What you Need to Know.
Background and Context
The link between chronic, non-resolving inflammation and cancer is well established, with inflammation-associated cancers among the most highly represented and frequently-occurring neoplasias worldwide. Investigation over the last several years has focused on determining critical pathways involved in this process, with a number of candidate molecules identified, including members of the interleukin-1 (IL-1) family that are particularly important in cancers of the GI tract.
New Findings
IL-33 (or IL-1F11), a member of the IL-1 family of cytokines, serves as an important mediator linking chronic inflammation and metaplasia by inducing the expansion and recruitment of activated eosinophils leading to advanced, intestinalized SPEM in gastritis-prone SAMP1/YitFc (SAMP) mice.
Limitations
Further studies are warranted to determine the precise inciting factors of increased IL-33 leading to intestinalized SPEM in SAMP mice, as well as in patients with gastric cancer.
Impact
The present manuscript contributes to a better understanding of potential mechanism(s) that promote the inflammation-metaplasia-dysplasia-carcinoma sequelae that can apply to several GI-related cancers.
Coronavirus disease 2019 (COVID-19) is a highly contagious and life-threatening infection caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 Identifying modifiable risk factors for COVID-19 would be of substantial public health benefit.
To date, several studies exploring the association between use of acid suppressants and COVID-19 have produced conflicting results,2, 3, 4, 5, 6 which makes it difficult to determine whether there is indeed an increased risk of SARS-CoV-2 infection and death for users of acid suppressants. Thus, we aimed to clarify the potential impact of acid-suppressant treatment on the risk of SARS-CoV-2 infection and death in patients with COVID-19.
Methods
The study included 9469 participants who had been tested for COVID-19 from March 16 to June 29, 2020, in UK Biobank.7 Medication data on UK Biobank patients were obtained through a verbal interview at time of enrollment (2006–2010). Data on short-term medications use were not collected, and only data on regular treatments were included in the database. The primary exposure of interest was acid-suppressive therapy. The 2 main types of acid inhibitors are proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2Ras). In this study, use of acid suppressants was defined as 0 = no and 1 = yes. The primary outcome was the rate of positive SARS-CoV-2 tests, and the secondary outcome was mortality in COVID-19–positive patients.
To reduce confounding effects of potential risk factors on outcomes, propensity score matching (PSM) was applied to match users of acid suppressants and nonusers. Furthermore, we selected patients with upper gastrointestinal diseases for subgroup analysis. The association between variables of interest and odds of SARS-CoV-2 infection was examined by logistic regression. The association between variables of interest and risk of death in COVID-19–positive patients was examined using Cox regression. The false discovery rate method was used for multiple comparisons correction, and an adjusted P value of <.1 was considered significant.8 We also performed a meta-analysis of our data with results of prior studies evaluating the association between acid suppressants use and risk of SARS-CoV-2 infection. Stata 14.0 software (StataCorp, College Station, TX) was used for all statistical analyses. Additional details can be found in the Supplementary Methods.
Results
Participant Characteristics
Among 9469 included participants, 1516 (16%) were regular users of acid suppressants, and 7953 (84%) were not. Regular users of acid suppressants had a higher proportion of patients aged <65 years and higher prevalence of comorbidities compared with nonusers (Supplementary Table 1). In addition, after 1:1 PSM, 1516 acid suppressants users and 1516 matched nonusers were selected for analysis. Participant characteristics in these 2 groups were well-balanced (all P > .1).
