Abstract
The H1N1 “Spanish influenza” pandemic of 1918–1919 caused the highest known number of deaths recorded for a single pandemic in human history. Several theories have been offered to explain the virulence and spread of the disease, but the environmental context remains underexamined. In this study, we present a new environmental record from a European, Alpine ice core, showing a significant climate anomaly that affected the continent from 1914 to 1919. Incessant torrential rain and declining temperatures increased casualties in the battlefields of World War I (WWI), setting the stage for the spread of the pandemic at the end of the conflict. Multiple independent records of temperature, precipitation, and mortality corroborate these findings.
Keywords: Spanish Flu, World War I, Climate Change, H1N1, Pandemic, Ice core
Key Points
Novel, high‐resolution climate record from Europe shows strong influx of marine air in a 1914–1918 climate anomaly unmatched in 100 years
Independent precipitation, temperature, and historical records corroborate the timing and extent of the anomaly
Historical and epidemiological records indicate that this climate anomaly affected the mortality in WWI as well as the “Spanish flu” pandemic
1. Main Text
A century ago, in the autumn of 1918, the deadliest wave of the H1N1 influenza pandemic, known as the “Spanish Flu”, claimed tens of millions of victims (Krammer et al., 2018; Taubenberger & Morens, 2006). It marked the beginning of the end of several years of unprecedented mortality throughout Europe, due to the horrors of World War I (WWI), and an unusually extreme, multiyear climate anomaly that brought torrential rains to battlefields as well as urban areas. The inescapable muddy landscapes of the war remain a common theme of surviving WWI eyewitness accounts and photographs. Despite recent work on the impact of climate change on the spread of viral infections and overall mortality, the role of environmental changes in the WWI‐Spanish flu period remains underexamined (Grant & Giovannucci, 2009; Mamelund, 2011). Here we present a new, high‐resolution climate proxy record from the high Alpine Monte Rosa (4,450 m a.m.s.l.) Colle Gnifetti (CG) glacier in the heart of Europe, indicating abnormally high influxes of North Atlantic marine air in the years 1914–1919. We evaluate the glaciochemical data from this ice core with a detailed monthly record of overall mortality in Europe for the same period and monthly precipitation and temperature measurements (Ansart et al., 2009; Bohleber et al., 2018; Bunle, 1954; Clifford et al., 2019; More et al., 2017, 2018; Schneider et al., 2014; Sneed et al., 2015; Willmott & Matsuura, 2001). The multiyear, extreme anomaly described here, in several independent but consilient records, had a significant impact in setting the stage for the onset, spread, and mutation of the H1N1 pandemic, while also increasing all‐cause deaths due to widespread harvest failures and worsening battlefield conditions.
The influenza pandemic of 1917–1919 claimed between 50 and 100 million lives (Krammer et al., 2018; Taubenberger & Morens, 2006). In Europe, the estimated death toll was 2.64 million (Ansart et al., 2009). Various theories have been proposed to document its spread from Asia to Europe. Historical research has recently focused on the recruitment of allied troops by the British in Asia in spring 1917 and their transport to Europe via Canada and the Atlantic crossing, as the most likely route of transmission (Humphries, 2014). Studies have also suggested that the use of chlorine gas on the battlefields of WWI may have caused the virus to mutate into its most virulent form (Erkoreka, 2009; Oxford et al., 2005; Taubenberger et al., 1997; Worobey et al., 2014). The environmental and especially climatic conditions in which the pandemic developed have received less attention in the scientific literature, even though historical accounts universally describe abnormally high precipitation and cold temperatures in the years preceding the onset of the pandemic, in 1917, and during its deadliest wave in 1918. In contrast, detailed research has shown how extreme weather conditions impacted the outcome of major battles on the western front, most notably during the battles of Verdun (1916–1917), the Somme (1916), the Chemin‐des‐Dames (1917), and the Third Battle of Ypres‐Passchendaele (1917) (Barbante et al., 2004; Hussey, 1997). Extreme weather anomalies in these years disrupted governmental efforts in collecting meteorological records (Savouret et al., 2011).
In this study, we combine a high‐resolution glaciochemical record reconstructed from CG marine air proxies (Na, Cl−) evaluated together with detailed monthly records of overall mortality from 13 European countries (Figures 1 and 2) and instrumental measurements of precipitation and temperature for the same period (Figure 3). Previous research on this CG record exposed in great detail variations detected in climate proxies and in the concentration of pollutants for this area due to its geographic proximity to events and its location under the influence of the Iceland low and Azores high pressure systems (Figure 4) (Barbante et al., 2004; Bohleber et al., 2018; Döscher et al., 1995; More et al., 2018). The glaciochemical records have been developed via discrete, inductively coupled plasma mass spectrometry (ICP‐MS, 465 data points for the period 1880–1980) and ion chromatography (IC, 581 data points for the period 1880–1980) analyses (More et al., 2017, 2018; Sneed et al., 2015).
Figure 1.
