Brucellosis is a debilitating disease of humans and animals globally, and there is currently no vaccine to combat human infection by Brucella spp. Moreover, effective antibiotic treatment in humans is extremely difficult and can lead to disease relapse. Therefore, it is imperative that systems and pathways be identified and characterized in the brucellae so new vaccines and therapies can be generated. In this study, we describe the impact of the endoribonuclease RNase E on the control of mRNA and small regulatory RNA (sRNA) levels in B. abortus, as well as the importance of RNase E for the full virulence of B. abortus. This work greatly enhances our understanding of ribonucleases in the biology and pathogenesis of Brucella spp.
KEYWORDS: Brucella abortus, ribonucleases, small RNAs
ABSTRACT
RNases are key regulatory components in prokaryotes, responsible for the degradation and maturation of specific RNA molecules at precise times. Specifically, RNases allow cells to cope with changes in their environment through rapid alteration of gene expression. To date, few RNases have been characterized in the mammalian pathogen Brucella abortus. In the present work, we sought to investigate several RNases in B. abortus and determine what role, if any, they have in pathogenesis. Of the 4 RNases reported in this study, the highly conserved endoribonuclease, RNase E, was found to play an integral role in the virulence of B. abortus. Although rne, which encodes RNase E, is essential in B. abortus, we were able to generate a strain encoding a defective version of RNase E lacking the C-terminal portion of the protein, and this strain (rne-tnc) was attenuated in a mouse model of Brucella infection. RNA-sequencing analysis revealed massive RNA dysregulation in B. abortus rne-tnc, with 122 upregulated and 161 downregulated transcripts compared to the parental strain. Interestingly, several mRNAs related to metal homeostasis were significantly decreased in the rne-tnc strain. We also identified a small regulatory RNA (sRNA), called Bsr4, that exhibited significantly elevated levels in rne-tnc, demonstrating an important role for RNase E in sRNA-mediated regulatory pathways in Brucella. Overall, these data highlight the importance of RNase E in B. abortus, including the role of RNase E in properly controlling mRNA levels and contributing to virulence in an animal model of infection.
IMPORTANCE Brucellosis is a debilitating disease of humans and animals globally, and there is currently no vaccine to combat human infection by Brucella spp. Moreover, effective antibiotic treatment in humans is extremely difficult and can lead to disease relapse. Therefore, it is imperative that systems and pathways be identified and characterized in the brucellae so new vaccines and therapies can be generated. In this study, we describe the impact of the endoribonuclease RNase E on the control of mRNA and small regulatory RNA (sRNA) levels in B. abortus, as well as the importance of RNase E for the full virulence of B. abortus. This work greatly enhances our understanding of ribonucleases in the biology and pathogenesis of Brucella spp.
INTRODUCTION
RNases are conserved throughout all forms of life, as they play the essential role of processing RNA molecules (1, 2). There are two types of RNases, endoribonucleases and exoribonucleases. Endoribonucleases are responsible for cleaving transcripts internally, whereas exoribonucleases cleave the ends of transcripts (3–5). As a group, RNases are involved in the maturation of RNA by processing pre-RNA molecules into their active, mature forms. This maturation process is well documented for Escherichia coli rRNAs, where the 30S pre-rRNA is initially fragmented by the endoribonuclease RNase III and further processed by other ribonucleases and enzymes to produce mature 23S, 16S, and 5S rRNAs (6). This maturation process is critical not only for rRNAs but also for tRNAs, mRNAs, and small regulatory RNAs (sRNAs) (6–9). RNases are also involved in the natural turnover of RNA in a cell and act as a “cleanup” network to dispose of unnecessary transcripts and recycle nucleotides (10).
Although largely beneficial, RNases must be controlled in their actions, as nonspecific degradation can be detrimental to an organism. Transcripts can be protected from RNase degradation through several mechanisms, such as through the presence of a secondary structure within the RNA, association of the RNA with a protein, or through coupling of the actions of transcription and translation (3, 4, 11). RNases themselves are also tightly regulated at the transcriptional, translational, and posttranslational levels to avoid nonspecific degradation. In addition, cellular localization of RNases, RNase-specific RNA sequences, and the ability or inability to cleave single-stranded and/or double-stranded RNA all add to the regulation of RNases (3, 4, 11). Overall, the activity of RNases depends on the status of the organism, as changes to the transcriptome are necessary for survival in a specific environmental niche.
One of the most well-conserved RNases in prokaryotes is the endoribonuclease RNase E, which is essential for cell viability (12). However, to date, RNase E has been linked to the pathogenesis of few organisms, including Salmonella and Yersinia. In Salmonella, RNase E is responsible for controlling the levels of mgtC mRNA, which encodes an inner membrane protein, to allow for survival of Salmonella in both macrophages and murine models of infection (13). Not surprisingly, a mutant of Salmonella rne led to attenuation in an insect model of infection (14). In Yersinia, RNase E is involved in regulating the type III secrection system and is critical for intracellular survival (15). Although the role of RNase E in processing RNA is well documented, the role of RNase E in bacterial pathogenesis remains poorly defined.
In the zoonotic pathogen Brucella, few studies have investigated and characterized RNases. One study found that mutation of rnaseR (also called vacB) did not affect the virulence of B. abortus, but no additional information was provided about the mechanism of action of RNase R in Brucella (16). In another study, the endoribonuclease RNase III in B. melitensis was biochemically characterized, but no evidence was reported about the requirement of RNase III for Brucella infection (17). More recently, our group described the endoribonuclease YbeY in B. abortus (18). YbeY is involved in maintaining appropriate mRNA levels of a wide variety of genes, and deletion of ybeY leads to aberrant cellular morphology and reduced virulence of B. abortus. Additionally, a comprehensive structural and biochemical analysis of the BrnT toxin of the BrnT/BrnA toxin-antitoxin system revealed that BrnT is an RNase in B. abortus, but specific RNA targets of BrnT remain to be defined (19). In the present study, we sought to determine the role of several Brucella RNases—RNase E (BAB_RS20380), RNase J (BAB_RS19930), RNase PH (BAB_RS16725), and a putative RNase referred to here as Hyp-RNase (BAB_RS18460). We aimed to determine the importance of each of these RNases in Brucella pathogenesis and in regulation of gene expression. Most importantly, this study demonstrates how the endoribonuclease RNase E plays a critical role in the stability of mRNAs and sRNAs and how RNase E is required for the full virulence of B. abortus.
RESULTS
The role of RNase PH, a hypothetical RNase, RNase J, and RNase E in Brucella virulence.