Primary Outcome
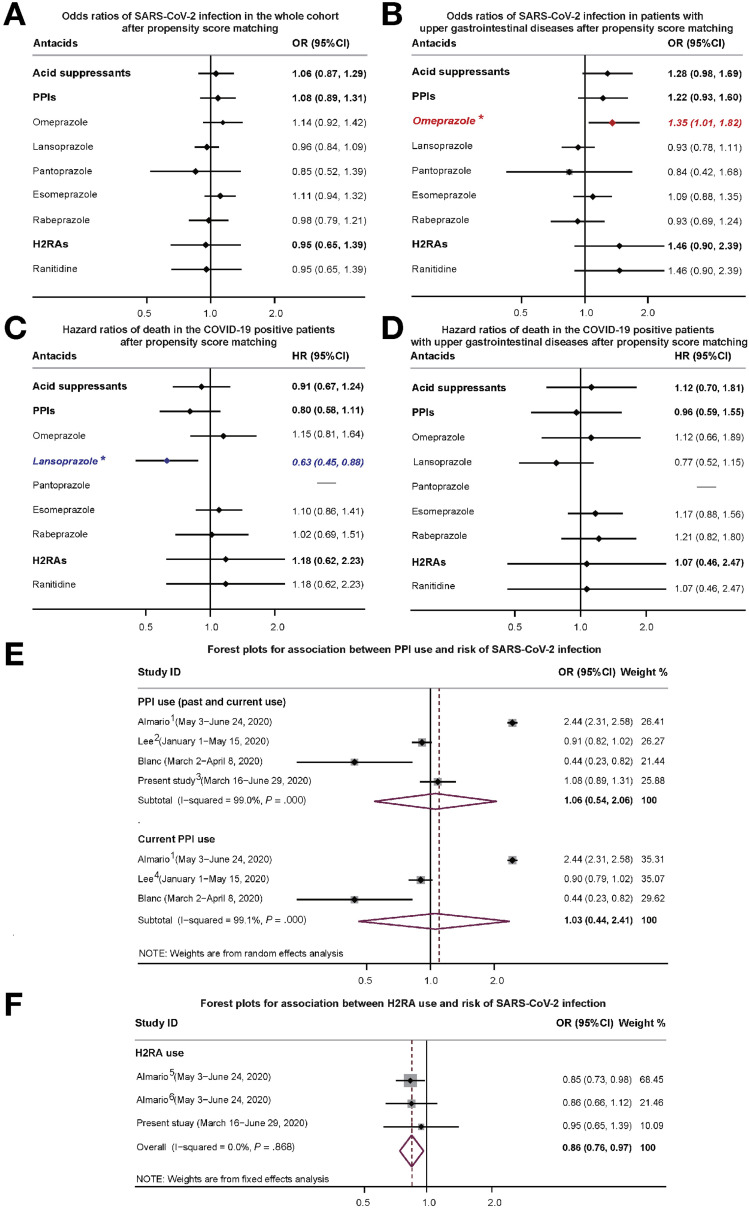
As shown in Figure 1 A, the odds ratio (OR) of testing positive for COVID-19 associated with PPI or H2RA therapy in the PSM cohort was 1.083 (95% confidence interval [CI], 0.892–1.315) and 0.949 (95% CI, 0.650–1.387), respectively. No single type of acid suppressant was associated with the risk of SARS-CoV-2 infection. Similar findings were also observed in the subgroup analysis, neither PPI nor H2RA use was associated with the risk of SARS-CoV-2 infection in patients with upper gastrointestinal diseases (Figure 1 B). However, we found omeprazole use alone was significantly related to an increased risk of SARS-CoV-2 infection from the subgroup analysis in patients with upper gastrointestinal diseases (OR, 1.353; 95% CI, 1.011–1.825; Figure 1 B). This was not observed with use of other types of PPIs.
Figure 1.
Logistic regression analysis of the association between acid-suppressive therapy and the risk of SARS-CoV-2 infection in the (A) whole cohort after PSM and (B) among participants with upper gastrointestinal diseases after PSM (∗P < .1). Cox regression analysis of the association between acid-suppressive therapy and the risk of death in (C) patients with COVID-19 after PSM (∗P < .1) and (D) in COVID-19–positive patients with upper gastrointestinal diseases after PSM. HR, hazard ratio. Forest plots for association between (E) PPI or (F) H2RA use and risk of SARS-CoV-2 infection. The gray boxes denote the effect sizes of studies and the size of each box is proportional to the weight given to each study. The diamonds represent the pooled OR of the meta-analysis, with the tips of the diamond indicating the 95% CI. 1. Almario et al2: the OR used in the meta-analysis was the pooled OR of reporting a positive COVID-19 test associated with once-daily and twice-daily use of PPIs from subgroup analysis. 2. Lee et al3: the OR used in the meta-analysis was the pooled ORs of testing positive for COVID-19 associated with past and current PPIs use from subgroup analysis. 3. In our study, the OR of testing positive for COVID-19 associated with PPIs therapy in the PSM cohort was used in the meta-analysis. 4. Lee et al3: the OR of testing positive for COVID-19 associated with current PPIs use from subgroup analysis was used in the meta-analysis. 5 and 6. Almario et al2: the ORs of reporting a positive COVID-19 test associated with once daily and twice daily use of H2RAs from subgroup analysis were pooled using the fixed effects meta-analysis.