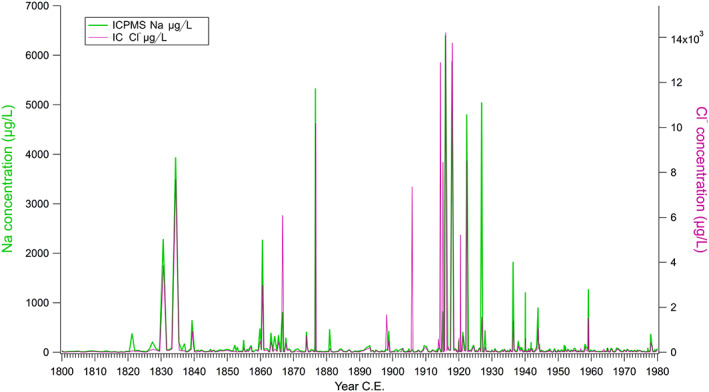
High marine air influx in the years of World War I and Spanish influenza 1914–1919. Concentrations of Na (ICPMS), Cl− (IC) marine air proxies in the Colle Gnifetti glacier (4,450 m.a.s.l. Swiss‐Italian Alps) in the years 1880–1980 (with a possible error of ±1 year in this segment of the core, per Bohleber et al., 2018). The years 1914–1918 show the highest concentrations in a century, indicating an extreme influx of cold marine air from the North Atlantic.
Figure 2.
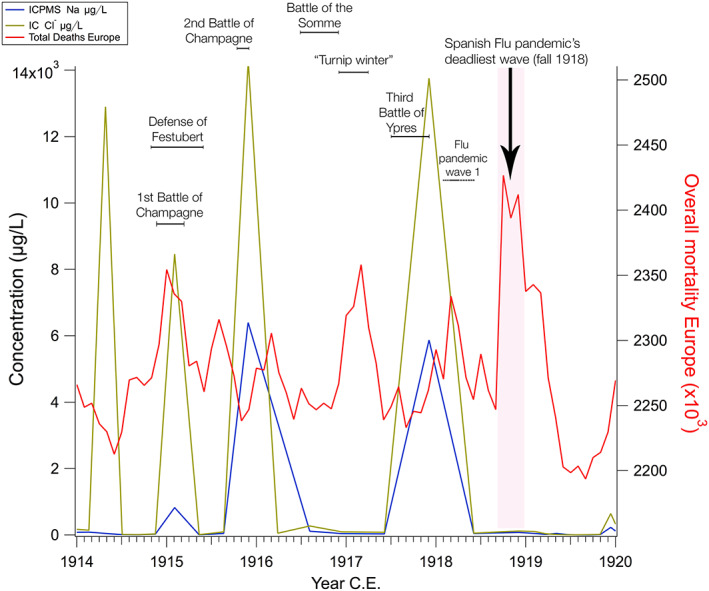
Marine air influx and total deaths in Europe 1914–1920. Concentrations of Na, Cl−, marine air proxies in the CG glacier and total mortality for 13 European countries 1914–1920 (Ansart et al., 2009; Bunle, 1954). In 1915, 1916, and 1918, overall mortality peaked during or immediately after periods of high marine air influx over Europe. Major battles of WWI where precipitation was a significant factor are labeled in black, along with the period of the three major waves of the Spanish Flu pandemic, the deadliest of which occurred in the autumn of 1918.
Figure 3.
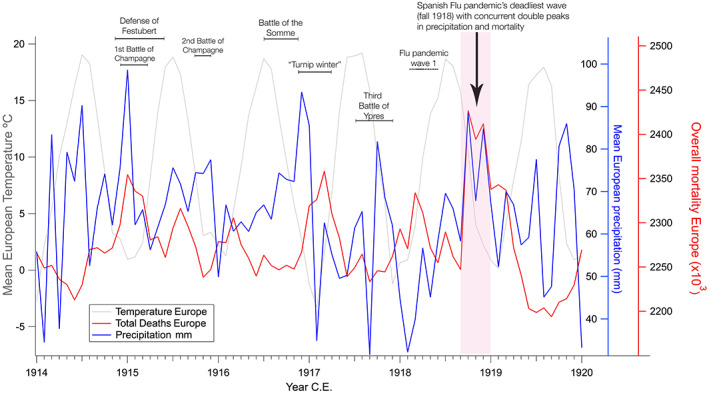
Mean temperature, precipitation, and total deaths in Europe, 1914–1920. Mean values of instrumental measurements of temperature and precipitation with overall deaths for 13 European countries (Ansart et al., 2009; Bunle, 1954; Schneider et al., 2014; Willmott & Matsuura, 2001). In the autumn and winter of 1918, mortality peaked together with high precipitation, with a peak in both records in the month of October, a slight decrease in November, and another peak in December of that year. The deadliest wave of the Spanish Influenza pandemic claimed most of its victims in the same months, where the arrow points to a double peak in both deaths and precipitation.
Figure 4.