To begin to gain insight into the importance of RNases in Brucella virulence, four RNases conserved among Brucella species were chosen for further characterization (see Fig. S1 in the supplemental material). For rnasePH (bab1_0172/BAB_RS16725) (Fig. S1A), a hypothetical rnase (hyp-rnase; bab1_0533/BAB_RS18460) (Fig. S1B), and rnaseJ (bab1_0837/BAB_RS19930) (Fig. S1C), unmarked, in-frame gene deletions were constructed in B. abortus 2308. It was reported previously that rne is essential in B. abortus (20), and therefore, a B. abortus strain harboring a deletion of the carboxyl-terminal region of rne (rne-tnc; bab1_0930/BAB_RS20380) (Fig. S1D) was constructed and utilized for all experiments (Fig. S2).
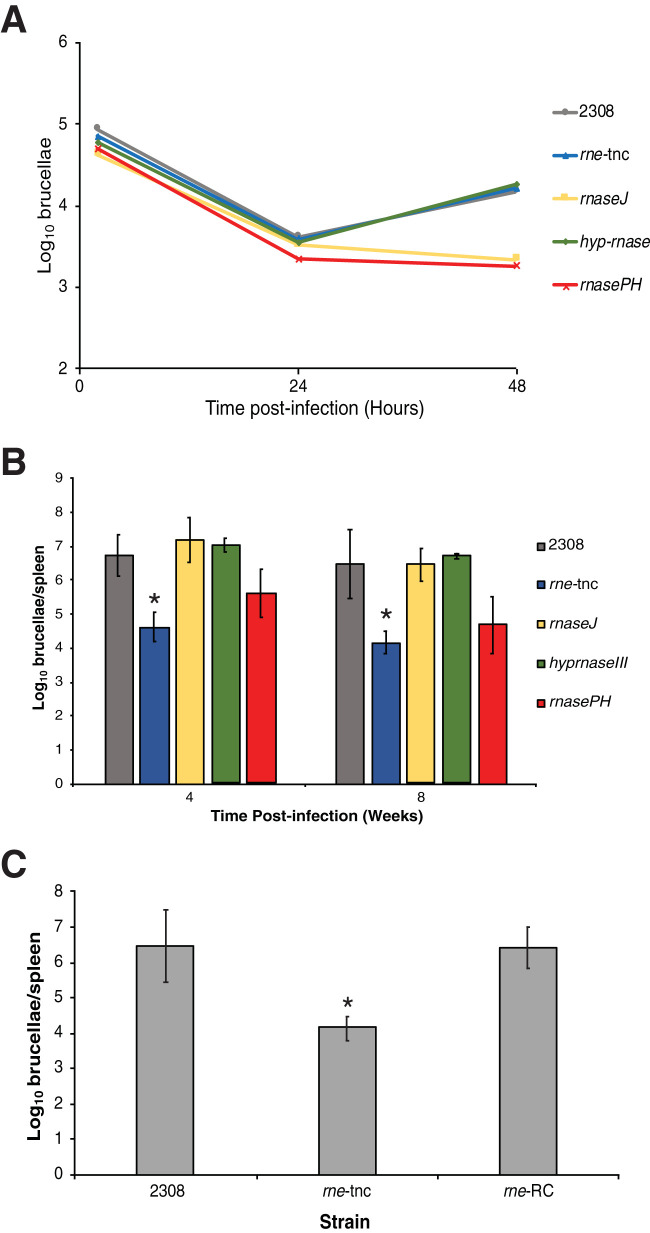
In order to assess the involvement of each RNase in Brucella virulence, primary peritoneal macrophages from BALB/c mice were experimentally infected with the isogenic rnase mutants (Fig. 1A). The Δhyp-rnase and rne-tnc strains exhibited similar levels of survival and replication in macrophages as the parental strain 2308. Interestingly, the ΔrnasePH and ΔrnaseJ strains exhibited significant attenuation at 48 h postinfection compared to the parental strain 2308, indicating that RNase PH and RNase J are required for the full capacity of B. abortus to survive and replicate in macrophages.
FIG 1.
RNase E is required for the full virulence of Brucella abortus 2308 in macrophages and mice. (A) Survival and replication of B. abortus strains in naive peritoneal macrophages. Peritoneal macrophages from BALB/c mice were infected with B. abortus 2308 or with B. abortus mutant strains, including rne-tnc, rnaseJ, hyp-rnase, and rnasePH strains. At the indicated time points, the macrophages were lysed, and the number of intracellular brucellae present was enumerated following serial dilution and plating on agar medium. The data are presented as the average number of brucellae plus or minus the standard deviation from three replicate wells of macrophages infected with a specific Brucella strain. The results are from a representative experiment that was performed independently three times. (B) Spleen colonization of experimentally infected mice by B. abortus strains. BALB/c mice were infected intraperitoneally with B. abortus 2308 or with B. abortus mutant strains, including rne-tnc, rnaseJ, hyp-rnase, and rnasePH strains. Mice were humanely euthanized 4 and 8 weeks postinfection, and the number of brucellae colonizing the spleens of the mice was determined. The data are presented as the average number of brucellae plus or minus the standard deviation from 5 mice colonized with a specific Brucella strain at a particular time point. The data are from a single experiment using 5 mice per bacterial strain per time point (i.e., n = 5). Statistical significance between the parental strain 2308 and the rne-tnc strain was determined by a t test (*, P < 0.05). (C) Genetic complementation of rne mutation in the mouse infection model. Mice were infected intraperitoneally as described above for panel B, but here, the mice were infected with B. abortus 2308, the rne truncation strain, and the genetically reconstructed rne strain (rne-RC). The data are from a single experiment using 5 mice per bacterial strain (i.e., n = 5), and the data depict the CFU per spleen after 8 weeks of infection. Statistical significance between the parental strain 2308 and the rne-tnc strain was determined by a t test (*, P < 0.05).
Next, in vivo infection experiments were carried out utilizing 6-week-old female BALB/c mice (Fig. 1B). Mice were infected intraperitoneally with approximately 1 × 105 CFU per mouse, and spleen colonization was assessed at 4 and 8 weeks postinfection. Although deletion of rnasePH resulted in decreased spleen colonization at 4 and 8 weeks postinfection compared the parental strain 2308, these differences were not statistically significant. However, the rne-tnc strain was significantly attenuated at both of these time points, and furthermore, reconstruction of the rne locus in the rne-tnc strain (rne-RC) restored the levels of brucella spleen colonization to those of the parental strain 2308 after 8 weeks of infection (Fig. 1C). Taken together, these data demonstrate that RNase E is required for the full virulence of B. abortus in an animal model of infection.
B. abortus rne-tnc has a small-colony phenotype and a growth defect in rich medium.
Following our determination that RNase E is important for the virulence of B. abortus in mice, the rne-tnc strain was evaluated for its ability to grow in rich medium (Fig. 2). Surprisingly, we observed that the B. abortus rne-tnc strain displayed a slow growth phenotype compared to the wild type and the reconstruction strains when the bacteria were grown in brucella broth. The rne-tnc strain was able to grow to the same cell density as the parental strain 2308, but it took longer for the rne-tnc strain to reach that same density. Additionally, the B. abortus rne-tnc strain displayed a small-colony phenotype when the bacteria were grown on agar medium (Fig. 2, inset). Taken together, these data demonstrate that RNase E is required for the appropriate growth of B. abortus in vitro.
