Abstract
Background
A direct comparison of severe acute respiratory syndrome coronavirus 2 positive patients with a severe acute respiratory syndrome coronavirus 2 negative control group undergoing an operative intervention during the current pandemic is lacking, and a reliable estimate of the assumed difference in morbidity and mortality between both patient categories remains unknown.
Methods
We included all consecutive patients with a confirmed pre- or postoperative severe acute respiratory syndrome coronavirus 2 positive status (operated in 27 hospitals) and negative control patients (operated in 4 hospitals) undergoing emergency or elective operations. A propensity score-matched comparison of clinical outcomes was performed between severe acute respiratory syndrome coronavirus 2 positive and negative tested patients (control group). Primary outcome was overall 30-day mortality rate between both groups. Main secondary outcomes were overall, pulmonary, and thromboembolic complications.
Results
In total, 161 severe acute respiratory syndrome coronavirus 2 positive and 342 control severe acute respiratory syndrome coronavirus 2 negative patients were included in this study. The 30-day overall postoperative mortality rate was greater in the severe acute respiratory syndrome coronavirus 2 positive cohort compared with the negative control group (16% vs 4% respectively; P = .007). After propensity score matching, the severe acute respiratory syndrome coronavirus 2 positive group consisted of 123 patients (median 70 years of age [interquartile range 59–77] and 55% male) were compared with 196 patients in the matched control group (median 69 years (interquartile range 58–75] and 53% male). The 30-day mortality rate and risk were greater in the severe acute respiratory syndrome coronavirus 2 positive group compared with the matched control group (12% vs 4%; P = .009 and odds ratio 3.4 [95% confidence interval 1.5–8.5]; P = .005, respectively). Overall, pulmonary and thromboembolic complications occurred more often in severe acute respiratory syndrome coronavirus 2 positive patients (P < .01).
Conclusion
Patients diagnosed with perioperative severe acute respiratory syndrome coronavirus 2 have an increased risk of 30-day mortality, pulmonary complications, and thromboembolic events. These findings serve as an evidence-based argument to postpone elective surgery and selected emergency cases.
Introduction
The worldwide pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 9 million registered coronavirus disease 2019 (COVID-19) cases and led to major disruptions of the global health care system.1 During the current peak of the pandemic, surgical theatres and recovery areas have been converted to intensive care unit (ICU) facilities to treat patients with SARS-CoV-2 infections.2 As a consequence, the global capacity for elective surgical care has decreased markedly with an estimated number of 2.4 million cases per week.3 Nevertheless, surgical emergency and urgent elective procedures needed to be continued in endemic areas. A recently published study of 1,128 patients with perioperative SARS-CoV-2 infections undergoing an operation in various health care systems across the world reported a 30-day postoperative overall mortality rate of 24%.4 Pulmonary complications were reported in 51% of patients. The 24% mortality rate in SARS-CoV-2 positive patients is unusually high compared with routine reported rates in similar elective and emergency operations before the pandemic.5 , 6 Recently, a retrospective, case-control study from Italy reported a 20% 30-day postoperative overall-mortality rate (odds ratio [OR] 9.5, 95% confidence interval [CI] 1.8–96.5) and a greater risk of pulmonary complications (OR 35.6, 95% CI 9.3–205.6) and thromboembolic complications (OR 13.2, 95% CI 1.5–∞) in 41 SARS-CoV-2 positive patients compared with a mainly historical control cohort.7 Although of relevance, this single-center study has limitations owing to the small sample of SARS-CoV-2 positive patients and the overall design resulting in inaccurate estimates. It is, therefore, important to provide clinicians with more accurate data to improve perioperative clinical decision-making for this patient category during the foreseen new waves of SARS-CoV-2 infections. This current multicenter, nationwide, matched-cohort study compares the 30-day postoperative morbidity and mortality rates between SARS-CoV-2 positive and negative patients undergoing elective or emergency operations in hospitals within a similar health care system and standardized surgical guidelines. Results of this study will provide a more reliable insight into the actual difference in overall mortality and complications between patients with and without perioperative SARS-CoV-2 infection.
Methods
Setting
This nationwide, multicenter, observational, cross-sectional retro- and prospective cohort study was conducted in the Kingdom of the Netherlands. The study protocol was designed at the University Medical Center Groningen (UMCG) and approved after an expedited review by the respective local ethical review committees of participating centers (METc 2020/170, non-WMO approval). Data transfer agreements between UMCG and participating centers were established. Informed consent of included patients was acquired in line with local regulations. Dutch surgeons were informed about this study with regular updates via the Dutch Surgical Society. From the first week of April, nationwide, routine, preoperative screening by quantitative reverse transcription polymerase chain reaction (RT-PCR) with or without a computed tomography (CT) of the chest was implemented per standard of care.8 The SARS-CoV-2 positive cohort was established from consecutive patients with a pre- or postoperative SARS-CoV-2 positive status who underwent an operation between February 27 and June 1, 2020 in 27 centers across the Kingdom of the Netherlands, covering 10 out of 12 provinces. The negative control group was recruited at 4 of the 27 centers (Hospital Bernhoven [Uden], Medical Center Leeuwarden [Leeuwarden], Radboud University Medical Center [Nijmegen], and UMCG [Groningen]) consisting of consecutive SARS-CoV-2 negative patients who underwent routine preoperative SARS-CoV-2 RT-PCR screening. These 4 centers (2 teaching hospitals and 2 tertiary referral centers) were chosen intentionally and spread across the country to include geographic areas with different incidence rates of SARS-CoV-2. When no 30-day follow-up was planned, patients were contacted per phone by coordinating researchers to acquire final follow-up status.
Inclusion and exclusion criteria
Patients eligible for inclusion in the SARS-CoV-2 positive cohort either had a SARS-CoV-2 positive RT-PCR test (nasopharyngeal or throat swab) or a strong clinical suspicion combined with a CT of the chest defined as suspect for SARS-CoV-2 infection 30 days before surgery or within 30 days postoperatively. Patients eligible for inclusion in the control group had a negative SARS-CoV-2 history, tested negative for SARS-CoV-2 during preoperative screening with RT-PCR, and remained negative during the 30-days of follow-up. The SARS-CoV-2 status during follow-up was assessed clinically based on symptomatology, without routine RT-PCR screening of asymptomatic patients. Patients of all ages and all surgical subspecialties were included in both cohorts on the condition of availability of completed 30-day follow-up. Patients were excluded when data were insufficient or follow-up information could not be completed.
Data acquisition and management
Data were collected and managed using the REDCap electronic data capture tool (version 8.10.18; Vanderbilt University, Nashville, TN).9 Pseudo anonymized data was entered manually from the electronic patient file in the electronic case report form by local researchers of the respective participating centers and cross-checked for inconsistencies and missing data by the coordinating researchers before data locking. Per site, a deidentification key was stored in an on-site, secured, digital data storage area. Center-specific data were accessible by local researchers and the coordinating researchers from the UMCG (S.K., P.K.C.J., W.Y.vdP., P.J.S., J.P.P.M.dV.). Before analysis, parameters were checked for completion per case, and data were curated by coordinating researchers.