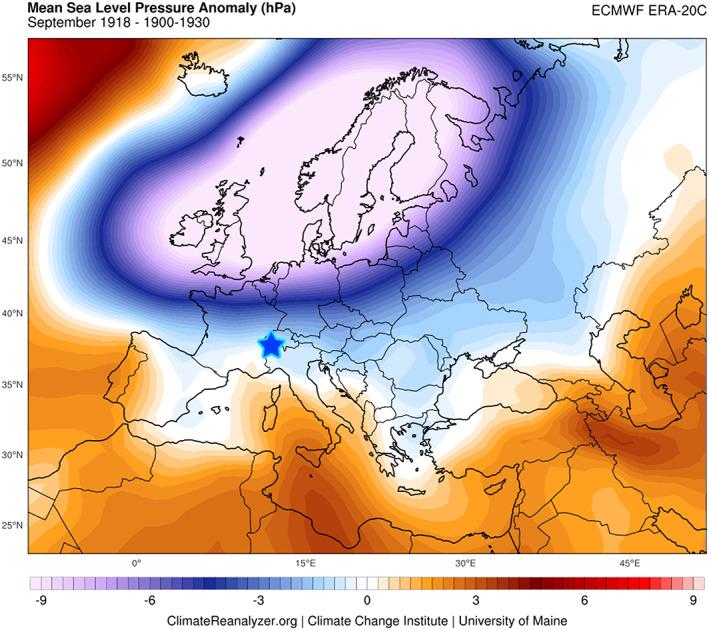
Atmospheric pressure anomaly over Europe. The CG ice core site (4,450 m.a.s.l.) is shown as a star. A mean sea level pressure difference map was calculated for September 1918 from an average for the 1900–1930 interval in the ECMWF ERA‐20C reanalysis data set via the Climate Reanalyzer™. A strong Iceland low pressure system influenced unusually cold and wet climate conditions during the month of September 1918.
Total European mortality peaked three times, concurrently or following cooling temperatures, increasing precipitation, and an extreme influx of cold marine air in winter 1915, 1916, and 1918 (Figure 2). The glaciochemical and instrumental records corroborate historical accounts of torrential precipitation on the battlefields of WWI—resulting in increased casualties due to drowning, exposure, pneumonia, and other infections—and its severity may have significantly altered the migration patterns of birds such as the mallard duck, the primary reservoir for the H1N1 avian influenza virus (Tucker et al., 2018).
The first significant increases in all marine air proxies occurred in spring 1914 and winter 1914–1915, with concurrent increases in precipitation and mortality in December 1914 and January–February 1915. Historical accounts of the Defense of Festubert (November 1914 to May 1915) and the First Battle of Champagne (December 1914 to March 1915, Figures S1–S3) highlight the impact of intensifying precipitation on British, French, and German military operations, where newly dug trenches and tunnels filled with rainwater, as bitter cold temperatures caused thousands to endure frostbite at night, while mud slowed down the movement of troops and artillery during the day (Barthas, 2014; Churchill et al., 1916; de Lécluse, 1998).
The highest increase in marine air proxies (Figures 1 and 2) occurred in the summer–autumn of 1915 and winter of 1915–1916 (Figure S4). Instrumental measurements of precipitation (Figure 3) show an increase from the spring of 1915 to December 1916 (Figure S5), with only a short interruption in July–August, and in December 1915. Extreme precipitation and winter conditions reached as far as Gallipoli (Turkey) in November 1915, famously affecting the ANZAC and British troops there, in one of the longest and deadliest campaigns of the war (Prior, 2009; Figure S4). Starting in January 1916, precipitation in Europe increased steadily throughout the year, with a peak in December 1916 (Figure S5), coinciding with the battles of the Somme and Verdun, where the mud and water‐filled trenches and bomb craters swallowed everything, from tanks, to horses and troops, becoming what eyewitnesses described as the “liquid grave” of the armies (Borden, 1917). Extended winter conditions delayed the start of the Chemin‐des‐Dames offensive until April 1917 (Attal & Rolland, 1993; Savouret et al., 2011). They also caused widespread harvest failures, resulting in the “turnip winter” of 1916–1917, whereby the German population resorted to root vegetables for their diet, due to failed potato and cereal harvests and the allied blockade (Chickering, 2004). After a brief respite, rain and cold returned again in the summer and autumn of 1917 (Figure S6), with widespread flooding of the trenches in the Third Battle of Ypres (Passchendaele) in July–November, often bringing military operations to a standstill and substantially increasing casualties (Barton et al., 2007; Lloyd, 2017; Reid et al., 1999).
The first wave of H1N1 influenza in Europe emerged in the spring of 1918, most likely originating in the fall and winter of 1917, among allied troops that had arrived from Asia and had established their base camp near Boulogne (Humphries, 2014). The combination of extremely high precipitation, the concentration of millions of troops on the battlefields, unsanitary conditions, and the use of chlorine gas as a chemical weapon have been cited as contributing factors to the mutation and emergence of the most lethal wave of the Spanish influenza pandemic in the fall and winter of 1918–1919 (Erkoreka, 2009; Oxford et al., 2005; Figure S7). Later peaks in marine air proxies in 1921–1922 and 1926 are connected to documented residual activity from North Atlantic hurricanes, particularly severe in those years, but not approximating the extremes of 1914–1918 (Landsea et al., 2012). The deadliest wave of the flu in Europe began in the autumn of 1918, closely following a period of extremely high precipitation and cold temperatures (Figure 3).