FIG 2.
Truncation of rne leads to a moderate growth defect in vitro. Brucella abortus strains, including the parental strain 2308, the rne truncation strain (ren-tnc), and the genetically reconstructed rne strain (rne-RC), were cultured in nutrient-rich brucella broth. At the indicated times, samples were removed from each culture, and serial dilutions were plated onto agar medium to determine the number of CFU per milliliter in the culture. Each strain was cultured in triplicate, and the experiment was performed independently three times. The data points represent the average CFU per milliliter at each time point plus or minus the standard deviation. (Inset) Colony morphology of B. abortus strains on agar medium after 15 h of growth in brucella broth.
Truncation of rne results in significant dysregulation of the Brucella transcriptome.
RNase E has been well documented as having a major role in the processing and degradation of RNA in prokaryotes (reviewed in reference 12). To determine what regulatory role RNase E has in B. abortus, RNA sequencing (RNA-seq) was performed to analyze the transcriptome of the rne-tnc strain (Table 1). For this, RNA was isolated from Brucella strains grown in nutrient-replete medium (i.e., brucella broth) to the late exponential phase. RNA-seq analysis revealed that 122 mRNAs were elevated (≥2-fold) in rne-tnc compared to the parental strain 2308, while 161 transcripts exhibited decreased levels (≤2-fold) in rne-tnc compared to 2308. The full list of dysregulated mRNAs can be found in Table S1.
TABLE 1.
RNA-seq analysis of transcript levels in B. abortus 2308 compared to the B. abortus rne-tnc straina
| Transcript level in B. abortus rne-tnc and BAB no. | BAB_RS no. | Log2 fold change | Description of gene product |
|---|---|---|---|
| Elevated | |||
| BAB_RS27990 | 2.5 | Hypothetical protein | |
| BAB2_0534 | BAB_RS28900 | 2.4 | Multicopper oxidase CueO |
| BAB1_0179 | BAB_RS16760 | 2.2 | Uroporphyrin-III c-methyltransferase |
| BAB1_0042 | BAB_RS16135 | 2.2 | Cytochrome c oxidase subunit IV |
| Decreased | |||
| BAB1_0393 | BAB_RS17810 | 4.4 | Fur family transcriptional regulator |
| BAB_RS17815 | 4.2 | Hypothetical protein | |
| BAB1_1579 | BAB_RS23450 | 4.2 | Membrane protein OmpW |
| BAB1_1460 | BAB_RS22885 | 3.5 | Divalent metal cation transporter MntH |
| BAB1_0308 | BAB_RS17400 | 3.0 | Dihydrorhizobitoxine desaturase |
| BAB1_0309 | BAB_RS17405 | 2.9 | Dihydropyrimidinase |
| BAB1_0313 | BAB_RS17425 | 2.9 | Dihydropyrimidine dehydrogenase subunit B |
| BAB1_0215 | BAB_RS16925 | 2.7 | Thiamine-phosphate pyrophosphorylase |
| BAB1_0214 | BAB_RS16920 | 2.5 | ABC transporter ATP-binding protein |
| BAB_RS28965 | 2.4 | Sugar transporter | |
| BAB1_0218 | BAB_RS16940 | 2.4 | Thiamine biosynthesis protein thio |
| BAB2_0348 | BAB_RS28030 | 2.3 | Major facilitator superfamily transporter |
| BAB1_0312 | BAB_RS17420 | 2.3 | Hypothetical protein |
| BAB2_0550 | BAB_RS28970 | 2.3 | Sugar ABC transporter permease |
| BAB2_0448 | BAB_RS28960 | 2.3 | Sugar ABC transporter permease |
| BAB1_0373 | BAB_RS17715 | 2.3 | ABC transporter substrate-binding protein |
| BAB1_0216 | BAB_RS16930 | 2.2 | Thiazole synthase |
| BAB2_0830 | BAB_RS30275 | 2.2 | Leu/Ile/Val-binding protein homolog 8 |
| BAB1_0219 | BAB_RS16945 | 2.2 | Phosphomethylpyrimidine kinase |
| BAB2_0277 | BAB_RS27685 | 2.2 | Glucose-methanol-choline oxidoreductase |
| BAB1_0967 | BAB_RS20555 | 2.2 | Aromatic hydrocarbon degradation protein |
| BAB2_0620 | BAB_RS29280 | 2.1 | Hypothetical protein |
| BAB1_0738 | BAB_RS19450 | 2.1 | Lactate permease |
| BAB_RS22440 | 2.1 | ATPase | |
| BAB1_0212 | BAB_RS16910 | 2.0 | ABC transporter permease |
| BAB1_0204 | BAB_RS16870 | 2.0 | Alcohol dehydrogenase |
| BAB2_0913 | BAB_RS30625 | 2.0 | Hypothetical protein |
| BAB2_0547 | BAB_RS28955 | 2.0 | Sugar-binding periplasmic protein |
| BAB1_1794 | BAB_RS24460 | 2.0 | Leu/Ile/Val-binding protein homolog 1 |
Shown are transcripts that exhibited ≥4-fold differential levels (i.e., log2 fold change ≥ 2) between the two strains.
When the transcriptome data were evaluated at a 4-fold cutoff or greater, 4 mRNAs were observed to be increased, and 29 mRNAs were decreased in the rne-tnc strain (Table 1). Interestingly, the transcripts whose levels were the most dysregulated belong to genes potentially involved in metal homeostasis in Brucella, and all of these mRNAs were significantly reduced in the rne-tnc strain. The gene bab_rs17810 encodes a Fur family transcriptional regulator, ompW encodes an outer membrane protein, and mntH encodes the high-affinity manganese transporter. The transcripts for these genes were decreased 8.8-fold, 8.4-fold, and 7.0-fold, respectively. Reverse transcriptase quantitative PCR (qRT-PCR) analysis was employed to validate the dysregulation of these genes, and indeed, bab_rs17810, ompW, and mntH mRNA levels were all significantly decreased in the rne-tnc strain compared to the parental strain 2308 (Fig. 3).
FIG 3.
Confirmation of gene dysregulation in the rne truncation strain. RNA was isolated from B. abortus 2308 or the B. abortus rne truncation strain, and following reverse transcription, quantitative PCR was used to assess the levels of cDNA corresponding to mRNAs from specific genes related to metal homeostasis in B. abortus (bab_rs17810 [encoding a Fur family regulator], ompW, and mntH). 16S rRNA levels were assessed as a control, and all relative gene expression values were normalized to B. abortus 2308. The graphic bars depict the average level of the specified gene for each strain, and the error bars represent the standard error of the mean (SEM).