Study parameters
Baseline patient demographics, comorbidity status, and drug use at initial presentation were recorded. The prevalence of SARS-CoV-2 positive people per 1,000 habitants per municipality on the first of May was acquired from the Dutch government.10 Clinical symptoms at SARS-CoV-2 diagnosis were scored. The Charlson comorbidity index was calculated.11 Operative procedures were graded with the surgical risk stratification from the University of California Los Angeles, a numerical value to reflect the risk level associated with the operation ranging from 1 (very low risk) to 5 (very high risk).12 Overall survival, SARS-CoV-2 infection status, and complication rates (according to the Clavien-Dindo classification and summarized by the Comprehensive Complication Index) were scored at 30 days from the index operation.13 , 14 Pulmonary complications were diagnosed either clinically (ie, respiratory insufficiency) or with imaging (ie, pneumonia, acute respiratory distress syndrome). Clinically diagnosed thromboembolic complications were confirmed with imaging.
Study end points
The primary endpoint was defined as the overall, 30-day postoperative mortality rate. As secondary outcomes, complication rate, complication severity, pulmonary complications, and thromboembolic events were compared between the matched cohorts.
Statistical analysis
Bivariate frequencies were calculated for the descriptive analysis. Missing data were included in the descriptive analyses. The χ2 test was used for categorical data, and logistic modeling was used for calculating ORs with 95% CIs. Propensity score matching was used to help control for differences at baseline between those patients undergoing an operation who had a preoperative SARS-CoV-2 infection or who developed a SARS-CoV-2 infection within the immediate 30 days postoperatively and those patients undergoing an operation without SARS-CoV-2 infection confirmed pre or postoperatively. Cohorts were matched by age, sex, body mass index, smoking status, preoperative comorbidities (diabetes, hypertension, congestive heart failure, myocardial infarction, peripheral vascular disease, cerebrovascular events, chronical renal disease, cancer, transplant), immunosuppressive medication, Eastern Cooperative Oncology Group score, Charlson comorbidity index, American Society of Anesthesiologists (ASA) classification, type of anesthesia, and surgical risk score. The individual propensities for infection with SARS-CoV-2 were estimated with the use of a multivariable logistic regression model that included all baseline covariates. In the propensity score-matching analysis, the nearest-neighbor method was applied with a caliper of .25 to create a matched control sample. Various matching strategies were explored. These strategies included matching with or without replacement of patients, with or without re-estimation of the propensity scores during matching, and matching smallest or largest distance first. Assessment of the covariate balance was done by comparing standardized differences before and after matching. The analysis of the primary and secondary outcomes was done by using the matched sample. Multiple imputation was used to handle missing data, and model estimates and standard errors were calculated with Rubin’s rules.15 Finally, a subgroup analysis was performed including only patients with a SARS-CoV-2 positive status diagnosed either 7 days pre- or postoperatively. Statistical analysis was performed using R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Imputation was done using the mice-package and matching was done using the MatchIt-package (R Foundation for Statistical Computing).16 , 17
Results
Description of the cohort
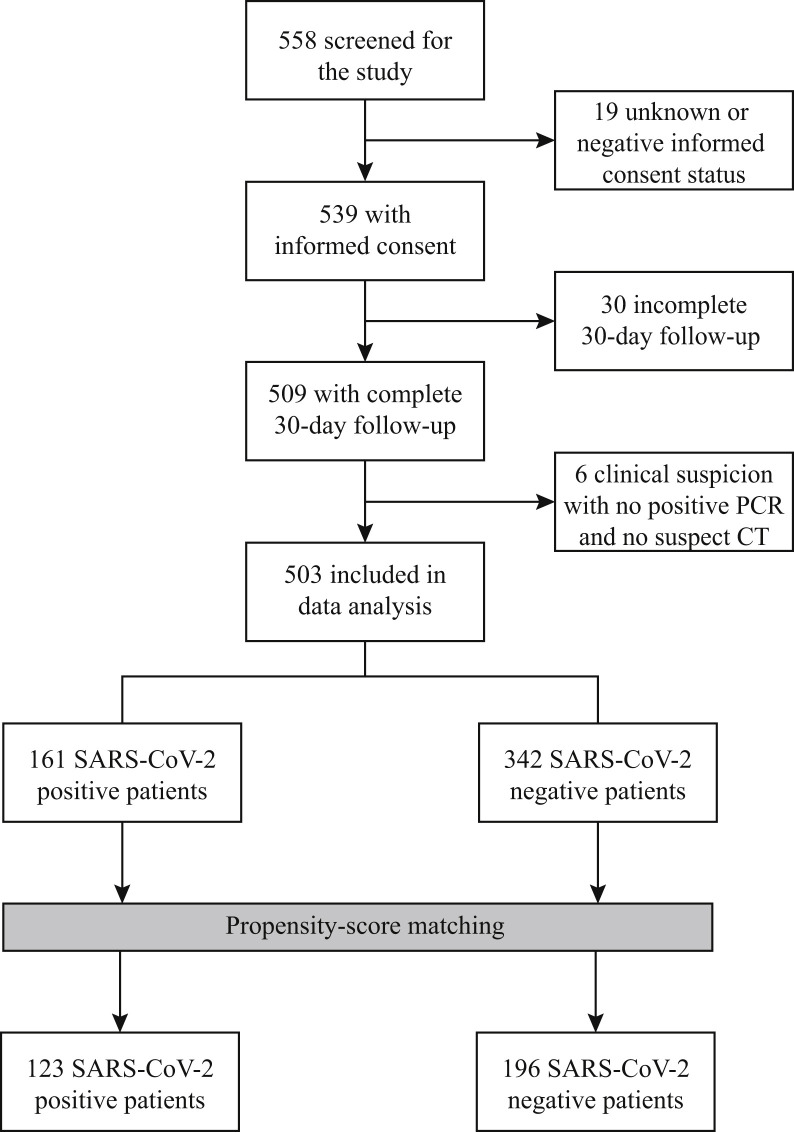
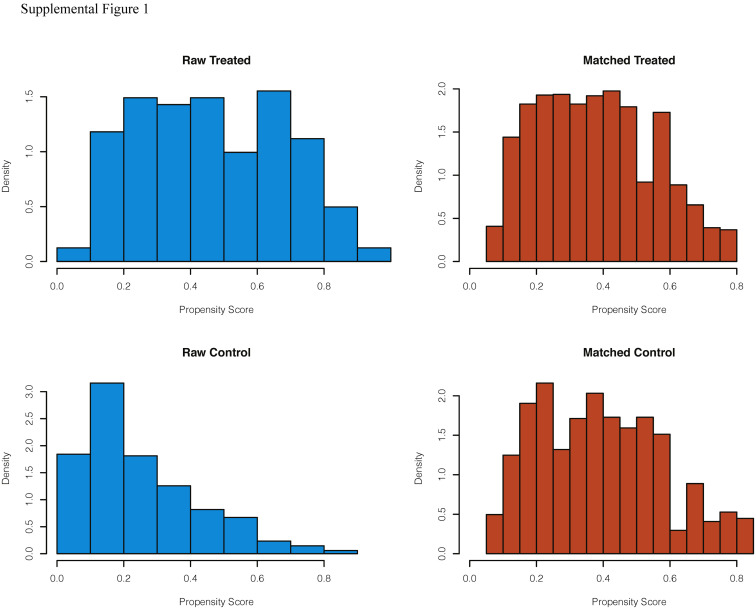
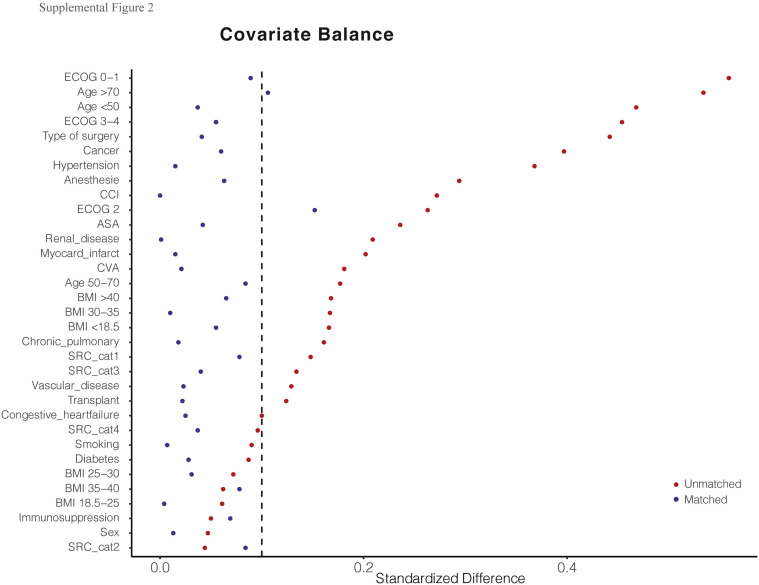
