12, 1–98. [Google Scholar]
- Courjaret R, Hodeify R, Hubrack S, Ibrahim A, Dib M, Daas S, & Machaca K (2016). The Ca2+-activated Cl- channel Ano1 controls microvilli length and membrane surface area in the oocyte. J Cell Sci, 129(13), 2548–2558. [DOI] [PubMed] [Google Scholar]
- Courtois A, Schuh M, Ellenberg J, & Hiiragi T (2012). The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J Cell Biol, 198(3), 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, … Pellman D (2012). DNA breaks and chromosome pulverization from errors in mitosis. Nature, 482(7383), 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder ME, Strzelecka M, Wilbur JD, Good MC, von Dassow G, & Heald R (2015). A comparative analysis of spindle morphometrics across metazoans. Curr Biol, 25(11), 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino M, Risselada HJ, Endter LJ, Comte-Miserez V, & Mayer A (2018). SNARE-mediated membrane fusion arrests at pore expansion to regulate the volume of an organelle. Embo J, 37(19), pii: e99193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro M, Hnia K, Gache V, Koch C, Gavriilidis C, Rodriguez D, … Laporte J (2015). Amphiphysin 2 Orchestrates Nucleus Positioning and Shape by Linking the Nuclear Envelope to the Actin and Microtubule Cytoskeleton. Dev Cell, 35(2), 186–198. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, & Hetzer MW (2009). Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell, 136(2), 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambournet D, Sochacki KA, Cheng AT, Akamatsu M, Taraska JW, Hockemeyer D, & Drubin DG (2018). Genome-edited human stem cells expressing fluorescently labeled endocytic markers allow quantitative analysis of clathrin-mediated endocytosis during differentiation. J Cell Biol, 217(9), 3301–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M, Jaensch S, Pozniakovsky A, Zinke A, O’Connell KF, Zachariae W, … Hyman AA (2011). Limiting amounts of centrosome material set centrosome size in C. elegans embryos. Curr Biol, 21(15), 1259–1267. [DOI] [PubMed] [Google Scholar]
- Deglincerti A, Etoc F, Guerra MC, Martyn I, Metzger J, Ruzo A, … Warmflash A (2016). Self-organization of human embryonic stem cells on micropatterns. Nat Protoc, 11(11), 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Brittingham GP, Pfeffer S, Surovtsev IV, Pinglay S, Kennedy KJ, … Holt LJ (2018). mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell, 174(2), 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, … Lammerding J (2016). Nuclear envelope rupture and repair during cancer cell migration. Science, 352(6283), 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desfougeres Y, Neumann H, & Mayer A (2016). Organelle size control - increasing vacuole content activates SNAREs to augment organelle volume through homotypic fusion. J Cell Sci, 129(14), 2817–2828. [DOI] [PubMed] [Google Scholar]
- Dinarina A, Pugieux C, Corral MM, Loose M, Spatz J, Karsenti E, & Nedelec F (2009). Chromatin shapes the mitotic spindle. Cell, 138(3), 502–513. [DOI] [PubMed] [Google Scholar]
- Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, … Field SJ (2009). GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell, 139(2), 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer TA, & Misteli T (2011). The lamin protein family. Genome Biol, 12(5), 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynska A, Gonzalo S, Shanahan C, & Askjaer P (2016). The nuclear lamina in health and disease. Nucleus, 7(3), 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundon SE, Chang SS, Kumar A, Occhipinti P, Shroff H, Roper M, & Gladfelter AS (2016). Clustered nuclei maintain autonomy and nucleocytoplasmic ratio control in a syncytium. Mol Biol Cell, 27(13), 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens LJ, Dilsaver MR, & Levy DL (2017). PKC-mediated phosphorylation of nuclear lamins at a single serine residue regulates interphase nuclear size in Xenopus and mammalian cells. Mol Biol Cell, 28(10), 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens LJ, & Levy DL (2014). cPKC regulates interphase nuclear size during Xenopus development. J Cell Biol, 206(4), 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, … Roca-Cusachs P (2017). Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell, 171(6), 1397–1410. [DOI] [PubMed] [Google Scholar]
- Eritano AS, Altamirano A, Beyeler S, Gaytan N, Velasquez M, & Riggs B (2017). The endoplasmic reticulum is partitioned asymmetrically during mitosis before cell fate selection in proneuronal cells in the early Drosophila embryo. Mol Biol Cell, 28(11), 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahati H, Pelham-Webb B, Blythe S, & Wieschaus E (2016). Nucleation by rRNA Dictates the Precision of Nucleolus Assembly. Curr Biol, 26(3), 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser G (1939). Polyploidy in the salamander, Eurycea bislineata. J Hered, 30, 379–388. [Google Scholar]
- Fankhauser G (1945a). The effects of changes in chromosome number on amphibian development. The Quarterly Review of Biology, 20(1), 20–78. [Google Scholar]
- Fankhauser G (1945b). Maintenance of normal structure in heteroploid salamander larvae, through compensation of changes in cell size by adjustment of cell number and cell shape. J Exp Zool, 100, 445–455. [DOI] [PubMed] [Google Scholar]
- Fankhauser G, & Watson RC (1942). Heat-Induced Triploidy in the Newt, Triturus Viridescens. Proc Natl Acad Sci U S A, 28(10), 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadifar R, Baer CF, Valfort AC, Andersen EC, Muller-Reichert T, Delattre M, & Needleman DJ (2015). Scaling, selection, and evolutionary dynamics of the mitotic spindle. Curr Biol, 25(6), 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, & Brangwynne CP (2013). A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat Cell Biol, 15(10), 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, … Brangwynne CP (2016). Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell, 165(7), 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes I, & Schock F (2014). The nebulin repeat protein Lasp regulates I-band architecture and filament spacing in myofibrils. J Cell Biol, 206(4), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Kriston-Vizi J, Metcalf DJ, Martin-Martin B, Freeman J, Burden JJ, … Cutler DF (2014). A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev Cell, 29(3), 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickentscher R, & Weiss M (2017). Physical determinants of asymmetric cell divisions in the early development of Caenorhabditis elegans. Sci Rep, 7(1), 9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, & Mitchison TJ (2018). Assembly of Spindles and Asters in Xenopus Egg Extracts. Cold Spring Harb Protoc, 2018(6). doi: 10.1101/pdb.prot099796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint Brodsly N, Bitman-Lotan E, Boico O, Shafat A, Monastirioti M, Gessler M, … Orian A (2019). The transcription factor Hey and nuclear lamins specify and maintain cell identity. eLife, 8, pii: e44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyamer IM, Gassler J, Imakaev M, Brandao HB, Ulianov SV, Abdennur N, … Tachibana-Konwalski K (2017). Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature, 544(7648), 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca CL, Malaby HLH, Sepaniac LA, Martin W, Byers C, Czechanski A, … Stumpff J (2019). Mitotic chromosome alignment ensures mitotic fidelity by promoting interchromosomal compaction during anaphase. J Cell Biol, 218(4), 1148–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, & Voeltz GK (2011). The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol, 21, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Bardai FH, & Feany MB (2016). Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol, 26(1), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta BJ, Jacobs KC, Fagerstrom CJ, & Rusan NM (2016). Asterless is required for centriole length control and sperm development. J Cell Biol, 213(4), 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic I, Boswell SA, & Mitchison TJ (2019). Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues. PLoS Biol, 17(4), e3000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Seelbinder B, Henderson JT, Watts RD, Scott AK, Veress AI, & Neu CP (2019). Deformation Microscopy for Dynamic Intracellular and Intranuclear Mapping of Mechanics with High Spatiotemporal Resolution. Cell Rep, 27(5), 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaux R, Acker R, Kitaoka M, Georgiou G, van Kruijsbergen I, Ford B, … Heald R (2018). Paternal chromosome loss and metabolic crisis contribute to hybrid inviability in Xenopus. Nature, 553(7688), 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, & Hyman AA (2012). Organelle growth control through limiting pools of cytoplasmic components. Curr Biol, 22(9), R330–339. [DOI] [PubMed] [Google Scholar]
- Goldstein B, & King N (2016). The Future of Cell Biology: Emerging Model Organisms. Trends Cell Biol, 26(11), 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MC, Vahey MD, Skandarajah A, Fletcher DA, & Heald R (2013). Cytoplasmic Volume Modulates Spindle Size During Embryogenesis. Science (New York, NY), 342(6160), 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopfrich K, Platzman I, & Spatz JP (2018). Mastering Complexity: Towards Bottom-up Construction of Multifunctional Eukaryotic Synthetic Cells. Trends Biotechnol, 36(9), 938–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, & Nystrom T (2017). The Upsides and Downsides of Organelle Interconnectivity. Cell, 169(1), 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal U, & Blackstone C (2013). Untangling the web: mechanisms underlying ER network formation. Biochim Biophys Acta, 1833(11), 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenan G, Brangwynne CP, Jaensch S, Gharakhani J, Julicher F, & Hyman AA (2010). Centrosome size sets mitotic spindle length in Caenorhabditis elegans embryos. Curr Biol, 20(4), 353–358. [DOI] [PubMed] [Google Scholar]
- Guan Y, Li Z, Wang S, Barnes PM, Liu X, Xu H, … Yang Q (2018). A robust and tunable mitotic oscillator in artificial cells. eLife, 7, e33549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Li D, Zhang S, Yang Y, Liu JJ, Wang X, … Li D (2018). Visualizing Intracellular Organelle and Cytoskeletal Interactions at Nanoscale Resolution on Millisecond Timescales. Cell, 175(5), 1430–1442. [DOI] [PubMed] [Google Scholar]
- Haider A, Wei YC, Lim K, Barbosa AD, Liu CH, Weber U, … Savage DB (2018). PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress. Dev Cell, 45(4), 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B, Mackmull MT, Machado P, Ronchi P, Bui KH, Schieber N, … Beck M (2016). Pre-assembled Nuclear Pores Insert into the Nuclear Envelope during Early Development. Cell, 166(3), 664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Iwabuchi M, Ohsumi K, & Kimura A (2013). Intranuclear DNA density affects chromosome condensation in metazoans. Mol Biol Cell, 24(15), 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, & Kimura A (2009). Cell-size-dependent spindle elongation in the Caenorhabditis elegans early embryo. Curr Biol, 19(18), 1549–1554. [DOI] [PubMed] [Google Scholar]
- Hara Y, & Kimura A (2013). An allometric relationship between mitotic spindle width, spindle length, and ploidy in Caenorhabditis elegans embryos. Mol Biol Cell, 24(9), 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, & Merten CA (2015). Dynein-Based Accumulation of Membranes Regulates Nuclear Expansion in Xenopus laevis Egg Extracts. Dev Cell, 33(5), 562–575. [DOI] [PubMed] [Google Scholar]
- Hardwick LJ, & Philpott A (2015). An oncologists friend: How Xenopus contributes to cancer research. Dev Biol, 408(2), 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MM, Jenkins BV, O’Connor-Giles KM, & Wildonger J (2014). A CRISPR view of development. Genes Dev, 28(17), 1859–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasley A, Chavez S, Danilchik M, Wuhr M, & Pelegri F (2017). Vertebrate Embryonic Cleavage Pattern Determination. Adv Exp Med Biol, 953, 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EM, Fischer AH, Deerinck TJ, & Hetzer MW (2013). Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell, 154(1), 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel J, Krutkramelis K, Mooney P, Tomschik M, Gerow K, Oakey J, & Gatlin JC (2013). Changes in Cytoplasmic Volume Are Sufficient to Drive Spindle Scaling. Science (New York, NY), 342(6160), 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, & Gibeaux R (2018). Subcellular scaling: does size matter for cell division? Curr Opin Cell Biol, 52, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke KJ, & Heald R (2014). TPX2 levels modulate meiotic spindle size and architecture in Xenopus egg extracts. J Cell Biol, 206(3), 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel NL, Thomson M, & Marshall WF (2018). Diffusion as a Ruler: Modeling Kinesin Diffusion as a Length Sensor for Intraflagellar Transport. Biophys J, 114(3), 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SJ, Driscoll TP, Thorpe SD, Nerurkar NL, Baker BM, Yang MT, … Mauck RL (2016). Differentiation alters stem cell nuclear architecture, mechanics, and mechano-sensitivity. eLife, 5, pii: 18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton LK, Gunawardane K, Kim JW, Schwarz MC, & Quarmby LM (2013). The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr Biol, 23(22), 2208–2214. [DOI] [PubMed] [Google Scholar]