The coincidence between increased precipitation and mortality in this pandemic wave in late 1918 (Figure 3) highlights the role of environmental conditions in a pandemic's morbidity and mortality, as already suggested in studies of recent H1N1 and other respiratory tract infections such as COVID‐19 in human populations (Kissler et al., 2020; Liu et al., 2020; Viboud et al., 2004) especially in regions with extensive, long‐term air pollution such as Europe (Clay et al., 2018; Morales et al., 2017; More et al., 2017). An additional exacerbating factor may have been the influence of the weather anomalies on established patterns of avian migration, similar to those documented for other species in response to modern, anthropogenic climate change (Tucker et al., 2018). Specifically, the migration of the mallard duck—one of the primary reservoirs of H1N1 avian influenza virus—may have been disrupted by the anomaly described here, resulting in increased presence of this species throughout Europe in the autumn of 1917 and 1918, in close proximity to both military and civilian populations, as well as domesticated animals (Oxford et al., 2005; Saunders‐Hastings & Krewski, 2016; Taubenberger et al., 1997; Worobey et al., 2014). Studies of disruptions in mallard migration have shown that changes in the environment—at start point and throughout the journey—can affect overall movement and direction (van Toor et al., 2013) interrupting their normal migratory route (Kleyheeg et al., 2019; Tolf et al., 2012). The transfer of H1N1 influenza virus from animals (avian and mammals) to humans (zoonosis) occurs primarily via water sources contaminated with fecal droppings from infected birds (Breban et al., 2009; Carter & Sanford, 2012; Pawar et al., 2018; Vandegrift et al., 2010; Vittecoq et al., 2017; Worobey et al., 2014). In autumn, the rate of influenza A viral infection can be as high as 60% in mallard populations, due to the exposure of immunologically naïve juveniles to the virus (Bengtsson et al., 2016; Tolf et al., 2012). Juveniles are especially prone to remain in the same area if their migration route is disrupted (van Toor et al., 2013). Exposure of mammalian hosts to the same infected bodies of water where mallard ducks may have remained may explain connections to the current seasonal recurrence of H1N1 in human populations as shown in recent studies (Belser & Terrence, 2019; Reid et al., 2004; Smith et al., 2009; Tang, 2009; Weingartl et al., 2009), suggesting that severe weather anomalies in 1917–1919 may have contributed to both the diffusion and mutation of the Spanish flu virus, as previous studies have suggested.
The influx of torrential rain accompanied by decreasing temperatures in the autumns of 1917 and 1918 provided ideal conditions for the survival and replication of the virus (Brown et al., 2009; Lowen & Steel, 2014; Reid et al., 2004; Tang, 2009; Weingartl et al., 2009). Prolonged exposure to decreasing temperatures may also have been a factor in increasing pneumococcal co‐infections, which recent studies have found to be more common in the WWI years than previously thought (Foxman et al., 2015, 2016; Iwasaki et al., 2017; Klugman & Chien, 2009; Meissner, 2016; Morens et al., 2008; Taubenberger et al., 1997; Worobey et al., 2014). Retrospective epidemiological studies have now shown that a significant contribution to high mortality during the Spanish Flu pandemic came from pneumococcal co‐infections, amounting to as much as one fifth of influenza victims, with a 34% mortality.
The data presented here show that extreme weather anomalies captured in glaciochemistry and reanalysis records brought unusually strong influxes of cold marine air from the North Atlantic, primarily between 1915 and 1919, resulting in unusually strong precipitation events, and that they exacerbated total mortality across Europe, due to the interplay of environmental, ecological, epidemiological, and human factors. As we experience increasingly severe weather anomalies brought about by global climate change, and with the persistent seasonal recurrence of H1N1, the contribution of environmental change and human action to pandemic morbidity, mortality, and diffusion cannot be underestimated. Indeed, the pandemic development history of the Spanish Influenza from 1917 to 1919 sends a warning into our own time, a century later, of the ongoing risks of war zones (including the use of chemical weapons), wildlife trade, unsanitary conditions, and humanitarian crises as incubators of disease, assisted by climate‐change triggers. Our past and current crisis highlights our continuing and growing need for robust local and global public health and environmental agencies, such as the CDC, WHO and UNEP, dedicated to reducing the risk that climate change will aggravate epidemic outbreaks. The role of climate anomalies such as the one described in this study must be assessed in relation to more recent pandemics such as COVID‐19.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
Recovery and analysis of the CG ice core, associated written records, and interpretation were supported by Arcadia, a charitable foundation of Lisbet Rausing and Peter Baldwin (grants AC3450, 3862, and 4190). This is contribution 15 of the Historical Ice Core Project funded by the Arcadia Foundation (AC4190), under the Initiative for the Science of the Human Past at Harvard in collaboration with the Climate Change Institute at the University of Maine. We thank Pascal Bohleber and Nicole E. Spaulding for their work on the CG ice core chronology and glaciochemical analysis and the late Dietmar Wagenbach for his initial support and innovative contributions to the project. The CG ice core was collected by a joint team effort from Institut für Umweltphysik, Universität Heidelberg, the Climate Change Institute at the University of Maine and the Climate and Environmental Physics Institute, University of Bern. We acknowledge the generosity of Hubertus Fischer, of the Climate and Environmental Physics Institute, University of Bern, in providing the drilling equipment. We particularly thank the drillers Remo Walther and Samuel Marending for their efforts during the drilling campaign. Additional support in ice core processing and analysis was provided by the Alfred‐Wegener‐Institute, Bremerhaven, and the Climate and Environmental Physics Institute, University of Bern. We gratefully acknowledge their support.