A total of 558 patients undergoing an operation during the pandemic were screened for study inclusion. Ultimately, 161 SARS-CoV-2 positive and 342 SARS-CoV-2 negative control patients with complete 30-day postoperative follow-up were included in the unmatched cohort (Fig 1 ). Baseline characteristics of the included patients are shown in Table I . The 342 SARS-CoV-2 negative patients underwent operative intervention in Hospital Bernhoven (23 [7%]), Medical Center Leeuwarden (124 [36%]), UMCG (93 [27%]), and at the Radboud University Medical Center (102 [30%]). After propensity matching, a matched cohort of 123 SARS-CoV-2 positive and 196 SARS-CoV-2 negative control patients was established (Table I). The distribution of the estimated propensity scores for a positive SARS-CoV-2 status among SARS-CoV-2 positive and negative control patients is shown in Supplemental Fig 1. ORs for a positive SARS-CoV-2 status according to all the variables included in the propensity score model are shown in Supplemental Table I. The C-statistic for the model was 0.78. The differences between SARS-CoV-2 status and baseline variables were attenuated in the propensity score-matched samples as compared with the unmatched samples (Supplemental Fig 2). An overview of operative procedures in the unmatched and matched cohorts is provided in Supplemental Table II.
Fig 1.
Study flowchart.
Table I.
Baseline characteristics of the unmatched and propensity score-matched patient groups
| Unmatched patients |
Propensity score-matched patients∗ |
|||
|---|---|---|---|---|
| SARS-CoV-2 positive patients n = 161 | SARS-CoV-2 negative patients n = 342 | SARS-CoV-2 positive patients n = 123 | SARS-CoV-2 negative patients n = 196 | |
| Age category–no (%) | ||||
| <50 y | 14 (8.7) | 89 (26.0) | 13 (10.6) | 19 (9.7) |
| 50–<70 y | 51 (31.7) | 138 (40.4) | 47 (38.2) | 80 (40.8) |
| ≥70 y | 95 (59.0) | 115 (33.6) | 63 (51.2) | 97 (49.5) |
| Female sex–no (%) | 72 (44.7) | 161 (47.1) | 56 (45.5) | 93 (47.4) |
| BMI–no (%) | ||||
| <18.5 | 7 (4.3) | 29 (8.5) | 6 (4.9) | 10 (5.1) |
| 18.5–<25 | 49 (30.4) | 117 (34.2) | 41 (33.3) | 65 (33.2) |
| 25–<30 | 46 (28.6) | 112 (32.7) | 39 (31.7) | 66 (33.7) |
| 30–<35 | 32 (19.6) | 49 (14.3) | 24 (19.5) | 38 (19.4) |
| 35–<40 | 12 (7.5) | 21 (6.1) | 10 (8.1) | 15 (7.7) |
| ≥40 | 5 (3.1) | 3 (0.9) | 3 (2.4) | 2 (1.0) |
| Missing | 10 (6.2) | 11 (3.2) | 0 | 0 |
| Current smoker–no (%) | 18 (11.2) | 47 (13.7) | 18 (14.6) | 33 (16.8) |
| Missing | 33 (20.5) | 71 (20.8) | 0 | 0 |
| Diabetes–no (%) | 44 (27.3) | 42 (12.3) | 22 (18) | 35 (18) |
| Hypertension–no (%) | 80 (49.7) | 105 (30.7) | 53 (43.1) | 87 (44.4) |
| Missing | 0 (0.0) | 13 (3.8) | 0 | 0 |
| Congestive heart failure–no (%) | 13 (8.1) | 19 (5.6) | 8 (6.5) | 13 (6.6) |
| Myocardial infarct–no (%) | 21 (13.0) | 24 (7.0) | 12 (9.8) | 19 (9.7) |
| Peripheral vascular disease–no (%) | 26 (16.1) | 40 (11.7) | 16 (13) | 27 (14) |
| Chronic pulmonary disease–no (%) | 20 (12.4) | 26 (7.6) | 10 (8.1) | 20 (10) |
| CVA–no (%) | 24 (14.9) | 31 (9.1) | 13 (11) | 21 (11) |
| Chronic renal disease–no (%) | 22 (13.7) | 25 (7.3) | 10 (8.1) | 16 (8.2) |
| Cancer–no (%) | 30 (18.6) | 123 (36.0) | 27 (22) | 47 (24) |
| Transplant–no (%) | 1 (0.6) | 7 (2.0) | 1 (0.8) | 1 (0.5) |
| Missing | 2 (1.2) | 2 (0.6) | 0 | 0 |
| Immunosuppression–no (%) | 10 (6.2) | 17 (5.0) | 9 (7.3) | 10 (5.1) |
| Missing | 0 | 6 (1.8) | 0 | 0 |
| ECOG score | ||||
| 0–1 | 93 (57.8) | 288 (84.2) | 92 (75) | 155 (79) |
| 2 | 26 (16.1) | 29 (8.5) | 21 (17) | 25 (13) |
| 3–4 | 30 (18.6) | 18 (5.3) | 10 (8.1) | 16 (8.2) |
| Missing | 12 (7.5) | 7 (2.0) | 0 | 0 |
| Charlson comorbidity index–median (IQR) | 4 (2–7) | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| Missing | 0 (0.0) | 1 (0.3) | 0 | 0 |
| ASA classification–no (%) | ||||
| I–II | 60 (37.3) | 169 (49.4) | 54 (44) | 87 (44) |
| III–IV | 97 (60.2) | 170 (49.7) | 69 (56) | 109 (56) |
| Missing | 4 (2.5) | 3 (0.9) | 0 | 0 |
| Anesthesia–no (%) | ||||
| General | 118 (73.3) | 291 | 98 (80) | 156 (80) |
| Other (including spinal) | 43 (26.7) | 51 | 25 (20) | 40 (20) |
| Missing | 0 (0.0) | 0 (0.0) | 0 | 0 |
| Type of operation–no (%) | ||||
| Elective | 87 (54.0) | 252 (73.7) | 76 (62) | 131 (67) |
| Emergency | 73 (45.3) | 84 (24.6) | 47 (38) | 65 (33) |
| Missing | 1 (0.6) | 6 (1.8) | 0 | 0 |
| Surgical risk score–no (%) | ||||
| 1–2 | 42 (26.1) | 72 (21.1) | 35 (29) | 48 (25) |
| 3 | 64 (39.8) | 135 (39.5) | 47 (38) | 86 (44) |
| 4 | 36 (22.4) | 100 (29.2) | 31 (25) | 48 (25) |
| 5 | 10 (6.2) | 31 (9.1) | 10 (8.1) | 14 (7.1) |
| Missing | 9 (5.6) | 4 (1.2) | 0 | 0 |
BMI, body mass index; CVA, cerebrovascular accident; ECOG, Eastern Cooperative Oncology Group.