- Hug CB, Grimaldi AG, Kruse K, & Vaquerizas JM (2017). Chromatin Architecture Emerges during Zygotic Genome Activation Independent of Transcription. Cell, 169(2), 216–228. [DOI] [PubMed] [Google Scholar]
- Iampietro C, Bergalet J, Wang X, Cody NA, Chin A, Lefebvre FA, … Lecuyer E (2014). Developmentally regulated elimination of damaged nuclei involves a Chk2-dependent mechanism of mRNA nuclear retention. Dev Cell, 29(4), 468–481. [DOI] [PubMed] [Google Scholar]
- Inoue D, Obino D, Pineau J, Farina F, Gaillard J, Guerin C, … Thery M (2019). Actin filaments regulate microtubule growth at the centrosome. Embo J, 38(11), pii: 99630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Nguyen PA, Wuhr M, Groen AC, Field CM, & Mitchison TJ (2014). Organization of early frog embryos by chemical waves emanating from centrosomes. Philos Trans R Soc Lond B Biol Sci, 369(1650), pii: 20130454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, & Marshall WF (2017). Testing the time-of-flight model for flagellar length sensing. Mol Biol Cell, 28(23), 3447–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Mori C, Kojidani T, Bunai F, Hori T, Fukagawa T, … Haraguchi T (2009). Two distinct repeat sequences of Nup98 nucleoporins characterize dual nuclei in the binucleated ciliate tetrahymena. Curr Biol, 19(10), 843–847. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Osakada H, Mori C, Fukuda Y, Nagao K, Obuse C, … Haraguchi T (2017). Compositionally distinct nuclear pore complexes of functionally distinct dimorphic nuclei in the ciliate Tetrahymena. J Cell Sci, 130(10), 1822–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajoo R, Jung Y, Huh D, Viana MP, Rafelski SM, Springer M, & Paulsson J (2016). Accurate concentration control of mitochondria and nucleoids. Science, 351(6269), 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayo A, Malboubi M, Antoku S, Chang W, Ortiz-Zapater E, Groen C, … Parsons M (2016). Fascin Regulates Nuclear Movement and Deformation in Migrating Cells. Dev Cell, 38(4), 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins H, Holman T, Lyon C, Lane B, Stick R, & Hutchison C (1993). Nuclei that lack a lamina accumulate karyophilic proteins and assemble a nuclear matrix. J Cell Sci, 106 (Pt 1), 275–285. [DOI] [PubMed] [Google Scholar]
- Jetta D, Gottlieb PA, Verma D, Sachs F, & Hua SZ (2019). Shear stress-induced nuclear shrinkage through activation of Piezo1 channels in epithelial cells. J Cell Sci, 132(11), pii: jcs226076. [DOI] [PubMed] [Google Scholar]
- Jevtic P, Edens LJ, Li X, Nguyen T, Chen P, & Levy DL (2015). Concentration-dependent Effects of Nuclear Lamins on Nuclear Size in Xenopus and Mammalian Cells. J Biol Chem, 290(46), 27557–27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, & Levy DL (2014). Mechanisms of nuclear size regulation in model systems and cancer. Adv Exp Med Biol, 773, 537–569. [DOI] [PubMed] [Google Scholar]
- Jevtic P, & Levy DL (2015). Nuclear size scaling during Xenopus early development contributes to midblastula transition timing. Curr Biol, 25(1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, & Levy DL (2017). Both Nuclear Size and DNA Amount Contribute to Midblastula Transition Timing in Xenopus laevis. Sci Rep, 7(1), 7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Mukherjee RN, Chen P, & Levy DL (2019). Altering the levels of nuclear import factors in early Xenopus laevis embryos affects later development. PLoS One, 14(4), e0215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Schibler AC, Wesley CC, Pegoraro G, Misteli T, & Levy DL (2019). The nucleoporin ELYS regulates nuclear size by controlling NPC number and nuclear import capacity. EMBO Rep, 20(6), e47283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H (2015). Cell size modulates oscillation, positioning and length of mitotic spindles. Sci Rep, 5, 10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, & Zheng Y (2015). Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell, 163(1), 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KM, Forer A, Yao C, Girton J, & Johansen J (2011). Do nuclear envelope and intranuclear proteins reorganize during mitosis to form an elastic, hydrogel-like spindle matrix? Chromosome Res, 19(3), 345–365. [DOI] [PubMed] [Google Scholar]
- Joseph SR, Palfy M, Hilbert L, Kumar M, Karschau J, Zaburdaev V, … Vastenhouw NL (2017). Competition between histone and transcription factor binding regulates the onset of transcription in zebrafish embryos. eLife, 6, pii: e23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AS, Huang X, Choudhary V, Levine TP, Hu J, & Prinz WA (2016). A family of membrane-shaping proteins at ER subdomains regulates pre-peroxisomal vesicle biogenesis. J Cell Biol, 215(4), 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AS, Zhang H, & Prinz WA (2017). Organelle biogenesis in the endoplasmic reticulum. Nat Cell Biol, 19(8), 876–882. [DOI] [PubMed] [Google Scholar]
- Jukam D, Shariati SAM, & Skotheim JM (2017). Zygotic Genome Activation in Vertebrates. Dev Cell, 42(4), 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakebeen A, & Wills A (2019). Advancing genetic and genomic technologies deepen the pool for discovery in Xenopus tropicalis. Dev Dyn, 248(8), 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifenheim D, Sun XM, D’Souza E, Ohira MJ, Magner M, Mayhew MB, … Rhind N (2017). Size-Dependent Expression of the Mitotic Activator Cdc25 Suggests a Mechanism of Size Control in Fission Yeast. Curr Biol, 27(10), 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelpsch DJ, Groen CM, Fagan TN, Sudhir S, & Tootle TL (2016). Fascin regulates nuclear actin during Drosophila oogenesis. Mol Biol Cell, 27(19), 2965–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieserman EK, & Heald R (2011). Mitotic chromosome size scaling in Xenopus. Cell Cycle, 10(22), 3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, & Schilling TF (1995). Stages of embryonic development of the zebrafish. Dev Dyn, 203(3), 253–310. [DOI] [PubMed] [Google Scholar]