A small RNA called Bsr4 is more abundant in the B. abortus rne-tnc strain.
RNase E is linked to the proper expression of small regulatory RNAs (sRNAs) in some bacteria (21, 22), and therefore, we hypothesized that RNase E is required for the efficient control of sRNA levels in B. abortus. Several groups have demonstrated the presence of sRNAs in many Brucella strains; however, to date, very few Brucella sRNAs have been studied mechanistically. Nonetheless, to test the hypothesis, we screened a wide array of sRNAs that have been reported in the literature, and we determined that one sRNA, called Bsr4, was significantly more abundant in the rne-tnc strain than the parental strain 2308 (Fig. 4; Fig. S3).
FIG 4.

The stability of the small regulatory RNA Bsr4 is impacted by RNase E and Hfq in B. abortus. (A) Schematic representation of the bsr4 locus in B. abortus 2308. The gene locations are depicted using both BAB and BAB_RS locus tag identifiers, and bsr4 is denoted. The predicted functions of the products of the genes flanking bsr4 are also shown. (B) Northern blot analysis of Bsr4 in the rne truncation strain. RNA as isolated from B. abortus 2308, rne-tnc, or rne-RC. The RNAs were probed by Northern blotting analysis for levels of Bsr4 (upper panel) or AbcR (lower panel). (C) Northern blot analysis of Bsr4 in the absence of hfq. RNA as isolated from B. abortus 2308, rne-tnc, hfq, or rne-tnc/hfq, and the RNAs were probed by Northern blotting analysis for levels of Bsr4 (upper panel) or 5S rRNA (lower panel).
Bsr4 is encoded on chromosome 1 in B. abortus 2308 in the intergenic region between bab1_0839 and bab1_0842, encoding a DUF1467-containing protein and a proline-tRNA ligase, respectively (Fig. 4A). Northern blot analysis revealed that Bsr4 levels were significantly elevated in the rne-tnc strain compared to the parental strain 2308, and complementation of rne expression in the truncation strain returned Bsr4 to wild-type levels (Fig. 4B). Importantly, levels of the AbcR sRNAs were unaffected by RNase E. Additionally, Bsr4 levels fluctuated slightly during the growth of B. abortus in rich medium, but truncation of RNase E resulted in sustained high levels of Bsr4 throughout in vitro growth (Fig. S4).
The RNA chaperone Hfq facilitates interactions between mRNAs and sRNAs in many bacteria, including Brucella. Therefore, we assayed for Bsr4 levels in a B. abortus hfq deletion strain, as well as in a strain carrying both the rne truncation and the deletion of hfq (rne-tnc/hfq) (Fig. 4C). These Northern blot analyses demonstrated that deletion of hfq leads to the complete abolishment of Bsr4 levels, indicating that Hfq is required for the expression and/or stability of Bsr4 in B. abortus.
Bsr4 has been described previously in B. suis and B. melitensis, where it is referred to as BSnc118 and BSR1141, respectively (23–25). In B. melitensis, deletion of this sRNA was shown to decrease the virulence of the bacteria in a mouse model of infection (25). In order to determine the role of Bsr4 in the virulence of B. abortus, we constructed a bsr4 deletion strain, and this strain was assessed for its ability to infect macrophages and mice (Fig. 5). The bsr4 deletion strain exhibited a pattern of intracellular infection comparable to that of the parental strain 2308 in primary murine peritoneal macrophages (Fig. 5A), and similarly, there were no significant differences observed in spleen colonization of BALB/c mice infected with the bsr4 deletion strain or 2308 at 4 and 8 weeks postinfection (Fig. 5B).
FIG 5.

Bsr4 is dispensable for B. abortus infection of macrophages and experimentally infected mice. (A) Survival and replication of B. abortus strains in naive peritoneal macrophages. Peritoneal macrophages from BALB/c mice were infected with B. abortus 2308 or with B. abortus bsr4. At the indicated time points, the macrophages were lysed, and the number of intracellular brucellae present was enumerated following serial dilution and plating on agar medium. The data are presented as the average number of brucellae plus or minus the standard deviation of three replicate wells of macrophages infected with a specific Brucella strain. The results are from a representative experiment that was performed independently three times. (B) Spleen colonization of experimentally infected mice by B. abortus strains. BALB/c mice were infected intraperitoneally with B. abortus 2308 or with B. abortus bsr4. Mice were euthanized 4 and 8 weeks postinfection, and the number of brucellae colonizing the spleens of the mice was determined. The data are presented as the average number of brucellae plus or minus the standard deviation from 5 mice colonized with a specific Brucella strain at a particular time point. The data are from a single experiment using 5 mice per bacterial strain per time point (i.e., n = 5).
DISCUSSION
In the present study, we characterized the contribution of several RNases to the virulence of B. abortus. Regarding the ability of B. abortus to survive and replicate in macrophages, we determined that deletion of rnasePH or rnaseJ led to attenuation compared to the parental strain 2308 (Fig. 1A), but both RNase PH and RNase J were dispensable for the chronic colonization of experimentally infected mice (Fig. 1B). This is an interesting observation, as often defective macrophage survival and replication lead to attenuation in an animal model of infection. In this case, the rnasePH and rnaseJ deletion strains may be less able to cope with the hostile environment of the macrophage in the short term, such as in our cellular infection assay, but the strains may be able to overcome this initial shortcoming in order to efficiently colonize and persist in mice. Regarding the rnaseJ deletion strain, we observed a decrease in macrophage infection compared to the parental strain (Fig. 1A), but the strain was not defective in a mouse model of chronic Brucella infection (Fig. 1B). However, it was recently reported that a B. abortus rnaseJ mutant is attenuated in mice (26). Therefore, we included RNA from the rnaseJ deletion strain in our transcriptomic analyses, and the RNA-seq experiments revealed a limited number of transcripts that were dysregulated in the absence of rnaseJ (see Table S2 in the supplemental material). The observed differences included several tRNAs and transcripts encoding hypothetical proteins, but more work is needed to determine the link between RNase J, specific RNAs, and virulence in Brucella.
It is worth noting that in addition to the RNases that we describe in this study, we also generated a deletion strain of rnaseR, encoding RNase R (bab2_0635/BAB_RS29350), that was described previously (16). Similar to the previous findings, a B. abortus rnaseR deletion strain was not defective in its ability to survive and replicate in macrophages, nor was it attenuated in a mouse model of infection (data not shown). Moreover, we made several attempts to construct strains carrying a deletion or truncations of rnaseIII (bab1_0681/BAB_RS19195), as RNase III was biochemically characterized previously (17). However, we failed to generate strains harboring rnaseIII modifications, indicating that rnaseIII may be essential in B. abortus (data not shown). Indeed, Sternon et al. determined that rnaseIII is essential (20).