Secondary Outcome
Among patients with COVID-19 in our study, 302 (19.0%) died before June 29, 2020. Neither PPI (hazard ratio, 0.804; 95% CI, 0.581–1.114) nor H2RA use (hazard ratio, 1.180; 95% CI, 0.624–2.232) was associated with the risk of death in patients with COVID-19 in the PSM cohort (Figure 1 C). Only lansoprazole use was potentially associated with a reduced risk of death; however, similar results were not obtained in subgroup analysis in patients with upper gastrointestinal diseases (Figure 1 D).
Meta-analysis
Finally, we performed a meta-analysis of our results with 3 prior studies2 , 3 , 6 on the risk of testing positive for COVID-19 with acid-suppressive therapy (Supplementary Table 2). The pooled ORs of testing positive for COVID-19 associated with PPI use (previous and current use, Figure 1 E), current PPI use only (Figure 1 E), and H2RA use (Figure 1 F) were 1.06 (95% CI, 0.54–2.06), 1.03 (95% CI, 0.44–2.41), and 0.86 (95% CI, 0.76–0.97), respectively.
Discussion
Our findings indicated that neither PPI nor H2RA use was associated with the risk of SARS-CoV-2 infection and death in patients with COVID-19. A notable exception was found in patients with upper gastrointestinal diseases taking omeprazole, who were more susceptible to SARS-CoV-2; this was not observed with use of other types of PPIs. In addition, no evidence of increased SARS-CoV-2 susceptibility was found with the use of PPI or H2RA in the meta-analysis.
Compared with other studies, our results are consistent with a study from South Korea in which PPI use was not associated with the risk of SARS-CoV-2 infection.3 In contrast, another study based on data from a self-administered survey in the United States found PPI but not H2RA use was associated with increased odds of reporting a positive COVID-19 test.2 In this United States survey study, self-reported COVID-19 status was the primary outcome. However, some asymptomatic infected individuals may not have received a SARS-CoV-2 test. Therefore, these individuals might have been classified as healthy participants, resulting in a certain selection bias. Moreover, Almario et al2 included participants who were not currently using PPIs as their reference group, so they were unable to determine the association between past PPI use and the odds of reporting a positive COVID-19 test.
The obvious advantage of our study compared with prior studies is the detailed and validated data in a well-characterized cohort including types of acid suppressants and potential confounding risk factors. As a result of the limitations of the data in the UK Biobank, we only know that participants regularly took acid suppressants at the time of enrollment. Whether participants were still taking acid suppressants is not known. To minimize the impact of this limitation, we conducted a subgroup analysis of patients with upper gastrointestinal diseases, because these patients are more likely to take antacids regularly over time. Further, our meta-analysis included studies with data on current use of antacids, and results were found to be almost identical in both our cohort study and meta-analysis.
Acknowledgments
Collaborators: Xiaoqin Wu1 and Kyle L Poulsen1; 1Department of Inflammation and Immunity, Cleveland Clinic, Cleveland, Ohio.
CRediT Authorship Contributions
Xiude Fan, MD (Conceptualization: Lead; Formal analysis: Lead; Funding acquisition: Supporting; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead); Daniel M. Rotroff, PhD (Funding acquisition: Supporting; Methodology: Supporting; Writing – review & editing: Equal); Srinivasan Dasarathy, MD (Funding acquisition: Supporting; Methodology: Supporting; Writing – review & editing: Supporting); Zhengwen Liu, MD, PhD (Formal analysis: Supporting; Writing – review & editing: Equal); Tasunori Miyata, MD (Formal analysis: Supporting; Writing – review & editing: Supporting); Xiaoqin Wu, PhD (Formal analysis: Supporting; Writing – review & editing: Supporting); Kyle L. Poulsen, PhD (Formal analysis: Supporting; Writing – review & editing: Supporting); Laura E. Nagy, PhD (Conceptualization: Lead; Formal analysis: Equal; Resources: Lead; Writing – original draft: Lead; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported in part by National Institutes of Health grants P50AA024333, U01AA021890, and U01AA026938 (L.E.N.); R01GM119174, R21AR71046, UO1DK061732, and R01DK113196, and the Mikati Foundation Grant (S.D.); and KL2TR002547 (D.M.R.) and K99AA026648 (K.L.P.). X.F. was supported by a fellowship from the China Scholarship Council (File:201806280215). T.M. was supported by a Japan Society for the Promotion of Science (JSPS) Overseas Research Fellowship 201960331.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.09.028
Supplementary Methods
Study Population From UK Biobank
The UK Biobank, a national health resource, recruited more than 500,000 participants aged 40 to 69 years (during 2006–2010) from 22 centers across the United Kingdom. Deidentified data from 502,566 participants were available for our study. We excluded participants without treatment or medication data at baseline (n = 923) and those who died before March 16, 2020 (n = 29,490). Our final analysis included 9469 participants who had been tested for COVID-19 from March 16 to June 29, 2020.