- Kita AM, Swider ZT, Erofeev I, Halloran MC, Goryachev AB, & Bement WM (2019). Spindle-F-actin interactions in mitotic spindles in an intact vertebrate epithelium. Mol Biol Cell, 30(14), 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka M, Heald R, & Gibeaux R (2018). Spindle assembly in egg extracts of the Marsabit clawed frog, Xenopus borealis. Cytoskeleton (Hoboken), 75(6), 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotadia S, Crest J, Tram U, Riggs B, & Sullivan W (2010). Blastoderm Formation and Cellularisation in Drosophila melanogaster Encyclopedia of Life Sciences (ELS). Chichester: John Wiley & Sons, Ltd. [Google Scholar]
- Kumar D, Golchoubian B, Belevich I, Jokitalo E, & Schlaitz AL (2019). REEP3 and REEP4 determine the tubular morphology of the endoplasmic reticulum during mitosis. Mol Biol Cell, 30(12), 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Cantwell H, Burrell A, & Nurse P (2019). Nuclear membrane protein Lem2 regulates nuclear size through membrane flow. Nat Commun, 10(1), 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Cantwell H, Neumann FR, Jones AW, Snijders AP, & Nurse P (2017). A systematic genomic screen implicates nucleocytoplasmic transport and membrane growth in nuclear size control. PLoS Genet, 13(5), e1006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyogoku H, & Kitajima TS (2017). Large Cytoplasm Is Linked to the Error-Prone Nature of Oocytes. Dev Cell, 41(3), 287–298. [DOI] [PubMed] [Google Scholar]
- Lacroix B, Letort G, Pitayu L, Salle J, Stefanutti M, Maton G, … Dumont J (2018). Microtubule Dynamics Scale with Cell Size to Set Spindle Length and Assembly Timing. Dev Cell, 45(4), 496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur AM, Dorn JF, & Maddox PS (2015). Mitotic chromosome length scales in response to both cell and nuclear size. J Cell Biol, 209(5), 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur AM, Ranjan R, Smith L, Fadero T, Heppert J, Goldstein B, … Maddox PS (2017). CENP-A and topoisomerase-II antagonistically affect chromosome length. J Cell Biol, 216(9), 2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SIR, & Jones KT (2017). Chromosome biorientation and APC activity remain uncoupled in oocytes with reduced volume. J Cell Biol, 216(12), 3949–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ZY, Prouteau M, Gotta M, & Barral Y (2016). Compartmentalization of the endoplasmic reticulum in the early C. elegans embryos. J Cell Biol, 214(6), 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Lee CM, Shugart EC, Benedetti M, Charo RA, Gartner Z, … Wilson KM (2019). Human organoids: a new dimension in cell biology. Mol Biol Cell, 30(10), 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao RM, & Kellogg DR (2017). The duration of mitosis and daughter cell size are modulated by nutrients in budding yeast. J Cell Biol, 216(11), 3463–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele TP, Dickinson RB, & Gundersen GG (2018). Mechanical principles of nuclear shaping and positioning. J Cell Biol, 217(10), 3330–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Michel V, Boutet de Monvel J, Cortese M, Bosch-Grau M, Aghaie A, … Petit C (2016). Class III myosins shape the auditory hair bundles by limiting microvilli and stereocilia growth. J Cell Biol, 212(2), 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL, & Heald R (2010). Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell, 143(2), 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SC, Uchiyama LF, & Nunnari J (2016). ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science, 353(6296), aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, & Jiang H (2017). Geometric Asymmetry Induces Upper Limit of Mitotic Spindle Size. Biophys J, 112(7), 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TL, Upadhyayula S, Milkie DE, Singh V, Wang K, Swinburne IA, … Betzig E (2018). Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science, 360(6386), pii: eaaq1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Fox DT, & Spradling AC (2013). Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol, 23(22), 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ, & Heald R (2011). Katanin contributes to interspecies spindle length scaling in Xenopus. Cell, 147(6), 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Schneider M, Neumann S, Jaeger VM, Taranum S, Munck M, … Karakesisoglou I (2012). Nesprin interchain associations control nuclear size. Cell Mol Life Sci, 69(20), 3493–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena R, Alcaide-Gavilan M, Schubert K, He M, Domnauer MG, Marquer C, … Kellogg DR (2018). Cell Size and Growth Rate Are Modulated by TORC2-Dependent Signals. Curr Biol, 28(2), 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitt MB, Price TS, Pizarro A, Wu W, Yocum AK, Seiler C, … Grosser T (2008). Analysis of the zebrafish proteome during embryonic development. Mol Cell Proteomics, 7(5), 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, & Barral Y (2005). Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol, 169(6), 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Liu Y, & Nassel DR (2013). Insulin/IGF-regulated size scaling of neuroendocrine cells expressing the bHLH transcription factor Dimmed in Drosophila. PLoS Genet, 9(12), e1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadangdang BR, Oberai A, Spektor T, Campos OA, Sheng F, Carey MF, … Kurdistani SK (2014). Evolution of histone 2A for chromatin compaction in eukaryotes. Elife (Cambridge), e02792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Falkowska KA, Li AY, Galanti SE, Kanuru RC, LaMont EG, … Chalker DL (2008). Nucleus-specific importin alpha proteins and nucleoporins regulate protein import and nuclear division in the binucleate Tetrahymena thermophila. Eukaryot Cell, 7(9), 1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley HR, Keightley MC, & Lieschke GJ (2018). The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function. Front Immunol, 9, 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF (2015). How Cells Measure Length on Subcellular Scales. Trends Cell Biol, 25(12), 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF (2016). Cell Geometry: How Cells Count and Measure Size. Annu Rev Biophys, 45, 49–64. [DOI] [PubMed] [Google Scholar]