More, A. F. , Loveluck, C. P. , Clifford, H. , Handley, M. J. , Korotkikh, E. V. , Kurbatov, A. V. , et al. (2020). The impact of a six‐year climate anomaly on the “Spanish flu” pandemic and WWI. GeoHealth, 4, e2020GH000277 10.1029/2020GH000277
Data Availability Statement
All data pertaining to this article and for the entire project are available in open access (https://dataverse.harvard.edu/dataverse/historicalicecore). Additional information may be obtained from A. F. M. (afmore@fas.harvard.edu).
References
- Ansart, S. , Pelat, C. , Boelle, P. Y. , Carrat, F. , Flahault, A. , & Valleron, A. J. (2009). Mortality burden of the 1918–1919 influenza pandemic in Europe. Influenza and Other Respiratory Viruses, 3(3), 99–106. 10.1111/j.1750-2659.2009.00080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal, R. , & Rolland, D. (1993). Ambleny, le temps d'une guerre. Journal d'Onézime Hénin (1914–1918) (p. 158). Soissons: Societé archéologique, historique et scientifique de Soissons. [Google Scholar]
- Barbante, C. , Schwikowski, M. , Döring, T. , Gäggeler, H. W. , Schotterer, U. , Tobler, L. , van de Velde, K. , Ferrari, C. , Cozzi, G. , Turetta, A. , Rosman, K. , Bolshov, M. , Capodaglio, G. , Cescon, P. , & Boutron, C. (2004). Historical record of European emissions of heavy metals to the atmosphere since the 1650s from alpine snow/ice cores drilled near Monte Rosa. Environmental Science and Technology, 38(15), 4085–4090. 10.1021/es049759r [DOI] [PubMed] [Google Scholar]
- Barthas, L. (2014). Poilu: The World War I Notebooks of Corporal Louis Barthas (Vol. 52, p. 65). New Haven: Yale University Press. [Google Scholar]
- Barton, P. , Doyle, P. , & Vandewalle, J. (2007). Beneath Flanders fields: The Tunnellers' war, 1914–1918 (Vol. 17, p. 76). Montreal: McGill–Queen's University Press. [Google Scholar]
- Belser, J. A. , & Terrence, M. (2019). The 1918 flu, 100 years later. Science, 359, 255 10.1126/science.aas9565 [DOI] [PubMed] [Google Scholar]
- Bengtsson, D. , Safi, K. , Avril, A. , Fiedler, W. , Wikelski, M. , Gunnarsson, G. , Elmberg, J. , Tolf, C. , Olsen, B. , & Waldenström, J. (2016). Does influenza a virus infection affect movement behaviour during stopover in its wild reservoir host? Royal Society Open Science, 3(2), 150633 10.1098/rsos.150633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohleber, P. , Erhardt, T. , Spaulding, N. , Hoffmann, H. , Fischer, H. , & Mayewski, P. (2018). Temperature and mineral dust variability recorded in two low‐accumulation Alpine ice cores over the last millennium. Climate of the Past, 14(1), 21–37. 10.5194/cp-14-21-2018 [DOI] [Google Scholar]
- Borden, M. (1917). At the Somme: The song of the mud. Current Opinion, 63, 275. [Google Scholar]
- Breban, R. , Drake, J. M. , Stallknecht, D. E. , & Rohani, P. (2009). The role of environmental transmission in recurrent avian influenza epidemics. PLoS Computational Biology, 5(4), e1000346 10.1371/journal.pcbi.1000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. D. , Goekjian, G. , Poulson, R. , Valeika, S. , & Stallknecht, D. E. (2009). Avian influenza virus in water: Infectivity is dependent on pH, salinity and temperature. Veterinary Microbiology, 136(1–2), 20–26. 10.1016/j.vetmic.2008.10.027 [DOI] [PubMed] [Google Scholar]
- Bunle, H. (1954). Le Mouvement naturel de la population dans le monde de 1906 à 1936. Paris, Institut national d'études démographiques, 432–438. [Google Scholar]
- Carter, R. W. , & Sanford, J. C. (2012). A new look at an old virus: Patterns of mutation accumulation in the human H1N1 influenza virus since 1918. Theoretical Biology & Medical Modelling, 9, 42 10.1186/1742-4682-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickering, R. (2004). Imperial Germany and the Great War, 1914–1918 (p. 141). Cambridge: Cambridge University Press. [Google Scholar]
- Churchill, A. L. , Miller, F. T. , & Reynolds, F. J. (1916). The Story of the Great War (p. 139). New York: P. F. Collier & Son. [Google Scholar]
- Clay, K. , Lewis, J. , & Severnini, E. (2018). Pollution, infectious disease, and mortality: Evidence from the 1918 Spanish influenza pandemic. The Journal of Economic History, 78(4), 1179–1209. 10.1017/S002205071800058X [DOI] [Google Scholar]