Medication data of individuals enrolled by the UK Biobank were obtained through a verbal interview by a trained nurse at the time of enrollment. If the participant indicated in the initial touch screen questionnaire that they were taking regular prescription medication, the nurse was prompted to record the name of the medication. Data on short-term medications use were not collected, only data on any regular treatments (eg, taken over weeks or months) were included in the database. In addition, doses and formulations of medication were not recorded in the UK Biobank.
The primary exposure of interest was acid-suppressive therapy. The 2 main types of acid inhibitors are PPIs and H2RAs. PPIs mainly consist of omeprazole, lansoprazole, pantoprazole, esomeprazole, and rabeprazole. H2RAs included ranitidine, cimetidine, famotidine, and nizatidine. In this study, we defined the use of acid suppressants as 0 = no and 1= yes.
Data obtained through the baseline touch screen questionnaire, genotype, inpatient hospital, and death register data were used to evaluate several potential confounders: age at time of the COVID-19 test, sex, race (classed as nonwhite and white ethnic background), body mass index, blood type extracted from imputed genotyped data, alcohol drinker status (never, previous, current), smoking status (no, only occasionally, most, or all days), and comorbidities (upper gastrointestinal diseases, chronic lower respiratory diseases, chronic heart diseases, diabetes mellitus, dementia, liver cirrhosis or liver failure, or both, renal failure, and acquired immunodeficiency syndrome).
Comorbidities were identified by medical records and death records. International Classification of Diseases, Tenth Revision (ICD-10) codes were used for cause of death for all participants and to identify comorbidities. Esophagitis, gastroesophageal reflux disease, peptic ulcer, and gastritis/duodenitis were uniformly classified as upper gastrointestinal disease events. Chronic lower respiratory diseases were defined as participants with chronic obstructive pulmonary disease, emphysema, bronchitis/bronchiectasis, or asthma. Chronic cardiac events were defined as participants with heart failure, hypertension, or chronic ischemic heart disease. Diabetes mellitus, dementia, liver cirrhosis or liver failure, renal failure, and acquired immunodeficiency syndrome were defined according to the related ICD-10 codes.
Ascertainment of Outcomes
The primary outcome used in the current study was rate of positive SARS-CoV-2 tests and the secondary outcome was mortality in COVID-19–positive patients. The UK Biobank began releasing COVID-19 test results from March 16, 2020 (http://biobank. ndph.ox.ac.uk/showcase/exinfo.cgi?src=COVID19_availability). Before April 27, 2020, testing for COVID-19 was initially limited to those with symptoms in hospitals. After April 27, 2020, hospitals could test patients admitted for overnight stays, including those without symptoms. In this study, we defined the result of the COVID-19 test as 0 = negative and 1= positive. COVID-19 death events were collected through certified death records with ICD-10 code U071 (n = 282) as well as all COVID-19–positive patients who died even without the corresponding code (n = 20).
Application of Propensity Score Matching Method in Analysis
To reduce the confounding effects of these diseases on outcomes, 1:1 ratio PSM was applied to match acid suppressants users and nonusers without replacement when we evaluated the associations between acid suppressants and the odds of SARS-CoV-2 infection. We included variables previously associated with higher risk for testing positive for COVID-19, including age, sex, race, body mass index categories, alcohol drinker status, smoking status, upper gastrointestinal diseases, chronic obstructive pulmonary disease, bronchitis/bronchiectasis, emphysema, asthma, heart failure, hypertension, chronic ischemic heart disease, diabetes, renal failure, liver cirrhosis or liver failure, dementia, and acquired immunodeficiency syndrome. Next, the 1:2 ratio PSM was applied to match acid-suppressants users and nonusers in the COVID-19–positive cohort. Matching factors for PSM were consistent as described above.