- Masui M, & Kominami T (2001). Change in the adhesive properties of blastomeres during early cleavage stages in sea urchin embryos. Develop Growth Differ, 43, 43–53. [DOI] [PubMed] [Google Scholar]
- Masui M, Yoneda M, & Kominami T (2001). Nucleus:cell volume ratio directs the timing of the increase in blastomere adhesiveness in starfish embryos. Develop Growth Differ, 43, 295–304. [DOI] [PubMed] [Google Scholar]
- Masui Y, & Wang P (1998). Cell cycle transition in early embryonic development of Xenopus laevis. Biol Cell, 90(8), 537–548. [PubMed] [Google Scholar]
- Meng D, & Pan J (2016). A NIMA-related kinase, CNK4, regulates ciliary stability and length. Mol Biol Cell, 27(5), 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M, Gao Z, Liu J, Wu JC, Malone CJ, & Starr DA (2009). Centrosome attachment to the C. elegans male pronucleus is dependent on the surface area of the nuclear envelope. Dev Biol, 327(2), 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TP, & Bjorklund M (2016). Cellular Allometry of Mitochondrial Functionality Establishes the Optimal Cell Size. Dev Cell, 39(3), 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TP, & Bjorklund M (2017). Mitochondrial Function and Cell Size: An Allometric Relationship. Trends Cell Biol, 27(6), 393–402. [DOI] [PubMed] [Google Scholar]
- Miettinen TP, Pessa HK, Caldez MJ, Fuhrer T, Diril MK, Sauer U, … Bjorklund M (2014). Identification of transcriptional and metabolic programs related to mammalian cell size. Curr Biol, 24(6), 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunovic-Jevtic A, Jevtic P, Levy DL, & Gatlin JC (2018). In vivo mitotic spindle scaling can be modulated by changing the levels of a single protein: the microtubule polymerase XMAP215. Mol Biol Cell, 29(11), 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc N, & Piel M (2012). Predicting division plane position and orientation. Trends Cell Biol, 22(4), 193–200. [DOI] [PubMed] [Google Scholar]
- Mitchison T, & Kirschner M (1984). Dynamic instability of microtubule growth. Nature, 312(5991), 237–242. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ (2019). Colloid osmotic parameterization and measurement of subcellular crowding. Mol Biol Cell, 30(2), 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, & Nakatogawa H (2015). Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature, 522(7556), 359–362. [DOI] [PubMed] [Google Scholar]
- Mogessie B, & Schuh M (2017). Actin protects mammalian eggs against chromosome segregation errors. Science, 357(6353), pii: eaal1647. [DOI] [PubMed] [Google Scholar]
- Moore DL, Pilz GA, Arauzo-Bravo MJ, Barral Y, & Jessberger S (2015). A mechanism for the segregation of age in mammalian neural stem cells. Science, 349(6254), 1334–1338. [DOI] [PubMed] [Google Scholar]
- Morgani SM, Metzger JJ, Nichols J, Siggia ED, & Hadjantonakis AK (2018). Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. eLife, 7, pii: 32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller RL (2015). Genome Biology and the Evolution of Cell-Size Diversity. Cold Spring Harb Perspect Biol, 7(11). doi: 10.1101/cshperspect.a019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller RL, Gregory TR, Gregory SM, Hsieh A, & Boore JL (2008). Genome size, cell size, and the evolution of enucleated erythrocytes in attenuate salamanders. Zoology (Jena), 111(3), 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee RN, & Levy DL (2019). Reticulon 4a promotes exocytosis in mammalian cells. Mol Biol Cell, 30(18), 2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji S, & O’Shea EK (2014). Mechanisms of organelle biogenesis govern stochastic fluctuations in organelle abundance. eLife, 3, e02678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu B, Chen HM, Moresco JJ, Orelo BD, Yang B, Gaspar JM, … Mango SE (2018). Regulated nuclear accumulation of a histone methyltransferase times the onset of heterochromatin formation in C. elegans embryos. Sci Adv, 4(8), eaat6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjundappa R, Kong D, Shim K, Stearns T, Brody SL, Loncarek J, & Mahjoub MR (2019). Regulation of cilia abundance in multiciliated cells. eLife, 8, e44039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann FR, & Nurse P (2007). Nuclear size control in fission yeast. J Cell Biol, 179(4), 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr GE, Terry RL, Lengefeld J, Bonney M, Brittingham GP, Moretto F, … Amon A (2019). Excessive Cell Growth Causes Cytoplasm Dilution And Contributes to Senescence. Cell, 176(5), 1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport JW, Wilson KL, & Dunphy WG (1990). A lamin-independent pathway for nuclear envelope assembly. J Cell Biol, 111(6 Pt 1), 2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niopek D, Wehler P, Roensch J, Eils R, & Di Ventura B (2016). Optogenetic control of nuclear protein export. Nat Commun, 7, 10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura YV, Shikanai M, Hoshino M, Ohshima T, Nabeshima Y, Mizutani K, … Kawauchi T (2014). Cdk5 and its substrates, Dcx and p27kip1, regulate cytoplasmic dilation formation and nuclear elongation in migrating neurons. Development, 141(18), 3540–3550. [DOI] [PubMed] [Google Scholar]
- Novakova L, Kovacovicova K, Dang-Nguyen TQ, Sodek M, Skultety M, & Anger M (2016). A Balance between Nuclear and Cytoplasmic Volumes Controls Spindle Length. PLoS One, 11(2), e0149535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakey J, & Gatlin JC (2018). Microfluidic Encapsulation of Demembranated Sperm Nuclei in Xenopus Egg Extracts. Cold Spring Harb Protoc. doi: 10.1101/pdb.prot102913. [DOI] [PubMed] [Google Scholar]
- Oda H, Shirai N, Ura N, Ohsumi K, & Iwabuchi M (2017). Chromatin tethering to the nuclear envelope by nuclear actin filaments: a novel role of the actin cytoskeleton in the Xenopus blastula. Genes Cells, 22(4), 376–391. [DOI] [PubMed] [Google Scholar]
- Oda T, Yanagisawa H, Kamiya R, & Kikkawa M (2014). A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science, 346(6211), 857–860. [DOI] [PubMed] [Google Scholar]
- Orhon I, Dupont N, Zaidan M, Boitez V, Burtin M, Schmitt A, … Codogno P (2016). Primary-cilium-dependent autophagy controls epithelial cell volume in response to fluid flow. Nat Cell Biol, 18(6), 657–667. [DOI] [PubMed] [Google Scholar]