Data for patients included in the propensity-score–matched analysis were multiply imputed.
SARS-CoV-2 positive patients
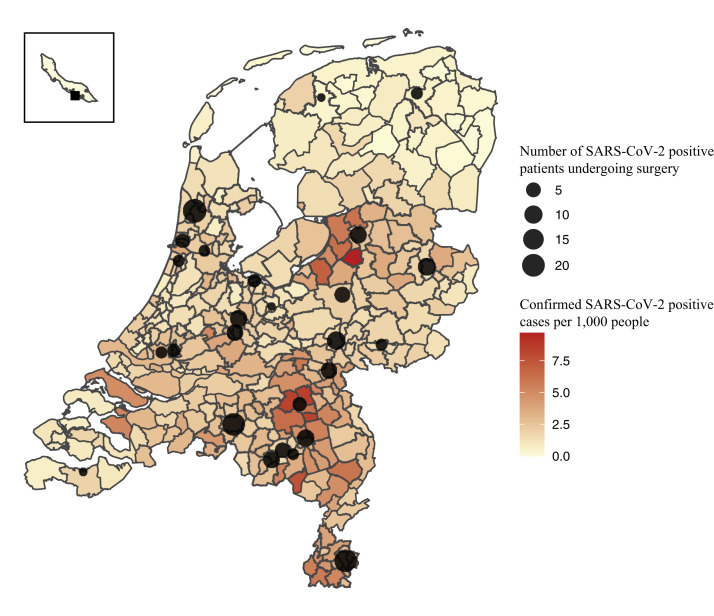
The 161 patients with perioperative SARS-CoV-2 positive status underwent operations in 27 centers. Distribution of participating centers across the Netherlands combined with the prevalence of confirmed SARS-CoV-2 positive people per municipality in May 2020 is presented in Fig 2 . The majority of SARS-CoV-2 positive patients was male (89 [55%]) with a median age of 72 years (interquartile range [IQR] 62–78). Patients with a perioperative SARS-CoV-2 infection underwent mainly elective operations (87 [54%]) with general anesthesia (118 [73%]), as shown in Table I; the majority of patients (92 [57%]) was diagnosed postoperatively with SARS-CoV-2. In case of preoperative diagnosis, median time from SARS-CoV-2 diagnosis to operation was 8 days (IQR 2–22 days). For the 92 patients diagnosed postoperatively, median time from the operation to diagnosis was 8 days (IQR 3–17 days). The diagnosis of SARS-CoV-2 was made with positive RT-PCR (150 [93%]) or a chest CT highly suspicious for SARS-CoV-2 in combination with clinical symptoms (11 [7%]). Eleven patients (7%) had an asymptomatic presentation at diagnosis but tested positive by RT-PCR. Eighty-seven percent of SARS-CoV-2 positive patients (n = 140) presented with 1 or more symptoms. The 5 most prevalent symptoms at diagnosis were fever (94 [58%]), cough (84 [52%]), tiredness (74 [46%]), dyspnea (66 [41%]), and abdominal pain (26 [23%]). SARS-CoV-2 positive patients received treatment on the ward (101 [63%]), in the ICU (40 [25%]), at home quarantine, in a nursing home, in a rehabilitation center (17 [11%]), or at an unknown location (3 [2%]). Endotracheal ventilation as treatment for SARS-CoV-2 was required in 33 patients (21%), and no extracorporeal membrane oxygenation was performed. According to the primary outcome (survivor versus nonsurvivor), more details of the unmatched SARS-CoV-2 group are listed in Table II . Of the nonsurvivor group (n = 26), patients were generally older. Deceased patients had diabetes, hypertension, or peripheral vascular disease more frequently. Additionally, they had a worse Eastern Cooperative Oncology Group performance status and higher ASA classification. No difference was found in the proportion of patients who underwent emergency operations or elective operations among the 2 groups (45% emergency surgery in the SARS-CoV-2 positive survivors versus 46% emergency surgery in the SARS-CoV-2 nonsurvivors; P = .99). Finally, there was no difference in the proportion of symptomatic patients in the nonsurvivor group compared with the patients who were alive at 30-days follow-up.
Fig 2.
Distribution of participating centers across the Netherlands and the prevalence of confirmed SARS-CoV-2 positive people per 1,000 habitants per municipality as per June 16, 2020.
Table II.
Description Of SARS-Cov-2 positive survivors and nonsurvivors (unmatched cohort)
| SARS-CoV-2 positive patients Survivors n = 135 |
SARS-CoV-2 positive patients Nonsurvivors n = 26 |
P value | |
|---|---|---|---|
| Patient characteristics | |||
| Age category–no (%) | <.001 | ||
| <50 y | 14 (10.4) | 0 (0.0) | |
| 50–<70 y | 49 (36.6) | 2 (7.7) | |
| ≥70 y | 71 (53.0) | 24 (92.3) | |
| Female sex–no (%) | 62 (45.9) | 10 (38.5) | .63 |
| BMI–no (%) | .93 | ||
| <18.5 | 5 (3.7) | 2 (7.7) | |
| 18.5–<25 | 40 (29.6) | 9 (34.6) | |
| 25–<30 | 40 (29.6) | 6 (23.1) | |
| 30–<35 | 26 (19.3) | 6 (23.1) | |
| 35–<40 | 10 (7.4) | 2 (7.7) | |
| ≥40 | 4 (3.0) | 1 (3.8) | |
| Current smoker–no (%) | 14 (10.4) | 4 (15.4) | .71 |
| Diabetes–no (%) | 32 (23.7) | 12 (46.2) | .04 |
| Hypertension–no (%) | 64 (47.4) | 16 (61.5) | |
| Congestive heart failure–no (%) | 7 (5.2) | 6 (23.1) | |
| Myocardial infarct–no (%) | 13 (9.6) | 8 (30.3) | .27 |
| Peripheral vascular disease–no (%) | 16 (11.9) | 10 (38.5) | .002 |
| Chronic pulmonary disease–no (%) | 14 (10.4) | 6 (23.1) | .14 |
| CVA–no (%) | 18 (13.3) | 6 (23.1) | .33 |
| Chronic renal disease–no (%) | 15 (11.1) | 7 (26.9) | .07 |
| Cancer–no (%) | 26 (19.3) | 4 (15.4) | .85 |
| Transplant–no (%) | 0 (0.0) | 1 (3.8) | 1.00 |
| Immunosuppression–no (%) | 9 (6.7) | 1 (3.8) | .92 |
| ECOG score | .002 | ||
| 0–1 | 84 (62.2) | 9 (34.6) | |
| 2 | 22 (16.3) | 4 (15.4) | |
| 3–4 | 19 (14.1) | 11 (42.3) | |
| Charlson comorbidity index–median (IQR) | 4.0 (2.0–6.0) | 6.5 (4.0–8.9) | .001 |
| Surgery characteristics | |||
| ASA classification–no (%) | .02 | ||
| I–II | 56 (41.5) | 4 (15.4) | |
| III–IV | 75 (55.6) | 22 (84.6) | |
| Anesthesia–no (%) | .79 | ||
| General | 100 (74.1) | 18 (69.2) | |
| Other (including spinal) | 35 (25.9) | 8 (30.8) | |
| Type of operation–no (%) | 1.00 | ||
| Elective | 73 (54.1) | 14 (53.8) | |
| Emergency | 61 (45.2) | 12 (46.2) | |
| Surgical risk score–no (%) | .06 | ||
| 1–2 | 39 (38.9) | 3 (11.5) | |
| 3 | 47 (34.8) | 17 (65.4) | |
| 4 | 31 (23.0) | 5 (19.2) | |
| 5 | 9 (6.7) | 1 (3.8) | |
| SARS-CoV-2 characteristics | |||
| Timing of diagnosis–no (%) | .001 | ||
| Preoperative | 67 (49.6) | 2 (7.7) | |
| Postoperative | 68 (50.4) | 24 (92.3) | |
| Symptomatology–no (%) | 1.00 | ||
| Asymptomatic | 9 (6.7) | 2 (7.7) | |
| ≥1 symptom | 117 (86.7) | 23 (88.5) | |
BMI, body mass index; CVA, cerebrovascular accident; ECOG, Eastern Cooperative Oncology Group.