- Clifford, H. M. , Spaulding, N. E. , Kurbatov, A. V. , More, A. , Korotkikh, E. V. , Sneed, S. B. , Handley, M. , Maasch, K. A. , Loveluck, C. P. , Chaplin, J. , McCormick, M. , & Mayewski, P. A. (2019). A 2000 year Saharan dust event proxy record from an ice core in the European Alps. Journal of Geophysical Research: Atmospheres, 124, 12,882–12,900. 10.1029/2019JD030725 [DOI] [Google Scholar]
- de Lécluse, H. (1998). Comrades‐in‐arms: The World War I memoir of captain Henri de Lécluse (Vol. 35, p. 36). Kent, OH: Kent State University Press. [Google Scholar]
- Döscher, A. , Gäggeler, H. W. , Schotterer, U. , & Schwikowski, M. (1995). A 130 years deposition record of sulfate, nitrate and chloride from a high‐alpine glacier. Water, Air, & Soil Pollution, 85(2), 603–609. 10.1007/BF00476895 [DOI] [Google Scholar]
- Erkoreka, A. (2009). Origins of the Spanish influenza pandemic (1918–1920) and its relation to the First World War. Journal of Molecular and Genetic Medicine: An International Journal of Biomedical Research, 3(2), 190–194. 10.4172/1747-0862.1000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman, E. F. , Storer, J. A. , Fitzgerald, M. E. , Wasik, B. R. , Hou, L. , Zhao, H. , Turner, P. E. , Pyle, A. M. , & Iwasaki, A. (2015). Temperature‐dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proceedings of the National Academy of Sciences, 112(3), 827–832. 10.1073/pnas.1411030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman, E. F. , Storer, J. A. , Vanaja, K. , Levchenko, A. , & Iwasaki, A. (2016). Two interferon‐independent double‐stranded RNA‐induced host defense strategies suppress the common cold virus at warm temperature. Proceedings of the National Academy of Sciences of the United States of America, 113(30), 8496–8501. 10.1073/pnas.1601942113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, W. B. , & Giovannucci, E. (2009). The possible roles of solar ultraviolet‐B radiation and vitamin D in reducing case‐fatality rates from the 1918–1919 influenza pandemic in the United States. Dermato‐endocrinology, 1(4), 215–219. 10.4161/derm.1.4.9063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, M. O. (2014). Paths of infection: The First World War and the origins of the 1918 influenza pandemic. War in History, 21(1), 55–81. 10.1177/0968344513504525 [DOI] [Google Scholar]
- Hussey, J. (1997). The Flanders battleground and the weather in 1917 In Liddle P. H. (Ed.), Passchendaele in Perspective: The Third Battle of Ypres (pp. 140–158). London: Leo Cooper. [Google Scholar]
- Iwasaki, A. , Foxman, E. F. , & Molony, R. D. (2017). Early local immune defenses in the respiratory tract. Nature Reviews Immunology, 17, 17–20. 10.1038/nri.2016.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler, S. M. , Tedijanto, C. , Goldstein, E. , Grad, Y. H. , & Lipsitch, M. (2020). Projecting the transmission dynamics of SARS‐CoV‐2 through the post‐pandemic period. Science, eabb5793 10.1126/science.abb5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyheeg, E. , Fiedler, W. , Safi, K. , Waldenström, J. , Wikelski, M. , & van Toor, M. L. (2019). A comprehensive model for the quantitative estimation of seed dispersal by migratory mallards. Frontiers in Ecology and Evolution, 7, 40 10.3389/fevo.2019.00040 [DOI] [Google Scholar]
- Klugman, K. P. , Chien, Y. W. , & Madhi, S. A. (2009). Pneumococcal pneumonia and influenza: A deadly combination. Vaccine, 27 Suppl 3, c9–c14. 10.1016/j.vaccine.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Krammer, F. , Smith, G. J. D. , Fouchier, R. A. M. , Peiris, M. , Kedzierska, K. , Doherty, P. C. , Palese, P. , Shaw, M. L. , Treanor, J. , Webster, R. G. , & García‐Sastre, A. (2018). Influenza. Nature Reviews, Disease Primers, 4(1), 3 10.1038/s41572-018-0002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsea, C. W. , Feuer, S. , Hagen, A. , Glenn, D. A. , Sims, J. , Perez, R. , et al. (2012). A reanalysis of the 1921–30 Atlantic hurricane database. Journal of Climate, 25(3), 865–885. 10.1175/JCLI-D-11-00026.1 [DOI] [Google Scholar]
- Liu, Q. , Tan, Z.‐M. , Sun, J. , Hou, Y. , Fu, C. , & Wu, Z. (2020). Changing rapid weather variability increases influenza epidemic risk in a warming climate. Environmental Research Letters, 15(4), 044004 10.1088/1748-9326/ab70bc [DOI] [Google Scholar]
- Lloyd, N. (2017). Passchendaele: The lost victory of World War I (Vol. 99, p. 73). New York: Basic Books. [Google Scholar]