- Osmanagic-Myers S, & Foisner R (2019). The structural and gene expression hypotheses in laminopathic diseases-not so different after all. Mol Biol Cell, 30(15), 1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, & O’Shea CC (2017). ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science, 357(6349), pii: eaag0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan KZ, Saunders TE, Flor-Parra I, Howard M, & Chang F (2014). Cortical regulation of cell size by a sizer cdr2p. eLife, 3, e02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JO, Rees P, & Nurse P (2019). Noisy Cell-Size-Correlated Expression of Cyclin B Drives Probabilistic Cell-Size Homeostasis in Fission Yeast. Curr Biol, 29(8), 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Gonzalez N, Tao J, Rochman ND, Vig D, Chiu E, Wirtz D, & Sun SX (2018). Cell tension and mechanical regulation of cell volume. Mol Biol Cell, 29(21), mbc.E18-04-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino M, & Veenstra GJ (2016). Chromatin Control of Developmental Dynamics and Plasticity. Dev Cell, 38(6), 610–620. [DOI] [PubMed] [Google Scholar]
- Peshkin L, Wuhr M, Pearl E, Haas W, Freeman RM Jr., Gerhart JC, … Kirschner MW (2015). On the Relationship of Protein and mRNA Dynamics in Vertebrate Embryonic Development. Dev Cell, 35(3), 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CR, Xia Y, Zhu K, Liu D, Irianto J, Garcia VMM, … Discher DE (2018). Constricted migration increases DNA damage and independently represses cell cycle. Mol Biol Cell, 29(16), 1948–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TT, Monnard A, Helenius J, Lund E, Lee N, Muller DJ, & Cabernard C (2019). Spatiotemporally Controlled Myosin Relocalization and Internal Pressure Generate Sibling Cell Size Asymmetry. iScience, 13, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre A, Salle J, Wuhr M, & Minc N (2016). Generic Theoretical Models to Predict Division Patterns of Cleaving Embryos. Dev Cell, 39(6), 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina FJ, Fleming T, Pogliano K, & Niwa M (2016). Reticulons Regulate the ER Inheritance Block during ER Stress. Dev Cell, 37(3), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony JW, England JM, & Attardi G (1977). Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol, 74(2), 468–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RE, Wang S, Liu TY, & Rapoport TA (2017). Reconstitution of the tubular endoplasmic reticulum network with purified components. Nature, 543(7644), 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, … Piel M (2016). ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science, 352(6283), 359–362. [DOI] [PubMed] [Google Scholar]
- Rafelski SM, Viana MP, Zhang Y, Chan YH, Thorn KS, Yam P, … Marshall WF (2012). Mitochondrial network size scaling in budding yeast. Science, 338(6108), 822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Munzig M, Kaneshiro K, Lee B, Strome S, Muller-Reichert T, & Cohen-Fix O (2015). C. elegans polo-like kinase PLK-1 is required for merging parental genomes into a single nucleus. Mol Biol Cell. 26(25), 4718–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D, Ward C, Potter C, Zhu W, Xue Y, Kefalov VJ, … Hodzic D (2016). Lamin B1 and lamin B2 are long-lived proteins with distinct functions in retinal development. Mol Biol Cell, 27(12), 1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SB, Baumgart J, Widlund PO, Pozniakovsky A, Howard J, Hyman AA, & Julicher F (2013). XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat Cell Biol, 15(9), 1116–1122. [DOI] [PubMed] [Google Scholar]
- Reisser M, Palmer A, Popp AP, Jahn C, Weidinger G, & Gebhardt JCM (2018). Single-molecule imaging correlates decreasing nuclear volume with increasing TF-chromatin associations during zebrafish development. Nat Commun, 9(1), 5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhoff EM, Ishihara K, & Brugues J (2019). How to tune spindle size relative to cell size? Curr Opin Cell Biol, 60, 139–144. [DOI] [PubMed] [Google Scholar]
- Robinson DO, Coate JE, Singh A, Hong L, Bush M, Doyle JJ, & Roeder AHK (2018). Ploidy and Size at Multiple Scales in the Arabidopsis Sepal. Plant Cell, 30(10), 2308–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanauska A, & Kohler A (2018). The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell, 174(3), 700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, & Walkowiak W (2015). The Influence of Genome and Cell Size on Brain Morphology in Amphibians. Cold Spring Harb Perspect Biol, 7(9), a019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Fulcher LJ, & Sapkota GP (2019). Advances in targeted degradation of endogenous proteins. Cell Mol Life Sci, 76(14), 2761–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, … Lammerding J (2013). Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem, 288(12), 8610–8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffi GT, & Botelho RJ (2019). Lysosome Fission: Planning for an Exit. Trends Cell Biol, 29(8), 635–646. [DOI] [PubMed] [Google Scholar]
- Samwer M, Schneider MWG, Hoefler R, Schmalhorst PS, Jude JG, Zuber J, & Gerlich DW (2017). DNA Cross-Bridging Shapes a Single Nucleus from a Set of Mitotic Chromosomes. Cell, 170(5), 956–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sater AK, & Moody SA (2017). Using Xenopus to understand human disease and developmental disorders. Genesis, 55(1–2), doi: 10.1002/dvg.22997. [DOI] [PubMed] [Google Scholar]
- Schlaitz AL, Thompson J, Wong CC, Yates JR 3rd, & Heald R (2013). REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Dev Cell, 26(3), 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoller KM, & Skotheim JM (2015). The Biosynthetic Basis of Cell Size Control. Trends Cell Biol, 25(12), 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert I, & Oud JL (1997). There is an upper limit of chromosome size for normal development of an organism. Cell, 88(4), 515–520. [DOI] [PubMed] [Google Scholar]
- Schuck S (2016). On keeping the right ER size. Nat Cell Biol, 18(11), 1118–1119. [DOI] [PubMed] [Google Scholar]