The data obtained during these studies clearly demonstrate that the endoribonuclease RNase E is critical for the colonization of mice by B. abortus (Fig. 1), and moreover, RNase E is involved in maintaining appropriate levels of a wide array of RNA transcripts (Table 1; Table S1). Strikingly, the mRNAs displaying the most reduced levels in the rne-tnc strain were those encoding proteins likely involved in metal homeostasis, including the Fur family regulator BAB_RS17910, the outer membrane protein OmpW, and the high-affinity manganese transporter MntH (Table 1; Fig. 3). It is still possible that decreased levels of specific mRNAs linked to metal homeostasis contribute to the reduction in virulence of the strain. For example, deletion of mntH severely attenuates B. abortus in animals, and thus, the significant reduction in mntH mRNA levels may play a part in the reduced virulence of the rne-tnc strain (27). However, for all of the RNAs that were identified as being dysregulated in the rne-tnc strain, the mechanism of RNase E-mediated RNA stability/turnover of these transcripts remains to be elucidated.
As noted earlier, rne is essential in B. abortus, and therefore, we generated a strain carrying a truncated allele of rnaseE (20). However, we are not the first to demonstrate that a deletion of a carboxy-terminal portion of rnaseE can be achieved in bacteria (28–32). In many bacteria, the carboxy-terminal region of RNase E functions as an interacting domain for proteins of the degradosome, while the amino-terminal portion of RNase E contains the catalytic domain (12). In several Gram-negative bacteria, such as E. coli, RNase E is the central component of the degradosome, where it performs the enzymatic cleavage of RNAs and also functions to bring together other proteins of the degradosome, including RhlB, PBPase, and enolase (33). However, the RNA helicase, RhlE, has also been reported to be part of the degradosome of Caulobacter crescentus, an alphaproteobacterium that is closely related to Brucella spp. (34). To date, the components of the Brucella degradosome remain to be defined. It is likely that RNase E serves an important role in the Brucella degradosome, and our group is currently carrying out experiments to identify and characterize the constituents of the Brucella degradosome.
Small regulatory RNAs are critical elements of bacterial gene expression, and several groups have identified sRNAs in Brucella spp. (23, 24, 35–41). Unfortunately, the nomenclature of Brucella sRNAs is not unified and is extremely difficult to navigate. In this study, we show a limited number of known Brucella sRNAs, and we have adopted “Bsr” nomenclature for “Brucella small RNA,” as the same sRNA may be present in several species (e.g., B. suis, B. abortus, and B. melitensis). For example, the sRNA BASRCI126 in B. abortus is the same sRNA from B. suis that is named BSnc159, and this sRNA is also found in B. melitensis (Fig. S3) (23, 40). Therefore, we have developed an sRNA Rosetta Stone of sorts to begin to organize Brucella sRNAs, and this database is freely available from our group. The database can be accessed at www.caswelllab.com. Our group will continue to maintain the most up-to-date list possible as literature is published in the area of Brucella sRNAs, and we are hopeful that this resource will be a useful tool for the community. More importantly, this database will assist in clarifying the nomenclature of sRNAs in Brucella spp.
Regarding a specific Brucella sRNA, we demonstrate here that Bsr4 is more abundant in the rne-tnc strain of B. abortus than in the parental strain 2308 and, moreover, that the RNA chaperone Hfq is required for Bsr4 stability (Fig. 4). Although deletion of bsr4 did not lead to attenuation of B. abortus during macrophage or mouse infections (Fig. 5), the lack of Bsr4 does not mirror what is occurring in the rnaseE-tnc strain. To further examine the impact of Bsr4 on pathogenesis, we are looking into generating a Brucella strain that constitutively expresses Bsr4. If this Bsr4 overexpression strain is attenuated in mice, we can conclude that this phenotype is RNase E independent and Bsr4 plays a role in the virulence of Brucella. We currently do not have information about specific genes controlled by Bsr4, but this work is ongoing in our laboratory. It should be noted that our infection data related to Bsr4 contrast with what has been demonstrated for the Bsr4 ortholog in B. melitensis, called BSR1141 (24, 25). The authors reported that deletion of bsr1141 led to a decreased infection compared to the wild-type strain in a mouse model of infection, while we report that deletion of bsr4 in B. abortus had no effect on virulence (Fig. 5). These differences may be due to the difference in Brucella species (B. melitensis versus B. abortus), or it is possible that differences in mouse strains or other variables account for the dissimilar phenotypes observed in these studies.
Another interesting point to underscore is the potential of Bsr4 to function in a “RyhB-like” manner. RyhB was first described in E. coli as an iron-responsive sRNA that regulates the expression of iron metabolism genes (42, 43). Subsequently, RyhB orthologs and functional homologs were identified in a number of bacteria, including Vibrio cholerae, Pseudomonas aeruginosa, Salmonella enterica, and Shigella dysenteriae (44–47). Given the metal gene dysregulation in the B. abortus rne-tnc strain and the significant elevation of Bsr4, it is tempting to speculate that Bsr4 may be a functional homolog of RyhB in B. abortus. However, at this time, this is merely conjecture, as much more work is needed to test this hypothesis.
In the end, this study highlights the importance of RNases in the biology and pathogenesis of Brucella spp., and more specifically, defines some of the regulatory pathways that link RNases, small regulatory RNAs, and virulence in the brucellae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Brucella abortus strains were grown on Schaedler agar (BD, Franklin Lakes, NJ) supplemented with 5% defibrinated bovine blood (Quad Five, Ryegate, MT) or in brucella broth (BD). For cloning, Escherichia coli strains (DH5α) were grown on tryptic soy agar (BD) or in Luria-Bertani (LB) broth. When appropriate, medium was supplemented with kanamycin (45 μg ml−1) or ampicillin (100 μg ml−1). All experiments utilizing Brucella strains were performed in a biosafety level 3 (BSL-3) facility.
Construction of Brucella abortus deletion and reconstruction strains.
The B. abortus rnasePH, hypothetical rnase, and rnaseJ deletion plasmids were constructed as described previously (18, 37, 48). Briefly, 1-kb fragments of the upstream and downstream regions of the genes were amplified by PCR using primers, genomic B. abortus 2308 DNA, and platinum Pfx polymerase (Invitrogen, Waltham, MA). All oligonucleotides listed in this study can be found in Table S3 in the supplemental material. The upstream fragments were digested with the BamHI restriction enzyme, and the downstream fragments were digested with PstI. Fragments were then phosphorylated with polynucleotide kinase (Monserate Biotechnology Group, San Diego, CA) and mixed in a single ligation mixture with BamHI/PstI-digested pNPTS138 and T4 DNA ligase (Monserate Biotechnology Group). The resulting plasmids, pLS033 (rnasePH), pLS032 (hyp rnase), and pLS031 (rnaseJ), were transformed into B. abortus 2308 via electroporation, and a merodiploid clone for each construct was obtained by selection with SBA with kanamycin. Kanamycin-resistant transformants were grown for 6 h in brucella broth and plated on SBA with 10% sucrose. Genomic DNA from sucrose-resistant, kanamycin-sensitive colonies was then isolated and screened via PCR for loss of the genes. The isogenic B. abortus deletions were named LS064 (ΔrnasePH), LS047 (Δhyp-rnase), and LS044 (ΔrnaseJ). Genotypes of mutant strains were confirmed by DNA sequence analysis. All plasmid and strains names in this study are listed in Table S4.