- Lowen, A. C. , & Steel, J. (2014). Roles of humidity and temperature in shaping influenza seasonality. Journal of Virology, 88(14), 7692–7695. 10.1128/jvi.03544-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelund, S.‐E. (2011). Geography may explain adult mortality from the 1918–20 influenza pandemic. Epidemics, 3(1), 46–60. 10.1016/j.epidem.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Meissner, H. C. (2016). Viral bronchiolitis in children. New England Journal of Medicine, 374(1), 62–72. 10.1056/NEJMra1413456 [DOI] [PubMed] [Google Scholar]
- Morales, K. F. , Paget, J. , & Spreeuwenberg, P. (2017). Possible explanations for why some countries were harder hit by the pandemic influenza virus in 2009—A global mortality impact modeling study. BMC Infectious Diseases, 17(1), 642 10.1186/s12879-017-2730-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, A. F. , Spaulding, N. E. , Bohleber, P. , Handley, M. J. , Hoffmann, H. , Korotkikh, E. V. , Kurbatov, A. V. , Loveluck, C. P. , Sneed, S. B. , McCormick, M. , & Mayewski, P. A. (2017). Next‐generation ice core technology reveals true minimum natural levels of lead (Pb) in the atmosphere: Insights from the Black Death. GeoHealth, 1, 211–219. 10.1002/2017gh000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, A. F. , Spaulding, N. E. , Bohleber, P. , Handley, M. J. , Hoffmann, H. , Korotkikh, E. V. , Kurbatov, A. V. , Loveluck, C. P. , Sneed, S. B. , McCormick, M. , Mayewski, P. A. , & Mayewski, P. A. (2018). The role of historical context in understanding past climate, pollution and health data in trans‐disciplinary studies: Reply to comments on More et al., 2017b. GeoHealth, 2, 162–170. 10.1029/2017gh000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens, D. M. , Taubenberger, J. K. , & Fauci, A. S. (2008). Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. The Journal of Infectious Diseases, 198(7), 962–970. 10.1086/591708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford, J. S. , Lambkin, R. , Sefton, A. , Daniels, R. , Elliot, A. , Brown, R. , & Gill, D. (2005). A hypothesis: The conjunction of soldiers, gas, pigs, ducks, geese and horses in northern France during the Great War provided the conditions for the emergence of the “Spanish” influenza pandemic of 1918–1919. Vaccine, 23(7), 940–945. 10.1016/j.vaccine.2004.06.035 [DOI] [PubMed] [Google Scholar]
- Pawar, S. D. , Pande, S. A. , Tare, D. S. , Keng, S. S. , Kode, S. S. , Singh, D. K. , & Mullick, J. (2018). Morphological and biochemical characteristics of avian faecal droppings and their impact on survival of avian influenza virus. Food and Environmental Virology, 10(1), 99–106. 10.1007/s12560-017-9323-3 [DOI] [PubMed] [Google Scholar]
- Prior, R. (2009). Gallipoli: The end of the myth (pp. 221–236). New Haven: Yale University Press. [Google Scholar]
- Reid, A. H. , Fanning, T. G. , Hultin, J. V. , & Taubenberger, J. K. (1999). Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proceedings of the National Academy of Sciences of the United States of America, 96(4), 1651–1656. 10.1073/pnas.96.4.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, A. H. , Taubenberger, J. K. , & Fanning, T. G. (2004). Evidence of an absence: The genetic origins of the 1918 pandemic influenza virus. Nature Reviews Microbiology, 2(11), 909–914. 10.1038/nrmicro1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders‐Hastings, P. R. , & Krewski, D. (2016). Reviewing the history of pandemic influenza: Understanding patterns of emergence and transmission. Pathogens, 66, 1–19. 10.3390/pathogens5040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouret, E. , Amat, J.‐P. , Cantat, O. , & Filippucci, P. (2011). Au temps météorologique de la grande guerre. Approche séquentielle des périodes contraignantes dans les tranchées sur le front de la Marne et de la Meuse, 1914‐1918. Climatologie, 8, 59–77. 10.4267/climatologie.276 [DOI] [Google Scholar]
- Schneider, U. , Becker, A. , Finger, P. , Meyer‐Christoffer, A. , Ziese, M. , & Rudolf, B. (2014). GPCC's new land surface precipitation climatology based on quality‐controlled in situ data and its role in quantifying the global water cycle. Theoretical and Applied Climatology, 115, 15–40. 10.1007/s00704-013-0860-x [DOI] [Google Scholar]