- Schuck S, Gallagher CM, & Walter P (2014). ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J Cell Sci, 127(Pt 18), 4078–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Prinz WA, Thorn KS, Voss C, & Walter P (2009). Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol, 187(4), 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KN, & Harrison MM (2019). Mechanisms regulating zygotic genome activation. Nat Rev Genet, 20(4), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seller CA, Cho CY, & O’Farrell PH (2019). Rapid embryonic cell cycles defer the establishment of heterochromatin by Eggless/SetDB1 in Drosophila. Genes Dev, 33(7–8), 403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D, & Linstedt AD (2011). Control of organelle size: the Golgi complex. Annu Rev Cell Dev Biol, 27, 57–77. [DOI] [PubMed] [Google Scholar]
- Shen X, Yu L, Weir JW, & Gorovsky MA (1995). Linker histones are not essential and affect chromatin condensation in vivo. Cell, 82(1), 47–56. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, & Rapoport TA (2006). Rough sheets and smooth tubules. Cell, 126(3), 435–439. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y, Tamura S, Masumoto H, & Maeshima K (2017). Nucleosomenucleosome interactions via histone tails and linker DNA regulate nuclear rigidity. Mol Biol Cell, 28(11), 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, & Brangwynne CP (2017). Liquid phase condensation in cell physiology and disease. Science, 357(6357), pii: eaaf4382. [DOI] [PubMed] [Google Scholar]
- Shindo Y, & Amodeo AA (2019). Dynamics of Free and Chromatin-Bound Histone H3 during Early Embryogenesis. Curr Biol, 29(2), 359–366. [DOI] [PubMed] [Google Scholar]
- Shintomi K, & Hirano T (2011). The relative ratio of condensin I to II determines chromosome shapes. Genes Dev, 25(14), 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Irianto J, Xia Y, Pfeifer CR, & Discher DE (2019). Constricted migration modulates stem cell differentiation. Mol Biol Cell, 30(16), 1985–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So C, Seres KB, Steyer AM, Monnich E, Clift D, Pejkovska A, … Schuh M (2019). A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science, 364(6447), pii: eaat9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen KF, & Merten CA (2019). Microfluidics as an Emerging Precision Tool in Developmental Biology. Dev Cell, 48(3), 293–311. [DOI] [PubMed] [Google Scholar]
- Spencer AK, Schaumberg AJ, & Zallen JA (2017). Scaling of cytoskeletal organization with cell size in Drosophila. Mol Biol Cell, 28(11), 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng B, Fleckenstein H, Kubler P, Di Biagio C, Benz M, Patra P, … Frischknecht F (2019). Microtubule number and length determine cellular shape and function in Plasmodium. Embo J, 38(15), e100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadhouders R, Filion GJ, & Graf T (2019). Transcription factors and 3D genome conformation in cell-fate decisions. Nature, 569(7756), 345–354. [DOI] [PubMed] [Google Scholar]
- Stegmaier J, Amat F, Lemon WC, McDole K, Wan Y, Teodoro G, … Keller PJ (2016). Real-Time Three-Dimensional Cell Segmentation in Large-Scale Microscopy Data of Developing Embryos. Dev Cell, 36(2), 225–240. [DOI] [PubMed] [Google Scholar]
- Stephens AD, Banigan EJ, & Marko JF (2019). Chromatin’s physical properties shape the nucleus and its functions. Curr Opin Cell Biol, 58, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TJ, Lando D, Basu S, Atkinson LP, Cao Y, Lee SF, … Laue ED (2017). 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature, 544(7648), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum DL (2017). Mechanisms of urodele limb regeneration. Regeneration (Oxf), 4(4), 159–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Mattie S, Prudent J, & McBride HM (2017). Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature, 542(7640), 251–254. [DOI] [PubMed] [Google Scholar]
- Sun T, Song Y, Dai J, Mao D, Ma M, Ni JQ, … Pastor-Pareja JC (2019). Spectraplakin Shot Maintains Perinuclear Microtubule Organization in Drosophila Polyploid Cells. Dev Cell, 49(5), 731–747. [DOI] [PubMed] [Google Scholar]
- Tadros W, & Lipshitz HD (2009). The maternal-to-zygotic transition: a play in two acts. Development, 136(18), 3033–3042. [DOI] [PubMed] [Google Scholar]
- Takemoto A, Kawashima SA, Li JJ, Jeffery L, Yamatsugu K, Elemento O, & Nurse P (2016). Nuclear envelope expansion is critical for proper chromosomal segregation during a closed mitosis. J Cell Sci., 129(6), 1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KL, Haelterman NA, Kwartler CS, Regalado ES, Lee PT, Nagarkar-Jaiswal S, … Bellen HJ (2018). Ari-1 Regulates Myonuclear Organization Together with Parkin and Is Associated with Aortic Aneurysms. Dev Cell, 45(2), 226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, … Ito K (2013). Cytoplasmic streaming velocity as a plant size determinant. Dev Cell, 27(3), 345–352. [DOI] [PubMed] [Google Scholar]
- Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, … Hetzer MW (2019). Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J Cell Biol, 218(2), 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran JR, Zheng X, & Zheng Y (2016). Lamin-B1 contributes to the proper timing of epicardial cell migration and function during embryonic heart development. Mol Biol Cell, 27(25), 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlaki E, & FitzHarris G (2016). Nucleus downscaling in mouse embryos is regulated by cooperative developmental and geometric programs. Sci Rep, 6, 28040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppaluri S, Weber SC, & Brangwynne CP (2016). Hierarchical Size Scaling during Multicellular Growth and Development. Cell Rep, 17(2), 345–352. [DOI] [PubMed] [Google Scholar]
- Vadia S, Tse JL, Lucena R, Yang Z, Kellogg DR, Wang JD, & Levin PA (2017). Fatty Acid Availability Sets Cell Envelope Capacity and Dictates Microbial Cell Size. Curr Biol, 27(12), 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey MD, & Fletcher DA (2014). The biology of boundary conditions: cellular reconstitution in one, two, and three dimensions. Curr Opin Cell Biol, 26, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valfort AC, Launay C, Semon M, & Delattre M (2018). Evolution of mitotic spindle behavior during the first asymmetric embryonic division of nematodes. PLoS Biol, 16(1), e2005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, … Lippincott-Schwartz J (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature, 546(7656), 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw NL, Cao WX, & Lipshitz HD (2019). The maternal-to-zygotic transition revisited. Development, 146(11), pii: dev161471. [DOI] [PubMed] [Google Scholar]