Primary outcome
Before propensity matching, the 30-day overall postoperative mortality rate in SARS-CoV-2 positive and negative patients was 16% and 4%, respectively (P = .007; Table III ). In the propensity score-matched cohort, 30-day overall mortality was associated with an OR of 3.4 (95% CI 1.5–8.5) for patients with a perioperative SARS-CoV-2 positive status compared with negative control patients. The overall 30-day postoperative mortality rates for both matched and unmatched cohorts are provided in Table III. In the subgroup of patients with a SARS-CoV-2 positive status diagnosed either 7 days pre- or postoperatively, an increase in mortality rate compared with SARS-CoV-2 negative patients was confirmed (n = 8 [12%] vs. n = 14 (4%); P = .009).
Table III.
30-d outcomes
| Unmatched patients |
P value | Propensity score-matched patients |
P value | |||
|---|---|---|---|---|---|---|
| SARS-CoV-2 positive patients n = 161 | SARS-CoV-2 negative patients n = 342 | SARS-CoV-2 positive patients n = 123 | SARS-CoV-2 negative patients n = 196 | |||
| 30-d postoperative mortality | ||||||
| 30-d follow-up–no (%) | <.001 | <.001 | ||||
| Alive at hospital | 26 (16.1) | 25 (7.3) | 21 (17.1) | 13 (6.6) | ||
| Alive at rehabilitation | 35 (21.7) | 21 (6.1) | 25 (20.3) | 16 (8.2) | ||
| Alive at home | 74 (46.0) | 282 (82.5) | 62 (50.4) | 158 (80.6) | ||
| Deceased | 26 (16.2) | 14 (4.1) | 15 (12.2) | 9 (4.6) | ||
| 30-d postoperative complications | ||||||
| Number of complications per patient, median (IQR) | 1 (0–3) | 0 (0–1) | <.0001 | 1 (0–2) | 0 (0–1) | <.001 |
| Comprehensive complication index, median (IQR) | 20.9 (0–42.7) | 0 (0–20.9) | <.0001 | 20.9 (0–39.5) | 0 (0–12.2) | <.0001 |
| Pulmonary complications–no (%) | 39 (24.2) | 11 (3.2) | <.0001 | 25 (20.3) | 6 (3.1) | <.0001 |
| Thromboembolic complications–no (%) | 11 (6.8) | 1 (0.3) | <.0001 | 8 (6.5) | 1 (0.5) | .004 |
| Hemorrhagic complications– no (%) | 22 (13.6) | 28 (8.2) | .052 | 16 (9.9) | 20 (10.2) | .49 |
| Infectious complications–no (%) | 13 (8.1) | 27 (7.8) | .97 | 11 (8.9) | 12 (6.1) | .42 |
Secondary outcomes
Patients with perioperative SARS-CoV-2 had more complications (1 [IQR 0–3] vs 0 [IQR 0–1]; P < .001) with a higher comprehensive complication index (21 [IQR 0–40]) vs 0 [IQR 0–12]; P < .001) compared with matched negative control patients. The number of grade II and grade IV complications was greater in the matched SARS-CoV-2 positive cohort (P < .01). Pulmonary complications occurred in 25 (20%) SARS-CoV-2 positive patients and in 6 (3%) matched SARS-CoV-2 negative patients (P < .001). Similarly, the number of patients with thromboembolic events was greater in the SARS-CoV-2 positive group (8 [7%]) compared with the matched negative control group (1 [0.5%]; P = .004). There was no difference in hemorrhagic or infectious complications between matched cohorts. An overview of the complications for matched and unmatched cohorts is provided in Table III. Supplemental Table III gives a detailed description of the diagnosed pulmonary complications and thromboembolic events.
Discussion
This nationwide, cohort study compares morbidity and mortality rates between matched patients with and without SARS-CoV-2 infection undergoing emergency or elective operations during the first wave of the pandemic. We found that a pre- or postoperative positive SARS-CoV-2 status was associated with a greater 30-day postoperative, overall mortality rate and a 3.4-fold (95% CI 1.5–8.5) increased overall mortality risk compared with matched control patients with a negative SARS-CoV-2 status. SARS-CoV-2 positive patients develop a greater number and more serious postoperative complications. Pulmonary complications and thromboembolic events are more prevalent in patients with perioperative SARS-CoV-2. Although several previous studies suggested this increase in mortality and morbidity, this is the first study to use a well-matched control group to provide good, evidence-based support for this clinical observation.
In this study, 30-day overall mortality rate in the unmatched SARS-CoV-2 positive cohort was 16% compared with 4% in the surgical control group. SARS-CoV-2 positive patients included in our study had a median age of 72 years. The worldwide COVID-19 overall case fatality rate for SARS-CoV-2 positive patients of the same age category 8%.18 The increased mortality rate of SARS-CoV-2 positive patients in this age category who underwent surgery might be attributed to a synergistic effect of SARS-CoV-2 and surgery. The 30-day mortality rate of surgical patients with a perioperative SARS-CoV-2 infection in our study is less than the previously reported 20.5–23.8% mortality rates.4 , 19 , 20 The difference may be attributed to the fact that we included patients treated in a health care system with negligible quality differences between hospitals with similar circumstances and time frame.