For the rne C terminus truncation (rne-tnc), mutagenesis was carried out as described above with several changes. An approximately 1.8-kb fragment of the rne gene (bp 1 to 1842) and a 1-kb fragment downstream of rne was amplified by PCR with primers, genomic B. abortus 2308 DNA, and platinum Pfx polymerase (Invitrogen). The fragment partially containing the rne gene was digested with BamHI, and the downstream region was digested with PstI. Fragments were then phosphorylated and added to a ligation mixture with BamHI/PstI-digested pNPTS138 and T4 DNA ligase (Monserate Biotechnology Group). The plasmid, pLS030, was then subjected to electroporation into B. abortus 2308, and mutagenesis was carried out as described above. The B. abortus rne truncation strain was given the designation LS042.
Reconstruction of rne-tnc was carried out as described above with modification. The wild-type rne gene was amplified using primers, genomic B. abortus DNA, and Taq polymerase (Monserate Biotechnology Group). The amplified fragments were then digested with the appropriate restriction enzymes and ligated into pNTPS138. The resulting plasmid, pLS034 (rne-RC), was electroporated into B. abortus LS042 (rne-tnc). Selection and screening were carried out as described above, and the reconstructed strain was named LS070 (rne-RC).
Deletion of the sRNA-encoding gene bsr4 was carried out similarly as described above. Briefly, 1-kb fragments of the upstream and downstream regions of bsr4 were amplified by PCR using the primers listed in Table S3, genomic B. abortus 2308 DNA, and platinum Pfx polymerase (Invitrogen, Waltham, MA). The upstream fragment was digested with BamHI, and the downstream fragment was digested with PstI. The fragments were then phosphorylated with polynucleotide kinase (Monserate Biotechnology Group, San Diego, CA) and mixed in a single ligation mixture with BamHI/PstI-digested pNPTS138 and T4 DNA ligase (Monserate Biotechnology Group). The resulting plasmid, pC3042, was transformed into B. abortus 2308 via electroporation, and selection and counterselection were carried out as described above.
RNA-sequencing analysis.
All RNA extractions were carried out as previously described (18, 37, 48). Brucella strains were grown to an optical density at 600 nm (OD600) of 0.15 with constant shaking at 37°C. An equal amount of 1:1 ethanol-acetone was added to cultures and stored at –80°C. RNA was then isolated by thawing the cell–ethanol-acetone mixtures and pelleted at 10,000 × g for 10 min. RNA was isolated from cells with TRIzol reagents (Invitrogen) followed by ethanol precipitation. Genomic DNA was removed with DNase I, and samples were cleaned up using phenol-chloroform extractions and precipitated with ethanol. RNA samples were then resuspended in nuclease-free H2O, and the purity of samples was checked with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). All samples had an A260/A280 ratio of ∼2.0 and a concentration yield of ∼1 μg μl−1. Ten micrograms of each RNA sample was submitted to the Biocomplexity Institute at Virginia Tech for RNA-seq analysis.
Following sequencing, data were trimmed for both adaptors and quality using a combination of ea-utils and Btrim (49, 50). Sequencing reads were then aligned to the genome using Tophat2/HISAT2 (51) and counted via HTSeq (52). Quality control (QC) summary statistics were examined to identify any problematic samples (e.g., total read counts, quality and base composition profiles [with or without trimming]), raw fastq formatted data files, aligned files (bam and text files containing sample alignment statistics), and count files (HTSeq text files). Following successful alignment, mRNA differential expression was determined using contrasts and tested for significance using the Benjamini-Hochberg corrected Wald test in the R-package DESeq2 (53).
Reverse transcriptase quantitative PCR (qRT-PCR).
Total RNA was isolated from B. abortus strains and treated with DNase I as described above. cDNA was generated from the final RNA preparation using a SuperScript III cDNA synthesis system (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol, and this cDNA was used for real-time PCR employing a SYBR green PCR supermix (Roche, Mannheim, Germany). For these experiments, primers for 16S rRNA were used as a control, and gene-specific primers were used for evaluating relative levels of mntH, ompW, and bab_rs17810 mRNAs. The steps for PCR consisted of a single denaturing step for 5 min at 95°C, followed by 40 cycles (denature for 15 s at 95°C, anneal for 15 s at 51°C, and extend for 15 s at 72°C) of amplification. Fluorescence from SYBR green incorporation into double-stranded DNA was measured with an iCycler machine (Bio-Rad), and the relative abundance of mRNA was determined using the Pfaffl equation (54).
Northern blot analysis.
RNA was isolated from Brucella cultures as described above. First, 15 μg of RNA was separated on a denaturing 10% polyacrylamide gel containing 7 M urea and 1× TBE (89 mM Tris-base, 89 mM boric acid, and 2 mM EDTA). To determine the size of the transcripts, a low-molecular-weight DNA ladder (New England BioLabs, Ipswich, MA) was labeled with [γ-32P]ATP (Perkin-Elmer, San Jose, CA) and polynucleotide kinase and separated on the polyacrylamide gel with RNA samples. Following electrophoresis, the ladder and RNA samples were transferred to an Amersham Hybond-N+ membrane (GE Healthcare, Piscataway, NJ) by electroblotting in 1× TBE buffer. The samples were then UV cross-linked to the membrane, and the membrane was prehybridized in ULTRAhyb oligonucleotide buffer (Ambion, Austin, TX) for 1 to 2 h at 45°C in a rotating hybridization oven. The oligonucleotide probes (Table S1) were end-labeled with [γ-32P]ATP and polynucleotide kinase. The radiolabeled probes were then incubated with the membranes overnight in the rotating hybridization oven. The following day, membranes were washed four times for 30 min each with 2× SSC (300 mM sodium chloride and 30 mM sodium citrate), 1× SSC, 0.5× SSC, and 0.025× SSC at 45°C in a rotating hybridization oven. Each SSC wash buffer contained 0.1% sodium dodecyl sulfate (SDS). Membranes were subsequently exposed to X-ray film and visualized via autoradiography.
Virulence of B. abortus strains in murine macrophages and experimentally infected mice.