Meta-analysis of Results With Prior Studies
We searched PubMed and Google Scholar from inception up to August 1, 2020. Three studies2 , 3 , 6 were identified and selected for analysis with the search terms (“acid-suppressive drugs” OR “acid suppressive therapy” OR “anti-ulcer agent” OR “antacid” OR “acid-suppressive medications” OR “gastric acid suppressants” OR “proton pump inhibitors” OR “proton pumps” OR “PPI or PPIs” OR “omeprazole” OR “lansoprazole” OR “rabeprazole” OR “pantoprazole” OR “esomeprazole” OR “histamine receptor 2 antagonists” OR “H2RAs” OR “ranitidine” OR “cimetidine” OR “famotidine” OR “nizatidine”) AND (“COVID-19” OR “SARS-CoV-2”).
Meta-analysis was applied to calculate pooled ORs with 95% CIs by integrating the results of previous studies with our own study. Adjusted ORs (AORs) obtained from studies were used for our analysis (Supplementary Table 2). We separately combined the ORs of reporting a positive COVID-19 test associated with low-dose and high-dose use of PPIs from subgroup analysis within the prior study2 and the ORs of risk of SARS-CoV-2 infection associated with past and current PPIs use from subgroup analysis within the prior study3 using the fixed-effects meta-analysis. The pooled ORs were then applied for the random-effects meta-analysis of the relationship between overall PPIs use and risk of infection.
As a result of the limitations of the data in the UK Biobank used in our analysis, we only know that participants regularly took acid suppressants at the time they completed their UK Biobank enrollment questionnaire. Data on current use are not available. Therefore, we considered the patients who took PPIs in the cohort of UK Biobank as overall PPI users (both previous and current use) in the meta-analysis. Heterogeneity among studies was evaluated using Cochran’s χ2 and the I 2 statistic. A P value <.10 from the χ2 test or an I 2 value >50%, or both, were considered significant for heterogeneity.
Supplementary Table 1.
Characteristics of Middle-Aged and Elderly Participants Who Underwent SARS-CoV-2 Testing in the UK Biobank Stratified by the Use of Acid Suppressants
| Variables | Cohort before PSM |
Cohort after PSM |
||||
|---|---|---|---|---|---|---|
| Non-user (n = 7953) | Anti-acids user (n = 1516) | Adjusted P-value |
Non-user (n = 1516) | Anti-acids user (n = 1516) | Adjusted P-value |
|
| Age (years), n (%) | <.001 | >.99 | ||||
| <65 | 2647 (33.3) | 250 (16.5) | 246 (16.2) | 250 (16.5) | ||
| ≥65 | 5306 (66.7) | 1266 (83.5) | 1270 (83.8) | 1266 (83.5) | ||
| Male, n (%) | 3869 (48.6) | 742 (48.9) | .854 | 758 (50.0) | 742 (48.9) | >.99 |
| Race, n (%) | .273 | >.99 | ||||
| White | 7318 (92.0) | 1409 (92.9) | 1398 (92.2) | 1409 (92.9) | ||
| No white | 635 (8.0) | 107 (7.1) | 118 (7.8) | 107 (7.1) | ||
| BMI categories, n (%) | <.001 | .617 | ||||
| Underweight (<18.5) | 45 (0.6) | 6 (0.4) | 9 (0.6) | 6 (0.4) | ||
| Normal weight (18.5-24.9) | 2425 (30.5) | 280 (18.5) | 246 (16.2) | 280 (18.5) | ||
| Overweight (25-29.9) | 3272 (41.1) | 560 (36.9) | 607 (40.0) | 560 (36.9) | ||