The underlying pathophysiologic mechanisms of the increased mortality rate of SARS-CoV-2 positive patients undergoing surgery is still unknown. Mechanical ventilation, anesthesia itself, or tissue damage caused by the operation may each provoke a proinflammatory cytokine and immunosuppressive response, potentially worsening the presentation of a pre- or postoperative SARS-CoV-infection.19 , 21 Surgery-related thromboembolic and pulmonary complications in addition to the underlying effects of the SARS-CoV-2 infection may further increase the risk of thrombotic effects in the pulmonary circulation, respiratory insufficiency, respiratory distress syndrome, and eventually death.20 , 22 We found a mortality rate of 4% in the SARS-CoV-2 negative control group, which is twice as high compared with the rate of 2% reported in a Dutch national registry that studied 3.7 million elective cases of patients undergoing surgery over a 15 year period.6 This difference may be caused by the inclusion of patients undergoing emergency surgery or the selection of patients with more urgent indications to undergo elective operations during the pandemic. The rate of pulmonary complications in SARS-CoV-2 positive patients included in our study (24%) is less than previous studies (41%–51%) in patients with perioperative SARS-CoV-2. The reason for this lesser rate is unclear but might be attributed to either under or over reporting or different characteristics of included patients. It is likely that pulmonary complications are a direct consequence of SARS-CoV-2, potentially increased in severity by an operation or anesthesia.4 , 7 Little is known about increased postoperative thromboembolic events in SARS-CoV-2. It is suggested that that the coagulopathy associated with COVID-19 might be attributed to a combination of disseminated intravascular coagulation and localized pulmonary thrombotic microangiopathy.23 Thromboembolic events have been described previously as a major risk factor for mortality in hospitalized patients with SARS-CoV2.24 , 25 Future studies with larger cohorts of surgical patients are needed to further confirm our observations and assess the association between thromboembolic events and postoperative mortality.
Comparing outcomes of surgery between hospitals within a uniform health care system allows for accurate assessment of differences in morbidity and mortality between SARS-CoV-2 positive and negative patients. We used propensity score matching in an attempt to account for a wide variety of baseline differences. Our study still, however, has limitations we need to address. Despite the propensity matching, it is still possible that some amount of unmeasured confounding remains. The SARS-CoV-2 positive cohort is a heterogeneous group, consisting of asymptomatic and symptomatic patients diagnosed pre- or postoperatively. Especially at the beginning of the first wave of SARS-CoV-2 infections in the Netherlands, testing capacity was limited, and patients could not be screened routinely before their operation. This reality is reflected by the proportion of patients with a postoperative SARS-CoV-2 status within the 30 days postoperatively. It is likely that many, perhaps most of the patients with a positive postoperative SARS-CoV-2 status, were already infected during the index operation; however, the exact number of patients with a preoperative SARS-CoV-2 status is unknown, because a proportion of preoperative SARS-CoV-2 infections was diagnosed postoperatively. Additionally, the median SARS-CoV-2 incubation period from time to symptom onset is 5 days, which may further bias the differentiation between preoperative, postoperative, in-hospital, and out-of-hospital infections.26 , 27 Unfortunately, we cannot dissect out these possibilities. Routine preoperative testing was implemented nationally as standard of care from April onward.8 Furthermore, because screening for SARS-CoV-2 was also not performed routinely after surgery, it is likely that patients with an unexpected adverse postoperative course were more prone to be tested for SARS-CoV-2, compared with patients with an unknown asymptomatic positive SARS-CoV-2 status, potentially leading to an over-reported mortality and morbidity rate. Additionally, the elective surgery capacity in the Netherlands during the initial wave of SARS-CoV-2 was severely impaired. Semiurgent operations, however, were still performed without evidence of the potential risk of a SARS-CoV-2 infection in combination with surgery. Therefore, patients who underwent surgical interventions in this timeframe are not representative of the general population undergoing elective surgery before the pandemic. Probably, based on the limited elective capacity in the hospitals, surgeons prioritized more urgent elective procedures such as semiacute cancer and trauma surgery.
The findings of this study have direct implications for the perioperative delivery of health care, because medical professionals across the world will be confronted continuously with SARS-CoV-2 until effective vaccination programs have been established or herd immunity is reached. The high morbidity and mortality risk among perioperative SARS-CoV2 positive patients should be an argument to postpone elective operations and even reconsider emergency operative interventions in selected patients, especially those at risk for pulmonary or thromboembolic complications. Surgeons on call will have to cope with acute dilemmas and at least consider alternative strategies to operative intervention. Surgical strategies in SARS-CoV-2 positive patients in need of emergency surgery may be changed toward a conservative approach; for instance, antibiotics for appendicitis or acute cholecystitis may be chosen instead of operative therapy. Results of our study further underline the relevance of preoperative testing of all patients in areas that have a high risk of SARS-CoV-2 infection. Current Dutch consensus documents advise to double the dosage of thromboembolic prophylaxis of SARS-CoV-2 positive patients during ICU admission with respect to the risk of bleeding.28 The high rate of postoperative thromboembolic events in SARS-CoV-2 positive patients undergoing operations might address the need for personalized protocols of thromboembolic prophylaxis in this patient category. Models of surgical risk stratification tailored to individual SARS-CoV-2 patients are needed. Previously established risk stratification systems, such as the Charlson comorbidity index, the ASA score, or performance scores might be useful tools to estimate potential postoperative mortality in SARS-CoV-2 positive patients diagnosed preoperatively. Therefore, it remains highly relevant to collaborate globally and to share and analyze outcomes of surgery in the SARS-CoV-2 positive population in future studies.4
In conclusion, this nationwide, matched cohort study shows that a pre- or postoperative SARS-CoV-2 positive status increases 30-day overall postoperative mortality rates, pulmonary complications, and thromboembolic events in patients undergoing operative care. The results of this study provide further evidence that elective operations in patients with preoperatively diagnosed SARS-CoV-2 should be postponed whenever possible and even emergency operative intervention for selected patients should be carefully reconsidered. Altered protocols of thromboembolic prophylaxis might be required to prevent thromboembolic complications in surgical patients with SARS-CoV-2, but the use of greater than the normal dosage of thromboembolic prophylaxis should be carefully weighed against the risk of (postoperative) bleeding.
Conflict of interest/Disclosures
MMRFS’s research group/department received (over the last 3 years) nonrelated research grants and nonrelated consultancy fees from The Medicines Company (Parsippany, NJ), Masimo (Irvine, CA), Becton Dickinson (Eysins, Switzerland), Fresenius (Bad Homburg, Germany), Dräger (Lübeck, Germany), Paion (Aachen, Germany), and Medtronic (Dublin, Ireland). He receives royalties on intellectual property from the Ghent University (Gent, Belgium). WFWB reports cost reimbursements from GSK and nonfinancial support from Janssen. All other authors have nothing to disclose.
Funding/Support
This study is funded by institutional and departmental funding only.