- Smith, G. J. D. , Bahl, J. , Vijaykrishna, D. , Zhang, J. , Poon, L. L. M. , Chen, H. , et al. (2009). Dating the emergence of pandemic influenza viruses. Proceedings of the National Academy of Sciences of the United States of America, 106(28), 11,709–11,712. 10.1073/pnas.0904991106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed, S. , Mayewski, P. , Sayre, W. , Handley, M. , Kurbatov, A. , Taylor, K. , et al. (2015). New LA‐ICP‐MS cryocell and calibration technique for sub‐millimeter analysis of ice cores. Journal of Glaciology, 61(226), 233–242. 10.3189/2015JoG14J139 [DOI] [Google Scholar]
- Tang, J. W. (2009). The effect of environmental parameters on the survival of airborne infectious agents. Journal of the Royal Society Interface, 6, S737–S746. 10.1098/rsif.2009.0227.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger, J. K. , & Morens, D. M. (2006). 1918 influenza: The mother of all pandemics. Emerging Infectious Diseases, 12(1), 15–22. 10.3201/eid1201.050979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger, J. K. , Reid, A. H. , Krafft, A. E. , Bijwaard, K. E. , & Fanning, T. G. (1997). Initial genetic characterization of the 1918 “Spanish” influenza virus. Science, 275(5307), 1793–1796. 10.1126/science.275.5307.1793 [DOI] [PubMed] [Google Scholar]
- Tolf, C. , Bengtsson, D. , Rodrigues, D. , Latorre‐Margalef, N. , Wille, M. , Figueiredo, M. E. , Jankowska‐Hjortaas, M. , Germundsson, A. , Duby, P. Y. , Lebarbenchon, C. , Gauthier‐Clerc, M. , Olsen, B. , & Waldenström, J. (2012). Birds and viruses at a crossroad—Surveillance of influenza a virus in Portuguese waterfowl. PLoS ONE, 7(11), e49002 10.1371/journal.pone.0049002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, M. A. , Böhning‐Gaese, K. , Fagan, W. F. , Fryxell, J. M. , Van Moorter, B. , Alberts, S. C. , et al. (2018). Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science, 359(6374), 466–469. 10.1126/science.aam9712 [DOI] [PubMed] [Google Scholar]
- van Toor, M. L. , Hedenström, A. , Waldenström, J. , Fiedler, W. , Holland, R. A. , Thorup, K. , & Wikelski, M. (2013). Flexibility of continental navigation and migration in European mallards. PLoS ONE, 8(8), e72629 10.1371/journal.pone.0072629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift, K. J. , Sokolow, S. H. , Daszak, P. , & Kilpatrick, A. M. (2010). Ecology of avian influenza viruses in a changing world. Annals of the New York Academy of Sciences, 1195, 113–128. 10.1111/j.1749-6632.2010.05451.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud, C. , Pakdaman, K. , Boëlle, P. Y. , Wilson, M. L. , Myers, M. F. , Valleron, A. J. , & Flahault, A. (2004). Association of influenza epidemics with global climate variability. European Journal of Epidemiology, 19(11), 1055–1059. 10.1007/s10654-004-2450-9 [DOI] [PubMed] [Google Scholar]
- Vittecoq, M. , Gauduin, H. , Oudart, T. , Bertrand, O. , Roche, B. , Guillemain, M. , & Boutron, O. (2017). Modeling the spread of avian influenza viruses in aquatic reservoirs: A novel hydrodynamic approach applied to the Rhône delta (southern France). Science of the Total Environment, 595, 787–800. 10.1016/j.scitotenv.2017.03.165 [DOI] [PubMed] [Google Scholar]
- Weingartl, H. M. , Albrecht, R. A. , Lager, K. M. , Babiuk, S. , Marszal, P. , Neufeld, J. , Embury‐Hyatt, C. , Lekcharoensuk, P. , Tumpey, T. M. , García‐Sastre, A. , & Richt, J. A. (2009). Experimental infection of pigs with the human 1918 pandemic influenza virus. Journal of Virology, 83(9), 4287–4296. 10.1128/JVI.02399-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott, C. J. , & Matsuura, K . (2001). Terrestrial air temperature and precipitation: Monthly and annual time series. http://climate.geog.udel.edu/~climate/html_pages/README.ghcn_ts2.html
- Worobey, M. , Han, G. Z. , & Rambaut, A. (2014). Genesis and pathogenesis of the 1918 pandemic H1N1 influenza a virus. Proceedings of the National Academy of Sciences of the United States of America, 111(22), 8107–8112. 10.1073/pnas.1324197111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
All data pertaining to this article and for the entire project are available in open access (https://dataverse.harvard.edu/dataverse/historicalicecore). Additional information may be obtained from A. F. M. (afmore@fas.harvard.edu).