| Obesity (≥30) | 2211 (27.8) | 670 (44.2) | 654 (43.1) | 670 (44.2) | ||
| Blood type, n (%) | .100 | .544 | ||||
| OO | 3183 (41.5) | 612 (42.1) | 614 (42.2) | 612 (42.1) | ||
| AA+AO | 3385 (44.2) | 655 (45.1) | 632 (43.4) | 655 (45.1) | ||
| BB+BO | 813 (10.6) | 152 (10.5) | 151 (10.4) | 152 (10.5) | ||
| AB | 283 (3.7) | 34 (2.3) | 59 (4.1) | 34 (2.3) | ||
| Alcohol drinker status, n (%) | <.001 | .360 | ||||
| Never | 438 (5.5) | 112 (7.4) | 118 (7.8) | 112 (7.4) | ||
| Previous | 374 (4.7) | 138 (9.1) | 99 (6.5) | 138 (9.1) | ||
| Current | 7141 (89.8) | 1266 (83.5) | 1299 (85.7) | 1266 (83.5) | ||
| Current smoking, n (%) | .693 | .597 | ||||
| No | 6914 (86.9) | 1307 (86.2) | 1299 (85.7) | 1307 (86.2) | ||
| Only occasionally | 273 (3.4) | 51 (3.4) | 38 (2.5) | 51 (3.4) | ||
| Most or all days | 766 (9.6) | 158 (10.4) | 179 (11.8) | 158 (10.4) | ||
| Comorbidities, n (%) | ||||||
| Upper gastrointestinal diseases | ||||||
| Oesophagitis | 271 (3.4) | 182 (12.0) | <.001 | 176 (11.6) | 182 (12.0) | >.99 |
| GERD | 598 (7.5) | 472 (31.1) | <.001 | 380 (25.1) | 472 (31.1) | .005 |
| Peptic ulcer | 189 (2.4) | 156 (10.3) | <.001 | 127 (8.4) | 156 (10.3) | .480 |
| Gastritis/duodenitis | 720 (9.1) | 448 (29.6) | <.001 | 438 (28.9) | 448 (29.6) | >.99 |
| Chronic lower respiratory diseases | ||||||
| COPD | 391 (4.9) | 217 (14.3) | <.001 | 190 (12.5) | 217 (14.3) | .664 |
| Emphysema | 82 (1.0) | 34 (2.2) | <.001 | 36 (2.4) | 34 (2.2) | .986 |
| Bronchitis/Bronchiectasis | 114 (1.4) | 46 (3.0) | <.001 | 48 (3.2) | 46 (3.0) | .957 |
| Asthma | 802 (10.1) | 328 (21.6) | <.001 | 296 (19.5) | 328 (21.6) | .787 |
| Chronic heart diseases | ||||||
| Heart failure | 291 (3.7) | 120 (7.9) | <.001 | 120 (7.9) | 120 (7.9) | >.99 |
| Hypertensive | 2561 (32.2) | 928 (61.2) | <.001 | 929 (61.3) | 928 (61.2) | .970 |
| Chronic ischaemic heart disease | 899 (11.3) | 431 (28.4) | <.001 | 399 (26.3) | 431 (28.4) | .552 |
| Diabetes mellitus | 854 (10.7) | 372 (24.5) | <.001 | 345 (22.8) | 372 (24.5) | .638 |
| Dementia | 64 (0.8) | 21 (1.4) | .037 | 22 (1.5) | 21 (1.4) | >.99 |
| Liver cirrhosis and/or liver failure | 52 (0.7) | 23 (1.5) | .001 | 28 (1.8) | 23 (1.5) | >.99 |
| Renal failure | 516 (6.5) | 237 (15.6) | <.001 | 232 (15.3) | 237 (15.6) | >.99 |
| AIDS | 7 (0.1) | 3 (0.2) | .248 | 3 (0.2) | 3 (0.2) | .984 |
| Medication, n (%) | ||||||
| PPIs | 0 | 1354 (89.3) | — | 0 | 1354 (89.3) | — |
| Omeprazole | 0 | 797 (52.6) | — | 0 | 797 (52.6) | — |
| Lansoprazole | 0 | 520 (34.3) | — | 0 | 520 (34.3) | — |
| Pantoprazole | 0 | 19 (1.3) | — | 0 | 19 (1.3) | — |
| Esomeprazole | 0 | 49 (3.2) | — | 0 | 49 (3.2) | — |
| Rabeprazole | 0 | 27 (1.8) | — | 0 | 27 (1.8) | — |
| H2RAs | 0 | 220 (14.5) | — | 0 | 220 (14.5) | — |
| Ranitidine | 0 | 219 (14.4) | — | 0 | 219 (14.4) | — |
| Cimetidine | 0 | 0 | — | 0 | 0 | — |
| Nizatidine | 0 | 2 (0.1) | — | 0 | 2 (0.1) | — |
| Famotidine | 0 | 0 | — | 0 | 0 | — |
| COVID-19, n (%) | 1341 (16.9) | 250 (16.5) | .754 | 238 (15.7) | 250 (16.5) | .939 |
NOTE: Data are presented as number (%). The adjusted P value was calculated by false discovery rate method.