Dutch Surgical COVID-19 Research Collaborative Co-Authors
| First Name | Middle Name(S) | Last Name | Affiliation |
|---|---|---|---|
| Djamila | Boerma | Department of Surgery, Antonius Ziekenhuis, Nieuwegein, The Netherlands | |
| Sarah | L | Gerritsen | Department of Surgery, Antonius Ziekenhuis, Nieuwegein, The Netherlands |
| Wout | van der Meij | Department of Surgery, Bernhoven, Uden, the Netherlands | |
| André | S | van Petersen | Department of Surgery, Bernhoven, Uden, the Netherlands |
| Charles | T | Stevens | Department of Surgery, Bernhoven, Uden, the Netherlands |
| Marc | van Sambeek | Department of Surgery, Catharina Ziekenhuis, Eindhoven, the Netherlands | |
| Marleen | Hölscher | Department of Surgery, Catharina Ziekenhuis, Eindhoven, the Netherlands | |
| Apollo | Pronk | Department of Surgery, Diakonessenhuis, Utrecht, the Netherlands | |
| Wouter | J | Bakker | Department of Surgery, Diakonessenhuis, Utrecht, the Netherlands |
| Patrick | WHE | Vriens | Department Of Surgery, Elisabeth Tweesteden Ziekenhuis, Tilburg, The Netherlands |
| Thymen | Houwen | Department of Surgery, Elisabeth Tweesteden Ziekenhuis, Tilburg, the Netherlands |
|
| Johannes | A | Wegdam | Elkerliek, Helmond, the Netherlands |
| Tammo | S | de Vries Reilingh | Elkerliek, Helmond, the Netherlands |
| Ellis | Schipper | Elkerliek, Helmond, the Netherlands | |
| Pascal | HE | Teeuwen | Elkerliek, Helmond, the Netherlands |
| Tessa | M | van Ginhoven | Department of Surgery, Erasmus Universitair MC, Rotterdam, the Netherlands |
| Charlotte | L | Viëtor | Department of Surgery, Erasmus Universitair MC, Rotterdam, the Netherlands |
| Mark | JW | van der Oest | Department of Plastic, Reconstructive and Hand Surgery, Erasmus MC, Rotterdam, The Netherlands Department of Rehabilitation Medicine, Erasmus MC, Rotterdam, The Netherlands Hand and Wrist Center, Xpert Clinic, the Netherlands |
| Sarah | L | Gans | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
| Peter | van Duijvendijk | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands | |
| Tanneke | Herklots | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands | |
| Tom | de Hoop | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
|
| Michelle | R | de Graaff | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
| Didi | AM | Sloothaak | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
| Marieke | J | Bolster-van Eenenennaam | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
| Jedidja | Baaij | Department of Surgery, Gelre Ziekenhuis, Apeldoorn, the Netherlands |
|
| Maarten | Vermaas | Department of Surgery, IJsselland Ziekenhuis, Capelle aan den IJssel, the Netherlands |
|
| Kelly | R | Voigt | Department of Surgery, IJsselland Ziekenhuis, Capelle aan den IJssel, the Netherlands |
| Gijs | A | Patijn | Department of Surgery, Isala Clinics, Zwolle, the Netherlands |
| Amarins | TA | Bransma | Department of Surgery, Isala Clinics, Zwolle, the Netherlands |
| Wouter | KG | Leclercq | Department of Surgery, Maxima Medisch Centrum, Veldhoven, the Netherlands |
| Julie | ML | Sijmons | Department of Surgery, Maxima Medisch Centrum, Veldhoven, the Netherlands |
| Martine | Uittenbogaart | Department of Surgery, Maxima Medisch Centrum, Veldhoven, the Netherlands |
|
| Paul | M | Verheijen | Department of Surgery, Meander Medisch Centrum, Amersfoort & Baarn, the Netherlands |
| Thijs | A | Burghgraef | Department of Surgery, Meander Medisch Centrum, Amersfoort & Baarn, the Netherlands |
| Marloes | Emous | Department of Surgery, Medical Center Leeuwarden, Leeuwarden, the Netherlands | |
| Ralph | Poelstra | Department of Surgery, Medical Center Leeuwarden, Leeuwarden, the Netherlands | |
| Manon | Teunissen | Department of Surgery, Medical Center Leeuwarden, Leeuwarden, the Netherlands | |
| Herman | Frima | Department of Surgery, Noordwest Ziekenhuisgroep, Alkmaar & Den Helder, the Netherlands | |
| Said | Bachiri | Department of Surgery, Noordwest Ziekenhuisgroep, Alkmaar & Den Helder, the Netherlands |
|
| Lennaert | CB | Groen | Department of Surgery, Noordwest Ziekenhuisgroep, Alkmaar & Den Helder, the Netherlands |
| Philip | R | de Reuver | Department of Surgery, Radboud University Medical Center, Nijmegen, the Netherlands |
| Floris | M | Thunnissen | Department of Surgery, Radboud University Medical Center, Nijmegen, the Netherlands |
| Britt | AM | Vermeulen | Department of Surgery, Radboud University Medical Center, Nijmegen, the Netherlands |
| Anna | Groen | Department of Surgery, Radboud University Medical Center, Nijmegen, the Netherlands |
|
| Ramon | RJP | van Eekeren | Department of Surgery, Rijnstate, Arnhem, the Netherlands |
| Ernst | J | Spillenaar Bilgen | Department of Surgery, Rijnstate, Arnhem, the Netherlands |
| Niels | J | Harlaar | Department of Surgery, Rode Kruis Ziekenhuis, Beverwijk, the Netherlands Department of Surgery, Noordwest Ziekenhuisgroep, Alkmaar & Den Helder, the Netherlands |
| Frederik | HW | Jonker | Department of Surgery, Rode Kruis Ziekenhuis, Beverwijk, the Netherlands Department of Surgery, Noordwest Ziekenhuisgroep, Alkmaar & Den Helder, the Netherlands |
| Sjirk | W | van der Burg | Department of Surgery, Rode Kruis Ziekenhuis, Beverwijk, the Netherlands |
| Lisanne | AE | Posma-Bouman | Department of Surgery, Slingeland Ziekenhuis, Doetinchem, the Netherlands |
| Steven | J | Oosterling | Department of Surgery, Spaarne Gasthuis, Haarlem & Hoofddorp, the Netherlands |
| Josephine | Franken | Department of Surgery, Spaarne Gasthuis, Haarlem & Hoofddorp, the Netherlands | |
| David | R | Nellensteijn | Department of Surgery, Curacao Medical Center, Willemstad, Curaçao |
| Elena Argia Bianca | Bensi | Department of Surgery, Curacao Medical Center, Willemstad, Curaçao | |
| Wim | van den Broek | Department of Surgery, Sint Anna Ziekenhuis, Geldrop, the Netherlands | |
| Eduard | R | Hendriks | Department of Surgery, Tergooi, Hilversum & Blaricum, The Netherlands |
| Anna | AW | van Geloven | Department of Surgery, Tergooi, Hilversum & Blaricum, The Netherlands |
| Schelto | Kruijff | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
|
| Jean-Paul | P.M. | de Vries | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Pieter | J | Steinkamp | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Pascal | KC | Jonker | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Willemijn | Y | van der Plas | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Wouter | FW | Bierman | Department of Internal Medicine and Infectious Diseases, University of Groningen, University |
| Medical Center Groningen, Groningen, the Netherlands | |||
| Michel | MRF | Struys | Department of Anesthesiology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Yester | F | Janssen | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Gooitzen | M | van Dam | Deptartments of surgery, nuclear medicine and molecular imaging, medical imaging center, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Frank | FA | IJpma | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Claire | van der Riet | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
|
| Elisabeth | A | Feitsma | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Kirsten | F | Ma | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Simone | F | Kleiss | Department of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands |
| Milan | C | Richir | Department of Surgery, University Medical Center Utrecht, Utrecht, the Netherlands |
| Menno | R | Vriens | Department of Surgery, University Medical Center Utrecht, Utrecht, the Netherlands |
| Mando | D | Filipe | Department of Surgery, University Medical Center Utrecht, Utrecht, the Netherlands |
| Frank | C | den Boer | Department of Surgery, Zaans Medisch Centrum, Zaandam, the Netherlands |
| Nicole | AM | Dekker | Department of Surgery, Zaans Medisch Centrum, Zaandam, the Netherlands |
| Tim | Verhagen | Department of Surgery, Ziekenhuisgroep Twente, Almelo & Hengelo, the Netherlands |
|
| Floor | ter Brugge | Department of Surgery, Ziekenhuisgroep Twente, Almelo & Hengelo, the Netherlands |
|
| Emmanuel | AGL | Lagae | Department of Surgery, Zorgsaam Zeeuws Vlaanderen, Terneuzen, the Netherlands |
| Evert-Jan | G | Boerma | Zuyderland Medical Center, Heerlen & Sittard/Geleen, the Netherlands |
| Donald | Schweitzer | Zuyderland Medical Center, Heerlen & Sittard/Geleen, the Netherlands | |
| Mark | HF | Keulen | Zuyderland Medical Center, Heerlen & Sittard/Geleen, the Netherlands |
| Shirley | Ketting | Zuyderland Medical Center, Heerlen & Sittard/Geleen, the Netherlands |
Acknowledgments
We thank all the participating centers for their contribution. We thank E.C. Schuinder, S.N. Schuurman, and D.A.B. de Vries-Werson for their valuable assistance in follow-up completion by phone. Finally, we would like to thank A.A. Phihos, J.S. Rowaihy, F.A. van der Zant, and M.E. Haveman for their support with the inclusion of SARS-CoV-2 negative patients in the control cohort.