To test the virulence of Brucella mutant strains, experiments utilizing peritoneal murine macrophages were carried out as previously described (18, 37, 48). Briefly, primary macrophages were harvested from mice and seeded into 96-well plates in Dulbecco’s modified Eagle’s medium with 5% fetal bovine serum. The next day, macrophages were infected with opsonized brucellae at a multiplicity of infection (MOI) of 50:1. Following incubation for 2 h, infected macrophages were treated with gentamicin (50 μg ml−1) for 1 h. Macrophages were then lysed with 0.1% deoxycholate in PBS, and serial dilutions were plated on SBA. For other time points, macrophages were washed with PBS following gentamicin treatment, and fresh cell culture medium containing gentamicin (20 μg ml−1) was added to the monolayer. At indicated time points, macrophages were lysed, serial diluted, and plated on SBA in triplicate.
The infection and colonization of mice by Brucella mutant strains was performed as previously described (18, 37, 48). Six-week-old female BALB/c mice (five mice per Brucella strain) were infected intraperitoneally with 5 × 104 CFU of each Brucella strain in PBS. Mice were then sacrificed at 4 and 8 weeks postinfection, spleens were homogenized, and serial dilutions were plated on SBA. All data were analyzed with the statistical software JMP 11.0.0 (SAS Institute, Cary, NC).
Data availability.
The Gene Expression Omnibus (GEO) accession number for the RNA-seq data is GSE155009.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the American Heart Association (15SDG23280044) and the National Institute of Allergy and Infectious Diseases (AI117648).
We are very grateful for the extremely helpful and constructive comments by Kevin Wang.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Condon C, Putzer H. 2002. The phylogenetic distribution of bacterial ribonucleases. Nucleic Acids Res 30:5339–5346. doi: 10.1093/nar/gkf691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui MP, Foley PL, Belasco JG. 2014. Messenger RNA degradation in bacterial cells. Annu Rev Genet 48:537–559. doi: 10.1146/annurev-genet-120213-092340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson AW. 1999. Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiol Rev 23:371–390. doi: 10.1111/j.1574-6976.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 4.Deutscher MP. 2015. How bacterial cells keep ribonucleases under control. FEMS Microbiol Rev 39:350–361. doi: 10.1093/femsre/fuv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matos RG, Casinhas J, Bárria C, Dos Santos RF, Silva IJ, Arraiano CM. 2017. The role of ribonucleases and sRNAs in the virulence of foodborne pathogens. Front Microbiol 8:910. doi: 10.3389/fmicb.2017.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher MP. 2015. Twenty years of bacterial RNases and RNA processing: how we’ve matured. RNA 21:597–600. doi: 10.1261/rna.049692.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viegas SC, Pfeiffer V, Sittka A, Silva IJ, Vogel J, Arraiano CM. 2007. Characterization of the role of ribonucleases in Salmonella small RNA decay. Nucleic Acids Res 35:7651–7664. doi: 10.1093/nar/gkm916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viegas SC, Arraiano CM. 2008. Regulating the regulators: how ribonucleases dictate the rules in the control of small non-coding RNAs. RNA Biol 5:230–243. doi: 10.4161/rna.6915. [DOI] [PubMed] [Google Scholar]
- 9.Viegas SC, Silva IJ, Saramago M, Domingues S, Arraiano CM. 2011. Regulation of the small regulatory RNA MicA by ribonuclease III: a target-dependent pathway. Nucleic Acids Res 39:2918–2930. doi: 10.1093/nar/gkq1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arraiano CM, Andrade JM, Domingues S, Guinote IB, Malecki M, Matos RG, Moreira RN, Pobre V, Reis FP, Saramago M, Silva IJ, Viegas SC. 2010. The critical role of RNA processing and degradation in the control of gene expression. FEMS Microbiol Rev 34:883–923. doi: 10.1111/j.1574-6976.2010.00242.x. [DOI] [PubMed] [Google Scholar]
- 11.Hammarlöf DL, Bergman JM, Garmendia E, Hughes D. 2015. Turnover of mRNAs is one of the essential functions of RNase E. Mol Microbiol 98:34–45. doi: 10.1111/mmi.13100. [DOI] [PubMed] [Google Scholar]
- 12.Mackie GA. 2013. RNase E: at the interface of bacterial RNA processing and decay. Nat Rev Microbiol 11:45–57. doi: 10.1038/nrmicro2930. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Groisman EA. 2010. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol Microbiol 76:1020–1033. doi: 10.1111/j.1365-2958.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viegas SC, Mil-Homens D, Fialho AM, Arraiano CM. 2013. The virulence of Salmonella enterica Serovar Typhimurium in the insect model Galleria mellonella is impaired by mutations in RNase E and RNase III. Appl Environ Microbiol 79:6124–6133. doi: 10.1128/AEM.02044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiano CA, Lathem WW. 2012. Post-transcriptional regulation of gene expression in Yersinia species. Front Cell Infect Microbiol 2:129. doi: 10.3389/fcimb.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi A, Rosinha GM, Camargo IL, Trant CM, Cardoso FC, Azevedo V, Oliveira SC. 2007. The role of the vacB gene in the pathogenesis of Brucella abortus. Microbes Infect 9:375–381. doi: 10.1016/j.micinf.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Wu CX, Xu XJ, Zheng K, Liu F, Yang XD, Chen CF, Chen HC, Liu ZF. 2016. Characterization of ribonuclease III from Brucella. Gene 579:183–192. doi: 10.1016/j.gene.2015.12.068. [DOI] [PubMed] [Google Scholar]
- 18.Budnick JA, Sheehan LM, Colquhoun JM, Dunman PM, Walker GC, Roop RM II, Caswell CC. 2018. Endoribonuclease YbeY is linked to proper cellular morphology and virulence in Brucella abortus. J Bacteriol 200:e00105-18. doi: 10.1128/JB.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton BE, Herrou J, Blackwell AE, Wysocki VH, Crosson S. 2012. Molecular structure and function of the novel BrnT/BrnA toxin-antitoxin system of Brucella abortus. J Biol Chem 287:12098–12110. doi: 10.1074/jbc.M111.332163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sternon JF, Godessart P, Gonçalves de Freitas R, Van der Henst M, Poncin K, Francis N, Willemart K, Christen M, Christen B, Letesson JJ, De Bolle X. 2018. Transposon sequencing of Brucella abortus uncovers essential genes for growth in vitro and inside macrophages. Infect Immun 86:e00312-18. doi: 10.1128/IAI.00312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalaouna D, Simoneau-Roy M, Lafontaine D, Massé E. 2013. Regulatory RNAs and target mRNA decay in prokaryotes. Biochim Biophys Acta 1829:742–747. doi: 10.1016/j.bbagrm.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Saramago M, Bárria C, Dos Santos RF, Silva IJ, Pobre V, Domingues S, Andrade JM, Viegas SC, Arraiano CM. 2014. The role of RNases in the regulation of small RNAs. Curr Opin Microbiol 18:105–115. doi: 10.1016/j.mib.