AA+AO indicates participants with type A blood, BB+BO indicates participants with type B blood.
Supplementary Table 2.
Characteristics of Observational Studies Reporting the Effects of Acid Suppressants Use on Risk of SARS-CoV-2 Infection
| Study | Country of participants | Study period | Study design | Total | Age, y | Male, % | Acid suppressants exposure | Types of acid suppressants | OR (95% CI)a |
|---|---|---|---|---|---|---|---|---|---|
| Almario2a | United States | May 3–June 24 2020 | Case control | 53,130 | Aged ≥60 (13.3% of participants) | 48 | Current use (at the time of survey) | Once-daily PPI use | 2.15 (1.90–2.44) |
| Twice-daily PPI use | 3.67 (2.93–4.60) | ||||||||
| Once-daily H2RA use | 0.85 (0.74–0.99) | ||||||||
| Twice-daily H2RA use | 0.86 (0.66–1.11) | ||||||||
| Lee3b | South Korea | January 1–May 15 2020 | Cohort | 132316 | mean age, 48 | 51 | Past use (31–365 days before the index date) | Past PPI use | 0.94 (0.77–1.15) |
| Current use (1–30 days before the index date) | Short-term PPI use | 0.94 (0.80–1.11) | |||||||
| Long-term PPI use | 0.85 (0.72–1.01) | ||||||||
| Blanc 6c | France | March 2–April 8 2020 | Case control | 179 | mean age, 84 | 31.8 | Current use (1–15 days before the index date) | PPI use | 0.43 (0.23–0.82) |
| Our studyd | United Kingdom | March 16–June 29 2020 | Cohort | 9469 | Aged ≥ 65 (69.4% of participants) | 48.7 | Ever use (no data on current use) | PPI use in whole cohort | 1.08 (0.89–1.31) |
| PPI use in patients with upper gastrointestinal diseases | 1.22 (0.93–1.60) | ||||||||
| H2RA use in whole cohort | 0.95 (0.65–1.39) | ||||||||
| H2RA use in patients with upper gastrointestinal diseases | 1.46 (0.90–2.39) |
In the Almario et al study, risk factors were adjusted for age, sex, race, education level, marital status, employment status, income, body mass index, current smoking, alcohol use, region, insurance status, usual source of care, and irritable bowel disease, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and acquired immunodeficiency syndrome.
In the Lee et al study, risk factors were adjusted for age, sex, region; history of diabetes, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, and chronic kidney disease; Charlson Comorbidity Index, and current use of systemic steroid, metformin, and aspirin.
In the Blanc et al study, adjusted factors were not clear.
In our study, PSM was performed before logistic regression analysis. Matching factors for PSM including age, sex, race, body mass index categories, alcohol drinker status, smoking status, upper gastrointestinal diseases, chronic obstructive pulmonary disease, emphysema, asthma, bronchitis/bronchiectasis, heart failure, hypertensive, chronic ischaemic heart disease, diabetes, renal failure, liver cirrhosis and/or liver failure, dementia, and acquired immunodeficiency syndrome.
Supplementary Material
References
- 1.Bergmann C.C. Cleve Clin J Med. 2020;87:321–327. doi: 10.3949/ccjm.87a.20047. [DOI] [PubMed] [Google Scholar]
- 2.Almario C.V. Am J Gastroenterol. 2020;115:1707–1715. doi: 10.14309/ajg.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.W. Gut. 2020 doi: 10.1136/gutjnl-2020-322248. [DOI] [Google Scholar]
- 4.Luxenburger H. J Intern Med. 2020 doi: 10.1111/joim.13121. [DOI] [Google Scholar]
- 5.Freedberg D.E. Gastroenterology. 2020;159:1129–1131.e3. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc F. Preprint. 2020 doi: 10.20944/preprints202005.0016.v1. [DOI] [Google Scholar]
- 7.Sudlow C. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamini Y. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.