Footnotes
This article was submitted on behalf of the Dutch Surgical COVID-19 Research Collaborative (please refer to Supplemental File 1 for the list of collaborators and contact details)
Pascal K.C. Jonker, Willemijn Y. van der Plas, and Pieter J. Steinkamp contributed equally to this work.
Contributor Information
Dutch Surgical COVID-19 Research Collaborative:
Djamilla Boerma, Sarah L. Gerritsen, Wout van der Meij, André S. van Petersen, Charles T. Stevens, Marc van Sambeek, Marleen Hölscher, Apollo Pronk, Wouter J. Bakker, Patrick Whe Vriens, Thymen Houwen, Johannes A. Wegdam, Tammo S. de Vries Reilingh, Ellis Schipper, Pascal HE. Teeuwen, Tessa M. van Ginhoven, Charlotte Viëtor, Mark JW. van der Oest, Sarah Gans, Peter van Duijvendijk, Tanneke Herklots, Tom de Hoop, Michelle de Graaff, Didi Sloothaak, Marieke Bolster - van Eenennaam, Jedidja Baaij, Maarten Vermaas, Kelly R. Voigt, Gijs A. Patijn, Amarins TA. Bransma, Wouter KG. Leclercq, Julie ML. Sijmons, Martine Uittenbogaart, Paul M. Verheijen, Thijs A. Burghgraef, Marloes Emous, Ralph Poelstra, Manon Teunissen, Herman Frima, Said Bachiri, Lennaert CB. Groen, Philip R. de Reuver, Floris M. Thunissen, Britt AM. Vermeulen, Anna Groen, Ramon RJP. van Eekeren, Ernst J. Spillenaar Bilgen, Niels J. Harlaar, Fredrik HW. Jonker, Sjirk W. van der Burg, Lisanne AE. Posma-Bouman, Steven J. Oosterling, Josephine Franken, David R. Nellensteijn, Elena Argia Bianca Bensi, Wim van den Broek, Eduard R. Hendriks, Anna AW. van Geloven, Schelto Kruijff, Jean-Paul P.M. de Vries, Pieter J. Steinkamp, Pascal KC. Jonker, Willemijn Y. van der Plas, Wouter FW. Bierman, Michel MRF. Struys, Yester F. Janssen, Gooitzen M. van Dam, Frank FA. IJpma, Claire van der Riet, Eline Feitsma, Kirsten Ma, Simone Kleiss, Milan C. Richir, Menno R. Vriens, Mando D. Filipe, Frank C. den Boer, Nicole AM. Dekker, Tim Verhagen, Floor ter Brugge, Emmanuel AGL. Lagae, Evert-Jan G. Boerma, Donald Schweitzer, Mark HF. Keulen, and Shirley Ketting
Supplementary data
Supplemental Figure 1.
Distribution of the estimated propensity score for a positive SARS-CoV-2 status among SARS-CoV-2 positive and SARS-CoV-2 negative patients. On the left, histograms of propensity scores for the unadjusted samples who were SARS-CoV-2 positive and who were SARS-CoV-2 negative. On the right, histograms of the propensity matched samples. Generated using the first imputed dataset. The other imputed datasets are similar and available on request.
Supplemental Fig 2.
References
- 1.Johns Hopkins University of Medicine COVID-19 Map. 2020. https://coronavirus.jhu.edu/map.html
- 2.Di Marzo F., Sartelli M., Cennamo R. Recommendations for general surgery activities in a pandemic scenario (SARS-CoV-2)[e-pub ahead of print] Br J Surg. 2020 doi: 10.1002/bjs.11652. Accessed April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans [e-pub ahead of print] Br J Surg. 2020 doi: 10.1002/bjs.11746. Accessed May 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiermeier A., Babidge W.J., McCulloch G.A.J., Maddern G.J., Watters D.A., Aitken R.J. National surgical mortality audit may be associated with reduced mortality after emergency admission. ANZ J Surg. 2017;87:830–836. doi: 10.1111/ans.14170. [DOI] [PubMed] [Google Scholar]
- 6.Noordzij P.G., Poldermans D., Schouten O., Bax J.J., Schreiner F.A., Boersma E. Postoperative mortality in the Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112:1105–1115. doi: 10.1097/ALN.0b013e3181d5f95c. [DOI] [PubMed] [Google Scholar]
- 7.Doglietto F., Vezzoli M., Gheza F. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:1–14. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nederlandse Federatie voor Medisch Specialisten. Pre-operative work-up for COVID-19 infection in asymptomatic patients scheduled for surgery under general anesthesia. 2020. https://www.nvtnet.nl/sites/thorax.productie.medonline.nl/files/bijlagen/Practice%20Guideline%20Preoperative%20work%20up%20on%20possible%20COVID-19%20infection%20in%20asymptomatic%20patients%20V3.0.pdf. Accessed April 2020.
- 9.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overheid.nl. Covid-19 cumulatieve aantallen per gemeente | Data overheid. 2020. https://data.overheid.nl/dataset/11508-covid-19-aantallen-gemeente-cumulatief#downloadable-files
- 11.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.UCLA Health Risk stratification: Risk stratification before elective surgery. https://www.uclahealth.org/anes/risk-stratification
- 13.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slankamenac K., Nederlof N., Pessaux P. The comprehensive complication index a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014;260:757–762. doi: 10.1097/SLA.0000000000000948. discussion 762-763. [DOI] [PubMed] [Google Scholar]
- 15.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. Hoboken (NJ): John Wiley & Sons, Inc; 2002.
- 16.van Buuren S., Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 17.Ho D.E., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 18.Worldometer Age. sex, existing conditions of COVID-19– cases and deaths. 2020. https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/#pre-existing-conditions
- 19.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;0:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myles P.S., Maswime S. Mitigating the risks of surgery during the COVID-19 pandemic. Lancet. 2020;396:2–3. doi: 10.1016/S0140-6736(20)31256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmy S.A.K., Wahby M.A., El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–738. doi: 10.1046/j.1365-2044.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 22.Assareh H., Chen J., Ou L., Hillman K., Flabouris A. Incidences and variations of hospital acquired venous thromboembolism in Australian hospitals: a population-based study. BMC Health Serv Res. 2016;16:511. doi: 10.1186/s12913-016-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e428–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.J., Ni Z.Y., Hu Y., and the China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federatie Medisch Specialisten Leidraad COVID-19 coagulopathie. 2020. https://www.demedischspecialist.nl/nieuws/leidraad-covid-19-coagulopathie
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.