- Vukovic LD, Jevtic P, Zhang Z, Stohr BA, & Levy DL (2016). Nuclear size is sensitive to NTF2 protein levels in a manner dependent on Ran binding. J Cell Sci, 129(6), 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, & Ron D (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science, 334(6059), 1081–1086. [DOI] [PubMed] [Google Scholar]
- Walters AD, Amoateng K, Wang R, Chen JH, McDermott G, Larabell CA, … Cohen-Fix O (2019). Nuclear envelope expansion in budding yeast is independent on cell growth and does not determine nuclear volume. Mol Biol Cell, 30(1), 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters AD, Bommakanti A, & Cohen-Fix O (2012). Shaping the nucleus: Factors and forces. J Cell Biochem, 113(9), 2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu X, Nguyen CM, Liu Y, Gao Y, Lin X, … Qi LS (2018). CRISPR-Mediated Programmable 3D Genome Positioning and Nuclear Organization. Cell, 175(5), 1405–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, & Rapoport TA (2019). Reconstituting the reticular ER network - mechanistic implications and open questions. J Cell Sci, 132(4), pii: jcs227611. [DOI] [PubMed] [Google Scholar]
- Wang S, Romano FB, Field CM, Mitchison TJ, & Rapoport TA (2013). Multiple mechanisms determine ER network morphology during the cell cycle in Xenopus egg extracts. J Cell Biol, 203(5), 801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nagarajan M, Uhler C, & Shivashankar GV (2017). Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression. Mol Biol Cell, 28(14), 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SC, & Brangwynne CP (2015). Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr Biol, 25(5), 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer KA, & Marshall WF (2007). Flagellar length control in chlamydomonas--paradigm for organelle size regulation. Int Rev Cytol, 260, 175–212. [DOI] [PubMed] [Google Scholar]
- West M, Zurek N, Hoenger A, & Voeltz GK (2011). A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. J Cell Biol, 193(2), 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijchers PJ, Krijger PHL, Geeven G, Zhu Y, Denker A, Verstegen M, … de Laat W (2016). Cause and Consequence of Tethering a SubTAD to Different Nuclear Compartments. Mol Cell, 61(3), 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom JD, Israeli T, Bachar-Wikstrom E, Swisa A, Ariav Y, Waiss M, … Leibowitz G (2013). AMPK regulates ER morphology and function in stressed pancreatic beta-cells via phosphorylation of DRP1. Mol Endocrinol, 27(10), 1706–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur JD, & Heald R (2013). Mitotic spindle scaling during Xenopus development by kif2a and importin alpha. eLife, 2, e00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilky H, Chari S, Govindan J, & Amodeo AA (2019). Histone concentration regulates the cell cycle and transcription in early development. Development, 146(19), pii: dev177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB (1925). The karyoplasmic ratio The Cell in Development and Heredity (Third ed, pp. 727–733). New York: The Macmillan Company. [Google Scholar]
- Windner SE, Manhart A, Brown A, Mogilner A, & Baylies MK (2019). Nuclear Scaling Is Coordinated among Individual Nuclei in Multinucleated Muscle Fibers. Dev Cell, 49(1), 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JB, Ferreira Gomes B, Widlund PO, Mahamid J, Honigmann A, & Hyman AA (2017). The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell, 169(6), 1066–1077. [DOI] [PubMed] [Google Scholar]
- Worman HJ, & Schirmer EC (2015). Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol, 34, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xu J, Liu B, Yao G, Wang P, Lin Z, … Sun Y (2018). Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature, 557(7704), 256–260. [DOI] [PubMed] [Google Scholar]
- Wueseke O, Zwicker D, Schwager A, Wong YL, Oegema K, Julicher F, … Woodruff JB (2016). Polo-like kinase phosphorylation determines Caenorhabditis elegans centrosome size and density by biasing SPD-5 toward an assembly-competent conformation. Biol Open, 5(10), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, & Mitchison TJ (2008). Evidence for an upper limit to mitotic spindle length. Curr Biol, 18(16), 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Horton ER, Salcini AE, Pocock R, Cox TR, & Erler JT (2018). Proteomic Characterization of Caenorhabditis elegans Larval Development. Proteomics, 18(2). doi: 10.1002/pmic.201700238. [DOI] [PubMed] [Google Scholar]
- Xiao S, Tong C, Yang Y, & Wu M (2017). Mitotic Cortical Waves Predict Future Division Sites by Encoding Positional and Size Information. Dev Cell, 43(4), 493–506. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chikka MR, Xia H, & Ready DF (2016). Ire1 supports normal ER differentiation in developing Drosophila photoreceptors. J Cell Sci, 129(5), 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JZ, Woo EM, Postow L, Chait BT, & Funabiki H (2013). Chromatin-bound Xenopus dppa2 shapes the nucleus by locally inhibiting microtubule assembly. Dev Cell, 27(1), 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Gandin V, Sasaki M, McCracken S, Li W, Silvester JL, … Mak TW (2014). Largen: a molecular regulator of mammalian cell size control. Mol Cell, 53(6), 904–915. [DOI] [PubMed] [Google Scholar]
- Zapata J, Dephoure N, Macdonough T, Yu Y, Parnell EJ, Mooring M, … Kellogg DR (2014). PP2ARts1 is a master regulator of pathways that control cell size. J Cell Biol, 204(3), 359–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, White MD, Templin RM, Parton RG, Thorn-Seshold O, Bissiere S, & Plachta N (2017). A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science, 357(6354), 925–928. [DOI] [PubMed] [Google Scholar]
- Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, … Pellman D (2015). Chromothripsis from DNA damage in micronuclei. Nature, 522(7555), 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, & Hu J (2016). Shaping the Endoplasmic Reticulum into a Social Network. Trends Cell Biol, 26(12), 934–943. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gong K, Denholtz M, Chandra V, Kamps MP, Alber F, & Murre C (2017). Comprehensive characterization of neutrophil genome topology. Genes Dev, 31(2), 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D, Fischer AH, & Nickerson JA (2004). Nuclear structure in cancer cells. Nat Rev Cancer, 4(9), 677–687. [DOI] [PubMed] [Google Scholar]