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Saadeh B, Caswell CC, Chao Y, Berta P, Wattam AR, Roop RM II, O’Callaghan D. 2016. Transcriptome-wide identification of Hfq-associated RNAs in Brucella suis by deep sequencing. J Bacteriol 198:427–435. doi: 10.1128/JB.00711-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ke Y, Xu J, Wang L, Wang T, Liang H, Zhang W, Gong C, Yuan J, Zhuang Y, An C, Lei S, Du X, Wang Z, Li W, Yuan X, Huang L, Yang X, Chen Z. 2015. Identification of a novel small non-coding RNA modulating the intracellular survival of Brucella melitensis. Front Microbiol 6:164. doi: 10.3389/fmicb.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Ke Y, Duan C, Ma X, Hao Q, Song L, Guo X, Sun T, Zhang W, Zhang J, Zhao Y, Zhong Z, Yang X, Chen Z. 2019. A small non-coding RNA facilitates Brucella melitensis intracellular survival by regulating the expression of virulence factor. Int J Med Microbiol 309:225–231. doi: 10.1016/j.ijmm.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Tian M, Qu J, Li P, Bao Y, Liu J, Ding C, Wang S, Li T, Qi J, Yu S. 2019. Identification of novel genes essential for Brucella abortus to establish infection by signature-tagged mutagenesis. Vet Microbiol 230:130–137. doi: 10.1016/j.vetmic.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS, Roop RM II. 2009. The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect Immun 77:3466–3474. doi: 10.1128/IAI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kido M, Yamanaka K, Mitani T, Niki H, Ogura T, Hiraga S. 1996. RNase E polypeptides lacking a carboxyl-terminal half suppress a mukB mutation in Escherichia coli. J Bacteriol 178:3917–3925. doi: 10.1128/jb.178.13.3917-3925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaberdin VR, Miczak A, Jakobsen JS, Lin-Chao S, McDowall KJ, von Gabain A. 1998. The endoribonucleolytic N-terminal half of Escherichia coli RNase E is evolutionarily conserved in Synechocystis sp. and other bacteria but not the C-terminal half, which is sufficient for degradosome assembly. Proc Natl Acad Sci U S A 95:11637–11642. doi: 10.1073/pnas.95.20.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pobigaylo N, Wetter D, Szymczak S, Schiller U, Kurtz S, Meyer F, Nattkemper TW, Becker A. 2006. Construction of a large signature-tagged mini-Tn5 transposon library and its application to mutagenesis of Sinorhizobium meliloti. Appl Environ Microbiol 72:4329–4337. doi: 10.1128/AEM.03072-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura M, Moore CJ, Cohen SN. 2013. Nutrient dependence of RNase E essentiality in Escherichia coli. J Bacteriol 195:1133–1141. doi: 10.1128/JB.01558-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgardt K, Charoenpanich P, McIntosh M, Schikora A, Stein E, Thalmann S, Kogel KH, Klug G, Becker A, Evguenieva-Hackenberg E. 2014. RNase E affects the expression of the acyl-homoserine lactone synthase gene sinI in Sinorhizobium meliloti. J Bacteriol 196:1435–1447. doi: 10.1128/JB.01471-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpousis AJ. 2007. The RNA degradosome of Escherichia coli: an mRNA degrading machine assembled on RNase E. Annu Rev Microbiol 61:71–87. doi: 10.1146/annurev.micro.61.080706.093440. [DOI] [PubMed] [Google Scholar]
- 34.Aguirre AA, Vicente AM, Hardwick SW, Alvelos DM, Mazzon RR, Luisi BF, Marques MV. 2017. Association of the cold shock DEAD-box RNA helicase RhlE to the RNA degradosome in Caulobacter crescentus. J Bacteriol 199:e00135-17. doi: 10.1128/JB.00135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottesman S. 2004. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu Rev Microbiol 58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- 36.Wagner EG, Romby P. 2015. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv Genet 90:133–208. doi: 10.1016/bs.adgen.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Caswell CC, Gaines JM, Ciborowski P, Smith D, Borchers CH, Roux CM, Sayood K, Dunman PM, Roop RM II. 2012. Identification of two small regulatory RNAs linked to virulence in Brucella abortus 2308. Mol Microbiol 85:345–360. doi: 10.1111/j.1365-2958.2012.08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu D, Song J, Li G, Cai W, Zong S, Li Z, Liu W, Hu S, Bu Z. 2018. A novel small RNA Bmsr1 enhances virulence in Brucella melitensis M28. Vet Microbiol 223:1–8. doi: 10.1016/j.vetmic.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhong Z, Xu X, Li X, Liu S, Lei S, Yang M, Yu J, Yuan J, Ke Y, Du X, Wang Z, Ren Z, Peng G, Wang Y, Chen Z. 2016. Large-scale identification of small noncoding RNA with strand-specific deep sequencing and characterization of a novel virulence-related sRNA in Brucella melitensis. Sci Rep 6:25123. doi: 10.1038/srep25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong H, Peng X, Wang N, Wu Q. 2014. Identification of novel sRNAs in Brucella abortus 2308. FEMS Microbiol Lett 354:119–125. doi: 10.1111/1574-6968.12433. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Dong H, Wu Q. 2015. A new cis-encoded sRNA, BsrH, regulating the expression of hemH gene in Brucella abortus 2308. FEMS Microbiol Lett 362:1–7. doi: 10.1093/femsle/fnu017. [DOI] [PubMed] [Google Scholar]
- 42.Massé E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A 99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massé E, Escorcia FE, Gottesman S. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev 17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis BM, Quinones M, Pratt J, Ding Y, Waldor MK. 2005. Characterization of the small untranslated RNA RyhB and its regulon in Vibrio cholerae. J Bacteriol 187:4005–4014. doi: 10.1128/JB.187.12.4005-4014.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML. 2004. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci U S A 101:9792–9797. doi: 10.1073/pnas.0403423101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellermeier JR, Slauch JM. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190:476–486. doi: 10.1128/JB.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy ER, Payne SM. 2007. RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect Immun 75:3470–3477. doi: 10.1128/IAI.00112-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheehan LM, Caswell CC. 2017. A 6-nucleotide regulatory motif within the AbcR small RNAs of Brucella abortus mediates host-pathogen interactions. mBio 8:e00473-17. doi: 10.1128/mBio.00473-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aronesty E. 2011. ea-utils: Command-line tools for processing biological sequencing data. https://github.com/ExpressionAnalysis/ea-utils.
- 50.Kong Y. 2011. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98:152–153. doi: 10.1016/j.ygeno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anders S, Pyl PT, Huber W. 2015. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Gene Expression Omnibus (GEO) accession number for the RNA-seq data is GSE155009.